-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFunctional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
Since the discovery of chromosomal inversions almost 100 years ago, how they are maintained in natural populations has been a highly debated issue. One of the hypotheses is that inversion breakpoints could affect genes and modify gene expression levels, although evidence of this came only from laboratory mutants. In humans, a few inversions have been shown to associate with expression differences, but in all cases the molecular causes have remained elusive. Here, we have carried out a complete characterization of a new human polymorphic inversion and determined that it is specific to East Asian populations. In addition, we demonstrate that it disrupts the ZNF257 gene and, through the translocation of the first exon and regulatory sequences, creates a previously nonexistent fusion transcript, which together are associated to expression changes in several other genes. Finally, we investigate the potential evolutionary and phenotypic consequences of the inversion, and suggest that it is probably deleterious. This is therefore the first example of a natural polymorphic inversion that has position effects and creates a new chimeric gene, contributing to answer an old question in evolutionary biology.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005495
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005495Summary
Since the discovery of chromosomal inversions almost 100 years ago, how they are maintained in natural populations has been a highly debated issue. One of the hypotheses is that inversion breakpoints could affect genes and modify gene expression levels, although evidence of this came only from laboratory mutants. In humans, a few inversions have been shown to associate with expression differences, but in all cases the molecular causes have remained elusive. Here, we have carried out a complete characterization of a new human polymorphic inversion and determined that it is specific to East Asian populations. In addition, we demonstrate that it disrupts the ZNF257 gene and, through the translocation of the first exon and regulatory sequences, creates a previously nonexistent fusion transcript, which together are associated to expression changes in several other genes. Finally, we investigate the potential evolutionary and phenotypic consequences of the inversion, and suggest that it is probably deleterious. This is therefore the first example of a natural polymorphic inversion that has position effects and creates a new chimeric gene, contributing to answer an old question in evolutionary biology.
Introduction
Polymorphic inversions have been known for a long time to segregate in the genomes of multiple species, ranging from insects to plants and animals, and constitute one of the most studied paradigms in evolutionary biology [1–3]. Given that they just change the orientation of a chromosomal segment and often do not result in gain or loss of DNA, inversions could easily be considered neutral variants, but far from this, their adaptive value and phenotypic effects are becoming increasingly clear. For example, in several species of Drosophila inversions affect characters like body size or developmental time [2], and some of them exhibit latitudinal or altitudinal clines that strongly suggest adaptation to different environmental conditions [1,4]. Inversions have also been attributed roles in flowering time and reproductive isolation between two ecotypes of monkeyflower [5], wing-pattern morphs in mimetic butterflies [6], stickleback fish freshwater adaptation [7], or in the social behavior and plumage color in sparrows [8], and even a human 900-kb inversion in chromosome 17 is thought to affect fertility and be positively selected in European populations [9].
However, the molecular mechanisms by which inversions are able to affect phenotype are not yet clear. Two main mechanisms have been proposed [10]. One is based on the effective suppression of recombination within the inverted sequence between standard and inverted chromosomes. This suppression can occur both through the reduced pairing and crossing over due to the formation of inversion loops in heterozygotes, and by selection against the unbalanced gametes resulting from unique crossovers within the inverted segment. In this case, the advantage of an inversion could be caused by the capture of a favorable combination of alleles maintained together in strong linkage disequilibrium (LD) due to the reduction of recombination [2,11,12]. Another option is that the phenotypic consequences of inversions depend on the mutational effect of their breakpoints on adjacent genes. Breakpoints can disrupt genes as happens in several disease-causing mutations in humans [13–15] or modify gene expression by separating coding regions from their regulatory elements or by providing new regulatory regions [16,17]. One striking example is the Rose-comb mutation in chickens, in which ectopic expression of a gene relocated by a 7.4-Mb inversion causes altered comb morphology and a gene disrupted by the same inversion has been associated to poor sperm motility [18]. Also, in humans, certain inversions mediated by repeats of complex structure seem to predispose to microdeletions that cause genetic disorders in the offspring [10,19,20], although in these cases, the disease-causing mutation is the deletion rather than the inversion. Nevertheless, very few cases of position effects have been identified for polymorphic inversions in natural populations [18,21].
Inversions represent a special challenge in complex genomes such as that of humans, not only due to their balanced nature, but also because their breakpoints are usually located within highly identical inverted repeated sequences that can reach large sizes (>100 kb). For these reasons, inversions have often been set aside in favor of other structural variants that are easier to detect, like copy number variants (CNVs). In recent years, the use of high-throughput techniques like paired-end mapping [22–24] has allowed the detection of multiple candidate regions to contain inversions in the human genome, but it is necessary to validate them since many represent different types of errors rather than real polymorphic variants [25]. Besides, large projects that try to characterize all human variation like the 1000 Genomes Project (1000GP) [26] might miss many inversions, either because short next-generation sequencing reads and paired-ends are not able to cross long repeats causing inversions [27], or due to the low coverage and difficulty of mapping reads containing simple breakpoint sequences.
So far, only a few polymorphic inversions not directly related to pathological processes have been studied in detail in humans [9,28–30]. Some of them have been associated to different expression levels of genes contained within the inverted sequence, such as the polymorphic inversion in chromosome 17q21 that is associated to a decreased MAPT expression, among other expression changes [31], or the 4.5-Mb inversion at 8p23 [29] and the 0.45-Mb inversion at chromosome 16p11 [32], where computationally-predicted inversion alleles correlate with expression levels of several genes. However, we do not know if these expression changes are consequence of position effects caused by the inversion breakpoints or of specific nucleotide changes captured by the inversion. These inversions have also been associated with certain phenotypes, such as recombination, fertility and several neurodegenerative diseases for the 17q21 inversion [9,33,34], or asthma and obesity for the 16p11 inversion [32], but the mechanisms linking the differentially expressed genes in the inverted region to their phenotypic consequences have not been completely elucidated. Other inversions have breakpoints located in positions where effects on adjacent genes would be expected. This is the case of a recently-described 16.5-kb polymorphic inversion that disrupts chymotrypsinogen precursor genes CTRB1 and CTRB2 by exchanging their respective first exons [30], although as both genes belong to the same family, complete copies are reconstructed in the inverted arrangement.
In this work, we describe a new polymorphic inversion found at a 4.7% frequency in East Asians, which represents one of the few inversions genotyped in several worldwide populations that show a limited geographical distribution. In addition, the inversion disrupts and inactivates a transcription factor gene and generates a new transcript in inverted chromosomes, thus providing mechanisms by which it may be directly affecting phenotype. Finally, we show that these changes are able to generate distinct genome-wide expression patterns and investigate the inversion’s functional and evolutionary impact.
Results
Identification of inversion breakpoints
Using available fosmid paired-end mapping data in humans [22], HsInv0379 inversion was predicted in chromosomal band 19p12 in a region close to the centromere [25]. This inversion was supported by five discordant fosmid clones belonging to a Japanese individual, NA18956, who also has concordant clones in the same genomic region. The analysis of available partial sequences from fosmid ABC9-45236600J18 insert obtained by 454 shotgun sequencing confirmed the presence of an inversion breakpoint, since these sequences map in two different regions separated by more than 400 kb on the GRCh37 (HG19) reference genome (Fig 1). Therefore, a fragment of 7 kb including the inversion breakpoint 1 (BP1) was amplified by PCR from the fosmid DNA and analyzed by restriction mapping with several enzymes (S1 Fig), which allowed us to locate the breakpoint within a 1.5-kb segment. Primers flanking this region were then used to amplify and sequence a 1,894-bp band containing the breakpoint directly from individual NA18956 genomic DNA. The alignment of this sequence in the inverted chromosome (Inv) with the reference genome (Std) revealed BP1 exact position, defining regions A (outside the inversion) and B and C (inside the inversion) (Fig 1). The mapping in the reference genome of sequence C allowed us to locate breakpoint 2 (BP2) and an 850-bp BD PCR product was amplified and sequenced from NA18956 genomic DNA.
Fig. 1. Inversion features and genotyping strategies. 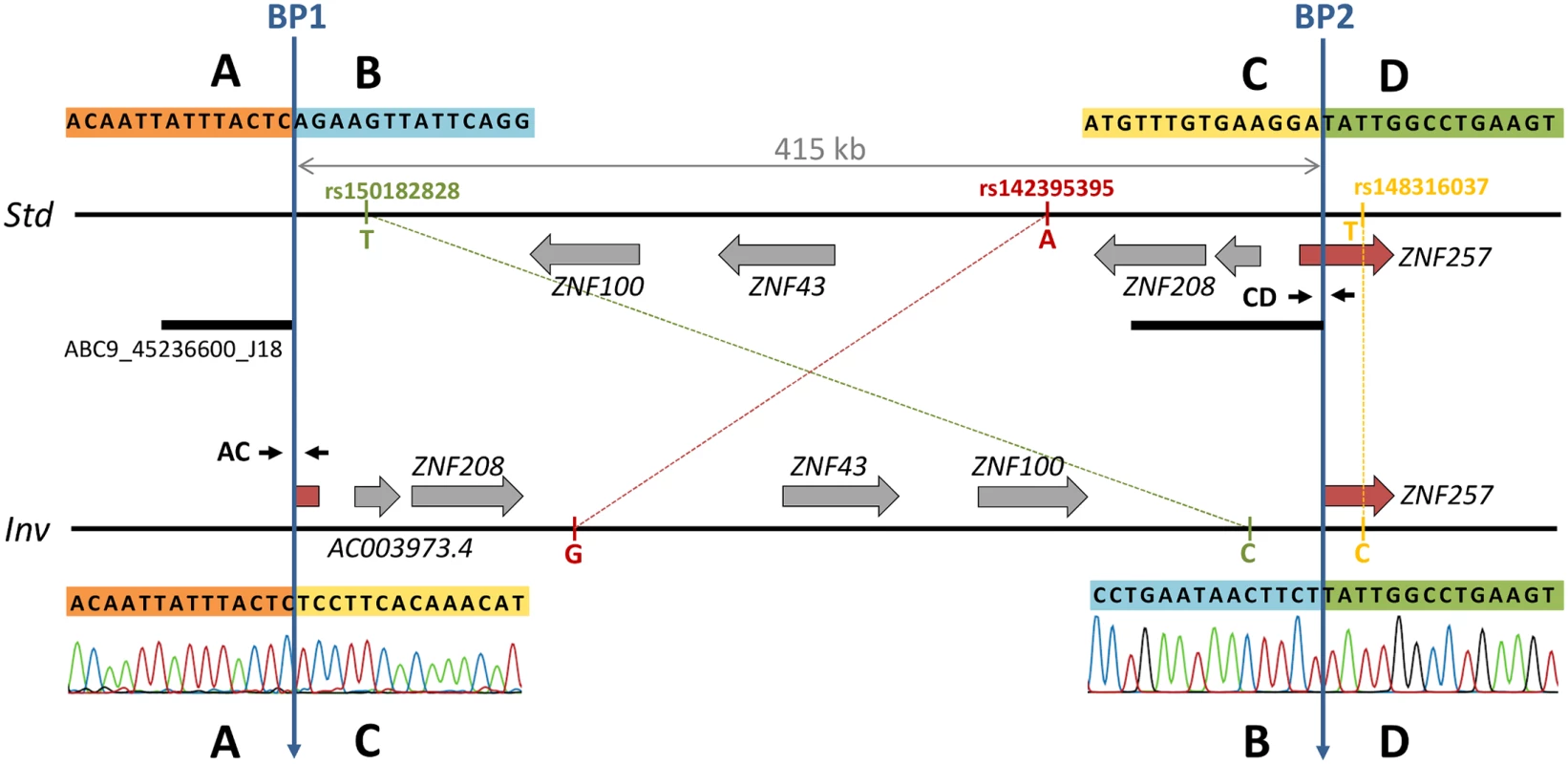
Horizontal lines represent the standard (Std, top) and inverted (Inv, bottom) arrangements. Genes are depicted as grey arrows indicating direction of transcription with the disrupted gene shown in red. Blue vertical arrows mark the two inversion breakpoints (BP1 and BP2). The black bar below the Std chromosome indicates the sequence included in the analyzed fosmid containing BP1 in the inverted orientation. Primers used for PCR genotyping are shown in the corresponding breakpoint of each arrangement as small black arrows. The three tag SNP alleles are shown next to the corresponding chromosome. Sanger chromatograms show the sequence of inversion breakpoints in the Inv chromosome. The analysis of BP1 and BP2 sequences revealed clean breaks with no insertions or deletions that were located at HG19 chr19 positions 21830133–21830134 (BP1) and 22245260–22245261 (BP2), resulting in a 415-kb inversion (Fig 1). BP1 falls within the first copy (42.9-kb long) of a pair of inverted segmental duplications located 24.5 kb apart, although they show only a 91.6% identity and present multiple indels that make the mapping of BP1 unequivocal. Both inversion breakpoints are located within blocks of transposable elements (TEs) of 14.3 kb in BP1 and 4.9 kb in BP2 formed by a LINE element with several Alu sequences inserted inside at different positions (S1 Fig). Even though the composition of both blocks is similar, the nucleotide identity between them is very low and the inversion breakpoints occurred within L1 elements of different subfamilies in a direct orientation with respect to each other, which suggests that the inversion was not generated by non-allelic homologous recombination (NAHR). No microhomology was detected at the breakpoints either. Therefore, the most plausible generation mechanism for this inversion is non-homologous end joining (NHEJ). These TE blocks also indicate that the Std chromosome corresponds to the ancestral arrangement because the L1 copies are abruptly interrupted at the breakpoint in the derived Inv sequence (S1 Fig). In addition, the Std arrangement is found in the chimpanzee genome sequence (PanTro4 assembly).
Inversion frequency and geographical distribution
To find out its distribution and frequency in different human populations, inversion HsInv0379 has been genotyped using three different strategies (Table 1). First, 534 individuals from seven human HapMap populations (CEU and TSI of European origin, YRI and LWK of African origin, and GIH, CHB and JPT of Asian origin; see Table 1 for details) were genotyped by multiplex PCR across the breakpoints (Fig 1 and S1 Fig). Only five individuals carrying the inversion in heterozygosis were found, all in East Asian populations (ASN), two CHB (2.2% Inv frequency) and three JPT (3.3% Inv frequency), and inversion genotypes fit perfectly Hardy-Weinberg equilibrium (P = 0.4636).
Tab. 1. HsInv0379 genotyping and frequency in different populations. 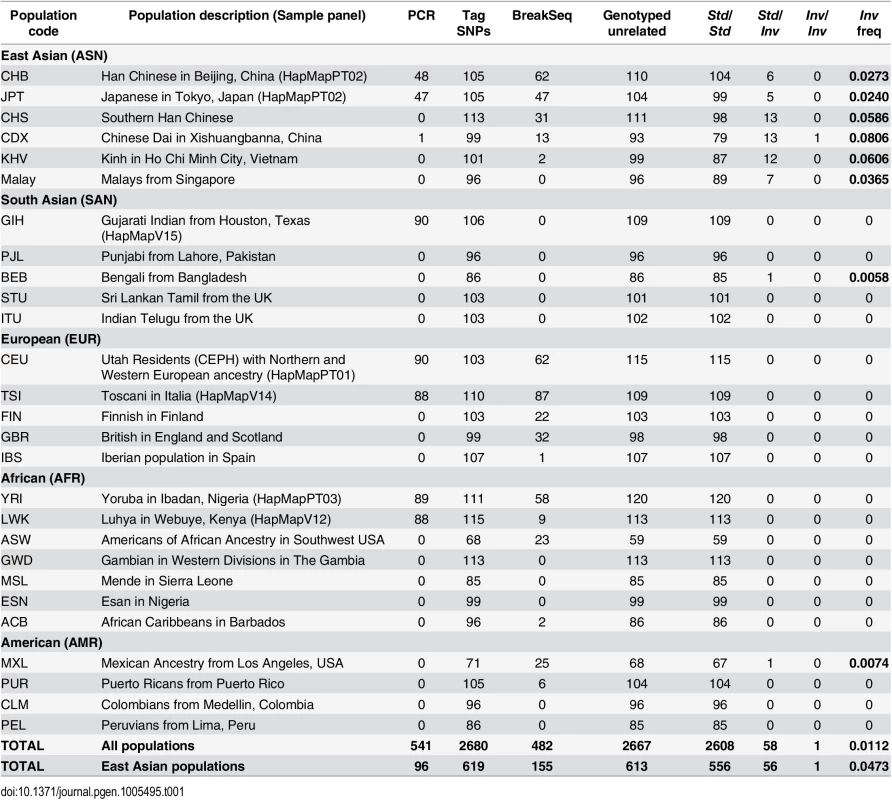
Number of individuals genotyped by PCR across inversion breakpoints, tag SNPs in high linkage disequilibrium with the inversion, and sequence reads spanning inversion breakpoints (BreakSeq). The number of unrelated individuals genotyped is also given together with genotype counts and allelic frequencies for each analyzed population. Second, taking advantage of the fact that many individuals genotyped by PCR had been sequenced in the 1000GP Phase 1 [26], we searched for SNPs in LD with the inversion. The comparison of the SNP genotypes in the inverted segment plus 20 kb of flanking sequence revealed three tag SNPs (rs150182828, rs142395395 and rs148316037) with alleles that segregate in complete LD with the inversion (r2 = 1). For these three SNPs, which span 400.8 kb, all Std chromosomes carry the ancestral reference alleles (Fig 1) while the five heterozygote individuals are the only ones with the alternative alleles, also in heterozygosis. Two tag SNPs are located within the inverted sequence in intergenic regions and the third one right outside, in an intron of gene ZNF257 (Fig 1).
The analysis of the recently released SNP calls for 2,535 genomes included in 1000GP Phase 3 (ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502/) together with the already published 1,092 genomes in Phase 1 [26], and 96 Malay genomes from the Singapore Sequencing Malay Project (SSMP) [35] allowed us to identify 56 additional candidate individuals to carry the inversion based on the tag SNPs (Table 1 and S1 Table). All these individuals carry the alternative alleles for the three SNPs except five individuals, which have only two of the alleles, and HG02152 (CDX), that is homozygous for two alternative SNP alleles. The predicted inversion genotypes were confirmed by PCR in six of the new heterozygotes and the Inv homozygote. Interestingly, almost all the potential inversion carriers belong to East Asian populations (CHB, JPT, CHS, CDX, KHV and Malays), but we also identified a Bengali (BEB) and a Mexican (MXL) individual that are clearly heterozygous for the three tag SNPs (S1 Table).
Finally, we performed similarity searches against the reads of 1,892 individuals from 19 populations available at the 1000GP ftp site on summer 2013 (including both mapped and unmapped reads) using 100-bp sequences spanning the two breakpoints in each arrangement as queries (S2 Table). Reads containing at least one of the inversion breakpoints (from either Std or Inv orientation) were retrieved for 482 individuals, with an average of just 3.6 reads/individual due to the low coverage of many of these genomes. Of those, Inv chromosomes were detected in 19 of 20 candidate Std/Inv heterozygotes according to the tag SNPs (the remaining individual having one Std read), whereas only reads corresponding to the Std arrangement were recovered from the 462 predicted Std/Std individuals (S1 Table). Std alleles were also detected in nine of the inversion carriers, validating their heterozygote status. In total, we have independent PCR and/or breakpoint-sequence confirmation of the Inv allele for 25 of the 61 individuals (41%) predicted by the tag SNPs to carry the inversion (59 unrelated; S1 Table). Therefore, we can confidently use these SNPs as proxies to detect Inv chromosomes. Overall, inversion genotypes were determined for 2,771 individuals (2,667 excluding related individuals; Table 1 and S1 Table), of which 197 individuals from different populations have been genotyped by the three methods and results are in complete agreement. These genotypes reveal an inversion frequency of 1.12% worldwide and of 4.73% in East Asia, which ranges from ~2.5% in the Northern populations to ~7% in the Southern populations (being Malays an exception with a frequency of 3.65%) (Table 1 and Fig 2).
Fig. 2. Geographical distribution of HsInv0379 inversion. 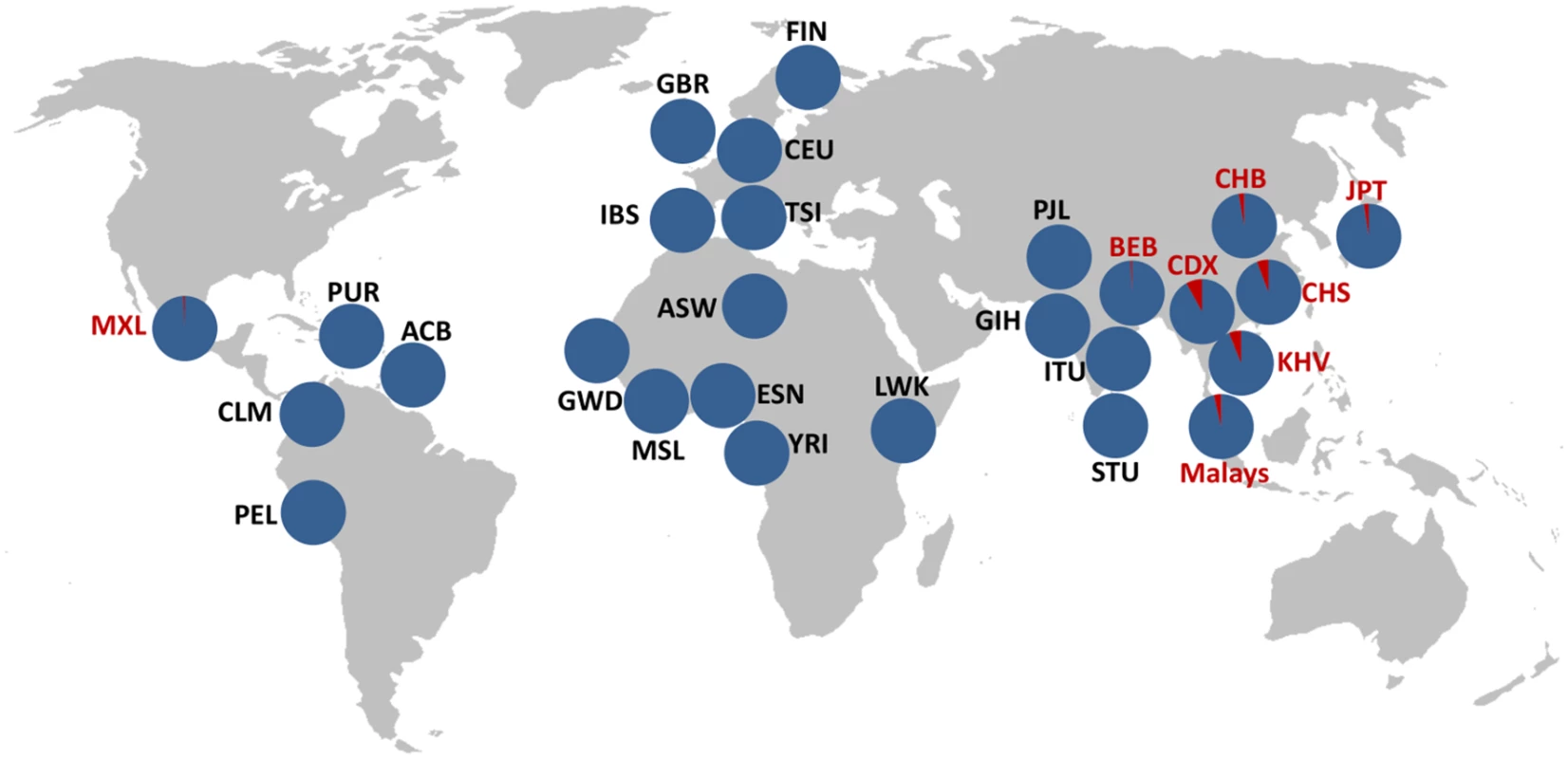
Inversion frequency in 27 different populations corresponding to 2,667 unrelated individuals analyzed by PCR, tag SNPs and reads spanning inversion breakpoints are shown. Blue corresponds to Std and red to Inv arrangement. Inversion frequencies and the origin of the different populations are listed in Table 1. Functional consequences of the inversion
The HsInv0379 inverted segment contains three protein-coding genes: ZNF100, ZNF43 and ZNF208 and at least one long intergenic non-coding RNA (reported in GENCODE v19 gene annotation). A fourth protein-coding gene, ZNF257, appears to be disrupted by BP2 (Fig 1). ZNF257 encodes a transcription factor with a Krüppel-associated box (KRAB) repressor domain and 13 zinc fingers. This gene has two main transcripts of 3,520 and 3,641 nucleotides, which are transcribed from the same promoter but differ in the presence of an extra non-coding second exon (Fig 3). The inversion removes the first two exons as well as the promoter of this gene, which are relocated 415 kb away in the Inv chromosomes. Thus, total ZNF257 expression (both isoforms included) was quantified by quantitative PCR (qPCR) in lymphoblastoid cell lines (LCLs) of 15 Std/Std and 11 Std/Inv individuals from the CHB and JPT populations, plus the Inv/Inv homozygote (CDX). A 2.9-fold decrease (P = 0.0047) was found in the inversion carriers compared to the homozygote Std individuals and no expression was detected in the Inv/Inv individual (Fig 3 and S2 Fig), indicating that, as expected, this gene is not expressed from the Inv chromosomes. However, five Std/Std individuals showed an extremely low ZNF257 expression, comparable to the levels in inversion carriers (S2 Fig). These individuals are all from the CHB population and most likely carry some regulatory variant in cis or trans that causes a lower expression of ZNF257 not related to the presence of the inversion. In fact, an analysis of Geuvadis RNA-Seq data [36] in LCLs from European and YRI populations (which include only Std/Std homozygotes) indicates that ZNF257 is among the top 10% genes with highest variation in expression among those with similar low expression levels (S3 Fig). In addition, several studies including the recent results from the GTEx project [36,37] suggest the existence of eQTLs for the gene, with 13 SNPs significantly associated to ZNF257 expression in different tissues, although due to the transformation of the data during the analysis the biological interpretation of the observed effects is not easy. In our data set, each of these SNPs explains 9.8–19.8% of the variation of ZNF257 expression in Std/Std homozygotes, which contrasts with the 27.6% explained by the inversion across all the samples assuming an additive model (S3 Table).
Fig. 3. ZNF257 gene structure and expression changes in inversion carriers. 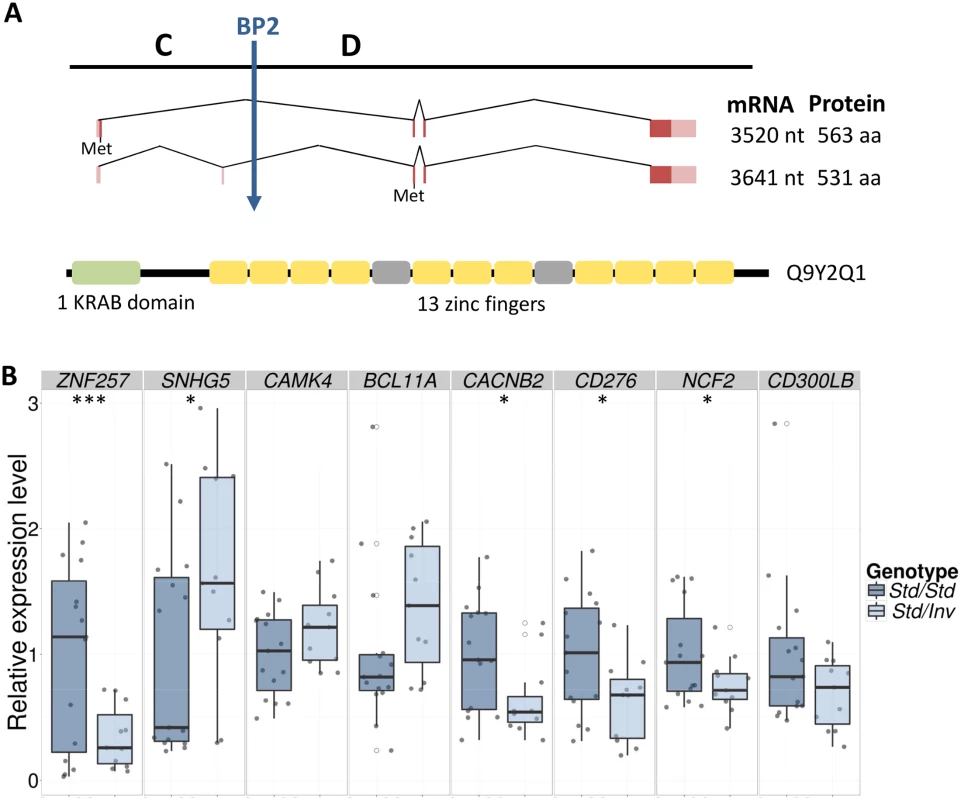
A. The two most reliable transcripts of gene ZNF257 are represented as dark and light-colored boxes corresponding to coding and non-coding exons, with their sizes and encoded proteins shown to the right. The longer transcript includes an extra 121-bp alternative non-coding exon in its 5’ UTR and in theory encodes a 32-aa shorter protein because of the shift in the methionine (Met) used as translation initiation from the three last nucleotides of the first exon to the third exon, common to both isoforms. The position of BP2 is indicated by a vertical arrow. A diagram of the longest protein domains (UniProt Q9Y2Q1) is represented by boxes below: green for the Krüppel-associated box (KRAB) domain, yellow for C2H2-type zinc fingers, and grey for degenerate zinc finger structures. B. Box plots of qPCR expression levels for ZNF257 and the seven genes with significant or marginally significant differences between LCLs of 15 Std/Std (dark blue) and 11 Std/Inv (light blue) individuals. For each gene, expression values have been normalized by the average of Std/Std individuals, with every sample represented by grey points and outliers by open circles. Horizontal black lines within each box indicate median values. *, P < 0.05; ***, P < 0.001. Since ZNF257 is a transcription factor that may be controlling the expression of other genes, we searched for genome-wide expression changes associated to the inversion by analyzing the RNA-Seq profiles of LCLs in eight of the previous Std/Std and Std/Inv individuals showing discrepant levels of ZNF257 expression (see S4 Table for RNA-Seq summary results). We detected 56 differentially expressed protein-coding genes and 27 non-coding RNAs (False Discovery Rate, FDR < 0.1), of which 49 were down-regulated and 34 up-regulated, although 60 of them (72.3%) have an FDR < 0.05. These genes exhibit moderate to high fold changes (1.2–8.7) with the greater variation corresponding to non-coding RNAs (S5 Table), and none of them are located within or close to the inverted sequence. Because only four individuals of each genotype were compared, the results could be confounded by differences between the two groups other than the presence of the inversion. Thus, we repeated the analysis for the 18 possible permutations exchanging two individuals from each genotype class (S4 Fig). The median proportion of genes differentially expressed between the two groups in the permutations was 0.17%, which is ~3 times less than those identified between Std/Std and Std/Inv individuals using the same criteria (0.47%, at a nominal FDR < 0.1). Besides, none of the genes identified in the permutations were in the differentially expressed list from the inversion genotype comparison. We also performed 400 simulations of read counts with the mean and dispersion of the real data and searched for differentially expressed genes with FDR < 0.1 in each case. Only 5.5% of the simulations had a higher number of differentially expressed genes than the comparison of individuals with different inversion genotypes.
Next, several enrichment analysis tools (see Methods) were used to try to detect functional relationships among the differentially expressed genes, but no statistically significant functional categories have been uncovered based on current knowledge. However, immune response was the most significant biological process gene-ontology category in the DAVID Functional Annotation Tool (P = 0.00091), although significance was lost after correcting for multiple testing. Thus, we selected 11 genes involved in the immune system with different fold-change values and FDRs to validate their expression in the whole set of available LCL RNAs (15 Std/Std, 11 Std/Inv and 1 Inv/Inv) using qPCR. All the genes except one showed good validation when considering only the eight individuals used in the RNA-Seq analysis (Table 2). In the large sample set, the expression difference was always in the expected direction (up or down regulated) except for one gene (NFATC1), but qPCR validation depended on the gene’s FDR. For FDR < 0.05, significant differences between Std/Std and Std/Inv were obtained for three genes, while the other three were marginally significant (Table 2 and Fig 3). Of the three genes with FDR = 0.05–0.10, only the expression difference of the CACNB2 gene (FDR = 0.077) could be validated, while none of the two genes tested with higher FDR showed significant differences. As for ZNF257, we also see a significant or marginally-significant correlation between the expression levels and inversion genotype (Std/Std or Std/Inv) for five genes (Table 2), in which inversion genotype explains between 10% and 19% of the variation in gene expression. Therefore, there is a clear association between inversion genotypes and gene expression of several genes in trans. In the case of the Inv/Inv sample, it behaves as expected from the expression change observed in heterozygotes in 7 of the 11 genes tested, although since expression levels for any given gene can be affected by many variables, it is difficult to draw reliable conclusions with a single individual.
Tab. 2. Analysis of RNA-Seq gene-expression changes between Std/Std and Std/Inv individuals by qPCR. 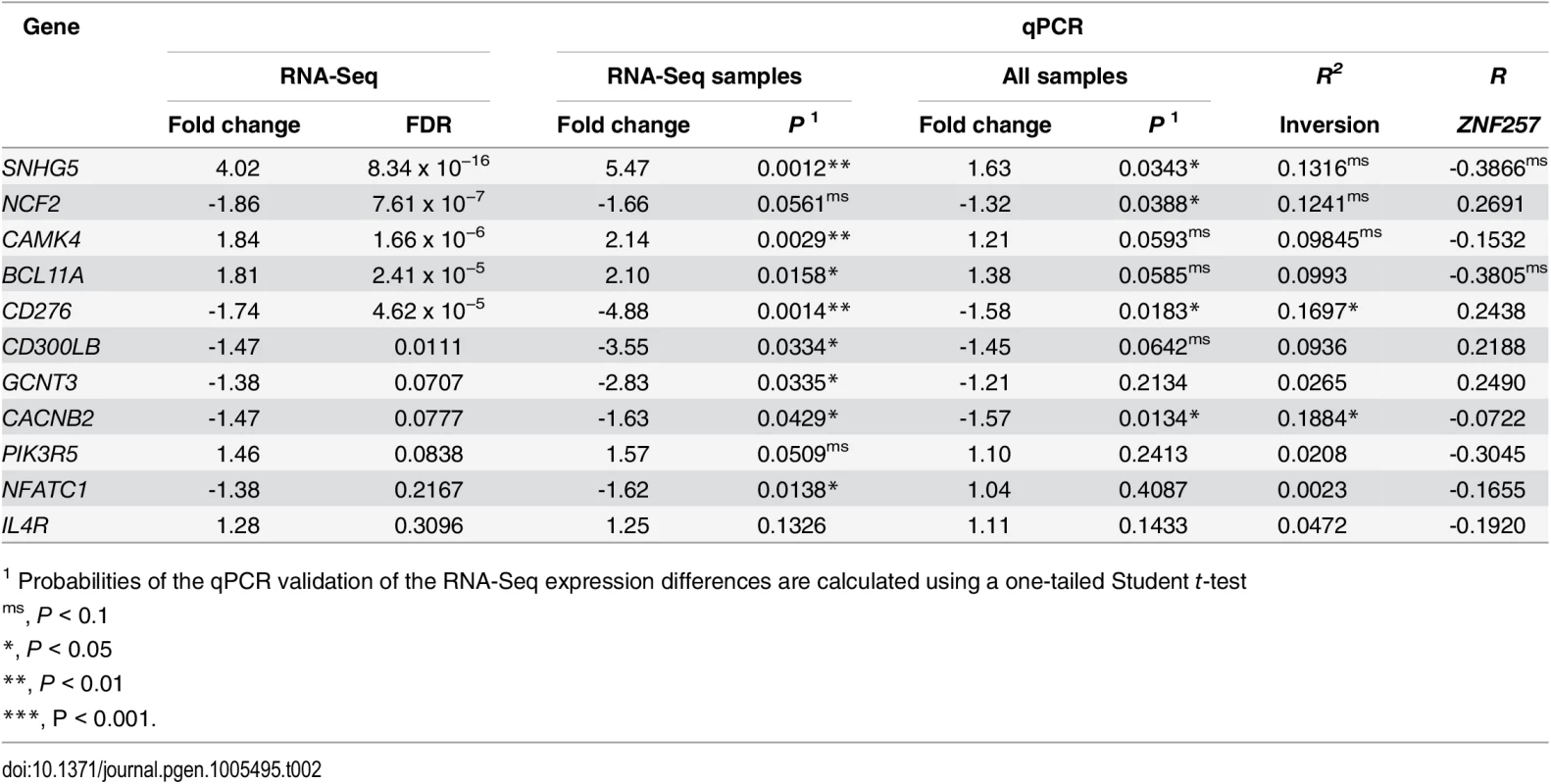
For each gene, fold change and differential expression p-value (P) between inversion genotypes are given both for only the eight samples also analyzed by RNA-Seq and for the total 26 samples, with the RNA-Seq results shown for comparison (a line separating genes with FDR < 0.05 from those with lower significance). R2 represents the percentage of gene-expression variation explained by the inversion genotype according to an additive model, and R the Pearson's correlation between the expression levels of ZNF257 and each analyzed gene. Since we found five Std/Std individuals with low expression levels of ZNF257 mRNA, we have an opportunity to test the relationship between this gene and the detected expression changes. A marginally significant correlation between the ZNF257 expression values and that of the other genes tested by qPCR in the 26 Std/Std or Std/Inv individuals was found for two genes, SNHG5 and BCL11A (Table 2), which indicates that a low ZNF257 expression level is associated to an increase in the two genes. Accordingly, for these genes the significance of the expression change between Std/Std and Std/Inv is higher when the five Std/Std with low ZNF257 expression are removed from the homozygous Std group (P = 0.0140 and P = 0.0096, respectively). Conversely, this does not happen for other genes like CACNB2, which shows no clear association with ZNF257, suggesting that these expression changes depend more on inversion genotype than on ZNF257 level.
Another important effect of the inversion is that it brings the ZNF257 promoter and first exons to a completely different part of the genome, where they might induce the transcription of new chimeric transcripts. Therefore, we specifically searched for transcripts generated by the ZNF257 promoter around BP1 in Inv chromosomes. By mapping RNA-Seq reads to a construct with the inverted sequence, we detected a spliced fusion transcript formed by the first exon of gene ZNF257 and a completely new 296-bp exon made up of fragments of LINE and Alu elements (Fig 4). We confirmed the existence of this RNA by amplifying most of it by RT-PCR and by sequencing the exon-exon junction in individual NA18956. Indeed, both by RT-PCR and qPCR the fusion transcript was found in the 11 inversion carriers analyzed, with 1.7-fold higher expression levels in the Inv/Inv individual, while it is not expressed in any of the Std/Std individuals tested (including three non-East Asian individuals) (Fig 4 and S2 Fig). The complete new transcript is short, with only 468 bases, and its longest ORF contains only 75 aa. It does not show homology to any other known transcript either. However, according to the RNA-Seq reads mapping to the ZNF257 exon 1 (common to the two genes) in inversion heterozygotes that express both transcripts and in Std homozygotes (Fig 4), the level of expression of the fusion transcript is at least 7.4 times higher than that of ZNF257.
Fig. 4. Analysis of the new fusion transcript in HsInv0379 breakpoint. 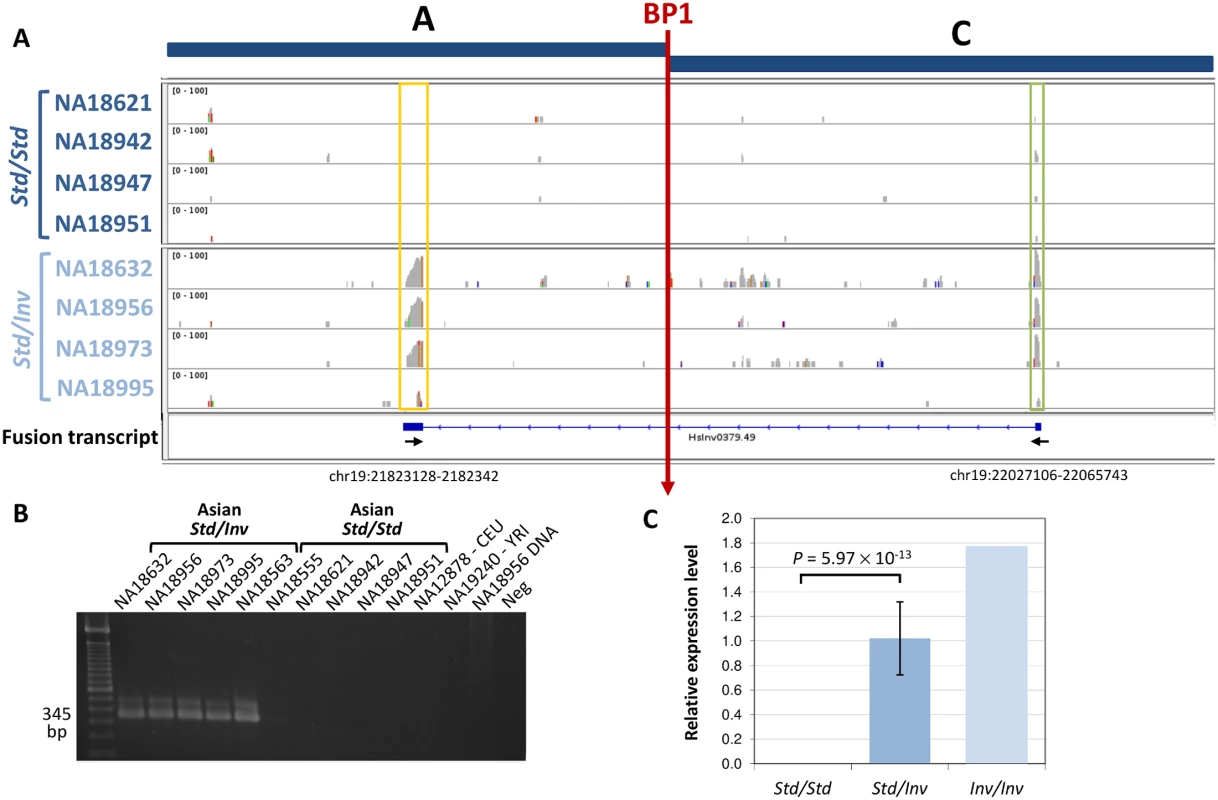
A. RNA-Seq reads mapped to an AC construct corresponding to BP1 (vertical red arrow) in an inverted chromosome reveal a fusion transcript present only in the four Std/Inv individuals (bottom) but not in the four Std/Std (top). Boxes highlight the new exon (yellow) and ZNF257 first exon (green). The structure of the fusion transcript reconstructed by Cufflinks [38] is shown below the RNA-Seq profiles. Small arrows indicate the approximate position of the primers used to validate the transcript. The coordinates of the new exon in the HG19 reference genome are also indicated. B. Analysis of fusion transcript expression by RT-PCR in several individuals. C. Quantification of the fusion transcript levels by qPCR in 15 Std/Std, 11 Std/Inv, and 1 Inv/Inv individuals. Because the inversion tag SNPs are not included in SNP genotyping arrays, the potential functional effects of the inversion have been missed by typical genome-wide association studies. Therefore, based on the lymphoblast transcriptomic results, we made an attempt to associate the inversion allele with general blood-related phenotypic traits by genotyping the inversion tag SNP rs148316037 in 3,787 Japanese individuals of the Nagahama cohort [39–41]. The inversion showed a frequency of 1.95% in this population with a single homozygote and 146 heterozygotes, which fits perfectly the Hardy-Weinberg equilibrium (P = 0.71). A regression analysis was performed to assess the effects of the inversion on immunological, hematological and metabolic measures from blood samples available for this cohort (S6 Table). However, no significant associations have been detected between HsInv0379 inversion alleles and the phenotypical traits analyzed, including disease indicators for diabetes and cardiovascular or liver disease, as well as alterations in blood cell counts or presence of certain antibodies (S6 Table). Only red blood cell count shows a decrease in inversion carriers (P = 0.036), but this association is lost after multiple testing correction.
Origin and evolutionary history of the inversion
The presence of tag SNPs and identical breakpoints in the reads of all Inv chromosomes indicate that the inversion was generated only once from the ancestral Std orientation. Thus, to investigate the evolutionary history of the inversion, we first estimated the age of the derived Inv allele. We used in this analysis the unphased Malay population SNP data set from the SSMP [35] because it provides accurate SNP genotypes for 96 individuals based on >30x coverage sequencing. In 381,827 bp of the inverted region we identified 30 SNPs that are polymorphic exclusively in the seven Std/Inv heterozygotes (out of 2,005 SNPs without missing values). Of these, one corresponds to the tag SNP rs142395395 and four SNPs are found only in three heterozygotes, so they most likely represent variants specific of the Inv chromosomes (the probability that one such variant belongs to Std chromosomes is P = 0.0001). The remaining 25 variants are singletons, and 22 were attributed to the seven Std chromosomes in heterozygotes (on average there are 3.12 singletons/chromosome), leaving 3 singletons in Inv chromosomes. Based on this variation, we determined a nucleotide diversity (π) of 8.86 × 10−4 for Std and 9.73 × 10−6 for Inv chromosomes. We used the divergence between the human and chimpanzee genomes (dH-C = 0.0134) to establish a local substitution rate for this region. Taking into account this substitution rate and the pairwise divergence between Std and Inv chromosomes (dS-I = 9.95 × 10−4), after subtracting the variation among ancestral Std haplotypes [42], we estimated the age of the inversion in 43,450 years (95% confidence interval (CI): 14,934–93,438) or 52,140 years (95% CI: 17,921–112,126), assuming a divergence time between human and chimpanzee of 5 or 6 mya, respectively.
Next, to understand better the effects of the inversion on the nucleotide variation patterns, we phased the SNPs for a 4-Mb region around the inverted segment in the 286 1000GP Phase 1 East Asian individuals (97 CHB, 100 CHS and 89 JPT) [26] to infer Std and Inv haplotypes. In this case, there are two main challenges for SNP phasing. First, the inversion has a low frequency and there are no Inv/Inv homozygotes in the 1000GP Phase 1 dataset from where to recognize SNP variants present exclusively in the derived arrangement (which are always found in Std/Inv individuals and are difficult to place in one of the chromosomes unequivocally). Second, inversion length increases the possibility of phasing errors [43]. To minimize these effects, we used the BEAGLE software [44] to filter and call the SNP genotypes based on their likelihoods in each individual, and then to phase the different variants (see Methods for details). A total of 15,799 high-quality SNPs in 570 phased haplotypes (excluding the two chromosomes of one heterozygote individual, in which a phasing error between inversion breakpoints was detected) were finally included in posterior analyses.
These haplotypes were used to estimate the proportion of each chromosome that belongs to a number K of hypothetical ancestral populations with the software STRUCTURE [45]. Notably, the Inv arrangement shows a single component even when a high number of ancestry components (K = 10) is considered (S5 Fig). At the well-supported value of K = 5, the proportion of the Inv component in Std chromosomes is 12.9% in JPT, 6.3% in CHB and 10.5% in CHS, which suggests that Inv haplotypes are similarly related to any group of Std chromosomes and probably appeared before the establishment of the different East Asian populations, as the estimated age suggests. In addition, this highlights the highly homogeneous composition of Inv haplotypes due to its relatively recent and unique origin as well as the lack of gene flow between arrangements. Similar results were obtained by building an haplotype network, in which all the haplotypes with the inversion form a closely related and monophyletic group (S5 Fig).
Although phasing and SNP imputation errors might affect nucleotide variation estimates, we have also examined the global variation patterns of π and Tajima's D statistic [46] along and beyond the inverted region in the 1000GP Phase 1 dataset (Fig 5). Consistent with the age estimates, there are low levels of nucleotide diversity and negative values of Tajima's D within the inverted haplotypes, which extend outside the breakpoints. We then tried to determine the effects of the inversion on recombination by inferring population-scaled recombination rates (4Ner/kb) between each pair of consecutive SNPs in the 24 Inv and 24 randomly sampled Std phased chromosomes from the three East Asian populations. As expected since most Inv chromosomes are found in heterozygotes, there is a marked reduction in recombination within the inverted segment (Fig 5), which shows a genetic length of 83 4Ner units in Std East Asian chromosomes and only 5 in Inv. Accordingly, the distribution of Fst values between SNPs in Std and Inv chromosomes shows increased differentiation within the inverted region, which is maximal at the breakpoints (Fig 5). This reduction of recombination in heterozygotes is apparent even taking into account that the phased chromosomes used in this analysis can include a higher proportion of shared variants due to the phasing problems explained above. In fact, in the high-coverage SSMP data only two SNPs with enough read support were found to be shared between arrangements and were likely generated by gene conversion.
Fig. 5. Recombination and nucleotide diversity patterns in the inversion region. 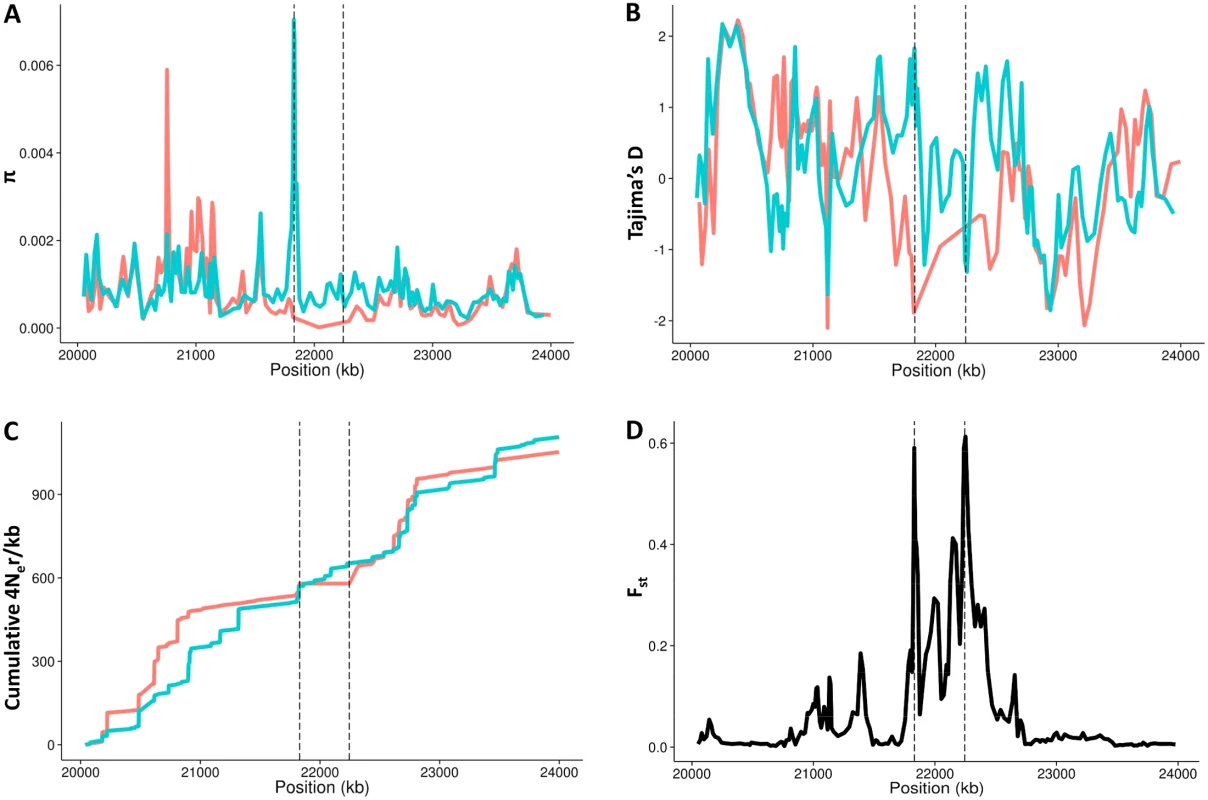
A. Nucleotide diversity (π). B. Tajima’s D test statistic. C. Cumulative genetic length in 4Ner units. D. Fst values between Std and Inv chromosomes. HG19 assembly coordinates are shown in the X-axis, including the inverted region (marked by vertical dashed lines) and 1.8 Mb of flanking sequence at each side. Blue lines correspond to East Asian Std chromosomes and red lines to Inv chromosomes. All values were estimated in non-overlapping windows of 80 SNPs each considering the 285 phased East Asian individuals, except the recombination rate that was calculated between consecutive SNPs in the 24 Inv chromosomes and 24 random East Asian Std chromosomes. Tajima’s D statistic was Z-transformed to counteract the loss of low frequency SNPs during the phasing process. Finally, we looked for evidences of natural selection acting on this inversion with two approaches. First, we searched for recent partial selective sweeps in this region by applying the integrated haplotype score (iHS) test [47] to the 5,890 SNPs with available ancestral state information (37.3% of the phased SNPs). Although this test may be affected by recombination inhibition, no significant signals of recent positive selection were detected for the proximal (P = 0.67) or distal (P = 0.83) breakpoints or any SNPs in high LD with the inversion. Second, we used forward-in-time simulations to compare the observed frequency of the inversion with that expected under a human demographic model [48,49] and different evolutionary scenarios (Fig 6). Population-scaled selection coefficient (Nes) values corresponding to positive (+10 and +5) and purifying selection (-5, -10, -15, -20, -25 and -30) acting on a mutation of ~43,450 years were considered, together with a neutral change (Nes = 0). The results show that the probabilities to observe the actual frequencies (2.4–8%) in positive selection simulations are lower than those from a neutral mutation (Fig 6). On the other hand, these probabilities are higher with increasingly negative selection coefficients (Fig 6), which suggests that the inversion could be evolving under purifying selection and therefore that it may have negative consequences for its carriers. However, the likelihoods of negative selection coefficients are not significantly different from that of a neutral allele according to the log-likelihood ratio test, and the possibility that the inversion is neutral cannot be ruled out.
Fig. 6. Evolutionary history of HsInv0379 inversion. 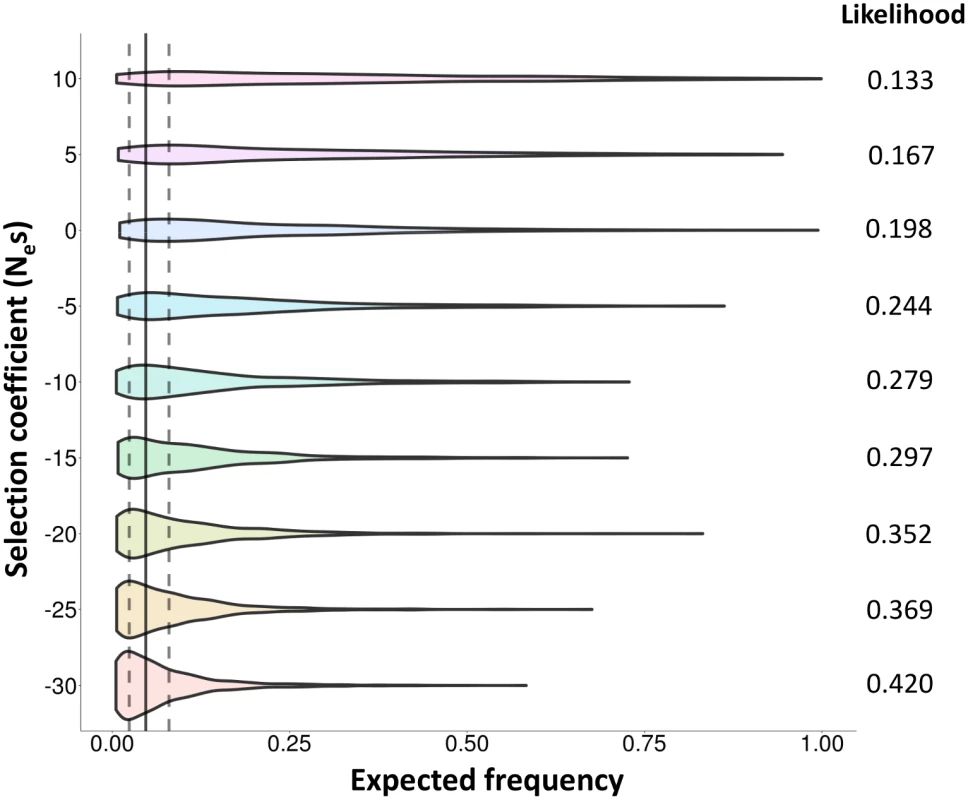
Distribution of expected frequencies for a mutation arising 43,450 years ago under a model of human demography and different evolutionary scenarios according to forward-in-time simulations. Violin plots show the allele frequencies in 1,000 simulations for each selection coefficient (Nes between -30 and +10). The vertical solid line corresponds to the average frequency of the inversion in East Asia (4.73%) and dotted lines mark the range of frequencies observed in actual East Asian populations (2.4–8%). The likelihood of each Nes value (probability to obtain the observed frequencies) is shown at the right of the graph. Discussion
In this work we analyze in detail a new human polymorphic inversion (HsInv0379) that is the first with a clear effect on genes. Unlike other previously described long human polymorphic inversions that are mediated by large and complex repeats [29,50], in the present variant breakpoints are very simple, without repeats or even microhomology sequences, which suggests that it was probably generated by non-homologous mechanisms [30,51,52] and has a unique origin. After genotyping 2,667 independent individuals from 27 worldwide populations using three methods (PCR across breakpoints, reads spanning inversion breakpoints, and tag SNPs), we have determined that this inversion is almost exclusively found in East Asian populations with an average frequency of 4.7%, although frequency varies between 2.4 and 8% with populations located at the southern part of China exhibiting a higher frequency of the Inv allele.
Although the low frequency of the inversion complicates age calculations, our estimates using high-coverage sequencing data from a single population indicate that the inversion is 40,000–50,000 years old, which is relatively young compared to other human polymorphic inversions [29,53]. Accordingly, we propose that this inversion was generated from the ancestral Std chromosome after the out-of-Africa event around the time of the split between Asian and European populations 20,000–40,000 years ago [54,55], which agrees well with its distribution across all the analyzed East Asian populations as well as in one Bengali individual [56]. The finding of the Inv tag SNPs in a single Mexican individual whose genome does not have any component indicative of a recent ancestor of Asian origin [57], is consistent with the inversion predating the migration into the Americas ~15,000–23,000 years ago [54,58] and opens the possibility that the inversion might be present at low frequency in some American populations as well. The low nucleotide variation within Inv chromosomes with only a few polymorphisms specific of this arrangement also supports a recent origin for inverted haplotypes, which have not had time to accumulate much variation.
Inversions are known to affect phenotype in many species [4–7,9], but the molecular mechanisms underlying these effects remain unclear [3]. In the case of HsInv0379, no expression differences have been detected for any of the genes captured within the inverted segment, but one of the inversion breakpoints completely disrupts a gene, thus raising the possibility of phenotypic consequences derived from this mutational effect. The decrease of ZNF257 expression level in inversion carriers and complete absence in the Inv/Inv individual indicate that this gene is not being expressed from the Inv chromosomes, although there is some degree of variation in the expression of the gene in Std chromosomes. In particular, variability is high among Std/Std individuals in available RNA-Seq data (S3 Fig), but it is not significantly different from that of other genes with low expression levels, which complicate their accurate quantification. However, despite the existence of other regulatory genetic variants, we have seen that the inversion is the main determinant of ZNF257 expression in the carriers (S3 Table).
ZNF257 belongs to the largest family of human transcription factors (KRAB-zinc finger proteins) [59] and it is positioned within a cluster of many similar genes. KRAB-zinc finger proteins are able to interact with chromatin-remodeling factors to repress transcription and have been involved in processes as diverse as development, differentiation, metabolism, apoptosis or cancer [59]. ZNF257 was first isolated in bone marrow [60] and appears to be expressed in multiple tissues, mainly in testis, LCLs, ovary and thyroid (according to RNA-Seq data on different human tissues from both GTEx [37] and Illumina Body Map (http://www.ebi.ac.uk/gxa/experiments/E-MTAB-513). The type of cellular process it regulates or any target genes under its control are not yet known, although different evidences point towards a non-essential role of this gene. Based on exome data, ZNF257 has been classified as a gene tolerant to functional genetic variation [61] and it shows loss-of-function mutations in heterozygosis in two individuals out of 1,092 in the 1000GP Phase 1 dataset. In addition, as expected by the inversion frequency, we have detected two Inv/Inv homozygotes where this gene is completely inactivated. Also, ZNF257 is conserved in non-human primates, where it appears to be under purifying selection (dN/dS = 0.5443 with chimpanzee), but does not present a one-to-one ortholog in other mammal species. Here we provide support for an association between ZNF257 down-regulation and expression changes in other genes in trans: (1) Expression differences in the same direction are observed independently using a different technique and a larger number of samples; and (2) Std/Std individuals with low ZNF257 expression show expression levels similar to those of inversion carriers in at least two of the genes tested by qPCR (and in both cases the genes are up-regulated in inversion carriers, which is consistent with a lower amount of a transcriptional repressor like ZNF257). It is important to note that the detection of expression changes in trans is complicated by several facts: (1) many factors can determine the level of expression of a given gene and the inversion just alters the levels of one transcription factor; (2) the effects of ZNF257 disruption in heterozygotes might be moderate due to buffering by ZNF257 functional copy and they are likely to be larger in Inv/Inv individuals; and (3) lymphoblasts may not be the tissue where ZNF257 expression reduction has its main effects. Still, this represents the best approach available to detect the functional consequences of this polymorphic inversion.
Biological relevance of ZNF257 decrease due to the inversion may be questioned in light of the range of expression levels detected for this gene in Std/Std individuals (S2 and S3 Figs). However, gene disruption is not the only mechanism by which this inversion may be affecting gene expression and having potential phenotypic consequences, since it also generates a fusion transcript at BP1. In fact, at least one of the differentially expressed genes validated by qPCR (CACNB2) showed a level of expression in Std/Std individuals with low ZNF257 closer to that in the other Std/Std individuals than to inversion carriers, suggesting that the expression change is associated with the inversion itself, rather than with ZNF257 decrease, and the fusion transcript could be involved. This transcript has no homology to known transcripts, but coding of short peptides [62] or binding to proteins or microRNAs cannot be ruled out [63,64]. The new exon is entirely transcribed from TE sequences, but it contains the sense strand for both Alu and L1 fragments and it should not bind RNAs from other TE copies. The higher expression level compared to ZNF257 when both are driven by the same promoter suggests that the fusion transcript is taking advantage of new regulatory elements in its new position or operating without the influence of negative regulators, supporting the effect of chromosomal position on gene regulation [16], although post-transcriptional mechanisms could play a role as well. Nevertheless, additional knockdown or overexpression experiments would be needed to really show a causal link between ZNF257 and the fusion gene to other gene-expression changes.
Although we tried to establish functional relationships among the differentially expressed genes in inversion carriers, no clear significant categories emerged that could indicate the cellular processes that may be affected. However, there are several genes that may have important functions in the cell (S5 Table), like BCL11A that acts as a switch between fetal and adult hemoglobin in erythrocytes, is essential for B-lymphocyte development [65] and has recently been involved in autism [66], or APP, a protein involved in autosomal dominant Alzheimer’s disease [67]. Moreover, several non-coding RNAs of unknown function show quite high fold changes in inversion carriers (S5 Table), such as the processed pseudogene RP11-343H5.4 (8.7-fold increase), or the lincRNA SNHG5 (4.0-fold increase), whose introns produce snoRNAs and is located at the breakpoint of a chromosomal translocation involved in B-cell lymphoma [68].
Assuming that any moderately long inversion should have some negative consequences due to the generation of unbalanced gametes by recombination in heterozygotes, the mutational effects at the breakpoints could result in several possible evolutionary scenarios. The inversion could behave as positively selected, neutral or nearly neutral depending on whether the potential beneficial effects of the ZNF257 disruption, fusion transcript and the associated expression changes overcome the negative fertility effects or if the fertility effects are very small. On the other hand, if both effects are negative, the inversion could range from slightly to strongly deleterious. Thus, we have simulated the evolution of a mutation arising ~43,000 years ago under a human demography model with neutrality and different strengths of positive and purifying selection. While the observed inversion frequencies are compatible with both neutrality and negative selection and the age uncertainty might alter the simulation outcomes, the actual values are more likely under the assumption that the inversion is a deleterious polymorphism that cannot rise in frequency in the population due to its negative effects.
A human individual typically carries ~20 genes completely inactivated, but in more than ¼ of these only some transcripts are affected [69]. Loss-of-function mutations tend to occur in genes that are part of a family and less evolutionary conserved and this type of changes show low frequencies, all of which fit perfectly our situation (although according to a recent study in the Icelandic population [70], 85% of loss-of-function mutations have a frequency below 0.5%, which is much lower than the 4.7% detected for the present inversion in East Asians). However, many genes involved in late-onset diseases will behave as neutral variants from an evolutionary point of view, but still may have functional consequences able to impact human health [71]. Alternatively, if we consider that recombination is not physically inhibited in the inverted region in heterozygotes, we can use its genetic length in Std chromosomes, 83 (in 4Ner units), as a proxy for the population-scaled selection coefficient (4Nes) acting on the inversion associated to the probability of harboring crossovers within the inverted sequence between Std and Inv chromosomes, which results in a Nes ≈ -21. Since similar (or even higher) negative coefficients seem to be the parameters that best fit the observed inversion frequencies, both mechanisms (recombination and gene disruption) may have a role in causing the negative effects of this inversion. In that case, gene surfing during the expansion of humans in Asia [72] could help to explain the observed frequencies of such a deleterious variant. So far we have been unable to detect an association of the inversion with disease markers and other phenotypic traits from blood tests in a Japanese cohort. Nevertheless, future association analysis with a higher number of individuals to overcome the reduced power to detect significant effects for low-frequency variants like HsInv0379 (just 1.95% in the analyzed population) and a more complete battery of phenotypes tested, should contribute to elucidate the possible functional consequences of this inversion.
Ethics statement
Procedures that involved the use of human samples were approved by the Animal and Human Experimentation Ethics Committee (CEEAH) of the Universitat Autònoma de Barcelona (Ref. 821H). Protocols for the Nagahama cohort project were approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (Ref. G278) and written informed consent was obtained from all of the participants.
Materials and Methods
Samples and DNA isolation
A total of 547 samples from eight populations of HapMap and 1000GP [26,73] projects have been used (see Table 1 for details). Ninety unrelated individuals were analyzed in each of these populations except for CHB (49) and JPT (47) plus a single CDX individual, and the CEU and YRI populations, which are organized in 30 parent-children trios. Genomic DNAs were obtained from Coriell Cell Repository (Camden, NJ, USA), except most of those from the CEU population and those of the samples used for the expression analysis, which were isolated from LCLs (Coriell Cell Repository, Camden, NJ, USA) as previously described [74] or using the PrepFiler Forensic DNA Extraction kit (Life Technologies). Fosmid ABC9-45236600J18 was obtained from Evan E. Eichler (University of Washington, WA, Seattle) and DNA was isolated from 1.5 ml of overnight bacterial TB culture following a typical alkaline lysis plasmid DNA extraction protocol [75].
PCR/RT-PCR amplification
For inversion genotyping, one primer in region C within the inverted segment was used in combination with primers in regions A and D outside the inversion (S7 Table) to generate products AC (Inv) and/or CD (Std). PCR amplifications were performed in a reaction volume of 25 μl including 1x buffer, 1.5 mM MgCl2, 200 μM each dNTP, 0.4 μM each primer, 1.5 U Taq DNA polymerase (Roche) and 50–100 ng genomic DNA. Typical cycling conditions were 2 min at 94°C of denaturation followed by 35 cycles of 30 sec at 94°C, 30 sec at 60–65°C and 30–60 sec at 72°C, and a final step of 7 min at 72°C. In long-range PCRs, the same reaction conditions were used except for 240 μM each dNTP, 2.5 U Pfu Turbo DNA Polymerase (Stratagene) and 1–10 ng of fosmid DNA, together with cycling steps of denaturation at 95°C and extension at 68°C for 9 min. For RT-PCR, total RNA from 27 East Asian LCLs (Coriell Cell Repository, Camden, NJ, USA) was isolated using Trizol (Life Technologies) and cDNA synthesis was carried out after DNase treatment (DNA-free, Ambion) using SuperScript First Strand Synthesis System for RT-PCR (Invitrogen) from 1 μg total RNA with a combination of oligodT and random primers. Controls without retrotranscriptase were performed for all samples to exclude DNA contamination. RT-PCR reactions were carried out in the same conditions described above with 1 μl of cDNA as template. When needed, PCR products were sequenced by the Sanger method at Macrogen (Seoul, Korea).
RNA-Seq and differential expression analysis
Total RNA was isolated from 15-ml cell cultures of eight East Asian LCLs collected before reaching saturation (4.5–8.6 × 106 cells), using miRNeasy Mini kit with on-column DNase digestion (Qiagen). RNA quality and quantity were measured by absorbance with Nanodrop (Thermo Scientific), fluorescence on a Qubit instrument (Life Technologies) and 2100 Bioanalyzer (Agilent Technologies). TruSeq cDNA libraries (Illumina) were prepared from 500 ng total RNA with polyA capture, and 2 × 100 bp paired-end sequencing was performed on the Illumina HiSeq 2000 platform at Beckman Coulter Genomics (Danvers, MA, USA). A total of 413 million single reads were produced (51.7 million per sample on average; S4 Table). We mapped fastq files of each sample against the GRCh37 (H19) human genome with TopHat2 (default parameters), adding the Ensembl release 73 (Gencode v18) gene annotation to guide the alignments, and HTSeq [76] was ran to count the number of reads for each gene based on the same gene annotation. To detect differentially expressed genes, we applied DESeq2 [77] and selected genes with FDR < 0.1. Protein coding genes and non-coding genes were analyzed separately. Permutations and simulations were carried out as detailed in S4 Fig. Functional relationships among differentially expressed genes (FDR < 0.05) were analyzed with the DAVID Functional Annotation tool [78]. Chimeric transcripts originated across the inversion breakpoints were detected with the IdeGen pipeline [79] using the gene annotation file (Ensembl 73, Gencode v18), inverted region sequence in GenBank format, human genome sequence (HG19) and the DESeq2 normalized fastq files for each sample. The sequence of the inverted region was created in silico by inverting the segment between breakpoints and adding 1 Mb of flanking region at each side.
Quantitative PCR (qPCR)
In quantitative real-time RT-PCR, 20-μl reactions were carried out in an ABI Prism 7500 Real-Time PCR System (Applied Biosystems) with iTaq SYBR Green Supermix with Rox (Bio-Rad), 75–125 nM of each primer (S7 Table) and 1 μl of a 1/10 dilution of the cDNA sample (except for gene ZNF257 that was amplified from 0.5 μl of undiluted cDNA due to its low expression levels). An independent RNA sample isolated from a different cell culture was used for those individuals previously analyzed by RNA-Seq. All samples were measured in triplicate by relative quantification with a standard curve and a final dissociation curve stage. Genes POLR2F and ACTB were amplified as reference genes to control for differences in cDNA concentration. Mean expression values for each genotype group (Std/Std vs. Std/Inv) were compared with a Student’s t-test.
In silico inversion genotyping
To search the Std and Inv sequences at inversion HsInv0379 breakpoints among the individuals sequenced by the 1000GP [26], we prepared a fasta file with the 100 bases surrounding the two breakpoints (S2 Table). If the 1000GP reads had already been aligned to the reference genome, only unmapped reads and reads mapped to the breakpoint regions were downloaded from the ftp server in SAM format with SAMtools [80], and then converted to fastq. Otherwise, the raw fastq files were downloaded, avoiding color-space sequences and exome reads. The reads were processed with a slightly modified version of BreakSeq [52] as described previously [81]. Reads overlapping at least 10 bases of either side of a breakpoint were retained (regardless of their length), and only those mapping uniquely to an Inv or Std breakpoint with no other hits in the reference genome were counted as allele observations. A total of 1,892 individuals from the 1000GP [26] were analyzed sequentially between January and August 2013. For inversion genotyping with the three tag SNPs, SNP calls were obtained from the 1000GP Phase 3 vcf files (20130502 release). For those individuals with alternative alleles, allele count was checked using mpileup function of SAMtools v 0.1.19 [80] with default parameters (minimum base quality Q = 13).
Nucleotide variation analysis
LD between the inversion and SNPs in the inverted region plus 20 kb of flanking sequence at each side from the 1000GP Phase 1 SNP data [26] was calculated with Haploview v4.1 [82] (both for each population separately and the seven HapMap populations together), using unrelated individuals for which the inversion had been genotyped by PCR. Only SNPs in perfect LD with the inversion (r2 = 1) were considered tag SNPs. Next, haplotypes were inferred using BEAGLE package v3.3.2 [44] from 1000GP Phase 1 SNP data [26] in the inverted region and 1.8 Mb of flanking sequence at each side for 286 East Asian individuals. Both inversion breakpoints were included in their corresponding genomic positions as extra variants with two alleles (Std and Inv) to assess the presence of switch errors in phasing. Two different phasing strategies were used. The first phasing run was carried out with default (fast) parameters including all 1000GP SNPs but using genotype likelihoods to call SNP genotypes. The second one was aimed to increase accuracy by excluding all markers with allelic R2 < 0.9 in the first run (larger values of allelic R2 indicate more accurate genotype imputation), and increasing to 20 both the number of iterations of the phasing algorithm and the number of sampled haplotype pairs for each individual during each iteration. In the accurate phasing, a switch error between breakpoints was detected in only one inversion carrier (compared to only two inverted chromosomes correctly retrieved using the first phasing run).
To determine the genetic substructure within the inverted region and the likely number of ancestral population groups (K) of the 570 phased East Asian chromosomes, we used a Bayesian genetic clustering algorithm (STRUCTURE v2.3) [45]. We assumed the admixture model and ran 100,000 Markov chain Monte Carlo iterations with a burn-in period of 100,000. We also ran STRUCTURE assuming the linkage model, and we obtained equivalent results. We did not use prior population information and K values between 1 and 10 were tested to estimate their posterior probability. Estimates of recombination rate were obtained using the rhomap program distributed within the LDHat package v2.2 [83] and the same strategy used in Alves et al. 2014 [84]. For each pair of adjacent SNPs we obtained five estimates of the population recombination rate (p = 4Ner/kb) and the median was used in the analysis. Recombination was estimated separately in a random sample of 24 Std phased chromosomes and the 24 phased Inv chromosomes. π [85] and Tajima's D [46] statistics were estimated at non-overlapping windows of 80 SNPs. The alteration of the site frequency spectrum produced by the phasing algorithm has a minor effect on recombination rate estimates or population structure inference, but it could have a big effect on polymorphism level estimates. Thus, the Tajima's D distribution was Z-transformed and centered at 0 to try to correct for the systematic overestimation caused by the preferential filtering of low frequency variants during the phasing procedure.
Inversion age estimation
A divergence-based estimate of the inversion age [42] was calculated using high-coverage SNP data from the SSMP [35]. Nucleotide variation was analyzed in the inverted region (chr19 : 22245260–21863433), excluding the segmental duplication copy at BP1 (chr19 : 22245260–21863433), which shows an increased nucleotide diversity that may alter final results (Fig 5), and 2.3% of SNPs with missing values for some individuals. We assumed that variants in heterozygotes found also in Std homozygotes belonged to the Std chromosome, while those variants found exclusively in more than one heterozygote had appeared in Inv chromosomes. A proportion of the singletons found in heterozygotes were assigned to Std chromosomes based on the observed singleton frequency in Std homozygotes, and the rest were attributed to Inv chromosomes. We calculated the net pairwise nucleotide differences between orientations (dA) by subtracting nucleotide diversity observed in Std sequences (πS) to the total nucleotide differences between Std and Inv sequences (dS-I) (equations 10.20, 10.21 and 10.22 in Nei 1987 [85]). The divergence time between Std and Inv chromosomes was estimated with the formula T = dA/2s (where T is time and s is the local substitution rate). The 95% confidence intervals for age estimates were obtained with 1,000 bootstrap resamplings of 96 individuals. The local substitution rate was estimated with the same formulas as before using chimpanzee as outgroup and assuming a divergence time with humans of 5–6 Mya. Alignment between human (HG19) and chimpanzee reference genomes (panTro4/CHIMP2.1.4) was retrieved from Ensembl database.
Detection of natural selection
The integrated haplotype score (iHS) test [47] was performed on those SNPs located within the inverted region ± 1.8 Mb with known ancestral state (5,890 SNPs). To determine if the inversion itself is undergoing positive selection we compared the standardized iHS value for the breakpoints to the overall iHS distribution. To further check the role of linked selection on inversion current frequency, LD with the inversion was calculated for the list of SNPs with significant iHS values (P < 0.05). The expected frequency distribution of hypothetical mutations with different selective effects that appeared ~43,450 ya under the human demographic history inferred by Gravel et al. 2011 [48] was estimated by forward-in-time simulations. The demographic history included an ancient African expansion (~177,000 ya), an out-of-Africa bottleneck (~62,000 ya), a founding of Asia bottleneck (~28,000 ya), an initial phase of exponential growth within Asia, and a recent explosive growth phase (starting ~5,000 ya). All simulations were performed using SFS_CODE [86] and the same parameters as in Maher et al. 2012 [87] were used except for the founding-of-Asia bottleneck which is more severe. Simulations consisted in 106 iterations of a gene with length 1 bp, initial population of 2,000 diploid individuals, random mating between males and females, without mutation in each generation, and sampling at the end 96 diploid individuals (from the terminal population size of 150,408 individuals) to calculate allele frequency. All extinct or fixed iterations were discarded and only the first 1,000 iterations still segregating were kept for analysis. Years were converted to generations by rescaling to the ancestral population size of 7,310 [48] and assuming 30 years per generation. The command line for SFS_CODE used here is "sfs_code 1 1000000-A-n 96-N 2000-P 2-t 0-L 1 2-I-r 0-W 0-Td 0 1.9800273598-Td 0.2211582307 0.1285753765-Tg 0.28499772 44.75089-Td 0.28499772 0.2976894143-Tg 0.3260373917 282.7192-TE 0.3374373005—mutation 0.238372093 S 0 G <selection coefficient>". Parameter likelihood was estimated as the probability to find the 2.4–8% observed frequency values in East Asian populations in each simulated scenario and selection coefficients were compared to the neutral model (Nes = 0) using a likelihood ratio test.
Phenotypic trait association
Inversion tag SNP rs148316037 was genotyped by TaqMan assay (Applied Biosystems) (S7 Table) from 5–10 ng of DNA of 3,824 Japanese subjects from the Nagahama Prospective Genome Cohort for Comprehensive Human Bioscience (The Nagahama Study), a community-based prospective multi-omics cohort study conducted by Kyoto University in Japan [39]. Complete linkage between the inversion and rs148316037 in this cohort was confirmed by genotyping the inversion by PCR in a total of 56 individuals (33 Std/Std, 22 Std/Inv and 1 Inv/Inv). We performed quantitative linear regression and logistic linear regression to analyze the associations between genotypes of rs148316037 tag SNP as a proxy for the inversion and clinical phenotypes (S6 Table). The quantitative traits were transformed by rank-based inverse normalization method to fit normality and used as dependent variables in the linear regression analyses. Age and sex were used as covariates.
Data availability
All the data described here have been deposited at the InvFEST Human Polymorphic Inversion Database (http://invfestdb.uab.cat/report.php?n=HsInv0379). DNA sequences have been deposited at GenBank (accession numbers KT592300-KT592304). RNA-Seq data are available at the Sequence Read Archive (SRA290330).
Supporting Information
Zdroje
1. Krimbas CB, Powell JR (1992) Drosophila inversion polymorphism. Boca Raton: CRC Press.
2. Hoffmann AA, Rieseberg LH (2008) Revisiting the impact of inversions in evolution: From population genetic markers to drivers of adaptive shifts and speciation? Annu Rev Ecol Evol Syst 39 : 21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532 20419035
3. Kirkpatrick M (2010) How and why chromosome inversions evolve. PLoS Biol 8: e1000501. doi: 10.1371/journal.pbio.1000501 20927412
4. Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffmann AA (2005) A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308 : 691–693. doi: 10.1126/science.1109523 15860627
5. Lowry DB, Willis JH (2010) A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biol 8: e1000500. doi: 10.1371/journal.pbio.1000500 20927411
6. Joron M, Frezal L, Jones RT, Chamberlain NL, Lee SF, et al. (2011) Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature 477 : 203–206. doi: 10.1038/nature10341 21841803
7. Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, et al. (2012) The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484 : 55–61. doi: 10.1038/nature10944 22481358
8. Thomas JW, Cáceres M, Lowman JJ, Morehouse CB, Short ME, et al. (2008) The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics 179 : 1455–1468. doi: 10.1534/genetics.108.088229 18562641
9. Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, et al. (2005) A common inversion under selection in Europeans. Nat Genet 37 : 129–137. doi: 10.1038/ng1508 15654335
10. Puig M, Casillas S, Villatoro S, Cáceres M (2015) Human inversions and their functional consequences. Brief Funct Genomics. doi: 10.1093/bfgp/elv020
11. Thompson MJ, Jiggins CD (2014) Supergenes and their role in evolution. Heredity 113 : 1–8. doi: 10.1038/hdy.2014.20 24642887
12. Kirkpatrick M, Barton N (2006) Chromosome inversions, local adaptation and speciation. Genetics 173 : 419–434. doi: 10.1534/genetics.105.047985 16204214
13. Lakich D, Kazazian HH, Antonarakis SE, Gitschier J (1993) Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat Genet 5 : 236–241. doi: 10.1038/ng1193-236 8275087
14. Bondeson ML, Dahl N, Malmgren H, Kleijer WJ, Tönnesen T, et al. (1995) Inversion of the IDS gene resulting from recombination with IDS-related sequences is a common cause of the Hunter syndrome. Hum Mol Genet 4 : 615–621. doi: 10.1093/hmg/4.4.615 7633410
15. Blake J, Riddell A, Theiss S, Gonzalez AP, Haase B, et al. (2014) Sequencing of a patient with balanced chromosome abnormalities and neurodevelopmental disease identifies disruption of multiple high risk loci by structural variation. PLoS One 9: e90894. doi: 10.1371/journal.pone.0090894 24625750
16. Kleinjan D-J, van Heyningen V (1998) Position effect in human genetic disease. Hum Mol Genet 7 : 1611–1618. doi: 10.1093/hmg/7.10.1611 9735382
17. Lettice LA, Daniels S, Sweeney E, Venkataraman S, Devenney PS, et al. (2011) Enhancer-adoption as a mechanism of human developmental disease. Hum Mutat 32 : 1492–1499. doi: 10.1002/humu.21615 21948517
18. Imsland F, Feng C, Boije H, Bed’hom B, Fillon V, et al. (2012) The Rose-comb mutation in chickens constitutes a structural rearrangement causing both altered comb morphology and defective sperm motility. PLoS Genet 8: e1002775. doi: 10.1371/journal.pgen.1002775 22761584
19. Sharp AJ, Cheng Z, Eichler EE (2006) Structural variation of the human genome. Annu Rev Genomics Hum Genet 7 : 407–442. doi: 10.1146/annurev.genom.7.080505.115618 16780417
20. Girirajan S, Eichler EE (2010) Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet 19: R176–87. doi: 10.1093/hmg/ddq366 20807775
21. Puig M, Cáceres M, Ruiz A (2004) Silencing of a gene adjacent to the breakpoint of a widespread Drosophila inversion by a transposon-induced antisense RNA. Proc Natl Acad Sci U S A 101 : 9013–9018. doi: 10.1073/pnas.0403090101 15184654
22. Kidd JM, Cooper GM, Donahue WF, Hayden HS, Sampas N, et al. (2008) Mapping and sequencing of structural variation from eight human genomes. Nature 453 : 56–64. doi: 10.1038/nature06862 18451855
23. Korbel JO, Urban AE, Affourtit JP, Godwin B, Grubert F, et al. (2007) Paired-end mapping reveals extensive structural variation in the human genome. Science 318 : 420–426. doi: 10.1126/science.1149504 17901297
24. Tuzun E, Sharp AJ, Bailey JA, Kaul R, Morrison VA, et al. (2005) Fine-scale structural variation of the human genome. Nat Genet 37 : 727–732. doi: 10.1038/ng1562 15895083
25. Martínez-Fundichely A, Casillas S, Egea R, Ràmia M, Barbadilla A, et al. (2014) InvFEST, a database integrating information of polymorphic inversions in the human genome. Nucleic Acids Res 42: D1027–32. doi: 10.1093/nar/gkt1122 24253300
26. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491 : 56–65. doi: 10.1038/nature11632 23128226
27. Lucas Lledó JI, Cáceres M (2013) On the power and the systematic biases of the detection of chromosomal inversions by paired-end genome sequencing. PLoS One 8: e61292. doi: 10.1371/journal.pone.0061292 23637806
28. Antonacci F, Kidd JM, Marques-Bonet T, Ventura M, Siswara P, et al. (2009) Characterization of six human disease-associated inversion polymorphisms. Hum Mol Genet 18 : 2555–2566. doi: 10.1093/hmg/ddp187 19383631
29. Salm MPA, Horswell SD, Hutchison CE, Speedy HE, Yang X, et al. (2012) The origin, global distribution, and functional impact of the human 8p23 inversion polymorphism. Genome Res 22 : 1144–1153. doi: 10.1101/gr.126037.111 22399572
30. Pang AWC, Migita O, Macdonald JR, Feuk L, Scherer SW (2013) Mechanisms of formation of structural variation in a fully sequenced human genome. Hum Mutat 34 : 345–354. doi: 10.1002/humu.22240 23086744
31. De Jong S, Chepelev I, Janson E, Strengman E, van den Berg LH, et al. (2012) Common inversion polymorphism at 17q21.31 affects expression of multiple genes in tissue-specific manner. BMC Genomics 13 : 458. doi: 10.1186/1471-2164-13-458 22950410
32. González JR, Cáceres A, Esko T, Cuscó I, Puig M, et al. (2014) A common 16p11.2 inversion underlies the joint susceptibility to asthma and obesity. Am J Hum Genet 94 : 361–372. doi: 10.1016/j.ajhg.2014.01.015 24560518
33. Skipper L, Wilkes K, Toft M, Baker M, Lincoln S, et al. (2004) Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am J Hum Genet 75 : 669–677. doi: 10.1086/424492 15297935
34. Webb A, Miller B, Bonasera S, Boxer A, Karydas A, et al. (2008) Role of the tau gene region chromosome inversion in progressive supranuclear palsy, corticobasal degeneration, and related disorders. Arch Neurol 65 : 1473–1478. doi: 10.1001/archneur.65.11.1473 19001166
35. Wong L-P, Ong RT-H, Poh W-T, Liu X, Chen P, et al. (2013) Deep whole-genome sequencing of 100 southeast Asian Malays. Am J Hum Genet 92 : 52–66. doi: 10.1016/j.ajhg.2012.12.005 23290073
36. Lappalainen T, Sammeth M, Friedländer MR, ‘t Hoen PAC, Monlong J, et al. (2013) Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501 : 506–511. doi: 10.1038/nature12531 24037378
37. Ardlie KG, Deluca DS, Segre A V., Sullivan TJ, Young TR, et al. (2015) The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348 : 648–660. doi: 10.1126/science.1262110 25954001
38. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28 : 511–515. doi: 10.1038/nbt.1621 20436464
39. Yoshimura K, Nakayama T, Sekine A, Matsuda F, Kosugi S, et al. (2013) Prevalence of postmicturition urinary incontinence in Japanese men: comparison with other types of incontinence. Int J Urol 20 : 911–916. doi: 10.1111/iju.12074 23305565
40. Terao C, Ohmura K, Ikari K, Kawaguchi T, Takahashi M, et al. (2014) Effects of smoking and shared epitope on the production of anti-citrullinated peptide antibody in a Japanese adult population. Arthritis Care Res 66 : 1818–1827. doi: 10.1002/acr.22385
41. Terao C, Ohmura K, Yamada R, Kawaguchi T, Shimizu M, et al. (2014) Association between antinuclear antibodies and the HLA class II locus and heterogeneous characteristics of staining patterns: the Nagahama study. Arthritis Rheumatol 66 : 3395–3403. doi: 10.1002/art.38867 25186300
42. Hasson E, Eanes WF (1996) Contrasting histories of three gene regions associated with In(3L)Payne of Drosophila melanogaster. Genetics 144 : 1565–1575. 8978045
43. Deng L, Tang X, Hao X, Chen W, Lin J, et al. (2011) Genetic flux between h1 and h2 haplotypes of the 17q21.31 inversion in European population. Genomics Proteomics Bioinformatics 9 : 113–118. doi: 10.1016/S1672-0229(11)60014-4 21802048
44. Browning BL, Yu Z (2009) Simultaneous genotype calling and haplotype phasing improves genotype accuracy and reduces false-positive associations for genome-wide association studies. Am J Hum Genet 85 : 847–861. doi: 10.1016/j.ajhg.2009.11.004 19931040
45. Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155 : 945–959. 10835412
46. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 : 585–595. 2513255
47. Voight BF, Kudaravalli S, Wen X, Pritchard JK (2006) A map of recent positive selection in the human genome. PLoS Biol 4: e72. doi: 10.1371/journal.pbio.0040072 16494531
48. Gravel S, Henn BM, Gutenkunst RN, Indap AR, Marth GT, et al. (2011) Demographic history and rare allele sharing among human populations. Proc Natl Acad Sci U S A 108 : 11983–11988. doi: 10.1073/pnas.1019276108 21730125
49. Tennessen JA, Bigham AW, O’Connor TD, Fu W, Kenny EE, et al. (2012) Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 337 : 64–69. doi: 10.1126/science.1219240 22604720
50. Steinberg KM, Antonacci F, Sudmant PH, Kidd JM, Campbell CD, et al. (2012) Structural diversity and African origin of the 17q21.31 inversion polymorphism. Nat Genet 44 : 872–880. doi: 10.1038/ng.2335 22751100
51. Kidd JM, Graves T, Newman TL, Fulton R, Hayden HS, et al. (2010) A human genome structural variation sequencing resource reveals insights into mutational mechanisms. Cell 143 : 837–847. doi: 10.1016/j.cell.2010.10.027 21111241
52. Lam HYK, Mu XJ, Stütz AM, Tanzer A, Cayting PD, et al. (2010) Nucleotide-resolution analysis of structural variants using BreakSeq and a breakpoint library. Nat Biotechnol 28 : 47–55. doi: 10.1038/nbt.1600 20037582
53. Zody MC, Jiang Z, Fung H-C, Antonacci F, Hillier LW, et al. (2008) Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat Genet 40 : 1076–1083. doi: 10.1038/ng.193 19165922
54. Schiffels S, Durbin R (2014) Inferring human population size and separation history from multiple genome sequences. Nat Genet 46 : 919–925. doi: 10.1038/ng.3015 24952747
55. Seguin-Orlando A, Korneliussen TS, Sikora M, Malaspinas A-S, Manica A, et al. (2014) Genomic structure in Europeans dating back at least 36,200 years. Science 346 : 1113–1118. doi: 10.1126/science.aaa0114 25378462
56. Stoneking M, Delfin F (2010) The human genetic history of East Asia: weaving a complex tapestry. Curr Biol 20: R188–93. doi: 10.1016/j.cub.2009.11.052 20178766
57. Ma J, Amos CI (2012) Principal components analysis of population admixture. PLoS One 7: e40115. doi: 10.1371/journal.pone.0040115 22808102
58. Raghavan M, Steinrucken M, Harris K, Schiffels S, Rasmussen S, et al. (2015) Genomic evidence for the Pleistocene and recent population history of Native Americans. Science. doi: 10.1126/science.aab3884
59. Lupo A, Cesaro E, Montano G, Zurlo D, Izzo P, et al. (2013) KRAB-Zinc Finger Proteins: A repressor family displaying multiple biological functions. Curr Genomics 14 : 268–278. doi: 10.2174/13892029113149990002 24294107
60. Han ZG, Zhang QH, Ye M, Kan LX, Gu BW, et al. (1999) Molecular cloning of six novel Krüppel-like zinc finger genes from hematopoietic cells and identification of a novel transregulatory domain KRNB. J Biol Chem 274 : 35741–35748. doi: 10.1074/jbc.274.50.35741 10585455
61. Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB (2013) Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet 9: e1003709. doi: 10.1371/journal.pgen.1003709 23990802
62. Bazzini AA, Johnstone TG, Christiano R, Mackowiak SD, Obermayer B, et al. (2014) Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J 33 : 981–993. doi: 10.1002/embj.201488411 24705786
63. Cech TR, Steitz JA (2014) The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157 : 77–94. doi: 10.1016/j.cell.2014.03.008 24679528
64. Guo X, Lin M, Rockowitz S, Lachman HM, Zheng D (2014) Characterization of human pseudogene-derived non-coding RNAs for functional potential. PLoS One 9: e93972. doi: 10.1371/journal.pone.0093972 24699680
65. Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, et al. (2013) An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 342 : 253–257. doi: 10.1126/science.1242088 24115442
66. De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, et al. (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515 : 209–215. doi: 10.1038/nature13772 25363760
67. van der Kant R, Goldstein LSB (2015) Cellular Functions of the Amyloid Precursor Protein from Development to Dementia. Dev Cell 32 : 502–515. doi: 10.1016/j.devcel.2015.01.022 25710536
68. Tanaka R, Satoh H, Moriyama M, Satoh K, Morishita Y, et al. (2000) Intronic U50 small-nucleolar-RNA (snoRNA) host gene of no protein-coding potential is mapped at the chromosome breakpoint t(3;6)(q27;q15) of human B-cell lymphoma. Genes to Cells 5 : 277–287. 10792466
69. MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, et al. (2012) A systematic survey of loss-of-function variants in human protein-coding genes. Science 335 : 823–828. doi: 10.1126/science.1215040 22344438
70. Sulem P, Helgason H, Oddson A, Stefansson H, Gudjonsson SA, et al. (2015) Identification of a large set of rare complete human knockouts. Nat Genet. doi: 10.1038/ng.3243
71. Subramanian S, Kumar S (2006) Evolutionary anatomies of positions and types of disease-associated and neutral amino acid mutations in the human genome. BMC Genomics 7 : 306. doi: 10.1186/1471-2164-7-306 17144929
72. Peischl S, Dupanloup I, Kirkpatrick M, Excoffier L (2013) On the accumulation of deleterious mutations during range expansions. Mol Ecol 22 : 5972–5982. doi: 10.1111/mec.12524 24102784
73. Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, et al. (2010) Integrating common and rare genetic variation in diverse human populations. Nature 467 : 52–58. doi: 10.1038/nature09298 20811451
74. Aguado C, Gayà-Vidal M, Villatoro S, Oliva M, Izquierdo D, et al. (2014) Validation and genotyping of multiple human polymorphic inversions mediated by inverted repeats reveals a high degree of recurrence. PLoS Genet 10: e1004208. doi: 10.1371/journal.pgen.1004208 24651690
75. Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. 3rd editio. Cold Spring Harbor Laboratory Press. doi: 10.1002/humu.1186.abs
76. Anders S, Pyl PT, Huber W (2014) HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics: btu638. doi: 10.1093/bioinformatics/btu638
77. Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol 15 : 550. doi: 10.1186/s13059-014-0550-8 25516281
78. Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37 : 1–13. doi: 10.1093/nar/gkn923 19033363
79. Pantano L, Puig M, Cáceres M (2014) IdeGen: Discovery of novel genes derived from known inversions using RNAseq data. In prep.
80. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079. doi: 10.1093/bioinformatics/btp352 19505943
81. Lucas-Lledó JI, Vicente-Salvador D, Aguado C, Cáceres M (2014) Population genetic analysis of bi-allelic structural variants from low-coverage sequence data with an expectation-maximization algorithm. BMC Bioinformatics 15 : 163. doi: 10.1186/1471-2105-15-163 24884587
82. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265. doi: 10.1093/bioinformatics/bth457 15297300
83. Auton A, McVean G (2007) Recombination rate estimation in the presence of hotspots. Genome Res 17 : 1219–1227. doi: 10.1101/gr.6386707 17623807
84. Alves JM, Chikhi L, Amorim A, Lopes AM (2014) The 8p23 inversion polymorphism determines local recombination heterogeneity across human populations. Genome Biol Evol 6 : 921–930. doi: 10.1093/gbe/evu064 24682157
85. Nei M (1987) Molecular evolutionary genetics. New York: Columbia University Press. 17246397
86. Hernandez RD (2008) A flexible forward simulator for populations subject to selection and demography. Bioinformatics 24 : 2786–2787. doi: 10.1093/bioinformatics/btn522 18842601
87. Maher MC, Uricchio LH, Torgerson DG, Hernandez RD (2012) Population genetics of rare variants and complex diseases. Hum Hered 74 : 118–128. doi:000346826. doi: 10.1159/000346826 23594490
88. Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, et al. (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35: W71–4. doi: 10.1093/nar/gkm306 17485472
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



