-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Rhombomeres (r) contribute to brainstem auditory nuclei during development. Hox genes are determinants of rhombomere-derived fate and neuronal connectivity. Little is known about the contribution of individual rhombomeres and their associated Hox codes to auditory sensorimotor circuitry. Here, we show that r4 contributes to functionally linked sensory and motor components, including the ventral nucleus of lateral lemniscus, posterior ventral cochlear nuclei (VCN), and motor olivocochlear neurons. Assembly of the r4-derived auditory components is involved in sound perception and depends on regulatory interactions between Hoxb1 and Hoxb2. Indeed, in Hoxb1 and Hoxb2 mutant mice the transmission of low-level auditory stimuli is lost, resulting in hearing impairments. On the other hand, Hoxa2 regulates the Rig1 axon guidance receptor and controls contralateral projections from the anterior VCN to the medial nucleus of the trapezoid body, a circuit involved in sound localization. Thus, individual rhombomeres and their associated Hox codes control the assembly of distinct functionally segregated sub-circuits in the developing auditory brainstem.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003249
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003249Summary
Rhombomeres (r) contribute to brainstem auditory nuclei during development. Hox genes are determinants of rhombomere-derived fate and neuronal connectivity. Little is known about the contribution of individual rhombomeres and their associated Hox codes to auditory sensorimotor circuitry. Here, we show that r4 contributes to functionally linked sensory and motor components, including the ventral nucleus of lateral lemniscus, posterior ventral cochlear nuclei (VCN), and motor olivocochlear neurons. Assembly of the r4-derived auditory components is involved in sound perception and depends on regulatory interactions between Hoxb1 and Hoxb2. Indeed, in Hoxb1 and Hoxb2 mutant mice the transmission of low-level auditory stimuli is lost, resulting in hearing impairments. On the other hand, Hoxa2 regulates the Rig1 axon guidance receptor and controls contralateral projections from the anterior VCN to the medial nucleus of the trapezoid body, a circuit involved in sound localization. Thus, individual rhombomeres and their associated Hox codes control the assembly of distinct functionally segregated sub-circuits in the developing auditory brainstem.
Introduction
The mammalian brainstem plays a crucial role in the regulation of many vital functions through a complex system of reflex arcs and relays information to higher brain centers through interconnected neuronal circuits. During development, the hindbrain becomes subdivided along the antero-posterior (A-P) axis into serially repeated, spatially segregated, modules of progenitor cells, the rhombomeres (r). Individual rhombomeres give rise to distinct portions of sensory and motor columns depending on the position of progenitors along the dorso-ventral (D-V) axis, respectively [1], thus generating nuclei of multi-segmental origin and topographic patterns of connectivity [2], [3], [4], [5], [6], [7]. For instance, in the somatosensory system, afferent innervation from mandibular or whisker pad (maxillary) facial dermatomes targets the r2 - or r3-derived components of the principal trigeminal sensory nucleus, respectively. In turn, the information is somatotopically relayed to the thalamus and somatosensory cortex, contributing to build a facial somatosensory map [7]. Vestibular nuclei also originate from different rhombomeres and display specific sets of axonal trajectories with distinct targets [3], [4], [8], [9]. Thus, regional patterning along the A-P axis and specific D-V determinants intersect to determine sub-circuit connectivity within functionally related longitudinal neuronal columns.
Topographic connectivity and employment of sensory and motor nuclei are also well described during formation of auditory-dependent circuits [10]. The auditory central pathway consists of sensory nuclei transmitting ascending acoustic information, and efferent motor neurons modulating primary afferent responses. The sensory organ for sound is the cochlea, which contains two types of receptors, namely the inner and outer hair cells. While the inner hair cells (IHCs) are the major detectors of auditory stimuli, the outer hair cells (OHCs) enhance low level sounds by increasing the amplitude and frequency selectivity of basilar membrane vibrations, a process called “cochlear amplification” [11], [12]. From the periphery, sound information travels through the VIIIth cranial nerve to the brainstem cochlear nuclear (CN) complex, which is the primary relay station for central auditory processing [13]. This cochlear complex originates from distinct portions of the r2–r5 region, which will give rise to the anteroventral (AVCN), posteroventral (PVCN) and dorsal (DCN) cochlear nuclei, as well as to the cochlear granule cell population of the microneuronal shell [6]. A significant portion of the CN complex derives from a dorsal rim of neuroepithelium referred to as the auditory lip [6], [14], which is part of the lower rhombic lip and selectively expresses the transcription factor Atoh1 (also known as Math1) [15], [16], [17]. Variously processed sound-related signals, ultimately leading to qualitative sound perception, travel from the cochlear nuclei through the lateral lemniscus (LL) complex to the inferior colliculus (IC; midbrain) and medial geniculate nucleus (MG) of the thalamus, which in turn relays auditory information to the auditory cortex. On the other hand, temporal and spatial sound localization are relayed by the cochlear nuclei through a parallel pathway in the ventral brainstem, before reaching high level brain structures [18]. This pathway includes the superior olivary complex (SOC), which is mostly derived from r5 and is partly composed of the corresponding Atoh1+ lineage [3], [16], [19].
Proper hearing function is also controlled by centrifugal (efferent) motor connections, which modulate the incoming afferent sensory auditory information. The major component is represented by the olivocochlear neurons (OC), a subpopulation of the inner ear efferent (IEE) neurons, which are born in ventral r4, cross the midline during early development and segregate from their vestibular counterpart around embryonic day 14.5 of gestation (E14.5) in mice [20], [21]. While lateral OC (LOC) motor neurons innervate afferent sensory neurons in contact with the IHCs, modulate cochlear nerve excitability and protect the cochlea from neuronal damage in acute acoustic injury [21], [22], medial OC (MOC) motor neurons are innervated by reflex neurons of the PVCN [23], [24] and regulate the vibrating OHCs in the cochlea, modulating in this way the “cochlear amplification” process [25], [26], [27]. This is known as the MOC reflex. Cochlear efferent motor neurons also play a role in the normal maturation of afferent responses, particularly during the early postnatal period [21], [28]. Another feedback reflex, the middle-ear muscle reflex (MEM), is closed by facial and trigeminal branchiomotor neurons (FBM, TBM) that activate the stapedius and tensor tympani muscles respectively, thus tensing the chain of tympanic ossicles and reducing the amplitude of sound transmission through the middle ear [29], [30]. Thus, the MOC and MEM reflexes represent two parallel sound-evoked feedback mechanisms acting on the auditory periphery to modulate incoming acoustic stimuli [29], [31], [32].
Little is known about the molecular determinants involved in the assembly of rhombomere-derived auditory sub-circuits. The Hox genes, a large family of homeobox-containing genes, display rhombomere-restricted expression patterns and provide early patterning information to progenitors and their neuronal derivatives [33], [34]. In turn, the expression of several Hox genes is maintained through later stages of circuit formation in distinct, rhombomere-derived neuronal subpopulations contributing to portions of hindbrain sensory and motor nuclei [4], [7], [35], [36]. In the developing hindbrain, Hoxb1 selectively expressed in r4 is required to pattern r4-derived ventral efferent neurons, such as IEE and FBM, and to maintain normal levels of Hoxb2 in r4 [37], [38]. Hoxb2 and Hoxa2, unlike Hoxb1, are expressed in r2 to r5 auditory derivatives. Moreover, the expression of Hoxb2 and Hoxa2 is maintained in the ventral CN, ventral nucleus of LL (VLL), and SOC nuclei throughout prenatal and postnatal developmental stages [36]. Thus, Hox genes are prime candidates to be involved in the assembly of sensorimotor functional circuits in the developing hindbrain.
In this study, we find that rostral rhombomeres and their associated Hox genes are required in establishing and maintaining two major functional circuits in the central auditory system, which have different rhombomeric origins. Firstly, we carried out a detailed fate map of r4 derivatives by generating a novel, highly restricted, r4-specific Cre-recombinase driver. We show that cells originating from r4 significantly contribute to the VLL, an important relay station in the sound perception pathway. Furthermore, we found that r4 largely supplies cells to the PVCN and DCN, a finding largely in agreement with previous work [6], [16], but not to the granule cells of the microneuronal shell. Secondly, in Hoxb1 and Hoxb2 mutants, the VLL, PVCN, and MOC motor neurons are selectively affected, though with different severities, leading ultimately to elevated auditory thresholds in adult mutant mice. Thirdly, Hoxb1 negatively modulates Hoxa2 during r4 patterning, whereas Hoxa2 is mainly required in r2/r3 AVCN-derived development. Moreover, early conditional Hoxa2 inactivation in rhombic-lip derivatives selectively perturbs the AVCN axonal pathfinding to the medial nucleus of the trapezoid body (MNTB), resulting in decreased contralateral and increased ipsilateral targeting of MNTB due to the down-regulation of Rig1, the main axon guidance receptor for midline crossing. Altogether, this study provides, for the first time, genetic and functional evidence for a Hox gene network in the establishment and maintenance of proper auditory rhombomere-specific circuitry during hindbrain development.
Results
Mapping of rhombomere 4-specific contribution to the auditory system
Taking advantage of specific r2 - and r3/5-Cre-expressing mouse lines [39], [40], previous studies have genetically mapped the contribution of the r2–r5 rhombic lip to distinct portions of the CN and SOC complexes [6], [16], [19]. However, previous attempts to generate r4-specific lines invariably resulted in additional Cre expression caudal to r4 [7], [16], [41]. In this study, we generated a novel mouse transgenic line, named b1r4-Cre, which allowed us to exclusively map r4 and its derivatives throughout the mature brainstem (Figure 1A and Figure S1). To this purpose, we used a well characterized enhancer from the Hoxb1 locus [42] to drive the Cre-recombinase gene exclusively in r4 (Figure S1A). In b1r4-Cre transgenic animals, onset of Cre expression in presumptive r4 occurs first in a mosaic fashion (Figure S1C), but from E9.0 onwards, Cre is expressed throughout r4 as shown in Figure S1D. To permanently label the polyclonal population of cells derived from r4, the b1r4-Cre transgenic line was mated to the ROSA26-YFP reporter mouse [43] and progenies positive for both alleles (herein called b1r4-Cre/YFP) were analyzed (Figure 1A). Accordingly, at E10.5 activation of YFP was observed exclusively in r4 and its cellular progeny, as shown by double staining of Cre and YFP (Figure 1B, 1C). No ectopic expression of Cre was detected at later stages (data not shown).
Fig. 1. Rhombomere 4 neuronal derivatives contribute to nuclei involved in auditory perception. 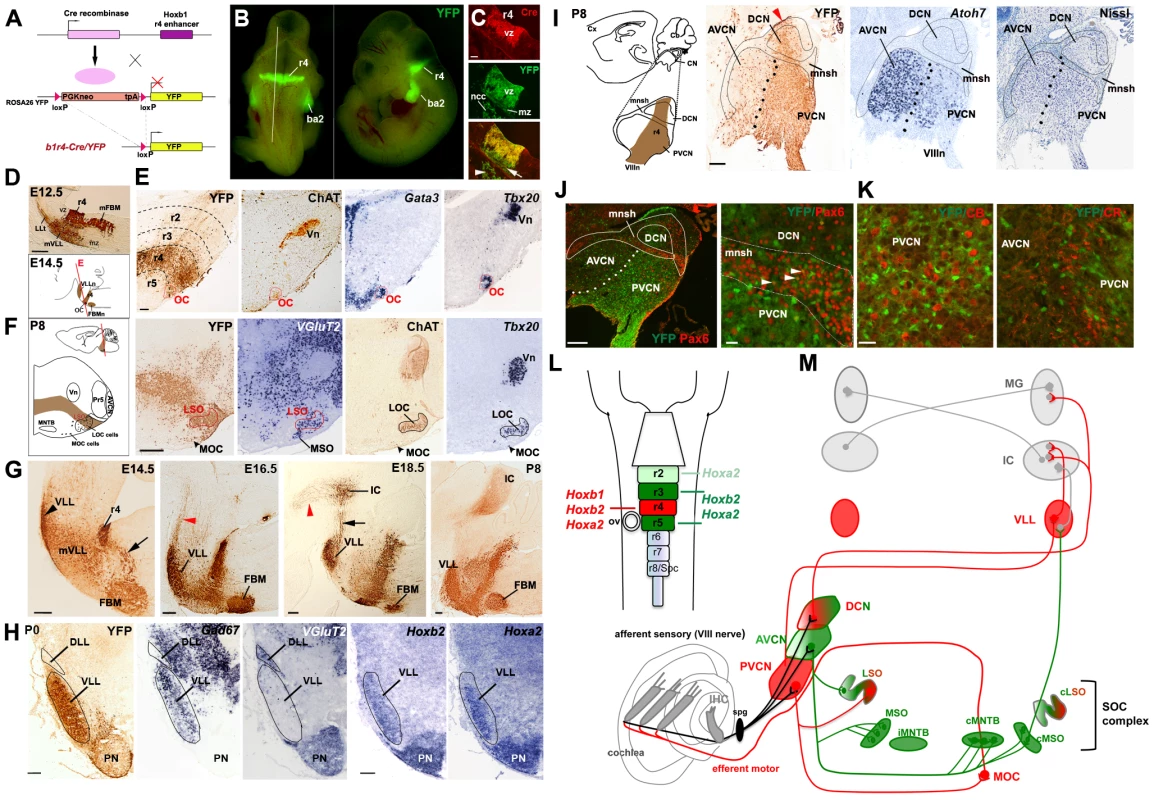
(A) Strategy for the Cre-loxP recombinase r4-fate map. Upon Cre-mediated recombination, the loxP sites surrounding the PGKneo cassette of the ROSA26 YY reporter line are excised and YFP is expressed exclusively in r4 and r4 derivatives. (B) Dorsal and lateral views of a E10.5 b1r4-Cre/YFP embryo show restricted expression of YFP in r4 and neural crest-derived cells (ncc) in the second branchial arch (ba2). The white line delineates the level and plane of sagittal section of panels in (C). (C) r4-restricted immunostaining of Cre-recombinase in progenitors of the ventricular zone (vz). YFP+/Cre− post-mitotic cells at the marginal zone (mz) originate from YFP+/Cre+ progenitors. (D) Sagittal sections of an E12.5 b1r4-Cre/YFP embryo immunostained with a GFP antibody reveal the r4 domain, the caudal migration of facial branchiomotor neurons (mFBM), the ventricular to pial migration of presumptive lateral lemniscus cells (mVLL) and the lateral lemniscus tract (LLt) projecting rostrally. Below, a schematic of an E14.5 sagittal section indicating the position of the various nuclei. The red line delineates the plane of section of panels (E). (E) The olivocochlear (OC) neurons (delimitated by a red contour) express choline acetyltransferase (ChAT), Gata3 and Tbx20. (F) Schematic coronal section of a P8 brain illustrating the positions of the various nuclei. The lateral superior olive (LSO) but not the medial superior olive (MSO) nucleus, has an r4 origin, as indicated by YFP and VGlut2 staining. ChAT and Tbx20 are expressed in lateral (LOC) and medial OC (MOC) neurons within the LSO and ventral to the LSO, respectively. (G) Sagittal sections at different ages indicating the YFP+ r4-derivatives: the ventral lateral lemniscus (VLL) (rostrally) and FBM (caudally) nuclei. VLL and cochlear neurons project rostrally to the inferior colliculus (IC) and some fibers continue to the thalamus (arrowheads). (H) Coronal sections of P0 pups indicate high contribution of r4/YFP+ cells to the VLL, positive for Gad67, but not to the dorsal LL (DLL), which is VGlut2+. Hoxb2 and Hoxa2 are expressed in the VLL and pontine nuclei (PN). A few dispersed YFP+ cells are identified in the PN. (I) Schematic of a P8 brain sagittal section showing the position of the cochlear nuclear complex (CN) and its subdivision into anteroventral (AVCN), posteroventral (PVCN) and dorsal (DCN) nuclei. Adjacent sagittal sections illustrate a high contribution of YFP+ cells to the PVCN and DCN. The arrowhead indicates the origin of r4-migrating cells. Dots delineate the presumptive boundary between AVCN and PVCN. Only a few YFP-positive cells label the AVCN, which is highly positive for Atoh7. The small intensely basofilic granule cells confined to the microneuronal shell (mnsh and outlined) and identified by Nissl and Pax6 expression (J) are not positive for YFP, indicating that cochlear granule cells do not have an r4-origin. (K) Similarly, YFP+ cells do not co-localize with calbindin- (CB) and calretinin- (CR) expressing cells in the PVCN region. (L) Schematic of the hindbrain in which rhombomeres 2 to 5 and their respective Hox genes are color-coded. The same code refers to (M). (M) Overview of the two main central auditory pathways, the MOC reflex and their rhombomeric origin. While r4-derivatives (in red) contribute mainly to the ascending sound perception pathway, which runs from the CN through the VLL to the IC, r2-, r3- and r5-derivatives (in green) contribute mainly (but not exclusively) to the pathway running through the superior olivary complex (SOC), which function in the localization of the temporal and spatial origins of sounds. The MOC reflex comprising of sensory PVCN interneurons and motor efferent OC neurons is also an r4-derivative. Vn, trigeminal motor nucleus; MNTB, medial nucleus of trapezoid body; Pr5 principal sensory trigeminal nucleus. Scale bars, 100 µm (C), 200 µm (D–J left), 20 µm (J right, K). See also Figures S1 and S2. We next analyzed the b1r4-Cre/YFP mouse line at different embryonic stages and early postnatal ages, using an anti-GFP antibody that cross-reacts with the YFP protein and labels proliferative and migrating/differentiating cells and their axonal projections. The r4 radial histogenetic territory itself is massively labeled and becomes morphogenetically deformed into a wedge-shaped, dorsally compressed configuration (Figure 1D, 1G). The first cohort of cells migrating out of r4 consists of the well-described caudomedial stream of tangentially migrating FBM neurons, which first move caudolaterally into r6 and then reach their definitive ventrolateral subpial position by radial migration [44], [45], [46] (Figure 1D, 1G). Another r4-derived efferent population is represented by OC neurons [20], [21], a subpopulation of IEE neurons. At E14.5 they form a compact superficial group of cells at the r4/r5 margin and selectively express the cholinergic marker ChAT [21] and the transcription factors Gata3 and Tbx20 [47], [48] (Figure 1E). At P8 the OC neurons split into lateral (LOC) and medial (MOC) components [49], which express ChAT and Tbx20 and become located within the lateral superior olive (LSO) and in the medioventral portion of the SOC as scattered cells, respectively (Figure 1F). A part of the LSO, expressing the glutamatergic marker VGlut2 [16], is included within the r4 domain (Figure 1F).
Another sizeable stream of labeled r4-derived cells, which was not previously described, migrates to the basal longitudinal zone and then moves rostrally along the growing lateral lemniscus tract, which courses obliquely through the rostral hindbrain (Figure 1D, 1G). These cells will contribute to the majority of the VLL from E14.5 onwards (Figure 1G). At E16.5, labeled ascending lateral lemniscus fibers, originating from the ipsilateral r4-derived VLL neurons and also from the r4-derived projection neurons of the contralateral CN [50], reach the IC. At E18.5, lateral lemniscus fibers also extend along the brachium of the IC into the medial geniculate nucleus of the thalamus, and collaterals can be distinctly seen in the superior colliculus intermediate layers at P8 (Figure 1G, and data not shown). Transverse sections at P0 clearly show a high density of YFP+ cells in the VLL but not in the dorsal nucleus of LL (DLL), which expresses the glutamatergic marker VGlut2 and originates from the Atoh1+ lineage [19], [51]. The majority of VLL neurons express the inhibitory GABAergic/glycinergic marker Gad67, a particular feature of this auditory structure, as previously described [52], and Hoxb2 and Hoxa2 [36]. In summary, our fate map study shows for the first time that r4 massively contributes to the VLL within the LL complex.
Next, we mapped precisely r4 contribution to the plurisegmental CN complex (Figure 1I). Previously, the contribution of r4 was indirectly inferred from the mapping of r3 and r5 derivatives, or from the mapping of the territory posterior to r3 [6], [16]. These studies indicated that the AVCN is derived from r2 and r3, the PVCN from r3 and r4, and the DCN from r4 and r5 (summarized in Figure 1L, 1M). A significant proportion of these nuclei were strongly affected in Atoh1 conditional and null mutants, supporting their origin from the Atoh1+ auditory lip [16], [17].
Our results are largely in agreement with previous data and further extend them. First, we show that at E10.5 the YFP+ domain includes Atoh1-expressing cells in the rhombic lip region of dorsal r4 (Figure S2A). However, at E14.5 when Atoh1+ cells migrating from r2 to r5 rhombic lip invade the presumptive cochlear complex [17], only a few r4-derived YFP+ cells overlap with Atoh1 expression domain, whereas no co-localization of YFP with the granule cell marker Barlh1 [6] is found in this region (Figure S2B). Secondly, we show that YFP+ cells contribute to an intermediate sector across the CN complex, which crosses dorsoventrally the magnocellular core portion of the DCN and then gives rise to the majority of the PVCN (Figure 1I and Figure S2C–S2E). Small portions of the DCN remain unlabeled, suggesting additional contribution from r5, as previously reported [6], [16], but also a likely contribution from r3 to the region of DCN anterior to the YFP+ domain. Thirdly, we found that only a small number of YFP+ cells are distributed in the AVCN at P8, which, unlike the PVCN, expresses high levels of Atoh7 (also known as Math5) [53] (Figure 1I). Finally, we started to characterize the cellular identity of YFP+ cells and found that at P8, r4-derived cells fail to co-express Pax6 (Figure 1J), a marker for the microneuronal granule cell population [15], [54]. Moreover, YFP signal is absent in calbindin - and calretinin-expressing neurons (Figure 1K), corresponding to octopus and globular-bushy cells, respectively [55], [56]. These data indicate that subpopulations of glutamatergic neurons, which normally derive from the Atoh1+ neuroepithelial domain [15], do not originate from r4. A full characterization of YFP+ cells in embryonic and adult stages will be reported elsewhere (M.D., L.P. and M.S., in preparation).
In summary, we show that r4 largely contributes to the motor cochlear efferent neurons, to the relay VLL neurons, and within the cochlear nucleus, to the majority of the PVCN, and part of the DCN. Thus, while r4 seems to be required for the structures involved in sound transmission, amplification and protection (i.e. PVCN, VLL, and OC), r2, r3 and r5 are likely contributing to components of the sound localization pathway that runs through the SOC and trapezoid body complex before reaching higher-order auditory structures (Figure 1L, 1M).
Overall, our r4 fate mapping, combined with previously published r2, r3 and r5 maps [6], [16] and patterns of early and late Hoxa2 and Hoxb2 expression profiles [36], strongly suggest rhombomere-specific and Hox-dependent assembly of the two main central auditory sub-circuits (Figure 1L, 1M).
Regulation of Hoxb1, Hoxb2, and Hoxa2 in sensory r4 of Hox mutant mice
Previous work dissected the genetic and regulatory network involved in establishing and maintaining the identity of r4 progenitors [34]. Hoxb1 plays a key role in patterning ventral r4 progenitors, partly through transcriptional regulation of Hoxb2 and Hoxa2 [37], [38], [57]. Maintenance of Hoxb1 expression in ventral r4 requires both Hoxb1 itself and Hoxb2 through auto - and cross-regulatory interactions, respectively [37], [58], [59], [60]; however, it is not known whether a similar mechanism is acting in dorsal/sensory r4. Unlike Hoxb1, Hoxb2 and Hoxa2 expression is maintained in differentiated r4-derivatives, such as the VLL and VCN ([36] and our study). Thus, Hoxb1 may pattern sensory r4-derivatives by regulating dorso-ventral Hoxb2/Hoxa2 expression in r4 progenitor cells, hence controlling post-mitotic specification during the development of the sensory system. To test this hypothesis and investigate the roles of Hoxb1, Hoxb2, and Hoxa2 in the development of auditory pathways, we analyzed the effects of their functional inactivation.
Hoxb1 functional deletion results in an early re-patterning of the ventral r4 territory into a more anterior identity [44], [61]. To bypass the early Hoxb1 role in r4 neuroepithelium and investigate its requirement during neurogenesis, we generated a novel Hoxb1 conditional mutant allele, Hoxb1flox (Figures S3 and S4; see Materials and Methods) and mated it to the b1r4-Cre driver. Conditional b1r4-Cre;Hoxb1flox/flox homozygous mutant mice (hereafter referred to as Hoxb1lateCKO) are viable and fertile. Mutant embryos retain Hoxb1 expression in r4 until E8.75–E9.0 (Figure 2A), in accordance with the timing of Cre expression and onset of Cre-mediated excision (Figure 1 and Figure S1). At E9.25, Hoxb1 expression is no longer present in mutant r4, while the remainder of its expression is unaltered (Figure 2A).
Fig. 2. Regulatory interactions between Hoxb1, Hoxb2, and Hoxa2 in r4. 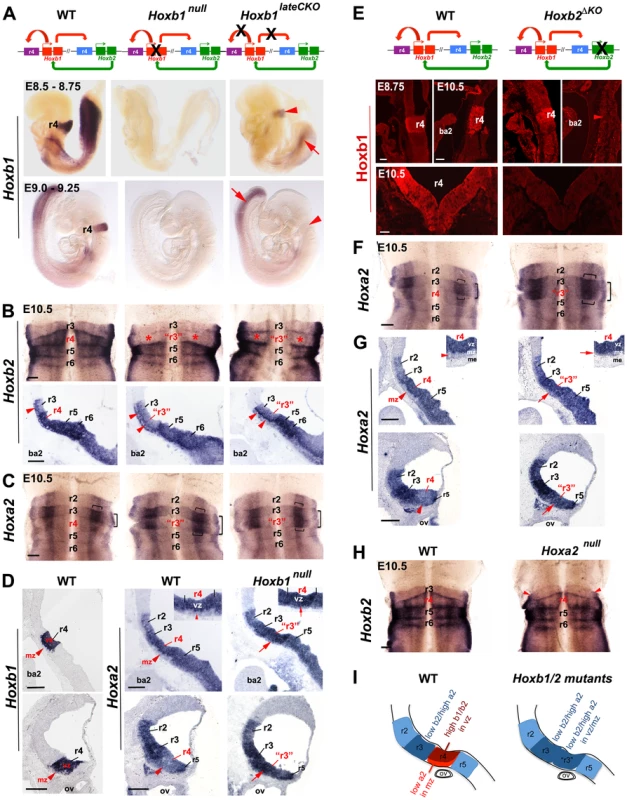
(A) The diagrams above the panels indicate the interactions between Hoxb1 and Hoxb2. While Hoxb1 auto-regulates its own expression in r4, it also binds to an Hoxb2 r4 enhancer to maintain Hoxb2 expression in r4. Hoxb2 maintains expression of Hoxb1 in r4. Crosses indicate loss of Hoxb1 protein in Hoxb1null embryos and loss of the auto- and cross-regulatory loops in Hoxb1lateCKO mutants. Lateral views of E8.5 to E9.25 embryos indicate that while Hoxb1 expression is still maintained in r4 (although at lower levels) of E8.75 Hoxb1lateCKO mutants, r4 expression is completely abolished in E9.25 mutant embryos (arrowheads). Expression in the posterior region is still maintained at both ages (arrows). (B) Ventricular views of flat-mount preparations of E10.5 WT, Hoxb1null and Hoxb1lateCKO hindbrains hybridized with Hoxb2. Expression of Hoxb2 is strongly decreased (but not abolished) in r4 of Hoxb1null and Hoxb1lateCKO embryos, at similar levels to r3 (asterisks). R4 acquires an expression pattern of r3, as indicated by “r3”. Down-regulation of Hoxb2 in r4 can also be appreciated in mid-sagittal sections of mutant embryos. The line of cells expressing high levels of Hoxb2 denotes early post-mitotic cells (arrowhead). (C) Ventricular views of flat-mount preparations of E10.5 WT, Hoxb1null and Hoxb1lateCKO hindbrains hybridized with Hoxa2. Expression of Hoxa2 is increased in r4 and the characteristic Hoxa2 expression profile of r3 is now duplicated in r4 of Hoxb1null and Hoxb1lateCKO embryos supporting an r4 to r3 change of identity. The horizontal and vertical brackets indicate higher expression domains of Hoxa2, respectively in a large intermediate stripe and in a thinner lateral stripe of the sensory column, the presumptive auditory column. (D) On the left, expression of Hoxb1 indicates the position of r4 on sagittal sections. On the right, increased expression of Hoxa2 is detected in the ventricular and mantle layers of r4 at two different dorsal levels in Hoxb1null embryos. In mutant embryos, the expression of Hoxa2 is maintained at levels comparable to r3 in the r4 mantle zone (mz) (i.e. post mitotic neurons) with respect to WT (arrows, see also insets). (E) In Hoxb2ΔKO mutants, lack of Hoxb2 (indicated with a cross) results in failure to maintain Hoxb1 expression in r4. Sagittal and coronal views show that Hoxb1 protein is present in r4 of E8.75 embryos, but not maintained in E10.5 Hoxb2ΔKO mutant embryos. (F) Flat-mounted hindbrain preparations hybridized with Hoxa2 show a duplication of r3 features in r4 in E10.5 Hoxb2ΔKO mutant embryos. Hoxa2 expression levels are increased in r4 in the absence of Hoxb2, similarly to Hoxb1 mutant embryos. (G) E10.5 sagittal sections in dorsal regions indicate increased expression of Hoxa2 in ventricular (vz) and mantle (mz) zones of r4 (arrows, see also insets) mimicking the expression profile of r3. (H) Flat-mounted hindbrain preparations of E10.5 WT and Hoxa2null embryos hybridized with Hoxb2. No particular expression changes can be observed in mutant embryos. Red arrowheads indicate a defect in alar r2/r3, as previously described [66]. (I) Schematics summarizing the expression of Hox genes in r4 of Hoxb1 or Hoxb2 mutants. Scale bars, 200 µm (B, C, F and H); 100 µm (E top); 50 µm (E bottom). ba2, second branchial arc; me, mesoderm. See also Figures S3, S4 and S5. We also obtained constitutive Hoxb1null homozygous mutants (Figures S3, S4; see Materials and Methods) that exhibit total loss of Hoxb1 expression from its onset (Figure 2A) and lack any selectable cassette that might interfere with adjacent Hox genes [62], [63]. To specifically trace r4 derivatives from the control and the two distinct Hoxb1 mutants throughout embryonic and postnatal brains, Hoxb1flox and Hoxb1null homozygous mice were mated with the b1r4-Cre/YFP reporter line (see Materials and Methods for mating schemes). In addition, we generated a novel, viable and fertile Hoxb2 homozygous mutant allele (hereafter referred to as Hoxb2ΔKO), that, similarly to the Hoxb1null and unlike previously described Hoxb2 knockout alleles [37], [58], [64], has no selectable marker left within the locus (see Materials and Methods). Finally, we made use of the previously described Hoxa2null and conditional Hoxa2flox alleles [7], [65].
Next, we assessed Hox cross-regulatory interactions within r4 in Hoxb1, Hoxb2 and Hoxa2 mutant alleles. Flat-mounted preparations and sagittal sections of control embryos show high Hoxb2 expression levels in r4 to r6 and low levels in r3 (Figure 2B). In E10.5 Hoxb1null and Hoxb1lateCKO hindbrain preparations, Hoxb2 expression is down-regulated throughout r4 to similar levels as in r3 (asterisks in Figure 2B), resulting in a duplication of r3-features (“r3” in Figure 2B). This is confirmed in mid-sagittal sections showing a decrease of Hoxb2 expression in r4 progenitors of Hoxb1null and Hoxb1lateCKO. Expression is still maintained in early differentiating cells, similarly to r3 (arrowheads in Figure 2B). In contrast, Hoxa2 is normally expressed at low levels in r2 and r4, and at high levels in r3, particularly in a wide intermediate stripe of the dorsal sensory column (horizontal bracket in Figure 2C) and in a thinner stripe laterally (vertical bracket in Figure 2C), the presumptive auditory column. In E10.5 Hoxb1 mutant embryos, expression of Hoxa2 is abnormally up-regulated in r4, predominantly in the two sensory stripes, resulting in a duplication of r3-typical features in r4 (Figure 2C). Sagittal sections at different alar plate levels of Hoxb1null mutant embryos confirm up-regulation of Hoxa2 in the ventricular zone, and, strikingly, also in the mantle zone of r4 (arrows in Figure 2D and inset), which normally expresses low levels of Hoxb1 and Hoxa2 (arrowheads in Figure 2D and inset). In mutant r4, Hoxa2 is maintained at high levels in the post-mitotic neurons, similarly as in r3. Thus, our data show that in the absence of Hoxb1, r4 acquires Hox features typical of r3, such as low levels of Hoxb2 and high levels of Hoxa2, indicating a re-patterning of r4 into r3. In addition, Hoxb1 differentially regulates Hoxb2 and Hoxa2 expression levels in r4, further supporting a complex regulatory interaction between Hoxb1 and Hoxb2/Hoxa2 in specifying r4 identity [37], [38], [57], [58], [59].
To investigate whether Hoxb2 acts similarly to Hoxb1 in r4 patterning, we assessed Hoxb1 and Hoxa2 expression in WT and Hoxb2ΔKO hindbrains from E8.75 to E10.5. Hoxb1 protein is lost in Hoxb2ΔKO at E10.5, though it is present at earlier embryonic stages (Figure 2E), indicating that Hoxb2 is required for maintaining Hoxb1 in r4. Similarly to Hoxb1 mutants, Hoxa2 expression is dramatically up-regulated throughout r4, with particular emphasis in the intermediate and lateral columns (Figure 2F), and in post mitotic neurons (arrows in Figure 2G and inset). In contrast, no changes are observed in Hoxa2null embryos (Figure 2H), apart from the r2/r3 alar defects previously described [66]. Taken together, our data show that within r4, Hoxb2 acts mainly in maintaining high expression of Hoxb1 and that in its absence, r4 acquires tr3-typical features, similarly to Hoxb1 mutant hindbrains. Importantly, we found increased Hoxa2 expression, at levels similar to r3, in post-mitotic r4 neurons of Hoxb1 and Hoxb2 mutants, implying that r4-derived sensory cells abnormally maintain Hoxa2 expression throughout hindbrain development (Figure 2I).
Finally, we investigated whether Hoxb1 and Hoxb2 are required in the differentiation process of the IEE and OC motor neurons, which normally differentiate in ventral r4 and interact with dorsally-derived sensory structures during the development of the auditory sensorimotor circuitry. At E10.5, IEE are located in the r4 mantle zone next to the floor plate and normally express Gata2/3, Isl1 and Phox2b [48], [59], [67] (Figure S5A, S5B). No IEE neurons are identified in E10.5 Hoxb1null and Hoxb1lateCKO embryos, as seen by the absence of Gata2/3 expression within the pool of Phox2b+/Isl1+ motor neurons (Figure S5B). This is not due to a delay in specification, since no OC neurons, positive for ChAT and Gata3, located in ventral peri-olivary positions can be distinguished in E14.5 Hoxb1 mutant embryos (Figure S5E). On the contrary, a few Gata3+ and Tbx20+ cells are preserved in Hoxb2ΔKO mutant embryos at E10.5 and E14.5, even if they are located in a slightly more dorsal position than in control embryos (Figure S5C, S5F). These data indicate that early IEE and late OC specification are maintained in Hoxb2ΔKO embryos despite the late absence of Hoxb1 in r4.
Hoxb1 is a key determinant gene in r4-derived VLL development
To investigate the requirement of Hox genes in the generation of sensory auditory structures, we first analyzed the size and position of the VLL with various markers on adjacent coronal sections at E18.5 and sagittal sections of P8 WT and mutant brains (Figure S6 and Figure 3). Very few cells contributing to the VLL are identified in E18.5 Hoxb1null mutant hindbrains, as shown by YFP and Gad67 staining (Figure S6B). Only some YFP+ cells with lemniscal projections, scattered rostral to the r4 wedge, are still maintained in Hoxb1null brains at P8 (Figure 3A). No detectable Hoxb2 or Hoxa2 expression can be found in sagittal sections at all levels, whereas reduced expression of Gata3 and Gad67, which both label the GABAergic/glycinergic cellular cohort of the VLL [52], [68], is still present in the remaining VLL, which is reduced by almost 90% in area (91.9±0.02) when compared to control VLL (Figure 3A, 3D). This indicates that the early absence of Hoxb1 function prevents normal r4-derived VLL specification and/or migration, and supports a major contribution of r4 to the formation of the VLL, particularly to its GABAergic cohort, which contributes to the majority of the VLL [52]. Moreover, absence of VGlut2 expression observed at E18.5, and confirmed at P8 (Figure S6B and Figure 3A), rules out any inhibitory to excitatory cell fate transformation within the VLL.
Fig. 3. The r4-derived VLL is affected in Hoxb1 and Hoxb2 mutant mice. 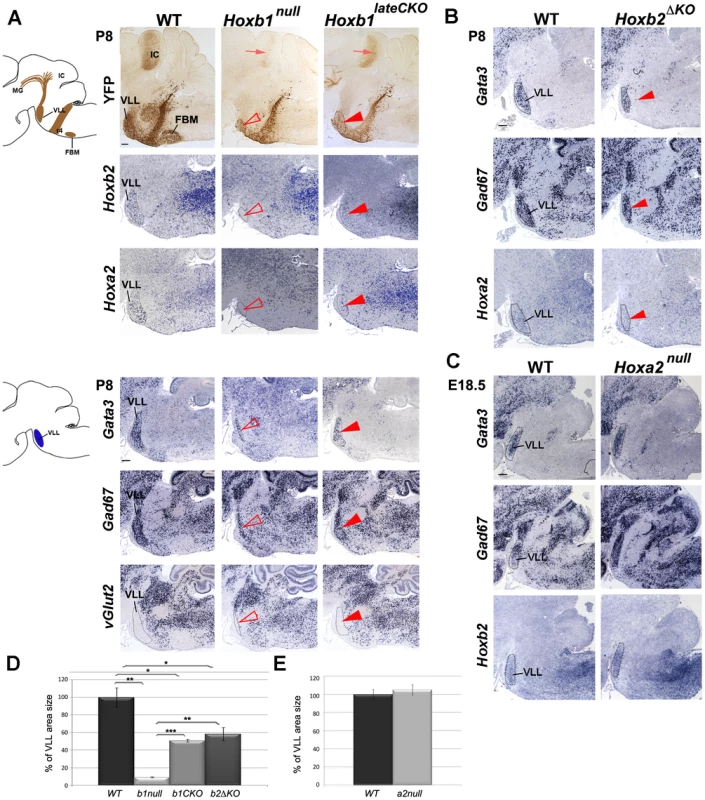
(A) Schematic view of a sagittal brain section indicating the YFP+ r4-derived nuclei and projections. A strong reduction of the YFP+ VLL nucleus (arrowhead) and projections (arrow) in Hoxb1 mutants is observed. In constitutive mutants the reduction is much more severe than in conditional mutants, as quantified in (D). Adjacent sagittal sections show no Hoxb2 and Hoxa2-expressing cells in Hoxb1null mutants, whereas cells in the reduced VLL of Hoxb1lateCKO still express Hoxb2 and Hoxa2. Adjacent sections of another P8 pup confirm reduction of the VLL and indicate persistence of Gata3- and Gad67-expressing cells in both Hoxb1 mutants. No ectopic expression of VGlut2 is detected in the VLL region. (B) The VLL is reduced in Hoxb2ΔKO mutant pups, similarly to Hoxb1lateCKO mutants, as indicated by expression of Gata3, Gad67, Hoxa2 and quantification in (D). (C) In contrast, Hoxa2null mutants show no significant changes in the VLL position and size quantified in (E). The apparently bigger shape is due to the slightly oblique sections in mutant compared to WT brains. (D) Histogram showing the percentage of the VLL area size in WT (set up to 100%) and in the different genotypes as indicated on the y-axis. Mutants show statistically significant differences when compared to WT, or when Hoxb1lateCKO and Hoxb2ΔKO are compared to Hoxb1null (inter-genotype comparison, ANOVA p<0.001; Hoxb1null versus WT: p = 0.001; Hoxb1lateCKO versus WT: p = 0.01; Hoxb2ΔKO versus WT: p = 0.04; Hoxb1lateCKO versus Hoxb1null: p<0.001; Hoxb2ΔKO versus Hoxb1null: p = 0.003). However, no statistically significant difference is found between Hoxb1lateCKO versus Hoxb2ΔKO (p = 0.39). (E) Histogram showing the percentage of the VLL area size in WT and Hoxa2null. No statistically significant difference is found (WT versus Hoxa2null: p = 0.56). FBM, facial branchiomotor nucleus; VLL, nucleus of lateral lemniscus; IC, inferior colliculus; MG, medial geniculate nucleus. Scale bars, 200 µm. See also Figure S6. A less severe reduction of the VLL was observed in Hoxb1lateCKO mutants (n = 3; 49.2±0.1% in area as compared to WT; Figure 3D and legend). This was mainly supported by the maintenance of Gata3-, Gad67-, Hoxb2 - and Hoxa2-expressing cells at E18.5 and P8, and by the presence, although reduced, of YFP+ VLL projections to the IC (Figure 3A and Figure S6B). Similarly, the VLL area is reduced by 41.7±0.3% in Hoxb2ΔKO (n = 3) when compared to WT (n = 3) brains (Figure 3D and legend), whereas no size reduction is measured in the VLL of Hoxa2null mutants (n = 3; 105±5.7%) at E18.5 before Hoxa2null perinatal death [69] (Figure 3C, 3E and legend). Together, these data indicate that Hoxb1 is an important determinant gene in r4-derived VLL development, because r4 to r3 change of identity occurring in Hoxb1 mutants prevent a proper VLL nucleus development. However, the persistence of some GABAergic cells in the VLL of Hoxb1null brains, suggests that other rhombomeres besides r4 might contribute to VLL formation. In contrast, Hoxb2 or Hoxa2 do not appear to play a predominant role in VLL specification. The reduction in VLL size in Hoxb2 mutant brains is most probably due to the failure of Hoxb2 maintaining Hoxb1 expression in r4, as suggested by the lack of statistically significant differences in VLL size between Hoxb1lateCKOand Hoxb2ΔKO mutant animals (Figure 3D).
Abnormal specification of r4-derived ventral cochlear structures in Hox mutants
We next investigated the involvement of Hoxb1, Hoxb2 and Hoxa2 in the development of the CN complex (Figure 4). In Hoxb1 mutant mice, no considerable changes in the overall size of the CN were observed at P8. However, careful analysis showed that r4-derived YFP+ cells massively invade the granule shell layer, normally derived from the Atho1+ lineage [15], [17], [19], and ectopically expressed Pax6, as confirmed by the abnormal presence of double YFP+/Pax6+ cells in the microneuronal shell (Figure 4A). This strongly indicates that r4-derived mutant cells have now acquired a granule cell identity, which r4 does not normally contribute to (Figure 1I–1J and Figure 4A). In addition, while Hoxb2 expression is maintained in the increased microneuronal shell of the Hoxb1 mutants, Hoxb2 and Hoxa2 expression levels are slightly affected in the ventral CN (Figure 4B). Namely, Hoxb2 is down-regulated in the PVCN (asterisks in Figure 4B), whereas Hoxa2 expression is slightly up-regulated in PVCN regions ventral to the microneuronal shell layer (arrows in Figure 4B), in line with their respective altered expression levels observed at E10.5 (Figure 2B–2D). Notably, Atoh7, predominantly expressed in specific Atoh1-derived post-mitotic glutamatergic populations of the AVCN [15], [53], is now strongly up-regulated in the PVCN of Hoxb1null and Hoxb1lateCKO mutants, indicating that the PVCN has acquired features of the r2/3-derived AVCN [6] (summarized in Figure 4E). Next, we asked whether Hoxb2 and Hoxa2 are also required in the specification of VCN components. Interestingly, Atoh7 and Hoxa2 expressions are also increased in the PVCN of Hoxb2ΔKO (arrows in Figure 4C). In contrast, Atoh7 and Hoxb2 expressions are strongly reduced in the AVCN of E18.5 Hoxa2null mutants (arrows in Figure 4D), in accordance with the early patterning defect previously described in the rostral hindbrain of these mutants [66].
Fig. 4. The cochlear nuclear complex is differently affected in Hoxb1, Hoxb2, and Hoxa2 mutants. 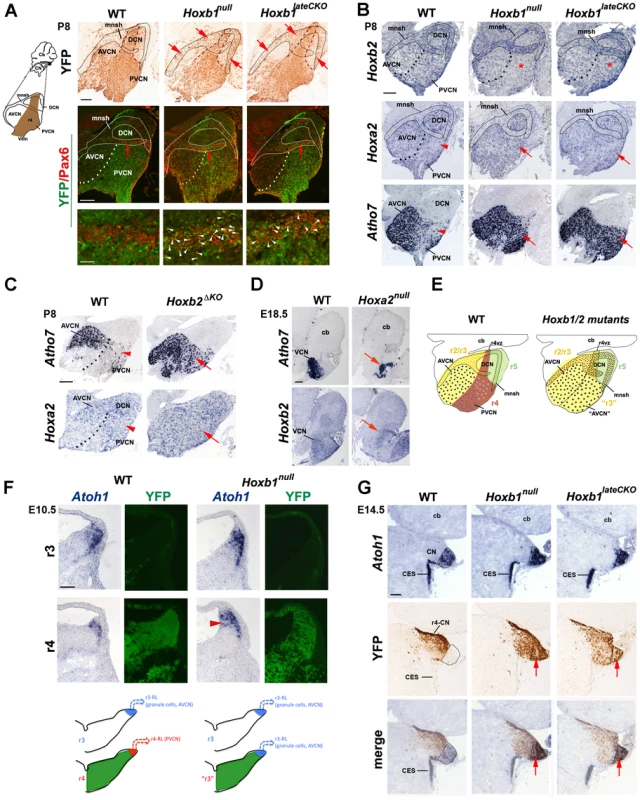
(A) Ectopic YFP+ r4-derived cells (arrows) are observed in the cochlear microneuronal shell (mnsh) (limited by solid line) of P8 Hoxb1null and Hoxb1lateCKO mutant sagittal sections. These cells now express Pax6 indicating that they are granule cells (white arrowheads). (B) Hoxb2 expression is decreased in the PVCN of Hoxb1null and in Hoxb1lateCKO mutants (asterisks). On the contrary, Hoxa2 expression is increased and Atoh7, normally expressed at high levels only in the AVCN, is dramatically up-regulated in the PVCN of P8 Hoxb1 (B) and Hoxb2ΔKO mutant pups (C) (arrows). Arrowheads in WT indicate Hoxa2 and Atoh7 low-expressing regions. (D) Formation of the AVCN is strongly affected in E18.5 Hoxa2null brains, as seen by decreased expression of Atoh7 and Hoxb2 (arrows). (E) Summary schematic indicating that in the absence of Hoxb1 and Hoxb2, the PVCN (r4-derived in brown) has acquired AVCN-like features (r2/3-derived in yellow) and YFP+ cell (brown) contribute to the shell. (F) The dorsal-most regions of WT and Hoxb1null hindbrains at r3 and r4 levels on adjacent coronal sections hybridized with Atoh1 and revealed by YFP epifluorescence (indicates r4 levels). Atoh1 is expressed in progenitors and differentiating cells migrating along the lateral ridge. The Atoh1-expressing domain is reduced in r4 compared to r3 in WT, whereas an enlarged Atoh1-expressing domain (arrowhead) is identified in r4 of Hoxb1null embryos. (G) On adjacent coronal sections at E14.5, YFP+ (r4-derived) cells located more laterally, do not overlap with Atoh1+ cells in the presumptive cochlear nucleus (CN), which originates from the r2–r5 auditory lip. In the absence of Hoxb1, YFP+ cells invade the Atoh1+ domain, thus acquiring the Atoh1 fate of adjacent rhombomeres. Cb, cerebellum; CES, caudal extramigratory stream. Scale bars, 200 µm (A up panels, B, C, D), 50 µm (A bottom panels), 100 µm (F, G). See also Figure S2. The dramatic increase of cells that express high levels of Atoh7 in the PVCN and the ectopic formation of YFP+ granule cells observed in Hoxb1 mutants (both are Atoh1+ lineage derivatives), led us to hypothesize that an increase of Atoh1 in r4 is the cause of the ectopic generation of glutamatergic neurons. Normally, the Atoh1+ domain in dorsal r4 (i.e. YFP+) of E10.5 embryos is smaller than in adjacent rhombomeres, such as r3 (Figure 4F). In contrast, in Hoxb1null mutant embryos this domain is enlarged to a similar extent as in control r3, suggesting an up-regulation of Atoh1 in r4, which may contribute to the acquisition of r3-like fate and hence to the ectopic generation of glutamatergic cell types (summarized in Figure 4F). This is even more pronounced at E14.5, when cells from the r2 to r5 rhombic lip contribute to the CN primordium. In WT embryos, the majority of YFP+ cells is identified lateral to the Atoh1+ region, whereas YFP+ cells abnormally express Atoh1 and clearly invade the Atoh1+ domain in Hoxb1null and Hoxb1lateCKO mutants, as demonstrated by the sizeable overlap of both domains (Figure 4G). Hence, Hoxb1 normally restricts the Atoh1+ domain in r4, impinging in this way on a specific r4 dorsal fate, which is different from the Atoh1+ lineage-related ones of adjacent rhombomeres. In summary, these data show that Hoxa2 is involved in the formation of the r2/3-derived AVCN, whereas Hoxb1 and Hoxb2 are required in the specification of the r4-derived PVCN by imposing an r4-specific identity during auditory development.
Axon pathfinding defects of cochlear nuclei in Hox mutant mice
We next assessed whether deletion of specific Hox genes may have direct consequences on the VCN connectivity pattern at postnatal stages. In P8 Hoxb1null and Hoxb1lateCKO mutants, we identified ectopic r4-derived YFP+ projections crossing the ventral midline and innervating the medial nucleus of the trapezoid body (MNTB), a normal contralateral target of r2/3 AVCN-derived fibers. These projections are never labeled by YFP in control mice, since they do not originate from the PVCN, the major source of r4-derived YFP+ CN projections (Figure 5A). Similarly, dextran injections in the PVCN of Hoxb2ΔKO label ectopic projections to the contralateral MNTB (cMNTB) (arrowhead in Figure 5B), whereas in control mice the very few axons projecting ventrally normally innervate contralateral MOC neurons as part of the MOC reflex (arrow in Figure 5B). This is in keeping with the finding that Atoh7 is increased in the PVCN of Hoxb1null, Hoxb1lateCKO and Hoxb2ΔKO mutants (Figure 4B, 4C), and the notion that AVCN Atoh7+ neurons normally target the MNTB [10], [53]. Thus, the molecular identity transformation of PVCN to AVCN observed in Hoxb1 and Hoxb2 mutant mice is further supported by abnormal connectivity to their respective targets (Figure 5C).
Fig. 5. Abnormal cochlear connectivity in Hoxb1, Hoxb2 and Hoxa2 mutants. 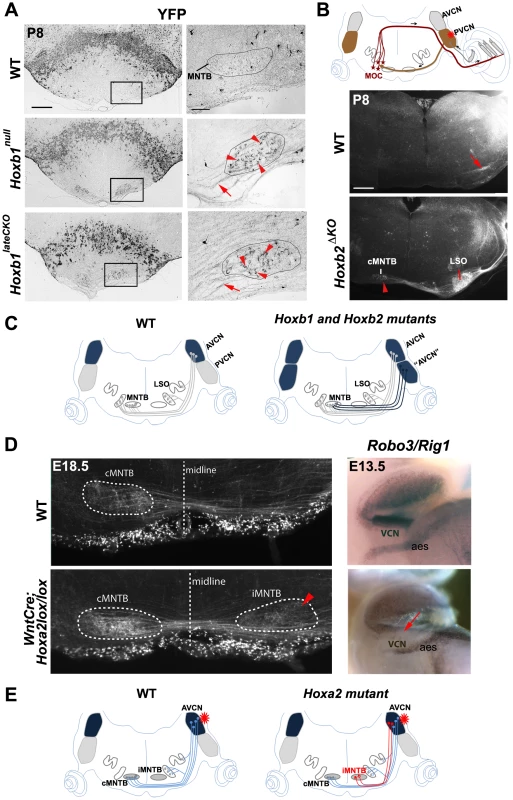
(A) Oblique P8 coronal sections show abnormal presence of YFP+ fibers projecting to the medial nuclei of the trapezoid body (MNTB) in Hoxb1null and Hoxb1lateCKO mutants. Right panels: enlarged views of the boxed areas to the left depicting abnormal YFP+ terminals (arrowheads) of labeled crossed fibers (arrows) surrounding cells of the MNTB (limited by solid line). (B) Schematic representation showing the position of the injected dextran at the level of the PVCN (asterisk). Normally, PVCN interneurons innervate contralateral MOC neurons, as indicated in the control coronal section (arrow). In Hoxb2ΔKO mutants, projections originating from the PVCN target now AVCN-specific targets, such as the contralateral MNTB (cMNTB) (arrowhead) and the lateral superior olivary (LSO) nuclei. (C) Schematics summarizing the normal connectivity pattern of cochlear AVCN neurons towards the nuclei of the superior olivary complex (SOC) complex, and the abnormal presence of YFP+ neurons behaving like AVCN neurons when Hoxb1 or Hoxb2 are inactivated. (D) A subpopulation of AVCN-labeled axons project abnormally to the ipsilateral MNTB (see arrowhead) when Hoxa2 is conditionally inactivated in the Wnt1+ (rhombic lip) domain. Expression of the Slit receptor Rig1 (or Robo3) is decreased in the VCN, indicating that absence of Rig1 affects midline crossing of AVCN projections. (E) Schematics summarizing the abnormal axonal projections of AVCN neurons in Wnt::Cre;Hoxa2lox/lox mice after dextran injection in the AVCN. AVCN, anterior ventral cochlear nucleus; PVCN, posterior ventral cochlear nucleus; PVCN IN, PVCN interneurons; MOC, medial olivocochlear neurons; aes, anterior extramural stream. Scale bars: 400 µm (A left panels, B), 50 µm (A right panels). To directly investigate the role played by Hoxa2 in VCN connectivity, we crossed the Hoxa2flox allele with a Wnt1::Cre driver [70] that allowed cell-autonomous inactivation of Hoxa2 at early stages in rhombic lip (and neural crest) derivatives. Wnt1::Cre;Hoxa2flox/flox mutants die around birth due to impaired neural crest development [71], thus preventing postnatal analysis of cochlear nuclei axon connectivity and functional impact on auditory function. Nonetheless, anterograde tracing by dextran injection in the AVCN of control and Wnt1::Cre;Hoxa2flox/flox brains at E18.5 reveal a neuronal population aberrantly innervating the ipsilateral MNTB (iMNTB) in mutant brains (arrowhead in Figure 5D). This phenotype is reminiscent, though less prominent, of the defects observed in the Robo3/Rig1 mutant mice, in which AVCN projections are prevented from crossing the midline and accumulate ipsilaterally [72]. Analysis in E13.5 control and Hoxa2 conditional mutant mice revealed that Rig1 expression is indeed selectively down-regulated, though not completely abolished, in the cochlear column (arrow, Figure 5D; and data not shown). Interestingly, Rig1 expression is not affected in the anterior extramural migratory stream (Figure 5D), derived from the precerebellar Wnt1+ lineage domain, where Hoxa2 regulates the expression of the slit receptor Robo2 [35]. Furthermore, Rig1 expression is not affected in Hoxb2ΔKO mutants (data not shown). Thus, our data show that Hoxa2 selectively regulates Rig1 expression during the guidance of contralateral AVCN projections.
Abnormal specification and innervation of olivocochlear neurons in Hoxb1 and Hoxb2 mutant mice
The OC neurons become subdivided into medial and lateral components (MOC, LOC). The MOC neurons support OHC maturation at early postnatal stages and regulate the prestin-induced vibration of OHCs in the cochlea [12], [21], and the LOC neurons, jointly with the MOC neurons, protect the cochlea from acoustic damage [22], [31], [32]. We next assessed whether the axonal behavior of efferent MOC neurons and synaptic MOC terminals on OHCs are affected in P8 and adult Hox mutant mice.
From E18.5 onwards, MOC motor axons have reached the contralateral cochlea. We thus injected DiI or dextran in Hoxb1null, Hoxb1lateCKO, Hoxb2ΔKO, or Hoxa2null mutant cochleae, and in their respective controls, to retrogradely label the fluorescent bundle of MOC efferent fibers crossing the midline (Figure 6A). No crossing axons are identified in P8 Hoxb1null and Hoxb1lateCKO mutants (Figure 6B), in keeping with the lack of OC molecular markers observed at earlier stages (Figure S5). While the MOC bundle develops normally in E18.5 Hoxa2null mutant brains, no axons cross the midline in Hoxb2ΔKO mutants (Figure 6C), albeit a few presumptive OC Gata3+ cells are detected at earlier stages (Figure S5C, S5F). This suggests that, either the few Hoxb2ΔKO mutant MOC neurons fail to target the cochlea, or that our axonal tracing procedure is not sufficiently sensitive to label just a few crossing axons.
Fig. 6. Affected connectivity of medial olivocochlear (MOC) neurons in Hoxb1 and Hoxb2 mutant mice. 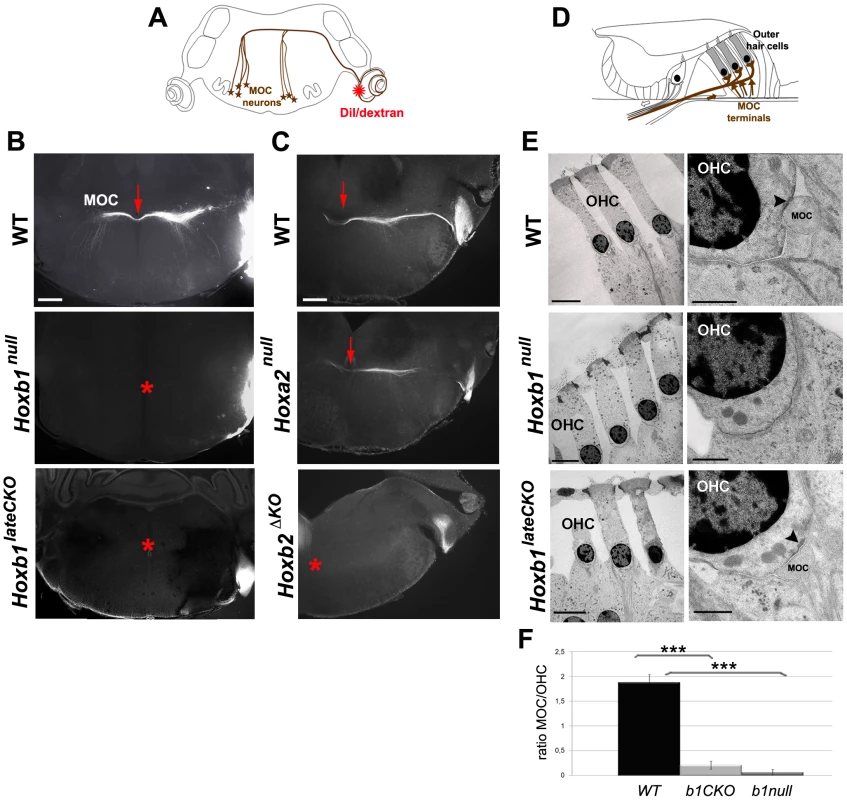
(A) Schematic view of a brain coronal section indicates the insertion of a DiI or dextran crystal into the cochlea to label controlateral MOC neurons. (B) Retrogradely-labeled MOC axons normally project across the midline as a compact bundle (arrow). No MOC axons crossing the midline are retrogradely labeled in Hoxb1null and Hoxb1lateCKO brains (asterisks). (C) Similarly, MOC fibers fail to cross the midline in Hoxb2ΔKO mutant brains (asterisk), whereas no obvious defect is observed in Hoxa2null mutants (arrow). (D) Schematic view of the organ of Corti, showing terminal innervation of the OHCs by the MOC efferent neurons (arrows). (E) Transmission electron microscopy of OHCs in adults WT, Hoxb1null and Hoxb1lateCKO cochleae. In high magnification views, MOC terminals synapse on OHCs in WT and Hoxb1lateCKO cochleae, even if at much reduced ratio (F); a sub-synaptic cisterna is visualized inside the OHC (arrowheads). No MOC terminals are detected in Hoxb1null cochleae. (F) Histogram showing the ratio of the number of MOC synaptic contacts on OHC visualized in TEM experiments in controls, Hoxb1null and Hoxb1lateCKO mutants. MOC/OHCs ratio: WT (n = 3; 32 OHCs): 1.9±0.2; Hoxb1lateCKO (n = 6; 64 OHCs): 0.2±0.2; Hoxb1null (n = 4; 40 OHCs): 0.1±0.1. Inter-genotype ANOVA p<0.001; Hoxb1lateCKO versus WT: p<0.001; Hoxb1null versus WT: p<0.001. Scale bars, 400 µm (B, C), 10 µm (E, left panel of WT), 50 µm (E, left panels of Hoxb1null and Hoxb1lateCKO), 1 µm (E, right panels). See also Figures S5 and S7. To further ascertain whether a few MOC axons may nonetheless reach the cochlea and establish synaptic contact in Hoxb1 and Hoxb2 mutant animals, we used transmission electron microscopy and looked for MOC terminals contacting OHCs in the organ of Corti (Figure 6D). In contrast to WT animals, in which 1 to 2 MOC terminals are normally seen in synaptic contact with individual OHCs (n = 4; 54 MOC terminals on 32 OHCs; Figure 6E, 6F), almost no MOC terminals are found in adult Hoxb1null cochleae (n = 4; 1 MOC terminal on 40 OHCs; Figure 6E, 6F). Similarly, a few residual synaptic contacts are identified in adult Hoxb1lateCKO (n = 6; 12 MOC terminals on 64 OHCs; Figure 6E, 6F) and Hoxb2ΔKO mutant cochleae (data not shown), indicating that, although in highly reduced number, some MOC neurons are able to innervate OHCs in these mutant mice (Figure 6F).
Next, we investigated whether LOC neurons were properly specified in P8 Hoxb1 mutant mice. While ChAT and Tbx20 label the cholinergic population of LOC neurons, VGlut2 labels the glutamatergic population of the LSO, which is primarily derived from the Atoh1+ lineage (Figure S7) [16]. No ChAT+ or Tbx20+ cells are found in the LSO of Hoxb1null brains, whereas very few positive cells can be identified in Hoxb1lateCKO mutant brains (Figure S7B). On the contrary, vGlut2 expression is only slightly decreased, particularly in Hoxb1null mutants, possibly because the LSO is only partially derived from r4, as previously shown [3], [16]. Thus, the populations primarily affected in Hoxb1 and Hoxb2 mutants are the cholinergic LOC neurons. Since the LSO largely forms within r5, this implies a previously unnoticed migration of some r4-derived cholinergic LOC cells into r5, possibly accompanying in part the migration of FBM neurons.
In summary, our molecular and cellular data confirm the absence of MOC and LOC efferent neurons in Hoxb1null mutants, but reveal the residual presence of a few MOC connections and some LOC neurons in Hoxb1lateCKO and Hoxb2ΔKO adult animals. This suggests that specification of OC neurons is primarily dependent on Hoxb1 expression in progenitor cells and on Hoxb2 expression in early post-mitotic neurons for their normal migratory and connectivity properties.
Abnormal morphology of cochlear hair cells in Hoxb1 and Hoxb2 mutant mice
The failure of MOC neurons to innervate OHCs and the absence of LOC neurons innervating IHCs might affect the correct development of cochlear hair cells and/or render them more susceptible to degenerative acoustic trauma [22], [25], [28], [32], [73]. To assess hair cell morphology in Hox mutant cochleae, we used scanning electron microscopy on the apical and basal turns of WT, Hoxb1null, Hoxb1lateCKO and Hoxb2ΔKO cochleae. Since the medial efferent system is mature prior to the onset of hearing [21], we performed this analysis at P8, when the interactions of MOC fibers with OHCs become established, and in 3-month-old animals, when the centrifugal cochlear connections are fully functional (Figure 7 and Figure S8). In this way, we could discriminate a developmental intrinsic defect of OHCs from a defect due to the absence of MOC/OHCs interactions.
Fig. 7. Late degeneration of OHCs in the apical turn of Hoxb1 and Hoxb2 mutant cochleae. 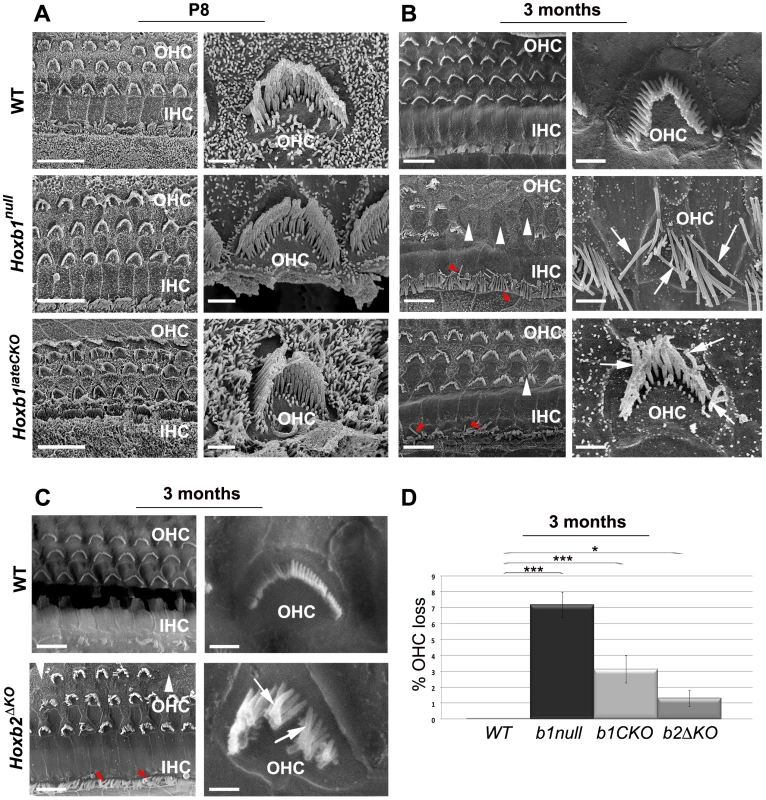
(A) Scanning electron microscopy (SEM) views of the cochlea at P8: an overview of the apical turns of WT, Hoxb1null and Hoxb1lateCKO cochleae showing three orderly arrayed rows of outer hair cells (OHCs) and one row of inner hair cells (IHCs). Representative high magnification images illustrate stereocilia of hair bundles of single OHCs arranged according to their different lengths. Shape and organization of OHCs in apical regions are normal at this stage in both mutants. (B) SEM views of 3-month-old WT, Hoxb1null and Hoxb1lateCKO cochleae and representative higher magnification images of OHCs. In Hoxb1null and Hoxb1lateCKO cochleae, OHCs have lost their regular organization and fail to develop in some areas (white arrowheads). Moreover, in Hoxb1null cochleae most stereocilia have completely lost their typical V-shaped morphology and their characteristic differences in lengths (arrows). OHCs are less severely affected in Hoxb1lateCKO cochleae. IHC cilia appeared weakly disarranged (red arrowheads). (C) SEM views of 3-months-old WT and Hoxb2ΔKO mutant cochleae and higher magnifications of representative OHCs. Note that, similarly to Hoxb1lateCKO cochleae, Hoxb2 mutants have occasional missing OHCs (white arrowheads), disarranged IHC cilia (red arrowheads) and disorganized OHC stereocilia (arrows). (D) Histogram quantifying the percentage of OHC loss in controls, Hoxb1null, Hoxb1lateCKO and Hoxb2ΔKO cochleae. While controls (n = 8) showed no OHC loss, in Hoxb1null mutants (n = 6) 7.2±0.8% of OHCs were absent, whereas 3.1±0.9% and 1.3±0.5% were lost in Hoxb1lateCKO (n = 6) and Hoxb2ΔKO (n = 3) cochleae, respectively. Inter-genotype ANOVA p<0.001; Hoxb1null versus WT: p<0.001; Hoxb1lateCKO versus WT: p<0.001; Hoxb2ΔKO versus WT: p = 0.02. Scale bars, 10 µm (A, B, C, left panels), 1 µm (A, B, C, right panels). See also Figure S8. Normally, three rows of OHCs and one row of IHCs are orderly arrayed along the entire organ of Corti at both ages. High magnification images of the hair bundles of individual WT OHCs illustrate the normal three rows of stereocilia of increasing height arranged in the characteristic V-shaped morphology; the latter appears slightly wider at the basal than apical turn (Figure 7 and Figure S8; n = 8). We found no obvious differences in the shape and organization of OHCs at the apical and basal cochlear turns in Hoxb1null (n = 6) and Hoxb1lateCKO (n = 4) pups at P8 (Figure 7A and Figure S8A), indicating a normal morphological development of the cochlea in young Hoxb1 mutant pups. However, when the architecture of the OHC area was assessed in 3-month-old animals, once the MOC/OHCs functional interactions are established and the cochlea has become fully responsive to sound, Hoxb1null adult mice show severely disorganized OHC rows with occasional loss of hair cells in the apical turn (white arrowheads in Figure 7B) (Hoxb1null, n = 6: on average 7.2/100.8 OHCs are missing, versus WT, n = 8 : 0/99 OHCs missing; Figure 7D). Furthermore, close observation of individual OHCs in Hoxb1null cochleae indicates that most stereocilia lose their typical V-shaped arrangement, as well as their organized structure and characteristic differences in ciliar length (arrows in Figure 7B). On the contrary, no IHCs are lost in these mutants, even if their cilia appear weakly disarranged (red arrowheads in Figure 7B). Only slight abnormalities in ciliar organization and orientation are observed in the basal turns of Hoxb1null cochleae (arrows in Figure S8B; n = 6), indicating that the major defects occur predominantly at the cochlear apex. In contrast, in Hoxb1lateCKO and Hoxb2ΔKO cochleae, morphological defects of OHCs are less severe compared to those in Hoxb1null mutants, although a loss of OHCs is still statistically significant, with occasionally missing cells (Hoxb1lateCKO, n = 6: average of 3.1/99.4 OHCs missing; Hoxb2ΔKO, n = 3 : 1.3/100.1 OHCs missing; Figure 7D) and moderate OHC and IHC ciliar malformations (Figure 7B, 7C). In any case, the major abnormalities observed in Hoxb1 and Hoxb2 mutants are the severe morphological defects in ciliar shape and organization, rather than the OHC loss, which was negligible, even if statistically different between the genotypes. Finally, no abnormalities are observed in basal regions of Hoxb1lateCKO and Hoxb2ΔKO cochleae (Figure S8B; and data not shown).
Taken together, we found strong late postnatal morphological defects of outer hair cells in Hoxb1null mutants, particularly in the apical region of the cochleae where low-frequency sounds are normally perceived [11]. In addition, minor defects were detected in the ciliar shape and number of hair cells in Hoxb1lateCKO and Hoxb2ΔKO mutants, indicating that abnormal development of r4-derivatives in the auditory brainstem can affect, with different severities, the long-term survival and organization of cochlear hair cells.
Hearing loss in Hoxb1 and Hoxb2 mutant mice
The observed defects of the different components of the central auditory pathway leading to sound perception prompted us to test general auditory function in Hoxb1 and Hoxb2 mutant mice. We measured the auditory brainstem response (ABR) in which a series of electrical potentials evoked by auditory stimuli ranging from 110 to 40 dB SPL (Sound Pressure Level) are analyzed, determining the lowest decibel level, or threshold, at which a response peak is reproducibly present [74]. The sequence of waves of the ABR reflects the synchronous short-latency synaptic activity of many neurons in successive nuclei along the central auditory pathway. We first analyzed the ABR response in control, Hoxb1null and Hoxb1lateCKO mice from 1 to 12 months of age (n = 307). While control mice have a normal 40 dB SPL threshold, the threshold is elevated to 90 dB SPL in Hoxb1null mice representative ABRs of 3-month-old mice (Figure 8A). Hoxb1lateCKO mutant mice also show a pathologically elevated average threshold, although lower than that of Hoxb1null mice (Figure 8A, 8C). In all ages examined, the threshold values of the responding Hoxb1 mutant mice are significantly higher than those of control mice (Figure 8C). Analyzing all data with regard to age, we observed a progressive increase of the hearing threshold for all three groups, although with different severities (doubled for Hoxb1null and 1.6 times for Hoxb1lateCKO with respect to WT), most likely due to a secondary degeneration of cochlear hair cells and/or related afferent neural structures [75]. Interestingly, our data do not show any differences in the latencies of the evoked waves (Figure 8B), suggesting that the auditory stimuli can seemingly travel normally along the successive nuclei of the central auditory pathway, once the decibel levels surpass the elevated threshold. Furthermore, we analyzed a group of Hoxb2ΔKO 3-month-old mutant mice (n = 5), together with their respective controls, and found an average threshold similar to that of Hoxb1lateCKO mice at the same age (Figure 8D).
Fig. 8. Elevated thresholds of auditory brainstem responses (ABR) in Hoxb1 and Hoxb2 mutant mice. 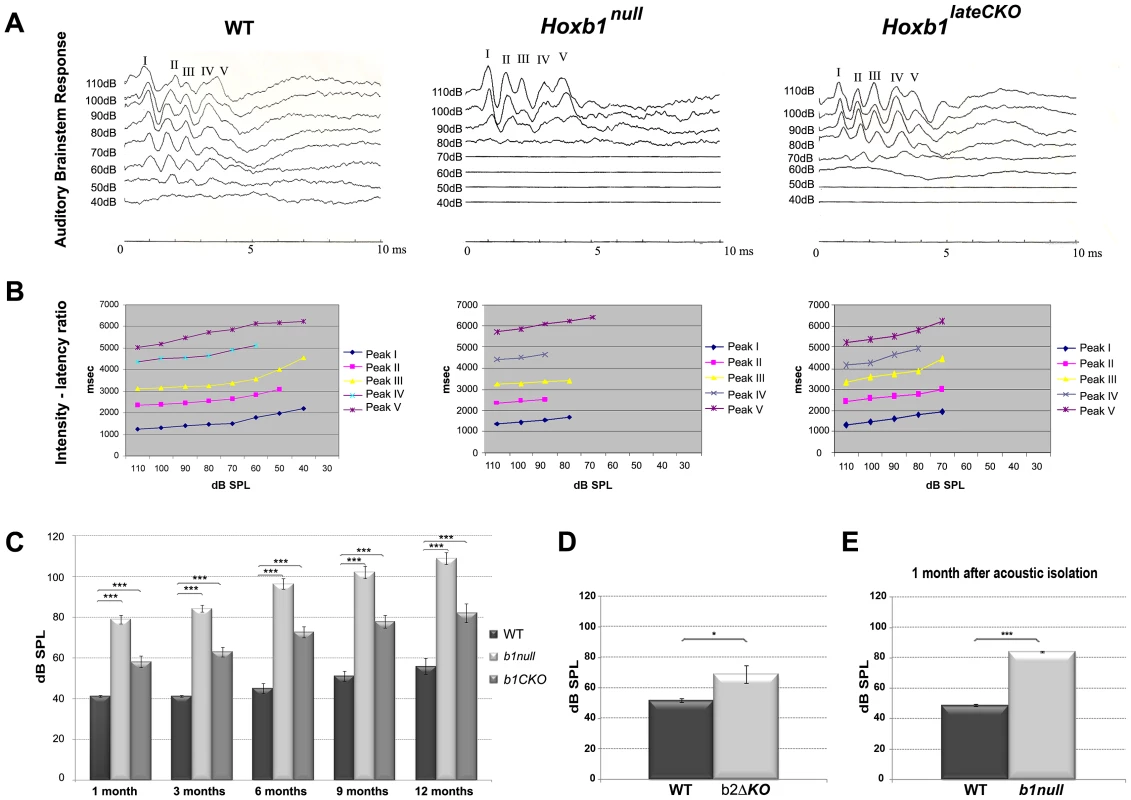
(A) Representative ABR measurements of 3 month-old WT, Hoxb1null and Hoxb1lateCKO mice. Five ABR peaks are the normal sequential responses evoked by an auditory stimulus at the level of the auditory nerve and along the series of auditory nuclei of the brainstem. The threshold, the lowest intensity of sound at which the response is present, is higher in Hoxb1 mutants than WT mice. (B) Latencies, the time intervals between the stimulus and the diverse response peaks, are normal at different intensities of sound. (C) Mean (± SE) thresholds measured in dB SPL (Sound Pressure Level) for WT, Hoxb1lateCKO and Hoxb1null mice at 1, 3, 6, 9 and 12 months of age. The differences between WT, Hoxb1lateCKO and Hoxb1null groups are statistically significant at all ages (1 month: WT, n = 24, 41.2±0.5; Hoxb1null, n = 25, 79.0±2.1; Hoxb1lateCKO, n = 23, 58.3±2.8; 3 months: WT, n = 28, 41.2±0.4; Hoxb1null, n = 23, 84.3±1.7; Hoxb1lateCKO, n = 33, 63.0±2.4; 6 months: WT, n = 22, 45.2±2.5; Hoxb1null, n = 17, 96.5±2.6; Hoxb1lateCKO, n = 25, 72.8±2.7; 9 months: WT, n = 20, 51.2±2.5, Hoxb1null, n = 14, 102.1±3.1, Hoxb1lateCKO, n = 17, 77.9±3.1; 12 months: WT, n = 10, 56±4, Hoxb1null, n = 12, 108.9±3.0, Hoxb1lateCKO, n = 14, 82.1±4.6; ANOVA p<0.001; post hoc t-test p<0.001; WT versus Hoxb1null; WT versus Hoxb1lateCKO: p<0.001 for all stages). A progressive increase of hearing thresholds in all groups with age is more prominent in Hoxb1null and Hoxb1lateCKO mutants than in the control mice. (D) Mean (± SE) thresholds of ABR for 3-month-old control and Hoxb2ΔKO mice. The differences between the two groups are statistically significant (WT, n = 5, 52±1.3; Hoxb2ΔKO, n = 5, 69±5.7; t-test p = 0.013); the threshold increase is similar to that of Hoxb1lateCKO mice at the same age. (E) ABR of 1 month-old WT and Hoxb1null mice put in an acoustically isolated environment from birth. Auditory thresholds are increased (WT: 48.9±0.2; Hoxb1null: 84.2±0.4; p<0,01) similarly to those of non-acoustically isolated mutants (C). dB, decibel. Taken together, our data demonstrate that in the absence of Hoxb1 or Hoxb2 function, low-amplitude sounds are not perceived in young and adult mice.
Environmental auditory stimuli are not the primary cause of auditory impairment in Hoxb1null mice
Previous studies have shown that the MOC and LOC neurons are required in protecting the cochlea against acoustic injury [22], [28], [31], [32]. Thus, the lack of OC neurons observed in Hoxb1null mutant mice may render these mice particularly hypersensitive to environmental sounds and lead to severe OHC damage and hearing defects. To directly address this issue, a group of WT and Hoxb1null pups (n = 5) were placed in an acoustically isolated environment at birth, and their hearing capacity was tested after one month of age. We found that acoustically isolated Hoxb1null mice have the same threshold shift as non-acoustically isolated mice (Figure 8E). This suggests that the environment is not the primary cause of the auditory threshold defects observed in Hoxb1null mice. However, the absence of protection from acoustic injury might determine secondarily the progressively more drastic increase of threshold observed with age in mutants compared to WT mice [76].
Discussion
Rhombomere 4 contribution to the auditory system
Our present r4-restricted fate map has confirmed previous studies, but also highlighted specific aspects that were not recognized before [6], [16]. In particular, we found that r4 contributes primarily to the generation and specification of auditory nuclei involved in sound transmission and amplification, as well as in the establishment of specific sensorimotor auditory circuitry during development (Figure 1M). Previous studies showed that ventral r4 is responsible for the specification of distinct subtypes of motor neurons, such as facial branchiomotor and inner ear efferent neurons [21], [44], [61], [77]; here, we show that dorsal r4 contributes to alar-plate-derived sensory components, such as the VLL, PVCN and DCN. In particular, cells migrating rostrally from r4 along the lateral lemniscus tract form the VLL nucleus, whereas dorsal sensory cells remaining within r4 contribute massively to the CN complex (PVCN and DCN), as well as to the vestibular and trigeminal sensory columns (M.D., L.P., M.S., unpublished). Interestingly, we find that r4-derived cochlear sensory neurons form jointly with basal-plate-derived motor structures two distinct auditory sensorimotor feedback sub-circuits essential for proper hearing. Some PVCN neurons together with auditory efferent MOC neurons generated within r4 support the sound-evoked MOC reflex, which terminates directly on the OHCs of the cochlea and modulates the gain of the cochlear amplifier [23], [24], [25], [26]. Interestingly, VCN neurons are also involved in the MEM reflex loop [29] through the action of the facial motor nucleus, originated in ventral r4 and strongly affected in Hoxb1 mutant mice [44], [61] (data not shown). This implies that a single rhombomere, in this case r4, contributes to various derivatives of the auditory pathway; these are distributed across several rhombomeres via selective migrations and structurally linked into functional circuits essential for proper hearing.
We also found that the majority of r4-derivatives do not overlap with the r3/r5-derived Atoh1+ lineage, which contributes to the AVCN and to the nuclei of the superior olivary complex involved in the spatial localization of sounds [16], [19]. Although further analyses are necessary to characterize the single populations derived from r4, our study suggests that r4 contributes more to inhibitory neurons (GABAergic and glycinergic) of the VLL and CN than to excitatory glutamatergic sub-populations. Firstly, we found that the VLL nucleus, which contains a majority of inhibitory Gad67+ neurons, is mainly an r4-derivative, differently from the VGlut2+ DLL, which is excitatory and originates primarily from the Atoh1+ lineage [17], [19], [51]. Secondly, r4-derived rhombic lip cells do not contribute to the cochlear granule cell populations, nor to octopus and globular bushy cells, all of which are glutamatergic Atoh1-derivatives [15], [53]. Thirdly, the change of r4 to r3 identity as a result of Hoxb1 inactivation in Hoxb1 and Hoxb2 mutants, leads to an increase of the excitatory populations (such as the cochlear granule cell population) and decrease of the inhibitory cell types (such as the Gad67 - and Gata3-expressing populations in the VLL). Fourthly, the Atoh7+ glutamatergic neurons, which derive from the Atoh1-expressing neuroepithelial regions, are massively increased in the PVCN of Hoxb1 mutant mice. Accordingly, mutant r4/YFP+ neurons ectopically project to the MNTB nucleus, as normally done by AVCN cells originating from the Atoh1+ lineage in r3 [16], [72]. Finally, we observed an increased dorsal r4 Atoh1+ domain in Hoxb1null mutants; this may be correlated to the ectopic production of glutamatergic granule cells and Atoh7+ neurons in the mutant r4-derived CN.
We therefore propose that Hoxb1 is indirectly involved in regulating and/or modulating the ratio between inhibitory and excitatory neurons in the r4-derived auditory circuits. Since r4 is changed to a more rostral identity (r3) in absence of Hoxb1 function, the rhombomere-specific ratio between GABAergic/glycinergic and glutamatergic neuronal fates might be consequently altered. Previous studies have shown that Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord and cerebellum [78], [79], [80], and is required for inhibitory GABAergic and glycinergic fate in the cochlear nucleus [15]; hence, it is plausible to speculate that Ptf1a expressed in the r4 ventricular zone may act downstream of, or together with Hoxb1 in the determination of r4-specific inhibitory features; altered Ptf1a expression may be responsible for the loss of GABAergic neurons in the VLL and/or for the ectopic expression of Atoh1, production of glutamatergic granule cells and Atoh7+ neurons in the r4-derived CN.
However, some Atoh1+ cells do derive normally from r4, even if the corresponding progenitor domain is reduced compared to that of other rhombomeres, and might thus also partially contribute to the glutamatergic lineage. Intersectional long-term fate mapping between r4 and neuronal subtype-specific mouse lines together with careful characterization of individual sub-populations will be required to fully elucidate this aspect.
Distinct regulation of Hoxb2 and Hoxa2 expression by Hoxb1 during patterning of sensory r4-derived neuronal structures
Previous studies reported that Hoxb1 together with Hoxb2 are crucial in impinging on an r4 identity during rhombomere patterning, and that ventral r4 is changed into a more anterior identity in the absence of Hoxb1, based on ectopic expression of markers and abnormal behavior of FBM neurons [34], [37], [38], [44], [61]. Here, we show that a similar regulation occurs also in dorsal r4 during the specification of auditory sensory derivatives. We found a re-patterning of r4 into r3 identity in Hoxb1 mutants, in which Hoxb1 is either constitutive (Hoxb1null) or conditionally (Hoxb1lateCKO) eliminated in r4, and in Hoxb2ΔKO mice, in which Hoxb1 expression fails to be maintained in r4. In the absence of Hoxb1, Hoxb2 expression is reduced and Hoxa2 expression is increased in r4 at levels similar to those in r3, leading ultimately to the loss of specific r4-derived auditory nuclei (VLL and PVCN) and the ectopic formation of r3-like derived structures (AVCN and cochlear granule cells).
We thus conclude that within this sensory system Hoxb1 and Hoxb2 are critically required during the specification of r4-derived structures, and that in their absence, r4 alar and rhombic lip derivatives largely acquire r3-like features. This is based on our data and is consistent with previous intersectional fate mapping with r2 and r3/r5-specific enhancers together with the selective inactivation of Atoh1 in r3 and r5 [6], [16].
In addition, we observed that Hoxb2ΔKO and Hoxb1lateCKO reproduce a similar phenotype. Indeed, although early Hoxb1 expression is able to partially specify r4 identity in Hoxb1lateCKO and Hoxb2ΔKO mutants, failure to maintain Hoxb1 at later stages inhibits further development of r4-derived structures, leading ultimately to a milder phenotype when compared to constitutive Hoxb1 null mutants. We observed slight differences in the phenotypic severity of these two mutant lines, which might essentially be due to the differences in timing of Hoxb1 inactivation in r4.
Importantly, our data show that in r4 Hoxb2, besides being involved in maintaining high levels of Hoxb1 in progenitor cells (our study and [37], [58]), also plays a key role in relaying r4-dependent regional fate to post-mitotic cells. In support of this, we show that Hoxb2 is expressed in both r4-derived progenitors and post-mitotic neurons, and that its expression is maintained in r4-derived structures, such as the VLL, VCN and OC.
A different situation applies for Hoxa2, which plays crucial roles in cell migration and axonal connectivity during hindbrain development, and whose expression is also maintained in the auditory sensory nuclei [36], [66]. Hoxb1 expression is not affected in Hoxa2null mutants, as previously reported [66] and, accordingly, no defects were observed in VLL and MOC development, two r4-derived structures. However, we found that Hoxa2 expression in r4 is increased in Hoxb1 and Hoxb2ΔKO embryos, both in progenitors and, importantly, in post-mitotic cells. This ectopic expression is maintained in the postnatal PVCN of these mutants together with higher expression of Atoh7, ultimately leading to a change of PVCN to AVCN identity, as confirmed by a corresponding abnormal connectivity pattern. Thus, we propose that Hoxb1 negatively modulates Hoxa2 expression levels in r4. In the absence of Hoxb1, ectopic up-regulation of Hoxa2 in PVCN changes r4-specific neuronal properties and drives cochlear neurons to innervate an inappropriate target, the MNTB cells, normally innervated by r3-derived AVCN axons [72].
Taken together, we show that while Hoxb1 and Hoxb2 establish and maintain the regional identity of r4 progenitors (Hoxb1 and Hoxb2) and possibly of their derivatives (Hoxb2), Hoxa2 is normally expressed at low levels in differentiating cells of r4 and thus plays only a minor role in patterning r4-derived sensory structures.
Hoxa2 regulates axon guidance receptor Rig1 expression in the auditory lip
In this study, we found that in AVCN, Hoxa2 controls the expression of the Slit receptor Rig1/Robo3, known to regulate crossing of the midline by commissural axons in the hindbrain [72]. In Wnt1::Cre;Hoxa2flox/flox mutants, in which Hoxa2 is conditionally inactivated solely in rhombic lip and neural crest cells, the CN complex is properly formed but many axonal projections from the AVCN fail to reach the contralateral MNTB, indicating that Rig1 is directly involved in these axonal defects.
Concerning possible ways by which Hoxa2 may regulate Rig1 expression, it is noteworthy to mention that Rig1 expression is not affected in the anterior extramural migratory stream derived from the posterior precerebellar Wnt1+ domain, where Hoxa2, instead, directly regulates the expression of another Slit receptor, Robo2 [35]. Thus, Hoxa2-mediated regulation of Rig1 is mostly evident in the most rostral domain of Hoxa2 activity (i.e. in the r2–r5-derived auditory lip), whereas in the r6–8-derived precerebellar lip, the role of Hoxa2 may be functionally compensated by other Hox factors of the paralogue groups 3–5. Alternatively, Hoxa2-dependent regulation of Rig1 might require a specific co-factor only present in r2–r5 rhombic lip derivatives. Finally, while Hoxa2 is required for the expression of Rig1 throughout the r2–r5 auditory lip column, Rig1 is normally expressed in Hoxb2ΔKO mutants (data not shown), strongly suggesting that Hoxa2 expression in Hoxb2 (and, likely, Hoxb1) knockout cells is sufficient to support normal Rig1 transcriptional regulation and thus, drive the contralateral ectopic projections of ‘PVCN’ mutant neurons. However, based on the available Rig1 functional data [72], we predict that the Rig1 function by itself is not sufficient to switch PVCN-to-AVCN target specificity (i.e. to target the MNTB), holding that Rig1 expression only confers the ability of axons to cross the midline. Thus, it is unlikely that the Hoxa2-mediated regulation of Rig1 alone could explain the target connectivity switch observed in Hoxb1 and Hoxb2 knockouts. Hoxa2 likely controls a larger downstream transcriptional program to provide r2/3 AVCN neurons with their proper regional identity and connectivity.
Assembly of a sensorimotor auditory sub-circuit by Hoxb1 and Hoxb2
We show that Hoxb1 and Hoxb2 mutants have increased auditory thresholds leading to severe hearing impairments. This phenotype is often associated with affected CN function [16] and/or alterations in the cochlear amplification mechanism executed by the OHCs [11], [12], [81]. Accordingly, we found defects in the CN complex and additional strong morphological damage of the OHCs. We exclude a direct role of Hoxb1 and Hoxb2 on hair cell development, since they are not expressed in presumptive hair cells [41], [82]. We also exclude that a defect in satellite glial cells, surrounding the spiral ganglion neurons and originating from r4-derived neural crest (Figure S9), can affect OHC development and/or contribute to the altered auditory threshold. Even if we observed a decrease of double YFP+/Sox10+ cells in Hoxb1null cochleae, spiral ganglion neurons seem to differentiate properly and appropriately express Gata3 (Figure S9). Moreover, type II ganglion fibers innervating the OHCs are unmyelinated, different from type I fibers innervating the IHCs, indicating that the r4-neural crest-derived YFP+ Schwann cells myelinize mainly type I fibers. Furthermore, we rule out abnormalities of the second arch-derived middle ear ossicles potentially contributing to the auditory phenotype observed in this study, since they are unaffected in both Hoxb1 and Hoxb2 mutant mice [58], [61]. Finally, we also exclude a involvement of LOC efferent neurons, which, even if affected in our mutants, appear to have no direct effect on cochlear thresholds measured by ABR [22].
Although we cannot ascertain the major structure responsible for the increased auditory threshold, we propose that abnormal development of MOC neurons, which are required for proper postnatal survival and functioning of OHCs during the hearing process [28], are involved in the hearing impairments of both mutants. In support of this, the strongest morphological hair cell abnormalities is found towards the apical region of the cochlea, where normally low frequency sounds are perceived [11]. Furthermore, early development of hair cells proceeds normally in the absence of efferent neurons but become affected at later stages when OHCs are dependent on proper MOC innervation [21]. Hence, degeneration of OHCs and consequently, altered hearing thresholds, might be caused by the absence of synaptic/trophic stimulation of cochlear hair cells from the centrifugal OC fibers during a postnatal critical period, which is essential for accurate maturation of OHCs [28]. More support comes from the observations that persistence of some MOC neurons innervating the OHCs in Hoxb1lateCKO and Hoxb2ΔKO mutant cochleae is sufficient to partially “rescue” the auditory threshold and OHC morphological defects. This occurs in the presence of seemingly comparable patterning and connectivity defects observed in the CN complex of Hoxb1 and Hoxb2 mutants. In addition, no differences in the latencies of the evoked responses are found in our ABR analysis, indicating that the auditory stimuli, when perceived, can travel normally along the successive nuclei of the central auditory pathway, even in the presence of abnormal CN and VLL.
Finally, we found altered hearing thresholds already in one-month-old Hoxb1null mice that were acoustically isolated at birth, and thus not exposed to noise. This rules out that the observed increased threshold is due to reduced function of the MOC and MEM reflexes, as well as LOC neurons that cannot protect the organ of Corti from noise-induced hearing damage. Nevertheless, this does not exclude that deficiencies in the efferent feedback systems are involved in the progressive age-related degeneration of hearing [76], which is more pronounced in mutant mice than in WT. Hence, the minor increase of threshold with age observed in the Hoxb1lateCKO compared to Hoxb1null might be due to the presence of a few efferent neurons in the conditional mice.
Thus, our data suggest that efferent innervations play an important postnatal role in the hearing impairment observed in Hoxb1 and Hoxb2 mutants, although we cannot completely rule out that altered development of other auditory structures might also contribute. Selective deletion of MOC efferents during development may further support this hypothesis.
Selective involvement of individual rhombomeres and Hox genes in patterning auditory circuits
Our data unravel a novel function for r4 and its crucial patterning genes, Hoxb1 and Hoxb2, in the ascending sound transmission pathway involving CN and VLL, as well as in the establishment of a sensorimotor reflex circuit formed by PVCN and MOC neurons. We found that within the cochlear nuclear complex, Hoxa2 expression is primarily maintained in the r2/r3-derived AVCN, whereas Hoxb2 is highly expressed in r4-derived structures, such as the PVCN, in the r2/r3-derived portion of AVCN and in r3/r5-derived granule cochlear cells. Both genes are expressed in the DCN, which is a r3/r4/r5 derivative ([6], [36] and our study). Such partially overlapping and complementary expression patterns, already observed at early stages, might reflect distinct functional rhombomere-specific pathways within the auditory circuit. In this study we show that the absence of Hoxa2 mainly affects development of the r2/3-derived AVCN and their respective projections to the contralateral MNTB nucleus, [6], [16]. This phenotype might alter a pathway crucial for sound localization [18]. In contrast, in the absence of Hoxb1 or Hoxb2 functions, PVCN acquires an r3-derived AVCN identity and the resultant projections fail to reach their normal targets, i.e. r4-derived VLL and MOC neurons, which are also affected. As a consequence, the efferent reflexes and the innervation of OHCs by MOC neurons are impaired and Hoxb1 and Hoxb2 mutants display hearing problems, although with different severities. Thus, Hoxb1 and Hoxb2 appear to act primarily upon r4-derived structures, contributing to the main pathway of sound perception, protection and amplification, whereas Hoxa2 seems to contribute to the sound localization circuitry centered in r3 and r5. In summary, our data support a model whereby rhombomere-specific (thus A-P) and alar - to basal-restricted pools of neurons (thus D-V) contribute to distinct functional pathways and circuits by maintaining differential expression levels of specific Hox gene combinations that, in turn, will continuously refine regional identity within the multi-segmental neuronal columns of the hindbrain (see also Model in Figure 1L, 1M).
Materials and Methods
Generation of Hox mouse mutant lines and matings
For the detailed generation of the b1r4-Cre transgenic line, the Hoxb1flox and Hoxb1null mutant mice, and the Hoxb2ΔKO line see the Protocol S1 section. Generation of the Hoxa2null and Hoxa2flox alleles are described in other studies [65], [83]. The b1r4-Cre mice were crossed with the ROSA26YY reporter line [43] to obtain double heterozygous b1r4-Cre/YFP mice in which r4 and r4-derivatives are selectively labeled. Hoxb1flox mice were mated to the b1r4-Cre or b1r4-Cre/YFP transgenic lines to obtain Hoxb1lateCKO in which Hoxb1 is inactivated exclusively in r4 at around E9.5. Similarly, Hoxb1null mice were mated to the b1r4-Cre/YFP line to permanently label r4 and r4-derivatives in a null background. All experiments were conducted following guidelines of the Institutional Animal Care and Use Committee of the Cardarelli Hospital, Naples, Italy, the University of Nice Sophia-Antipolis, Nice, France and the Friedrich Miescher Institute, Basel, Switzerland.
Tissue preparation
Adult and P8 mice were perfused with 4% paraformaldehyde (PFA). Embryos, brains and cochleae were fixed overnight in 4% paraformaldehyde (PFA) in phosphate-buffered saline, pH 7.4 (PBS). Tissues were cryoprotected with 10, 20 and 30% sucrose in PBS and frozen in OCT embedding matrix (Kaltek) and sectioned at 14 µm (E10.5 brains; transversal plane), 16 µm (E10.5 embryos sagittal plane; E14.5 to E18.5 brains), or 20 µm (P8 and adult brains).
Immunohistochemistry and Nissl staining
After inactivation of endogenous peroxidase with 0.5% H2O2, tissue cryosections were blocked in blocking buffer (0.05% Tween 20, 20% newborn calf serum, NBCS, in PBS) and incubated overnight at 4°C with primary antibodies diluted in hybridization buffer (0.05% Tween 20, 5% NBCS in PBS): anti-GFP rabbit polyclonal antibody (1∶1500/1∶500, Molecular Probes), anti-ChAT goat polyclonal (1∶100, Chemicon), anti-Islet mouse monoclonal (1∶300 clone 39.4D5; Developmental Studies Hybridoma Bank). Sections were washed in blocking buffer and incubated for an hour and a half at room temperature with secondary antibodies: biotinylated rabbit anti-goat (1∶300), goat anti-rabbit or goat anti-mouse (1∶200). The Vectastain Elite ABC kit and DAB substrate kit for peroxidase (Vector) were used for immunohistochemical staining. Nissl staining of frozen sections was performed using standard procedures and the slides were mounted with EUKITT mounting medium. Embryos/pups for each genotype were tested at least twice with the antibodies listed above.
Immunofluorescence
Tissue cryosections or whole mount dissected cochleae were incubated overnight at 4°C with primary antibodies diluted in blocking buffer (5% goat serum, 6% BSA, 20 mM MgCl2, 0.3% Tween 20 in PBS): rabbit anti-GFP (1∶500, Chemicon), mouse anti-Cre (1∶200, BABCO), rabbit anti-Pax6 (1∶100, Chemicon), rabbit anti-Hoxb1 (1∶200, Covance), rabbit anti-Phox2b (Pattyn et al., 1997) (1∶750, gift from C.Goridis), mouse anti-Gata3 (1∶50, Santa Cruz), mouse anti-Islet (1∶300 clone 39.4D5; Developmental Studies Hybridoma Bank), rabbit anti-calbindin (1∶2500, Swant), rabbit anti-calretinin (1∶3000, Swant), guinea pig anti-Sox10 (1∶1000, gift from M. Wegner [84]), rabbit anti-Atoh1 (1∶500, gift from J. Johnson). For Atoh1 antibody immunofluorescence, a particular blocking buffer was used (1% goat serum, 0.1% Triton-X). Tissue was washed in PBS 0.1% Triton-X and incubated for an hour at room temperature with secondary antibodies: Alexa Fluor 488 (green) goat anti-mouse and goat anti-rabbit, Alexa Fluor 594 (red) goat anti-mouse, goat anti-rabbit and goat anti-guinea pig (1∶400, Molecular Probes). The slides were mounted with the Vectastain elite ABC kit (Vector). Embryos/pups for each genotype were tested at least twice with the antibodies listed above.
In situ hybridization
In situ hybridization on cryosections or whole-mount preparations of embryos, as well as combined in situ hybridization and immunohistochemistry, were performed as previously described [85]. The tissue was hybridized using digoxigenin-labeled (Roche labeling kit) riboprobes for Gata2, Gata3, Tbx20, VGlut2, Gad67, Hoxb1, Hoxb2, Hoxa2, Atoh7, Atoh1, Barhl1, Robo3, Cre-recombinase. Embryos/pups for each genotype were tested at least twice with the riboprobes listed above.
Retrograde tracing from the cochlea
P8 heads of WT (n = 4) and Hoxb1 mutants (n = 4) were dissected and fixed overnight in 4% PFA. A crystal of fluorescent carbocyanine dye DiI (Molecular Probes) was placed unilaterally in the cochlea (exposed through the base of the cranium) and allowed to diffuse for 1 to 3 months (the first two months at 37°C; afterwards at RT) in PBS containing 0.025% sodium azide. Rhodamine-conjugated dextran (Molecular Probes, Eugene, Oregon) was employed for tracing the MOC, AVCN and PVCN projections of WT (n = 5), Hoxb2 (n = 3) and Hoxa2 (n = 2) mutants. E18.5 and P8 heads (MOC tracing) and brains (AVCN and PVCN tracing) were dissected and dextran crystals were inserted unilaterally into cochlea, AVCN and PVCN regions, respectively. The embryos were incubated for 8–12 hours as described in [7]. All brains were fixed in 4% PFA, embedded in 3% agarose in PBS and vibratome-sectioned (100 µm-thick).
Auditory brainstem response (ABR) and statistical analysis
ABR recordings were performed as previously reported [86] and described in the Protocol S1. The graph plot mean ± SE (standard error) statistics for dual comparisons and were generated using Student's t-tests, whereas statistics for multiple comparisons were generated using one-way analysis of variance (ANOVA) followed by a suitable post hoc t-test; *0.01≤p<0.05, **0.001≤p<0.01, ***p<0.001 for all statistics herein.
Scanning electron microscopy (SEM)
For SEM, cochleae were fixed in 2.5% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS) pH 7.4 (19 ml of 0.2 M sodium phosphate monobasic NaH2PO4 and 81 ml of 0.2 M sodium phosphate bibasic Na2HPO4) for 4 h at 4°C and rinsed in PBS over night. The organs of Corti were isolated, rinsed in PBS and post-fixed in 1% OsO4 in the same buffer for 1 h at 4°C. After several rinses in PBS, the cochleae were separated in apical and basal turn and then the specimens were subjected to serial dehydration followed by critical point drying. The samples were mounted on aluminum stubs and sputter coated with gold. The processed specimens were investigated and photographed using a JEOL 6700F SEM operated at 5 kV and at a 8.3 mm working distance. SEM images were collected digitally.
Transmission electron microscopy (TEM)
For TEM, cochleae were fixed in 2.5% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS) pH 7.4 for 4 h at 4°C and rinsed in PBS over night. The organs of Corti were isolated, rinsed in PBS and postfixation was based on 1% osmium tetroxide solution (Fluka) in a 0.05 M PBS at pH 7.4 cooled on ice for 1 h. Specimens were dehydrated with ethanol and then, with propylene oxide and embedded in Epon 812 resin (Fluka). The blocks containing cells were cut using a Super Nova Leica Ultratome. Semithin sections at 2 µm thickness were studied with a light microscope (Polivar Reichert-Jung) after staining with 1% toluidine blue (Carlo Erba). Ultrathin sections (80 nm) were stained with 2% uranyl acetate (Electron Microscopy Sciences) for 10 min at room temperature and 2.66% lead citrate (Electron Microscopy Sciences) for 8 min at room temperature. Grids were examined by using a Philips EM 208 S transmission electron microscope (Philips) operating at 80 kV.
Quantification and statistical analysis
The VLL size was quantified on adjacent sections of P8 WT, Hoxb1null, Hoxb1lateCKO and Hoxb2ΔKO and of E18.5 WT and Hoxa2null brainstems hybridized with Gad67. The area of the VLL was measured using the MacBiophotonics Image J software. The sum of the area of all sections was expressed as a percentage of the VLL area in wt.
The loss of outer hair cells (OHCs) on SEM sections was quantified on 10 non-overlapping areas of 3060 µ2, considering at least one area per sample. Quantitative data are depicted as mean with standard error (SE) of the mean obtained from at least 3 pups or animals tested for significance by the unpaired Student's t-test or for multiple comparisons by the one-way analysis of variance (ANOVA) followed by a suitable post hoc t-test; *0.01≤p<0.05, **0.001≤p<0.01, ***p<0.001 for all statistics herein.
Supporting Information
Zdroje
1. KieckerC, LumsdenA (2005) Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci 6 : 553–564.
2. ClarkeJD, LumsdenA (1993) Segmental repetition of neuronal phenotype sets in the chick embryo hindbrain. Development 118 : 151–162.
3. MarinF, PuellesL (1995) Morphological fate of rhombomeres in quail/chick chimeras: a segmental analysis of hindbrain nuclei. Eur J Neurosci 7 : 1714–1738.
4. PasqualettiM, DiazC, RenaudJS, RijliFM, GloverJC (2007) Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo. J Neurosci 27 : 9670–9681.
5. CramerKS, FraserSE, RubelEW (2000) Embryonic origins of auditory brain-stem nuclei in the chick hindbrain. Dev Biol 224 : 138–151.
6. FaragoAF, AwatramaniRB, DymeckiSM (2006) Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron 50 : 205–218.
7. OuryF, MurakamiY, RenaudJS, PasqualettiM, CharnayP, et al. (2006) Hoxa2 - and rhombomere-dependent development of the mouse facial somatosensory map. Science 313 : 1408–1413.
8. CambroneroF, PuellesL (2000) Rostrocaudal nuclear relationships in the avian medulla oblongata: a fate map with quail chick chimeras. J Comp Neurol 427 : 522–545.
9. DiazC, GloverJC, PuellesL, BjaalieJG (2003) The relationship between hodological and cytoarchitectonic organization in the vestibular complex of the 11-day chicken embryo. J Comp Neurol 457 : 87–105.
10. Malmierca MS, Merchan MA (2004) Auditory System. In: Paxinos G, editor. The Rat Nervous System. Third Edition ed. San Diego, USA: Elsevier Academic Press. pp. 997–1082.
11. FettiplaceR, HackneyCM (2006) The sensory and motor roles of auditory hair cells. Nat Rev Neurosci 7 : 19–29.
12. DallosP (2008) Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 18 : 370–376.
13. RubelEW, FritzschB (2002) Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 25 : 51–101.
14. NicholsDH, BruceLL (2006) Migratory routes and fates of cells transcribing the Wnt-1 gene in the murine hindbrain. Dev Dyn 235 : 285–300.
15. FujiyamaT, YamadaM, TeraoM, TerashimaT, HiokiH, et al. (2009) Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development 136 : 2049–2058.
16. MaricichSM, XiaA, MathesEL, WangVY, OghalaiJS, et al. (2009) Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J Neurosci 29 : 11123–11133.
17. WangVY, RoseMF, ZoghbiHY (2005) Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48 : 31–43.
18. Willott J (2001). Handbook of Mouse Auditory Research:From Behavior to Molecular Biology: Boca Raton:CRC Press.
19. RoseMF, AhmadKA, ThallerC, ZoghbiHY (2009) Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci U S A 106 : 22462–22467.
20. BruceLL, KingsleyJ, NicholsDH, FritzschB (1997) The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci 15 : 671–692.
21. SimmonsDD (2002) Development of the inner ear efferent system across vertebrate species. J Neurobiol 53 : 228–250.
22. DarrowKN, MaisonSF, LibermanMC (2007) Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol 97 : 1775–1785.
23. BrownMC, de VeneciaRK, GuinanJJJr (2003) Responses of medial olivocochlear neurons. Specifying the central pathways of the medial olivocochlear reflex. Exp Brain Res 153 : 491–498.
24. de VeneciaRK, LibermanMC, GuinanJJJr, BrownMC (2005) Medial olivocochlear reflex interneurons are located in the posteroventral cochlear nucleus: a kainic acid lesion study in guinea pigs. J Comp Neurol 487 : 345–360.
25. GuinanJJJr (2006) Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27 : 589–607.
26. GuinanJJJr (2010) Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg 18 : 447–453.
27. GuinanJJJr, StankovicKM (1996) Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am 100 : 1680–1690.
28. WalshEJ, McGeeJ, McFaddenSL, LibermanMC (1998) Long-term effects of sectioning the olivocochlear bundle in neonatal cats. J Neurosci 18 : 3859–3869.
29. LeeDJ, de VeneciaRK, GuinanJJJr, BrownMC (2006) Central auditory pathways mediating the rat middle ear muscle reflexes. Anat Rec A Discov Mol Cell Evol Biol 288 : 358–369.
30. LibermanMC, GuinanJJJr (1998) Feedback control of the auditory periphery: anti-masking effects of middle ear muscles vs. olivocochlear efferents. J Commun Disord 31 : 471–482; quiz 483; 553.
31. KujawaSG, LibermanMC (1997) Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol 78 : 3095–3106.
32. MaisonSF, LuebkeAE, LibermanMC, ZuoJ (2002) Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci 22 : 10838–10846.
33. KrumlaufR, MarshallH, StuderM, NonchevS, ShamMH, et al. (1993) Hox homeobox genes and regionalisation of the nervous system. J Neurobiol 24 : 1328–1340.
34. TumpelS, WiedemannLM, KrumlaufR (2009) Hox genes and segmentation of the vertebrate hindbrain. Curr Top Dev Biol 88 : 103–137.
35. GeisenMJ, Di MeglioT, PasqualettiM, DucretS, BrunetJF, et al. (2008) Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol 6: e142 doi:10.1371/journal.pbio.0060142.
36. NaritaY, RijliFM (2009) Hox genes in neural patterning and circuit formation in the mouse hindbrain. Curr Top Dev Biol 88 : 139–167.
37. GavalasA, RuhrbergC, LivetJ, HendersonCE, KrumlaufR (2003) Neuronal defects in the hindbrain of Hoxa1, Hoxb1 and Hoxb2 mutants reflect regulatory interactions among these Hox genes. Development 130 : 5663–5679.
38. MaconochieMK, NonchevS, StuderM, ChanSK, PopperlH, et al. (1997) Cross-regulation in the mouse HoxB complex: the expression of Hoxb2 in rhombomere 4 is regulated by Hoxb1. Genes Dev 11 : 1885–1895.
39. AwatramaniR, SorianoP, RodriguezC, MaiJJ, DymeckiSM (2003) Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat Genet 35 : 70–75.
40. VoiculescuO, CharnayP, Schneider-MaunouryS (2000) Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis 26 : 123–126.
41. ArenkielBR, GaufoGO, CapecchiMR (2003) Hoxb1 neural crest preferentially form glia of the PNS. Dev Dyn 227 : 379–386.
42. StuderM, PopperlH, MarshallH, KuroiwaA, KrumlaufR (1994) Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science 265 : 1728–1732.
43. SrinivasS, WatanabeT, LinCS, WilliamCM, TanabeY, et al. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1 : 4.
44. StuderM, LumsdenA, Ariza-McNaughtonL, BradleyA, KrumlaufR (1996) Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature 384 : 630–634.
45. GaufoGO, FlodbyP, CapecchiMR (2000) Hoxb1 controls effectors of sonic hedgehog and Mash1 signaling pathways. Development 127 : 5343–5354.
46. AuclairF, ValdesN, MarchandR (1996) Rhombomere-specific origin of branchial and visceral motoneurons of the facial nerve in the rat embryo. J Comp Neurol 369 : 451–461.
47. KarisA, PataI, van DoorninckJH, GrosveldF, de ZeeuwCI, et al. (2001) Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol 429 : 615–630.
48. PataI, StuderM, van DoorninckJH, BriscoeJ, KuuseS, et al. (1999) The transcription factor GATA3 is a downstream effector of Hoxb1 specification in rhombomere 4. Development 126 : 5523–5531.
49. BrownMC, LevineJL (2008) Dendrites of medial olivocochlear neurons in mouse. Neuroscience 154 : 147–159.
50. GurungB, FritzschB (2004) Time course of embryonic midbrain and thalamic auditory connection development in mice as revealed by carbocyanine dye tracing. J Comp Neurol 479 : 309–327.
51. MacholdR, FishellG (2005) Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48 : 17–24.
52. RiquelmeR, SaldanaE, OsenKK, OttersenOP, MerchanMA (2001) Colocalization of GABA and glycine in the ventral nucleus of the lateral lemniscus in rat: an in situ hybridization and semiquantitative immunocytochemical study. J Comp Neurol 432 : 409–424.
53. SaulSM, BrzezinskiJAt, AltschulerRA, ShoreSE, RudolphDD, et al. (2008) Math5 expression and function in the central auditory system. Mol Cell Neurosci 37 : 153–169.
54. YamasakiT, KawajiK, OnoK, BitoH, HiranoT, et al. (2001) Pax6 regulates granule cell polarization during parallel fiber formation in the developing cerebellum. Development 128 : 3133–3144.
55. FrisinaRD, ZettelML, KelleyPE, WaltonJP (1995) Distribution of calbindin D-28k immunoreactivity in the cochlear nucleus of the young adult chinchilla. Hear Res 85 : 53–68.
56. PorA, PocsaiK, RusznakZ, SzucsG (2005) Presence and distribution of three calcium binding proteins in projection neurons of the adult rat cochlear nucleus. Brain Res 1039 : 63–74.
57. TumpelS, CambroneroF, FerrettiE, BlasiF, WiedemannLM, et al. (2007) Expression of Hoxa2 in rhombomere 4 is regulated by a conserved cross-regulatory mechanism dependent upon Hoxb1. Dev Biol 302 : 646–660.
58. BarrowJR, CapecchiMR (1996) Targeted disruption of the Hoxb-2 locus in mice interferes with expression of Hoxb-1 and Hoxb-4. Development 122 : 3817–3828.
59. PattynA, VallstedtA, DiasJM, SamadOA, KrumlaufR, et al. (2003) Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev 17 : 729–737.
60. PopperlH, BienzM, StuderM, ChanSK, AparicioS, et al. (1995) Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 81 : 1031–1042.
61. GoddardJM, RosselM, ManleyNR, CapecchiMR (1996) Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve. Development 122 : 3217–3228.
62. RenSY, AngrandPO, RijliFM (2002) Targeted insertion results in a rhombomere 2-specific Hoxa2 knockdown and ectopic activation of Hoxa1 expression. Dev Dyn 225 : 305–315.
63. RijliFM, DolleP, FraulobV, LeMeurM, ChambonP (1994) Insertion of a targeting construct in a Hoxd-10 allele can influence the control of Hoxd-9 expression. Dev Dyn 201 : 366–377.
64. DavenneM, MaconochieMK, NeunR, PattynA, ChambonP, et al. (1999) Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron 22 : 677–691.
65. RenSY, PasqualettiM, DierichA, Le MeurM, RijliFM (2002) A Hoxa2 mutant conditional allele generated by Flp - and Cre-mediated recombination. Genesis 32 : 105–108.
66. GavalasA, DavenneM, LumsdenA, ChambonP, RijliFM (1997) Role of Hoxa-2 in axon pathfinding and rostral hindbrain patterning. Development 124 : 3693–3702.
67. TiveronMC, PattynA, HirschMR, BrunetJF (2003) Role of Phox2b and Mash1 in the generation of the vestibular efferent nucleus. Dev Biol 260 : 46–57.
68. ZhaoGY, LiZY, ZouHL, HuZL, SongNN, et al. (2008) Expression of the transcription factor GATA3 in the postnatal mouse central nervous system. Neurosci Res 61 : 420–428.
69. RijliFM, MarkM, LakkarajuS, DierichA, DolleP, et al. (1993) A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75 : 1333–1349.
70. DanielianPS, MuccinoD, RowitchDH, MichaelSK, McMahonAP (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 8 : 1323–1326.
71. SantagatiF, MinouxM, RenSY, RijliFM (2005) Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development 132 : 4927–4936.
72. RenierN, SchonewilleM, GiraudetF, BaduraA, Tessier-LavigneM, et al. (2010) Genetic dissection of the function of hindbrain axonal commissures. PLoS Biol 8: e1000325 doi:10.1371/journal.pbio.1000325.
73. MaisonSF, AdamsJC, LibermanMC (2003) Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol 455 : 406–416.
74. HenryKR (1979) Auditory brainstem volume-conducted responses: origins in the laboratory mouse. J Am Aud Soc 4 : 173–178.
75. ShaSH, KanickiA, DootzG, TalaskaAE, HalseyK, et al. (2008) Age-related auditory pathology in the CBA/J mouse. Hear Res 243 : 87–94.
76. ZhuX, VasilyevaON, KimS, JacobsonM, RomneyJ, et al. (2007) Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol 503 : 593–604.
77. SimonH, LumsdenA (1993) Rhombomere-specific origin of the contralateral vestibulo-acoustic efferent neurons and their migration across the embryonic midline. Neuron 11 : 209–220.
78. GlasgowSM, HenkeRM, MacdonaldRJ, WrightCV, JohnsonJE (2005) Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development 132 : 5461–5469.
79. HoshinoM, NakamuraS, MoriK, KawauchiT, TeraoM, et al. (2005) Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron 47 : 201–213.
80. PascualM, AbasoloI, Mingorance-Le MeurA, MartinezA, Del RioJA, et al. (2007) Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci U S A 104 : 5193–5198.
81. van der WeesJ, van LooijMA, de RuiterMM, EliasH, van der BurgH, et al. (2004) Hearing loss following Gata3 haploinsufficiency is caused by cochlear disorder. Neurobiol Dis 16 : 169–178.
82. SzetoIY, LeungKK, ShamMH, CheahKS (2009) Utility of HoxB2 enhancer-mediated Cre activity for functional studies in the developing inner ear. Genesis 47 : 361–365.
83. PasqualettiM, RenSY, PouletM, LeMeurM, DierichA, et al. (2002) A Hoxa2 knockin allele that expresses EGFP upon conditional Cre-mediated recombination. Genesis 32 : 109–111.
84. BreuskinI, BodsonM, ThelenN, ThiryM, BorgsL, et al. (2010) Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. J Neurochem 114 : 1827–1839.
85. TiveronMC, HirschMR, BrunetJF (1996) The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci 16 : 7649–7660.
86. FranzeA, SequinoL, SaulinoC, AttanasioG, MarcianoE (2003) Effect over time of allopurinol on noise-induced hearing loss in guinea pigs. Int J Audiol 42 : 227–234.
87. DanielianPS, EchelardY, VassilevaG, McMahonAP (1997) A 5.5-kb enhancer is both necessary and sufficient for regulation of Wnt-1 transcription in vivo. Dev Biol 192 : 300–309.
88. BodeJ, SchlakeT, IberM, SchubelerD, SeiblerJ, et al. (2000) The transgeneticist's toolbox: novel methods for the targeted modification of eukaryotic genomes. Biol Chem 381 : 801–813.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



