-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
Vertebrate females transfer antibodies via the placenta, colostrum and milk or via the egg yolk to protect their immunologically immature offspring against pathogens. This evolutionarily important transfer of immunity is poorly documented in invertebrates and basic questions remain regarding the nature and extent of parental protection of offspring. In this study, we show that a lipopolysaccharide binding protein/bactericidal permeability increasing protein family member from the invertebrate Biomphalaria glabrata (BgLBP/BPI1) is massively loaded into the eggs of this freshwater snail. Native and recombinant proteins displayed conserved LPS-binding, antibacterial and membrane permeabilizing activities. A broad screening of various pathogens revealed a previously unknown biocidal activity of the protein against pathogenic water molds (oomycetes), which is conserved in human BPI. RNAi-dependent silencing of LBP/BPI in the parent snails resulted in a significant reduction of reproductive success and extensive death of eggs through oomycete infections. This work provides the first functional evidence that a LBP/BPI is involved in the parental immune protection of invertebrate offspring and reveals a novel and conserved biocidal activity for LBP/BPI family members.
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003792
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003792Summary
Vertebrate females transfer antibodies via the placenta, colostrum and milk or via the egg yolk to protect their immunologically immature offspring against pathogens. This evolutionarily important transfer of immunity is poorly documented in invertebrates and basic questions remain regarding the nature and extent of parental protection of offspring. In this study, we show that a lipopolysaccharide binding protein/bactericidal permeability increasing protein family member from the invertebrate Biomphalaria glabrata (BgLBP/BPI1) is massively loaded into the eggs of this freshwater snail. Native and recombinant proteins displayed conserved LPS-binding, antibacterial and membrane permeabilizing activities. A broad screening of various pathogens revealed a previously unknown biocidal activity of the protein against pathogenic water molds (oomycetes), which is conserved in human BPI. RNAi-dependent silencing of LBP/BPI in the parent snails resulted in a significant reduction of reproductive success and extensive death of eggs through oomycete infections. This work provides the first functional evidence that a LBP/BPI is involved in the parental immune protection of invertebrate offspring and reveals a novel and conserved biocidal activity for LBP/BPI family members.
Introduction
The existence of complex immune systems implies that interactions with pathogens represent major selective forces shaping the evolution of animal and plant species [1]. Vertebrate immune systems not only protect the adult organism against infections but also increase reproductive success through parental transfer of innate and adaptive immune factors via the placenta, colostrum and milk or via the egg yolk [2]–[4]. This maternal transfer of immunity is critical for species survival as embryos and neonates are immunologically immature and unable to fight off infections at early life stages. Parental transfer of protection has also been found in invertebrates hosting mutualists and many vertically transmitted arthropod symbionts are able to protect offspring against specific infections [5], [6]. Despite the impressive advances recently made in characterizing invertebrate immune systems [7], [8], data on the nature of the symbiont-mediated or parentally transmitted protection across generations are scarce [9]–[11]. How the estimated 1.3 million of invertebrate species [12] protect their offspring against pathogens remains therefore an intriguing question.
The freshwater snail Biomphalaria glabrata is particularly well studied as it is the intermediate host of the human blood fluke Schistosoma mansoni, responsible for schistosomiasis affecting millions of people in developing countries [13]. Biomphalaria snails live in various resting water biotopes such as, ponds, marshes, irrigation channels or open sewer drains that are particularly rich in pathogenic organisms. Egg masses are laid on solid substrates under water where they remain for approximately a week before hatching [14]. In a proteomic study on the content of B. glabrata egg masses, 16 defense-related polypeptides were partially identified, among which a lipopolysaccharide binding protein/bactericidal permeability increasing protein (LBP/BPI) representing a major protein band [15]. LBP/BPIs are structurally related proteins belonging to the lipid transfer/binding protein (LT/LBP) family [16], which represent important components of the innate immune system against Gram-negative bacterial infections [17]. In mammals, LBPs and BPIs have been extensively studied due to their role in regulating transducing cellular signals from Lipopolysaccharide (LPS) [18], [19]. LBP functions as a carrier of LPS monomers onto CD14 and together with the TLR4-MD2 receptor complex, mediates the activation of monocytes and macrophages, which produce inflammatory mediators [20]. BPI is an antibacterial protein specifically active against Gram-negative bacteria that acts by damaging bacterial membranes [21]. BPI also enhances adaptive immune responses by promoting LPS uptake and presentation to dendritic cells [22]. Although these two proteins present similarities in sequence and activities, they exert different effects on interactions of the host with Gram-negative bacteria [23]. BPI neutralizes the inflammatory properties of LPS decreasing its uptake by LBP whereas LBP is an acute phase protein with LPS-dependent cell stimulatory activity [24], [25]. These antagonist functions efficiently regulate host response to bacterial invasion and allow the host immune system to return to its normal resting state.
The distinction between LBPs and BPIs has not been established in invertebrates. LBP/BPI family members have been reported only in a few invertebrate phyla such as annelids [26] and molluscs [27], [28]. To date, a single functional study of LBP/BPI has been performed, showing that the oyster Crassostrea gigas expresses a BPI-like protein endowed with the conserved LPS-binding and bacterial permeability increasing activity [27].
As B. glabrata snails apparently heavily invest in the production of LBP/BPI in their eggs [15], we investigated whether this protein showed the expected anti-bacterial activity and whether it could provide protection against other pathogens.
Results
BgLBP/BPI1 characterization
We first characterized the complete coding sequence of BgLBP/BPI1 (genbank accession number KC206037) from a partial transcript that we had previously identified in an albumen gland cDNA library [29], as this organ is known in gastropod snails to produce many components of the egg masses, among which the egg perivitelline fluid [30]. Interestingly, BLAST searches against non-redundant protein databases using the BLASTp program revealed that BgLBP/BPI1 corresponded to the “Developmentally regulated albumen gland protein” (partial sequence; genbank accession number AAB00448.1) previously identified as being over-expressed in Schistosoma mansoni resistant snails [31]. The sequence of BgLBP/BPI1 displayed the typical features of LBP/BPI family members, including a N-terminal LBP/BPI domain (pfam PF01273, Interpro IPR017942) containing conserved lysines involved in the interaction with LPS, a central proline-rich domain, and a C-terminal LBP/BPI domain (Figure S1) [17], [32]. Expression studies showed that the major site of expression for BgLBP/BPI1 was the albumen gland (Figure 1, A and B). We confirmed, using a specific antibody and western blot analysis followed by mass spectrometry, that the BgLBP/BPI1 gene product is the major protein found in egg masses of B. glabrata (Figure 1, C and D).
Fig. 1. BgLBP/BPI1 is expressed in the albumen gland and is the major protein of B. glabrata egg masses. 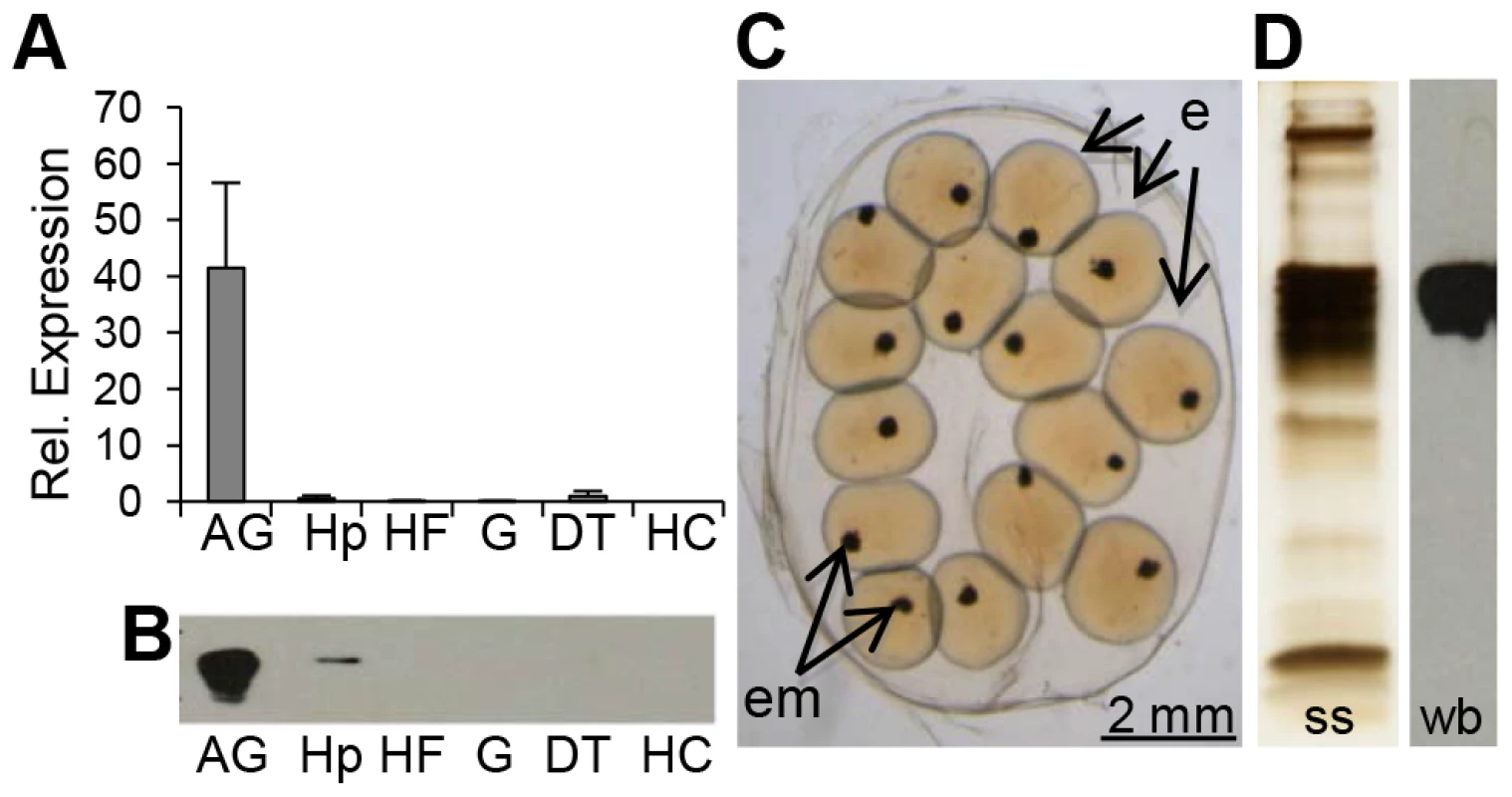
(A) Relative expression levels of BgLBP/BPI1 transcripts in the albumen gland (AG), hepatopancreas (Hp), Headfoot (HF), gonads (G), digestive tract (DT) and circulating hemocytes (HC) determined using real-time quantitative PCR (data from 3 independent experiments). Expression has been normalized to expression of S19 and EEF1-α reference genes. (B) Representative western blot showing the content in BgLBP/BPI1 protein in 15 µg total protein from the corresponding snail tissues (legend as in (A). (C) Typical live 2-days old B. glabrata egg mass with eggs (e) containing embryos (em) observed under a phase-contrast reversed microscope. (D) Total egg mass protein content as revealed by silver staining (ss) and egg mass BgLBP/BPI1 revealed by western blot (wb) using a custom-made antibody raised against two BgLBP/BPI1 peptides. In vitro biological activities
We purified the native BgLBP/BPI1 protein from fresh egg masses and estimated its physiologic concentration around 100 µg/ml of egg mass extract, representing 60% of the total protein dry weight. In order to control for trace contamination of the purified native protein by biologically active polypeptides, we produced a recombinant protein in Drosophila S2 cell culture and compared both the native and the recombinant proteins in our assays. Plasmon resonance analysis confirmed that both BgLBP/BPI1 bind LPS and the Lipid A region, which are common to all LPS's, with a range of affinity similar to that of human BPI protein, as shown by their dissociation constants (Figure 2) [33]. In addition, both proteins showed the typical membrane permeabilizing activity leading to bacterial death (Figure 3) that LBP/BPI proteins present toward short-LPS strains of E. coli [34], demonstrating that the biocidal activity of LBP/BPI proteins against Gram-negative bacteria is conserved in BgLBP/BPI1 [21], [35], [36].
Fig. 2. BgLBP/BPI1 bind to LPS and lipid A. 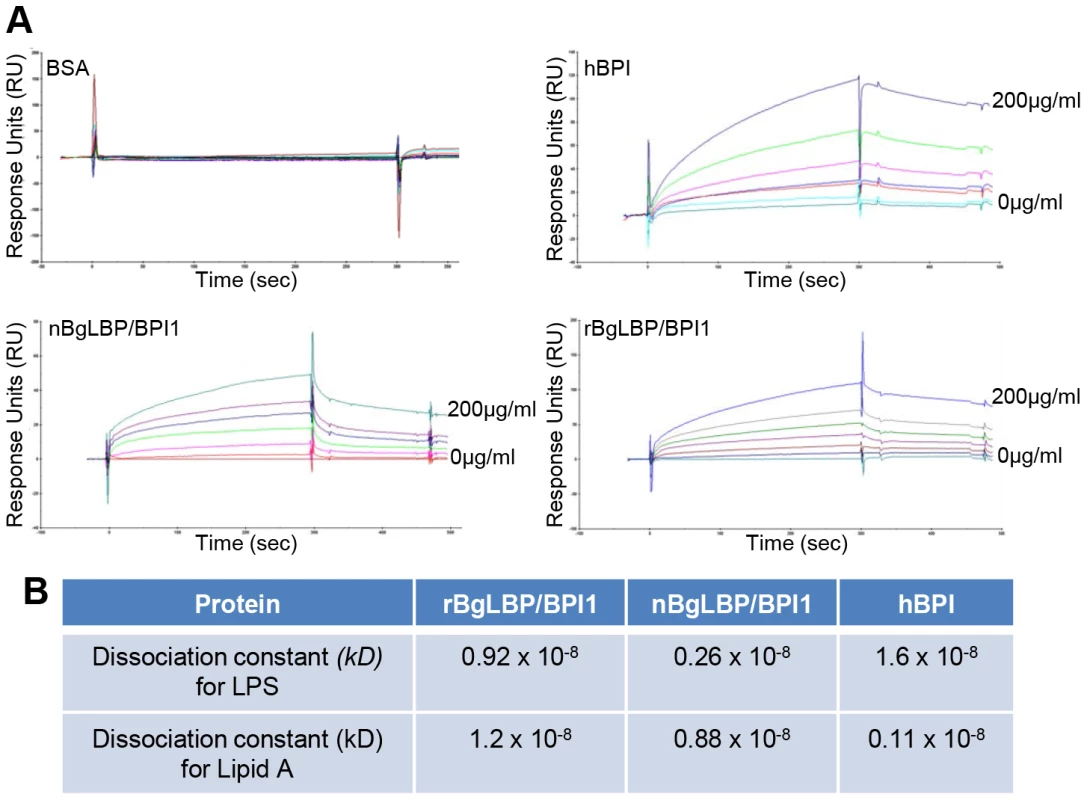
(A) Representative plasmon resonance sensorgrams showing the interactions of BSA (negative control), hBPI (positive control), nBgLBP/BPI1 and rBgLBP/BPI1 with LPS. Purified proteins were immobilized on a CM5 sensor chip and serial LPS dilutions (5, 10, 25, 50, 100 and 200 µg/ml) were injected at a flow rate of 50 ul/min. The responses in RU were recorded as a function of time. Data were fitted to a 1∶1 Langmuir binding model. Similar sensorgrams were obtained with lipid A. (B) Dissociation constants (kD) calculated for each protein. Fig. 3. BgLBP/BPI1s permeabilize membranes and induce death of short LPS E. coli. 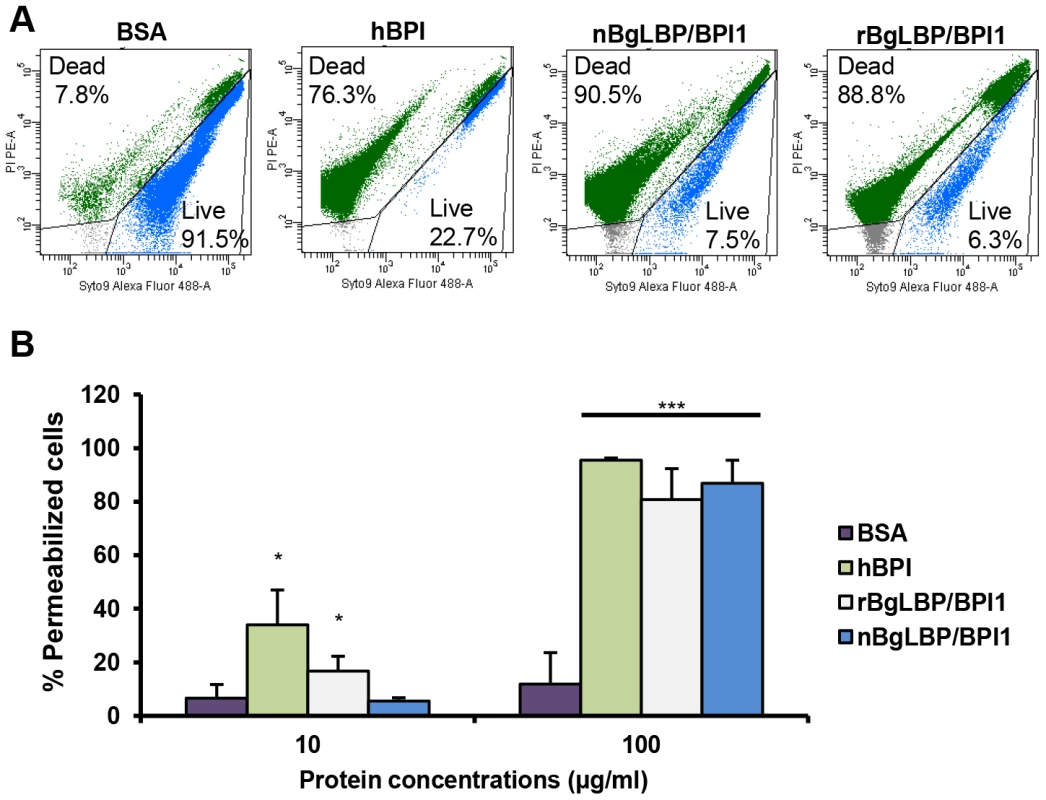
(A) Representative flow cytometry profiles of E. coli SBS363 cells exposed to BSA (negative control), hBPI (positive control), native (n) BgLBP/BPI1 and recombinant (r) BgLBP/BPI1 at a concentration of 100 µg/ml, for 1 h. Cells have been stained with the Baclight kit (SYTO 9 and PI) and a total of 60,000 events were recorded for each sample. (B) Quantification of the effect of the four proteins on E. coli SBS363 cell death (as measured in (A)). Cell death has been measured by flow cytometry after 1 hour exposure to 10 or 100 µg/ml proteins. Results are the mean percentages of permeabilized cells ± SE of three independent experiments. Asterisks indicate significant differences with negative control (*p<0.05, ***p<0.001). Exposure of helminths (S. mansoni), Gram-positive bacteria (Micrococcus luteus, Bacillus cereus), Gram-negative bacteria (Citrobacter freundii, and Pseudomonas aeruginosa) and fungi (Candida albicans and Saccharomyces cerevisiae) to increasing concentrations of the recombinant BgLBP/BPI1 (up to the physiological concentration of 100 µg/ml) had no significant effect on the viability of the microorganisms (P>0.1, Figure S2 and S3). With the exception of helminths such as S. mansoni, information on natural pathogens of B. glabrata is scarce. Therefore we also wanted to investigate whether oomycetes are sensitive to BgLBP/BPI1. Oomycetes or water molds are a large group of eukaryotic microbes that can infect plants and animals and can cause devastating diseases in agriculture, aquaculture, and natural (aquatic) ecosystems [37], [38]. The motile zoospores (infective stage) and cysts (germinal stage) of both Saprolegnia parasitica and Saprolegnia diclina, two well-known pathogens of fresh water fish and their eggs [38], were exposed to increasing concentrations of BgLBP/BPI1 proteins for 30 min. No effect was observed on Saprolegnia cysts (not shown), but a strong biocidal activity was observed on the infective stage of these pathogens at all protein concentrations (Figure 4). The viability of S. parasitica or S. diclina zoospores was significantly reduced to 50.3% and 68.4% or 55.8% and 39% with 100 µg/ml of native BgLBP/BPI1 and recombinant BgLBP/BPI1, respectively (Figure 4). Interestingly, human BPI also strongly decreased the viability of both oomycete species (Figure 4), revealing a yet unsuspected activity of this well-studied human immune protein [39]. To better assess the LBP/BPI-dependent anti-oomycete activity, we also tested a plant pathogenic species, Phytophthora parasitica that has the ability to form biofilms [40] like Gram-negative bacteria. Biomphalaria proteins were tested on the three stages of biofilm formation; zoospores, cysts and microcolonies (Figure S4A). Similarly to the Saprolegnia species, only zoospores were affected by the BgLBP/BPI proteins. Both the snail and the human LBP/BPI proteins showed a strong biocidal activity of zoospores from P. parasitica in a dose dependent manner (Figure 5 and S4B). The effect of LBP/BPIs was observed as early as 10 min and resulted in 100% zoospore mortality after one or two hours of exposure to physiological concentrations of 100 µg/ml LBP/BPI proteins (Figure 5), confirming the strong biocidal activity of LBP/BPIs against oomycete zoospores.
Fig. 4. BgLBP/BPI1 and hBPI display anti-oomycete activity against Saprolegnia parasitica and S. diclina. 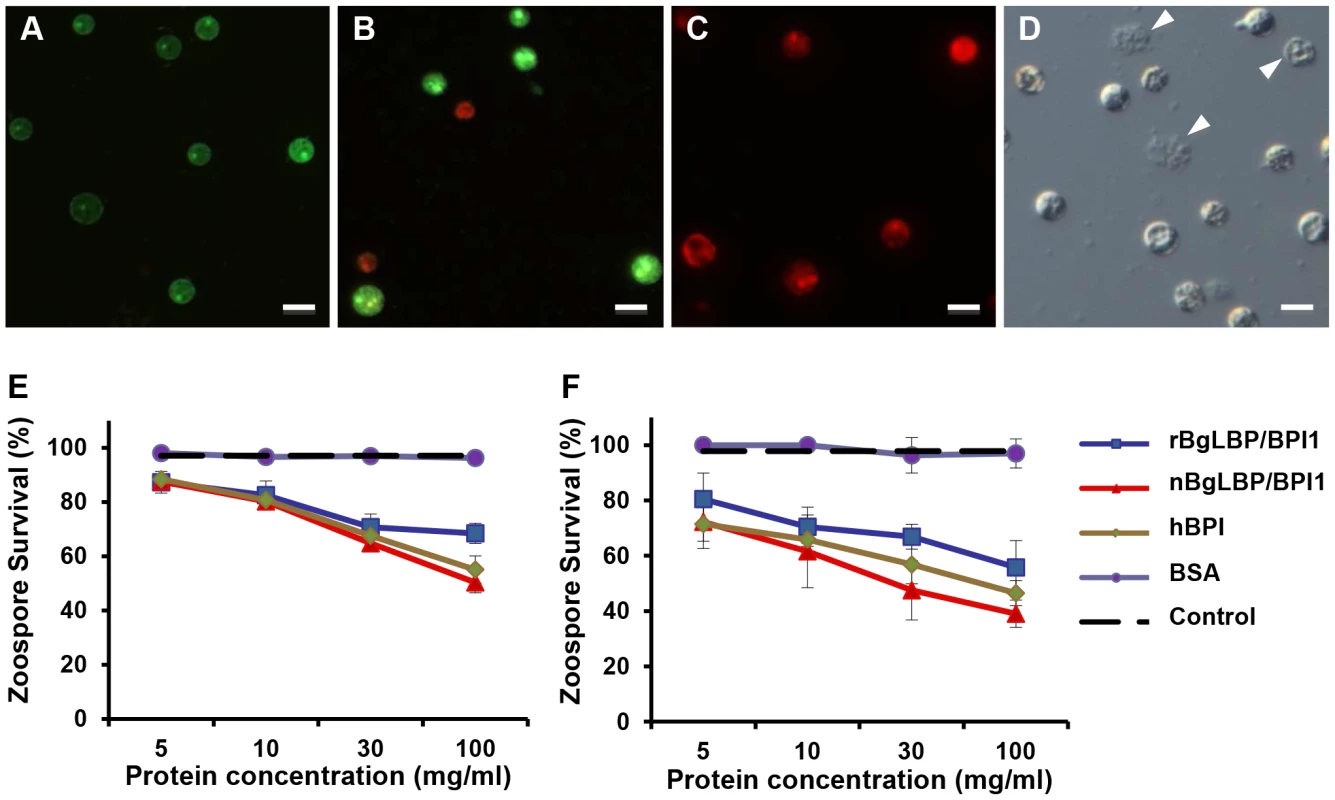
(A–C) Pictures of live (green) or dead (red) Saprolegnia zoospores after exposure to BSA (A), or BgLBP/BPI proteins at 10 µg/ml (B) or 100 µg/ml (C). Zoospores have been stained by the live/dead cell assay (Abcam). Scale bar is 20 µm. (D) Phase contrast observation of zoospores exposed to 10 µg/ml BgLBP/BPI. Arrows show examples of dead cells or cell debris. (E, F) Survival rate of Saprolegnia parasitica (E) and S. diclina (F) after 30 min exposure to buffer alone (Control) or increasing concentrations of rBgLBP/BPI1, nBgLBP/BPI1, hBPI and BSA. Results are mean percentages (± SE) of three independent experiments. Fig. 5. BgLBP/BPI1s and hBPI display anti-oomycete activity against P. parasitica zoospores. 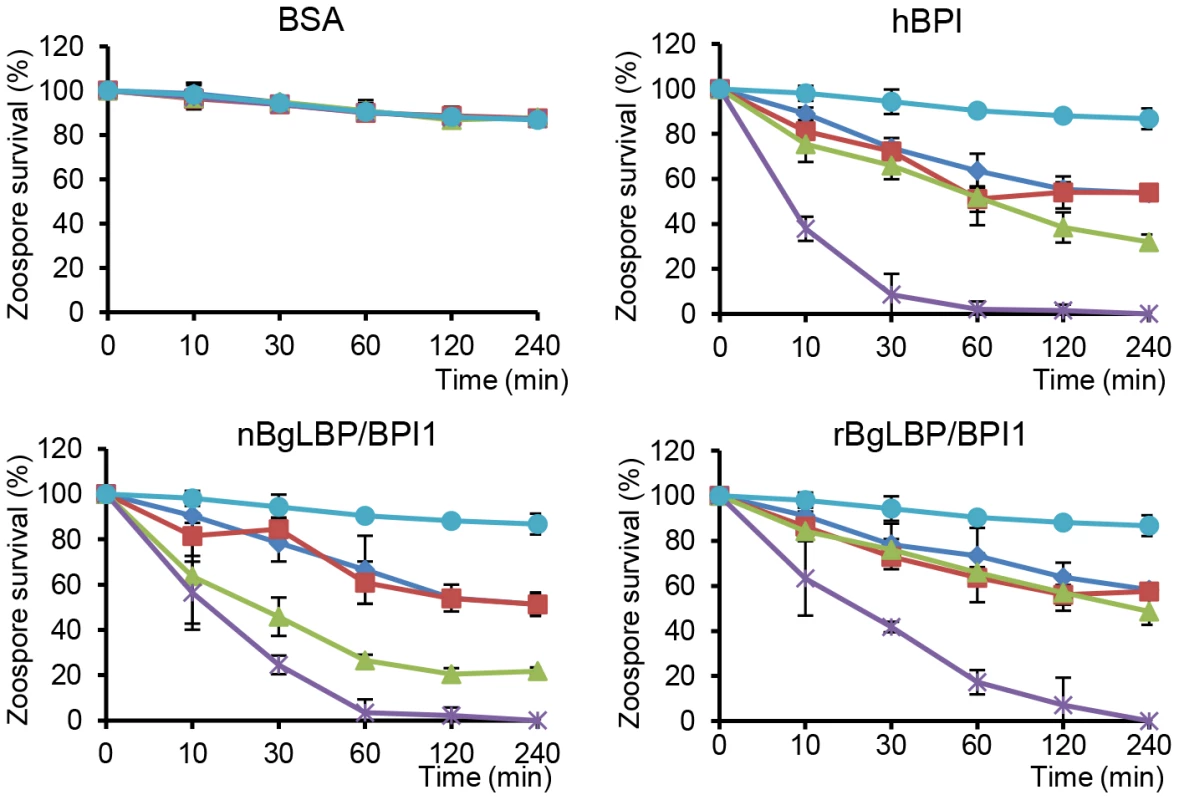
Survival rate of P. parasitica zoospores after exposure to BSA, hBPI, rBgLBP/BPI1 and nBgLBP/BPI1. Zoospores were incubated with proteins at 5 (dark blue), 10 (red), 30 (green) and 100 µg/ml (purple). Negative controls (light blue) are zoospores without treatment. Results are mean percentages (± SE) of three independent experiments. Demonstration of the anti-oomycete activity in vivo
In order to assess the role of BgLBP/BPI1 in vivo, we undertook to decrease its expression by using RNA-interference mediated knock-down. Following double strand dsRNA injections, BgLBP/BPI1 protein abundance was analyzed by western blotting and showed a significant decrease in the albumen gland and in the egg masses after 12 and 18 days, respectively (Figure 6). The number of eggs per clutch collected over 28 days from parents treated with dsRNA of BgLBP/BPI1 was significantly lower than in the control experiment, whereby dsRNA of the luciferase gene was injected. Furthermore, the egg masses of the BgLBP/BPI1 dsRNA-treated snails showed a significant decrease in fecundity (Table 1). Thereby confirming that the albumen gland, the site of expression of BgLBP/BPI1, is directly involved in egg mass production [41]. After exposure to zoospores of S. diclina, eggs from parents silenced for BgLBP/BPI1 expression suffered an important decrease in their hatching rate, when compared to snails injected with control dsRNA (Table 1).
Fig. 6. Injection of BgLBP/BPI1 dsRNA results in a substantial decrease in BgLBP/BPI1 protein in the albumen gland and in egg masses. 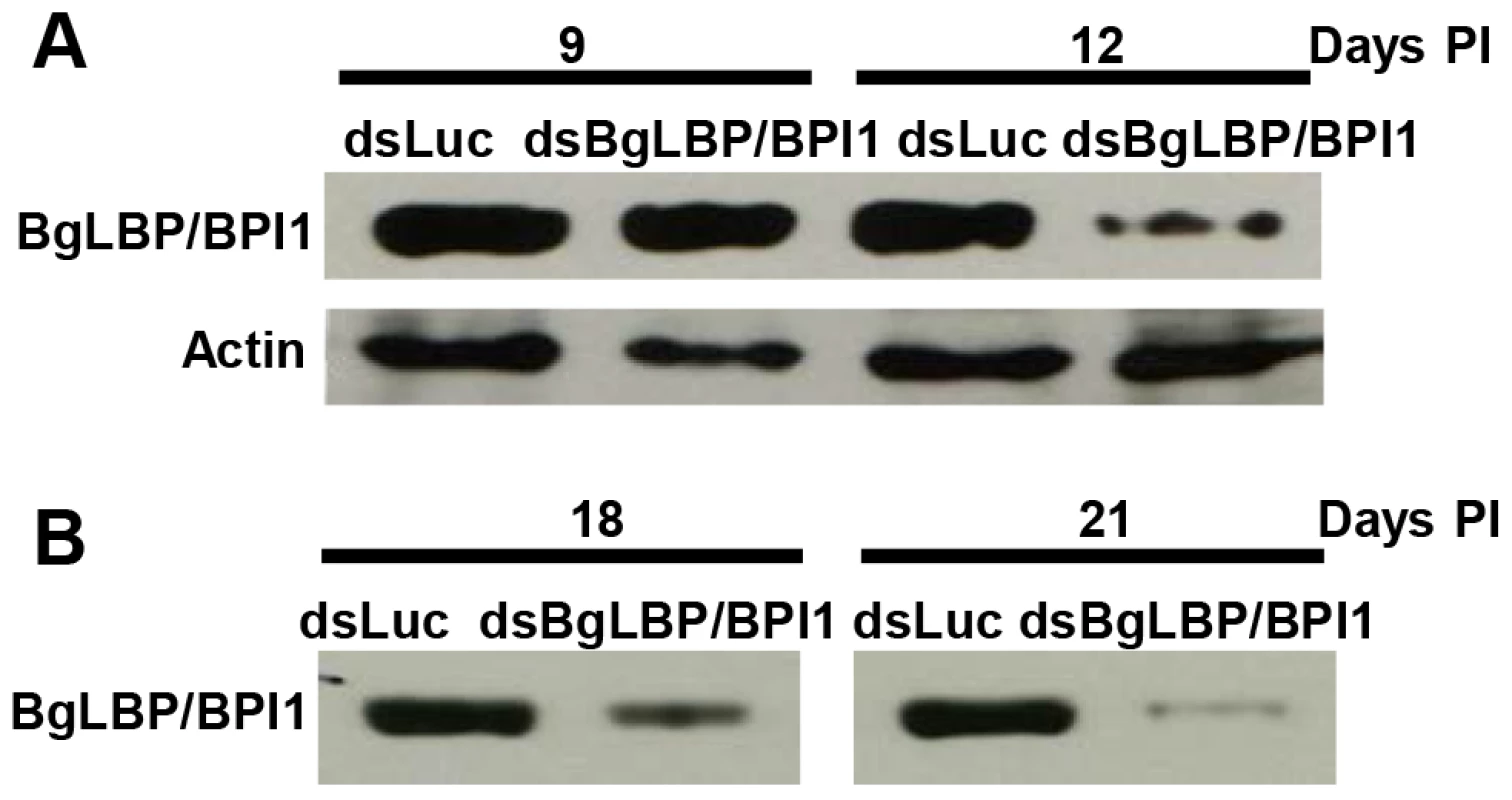
(A) Western blots of albumen glands from snails at 9 days and 12 days post injection (Days PI) of dsRNA of luciferase (non-relevant dsRNA) (dsLuc) or BgLBP/BPI1 (dsBgLBP/BPI1). Actin was used as an endogenous control for protein loads. (B) Western blots of egg masses laid by snails at 18 and 21 days post injection of dsRNAs. Note that control for actin or total protein contents were not appropriate for egg masses and that protein loads have been standardized towards egg mass dry weights. Western Blots were performed using a custom-made antibody raised against two BgLBP/BPI1 peptides. Tab. 1. BgLBP/BPI1 expression affects snail fecundity and egg survival to oomycete exposure. 
Fecundity was assessed as the number of egg masses and the number of eggs per egg mass laid during the first 28 days following the first injection of non-relevant luciferase dsRNA (dsLUC) or BgLBP/BPI1 dsRNA (dsBgLBP/BPI1). Egg hatching rate was assessed from egg masses collected from 12 to 21 days post-injection (period of optimum gene expression knock-down). The eggs from control-treated parents appeared healthy with a normal development of the embryos (Figure 7A), whereas the egg masses from parents treated with BgLBP/BPI1 dsRNA were covered by oomycete hyphae and the resulting infection impaired dramatically the survival of the snail embryos (Figure 7B).
Fig. 7. BgLBP/BPI1 is essential to protect eggs from oomycete infection. 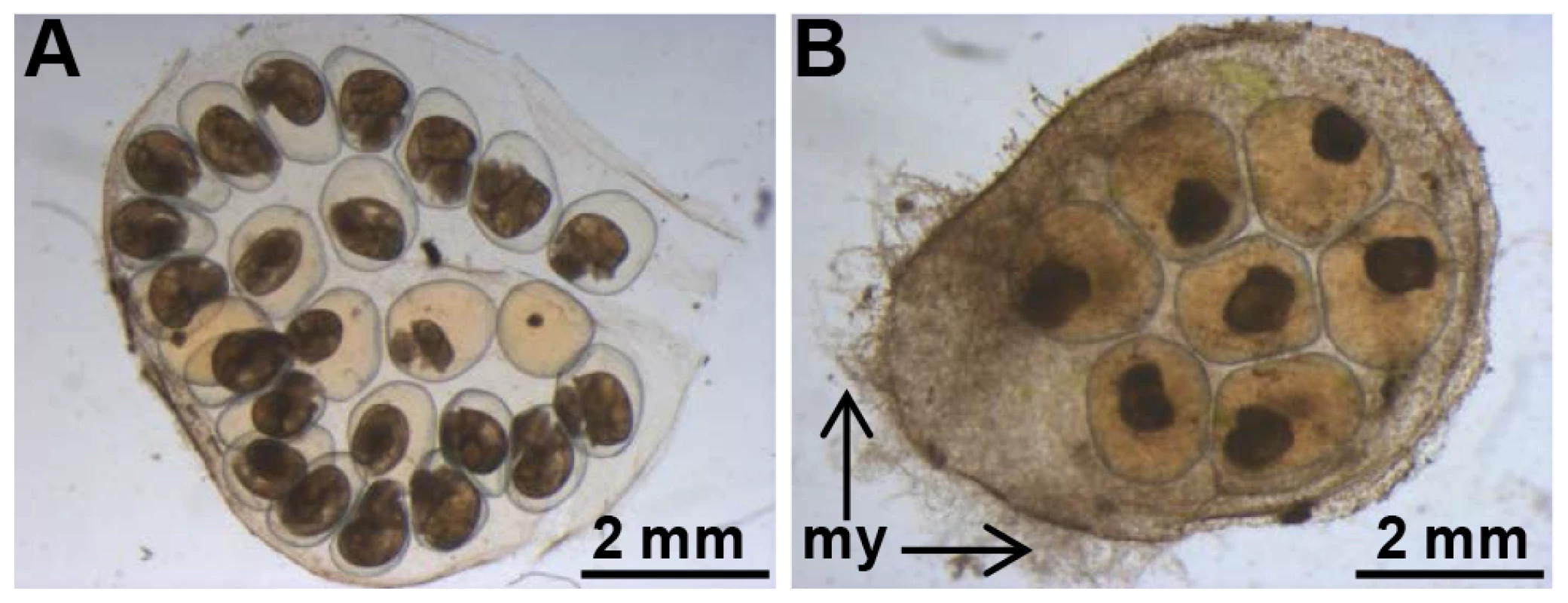
Typical egg mass from Luc- (A) or BgLBP/BPI1 (B) dsRNA-injected parents, after exposure to S. diclina zoospores. Note the well-developed mycelium (my). Egg masses from (A) and (B) have been laid 19–21 days after dsRNA injection to parents and have been exposed to zoospores at day 22. Observation was made after 8 days exposure to S. diclina zoospores. Discussion
Many invertebrate species lay fertilized eggs in nutritive egg masses that are highly suitable to the development of microorganisms [42], [43]. Although parental protection of eggs seems crucial to the survival of species, studies on the immune protection of invertebrate eggs are scarce. For example, an antibacterial activity was shown in eggs from 32 mollusk, 2 polychaete and 1 coral species, out of, respectively 34, 4 and 11 species tested [9], [42]. An antibacterial protein, the aplysianin-A was identified from eggs of the gastropod Aplysia kurodai [44], and an N-acetyl-galactosamine-binding lectin that agglutinates bacteria was identified in eggs from the pulmonate snail Helix pomatia [45]. Together with peptides of aplysianin, peptides of LBP/BPI proteins were identified in a proteomic study on Biomphalaria glabrata egg masses [15]. Here we characterized a B. glabrata LBP/BPI family member that is produced in the albumen gland and abundantly loaded into the egg masses. Consistent with the study on the BPI-like protein from Crassostrea gigas [27], we showed that the LPS and lipid A-binding activities against Gram-negative bacteria are conserved in BgLBP/BPI1. BgLBP/BPI1s did not exert any effect against the panel of microorganism tested. However, bacterial permeability activity was observed against E. coli SBS363, a mutant strain containing short-chain LPS. This is in agreement with previous studies reporting the resistance of Gram-negative bacteria harboring long lipopolysaccharide chain to the activity of hBPI [34], [46].
In addition to this expected anti-bacterial activity, we discovered a yet unsuspected anti-oomycete activity and demonstrated that BgLBP/BPI1 is a major fitness-related protein affecting both egg production under control conditions and offspring survival in the presence of pathogens. It is possible that the positive effect of BgLBP/BPI1 on the number of eggs produced is related to the glycoprotein nature of the molecule rather than to its antimicrobial activities. Interestingly, the glycoprotein HdAGP, identified from the snail Helisoma duryi albumen gland, was reported as the major nutritive glycoprotein secreted in the perivitelline fluid, and is also sharing sequence similarities with LBP/BPIs [43]. The content in BgLBP/BPI1 may therefore affect egg production as a major nutritive egg mass compound, independently of its antimicrobial action. However, once the egg masses are laid, we demonstrated that the biocidal activity of BgLBP/BPI1 affects offspring survival in the presence of oomycete pathogens.
LBP and BPIs are pleiotropic molecules, well characterized for their interactions with LPS from Gram-negative bacteria, but also reported to interact with other organisms such as Gram-positive bacteria and fungi [18], [47], [48]. A wide range of lipidic ligands have been reported for human LBP and BPI [18], [47], [48]. Our results further evidence the diversity of binding capabilities of LBP/BPIs as both BgLBP/BPI1 and hBPI can interact with an oomycete lipidic ligand that remains to be identified. Oomycetes do share physical characteristics with true fungi, including polarized hyphal extensions but they have a distinct evolutionary history and belong to the kingdom Stramenopila, which also includes brown algae and diatoms [49]. In contrast to fungi, they produce bi-flagellated swimming spores (zoospores) and the cell-wall of their cysts is composed of cellulose, β-glucans and hardly any chitin [49]. Oomycetes include some of the most devastating animal and plant pathogens. A few species cause Saprolegniosis in the aquaculture industry [38]. Saprolegnia species are endemic to freshwater habitat worldwide and are partly responsible for declining natural populations of salmonids and amphibians [50], [51]. Furthermore, the potato and tomato late-blight pathogen, Phytophthora infestans triggered the Irish Famine in the mid-1840s [37], [50], [52]. The potent oomycete killing activity of both BgLBP/BPI1 and hBPI was observed with three species belonging to two major oomycete orders, the Perenosporales and Saprolegniales [52]. Our observations demonstrate a conserved and broad-spectrum oomycete killing activity of BgLBP/BPI1, which may be of interest for both the agricultural and aquacultural sectors [53]. Interestingly, the specificity of this biocidal activity for the zoospore stage suggests that the ligand may be expressed specifically at this developmental stage. To date, despite the economic impact of oomycetes, there is no biochemical information on the membrane compounds of zoospores as studies have focused on identifying surface components of the cell wall of cysts [54], [55].
Collectively, our results significantly expand our knowledge of the multiple functions of LBP/BPI and highlight their importance in invertebrate biology. We demonstrated that LBP/BPI proteins display a conserved, potent and so far unexpected biocidal activity against zoospores from different oomycete orders. The precise binding and killing activity of the zoospores is unknown, but it is clear that BgLBP/BPI1 represents a major fitness-related protein transferred from parents to their clutches protecting snail eggs from widespread and lethal oomycete infections.
Materials and Methods
Characterization of BgLBP/BPI1
A partial cDNA sequence (EST GenBank accession number EB709540) was used to design specific primers and perform 5′ - and 3′-RACE amplification (5′3′ RACE kit, 2nd generation - Roche) according to the manufacturer's instructions. PCR products were cloned into pCR4-TOPO vector (Invitrogen) for sequencing. Sequence similarity searches were carried out using NCBI's BLAST-X program [56] against non-redundant databases with default parameters. Global sequence alignments were performed with Clustal W software [57]. The protein domains and signal peptide were predicted with the SMART [58] and SignalP [59] softwares, respectively.
Animals and microorganisms
Adult Biomphalaria glabrata snails (albino strain) were raised in pond water and fed leaf lettuce ad libitum according to previously described procedures [60]. Bacterial and yeast strains used in this study were Micrococcus luteus (CIP A270), Pseudomonas aeruginosa (PA14) [61], Bacillus cereus (ATCC 11778), Citrobacter freundii (ATCC 8090), Candida albicans (a pathogenic strain isolated in patient no. 3 by Pr M. Koenig, CHU Strasbourg-Hautepierre) and Saccharomyces cerevisiae (Bioreference Laboratory – Institut Pasteur (Lille, France) as well as the E. coli SBS363, a Trp+ galU129 (truncated LPS) derivative of E. coli K12 strainD22 (gift from D. Destoumieux-Garzón, Université Montpellier 2). Bacterial strains were maintained in LB medium at 37°C and yeast in YPD medium at 28°C under standard conditions.
S. mansoni miracidia (swimming infective stage) were hatched from eggs axenically recovered from 50-days infected hamster livers according to previously described procedures [62]. Oomycete species used in the study were Saprolegnia parasitica, S. diclina and Phytophthora parasitica. Saprolegnia zoospores were obtained as described previously [63]. The average number of zoospores released was approximately 104 zoospores per ml. Phytophthora parasitica was grown in 90 mm-diameter Petri dishes on 20% V8 agar media (350 ml V8 juice, 5 g CaCO3, 3.5 g agar) at 25°C for 6–8 days under continuous light. To induce zoospore release, Phytophthora isolates were placed at 4°C for 30 min. Mycelial cultures were then flooded with 10 ml of warm sterile water and left at 28°C for 30 min [40]. The average number of zoospores obtained was approximately 106 zoospores per ml.
Expression studies
Snail organs or tissues, namely albumen gland, hepatopancreas, headfoot, digestive tract and gonads were dissected under a binocular microscope, pooled from 10 individuals and frozen in liquid nitrogen. Snail circulating hemocytes were recovered from hemolymph collected prior to tissue dissections according to previously described procedures [62]. Total RNA and protein were simultaneously isolated using TRIZOL LS Reagent (Invitrogen) according to the manufacturer's instructions. Total RNA was quantified using a NanoDrop Spectrophotometer ND-1000 (Thermo Scientific). For cDNA synthesis, 50 ng of RNA from dissected tissues and hemocytes were used for reverse transcription using iScript cDNA Synthesis kit (Bio-Rad) and the oligo(dT) 20 primer.
The relative expression of BgLBP/BPI1 was monitored using Quantitative Real-time PCR on a DNA engine opticon 2 system (Biorad). Primers specific for B. glabrata ribosomal protein S19 (Genbank accession number CK988928) [64], elongation factor EEF1 - α (Genbank accession number ES482381.1) and BgLBP/BPI1, were designed with primer 3 software and used for amplification in triplicate assays. The PCR cycling procedure was as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of amplification 95°C for 30 s, 60°C for 30 s and 68°C for 30 s for signal collection in each cycle. To assess the specificity of the PCR amplification, a melting curve analysis of the amplicon was performed at the end of each reaction and a single peak was always observed.
To examine the distribution of BgLBP/BPI1 protein in snail dissected tissues or egg masses, an anti-BgLBP/BPI1 antiserum was produced in a rabbit using the LAKAHIEKNRLIPDLLSYD and AQDKPGAVLRLNQEALDYGSR peptides and the polyclonal sera were purified using a peptide linked resin column (Proteogenix). Total protein contents of tissues were first determined by the BCA method (BC assay kit, Uptima) using albumin as a standard. 15 ug of tissue or egg mass proteins were loaded onto 10% SDS-PAGE gels and either silver stained using standard procedures, or transferred to a PVDF membrane (0.2 mm pore size) using a semi-dry blotting system. Western blots were performed using the custom anti-BgLBP/BPI1 antisera (Proteogenix).
Production of recombinant and native BgLBP/BPI1 proteins
cDNA corresponding to the open-reading frame of BgLBP/BPI1 was ligated into the pMT/V5/His-A expression vector (Invitrogen). The Drosophila expression system with Schneider 2 (S2) cells (Invitrogen) was used to express recombinant C-terminally His-tagged full-length BgLBP/BPI1 (rBgLBP/BPI1) as described previously [65], [66]. Briefly, S2 cells were transiently transfected by calcium phosphate method with 1 µg of pMT/BgLBP/BPI1/V5/His-A vector and its expression was monitored by SDS-PAGE and western blotting after 3 days of induction with CuSO4 (500 mM). After confirmation of transient rBgLBP/BPI1 expression, stable cell lines were generated performing co-transfections along with 0.1 µg of PJL3 selection vector and 1 µg/ml puromycin. Establishment of stable cell lines and production of rBgLBP/BPI1 were carried out as described previously [66]. Nickel (II)-based immobilized metal affinity chromatography (Qiagen) in native conditions was performed to purify the recombinant BgLBP/BPI1 protein according to the manufacturer's protocol.
The native BgLBP/BPI1 protein (nBgLBP/BPI1) was purified from two-days old B. glabrata egg masses. Egg masses were homogenized in 20 mM acetate buffer, pH 4.5. The crude homogenate was centrifuged at 13000 rpm for 10 min to remove the gelatinous and solid debris. Supernatant containing nBgLBP/BPI1 was loaded onto SP Trisacryl M cation-exchange resin (BioSepra) equilibrated in 20 mM acetate buffer, pH 4.5. After washing 3 times with equilibration buffer, nBgLBP/BPI1 was eluted with 1 M NaCl, 20 mM acetate buffer, pH 4.5 and quantified by Bradford method. In order to analyze the sequence of the nBgLBP/BPI1, the purified protein was excised from a 10% SDS-PAGE gel and subjected to a MALDI TOF/TOF-MS analysis (Proteomic facility, University of Strasbourg, France). Protein identification was performed by subjecting the m/z values to Mascott software at an adjusted peptide mass tolerance of 50.000.000 ppm and/or 0.5 Da and at a fragment mass tolerance of 0.4 Da. For the subsequent activity assays, the purity of the purified nBgLBP/BPI1 protein was assessed after SDS-PAGE and silver staining. Assessments of both the egg mass volumes used for purification and the final concentration of the purified protein allowed to determine that the natural concentration of nBgLBP/BPI1 is in the range of 100 µg/ml of fresh 2 days old egg masses.
LPS-binding activity
Binding of LPS or lipid A to rBgLBP/BPI1 and nBgLBP/BPI1 was assessed with a Biacore 3000 system (Biacore, GE Healthcare). RBgLBP/BPI1 and nBgLBP/BPI1 were immobilized at 7000 response units (RU) onto an activated CM5 sensor chip (Biacore) according to the manufacturer's instructions. Human BPI (hBPI - Athens Research, USA) and BSA proteins were immobilized using the same conditions as positive and negative control proteins, respectively. An activated and blocked flow-cell without immobilized ligand was used as a reference to evaluate nonspecific binding. HBS-EP running buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% Tween 20, pH 7.4) was used for sample dilution and analysis. Purified diphosphoryl lipid A from E. coli F583 Rd mutant and LPS from E. coli O26:B6 (Sigma) were sonicated 15 min at 25°C and injected at various concentrations. LPS was diluted at 50, 100, 250, 500, 1000 and 2000 nanograms and lipid A at 30, 60, 100, 150, 200, 300, 400, 500 nanograms and passed over the sensor chip at a flow rate of 50 µl/min. Regeneration was achieved with two washes of 20 mM NaOH for 5 min and 150 mM NaOH for 5 min for LPS and lipid A, respectively. Sensor chip was finally equilibrated with HBS-EP buffer for 2 min. All analyses were done at a constant temperature of 25°C. Data analysis was performed after subtraction of the uncoated flow-cell values by using BIAevaluation software version 4.1 (BIAcore). The association and dissociation phases of all sensor-grams were fitted globally. Kinetic parameters were then determined using a 1∶1 Langmuir binding model.
Bacterial membrane permeability assays
The effect of proteins on the permeability of bacterial membranes was determined by flow cytometry using E. coli SBS363 and the LIVE/DEAD BacLight Bacterial Viability Kit (Molecular probes). This kit enables assessment of bacterial viability based on membrane integrity by differentiating between bacteria with intact and damaged cytoplasmic membranes [67]. Bacterial culture in mid-logarithmic phase was adjusted to an optical density of A600 = 0.003 with poor-broth nutrient medium and treated with 10, 30, or 100 µg/ml of rBgLBP/BPI1 and nBgLBP/BPI1. BSA and hBPI were used at similar concentrations as a negative and positive control, respectively. Samples were incubated 1, 2 and 6 h at 28°C under vigorous shaking. Bacterial suspensions were stained with LIVE/DEAD BacLight staining reagent mixture (SYTO 9 and propidium iodide - PI) as described by the manufacturer. Staining was allowed for 5 min at room temperature in the dark. Flow cytometric measurements were performed on a FACSCanto II flow cytometer (BD Biosciences) with a 488 nm argon excitation laser. A total of 60,000 events were acquired and analyzed in each sample, using BD FACSDiVa software version 6.1.3 (BD Biosciences). Results are displayed as a percentage of permeabilized cells with respect to the negative control. The experiments were carried out three times independently.
Assays for biocidal activity
The antibacterial activity was tested on Micrococcus luteus, Bacillus cereus, Citrobacter freundii, and Pseudomonas aeruginosa using the liquid growth inhibition method as previously described [68]. For antifungal activity assays, a similar liquid growth inhibition assay was performed using YPD medium. Microbial growth was controlled by measurement of the optical density at A600 after 6, 16 and 24 h incubation in proteins at 10, 50, 100 ug/ml. Fungal growth was additionally evaluated at 48 h. The percentage of growth (% growth) was deduced from the absorbance (OD) at 600 nm as previously described [69].
The effect on S. mansoni viability was tested on groups of 10–15 miracidia placed in 24-well plates. Miracidia were exposed to proteins at 10, 50, 100, 200 µg/ml at 28°C. Microscopic observations were performed at 30 min, 1, 2, 4, 6, 8 and 24 h of incubation.
The anti-oomycete assays were adjusted to the characteristics and life-cycles of the oomycete species. Zoospores of S. parasitica and S. diclina were adjusted to 10000 cells/ml and exposed to increasing concentrations (5, 10, 30, 100 µg/ml final concentration) of proteins. Because of the rapid encystment of zoospores into cysts, assessment of mortality was only performed at 30 min (prior to encystment of live zoospores). Zoospores of Phytophthora parasitica were adjusted to 500000 cells/ml and exposed to identical concentrations of proteins (5, 10, 30, 100 ug/ml final concentration). Microscopic observations of the number of live and dead zoospores were performed after 10, 30, 60, 120, 240 min of treatment. Zoospore-treated suspensions were stained immediately after incubation with the Live/Dead Cell Assay kit (Abcam) as described in the manufacturer's protocol. All conditions were tested in triplicate and assays were performed 3 times independently.
RNA interference experiments
BgLBP/BPI1-specific primers containing a T7 promoter sequence were designed to amplify a 420-bp region of BgLBP/BPI1 used as a template for double stand RNA (dsRNA) synthesis according to manufacturer's instructions (MEGAScript T7 kit, Ambion). The firefly (Photinus pyralis) luciferase gene dsRNA (pGL3 vector, Promega) was produced and used as a non-relevant dsRNA control.
Each dsRNA (15 µg in 10 µl of sterile Chernin's Balanced Salt Solution - CBSS) was injected into the cardiac sinus of individual snails using a 10 µl Hamilton syringe with a 26 s needle [62]. A second injection was performed 12 days after the first dsRNA injection in order to optimize the knock-down efficiency. Groups of 10 snails were injected either with BgLBP/BPI1 or Luc dsRNA and were maintained under standard conditions. Egg masses produced during 28 days following the first dsRNA injection were scored, collected and observed under a stereoscopic microscope for assessment of the number of eggs in each egg mass. Egg masses laid from day 12 to 21 after the first dsRNA injection were either maintained under control conditions, or exposed to Saprolegnia diclina zoospores at a final concentration of 104cells/ml. Egg masses were microscopically observed during the following 10 days to assess the egg hatching rate. Results are shown as the percentage of eggs hatched. RNA interference experiments were performed three times independently.
Statistical analyses
All data were expressed as mean of three independent experiments plus or minus SE. Differences in relative BgLBP/BPI1 gene expression were tested for statistical significance by one-way ANOVA and the tukey-Kramer test (Software Prism v.5.0, GraphPad). Data from membrane-permeabilizing and antimicrobial (antibacterial, anti-oomycete) assays were analyzed by the chi-square test of independence [70] between treatments (rBgLBP/BPI1, nBgLBP/BPI1, hBPI and BSA) and the proportion of dead cells, using the computing environment R [71]. Schistosoma mansoni survival curves were analyzed by the Mantel-Cox log-rank test (Software Prism v.5.0, GraphPad). Results on snail fecundity and on egg viability were statistically analyzed by the likelihood ratio test between nested models [71]. Briefly, three variables were considered in these models; the mean number of egg per snail, the mean number of egg masses per snail and the mean number of eggs per egg mass. To test the effect of the BgLBP/BPI1 silencing on snail fecundity for each of the three variables, linear mixed models were fitted with the function lmr (LML 4 package of R software). In each case the model contains the BgLBP/BPI1 silencing as fixed effect and the time and the replicates as random effects. To normalize the data the mean number of eggs and egg mass per snail were log transformed. To assess the effect of the oomycete infection on the hatching rate of BgLBP/BPI1 silenced eggs, saturated binomial generalized linear mixed models were fitted. This model contained as fixed effects the BgLBP/BPI1 silencing, the oomycete infection, the time and all interactions among these variables; and as random effects, the replicates. To account for over-dispersion, individual level of variability was added. From this model variable selection of fixed effect was based on the AICc (dredge function of MuMIn package of R software) then the selected fixed effect was analyzed by the likelihood ratio test. A P value of <0.05 was considered statistically significant. Where indicated in figures: *P<0.05, **P<0.01, ***P<0.001.
Supporting Information
Zdroje
1. FlajnikMF, Du PasquierL (2004) Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol 25 : 640–644.
2. HasselquistD, NilssonJA (2009) Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philos Trans R Soc Lond B Biol Sci 364 : 51–60.
3. ChucriTM, MonteiroJM, LimaAR, SalvadoriML, KfouryJRJr, et al. (2010) A review of immune transfer by the placenta. J Reprod Immunol 87 : 14–20.
4. SwainP, NayakSK (2009) Role of maternally derived immunity in fish. Fish Shellfish Immunol 27 : 89–99.
5. HaineER (2008) Symbiont-mediated protection. Proc Biol Sci 275 : 353–361.
6. JaenikeJ, UncklessR, CockburnSN, BoelioLM, PerlmanSJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329 : 212–215.
7. HoffmannJA, ReichhartJM (2002) Drosophila innate immunity: an evolutionary perspective. Nat Immunol 3 : 121–126.
8. DishawLJ, LitmanGW (2009) Invertebrate allorecognition: the origins of histocompatibility. Curr Biol 19: R286–288.
9. MarquisCP, BairdAH, de NysR, HolmströmC, KoziumiN (2005) An evaluation of the antimicrobial properties of the eggs of 11 species of scleractinian corals. Coral Reefs 24 : 248–253.
10. LiangY, ZhangS, WangZ (2009) Alternative complement activity in the egg cytosol of amphioxus Branchiostoma belcheri: evidence for the defense role of maternal complement components. PLoS One 4: e4234.
11. FrauneS, AugustinR, BoschTC (2011) Embryo protection in contemporary immunology: Why bacteria matter. Commun Integr Biol 4 : 369–372.
12. Groombridge B, Jenkins MD (2002) World Atlas of Biodiversity: earth's living resources in the 21st century: University of California Press, Berkeley, USA. 360 p.
13. GryseelsB, PolmanK, ClerinxJ, KestensL (2006) Human schistosomiasis. Lancet 368 : 1106–1118.
14. PimentelD (1957) Life History of Australorbis glabratus, The Intermediate Snail Host of Schistosoma mansoni in Puerto Rico. Ecology 38 : 576–580.
15. HathawayJJ, AdemaCM, StoutBA, MobarakCD, LokerES (2010) Identification of protein components of egg masses indicates parental investment in immunoprotection of offspring by Biomphalaria glabrata (gastropoda, mollusca). Dev Comp Immunol 34 : 425–435.
16. BingleCD, CravenCJ (2004) Meet the relatives: a family of BPI - and LBP-related proteins. Trends Immunol 25 : 53–55.
17. BeamerLJ, FischerD, EisenbergD (1998a) Detecting distant relatives of mammalian LPS-binding and lipid transport proteins. Protein Sci 7 : 1643–1646.
18. CannyG, LevyO (2008) Bactericidal/permeability-increasing protein (BPI) and BPI homologs at mucosal sites. Trends Immunol 29 : 541–547.
19. ElsbachP, WeissJ (1993) The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology 187 : 417–429.
20. FentonMJ, GolenbockDT (1998) LPS-binding proteins and receptors. J Leukoc Biol 64 : 25–32.
21. ElsbachP, WeissJ (1998) Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol 10 : 45–49.
22. SchultzH, HumeJ, Zhang deS, GioanniniTL, WeissJP (2007) A novel role for the bactericidal/permeability increasing protein in interactions of gram-negative bacterial outer membrane blebs with dendritic cells. J Immunol 179 : 2477–2484.
23. WeissJ (2003) Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans 31 : 785–790.
24. MarraMN, WildeCG, CollinsMS, SnableJL, ThorntonMB, et al. (1992) The role of bactericidal/permeability-increasing protein as a natural inhibitor of bacterial endotoxin. J Immunol 148 : 532–537.
25. SchumannRR, LeongSR, FlaggsGW, GrayPW, WrightSD, et al. (1990) Structure and function of lipopolysaccharide binding protein. Science 249 : 1429–1431.
26. AltincicekB, VilcinskasA (2007) Analysis of the immune-related transcriptome of a lophotrochozoan model, the marine annelid Platynereis dumerilii. Front Zool 4 : 18.
27. GonzalezM, GueguenY, Destoumieux-GarzonD, RomestandB, FievetJ, et al. (2007) Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc Natl Acad Sci U S A 104 : 17759–17764.
28. KrasityBC, TrollJV, WeissJP, McFall-NgaiMJ (2011) LBP/BPI proteins and their relatives: conservation over evolution and roles in mutualism. Biochem Soc Trans 39 : 1039–1044.
29. GuillouF, MittaG, GalinierR, CoustauC (2007) Identification and expression of gene transcripts generated during an anti-parasitic response in Biomphalaria glabrata. Dev Comp Immunol 31 : 657–671.
30. de Jong-Brink M, Goldschmeding J (1983) Endocrine and nervous regulation of female reproductive activity in the gonad and albumen gland of Lymnaea stagnalis. In: Lever JB, HH., editor. Molluscan Neuroendocrinology. Amsterdam: North Holland Press pp. 126–131.
31. MillerAN, OforiK, LewisF, KnightM (1996) Schistosoma mansoni: use of a subtractive cloning strategy to search for RFLPs in parasite-resistant Biomphalaria glabrata. Exp Parasitol 84 : 420–428.
32. BeamerLJ, CarrollSF, EisenbergD (1998b) The BPI/LBP family of proteins: a structural analysis of conserved regions. Protein Sci 7 : 906–914.
33. TanNS, HoB, DingJL (2000) High-affinity LPS binding domain(s) in recombinant factor C of a horseshoe crab neutralizes LPS-induced lethality. FASEB J 14 : 859–870.
34. CapodiciC, ChenS, SidorczykZ, ElsbachP, WeissJ (1994) Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect Immun 62 : 259–265.
35. Gazzano-SantoroH, ParentJB, GrinnaL, HorwitzA, ParsonsT, et al. (1992) High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun 60 : 4754–4761.
36. LampingN, HoessA, YuB, ParkTC, KirschningCJ, et al. (1996) Effects of site-directed mutagenesis of basic residues (Arg 94, Lys 95, Lys 99) of lipopolysaccharide (LPS)-binding protein on binding and transfer of LPS and subsequent immune cell activation. J Immunol 157 : 4648–4656.
37. KamounS (2003) Molecular genetics of pathogenic oomycetes. Eukaryot Cell 2 : 191–199.
38. PhillipsAJ, AndersonVL, RobertsonEJ, SecombesCJ, van WestP (2008) New insights into animal pathogenic oomycetes. Trends Microbiol 16 : 13–19.
39. BalakrishnanA, MaratheSA, JoglekarM, ChakravorttyD (2012) Bactericidal/permeability increasing protein: A multifaceted protein with functions beyond LPS neutralization. Innate Immun 19(4): 339–47.
40. GalianaE, FourreS, EnglerG (2008) Phytophthora parasitica biofilm formation: installation and organization of microcolonies on the surface of a host plant. Environ Microbiol 10 : 2164–2171.
41. BaiG, LiJ, ChristensenBM, YoshinoTP (1996) Phenoloxidase activity in the reproductive system and egg masses of the pulmonate gastropod, Biomphalaria glabrata. Comp Biochem Physiol B Biochem Mol Biol 114 : 353–359.
42. BenkendorffK, DavisAR, BremnerJB (2001) Chemical defense in the egg masses of benthic invertebrates: an assessment of antibacterial activity in 39 mollusks and 4 polychaetes. J Invertebr Pathol 78 : 109–118.
43. MukaiST, HoqueT, MorishitaF, SaleuddinASM (2004) Cloning and Characterization of a Candidate Nutritive Glycoprotein from the Albumen Gland of the Freshwater Snail, Helisoma duryi (Mollusca: Pulmonata). Invertebrate Biology 123 : 83–92.
44. TakamatsuN, ShibaT, MuramotoK, KamiyaH (1995) Molecular cloning of the defense factor in the albumen gland of the sea hare Aplysia kurodai. FEBS Lett 377 : 373–376.
45. SanchezJF, LescarJ, ChazaletV, AudfrayA, GagnonJ, et al. (2006) Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J Biol Chem 281 : 20171–20180.
46. WeissJ, Beckerdite-QuagliataS, ElsbachP (1980) Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest 65 : 619–628.
47. SchroderNW, SchumannRR (2005) Non-LPS targets and actions of LPS binding protein (LBP). J Endotoxin Res 11 : 237–242.
48. ZweignerJ, GrammHJ, SingerOC, WegscheiderK, SchumannRR (2001) High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 98 : 3800–3808.
49. BeakesGW, GlocklingSL, SekimotoS (2012) The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 249 : 3–19.
50. van WestP (2006) Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challenges for an old problem. Mycologist 20 : 99–104.
51. RomansicJM, DiezKA, HigashiEM, JohnsonJE, BlausteinAR (2009) Effects of the pathogenic water mold Saprolegnia ferax on survival of amphibian larvae. Dis Aquat Organ 83 : 187–193.
52. JudelsonHS (2012) Dynamics and innovations within oomycete genomes: insights into biology, pathology, and evolution. Eukaryot Cell 11 : 1304–1312.
53. Baron O, Reichhart JM, Ponchet M, Coustau C (2013) Anti-oomycete activity fo lipopolysaccharide (LPS)-binding proteins/bactericidal/permeability-increasing proteins. WO/2013/120619. In: INRA/CNRS, editor. WIPO Patentscope. France.
54. TylerBM (2009) Entering and breaking: virulence effector proteins of oomycete plant pathogens. Cell Microbiol 11 : 13–20.
55. MelidaH, Sandoval-SierraJV, Dieguez-UribeondoJ, BuloneV (2013) Analyses of extracellular carbohydrates in oomycetes unveil the existence of three different cell wall types. Eukaryot Cell 12 : 194–203.
56. AltschulSF, MaddenTL, SchafferAA, ZhangJ, ZhangZ, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402.
57. LarkinMA, BlackshieldsG, BrownNP, ChennaR, McGettiganPA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 : 2947–2948.
58. SchultzJ, CopleyRR, DoerksT, PontingCP, BorkP (2000) SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res 28 : 231–234.
59. NielsenH, KroghA (1998) Prediction of signal peptides and signal anchors by a hidden Markov model. Proc Int Conf Intell Syst Mol Biol 6 : 122–130.
60. GuillouF, MittaG, DissousC, PierceR, CoustauC (2004) Use of individual polymorphism to validate potential functional markers: case of a candidate lectin (BgSel) differentially expressed in susceptible and resistant strains of Biomphalaria glabrata. Comp Biochem Physiol B Biochem Mol Biol 138 : 175–181.
61. LimmerS, HallerS, DrenkardE, LeeJ, YuS, et al. (2011) Pseudomonas aeruginosa RhlR is required to neutralize the cellular immune response in a Drosophila melanogaster oral infection model. Proc Natl Acad Sci U S A 108 : 17378–17383.
62. Baeza GarciaA, PierceRJ, GourbalB, WerkmeisterE, ColinetD, et al. (2010) Involvement of the cytokine MIF in the snail host immune response to the parasite Schistosoma mansoni. PLoS Pathog 6: e1001115.
63. van WestP, de BruijnI, MinorKL, PhillipsAJ, RobertsonEJ, et al. (2010) The putative RxLR effector protein SpHtp1 from the fish pathogenic oomycete Saprolegnia parasitica is translocated into fish cells. FEMS Microbiol Lett 310 : 127–137.
64. MittaG, GalinierR, TisseyreP, AllienneJF, Girerd-ChambazY, et al. (2005) Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Dev Comp Immunol 29 : 393–407.
65. TauszigS, JouanguyE, HoffmannJA, ImlerJ-L (2000) Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proceedings of the National Academy of Sciences 97 : 10520–10525.
66. GardsvollH, HansenLV, JorgensenTJ, PlougM (2007) A new tagging system for production of recombinant proteins in Drosophila S2 cells using the third domain of the urokinase receptor. Protein Expr Purif 52 : 384–394.
67. BerneyM, HammesF, BosshardF, WeilenmannHU, EgliT (2007) Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl Environ Microbiol 73 : 3283–3290.
68. HetruC, BuletP (1997) Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. Methods Mol Biol 78 : 35–49.
69. BarbaultF, LandonC, GuenneuguesM, MeyerJP, SchottV, et al. (2003) Solution structure of Alo-3: a new knottin-type antifungal peptide from the insect Acrocinus longimanus. Biochemistry 42 : 14434–14442.
70. HopeACA (1968) A Simplified Monte Carlo Significance Test Procedure. Journal of the Royal Statistical Society Series B (Methodological) 30 : 582–598.
71. Team RDC (2005) R: A language and environment for statistical computing. In: Computing FfS, editor. 2.11.1 ed. Vienna, Austria.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



