-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHost Defense via Symbiosis in
article has not abstract
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003808
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003808Summary
article has not abstract
Drosophila and Their Defensive Symbionts
Host-associated microbes have often been studied as pathogens and the causes of disease, but symbiotic microbes that benefit their hosts are now known to be ubiquitous. In particular, insects possess a diversity of bacteria that can defend against natural enemies—Anopheles mosquitoes, for example, were recently shown to host a gut bacterium that confers refractoriness to malaria parasites [1]. In Drosophila, a key model of infection and immunity, fascinating examples of defense are accumulating, and two lineages of bacteria that infect the genus are now known to be defensive: Wolbachia and Spiroplasma (Figure 1). Both are vertically transmitted, both are facultative in Drosophila in that they are not strictly required by the host, and both infect Drosophila melanogaster. Here, we summarize what is known of Drosophila as an intriguing and emerging model of defensive symbiosis.
Fig. 1. The inherited symbionts of <i>Drosophila</i>. 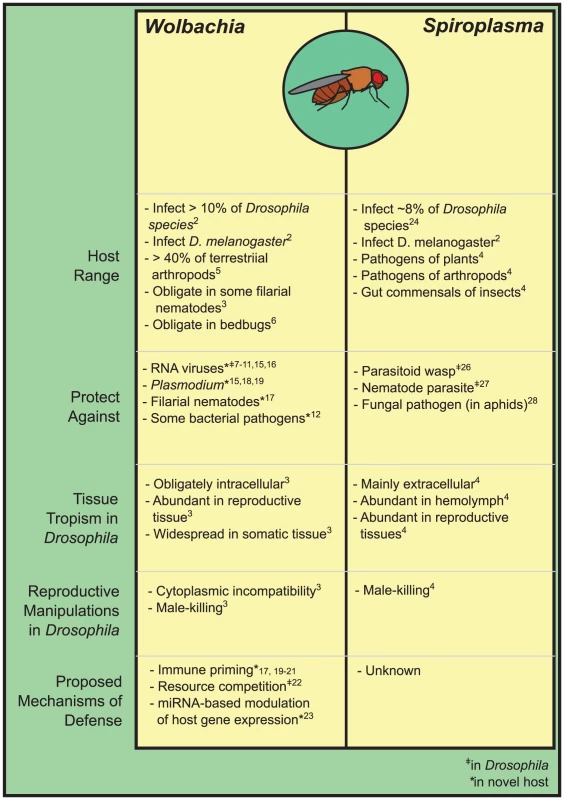
Drosophila is an incredibly diverse genus with thousands of species, many of which are infected by Wolbachia and Spiroplasma [2]. As maternally transmitted symbionts, Wolbachia and Spiroplasma came to attention in Drosophila through their ability to manipulate host reproduction to favor their own transmission. Wolbachia are notorious for doing this by inducing cytoplasmic incompatibility (CI), whereby matings between Wolbachia-infected males and uninfected females result in the production of few to no offspring [3], providing selective pressure to maintain and rapidly spread Wolbachia in host populations. Though Spiroplasma are not known to induce CI, both Spiroplasma and Wolbachia can selfishly distort host sex ratios through male-killing in Drosophila, selectively killing the male offspring of infected females [3],[4]. Many strains of Wolbachia and Spiroplasma, though, do not have such manipulative tendencies, and it has largely been a mystery how they are maintained in host populations. The discovery that they can defend against enemies has gone a long way in explaining their persistence, and has begun to shift our perception of many facultative inherited symbionts from that of manipulative parasites toward helpful mutualists.
Wolbachia: The Master Manipulator
Wolbachia are gram-negative α-proteobacteria and are the most widespread and probably best-studied of insect symbionts, infecting upwards of 40% of arthropod species [5]. Most Wolbachia are facultative insect symbionts, but the genus is ancient and divergent lineages are obligate symbionts of bedbugs and filarial worms (e.g., [3],[6]). In Drosophila, screens have uncovered Wolbachia in 8–12% of species, although this probably underrepresents the true frequency of infection [2].
Defensive properties of Wolbachia were discovered when screens of D. melanogaster for resistance to RNA viruses uncovered an association between resistance and Wolbachia infection, with Wolbachia inhibiting viral replication and decreasing virus-induced mortality [7],[8]. Strains of Wolbachia have since been shown to defend D. melanogaster, D. simulans, and D. innubila against multiple RNA viruses [7]–[11]. As yet, though, Wolbachia are not known to defend against other enemies of Drosophila, with a lack of defense demonstrated against DNA viruses [7], bacterial pathogens [12],[13], and parasitoid wasps [14]. It remains possible however, that Wolbachia may defend against some of these parasites or pathogens in the wild, as laboratory challenges do not always use pathogens that naturally infect Drosophila (e.g., the DNA virus used in [7]).
Spreading the Love: Transforming Disease Vectors with Wolbachia
In contrast to Wolbachia's limited defensive effects in Drosophila, strains introduced from Drosophila into Aedes and Anopheles mosquitoes (“heterologous” infections) more broadly inhibit the development of diverse parasites and pathogens. These include dengue virus, chikungunya virus, Plasmodium spp., and filarial nematodes [15]–[19]—suggesting the possibility of transforming vectors to limit transmission of human disease. Wolbachia also causes CI in these novel hosts, providing a ready mechanism for the drive of Wolbachia and defensive traits into naïve vector populations. This possibility has spurred interest in the mechanistic basis of Wolbachia's defense, but has also led to a focus on heterologous infections in mosquitoes rather than native infections in Drosophila.
In these novel hosts, it is commonly observed that Wolbachia causes a barrage of immunological effects that are apparently responsible for defense: these include the induction of the Toll and other host immune pathways, and the production of toxic reactive oxygen species (e.g., [15],[18]–[20]), and are typically accompanied by substantial cost to the host (i.e., Wolbachia infections are virulent) (e.g., [19]). Conversely, such effects are absent or attenuated in native Drosophila infections that are also defensive [21], raising the question of whether the same defensive mechanisms underlie Wolbachia's effects in native and novel hosts, and whether the dramatic immune induction observed in novel mosquito hosts is the cause of defense, a corollary of a new and virulent Wolbachia infection, or both—but as yet it is essentially completely unknown how Drosophila are defended. Recently though, Wolbachia competition with RNA viruses for cholesterol has been argued to contribute to defense in D. melanogaster [22], while Wolbachia modulation of a host miRNA to regulate a methyltransferase appears to contribute to viral defense in Aedes [23]. Studies have also observed that levels of defense are related to Wolbachia density—for example, the protection conferred by Wolbachia that naturally infect Drosophila simulans is limited to strains that achieve higher densities within the host [9], suggesting that competitive effects or tissue tropism (e.g., overlap in symbiont and parasite infection in host tissues) may be important. Further work is clearly needed to untangle the relative contributions of divergent mechanisms to defense, both in Drosophila and in novel hosts that we might seek to transform.
Based on findings of defense and the recapitulation of CI in mosquitoes, researchers have engineered the mass release of Wolbachia-infected Aedes mosquitoes in Australia to limit the transmission of dengue, and observed the rapid spread of Wolbachia through CI [24], serving as a proof-of-concept of vector transformation with Wolbachia. While exciting, it remains to be seen how the incidence of dengue in humans will be affected in the study area.
Wolbachia have also been introduced into the anopheline vectors of malaria, which they do not naturally infect, and can substantially inhibit the development of Plasmodium falciparum in Anopheles gambiae [17]. However, efforts at using Wolbachia in the biocontrol of malaria have been hampered by difficulty in establishing infections that are efficiently vertically transmitted. Intriguingly though, a Wolbachia strain native to Aedes albopictus has recently been introduced into Anopheles stephensii; in this host it inhibits Plasmodium development, is vertically transmitted with high fidelity, and induces CI, suggesting that biocontrol methods using Wolbachia in Anopheles could be on the horizon [18]. Still, this infection is highly virulent, substantially decreasing the hatch rate of host eggs [18], and whether CI traits are strong enough to offset such costs to maintain Wolbachia in this host in the long term is unclear.
Spiroplasma: Under the Radar
Spiroplasma are cell-wall-less gram-positive bacteria of the class Mollicutes, and are known as plant and arthropod pathogens, and gut commensals and inherited symbionts in insects [3]. In Drosophila, Spiroplasma are primarily known as male-killers and are widespread, infecting ∼8% of screened species [25].
Different Spiroplasma strains have now been shown to be defensive in two Drosophila species [26],[27], as well as in aphids [28], in what appear to be the first examples of Spiroplasma behaving mutualistically. In Drosophila hydei, Spiroplasma increases the survival rate of flies attacked by a parasitoid wasp [26]. In Drosophila neotestacea, Spiroplasma decreases the size and transmission of a common and virulent nematode parasite of the fly, and restores the fertility of nematode-parasitized flies that are normally sterilized by infection [27]. This strong protective effect has lead to Spiroplasma's rapid continent-wide spread through North American D. neotestacea in recent decades [29]. This Spiroplasma strain causes no reproductive manipulation, apparently relying solely on the selective advantage of the defense it confers to spread, providing one of the more compelling examples of the importance of defensive symbioses in the wild.
Little is known of the mechanisms by which Spiroplasma provide defense, but Spiroplasma are phylogenetically distant from Wolbachia, underscoring many differences in their biology. Spiroplasma typically occur extracellularly in the host hemolymph while Wolbachia are predominately intracellular, and Spiroplasma cause little apparent immune activation in their hosts [30],[31]. Spiroplasma may be more susceptible to immune effectors active in the host hemolymph due to their extracellular lifestyle, and thus under selection to avoid or even suppress host immune activation (e.g., [30]). Mechanisms other than host immune priming might therefore account for Spiroplasma-mediated defense. In other defensive symbioses, symbiont-encoded or associated toxins have been implicated [32], and it remains possible that toxins are involved in Spiroplasma defensive symbioses.
A recent study also found that D. melanogaster infection by a male-killing Spiroplasma actually increased mortality from infection by a gram-negative pathogen, while not affecting host survival after challenge by gram-positive or fungal pathogens, demonstrating the complexity and contingency of interactions between hosts, symbionts, and enemies in heritable symbioses [32]. The co-occurrence of Wolbachia and Spiroplasma in the same Drosophila species, and even in the same individuals, provides a powerful opportunity for comparative study of the factors underlying apparently independently evolved defensive symbioses in order to untangle some of this complexity.
Conclusions and Perspectives
The recent surge of interest in defensive symbioses of insects has roots in our growing awareness of the importance of host microbiomes to health and disease, and in our desire to control disease through engineering the frequency or defensive characteristics of insect symbionts. Despite our yet-limited understanding of specific mechanisms underlying defense, the goal of using symbionts as biological control agents points to unresolved questions regarding the nature of defensive symbiosis, typified by Wolbachia's incongruent effects in heterologous and native infections.
In novel mosquito hosts, upregulation of immune pathways by Wolbachia appears to entail increased resistance to diverse infectious agents. In native Drosophila hosts, defense appears specific and finely tuned by natural selection, possibly conferring a selective advantage that maintains symbiont infection in host lineages. Understanding whether these observations comprise qualitatively different means of defense or are only superficially divergent and rely on the same underlying mechanisms will be necessary. It also raises the question of the specificity of defense, both in different hosts and against different enemies. Mechanism will also have consequences for evolutionary stability; for instance, symbionts that achieve defense through immune priming with high collateral cost to the host will place strong selective pressure not only natural enemies, but also on the host and symbiont to suppress defensive traits.
If defense does in fact turn out to be a relatively nonspecific consequence of symbiont infection, it raises the question: what infection isn't defensive? Interactions between coinfecting parasites and pathogens abound, some positive, some negative (e.g., [33]). Defining any infection with a negative effect on a coinfection as defensive may not ultimately reflect relationships well. Nonetheless, symbiont-mediated defense in Drosophila can be both adaptive and ecologically relevant [26]. Deciding if a symbiont should be considered defensive will require integrating an understanding of the strength of symbiont-mediated protection and costs of symbiont infection with the frequency and effects of parasites and pathogens in the wild. Unfortunately, such data are often lacking.
Other microbes may also be important. In bumblebees, gut microbes can protect against trypanosomatid parasites [34], and, as mentioned, a gut bacterium of Anopheles provides refractoriness to Plasmodium [1]. That such defensive effects result from members of the Drosophila microbiome seems probable, but their study is only in its infancy. Still, in the past decade, great strides have been made in understanding the importance, evolutionary consequences, and possible mechanisms of defensive symbioses. We hope that further work in Drosophila will continue to drive this trend.
Zdroje
1. CirimotichCM, DongY, ClaytonAM, SandifordSL, Souza-NetoJA, et al. (2011) Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332 : 855–858.
2. MateosM, CastrezanaSJ, NankivellBJ, EstesAM, MarkowTA, et al. (2006) Heritable endosymbionts of Drosophila. Genetics 174 : 363–376.
3. WerrenJ, BaldoL, ClarkM (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiology 6 : 741–751.
4. AnbutsuH, FukatsuT (2011) Spiroplasma as a model insect endosymbiont. Env Microb Reports 3 : 144–153.
5. ZugR, HammersteinP (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7: e38544 doi:10.1371/journal.pone.0038544
6. HosokawaT, KogaR, KikuchiY, MengX-Y, FukatsuT (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A 107 : 769–774.
7. TeixeiraL, FerreiraÁ, AshburnerM (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e1000002 doi:10.1371/journal.pbio.1000002
8. HedgesLM, BrownlieJC, O'NeillSL, JohnsonKN (2008) Wolbachia and virus protection in insects. Science 322 : 702.
9. OsborneSE, LeongYS, O'NeillSL, JohnsonKN (2009) Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5: e1000656 doi:10.1371/journal.ppat.1000656
10. UncklessRL, JaenikeJ (2011) Maintenace of a male-killing Wolbachia in Drosophila by male-killing dependent and male-killing independent mechanisms. Evolution 66 : 678–690.
11. GlaserRL, MeolaMA (2010) The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resitance to West Nile Virus infection. PLoS ONE 5: e11977 doi:10.1371/journal.pone.0011977
12. WongZS, HedgesLM, BrownlieJC, JohnsonKN (2012) Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS ONE 6: e25430 doi:10.1371/journal.pone.0025430
13. YeYH, WoolfitM, RancèsE, O'NeillSL, McGrawEA (2013) Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Negl Trop Dis 7: e2362 doi:10.1371/journal.pntd.0002362
14. LongdonB, FabianDK, HurstGD, JigginsFM (2012) Male-killing Wolbachia do not protect Drosophila bifasciata against viral infection. BMC Microbiol 122(s1): S8.
15. MoreiraLA, Iturbe-OrmaetxeI, JefferyJA, LuG, PykeAT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139 : 1268–1278.
16. BianG, XuY, LuP, XieY, XiZ (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833 doi:10.1371/journal.ppat.1000833
17. KambrisZ, CookPE, PhucHK, SinkinsSP (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326 : 134–136.
18. HughesGL, KogaR, XueP, FukatsuT, RasgonJL (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7: e1002043 doi:10.1371/journal.ppat.1002043
19. BianG, JoshiD, DongY, LuP, ZhouG, et al. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340 : 748–751.
20. PanX, ZhouG, WuJ, BianG (2012) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109: E23–31.
21. RancèsE, YeYH, WoolfitM, McGrawEA, O'NeillSL (2012) The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8: e1002548 doi:10.1371/journal.ppat.1002548
22. CaragataEP, RancèsE, HedgesLM, GoftonAW, JohnsonKN, et al. (2013) Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9: e1003459 doi:10.1371/journal.ppat.1003459
23. ZhangG, HussainM, O'NeillSL, AsgariS (2013) Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci U S A 110 : 10276–10281.
24. HoffmannAA, MontgomeryBL, PopoviciJ, Iturbe-OrmaetxeI, JohnsonPH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 : 454–457.
25. WattsT, HaselkornTS, MoranNA, MarkowTA (2009) Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS ONE 4: e5703 doi:10.1371/journal.pone.0005703
26. XieJ, VilchezI, MateosM (2010) Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 5: e12149 doi:10.1371/journal.pone.0012149
27. JaenikeJ, UncklessR, CockburnSN, BoelioLM, PerlmanSJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329 : 212–215.
28. ŁukasikP, van AschM, GuoH, FerrariJ, GodfrayHCJ (2013) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16 : 214–218.
29. CockburnSN, HaselkornTS, HamiltonPT, LandzbergE, JaenikeJ, et al. (2013) Dynamics of the continent-wide spread of a Drosophila defensive symbiont. Ecol Lett 16 : 609–616.
30. HutchenceKJ, FischerB, PatersonS, HurstGDD (2011) How do insects react to novel inherited symbionts? A microarray analysis of Drosophila melanogaster response to the presence of natural and introduced Spiroplasma. Mol Ecol 20 : 950–958.
31. HerrenJK, LemaitreB (2011) Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol 13 : 1385–1396.
32. MoranNA, DegnanPH, SantosSR, DunbarHE, OchmanH (2005) The players in a mutualistic symbiosis: Insects, bacteria, viruses, and virulence genes. Proc Natl Acad Sci U S A 102 : 16919–16926.
33. TelferS, LambinX, BirtlesR, BeldomenicoP, BurtheS, et al. (2010) Species interactions in a parasite community drive infection risk in a wildlife population. Science 330 : 243–246.
34. KochH, Schmid-HempelP (2011) Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci U S A 108 : 19288–19292.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



