-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
Chronic infections with human viruses, such as HIV and HCV, or mouse viruses, such as LCMV or Friend Virus (FV), result in functional exhaustion of CD8+ T cells. Two main mechanisms have been described that mediate this exhaustion: expression of inhibitory receptors on CD8+ T cells and expansion of regulatory T cells (Tregs) that suppress CD8+ T cell activity. Several studies show that blockage of one of these pathways results in reactivation of CD8+ T cells and partial reduction in chronic viral loads. Using blocking antibodies against PD-1 ligand and Tim-3 and transgenic mice in which Tregs can be selectively ablated, we compared these two treatment strategies and combined them for the first time in a model of chronic retrovirus infection. Blocking inhibitory receptors was more efficient than transient depletion of Tregs in reactivating exhausted CD8+ T cells and reducing viral set points. However, a combination therapy was superior to any single treatment and further augmented CD8+ T cell responses and resulted in a sustained reduction in chronic viral loads. These results demonstrate that Tregs and inhibitory receptors are non-overlapping factors in the maintenance of chronic viral infections and that immunotherapies targeting both pathways may be a promising strategy to treat chronic infectious diseases.
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003798
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003798Summary
Chronic infections with human viruses, such as HIV and HCV, or mouse viruses, such as LCMV or Friend Virus (FV), result in functional exhaustion of CD8+ T cells. Two main mechanisms have been described that mediate this exhaustion: expression of inhibitory receptors on CD8+ T cells and expansion of regulatory T cells (Tregs) that suppress CD8+ T cell activity. Several studies show that blockage of one of these pathways results in reactivation of CD8+ T cells and partial reduction in chronic viral loads. Using blocking antibodies against PD-1 ligand and Tim-3 and transgenic mice in which Tregs can be selectively ablated, we compared these two treatment strategies and combined them for the first time in a model of chronic retrovirus infection. Blocking inhibitory receptors was more efficient than transient depletion of Tregs in reactivating exhausted CD8+ T cells and reducing viral set points. However, a combination therapy was superior to any single treatment and further augmented CD8+ T cell responses and resulted in a sustained reduction in chronic viral loads. These results demonstrate that Tregs and inhibitory receptors are non-overlapping factors in the maintenance of chronic viral infections and that immunotherapies targeting both pathways may be a promising strategy to treat chronic infectious diseases.
Introduction
Cytotoxic CD8+ T cells are crucial for the control of most virus infections. However, in several chronic virus infections, like HIV or hepatitis C virus (HCV) in humans, the virus evades destruction by CD8+ T cells. Mostly these infections are associated with an appearance of functionally exhausted virus-specific effector cells, which reflects an important mechanism of immune evasion and likely contributes to the inability of the host to eliminate the pathogen. There are two main mechanisms described in the context of functional disability of CD8+ T cells. One of these mechanisms appears to be the induction of Tregs, a specialized CD4 - and Foxp3-expressing T cell subset that controls immune responses by suppressing the proliferation and functions of effector T cells. The mechanism of viral immune escape by induction of Tregs was first described in studies using the Friend retrovirus (FV) infection of mice [1]. We demonstrated that acute FV infection induces expansion of two distinct Treg subpopulations [2]. The expansion was partly dependent on the magnitude of the virus-specific CD8+ T cell response. In turn the Tregs negatively influenced the peak CD8+ T cell response contributing to the establishment and maintenance of long-term chronic FV infections [2], [3]. The depletion of Tregs during the acute phase of infection resulted in enhanced effector T cell function and decreased viral loads [3], [4]. In an established chronic infection the Treg pool is reduced compared to its peak expansion after acute infection, but still significantly enlarged as compared to the pool of naive mice (data not shown and [3]). A transient depletion of Tregs in an established chronic infection improved anti-viral immune responses in part by reactivating previously suppressed and functionally exhausted CD8+ T cells and thereby significantly reduced chronic viral set points [5].
Another important mechanism associated with the appearance of dysfunctional CD8+ T cells is the signaling of inhibitory receptors, which induces CD8+ T cell exhaustion. One of the prototypic inhibitory receptors described as an important mediator of T cell exhaustion in chronic viral infections is programmed death-1 (PD-1). The PD-1 receptor is a negative regulator of T cell proliferation and activation and is known to mediate suppressive functions after binding to its ligands PD-L1 or PD-L2 [6], [7]. Another important inhibitory receptor known to play a role in CD8+ T cell dysfunction is T cell immunoglobulin and mucin domain 3 (Tim-3), which is thought to recognize the ligand galectin-9 (galactose-specific soluble lectin 9) [8]. Increased levels of these inhibitory receptors were found on the surface of exhausted CD8+ T cells from patients chronically infected with HCV, HIV or hepatitis B virus (HBV) [9], [10], [11]. In addition, high levels of these inhibitory receptors were also found on CD8+ T cells from mice chronically infected with LCMV or FV [12], [13] as well as on CD8+ T cells from rhesus macaques chronically infected with simian immunodeficiency virus (SIV) [14]. In several mouse and monkey studies a temporary blockade of the interactions between these receptors and their ligands resulted in a partial reconstitution of effector functions of exhausted virus-specific CD8+ T cells and reduced viral loads during chronic infections [6], [7], [12], [14], [15]. Additionally, a successful clinical trial of PD-1–blocking was recently completed in patients with different types of cancer [16]. Despite these encouraging results the current studies indicate that blocking inhibitory receptors or manipulating Tregs only partially reactivates CD8+ T cells and do not completely eliminate chronic virus. We therefore decided to combine these two therapeutic approaches, which until now has only been done in a study investigating the pathogenesis of acute murine AIDS [17], but never been performed in an established chronic virus infection. As a model we choose the chronic infection of mice with FV, a life-long persistent infection that is characterized by constant low-level virus replication and profound functional exhaustion of CD8+ T cells [18].
Results and Discussion
DEREG mice [19] were infected with FV and rested for >60 days to establish chronic infection. During chronic FV infection specific CD8+ T cells are present with an exhausted phenotype [20] and expressed high levels of the inhibitory receptors PD-1 and Tim-3 (Fig. S2). In addition, they have expanded populations of activated, but non-virus-specific Tregs in lymphoid tissues [3], [21], [22]. The viral set points are low (mean of about 10 infected cells per 106 spleen cells) and relatively consistent between individual animals. After 60 days of infection, one group of mice received diphtheria toxin (DT), which resulted in over 97% depletion of eGFP+ Tregs [3], [4]. Another group of animals was injected with monoclonal antibodies against PD-L1 and Tim-3 to block inhibitory receptor signaling on CD8+ T cells. After depletion of Tregs a more than 5-fold increase in the mean frequency of total activated (CD43+) CD8+ T cells was detected in the spleen of chronically infected mice (Fig. 1A). A 4-fold expansion of CD43+ CD8+ T cells was also found in mice treated with α-PD-L1 and α-TIM-3 but it was significantly lower than in the group of Treg depleted mice. Activated CD43+ CD8+ T cells also had increased expression of CD44 and were negative for CD62L demonstrating their effector phenotype (data not shown and [22]). Tetramers specific for the immunodominant FV epitope Db-GagL [23] were then used to determine augmentation of virus-specific CD8+ T cell responses. Treg ablation resulted in a 2.5-fold increase in the mean frequency of tetramer positive CD8+ T cells compared to non-depleted controls (Fig. 1B). Interestingly, in this assay the increased frequencies in FV-specific T cells was significantly higher in the group of mice receiving the blocking antibodies (7-fold) than those receiving DT. Hence, Treg depletion resulted in a higher expansion of the total population of activated CD8+ T cells compared to inhibitory receptor blockage, whereas it was the other way around for the expansion of the tetramer+ cells recognizing the immunodominant FV CD8+ T cell epitope (Fig. 1A and B). From studies with LCMV it is known that CD8+ T cells specific for immunodominant epitopes tend to expand more efficiently than the ones recognizing subdominant epitopes after treatment with blocking antibodies [6], [24]. In contrast, Treg depletion most likely affects all activated T cells rather equally. Thus, blocking inhibitory receptors seems to be most effective in reactivating terminally differentiated T cells because those are the ones that express the highest levels of inhibitory receptors [13], whereas Treg depletion may be more efficient to reactivate the total CD8+ T cell effector population. Since virus-specific CD8+ T cells are exhausted during chronic FV infection [20], we determined the level of functional reactivation after Treg depletion or inhibitory receptor signaling blockade. The cytolytic capacity of CD8+ T cells is a key factor in FV control [25], so we focused on analyzing the production of the cytotoxic molecule granzyme B and the in vivo killing activity of CD8+ T cells after treatment. Activated CD8+ T cells from chronically infected mice expressed very low levels of granzyme B, which were indistinguishable from those of naive mice. Consequently, no killing of splenocytes loaded with the FV immunodominant epitope [3], [23] was found in an in vivo CTL assay. Following Treg depletion as well as receptor blockade significantly more of the total activated CD8+ T cells expressed granzyme B (Fig. 2A). This was also the case for the subset of tetramer+ CD8+ T cells but for these cells recognizing the immunodominant FV epitope the treatment with α-PD-L1 and α-TIM-3 was more efficient for granzyme B induction than Treg depletion (Fig. 2B). Similar findings were made in the in vivo CTL assay. Treg-depleted mice averaged 46% killing of peptide-loaded targets whereas the killing activity was with almost 86% significantly higher in chronically infected mice receiving the blocking antibodies (Fig. 2C and D). These data demonstrate that reactivation of cytotoxic CD8+ T cell function in chronic retroviral infection is more pronounced when inhibitory receptors on CD8+ T cells were blocked compared to depletion of Tregs.
Fig. 1. CD8+ T cell functions after Treg depletion and/or inhibitory pathway blockage in chronically infected mice. 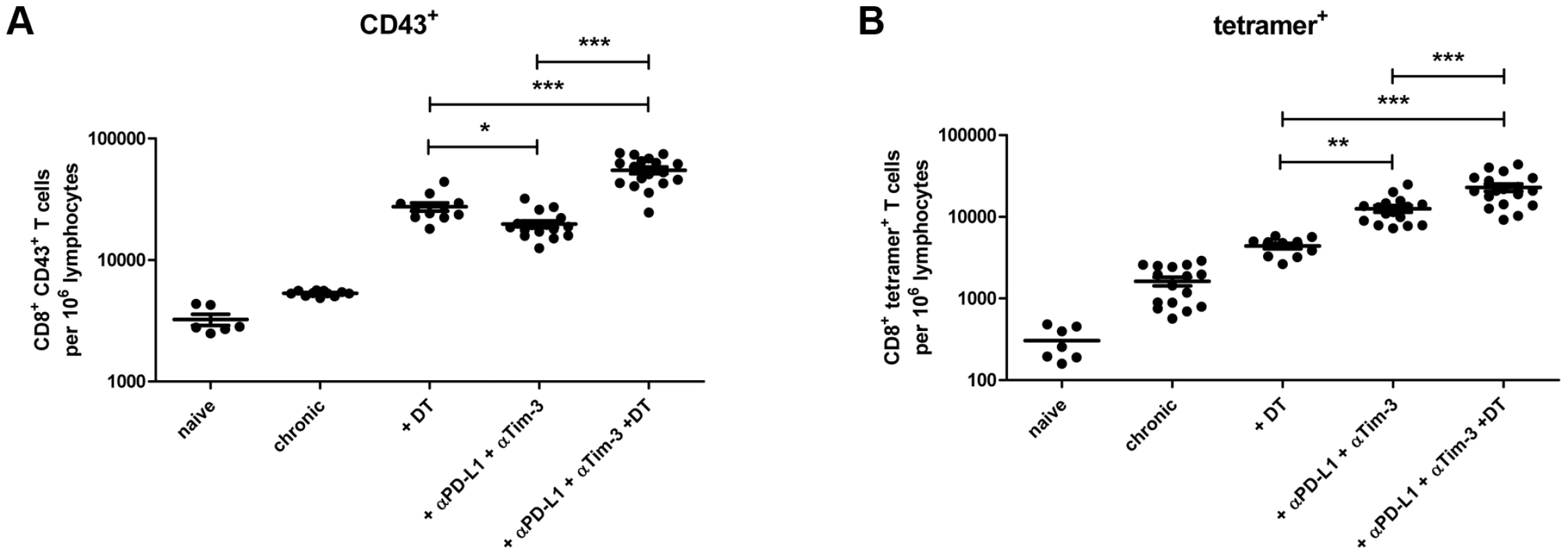
Mice chronically infected with FV were treated with DT (DEREG mice) and/or blocking antibodies against PD-L1 and TIM-3 as indicated. Frequencies of (A) total activated (expressing the activation-induced isoform of CD43) CD8+ T cells and (B) virus-specific class I tetramer+ CD8+ T cells are shown as calculated by flow cytometry. Each dot represents an individual mouse. Data were pooled from 3 to 5 independent experiments with similar results. Statistically significant differences are indicated by asterisks (*<0.05; **<0.005; ***<0.0005; analysis of variance [ANOVA], Newman-Keuls multiple comparison test). Fig. 2. CD8+ T cell activity in chronically infected mice after Treg depletion and/or blocking of inhibitory pathways. 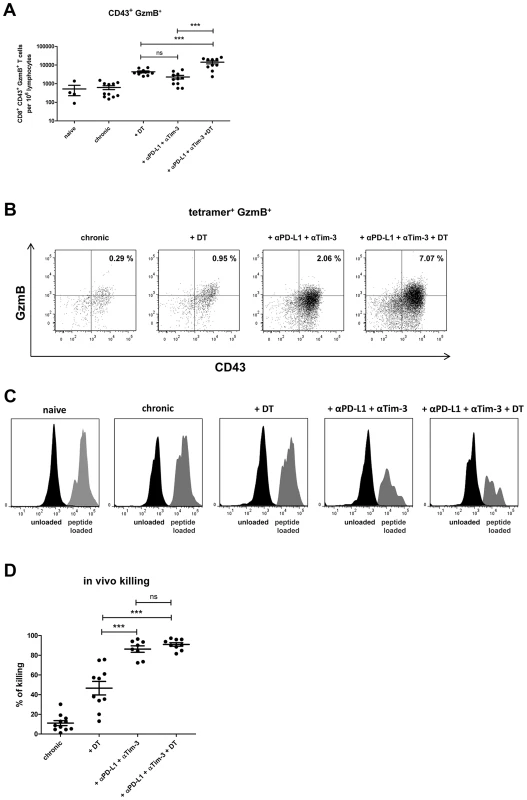
Mice chronically infected with FV were treated with DT (DEREG mice) and/or blocking antibodies against PD-L1 and TIM-3 as indicated. (A) Frequencies of CD8+ CD43+ granzyme B+ (GzmB) T cells are shown as calculated by flow cytometry. Each dot represents an individual mouse. Data were pooled from 3 to 5 independent experiments with similar results. Statistically significant differences are indicated by asterisks (*<0.05; **<0.005; ***<0.0005; analysis of variance [ANOVA], Newman-Keuls multiple comparison test). (B) Representative dot plots for GzmB production in virus-specific (tetramer+) CD8+ T cells. The percentages of tetramer+ CD8+ T cells that were CD43+ and expressed GzmB are given in the upper right quadrants. (C) Representative histograms showing differential killing of unloaded cells (CFSElow, black) versus target cells loaded with the FV Db-GagL peptide (CFSEhi, grey) in spleens using an in vivo cytotoxicity assay. (D) Percentages of target cell killing in spleens of mice from the differently treated groups. Data were pooled from spleen cells of 8–11 mice per group from 2 independent experiments. Statistically significant differences are indicated by asterisks (***<0.0005; analysis of variance [ANOVA], Newman-Keuls multiple comparison test). This suggested that α-PD-L1 and α-TIM-3 treatment might be more potent than ablation of Tregs in diminishing chronic virus loads. Indeed, we found a significant difference in viral loads between these two groups one day post treatment (Fig. 3A) indicating that the strongly enhanced cytotoxic activity of FV-specific CD8+ T cells after blockage of inhibitory receptors correlated with superior virus control. As previously published the therapeutic effect of Treg depletion was sustained [5] with reduced viral loads still detectable at 21 days post treatment (Fig. 3B). In addition, the viral set points remained also reduced 21 days after cessation of antibody blockage (Fig. 3B).
Fig. 3. Viral loads in chronic infection after Treg depletion and/or blocking of inhibitory pathways. 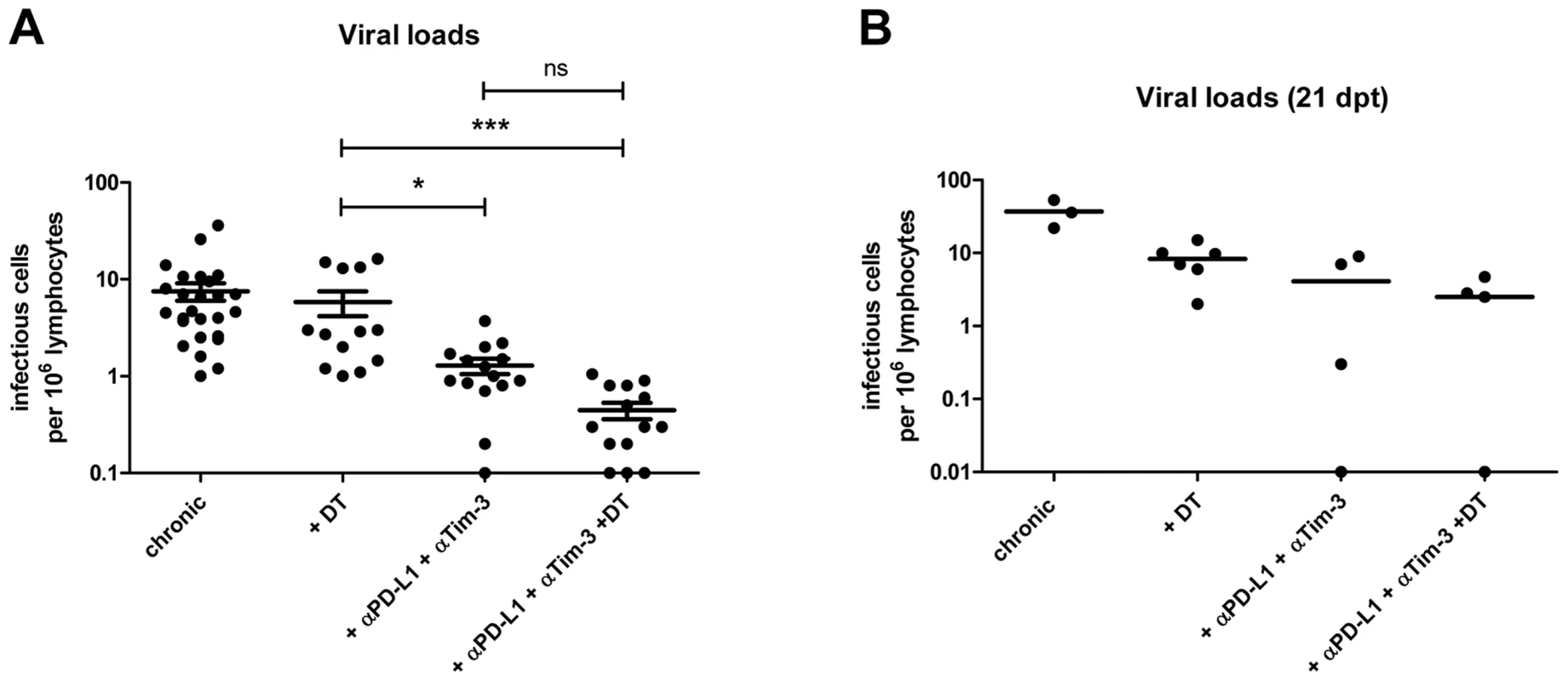
(A) Spleens of chronically FV-infected mice from the different treatment groups were analyzed for viral loads by infectious center assays 1 day after termination of treatment. Each dot represents an individual mouse. Data were pooled from 4 to 6 independent experiments with similar results. Statistically significant differences are indicated by asterisks (*<0.05; ***<0.0005; analysis of variance [ANOVA] nonparametric, Dunn's multiple comparison test). (B) To study long-term effects of the treatment spleens of mice from the different groups were analyzed for viral loads by infectious center assay at 21 days post treatment (dpt). Each dot represents an individual mouse. As a next step we performed a combination therapy with DT injection plus antibody treatment in chronically infected mice. The results demonstrated an additive therapeutic effect with significant higher frequencies of total activated (CD43+) and tetramer+ CD8+ T cells than after any of the two single treatments (Fig. 1A and B). Further characterization of the CD8+ T cells revealed that the combination therapy induced proliferation (Fig. S3A) and increased IFN-γ production by activated CD8+ T cells (Fig. S3B and C). Virus-specific CD8+ T cells were high in KLRG1 (Killer cell lectin-like receptor G1) expression after combination therapy confirming their terminally differentiated effector phenotype (Fig. S3D). Granzyme B expression was also further enhanced in total activated CD8+ T cells (Fig. 2A) as well as in virus-specific CD8+ T cells (Fig. 2B) after combination therapy. However, this was only reflected in augmented FV-specific target cell killing when Treg depleted mice were compared to the animals receiving DT plus α-PD-L1 and α-TIM-3. No significant difference was found between mice treated with antibodies plus DT and antibodies alone, most likely because the target cell killing in this group was already at a mean of 86% (Fig. 2D). The significantly enhanced CD8+ T cell killing resulted in significantly reduced chronic viral loads when Treg ablated mice were compared to the mouse group receiving combination therapy (Fig. 3A). The combined therapy also reduced the viral set points in comparison to the group of mice in which only the inhibitory receptors were blocked, but this difference was not significant using the one-way ANOVA and Newman-Keuls multiple comparison test. However, when only the α-PD-L1 and α-TIM-3 versus the α-PD-L1 and α-TIM-3 plus DT group were compared with a Student's t-test the reduction was highly significant (p = 0.0009) (Fig. 3A). Furthermore, an analysis performed three weeks after termination of treatment demonstrated a sustained reduction in chronic viral loads after combination therapy (Fig. 3B). At that time, CD8+ T cells still possessed slightly enhanced functional properties such as higher activation levels (CD62L− CD43+) and increased IFN-γ production, although the Treg compartment was fully restored 21 days after the last DT injection (data not shown).
The current experiments show that both Tregs and inhibitory receptors are independent factors mediating T cell exhaustion during chronic infection. Until now the exact molecular mechanism of CD8+ T cell suppression by Tregs has not been unraveled for most chronic viral infections, but studies suggest that it is cell-to-cell contact dependent [26], [27]. Since ligands for inhibitory receptors were found to be highly expressed on Tregs [28] these cells might be able to inhibit effector T cells by direct interaction of their ligands with inhibitory receptors. In this scenario, depleting Tregs and blocking inhibitory signals with antibodies would target the same suppressive mechanism. However, our current data show that Tregs and inhibitory receptors are mostly non-overlapping mechanisms of T cell exhaustion and that blocking both pathways has an additive therapeutic effect during chronic retroviral infection. Another cell-to-cell contact dependent mechanism of Treg suppression, like the cyclic AMP (Adenosine monophosphate) that has been described in HIV infection [27], are therefore more likely contributing to functional T cell exhaustion.
Our findings have obvious implications for therapeutic strategies to treat chronic infections. A reduction of chronic viral loads following a temporary treatment could be beneficial for many chronic viral infections in humans. This is especially true for HIV infection where chronic viral set points strongly influence disease progression [29]. Similar to chronic FV infection, Tregs accumulate in lymphoid tissues of HIV-infected patients [30], [31] and the effector T cells upregulate inhibitory receptors [32], which correlates with CD8+ T cell exhaustion and local viral loads [33]. Similar findings were made in studies with HCV-infected individuals [34], [35], [36], suggesting that these patients could benefit from a combination therapy of Treg manipulation and inhibitory receptor blockade. One obvious concern with Treg manipulation is that this may result in immunopathology or autoimmunity. However, in the current and in previous experiments transient ablation of Tregs in mice infected with FV did not result in any clinical symptoms of immunopathology ([3] and data not shown). Furthermore, clinical trials with melanoma patients in which a recombinant IL-2-diphteria toxin fusion protein (ONTAK®) was administered [37], demonstrated that temporary Treg depletion did not induce serious clinical side effects in humans.
These results demonstrate that Tregs and inhibitory receptors may both be targeted at the same time in new combination therapies as a promising strategy to treat chronic infectious diseases.
Methods
Ethics statement
Animal experiments were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV-SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). The protocol was approved by the North Rhine-Westphalia State Agency for Nature, Environment and Consumer Protection (LANUV) (Permit number: G 1208/11 and G 1341/12). All efforts were made to minimize suffering.
Mice
Inbred C57BL/6 (B6) and DEREG [19] mice were maintained under pathogen free conditions. Experiments were done using mice (H-2b/b, Fv1b/b, Fv2r/r) or transgenic mice backcrossed on C57BL/6 background that are resistant to FV-induced leukemia. All mice were females of 8–12 weeks of age at the beginning of the experiments.
Virus and viral infection
The FV stock used in these experiment was FV complex containing B-tropic Friend murine leukemia helper virus (F-MuLV) and polycythemia-inducing spleen focus-forming virus [38]. The stock was prepared as a 10% spleen cell homogenate from BALB/c mice infected 14 days previously with 3,000 spleen focus-forming units of non-cloned virus stock. Experimental mice were injected intravenously with 60,000 spleen focus-forming units of FV complex and additional 2×105 units of F-MuLV to enhance chronic FV loads.
The virus stock did not contain lactate dehydrogenase-elevating virus.
Infectious center assays
Infectious center assays were performed as described previously [39].
Cell surface and intracellular staining by flow cytometry
Surface and intracellular staining were performed as described previously [5]. Data were acquired on a LSR II flow cytometer (Becton Dickinson) from 350,000–500,000 lymphocyte-gated events per sample. Analyses were done using FACSDiva software (Becton Dickinson) and FlowJo software (Treestar).
Lymphocyte depletion
To deplete Tregs, chronically FV-infected DEREG mice were injected intraperitoneally with diphtheria toxin (Merck, Darmstadt, Germany), diluted in endotoxin-free PBS. 0.5 µg DT was inoculated every third day for 3 times. The treatment depleted over 97% of the CD4+ eGFP+ T cells in all investigated organs of DEREG mice. The T cell responses and viral loads were analyzed 1 or 21 days post treatment.
In vivo blockade
For blockade of the PD-1 pathway in chronically FV-infected mice, 200 µg rat anti-mouse PD-L1 Ab (10F.9G2; BioXCell) was administered intraperitoneally every third day for 4 times. To block the Tim-3 pathway, 100 µg rat anti-mouse Tim-3 Ab (RMT3-23; BioXCell) was administered intraperitoneally every other day for 4 times. The anti-PD-L1 treatment did not influence the PD-1 expression on CD8+ T cells from chronically infected mice, whereas the direct blocking of the Tim-3 receptor could be nicely demonstrated by flow cytometry staining (Fig. S2). The T cell responses and viral loads were analyzed one day post treatments or 21 days post treatments (Fig. S1)
Tetramers and tetramer staining
For detection of Db-GagL-specific CD8+ T cells, nucleated spleen cells were stained with PE labeled MHC class I H2-Db tetramers specific for FV GagL peptide [23], [40] (Beckman Coulter, Marseille, France).
In vivo cytotoxicity assay
The in vivo CTL assay described by Barber et al. [41] was modified to measure cytotoxicity in FV-infected mice [3].
Statistical analysis
Statistical data were derived by using the GraphPad Prism software (GraphPad Software). Data were analyzed using one-way ANOVA and Newman-Keuls multiple comparison test, non-parametric one-way ANOVA and Dunn's multiple comparison test or Student's t-test.
Supporting Information
Zdroje
1. IwashiroM, MesserRJ, PetersonKE, StromnesIM, SugieT, et al. (2001) Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proceedings of the National Academy of Sciences of the United States of America 98 : 9226–9230.
2. MyersL, JoedickeJJ, CarmodyAB, MesserRJ, KassiotisG, et al. (2013) IL-2-independent and TNF-alpha-dependent expansion of Vbeta5+ natural regulatory T cells during retrovirus infection. Journal of immunology 190 : 5485–5495.
3. ZelinskyyG, DietzeKK, HuseckenYP, SchimmerS, NairS, et al. (2009) The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood 114 : 3199–3207.
4. ZelinskyyG, DietzeK, SparwasserT, DittmerU (2009) Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog 5: e1000406.
5. DietzeKK, ZelinskyyG, GibbertK, SchimmerS, FrancoisS, et al. (2011) Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proceedings of the National Academy of Sciences of the United States of America 108 : 2420–2425.
6. BarberDL, WherryEJ, MasopustD, ZhuB, AllisonJP, et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439 : 682–687.
7. BlackburnSD, ShinH, HainingWN, ZouT, WorkmanCJ, et al. (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10 : 29–37.
8. JonesRB, NdhlovuLC, BarbourJD, ShethPM, JhaAR, et al. (2008) Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. The Journal of experimental medicine 205 : 2763–2779.
9. UrbaniS, AmadeiB, TolaD, MassariM, SchivazappaS, et al. (2006) PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. Journal of virology 80 : 11398–11403.
10. DayCL, KaufmannDE, KiepielaP, BrownJA, MoodleyES, et al. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443 : 350–354.
11. MaierH, IsogawaM, FreemanGJ, ChisariFV (2007) PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. Journal of immunology 178 : 2714–2720.
12. JinHT, AndersonAC, TanWG, WestEE, HaSJ, et al. (2010) Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America 107 : 14733–14738.
13. ZelinskyyG, MyersL, DietzeKK, GibbertK, RoggendorfM, et al. (2011) Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. Journal of immunology 187 : 3730–3737.
14. VeluV, KannanganatS, IbegbuC, ChennareddiL, VillingerF, et al. (2007) Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. Journal of virology 81 : 5819–5828.
15. PalmerBE, NeffCP, LecureuxJ, EhlerA, DsouzaM, et al. (2013) In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. Journal of immunology 190 : 211–219.
16. BrahmerJR, TykodiSS, ChowLQ, HwuWJ, TopalianSL, et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine 366 : 2455–2465.
17. LiW, GreenWR (2011) Immunotherapy of murine retrovirus-induced acquired immunodeficiency by CD4 T regulatory cell depletion and PD-1 blockade. Journal of virology 85 : 13342–13353.
18. DittmerU, HeH, MesserRJ, SchimmerS, OlbrichAR, et al. (2004) Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity 20 : 293–303.
19. LahlK, LoddenkemperC, DrouinC, FreyerJ, ArnasonJ, et al. (2007) Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 204 : 57–63.
20. ZelinskyyG, RobertsonSJ, SchimmerS, MesserRJ, HasenkrugKJ, et al. (2005) CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J Virol 79 : 10619–10626.
21. AntunesI, TolainiM, KissenpfennigA, IwashiroM, KuribayashiK, et al. (2008) Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology. Immunity 29 : 782–794.
22. ZelinskyyG, KraftAR, SchimmerS, ArndtT, DittmerU (2006) Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur J Immunol 36 : 2658–2670.
23. ChenW, QinH, ChesebroB, CheeverMA (1996) Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J Virol 70 : 7773–7782.
24. BlattmanJN, WherryEJ, HaSJ, van der MostRG, AhmedR (2009) Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. Journal of virology 83 : 4386–4394.
25. ZelinskyyG, BalkowS, SchimmerS, SchepersK, SimonMM, et al. (2004) Independent roles of perforin, granzymes, and Fas in the control of Friend retrovirus infection. Virology 330 : 365–374.
26. RobertsonSJ, MesserRJ, CarmodyAB, HasenkrugKJ (2006) In vitro suppression of CD8+ T cell function by Friend virus-induced regulatory T cells. J Immunol 176 : 3342–3349.
27. Moreno-FernandezME, RuedaCM, RusieLK, ChougnetCA (2011) Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 117 : 5372–5380.
28. FranciscoLM, SalinasVH, BrownKE, VanguriVK, FreemanGJ, et al. (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine 206 : 3015–3029.
29. MellorsJW, RinaldoCRJr, GuptaP, WhiteRM, ToddJA, et al. (1996) Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272 : 1167–1170.
30. NilssonJ, BoassoA, VelillaPA, ZhangR, VaccariM, et al. (2006) HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108 : 3808–3817.
31. BettsMR, NasonMC, WestSM, De RosaSC, MiguelesSA, et al. (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107 : 4781–4789.
32. D'SouzaM, FontenotAP, MackDG, LozuponeC, DillonS, et al. (2007) Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. Journal of immunology 179 : 1979–1987.
33. AnderssonJ, BoassoA, NilssonJ, ZhangR, ShireNJ, et al. (2005) The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol 174 : 3143–3147.
34. McMahanRH, Golden-MasonL, NishimuraMI, McMahonBJ, KemperM, et al. (2010) Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. The Journal of clinical investigation 120 : 4546–4557.
35. SeigelB, BengschB, LohmannV, BartenschlagerR, BlumHE, et al. (2012) Factors That Determine the Antiviral Efficacy of HCV-Specific CD8(+) T Cells Ex Vivo. Gastroenterology 144 (2): 426–36.
36. ValiB, JonesRB, SakhdariA, ShethPM, ClaytonK, et al. (2010) HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. European journal of immunology 40 : 2493–2505.
37. DannullJ, SuZ, RizzieriD, YangBK, ColemanD, et al. (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115 : 3623–3633.
38. LillyF, SteevesRA (1973) B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology 55 : 363–370.
39. DittmerU, BrooksDM, HasenkrugKJ (1998) Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J Virol 72 : 6554–6558.
40. SchepersK, ToebesM, SotthewesG, Vyth-DreeseFA, DellemijnTA, et al. (2002) Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J Immunol 169 : 3191–3199.
41. BarberDL, WherryEJ, AhmedR (2003) Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol 171 : 27–31.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication FidelityČlánek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



