-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
Single-stranded DNA binding proteins (SSBs) regulate multiple DNA transactions, including replication, transcription, and repair. We recently identified SSB1 as a novel protein critical for the initiation of ATM signaling and DNA double-strand break repair by homologous recombination. Here we report that germline Ssb1−/− embryos die at birth from respiratory failure due to severe rib cage malformation and impaired alveolar development, coupled with additional skeletal defects. Unexpectedly, Ssb1−/− fibroblasts did not exhibit defects in Atm signaling or γ-H2ax focus kinetics in response to ionizing radiation (IR), and B-cell specific deletion of Ssb1 did not affect class-switch recombination in vitro. However, conditional deletion of Ssb1 in adult mice led to increased cancer susceptibility with broad tumour spectrum, impaired male fertility with testicular degeneration, and increased radiosensitivity and IR–induced chromosome breaks in vivo. Collectively, these results demonstrate essential roles of Ssb1 in embryogenesis, spermatogenesis, and genome stability in vivo.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003298
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003298Summary
Single-stranded DNA binding proteins (SSBs) regulate multiple DNA transactions, including replication, transcription, and repair. We recently identified SSB1 as a novel protein critical for the initiation of ATM signaling and DNA double-strand break repair by homologous recombination. Here we report that germline Ssb1−/− embryos die at birth from respiratory failure due to severe rib cage malformation and impaired alveolar development, coupled with additional skeletal defects. Unexpectedly, Ssb1−/− fibroblasts did not exhibit defects in Atm signaling or γ-H2ax focus kinetics in response to ionizing radiation (IR), and B-cell specific deletion of Ssb1 did not affect class-switch recombination in vitro. However, conditional deletion of Ssb1 in adult mice led to increased cancer susceptibility with broad tumour spectrum, impaired male fertility with testicular degeneration, and increased radiosensitivity and IR–induced chromosome breaks in vivo. Collectively, these results demonstrate essential roles of Ssb1 in embryogenesis, spermatogenesis, and genome stability in vivo.
Introduction
Appropriate and timely repair of damaged DNA is critical for maintaining genomic integrity and tumour suppression [1], [2]. DNA double-strand breaks (DSBs) are the most cytotoxic genomic lesions, and can arise from exogenous genotoxic insult, stalled replication forks, or during physiological processes such as meiosis and B and T cell maturation. Organisms have evolved two main pathways for DSB repair: non-homologous end joining (NHEJ) and homologous recombination (HR). In the initial step of HR, DSBs are resected to generate 3′ single-stranded DNA (ssDNA) tails. The ssDNA intermediates are protected from further degradation by ssDNA-binding proteins (SSBs).
The SSB family of proteins are conserved in all three kingdoms of life [3] and are characterised structurally by their oligonucleotide-binding (OB) folds that bind ssDNA. SSB proteins can be subdivided into two sub-groups. First, simple SSBs, typified by the Escherichia coli (E. coli) SSB, contain a single OB-fold. The second sub-group includes the higher ordered replication protein A (RPA), which contains multiple OB-folds and is conserved in yeast and higher eukaryotes [3]. Human RPA is a heterotrimeric polypeptide, widely believed to be a central component of both DNA replication and DNA repair pathways [4], [5], [6]. Recently, we identified two novel SSB proteins, named SSB1 (also known as OBFC2B, NABP2 or SSOS-B1) and SSB2 (also known as OBFC2A, NABP1 or SOSS-B2) [7], which are conserved in vertebrates but not in lower eukaryotes. These SSBs are more closely related to the bacterial and archaeal SSB sub-group than to RPA [3]. Both SSBs encode a conserved single OB-fold followed by a divergent spacer domain and a conserved C-terminal motif, suggesting functional overlap between these proteins. The spacer region is the only significant difference between human SSB1 and SSB2.
Our functional characterization of SSB1 revealed that it is stabilised following exposure of cells to ionizing radiation (IR) forming distinct foci at DSB sites [7]. Depletion of SSB1 compromises the DNA damage checkpoints and HR, resulting in an increased sensitivity to IR. Further studies showed that human SSB1 and SSB2 exist in two separate sub-complexes that also contain IntS3 and C9orf80 (also known as SSBIP1/MISE) [8], [9], [10], [11]. Similar to depletion of SSB1, silencing of INTS3 and C9orf80 results in defects in ATM signalling and HR as well as hypersensitivity to IR [8], [9], [10].
Here, we describe the generation of Ssb1 knockout mice to define the physiological role of Ssb1. We report that germline deficiency for Ssb1 causes perinatal lethality due to aberrant rib-cage formation and improper lung differentiation. Furthermore, conditional knockout of Ssb1 in adult mice leads to reduced fertility in male mice, increased sensitivity to γ-irradiation and a predisposition to tumorigenesis. Taken together, our data demonstrate that Ssb1 is essential for embryogenesis and the maintenance of genomic stability in vivo.
Results
Ssb1 deficiency results in perinatal lethality
The murine Ssb1 gene is located on chromosome 10 and spans 7 exons. We engineered a “floxed” Ssb1 allele with unidirectional loxP sites flanking its major protein coding exons 3–6, including the OB-fold domain critical for its DNA binding activity (Figure S1A). Correct targeting was confirmed by Southern blot (Figure S1B) and genotyping PCR (Figure S1C). Evaluation of the growth of Ssb1 heterozygous mice (Ssb1+/−) relative to wild-type littermates (Ssb1+/+) revealed no apparent physiological abnormalities in Ssb1+/− mice monitored for up to 2 years. To generate mice with targeted deletion of Ssb1, we intercrossed Ssb1+/− breeding pairs, with the expectation that approximately 25 percent of the offspring would be of an Ssb1−/− genotype. Interestingly, no viable Ssb1−/− mice were detected amongst more than one hundred offspring from these intercrosses genotyped at 12 days post-partum (Table I). These results suggested that Ssb1 deletion might result in lethality during embryogenesis.
Tab. 1. Impact of deletion of <i>Ssb1</i> on embryonic survival in <i>Ssb1</i><sup>+/−</sup> intercrosses. 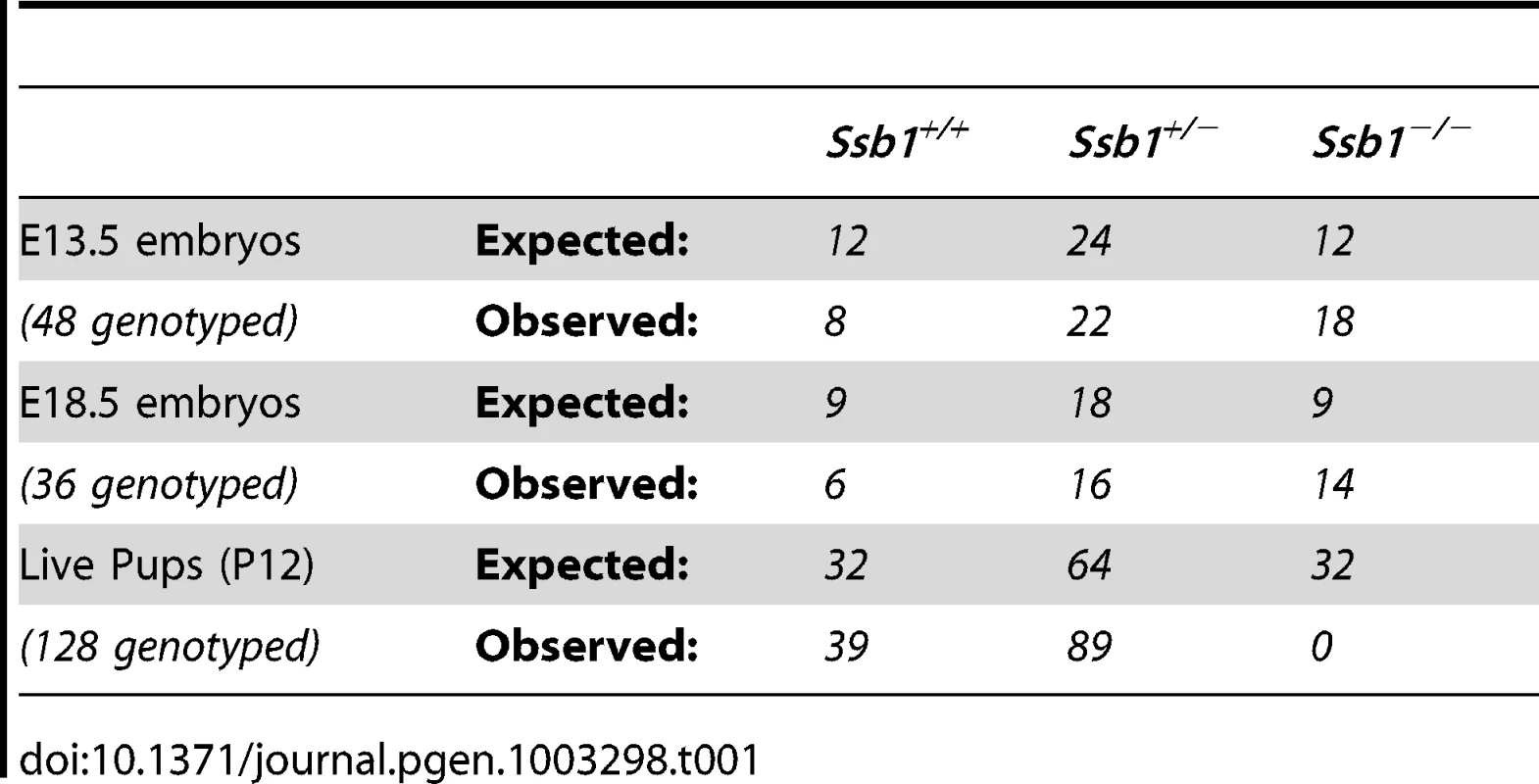
In order to define the time point of embryonic lethality caused by Ssb1 ablation, we collected embryos from Ssb1+/− intercrosses at different gestational days, assessed by the presence of a vaginal plug at E0.5. Ssb1−/− embryos were recovered at near-Mendelian ratios at E13.5 and E18.5 (Table I), but were significantly growth retarded in terms of both body weight and length at the latter time-point, when compared to wild-type and heterozygous littermates (Figure 1A, 1B; Figure S2A, S2B). Ssb1+/+ and Ssb1+/− embryos were morphologically indistinguishable, in terms of both body size and body length. Ssb1−/− embryos also displayed craniofacial abnormalities, including a recessed mandible (lower jaw) and misshapen snout (Figure 1A, arrowheads; Figure 2C, 2D, Figure S2C). Furthermore, there was a defect in the outgrowth of both fore - and hindlimbs, as well as hindlimb-specific oligodactyly (missing digits) (Figure 1A, arrows). However, these embryos appeared otherwise grossly normal, suggesting that Ssb1 ablation may cause lethality during the perinatal period.
Fig. 1. Ssb1 deletion causes perinatal lethality due to severe respiratory failure. 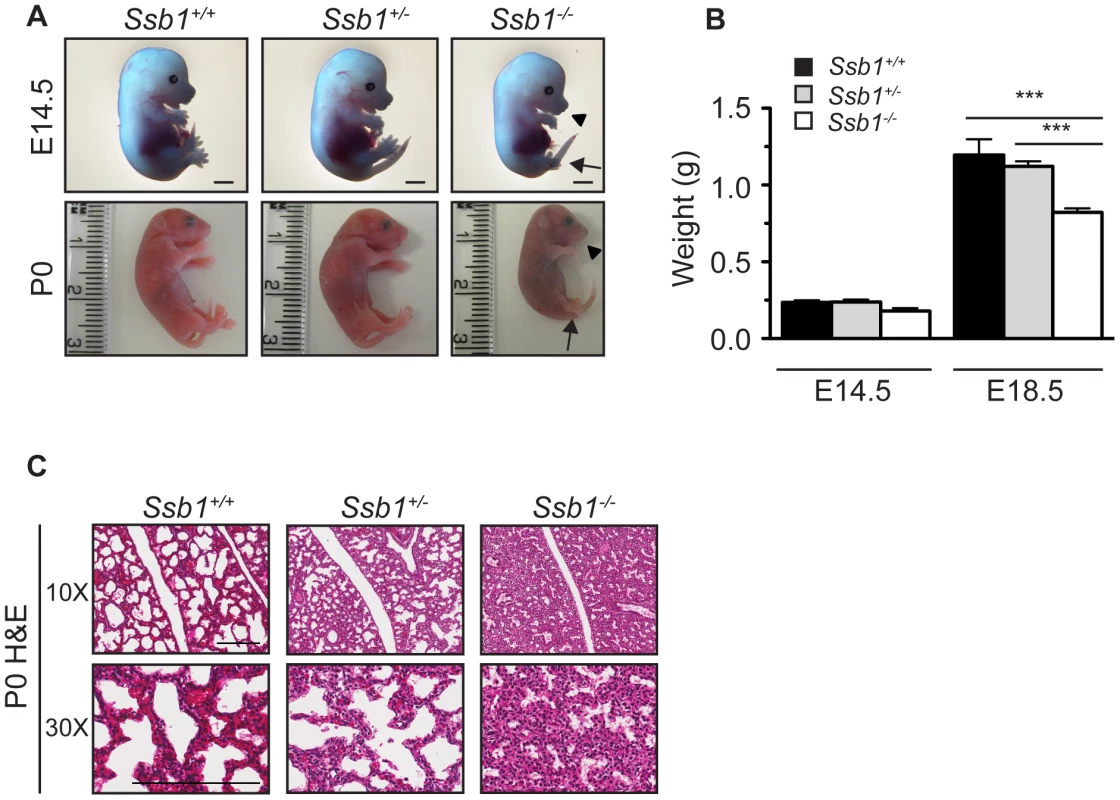
(A) Comparison of size and appearance of E14.5 and P0 Ssb1+/+, Ssb1+/−, and Ssb1−/− offspring. Note the cyanosis in P0 Ssb1−/− pups. Scale = 2 mm. (B) Comparison of weights of E14.5 and E18.5 Ssb1+/+, Ssb1 +/− and Ssb1−/− embryos. Data represent the mean ± SEM, n = 3–16 embryos per group from a minimum of 3 litters per timepoint (***P<0.001; student's t-test). (C) Haematoxylin and eosin staining of P0 lungs of Ssb1+/+, Ssb1+/− and Ssb1−/− pups delivered by caesarian section. Fig. 2. Ssb1 deletion causes multiple skeletal defects. 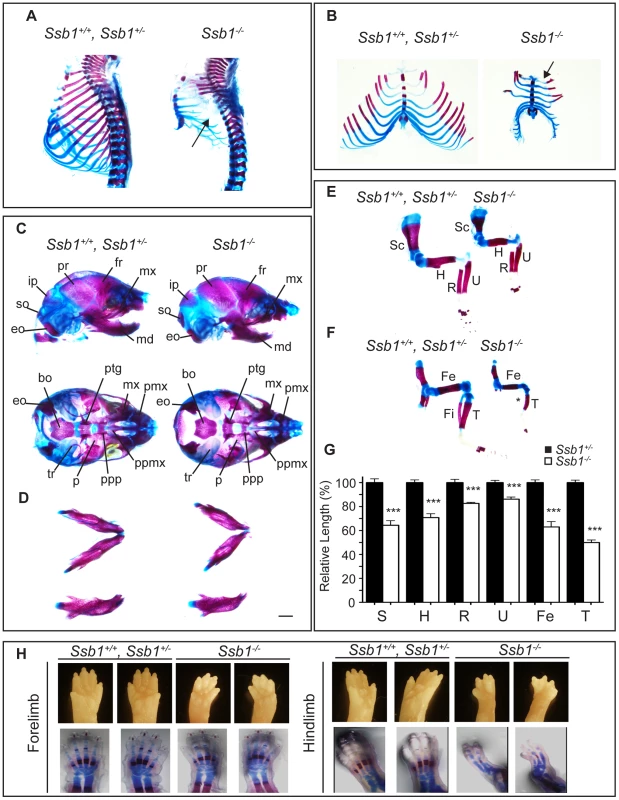
(A) Alcian blue and Alizarin red staining of control (Ssb1+/+, Ssb1+/−) and Ssb1−/− E18.5 ribcages. (B) Butterfly rib-spread of Ssb1 control and Ssb1−/− E18.5 ribcages. (C) Comparison of skull anatomy of Ssb1 control and Ssb1−/− E18.5 embryos. eo, exoccipital; so, supraoccipital; ip interparietal; pr, parietal; fr, frontal; mx, maxilla; md, mandible; bo, basioccipital; ptg, pterygoid; pmx, premaxilla; ppmx, palatal process maxilla; ppp, palatal process palatine; p, palatine; tr, tympanic ring. (D) Comparison of Ssb1 control and Ssb1−/− mandibles (Scale = 1 mm). (E) Forelimb and (F) Hindlimb of E18.5 Ssb1 control and Ssb1−/− embryos. Sc, scapula; H, humerus; R, radius; U, ulna; F, femur; T, tibia. * designates missing fibula. (G) Quantification of long bone measurements of the forelimb and hindlimb of Ssb1+/− and Ssb1−/− E18.5 limbs. Data represent the mean ± SEM of bone length (n = 3 per condition, ***P<0.001, student's t-test). (H) Whole-autopod (top) and skeletal preparation (bottom) of E18.5 forelimbs (left) and hindlimbs (right) from Ssb1 control and Ssb1−/− embryos. To further investigate the cause of Ssb1−/− lethality, we performed caesarian recovery of embryos at E18.5 or at the time of birth (P0), and stimulated breathing by clearing the facial orifices and gentle stroking of the snout. In the litters examined, all Ssb1+/+ and Ssb1+/− pups established rhythmic breathing, a healthy pink skin color and movement within minutes. However, Ssb1−/− pups rapidly became asphyxic and typically died between 10∼30 min post caesarian excision, despite taking short, sporadic gasping breaths, suggesting that they could not breathe and oxygenate their blood properly (Figure 1A, Figure S2A). Haematoxylin and eosin (H&E) staining on these embryos suggested that atelectasis was the primary cause of respiratory failure (Figure 1C). These results suggest that Ssb1−/− embryos survive the entire course of development in utero but die at the perinatal stage.
Ssb1 ablation results in aberrant skeletal patterning
To further investigate the abnormalities we observed in the craniofacial region and hindlimb of Ssb1−/− embryos, we next sought to determine if their skeletal architecture was altered by performing whole-mount cartilage and mineralized bone staining with alcian blue and alizarin red. Strikingly, we observed a number of defects in formation of both the axial and appendicular skeleton. Most notably, the ribcage of Ssb1−/− embryos was poorly formed, small in size, and exhibited an almost complete lack of ossification when compared to control littermates (Ssb1+/+, Ssb1+/−) (Figure 2A). This defect led to the appearance of “floating ribs”, with no evidence of ossification in all but the four most anterior rib pairs. In addition, the ribcage of Ssb1−/− embryos was misshapen, with a lack of curvature in the anterior ribs, and horizontally orientated rib-sternum attachments (Figure 2A, Figure 2B, arrow). The more posterior “floating” ribs in these embryos were also rudimentary and abnormally shaped, contributing to a general decrease in size of the rib-cage (Figure 2A, Figure 2B). The lack of structural support from the misshapen and poorly developed rib-cage in Ssb1−/− embryos would have significantly contributed to the respiratory distress evident in these embryos at birth, and resulted in rapid atelectasis and perinatal death.
Examination of the skull of E18.5 embryos revealed normal formation of major bone structures, including the parietal (pr), intraparietal (ip), frontal (fr) and supraoccipital (so) bones. We noted a modest elongation of the premaxillary bone (pmx), consistent with the pointed snout seen in these embryos, and a shortened mandible (micrognathia), which was set at a wider angle than in control embryos (Ssb1+/+, Ssb1+/−) (Figure 2C, 2D). The tympanic ring (tr), which supports the eardrum, was also poorly formed in Ssb1−/− embryos (Figure 2C). Furthermore, we observed evidence of a variably penetrant cleft palate (n = 2 of 5 embryos), which was evident even between Ssb1−/− mice of the same litter (Figure S2D–S2F; arrows, arrowheads). Together, these data suggest a spectrum of craniofacial abnormalities in Ssb1−/− embryos.
The limb skeleton of Ssb1−/− E18.5 embryos showed a significant decrease in the length of all long bones, including humerus, radius, ulna, femur and tibia, as well as the scapula (Figure 2E–2G), indicating a limb outgrowth defect (***P<0.001, Figure 2G). Overall, this phenotype was more pronounced in the hindlimbs, where we observed varying degrees of abnormalities in these structures, including absent fibulas (Figure 2F). Finally, although the forelimbs of Ssb1−/− embryos were properly patterned (albeit smaller in size), hindlimbs displayed aberrant bone mineralization and severe defects in patterning along the anterior-posterior axis, which always manifested as oligodactyly (Figure 2H). Interestingly, this phenotype was variable in penetrance, with between two to a maximum of four digits present, and we often observed variation of patterning defects between the left and right hindlimb autopods within the one embryo. Taken together, these data indicate that Ssb1 is necessary for skeletogenesis and hindlimb digit specification in the embryo, and that it is of particular importance for the later steps of chondrogenesis involving bone ossification. These data highlight a novel and unexpected role for Ssb1 during embryogenesis.
Ssb1−/− embryos exhibit distal lung differentiation defects
To determine if other causative factors may have contributed to the perinatal lethality in Ssb1−/− embryos, we next performed histological analysis of sagittal sections from E18.5 embryos. We observed grossly normal morphology for major organs including the brain, heart, thymus, intestine, and liver (Figure S3). However, consistent with the respiratory distress phenotype, we observed immature lung morphology in these sections (Figure S3). To more closely examine this, we dissected lungs from E18.5 Ssb1+/+, Ssb1+/− and Ssb1−/− embryos (Figure 3A) and confirmed complete deletion of the Ssb1 protein by western blot (Figure 3B). Interestingly, we also noted an increase in the protein level of Ssb2 in Ssb1−/− lungs (Figure 3B), similar to what has been observed based on siRNA depletion in human cells [8], [9], [10]. A comparison of the gross morphology of the lungs revealed that the lungs of Ssb1−/− embryos were consistently smaller than their Ssb1+/+ and Ssb1+/− counterparts when measured in terms of lobe length and width (Figure 3A, data not shown), although this was in proportion to the overall growth retardation in these embryos. In addition, lungs of Ssb1−/− embryos were correctly lobulated, with four right lobes and a single left lobe flanking the heart, suggesting that early lung development patterning in these embryos is intact (Figure 3A). However, H&E analysis on coronal sections of these lungs revealed aberrant late-stage lung development, with reduced alveolar lumens and thickened, hypercellular alveolar walls in Ssb1−/− lungs when compared to control (Ssb1+/− and Ssb1+/−) littermates (Figure 3C–3E ***P<0.001). During lung development, regression of the mesenchyme occurs from approximately E15.5 onwards by apoptosis to form the air-blood barrier, necessary for efficient respiration. To determine if the higher cell density in Ssb1−/− lungs results from either a decrease in apoptosis during development or increased proliferation, we performed immunohistological staining on E14.5 and E18.5 lung sections for ApopTag and Ki67, respectively. However, no differences in the levels of Ki67 or ApopTag were observed at these developmental stages (Figure S4).
Fig. 3. Distal lung differentiation defects in Ssb1−/− embryos. 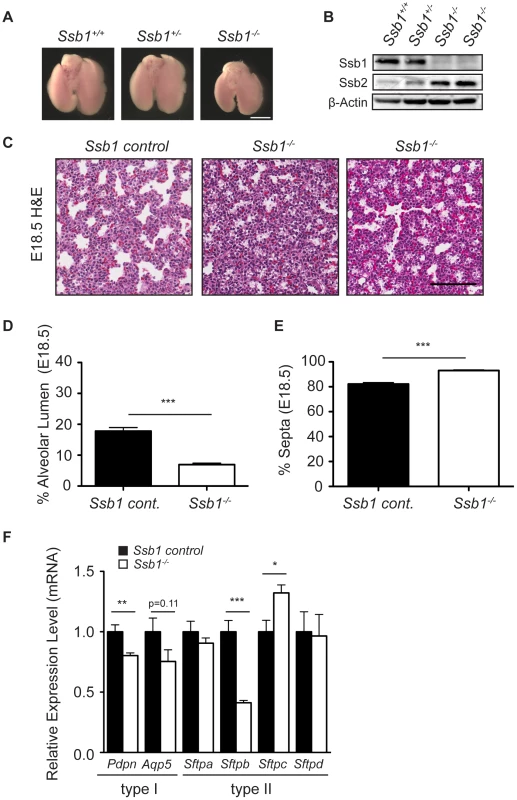
(A) Representative image showing the comparison of lung size and morphology of E18.5 Ssb1+/+, Ssb1+/− and Ssb1−/− embryos. Scale bar = 2 mm. (B) Protein level of Ssb1, Ssb2 and Actin in Ssb1+/+, Ssb1+/− and Ssb1−/− E18.5 lungs. (C) Haematoxylin and eosin staining showing morphology of alveolar lumen formation and intra-alveolar septae thickness in Ssb1 control (Ssb1+/+, Ssb1+/−) and Ssb1−/− E18.5 embryos. (D) Quantitation of alveolar lumen area and (E) intra-alveolar septa area in Ssb1 control (Ssb1+/+, Ssb1+/−) and Ssb1−/− embryos. Data represent the mean ± SEM (n = 3 control and 4 Ssb1−/− embryos from 2 litters. ***P<0.001, student's t-test). (F) qRT-PCR of Type I and Type II alveolar epithelial cell markers in Ssb1 control (Ssb1+/+, Ssb1+/−) and Ssb1−/− E18.5 lungs. Data represent mean ± SEM from 4 embryos per condition (*P<0.05, **P<0.01,***P<0.001; student's t-test). Perinatal death due to respiratory failure can be caused by impaired differentiation of the proximal and/or distal airway epithelium. To determine if proximal airway epithelium was properly differentiated, we examined levels of Cc10 (also known as Scgb1a1/Ccsp), a marker for secretory Clara cells, as well as Foxj1 (also known as Hfh-4), a marker of ciliated epithelial cells in the proximal epithelium by quantitative real-time PCR (qPCR) in Ssb1 control (Ssb1+/+, Ssb1+/−, n = 4) and Ssb1−/− (n = 4) lung tissue at E18.5. In addition, we also examined transcript levels of Cd31, a marker of endothelial cells. These analyses revealed no significant differences in the mRNA levels of these markers, suggesting that both proximal airway differentiation and blood vessel formation of Ssb1−/− lungs are intact (Figure S5A). Furthermore, immunohistological staining of smooth muscle actin revealed normal bronchi and bronchioli development in these embryos (Figure S5B). Next, we examined differentiation of the distal saccules which contain alveolar epithelial type I and type II cells (AECs), responsible for gas exchange and the maintenance of surface tension through surfactant protein secretion, respectively. To determine if Ssb1−/− embryos exhibited defective differentiation in either of these cell types, we performed qPCR on E18.5 control and Ssb1−/− embryos to assess the transcript levels of Aqp5 and Pdpn, as markers of type I AECs, as well as the surfactant protein transcripts Sftpa, Sftpb, Sftpc and Sftpd, as markers of type II AECs. Although Sftpa and Sftpd were unaffected by Ssb1 ablation, we observed a −2.4 fold change in Sftpb expression, suggesting aberrant type II AEC differentiation (***P<0.001, Figure 3F). Notably, deletion of Sftpb in the mouse has been shown to result in severe neonatal respiratory distress syndrome, and is the only surfactant protein that is indispensable for neonatal survival [12], [13], [14]. In addition to the decrease in Sftpb levels, we also observed a smaller (−1.25 fold) change in Pdpn, a type I AEC marker (**P<0.01), as well as small, but statistically non-significant decrease in Aqp5, another type I AEC marker (Figure 3F). As type II cells are thought to trans-differentiate to type I cells, this may be a secondary effect of improper type II AEC differentiation [15], [16]. Interestingly, we also observed a 1.3 fold increase in Sftpc mRNA (*P = 0.01, Figure 3F). As pro-SPC is expressed from E11.5 to E17.5 in lung epithelial progenitor cells, the relative increase in this transcript may simply represent developmental immaturity of Ssb1−/− lungs [16]. This is in accordance with blinded assessment by an independent pathologist, who observed an increase of immature type II AECs in the lungs of Ssb1−/− P0 embryos. These data indicate that Ssb1 is necessary for proper lung differentiation in the late stages of embryogenesis. Taken together, our results point to an important and novel role of Ssb1 in skeletal and lung differentiation.
Ssb1 is not required for the response to DNA double-strand breaks in mouse embryonic fibroblasts or during class switch recombination
Mouse embryonic fibroblasts (MEFs) from Ssb1+/+ and Ssb1−/− E13.5 embryos were isolated to investigate the role of Ssb1 in DSB repair and signaling in the mouse. Early passage Ssb1+/+ and Ssb1−/− MEFs exhibited similar cell-cycle profiles, but Ssb1−/− MEFs had a slightly diminished proliferative capacity and more rapidly reached the plateau phase when compared with Ssb1+/+ MEFs (Figure S6A, S6B). As we and others had previously described a role of SSB1 in the activation of ATM signaling in response to IR based on siRNA depletion in human cells [7], [9], [10], we assessed activation of this pathway in MEFs. Although we observed stabilization of Ssb1 in response to IR, interestingly, no attenuation of Atm activation was detected when we assessed autophosphorylation of Atm on serine1987 (serine1981 in human) or phosphorylation of its downstream activation target p53 on serine18 (serine15 in human) (Figure S6C). Similar to what we observed in Ssb1−/− lungs, Ssb2 protein levels were upregulated in Ssb1−/− MEFs. These results suggest that deletion of Ssb1 does not abrogate Atm activation in MEFs, and may highlight potential redundancy between Ssb1 and Ssb2 in these cells. To determine if the response to ionizing radiation was intact in Ssb1−/− cells, we also assessed the dynamics of γ-H2ax foci formation in Ssb1+/+ and Ssb1−/− MEFs by immunofluorescence. These studies revealed no significant differences in the baseline level of γ-H2ax foci induction nor in the clearance of IR-induced γ-H2ax foci (Figure S6D, S6E), indicating that these cells did not exhibit higher levels of endogenous DNA damage and/or defective repair of IR-induced DSBs.
Next, we sought to utilise an in vivo model of DSB repair to interrogate if Ssb1 is necessary for DSB repair in the mouse. Class switch recombination (CSR) involves a programmed Ig heavy gene rearrangement in B-lymphocytes that requires repair of physiological DSBs generated as a result of activation-induced deaminase (AID) catalysed DNA base damage. In B-lymphocytes, the initial secreted antibodies contain heavy chains of the IgM class (or IgD formed via alternative splicing). Upon stimulation of these B-lymphocytes by antigen, the original IgM class heavy chain gene undergoes CSR to encode heavy chains of IgG, IgE, or IgA classes [17]. Several proteins involved in DSB repair including ATM, H2AX and 53BP1 have been suggested to have a role in CSR, to different extents, probably due to their roles in synapsis and/or DNA repair [17].
To assess whether loss of Ssb1 affects CSR, we generated B cell specific conditional Cd19-Cre+: Ssb1−/− mice. Western blotting of whole cell extracts showed loss of Ssb1 protein in B cells from Cd19-Cre+: Ssb1−/− mice (Figure 4A) and upregulation of Ssb2 protein levels (Figure 4B), similar to what we observed in Ssb1−/− lungs and MEFs (Figure 3B, Figure S6C). Mice lacking Ssb1 in the B lineage produced normal numbers of mature IgM+ lymphocytes in the bone marrow and had spleens of normal size and cellularity. Upon in vitro stimulation of B cells isolated from spleens with anti-CD40 antibody plus IL-4 over 3 days, the extent of IgM to IgG1 switching and cell viability in wild-type and Ssb1-deficient B cells was also comparable (Figure 4C, 4D). No difference was found in the percentage and total numbers of direct or microhomology-mediated joins in switch region junctions from IL4 plus anti-CD40 stimulated primary Ssb1−/− B cells and wild-type B cells (Figure 4E). These results suggest that Ssb1 is dispensable for DSB repair by class-switch recombination.
Fig. 4. Class switch recombination activity in B-cell specific Ssb1-deleted mice. 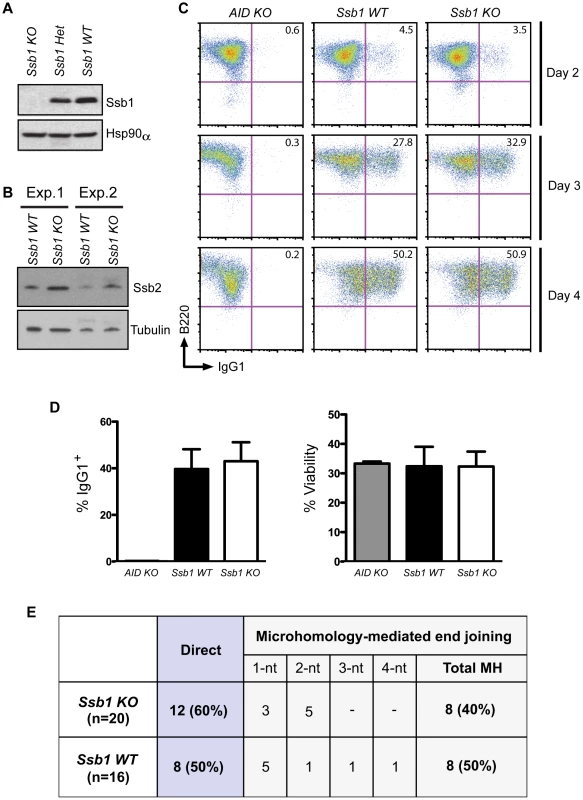
Ssb1 fl/fl mice were crossed with a Cd19-Cre transgene expressing C57BL/6J mice to specifically delete Ssb1 in B cells. Splenic B cells were isolated and stimulated for 2, 3, and 4 days using anti-Cd40 antibodies plus IL-4 to induce CSR to IgG1. (A) Western blotting of whole cell extracts showed loss of Ssb1 protein in stimulated B cells from Ssb1 knockout (Ssb1 KO; Cd19Cre+: Ssb1−/−) mice. Equal amounts of stimulated B cell extracts from heterozygous Ssb1 (Ssb1 Het; Cd19Cre+: Ssb1+/−) and wild-type Ssb1 (Ssb1 WT; Cd19Cre+: Ssb1+/+) mice were included for comparison. Equal loading was confirmed by probing for Hsp90α. (B) Western blotting of Ssb2 levels in B cells from Ssb1 WT and Ssb1 KO mice. (C) FACS analysis of CSR to IgG1 over time in stimulated B cells from mice of the indicated genotypes. Stimulated splenic B cells from AID−/− (AID KO) mice served as a negative control. (D) Summary statistics of CSR activity to IgG1 and viability on day 3 of stimulation. Mean and S.E.M. from three independent experiments are shown. No statistically significant differences (two-tailed unpaired t-test) were found. (E) Switch region junction analysis. Sm-Sg1 junctions were amplified from IL4 plus anti-CD40 stimulated primary B cells (day 4) and sequenced. Percentage and total numbers of direct or microhomology-mediated joints are indicated. nt, nucleotides. Conditional Ssb1 gene deletion in adult mice
Given the perinatal lethality we observed in constitutive Ssb1−/− mice, we next employed a conditional approach to ubiquitously ablate Ssb1 postnatally using a tamoxifen-inducible Cre system by interbreeding Ssb1fl/fl mice with the Rosa26-CreERT2 strain (Figure S7) [18]. Efficiency of Ssb1 deletion in adult mice (4 weeks old) following a series of tamoxifen injections was confirmed by both PCR for genomic recombination, and western blot analysis for protein depletion in various tissues (Figure S8A–S8C). The floxed Ssb1 allele was efficiently deleted in bone marrow (BM), thymus, spleen, testes and small intestine, partially deleted in lung, kidney, liver and heart, but not in the brain (Figure S8A). Dramatically decreased Ssb1 protein levels were confirmed in multiple tissue samples from tamoxifen induced Rosa26-CreERT2: Ssb1−/− mice, with undetectable levels of Ssb1 protein in splenocytes and thymocytes as early as 10 days after the final tamoxifen induction (Figure S8B). Interestingly, we observed a dramatic up-regulation of Ssb2 in response to Ssb1 ablation in the bone marrow and spleen, but not in the testes and thymus of Rosa26-CreERT2: Ssb1−/− mice (Figure S8C).
Impaired fertility in conditional Rosa26-CreERT2: Ssb1−/− male mice
Monitoring of Rosa26-CreERT2: Ssb1−/− mice and control Rosa26-CreERT2: Ssb1+/− mice revealed no significant differences in body weight over a period of up to 90 weeks (Figure S9). In addition, histological analysis of all major organs, including the brain, thymus, lung, heart, liver, kidney and small intestine revealed no gross abnormalities. The abrogation of many DNA repair factors, (such as Atm [19], H2ax [20], Mdc1 [21] and Mcph1/Brit1 [22]) has been shown to result in impaired fertility, due to important roles of these proteins in DSB repair during meiosis. To determine the impact of Ssb1 deficiency on fertility, we examined ovaries and testes of Rosa26-CreERT2: Ssb1−/− mice six weeks after induction with tamoxifen. Whereas Rosa26-CreERT2: Ssb1−/− ovaries were morphologically normal in females, the testes from Rosa26-CreERT2: Ssb1−/− males were reduced in size (Figure 5A), in terms of both absolute weight (n = 8, ***P<0.001, Figure 5B) and gonado-somatic index (GSI) [23], an indicator of gonad weight as a proportion of total body mass (n = 8, ***P<0.001, Figure 5C), when compared to their Rosa26-CreERT2: Ssb1+/− littermates.
Fig. 5. Testicular degeneration and impaired fertility in conditional Rosa26-CreERT2: Ssb1−/− male mice. 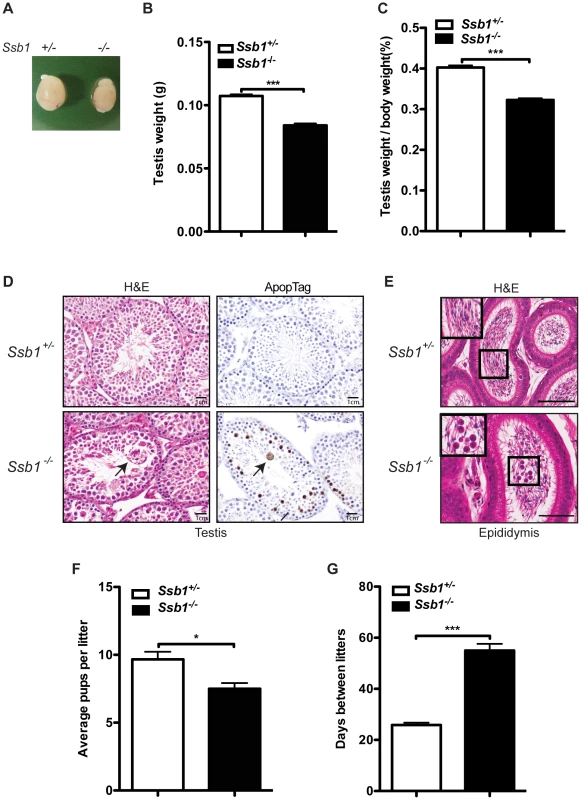
(A) Representative image of testes from Rosa26-CreERT2: Ssb1−/− mice compared with those from Rosa26-CreERT2: Ssb1+/− littermates at 10 weeks of age. (B) Testis weight and (C) the gonado-somatic index (GSI) in conditional Ssb1 deleted male mice compared with their littermates. Data represents the mean ± SEM of testis weight (n = 8, ***P<0.001; student's t-test) and the GSI (C) from 8 mice in each group. (D) Representative images of testis sections stained with Haematoxylin and eosin (left panels) and ApopTag for the detection of apoptotic cells (right panels). Note the multinucleated giant cells (black arrow) frequently present in the lumen in the testes from Rosa26-CreERT2: Ssb1−/− mice. Spermatogenic cells in Rosa26-CreERT2: Ssb1−/− testes showed an elevated apoptotic marker (black arrows, lower panel, right, Scale = 25 µm). (E) Haematoxylin and eosin staining showing epididymides of Rosa26-CreERT2: Ssb1−/− mice containing prematurely sloughed developing germ cells. The upper panel displays maturing spermatozoa in the wild type epididymis. In contrast, immature germ cells (black arrow) are present in the epididymis of Rosa26-CreERT2: Ssb1−/− mice (lower panel). Embedded images in the left corners show magnified views of the selected areas (Scale = 50 µm). (F) Litter size from Rosa26-CreERT2: Ssb1−/− male breeders (n = 4) with wild type female mice compared with those from Rosa26-CreERT2: Ssb1+/− breeders (n = 6, *P<0.05; student's t-test). (G) Litter interval in Rosa26-CreERT2: Ssb1−/− male mice (average of 63 days, n = 4) and Rosa26-CreERT2: Ssb1+/− males (average of 27 days, n = 6, ***P<0.001; student's t-test). Histological examination of testes from 3-month-old Rosa26-CreERT2: Ssb1−/− male mice showed bilateral testicular degeneration with a spectrum of alterations in spermatogenesis. Testicular tubules showed degenerate, sometimes vacuolated, or necrotic spermatogenic cells, the latter with pyknotic nuclei and hypereosinophilic cytoplasm, or apoptotic body formation. Multinucleated giant cells were also frequently present in the lumen, either derived from spermatocytes with arrested development or the coalescence of spermatids (Figure 5D, left panel). Increased apoptosis at approximately the same stage, equivalent to stage IV of the normal seminiferous epithelial cycle has been reported in a number of mutants defective for meiotic recombination and/or meiosis-specific chromosome structures [24]. We performed ApopTag staining to determine the rate of spermatocyte apoptosis in testes from Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− littermates. As expected, the spermatocytes of Rosa26-CreERT2: Ssb1−/− testes exhibited increased ApopTag staining, compared to Rosa26-CreERT2: Ssb1+/− spermatocytes that were uniformly immunonegative for apoptosis (Figure 5D, right panel). As newly formed spermatozoa are released for passage into the epididymis for further maturation, we examined epididymides from Rosa26-CreERT2: Ssb1−/− mice for developing germ cells that were prematurely sloughed from the seminiferous epithelium and passed into the epididymis. The presence of round germ cells within the lumen of the epididymis (Figure 5E) suggests that, in addition to apoptosis, a significant number of germ cells were being lost via premature sloughing from the supporting Sertoli cells. Taken together, these results reveal a spectrum of testicular degenerations in the Rosa26-CreERT2: Ssb1−/− mice.
To further characterize the consequences of Ssb1 ablation on fertility, we interbred induced Rosa26-CreERT2: Ssb1−/− mice with wild-type mice. Consistent with the normal physiological appearance of their ovaries, induced female Rosa26-CreERT2: Ssb1−/− mice at ten weeks of age were found to be fertile. In contrast, only 4 out of 6 pairings of male Rosa26-CreERT2: Ssb1−/− mice with wild-type females led to successful pregnancies. In addition, in the 4 successful breeding pairs, we observed significantly smaller litter sizes (*P<0.05, Figure 5F) and much longer litter intervals (63 days vs. 27 days, ***P<0.001, Figure 5G) for Rosa26-CreERT2: Ssb1−/− breeders compared to Rosa26-CreERT2: Ssb1+/− control males. Histological analysis of testes sections revealed a dramatically decreased number of elongated spermatids in the infertile compared to the fertile Ssb1-deleters. Thus, post-natal Ssb1 deletion leads to a spectrum of partial to complete male fertility defects, demonstrating the importance of this protein for spermatogenesis.
Conditional Ssb1 deletion leads to increased radiation sensitivity in vivo
To assess if conditional deletion of Ssb1 in mice causes a DNA damage response defect in vivo, we challenged Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice with 8 Gy of total body irradiation (TBI) at 4 weeks post tamoxifen-induction and monitored them for up to 30 days post-IR (Figure 6A). Although we observed comparable progressive weight loss in all 3 groups within the first few days of radiation exposure, death events started to occur in the group of irradiated Rosa26-CreERT2: Ssb1−/− mice by the 10th day. By day 19, 92% (11 out of 12) of Rosa26-CreERT2: Ssb1−/− mice had died. In contrast, in Rosa26-CreERT2: Ssb1+/+ and Rosa26-CreERT2: Ssb1+/− groups, the first death event occurred on the 13th day and more than 50% of mice survived for at least 30 days after irradiation (Figure 6B). Thus, in vivo radiation sensitivity was significantly increased in Rosa26-CreERT2: Ssb1−/− mice compared to Rosa26-CreERT2: Ssb1+/+ or Rosa26-CreERT2: Ssb1+/− controls based on Kaplan-Meier survival analysis (**P<0.01) (Figure 6B). As injury of the small intestine or bone marrow are the most common causes of death in irradiated mice, we examined these tissues to establish the cause of death in induced Rosa26-CreERT2: Ssb1−/− mice. At 24 h and 3 days post TBI, the histology of the small intestine was comparable across induced Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice, as assessed by Haematoxylin and eosin, Ki67 and ApopTag immunohistochemical staining (Figure S10A and data not shown). However, at 5 days post TBI, we observed some pathological abnormalities in Rosa26-CreERT2: Ssb1−/− mice, including distended crypt lumina lined by attenuated enterocytes and containing desquamated necrotic cellular debris as well as a small increase of cells near deep crypts with apoptotic bodies (Figure 6C).
Fig. 6. Conditional Ssb1-deleted mice are sensitive to IR. 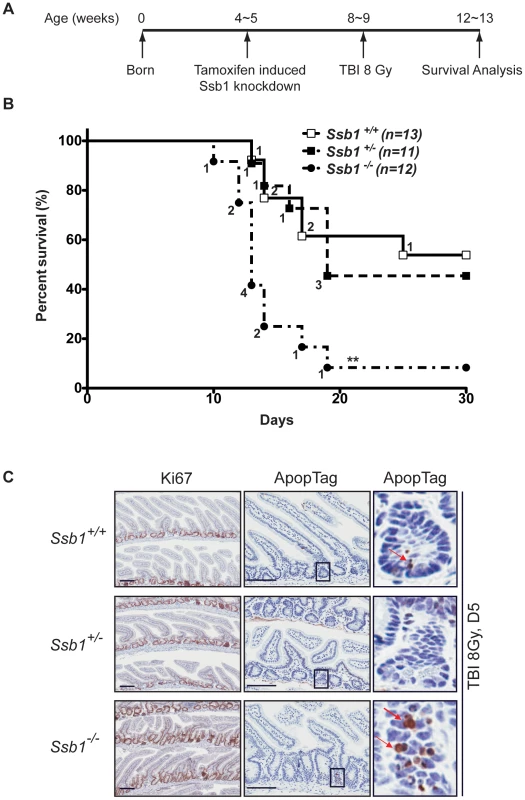
(A) Schematic diagram of the radiation challenge assay in Ssb1 deleted mice. Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice were challenged with 8 Gy of total body irradiation (TBI). The acute lethal response of mice to TBI was evaluated over a 30-day observation period. (B) Kaplan-Meier survival analysis of irradiated mice. Kaplan-Meier survival curves compared by log-rank (Mantel-Cox) analysis showed significant difference between Rosa26-CreERT2: Ssb1−/− mice and the other two groups (**P<0.01), while no difference was found between Rosa26-CreERT2: Ssb1+/+ and Rosa26-CreERT2: Ssb1+/− groups. (C) Representative images of Haematoxylin and eosin, Ki67 (cell proliferation) and ApopTag (cell death) staining on small intestine sections from mice at Day 5 post 8 Gy of TBI. Further, we performed complete blood count (CBC) analysis on peripheral blood of these mice to assess hematologic radiation toxicity, but no significant difference between the groups was observed (Figure S10B).
To assess whether Ssb1 deficiency affects radiosensitivity in other tissues, we also isolated and exposed thymocytes to various doses of IR (1–6 Gy). We observed increased radiosensitivity in Ssb1−/− thymocytes as assessed by Annexin V and 7-AAD staining (Figure S11). Taken together, these data indicate that depletion of Ssb1 leads to increased radiosensitivity in vivo and in thymocytes in vitro.
Increased genomic instability in conditional Rosa26-CreERT2: Ssb1−/− mice
To further investigate the increased radiation sensitivity of conditional Ssb1 null mice, we cytologically examined bone marrow metaphase spreads from Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice at 24 h after 2 and 6 Gy of TBI to assess chromosomal abnormalities. We observed a significant increase in chromosomal breakage, fragmentation and fusion in Rosa26-CreERT2: Ssb1−/− bone marrow metaphases upon irradiation, as assessed by fluorescence in situ hybridization (FISH) analysis (Figure 7). These results provide in vivo evidence that Ssb1 functions to maintain genomic stability.
Fig. 7. Increased genomic instability in irradiated bone marrow metaphases from total body irradiated mice. 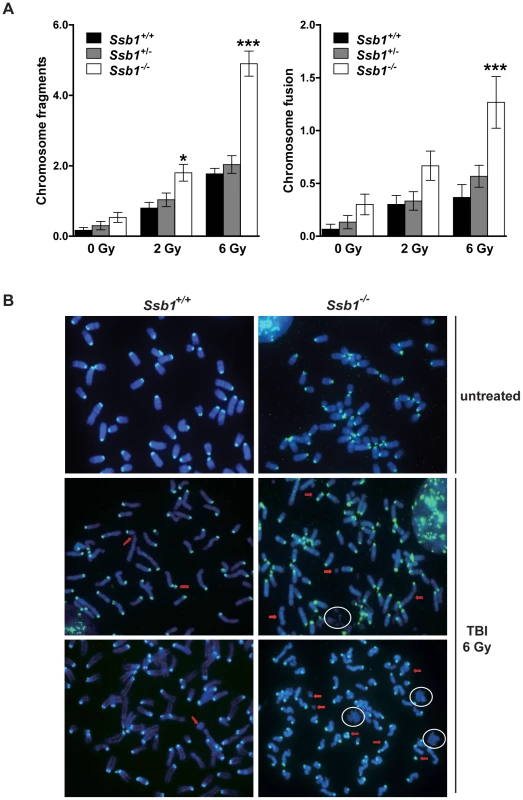
Five weeks after tamoxifen induction, nine-week-old Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice were given 2 or 6 Gy of TBI and kept for 24 h before bone marrow collection. Metaphases were prepared directly from bone marrow cells of demicolcine-treated mice for fluorescence in situ hybridization (FISH) analysis. (A) Quantification of chromosomal breakage (fragmentation and fusion) in bone marrow metaphase spreads from Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice at 24 h after 2 and 6 Gy of TBI (n = 3 mice per genotype for each condition). (B) Representative images of bone marrow metaphases from mice with indicted genotypes. Red arrows mark some of the chromosomal breakages. Note the presence of DNA debris marked with circles. Broad spontaneous tumour spectrum in conditional Rosa26-CreERT2: Ssb1−/− mice
To assess whether conditional Ssb1 deletion would lead to increased cancer susceptibility, we monitored tumour development in age - and gender-matched long-term survival cohorts of Rosa26-CreERT2: Ssb1+/− (n = 35) and Rosa26-CreERT2: Ssb1−/− (n = 35) mice. No significant difference in body weight was found between Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice over the 86 week observation period post-Ssb1 deletion (Figure S9). However, during this period, 11 out of 35 (31.4%) Rosa26-CreERT2: Ssb1−/− mice developed tumours, in contrast to only 2 out of the 35 (5.7%) Ssb1+/− mice, revealing a statistically significant difference (**P<0.01) in tumour-free Kaplan-Meier survival analysis (Figure 8A). No tumours were observed in a Cre-negative control group (Ssb1fl/fl mice, n = 10) treated with an identical tamoxifen dose or in a vehicle (olive oil: ethanol at 19∶1 ratio) treated Rosa26-CreERT2: Ssb1+/− control group (n = 5). In the 11 Rosa26-CreERT2: Ssb1−/− mice that developed tumours, we observed a broad tumour spectrum (Figure 8B) including splenic and metastatic B lymphomas, T cell lymphoma in thymus (Figure 8C), hepatocellular carcinoma, (HCC, Figure 8D) and B or T lymphoblastic leukemia (Figure S12). We also observed p53 stabilization, which is most likely an indication of the presence of mutated p53, in a high proportion of tumours (9 of 11 Ssb1−/− tumours and 2 of 2 Ssb1+/− tumours) when compared with adjacent normal tissue from the same mice (Figure S13 and Figure S14). Moreover, in the two tumours observed in Rosa26-CreERT2: Ssb1+/− mice, the Ssb1 protein was undetectable by immunohistochemical staining, indicating possible loss of heterozygosity (LOH) of the other Ssb1 allele in these tumors (Figure S14). Taken together, these data indicate that Ssb1 prevents tumor formation in vivo.
Fig. 8. Broad tumour spectrum in conditional Ssb1-deleted Rosa26-CreERT2: Ssb1−/− mice. 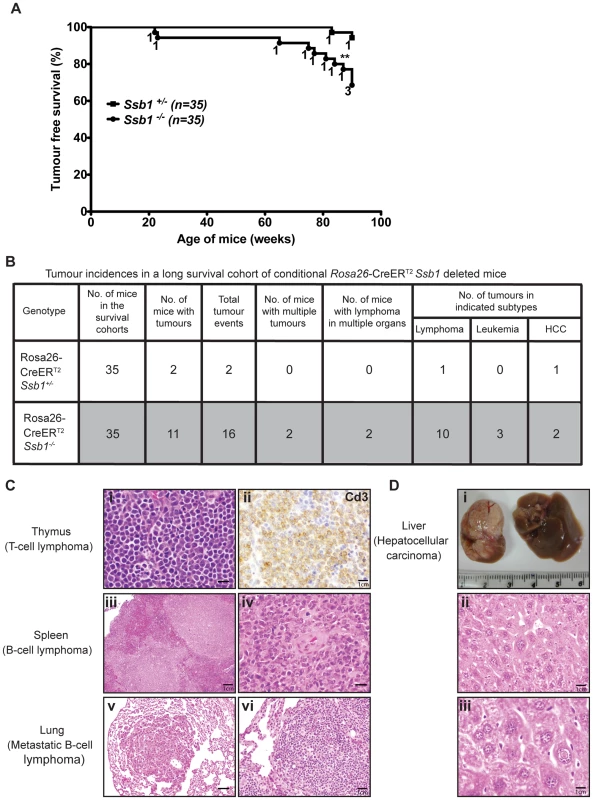
(A) Long time survival cohort of Rosa26-CreERT2: Ssb1+/− (n = 35) and Rosa26-CreERT2: Ssb1−/− (n = 35) mice were monitored for 86 weeks after Ssb1 deletion for tumour development. (A) Kaplan-Meier survival analysis showed tumour free survival rate of indicated genotypes over 90 weeks (**P<0.01). (B) Tumour incidence and a spectrum in Rosa26-CreERT2: Ssb1−/− mice compared with Rosa26-CreERT2: Ssb1+/− mice. (C) Representative sections have T lymphoma in thymus (top panel) and B lymphomas in spleen (middle panel), which spread to lung in a Rosa26-CreERT2: Ssb1−/− mice (bottom panel). Representative images of Haematoxylin and eosin stained sections of tumours in indicated organs are shown. Immunohistochemical staining of Cd3 (top panel, right) confirmed T-cell lymphoma in thymus (Scale bars, i = 40 µm, ii = 120 µm, iii = 20 µm, iv = 40 µm, v = 60 µm, vi = 80 µm). (D) Representative image of liver cancer (hepatocellular carcinoma, HCC) in a Rosa26-CreERT2: Ssb1−/− mouse. Representative images of tumour mass (upper panel) and Haematoxylin and eosin-stained liver section (middle and lower panels) are shown (Scale bars, ii = 100 µm, iii = 140 µm). Discussion
Previous studies using siRNA depletion in human cells have reported a role for SSB1 in the proper co-ordination of DNA repair in response to IR [7], [8], [9], [10]. By disrupting the major protein coding exons of Ssb1 in mice, including the OB-fold domain, we have created mouse models to study the physiological function of Ssb1 in vivo, and describe a wide spectrum of phenotypes upon Ssb1 deletion during embryogenesis and in adult and aged mice.
Major unexpected findings include novel roles of Ssb1 in the regulation of lung and skeletal development, as constitutive germline ablation of Ssb1 resulted in immature alveolar differentiation and multiple skeletal defects encompassing the ribs, craniofacial skeleton, and limbs. Interestingly, a handful of other DNA repair factors have been linked to roles in skeletogenesis: patients with Rothmund-Thompson and Rapadilino syndrome, who have mutations in the DNA helicase RECQ4, exhibit some skeletal defects in the limb [25]; patients with mutations in the repair-associated proteins Ctbp-interacting protein (CtIP/RBBP8), Centrosomal Protein 152 (CEP152), microcephalin1 (MCPH1), or Ataxia-Telangiectasia Related (ATR) exhibit dwarfism and a characteristic “bird-shaped” face with micrognathia, which is similar to the craniofacial phenotype we observe in Ssb1−/− mice [26], [27], [28], [29]. Similarly, patients with Nijmegen Breakage Syndrome (NBS), who have mutations in the MRN complex protein NBS-1, also have similar craniofacial abnormalities [30]. Previously, we demonstrated an interaction between SSB1 and NBS1, which, in in vitro studies, was abrogated by NBS-1 mutations observed in patients [31]; therefore it is tempting to speculate that SSB1 may be involved in some of the craniofacial phenotypes of this disorder. However, the broad spectrum of skeletal phenotypes in Ssb1-deficient mice is more pronounced than those reported for any of these human syndromes. This, together with the absence of obvious defects in signalling and repair of IR-induced DNA damage in both MEFs and absence of CSR defects in B cell-specific Ssb1−/− mice, may suggest additional functions of Ssb1 during embryogenesis that are outside of DNA repair.
Skeletal patterning is a complex process, and involves the spatial and temporal co-ordination of a number of developmental signalling pathways, including Hedgehog (in particular Indian Hedgehog [Ihh] and Sonic Hedgehog [Shh]), Bone Morphogenic Protein (BMP) and the Transforming Growth Factor Beta (TGF-β) family, Fibroblast Growth Factor (FGF) and Wnt signalling [32], [33]. Not surprisingly, a plethora of proteins have been implicated in skeletogenesis. During vertebrate skeletal development, mesenchymal condensations (known as somites) differentiate into the sclerotome and dermomyotome [34], [35]. Whilst the sclerotome differentiates into chondrocytes, which form the ribcage and axial skeleton, the dermomyotome further differentiates into the skin (dermatome) and muscle (myotome). Correct outgrowth and differentiation has been shown to be dependent on signalling from each of these compartments [33], [34], [35].
Interestingly, the rib-cage phenotype we observe in Ssb1−/− skeletons bears striking similarity to that of targeted disruption of the myotome regulator Myf5 [36], [37]. In Myf5-deficient mice, a similar lack of ossification in the ribcage and “floating-rib” phenotype is observed, with a partial or complete lack of ossification of the dorsal region of the ribcage, combined with micrognathia [36], [37]. Myf5−/− mice also die perinatally, but do not show the same degree of hindlimb defects that we observe in Ssb1−/− mice. Intriguingly, Myf5 is one of the genes hypothesized to have a causal role in cerebro-costo-mandibular syndrome, a rare multiple congenital anomaly syndrome characterized by absent ossification of the posterior rib-cage and micrognathia [38], [39]. Strikingly, cerebro-costo-mandibular syndrome patients also usually exhibit lung hypoplasia, due to improper development of the lung inside a poorly formed rib-cage, and have a poor prognosis for survival [40]. In addition, this disorder has also been associated with hearing defects, variable palate clefting, and sometimes mental retardation [38], [39], [40]. Although limb-patterning defects have not been described for this disorder, given the striking similarity in other phenotypes, Ssb1 may prove an interesting new candidate gene for this disorder.
Bone development can occur through two major processes, endochondral ossification, where a cartilage precursor template is laid down prior to bone formation, or intramembranous ossification, where mesenchymal cells condense and directly transition to form bone [41], [42]. Whilst endochondral ossification is the process responsible for skeletal formation in the majority of the axial and appendicular skeleton, intramembranous ossification is restricted to parts of the skull, including the cranial vault, and maxillo-mandibular bones [41], [42]. The skeletal outgrowth and patterning defects observed in Ssb1−/− mice suggest that Ssb1 is important for endochondral ossification. During the preparation of this manuscript, another report of the critical role of Ssb1 in skeletogenesis was published, where the authors had used a similar genetic targeting approach to delete Ssb1 in the mouse [43]. Interestingly, the authors described an almost identical skeletal phenotype to this report, with a similar lack of ossification of the rib-cage, micrognathia, timpanic ring malformation and variably-penetrant oligodactyly. In addition, they also reported clefting of the palate, which we observed in two cases but not in others. However, although both mouse models were generated in C57BL/6 mice, craniofacial phenotypes can be heavily affected by sometimes-subtle strain differences [44]. Intriguingly, the role of Ssb1 in skeletogenesis was attributed to p53-dependent apoptosis at E12.5 throughout the somites and limb, and a partial rescue of these phenotypes was observed upon crossing to a p53−/− background. In the case of combined Ssb1 and p53 ablation, however, although the hindlimb digit patterning and ribcage structure was substantially rescued, a distinctive lack of ossification was still evident, particularly in the dorsal extremities of the ribs abutting the vertebrae [43]. This suggests that the Ssb1−/− phenotype cannot be solely attributed to apoptosis, and that some steps in the later stages of endochondral ossification are dependent on Ssb1. Interestingly, the authors did not observe differences in canonical chondrogenic and osteogenic markers by microarray analysis on E18.5 sternum chondrocytes and calvarial osteoblasts [43]. However, the late time point of analysis and tissue origin of these cell lines may have affected the outcome of this study. Indeed, calvarial osteoblasts form through intramembranous, not endochondral ossification [41], and sternum development was not as severely affected as the rest of the rib-cage in Ssb1−/− embryos. It will therefore be of great interest to more rigorously investigate the role of Ssb1 in bone development, and to determine the precise mechanisms that lead to bone-specific apoptosis observed in these mice.
While the development defects in germline Ssb1 knockout mice were surprising, effects of inducible ablation of Ssb1 in adult mice revealed phenotypes more relevant to the proposed role of Ssb1 in maintaining genomic stability, as we observed defects in spermatogenesis, increased radiation sensitivity, increased genomic instability as well as an increased tumour incidence in induced Ssb1−/− mice. Spermatogenesis in the mouse commences postnatally at day 7 and by day 35 post-natal mature sperm can be found within the seminiferous tubules. One round of spermatogenesis takes approximately 28 days and it is a continuous process within the testes. The major phases of spermatogenesis are mitosis, meiosis, and post-meiotic germ cell maturation, which last 11, 10 and 14 days, respectively [45]. We commenced induction of Ssb1 deletion at the age of 28 days, which is at the late stage of meiosis during the first wave of spermatogenesis. We observed a variable degree of testicular degeneration and defective spermatogenesis, which led to smaller sized testes and reduced fertility in conditional Ssb1−/− adult male mice. The increased number of apoptotic spermatocytes in testes and premature sloughing of germ cells into the epididymis may be the cause of reduced fertility. The observation of a degree of phenotypic variation between conditional Ssb1−/− mice suggests that the severity of the fertility defects was dependent on the degree of testicular degeneration, which may be correlated with the variation in the residual amount of Ssb1 protein between different mice after Cre-recombination. Further investigation of the function of Ssb1 in spermatogenesis is beyond the scope of this first report, but it would be of great interest to study testicular defects in testis-specific Ssb1-deleted mice.
Aside from meiotic chromosome rearrangement, physiological programmed DSBs are also generated during Class Switch Recombination (CSR) in mature antigen-stimulated B lymphocytes [17]. CSR involves programmed DNA rearrangements within the Ig heavy chain locus of B-lymphocytes to switch from IgM to other Ig isotypes [17]. Splenic B cells with Ssb1−/− specific deletion showed similar ex vivo induced switching from IgM to IgG1. This lack of a CSR defect in B-cell specific Ssb1 knockout mice was unexpected, and may be due to functional compensation by Ssb2 as we observed dramatically up-regulated Ssb2 protein levels in bone marrow and spleen from Rosa26-CreERT2: Ssb1−/− mice and B cells from Cd19-Cre+: Ssb1−/− mice. However, we suspect the potential compensation of Ssb2 might not be sufficient to compensate for all lost Ssb1 functions in the long term or in tissues such as testes, where Ssb2 is already abundantly expressed. To investigate this aspect, a double inducible knockout mouse model of Ssb1 and Ssb2 is under investigation in our group, which will provide insight into how the Ssbs are functionally related in DNA repair.
A major role of DDR proteins, particularly crucial HR proteins, in mammalian cells is to maintain genomic integrity [46]. Not surprisingly, the impairment of this process increases cancer risk [19], [20], [21], [22], [47], [48], [49], [50]. Interestingly, the increased radiation sensitivity and chromosomal instability in total body irradiated conditional Ssb1−/− mice demonstrate the importance of Ssb1 in the maintenance of genomic integrity. Further confirming the role of Ssb1 in genomic stability was the increased incidence of spontaneous tumor formation in aged conditional Ssb1−/− mice compared with their heterozygous littermates, revealing a potential tumor suppressor function of Ssb1 in vivo. Notably, we were unable to observe a defect in γ-H2ax induction or clearance, nor in Atm signaling in response to ionizing radiation in isolated Ssb1−/− MEFs; however, this does not rule out a potential role of Ssb1 in these processes in a context - or tissue-specific manner.
In conclusion, our results highlight a novel, and non-redundant role of Ssb1 in embryonic development, which may be due to a function independent of its previously described role in DNA repair. Furthermore, our conditional deletion studies of Ssb1 in adult mice highlight the importance of Ssb1 in maintaining some aspects of genome stability and may represent tissue-specific and context-dependent roles of this protein in the adult mouse.
Materials and Methods
Generation of targeting construct
To target the mouse Ssb1 allele, a targeting construct was engineered with unidirectional lox-P sites flanking exons 3–6 of mouse Ssb1, which encompasses the DNA-binding OB-fold domain of the protein. A neomycin resistant cassette (PGK-NEO), necessary for gene targeting in mouse ES cells, was flanked by FRT recombination sites and situated within the lox-P flanked region (Figure S1A). Genomic targeting of the construct was performed in C57BL/6J ES cells using standard homologous recombination and blastocyst manipulation techniques. Gene targeting was confirmed by Southern blot using 5′ and 3′ probes situated outside the targeting vector, in addition to an internal neo probe following restriction digest of genomic DNA using HindIII, SacI or ScaI restriction enzymes. Generation of Ssb1 floxed/neo (flneo) mice was a contracted service performed by Ozgene Pty Ltd (Perth, Australia).
Generation of constitutive Ssb1 knockout mice
Ssb1 floxed (fl) mice were generated by first crossing Ssb1 targeted mice against FLPe recombinase transgenic mice to remove the neomycin cassette, and subsequently backcrossed onto a C57BL/6J strain to remove the FLP transgene. To generate constitutive germline deletion of Ssb1, Ssb1fl/fl mice were crossed against CMV-Cre (TgN(CMV-cre)1Cgn) transgenic mice that have been described previously [51]. Offspring containing the Ssb1 null (−) allele were backcrossed to the C57BL/6J strain to segregate the Ssb1 null allele and Cre transgene. Ssb1+/− heterozygous mice were intercrossed to generate Ssb1−/− animals. Ssb1+/+ and Ssb1+/− embryos were indistinguishable at the phenotypic level and were used interchangeably for some experiments as explicitly stated in the text.
Generation of conditional Ssb1 knockout mice
To generate conditional and ubiquitous Ssb1−/− mice, Ssb1fl/fl mice were crossed against Rosa26-CreERT2 transgenic mice (Figure S7) [18], [52]. Double transgenic progeny carrying both the floxed and Cre transgenes (Rosa26-CreERT2: Ssb1fl/+) were subsequently crossed to the Ssb1fl/fl mouse line to generate experimental animals (Rosa26-CreERT2: Ssb1fl/+ and Rosa26-CreERT2: Ssb1fl/fl). Induction of Ssb1 knockout was performed by intraperitoneal (IP) injection of 1 mg tamoxifen/mouse for 5 consecutive days into 4 week-old experimental animals. Cre-mediated excision was verified in a number of tissues by both genotyping PCR and western-blot (Figure S8).
To determine if Ssb1 plays a role in class switch recombination (CSR), we crossed Ssb1fl/fl mice with Cd19-Cre transgenic mice to conditionally delete Ssb1 in B cells [53].
Animal husbandry and ethics
All experimental animals were maintained on a C57BL/6J strain, and were housed at 25°C with a 12 h light/12 h dark cycle. All experiments were performed in accordance with the Queensland Institute of Medical Research animal ethics guidelines.
Genotype analysis
Genotyping was performed using genomic DNA extracted from tails. The sequences of PCR primers for genotyping Rosa26-CreERT2 mice are: 5′-TGTGGACAGAGGAGCCATAAC-3′ (forward primer) and 5′-CATCACTCGTTGCATCGACC-3′(reverse primer). As expected, PCR amplification of the 356-bp Rosa26-CreERT2-specific product reliably identified transgenic mice. Assessment of the Ssb1 gene before and after Cre recombination was performed by PCR designed to detect if the floxed-sequence had been deleted via Cre/loxP recombination. Two different reverse PCR primers were used, together with a common forward primer, result in 482, 360 and 118-bp PCR products, specific for Ssb1 floxed, wild-type, and null alleles, respectively (Figure 2A). The sequences of the common forward primer for Ssb1 wild type, floxed and null allele is: 5′-GCTTTGCTTCTGTTCCTTTACCT-3′. The reverse primer for Ssb1 wide-type and floxed alleles is 5′-ACAACCTTTGAACACTGAAGC-3′and for the Ssb1 null allele is 5′-GAAATGGATTCCGAGCTCAA-3′.
Skeletal preparations
Alcian Blue and Alizarin Red whole-mount skeletal preparations were performed as described previously [54] on E18.5 embryos. Skeletal Preparations were imaged on a Nikon SMZ45 dissecting microscope equipped with a Nikon 5MP colour camera.
Western blot
For protein extraction, tissue samples were homogenized in RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP40, 0.25% Na-deoxycholate, 1 mM PMSF, 1× Roche complete mini protease inhibitor cocktail, 1× Pierce phosphatase inhibitor cocktail). Western blotting was performed as described previously [3] with the following antibodies: sheep anti-SSB1 (1∶1000), rabbit anti-SSB2 (1∶250), mouse anti-β-actin (Sigma, 1∶10,000), mouse anti-phosphorylated-ATM serine1981 (Cell Signaling, 1∶1000) and rabbit anti phosphorylated-p53 serine 15 (Cell Signaling, 1∶1000). Detection of the signals with the chemiluminiscence reagent (Super Signal West Pico, Pierce) was carried out using the LAS-4000 imaging system (Fujifilm Life Science).
Alveolar lumen and septa quantitation
Images of Haematoxylin and eosin stained lung sections from Ssb1 control (Ssb1+/+, Ssb1+/−, n = 3) and Ssb1−/− (n = 4) were analysed using Image J software (rsbweb.nih.gov/ij/) on four representative images for each lung, with care taken not to include areas with conducting airway. Briefly, images were converted to greyscale and thresholded equally across images from control and Ssb1−/− lungs to highlight alveolar septa. The average area occupied by septa and airspace was calculated and subjected to statistical analysis.
Quantitative real-time PCR on lungs
The right lobes of Ssb1+/+, Ssb1+/− and Ssb1−/− lungs were homogenized and RNA extracted using the RNeasy mini kit (Qiagen), followed by DNAse I (New England Biolabs) digestion to remove genomic DNA contamination. 2 µg of RNA was used for first-strand cDNA synthesis using random primers (Life Technologies) and Superscript III reverse transcriptase (Life Technologies). qRT-PCR was performed using Light Cycler 480 Sybr green I mastermix (Roche Applied Science) on a Light Cycler 480 Real-time PCR system (Roche Applied Science). Primer sequences for Cc10, Foxj1, Cd31, Pdpn, Sftpa, Sftpb, Sftpc and Sftpd have been described previously [55], [56]. Aqp5 and β-Actin primer sequences were as follows: Aqp5, 5′-CTGCGGTGGTCATGAATC-3′ (forward) and 5′-CTACCCAGAAGACCCAGTGA-3′ (reverse); β-Actin, 5′-GGCTGTATTCCCCTCCATCG-3′ (forward) and 5′-CCAGTTGGTAACAATGCCATGT-3′ (reverse). Negative controls with no template and no reverse transcriptase were also included and used in qRT-PCR reactions to ensure no contaminating genomic DNA was present.
Mouse embryonic fibroblast isolation and 3T3 growth assays
Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 embryos from Ssb1+/− intercrosses as described previously [57]. At least three independent embryos per condition were used for analysis. For 3T3 fibroblast growth assays, Ssb1+/+ and Ssb1−/− cell lines were seeded at passage 2 at a concentration of 0.5×106 cells/10 cm dish. Cells were trypsinised, counted and re-seeded every 3 days at the same concentration to monitor relative changes in growth at each passage.
Immunofluorescence and microscopy
Cells were plated on glass coverslips and used at approximately 70 percent confluency. Immunofluorescence with the γ-H2AX antibody (Millipore) was performed as described previously [7]. For γ-H2ax foci quantitation, 50 cells for each MEF cell line (n = 2 Ssb1+/+, 3 Ssb1−/−) were scored for those containing >10 foci/cell at the indicated timepoints following 2 Gy of gamma-irradiation, across two independent experiments.
Gonado-somatic index analysis
The testes from Rosa26-CreERT2: Ssb1 mice were dissected out and weighed with an analytical balance (Mettler AT261). The gonado-somatic index was determined according to the formula: Gonado-Somatic Index (GSI) = (Gonad weight/total body weight) X 100, where gonad weight = (weight of the right testis+ weight of the left testis)/2 [23].
Class switch recombination (CSR) analysis
Splenic B cells were stimulated for IgH CSR to IgG1 using anti-CD40 antibodies plus IL-4 and analyzed by flow cytometry as described previously [58].
Total body irradiation
Total body irradiation (TBI) was performed using a 137Cs source at 108 cGy/min. Mice were placed in plexiglass cages and irradiated in groups of five simultaneously with the indicated doses.
Florescence in situ hybridization (FISH) analysis of chromosomal aberrations
Metaphases were prepared directly from bone marrow cells in demicolcine-treated mice for FISH analysis. Five weeks after tamoxifen induction, nine-week-old Rosa26-CreERT2: Ssb1+/+, Rosa26-CreERT2: Ssb1+/− and Rosa26-CreERT2: Ssb1−/− mice were given 2 or 6 Gy of TBI and kept for 24 h before bone marrow collection. Demicolcine (Sigma, 250 µl of a 200 µg/ml solution) was administered by intraperitoneal injection into each mouse 1 h prior to bone marrow collection. Bone marrow was flushed from each femur and tibia with pre-warmed potassium chloride solution (0.06 M). Fluorescence in situ hybridization (FISH) analysis was performed on metaphases using a biotinylated centromere-specific minor satellite probe. Three mice were analyzed for each genotype per condition and thirty metaphases were analyzed per case for chromosome breaks. Within each spread, the number of chromosomal fragments and fusions (identified by the presence of more than one centromere signals) was determined.
Lymphocyte surface markers staining
Lymphocyte surface makers were measured in peripheral blood samples by flow-cytometric analysis. Following lysis with 0.145 M ammonium chloride to remove red blood cells, the remaining lymphocytes were washed and incubated with APC conjugated anti-Cd3, PerCP-conjugated anti-Cd8, FITC-conjugated anti-Cd4, and PE-conjugated anti-Cd19 (BD Pharmingen), at 4°C for 30 minutes. Cells were washed, resuspended in PBS, and acquired on a FACS Canto II. Data were analyzed with Flowjo software (Ashland, OR, USA).
Histopathological analysis and immunohistochemistry
Tissues were collected and fixed in 10% buffered formalin fixative or 4% Paraformaldehyde, embedded in paraffin blocks, and 5-µm-thick sections were stained with Haematoxylin and eosin for histological examination. Slides were coded and examined in a blinded fashion by an independent veterinary pathologist. Immunohistochemistry staining was performed following standard procedures. Apoptosis was assessed using the ApopTag peroxidase in situ apoptosis detection kit (Chemicon International), according to the manufacturer's instructions. Stained slides were scanned on Aperio ScanScope XT Slide Scanner and the images were analyzed with Image Scope software.
Statistical analysis
Data were analyzed with GraphPad Prism software. The student's t-test was used for the statistical analysis of embryo weight and length, long bone comparison, qPCR, lung airspace analysis, testis weight, GSI, litter interval, litter size, chromosome breaks and blood cell counting data. Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank (Mantel-Cox) analysis. P values less than 0.05 were considered statistically significant.
Supporting Information
Zdroje
1. BhattiS, KozlovS, FarooqiAA, NaqiA, LavinM, et al. (2011) ATM protein kinase: the linchpin of cellular defenses to stress. Cell Mol Life Sci 68 : 2977–3006.
2. HelledayT, LoJ, van GentDC, EngelwardBP (2007) DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 6 : 923–935.
3. RichardDJ, BoldersonE, KhannaKK (2009) Multiple human single-stranded DNA binding proteins function in genome maintenance: structural, biochemical and functional analysis. Crit Rev Biochem Mol Biol 44 : 98–116.
4. IftodeC, DanielyY, BorowiecJA (1999) Replication protein A (RPA): the eukaryotic SSB. Crit Rev Biochem Mol Biol 34 : 141–180.
5. WoldMS (1997) Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem 66 : 61–92.
6. ZouY, LiuY, WuX, ShellSM (2006) Functions of human replication protein A (RPA): from DNA replication to DNA damage and stress responses. J Cell Physiol 208 : 267–273.
7. RichardDJ, BoldersonE, CubedduL, WadsworthRI, SavageK, et al. (2008) Single-stranded DNA-binding protein hSSB1 is critical for genomic stability. Nature 453 : 677–681.
8. HuangJ, GongZ, GhosalG, ChenJ (2009) SOSS complexes participate in the maintenance of genomic stability. Mol Cell 35 : 384–393.
9. LiY, BoldersonE, KumarR, MuniandyPA, XueY, et al. (2009) HSSB1 and hSSB2 form similar multiprotein complexes that participate in DNA damage response. J Biol Chem 284 : 23525–23531.
10. SkaarJR, RichardDJ, SarafA, ToschiA, BoldersonE, et al. (2009) INTS3 controls the hSSB1-mediated DNA damage response. J Cell Biol 187 : 25–32.
11. ZhangF, WuJ, YuX (2009) Integrator3, a partner of single-stranded DNA-binding protein 1, participates in the DNA damage response. J Biol Chem 284 : 30408–30415.
12. NogeeLM, de MelloDE, DehnerLP, ColtenHR (1993) Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med 328 : 406–410.
13. NogeeLM, GarnierG, DietzHC, SingerL, MurphyAM, et al. (1994) A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest 93 : 1860–1863.
14. MeltonKR, NessleinLL, IkegamiM, TichelaarJW, ClarkJC, et al. (2003) SP-B deficiency causes respiratory failure in adult mice. Am J Physiol Lung Cell Mol Physiol 285: L543–549.
15. FlecknoeS, HardingR, MaritzG, HooperSB (2000) Increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep. Am J Physiol Lung Cell Mol Physiol 278: L1180–1185.
16. WuM, WeiYQ (2004) Development of respiratory stem cells and progenitor cells. Stem Cells Dev 13 : 607–613.
17. ChaudhuriJ, AltFW (2004) Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol 4 : 541–552.
18. FeilR (2007) Conditional somatic mutagenesis in the mouse using site-specific recombinases. Handb Exp Pharmacol 3–28.
19. BarlowC, HirotsuneS, PaylorR, LiyanageM, EckhausM, et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86 : 159–171.
20. CelesteA, PetersenS, RomanienkoPJ, Fernandez-CapetilloO, ChenHT, et al. (2002) Genomic instability in mice lacking histone H2AX. Science 296 : 922–927.
21. LouZ, Minter-DykhouseK, FrancoS, GostissaM, RiveraMA, et al. (2006) MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell 21 : 187–200.
22. LiangY, GaoH, LinSY, PengG, HuangX, et al. (2010) BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet 6: e1000826 doi:10.1371/journal.pgen.1000826.
23. LatifR, LodhiGM, AslamM (2008) Effects of amlodipine on serum testosterone, testicular weight and gonado-somatic index in adult rats. J Ayub Med Coll Abbottabad 20 : 8–10.
24. BarchiM, MahadevaiahS, Di GiacomoM, BaudatF, de RooijDG, et al. (2005) Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol 25 : 7203–7215.
25. HanadaK, HicksonID (2007) Molecular genetics of RecQ helicase disorders. Cell Mol Life Sci 64 : 2306–2322.
26. KalayE, YigitG, AslanY, BrownKE, PohlE, et al. (2011) CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet 43 : 23–26.
27. O'DriscollM, Ruiz-PerezVL, WoodsCG, JeggoPA, GoodshipJA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33 : 497–501.
28. FarooqM, BaigS, TommerupN, KjaerKW (2010) Craniosynostosis-microcephaly with chromosomal breakage and other abnormalities is caused by a truncating MCPH1 mutation and is allelic to premature chromosomal condensation syndrome and primary autosomal recessive microcephaly type 1. Am J Med Genet A 152A: 495–497.
29. QvistP, HuertasP, JimenoS, NyegaardM, HassanMJ, et al. (2011) CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS Genet 7: e1002310 doi:10.1371/journal.pgen.1002310.
30. ChrzanowskaKH, KleijerWJ, Krajewska-WalasekM, BialeckaM, GutkowskaA, et al. (1995) Eleven Polish patients with microcephaly, immunodeficiency, and chromosomal instability: the Nijmegen breakage syndrome. Am J Med Genet 57 : 462–471.
31. RichardDJ, CubedduL, UrquhartAJ, BainA, BoldersonE, et al. (2011) hSSB1 interacts directly with the MRN complex stimulating its recruitment to DNA double-strand breaks and its endo-nuclease activity. Nucleic Acids Res 39 : 3643–3651.
32. LefebvreV, BhattaramP (2010) Vertebrate skeletogenesis. Curr Top Dev Biol 90 : 291–317.
33. YangY (2009) Skeletal morphogenesis during embryonic development. Crit Rev Eukaryot Gene Expr 19 : 197–218.
34. MarotoM, BoneRA, DaleJK (2012) Somitogenesis. Development 139 : 2453–2456.
35. TajbakhshS, BuckinghamM (2000) The birth of muscle progenitor cells in the mouse: spatiotemporal considerations. Curr Top Dev Biol 48 : 225–268.
36. BraunT, RudnickiMA, ArnoldHH, JaenischR (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71 : 369–382.
37. TallquistMD, WeismannKE, HellstromM, SorianoP (2000) Early myotome specification regulates PDGFA expression and axial skeleton development. Development 127 : 5059–5070.
38. HennekamRC, BeemerFA, HuijbersWA, HustinxPA, van SprangFJ (1985) The cerebro-costo-mandibular syndrome: third report of familial occurrence. Clin Genet 28 : 118–121.
39. KirkEP, ArbuckleS, RammPL, AdesLC (1999) Severe micrognathia, cleft palate, absent olfactory tract, and abnormal rib development: cerebro-costo-mandibular syndrome or a new syndrome? Am J Med Genet 84 : 120–124.
40. KangYK, LeeSK, ChiJG (1992) Maxillo-mandibular development in cerebrocostomandibular syndrome. Pediatr Pathol 12 : 717–724.
41. Franz-OdendaalTA (2011) Induction and patterning of intramembranous bone. Front Biosci 16 : 2734–2746.
42. MackieEJ, TatarczuchL, MiramsM (2011) The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol 211 : 109–121.
43. FeldhahnN, FerrettiE, RobbianiDF, CallenE, DeroubaixS, et al. (2012) The hSSB1 orthologue Obfc2b is essential for skeletogenesis but dispensable for the DNA damage response in vivo. EMBO J
44. DixonJ, DixonMJ (2004) Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle. Dev Dyn 229 : 907–914.
45. EddyEM (2002) Male germ cell gene expression. Recent Prog Horm Res 57 : 103–128.
46. AguileraA, Gomez-GonzalezB (2008) Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet 9 : 204–217.
47. Minter-DykhouseK, WardI, HuenMS, ChenJ, LouZ (2008) Distinct versus overlapping functions of MDC1 and 53BP1 in DNA damage response and tumorigenesis. J Cell Biol 181 : 727–735.
48. KimHS, VassilopoulosA, WangRH, LahusenT, XiaoZ, et al. (2011) SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20 : 487–499.
49. LiL, HalabyMJ, HakemA, CardosoR, El GhamrasniS, et al. (2010) Rnf8 deficiency impairs class switch recombination, spermatogenesis, and genomic integrity and predisposes for cancer. J Exp Med 207 : 983–997.
50. MoynahanME, JasinM (2010) Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol 11 : 196–207.
51. TonksID, HackerE, IrwinN, MullerHK, KeithP, et al. (2005) Melanocytes in conditional Rb−/ − mice are normal in vivo but exhibit proliferation and pigmentation defects in vitro. Pigment Cell Res 18 : 252–264.
52. FeilS, ValtchevaN, FeilR (2009) Inducible Cre mice. Methods Mol Biol 530 : 343–363.
53. RickertRC, RoesJ, RajewskyK (1997) B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 25 : 1317–1318.
54. BruceSJ, ButterfieldNC, MetzisV, TownL, McGlinnE, et al. (2010) Inactivation of Patched1 in the mouse limb has novel inhibitory effects on the chondrogenic program. J Biol Chem 285 : 27967–27981.
55. BirdAD, FlecknoeSJ, TanKH, OlssonPF, AntonyN, et al. (2011) cAMP response element binding protein is required for differentiation of respiratory epithelium during murine development. PLoS ONE 6: e17843 doi:10.1371/journal.pone.0017843.
56. O'BrienKB, Alberich-JordaM, YadavN, KocherO, DiruscioA, et al. (2010) CARM1 is required for proper control of proliferation and differentiation of pulmonary epithelial cells. Development 137 : 2147–2156.
57. JuradoS, SmythI, van DenderenB, TenisN, HammetA, et al. (2010) Dual functions of ASCIZ in the DNA base damage response and pulmonary organogenesis. PLoS Genet 6: e1001170 doi:10.1371/journal.pgen.1001170.
58. ChengHL, VuongBQ, BasuU, FranklinA, SchwerB, et al. (2009) Integrity of the AID serine-38 phosphorylation site is critical for class switch recombination and somatic hypermutation in mice. Proc Natl Acad Sci U S A 106 : 2717–2722.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



