-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTransposon Variants and Their Effects on Gene Expression in
Transposable elements (TEs) make up the majority of many plant genomes. Their transcription and transposition is controlled through siRNAs and epigenetic marks including DNA methylation. To dissect the interplay of siRNA–mediated regulation and TE evolution, and to examine how TE differences affect nearby gene expression, we investigated genome-wide differences in TEs, siRNAs, and gene expression among three Arabidopsis thaliana accessions. Both TE sequence polymorphisms and presence of linked TEs are positively correlated with intraspecific variation in gene expression. The expression of genes within 2 kb of conserved TEs is more stable than that of genes next to variant TEs harboring sequence polymorphisms. Polymorphism levels of TEs and closely linked adjacent genes are positively correlated as well. We also investigated the distribution of 24-nt-long siRNAs, which mediate TE repression. TEs targeted by uniquely mapping siRNAs are on average farther from coding genes, apparently because they more strongly suppress expression of adjacent genes. Furthermore, siRNAs, and especially uniquely mapping siRNAs, are enriched in TE regions missing in other accessions. Thus, targeting by uniquely mapping siRNAs appears to promote sequence deletions in TEs. Overall, our work indicates that siRNA–targeting of TEs may influence removal of sequences from the genome and hence evolution of gene expression in plants.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003255
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003255Summary
Transposable elements (TEs) make up the majority of many plant genomes. Their transcription and transposition is controlled through siRNAs and epigenetic marks including DNA methylation. To dissect the interplay of siRNA–mediated regulation and TE evolution, and to examine how TE differences affect nearby gene expression, we investigated genome-wide differences in TEs, siRNAs, and gene expression among three Arabidopsis thaliana accessions. Both TE sequence polymorphisms and presence of linked TEs are positively correlated with intraspecific variation in gene expression. The expression of genes within 2 kb of conserved TEs is more stable than that of genes next to variant TEs harboring sequence polymorphisms. Polymorphism levels of TEs and closely linked adjacent genes are positively correlated as well. We also investigated the distribution of 24-nt-long siRNAs, which mediate TE repression. TEs targeted by uniquely mapping siRNAs are on average farther from coding genes, apparently because they more strongly suppress expression of adjacent genes. Furthermore, siRNAs, and especially uniquely mapping siRNAs, are enriched in TE regions missing in other accessions. Thus, targeting by uniquely mapping siRNAs appears to promote sequence deletions in TEs. Overall, our work indicates that siRNA–targeting of TEs may influence removal of sequences from the genome and hence evolution of gene expression in plants.
Introduction
While transposable elements (TEs) constitute a large fraction of plant, animal and human genomes [1]–[3], their contribution to genome size can change rapidly during evolutionary time. In some taxa, TEs have been responsible for two-fold differences in genome size that arose over a few million years or less. These rapid fluctuations, which may be due to TEs being either more active or more efficiently deleted in certain species, indicate that control of TEs can differ greatly between closely related plant species [4]–[7]. The balance between TE transpositions and selection against TEs is influenced by factors ranging from mating system to silencing by short interfering RNAs (siRNAs) and chromatin modification. Therefore the control of TE activity and the removal of transposed copies can be considered key factors in the evolution of genomes.
TEs are often regarded as genomic parasites due to the potentially detrimental effects of insertional inactivation of genes and ectopic recombination of DNA [8]. Twenty-four nt long siRNAs are associated with most TEs as part of a ‘double-lock’ mechanism of siRNA-mediated DNA methylation that controls transposition via transcriptional repression, with a reinforcement loop between DNA methylation, histone methylation and siRNAs [reviewed in 9]. siRNAs are a robust proxy for DNA methylation at TEs, with unmethylated TEs generally lacking matching 24 nt siRNAs [10]–[13]. Most plant TEs have cytosine methylation at CG, CHG and CHH sites, but a quarter is unmethylated and a further 15% have atypical methylation patterns. In the TE-dense heterochromatin, DNA methylation can spread about 500 bp into neighboring unmethylated TEs [13]. In the euchromatin, methylation spreads from TEs to approximately 200 bp beyond the siRNA target sites [13], consistent with the effect of siRNAs on expression of proximal genes dissipating by 400 bp [14]. siRNA-targeted, methylated TEs are, on average, located farther away from expressed genes than TEs that are not strongly methylated or associated with siRNAs [13], [15]. As expected from this correlation, siRNA-targeted TEs have more effects on nearby gene expression than those without [14], [15].
Most poorly methylated TEs are short and have few CG dinucleotides [13]. This indicates a progression over evolutionary time from TEs that are active and targeted by siRNA-mediated DNA methylation, to inactive, degenerate relics that have changed through deletions and nucleotide substitutions initiated by deamination of methylated cytosines. These inactive TEs are then no longer targeted by siRNA-mediated DNA methylation.
Presumably because of interference with cis-regulatory elements, Arabidopsis TEs reduce the average expression levels of adjacent genes, although the distance over which these effects are noticeable varies between A. thaliana and A. lyrata [14]. Differences in TEs next to genes contribute to the divergence of gene expression levels between orthologs in these closely related species [14], and gene expression is negatively correlated with the number of nearby siRNA-targeted, methylated TEs [15].
In the selfing species A. thaliana, TEs account for only a fifth of the genome [7], [13], [16], making it relatively depauperate of TEs. Given that the A. thaliana genome is small relative to other members of the family and that its close relative A. lyrata, an outcrosser, contains approximately three times as many TEs [14], deletion of TEs in A. thaliana is likely an ongoing, active process. In accordance with this hypothesis, intraspecific polymorphisms and deletions in A. thaliana are disproportionately located within TEs and, to a lesser extent, intergenic regions [17]–[19].
A reference-guided assembly approach has been applied to accurately characterize complex sequence variation in several A. thaliana accessions [19]. Here, we exploit this information to examine TE variants and their effect on the expression of nearby genes in three divergent accessions. We report that TEs are more likely to be located in polymorphic regions of the genome. Where TEs are present in less polymorphic regions, they also tend to be less polymorphic themselves. Although polymorphic TE variants are less abundantly targeted by siRNAs, uniquely mapping siRNAs targeting polymorphic TE variants are strongly correlated with the TE regions that vary between accessions. These findings suggest a link between the ability to tolerate TE insertions, siRNA-mediated silencing and purging of TEs by deletion.
Results
TE variation across the genome
We annotated the sets of genes and TEs in three A. thaliana accessions: Col-0, Bur-0 and C24 [19], [20]. For reference accession Col-0, we used the TAIR9 annotation of TEs and protein-coding genes. Excluding centromeric sequences, 21,913 full-length and degenerate TEs and 26,541 genes were considered further. We built genome templates of Bur-0 and C24 from re-sequencing data using the SHORE pipeline [21]. The reference coordinates of TEs and genes were projected onto these genome templates, and variation in TEs and genes was determined based on single nucleotide polymorphisms (SNPs), 1 to 3 bp insertions/deletions (indels) and larger deletions of 4 to 11,464 bp (median 30 bp, mean 113 bp). Larger insertions were not included because of the high false-negative rate [17].
Comparison of polymorphism densities confirmed that coding regions were relatively depauperate of SNPs, indels and large deletions compared to intergenic regions and TEs (binomial test, p[Coding Regions/Intergenic Region] = 0 and p[Coding Regions/TE] = 0 for SNPs, indels or large deletions). Large deletions were significantly over-represented in TEs compared to intergenic regions, while SNPs and indels were not (Figure S1a; binomial test, p[TE/Intergenic Region] = 0 for large deletions). Over 6% of reference TEs differed by at least 10% of total length in each of the two accessions, Bur-0 and C24, compared to Col-0 (Figure 1a and Figure S2). Almost all of this variation, 93%, was due to large deletions (Figure S1b; for distribution of large deletion sizes see Figure S1c). We defined TEs with at least 10% variation by length (SNPs, indels and larger deletions combined), but not completely missing in Bur-0 or C24, as TE variants or VarTEs (please also see Figure S3 for abbreviation definitions). Close to 40% of VarTEs were shared between Bur-0 and C24 (Figure S4a).
Fig. 1. TE variation and its relation to coding gene proximity and genomic region. 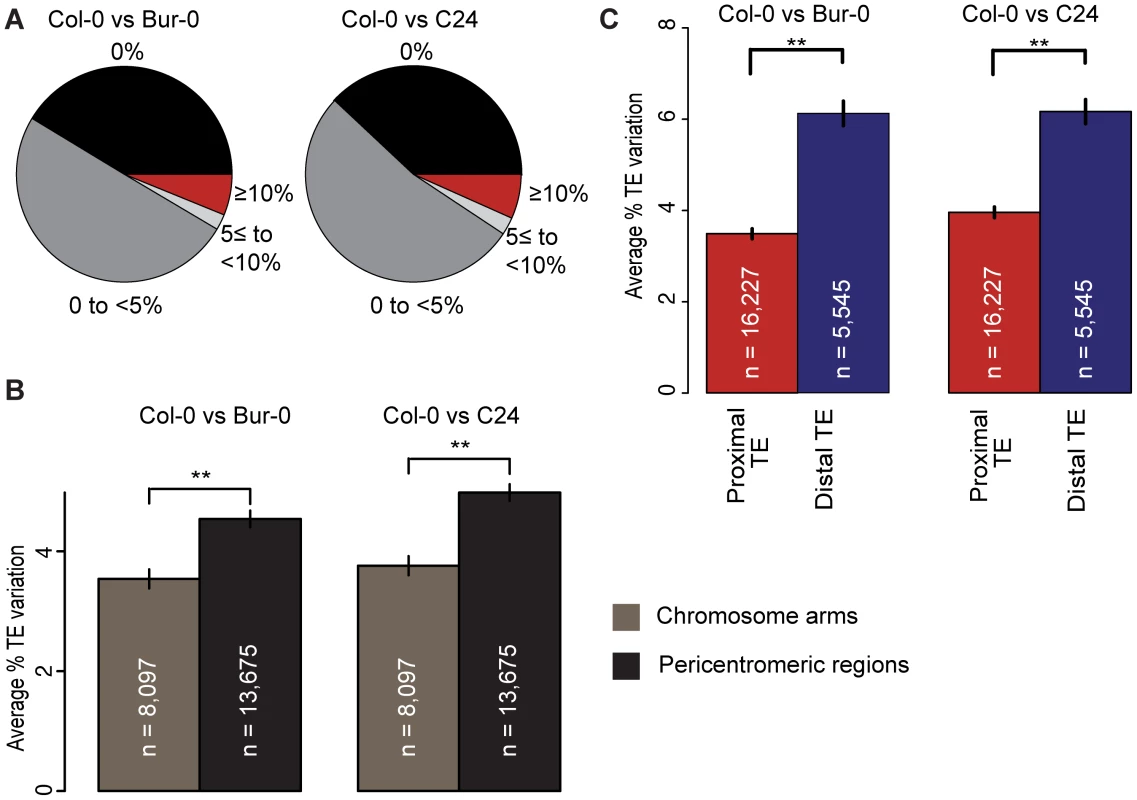
(a) TE variation between Col-0 and Bur-0 or C24, calculated as the percentage of total TE length that differs between two accessions. (b) for TEs on the chromosome arms vs the pericentromeric regions between Col-0 and Bur-0 or C24. Pericentromeric regions are defined as 8 MB regions flanking the centromeric regions (20). Mann-Whitney U [MWU] test p[Col-0/Bur-0] = 0.001, p[Col-0/C24]<6×10−5. ** = p<0.01. Standard errors are shown. (c) Average variation of proximal TEs and distal TEs between Col-0 and Bur-0 or C24 (MWU, p<2×10−16 for Col-0 versus Bur-0/C24). TE density is highest in and next to the centromeres, where there are few genes. The fraction of VarTEs and the average level of TE variation were higher in the pericentromeric regions than on the gene-dense chromosome arms (Figure 1b; Mann-Whitney U [MWU] test, p<2×10−16 for Col-0 versus Bur-0/C24, Table S1 and Figures S5 and S6). To examine whether gene proximity biases TE variation across the chromosomes, we calculated the distance between TEs and protein-coding genes for Col-0. TEs were separated into two subsets: TEs within 2 kb of any gene, subsequently called proximal TEs, and TEs at least 2 kb away from the closest gene, called distal TEs. Distal TEs were on average more variable than proximal TEs (Figure 1c; Figures S7 and S8; MWU p[Col-0/Bur-0] = 0.001, p[Col-0/C24]<6×10−5). Proximity to protein-coding genes may therefore influence TE variation, consistent with TEs closer to genes likely being under stronger selective constraint [15], [22].
The correlation between TE variation and proximity to genes was compared among TE superfamilies [23], [24]. For non-centromeric TEs, LTR retrotransposons were more distal from genes, while no significant difference in distance to genes was observed for other TE superfamilies (Table S2). However, for proximal TEs there were differences among TE superfamilies in distance to genes and, as expected, TE superfamilies that are closer to genes (e.g. CACTA, MITE) were less variable than superfamilies located farther away from genes, e.g. non-LTR retrotransposons (Table S2).
To investigate the link between TE and proximal gene variation, we examined whether TE variation and location correlated with the polymorphism level of neighboring genes. We used the small-scale mutations to calculate the polymorphism level of non-centromeric genes. For each accession, genes were separated into two subsets; TE+ genes included genes within 2 kb of a TE and genes with TEs anywhere within the transcribed region, while TE - genes were at least 2 kb from the closest TE (Table S3). To be conservative, any TEs in Bur-0 or C24 with predicted deletions of at least 10% of the reference length were annotated as deleted. TE+ genes were on average more polymorphic than TE − genes in each accession (Figure 2a; MWU p<2×10−16 for Col-0, Bur-0 and C24). The same analysis was repeated for 80 resequenced A. thaliana accessions [17]; we could confirm the correlations observed with Bur-0 and C24 in these accessions.
Fig. 2. TE presence, variation, and the polymorphism level of proximal genes. 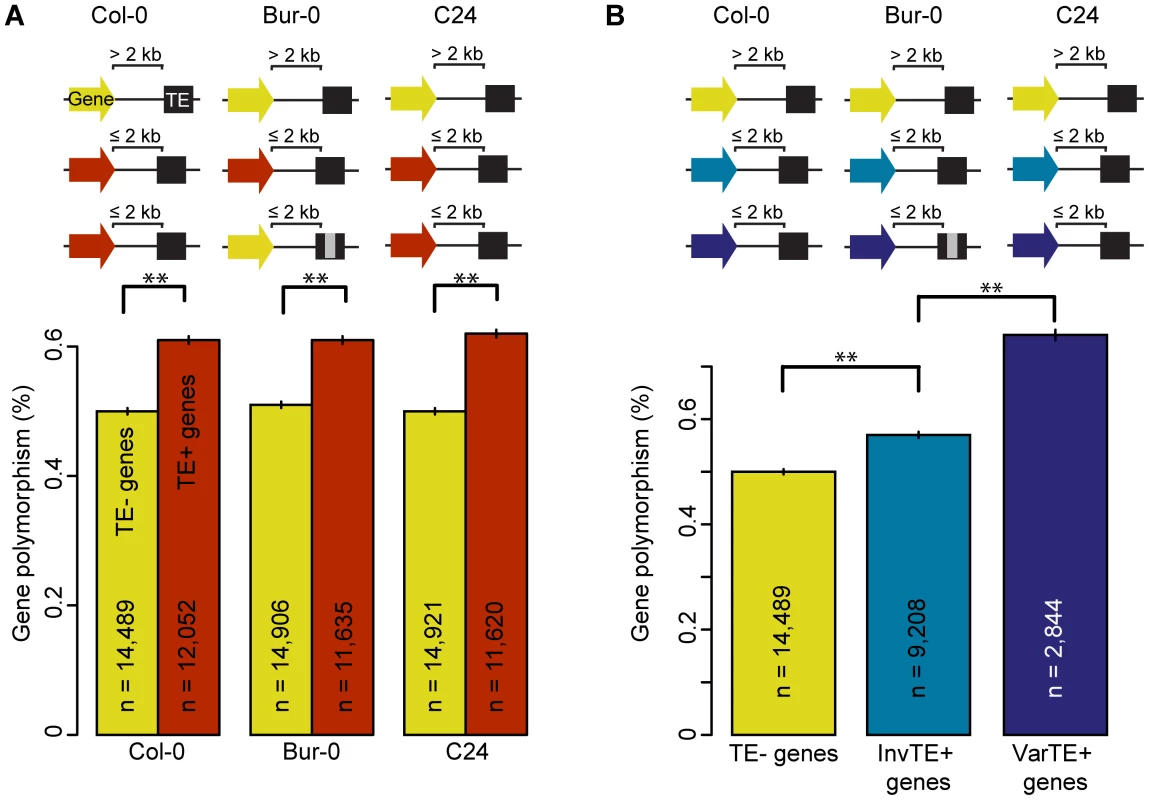
(a) Gene polymorphism levels in Bur-0 and C24 for TE− genes (yellow) vs TE+ genes (red). MWU p<2×10−16 for Col-0, Bur-0 and C24. Grey regions in the schema represent variations such as deletions. (b) Gene polymorphism levels for TE− genes (yellow), InvTE+ genes (cyan) or VarTE+ genes (navy). MWU p(InvTE+/VarTE+) = 0.005. ** = p<0.01. Standard errors are shown. Since polymorphism levels vary enormously among gene families, we further investigated whether there is a correlation of TE proximity with gene family using small-scale mutations from the 80 A. thaliana accessions (20, 61), and Col-0, C24 and Bur-0. Genes from highly polymorphic families such as those encoding NBS-LRR, F-box and Cytochrome P450s proteins were, on average, closer to TEs in all accessions (Figure S9; distance is negatively correlated with gene polymorphism, Spearman's ρ(Col-0) = −0.11, ρ(Bur-0) = −0.11, ρ(C24) = −0.10; p<2×10−16), including a higher proportion of genes having proximal TEs (Figure S10). TEs are therefore either more likely to insert into or near polymorphic genes, or are less efficiently purged from such regions.
To further examine the effects of TE variants on proximal genes, we divided TE+ genes into two subsets: genes where flanking TEs were <10% variant (Invariant TEs: InvTE) among the three accessions (InvTE+ genes), and genes where at least one flanking TE showed ≥10% sequence (VarTE) variation between accessions (VarTE+ genes; Table S3). Three quarters of VarTE+ genes were shared in comparisons between Col-0 and Bur-0 or Col-0 and C24 (Figure S4b). The VarTE+ genes were on average more polymorphic than InvTE+ genes (Figure 2b; MWU p = 0.005), also in the 80 accessions dataset [17]. We conclude that TEs close to genes are less polymorphic, while genes close to polymorphic TEs are themselves more polymorphic.
A correlation between polymorphism levels of TEs and nearby genes is insufficient to address whether this is a direct link as opposed to high directional selection pressure on the genomic region in general. To address this question, we therefore compared the polymorphism level of TEs, the flanking regions and nearby genes. TEs in highly polymorphic regions are themselves more polymorphic than TEs in regions of low divergence (Figure S11a; binomial test, p = 0), with the exception that TEs in highly polymorphic regions with nearby lowly polymorphic genes show a similar level of divergence as TEs in regions of low polymorphism with no coding genes. Moreover, TEs in gene-free regions show significantly higher divergence than TEs within 4 kb of a gene, especially if those genes are less polymorphic. TEs are generally more polymorphic than their flanking sequences (binomial test, p = 0), with the exception of TEs in highly polymorphic regions with lowly polymorphic gene. The results for large deletions (Figure S11b) are consistent with our observation from Figure S1 that large deletions are over-represented in TEs compared to intergenic regions. Notably, there is no significant difference in the level of small-scale mutations between TEs and flanking regions (Figure S11c). Taken together, TE variation through large deletions shows a positive correlation with flanking region polymorphism level, but is also strongly influenced by the conservation and presence/absence of nearby genes. The frequency of large deletions is however generally higher in TEs than in the flanking regions, indicating positive selection for large deletions within TEs.
TEs, siRNAs, and their effects on expression of adjacent genes
Genes that are close to TEs (TE+ genes) tend to have a lower expression average than TE − genes in the Col-0 reference accession [15]. We set out to determine whether this was true for the accessions studied here as well. Gene expression was measured using Affymetrix tiling arrays and RNA extracted from floral tissue of each accession. We considered presence/absence of TEs in the flanking regions of genes, taking into account the number of linked TE insertions and the distance from each gene to the closest TE. We confirmed the reported pattern for Col-0 [15], and found that it applies to Bur-0 and C24 as well. In all three accessions, genes with proximal TEs (TE+ genes) were on average expressed at lower levels than those without proximal TEs (TE − genes; Figure 3a; MWU p<2×10−16 for Col-0, Bur-0 and C24). This effect was even stronger if TEs were located simultaneously within, upstream and downstream of the gene (Figure 3a; MWU p≤2×10−14 for Col-0, Bur-0 and C24). Moreover, the average expression level of neighboring genes was positively correlated with the distance to the nearest TE (Figure 3b; Spearman's ρ(Col-0) = 0.15, ρ(Bur-0) = 0.13, ρ(C24) = 0.13; p<2×10−16), and negatively correlated with the number of proximal TEs (Figure 3c; df = 55, chi-square sums 915, 588 and 553 for Col-0, Bur-0 and C24, respectively, p<2×10−16). Thus, gene expression is suppressed by proximal TEs, especially if they are close to the gene and numerous.
Fig. 3. TEs and neighboring gene expression. 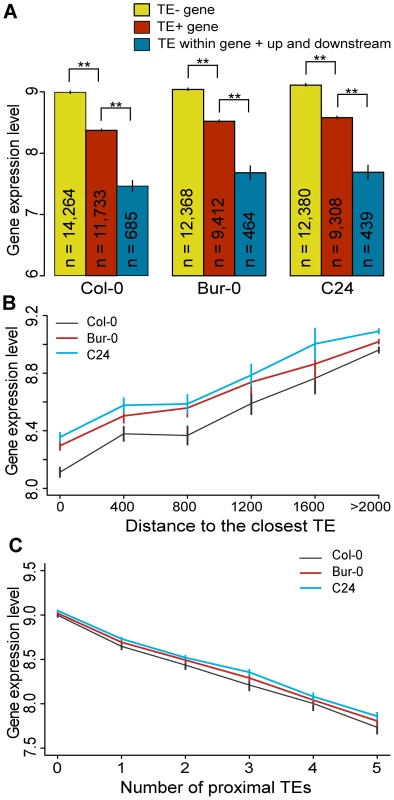
(a) Average gene expression levels for TE− genes (yellow), TE+ genes (red) and genes where TEs are located simultaneously within, upstream and downstream of the genes (cyan). MWU [TE+/TE−] p<2×10−16 for Col-0, Bur-0 and C24, MWU [TE+/TE within gene + up and downstream] p≤2×10−14 for Col-0, Bur-0 and C24. (b) Average gene expression as a function of the distance to the nearest TE. Distance was binned into 400 bp windows. A distance of 0 indicates genes that contain a TE. Spearman's ρ(Col-0) = 0.15, ρ(Bur-0) = 0.13, ρ(C24) = 0.13; p<2×10−16). (c) Average gene expression as a function of the number of proximal TEs. df = 55, chi-square sums 915, 588 and 553 for Col-0, Bur-0 and C24, respectively, p<2×10−16. ** = p<0.01. Standard errors are shown. Since TE superfamilies may have different effects on proximal genes, we examined gene expression according to the TE superfamily of the closest proximal TE. TE+ genes are expressed differentially depending on the TE superfamily of the proximal TE. TE+ genes with DNA transposons are on average expressed at a higher level compared to TE+ genes surrounded by retrotransposons (Figure S12; MWU, p = 0.02 for Col-0, Bur-0 and C24). However, this is solely due to the higher expression level of genes proximal to CACTA elements. Indeed, we did not find evidence for CACTA TEs having any effect on gene expression (Figure S12, MWU, p(CACTA TE+ genes/TE − genes) = 0.7, 0.6 and 0.8 for Col-0, Bur-0 and C24, respectively), which may explain why they are on average closer to genes than TEs from other families. Within the retrotransposons, LTR retrotransposons are younger on average than non-LTR retrotransposons and have a greater suppressive effect on proximal genes (Table S2; [25]). Therefore TE superfamilies can differ considerably in their effects on proximal genes.
TEs suppress the expression of neighboring genes at least partially through DNA methylation, which in turn is linked to 24-nt long siRNAs [12], [15], [22], [26], [27]. To investigate the influence of siRNAs on TE silencing, we sequenced siRNAs from mixed inflorescence tissue (shoot meristem plus flowers, stages 1–14) of each accession and mapped the reads to all possible positions of the respective genomes without any mismatches. As expected from previous work, the density of siRNAs over TEs was about four times higher than the genome average (Table S4; Figure S13).
We have reported before that siRNA-targeted TEs are more effective in suppressing expression of neighboring genes than are non-siRNA-targeted TEs, and that they are farther from genes [15]. We determined whether this held true in the current, more comprehensive dataset. If at least one 24-nt siRNA mapped to a TE it was labeled as siRNA+ (Table S5). siRNA+ and siRNA − TEs were overall similar in number, but retrotransposons were targeted by siRNAs more frequently than DNA transposons (Figure S14; binomial test, p = 0 for Col-0, Bur-0 and C24). siRNA+ TEs were farther from genes (Figure 4a; Figure S15a; MWU p<2.2×10−16 for Col-0, Bur-0 and C24), and this bias was consistent among TE superfamilies (Figure S16). To examine the effects of siRNA-targeting on the expression of flanking genes, we classified genes by whether the nearest TE was siRNA+ or siRNA − (Table S5). In each accession, genes flanked by siRNA+ TEs had lower average expression levels than genes with adjacent siRNA − TEs (Figure 4b; Figure S15b; MWU p[Col-0] = 0.0001, p[Bur-0] = 0.002, p[C24] = 2×10−6). The effect of suppression was stronger if the closest siRNA+ TE was within 2 kb of the gene (Figure 4b; Figure S15b; MWU p<2×10−16 for Col-0, Bur-0 and C24). Therefore, as found previously for Col-0, siRNA-targeting of TEs represses nearby genes and TEs that are close to genes are less likely to be targeted by siRNAs, either due to stronger selection for deletion of siRNA-targeted TEs close to genes or selection against siRNA-targeting of these TEs.
Fig. 4. Relationship of TE siRNA–targeting to distance from genes and its effect on gene expression in Col-0. 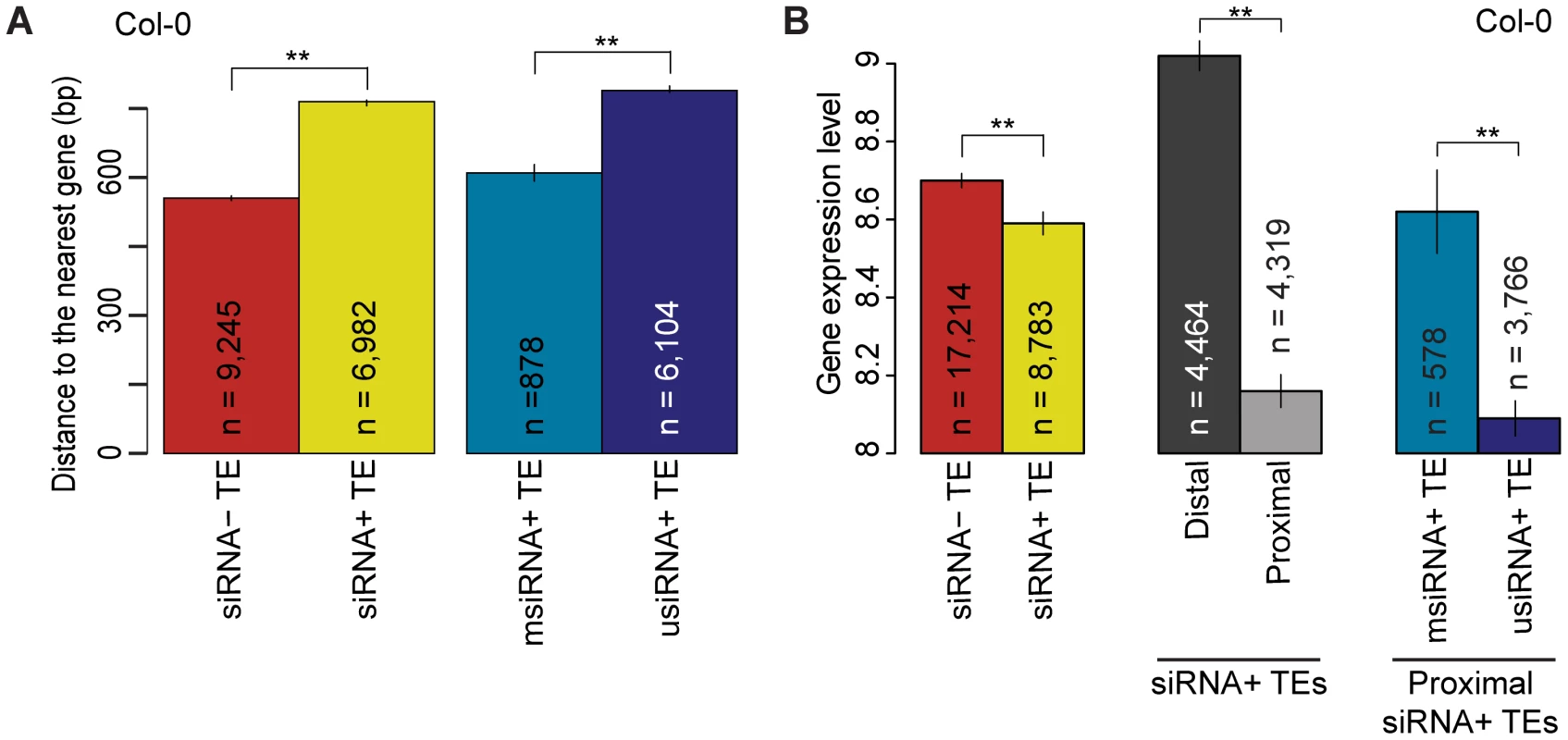
(a) Average distance of siRNA− (red) and siRNA+ (yellow) proximal TEs to the nearest gene. For siRNA+ proximal TEs, distance to the closest gene is compared between msiRNA+ TEs (cyan) and usiRNA+ TEs (navy). MWU [siRNA+/siRNA−] p<2.2×10−16 for Col-0, Bur-0 and C24. MWU [msiRNA/usiRNA] p[Col-0]<2×10−16, p[Bur-0] = 6×10−13 and p[C24] = 2×10−6. (b) Average expression level of genes when neighboring TEs are siRNA− (red) or siRNA+ (yellow). For siRNA+ TEs, average gene expression levels are given for when the nearest TE is distal (greater than 2 kb from gene; dark gray) or proximal (within 2 kb; light gray). For genes with proximal siRNA+ TEs, expression levels were further compared between msiRNA+ TEs (cyan) and usiRNA+ TEs (navy). See Figure S12 for Bur-0 and C24. MWU [siRNA+/siRNA−] p[Col-0] = 0.0001, p[Bur-0] = 0.002, p[C24] = 2×10−6. MWU [siRNA+ distal/proximal] p<2×10−16 for Col-0, Bur-0 and C24. MWU [msiRNA/usiRNA] p[Col-0] = 3×10−6, p[Bur-0] = 5×10−5, p[C24] = 0.01). ** = p<0.01. Standard errors are shown. Because siRNAs that map to unique positions in the genome (usiRNAs) correlate more closely with DNA methylation than siRNAs that map to multiple positions (msiRNAs; [12]), we investigated whether usiRNAs and msiRNAs target TEs differentially, and how usiRNA − and msiRNA-targeted TEs might affect the expression of nearby genes. All TEs with at least one usiRNA were labeled as usiRNA+ (Table S5). In both Bur-0 and C24, over 83% of siRNA+ TEs were usiRNA+, similar to what has been reported for Col-0 [14]. usiRNA+ TEs were farther away from genes than msiRNA+ TEs (Figure 4a; Figure S15a; MWU p[Col-0]<2×10−16, p[Bur-0] = 6×10−13 and p[C24] = 2×10−6). We also observed that the average expression level of genes within 2 kb of usiRNA+ TEs was lower than the expression of genes within 2 kb of msiRNA+ TEs (Figure 4b; Figure S15b; MWU p[Col-0] = 3×10−6, p[Bur-0] = 5×10−5, p[C24] = 0.01). Therefore, even though TEs targeted by usiRNAs and msiRNAs are on average farther from genes, they more strongly reduce expression of proximal genes compared to TEs targeted by only msiRNAs. Overall, we confirmed that siRNA+ TEs, especially usiRNA+ TEs, suppress neighboring gene expression, consistent with a trade-off between reduced TE mobility and deleterious effects on neighboring gene expression [14], [15].
Links between variation in TEs, siRNA–targeting, and gene expression differences
If TEs suppress the expression of adjacent genes, presence of gene-proximal TEs in the different accessions should be associated with differences in expression levels of proximal genes. We found that expression of TE − genes varied less between accessions than TE+ genes, and further that expression varied less between genes proximal to invariant TEs (InvTE+ genes) than genes proximal to variant TEs (VarTE+ genes; Figure 5a; MWU p[TE−/TE+]<2×10−16, p[InvTE+/VarTE+] = 2×10−5). However, because TEs, and especially VarTEs, are found more often next to polymorphic genes, these conclusions could be confounded by correlated differences in genic polymorphisms. We therefore classified genes based on the extent of sequence variation (Table S6). Regardless of degree of genic polymorphism, VarTE+ genes were the ones that varied most in expression between accessions (Figure 5b), indicating that TE variation increases variance in gene expression.
Fig. 5. TE variation, siRNA–targeting, and differences in proximal gene expression. 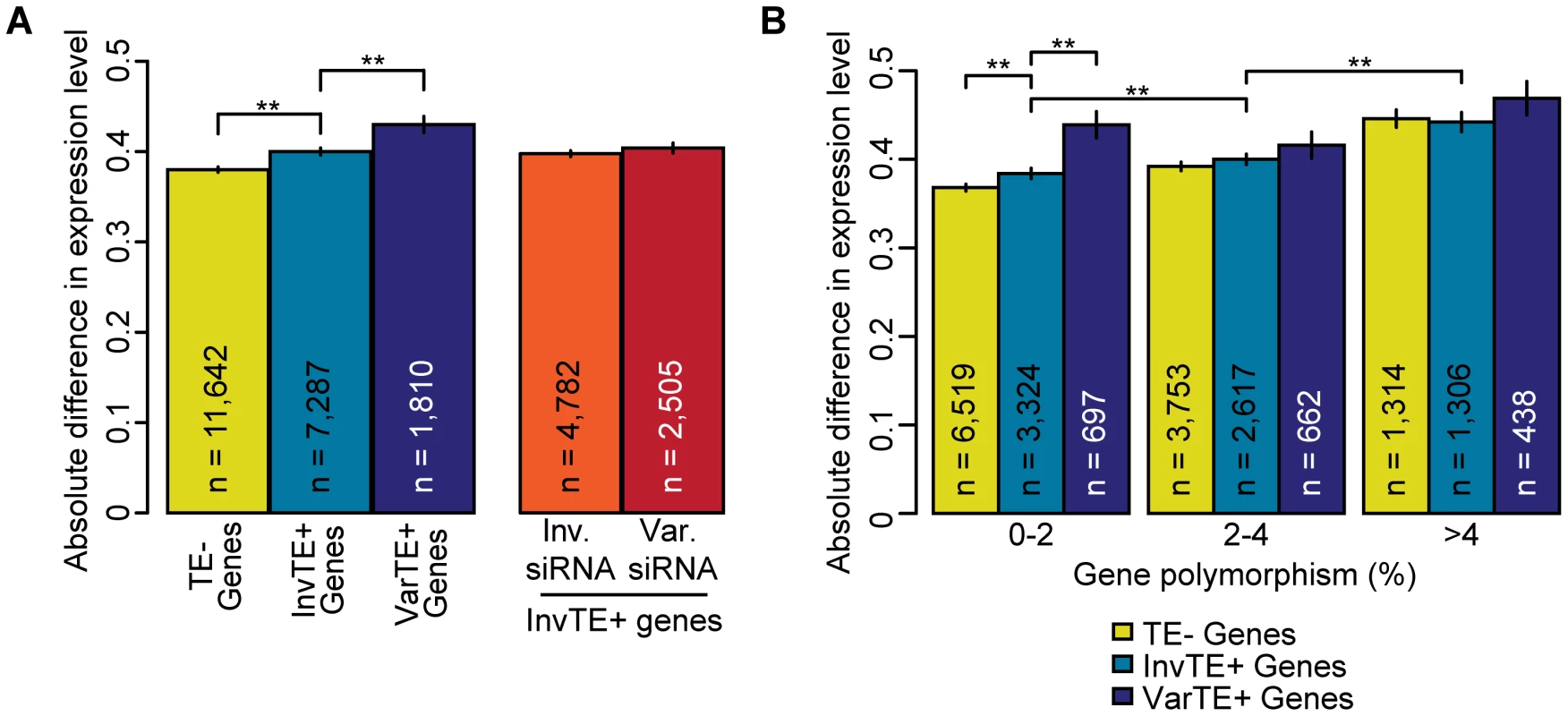
(a) Average absolute difference in gene expression for TE− genes (yellow), InvTE+ genes (cyan) and VarTE+ (navy) genes. MWU p[TE−/TE+]<2×10−16, p[InvTE+/VarTE+] = 2×10−5. Expression divergence is also shown for InvTE+ genes divided by whether the proximal TEs are invariably (orange) or variably (red) targeted by siRNA. (b) TE− genes (yellow), InvTE+ (cyan) genes and VarTE+ (navy) genes were divided into subgroups depending on their polymorphism levels. Genes were binned by polymorphism levels into 0–2%, 2–4% and >4% groups. The average absolute change in expression level for each subgroup of genes is shown. ** = p<0.01. Standard errors are shown. We next determined whether differential siRNA-targeting influences gene expression. To remove the potentially confounding effects of variation in TEs themselves, we focused on InvTE+ genes and grouped these based on whether siRNAs for the adjacent TE could be detected in either all or none of the three accessions, or whether accessions differed in siRNA-targeting of the adjacent TE. We found that while variation in siRNA-targeting increased expression differences between accessions, this increase was not statistically significant (Figure 5a). It should be noted that in our analysis we could not distinguish between the effects of differential siRNA-targeting and any perturbations of cis-regulatory sequences.
Since each TE that differs in presence/absence or each siRNA-targeting variant between accessions represents a natural mutagenesis experiment, this offers an opportunity to study the effects on individual genes, to confirm the inferences drawn from averaging over all genes. We selected siRNA+ TE+ genes in Col-0 that are siRNA − TE+ or TE − in Bur-0 or C24 and tested for differential expression between Bur-0 or C24 and Col-0. To remove the potential confounding effect of genic polymorphism, we excluded genes with a polymorphism level greater than 2%. Overall 706 genes were retained for this analysis. The effect of siRNA-targeting on gene expression was further verified by comparing expression profiles among wild-type, rdr2-1 and a ddc (drm1drm2cmt3) DNA methyltransferase triple mutant [28]. Fifteen genes out of 706 showed significant up-regulation (top 5% ranking) in Bur-0 or C24 and in at least one of the RNA silencing mutants (Table S7). Although not statistically significant, this observation is consistent with siRNA-targeting and TE presence affecting gene expression. Moreover, it is likely an underestimate of TE effects on gene expression, given our stringent selection criteria.
siRNA–targeting and TE evolution
Because siRNA+ TEs suppress neighboring gene expression particularly efficiently, we asked whether targeting of different regions of TEs was reflected in the expression of adjacent genes. We first investigated whether invariant and variant TEs (InvTEs and VarTEs) differed in siRNA-targeting, normalized by TE length, and whether there were differences between invariable and variable regions of VarTEs (Figure 6a; Table S8). Fewer siRNAs mapped to siRNA+ VarTEs than to siRNA+ InvTEs (Figure 6a; MWU p<2×10−16 for Col-0 versus Bur-0/C24), but there were more siRNAs in variable regions than invariable regions of siRNA+ VarTEs in Col-0 (Figure 6a; MWU p[Col-0/Bur-0] = 1×10−5, p[Col-0/C24]<2×10−16). Furthermore, usiRNAs were overrepresented in variable regions (binomial test, p[Col-0/Bur-0] = 7×10−18, p[Col-0/C24] = 0), while msiRNAs were biased towards invariable regions (p[Col-0/Bur-0] = 1×10−6, p[Col-0/C24] = 0). Therefore, usiRNAs strongly correlate with variability of TE sequences and are over-represented in the variable regions of variant TEs.
Fig. 6. siRNA–targeting of VarTEs and the effect on proximal gene expression. 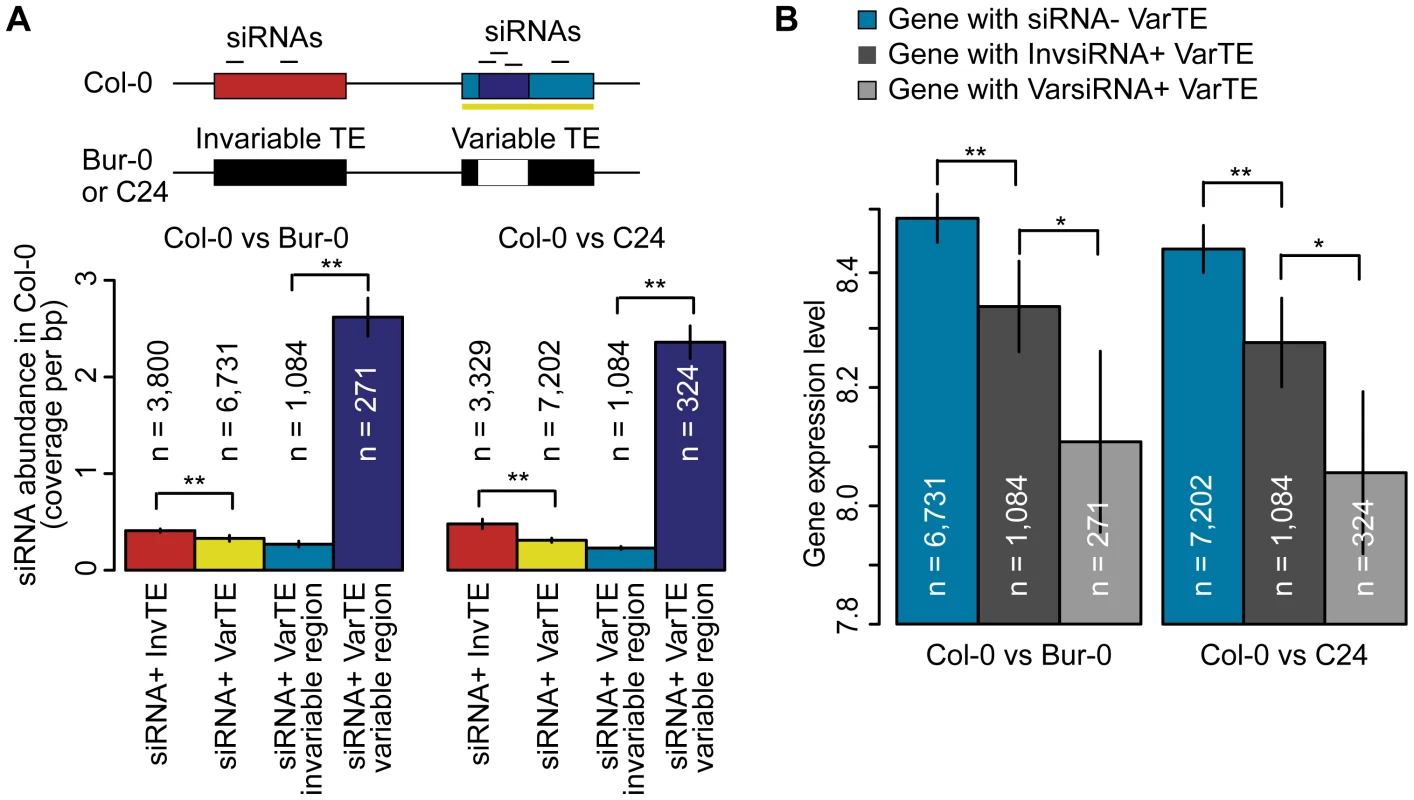
(a) Upper panel depicts siRNA-targeting of variable and invariable regions of VarTEs defined between Col-0 and Bur-0 or C24. Lower panel shows abundance of Col-0 siRNA in siRNA+ InvTEs (red) and siRNA+ VarTEs (yellow) between Col-0 and Bur-0 or C24. Within siRNA+ VarTEs, the abundance of Col-0 siRNA was compared between invariable (cyan) and variable regions (navy). MWU [InvTE/VarTE] p<2×10−16 for Col-0 versus Bur-0/C24. MWU [variable/invariable regions of VarTEs] p[Col-0/Bur-0] = 1×10−5, p[Col-0/C24]<2×10−16 (b) VarTE+ genes were divided into subgroups based on whether the closest proximal TE was siRNA− (cyan), InvsiRNA+ (dark gray) or VarsiRNA+ (light gray). The average expression level of each gene group is shown. MWU [siRNA− VarTE+/InvsiRNA+ VarTE+] p[Col-0/C24] = 0.01, p[Col-0/Bur-0] = 0.01; MWU [siRNA− VarTE+/VarsiRNA+ VarTE+] p[Col-0/C24] = 9×10−5, p[Col-0/Bur-0] = 0.003; MWU [VarsiRNA+ VarTE+/InvsiRNA VarTE+] p[Col-0/C24] = 0.01, p[Col-0/Bur-0] = 0.04; * = p<0.05, ** = p<0.01. Standard errors are shown. This finding raised the question whether TE regions that varied between accessions and were targeted by siRNAs had a particularly large effect on expression of adjacent genes. We therefore separated Col-0 genes within 2 kb of variable TEs into three subsets: genes next to siRNA − VarTEs (siRNA − VarTE+ genes); genes next to VarTEs with an siRNA-targeting bias towards invariable TE regions (InvsiRNA+ VarTE+ genes); and genes next to VarTEs with an siRNAs targeting bias towards variable TE regions (VarsiRNA+ VarTE+ genes; Table S8). As expected, siRNA − VarTE+ genes had a higher average expression level compared to InvsiRNA+ VarTE+ genes (Figure 6b; MWU p[Col-0/C24] = 0.01, p[Col-0/Bur-0] = 0.01) or VarsiRNA+ VarTE+ genes (MWU p[Col-0/C24] = 9×10−5, p[Col-0/Bur-0] = 0.003). The InvsiRNA+ VarTE+ genes, however, were expressed on average more highly than the VarsiRNA+ VarTE+ set (MWU p[Col-0/C24] = 0.01, p[Col-0/Bur-0] = 0.04). This indicates that gene suppression by neighboring TEs may not only be influenced by siRNA presence or absence at the TEs, but may also depend on which TE regions are targeted by siRNAs. We speculate that siRNA-targeting of particular TE regions suppresses the expression of nearby genes to such an extent that there is significantly higher selection pressure for these regions to be excised or mutated. Alternatively, due to the skew of usiRNA mapping towards variable regions, and the greater correlation between usiRNAs and TE methylation, the lower expression level of VarsiRNA+ VarTE+ genes may reflect a higher degree of epigenetic silencing of these elements compared to InvsiRNA+ VarTE+ genes.
Discussion
TEs constitute the majority of DNA in many plant genomes [2], [3]. Evolutionary dynamics vary among TE types and they are affected, for example, by species demography and mating system [29]. A number of measures counteract the proliferation of TEs including TE silencing and removal. Because TE deletions via illegitimate recombination and unequal intra-strand homologous recombination are common [30]–[33], it is important to understand how changes in TE composition affect nearby gene expression. We have studied the interactions of TE variants, genic polymorphism, gene expression, and siRNA-targeting in Arabidopsis thaliana. We have shown that there is substantial variation in TEs between accessions primarily through large deletions, with invariant TEs on average closer to genes than variant TEs. We have confirmed that gene expression is positively correlated with distance to the nearest TE, and negatively correlated with the number of proximal TEs. While variation within a TE has some effect on the expression of adjacent genes, genes close to TEs are also on average more polymorphic than those that are not. Perhaps our most interesting observation is the increased usiRNA-targeting in TE regions that are variable between accessions compared to TE regions that are invariant.
TE variation between accessions
TEs may be prevented from reaching fixation within a population through negative selection, especially for gene-proximal, methylated TEs [13], [15], [34]. Therefore, it is perhaps unsurprising that TEs are over-represented in analyses of structural variants among accessions and between species [17], [18], [35], [36], and that a recent comparison of 80 A. thaliana genomes reported evidence of structural variation in 80% of TEs [17]. Similarly, Hollister and Gaut [15] found that 44% of over 600 TE insertions were polymorphic among 48 accessions. Since most TEs in A. thaliana are relatively old [7], the simplest way to explain these patterns is ongoing deletion of TEs, which is also consistent with TEs in A. thaliana being on average farther from genes than in the closely related but outcrossing A. lyrata [7]. This may, however, be too simplistic an explanation as non-LTR retrotransposons are skewed towards an older insertion distribution than LTR retrotransposons [25], even though they are not significantly more variable (Table S2). While TE presence/absence polymorphisms in different accessions have been previously characterized [17], we have shown that there is substantial sequence variation in about 6% of TEs when comparing accessions (Figure 1a). These TE variants are equally distributed throughout the genome (Figure 1b).
TE effects on nearby genes
TEs can affect the expression of proximal genes via mechanisms including disruption of promoter sequences, reduction of transcription through the spread of epigenetic silencing [13], or read-though antisense transcription [37]. Often TEs suppress the expression of proximal coding genes [15], [22], [38] however, TEs can also introduce new promoter sequences, leading to up-regulation of proximal genes [37]. In both plants and animals, TE-derived sequences have been recruited to form regulatory sequences and have contributed to coding regions [8], [39]–[42].
Methylated TEs suppress expression of proximal genes in A. thaliana, regardless of insertion upstream or downstream of the coding region. Purifying selection is therefore greatest for methylated TEs proximal to genes [15]. Notably, the effects of siRNAs on expression of proximal genes can only be detected up to 400 bp [14], while measurable TE effects extend to 2 kb [14]. This supports the assertion that TEs either directly affect gene expression by disruption of positive regulatory sequences, or otherwise act through DNA structure and epigenetic marks to affect genes over longer distances.
We found that TEs that with variable siRNA-targeting do not affect proximal genes more strongly than TEs that are targeted in all three accessions (Figure 5). It is possible that siRNA-targeting varies independently of TE sequence variation, as observed recently for DNA methylation [43], and that such TEs mask more subtle differences between the TE classes examined. However, the region of the TE targeted by siRNAs does seem to matter, with siRNA-targeting of TE sequences within an accession that are variant/absent in other accessions showing a greater suppression of proximal genes (Figure 6). This agrees with the observation that genes close to usiRNA-targeted TEs have a lower expression average than those close to msiRNA-targeted TEs, and that usiRNAs are over-represented in the variable regions of transposons. A recent study of hybrids between parents of different ploidy found that a reduction in 24 nt siRNAs is associated with up-regulation of more TE-associated genes than when there is no significant change in siRNA levels [44]. This result supports the hypothesis that siRNAs, or linked epigenetic changes, can affect the expression of nearby genes, with deletion of the siRNA-targeted regions alleviating repression of adjacent genes.
While TEs in the euchromatin are often found close to genes, methylated TEs are underrepresented upstream of genes, likely because changes in the promoter more easily affect gene expression than variation in the 3′ region [13]. In agreement, methylated TEs have a skewed distribution, with older elements farther from genes, but unmethylated TEs do not show such a bias [15]. In a comparison of humans and chimpanzees, TE insertion site preference appears to be the main cause for TEs being found more often in the vicinity of genes with increased interspecific expression variation [45]. This is reminiscent of what we have observed, with additive effects of polymorphism, TE presence and TE variance on the variability of orthologous gene expression (Figure 2 and Figure 4). In a comparison of two rice subspecies, TE presence/absence polymorphisms were also found to be underrepresented in SNP deserts [35]. There are several possible explanations for these observations: some genomic regions may suffer from generally elevated mutation rates TEs near highly conserved genes are more efficiently purged; or TE integration into more mutable genomic regions is favored. In the latter case, new mutations may destabilize DNA packing and facilitate TE insertions, similar to the TE insertion preference for transcribed genomic regions [42].
TE evolution through silencing and deletions
With our observation of TE deletions correlating with siRNA-targeting, we can expand the current model for TE evolution [15]. Our model starts with the duplication of a TE that is already present and targeted by siRNAs within the genome (Figure 7a and 7b), leading to all siRNAs produced by and targeting the original TE now being multiply-mapping siRNAs (msiRNAs). As the two copies of the duplicated TE gain mutations (enhanced by deamination of methylated cytosines), uniquely-mapping siRNAs (usiRNAs) are produced in addition to msiRNAs (Figure 7c). Hollister and colleagues [14] noted that usiRNA-targeting increases with TE age, while msiRNA-targeting decreases, and that TEs are expressed at lower levels when also targeted by usiRNAs. Furthermore, usiRNAs are more closely correlated with DNA methylation than are msiRNAs [12] and they are expressed at higher levels than msiRNAs [14]. With usiRNAs, the duplicated TEs will therefore be more effectively silenced, probably with a concurrent increase in methylation, a further reduction in the expression level of proximal genes, and thus increased selection against the TEs.
Fig. 7. Hypothesis for the role of siRNA–targeting in TE evolution. 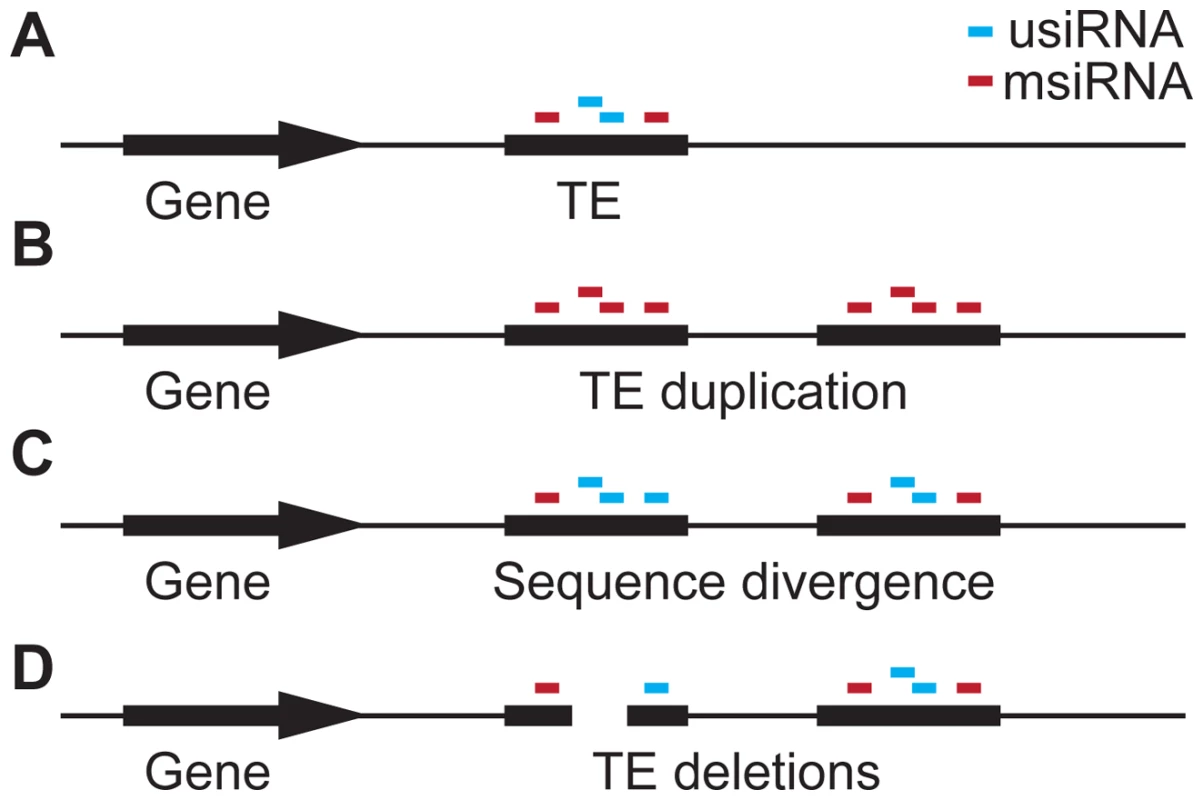
(a) A gene with an adjacent TE targeted by siRNAs that are either unique to this TE (usiRNAs) or that are shared with multiple locations in the genome (msiRNAs). (b) Duplication of the TE causes all usiRNAs to become msiRNAs. (c) Sequence divergence between the duplicated TEs, e.g. through deamination of methyl-cytosines, which causes C:T transition mutations. As a consequence, msiRNAs are converted to usiRNAs again. (d) TE regions that are enriched for siRNAs, especially usiRNAs, are deleted, reducing the effect of the TE on adjacent genes. usiRNA-targeting may then facilitate TE inactivation through preferential deletion of usiRNA-targeted regions (Figure 6 and Figure 7d). This may be actively promoted by the usiRNAs and attendant epigenetic marks, in a mechanism analogous to the siRNA-guided removal of “internal eliminated sequences” including TEs in Tetrahymena [46], [47]. In favor of such a scenario, small deletions within TEs have been shown to occur more frequently than ectopic recombination events at the LTRs [31], [48]. Ectopic recombination appears to be less important for TE elimination in A. thaliana, as TE density and recombination rate are not correlated in this species [48], and because ectopic recombination is lower in homozygotes [49]. No matter what the mechanism, deletions within TEs would reduce selection pressure by removing usiRNA target sites, inactivating TEs so they are no longer transposition-competent, and relieving proximal gene repression.
In apparent contrast to the majority of TEs, some are under positive selection [50], [51], and TEs can also contribute to new regulatory networks [52]. Our model is only appropriate for TEs under neutral or negative selection. Modeling of TE dynamics suggests that transposition events occur in a cyclical manner [53], [54], with some activation events creating new favorable genetic variants. One such example is provided by transposition of a TE that is induced upon heat stress in genetic backgrounds impaired in siRNA biogenesis confers heat-responsiveness to proximal genes [55].
Conclusions
We have exploited high-quality genome information from multiple accessions of a single species to study the effects of TE variation on proximal gene expression. We discovered a link between siRNA-targeting and TE variation that illuminates how epigenetic mechanisms may help to shape genomes, but several questions remain: Do usiRNAs directly facilitate TE deletions or do they act indirectly through differences in selection for deletions? Are TE deletions in other species also associated with regions of increased usiRNA-targeting? And do species differ in the rate of TE deletion via this mechanism? Because of the rarity of TE deletions, this is a challenging process to dissect. Genomes with a large fraction of TEs, such as those of many crop plants, might therefore prove more tractable systems for studying mechanism of TE removal than the TE poor A. thaliana genome.
Methods
Annotation of genes and TEs in Col-0, Bur-0, and C24
We extracted positions of genes and TEs from the A. thaliana Col-0 genome sequence TAIR version 9 from http://www.arabidopsis.org. We excluded genes and TEs within the centromeric regions [56]. To define gene and TE sets in Bur-0 and C24, we built genome templates using published Illumina paired-end reads of Bur-0 and C24 [19]. We used the SHORE pipeline [21] to align the reads to the Col-0 reference genome and extracted the consensus sequences as genome templates by calling bases with quality>24, support>6, concordance>0.7 and average hits = 1. We then applied a naïve projection of the coordinates of genes and TEs from Col-0 onto the genome templates to define the gene and TE sets of Bur-0 and C24. SHORE was also used to detect genomic variations by calling SNPs, small (1–3 bp) insertions/deletions and larger deletions from the genome templates of Bur-0 and C24 compared to the Col-0 genome using the same parameters for quality control. The distance between TEs and genes in Bur-0 and C24 was estimated from Col-0 using the annotated TE and gene coordinates, and adjusted to account for insertions and deletions between TEs and genes.
Comparison of polymorphism densities
For each polymorphism type (i.e., SNPs, small indels, and large deletions), we compared the densities pairwise across coding regions, intergenic regions and TEs. To test whether a higher density was significant in a particular genomic region (e.g. TE) compared to others (e.g. coding region), a cumulative binomial probability distribution was applied:
p is the polymorphism density in coding regions, and k and n are the total number of polymorphic sites in TEs and the total length of TEs, respectively.We calculated gene polymorphism levels as the fraction of genic region containing small-scale variations in at least C24 or Bur-0, or one of the 80 A. thaliana accessions [17]. Genes with more than 20% zero sequencing coverage or no base calls among 80 accessions were excluded from the analysis.
4 kb 5′ and 3′ flanking regions for each TE were extracted. For each flanking region (FR), or genic regions (GR) within the FR, small-scale mutations and large deletion polymorphisms between Col-0 and Bur-0/C24 were calculated. Using all mutations, the polymorphism levels of TEs, FRs and GRs were ranked. A threshold of 50% was used to split FRs and GRs into high or low polymorphism datasets and thereby classify the TEs by genomic environment. The polymorphism levels of the FRs were calculated in 200 bp bins for each group of TEs, with binomial tests to compare polymorphism levels between TEs and FRs, and between different TE groups.
Gene expression
Inflorescences (meristem and flowers up to stage 14) were pooled from five plants of each accession grown at 23°C. Triplicate samples were collected between 7 and 8 hours into a 16 hour light cycle. RNA was extracted using the Qiagen (Hilden, Germany) Plant RNeasy Mini kit. Each biological replicate was analyzed with Affymetrix (Santa Clara, CA, USA) tiling 1.0R arrays and the data were processed according to published methods [57], [58]. Tiling array probes that were polymorphic for C24 or Bur-0 were removed from the dataset for the affected accession(s). For gene expression estimates, ≥70% and at least 3 probes had to be present; all other genes were not considered.
Tilling array data from Arabidopsis Col-0 and the RNA silencing mutants rdr2-1 and ddc (drm1-1;drm2-2;cmt3-11) mutants were downloaded from GEO (GSE12549; [28]) and processed according to published methods [57], [58]. Expression level changes for each dataset were estimated by fold-change differences between Bur-0/C24 and Col-0, and between the RNA silencing mutants and wild type Col-0. Background distributions of fold-change were calculated and genes, with a fold-change exceeding a one-sided 95% quantile in each dataset were considered as significantly up-regulated in Bur-0/C24 or the mutants.
siRNA analyses
The siRNA datasets have been published [19] (GEO accession number GSE24569). We mapped the 24-nt siRNA reads onto both strands of the genome templates (see below) and the TEs of Col-0, Bur-0 and C24, respectively, using the Vmatch package (http://www.vmatch.de). Only reads with perfect matches were considered.
Comparison of usiRNA – and msiRNA–targeting
The statistical significance of over-representation of usiRNAs or msiRNAs within the variable regions of siRNA+ VarTEs in comparison to all siRNAs was tested using the cumulative binomial probability distribution given above. p, expected frequency, is the ratio between the number of siRNAs mapped to the variable regions the total number of siRNAs mapped to any region of siRNA+ VarTEs, and n and k are the total number of usiRNAs/msiRNAs mapped to any region and the number of usiRNAs/msiRNAs mapped to the variable regions, respectively.
Determination of InvsiRNA+ and VarsiRNA+ VarTEs
We defined an siRNA+ VarTE as either InvsiRNA+ or VarsiRNA+ if siRNAs are overrepresented in the invariable regions and variable regions, respectively. For siRNA+ VarTEs that contain siRNAs in both variable and invariable regions, we employed the cumulative binomial probability distribution described above to test whether siRNA-targeting shows statistically significant bias towards variable or invariable regions. For each siRNA+ VarTE, p in the formula above is the abundance of siRNA-targeting at the TE. To test the bias towards variable regions, n and k represent the genomic length of variable regions and the number of siRNAs targeting variable regions, respectively. Similarly, to test the bias towards invariable regions, n and k represent the genomic length of invariable regions and the number of siRNAs targeting invariable regions, respectively. P-values were adjusted for multiple hypothesis testing with the Benjamini-Hochberg method to control for a false discovery rate of 5% [59].
Data deposition
The siRNA and microarray data reported in this paper have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE24569 and GSE24669. The genome assemblies are available from http://1001genomes.org/projects/MPIWang2012/ while the transposable element annotations for Bur-0 and C24 are available from Dryad under doi 10.5061/dryad.8674d.
Supporting Information
Zdroje
1. LanderES, LintonLM, BirrenB, NusbaumC, ZodyMC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409 : 860–921.
2. MessingJ, BhartiAK, KarlowskiWM, GundlachH, KimHR, et al. (2004) Sequence composition and genome organization of maize. Proc Natl Acad Sci USA 101 : 14349–14354.
3. SanMiguelPJ, RamakrishnaW, BennetzenJL, BussoCS, DubcovskyJ (2002) Transposable elements, genes and recombination in a 215-kb contig from wheat chromosome 5A(m). Funct Integr Genomics 2 : 70–80.
4. TenaillonMI, HuffordMB, GautBS, Ross-IbarraJ (2011) Genome size and transposable element content as determined by high-throughput sequencing in maize and Zea luxurians. Genome Biol Evol 3 : 219–229.
5. HawkinsJS, KimH, NasonJD, WingRA, WendelJF (2006) Differential lineage-specific amplification of transposable elements is responsible for genome size variation in Gossypium. Genome Res 16 : 1252–1261.
6. PieguB, GuyotR, PicaultN, RoulinA, SaniyalA, et al. (2006) Doubling genome size without polyploidization: dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res 16 : 1262–1269.
7. HuTT, PattynP, BakkerEG, CaoJ, ChengJF, et al. (2011) The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat Genet 43 : 476–481.
8. LocktonS, GautBS (2009) The Contribution of Transposable Elements to Expressed Coding Sequence in Arabidopsis thaliana. J Mol Evol 68 : 80–89.
9. LischD (2009) Epigenetic regulation of transposable elements in plants. Annu Rev Plant Biol 60 : 43–66.
10. ZhangX, YazakiJ, SundaresanA, CokusS, ChanSWL, et al. (2006) Genome-wide High-Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 126 : 1189–1201.
11. CokusSJ, FengS, ZhangX, ChenZ, MerrimanB, et al. (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452 : 215–219.
12. ListerR, O'MalleyRC, Tonti-FilippiniJ, GregoryBD, BerryCC, et al. (2008) Highly Integrated Single-Base Resolution Maps of the Epigenome in Arabidopsis. Cell 133 : 523–536.
13. AhmedI, SarazinA, BowlerC, ColotV, QuesnevilleH (2011) Genome-wide evidence for local DNA methylation spreading from small RNA-targeted sequences in Arabidopsis. Nucleic Acids Res 39 : 6919–6931.
14. HollisterJD, SmithLM, GuoYL, OttF, WeigelD, et al. (2011) Transposable elements and small RNAs contribute to gene expression divergence between Arabidopsis thaliana and Arabidopsis lyrata. Proc Natl Acad Sci USA 108 : 2322–2327.
15. HollisterJD, GautBS (2009) Epigenetic silencing of transposable elements: A trade-off between reduced transposition and deleterious effects on neighboring gene expression. Genome Res 19 : 1419–1428.
16. BuisineN, QuesnevilleH, ColotV (2008) Improved detection and annotation of transposable elements in sequenced genomes using multiple reference sequence sets. Genomics 91 : 467–475.
17. CaoJ, SchneebergerK, OssowskiS, GuntherT, BenderS, et al. (2011) Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43 : 956–963.
18. GanX, StegleO, BehrJ, SteffenJG, DreweP, et al. (2011) Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477 : 419–423.
19. SchneebergerK, OssowskiS, OttF, KleinJD, WangX, et al. (2011) Reference-guided assembly of four diverse Arabidopsis thaliana genomes. Proc Natl Acad Sci USA 108 : 10249–10254.
20. The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408 : 796–815.
21. OssowskiS, SchneebergerK, ClarkRM, LanzC, WarthmannN, et al. (2008) Sequencing of natural strains of Arabidopsis thaliana with short reads. Genome Res 18 : 2024–2033.
22. LippmanZ, GendrelAV, BlackM, VaughnMW, DedhiaN, et al. (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430 : 471–476.
23. FeschotteC, JiangN, WesslerSR (2002) Plant transposable elements: where genetics meets genomics. Nat Rev Genet 3 : 329–341.
24. Feschotte C, Zhang X, Wessler SR (2002) Miniature inverted-repeat elements and their relationship to established DNA transposons. Mobile DNA II. Washington, D.C.: ASM Press.
25. de la ChauxN, TsuchimatsuT, ShimizuKK, WagnerA (2012) The predominantly selfing plant Arabidopsis thaliana experienced a recent reduction in transposable element abundance compared to its outcrossing relative Arabidopsis lyrata. Mob DNA 3 : 2.
26. MatzkeMA, MetteMF, MatzkeAJM (2000) Transgene silencing by the host genome defense: implications for the evolution of epigenetic control mechanisms in plants and vertebrates. Plant Mol Biol 43 : 401–415.
27. ZilbermanD, GehringM, TranRK, BallingerT, HenikoffS (2007) Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39 : 61–69.
28. KuriharaY, MatsuiA, KawashimaM, KaminumaE, IshidaJ, et al. (2008) Identification of the candidate genes regulated by RNA-directed DNA methylation in Arabidopsis. Biochem Biophys Res Commun 376 : 553–557.
29. LocktonS, GautBS (2010) The evolution of transposable elements in natural populations of self-fertilizing Arabidopsis thaliana and its outcrossing relative Arabidopsis lyrata. BMC Evol Biol 2010/01/14 ed. pp. 10.
30. PereiraV (2004) Insertion bias and purifying selection of retrotransposons in the Arabidopsis thaliana genome. Genome Biol 2004/10/06 ed. pp. R79.
31. DevosKM, BrownJK, BennetzenJL (2002) Genome size reduction through illegitimate recombination counteracts genome expansion in Arabidopsis. Genome Res 12 : 1075–1079.
32. LeQH, WrightS, YuZ, BureauT (2000) Transposon diversity in Arabidopsis thaliana. Proc Natl Acad Sci USA 97 : 7376–7381.
33. SabotF, PicaultN, El-BaidouriM, LlauroC, ChaparroC, et al. (2011) Transpositional landscape of the rice genome revealed by paired-end mapping of high-throughput re-sequencing data. Plant J 66 : 241–246.
34. LocktonS, Ross-IbarraJ, GautBS (2008) Demography and weak selection drive patterns of transposable element diversity in natural populations of Arabidopsis lyrata. Proc Natl Acad Sci USA 105 : 13965–13970.
35. HuangX, LuG, ZhaoQ, LiuX, HanB (2008) Genome-wide analysis of transposon insertion polymorphisms reveals intraspecific variation in cultivated rice. Plant Physiol 148 : 25–40.
36. VaughnMW, TanurdzicM, LippmanZ, JiangH, CarrasquilloR, et al. (2007) Epigenetic natural variation in Arabidopsis thaliana. PLoS Biol 5: e174 doi:10.1371/journal.pbio.0050174.
37. KashkushK, FeldmanM, LevyAA (2003) Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat Genet 33 : 102–106.
38. ZhangX, ShiuSH, CalA, BorevitzJO (2008) Global analysis of genetic, epigenetic and transcriptional polymorphisms in Arabidopsis thaliana using whole genome tiling arrays. PLoS Genet 4: e1000032 doi:10.1371/journal.pgen.1000032.
39. QuesnevilleH, NouaudD, AnxolabehereD (2005) Recurrent recruitment of the THAP DNA-binding domain and molecular domestication of the P-transposable element. Mol Biol Evol 22 : 741–746.
40. CasacubertaE, PardueML (2003) Transposon telomeres are widely distributed in the Drosophila genus: TART elements in the virilis group. Proc Natl Acad Sci USA 100 : 3363–3368.
41. JordanIK, RogozinIB, GlazkoGV, KooninEV (2003) Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet 19 : 68–72.
42. BennetzenJL (2000) Transposable element contributions to plant gene and genome evolution. Plant Mol Biol 42 : 251–269.
43. BeckerC, HagmannJ, MullerJ, KoenigD, StegleO, et al. (2011) Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature 480 : 245–249.
44. LuJ, ZhangC, BaulcombeDC, ChenZJ (2012) Maternal siRNAs as regulators of parental genome imbalance and gene expression in endosperm of Arabidopsis seeds. Proc Natl Acad Sci USA 109 : 5529–5534.
45. WarneforsM, PereiraV, Eyre-WalkerA (2010) Transposable elements: insertion pattern and impact on gene expression evolution in hominids. Mol Biol Evol 27 : 1955–1962.
46. MochizukiK, GorovskyMA (2004) Small RNAs in genome rearrangement in Tetrahymena. Curr Opin Genet Dev 14 : 181–187.
47. MochizukiK, FineNA, FujisawaT, GorovskyMA (2002) Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in tetrahymena. Cell 110 : 689–699.
48. WrightSI, AgrawalN, BureauTE (2003) Effects of recombination rate and gene density on transposable element distributions in Arabidopsis thaliana. Genome Res 13 : 1897–1903.
49. MontgomeryEA, HuangSM, LangleyCH, JuddBH (1991) Chromosome rearrangement by ectopic recombination in Drosophila melanogaster: genome structure and evolution. Genetics 129 : 1085–1098.
50. SchlenkeTA, BegunDJ (2004) Strong selective sweep associated with a transposon insertion in Drosophila simulans. Proc Natl Acad Sci USA 101 : 1626–1631.
51. MillerWJ, McDonaldJF, NouaudD, AnxolabehereD (1999) Molecular domestication–more than a sporadic episode in evolution. Genetica 107 : 197–207.
52. NaitoK, ZhangF, TsukiyamaT, SaitoH, HancockCN, et al. (2009) Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature 461 : 1130–1134.
53. Le RouzicA, CapyP (2006) Population genetics models of competition between transposable element subfamilies. Genetics 174 : 785–793.
54. Le RouzicA, BoutinTS, CapyP (2007) Long-term evolution of transposable elements. Proc Natl Acad Sci USA 104 : 19375–19380.
55. ItoH, GaubertH, BucherE, MirouzeM, VaillantI, et al. (2011) An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 472 : 115–119.
56. ClarkRM, SchweikertG, ToomajianC, OssowskiS, ZellerG, et al. (2007) Common Sequence Polymorphisms Shaping Genetic Diversity in Arabidopsis thaliana. Science 317 : 338–342.
57. NaouarN, VandepoeleK, LammensT, CasneufT, ZellerG, et al. (2009) Quantitative RNA expression analysis with Affymetrix Tiling 1.0R arrays identifies new E2F target genes. Plant J 57 : 184–194.
58. LaubingerS, ZellerG, HenzSR, SachsenbergT, WidmerCK, et al. (2008) At-TAX: a whole genome tiling array resource for developmental expression analysis and transcript identification in Arabidopsis thaliana. Genome Biol pp. R112.
59. BenjaminiY, DraiD, ElmerG, KafkafiN, GolaniI (2001) Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125 : 279–284.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




