-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
Ovulation is the process of releasing fertilizable oocytes from the ovary and is essential for metazoan reproduction. Our recent work has demonstrated principles governing ovulation process that are highly conserved across species, such that both mammals and Drosophila utilize matrix metalloproteinase (Mmp) to degrade extracellular matrix and weaken the follicle wall for follicle rupture. However, a fundamental question remaining in the field is how Mmp activity is precisely regulated during ovulation. This paper reports that Drosophila octopamine (OA), the insect equivalent of norepinephrine (NE), is the signal to induce Mmp activity through activating its receptor Oamb on mature follicle cells and that this may induce ovulation. These findings allow us to develop the first ex vivo follicle rupture assay for Drosophila, which gives us unprecedented ability to characterize the entire follicle rupturing process ex vivo and to identify essential factors for ovulation. Furthermore, we show that NE partially fulfills OA’s role in inducing follicle rupture ex vivo, indicating that follicular adrenergic signal is a conserved signal to regulating Mmp activity and ovulation. Our work not only sheds light on the long-standing question of Mmp regulation, but also may lead to a better understanding of Mmp and NE linked pathological processes including cancer metastasis and polycystic ovary syndrome.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005604
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005604Summary
Ovulation is the process of releasing fertilizable oocytes from the ovary and is essential for metazoan reproduction. Our recent work has demonstrated principles governing ovulation process that are highly conserved across species, such that both mammals and Drosophila utilize matrix metalloproteinase (Mmp) to degrade extracellular matrix and weaken the follicle wall for follicle rupture. However, a fundamental question remaining in the field is how Mmp activity is precisely regulated during ovulation. This paper reports that Drosophila octopamine (OA), the insect equivalent of norepinephrine (NE), is the signal to induce Mmp activity through activating its receptor Oamb on mature follicle cells and that this may induce ovulation. These findings allow us to develop the first ex vivo follicle rupture assay for Drosophila, which gives us unprecedented ability to characterize the entire follicle rupturing process ex vivo and to identify essential factors for ovulation. Furthermore, we show that NE partially fulfills OA’s role in inducing follicle rupture ex vivo, indicating that follicular adrenergic signal is a conserved signal to regulating Mmp activity and ovulation. Our work not only sheds light on the long-standing question of Mmp regulation, but also may lead to a better understanding of Mmp and NE linked pathological processes including cancer metastasis and polycystic ovary syndrome.
Introduction
Ovaries in organisms ranging from humans to insects are extensively innervated [1–4], and neuronal inputs likely play important roles in ovarian physiology [5]. In mammals, ovaries are predominantly innervated by sympathetic fibers from the ovarian plexus nerve and the superior ovarian nerve [6], which release norepinephrine (NE) locally and contribute to follicle development [7]. Deregulation of sympathetic nerve outflow to ovaries is associated with polycystic ovary syndrome (PCOS), a common endocrine disorder leading to anovulatory infertility [8,9]. Despite the apparent importance of sympathetic innervation, however, it is not yet clear how the neuronal modulators/transmitters released from nerve termini affect ovulation [10–16].
In Drosophila and other insects, the biogenic monoamines tyramine (TA) and octopamine (OA) act as functional counterparts to mammalian epinephrine and norepinephrine and regulate a variety of behaviors, including the fight-or-flight response, motivation, aggression, and reproduction [17,18]. Analogous to the adrenergic innervation in mammalian ovaries, Drosophila octopaminergic neurons innervate ovaries and the female reproductive tract (Fig 1A; [3,19,4]). OA released from these neurons is essential for ovulation, as mutations that disrupt the enzymes required for OA synthesis, tyrosine decarboxylase 2 (Tdc2) and tyramine β-hydroxylase (TβH), completely block ovulation [20–22].
Fig. 1. A novel ex vivo follicle rupture assay in Drosophila. 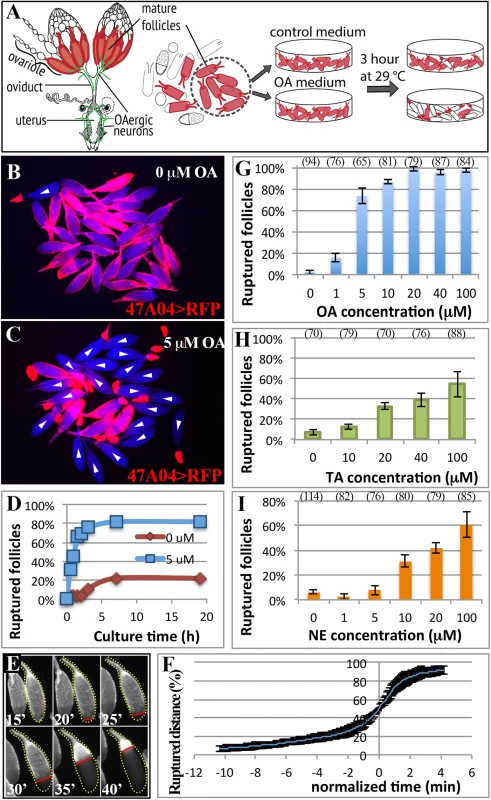
(A) A schematic diagram representing the female reproductive system and ex vivo experiments. Mature follicle cells are marked by fluorescent proteins (red), and octopaminergic neurons are shown in green [3]. (B-C) Representative images show mature follicles after three-hour culture without (B) or with (C) OA. Follicles are imaged with incident light shown in blue and follicle cells are marked by R47A04-Gal4 driving UAS-RFP (47A04>RFP) expression in red. White arrowheads indicate ruptured follicles here and in subsequent figures. (D) The cumulative percentage of ruptured follicles throughout the 19-hour culture period. Twenty-seven and 50 mature follicles were used in the control (0 μM) and experimental (5 μM) group, respectively. (E) A time-lapse image shows the entire follicle rupturing process after 20 μM of OA stimulation. The dotted yellow line outlines the rupturing follicle and the straight red line marks the posterior leading edge of the follicle-cell layer. Time is in minutes. (F) The kinetics of the rupturing process is similar between follicles. Data were pooled from two independent experiments, and nine out of 28 follicles isolated from ten females were analyzed. (G-I) Percentage of ruptured follicles after three-hour culture with different concentrations of OA (G), TA (H), and NE (I). Errors are standard deviations. The number of follicles analyzed was in the parenthesis above the charts in this figure and all the following figures. All conditions have three replicates except for 0 μM NE, which has four replicates. Four OA receptors have been identified in Drosophila: Oamb, Octβ1R, Octβ2R, and Octβ3R. Oamb is most closely related to mammalian α-adrenergic receptors, and the other three to β-adrenergic receptors [17,23]. Recent work demonstrated that Oamb and Octβ2R are important in egg laying and ovulation [24–26]. Oamb is widely expressed in the female reproductive system, including the ovary, with strongest expression observed in the oviduct [24]. It is currently believed that OA activates receptors in the oviduct, inducing oviduct contraction and secretion, which ultimately regulates ovulation through an unknown mechanism [19,27,25]. In addition to OA signaling, ovulation in Drosophila is affected by female reproductive gland secretions [28] and by mating, which increases the ovulation rate by stimulating afferent nerve activity in the female reproductive tract [29–33,4]. In particular, Ovulin transferred into the female reproductive tract after mating was recently shown to increase octopaminergic signaling and relax oviduct muscle [34], consistent with the role of OA signaling in regulating muscle contraction. It is, however, not clear whether OA plays any direct roles in the ovary to control ovulation.
In addition to above important work on Drosophila ovulation (also see review [35]), recent studies from our lab also showed significant conservation of the basic cellular and molecular mechanisms of ovulation from flies to mammals. Drosophila female contains two ovaries that are connected by the oviduct. Each ovary is organized into ovarioles, which have mature follicles (stage-14 egg chambers) at the posterior end toward the oviduct (Fig 1A; [36]). Each mature follicle has one layer of epithelial follicle cells surrounding the oocyte. During ovulation, posterior follicle cells are first trimmed to break the follicle-cell layer and to allow the oocyte to be released into the oviduct. The rest of the follicle cells remain at the end of the ovariole and form a corpus luteum [37]. Similar to vertebrate ovulation [38–40], the entire follicle rupture requires matrix metalloproteinase 2 (Mmp2), a proteolytic enzyme expressed in posterior follicle cells of mature egg chambers but only activated during follicle rupture [37]. It is not yet clear what signals control Mmp2 activity, but it is clear that studying this question in Drosophila could yield important insights into the fundamental mechanism of ovulation.
Here, we developed the first ex vivo assay for follicle rupture in Drosophila and used it to investigate the role of octopaminergic signaling in this process. We found that OA directly activates its receptor Oamb on mature follicle cells to induce the breakdown of posterior follicle wall and ovulation. In addition, NE could partially substitute for OA, indicating an evolutionary conserved role for follicular adrenergic signaling in ovulation. Finally, we demonstrated that follicular adrenergic signaling activates Mmp2 activity to control ovulation via the intracellular Ca2+ as the second messenger. This is the first demonstration of a direct role of a neuromodulator in the control of follicle rupture during ovulation.
Results
Octopamine is sufficient to induce follicle rupture ex vivo
Octopaminergic neurons innervate ovarioles extensively [21], and OA receptor Oamb is transcribed in mature follicle cells according to in situ hybridization [24], microarray analysis (S1 Fig; [41]), and the expression of R47A04-Gal4 [42], an Oamb enhancer element-regulated Gal4 driver, in mature follicle cells [37]. We examined whether OA activates Oamb directly in mature follicle cells to induce follicle rupture. Mature follicles with an intact layer of follicle cells marked by R47A04-Gal4 were isolated from ovaries (see methods) and cultured with OA or control media (Fig 1A). After three hours, follicles in control medium maintained an intact follicle-cell layer (Fig 1B). In contrast, about 80% of the follicles cultured with 5 μM of OA had shed their follicle-cell layer to the dorsal appendage at the anterior tip of the oocytes (Fig 1C); some were completely detached from the oocyte and floating in the medium. This phenomenon of shedding the follicle-cell layer, which we called follicle rupture in our ex vivo culture system, is reminiscent of what occurs during the ovulation process in vivo [37]. The percentage of ruptured follicles with OA stimulation increased dramatically in the first two hours and reached a plateau at about three hours (Fig 1D). Extending the culture period neither increased the percent of ruptured follicles to 100% in the OA medium, nor allowed follicles in the control medium to reach the same level of rupture as OA-stimulated follicles (Fig 1D).
To validate that the follicle rupture in our ex vivo assay mimics the in vivo process, we video-recorded the entire rupturing process (Fig 1E and S1 Movie). We found that posterior follicle cells were first trimmed, as we previously observed in vivo [37]. The remaining follicle-cell layer was then squeezed toward the anterior dorsal appendage (Fig 1E and S1 Movie). The entire rupturing process took 13.1 ± 5.0 minutes (S1 Table), resembling the estimated in vivo ovulation time of 11.2 ± 2.5 minutes (Table 1; [37]). Each mature follicle initiated the follicle rupture asynchronously, likely reflecting their asynchronous developmental stages; however, the kinetics of all ruptures was similar, with a very slow initial speed (Fig 1F). It took about 10 minutes to rupture through the posterior half of the oocyte, but only four minutes for the rest of the area (Fig 1E and 1F).
Tab. 1. The effect of follicular adrenergic signaling on egg laying, egg distribution in the reproductive tract, and egg laying time. 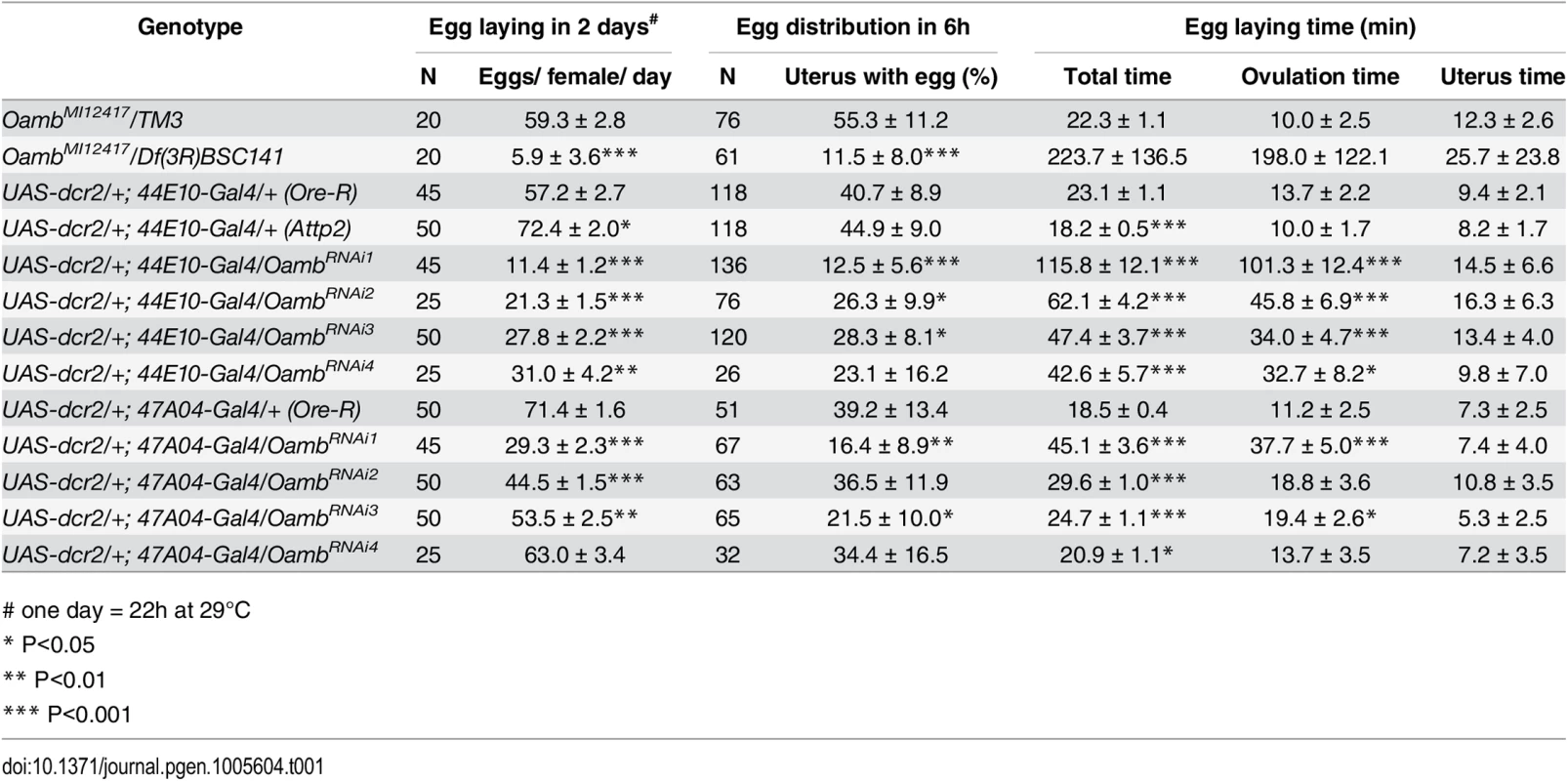
# one day = 22h at 29°C All data are mean ± 95% confidence interval. Student's T-test was used for egg laying, Chi-square test was used for egg distribution, and Z Score test was used for egg laying time assuming normal distribution
To further examine the quality of ex vivo ruptured oocytes, we determined whether these oocytes were activated. Mature oocytes released into the oviduct are activated and resistant to bleach treatment because their egg shells are hardened through cross-linking [43]. This activation process can also be mimicked in vitro by culturing oocytes in hypotonic activation buffer [44,45]. Using the established bleach assay (see methods), we found that oocytes from our ex vivo assay dissolved immediately after bleach treatment (n = 96), indicating that they were not fully activated and their eggshells were not hardened. However, treatment with hypotonic activation buffer for 15 minutes can efficiently activate these ruptured oocytes (95%, n = 150; S2A and S2B Fig), indicating these oocytes from our ex vivo system are of good quality and responsive to egg activation stimuli.
OA-induced follicle rupture is dose-dependent. Stimulation with 1 μM of OA had a minimal effect on follicle rupture, while stimulation with 20 μM of OA reached the maximal effect (Fig 1G), which led us to use 20 μM for all the following experiments. In contrast, stimulation with 20 μM of tyramine (TA), the immediate precursor of OA, had a much weaker effect on follicle rupture (Fig 1H), consistent with a previous report that OA, but not TA, is responsible for inducing ovulation [20]. Since NE is the counterpart of OA in mammals, we tested whether NE can also induce follicle rupture in our ex vivo assay. NE had only a minimal effect at lower doses (Fig 1I). Higher doses of NE could induce follicle rupture (Fig 1I), likely reflecting a differential binding properties of OA and NE to their respective receptors [18]. Nevertheless, these data suggest that OA and NE play a conserved role in regulating follicle rupture. In summary, we developed the first ex vivo assay to study follicle rupture in Drosophila, and our data suggest that OA is sufficient to induce follicle rupture in the absence of the oviduct and muscle function, as these tissues were excluded from our culture assay (68 mature follicles examined and none had ovariole muscle; S3A and S3B Fig).
Follicular Oamb is essential for OA/NE-induced follicle rupture
To identify the receptor responsible for OA/NE-induced follicle rupture, we focused on Oamb, which is essential for ovulation [24] and is the most highly expressed OA receptor in mature follicles (S1 Fig). We verified the requirement of Oamb in ovulation with a new mutant allele (OambMI12417), in which a MiMIC vector with a splice acceptor [46] was inserted in the coding intron of Oamb gene to disrupt the correct mRNA splicing (S4 Fig). Females bearing this mutant allele laid significantly fewer eggs and took a much longer time to ovulate an egg (Table 1). We then isolated mature follicles from these females and applied OA stimulation ex vivo. Oamb mutant follicles showed severe defects in OA-induced follicle rupture compared to control follicles (Fig 2A, 2B and 2E). In addition, the Oamb mutation abolished the NE-induced follicle rupture (Fig 2C–2E). The defective response of Oamb mutant follicles to OA/NE stimulation is not likely due to defective OA signaling in the oviduct or other organs, because follicles from TβH or Tdc2 mutant females are fully competent to OA/NE-induced follicle rupture (Fig 2F and 2G). These data indicate that Oamb in mature follicles is likely responsible for OA/NE-induced follicle rupture.
Fig. 2. Follicular Oamb is required for OA/NE-induced follicle rupture. 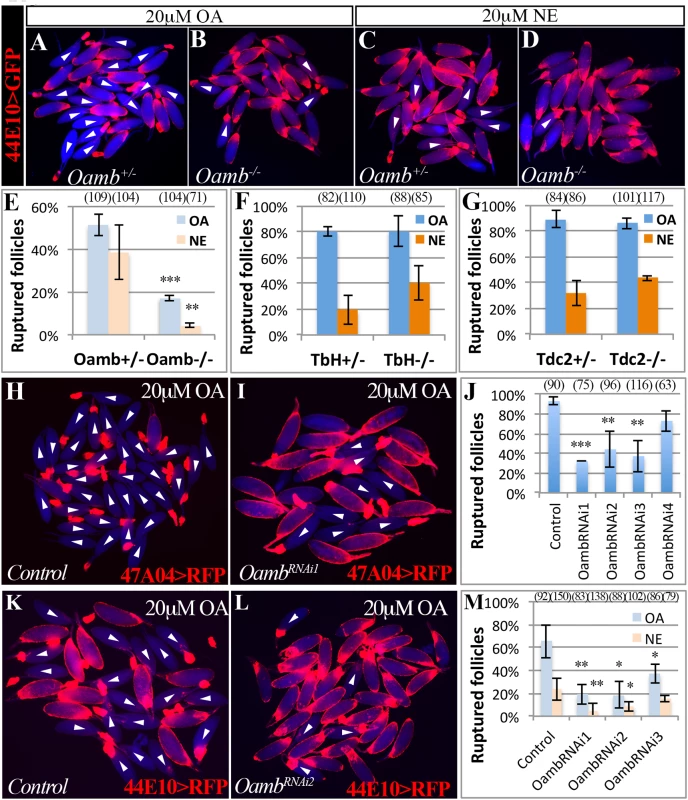
(A-D) Representative images show mature follicles (marked by R44E10>GFP in follicle cells in red) after three-hour culture with 20 μM of OA (A-B) or NE (C-D). Mature follicles are from control (A and C) and Oamb mutant (B and D) females. (E) Quantification of Oamb mutant mature follicles in response to OA or NE stimulation. Four replicates were used for each genotype, except in Oamb-/- group with NE treatment, which has three replicates. (F-G) Quantification of follicle rupture after three-hour OA or NE treatment (20 μM). Mature follicles were derived from TβH (F) or Tdc2 (G) mutant females and marked by 47A04>RFP. All treatments have three replicates except for TβH+/- with NE treatment and Tdc2-/-, which have four replicates. (H-J) Oamb knockdown with R47A04-Gal4 blocks follicle rupture. Representative images show control (H) and OambRNAi1 (I) mature follicles after three-hour culture with 20 μM of OA. Quantification of follicle rupture (J). The number of replicates for each condition in (J) is 3, 3, 3, 4, and 2. (K-M) Oamb knockdown with R44E10-Gal4 blocks follicle rupture induced by OA or NE. Representative images show control (K) and OambRNAi2 (L) mature follicles after a three-hour culture with 20 μM of OA. Quantification of follicle rupture (M). The number of replicates for each condition in (M) is 6, 5, 4, and 3. Student’s T-test was used (*** P<0.001; ** P<0.01; * P<0.05). To test if Oamb functions directly in mature follicle cells, we knocked down Oamb specifically in these cells with RNA interference (RNAi) and then performed OA stimulation ex vivo. Oamb knockdown in mature follicle cells with R47A04-Gal4 severely disrupted OA-induced follicle rupture (Fig 2H–2J). Since R47A04-Gal4 is regulated by an Oamb enhancer element [42], it could potentially be expressed in other Oamb-expressing cells, which may facilitate follicle maturation and ovulation. To exclude this possibility, we identified another Gal4 driver (R44E10-Gal4) expressed in mature follicle cells (S5B–S5D Fig). Compared to R47A04-Gal4, which is only expressed in late stage-14 follicles (S5A Fig), R44E10-Gal4 was expressed in all stage-14 follicles, slightly earlier than R47A04-Gal4. R44E10-Gal4 was not expressed in any tissues in the lower reproductive tract, nor in the neurons innervating the reproductive tract (S5B, S5E and S5F Fig). Like mature follicles isolated using R47A04-Gal4, follicles isolated using R44E10-Gal4 were also responsive to OA/NE-induced follicle rupture (S5G and S5H Fig). In addition, mature follicles with R44E10-Gal4 driving OambRNAi showed similar unresponsiveness to OA or NE stimulation (Fig 2K–2M). Taken together, these data suggest that follicular Oamb is required for OA/NE-induced follicle rupture ex vivo.
Follicular adrenergic signaling is required for ovulation in vivo
To determine whether follicular adrenergic signaling is required for ovulation in vivo, we first analyzed the fecundity of females lacking follicular Oamb. Follicular Oamb-knockdown females with either R47A04-Gal4 or R44E10-Gal4 drivers laid significantly fewer eggs than control flies (Fig 3A and Table 1). The egg-laying defect is not caused by oogenesis problems, as mature follicles are abundant in these ovaries. In fact, Oamb-knockdown flies generally had more mature follicles in their ovaries (Fig 3B), indicating an ovulation defect. Indeed, Oamb-knockdown flies had a much longer ovulation time compared to control flies but did not show defects in transporting ovulated eggs into the uterus or ejecting them out of the uterus (Fig 3C and Table 1). These data strongly suggest that follicular Oamb is required for ovulation in vivo.
Fig. 3. Follicular adrenergic signaling is required for ovulation and follicle cell trimming in vivo. 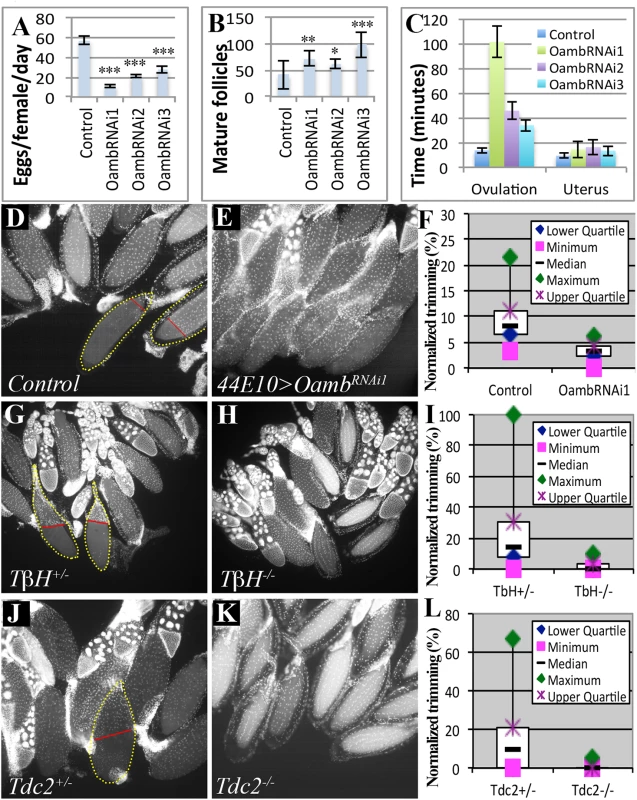
(A-C) Egg laying (A), mature follicles in ovary (B), and the average ovulation and uterus time (C) is shown for control females or those expressing OambRNAi in mature follicle cells driven by R44E10-Gal4. Student’s T-test was used (A-B; *** P<0.001; **P<0.01; * P<0.05). (D-F) Follicle cell trimming is significantly reduced when follicular Oamb is knocked down by R44E10-Gal4 driving OambRNAi1 expression (44E10>OambRNAi1). Representative images show trimmed follicles in control (D) but not Oamb-knockdown (E) ovaries. Trimmed follicles are outlined with dashed yellow lines, and the posterior leading edge of the follicle-cell layer is marked by a straight red line. Quantification of trimmed follicles (F). (G-L) Follicle cell trimming is also significantly reduced in TβH (G-I) or Tdc2 (J-L) mutant females. See Tables 1 and 2 for the number of females analyzed and statistics. Tab. 2. The effect of follicular adrenergic signaling for follicle trimming. 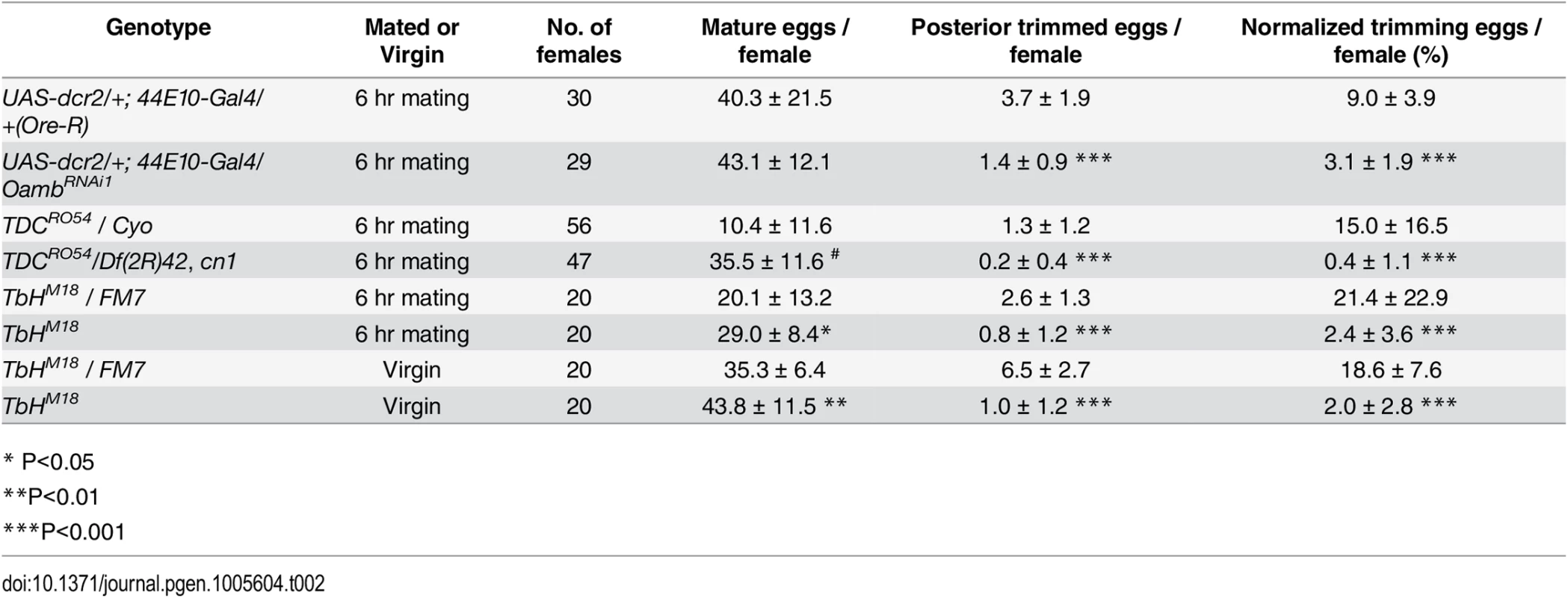
* P<0.05 All data are mean ± SD. Student’s T-test was used.
Trimming of posterior follicle cells is essential for ovulation and precedes follicle rupture [37]. We investigated the role of follicular adrenergic signaling in this trimming process. Posterior trimmed follicles were readily observed in the ovaries of control females six hours after mating, and they account for 9% of the total mature follicles in each female (Fig 3D and 3F and Table 2), consistent with our previous analysis [37]. In contrast, the percentage of posterior trimmed follicles was reduced three fold in females lacking follicular Oamb (Fig 3E and 3F and Table 2), indicating its essential role in follicle trimming. This is consistent with our observation that posterior follicle cells remain intact in Oamb-knockdown follicles even after three hours of OA stimulation ex vivo (Fig 2I and 2L). Furthermore, the percentage of trimmed follicles also decreased in flies that lacked the ability to produce OA; we saw a reduction to 2.4% and 0.4% in TβH and Tdc2 mutant females, respectively (Fig 3G–3L and Table 2). This reduction of trimmed follicles was not only observed in mated females, but also in virgin females (Table 2). Taken together, these data suggest that follicular adrenergic signaling is required for posterior follicle cell trimming.
Adrenergic signaling activates Mmp2 to regulate ovulation
The crucial role of Mmp2 in trimming of posterior follicle cells [37] prompted us to investigate the relationship between follicular adrenergic signaling and Mmp2 activity. It is unlikely that adrenergic signaling regulates Mmp2 expression, as Mmp2 was readily detected in the posterior follicle cells of TβH mutants (S6A and S6B Fig). To test whether OA regulates Mmp2 activity, we examined gelatinase enzymatic activity in the OA-induced ex vivo ovulation assay using in situ zymography [37,38]. About 20% of mature follicles cultured in a control medium had gelatinase activity at their posterior end (Figs 4A, 4C, S6C and S6G). In contrast, more than 70% of mature follicles stimulated with OA had gelatinase activity (Figs 4B, 4C, S6D and S6G). The entire eggshells of ruptured oocytes were coated with Mmp-activated gelatin-fluorescein (Figs 4B and S6D), as we observed in vivo [37]. In addition, OA-induced gelatinase activity was blocked in mature follicles with Oamb knockdown or misexpression of Timp, an endogenous inhibitor of Mmp2 [47], in follicle cells (S6E–S6G Fig). These data indicate that OA-Oamb signaling is sufficient to induce Mmp2 activation.
Fig. 4. Adrenergic signaling activates Mmp2 to regulate ovulation. 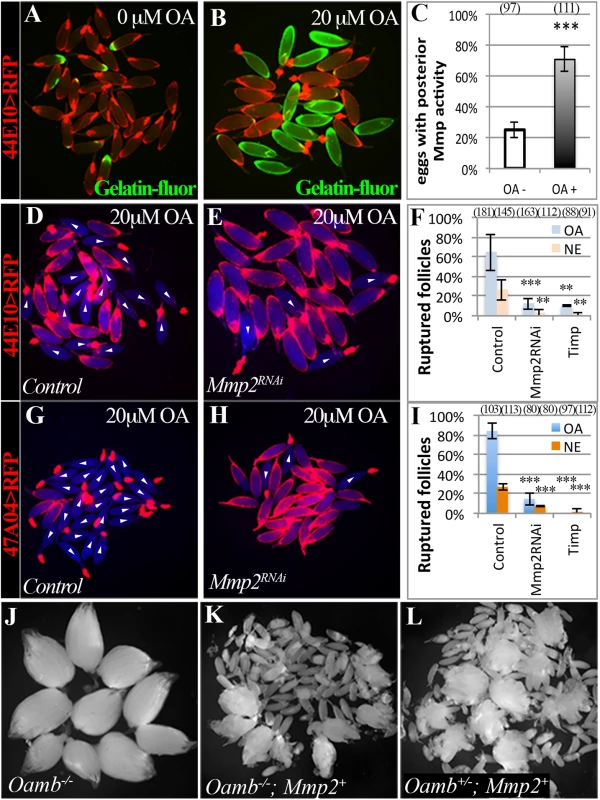
(A-C) In situ zymography shows increased Mmp activity in mature follicles after three-hour culture with 20 μM of OA. Mmp activity is indicated by Gelatin-fluorescein (green in A and B). The percentage of follicles with posterior Mmp activity is quantified in (C; *** P < 0.001). Three and four replicates were used for OA- and OA+ groups, respectively. (D-F) Expression of Mmp2RNAi or Timp driven by R44E10-Gal4 prevents follicle rupture in response to OA or NE (*** P <0.001 and ** P < 0.01). The number of replicates used for each condition is 6, 5, 6, 4, 3, and 3. (G-I) Expression of Mmp2RNAi or Timp driven by R47A04-Gal4 prevents follicle rupture in response to OA or NE. All experiments were performed in four replicates except Mmp2RNAi, which have three replicates. (J-L) Ovaries are shown for the Oamb mutant (J), the Oamb mutant with ectopic expression of Mmp2 driven by R44E10-Gal4 (K), and the Oamb heterozygous with ectopic Mmp2 expression (L). Mature eggs were released into the female abdominal cavity. To determine whether Mmp2 activity is required for OA-induced follicle rupture, we isolated mature follicles containing follicle cell-specific Mmp2 knockdown and cultured them in the OA medium. These follicles did not respond to OA stimulation, and their posterior follicle cells remained intact (Fig 4D–4I). In addition, Mmp2 knockdown in follicle cells also abolished the NE-induced follicle rupture (Fig 4F and 4I). Furthermore, misexpression of Timp in mature follicle cells completely prevented follicle rupture ex vivo (Fig 4F and 4I). Therefore, Mmp2 activity in mature follicle cells is essential for OA/NE-induced follicle rupture ex vivo, consistent with its essential role in follicle trimming and ovulation in vivo [37].
To confirm that Mmp2 acts downstream of adrenergic signaling in follicle trimming and rupture, we attempted to rescue the defect of follicle rupture in Oamb mutant flies with ectopic expression of Mmp2 in mature follicle cells. Oamb mutant females had two intact ovaries, which contain a large number of mature follicles (Fig 4J). In contrast, follicular misexpression of Mmp2 in Oamb mutant females caused the breakdown of the ovariole muscle sheath and the release of mature follicles into the abdominal cavity (Fig 4K). Further examination of these released follicles demonstrated that 99% of them (n = 70) had no follicle-cell covering, similar to follicles released upon misexpression of Mmp2 in Oamb heterozygous or wild-type females (Fig 4L; [37]). Therefore, Mmp2 is sufficient to induce follicle rupture in the absence of adrenergic signaling. Together, our data indicate that follicular adrenergic signaling activates Mmp2 to control follicle trimming and ovulation.
Intracellular Ca2+ acts as the second messenger downstream of follicular adrenergic signaling to induce follicle rupture
OA-Oamb interaction can induce transient increase of intracellular Ca2+ concentration ([Ca2+]i) [23]. To determine whether OA evokes Ca2+ signaling in mature follicle cells to induce follicle rupture, we first monitored the [Ca2+]i using a genetically encoded calcium sensor (see methods). Fluorescent intensity of the calcium sensor expressed in mature follicle cells rose significantly around six minutes after OA administration in our ex vivo culture system (S7 Fig and S2 Movie). To determine whether Ca2+ is required for OA-induced follicle rupture, we pretreated mature follicles with BAPTA-AM, an intracellular Ca2+ chelator, before OA stimulation. Two hundred μM BAPTA-AM treatment significantly perturbed the OA-induced follicle rupture (Fig 5A–5C). To determine whether Ca2+ is sufficient to induce follicle rupture, we stimulated mature follicles with ionomycin, a potent ionophore for increasing [Ca2+]i. Ionomycin is potent to induce follicle rupture even at 5 μM concentration (Fig 5D–5F), lower than the dose typically used in the field [48]. Taken together these data suggest that the increase of [Ca2+]i is both necessary and sufficient to induce follicle rupture.
Fig. 5. Intracellular Ca2+ is the second messenger downstream of follicular adrenergic signaling. 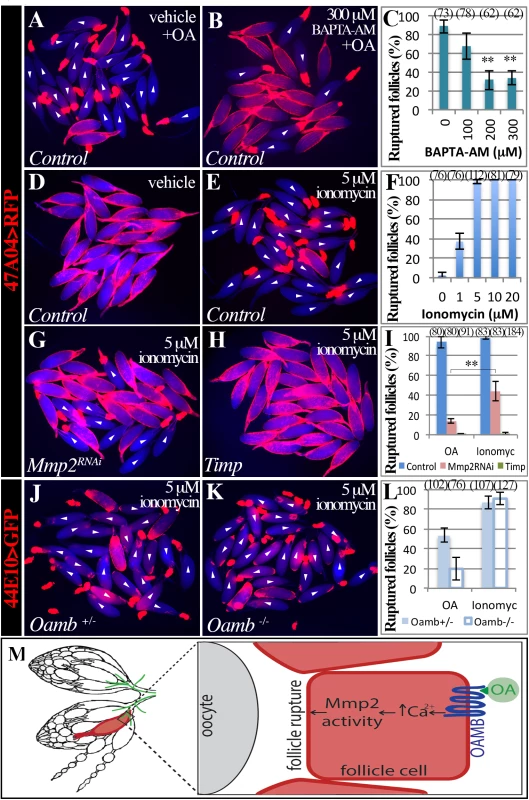
(A-C) Pretreatment of BAPTA-AM blocks OA-induced follicle rupture. Representative images show mature follicles treated with DMSO (A) or BAPTA-AM (B) followed a three-hour stimulation with 20 μM of OA. Ruptured follicles were quantified in C. Three replicates are used for each condition. (D-F) Ionomycin is sufficient to induced follicle rupture. Representative images show follicles after three-hour culture with ethanol (D) or 5 μM of ionomycin. Ruptured follicles after different doses of ionomycin treatment are quantified in F. All conditions have three replicates except in 5 μM, which has four replicates. (G-H) Representative images of Mmp2-knockdown (G) and Timp-overexpressing (H) follicles treated with 5 μM of ionomycin for three hours. (I) Quantification of ruptured follicles with Mmp2 knockdown or Timp overexpression in mature follicle cells in response to 20 μM of OA or 5 μM of ionomycin stimulation. All conditions have three replicates except for Timp overexpression with ionomycin treatment, which has six replicates. (J-L) Ionomycin, but not OA, is sufficient to induce rupture in Oamb mutant follicles. Representative images show Oamb+/- (J) and Oamb-/- (K) follicles after three-hour culture with ionomycin. (L) Quantification of ruptured follicles after three-hour culture with 20 μM of OA or 5 μM of ionomycin. The number of replicates for each condition is 4, 4, 3, and 5. (M) A cartoon showing the model of follicular adrenergic signaling in Mmp activity and follicle rupture. Octopaminergic neurons are shown in green. To further test whether Ca2+ is the second messenger of follicular adrenergic signaling for Mmp2 activation and follicle rupture, we set to examine whether ionomycin is sufficient to induce rupture of follicles lacking follicular Mmp2 or Oamb, which do not respond to OA stimulation. Ionomycin only partially induces follicle rupture when Mmp2 is knocked down in mature follicle cells and is not able to induce any rupture when Timp is overexpressed (Fig 5G–5I). In contrast, ionomycin is able to induce follicle rupture in both control and Oamb mutant follicles at the equal efficiency (Fig 5J–5L). All these data indicate that Ca2+ acts downstream of Oamb but upstream of Mmp2 during follicle rupture. Together, we conclude that follicular adrenergic signaling activates Mmp2 to control follicle trimming and ovulation likely via intracellular Ca2+ (Fig 5M).
Discussion
The first ex vivo follicle rupture assay in Drosophila
Ovulation, an essential step in metazoan reproduction, has been extensively studied in mammals over the past several decades [49–51]. However, progress in the field has been hindered by the limited ability of mammalian model systems to be genetically manipulated. Thus it is still unclear how follicles break their wall in a highly regulated spatio-temporal manner to allow release of oocytes. The model organism Drosophila offers a wealth of tools for genetic manipulation, but to date, few specific readouts for Drosophila ovulation has been developed. Previous studies of Drosophila ovulation have used readouts such as egg laying, percentage of females with eggs in the reproductive tract, or egg retention [21,25–27,33]. We recently combined these parameters to estimate ovulation time [28,37]. In the present study, we developed the first ex vivo follicle rupture assay in Drosophila and demonstrated that OA-induced follicle rupture in this assay is similar to the rupturing process in vivo. This assay gave us the unprecedented ability to visualize the entire process of follicle rupture and quantify its kinetics. Further genetic evidence illustrated that genes required for ex vivo follicle rupture are also required for in vivo ovulation, including Oamb and Mmp2. Our ex vivo assay represents a simple, specific, and reliable method for measuring rupturing ability of mature follicles. In conjunction with the powerful genetic tools available in Drosophila, this ex vivo assay will allow genetic screens to identify candidate genes involved in follicle rupture, thus opening new avenues for ovulation research.
A direct role for octopamine signaling in Drosophila ovulation
Octopamine, a biogenic amine derived from tyrosine, has been identified as essential for ovulation in Drosophila [20]. The major source of OA is octopaminergic neurons innervating the female reproductive system, and previous studies showed that restoring TβH specifically in these neurons rescues the ovulation defect caused by TβH mutation [21]. Due to its effects on muscle contraction, OA was proposed to regulate ovulation by inducing the contraction of ovarian muscle and relaxation of oviduct muscle [3,19,25,27,34].
Ovarian smooth muscle contraction was also proposed to regulate ovulation in mammals in the early 1980’s [11,52,53]. However, subsequent work suggest that ovulation requires the active proteolytic degradation of the follicle wall rather than passive muscle contraction [54,40,55]. At least three families of proteolytic enzymes are involved in this process, including matrix metalloproteinases [56,57]. Pharmacological blockage of any of these enzymes results in inhibition of follicle rupture.
Our recent work suggested that Drosophila also requires proteolysis for breaking the follicle wall and ovulation [37], and in this way shares similarities with mammalian ovulation at both the cellular and molecular level [28,37]. These new insights into Drosophila ovulation process lead to the speculation that octopaminergic signaling may play a direct role on the follicle in controlling ovulation in addition to its role on muscle contraction. Here, we demonstrate that OA-Oamb signaling in mature follicle cells directly regulates follicle wall degradation, follicle rupture, and ovulation by activating key enzyme Mmp2. Furthermore, our pharmacological data suggest that OA-Oamb signaling likely fulfill these functions via intracellular Ca2+ as the second messenger. However, it is still unclear how OA-Oamb-Ca2+ regulates Mmp2 activity. Lacking a method to detect Mmp2 protein prevents us to test whether OA-Oamb-Ca2+ regulates Mmp2 protein secretion. The Mmp2::GFP fusion allele we previously generated [37] is good to detect Mmp2::GFP expression but not good to track Mmp2 secretion because Mmp2::GFP fusion proteins are not properly processed and secreted to the extracellular space (S8 Fig) and Mmp2::GFP homozygous flies are lethal as Mmp2 mutant females do [47]. Alternatively, Ca2+ signaling may indirectly regulate Mmp2 activity via its inhibitor or other regulatory processes. Despite that, it is intriguing that [Ca2+]i also rises after NE and gonadotropin stimulation in human granulosa cells [58] and that perfusion of a Ca2+ chelator in rabbits significantly reduces gonadotropin-induced ovulatory efficiency [59]. Given adrenergic innervation of ovaries observed throughout metazoans, it is plausible to speculate that follicular adrenergic signaling plays conserved roles in regulating Mmp activity and ovulation (See below).
Conservation of ovarian adrenergic signaling in ovulation
Adrenergic innervation of the ovary has long been found in mammals including humans. The role of adrenergic signaling in ovulation has been studied as early as the 1970’s. The neurotransmitter norepinephrine (NE) reaches the highest level in peripheral plasma during ovulation [60] and is enriched in the follicular fluid of preovulatory follicles compared to in peripheral plasma in healthy women [16,61,62]. Functional adrenergic receptors are expressed in mammalian ovarian follicular cells [13,58,63]. Ovarian perfusion of adrenergic agonists or antagonists influences the ovulation rate in rabbits and rats [12,14]. It has been speculated that adrenergic signaling regulates ovulation by stimulating muscle contraction or by increasing production of reactive oxygen species [16,53]. In contrast to this view, ovarian sympathetic denervation does not affect ovulation in rabbits and rats [10,15]; instead, it rescues ovulation defect in a rat model of PCOS [64,65], which is associated with increased sympathetic inputs to the ovary [8,9]. It is not clear why a discrepancy exists between the effects of surgical denervation and of pharmacological agents. Thus, no consensus has been reached in regard to the role of ovarian adrenergic signaling in mammalian ovulation.
Instead of regulating ovarian smooth muscle contraction, the results of the present study suggest an alternative pathway for ovarian NE to regulate ovulation. NE likely activates adrenergic receptors in granulosa and theca cells (equivalent to Drosophila follicle cells) in mammalian periovulatory follicles, which activates Mmp enzymatic activity at the apex [38], where mature oocytes rupture through. A surgical denervation may cause tissue damage and activate Mmps directly, bypassing the requirement of follicular adrenergic signaling. Future studies, using both mammalian and Drosophila genetic tools, will identify fundamental mechanisms of adrenergic signaling in ovulation.
Materials and Methods
Drosophila genetics
Flies were reared on standard cornmeal-molasses food at 25°C unless otherwise indicated. OambMI12417 is a MiMIC line inserted in the coding intron of both Oamb spicing isoforms (S4 Fig) [46], and OambMI12417/Df(3R) BSC141 was used to characterize the Oamb mutant phenotype. TbHM18 [20] and Tdc2RO54 [22] were kindly provided by Dr. Mariana Wolfner. All RNAi-knockdown experiments were performed at 29°C with UAS-dcr2 to increase the efficiency of RNAi. R47A04-Gal4 (Oamb) and R44E10-Gal4 (lilli) from the Janelia Gal4 collection [42] were used for misexpressing genes or RNAi in mature follicle cells. The following RNAi or overexpressing lines were used: UAS-OambRNAi1 (V2861) and UAS-OambRNAi2 (V106511) from the Vienna Drosophila Resource Center; UAS-OambRNAi3 (B31233) and UAS-OambRNAi4 (B31171) from the Bloomington Drosophila Stock Center; UAS-Mmp2RNAi [66]; UAS-Mmp2 [47]; and UAS-GCaMP5G [67]. UASpGFP-act79B; UAS-mCD8-GFP[37] was used to analyze Gal4 expression in both germline and somatic cells, as well as neurons. UAS-GFPnls and UAS-RFP were used for follicle isolation. Control flies were derived from specific Gal4 drivers crossed to Oregon-R or yv; attP2 (B36303). The Mmp2::GFP fusion allele in the Mmp2 endogenous locus was used for detecting Mmp2 protein expression [37].
Ex vivo follicle rupture, Ca2+ imaging, in situ zymography, and egg activation assays
For the ex vivo follicle rupture assay, 4–6-day-old virgin females were used to isolate mature follicles, and follicle cells were fluorescently labeled using R47A04-Gal4 or R44E10-Gal4. Ovaries were dissected in Grace’s medium and ovarioles were separated from each other using forceps. This process will partially break the ovariole muscle sheath and release mature follicles. Mature follicles with an intact follicle-cell layer and completely dissociated from younger follicles were immediately transferred to new Grace’s medium to minimize their exposure to endogenous biogenic amines during dissection. With this method, we can isolate about 10 mature follicles/female and isolated mature follicles are no longer surrounded by ovariole or oviduct muscle sheaths (S3 Fig). Within one hour, isolated mature follicles were subsequently cultured in culture media (Grace’s medium, 10% fetal bovine serum, and 1X penicillin/streptomycin) supplemented with the indicated concentration of OA, TA, NE (Sigma), or ionomycin (dissolved in ethanol; Cayman Chemical). For chelating intracellular Ca2+, isolated mature follicles were treated with BAPTA-AM (dissolved in DMSO; Cayman Chemical) for 30 minutes before OA culture. All cultures were performed at 29°C, the same condition as flies were maintained, to enhance Gal4/UAS expression. About 25–30 follicles were used for each culture group and the percentage of ruptured follicles was then calculated as one data point. Typically three-six replicates were used for each genotype or treatment; data were represented as mean percentage ± standard deviation (SD); and Student’s T-test was used for statistic analysis. Ruptured follicles were defined as those losing more than 80% follicle-cell covering. With the exception of Fig 1D, all data were collected at the end of the three-hour culture.
For Ca2+ imaging and follicle rupture kinetics, video images were captured at 0.2 frame/second (FPS) with a sCOMS camera (PCO.Edge) installed in a Leica MZ10F fluorescent stereoscope. To examine the kinetics of follicle rupture, mature follicles were cultured in 20 μM of OA medium for 20 minutes at 29°C before recording. Unruptured follicles were then transferred into a home-made slide for video recording at room temperature. Each ruptured follicle was analyzed frame-by-frame manually to determine the ruptured distance between the posterior tip of the oocyte and the posterior leading edge of the follicle-cell layer using ImageJ. The percent of ruptured distance was then calculated as the ruptured distance divided by the length of the oocyte from the anterior to posterior tip. Because of the asynchronous onset of follicle rupture, data were normalized at the time point when follicles reach 50% ruptured distance.
In situ zymography for detecting gelatinase activity was performed as previously reported with minor modifications [37]. 50 μg/ml of DQ-gelatin conjugated with fluorescein (Invitrogen) was added into the culture media with or without OA for three hours. After a quick rinse, mature follicles with posterior fluorescent signal were directly counted. For egg activation, ruptured oocytes were treated with hypotonic activation buffer [45] for 15 minutes and treated with 50% bleach for three minutes. The number of unbroken oocytes was counted.
Egg laying, ovulation time, and follicle cell trimming
Egg laying, ovulation time, and follicle cell trimming were performed as previously described [28,37]. In brief, 4–6-day-old virgin females fed with wet yeast for one day were used. For egg laying, five females were housed with ten Oregon-R males in one bottle to lay eggs on grape juice-agar plates for two days at 29°C. After egg laying, ovaries were dissected and mature follicles in these ovaries were counted. The number of eggs on the plates was then counted, which was used to calculate the average time for laying an egg (egg-laying time). The egg-laying time was partitioned into the ovulation time and the uterus time (the time egg spent in the uterus and during oviposition). The partition ratio was determined based on the percentage of females having eggs in the uterus at six hours after mating. To do so, ten virgins were placed in a vial with 15 Oregon R males for six hours at 29°C, frozen for 4.5 minutes at -80°C, and then dissected to examine the reproductive tract. For follicle cell trimming, virgin or mated females were frozen for 4.5 minutes at -80°C, and ovary pairs were dissected, fixed, stained with DAPI, and mounted carefully to preserve the posterior end of mature follicles. Trimmed follicles were defined as more than a quarter of oocytes at the posterior end lacking follicle cell covering. Normalized trimming follicles were then calculated by the number of trimming follicles divided by the number of mature follicles in each female.
Immunostaining and microscopy
Immunostaining was performed following a standard procedure [68], including fixation in 4% EM-grade paraformaldehyde for 15 minutes, blocking in PBTG (PBS+ 0.2% Triton+ 0.5% BSA+ 2% normal goat serum), and primary and secondary antibody staining. For Mmp2::GFP localization, dissected tissues were stained in primary antibodies for 45 minutes at 4°C before the fixation treatment followed with the secondary antibody staining. Mouse anti-Hnt (1 : 75; Developmental Study Hybridoma Bank) and rabbit anti-GFP (1 : 4000; Invitrogen) were used as primary antibodies, and Alexa 488 goat anti-rabbit and 546 goat anti-mouse (1 : 1000, Invitrogen) were used as secondary antibodies. Images were acquired using a Leica TCS SP8 confocal microscope or Leica MZ10F fluorescent stereoscope with a sCOMS camera (PCO.Edge), and assembled using Photoshop software (Adobe, Inc.).
Supporting Information
Zdroje
1. Wojtkiewicz J, Jana B, Kozłowska A, Crayton R, Majewski M, Zalecki M, et al. Innervation pattern of polycystic ovaries in the women. J Chem Neuroanat. 2014;61–62 : 147–152. doi: 10.1016/j.jchemneu.2014.05.003 24905277
2. Cisint S, Crespo CA, Medina MF, Iruzubieta Villagra L, Fernández SN, Ramos I. Innervation of amphibian reproductive system. Histological and ultrastructural studies. Auton Neurosci Basic Clin. 2014;185 : 51–58.
3. Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJ. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4 : 17. 16768790
4. Heifetz Y, Lindner M, Garini Y, Wolfner MF. Mating Regulates Neuromodulator Ensembles at Nerve Termini Innervating the Drosophila Reproductive Tract. Curr Biol CB. 2014.
5. Gerendai I, Banczerowski P, Halász B. Functional significance of the innervation of the gonads. Endocrine. 2005;28 : 309–318. 16388121
6. Aguado LI. Role of the central and peripheral nervous system in the ovarian function. Microsc Res Tech. 2002;59 : 462–473. 12467021
7. Mayerhofer A, Dissen GA, Costa ME, Ojeda SR. A Role for Neurotransmitters in Early Follicular Development: Induction of Functional Follicle-Stimulating Hormone Receptors in Newly Formed Follicles of the Rat Ovary. Endocrinology. 1997;138 : 3320–3329. 9231784
8. Greiner M, Paredes A, Araya V, Lara HE. Role of stress and sympathetic innervation in the development of polycystic ovary syndrome. Endocrine. 2005;28 : 319–324. 16388122
9. Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf). 2012;77 : 791–801.
10. Weiner S, Wright KH, Wallach EE. Studies on the function of the denervated rabbit ovary: human chorionic gonadotropin-induced ovulation. Fertil Steril. 1975;26 : 363–368. 1116631
11. Walles B, Edvinsson L, Owman C, Sjöberg NO, Sporrong B, Stefenson A. Influence of sympathetic nerves, amine receptors and anti-adrenergic drugs on follicular contractility and ovulation. Acta Physiol Scand Suppl. 1977;452 : 113–120. 273368
12. Kobayashi Y, Sjöberg NO, Walles B, Owman C, Wright KH, Santulli R, et al. The effect of adrenergic agents on the ovulatory process in the in vitro perfused rabbit ovary. Am J Obstet Gynecol. 1983;145 : 857–864. 6837665
13. Kannisto P, Owman C, Walles B. Involvement of local adrenergic receptors in the process of ovulation in gonadotrophin-primed immature rats. J Reprod Fertil. 1985;75 : 357–362. 2999381
14. Schmidt G, Owman C, Sjöberg NO, Walles B. Influence of adrenoreceptor agonists and antagonists on ovulation in the rabbit ovary perfused in vitro. J Auton Pharmacol. 1985;5 : 241–250. 4055819
15. Wylie SN, Roche PJ, Gibson WR. Ovulation after sympathetic denervation of the rat ovary produced by freezing its nerve supply. J Reprod Fertil. 1985;75 : 369–373. 4067920
16. Saller S, Merz-Lange J, Raffael S, Hecht S, Pavlik R, Thaler C, et al. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology. 2012;153 : 1472–1483. doi: 10.1210/en.2011-1769 22234472
17. Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophilaβ-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94 : 547–560. 15998303
18. Roeder T. TYRAMINE AND OCTOPAMINE: Ruling Behavior and Metabolism. Annu Rev Entomol. 2005;50 : 447–477. 15355245
19. Rodriguez-Valentin R, Lopez-Gonzalez I, Jorquera R, Labarca P, Zurita M, Reynaud E. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 2006;209 : 183–98. 16826564
20. Monastirioti M, Linn CE Jr, White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16 : 3900–3911. 8656284
21. Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264 : 38–49. 14623230
22. Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280 : 14948–55. 15691831
23. Han K-A, Millar NS, Davis RL. A Novel Octopamine Receptor with Preferential Expression inDrosophila Mushroom Bodies. J Neurosci. 1998;18 : 3650–3658. 9570796
24. Lee H-G, Seong C-S, Kim Y-C, Davis RL, Han K-A. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264 : 179–190. 14623240
25. Lim J, Sabandal PR, Fernandez A, Sabandal JM, Lee H-G, Evans P, et al. The Octopamine Receptor Octβ2R Regulates Ovulation in Drosophila melanogaster. PLoS ONE. 2014;9: e104441. doi: 10.1371/journal.pone.0104441 25099506
26. Li Y, Fink C, El-Kholy S, Roeder T. THE OCTOPAMINE RECEPTOR octß2R IS ESSENTIAL FOR OVULATION AND FERTILIZATION IN THE FRUIT FLY Drosophila melanogaster. Arch Insect Biochem Physiol. 2015;88 : 168–178. doi: 10.1002/arch.21211 25353988
27. Lee HG, Rohila S, Han KA. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One. 2009;4: e4716. doi: 10.1371/journal.pone.0004716 19262750
28. Sun J, Spradling AC. Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. eLife. 2013;2: e00415. doi: 10.7554/eLife.00415 23599892
29. Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451 : 33–7. 18066048
30. Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61 : 511–8. doi: 10.1016/j.neuron.2009.01.009 19249272
31. Yang C, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, et al. Control of the Postmating Behavioral Switch in Drosophila Females by Internal Sensory Neurons. Neuron. 2009;61 : 519–526. doi: 10.1016/j.neuron.2008.12.021 19249273
32. Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually Dimorphic Octopaminergic Neurons Modulate Female Postmating Behaviors in Drosophila. Curr Biol. 2014
33. Heifetz Y, Vandenberg LN, Cohn HI, Wolfner MF. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci U A. 2005;102 : 743–8.
34. Rubinstein CD, Wolfner MF. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc Natl Acad Sci. 2013;110 : 17420–17425. doi: 10.1073/pnas.1220018110 24101486
35. Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in drosophila melanogaster. Dev Biol. 2003;256 : 195–211. 12679097
36. Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70.
37. Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in Drosophila. PLoS Genet. 2015;11: e1004989. doi: 10.1371/journal.pgen.1004989 25695427
38. Curry TE, Osteen KG. The Matrix Metalloproteinase System: Changes, Regulation, and Impact throughout the Ovarian and Uterine Reproductive Cycle. Endocr Rev. 2003;24 : 428–465. 12920150
39. Ogiwara K, Takano N, Shinohara M, Murakami M, Takahashi T. Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proc Natl Acad Sci U S A. 2005;102 : 8442–8447. 15941829
40. Espey LL, Richards JS. Ovulation. In: Neill JD, editor. Physiology of Reproduction. 3rd ed. Amsterdam: Academic Press; 2006. pp. 425–474.
41. Tootle TL, Williams D, Hubb A, Frederick R, Spradling A. Drosophila Eggshell Production: Identification of New Genes and Coordination by Pxt. PLoS ONE. 2011;6: e19943. doi: 10.1371/journal.pone.0019943 21637834
42. Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci. 2008;105 : 9715–9720. doi: 10.1073/pnas.0803697105 18621688
43. Heifetz Y, Yu J, Wolfner MF. Ovulation Triggers Activation of Drosophila Oocytes. Dev Biol. 2001;234 : 416–424. 11397010
44. Mahowald AP, Goralski TJ, Caulton JH. In vitro activation of Drosophila eggs. Dev Biol. 1983;98 : 437–445. 6409691
45. Page AW, Orr-Weaver TL. Activation of the Meiotic Divisions inDrosophilaOocytes. Dev Biol. 1997;183 : 195–207. 9126294
46. Nagarkar-Jaiswal S, Lee P-T, Campbell ME, Chen K, Anguiano-Zarate S, Gutierrez MC, et al. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife. 2015;4: e05338.
47. Page-McCaw A, Serano J, Sante JM, Rubin GM. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell. 2003;4 : 95–106. 12530966
48. Wong R, Hadjiyanni I, Wei H-C, Polevoy G, McBride R, Sem K-P, et al. PIP2 Hydrolysis and Calcium Release Are Required for Cytokinesis in Drosophila Spermatocytes. Curr Biol. 2005;15 : 1401–1406. 16085493
49. Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356 : 65–73. doi: 10.1016/j.mce.2011.11.002 22101318
50. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324 : 938–41. doi: 10.1126/science.1171396 19443782
51. Fan H-Y, Liu Z, Mullany LK, Richards JS. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol Cell Endocrinol. 2012;356 : 74–79. doi: 10.1016/j.mce.2011.12.005 22197887
52. Martin GG, Talbot P. The role of follicular smooth muscle cells in hamster ovulation. J Exp Zool. 1981;216 : 469–482. 7276896
53. Walles B, Edvinsson L, Owman C, Sjöberg NO, Svensson KG. Mechanical response in the wall of ovarian follicles mediated by adrenergic receptors. J Pharmacol Exp Ther. 1975;193 : 460–473. 238021
54. Tsafriri A. Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Adv Exp Med Biol. 1995;377 : 121–40. 7484419
55. Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, et al. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83 : 549–57. doi: 10.1095/biolreprod.110.084434 20592310
56. Curry T, Smith M. Impact of Extracellular Matrix Remodeling on Ovulation and the Folliculo-Luteal Transition. Semin Reprod Med. 2006;24 : 228–241. 16944420
57. Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochim Biophys Acta BBA—Proteins Proteomics. 2005;1751 : 95–109. 15950557
58. Föhr KJ, Mayerhofer A, Sterzik K, Rudolf M, Rosenbusch B, Gratzl M. Concerted action of human chorionic gonadotropin and norepinephrine on intracellular-free calcium in human granulosa-lutein cells: evidence for the presence of a functional alpha-adrenergic receptor. J Clin Endocrinol Metab. 1993;76 : 367–373. 8381798
59. Kitai H, Santulli R, Wright KH, Wallach EE. Examination of the role of calcium in ovulation in the in vitro perfused rabbit ovary with use of ethyleneglycol-bis(beta-aminoethyl ether)-n,n’-tetraacetic acid and verapamil. Am J Obstet Gynecol. 1985;152 : 705–708. 3927731
60. Blum I, Lerman M, Misrachi I, Nordenberg Y, Grosskopf I, Weizman A, et al. Lack of plasma norepinephrine cyclicity, increased estradiol during the follicular phase, and of progesterone and gonadotrophins at ovulation in women with premenstrual syndrome. Neuropsychobiology. 2004;50 : 10–15. 15179014
61. Bòdis J, Bognàr Z, Hartmann G, Török A, Csaba IF. Measurement of noradrenaline, dopamine and serotonin contents in follicular fluid of human graafian follicles after superovulation treatment. Gynecol Obstet Invest. 1992;33 : 165–167. 1612529
62. Itoh MT, Ishizuka B, Kuribayashi Y, Abe Y, Sumi Y. Noradrenaline concentrations in human preovulatory follicular fluid exceed those in peripheral plasma. Exp Clin Endocrinol Amp Diabetes. 2000;108 : 506–509.
63. Itoh MT, Ishizuka B. α1-Adrenergic receptor in rat ovary: Presence and localization. Mol Cell Endocrinol. 2005;240 : 58–63. 16026926
64. Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: role of sympathetic innervation. Endocrinology. 1993;133 : 2696–2703. 8243293
65. Morales-Ledesma L, Linares R, Rosas G, Morán C, Chavira R, Cárdenas M, et al. Unilateral sectioning of the superior ovarian nerve of rats with polycystic ovarian syndrome restores ovulation in the innervated ovary. Reprod Biol Endocrinol RBE. 2010;8 : 99.
66. Uhlirova M, Bohmann D. JNK-and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25 : 5294–5304. 17082773
67. Akerboom J, Chen T-W, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J Neurosci. 2012;32 : 13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012 23035093
68. Sun J, Spradling AC. NR5A Nuclear Receptor Hr39 Controls Three-Cell Secretory Unit Formation in Drosophila Female Reproductive Glands. Curr Biol. 2012;22 : 862–871. doi: 10.1016/j.cub.2012.03.059 22560612
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



