-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
article has not abstract
Published in the journal: . PLoS Pathog 9(12): e32767. doi:10.1371/journal.ppat.1003760
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003760Summary
article has not abstract
Coronaviruses Contain the Largest Known RNA Genomes
Coronaviruses (CoVs) are positive-sense single-stranded RNA viruses and contain the largest known RNA genomes, ranging from 27 to 32 kilobases in length. CoVs are capable of trans-species movement as evidenced by the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) epidemic in 2002–2003 [1], [2]. Additionally, the emergence of Middle East Respiratory Syndrome coronavirus (MERS-CoV) in 2012 demonstrates that CoVs continue to cause severe and lethal human disease [3]. Exactly how CoVs maintain the integrity of their large genomes while generating the population diversity required for emergence and adaptation has been a major question in RNA virology. The discovery of 3′-to-5′ exoribonuclease (ExoN) activity within CoV nonstructural protein 14 (nsp14-ExoN), which is critical for CoV high-fidelity replication, has challenged the long-held paradigm that RNA viruses cannot proofread and raises the possibility of an entirely new model for how RNA viruses regulate replication fidelity. We will summarize: 1) the data supporting proofreading during CoV replication; 2) the possibility of a multi-protein fidelity complex, using E. coli DNA polymerase III as a conceptual framework; and 3) the promise of genetic and therapeutic interference with fidelity regulation as an approach for attenuation and treatment of CoVs.
CoVs Encode a Proofreading 3′-to-5′ Exoribonuclease Distinct from the Viral RNA-Dependent RNA Polymerase
RNA viruses rely primarily on low-fidelity replication by RNA-dependent RNA polymerases (RdRps) to facilitate viral adaptation to complex host environments [4]. Because of the lack of proofreading and repair functions, the average mutation rate of RNA viruses is estimated to be around one mutation per genome per round of replication [5], [6]. Much beyond this rate, RNA viruses risk crossing an “error threshold,” or the point at which there are too many deleterious mutations for the viral population to reproduce faithfully [4]. Thus, while allowing for enormous population diversity, the low-fidelity of RdRp-mediated replication imposes constraints on both viral genome size and maintenance of genomic integrity, theoretically limiting the size of RNA virus genomes to around ∼15 kb (reviewed in [4]). RNA viruses have evolved several mechanisms to partially circumvent these constraints, including large population sizes, rapid replication cycles, robustness to mutations, recombination, and compact genomes [7], [8].
In addition to these mechanisms, CoVs encode several additional RNA-processing functions within their 16 nonstructural proteins (nsp1–16), including a 3′-to-5′ exoribonuclease (nsp14-ExoN; Figure 1). Many of these enzymatic functions likely have facilitated the expansion of the CoV genome beyond the ∼15 kb upper limit. CoV nsp14-ExoN contains four conserved DE-D-D acidic residues (Figure 1B; [9]), which are the hallmark of the DEDDh superfamily of DNA and RNA exonucleases. Many of these DEDDh exonucleases are involved in proofreading [10]. Alanine substitutions at the CoV DE-D-D residues significantly reduces or abolishes the nucleolytic activity of nsp14-ExoN in vitro [9] and results in CoVs with up to 20-fold reduced fidelity (increased mutation rate) in vitro [11], [12] and in vivo [13]. A recent biochemical study demonstrated that nsp14-ExoN is capable of removing single 3′ mismatched nucleotides and that this activity is stimulated by the non-enzymatic protein nsp10 [14]. Thus, all biochemical and genetic studies indicate that CoV nsp14-ExoN performs a proofreading function during CoV replication.
Fig. 1. CoV genomic architecture and nonstructural proteins (nsps). 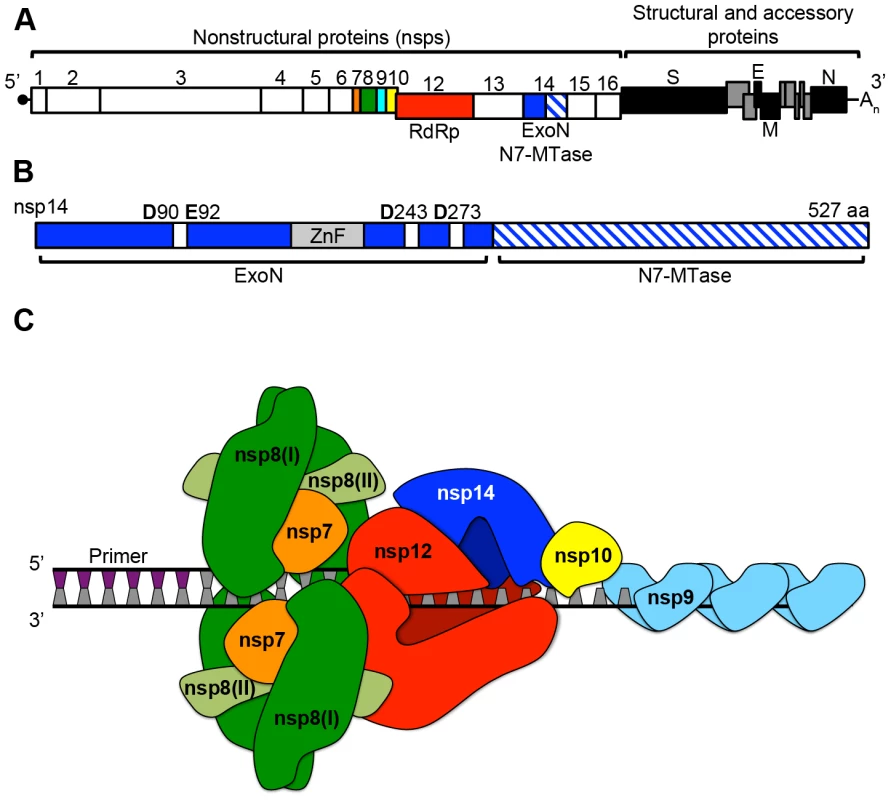
A. Shown is a linear schematic of the SARS-CoV genome containing both the nonstructural protein (nsp) and the structural and accessory protein open reading frames. The −1 ribosomal frameshift between nsps 10 and 12 is shown by the offset boxes. Boxes denoting each individual nsp are scaled according to amino acid length, and products of polyprotein 1a (nsps1–10) and 1ab (nsps12–16) processing are shown. Colors are as follows: orange (nsp7), green (nsp8), cyan (nsp9), yellow (nsp10), red (nsp12: RNA-dependent RNA polymerase [RdRp]), and blue (nsp14: 3′-to-5′ exoribonuclease [ExoN] and N7-methyltranferase [N7-MTase]). B. A linear schematic of nsp14 is shown. The ExoN domain is colored solid blue, while the N7-MTase domain is hatched blue and white. The zinc-finger domain (ZnF) is shown in gray. DE-D-D residues characteristic of the DEDDh exonuclease superfamily [10] are shown as white boxes. C. A model of how nsps7–8 could assemble on viral dsRNA and interact with the putative multi-subunit CoV polymerase complex consisting of nsps10, 12, and 14. Colors are the same as in panel A, except the two forms of nsp8 (I and II) are shown in green and light green respectively. A short (∼6 n.t.) primer generated by the non-canonical RdRp activity of nsp8 is shown. Binding of ssRNA by nsp9 is also shown. Is nsp14-ExoN the Proofreading Component of a Multi-Subunit Polymerase Complex Containing nsp10 and nsp12-RdRp?
The identification of a proofreading exoribonuclease distinct from the CoV RNA-dependent RNA polymerase (nsp12-RdRp) suggests that CoVs might use a multi-protein complex for RNA synthesis and regulation of fidelity. A possible analogy would be the E. coli DNA polymerase III (pol III) core (αεθ), which contains a polymerase (α subunit), a DEDDh exonuclease (ε subunit), and an exonuclease stimulatory protein (θ subunit) [15]. Is there evidence to support or to even suggest the possibility that CoVs contain a DNA pol III–like multi-subunit polymerase complex? Several lines of evidence support the hypothesis that regulation of CoV fidelity involves, at minimum, three nsps: nsp12-RdRp, nsp14-ExoN, and nsp10 (Figure 1C). The presence of nsp12-RdRp would be critical as nsp14-ExoN has not been demonstrated to possess polymerase activity, and thus error recognition, removal, and repair would require interactions between nsp14-ExoN and nsp12-RdRp on elongating RNA. Several studies with poliovirus, chikungunya virus, and Coxsackievirus have demonstrated that point mutations within the viral RdRp can decrease or increase fidelity [16]–[19]. While the fidelity of nucleoside triphosphate (NTP) incorporation for nsp12-RdRp has not been determined, CoV nsp12-RdRp would provide essential polymerase activity and likely function to regulate fidelity primarily through NTP discrimination.
The CoV nsp10 is a ∼130 amino acid protein with no identified enzymatic function that has been demonstrated to bind and enhance nsp14-ExoN activity in vitro by up to 35-fold [14]. While the precise mechanism and function of this stimulation during virus replication remain unknown, nsp10 also plays a critical yet undefined role in viral polyprotein processing and RNA synthesis [20], [21]. Unlike prototypical members of the DEDDh exonuclease superfamily, nsp14-ExoN contains a zinc-finger (ZnF) domain that is likely critical for associating with viral RNA, though the affinity of nsp14 for RNA has not been determined (Figure 1B). Nsp10 also has two ZnF domains [22]. Thus, nsp10 might increase the affinity of nsp14 for RNA, or directly affect ExoN activity through allosteric mechanisms. Extending our analogy of CoV nsp10 to the theta (θ) subunit of the E. coli pol III core, theta is a ∼76 amino acid protein with no known enzymatic function that has been shown to both bind and stabilize the pol III ε subunit and stimulate ε-mediated removal of terminal mismatches [23], [24]. Thus, nsp10 could act to increase nsp14-ExoN stability, or possibly alter the capacity or preference of nsp14 to remove certain types of mismatches. Why CoVs encode an enhancer of ExoN activity remains unknown. However, the presence of such a complex might allow for greater control, and potentially active regulation, of replication fidelity.
CoVs Encode Primase, Single-Strand RNA Binding, and Helicase Activities within nsps 8, 9 and 13
Might other CoV nonstructural proteins be associated with this putative multi-subunit polymerase? And how would such a complex be assembled? Again using E. coli pol III as a model, in addition to the αεθ core, the pol III holoenzyme requires additional subunits including a processivity factor (β sliding clamp), single-strand DNA-binding proteins (SSB), and a helicase (DnaB; reviewed in [15]). Remarkably, ssRNA-binding, helicase, and NTPase (e.g., ATPase) functions have been identified within the CoV replicase. SARS-CoV nsp9 dimerizes and binds both ssDNA and ssRNA in a sequence-independent manner [25]. The capacity of nucleic acid to be bound by multiple nsp9 dimers strongly suggests that nsp9 could function as an SSB-like molecule, thus protecting the CoV genome from degradation during replication (Figure 1C). NTPase and helicase activity have been identified within nsp13 of human coronavirus 229E [26], [27]. Because dsRNA is likely an abundant replicative intermediate, nsp13 could function ahead of the polymerase complex and unwind the dsRNA, which would then be subsequently bound by nsp9. More recent work has shown that SARS-CoV nsp8 has non-canonical RdRp activity and likely functions as a primase [28]. Additionally, two distinct folds of nsp8, named I and II, participate in a hexadecameric nsp7-nsp8 supercomplex capable of primer extension and binding RNA, likely due to the presence of a ∼30 Å central channel lined with positively charged amino acids (Figure 1C) [29], [30]. Combined, these studies suggest that the nsp7-nsp8 supercomplex could be analogous to the β sliding clamp within the pol III holoenzyme, which increases both the processivity and speed of the α subunit [15].
Is Fidelity Regulation a Target for CoV Inhibitors?
Ribavirin (RBV) and 5-fluorouracil (5-FU) have been shown to be mutagenic for many RNA viruses (reviewed in [31]). Nucleoside analogs, including both mutagens and chain terminators, have been tested or are used to treat many RNA and DNA viruses, including herpesviruses, HIV, hepatitis C virus, and hepatitis B virus. However, in vitro and in vivo studies demonstrate the limited antiviral activity and variable effectiveness of RBV against SARS-CoV infection [32], [33]. Our recent study shows that nsp14-ExoN is responsible for CoV resistance to RNA mutagens. SARS-CoV and murine hepatitis virus (MHV) lacking ExoN activity (ExoN−) demonstrate 160 - to 300-fold increased sensitivity to 5-FU [34]. While 5-FU treatment significantly increases the number of mutations present within both wild-type (ExoN+) and ExoN − viruses, almost 4,000 mutations were present in the ExoN − population following a single round of replication (Figure 2). This suggests that small-molecule inhibitors of ExoN could reproduce this genetic defect, and render CoVs vulnerable to treatment with RNA mutagens and obligate or non-obligate chain-terminating nucleoside analogs. The complete conservation of the ExoN − genotype and phenotype in CoVs studied to date [11]–[13], and the lack of redundancy or complementation of ExoN activity, further suggests that ExoN inhibition is a broadly applicable approach to therapeutically target CoVs. Furthermore, ExoN − viruses are highly attenuated in vivo, suggesting that small-molecule inhibition of ExoN activity would also significantly impact virus infection and virulence [13]. Finally, the probability that fidelity is regulated by a multi-protein complex involving nsp12-RdRp, nsp14-ExoN, and nsp10 suggests that interference with fidelity could be achieved through the disruption of multiple enzymatic activities and/or protein-protein interactions. The opportunity to disrupt the process of CoV fidelity regulation through the simultaneous targeting of multiple proteins, instead of a single protein or enzymatic function, has the potential for broad applicability and for potentially thwarting the emergence of resistance, particularly in combination with mutagens or other nucleoside analogs.
Fig. 2. Loss of ExoN activity dramatically increases the sensitivity of CoVs to RNA mutagens. 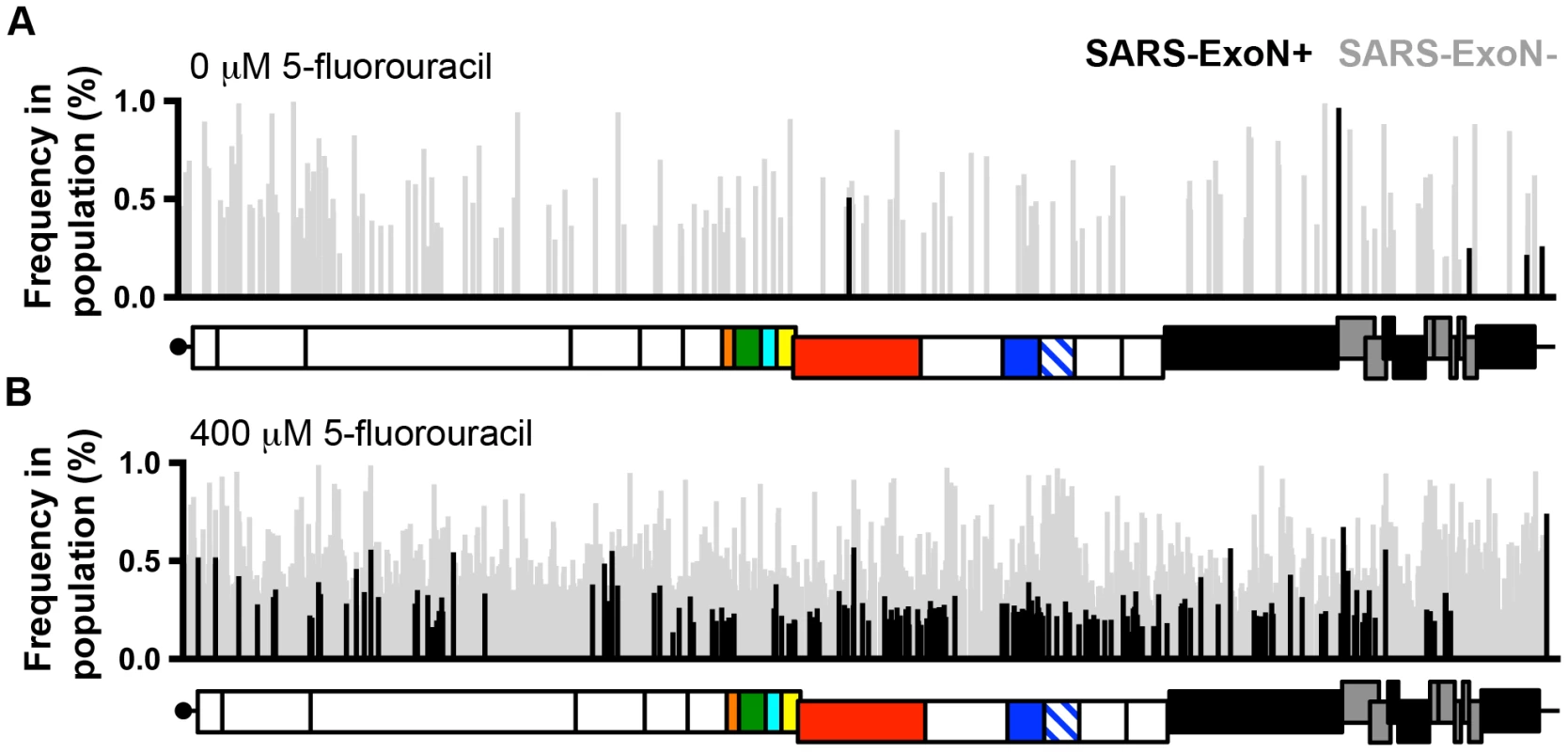
The distribution of characteristic 5-fluorouracil (5-FU)-mediated mutations (A-to-G and U-to-C) across the genomes of ExoN+ (wild-type; black) and ExoN− (gray) SARS-CoV following treatment with 0 µM (A) or 400 µM (B) 5-FU during single-cycle replication as determined by Illumnia deep sequencing. Each mutation is denoted as a vertical line, with line height representing the frequency of each mutation within the viral population. The genomic schematic below each panel show the approximate position of each mutation. Data are originally from [34]. Summary
Coronaviruses encode a proofreading exoribonuclease that is responsible for genome expansion, increased robustness to mutations, and resistance to mis-incorporations during RNA synthesis, as well as being required for virulence. The stability of the ExoN − genotype and phenotype provides a powerful model for the study of additional CoV fidelity determinants and of the effects of altered fidelity on virus replication, fitness, host-species range, and response to environmental changes. Experiments testing the proposed multi-subunit fidelity complex will yield exciting new insights into how CoVs ignore the RNA virus playbook and instead seem to play by their own rules while dancing on the edge of genetic disaster.
Zdroje
1. DrostenC, GuntherS, PreiserW, van der WerfS, BrodtHR, et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348 : 1967–1976.
2. KsiazekTG, ErdmanD, GoldsmithCS, ZakiSR, PeretT, et al. (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348 : 1953–1966.
3. ZakiAM, van BoheemenS, BestebroerTM, OsterhausAD, FouchierRA (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367 : 1814–1820.
4. DomingoE, SheldonJ, PeralesC (2012) Viral quasispecies evolution. Microbiol Mol Biol Rev 76 : 159–216.
5. MalpicaJM, FraileA, MorenoI, ObiesCI, DrakeJW, et al. (2002) The rate and character of spontaneous mutation in an RNA virus. Genetics 162 : 1505–1511.
6. DrakeJW, HollandJJ (1999) Mutation rates among RNA viruses. Proc Natl Acad Sci U S A 96 : 13910–13913.
7. GraciJD, GnadigNF, GalarragaJE, CastroC, VignuzziM, et al. (2012) Mutational robustness of an RNA virus influences sensitivity to lethal mutagenesis. J Virol 86 : 2869–2873.
8. LauringAS, FrydmanJ, AndinoR (2013) The role of mutational robustness in RNA virus evolution. Nat Rev Microbiol 11 : 327–336.
9. MinskaiaE, HertzigT, GorbalenyaAE, CampanacciV, CambillauC, et al. (2006) Discovery of an RNA virus 3′→5′ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc Natl Acad Sci U S A 103 : 5108–5113.
10. ZuoY, DeutscherMP (2001) Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res 29 : 1017–1026.
11. EckerleLD, BeckerMM, HalpinRA, LiK, VenterE, et al. (2010) Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog 6: e1000896 doi:10.1371/journal.ppat.1000896
12. EckerleLD, LuX, SperrySM, ChoiL, DenisonMR (2007) High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J Virol 81 : 12135–12144.
13. GrahamRL, BeckerMM, EckerleLD, BollesM, DenisonMR, et al. (2012) A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease. Nat Med 18 : 1820–1826.
14. BouvetM, ImbertI, SubissiL, GluaisL, CanardB, et al. (2012) RNA 3′-end mismatch excision by the severe acute respiratory syndrome coronavirus nonstructural protein nsp10/nsp14 exoribonuclease complex. Proc Natl Acad Sci U S A 109 : 9372–9377.
15. JohnsonA, O'DonnellM (2005) Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem 74 : 283–315.
16. GnadigNF, BeaucourtS, CampagnolaG, BorderiaAV, Sanz-RamosM, et al. (2012) Coxsackievirus B3 mutator strains are attenuated in vivo. Proc Natl Acad Sci U S A 109: E2294–2303.
17. CoffeyLL, BeeharryY, BorderiaAV, BlancH, VignuzziM (2011) Arbovirus high fidelity variant loses fitness in mosquitoes and mice. Proc Natl Acad Sci U S A 108 : 16038–16043.
18. VignuzziM, StoneJK, ArnoldJJ, CameronCE, AndinoR (2006) Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439 : 344–348.
19. PfeifferJK, KirkegaardK (2003) A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc Natl Acad Sci U S A 100 : 7289–7294.
20. DonaldsonEF, SimsAC, GrahamRL, DenisonMR, BaricRS (2007) Murine hepatitis virus replicase protein nsp10 is a critical regulator of viral RNA synthesis. J Virol 81 : 6356–6368.
21. DonaldsonEF, GrahamRL, SimsAC, DenisonMR, BaricRS (2007) Analysis of murine hepatitis virus strain A59 temperature-sensitive mutant TS-LA6 suggests that nsp10 plays a critical role in polyprotein processing. J Virol 81 : 7086–7098.
22. JosephJS, SaikatenduKS, SubramanianV, NeumanBW, BroounA, et al. (2006) Crystal structure of nonstructural protein 10 from the severe acute respiratory syndrome coronavirus reveals a novel fold with two zinc-binding motifs. J Virol 80 : 7894–7901.
23. Studwell-VaughanPS, O'DonnellM (1993) DNA polymerase III accessory proteins. V. Theta encoded by holE. J Biol Chem 268 : 11785–11791.
24. Taft-BenzSA, SchaaperRM (2004) The theta subunit of Escherichia coli DNA polymerase III: a role in stabilizing the epsilon proofreading subunit. J Bacteriol 186 : 2774–2780.
25. EgloffMP, FerronF, CampanacciV, LonghiS, RancurelC, et al. (2004) The severe acute respiratory syndrome-coronavirus replicative protein nsp9 is a single-stranded RNA-binding subunit unique in the RNA virus world. Proc Natl Acad Sci U S A 101 : 3792–3796.
26. SeybertA, HegyiA, SiddellSG, ZiebuhrJ (2000) The human coronavirus 229E superfamily 1 helicase has RNA and DNA duplex-unwinding activities with 5′-to-3′ polarity. RNA 6 : 1056–1068.
27. SeybertA, ZiebuhrJ (2001) Guanosine triphosphatase activity of the human coronavirus helicase. Adv Exp Med Biol 494 : 255–260.
28. ImbertI, GuillemotJC, BourhisJM, BussettaC, CoutardB, et al. (2006) A second, non-canonical RNA-dependent RNA polymerase in SARS coronavirus. EMBO J 25 : 4933–4942.
29. ZhaiY, SunF, LiX, PangH, XuX, et al. (2005) Insights into SARS-CoV transcription and replication from the structure of the nsp7-nsp8 hexadecamer. Nat Struct Mol Biol 12 : 980–986.
30. te VelthuisAJ, van den WormSH, SnijderEJ (2012) The SARS-coronavirus nsp7+nsp8 complex is a unique multimeric RNA polymerase capable of both de novo initiation and primer extension. Nucleic Acids Res 40 : 1737–1747.
31. PeralesC, MartinV, DomingoE (2011) Lethal mutagenesis of viruses. Curr Opin Virol 1 : 419–422.
32. StockmanLJ, BellamyR, GarnerP (2006) SARS: systematic review of treatment effects. PLoS Med 3: e343 doi:10.1371/journal.pmed.0030343
33. BarnardDL, DayCW, BaileyK, HeinerM, MontgomeryR, et al. (2006) Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res 71 : 53–63.
34. SmithEC, BlancH, VignuzziM, DenisonMR (2013) Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog 9: e1003565 doi:10.1371/journal.ppat.1003565
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete InfectionsČlánek Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?Článek CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial PathogensČlánek The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite PersistenceČlánek The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Host Susceptibility Factors to Bacterial Infections in Type 2 Diabetes
- LysM Effectors: Secreted Proteins Supporting Fungal Life
- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Innate Lymphoid Cells: New Players in IL-17-Mediated Antifungal Immunity
- Cytoplasmic Viruses: Rage against the (Cellular RNA Decay) Machine
- Balancing Stability and Flexibility within the Genome of the Pathogen
- The Evolution of Transmissible Prions: The Role of Deformed Templating
- Parental Transfer of the Antimicrobial Protein LBP/BPI Protects Eggs against Oomycete Infections
- Host Defense via Symbiosis in
- Regulatory Circuits That Enable Proliferation of the Fungus in a Mammalian Host
- Immune Therapeutic Strategies in Chronic Hepatitis B Virus Infection: Virus or Inflammation Control?
- Burning Down the House: Cellular Actions during Pyroptosis
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens
- Combining Regulatory T Cell Depletion and Inhibitory Receptor Blockade Improves Reactivation of Exhausted Virus-Specific CD8 T Cells and Efficiently Reduces Chronic Retroviral Loads
- Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens
- Identification of the Virulence Landscape Essential for Invasion of the Human Colon
- Nodular Inflammatory Foci Are Sites of T Cell Priming and Control of Murine Cytomegalovirus Infection in the Neonatal Lung
- Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis
- Mycobacterial MazG Safeguards Genetic Stability Housecleaning of 5-OH-dCTP
- Systematic MicroRNA Analysis Identifies ATP6V0C as an Essential Host Factor for Human Cytomegalovirus Replication
- Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion
- The CD8-Derived Chemokine XCL1/Lymphotactin Is a Conformation-Dependent, Broad-Spectrum Inhibitor of HIV-1
- Cyclin A Degradation by Primate Cytomegalovirus Protein pUL21a Counters Its Innate Restriction of Virus Replication
- Genome-Wide RNAi Screen Identifies Novel Host Proteins Required for Alphavirus Entry
- Zinc Sequestration: Arming Phagocyte Defense against Fungal Attack
- The Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence
- Biphasic Euchromatin-to-Heterochromatin Transition on the KSHV Genome Following Infection
- The Malarial Serine Protease SUB1 Plays an Essential Role in Parasite Liver Stage Development
- HIV-1 Vpr Accelerates Viral Replication during Acute Infection by Exploitation of Proliferating CD4 T Cells
- A Human Torque Teno Virus Encodes a MicroRNA That Inhibits Interferon Signaling
- The ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis
- An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients
- Enterohemorrhagic Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis
- Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1
- The Type-Specific Neutralizing Antibody Response Elicited by a Dengue Vaccine Candidate Is Focused on Two Amino Acids of the Envelope Protein
- Tmprss2 Is Essential for Influenza H1N1 Virus Pathogenesis in Mice
- Signatures of Pleiotropy, Economy and Convergent Evolution in a Domain-Resolved Map of Human–Virus Protein–Protein Interaction Networks
- Interference with the Host Haemostatic System by Schistosomes
- RocA Truncation Underpins Hyper-Encapsulation, Carriage Longevity and Transmissibility of Serotype M18 Group A Streptococci
- Gene Fitness Landscapes of at Important Stages of Its Life Cycle
- Phagocytosis Escape by a Protein That Connects Complement and Coagulation Proteins at the Bacterial Surface
- t Is a Structurally Novel Crohn's Disease-Associated Superantigen
- An Increasing Danger of Zoonotic Orthopoxvirus Infections
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
- Transcriptional Analysis of Murine Macrophages Infected with Different Strains Identifies Novel Regulation of Host Signaling Pathways
- Serotonergic Chemosensory Neurons Modify the Immune Response by Regulating G-Protein Signaling in Epithelial Cells
- Genome-Wide Detection of Fitness Genes in Uropathogenic during Systemic Infection
- Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages
- Intestinal CD103+ Dendritic Cells Are Key Players in the Innate Immune Control of Infection in Neonatal Mice
- Emerging Functions for the RNome
- KSHV MicroRNAs Mediate Cellular Transformation and Tumorigenesis by Redundantly Targeting Cell Growth and Survival Pathways
- HrpA, an RNA Helicase Involved in RNA Processing, Is Required for Mouse Infectivity and Tick Transmission of the Lyme Disease Spirochete
- A Toxin-Antitoxin Module of Promotes Virulence in Mice
- Real-Time Imaging of the Intracellular Glutathione Redox Potential in the Malaria Parasite
- Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism
- Novel Strategies to Enhance Vaccine Immunity against Coccidioidomycosis
- Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic
- —What Makes the Species a Ubiquitous Human Fungal Pathogen?
- αvβ6- and αvβ8-Integrins Serve As Interchangeable Receptors for HSV gH/gL to Promote Endocytosis and Activation of Membrane Fusion
- -Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite
- Semen CD4 T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology
- Host Defense via Symbiosis in
- Coronaviruses as DNA Wannabes: A New Model for the Regulation of RNA Virus Replication Fidelity
- Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4 T Cells
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



