-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
Among the RNA/protein bodies within the nucleus, nucleoli are essential factories for ribosome production and assembly. The size and morphology of the nucleolus is thus a cytological manifestation of protein biosynthesis and is closely coordinated with cell biology and even malignancy. However, without membrane delimitation, the principles that define nucleoli size are poorly understood. Caenorhabditis elegans represents an ideal model to address this question owing to distinct tissue distribution of nucleolar sizes and a mutant, ncl-1, which exhibits larger-than-normal nucleoli. We report here a genetic cascade of microRNA let-7 and translation repressor NCL-1, which tightly controls abundance of FIB-1/fibrillarin. This network ultimately contributes to developmental control of nucleolar size and function.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005580
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005580Summary
Among the RNA/protein bodies within the nucleus, nucleoli are essential factories for ribosome production and assembly. The size and morphology of the nucleolus is thus a cytological manifestation of protein biosynthesis and is closely coordinated with cell biology and even malignancy. However, without membrane delimitation, the principles that define nucleoli size are poorly understood. Caenorhabditis elegans represents an ideal model to address this question owing to distinct tissue distribution of nucleolar sizes and a mutant, ncl-1, which exhibits larger-than-normal nucleoli. We report here a genetic cascade of microRNA let-7 and translation repressor NCL-1, which tightly controls abundance of FIB-1/fibrillarin. This network ultimately contributes to developmental control of nucleolar size and function.
Introduction
Among the RNA/protein bodies within the nucleus, nucleoli bear the essential function of being the factories for ribosome subunit production and assembly, a stress sensor for cell cycle control, as well as a site for hepatitis D virus (HDV) replication and adenovirus-associated virus (AAV) assembly [1–3]. The size and morphology of the nucleolus is a cytological manifestation of ribosome biogenesis and therefore protein biosynthesis and is closely coordinated with cell growth and development [4]. Accordingly, these attributes sometimes are also physiological indicators of cell cycle, cancer growth and malignancy as well as stem cells differentiation and pluripotency [5, 6]. However, without membrane delimitation, the principles that define nucleoli size and shape are poorly understood. Furthermore, spatiotemporal regulation of nucleolar size and output, particularly in coordination with development and in non-dividing cells, are not fully characterized.
Caenorhabditis elegans represents an exploitable model for further interrogating nucleolus biology owing to distinct distribution of nucleolar sizes in different cell types. A C. elegans mutant, ncl-1, described as a recessive mutation with enlarged nucleoli in nearly all cells of the worm [7, 8], has this phenotype consistent with its role as a suppressor of rRNA biosynthesis. C. elegans ncl-1 phenotypes can be rescued by its Drosophila homolog, brat [9, 10]. Mutations in the fly brat gene have a similar phenotype to the defect of ncl-1 mutants in C. elegans, affecting nucleolar size. In addition, brat mutants induce brain tumor formation [11]. These homologous proteins belong to a TRIM/RBCC/NHL (NCL-1, HT2A, and LIN-41] family characterized by the presence of a RING domain, a B-box zinc finger, and a coil-coiled domain [12, 13]. Because its lack of an RNA binding motif, Brat protein was previously shown to associate with the 3’UTR of the hunchback transcript in partnership with two RNA-binding proteins Pumillio (PUF) and Nanos (NOS), and suppress expression of Hunchback protein at the translational level [14].
In this study, we dissected the molecular mechanism through which NCL-1 controls nucleolar size and function, and pinpointed fibrillarin—the rRNA 2’-O-methyltransferase and pre-rRNA processing factor [1, 15–17]–as a downstream effector. Further, this regulation is dynamically coordinated with development as part of a functional axis driven by let-7, a critical developmental regulator of heterochronic development in worms and flies [18–20] and of cancer formation and stem cell maintenance in the mammals [21].
Results
Suppression of nucleolar size and rRNA expression by NCL-1 is associated with nucleolar protein FIB-1
Although abundantly expressed in the gonads of C. elegans [9], the effect of ncl-1 on the nucleoli of germ cells was not characterized. In intact gonads of wild-type (N2) young adult worms, nucleolar structure is nearly absent in the -1 oocyte, which is immediately adjacent to the spermatheca (Fig 1A, upper panel). In contrast, the nucleolus was readily detectable in the -1 oocyte of ncl-1(e1942) mutant (Fig 1A, lower panel). While nucleoli were evident in the germ cells and the -3 and -2 oocytes of both worms, ncl-1 worms exhibited considerably larger average nucleoli size ranging from 119% to 176% of wild-type diameter (Fig 1A and 1B). Profiling of the ncl-1 mRNA expression by RT-qPCR revealed a progressive decline in mRNA abundance from the embryo to and throughout the four larva stages, followed by subsequent up-regulation in the adult (S1 Fig). This developmental stage-specific expression is consistent with previous in situ immuno-staining of NCL-1 that demonstrated its expression in the proximal gonad and early embryos and the subsequent gradual disappearance in the late stages of embryos [9]. Further, this expression is in line with the non-detectable to small sizes of nucleoli in the -1 oocyte and early embryos (Fig 1C, left panel), supporting the notion that NCL-1 is a negative regulator of nucleolar size.
Fig. 1. NCL-1 acting as a restriction factor for nucleolar size and rRNA expression is associated with nucleolar protein, FIB-1. 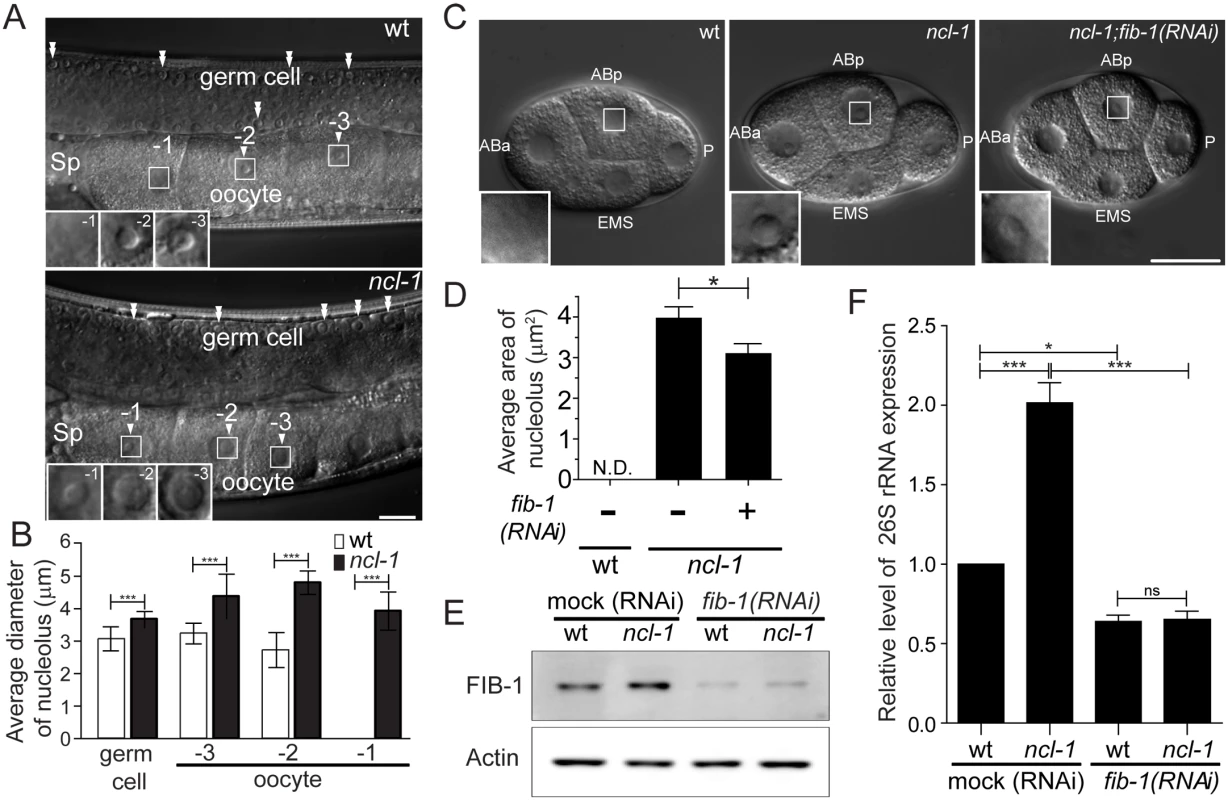
(A) Differential contrast interference (DIC) microscopy of the gonads of the wild-type (wt) or ncl-1(e1942) worms. Numbers mark the different oocytes and arrowheads indicate the location of nucleoli. Double arrowheads indicate the nucleoli of germ cells. Insets at lower left are enlargement from the square areas of oocytes. Sp, spermatheca. Scale bar, 20 μm. (B) Diameters of the nucleoli in the gonadal cells, as shown in (A), were quantitatively determined. Asterisks signify differences between the two worms: ***P < 0.0001; n = 8–14 gonad arms. (C) DIC microscopy of the blastomeres of wild-type (wt) and the ncl-1 embryos, with or without fib-1 RNAi. Each blastomere is indicated as ABa, ABp, EMS and P. Insets represent enlarged versions of the boxed regions of ABp cells to highlight the nucleoli. Scale bar, 20 μm. (D) Quantitative representation of the results shown in (C), which illustrates the distribution of nucleolar areas in the four blastomeres. Asterisk signifies difference between the indicated strains: *P < 0.05; n ≥ 31 embryos. (E) Knockdown of fib-1 was done in the indicated worms. The expression of Actin (lower panel) and the endogenous FIB-1 (upper panel) were examined by Western blot analysis. (F) RT-qPCR analysis of 26S rRNA expression in the indicated strains of worms as shown in (E) *P < 0.05; ***P < 0.001; ns, no significant; n = 3. We also examined nucleolar morphology in worms devoid of functional fib-1. Consistent with its significance, fib-1 mutation led to lethality [22]; we thus characterized fib-1 mutant larvae (L1 stage) and found that nucleoli therein displayed size reduction (S2 Fig). To next determine if FIB-1/fibrillarin is involved in the nucleolar appearance and size, we depleted fib-1 in ncl-1(e1942) worms by RNAi feeding and measured the nucleolar size. The increase in nucleolar size in the blastomeres of ncl-1(e1942) worms (Fig 1C, middle panel) was significantly reversed by fib-1 abrogation as shown by image analysis (Fig 1C, right panel, and 1D). This observation supports a notion that the amount of FIB-1 expression is directly associated with the control of nucleolar size by NCL-1. Moreover, Western blot and RT-qPCR analyses showed that worms expressing a greater amount of FIB-1 generally had a higher level of rRNA abundance (Fig 1D and 1F). Conversely, knockdown of FIB-1 led to an overall reduction in the rRNA levels, further indicating a positive role of FIB-1 in this functional regard.
NCL-1 is a suppressor of FIB-1 expression
To examine whether NCL-1-mediated nucleolar size alternations is through the regulation of FIB-1 expression, we generated a pair of transgenic worms that express FIB-1::GFP chimeric protein in both the N2 and ncl-1 backgrounds [respectively designated as cguIs1 (strain SJL1) and ncl-1(e1942); cguIs1 (strain SJL14), see S1 Table]. Time-lapse fluorescence microscopy of embryos was performed to trace the level of GFP expression during early stages, and showed progressively higher GFP signals (Fig 2A and S1–S3 Movies). Dynamic up-regulation of GFP levels was more prominent in the ncl-1(e1942); cguIs1 embryos (62.8%) than in cguIs1 (26.9%) (Fig 2B). Random collections of embryos from both transgenic worms were further examined to quantify the GFP intensity of each embryo in the same field (Fig 2C) and subsequently revealed that the embryos in the absence of NCL-1 exhibited higher levels of FIB-1::GFP (about 2 fold) (Fig 2D). Further expression analyses consistently showed elevated levels of FIB-1 in ncl-1(e1942) embryos (5.2 fold) and adult worms (1.7 fold) (Fig 2E). Unexpectedly, RT-qPCR analysis revealed comparable levels of fib-1 mRNA in wild type and ncl-1(e1942) in embryo and adult stages (Fig 2F). Taken together, these findings indicate that ncl-1 is an upstream negative regulator of fib-1 expression at the post-transcriptional/translational stage.
Fig. 2. NCL-1 is a negative regulator of FIB-1 expression. 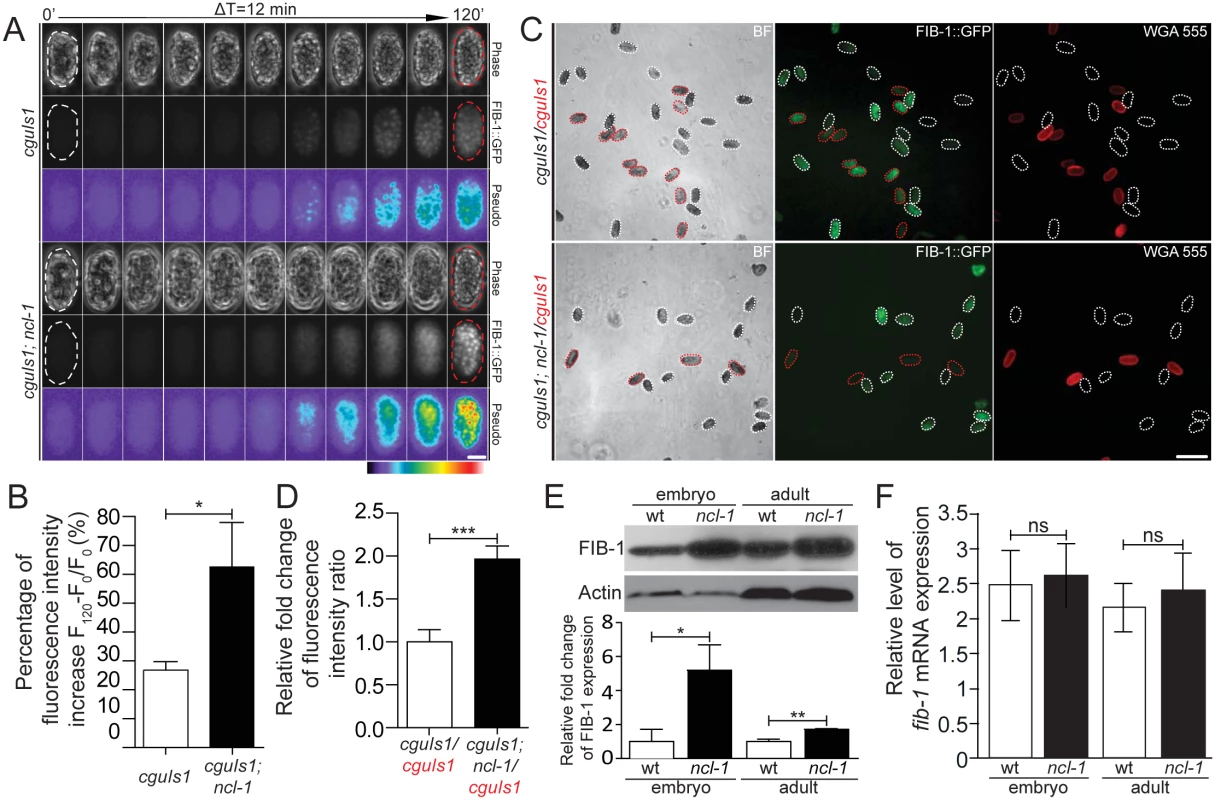
(A) Time-lapse imaging of embryos from the two indicated transgenic worms during early development, taken every 12 minutes. Both phase-contrast and green fluorescence (FIB::GFP) images are shown, with pseudo-colors depicting the degree of fluorescence intensity as indicated by a color bar. Scale bar, 20 μm. (B) Average fluorescence levels were quantitatively determined from the images in (A). The bar graph shows the percentage of fluorescence intensity increase between the initial and end-point time-lapse images for the indicated strains. *P < 0.05; n = 20–23 embryos. (C) Fluorescence images shown the FIB-1::GFP expression in different transgenic embryo pairs. Embryos of the indicated strains were labeled either with or without WGA 555 prior to mixture at an equal ratio (labeled strains are marked in red). Representative images are shown, with white and red dotted contours respectively marking WGA-negative and WGA-positive embryos (“BF”, bright field). Scale bar, 100 μm. (D) Quantitative image analysis for FIB-1::GFP expression in embryos shown in (C), illustrating the ratios of average fluorescence intensity between the indicated strains. Asterisk signifies the difference: ***P < 0.0001; n = 129–222 embryos. (E) and (F) Western blot and RT-qPCR analyses of FIB-1/fib-1 mRNA and Actin/actin mRNA (as control) in the wild-type (wt) or ncl-1 animals, at the embryo or adult stage. Relative levels of normalized FIB-1/fib-1 mRNA expression are quantified and presented below. The bar graph depicts means ± S.E.M.; *P < 0.05; **P < 0.005; ns, no significance; n = 3–5. NCL-1 cooperates with PUF and NANOS to modulate fib-1 mRNA translation
We next aimed to test whether NCL-1 acts as its fly homologue Brat, which suppresses its target gene at the translational level by binding to the 3' UTR of transcripts [14]. Towards this end, we created two more pairs of transgenic worms [cguIs2 and ncl-1(e1942); cguIs2 (strain SJL2/strain SJL15), and cguIs19 and ncl-1(e1942); cguIs19 (strain SJL34/strain SJL38), see S1 Table]; SJL2 and SJL15 harbored a plasmid similar to cguIs1 worms that contains the full-length fib-1 3' UTR, while in SJL34 and SJL38 the fib-1 3' UTR was replaced with unc-54 3' UTR sequence (Fig 3A). In agreement with the above observations, enlarged nucleoli and a significantly increased levels of FIB-1::GFP expression were both evident in the tail hypodermis of ncl-1(e1942); cguIs1 and ncl-1(e1942); cguIs2 worms (Fig 3B, top two panels at right, and S3 Fig). In contrast, for the transgene harboring the unc-54 3' UTR, ncl-1 inactivation did not lead to discernable difference in GFP intensity, despite the occurrence of enlarged nucleoli of cells in cguIs19; ncl-1 transgenic worms (Fig 3C). These observations and the quantitative data for the whole worms (Fig 3D and 3E) strongly support the notion that, rather than being the consequence of altered nucleolus, the suppression of FIB-1 may arise from direct targeting of its 3' UTR by NCL-1.
Fig. 3. The 3’ UTR of fib-1 is under the control of NCL-1. 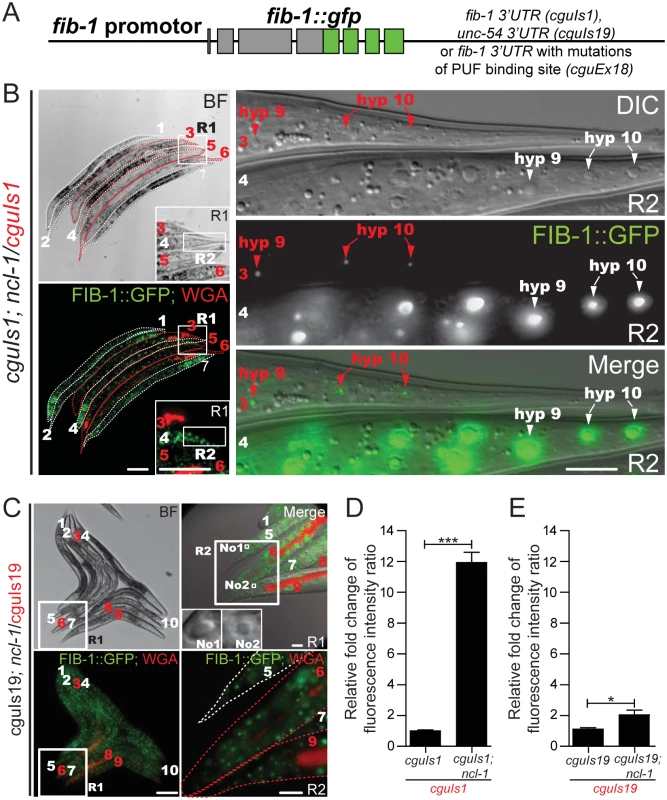
(A) Schematic for a FIB-1::GFP transgene construct that harbors 3' UTR sequence from either the fib-1 or unc-54 gene, or with mutated PUF binding site. (B) Before image acquisition, worms of different strains were first labeled with or without WGA 555 prior to mixture at an equal ratio. Expression of the fib-1 3' UTR reporter (FIB-1::GFP) was examined in the N2 (cguIs1; WGA-positive and indicated by numbers and contours in red) and ncl-1 mutant (ncl-1(e1942); cguIs1; WGA-negative and indicated by numbers and contours in white) backgrounds. Insets in the left panels represent enlarged versions of the boxed regions (R1); images on the right denote magnified versions of the boxed regions in the corresponding images on the left (R2), and represent the tail hypodermis. Red and white arrowheads pinpoint the hyp9 and hyp10 cells of respectively the cguIs1 and ncl-1(e1942); cguIs1 worms. Scale bar, 100 μm in images on the left and 10 μm in images on the right. (C) Comparison of FIB-1::GFP expression of a pair of worms carrying a reporter with unc-54 3' UTR (cguIs19 and ncl-1(e1942); cguIs19, respectively WGA-positive and WGA-negative, and indicated by numbers in red and in white). Image on the upper right denotes a magnified version of the boxed region in the corresponding image on the left (R1) and represents the tail region. Insets of upper right panel represent the nucleolus (labeled with No1 and No2) of tail hypodermis from worm 5 and worm 9, respectively. Note the enlargement of nucleolus in ncl-1(e1942); cguIs19 but similar fluorescence intensity as cguIs19 (lower right image, which is an enlarged version of the R2 box region from upper right image). Scale bar: 100 μm (left panels) and 10 μm (right panels). (D)-(E) Quantitative image analysis for FIB-1::GFP reporter expression in the whole worms. Fluorescence in images shown in Fig 3B (the cguIs1 and ncl-1(e1942); cguIs1 worm pair containing a full-length of fib-1 3' UTR) (D), in Fig 3C (the cguIs19 and ncl-1(e1942); cguIs19 worm pair containing the unc-54 3' UTR) (E), were quantitatively determined, with ratios between the indicated strains being shown in the bar graph. Asterisk signifies the difference: *P < 0.05; ***P < 0.001; n = 148–215 animals. Since Brat mediates its repressive role through other RNA-binding factors, we further tested the roles of C. elegans pumillio and nanos in the translational suppression of fib-1. A potentially direct involvement of these RNA-binding proteins was first supported by the sequence analysis of the fib-1 3’UTR, which revealed a consensus PUF binding motif (Fig 4A). To demonstrate the link between this 3’UTR element and NCL-1-dependent control, we then generated worms with 3’UTR reporter carrying mutations in the PUF binding sequence (cguEx18; Figs 3A and 4A) [23, 24]. Fluorescence microscopy showed that, in comparison to the wild-type reporter (Fig 3B and 3D), this particular transgene exhibited considerably diminished responsiveness to the loss of ncl-1 (Fig 4B and 4C), giving rise to a lower level of fluorescence intensity. In further support to the roles of the PUF proteins, RNAi knockdown puf-5, puf-8 and puf-9 and nos-2 in cguIs1 worms resulted in the appearance of brighter GFP signals (Fig 4D and 4E). However, such effect of nos/puf knockdown (puf-8 and puf-9 in particular) on the GFP reporter was reduced in the cguEx18 worms, in which the PUF binding sequence was altered (Fig 4F). Consistently with the ncl-1 knockdown and mutant worms, immunoblotting showed a rise in FIB-1::GFP abundance in these knockdown worms (Fig 4G). Collectively, these data imply that ncl-1 may coordinate with puf-5, -8, -9 and nos-2 to act directly on the 3' UTR element of fib-1, likely through a similar regulatory mechanism exhibited by brat, pumillio and nanos in the fly [14]. This demonstration of a response element in the fib-1 3’UTR and its regulatory relevance would certainly strengthen a specific and direct control mechanism.
Fig. 4. NCL-1 cooperates with PUF and NANOS to modulate FIB-1 expression. 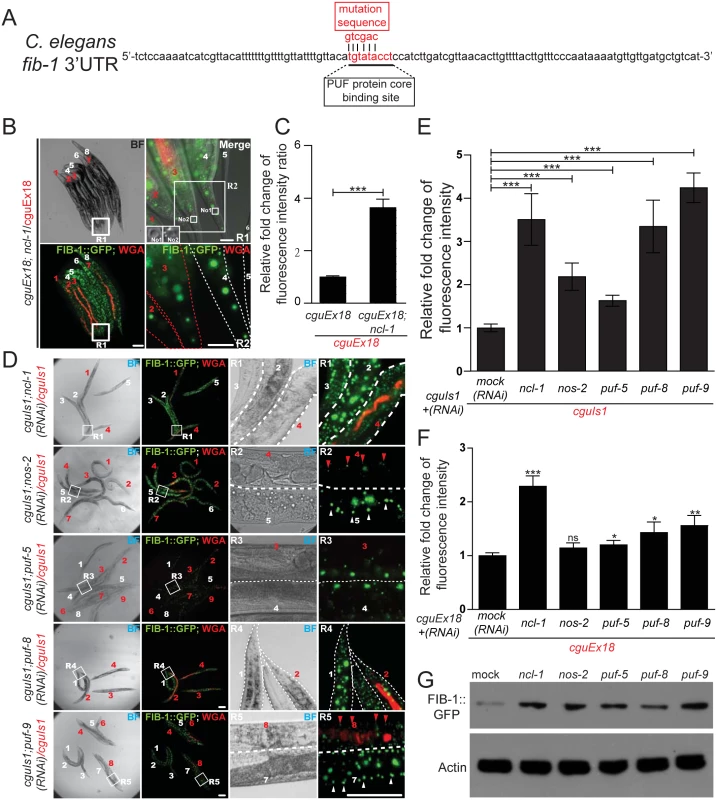
(A) Putative PUF target sites within the fib-1 3' UTR (underlined). Mutant nucleotide sequence used to generate the altered reporter in cguEx18 worms is shown above the sequence (mutation sequence). (B) Comparison of FIB-1::GFP expression of a pair of worms carrying a reporter with mutated fib-1 3' UTR (cguEx18 and ncl-1(e1942); cguEx18), respectively WGA-positive and WGA-negative, and indicated by numbers in red and in white). Image on the upper right denotes a magnified version of the boxed region in the corresponding image on the left (R1) and represents the tail region. Insets of upper right panel represent the nucleolus (labeled with No1 and No2) of tail hypodermis from worm 4 and worm 3, respectively. Lower right image is an enlarged version of the R2 box region from upper right image. Scale bar: 100 μm (left panels) and 10 μm (right panels). (C) In Fig 4C (the cguEx18 and ncl-1(e1942); cguEx18 worm pair containing the mutated fib-1 3' UTR) were quantitatively determined, with ratios between the indicated strains being shown in the bar graph. Asterisk signifies the difference: ***P < 0.001; n = 136–156 animals. (D) cguIs1 worms with RNAi targeting ncl-1, nos-2, puf-5, puf-8, or puf-9 were assessed for FIB-1::GFP expression as in (B). Scale bar: 100 μm (left panels) and 10 μm (right panels). (E) Quantitative image analysis for the results shown in (D), showing the relative ratios of average FIB-1::GFP signals between the indicated worm pairs. The bar graph depicts means ± S.E.M.; ***P < 0.001; n = 30–198 animals. (F) Quantitative image analysis for the FIB-1::GFP reporter expression in worm strains derived from cguEx18, showing the relative ratios of average FIB-1::GFP signals between the indicated worm pairs. The bar graph depicts means ± S.E.M.; *P < 0.05; **P < 0.01;***P < 0.001; ns, no significance; n = 30–198 animals. (G) Expression of the FIB-1::GFP reporter in worms with RNAi targeting the indicated genes was monitored by anti-GFP immunoblotting. The let-7-ncl-1-fib-1 pathway controls the nucleolus size and rRNA pool
Since another TRIM/RBCC/NHL family protein, LIN-41, is regulated by let-7 [13, 25], we tested for the potential involvement of microRNAs in the regulation of NCL-1. Indeed, potential let-7 and mir-49 target sequences were identified in the 3' UTR of ncl-1 (S4 Fig). As one of the best-known and evolutionarily conserved microRNAs [18, 26, 27], let-7 was selected for further investigation of possible role in ncl-1 expression. Two transcriptional reporters–Pncl-1::gfp::3' UTRncl-1 and Pncl-1::gfp::3' UTRncl-1(m), respectively harboring the wild type and mutated let-7 presumptive sites (Fig 5A), were constructed to each generate multiple independent integration lines of transgenic worms (S1 Table). Immunoblotting results revealed that transgenic worms bearing Pncl-1::gfp::3' UTRncl-1 (strain SJL8) expressed less GFP than those of Pncl-1::gfp::3' UTRncl-1(m) (strain SJL12) (Fig 5B), despite comparable copy numbers and mRNA levels of the transgene between the two strains (Fig 5C). These results thus indicated a loss of responsiveness to let-7 suppression. In further support to the let-7-ncl-1 link, fluorescence microscopic analysis of the seam cells and vulva, which are known to express let-7 [28, 29], indeed showed pronounced GFP reporter expression in the context of defective let-7 targeting (Fig 5D and 5E; S4 and S5 Movies).
Fig. 5. The let-7-ncl-1-fib-1 pathway controls the nucleolus size and function. 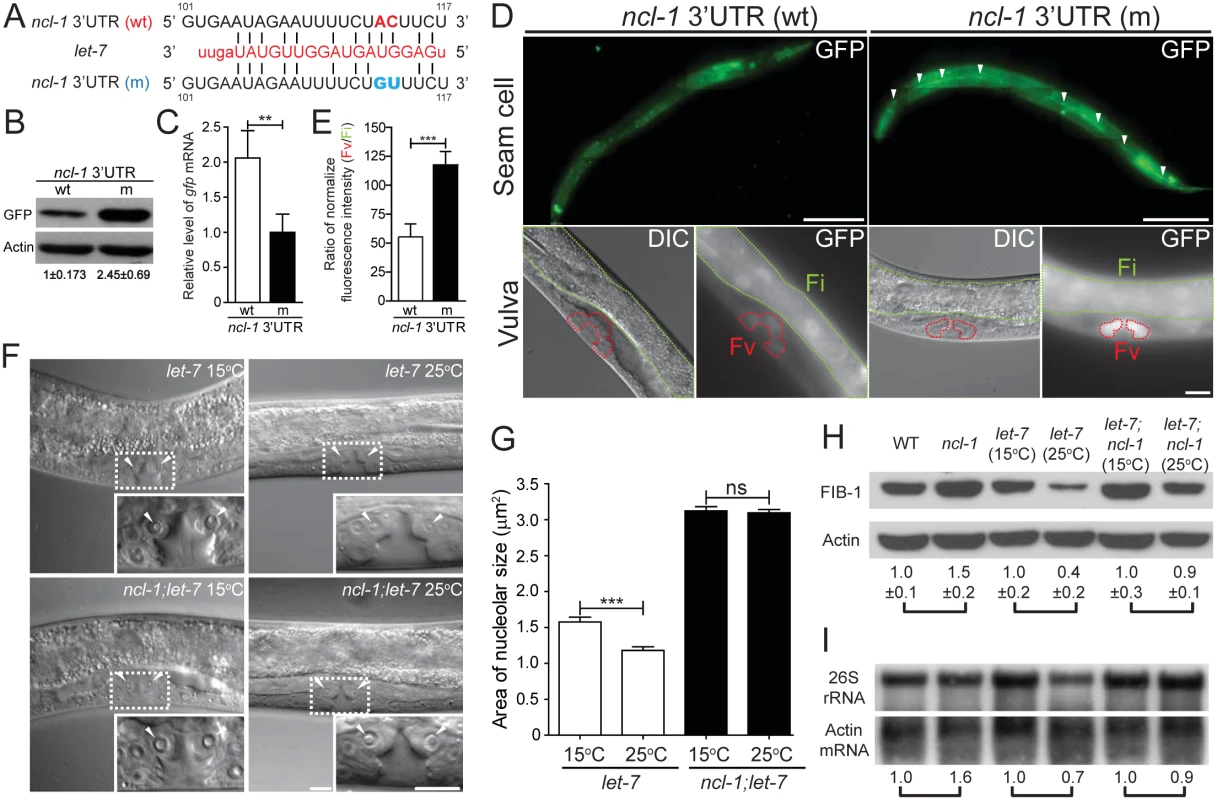
(A) Putative miRNA target sites within the ncl-1 3' UTR. The alignment on the right indicates the ncl-1 3' UTR/let-7 complement, with numbers denoting sequences relative to the stop codon. GFP reporter construct (driven by ncl-1 promoter) that contains wild-type 3' UTR sequence (wt) or mutated let-7 target sites (m) was used to generate transgenic worms. (B) Expression of the GFP protein and mRNA expression in worms carrying the reporter gene with wild-type (wt) vs. mutant (m) ncl-1 3' UTR. (A) Immunoblots of GFP reporter and the control Actin in the indicated worms. Numbers below represent relative levels of normalized GFP expression, based on quantified intensity of immunoblotting signals from three independent experiments. (C) Quantitative RT-qPCR analysis of gfp mRNA expression in the indicated transgenic worms. **P < 0.01; n = 4. (D) Characterization of GFP reporter expression in the seam (top; scale bar of 100 μm) and vulva (bottom; scale bar of 10 μm) cells. Arrowheads point to the seam cells, while red and green contours respectively denote the vulva (Fv) and intestinal (Fi) areas in the bottom images. (E) Quantitative depiction of the ratios of average fluorescence intensity in the indicated strains. Ratios were derived from the vulva (Fv) vs. intestinal (Fi) comparison, as shown in (D). ***P < 0.001; n = 46 animals. (F) DIC microscopy of the vulva cells of the let-7(n2853) and let-7(n2853); ncl-1(e1942) worms, at the permissive (15°C) or non-permissive (25°C) temperature. Insets represent enlarged images of the boxed regions in the corresponding figures. Arrowheads point to the nucleoli of the vulva cells. Scale bar, 10 μm. (G) Quantitative representation of the results shown in (F), illustrating the distribution of nucleolar areas in the vulva. Asterisk signifies difference between the indicated strains: ***P < 0.001; ns, no significance; n = 22–36 [for let-7(n2853) worms] or 100–110 [for ncl-1(e1942); let-7(n2853) worms]. (H) Western blot analysis of FIB-1 and Actin (as control) in the indicated strains. Numbers below represent the relative levels of FIB-1 protein expression (normalized to the control sample of each pair-wise comparison), calculated from five independent experiments. (I) Northern blot analysis of 26S rRNA and Actin mRNA expression in the indicated strains of worms as shown in (H). Numbers below represent the relative levels of 26S rRNA, with control sample of each pair-wise comparison being represented as 1. We next interrogated the significance of let-7 in the regulation of nucleolar size by assessing vulva cells in the temperature-sensitive, loss-of-function let-7(n2853) mutants. Mutants grown at non-permissive temperature (25°C) displayed a significant reduction in nucleolar size in these cells, by 25% as compared to those at permissive condition (15°C) (Fig 5F and 5G). However, such temperature-sensitive nucleolar size alteration was not observed in a double mutant let-7; ncl-1 (strain SJL39) (Fig 5F and 5G), implying that let-7 acts upstream of ncl-1 transcript to directly suppress NCL-1 translation and regulate nucleolar sizes of the vulva cells. We further verified the link of let-7 to NCL-1-mediated regulation by assessing downstream FIB-1 expression and rRNA abundance in let-7(n2853) and let-7(n2853); ncl-1(e1942) worms. To this end, expression profiling revealed higher amounts of both FIB-1 (Fig 5H) and ribosomal RNA species (Fig 5H and 5I and S5 Fig) in let-7(n2853) worms grown at 15°C vs. 25°C, in contrast to a lack of discernable differences in the let-7(n2853); ncl-1(e1942) worms between these rearing temperatures (Fig 5H and 5I, and S5 Fig). Such loss of phenotypes in the ncl-1(e1942) background is in agreement with let-7-ncl-1 interaction and functional antagonism. Based on these findings, we hypothesize that the genetic circuit of let-7-ncl-1-fib-1 constitutes a critical determinant in the regulation of nucleolar size and rRNA pool (Fig 6).
Fig. 6. A schematic model of the let-7-ncl-1-fib-1 circuit and its regulation of nucleolus size and function. 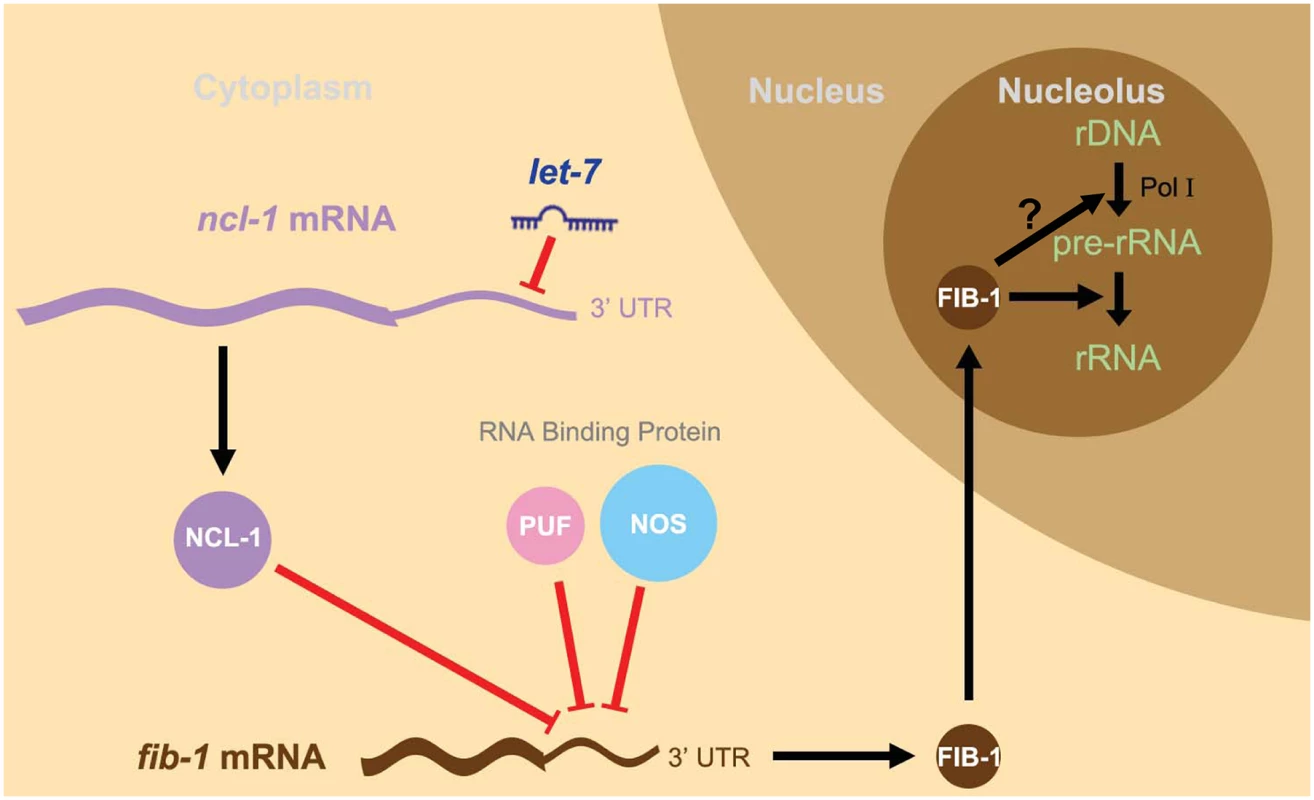
Since let-7 is a heterochronic gene linked to the control of vulva formation in the L4 larva stage, this model depicts a novel let-7-driven regulatory cascade—the let-7-ncl-1-fib-1 pathway—that regulates the nucleolus size and rRNA expression in the vulva cells. In this context, let-7 increases in the L4 larva and targets the 3’ UTR of ncl-1 transcript to suppress NCL-1 translation. In other types of cells with low levels of let-7, such as hypodermis for example, NCL-1 may be accumulated and cooperates with two other RNA binding proteins, PUF and NOS, to suppress translation of a nucleolar protein FIB-1 and consequently the size of the nucleolus (see Fig 4B and 4C). However, in the vulva cells in which NCL-1 is down-regulated, a higher abundance of FIB-1 enters the nucleolus to facilitate rRNA processing and likely contributes to enlarged nucleolus exhibited by this particular cell type (see Fig 5F and 5G). Possible FIB-1 action on Pol I activity is not resolved in this study (the question mark in the scheme), although one recent study (Tessarz et al., Nature 505, 564–568, 2014) [47] has shown that FIB-1 impacts Pol I transcription through an epigenetic control. Discussion
let-7 is known as a critical regulator of heterochronic development in worms and flies [18, 29]. Our studies outlined for the first time a genetic cascade through which the coordinated actions of let-7 and NCL-1 modulate the expression of a major nucleolar protein FIB-1, thereby fine-tuning the size and function of the nucleolus (Fig 6). This circuit of let-7-ncl-1-fib-1 and nucleolus size may represent an adaptive mechanism that couple cellular protein production capacity to the metabolic state of individual cell types. Interestingly, in a recent genome-wide RNAi-based screening for molecular networks underlying nucleolus size regulation in Drosophila, both brat and fib were identified [4], substantiating the possibility that these factors constitute a conserved core of regulatory network. Moreover, Vogt et al. has demonstrated that nucleolus maturation during early embryonic development in mice is dependent on the pluripotency factor LIN28 [30], which is known as an essential regulator of let-7 biogenesis [19, 20, 31]. Intriguingly, Chan and Slack have also shown that ribosomal protein RPS-14 is able to modulate let-7 function [32], which hints at the possibility for a feedback regulation between let-7 and nucleolar dynamics. Our work thus contributes to these findings by reinforcing the relevance of hierarchical organization of post-transcriptional regulators in the fundamental process of nucleogenesis. As FIB-1 expression in C. elegans is also regulated by the die-1 and let363/TOR pathways [33, 34], our findings further support the notion that intricate integration of multiple mechanisms underpins nucleolus integrity.
NCL-1 is a member of TRIM/RBCC-NHL protein family, which has been implicated in the regulation of tumor suppression, cell growth, and cell differentiation. In Drosophila larval neuroblasts (stem cell-like precursors), the Brat homologue is distributed to only one daughter cell through asymmetric cell division and acts as an inhibitor of its self-renewal through post-transcriptional suppression of Myc expression. In Brat mutant, both daughter cells grow and lead to the formation of larval brain tumor [35]. Similarly, the mammalian homologue TRIM3 has been reported as a tumor suppressor in human glioblastoma (GBM), a highly malignant human brain tumor, through its suppression on Myc [36]. Our study complements these findings on the NCL-1 homologues and further provides significant insight into understanding how microRNA cooperates with TRIM/RBCC-NHL proteins to suppress tumor formation.
Despite the prevalent requirement for proper maintenance of nucleolus size, our data did not exclude the possibility that the NCL-1-dependent control mechanism may have tissue - and developmental stage-specific relevance. First, while elevated FIB-1::GFP expression was robustly observed in the ncl-1 mutant, the extent to which it was up-regulated was varied between cells/tissues. A strong evidence for this phenotype is shown in Fig 3B, in which we observed variation in nucleolar size changes between hyp 9 and hyp 10 cells. Second, and perhaps more intriguingly, even in the absence of putative PUF binding site, loss of ncl-1 led to a prominently up-regulated GFP reporter expression in the head region of the cguEx18 worms (Fig 4B). This observation of differential regulation thus implies that 1) there is additional cis-acting element(s) in the fib-1 3' UTR, through which a yet unknown protein mediates brain-specific expression suppression, and/or 2) NCL-1 may functionally cooperates with other neuronal RNA-binding protein(s) to exert a context-dependent regulation of fib-1. This possibility of a modular organization of NCL-1-based regulatory network, as well as its developmental implications, may be further resolved by genetic screens and/or biochemical characterization of NCL-1-interacting factors.
Materials and Methods
Strains and mutant alleles of C. elegans
N2 Bristol C. elegans (used as a wild-type animal control) and mutant strains were obtained from Caenorhabditis Genetic Center (CGC, Minnesota). Alleles of the mutants are as follows: ncl-1(e1942) III, fib-1(ok2527) V, unc-119(ed3) III and let-7(n2853) X. Transgenic worms generated in this study are listed in S1 Table. Five additional strains for this study were generated by the following crosses: SJL14 ncl-1(e1942); cguIs1, SJL15 ncl-1(e1942); cguIs2, SJL38 ncl-1(e1942); cguIs19, SJL39 let-7(n2853); ncl-1(e1942) and SJL118 ncl-1(e1942); cguEx18. Worms were cultured at 15°C or 20°C on NGM plate (1.7% agar, 2.5 mg/mL peptone, 50 mM KH2PO4 pH 6.0, 25 mM NaCl, 5 mg/mL cholesterol, 1 mM MgSO4, 1 mM CaCl2) with fresh Escherichia coli OP50 as food source [37], and synchronized by the protocol with alkaline hypochlorite treatment and the resulting eggs were seeded onto NGM agar plates [38]. Temperature-sensitive mutants (let-7(n2853) X and SJL39 let-7(n2853); ncl-1(e1942)) were maintained at 15°C and shifted to 25°C at the larval 1 (L1) stage and harvested in L4 stage.
Plasmid constructions
Plasmids containing the genomic region of fib-1, including the promoter and 3' UTR of fib-1, were constructed from the PCR amplified DNA fragment from the C. elegans operon CEOP5428 [39]. The gfp gene was inserted into the last codon of fib-1 open reading frame to obtain a translational reporter, Pfib-1::fib-1::gfp::3' UTR fib-1, which encodes a fusion protein of FIB-1::GFP. Another derivative Pfib-1::fib-1::gfp::3' UTRunc-54 was generated by replacing the fib-1 3' UTR with unc-54 3' UTR sequence, and the construct of Pfib-1::fib-1::gfp::3' UTRfib-1(m) was done by site-direct mutagenesis of PUF binding site. To construct two transcriptional reporters, the 1.0-kb promoter and 3' UTR of ncl-1 were cloned into a vector to then generate Pncl-1::gfp::3' UTRncl-1 and Pncl-1::gfp::3' UTRncl-1(m), which differ in the let-7 presumptive targeting sites.
Worm transformation (microinjection and bombardment)
Germ line transformation by microinjection was performed as described by Mello and Fire [40]. Plasmids at the concentration of 100 ng/μl were injected into young adult N2 worms. An integrated line containing the plasmid of Pfib-1::fib-1::gfp::3' UTR fib-1 in about a hundred copies (determined by RT-qPCR) was first obtained in the wild-type background (designated as SJL1 cguIs1). A male of cguIs1 was then crossed with ncl-1(e1942) hermaphrodites, and GFP positive worms were selected. This was followed by hermaphrodite selfing to generate a homozygote worms [SJL14 ncl-1(e1942); cguIs1]. The same method was used to generate the other integration lines (see S1 Table), whereas strains of SJL6 to SJL12 (S1 Table) were obtained by the bombardment method [41].
RNAi treatment
The RNAi library was obtained from Julie Ahringer's group [42–44]. Bacteria clones producing double-stranded RNA to each target gene were grown in LB broth containing ampicillin and tetracycline for 7 to 8 hrs, and subsequently induced to produce double-stranded RNA by 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 2 hrs. Concentrated bacteria were then seeded on RNAi plates (NGM agar, 1 mM IPTG, 100 mg/ml ampicillin, and 5 mg/ml tetracycline), onto which synchronized L1 - L2 stage worms were placed and cultured for 36 hrs at 25°C. Young adult worms were collected for microscopy, RT-qPCR, and/or Western blot analyses.
Western blot
Protein extracts from embryos or worms at L4 or young adult stage were prepared by sonication and separated on 10% or 15% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes. Blocked membranes were then incubated with anti-FIB-1 (1 : 2,000 dilution, Santa Cruz) or anti-Actin (1 : 200,000 dilution, Millipore) antibody overnight at 4°C, and subsequently probed with secondary antibody-horseradish peroxidase conjugate (1 : 5,000 dilution, Sigma). Signals were detected with the ECL Western blot detection system (Thermo Scientific Inc., Waltham, MA).
RT-qPCR
Synchronized worms were collected by washing with M9 buffer and then subjected to sucrose density centrifugation to remove OP50 (E. coli) contamination. Total RNA was isolated from a frozen 1 ml aliquot (100 μl worm pellet dissolved in 1 ml TRIzol) by thawing and vigorous mixing according to the manufacturer’s instructions. The genomic DNA was digested by DNase I (Promega). Reverse transcription reactions were performed with iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad) with 1 μg of RNA. Fifty ng of cDNA was used for each real-time PCR reaction, which was performed with the iCycler IQ real-time PCR detection system (Bio-Rad). For the quantitative detection of ncl-1, fib-1, act-1, gfp and 26S rRNA transcripts, the following primer pairs were respectively used (the act-1 transcript was simultaneously quantified as an internal control):
Qncl-1F’: 5’CAAATCGGAGGCGAGGGAGT3’
Qncl-1R’: 5’CGGAAGGAAGCGGTAGAGGTA3’
Qfib-1F’: 5’ CGTCGTTGGACCAGAAGGAAT 3’
Qfib-1R’: 5’ CACCGTTGCGAAGGAAGTTTT 3’
Qact-1F’: 5’GTGTGACGACGAGGTTGCCGCTCT3’
Qact-1R’: 5’GGTAAGGATCTTCATGAGGTAATC3’
QgfpF’: 5’ CATTGAAGATGGAAGCGTTC 3’
QgfpR’: 5’ ATAGTTCATCCATGCCATGT 3’
Q26S rRNAF’: 5’ GGAGTGCTTGTCTACTGCGAG 3’
Q26S rRNAR’: 5’ CCTCTGCACAGTCACAAGTG 3’
Northern blot analysis
Synchronized late L4 worms grown at 15°C or 25°C as indicated were homogenized by a bead-beating homogenizer (FastPrep-24, MP Biomedicals) and total RNA was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction [45]. Total RNA was subjected to 1.2% agarose-formaldehyde gel electrophoresis (5 μg/lane) and transferred to a Hybond-N+ membrane (GE Healthcare). DNA probes were generated from PCR products amplified from C. elegans genomic DNA and labeled with 32P-dCTP (Perkin Elmer, PK-BLU513H) by hexamer priming. Primers for generating the ribosomal RNA species and actin probes were performed as described by Voutev et al. [46]. Hybridization was carried out at 55°C in 0.36 M Na2HPO4, 0.14 M NaH2PO4, 1 mM EDTA, 10% SDS, 25% formamide and 0.1 mg/ml salmon sperm DNA. Washes were done at 55°C sequentially in 4× SSPE, 4% SDS and 0.1× SSC, 0.1% SDS. Membranes were exposed to Kodak BioMax MS film.
Light microscopy and quantitative image analysis
To observe the FIB-1::GFP expression, embryos or young adult worms of cguIs1 were pre-stained with WGA 555 (50 μg/ml) (Alexa Fluor 555 conjugate of wheat germ agglutinin, Invitrogen) at room temperature for 30 mins (embryos) or 4 h (worms) and collected by washing 3 times with M9 buffer. They were then mixed with embryos or worms of ncl-1(e1942); cguIs1 in an equal ratio and mounted onto 5% agar pad (worms) or a chamber coverglass (embryo) (Thermo) for image acquisition. Bright field and fluorescence images were captured on an inverted or upright microscopy (Leica DMIRE2 and DM2500) using a 10×/NA 0.3 air immersion objective lens and a cool CCD (CoolSNAP K4, Roper Scientific). In order to distinguish the levels of GFP in the experimental and control embryos or worms under a same fluorescence microscope field, the average fluorescence intensity of different strains in the same images was measured using Metamorph 7.7.10.0 offline (Molecular Devices) and quantitatively determined by using Microsoft Excel software. For visualization of FIB-1::GFP expression and nucleolus size in worms, a upright microscope (Leica DM2500) with high-magnification, differential interference contrast (DIC) and fluorescence channels was used; images (shown in enlarged insets) were captured using a 63×/NA 1.4 oil immersion objective lens and a cool CCD (CoolSNAP K4). Metamorph 7.7.10.0 and Microsoft Excel software were used to measure the nucleolus size.
Deconvolution microscopy
For visualization of GFP signals in the vulva and seam cells, transgenic worms at the L4 stage were paralyzed and mounted onto 5% agar pad for z-series image recording. The DIC and fluorescence signals were collected on a Deltavision deconvolution microscope (PersonalDV, Applied Precision) using a 60×/NA 1.4 oil immersion objective lens and a cool CCD (CoolSNAP HQ2, Roper Scientific). The Metamorph software version 7.7.10.0 offline was used in image analysis.
Time-lapse images recording
Embryos of cguIs1 or ncl-1(e1942); cguIs1 as described above were plated onto a chamber coverglass for image acquisition. Phase contrast and fluorescence images were captured on an inverted microscope (Leica DMIRE2) using a 25×/NA 0.95 water immersion objective lens and an electron multiplying (EM) CCD (iXon ultra 897, Andor Technology). Images were recorded at 30s intervals and converted to pseudo-color using Metamorph software.
Statistical analysis
Statistical analyses were performed with a two-tailed Student’s t-test for independent samples by using GraphPad Prism 5 software. P<0.05 was considered statistically significant.
Supporting Information
Zdroje
1. Lo SJ, Lee CC, Lai HJ (2006) The nucleolus: reviewing oldies to have new understandings. Cell Res. 16 : 530–538. 16775624
2. Pederson T. (2010) "Compact" nuclear domains: reconsidering the nucleolus. Nucleus 1(5):444–5. doi: 10.4161/nucl.1.5.13056 21326828
3. Powell K. (2015) Thoru Pederson: Spotting novel roles for the nucleolus. J. Cell Biol. 208(4):384–5. doi: 10.1083/jcb.2084pi 25688131
4. Neumuller RA, Gross T, Samsonova AA, Vinayagam A, Buckner M, Founk K, et al. (2013) Conserved regulators of nucleolar size revealed by global phenotypic analyses. Sci. Signal 6:ra70. doi: 10.1126/scisignal.2004145 23962978
5. Tsai RY, Pederson T (2014) Connecting the nucleolus to the cell cycle and human disease. FASEB J. 28(8):3290–6. doi: 10.1096/fj.14-254680 24790035
6. Watanabe-Susaki K, Takada H, Enomoto K, Miwata K, Ishimine H, Intoh A, et al. (2014) Biosynthesis of ribosomal RNA in nucleoli regulates pluripotency and differentiation ability of pluripotent stem cells. Stem Cells 12 : 3099–111.
7. Lee LW, Lee CC, Huang CR, Lo SJ (2012) The nucleolus of Caenorhabditis elegans. J. Biomed. Biotechnol. 2012 : 601274. doi: 10.1155/2012/601274 22577294
8. Hedgecock EM, Herman RK (1995) The ncl-1 gene and genetic mosaics of Caenorhabditis elegans. Genetics 141 : 989–1006. 8582642
9. Frank DJ, Roth MB (1998) ncl-1 is required for the regulation of cell size and ribosomal RNA synthesis in Caenorhabditis elegans. J. Cell Biol. 140 : 1321–1329. 9508766
10. Frank DJ, Edgar BA, Roth MB (2002) The Drosophila melanogaster gene brain tumor negatively regulates cell growth and ribosomal RNA synthesis. Development 129 : 399–407. 11807032
11. Arama E, Dickman D, Kimchie Z, Shearn A, Lev Z (2000) Mutations in the beta-propeller domain of the Drosophila brain tumor (brat) protein induce neoplasm in the larval brain. Oncogene 19 : 3706–3716. 10949924
12. Slack FJ, Ruvkun G (1998) A novel repeat domain that is often associated with RING finger and B-box motifs. Trends Biochem. Sci. 23 : 474–475. 9868369
13. Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G (2000) The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol. Cell. 5 : 659–669. 10882102
14. Sonoda J, Wharton RP (2001) Drosophila Brain Tumor is a translational repressor. Genes Dev. 15 : 762–773. 11274060
15. Pi H, Lee LW, Lo S.J. (2009) New insights into polycistronic transcripts in eukaryotes. Chang Gung Med. J. 32 : 494–498. 19840506
16. Marcel V, Ghayad SE, Belin S, Therizols G, Morel AP, Solano-Gonzàlez E, et al. (2013) p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell. 24(3):318–30. doi: 10.1016/j.ccr.2013.08.013 24029231
17. Rodriguez-Corona U, Sobol M, Rodriguez-Zapata LC, Hozak P, Castano E. (2015) Fibrillarin from Archaea to human. Biol. Cell Jun;107(6):159–74. doi: 10.1111/boc.201400077 25772805
18. Ambros V (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113 : 673–676. 12809598
19. Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, et al. (2008) A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell. Biol. 10 : 987–993. doi: 10.1038/ncb1759 18604195
20. Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, et al. (2011) Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 147 : 1066–1079. doi: 10.1016/j.cell.2011.10.039 22118463
21. Bussing I, Slack FJ, Grosshans H (2008) let-7 microRNAs in development, stem cells and cancer. Trends Mol. Med. 14 : 400–409. doi: 10.1016/j.molmed.2008.07.001 18674967
22. Piano F, Schetter AJ, Morton DG, Gunsalus KC, Reinke V, Kim SK, et al. (2002) Gene clustering based on RNAi phenotypes of ovary-enriched genes in C. elegans. Curr Biol. 12(22):1959–64. 12445391
23. Bernstein D, Hook B, Hajarnavis A, Opperman L, Wickens M (2005) Binding specificity and mRNA targets of a C. elegans PUF protein, FBF-1. RNA 11 : 447–458. 15769874
24. Wickens M, Bernstein DS, Kimble J, Parker R (2002) A PUF family portrait: 3'UTR regulation as a way of life. Trends Genet. 18(3):150–7. 11858839
25. Stadler M, Artiles K, Pak J, Fire A (2012) Contributions of mRNA abundance, ribosome loading, and post - or peri-translational effects to temporal repression of C. elegans heterochronic miRNA targets. Genome Res. 12 : 2418–26.
26. Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. (2000) Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408 : 86–89. 11081512
27. Ambros V (2011) MicroRNAs and developmental timing. Curr. Opin. Genet. Dev. 21 : 511–517. doi: 10.1016/j.gde.2011.04.003 21530229
28. Johnson SM, Lin SY, Slack FJ (2003) The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev. Biol. 259 : 364–379. 12871707
29. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403 : 901–906. 10706289
30. Vogt EJ, Meglicki M, Hartung KI, Borsuk E, Behr R (2012) Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development. Development 139 : 4514–4523. doi: 10.1242/dev.083279 23172912
31. Viswanathan SR, Daley GQ, Gregory RI (2008) Selective blockade of microRNA processing by Lin28. Science 320 : 97–100. doi: 10.1126/science.1154040 18292307
32. Chan SP, Slack FJ (2009) Ribosomal protein RPS-14 modulates let-7 microRNA function in Caenorhabditis elegans Developmental Biology 334(1):152–60. doi: 10.1016/j.ydbio.2009.07.011 19627982
33. Sheaffer KL, Updike DL, Mango SE (2008) The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr. Biol. 18 : 1355–1364. doi: 10.1016/j.cub.2008.07.097 18804378
34. Goldsmith AD, Sarin S, Lockery S, Hobert O (2010) Developmental control of lateralized neuron size in the nematode Caenorhabditis elegans. Neural Dev. 5 : 33. doi: 10.1186/1749-8104-5-33 21122110
35. Betschinger J, Mechtler K, Knoblich JA. (2006) Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 24;124(6):1241–53.
36. Chen G, Kong J, Tucker-Burden C, Anand M, Rong Y, Rahman F, Moreno CS, et al. (2014) Human Brat ortholog TRIM3 is a tumor suppressor that regulates asymmetric cell division in glioblastoma. Cancer Res. 15; 74(16):4536–48.
37. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94. 4366476
38. Stiernagle T (2006) Maintenance of C. elegans. WormBook 11 : 1–11
39. Lee LW, Lo HW, Lo SJ (2010) Vectors for co-expression of two genes in Caenorhabditis elegans. Gene 455 : 16–21. doi: 10.1016/j.gene.2010.02.001 20149852
40. Mello C, Fire A (1995) DNA transformation. Methods Cell Biol. 48 : 451–482. 8531738
41. Praitis V, Casey E, Collar D, Austin J (2001) Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157 : 1217–1226. 11238406
42. Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 : 103–112. 11223248
43. Timmons L, and Fire A (1998) Specific interference by ingested dsRNA. Nature 395 : 854. 9804418
44. Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 : 325–330. 11099033
45. Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162 : 156–159. 2440339
46. Voutev R, Killian DJ, Ahn JH, Hubbard EJ (2006) Alterations in ribosome biogenesis cause specific defects in C. elegans hermaphrodite gonadogenesis. Dev. Biol. 298 : 45–58. 16876152
47. Tessarz P, Santos-Rosa H, Robson SC, Sylvestersen KB, Nelson CJ, Nielsen ML, Kouzarides T. (2014) Glutamine methylation in histone H2A is an RNA-polymerase-I-dedicated modification. Nature 505(7484):564–8. doi: 10.1038/nature12819 24352239
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



