-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
Retrograde axonal transport requires an intricate interaction between the dynein motor and its cargo. What mediates this interaction is largely unknown. Using forward genetics and a novel in vivo imaging approach, we identified JNK-interacting protein 3 (Jip3) as a direct mediator of dynein-based retrograde transport of activated (phosphorylated) c-Jun N-terminal Kinase (JNK) and lysosomes. Zebrafish jip3 mutants (jip3nl7) displayed large axon terminal swellings that contained high levels of activated JNK and lysosomes, but not other retrograde cargos such as late endosomes and autophagosomes. Using in vivo analysis of axonal transport, we demonstrated that the terminal accumulations of activated JNK and lysosomes were due to a decreased frequency of retrograde movement of these cargos in jip3nl7, whereas anterograde transport was largely unaffected. Through rescue experiments with Jip3 engineered to lack the JNK binding domain and exogenous expression of constitutively active JNK, we further showed that loss of Jip3–JNK interaction underlies deficits in pJNK retrograde transport, which subsequently caused axon terminal swellings but not lysosome accumulation. Lysosome accumulation, rather, resulted from loss of lysosome association with dynein light intermediate chain (dynein accessory protein) in jip3nl7, as demonstrated by our co-transport analyses. Thus, our results demonstrate that Jip3 is necessary for the retrograde transport of two distinct cargos, active JNK and lysosomes. Furthermore, our data provide strong evidence that Jip3 in fact serves as an adapter protein linking these cargos to dynein.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003303
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003303Summary
Retrograde axonal transport requires an intricate interaction between the dynein motor and its cargo. What mediates this interaction is largely unknown. Using forward genetics and a novel in vivo imaging approach, we identified JNK-interacting protein 3 (Jip3) as a direct mediator of dynein-based retrograde transport of activated (phosphorylated) c-Jun N-terminal Kinase (JNK) and lysosomes. Zebrafish jip3 mutants (jip3nl7) displayed large axon terminal swellings that contained high levels of activated JNK and lysosomes, but not other retrograde cargos such as late endosomes and autophagosomes. Using in vivo analysis of axonal transport, we demonstrated that the terminal accumulations of activated JNK and lysosomes were due to a decreased frequency of retrograde movement of these cargos in jip3nl7, whereas anterograde transport was largely unaffected. Through rescue experiments with Jip3 engineered to lack the JNK binding domain and exogenous expression of constitutively active JNK, we further showed that loss of Jip3–JNK interaction underlies deficits in pJNK retrograde transport, which subsequently caused axon terminal swellings but not lysosome accumulation. Lysosome accumulation, rather, resulted from loss of lysosome association with dynein light intermediate chain (dynein accessory protein) in jip3nl7, as demonstrated by our co-transport analyses. Thus, our results demonstrate that Jip3 is necessary for the retrograde transport of two distinct cargos, active JNK and lysosomes. Furthermore, our data provide strong evidence that Jip3 in fact serves as an adapter protein linking these cargos to dynein.
Introduction
Active transport of proteins and organelles between the neuronal cell body and axon terminals is necessary for the formation and maintenance of functional neural circuits. Anterograde (to axon terminals) and retrograde (to the cell body) transport rely on motor proteins of the Kinesin and Dynein families respectively. These motors use the energy of ATP hydrolysis to walk along microtubule tracks, carrying cargo to its proper destination. Though 15 kinesin families exist in mammals [1], only 1 retrograde microtubule based motor protein, cytoplasmic dynein, is responsible for the majority of retrograde cargo transport in axons [2]–[4], leading to intriguing questions about the nature of dynein-cargo interaction specificity which have been largely unexplored [5].
The core cytoplasmic dynein motor is composed of an array of proteins that includes two motor domain-containing heavy chains, two intermediate chains, two light intermediate chains, and four light chains which bind the intermediate chains [6]. Though recombinant dynein heavy chain can function in microtubule sliding assays in vitro [7], dynein complex interacting proteins have been shown to be essential for the initiation of retrograde cargo movement in vivo. Dynactin, a large dynein-interacting protein complex, and Lis1 have been separately shown to be co-factors that are necessary for the initiation of retrograde transport [8]–[10]. Loss of either of these factors leads to decreased retrograde transport frequency of some cargo and can lead to the accumulation of dynein components as well as cargo in axon terminals [9]. Retrograde cargo is thought to either bind directly to the core dynein complex proteins or, alternatively, to additional adapter proteins. It is tempting to speculate that the use of distinct adapter proteins may confer specificity to motor-cargo interactions in the dynein motor system. Despite their importance for the understanding of dynein-based cargo transport, the identity of specific dynein cargo adapters is dramatically lacking [5].
We used the advantages of the zebrafish system, including its amenity to forward genetics and live imaging, to identify Jip3 (JNK-interacting protein 3) as a cargo-specific adapter for dynein-based axonal transport. Through a forward genetic screen, we isolated a mutant strain (jip3nl7) that exhibited swellings in axon terminals of long sensory axons, a potential sign of interrupted retrograde transport. jip3nl7 carried a mutation in Jip3, a scaffold protein shown previously to serve as an adapter and facilitator of synaptic cargo anterograde transport through its interaction with Kinesin-1 [11]–[13]. In addition to anterograde transport machinery, Jip3 interacts with components of the dynein motor complex and c-Jun N-terminal Kinase (JNK). Indeed, Jip3 was first identified as a scaffold protein that links JNK to its upstream activating kinases, facilitating JNK activation [14]. Interestingly, Cavalli and colleagues demonstrated that Jip3 and activated JNK (pJNK) colocalized with p150glued (dynactin complex protein) distal to sciatic nerve injury. Based on this data, they postulated that Jip3-JNK-dynein interaction may be important during retrograde damage signaling [15]. Furthermore, in this and other studies, Jip3 has been shown to biochemically interact with components of the retrograde motor complex, specifically p150glued [15] and dynein light intermediate chain (DLIC; [13]). Thus, an intriguing possibility is that Jip3 could serve as an adapter for dynein-mediated retrograde transport of JNK and other cargo; however, neither this hypothesis nor the possibility that Jip3 is required for retrograde transport of any cargo, has been directly addressed to date.
Our work reveals discrete and direct roles for Jip3 in the retrograde transport of two cargos, pJNK and lysosomes. Using an in vivo imaging technique we developed for use in the zebrafish, we found specific retrograde transport defects in jip3nl7: frequencies of lysosome and pJNK retrograde transport were decreased causing accumulation of both cargos in axon terminals. Further analyses showed that direct Jip3-JNK interaction was necessary for retrograde clearance of pJNK from axon terminals and provided evidence that increased levels of pJNK were directly responsible for axon terminal swellings. Surprisingly, JNK activity and Jip3-JNK interaction had no impact on lysosome localization. Rather, co-transport analysis of lysosomes with both Jip3 and DLIC provided strong evidence that DLIC-lysosome interaction during retrograde transport relies on Jip3. Thus, based on our data we posit that Jip3 serves as an adapter protein for the retrograde transport of two distinct cargos, pJNK and lysosomes, and that failed retrograde clearance of pJNK contributes to the dysmorphic axon terminals in jip3nl7 mutants.
Results
jip3nl7 displays phenotypes consistent with impaired retrograde transport
jip3nl7 was isolated in a forward genetics screen for which we utilized the TgBAC(neurod:EGFP)nl1 transgenic zebrafish (hereafter referred to as neurod:EGFP; [16]). This transgenic strain expresses an EGFP reporter in the central and peripheral nervous systems, including the posterior lateral line (pLL) ganglion and the long sensory axons emanating from it (Figure 1A, 1B; for screen details consult the Materials and Methods). We focused our screen on the long sensory axons of the pLL because of their planar character and superficial localization. These axons originate from the pLL ganglion, located just posterior to the ear, and extend along the trunk, branching to innervate mechanosensory hair cells that reside within surface sensory organs called neuromasts (NMs; axon terminals innervating NM3 and terminal NMs are shown in Figure 1B′ and 1B″ respectively). Initial pLL nerve extension and NM formation is complete by 2 dpf (days post-fertilization), and by 5 dpf a functional neural circuit has developed between NM hair cells and afferent pLL axons [17]. The recessive jip3nl7 mutant (Figure 1C) was isolated because it displayed truncation of pLL axons (incomplete penetrance; Figure 1C″) and swollen axon terminals innervating all trunk NMs (penetrance 100%; NM3 in Figure 1C′ and data not shown). To determine if long central nervous system axons were also affected by loss of Jip3, we analyzed axons of the reticulospinal tract as well as the efferent axons that project from the CNS to innervate the pLL NMs by crossing the jip3nl7 mutation into the TgBAC(phox2b:EGFP)w37 transgenic line [18]. Similar to pLL afferents, both reticulospinal tract and pLL efferent axons were truncated in jip3nl7 mutants (Figure 1D, 1E). jip3nl7 mutants were homozygous viable and the pLL axonal phenotype did not have a maternal component, as progeny derived from homozygous crosses displayed identical phenotypes to that of progeny derived from heterozygous crosses (data not shown).
Fig. 1. Jip3, an actively transported protein, was necessary for axon extension and the prevention of axon terminal swellings. 
(A) Schematic of a larval zebrafish illustrating the basic anatomy of the primary posterior lateral line (pLL) system. Neuromasts (NMs; terminal NM cluster-ter) are innervated by the pLL nerve (green), which emanates from the pLL ganglion (pLLg). (B) Wildtype neurod:EGFP transgenic at 5 dpf with the pLLg and pLL nerve (pLLn) indicated. (B′,B″) Panels illustrate pLL axon terminals that innervate NM3 (B′) and the distal end of the pLL nerve including the axon terminals at the terminal NM cluster (ter; B″; red arrowheads point to axon terminals). (C) jip3nl7 mutants displayed truncated pLL nerves and distal pLL nerve thinning (C″) as well as swollen axon terminals in all NMs (NM3 shown in C′). Scale bars B and C = 100 µm. Scale bars in B′, B″, C′ and C″ = 10 µm. (D, E) Long central nervous system axons of the reticulospinal tract (arrowhead) and pLL efferent axons (arrow), visualized by the phox2b:EGFP transgenic reporter, were also truncated in jip3nl7 mutants. End of trunk indicated by the asterisk. (F) Schematic of the zebrafish Jip3 protein showing conserved structural and binding domains. The red arrowhead indicates the location of the jip3nl7 mutation, which generates a premature stop codon at amino acid 184. (G,H) In situ hybridization analysis revealed that jip3 was expressed in the central and peripheral nervous systems at 2 dpf in wildtype but was lost in jip3nl7. (I) Schematic of the paradigm designed to image axon transport in the pLL nerve. (J) Transient expression of Jip3-mCherry in 1 neuron of the pLL ganglion at 30 hpf. (K) Jip3-mCherry was localized to a growth cone of an extending axon at 30 hpf. The pLL ganglion and nerve were visualized by expression of the neurod:EGFP transgene. (L) Jip3-mCherry is actively transported in pLL axons (Video S1). Arrowhead (pink) and arrow (yellow) indicate anterograde and retrograde particle movement respectively. (M) Kymograph of time-lapse imaging in J. Scale bars in J–L = 10 µm. We used a positional cloning approach to isolate the genomic locus containing the jip3nl7 gene mutation. Zebrafish Jip3, which mapped to this locus, is similar to its mammalian orthologs and contains two coiled coil domains, one leucine zipper deemed integral for Kinesin Light Chain (KLC) and dynactin binding [19], [20], and a JNK binding domain (Figure 1F). Sequencing of jip3 from jip3nl7 mutants revealed a mutation at nucleotide 552 which created a premature stop codon, truncating the Jip3 protein at amino acid 184 (Figure 1F). In situ hybridization analysis showed that, similar to mouse [21], jip3 was expressed in the central and peripheral nervous systems of the zebrafish embryo (Figure 1G). jip3 expression was lost in jip3nl7, perhaps due to nonsense-mediated mRNA decay (Figure 1H). Consequently, jip3nl7 is likely a Jip3 null. Initial investigations revealed the pLL nerve phenotypes were not due to impaired pLL patterning, neuronal cell death, abnormal glial support/myelination, or gross cytoskeletal abnormalities (Figure S1). As Jip3 has been shown to interact with members of the anterograde and retrograde motor complexes [11]–[13], [22], [23] and interruptions in transport have been associated with axon swellings like those observed in jip3nl7 [24], [25], we next focused our investigations on the potential function of Jip3 in axonal transport.
In vivo analysis of Jip3 transport in the zebrafish pLL nerve
To study the function of Jip3 in axonal transport, we developed methods to visualize microtubule-based axonal transport in the pLL system in vivo, in intact zebrafish embryos and larvae (Figure 1I). Zebrafish are ideal for such a preparation as they are transparent through early embryonic and larval development, facilitating in vivo live imaging, and transient transgenesis can be used reliably to express tagged cargos of interest mosaically. Using these advantages, we developed a protocol that requires no surgical or invasive techniques to visualize protein or organelle transport in the long and planar axons of the pLL. To image axonal transport in zebrafish pLL axons, zygotes are injected with DNA encoding a cargo of interest tagged with a fluorescent reporter. Expression of these constructs is controlled by a neuron-specific 5 kilobase portion of the neurod promoter (5kbneurod; [26]). This results in mosaic expression of the desired cargo in the pLL ganglion, which, in ideal preparations, labels 1 to 2 neurons. Neurons expressing cargo are then monitored for full axon extension, innervation of NMs, and the absence of cargo accumulation in neuronal cell bodies and axons to assess optimal concentrations of DNA for injection. Using this approach, cargo transport can be visualized in individual pLL axons during axon extension (1–2 dpf), post-extension (after 2 dpf), and after functional synaptic connections are established (5 dpf).
We first utilized this technique to observe the localization and transport of a Jip3-mCherry fusion in pLL neurons and their axons. During axon extension (30 hpf), Jip3-mCherry localized to the neuronal cell body and axon growth cones (Figure 1J, 1K), similar to Jip3 localization in cultured neurons [27]. We then visualized Jip3 transport at 2 dpf, just after pLL nerve extension completes, and analyzed transport parameters using kymograph analysis (Figure 1L, 1M and Video S1). Jip3-containing cargo traveled at average velocities of 1.60 µm/sec in the anterograde direction and 1.35 µm/sec when moving in the retrograde direction (N = 7 larvae); these parameters are consistent with fast anterograde and retrograde transport [1].
Defects in organelle transport in jip3nl7 mutants
Next, we assayed the localization and transport of ssNPY-mCherry [28], a marker of Golgi-derived vesicles, to determine if loss of Jip3 affects the axonal transport of this generalized cargo. At 5 dpf, we observed large accumulations of mCherry positive puncta in axon terminals of jip3nl7 mutants but not in wildtype siblings (Figure S2A, S2B; for this and other experiments, mutants were identified using the genotyping protocol described in the Materials and Methods, except where otherwise indicated). In vivo imaging and kymograph analysis demonstrated bidirectional movement of mCherry-positive puncta in wildtype and jip3nl7 mutants (Figure S2C–S2F; Videos S2 and S3) with decreased frequency of anterograde and retrograde transport of this cargo in jip3nl7 at 2 dpf with a tendency toward a decrease at 5 dpf (Figure S2G). Neither distance nor velocity of cargo movement were altered (Figure S2H, S2I), potentially implicating Jip3 in cargo-motor attachment, rather than modulation of motor activity.
Next, we set out to determine the identity of the mCherry labeled retrograde cargo(s) by looking for accumulation of commonly transported retrograde cargos in jip3nl7 axon terminals using immunofluorescence [29], [30]. Neither late endosomes (Rab7-positive) nor autophagosomes (LC3-positive) accumulated in jip3nl7 axon terminals (Figure S3A–S3D). Consistent with a previous study on Jip3's role in anterograde transport of TrkB [13], TrkB levels were decreased in jip3nl7 axon terminals, as assayed by TrkB antibody labeling (Figure S3E, S3F). In contrast, the axon terminal swellings in jip3nl7 were rich in lysosomes that were visualized using two separate markers, Lamp1 (detected by immunofluorescence; Figure 2A, 2B) and Lysotracker red (vital dye; Figure S3G, S3H).
Fig. 2. Lysosomes accumulated in jip3nl7 axon terminal swellings. 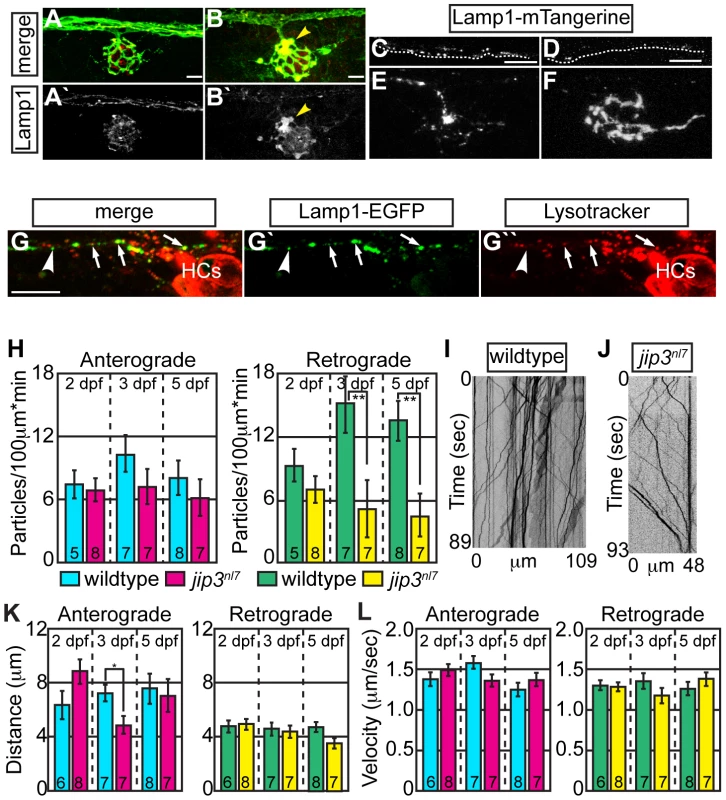
(A,B) Lysosome density, as assayed by Lamp1 immunolabeling (A,B-red, A′B′-white) was increased in jip3nl7 NM3 axon terminals at 5 dpf (arrowhead). Larvae carried the neurod:EGFP transgene to label pLL axons. (C,D) Stills from imaging sessions of Lamp1-mTangerine transport in the pLL nerve of wildtype (C) and jip3nl7 mutant (D) embryos at 3 dpf (Videos S4 and S5). Dotted lines denote the lower bound of the axons imaged. (E,F) Lamp1-mTangerine accumulated in the axon terminals of jip3nl7 mutants (F) but not wildtype siblings (E) at 3 dpf (NM1 shown for both). (G) The majority of Lamp1-EGFP positive vesicles in axons co-labeled with Lysotracker red, indicating they were lysosomes. Arrows denote a subset of the Lamp1-EGFP/Lysotracker red co-labeled vesicles. Arrowhead points to a small Lamp1 positive vesicle that was not acidified. HC denotes hair cells rich in Lysotracker red positive, acidic vesicles. (H) Retrograde, but not anterograde, lysosome transport frequency was decreased in jip3nl7 mutants at 3 and 5 dpf (Wilcoxon rank-sum; **-p<0.005). Number of embryos analyzed is indicated on the graph for this and all subsequent bar graphs. (I,J) Kymographs of wildtype (I) and jip3nl7 (J) Lamp1-mTangerine transport shown in C and D. (K,L) Neither distance moved in individual bouts (K) nor velocity of movement (L) were altered in jip3nl7 mutants, save a decrease in anterograde transport distance at 3 dpf (Wilcoxon rank-sum; *p<0.05). Scale bars = 10 µm. We then asked whether abnormalities in lysosomal transport caused lysosome accumulations in axon terminals by employing our in vivo imaging approach, using a Lamp1-mTangerine fusion [31] to mark lysosomes in pLL axons (Figure 2C–2F; see Videos S4 and S5). The ability of a Lamp1-EGFP fusion construct to label lysosomes was confirmed by double labeling with the vital dye Lysotracker red (Figure 2G). Similar to our immunolabeling results, Lamp1-mTangerine accumulated in the axon terminals of jip3nl7 mutants but not wildtype controls (Figure 2E, 2F). Live imaging analysis demonstrated that, though Lamp1-mTangerine transport parameters were not altered at 2 dpf, the number of lysosomes moving in the retrograde direction was significantly decreased at 3 dpf in jip3nl7 axons (Figure 2H–2J; WT = 15.08±2.71 vs. jip3nl7 = 5.14±2.71 particles/100 µm*min, p≤0.002; Wilcoxon rank-sum). A similarly reduced frequency of lysosome retrograde transport was also observed at 5 dpf, while distance and velocity of movement were largely unaffected at all stages (Figure 2K, 2L). These data show that retrograde lysosome transport relies on Jip3.
Jip3 is necessary for retrograde pJNK transport
Jip3 has been shown to interact with components of the Kinesin-1 motor to regulate anterograde transport [11]–[13], but a role for Jip3 in retrograde transport has not been described previously. Therefore, we next sought to address how Jip3 functioned to regulate retrograde axonal transport. Jip3 was originally identified as a JNK-interacting protein and has been shown to facilitate JNK activation in vitro [14]. Thus, we would predict that loss of Jip3 would lead to decreased JNK activation. As JNK activity can impact numerous intracellular processes that could potentially affect axonal transport machinery [32], [33], we assayed levels and localization of active JNK (pJNK) using pan-pJNK immunolabeling. Surprisingly, instead of a decrease, we found elevated levels of pJNK in the mutant axon terminals innervating all NMs from 2 dpf onward (Figure 3A–3I and data not shown; see Materials and Methods for an explanation of fluorescent intensity measurement). In contrast, total JNK (tJNK) levels in jip3nl7 were comparable to controls (Figure 3J and Figure S4A–S4D). Western blot analysis of whole embryo extracts revealed no increase in overall tJNK or pJNK levels in jip3nl7 (Figure S4E, S4F), pointing to a change in localization of pJNK rather than overall JNK expression or activity.
Fig. 3. pJNK levels were elevated in jip3nl7 axon terminals. 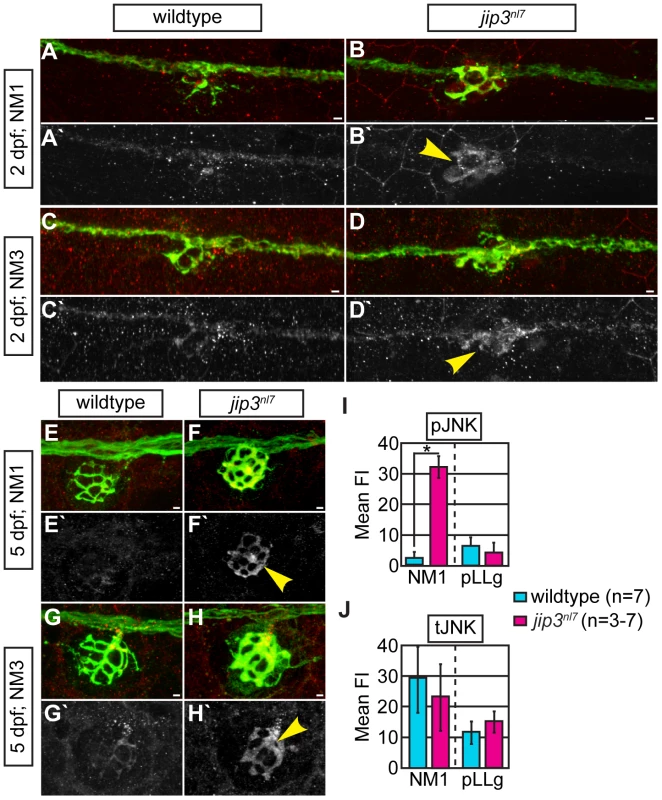
(A–H) Immunolabeling for active JNK (pJNK; red in merge; white in single channel) in proximal (NM1) and distal (NM3) neuromasts at 2 and 5 dpf. pJnk levels were elevated in all axon terminals in jip3nl7 mutants (A–I; arrowheads). (I,J) Mean fluorescent intensity (background subtracted; see Materials and Methods for details) of pJNK and total JNK (tJNK) labeling in NM1 axon terminals and the pLL ganglion (pLLg) at 5 dpf. (ANOVA, post-hoc contrasts; *-p<0.001). Scale bars = 10 µm. Given the ability of Jip3 to bind components of the retrograde motor and pJNK [14], [15], we reasoned that Jip3 might directly mediate pJNK retrograde transport/clearance from axon terminals by attaching this active kinase to the dynein motor complex. To determine if Jip3 has a specific role in pJNK transport, we used two complimentary approaches. First, we developed an axon injury model for use in the zebrafish pLL nerve to indirectly assay pJNK transport, similar to a protocol previously used in mouse sciatic nerve (Figure 4A; see Materials and Methods for procedure details; [15]). Following injury, cargos that are transported in the anterograde direction will accumulate proximal to the injury site, whereas retrograde cargos will accumulate distal to the injury site. Severing the pLL nerve between NM2 and NM3 at 5 dpf resulted in accumulation of pJNK in the pLL nerve proximal and distal to the site of injury in wildtype larvae by 3 hours post-injury. In contrast, pJNK failed to accumulate distal to the site of injury in jip3nl7 mutants (Figure 4B–4E, 4J), indicating failed retrograde pJNK transport in mutant axons. Total JNK levels were not significantly different proximal or distal to injury site in jip3nl7 mutants (Figure 4F–4I, 4K), though there was a strong trend towards decreased levels of the tJNK anterograde pool (proximal to the injury site) in jip3nl7 mutants. This data supports the hypothesis that loss of Jip3 inhibits pJNK retrograde transport, which would lead to accumulations of this kinase in axon terminals.
Fig. 4. pJNK failed to accumulate distal to injury. 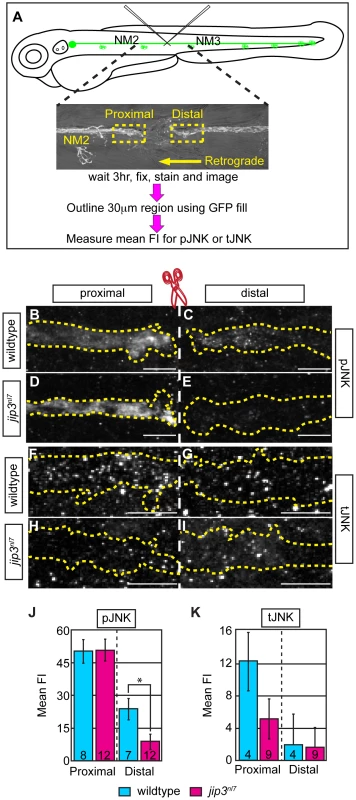
(A) Schematic and time-line of the injury model experiment and fluorescent intensity quantification. pLL nerve (identified using the neurod:EGFP transgene) was severed using finely pulled glass capillaries. DIC image of a representative injury illustrates peripheral tissue remained mostly intact. Three hours post-injury, larvae were fixed and stained for GFP (to identify the nerve) and either pJNK or tJNK. Thirty µm areas immediately proximal or distal to the injury were imaged and the mean fluorescent intensity of pJNK or tJNK was determined in summed projected stacks through the nerve only in areas that overlapped with GFP expression (outlined by dotted lines in B–I). Background mean fluorescent intensity was determined in adjacent tissue. (B–I) Proximal and distal nerve (dotted outline) adjacent to site of injury (dashed line) in wildtype and jip3nl7 larvae immunolabled for pJNK (B–E) and tJNK (F–I). (J) Levels of pJNK were decreased distal to nerve injury in jip3nl7 but proximal levels were comparable to wildtype (ANOVA, post-hoc contrasts; *-p<0.05). (K) tJNK levels trended towards a decrease proximal to the site of injury in jip3nl7 (ANOVA, post-hoc contrasts; p<0.1790) but were not different in the retrograde pool, distal to axonal severing. Next, we asked whether dynein motor components were normally transported to axon terminals in jip3nl7 mutants, as the perturbation of this transport could indirectly affect retrograde cargo movement. Using immunolabeling for two components of the dynein complex (Dynein heavy chain and p150glued), we demonstrated proper localization of these core dynein motor proteins to jip3nl7 mutants, confirming that the retrograde motor can reach axon terminals in jip3nl7 mutants (Figure S5A–S5G). From this data, we can also infer that even in the absence of Jip3, the initiation of dynactin-mediated, dynein movement was intact since these retrograde motor components did not accumulate in axon terminals [9], [10].
Finally, we used our in vivo live imaging to concretely determine if retrograde JNK transport was impaired in jip3nl7 mutant pLL axons using transient expression of JNK3 tagged with mEos. We chose to use JNK3 for our in vivo analysis because Jip3 has been shown to bind most strongly to the JNK3 homolog [14], and jnk3 is strongly expressed in the zebrafish nervous system (Figure S6A, S6B). Phospho-JNK immunolabeling of embryos expressing JNK3-mEos driven by the 5kbneurod promoter in pLL axons demonstrated that a large portion of JNK3-mEos positive vesicles carried the active form of this kinase (Figure 5A). Live imaging experiments revealed JNK3-mEos positive puncta traveled bidirectionally in wildtype and jip3nl7 mutants at 2 dpf (Figure 5B, 5C; Videos S6 and S7). Using kymograph analysis (Figure 5D, 5E), we found a decrease in the number of JNK3-mEos positive puncta moving in the retrograde direction at 2 dpf in jip3nl7 mutants (Figure 5F; wildtype:2.99±0.48 vs. jip3nl7:1.15±0.58 particles/100 µm*min, p≤0.05; Wilcoxon rank-sum) while retrograde movement distance and velocity were largely unchanged (Figure 5G, 5H). Taken together with the results from our injury model, these data confirmed that the frequency of retrograde pJNK transport was hindered in jip3nl7 mutants.
Fig. 5. Retrograde JNK3 transport frequency was decreased in jip3nl7 mutants. 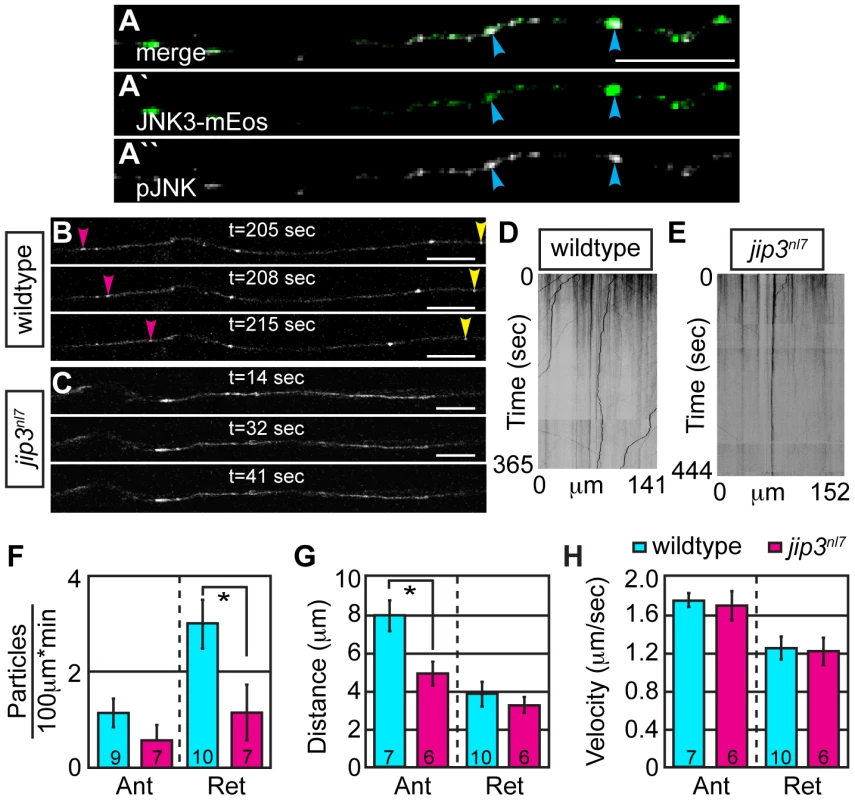
(A) Immunolabeling for pJNK in an axon expressing JNK3-mEos showed a high degree of colocalization (arrowheads) indicating that a large percentage of axonal JNK3-mEos is activated. (B,C) Representative stills from a live imaging session showing axonal transport of JNK3-mEos in a pLL axon of a wildtype (B) and jip3nl7 mutant (C) at 2 dpf (see Videos S6 and S7). Pink arrowhead denotes anterograde movement, yellow retrograde movement. (D,E) Kymographs generated from these imaging sessions. (F) Number of retrograde JNK3-mEos puncta (corrected for size of analyzed region and time of imaging session) was decreased in jip3nl7 (ANOVA, post-hoc contrasts; *-p<0.05). Distance of individual retrograde movement bouts (G) and velocity (H) were unaltered in jip3nl7. Anterograde transport distance was decreased (*-p<0.05; Ant = anterograde; Ret = retrograde). Scale bars = 10 µm. Jip3-JNK interaction is necessary for pJNK retrograde transport
Based on our data and previous work showing that Jip3 can bind components of the dynein motor complex [15], we hypothesized that direct Jip3-JNK interaction was necessary for the retrograde transport of pJNK. To address this, we first asked whether Jip3 and JNK3 were transported together in pLL axons using a dual cargo transport assay. We co-injected Jip3-mCherry and JNK3-mEos plasmids and identified embryos in which both constructs were expressed in the same pLL neuron. Notably, co-injection of these and other cargos used for dual transport analysis (see below) resulted in almost 100% co-expression. Sequential imaging of Jip3 and JNK3 positive vesicles at 2 dpf revealed a high degree of co-transport, primarily in the retrograde direction (Video S8). While only 16% of vesicles in the anterograde pool were positive for both Jip3 and JNK3, 87% of vesicles in the retrograde pool carried both proteins (N = 5 embryos). This data supported a role for Jip3 in the retrograde transport of activated JNK. Importantly, since mEos is a green to red photoconvertable molecule, we used extreme caution during these dual imaging experiments to prevent accidental photoconversion and noted no green to red shift in the vesicles imaged during these sessions (data not shown).
Next, we addressed whether the direct interaction between Jip3 and JNK was necessary for retrograde pJNK transport by asking whether the pJNK accumulation in jip3nl7 could be rescued with a Jip3 variant that lacked the JNK binding domain (Jip3ΔJNK: amino acids 202–214; [32]). DNA constructs were injected into zygotes to mosaically express Jip3-mCherry or Jip3ΔJNK-mCherry in individual pLL ganglion neurons. At 4 dpf, axon terminals expressing the respective fusions were imaged live and scored for axon morphology before larvae were individually immunolabeled for pJNK and the same axon terminals were re-imaged. As each NM is innervated by 2 axons and this innervation is segregated in space [34], we could use the non-expressing half of the NM to identify which larvae were jip3nl7 mutants as well as utilize it as a normalizing factor for the quantification of pJNK immunofluorescence. Though full-length Jip3 rescued axon terminal swellings and the accumulation of pJNK, Jip3ΔJNK was unable to rescue either phenotype (Figure 6A–6E). Importantly, expression of Jip3ΔJNK by mRNA injection rescued axon length, providing evidence that deletion of this region did not result in protein instability or failed processing, and pointing to a JNK-independent mechanism for Jip3's role in axon outgrowth (Figure S7). In summary, these data show that direct interaction between Jip3 and JNK is necessary for pJNK retrograde transport and also revealed a correlation between the accumulation of pJNK due to loss of Jip3-JNK interaction and the generation of axon terminal swellings.
Fig. 6. Jip3 interaction with JNK was necessary for pJNK clearance and the prevention of axon swellings. 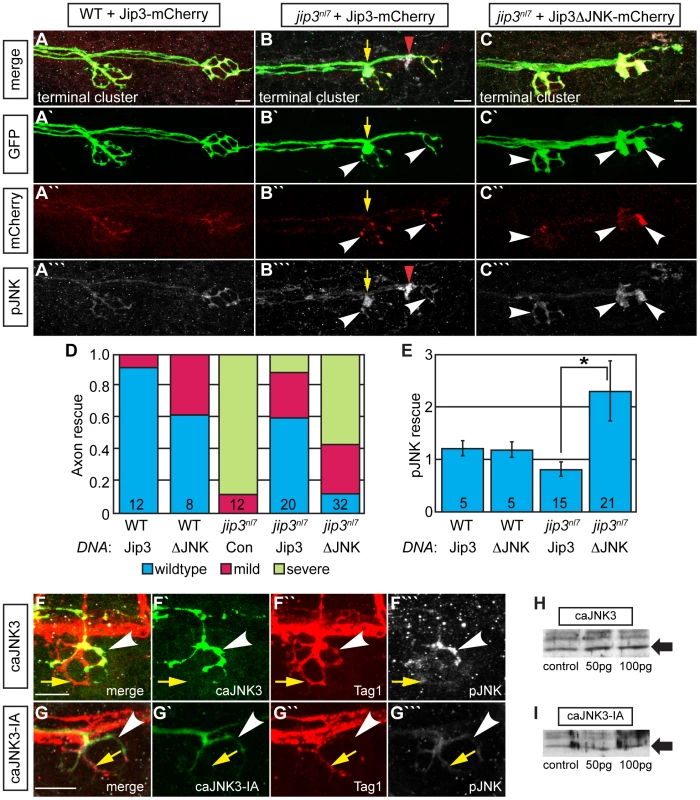
(A–C) Axon terminal swellings and pJNK accumulation were rescued by Jip3 but not by Jip3ΔJNK. Axonal swellings were visualized live by neurod:EGFP transgene expression; following live imaging, pJNK was assayed by individual larva immunolabeling at 4 dpf. White arrowheads mark axon terminals expressing the DNA constructs. Yellow arrow points to a swelling in an axon not expressing Jip3-mCherry. Red arrowhead denotes an underlying pJNK positive cell not expressing Jip3-mCherry. (D) Ratio of axon terminal swellings in each class (mild = small swellings, severe = large swellings) show rescue of axonal swellings by full-length Jip3 but not Jip3ΔJNK. DNA indicates the rescue construct injected; Jip3 = full-length Jip3-mCherry; ΔJNK = Jip3ΔJNK-mCherry; Con = uninjected control. Embryo genotype, determined by control axon terminal morphology, is indicated below each bar. (E) Ratio of pJNK levels in Jip3 or Jip3ΔJNK expressing axon terminals to those not expressing the rescue construct at 4 dpf. Jip3, but not Jip3ΔJNK, suppressed increased pJNK levels in jip3nl7 (Wilcoxon rank-sum; *-p<0.01). (F) Induction of constitutively active JNK3 tagged with EGFP (caJNK3; green) for 15 hours at 4 dpf increased the level of pJNK immunofluorescence concomitant with the induction of swellings shown by both the caJNK3-EGFP fill and Tag1 immunolabeling of neuronal membranes. Arrowhead points to a caJNK3-EGFP expressing axon. Yellow arrow indicates an axon terminal in the same NM not expressing this construct. (G) Axon terminal swellings were absent in axon terminals expressing an inactive form of the same construct (caJNK3-IA), indicating that JNK activation was necessary to induce swellings. (H,I) Efficacy of both caJNK3 and caJNK3-IA were assayed by Western blot analysis of phospho-cJun, a downstream target of active JNK. While whole embryo overexpression of caJNK3-EGFP by RNA injection induced elevated levels of phospho-cJun at 24 hpf (H), similar expression of the inactive form of caJNK3-EGFP (caJNK3-IA) failed to induce a similar increase (I). Scale bars = 10 µm. Elevated pJNK is sufficient to induce axon terminal swellings
To determine if high levels of pJNK in axon terminals were sufficient to cause axon terminal swellings, we conditionally and mosaically expressed a constitutively active form of JNK3 (caJNK3; [35], [36]) fused to EGFP under the control of a heat shock promoter in pLL neurons of wildtype larvae. Fifteen hours after activation at 4 dpf, we identified larvae that were expressing this construct in pLL axon terminals. Subsequently, these larvae were individually immunolabeled using anti-pJNK and anti-GFP antibodies to determine if caJNK3 could alter axonal morphology and additionally determine if axonal swellings correlated with elevated pJNK levels. Using this assay, we found that increased pJNK levels by expression of caJNK3 correlated with the presence of axon terminal swellings (Figure 6F). Interestingly, expression of caJNK3 did not always elevate pJNK levels (8 out of 17 larvae) and axon terminals were not swollen in these instances (data not shown). To test if axon terminal swellings were a result of JNK activity, we mutated the site phosphorylated by the upstream activating MAPKK to render caJNK3 inactive (caJNK3-IA; [37]). To assay the efficacy of the caJNK3 and caJNK3-IA constructs, we expressed both individually using RNA-mediated whole embryo expression and assayed phospho-cJun levels, a direct downstream JNK target, by Western blot analysis. As predicted, caJNK3 elevated levels of p-cJun (Figure 6H) while caJNK3-IA did not (Figure 6I). Induction of caJNK3-IA using a protocol identical to that used of caJNK3 did not cause axonal swellings in any of the 16 larvae we imaged (Figure 6G), confirming that JNK activity was indeed required for the generation of axon terminal swellings. These experiments demonstrated that high JNK activity is sufficient to induce axonal swellings and provided strong evidence that the axon terminal swellings in jip3nl7 mutants are due to increased pJNK levels at axon terminals.
Lysosome accumulation is independent of pJNK levels and Jip3-JNK interaction
Our data demonstrated that lysosomes accumulate in jip3nl7 mutant axon terminals (see Figure 2) and elevated pJNK levels cause axon terminal swellings (see Figure 6). Next, we asked whether elevated pJNK could cause lysosomal accumulation. To test this, we used the approach described above to conditionally expressed caJNK3 at 4 dpf in wildtype larvae. Larvae expressing caJNK3 in pLL neurons were immunolabeled with an anti-Lamp1 antibody and axon terminals were imaged. This analysis demonstrated that elevation of pJNK levels did not increase Lamp1 levels above controls (Figure 7A, 7B). Importantly, lysosome number and dynamics appeared normal in the presence of activated JNK, as Lysotracker red vital dye labeling was similar between caJNK3 expressing axons and non-expressing neighboring axons (Figure 7C, 7D).
Fig. 7. Increased levels of pJNK did not cause lysosome accumulation in jip3nl7. 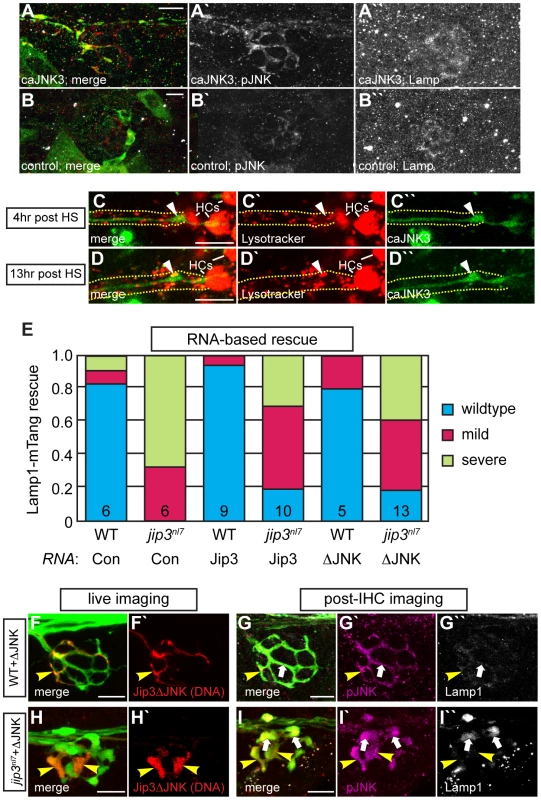
(A) Induction of caJNK3-EGFP at 4 dpf increased the level of pJNK immunofluorescence (middle) in a subset of axon terminals but did not lead to lysosome accumulation as compared to control (B). Scale bars = 10 µm. (C, D) This result was confirmed by Lysotracker red labeling. Surrounding, non-caJNK3-EGFP positive axons show similar numbers, size and density of lysosomes both 4 hours and 13 hours after induction of caJNK3. The pLL nerve was visualized by phase contrast optics and is outlined. Arrowhead indicates axonal swellings caused by high levels of activated JNK. HC denotes neuromast hair cells that strongly label with Lysotracker red. (E) Whole embryo expression of Jip3 and Jip3ΔJNK by mRNA injection partially suppressed the accumulation of lysosomes in jip3nl7 mutant axon terminals at 3 dpf as assayed by expression of Lamp1-mTangerine in pLL neurons. Wildtype – Lamp1-mTangerine positive small puncta only; Mild – small puncta and aggregates visible; Severe - few to no small puncta apparent and large aggregations of Lamp1-mTangerine. (F–I) Injection of 10 pg of a DNA construct encoding Jip3ΔJNK-mCherry rescued lysosome accumulation in jip3nl7 axon terminals. Larvae that expressed Jip3ΔJNK-mCherry (red) in pLL axons and carried the neurod:EGFP transgene were first imaged live (F,H) to identify expressing axon terminals. They were then individually fixed, stained for pJNK (pseudo-colored magenta) and Lamp1 (white), and subsequently the same axon terminals were reimaged (G,I). Arrowheads point to axon terminals in wildtype (F,G; NM1) and jip3nl7 (H,I; NM5) that express Jip3ΔJNK-mCherry (red) at 5 dpf. Arrows point to axon terminals in the same NMs that did not express this construct. Note that expression of Jip3ΔJNK-mCherry in jip3nl7 completely rescued lysosome accumulation (yellow arrowheads in I″) but failed to rescue high levels of pJNK (yellow arrowheads in I′). Based on genetic work in Drosophila, JNK has been postulated to act as a “switch”, controlling anterograde vs. retrograde motor activity for cargo transport [38]. Thus, we asked whether Jip3-JNK interaction could be a potential regulator of directional lysosome transport. First, we used sequential imaging to determine if JNK3 and lysosomes were co-transported by co-expressing JNK3-mEos and Lamp1-mTangerine in pLL axons and imaging their transport at 2 dpf (N = 6; Video S9). This analysis demonstrated that only ∼19% of Lamp1-positive vesicles moving in the anterograde or retrograde direction were co-labeled with JNK3-mEos. Interestingly, 72% of JNK3 positive retrograde vesicles label with Lamp1-mTangerine, suggesting that, though lysosomes do not rely on JNK3 for their movement, JNK3 was transported with lysosomes towards the cell body.
Finally, we tested whether Jip3-JNK interaction had any function in lysosome transport, which, if disrupted, could lead to lysosome accumulation in axon terminals in the absence of Jip3. To address this, we assayed whether lysosome accumulation in jip3nl7 mutants could be rescued by expressing Jip3ΔJNK (and Jip3 as a control) by RNA injection. For this assay, RNA was co-injected with the Lamp1-mTangerine DNA construct to visualize lysosomes in individual axons (see Figure 2E, 2F). Rescue score was determined as the average of the scores recorded by 2 blind, independent raters and was based on the ratio of punctate lysosomes (similar to wildtype in Figure 2E) vs. aggregates (as in mutants in Figure 2F). This analysis determined that Jip3ΔJNK was as effective as full-length Jip3 at suppressing lysosome accumulation in jip3nl7 mutants (Figure 7E). We did not, however, observe complete rescue, potentially due to RNA degradation by 3 dpf. To complement this analysis, we implemented a DNA-based expression strategy that would allow expression of the rescue constructs at later stages. We expressed Jip3-mCherry and Jip3ΔJNK-mCherry in pLL axons using the 5kbneurod promoter and assayed larvae for lysosome accumulation using Lamp1 immunolabeling at 4 dpf. Larvae were imaged live at 4 dpf to identify the axon terminals expressing these constructs and to identify mutant and wildtype siblings based on axonal phenotype of mCherry negative axons. Subsequently, larvae were individually immunolabeled for pJNK and Lamp1 and the same axon terminals were reimaged. Consistent with our previous results (see Figure 6), Jip3ΔJNK failed to rescue axon terminal swellings or pJNK accumulation in jip3nl7 mutants but was capable of suppressing the elevation of Lamp1 levels similar to full-length Jip3 (Figure 7F–7I and data not shown; N = 5 out of 8 jip3nl7 mutants injected with Jip3ΔJNK showed full rescue). Together, these data argue that Jip3-JNK interaction is not necessary for retrograde lysosome transport and supports a JNK-independent role for Jip3 in lysosome clearance from axon terminals.
Jip3 functions in lysosome-dynein light intermediate chain (DLIC) association during retrograde lysosome transport
In cultured cells, DLIC, a dynein accessory protein, functions in dynein-dependent lysosome transport [30]. As Jip3 has been shown to interact with DLIC [22], we hypothesized that Jip3 might serve as an adapter for lysosome-DLIC attachment during retrograde lysosome transport in axons. To ascertain whether Jip3 co-localized with moving lysosomes and could function in such a direct role, we performed sequential imaging of axons expressing both Jip3-mCherry and Lamp1-EGFP cargos at 2 and 3 dpf. Co-transport analysis revealed that Jip3 is present on lysosomes moving in the retrograde direction at both time-points (Figure 8A–8E; Video S10). Interestingly, the percentage of lysosomes that were transported in the retrograde direction labeled with Jip3 was higher at 3 dpf than at 2 dpf (2 dpf: 15%±3.8%, N = 5 vs. 3 dpf: 37%±4.2%, N = 4). This may indicate a differential reliance on Jip3 for the transport of this organelle beyond 2 dpf, leading to the decrease in lysosome retrograde transport frequency only after 2 dpf in jip3nl7 (see Figure 2).
Fig. 8. Jip3 scaffolds lysosomes to DLIC for retrograde transport. 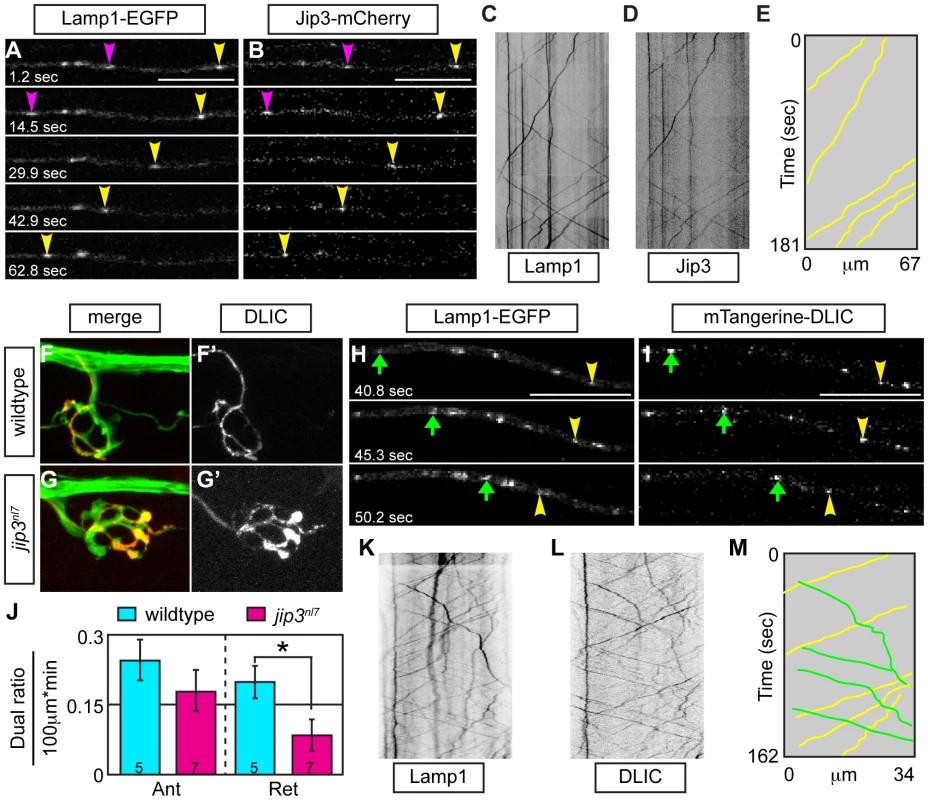
(A,B) Stills from a wildtype imaging session at 3 dpf in which Lamp1-EGFP (A) and Jip3-mCherry (B) co-transport was analyzed (Video S10). Pink and yellow arrowheads point to two retrograde Jip3/Lamp1 positive cargos. (C,D) Kymographs generated from this imaging session for individual cargos. (E) Schematized kymograph of co-transport. Yellow lines denote Jip3-positive lysosomes moving in the retrograde direction. (F,G) mTangerine-DLIC expression in a wildtype (F) and jip3nl7 mutant (G) NM1 axon terminal at 3 dpf. (H,I) Stills from analysis of Lamp1 (H) and DLIC (I) co-transport at 3 dpf in a wildtype (Video S11). Green arrow-anterograde co-labeled puncta. Yellow arrowhead-DLIC positive lysosome undergoing retrograde transport. (J) The ratio of DLIC positive lysosomes moving in the retrograde direction was significantly decreased in jip3nl7 mutants (ANOVA, *-p<0.05; Anterograde-Ant; Retrograde-Ret). (K–M) Kymographs from this imaging session and schematized kymograph depicting co-labeled anterograde lysosomes in green and retrograde in yellow. Finally, we co-expressed DLIC tagged with mTangerine (mTangerine-DLIC) and Lamp1-EGFP to characterize DLIC localization and co-transport with lysosomes and determine if this association is lost in jip3nl7 mutants. At 3 dpf, mTangerine-DLIC localized to discrete puncta along the axon and in axon terminals in wildtype larvae (Figure 8F). In contrast, in jip3nl7 mutants, DLIC accumulated in axon terminals, similar to lysosomes and pJNK (Figure 8G). Co-transport analysis of mTangerine-DLIC and Lamp1-EGFP cargos revealed a decrease in the ratio of DLIC-positive lysosomes moving in the retrograde direction in jip3nl7 mutants (Figure 8H–8M; Video S11). This observation points to a failure of lysosome-dynein interaction during transport with loss of Jip3. Interestingly, there was a slight decrease in DLIC-Lamp1 vesicle co-transport in the anterograde direction as well in jip3nl7 mutants suggesting that this complex may move bidirectionally. In summary, our data supports a model where the independent interaction of Jip3 with pJNK and lysosomes is required for the attachment of these cargoes to the dynein motor for clearance from axon terminals (Figure 9).
Fig. 9. Model of Jip3's role in retrograde transport of lysosomes and pJNK. 
Our data support a model in which Jip3 (green star) serves as a necessary adapter for retrograde transport of pJNK (yellow hexagon) and lysosomes (red oval). This interaction serves to attach these cargoes to the dynein motor complex and, in the case of lysosomes, likely requires interaction with dynein light intermediate chain (DLIC). Global retrograde transport initiation is unaffected with loss of Jip3 as dynein heavy chain, dynactin and other dynein cargos (late endosomes and autophagosomes) do not accumulate in jip3nl7 mutant axon terminals. Discussion
Our results revealed a novel role for Jip3 in retrograde axonal transport. We provided evidence that loss of Jip3 led to a decreased frequency of retrograde transport of an active kinase (pJNK) and lysosomes but not other components of the endosomal or autophagocytic system. We demonstrated that direct interaction of Jip3 and JNK was necessary to prevent pJNK accumulation and the axon terminal swellings characteristic of the jip3nl7 mutant but had no effect on lysosome accumulation. Additionally, exogenous expression of activated JNK phenocopied the jip3nl7 mutant axon terminal swellings but did not cause lysosome accumulation, providing evidence that high levels of active JNK cause this phenotype in a lysosome-independent manner. Finally, our co-transport analysis suggested that Jip3 directly facilitated lysosome interaction with the dynein motor through binding to the accessory protein DLIC. Given the decrease in frequency of cargo movement, the normal distribution of dynein components in jip3nl7 mutant axon terminals, and the high rate of Jip3-lysosome and Jip3-JNK3 co-transport, we posit that Jip3 likely serves as an adapter protein that mediates attachment of these cargos to the dynein motor (Figure 9).
Jip3 has been implicated in anterograde axonal transport in several studies through its interaction with both Kinesin light chain and Kinesin heavy chain components of the Kinesin-1 motor [12], [13], [23]. We became interested specifically in Jip3's function in retrograde transport as jip3nl7 demonstrated the unusual quality of extreme swellings in axon terminals, the end of the line for anterograde transport. A function for Jip3 in retrograde transport has indeed been posited by Cavalli et al. as they demonstrated that Jip3 co-localized with pJNK distal to nerve ligation and co-purified from similar membrane fractions as dynein components [15]; however, our study is the first to provide conclusive evidence that Jip3 is required for retrograde transport of pJNK, as pJNK accumulates in axon terminals in jip3nl7 mutants, Jip3 and JNK3 are co-transported, and direct Jip3-JNK interaction is functionally required for pJNK retrograde transport. Thus, our work identifies pJNK as a Jip3-dependent retrograde cargo. In addition, through the implementation of our in vivo imaging approach, we found that the frequency of retrograde JNK3 transport was decreased with loss of Jip3, but the processivity of the motor (reflected by run length) and velocity of movement were unchanged. This data, in combination with previous biochemical studies of Jip3-JNK and Jip3-dynein interaction [15], provide strong evidence that Jip3 functions as an adapter for pJNK, linking it to the dynein complex for transport, while not affecting motor movement itself.
Using a combination of immunolabeling and in vivo imaging techniques, we further show that Jip3 is necessary for retrograde transport of lysosomes through interaction with the dynein accessory protein DLIC. DLIC has been shown to be an important mediator of dynein-based lysosome movement in culture systems [30] and was shown to biochemically interact with Jip3 in another system [22]. Thus, Jip3 could provide a link between lysosomes and dynein through its interaction with DLIC. In support of this, Jip3 is co-transported with lysosomes, the retrograde transport velocities for Jip3 alone were highly similar to those observed for lysosomes, and DLIC-lysosome co-transport was significantly decreased in jip3nl7 mutants. Together, these data provides strong evidence that Jip3 serves as an important adapter protein for lysosome-DLIC interaction and subsequent retrograde lysosome transport. Notably, Jip3 was implicated in the anterograde transport of DLIC to axon terminals in C. elegans [22]. However, instead of a decrease, we observed increased levels of DLIC in jip3nl7 axon terminals, arguing that this Jip3 function may not be conserved in vertebrates or is compensated for by another member of the Jip family [39].
Elevated levels of activated JNK, lysosome accumulation and axonal dysmorphology have been co-associated with neurodegenerative disorders [40]. Interestingly, though our studies indicated that Jip3-JNK interaction was not required for lysosome retrograde transport, JNK3 was frequently present on lysosomes moving in the retrograde direction, suggesting that Jip3 could serve to attach both cargos to the dynein motor simultaneously. Furthermore, our results point to a lysosome-independent etiology of axon terminal swellings in jip3nl7 mutants. Evidence to support a lysosome-independent mechanism includes: 1) the ability to induce axonal swellings without lysosome accumulation by exogenous expression of constitutively active JNK; 2) the absence of axon morphological changes following expression of an inactivated form of the constitutively active JNK; and 3) rescue of lysosome accumulation, but not pJNK levels or axonal swellings, in jip3nl7 mutant axon terminals by Jip3ΔJNK expression. Thus, our work provides evidence that axonal swellings can occur downstream of this active kinase without causing concomitant accumulation of organelles in the autolysosomal pathway. The exact etiology of axonal swellings in jip3nl7 mutants due to elevated levels of activated JNK remains to be determined.
Importantly, jip3nl7 mutants did not exhibit a global disruption of retrograde axonal transport, which would indirectly lead to cargo accumulations. Evidence supporting the specificity of transport disruptions includes: 1) absence of the accumulation of other cargo (late endosomes, autophagosomes, and signaling endosomes) in jip3nl7 axon terminals; and 2) normal localization of dynein heavy chain and p150glued in jip3nl7 axon terminals, indicating that dynactin-based initiation of dynein transport is not hindered [9], [10]. Thus, our data supports a direct role for Jip3 as an adapter for the transport of two specific retrograde cargos, pJNK and lysosomes.
In summary, our data demonstrate novel and separate roles for Jip3 in the retrograde axonal transport of activated JNK and lysosomes. It is tempting to speculate that Jip3-dependent retrograde clearance of activated JNK may be a novel and crucial strategy for the removal of this active kinase from axon terminals, bypassing traditional phosphatase pathways. Furthermore, we show that enhanced JNK activity can indeed cause axon terminal swellings, similar to those observed in the jip3nl7 mutant, in the absence of lysosome accumulation. Thus, we have shown that there can be an independent etiology for these tightly coupled events observed in disease models. The similarities between the axonal swellings, high levels of pJNK, and accumulation of lysosomes in jip3nl7 and neurodegenerative diseases such as Alzheimer's Disease points to an intricate relationship between these phenotypes during pathogenesis. Our studies begin to unravel how Jip3-dependent regulation of retrograde axonal transport may underlie or modulate such disease states.
Materials and Methods
Zebrafish husbandry
Adult *AB and WIK zebrafish and *AB/WIK hybrids were maintained at 28.5°C and staged as described [41]. Embryos were derived from natural matings or in vitro fertilization, raised in embryo media, and developmentally staged using previously established methods [42]. Strains utilized included TgBAC(neurod:EGFP)nl1 [16], TgBAC(phox2b:EGFP)w37 [18], TgBAC(neurog:DsRed)nl6, TgBAC(foxd3:EGFP)nl5 transgenics and mitfaw2 [43], and mapk8ip3nl7 (jip3nl7) mutants.
Generation of transgenic lines
We used Escherichia coli-based homologous recombination to modify a neurog1 - and foxd3-containing bacterial artificial chromosome (BAC) clones [44]. The neurog1 BAC clone zK171N3 contains 63.8 kb of upstream and 106.1 kb of downstream sequence of neurog1, while the foxd3 BAC clone zC137J12 contains 66.2 kb of upstream and 122.1 kb of downstream sequence of foxd3 (http://www.sanger.ac.uk/Projects/D_rerio/mapping.shtml). After recombination, the modified BAC clones contained DSRedExpress-1 and EGFP positioned at the endogenous start site of neurog1 or foxd3, respectively. The accuracy of recombination was evaluated by PCR, sequencing, and analysis of transient expression. To obtain germline transgenics, we microinjected 20–80 pg of BAC DNA (linearized with Srf I for neurog1 BAC and supercoiled for foxd3 BAC) into zebrafish zygotes, raised injected fish to adulthood, and screened their progeny for reporter gene expression. The germline transmission rate was 2.3% for neurog1 BAC and 1.4% for the foxd3 BAC. The TgBAC(neurog1:DSRed)nl6 and TgBAC(foxd3:EGFP)nl5 strains have been outcrossed for multiple generations and transfmitted the transgenes in a Mendelian manner.
ENU mutagenesis and positional cloning
The jip3nl7 mutant was identified in a standard three-generation N-ethyl-N-nitrosourea (ENU) mutagenesis screen [45], [46]. For this screen, TgBAC(neurod:EGFP)nl1-positive larvae were screened at 4 dpf for axon truncation and the presence of axonal swellings under epifluorescence. For genetic mapping, heterozygous carriers of jip3nl7 on a polymorphic *AB/WIK background were incrossed to produce homozygous, heterozygous and wildtype progeny. Initial chromosome assignment was done by bulk segregate analysis of DNA pools from 20 wildtype and 20 mutant individuals using microsatellite markers (http://zfin.org/ZFIN). Flanking regions were identified using individual wildtype and mutant larva and markers z15457, z21697, and a designed marker, CA50 (forward: 5′-TTACACACTTTCAGCCTGTC, reverse: 5′-CCTTTATGCCACGGTCACA).
Genomic DNA isolation, cDNA generation, gene cloning, and genotyping
Genomic DNA was isolated from larvae by incubating it overnight at 55°C in PCR Extraction Buffer (10 mM Tris pH = 8, 2 mM EDTA, 0.2% Triton X-100, 200 µg/ml Proteinase K). Total RNA was isolated from larvae using Trizol according to the manufacture's protocol (Invitrogen) and cDNA was generated using Superscript II reverse transcriptase and oligo dT primers (Invitrogen). The full mapk8ip3 (jip3) cDNA was amplified using following primers (forward: 5′-CGTTAAACGAGCTTCGGACA, reverse: 5′-GCGTGTCACTTTGAGTTTGG) based on the predicted sequence and subsequently entered into GenBank (KC170712). Full-length jnk3 was amplified using primers (forward: 5′-ATGAACAGACGTTTCTTATATAACTGC, reverse: 5′-CACGGCTGCACCTGCGCTG) designed against the annotated sequence (NM 001037701). Full-length dynein light intermediate chain was amplified using primers (forward: 5′-TGTCACTCAAGCCTGCGAAG, reverse: 5′-GGATTTGTCGTTTTCAGCAG) designed against the annotated sequence (NM 001017669). To genotype jip3nl7 carriers, a 385 bp region around the mutation was amplified from genomic DNA by PCR using annealing T = 55°C and the following primers (forward: 5′-TTTGTCTGTTGAAATTGCT, reverse: 5′-ACGGTCCATACCCATGATT). PCR products were then digested with SpeI, as the single nucleotide change generates this restriction site in the jip3nl7 allele, producing two bands, 243 and 142 bp.
In situ hybridization, immunohistochemistry, TUNEL, and Lysotracker staining
RNA in situ hybridization was performed as described [47]. Digoxygenin-labeled antisense RNA probes were generated for jip3 and jnk3 using the full-length cDNA cloned. Whole mount immunohistochemistry was performed following established protocols [48]. The following antibodies were used: anti-GFP (1∶1000; Invitrogen #A11122), anti-pJNK (1∶100; Cell Signaling #9251S), anti-tJNK (1∶100; Cell Signaling #9252), anti-p150glued (1∶100; Signal Transduction Labs #610473), anti-dynein heavy chain (1∶100; gift of R. Vallee; [49]), anti-Rab7 (1∶100; Sigma #R8779), anti-Lamp1 (1∶100; Developmental Studies Hybridoma Bank), anti-LC3 (1∶100; Novus #NB100-2331), anti-TrkB (1∶100; Santa Cruz Biotechnology #sc-12) and Alexa-488/568/647 (1∶750; Invitrogen). Antibodies not used previously in zebrafish were validated by Western blot analysis (see below: Figure S3I–S3L; Rab7–24 kD, LC3–14.5 kD, TrkB-69 kD and 18 kD, Lamp1–27 kD). For TUNEL labeling, embryos were processed as previously described [50] with minor modifications according to the manufacturer's instructions (In situ cell death kit, Roche). For Lysotracker red vital dye staining, 4–5 dpf larvae were incubated in Lysotracker red (1∶10,000; Invitrogen) for 15 minutes in embryo media, washed briefly, embedded in 1.2% low-melt agarose, and imaged. All fluorescently labeled embryos were imaged using a FV1000 laser scanning confocal system (Olympus). Brightfield or Nomarski microscopy images were collected using a Zeiss Imager Z1 system. Images were processed using ImageJ software [51]. Brightness and contrast were adjusted in Adobe Photoshop and figures were compiled in Adobe Illustrator.
Western blotting
For western blot analysis, protein was isolated from wildtype and jip3nl7 3 dpf larvae by homogenizing individuals in extraction buffer (150 mM NaCl, 50 mM Tris pH = 7.4, 5 mM EDTA, 0.05% NP40, 25 mM NaF, 10 mM Na3VO4, 1 mM DTT, 10 µL/mL protease inhibitor) at a ratio of 4 µL buffer per embryo. The equivalent of 4 embryos was run in each lane on a 12% SDS-PAGE gel and transferred onto a PVDF membrane (Millipore). Primary antibodies were applied overnight: anti-pJNK (1∶1000; Cell Signaling #9251S), anti-tJNK (1∶1000; Cell Signaling #9252), anti-p150glued (1∶1000; Signal Transduction Labs #610473), anti-dynein heavy chain (1∶1000; gift of R. Vallee; [49]), anti-Rab7 (1∶2000; Sigma #R8779), anti-Lamp1 (1∶4000; Developmental Studies Hybridoma Bank), anti-LC3 (1∶500; Novus #NB100-2331), anti-TrkB (1∶100; Santa Cruz Biotechnology #sc-12), and anti-p-cJun (1∶1000; Cell Signaling 9164S). After washing, an anti-rabbit*HPR, anti-mouse*HRP, or anti-rat*HRP secondary (1∶10,000; Jackson Immuno) was applied for 90 minutes. Protein was visualized using SuperSignal West Pico Chemiluminescent Substrate according to the manufacture's specification (Thermo Scientific). If necessary, the blot was then stripped with 25 mM glycine (pH = 2.5) and re-probed with rabbit anti-α-actin (1∶10,000; Sigma).
Generation of caJNK3 and caJNK3-IA constructs and heat-shock activation
To generate constitutively active JNK3 that could be activated in a temporally specific manner, we fused MKK7 to JNK3 and placed this fusion behind a heat-shock inducible promoter (hsp70:mkk7-JNK3-egfp, referred to as caJNK3 in the text). To generate an inactive form of the same construct (referred to as caJNK3-IA in the text), two amino acids were mutated (T221A and Y223F) to render JNK3 not able to be phosphorylated, which is required for its activity [37]. For induction of transcription of both constructs, 4 dpf larvae injected with 10 pg of the caJNK3 or caJNK3-IA constructs were heat-shocked at 38°C for 1 hour. Larvae were then transferred to 28.5°C prior to analysis.
Axon transport analysis
Zygotes were injected with plasmid DNA encoding fluorescently tagged cargos of interest with expression driven by the 5kbneurod promoter [26]. At 30 hpf, 2 dpf, or 5 dpf, embryos or larvae were sorted under epifluorescence to identify individuals with tagged cargo expression in a few cells of the pLL ganglion. For imaging, embryos were mounted in 1.2% low melting point agarose on a glass coverslip, submerged in embryo media containing 0.02% tricaine and imaged using a 60X/NA = 1.2 water objective on an upright Fluoview1000 confocal microscope (Olympus). For each embryo, a region of interest (30–200 µm) was selected in the pLL nerve in which a long stretch of axon was observable in a single plane. Scans were taken at the fastest possible speed (3–5 frames per second) for 600 to 2500 frames. Embryos were subsequently released from agarose and processed for genotyping. For co-transport, embryos expressing both constructs in a single cell were selected and imaged as described above using sequential imaging of the 488 and 568 nm excitation channels. 600 frames were collected at 2–3 frames per second.
Transport parameters were analyzed using kymograph analysis in the MetaMorph software package (Molecular Devices, Inc.). Kymographs were generated from each imaging session and used to determine distance moved in individual bouts of movement (uninterrupted straight lines) and velocity of movement (slope of uninterrupted straight lines). Typically, 10–50 traces were analyzed in each kymograph and these were averaged within individual embryos for statistical analysis. The number of particles moving in each direction was estimated based on traces on the kymographs and then normalized to length of axonal segment and total imaging time.
Axotomy and image acquisition
Five day old zebrafish larva (neurod:EGFP carriers) were anesthetized in 0.02% tricaine (MS-222; Sigma) and embedded in 3% methylcellulose on a slide. Pulled thick-walled glass capillaries were used to sever the nerve between NMs 2 and 3. Slides were immersed in Ringer's solution (116 mM NaCl, 2.9 mM KCl, 1.8 mM CaCl2, 5 mM HEPES pH = 7.2, 1% Pen/Strep) and incubated at 28.5°C for 3 hours. Larva were then collected and immunolabeled for pJNK or tJNK and EGFP. Details of image and statistical analyses are described below.
Quantification of immunofluorescence
For analysis of pJNK and tJNK intensity in axon terminals and after nerve injury, individuals were immunolabeled as described above. For consistency of labeling, larvae that were directly compared were processed in the same batch. Confocal Z-stacks (0.5 µm between planes) were taken of the area of interest using a 40X/NA = 1.3 oil objective with identical settings. Images were analyzed using ImageJ [51]. For fluorescent intensity measurements of pJNK or tJNK in wildtype and mutant axon terminals, summed projections of the regions of interest were generated only through regions that contained the neurod:EGFP signal and converted to 8 bit in ImageJ. In the pLL nerve injury analysis, a 30 µm, neurod:EGFP-positive region encompassing the proximal or distal edge of the severed axon was selected and summed projections through only this segment were compiled for analysis. By restricting our analysis to the neurod:EGFP axons we eliminated a majority of the fluorescent signal from the surrounding tissue. Prior to statistical comparison, the mean background fluorescent intensity, measured in a region adjacent to the NM axon terminal or injury site, was subtracted from the values generated.
For analysis of pJNK levels in the DNA rescue experiment, axon terminals expressing Jip3-mCherry or Jip3ΔJNK-mCherry (typically innervating half a NM) and control terminals not expressing these constructs (the alternate half of the NM) were outlined in similar summed confocal projections and the mean fluorescent intensity was measured. The ratio of pJNK fluorescence in the axons expressing the rescue construct to those not expressing the rescue construct were compared for statistical analysis.
Statistical analysis
Statistical analysis was performed using the JMP software package. Data suitable for parametric analysis were analyzed using ANOVA, with Tukey-Kramer highly significant difference post-hoc contrasts for more than two variables. Data not suitable for parametric analysis were analyzed using Wilcoxon rank-sum analysis. Ordinal data was analyzed using Chi Square test. In all cases, data from individual embryos was averaged prior to analysis making each N equivalent to an embryo.
Ethics statement
All animal work was approved by and conducted according to guidelines of the Oregon Health & Science University IACUC.
Supporting Information
Zdroje
1. HirokawaN, NodaY, TanakaY, NiwaS (2009) Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10 : 682–696.
2. MikamiA, PaschalBM, MazumdarM, ValleeRB (1993) Molecular cloning of the retrograde transport motor cytoplasmic dynein (MAP 1C). Neuron 10 : 787–796.
3. PaschalBM, ValleeRB (1987) Retrograde transport by the microtubule-associated protein MAP 1C. Nature 330 : 181–183.
4. SchnappBJ, ReeseTS (1989) Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci U S A 86 : 1548–1552.
5. TwelvetreesA, HendricksAG, HolzbaurEL (2012) SnapShot: Axonal Transport. Cell 149 : 950–950 e951.
6. KardonJR, ValeRD (2009) Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10 : 854–865.
7. MazumdarM, MikamiA, GeeMA, ValleeRB (1996) In vitro motility from recombinant dynein heavy chain. Proc Natl Acad Sci U S A 93 : 6552–6556.
8. EganMJ, TanK, Reck-PetersonSL (2012) Lis1 is an initiation factor for dynein-driven organelle transport. J Cell Biol 197 : 971–982.
9. LloydTE, MachamerJ, O'HaraK, KimJH, CollinsSE, et al. (2012) The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron 74 : 344–360.
10. MoughamianAJ, HolzbaurEL (2012) Dynactin is required for transport initiation from the distal axon. Neuron 74 : 331–343.
11. BowmanAB, KamalA, RitchingsBW, PhilpAV, McGrailM, et al. (2000) Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell 103 : 583–594.
12. ByrdDT, KawasakiM, WalcoffM, HisamotoN, MatsumotoK, et al. (2001) UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32 : 787–800.
13. HuangSH, DuanS, SunT, WangJ, ZhaoL, et al. (2011) JIP3 mediates TrkB axonal anterograde transport and enhances BDNF signaling by directly bridging TrkB with kinesin-1. J Neurosci 31 : 10602–10614.
14. ItoM, YoshiokaK, AkechiM, YamashitaS, TakamatsuN, et al. (1999) JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway. Mol Cell Biol 19 : 7539–7548.
15. CavalliV, KujalaP, KlumpermanJ, GoldsteinLS (2005) Sunday Driver links axonal transport to damage signaling. J Cell Biol 168 : 775–787.
16. ObholzerN, WolfsonS, TrapaniJG, MoW, NechiporukA, et al. (2008) Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci 28 : 2110–2118.
17. GhysenA, Dambly-ChaudiereC (2004) Development of the zebrafish lateral line. Curr Opin Neurobiol 14 : 67–73.
18. NechiporukA, LinboT, PossKD, RaibleDW (2007) Specification of epibranchial placodes in zebrafish. Development 134 : 611–623.
19. MontagnacG, SibaritaJB, LouberyS, DavietL, RomaoM, et al. (2009) ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol 19 : 184–195.
20. NguyenQ, LeeCM, LeA, ReddyEP (2005) JLP associates with kinesin light chain 1 through a novel leucine zipper-like domain. J Biol Chem 280 : 30185–30191.
21. MiuraE, FukayaM, SatoT, SugiharaK, AsanoM, et al. (2006) Expression and distribution of JNK/SAPK-associated scaffold protein JSAP1 in developing and adult mouse brain. J Neurochem 97 : 1431–1446.
22. ArimotoM, KoushikaSP, ChoudharyBC, LiC, MatsumotoK, et al. (2011) The Caenorhabditis elegans JIP3 protein UNC-16 functions as an adaptor to link kinesin-1 with cytoplasmic dynein. J Neurosci 31 : 2216–2224.
23. SunF, ZhuC, DixitR, CavalliV (2011) Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility. EMBO J 30 : 3416–3429.
24. HurdDD, SaxtonWM (1996) Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics 144 : 1075–1085.
25. MartinM, IyaduraiSJ, GassmanA, GindhartJGJr, HaysTS, et al. (1999) Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell 10 : 3717–3728.
26. MoW, NicolsonT (2011) Both pre - and postsynaptic activity of nsf prevents degeneration of hair-cell synapses. PLoS ONE 6: e27146 doi:10.1371/journal.pone.0027146
27. KelkarN, GuptaS, DickensM, DavisRJ (2000) Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol Cell Biol 20 : 1030–1043.
28. FangC, BourdetteD, BankerG (2012) Oxidative stress inhibits axonal transport: implications for neurodegenerative diseases. Mol Neurodegener 7 : 29.
29. CaiQ, LuL, TianJH, ZhuYB, QiaoH, et al. (2010) Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron 68 : 73–86.
30. TanSC, SchererJ, ValleeRB (2011) Recruitment of dynein to late endosomes and lysosomes through light intermediate chains. Mol Biol Cell 22 : 467–477.
31. ShererNM, LehmannMJ, Jimenez-SotoLF, IngmundsonA, HornerSM, et al. (2003) Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4 : 785–801.
32. MorfiniGA, YouYM, PollemaSL, KaminskaA, LiuK, et al. (2009) Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12 : 864–871.
33. OlivaAAJr, AtkinsCM, CopenagleL, BankerGA (2006) Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci 26 : 9462–9470.
34. NagielA, Andor-ArdoD, HudspethAJ (2008) Specificity of afferent synapses onto plane-polarized hair cells in the posterior lateral line of the zebrafish. J Neurosci 28 : 8442–8453.
35. SeoJ, AsaokaY, NagaiY, HirayamaJ, YamasakiT, et al. (2010) Negative regulation of wnt11 expression by Jnk signaling during zebrafish gastrulation. J Cell Biochem 110 : 1022–1037.
36. ZentrichE, HanSY, Pessoa-BrandaoL, ButterfieldL, HeasleyLE (2002) Collaboration of JNKs and ERKs in nerve growth factor regulation of the neurofilament light chain promoter in PC12 cells. J Biol Chem 277 : 4110–4118.
37. GuptaS, CampbellD, DerijardB, DavisRJ (1995) Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267 : 389–393.
38. HoriuchiD, CollinsCA, BhatP, BarkusRV, DiantonioA, et al. (2007) Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol 17 : 1313–1317.
39. KoushikaSP (2008) “JIP”ing along the axon: the complex roles of JIPs in axonal transport. Bioessays 30 : 10–14.
40. De VosKJ, GriersonAJ, AckerleyS, MillerCC (2008) Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci 31 : 151–173.
41. KimmelCB, BallardWW, KimmelSR, UllmannB, SchillingTF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203 : 253–310.
42. Westerfield M (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). Eugene: Univ. of Oregon Press.
43. ListerJA, RobertsonCP, LepageT, JohnsonSL, RaibleDW (1999) nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126 : 3757–3767.
44. ZhangY, BuchholzF, MuyrersJP, StewartAF (1998) A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet 20 : 123–128.
45. MullinsMC, HammerschmidtM, HaffterP, Nusslein-VolhardC (1994) Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol 4 : 189–202.
46. MullinsMC, Nusslein-VolhardC (1993) Mutational approaches to studying embryonic pattern formation in the zebrafish. Curr Opin Genet Dev 3 : 648–654.
47. AndermannP, UngosJ, RaibleDW (2002) Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol 251 : 45–58.
48. UngosJM, KarlstromRO, RaibleDW (2003) Hedgehog signaling is directly required for the development of zebrafish dorsal root ganglia neurons. Development 130 : 5351–5362.
49. SuzukiSO, McKenneyRJ, MawatariSY, MizuguchiM, MikamiA, et al. (2007) Expression patterns of LIS1, dynein and their interaction partners dynactin, NudE, NudEL and NudC in human gliomas suggest roles in invasion and proliferation. Acta Neuropathol 113 : 591–599.
50. NechiporukA, LinboT, RaibleDW (2005) Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development 132 : 3717–3730.
51. AbramoffMD, MagalhaesPJ, RamSJ (2004) Image Processing with ImageJ. Biophotonics International 11 : 36–42.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



