-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
The semidominant Danforth's short tail (Sd) mutation arose spontaneously in the 1920s. The homozygous Sd phenotype includes severe malformations of the axial skeleton with an absent tail, kidney agenesis, anal atresia, and persistent cloaca. The Sd mutant phenotype mirrors features seen in human caudal malformation syndromes including urorectal septum malformation, caudal regression, VACTERL association, and persistent cloaca. The Sd mutation was previously mapped to a 0.9 cM region on mouse chromosome 2qA3. We performed Sanger sequencing of exons and intron/exon boundaries mapping to the Sd critical region and did not identify any mutations. We then performed DNA enrichment/capture followed by next-generation sequencing (NGS) of the critical genomic region. Standard bioinformatic analysis of paired-end sequence data did not reveal any causative mutations. Interrogation of reads that had been discarded because only a single end mapped correctly to the Sd locus identified an early transposon (ETn) retroviral insertion at the Sd locus, located 12.5 kb upstream of the Ptf1a gene. We show that Ptf1a expression is significantly upregulated in Sd mutant embryos at E9.5. The identification of the Sd mutation will lead to improved understanding of the developmental pathways that are misregulated in human caudal malformation syndromes.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003205
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003205Summary
The semidominant Danforth's short tail (Sd) mutation arose spontaneously in the 1920s. The homozygous Sd phenotype includes severe malformations of the axial skeleton with an absent tail, kidney agenesis, anal atresia, and persistent cloaca. The Sd mutant phenotype mirrors features seen in human caudal malformation syndromes including urorectal septum malformation, caudal regression, VACTERL association, and persistent cloaca. The Sd mutation was previously mapped to a 0.9 cM region on mouse chromosome 2qA3. We performed Sanger sequencing of exons and intron/exon boundaries mapping to the Sd critical region and did not identify any mutations. We then performed DNA enrichment/capture followed by next-generation sequencing (NGS) of the critical genomic region. Standard bioinformatic analysis of paired-end sequence data did not reveal any causative mutations. Interrogation of reads that had been discarded because only a single end mapped correctly to the Sd locus identified an early transposon (ETn) retroviral insertion at the Sd locus, located 12.5 kb upstream of the Ptf1a gene. We show that Ptf1a expression is significantly upregulated in Sd mutant embryos at E9.5. The identification of the Sd mutation will lead to improved understanding of the developmental pathways that are misregulated in human caudal malformation syndromes.
Introduction
The Danforth's short tail (Sd) mouse mutation arose spontaneously in the early 1920s in an inbred mouse colony maintained in the laboratory of C.H. Danforth [1], [2]. The inbred line in which the Sd mutation arose was being maintained for study of a dominant but incompletely penetrant posterior duplication phenotype. Danforth shared four mice with shortened tails (2 males and 2 females) with L.C. Dunn and S. Gluecksohn-Schoenheimer. None of the offspring of these shared mice displayed the posterior duplication phenotype, indicating that Danforth's original line was segregating two different mutations [1], [2]. The new short tailed line was named short-Danforth or Sd. Dunn et al. determined that the Sd mutation was inherited in a semi-dominant manner with complete penetrance and was not allelic to Brachyury (T) [2].
The Sd mutation causes severe defects in development of the axial skeleton, urogenital, and gastrointestinal systems [3]. Homozygous mutant mice (Sdsd/sd, to be denoted herein as Sd/Sd) are born live with viability equal to their littermates, although death occurs within 24 hours of birth [2], [3], [4]. The phenotype of homozygous mutants includes complete lack of tail development with a shortened spinal column due to absence of caudal and sacral vertebrae. The kidneys are completely absent, though occasionally a single small kidney rudiment can be identified medially. The bladder is present, but there is no urethral opening. The cloaca persists due to lack of proper growth of the urorectal septum to divide the cloaca into the primary urogenital sinus (ventrally) and the rectum (dorsally). This septation defect also results in a blind ending intestine and anal atresia.
Heterozygous animals (Sdsd/+, denoted herein as Sd/+) are less severely affected than homozygotes, and survive into adulthood [2], [3]. Adult heterozygotes are fertile, but fecundity is reduced. The heterozygous phenotype is 100% penetrant and includes caudal and sacral skeletal malformations and the characteristic extremely shortened tail. Both urogenital and anal openings are present. The kidneys are variably affected and are generally smaller in size (unilaterally or bilaterally), and unilateral kidney agenesis is not uncommon [4]. However, histologically the kidney tissue is normal. Despite this knowledge, it has been hypothesized that the kidney defects are the cause of reduced lifespan in Sd/+ mice [2].
During embryogenesis Sd/Sd mutants develop normally through approximately embryonic day (E)10.0–10.5, when the first external manifestation of the phenotype is shortening of the tail [4], [5], [6]. Histologically the phenotype first observed in Sd/Sd embryos at E9.5 is disintegration of the notochord [4], [6], [7], and no new notochord is formed caudally from this point [6], [7]. Additionally, the chordal cells remain abnormally close to the neural tube. By E14 the notochord has completely disintegrated except for a few fragments remaining in the sacral region. However, any floor plate that was properly formed remains. The notochord degeneration is equally severe in both homozygous mutant and heterozygous embryos, but it starts slightly earlier in Sd/Sd animals (E9.5) than in Sd/+ embryos (E10.5) [6], [7].
The phenotype of Sd mice resembles several human caudal developmental disorders characterized by malformations of the spine, lower gastrointestinal, and urogenital systems. These include caudal regression syndrome (CRS, OMIM #600145), urorectal septal malformation sequence (URSMS, also known as persistent cloaca), Currarino syndrome (OMIM #176450), and VACTERL (Vertebral-Anal-Cardiac-Tracheo-Esophageal fistula-Renal-Limb anomalies) association (OMIM #192350). Currarino syndrome, characterized by the triad of partial sacral agenesis, presacral mass, and anorectal malformation, is caused by mutations in the MNX1 (HLXB9) gene in 50% of sporadic and 90% of familial cases with the classic phenotype [8], [9], [10]. In addition, private mutations in VANGL1 [11], ZIC3 [12], HOXD13 [13], and PTEN [14] have been described in single cases of caudal dysgenesis and/or VACTERL phenotypes. However, the developmental mechanisms that lead to caudal malformations in humans are still largely unknown. Because of the significant overlap in phenotype, Sd mice are an ideal model to improve our understanding of the genetic and pathophysiologic mechanisms that lead to human caudal malformations.
Although the Sd mutation arose over 90 years ago and the mutation has been genetically mapped to a small region on mouse chromosome 2 [15], the specific genetic lesion has not yet been identified. Here, we report the identification of the Danforth's short tail mutation. We used the flanking markers from the previously published genetic map to delineate the corresponding physical map of the Sd critical region. Direct DNA sequencing of all the exons and exon/intron boundaries of the positional candidate genes and expressed sequence tags (ESTs) did not reveal any mutations. Since direct sequencing of the exonic DNA only provided ∼1% coverage of the Sd critical region, we performed next-generation sequencing (NGS) of the entire Sd genomic interval. Standard bioinformatic analysis of our NGS data did not reveal any causative mutations; therefore, we interrogated reads for which only a single end of a paired-end sequence mapped correctly to the Sd locus. Using this innovative technique, we identified an early transposon (ETn) retroviral-like insertion at the Sd locus. Expression analysis at E9.5 revealed that the Sd ETn causes inappropriate expression of the Ptf1a gene at this developmental timepoint.
Results
The Sd critical region and candidate gene analysis
We examined the Sd mutation on the recombinant inbred RSV/LeJ mouse line, and have confirmed the previously published phenotype of Sd/+ and Sd/Sd mice (Figure 1). The Danforth's short tail mouse phenotype is characterized by significant anomalies of the urogenital, digestive, and skeletal systems, and is 100% penetrant. Using backcrossing methods, Alfred et al. previously mapped the Sd critical region to an ∼0.9 cM region on mouse chromosome 2qA3 [15]. Two groups of flanking markers defined the proximal and distal borders; however, the mapping was not able to distinguish between the individual markers in each group. We used the flanking marker groups from the published genetic map to identify the corresponding Sd critical region on the mouse physical map. Using the UCSC Genome browser (July 2007; NCBI37/mm9) we defined the Sd critical region as 1.5 megabase pairs (Mb) spanning Chr2 : 18,901,614–20,456,182 flanked by the genetic markers D2Mit362 and D2Mit364 (Figure 2). The critical region contains 9 annotated RefSeq genes, representing both coding and non-coding genes. In addition, we identified 3 candidate expressed sequence tags (ESTs) not represented by RefSeq genes in the critical region (Figure 2; Table 1).
Fig. 1. The Sd phenotype. 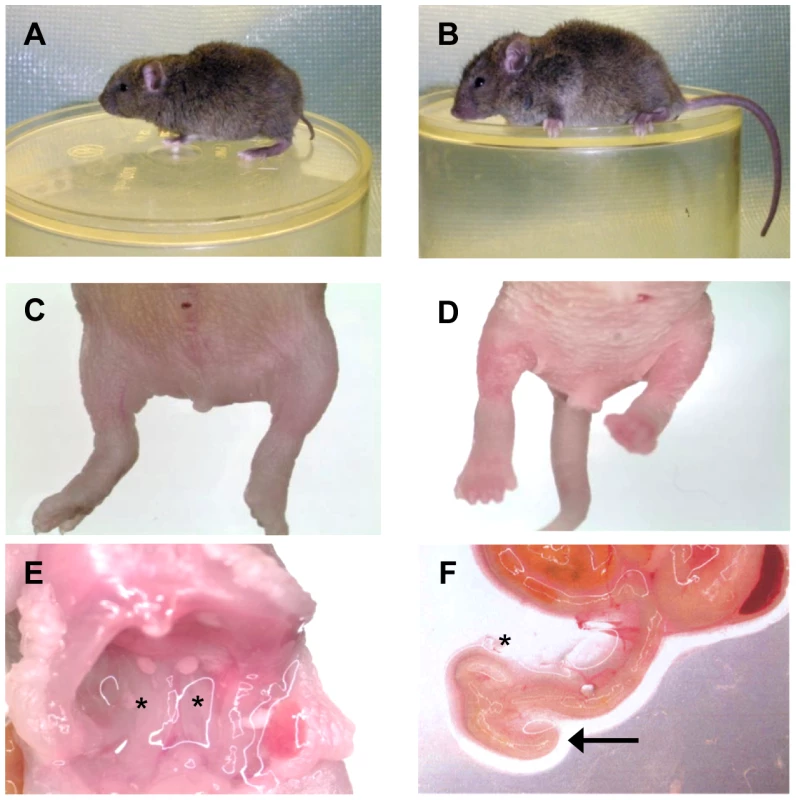
A) Heterozygous (Sd/+) mouse. B) Wildtype (+/+) mouse. C) Homozygous (Sd/Sd) P0 neonate. Note the lack of urogenital openings and tail. D) Wildtype (+/+) P0 neonate. E) Homozygous (Sd/Sd) mutant neonate showing bilateral kidney agenesis. Kidney location indicated by asterisks below the normally formed adrenal glands. F) Homozygous (Sd/Sd) mutant intestine. The colon ends blindly (indicated by arrow), never connecting to the rectum which is never formed (see panel C). The asterisk indicates normal formation of the caecum. Fig. 2. Physical map of the Sd critical region as viewed on the UCSC genome browser ( 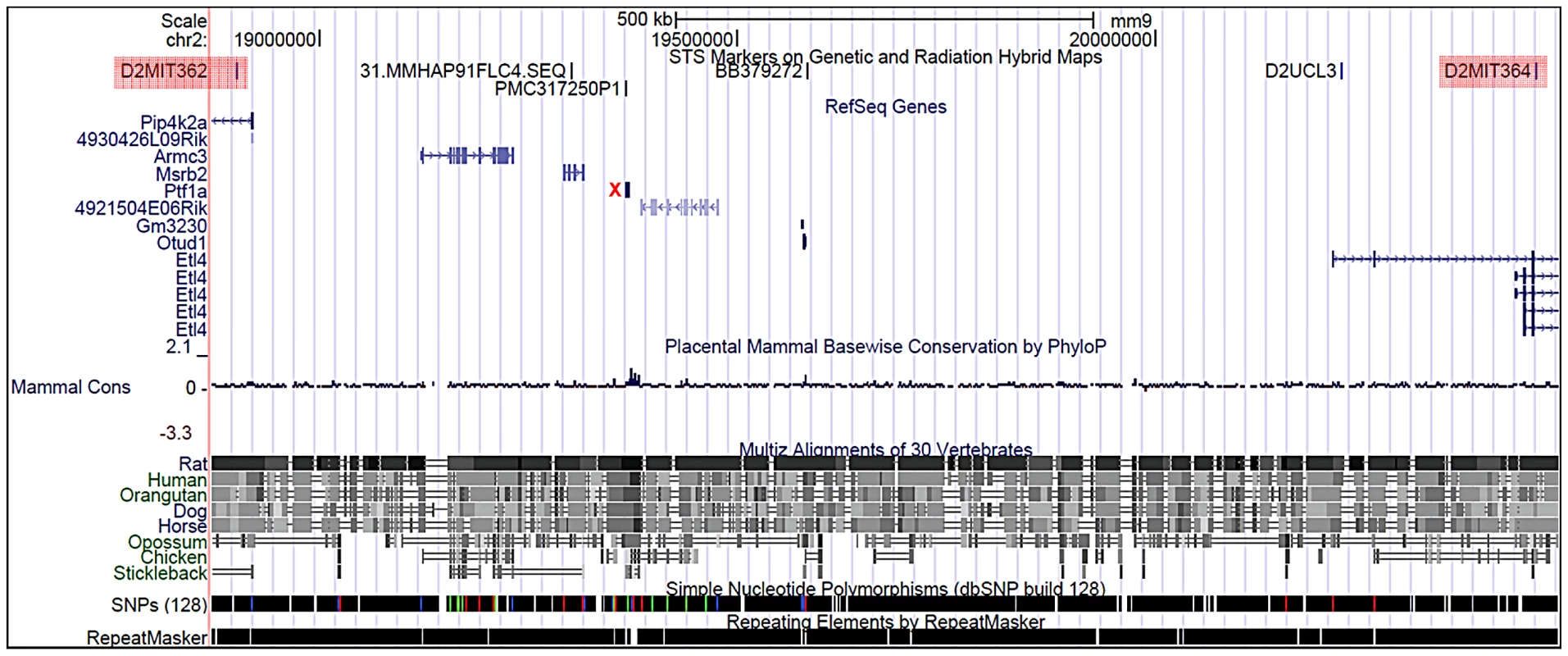
http://www.genome.ucsc.edu). The Sd region on chromosome 2 is defined by the flanking genetic markers D2Mit362 proximally and D2Mit364 distally (markers are highlighted in red) and represented by bases 18,686,882–20,915,358 on the July 2007 genome build (mm9). The Sd critical region contains 11 known genes. The mutation location is denoted by the red X. Tab. 1. Genes and ESTs mapping to the <i>Sd</i> critical region. 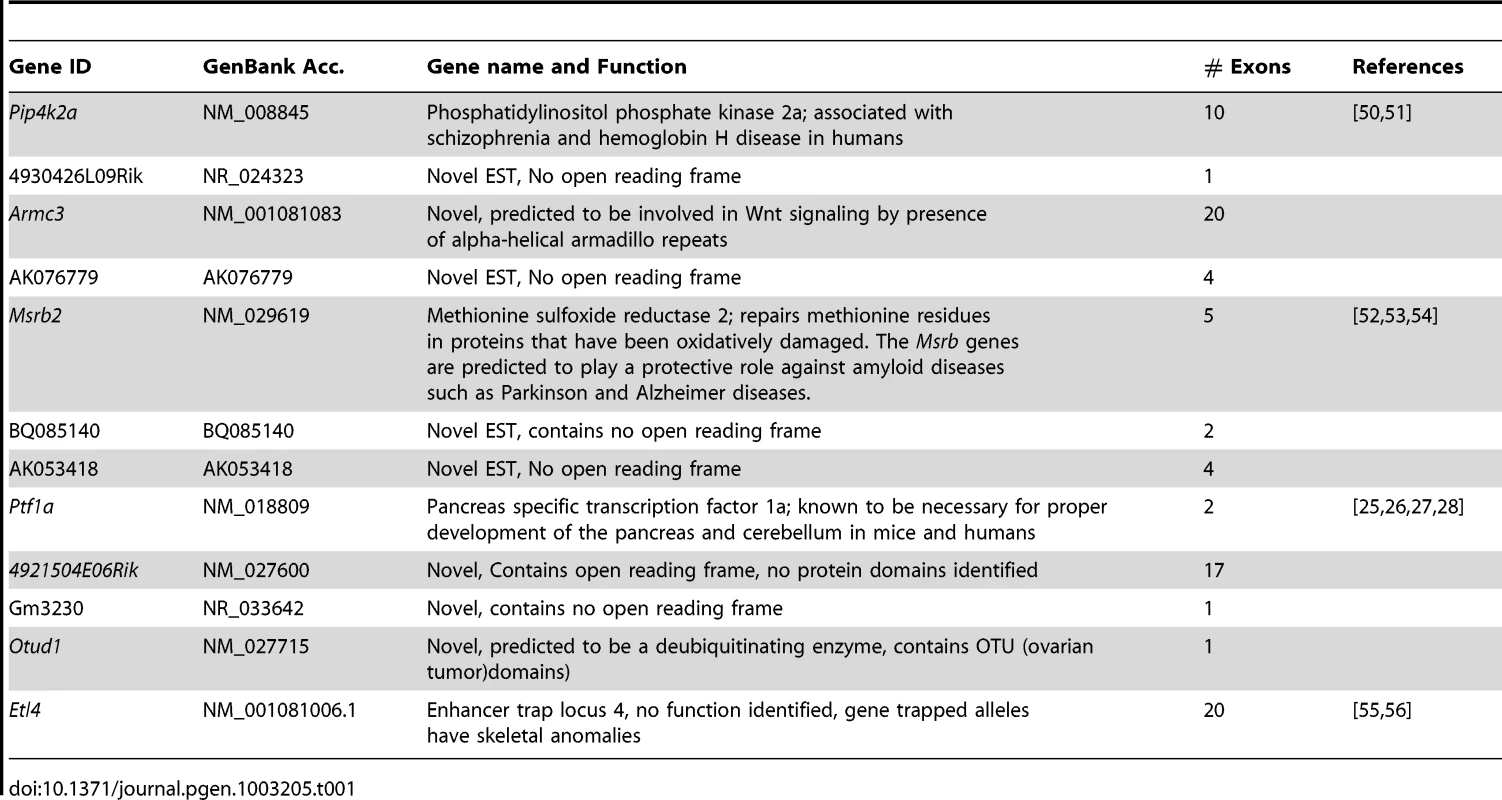
Using template Sd/Sd DNA from the RSV/LeJ line we individually sequenced a total of 87 annotated exons and intron/exon boundaries mapping to the Sd region. We compared sequences from the PCR products to the C57BL/6J (B6) mouse reference genome sequence and identified only 6 DNA changes. Among these changes, we identified a novel microsatellite in intron 9 of the 4921504E06Rik gene (IVS9+76_77DupTATC). Homozygous Sd mice have 16 copies of a TATC repeat, while Sd/+ mice have one allele with 16 copies and one with 15 copies, and wildtype mice from the RSV/LeJ line and the B6 reference genome have 15 copies. This microsatellite segregated with the mutant phenotype in all mice/embryos tested (26/26). Although linked to the Sd mutation and serving as a genetic marker for the Sd mutation, the microsatellite itself is unlikely to be mutagenic since it does not change any coding sequence or uncover a cryptic splice site. In addition, we tested 11 mouse lines and found that the A/J, CBA, CD-1, and DBA mouse lines also carry 16 copies of the TATC repeat, and the CAST/EiJ line has 18 copies, indicating that this TATC repeat is a polymorphic microsatellite (data not shown).
Our exon sequence analysis of homozygous mutant Sd DNA from the RSV/LeJ recombinant inbred line also identified 5 SNPs in exon 2 of the Etl4 gene. Three of these changes were represented in the SNP database (dbSNP: rs33065171, rs33061041, rs32925809), and two changes were novel (c.207A>G and c.210G>A). These 5 SNPs were homozygous in all mice from the RSV/LeJ recombinant inbred line regardless of phenotype (Sd/Sd, Sd/+, and +/+). Thus, no mutagenic DNA changes were identified in direct sequencing of the annotated exons mapping to the Sd critical region.
DNA capture/enrichment of the Sd critical region and next-generation sequencing
Since PCR and direct DNA sequencing of all known exons mapping to the critical region failed to identify the Sd mutation, we employed next-generation sequencing (NGS) technology for mutation detection. DNA capture/enrichment allowed us to focus only on the Sd genomic interval on mouse chromosome 2. An oligonucleotide based array was designed to capture the DNA mapping between bases 18,883,257 and 20,481,194 on mouse chromosome 2. Repeat masking software was employed to ensure only unique DNA sequences would be present on the array. Total genomic DNA isolated from a phenotypically heterozygous (Sd/+) neonatal mouse was hybridized to the capture array.
Prior to performing the NGS reaction we confirmed success of the DNA capture by quantitative real time PCR (qRT-PCR) experiments (Figure S1). Using two sets of primer pairs, one mapping within the captured region (to Etl4, exon 2), and the other mapping outside the captured region (to mouse β-Actin) we demonstrated an ∼20 fold increase in captured DNA, indicating a successful enrichment of the Sd interval genomic DNA sequences.
We performed 36 base pair paired-end sequencing using one lane on an Illumina Genome Analyzer IIx. The sequencing reaction produced 1.8 Gb of DNA sequence. Assembly and alignment of our NGS data was performed using the Bowtie software program [16]. We used the B6 genome as a template for the alignment and analysis of the NGS data. 93% of the obtained sequence reads mapped to the captured region on mouse chromosome 2 with both paired-ends mapping to the proper position and in the proper orientation. The sequence reads obtained cover 811,821 bases (51%) of the 1.5 Mb Sd critical interval as defined by Alfred et al. [15], and the average coverage of sequence reads across the interval was 443×. The Sd critical region is highly repetitive, and ∼49% of the region is made up of LINE, SINE and other highly conserved repetitive DNA sequences. In order for optimal capture and sequence alignment these DNA sequences were excluded from our capture array and are not fully represented in our data. Since we used heterozygous DNA as a template for sequencing, we set an initial variant threshold for our mutation analysis to be 40% of reads (meaning any change from reference showing up at least 40% of the time would trigger a heterozygous call). In the initial data analysis we did not identify any point mutations or small insertions or deletions. We increased the heterozygous call ratio to 25%/75% (where if 25% of reads differed from the reference a heterozygous call would be denoted) and still did not identify any mutations.
We hypothesized that the Sd mutation might be due to insertion of a retrotransposon. Retrotransposon insertions are frequently mutagenic in mice and are estimated to be the cause of 10% of spontaneous mouse mutations [17], [18]. In order to search for novel retrotransposon insertions, we sought to align separately individual ends of each pair from our NGS data. Reads for which one of the paired-ends mapped to the Sd critical region and the other end failed to align to unique (non-repeat masked) DNA were filtered to remove failed reads and low complexity sequences and screened for repetitive elements. Although our capture array was designed to exclude repetitive DNA, we expected some carry over, especially of paired-end reads where one end of the fragment mapped to unique DNA. New Perl scripts were written (available from the authors) to accomplish the analysis. Using this method we discovered a region in which one end of 166 independent paired-end reads mapped in the proper orientation to a consistent chromosome 2 position, and the other end of the pair mapped to the 5′ end of an ETn specific retrotransposon long terminal repeat (LTR) sequence. In addition, since the DNA sample sequenced came from a heterozygous mouse, we were also able to identify 1127 reads from the wildtype allele at the same location showing no variation from the reference genome. There are a larger proportion of reads representing the wildtype allele likely due to reduced capture efficiency from the mutant allele since repetitive sequences were excluded from the capture array. The method presented here provides a way to utilize “unmapped” reads which would normally be discarded. These unmapped data are important to understand the complexity of the genome, and the variation in individuals or strains from a reference sequence.
The Sd mutation is an ETn insertion
To confirm the presence of an ETn insertion at the Sd locus we performed both Southern analysis and long range PCR (Figure 3A). EcoRI and BamHI digested DNA isolated from Sd/Sd, Sd/+, and +/+ mice was hybridized with an ∼1000 bp probe from non-repetitive DNA flanking the location of the ETn insertion. The probe hybridized to a 7,300 bp fragment in wildtype DNA digested with EcoRI, which was predicted from the mouse genome reference sequence. However, hybridization of the probe to EcoRI digested Sd/Sd DNA resulted in detection of two bands of ∼4,000 bp and ∼6,000 bp, and hybridization to heterozygous DNA showed all three bands (Figure 3A). Similarly in BamHI digested DNA from a +/+ mouse, an expected band of 3,500 bp was detected. In an Sd/Sd mutant a single fragment of ∼5,000 bp was identified, and both BamHI fragments were detected in DNA from the Sd/+ mouse (Figure 3A).
Fig. 3. Confirmation and mapping of the Sd mutation. 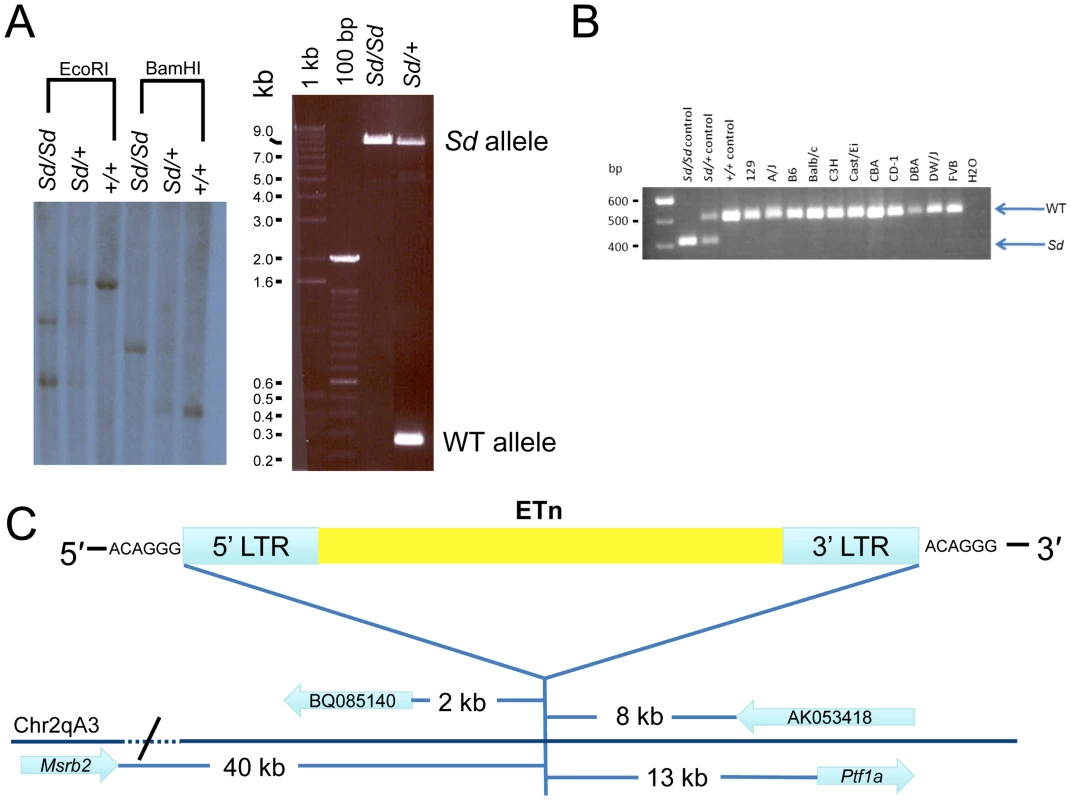
A) Southern analysis and PCR showing the presence of a large DNA insertion at the Sd locus. B) Multiplex PCR from inbred mouse lines showing the mutation is only present in Sd mice, and is not a polymorphism. In this three primer PCR reaction the Sd amplimer is 406 bp, while the WT amplimer is 510 bp. C) Mapping of the Sd mutation which we identified as an ETn (early transposon) in relation to nearby gene/ESTs, figure not to scale. To further confirm the presence of an ETn insertion at the Sd locus, long range PCR was utilized with PCR primers designed flanking the insertion site. Using wildtype genomic DNA as a template, the primers amplified a product of 253 bp as expected (Figure 3A). When Sd/Sd DNA was used as a template, the 253 bp product was absent, and instead a product of ∼9,000 bp was amplified. In heterozygous DNA, both fragments were amplified (Figure 3A). These data, in conjunction with the Southern analysis, confirmed insertion of a large piece of DNA at the Sd locus. The long range PCR product was cloned and sequenced using primer walking. The Sd insertion was identified as an early transposon (ETn) at mouse chromosome 2 position 19,355,026. There was no loss of wildtype DNA at the insertion site. However, the insertion resulted in a 6 base pair terminal duplication sequence (TSD) flanking the ETn. TSD sequences are a hallmark of retroviral insertion. Sequencing data showed that the long terminal repeats (LTRs) of the ETn were 847 base pairs in length and 100% identical to each other. The internal sequence of the ETn is 6,834 bp in length and does not contain any open reading frames. The total length of the Sd ETn is 8,528 bp (GenBank Accession JX863104).
Rigorous comparison of our next-generation sequencing data across the Sd critical region to the reference B6 genome, and available sequence from the CBA genome (CBA is the last known outcross of the RSV/LeJ line), did not reveal any significant sequence changes. Thus, we were not able to identify the mouse strain on which the Sd mutation originally arose in the 1920s. In order to rule out the possibility that the Sd ETn is a common polymorphism, we assembled a panel of DNA from 10 inbred and 1 outbred mouse strains for analysis. These strains were chosen to include a diverse number of strains spanning many arms of the inbred mouse genealogy chart [19]. We specifically included the A/J line since ETn sequences are reported to be highly active in this strain [17]. We designed a locus specific multiplex PCR and screened the 11 additional mouse strains (Figure 3B). The Sd ETn was not identified in any of the strains, indicating that it is not a common strain polymorphism.
q–RT PCR and 5′ RACE analysis
ETn sequences are known to affect gene expression when they insert within a gene and are predicted to affect expression of genes when they land upstream [17]. The Sd ETn insertion is located 12,463 bases upstream of the Ptf1a start codon and within the gene's previously defined 15.6 kb promoter/enhancer region and in the same orientation as the Ptf1a gene (Figure 3C; Figure S2) [20]. Thus, we hypothesized that Ptf1a expression is affected by the ETn insertion. qRT-PCR on RNA isolated from whole embryos at E9.5, when the first manifestation of the Sd phenotype becomes apparent, indicated the Ptf1a gene is upregulated at ∼9 times normal levels in Sd/Sd embryos at this timepoint (Figure 4). This over expression was also seen in Sd/+ embryos where the Ptf1a is expressed ∼5 fold that of wildtype (Figure 4). These data demonstrate a dose-dependent up regulation of Ptf1a expression that correlates with the number of mutant alleles and the severity of the Sd phenotype.
Fig. 4. Gene expression at E9.5 in Sd mutant embryos. 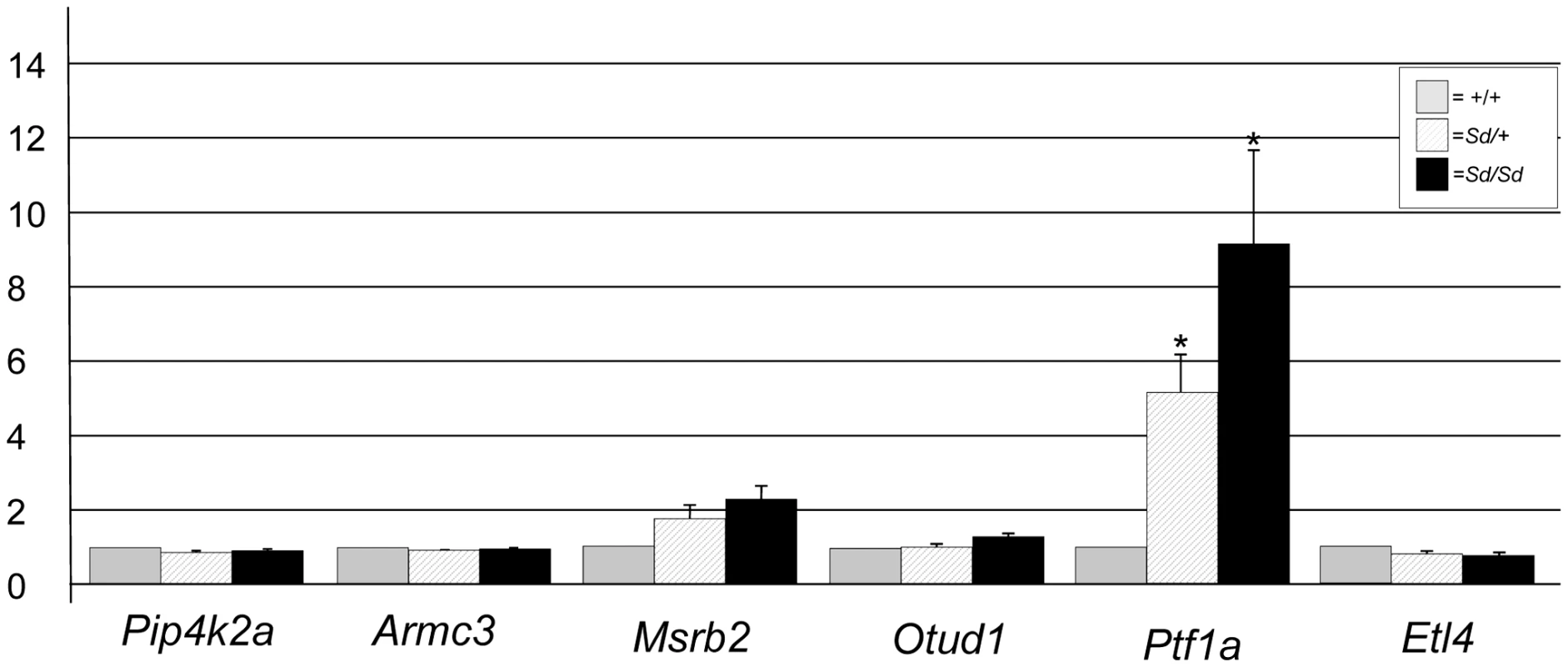
Quantitative real time PCR plot of gene expression in RNA isolated from Sd/Sd (black bars), Sd/+ (hatched bars), and +/+ (grey bars) embryos of RefSeq genes mapping to the Sd interval. Y-axis shows fold change normalized to 1 for wildtype (+/+) expression. * denotes significance in expression levels where p<0.05 (For Ptf1a; Sd/+ versus +/+ p = 0.029, Sd/Sd versus +/+ p = 0.044). RNA expression levels of other genes mapping to the Sd critical interval, including Pip4k2a, Armc3, Otud1, and Etl4 were equivalent in Sd/Sd, Sd/+, and +/+ embryos (Figure 4). The Msrb2 gene showed an ∼2 fold change that was not statistically significant (Figure 4). This increased expression change in Msrb2 is likely due to our small sample size (n = 3/genotype). We were unable to amplify message from the 4921504E06Rik gene at this timepoint. In addition, we identified an EST (BQ085140) that is not represented in the RefSeq data located 2 kb from the 5′ end of the ETn. BQ085140 is the closest expressed sequence to the ETn, however, its orientation is opposite that of the retrotransposon. We were unable to amplify BQ085140 from RNA isolated from E9.5 embryos of any genotype. EST AK053418 maps between the ETn insertion site and the Ptf1a gene, 8 kb from the 3′ end of the ETn and also in the opposite orientation. We were unable to amplify AK053418 from RNA isolated at this timepoint in either mutant or wildtype RNA. These data indicate that the two closest expressed sequences are not expressed at E9.5, and are not affected by insertion of the ETn at this timepoint even though Ptf1a is overexpressed. We did not test the expression of AK076779, 4930436L09Rik, and Gm3230 based on their distance from the ETn and lack of open reading frames.
To determine whether the ETn was acting as a promoter/enhancer or resulting in a dominant negative fusion RNA we performed 5′ RACE (rapid amplification of cDNA ends). Using RNA isolated from E9.5 Sd/Sd embryos, our 5′ RACE reactions only amplified the 5′ end of the Ptf1a gene (represented by GenBank Accession 007922). Thus, there is no incorporation of ETn sequence in the RACE products from homozygous mutant RNA. These data indicate that the Sd ETn is acting as an enhancer to strongly augment expression of normal Ptf1a transcripts.
Ptf1a transgenic mouse lines
We generated two transgene constructs in an attempt to recapitulate the Sd phenotype (Text S1). In the first experiment the Ptf1a coding sequence was cloned into the pCAGGS vector [21]. The pCAGGS vector contains the cytomegalovirus immediate early enhancer and the chicken β-actin promoter, resulting in ubiquitous expression of cloned sequences in mammalian cells [21], [22]. Among 116 harvested embryos only 6 carried the pCAGGS-mPtf1a transgene, compared with a typical yield of 10 to 20% transgenic offspring [23] (Table S1). Three of the six transgenic embryos did not express Ptf1a ectopically and were phenotypically normal at E16.5. The remaining three transgenic embryos had arrested growth and were dead and in the process of resorbing (Table S1). These data are consistent with a negative effect of Ptf1a overexpression on development.
In the second experiment we used recombineering [24] to create a BAC-based genomic clone containing both the Ptf1a gene and the Sd ETn (Text S1). Using gap repair we sub-cloned a 31 kb DNA fragment containing the Ptf1a gene and the previously reported known promoter, 5′ enhancer, and 3′ control region sequences (Figure S2) [20]. We inserted the ETn into the precise Sd location 12 kb upstream of Ptf1a to create an ETn/Ptf1a-containing genomic transgene. Both the ETn-containing transgene and the control BAC-based transgene lacking the ETn were injected into fertilized mouse eggs. Founder (G0) embryos were dissected between E14.5 and E15.5. No abnormal phenotype was apparent in the transgenic embryos with or without the Sd ETn insertion. However, there were significantly fewer transgenic embryos (3 of 23; p = 0.0086, Fishers exact test) carrying the ETn-containing transgene than the associated control transgene (16 of 32) (Table S1).
Discussion
Our data reveal that the Sd phenotype is caused by an insertion of an early transposon (ETn) at the Sd locus. The Sd ETn maps upstream of the promoter of the Ptf1a gene causing overexpression of normal Ptf1a transcripts at E9.5. No fusion transcript of the ETn and the Ptf1a gene was detected, indicating that the ETn is functioning as a strong enhancer. Ptf1a (originally termed p48) is a basic-helix-loop helix transcription factor that was first cloned from a rat exocrine-specific pancreatic cell line [25]. Northern blot analysis in adult rat tissues revealed expression limited to the exocrine pancreas [25]. Thus, p48 was given the name “pancreas specific transcription factor 1a.” Northern and PCR-based analysis in developing mouse pancreas initially showed onset of Ptf1a expression at embryonic day 12 (E12) [25]. However, further examination of whole mouse embryo by RT-PCR indicated that Ptf1a expression is highest at E10.5 [26]. Analysis of the expression pattern of Ptf1a using whole mount in situ hybridization indicated that expression of the gene is not restricted to the developing pancreas. High levels of expression were found at E10.5 in the cerebellar primordium and throughout the length of the developing neural tube in addition to the developing pancreatic buds [26]. In humans, recessive mutations of PTF1A cause cerebellar agenesis and permanent neonatal diabetes due to pancreatic agenesis [27].
Ptf1a null mice are born in Mendelian ratios; however, they lack a pancreas and die shortly after birth [28]. The exocrine cells of the developing pancreas fail to form in null embryos, and exocrine specific genes (amylase, etc) are never expressed. However, some endocrine cells do form in null embryos and express endocrine specific genes (insulin, etc.); interestingly, these endocrine cells reside in the spleen. Based on the null phenotype it was thought that Ptf1a was essential for development of exocrine-specific pancreas cells and that the exocrine cells were secondarily crucial for the proper spatial organization of the endocrine pancreas [28]. Fate mapping using a Ptf1a-Cre locus specific knock-in and cre-mediated LacZ expressing mice showed that Ptf1a expression was present in all early pancreatic precursor cells as nearly all acinar, ductal, and islet cells showed a history of Ptf1a expression [29]. Sd mice do not have a reported pancreatic phenotype. Our analysis of Ptf1a overexpression in Sd mice only focused on total RNA isolated from E9.5 embryos, which is ∼1 day prior to pancreatic bud formation. It is unknown whether the ETn insertion influences Ptf1a expression occurring in the pancreatic primordia, or older pancreas.
Sd mice exhibit neuronal patterning abnormalities, and there is a significant decrease in the number of motor neurons in the most caudal portions of the developing embryo [30]. This could be secondary to failure of development of multiple caudal tissues in Sd mice, or could result from up regulation of Ptf1a. Mouse Ptf1a mRNA was injected into one blastomere of two cell stage Xenopus embryos [26]. Presence of the mouse Ptf1a mRNA in the cells of the developing Xenopus embryos suppressed growth in the cells which become interneurons and primary sensory neurons [26]. Injected embryos were not analyzed beyond neural development at early stages, so it is unknown whether overexpression of mouse Ptf1a in Xenopus would have any caudal phenotypic characteristics similar to Sd mice. Overexpression of Ptf1a has not yet been studied in other model organisms. Thus, the Sd mouse is the first report of a phenotype caused by ectopic Ptf1a expression.
Ptf1a is a transcription factor required in several tissue lineages and which interacts with tissue specific co-factors [31]. Ptf1a is part of a heterotrimeric complex called PTF1 [25], [26], [32]. The PTF1 complex includes Ptf1a, RPB-J or RPB-L, and a class A bHLH protein (HEB, E2, or E47) [31]. The RPB genes act as both repressors and co-activators [33]. RPB-J is ubiquitously expressed and responsive to the Notch intracellular domain, while RPB-L is tissue-specific in the developing pancreas, neural tube, and cerebellum, and is Notch-independent [33]. Ectopically increased Ptf1a could act to affect downstream gene expression through heterodimeric binding to RBP-J and/or heterotrimeric binding with RPB-J and a class A bHLH protein, as both heterotrimeric and heterodimeric complexes have the ability to bind DNA [31]. It is also possible that excessive Ptf1a may lead to de-repression of RPB-J regulated genes. However, it is unclear whether this would occur without specific signals from the Notch intracellular domain. It will be interesting to determine whether ectopic overexpression of Ptf1a causes misexpression of downstream genes, possibly through interaction with RBP-J.
Endogenous retrovirus (ERV) sequences containing long terminal repeats (LTRs) make up ∼8–10% of the human and mouse genomes [17]. ERV elements transpose in a copy and paste method via transcribed RNA intermediates. In humans, LTR mediated ERV retrotransposition has not been described and these elements have never been implicated in human disease. However, LTR mediated ERVs are mobile in mice and account for ∼10–15% of all spontaneous mouse mutations [17]. Early transposons (ETn) are a subfamily of the ERVs which also include intracisternal A particles (IAP) and MusD elements [34]. ETn elements range in size from 2 kb to 8 kb, do not contain open reading frames, and are non-autonomous [34], [35]. Rather, retrotransposition of the ETn elements require proteins encoded by the related MusD ERV [34], [36].
The ETn elements are known to be very highly expressed between E3.5 and E7.5 in all cells [37]. This high level of ETn expression likely accounts for the high rate of transposition of these elements in mice. After E7.5, global ETn transcription decreases to less than 5% of that seen in the undifferentiated cells of the early embryo. The expression pattern post-implantation (between E7.5–13.5) becomes more limited to mostly non-differentiated cells [38]. However, it is interesting to note that at E9.5 there is intense expression of ETn sequences in the caudal portion of the neural tube and caudal somites. [38]. It is tempting to hypothesize that this caudally localized ETn driven overexpression of Ptf1a at E9.5 results in the severe caudal defects observed in Sd mutant mice.
Luciferase assays have been used in cultured cells to study the transcriptional activity of LTR sequences contained in ETn elements [39]. Promoter activity can be up to 200-fold higher from the same LTR cloned into undifferentiated compared to differentiated cells [39]. Mutagenic ETn elements published to date have all landed intronically within the genes they affect [17], [18]. These ETn elements cause aberrant splicing of the gene in which the ETn inserted, resulting in premature polyadenylation and protein truncation. The related IAP elements are known to have transposed upstream of genes and act as strong promoters, as in the agouti locus [40]. Similarly, the Dactylaplasia (Dac) mouse is characterized by an incompletely penetrant limb phenotype consisting of missing central digital rays. The Dac mutation has been identified as a MusD element that maps downstream of the Fgf8 gene, and Fgf8 expression is downregulated during limb development in homozygous mutants [41]. The Sd ETn landed within the well-studied promoter/enhancer region of the Ptf1a gene [20]. It is plausible that insertion of the ETn within Ptf1a could usurp endogenous promoter activity until the ETn is methylated, thus suppressing endogenous Ptf1a promoter activity at early times. Our data do suggest that the Sd ETn is acting as a strong enhancer by causing a dose-dependent increase in Ptf1a expression that correlates with the severity of the Sd phenotype.
We attempted to recapitulate the Sd phenotype using two different transgene constructs; one with the ubiquitously expressed strong pCAGGS promoter driving the Ptf1a cDNA, and one BAC-based transgene with insertion of the ETn element upstream of the Ptf1a gene. Neither of these transgenes recapitulated the Sd phenotype. We hypothesize that the pCAGGS-driven expression of Ptf1a was more deleterious than the Sd mutation because of more widespread, and possibly higher level, of Ptf1a expression. The BAC-based ETn-containing transgene could also be expressed in novel spatial and temporal patterns that do not recapitulate the Sd mutation. Our ETn-containing BAC-based transgene may also be lethal since there were significantly fewer embryos carrying the ETn-containing transgene than the control. Although we designed our BAC-based genomic transgene to contain the known upsteam and downstream regulatory elements of the Ptf1a gene [20], it is possible that additional sequences necessary for recapitulation of the Sd phenotype are required and were unknowingly excluded in the design of our experiment (Figure S2). Passage through the germline might also be required for proper methylation of the ETn element to mirror the mutagenic effects of the Sd ETn.
Development of the notochord is severely affected in Sd mutant embryos [4], [7]. The notochord and associated floor plate develop normally through E9.5 in Sd/Sd embroys and E10.5 in Sd/+ embryos. No new notochord is formed caudally from these timepoints forward. The notochord that has developed degenerates completely in both Sd/Sd and Sd/+ embryos by E14 [6], [7]. However, the floorplate that developed alongside the notochord prior to notochord degradation remains intact. Sonic hedgehog (Shh) expression is affected by the lack of notochord, though the floorplate that remains after notochord degeneration still expresses Shh normally [42]. The lack of notochord and floorplate in the most caudal portions of the embryo is a plausible explanation for some aspects of the characteristic Sd phenotype. However, the lack of notochord is the same in both homozygous and heterozygous embryos even though the phenotype is less severe in heterozygous animals, suggesting that simple lack of Shh in the caudal portion of the embryo is unlikely the sole cause of the Sd phenotype. It is more likely that a combination of lack of Shh from the absent notochord as well as misexpression of other genes due to the ectopic overexpression of Ptf1a play a significant role in the phenotypic etiology. Global gene expression analysis of various timepoints in developing Sd mutants will be helpful to characterize these changes.
Mouse models have been invaluable in the study of human development, and the Danforth's short tail mouse is a striking model of human caudal birth defects. The Sd mouse exhibits features seen in caudal regression syndrome (CRS), urorectal septum malformation sequence/persistent cloaca (URSMS), VACTERL association, and Currarino syndrome. Shared phenotypic characteristics include kidney dysgenesis (or agenesis), vertebral anomalies, and anorectal malformations. The overlap of phenotypic characteristics suggests that there are similar pathways or related pathways that are disrupted in these disorders. Interestingly, Currarino syndrome, characterized by the triad of hemisacrum, anorectal malformation and pre-sacral mass, is caused by mutations in the HLXB9 gene (also known as MNX1), which is known to have a role in pancreas and motor neuron development [8], [9]. Recently, it has been reported that Ptf1a is a strong regulator driving Mnx1 expression during pancreatic development [43]. Although we identified a significant increase in Ptf1a in mutant Sd embryos at E9.5, we did not observe a concurrent change in Mnx1 expression in mutant embryos (data not shown). Further analysis of expression patterns may be needed at later timepoints to identify downstream changes due to Ptf1a misexpression. Importantly, mice lacking Mnx1 do not mirror the caudal phenotype seen in human patients with Currarino syndrome [44]. Thus, the genetic pathways leading to caudal dysgenesis via misexpression of Ptf1a and/or Mnx1 may differ between these species.
It is hypothesized that caudal malformation disorders result from failure of induction and proliferation of the caudal mesoderm [45]. In URSMS it is specifically postulated that the phenotype is caused by failure of the meosdermally derived urorectal septum to properly grow and divide the cloaca into the primary urogenital sinus (ventrally) and the rectum (dorsally) [46]. Studies of chimeric Sd mice in which Sd mutant cells carrying the LacZ gene were injected into wildtype blastocysts show specific exclusion of all Sd cells from the dorsal side of the urorectal septum (which overlaps the ventral portion of the hindgut), indicating a failure of this structure to grow in mutant animals [47]. Whether Ptf1a is overexpressed due to the ETn insertion in the developing mesoderm of the urorectal septum in Sd embryos is unknown and will need further investigation. Now that the genetic lesion has been identified, the study of downstream gene expression changes in Sd mice will undoubtedly lead to an improved understanding of human caudal development disorders.
Materials and Methods
Ethics statement
All experiments involving mice have been approved by The University of Michigan University Committee on Use and Care of Animals.
Mice
The Sd mutation has been maintained at the Jackson Laboratories on a recombinant inbred strain (RSV/LeJ, stock# 000268) for over 127 generations, and we have established an Sd breeding colony at the University of Michigan. Data presented herein are from mice from the RSV/LeJ line, or from outcrossing the Sd mutation to CD-1 mice (Charles River Laboratories, Wilmington, MA). An outbred/mixed background was maintained by crossing inbred RSV/LeJ mice to CD-1 and harvesting embryos only from intercrossed F1 mice. The Sd phenotype is consistent between the inbred and outbred mice. Mice were housed in environmentally controlled conditions with 14 hour light/10 hour dark cycles with food and water provided ad libitum.
Timed pregnancies
Matings for timed embryo isolation were set up using standard animal husbandry techniques. Noon on the day of vaginal plug observation was considered embryonic day (E) 0.5. DNA for embryo genotyping was isolated from yolk sacs via the HotSHOT extraction method.
Genomic DNA isolation
Genomic DNA for next-generation sequencing and Southern analysis was isolated using phenol∶chloroform extraction and ethanol precipitation, and DNA was quantified on a NanoDrop spectrophotometer (ThermoFisher, Asheville, NC).
Targeted capture, library creation, and next-generation sequencing
5 µg (at 50 ng/µl) of heterozygous Sd DNA was sheared into 300 bp fragments on a Covaris S-series sonicator (Covaris, Woburn, MA) and was subsequently used to create a total genomic DNA library for next-generation sequencing using standard Illumina protocols. Prior to sequencing, the DNA library was enriched for the Sd critical region (between chromosome 2 bases 18,883,257–20,481,194 on build 37 of the mouse genome (July 2007; mm9) as visualized on the UCSC genome browser (http://www.genome.ucsc.edu) using a 244 K Agilent SureSelect DNA oligo microarray designed utilizing Agilent eArray software (https://earray.chem.agilent.com/). Capture was performed by following the Agilent SureSelect Array protocol version 1. The captured DNA was tested for proper enrichment via quantitative PCR with locus specific primers and then subject to 36 bp paired-end sequencing on an Illumina Genome Analyzer IIx at the University of Michigan DNA Sequencing Core.
Bioinformatic analysis of next-generation sequencing data
Next-generation sequence data were aligned to the corresponding region of chromosome 2 represented on the NCBI37/mm9 build of the mouse genome. Sequences were aligned using the Bowtie and MAQ software applications [16]. Overall assembly of the region was done using the Mosaik software suite and assembled discrepancies were mined using Consed software [48]. Sequences were also aligned using the Bowtie software application. Output files were produced in SAM and BAM format and converted to BED format for visualization on the UCSC Genome Browser. Histogram graphs to analyze coverage (copy number variation and deletions and duplications) were created.
The unmapped reads contain a combination of poor quality sequence and reads which couldn't be mapped to the existing reference sequence. Reasons for not mapping include repetitive and low complexity sequence as well as novel elements. Using the paired-end reads, and a combination of existing tools, and custom methods, we identified read pairs where there were at least 5 sequence reads where one end mapped uniquely to Chromosome 2 and the other end did not map but contained a repetitive element. We compared these repetitive sequences (LTR) with known locations of repeat sequences in the mouse genome to identify novel insertions and their locations.
Genotyping
Multiplex PCR was used for genotyping with primers CNV577 (5′-TTTCCACGGCCATTCTTTAC-3′), CNV578 (5′-GCTCAACCAGAACAATACATTCAG-3′) and CNV580 (5′-GCCAATCAGGAGACTGAAGC-3′). PCR was performed in 20 µl reactions containing 1 µM of each primer, and 1× TaqProComplete (Denville Scientific Inc., Metuchen, NJ) in an Eppendorf Mastercycler (Eppendorf North America, Hauppauge, NY). Cycling conditions were 94°C for 5 minutes, followed by 30 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds, and a final extension of 72°C for 10 minutes. PCR products were resolved on a 2%TAE gel stained with ethidium bromide. The resulting wildtype amplimer is 512 bp and the Sd allele amplimer is 405 bp.
Southern analysis
6 µg of genomic DNA from wildtype, Sd/+, and Sd/Sd was digested with 4 U/µg of either EcoRI or BamHI (New England Biolabs, Ipswich MA) supplemented with 2.5 mM spermidine overnight at 37°C. Digested DNA was electrophoresed on a 1% TAE gel and transferred to Hybond-N+ nylon membranes as previously described. Digoxigenin labeled probe was created using the Roche PCR DIG probe synthesis kit (Roche Applied Science, Indianapolis, IN) per manufacturer instructions with primers CNV551 (5′-AACCACAGGAAAGGTTGCAG-3′) and CNV552 (5′-TCTGGGTACCAGCTTCAGTG-3′) using mouse genomic DNA as a template. Southern analysis was performed using the Roche DIG Easy Hyb system per manufacturer instructions (Roche Applied Science, Indianapolis, IN).
Long-range PCR and cloning
PCR primers flanking the Sd ETn insertion were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/). The ETn was amplified using the Roche Expand Long Template PCR system (Roche Applied Science, Indianapolis, IN) per manufacturer instructions with primers CNV559 (5′-GAAGCTCTGCAGGCTGAAAGCAAAG-3′) and CNV560 (5′-GAATGAGGACTCTGCCCTTGAGTGG-3′). Amplified PCR product was cloned using the TOPO XL PCR cloning kit (Invitrogen/Life Technologies, Grand Island, NY). Sequencing of the cloned ETn was performed by primer walking (see GenBank Accession JX863104)
Quantitative real-time PCR
Primers for qRT-PCR were identified via PrimerBank (http://pga.mgh.harvard.edu/primerbank/) for Ptf1a, Msrb2, Otud1, Etl4, Pip4k2a, and Armc3. Primer3 was used to design primer pairs for 4921504E06Rik (CNV496 5′-TACCTAGCATGGTGCCTGAAGA-3′/CNV497 5′-GTCATTGCATACTGCCGGTAAA-3′), BQ085140 (CNV788 5′-GTGCTGGACCCAAACATAGC-3′/CNV789 5′-TGGGGAATCAACGAACTCTG-3′), and AK053418 (CNV792 5′-TAAGGGGATGGGAAGGTGTC/CNV793 5′-AGGTGCATCATCATGGCTTC-3′). cDNA template for qRT-PCR was synthesized using the First Strand cDNA synthesis kit from Invitrogen/Life Technologies (Grand Island, NY) from 1 µg RNA isolated from E9.5 Sd/Sd, Sd/+, and +/+ embryos (3 from each genotype) using the RNeasy Micro Kit (Qiagen, Valencia, CA). qRT-PCR reactions were performed in triplicate using 1× Applied Biosystems Power SYBR mix and run on an Applied Biosystems StepOne Plus PCR system (Applied Biosystems/Life Technologies, Grand Island, NY). Cycling conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 57°C for 30 seconds, 72°C for 30 seconds. The cycle threshold for each reaction was automatically calculated using StepOne Software v2.2. Fold change of the test genes was calculated for each genotype by normalization to β-actin using the Pfaffl method [49]. Statistical analysis was performed via Student's T-test with significance p<0.05.
5′ RACE
5′ RACE was performed using the FirstChoice RLM-RACE kit (Ambion/Life Technologies, Grand Island, NY) per manufacturer instructions with 1 µg template Sd/Sd RNA as input. RACE products were sequenced at the University of Michigan DNA Sequencing Core.
Supporting Information
Zdroje
1. DanforthC (1930) Developmental Anomalies in a special starin of mice. Am J Anat 45 : 275–287.
2. DunnLC, SG-S, VB (1940) A new mutation in the mouse affecting spinal column and urogenital system. The Journal of Heredity 31 : 343–348.
3. Gluecksohn-SchoenheimerS (1943) The Morphological Manifestations of a Dominant Mutation in Mice Affecting Tail and Urogenital System. Genetics 28 : 341–348.
4. Gluecksohn-SchoenheimerS (1945) The Embryonic Development of Mutants of the Sd-Strain in Mice. Genetics 30 : 29–38.
5. GrunebergH (1958) Genetical studies on the skeleton of the mouse. XXII. The development of Danforth's short-tail. J Embryol Exp Morphol 6 : 124–148.
6. PaavolaLG, WilsonDB, CenterEM (1980) Histochemistry of the developing notochord, perichordal sheath and vertebrae in Danforth's short-tail (sd) and normal C57BL/6 mice. J Embryol Exp Morphol 55 : 227–245.
7. WilsonDB, FintaLA, CenterEM, PaavolaLG (1982) An electron microscopic analysis of notochordal and mesenchymal cell abnormalities in embryos of Danforth's short-tail (Sd) mice. Virchows Arch B Cell Pathol Incl Mol Pathol 39 : 101–110.
8. LynchSA, WangY, StrachanT, BurnJ, LindsayS (2000) Autosomal dominant sacral agenesis: Currarino syndrome. J Med Genet 37 : 561–566.
9. RossAJ, Ruiz-PerezV, WangY, HaganDM, SchererS, et al. (1998) A homeobox gene, HLXB9, is the major locus for dominantly inherited sacral agenesis. Nat Genet 20 : 358–361.
10. CretolleC, PeletA, SanlavilleD, ZerahM, AmielJ, et al. (2008) Spectrum of HLXB9 gene mutations in Currarino syndrome and genotype-phenotype correlation. Hum Mutat 29 : 903–910.
11. KibarZ, TorbanE, McDearmidJR, ReynoldsA, BerghoutJ, et al. (2007) Mutations in VANGL1 associated with neural-tube defects. N Engl J Med 356 : 1432–1437.
12. WesselsMW, KuchinkaB, HeydanusR, SmitBJ, DooijesD, et al. (2010) Polyalanine expansion in the ZIC3 gene leading to X-linked heterotaxy with VACTERL association: a new polyalanine disorder? J Med Genet 47 : 351–355.
13. Garcia-BarceloMM, WongKK, LuiVC, YuanZW, SoMT, et al. (2008) Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A 146A: 3181–3185.
14. ReardonW, ZhouXP, EngC (2001) A novel germline mutation of the PTEN gene in a patient with macrocephaly, ventricular dilatation, and features of VATER association. J Med Genet 38 : 820–823.
15. AlfredJB, RanceK, TaylorBA, PhillipsSJ, AbbottCM, et al. (1997) Mapping in the region of Danforth's short tail and the localization of tail length modifiers. Genome Res 7 : 108–117.
16. LangmeadB (2010) Aligning short sequencing reads with Bowtie. Curr Protoc Bioinformatics Chapter 11: Unit 11 17.
17. MaksakovaIA, RomanishMT, GagnierL, DunnCA, van de LagemaatLN, et al. (2006) Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS Genet 2: e2 doi:10.1371/journal.pgen.0020002
18. BaustC, BaillieGJ, MagerDL (2002) Insertional polymorphisms of ETn retrotransposons include a disruption of the wiz gene in C57BL/6 mice. Mamm Genome 13 : 423–428.
19. BeckJA, LloydS, HafezparastM, Lennon-PierceM, EppigJT, et al. (2000) Genealogies of mouse inbred strains. Nat Genet 24 : 23–25.
20. MasuiT, SwiftGH, HaleMA, MeredithDM, JohnsonJE, et al. (2008) Transcriptional autoregulation controls pancreatic Ptf1a expression during development and adulthood. Mol Cell Biol 28 : 5458–5468.
21. NiwaH, YamamuraK, MiyazakiJ (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108 : 193–199.
22. KeeganCE, HutzJE, ElseT, AdamskaM, ShahSP, et al. (2005) Urogenital and caudal dysgenesis in adrenocortical dysplasia (acd) mice is caused by a splicing mutation in a novel telomeric regulator. Hum Mol Genet 14 : 113–123.
23. Nagy A, Gertsentein M, Vintersten K, Behringer R (2003) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, New york: Cold Spring Harbor Press.
24. WarmingS, CostantinoN, CourtDL, JenkinsNA, CopelandNG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33: e36.
25. KrappA, KnoflerM, FrutigerS, HughesGJ, HagenbuchleO, et al. (1996) The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J 15 : 4317–4329.
26. ObataJ, YanoM, MimuraH, GotoT, NakayamaR, et al. (2001) p48 subunit of mouse PTF1 binds to RBP-Jkappa/CBF-1, the intracellular mediator of Notch signalling, and is expressed in the neural tube of early stage embryos. Genes Cells 6 : 345–360.
27. SellickGS, BarkerKT, Stolte-DijkstraI, FleischmannC, ColemanRJ, et al. (2004) Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet 36 : 1301–1305.
28. KrappA, KnoflerM, LedermannB, BurkiK, BerneyC, et al. (1998) The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 12 : 3752–3763.
29. KawaguchiY, CooperB, GannonM, RayM, MacDonaldRJ, et al. (2002) The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32 : 128–134.
30. BovolentaP, DoddJ (1991) Perturbation of neuronal differentiation and axon guidance in the spinal cord of mouse embryos lacking a floor plate: analysis of Danforth's short-tail mutation. Development 113 : 625–639.
31. BeresTM, MasuiT, SwiftGH, ShiL, HenkeRM, et al. (2006) PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol 26 : 117–130.
32. MasuiT, LongQ, BeresTM, MagnusonMA, MacDonaldRJ (2007) Early pancreatic development requires the vertebrate Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev 21 : 2629–2643.
33. TanigakiK, HonjoT (2010) Two opposing roles of RBP-J in Notch signaling. Curr Top Dev Biol 92 : 231–252.
34. MagerDL, FreemanJD (2000) Novel mouse type D endogenous proviruses and ETn elements share long terminal repeat and internal sequences. J Virol 74 : 7221–7229.
35. McCarthyEM, McDonaldJF (2004) Long terminal repeat retrotransposons of Mus musculus. Genome Biol 5: R14.
36. RibetD, DewannieuxM, HeidmannT (2004) An active murine transposon family pair: retrotransposition of “master” MusD copies and ETn trans-mobilization. Genome Res 14 : 2261–2267.
37. BruletP, CondamineH, JacobF (1985) Spatial distribution of transcripts of the long repeated ETn sequence during early mouse embryogenesis. Proc Natl Acad Sci U S A 82 : 2054–2058.
38. LoebelDA, TsoiB, WongN, O'RourkeMP, TamPP (2004) Restricted expression of ETn-related sequences during post-implantation mouse development. Gene Expr Patterns 4 : 467–471.
39. MaksakovaIA, MagerDL (2005) Transcriptional regulation of early transposon elements, an active family of mouse long terminal repeat retrotransposons. J Virol 79 : 13865–13874.
40. WolffGL, KodellRL, MooreSR, CooneyCA (1998) Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12 : 949–957.
41. KanoH, KurahashiH, TodaT (2007) Genetically regulated epigenetic transcriptional activation of retrotransposon insertion confers mouse dactylaplasia phenotype. Proc Natl Acad Sci U S A 104 : 19034–19039.
42. AsakuraA, TapscottSJ (1998) Apoptosis of epaxial myotome in Danforth's short-tail (Sd) mice in somites that form following notochord degeneration. Dev Biol 203 : 276–289.
43. ThompsonN, GesinaE, ScheinertP, BucherP, Grapin-BottonA (2012) RNA profiling and chromatin immunoprecipitation-sequencing reveal that PTF1a stabilizes pancreas progenitor identity via the control of MNX1/HLXB9 and a network of other transcription factors. Mol Cell Biol 32 : 1189–1199.
44. HarrisonKA, ThalerJ, PfaffSL, GuH, KehrlJH (1999) Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat Genet 23 : 71–75.
45. PauliRM (1994) Lower mesodermal defects: a common cause of fetal and early neonatal death. Am J Med Genet 50 : 154–172.
46. EscobarLF, WeaverDD, BixlerD, HodesME, MitchellM (1987) Urorectal septum malformation sequence. Report of six cases and embryological analysis. Am J Dis Child 141 : 1021–1024.
47. MaatmanR, ZachgoJ, GosslerA (1997) The Danforth's short tail mutation acts cell autonomously in notochord cells and ventral hindgut endoderm. Development 124 : 4019–4028.
48. GordonD, AbajianC, GreenP (1998) Consed: a graphical tool for sequence finishing. Genome Res 8 : 195–202.
49. PfafflMW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45.
50. ThiseltonDL, MaherBS, WebbBT, BigdeliTB, O'NeillFA, et al. (2010) Association analysis of the PIP4K2A gene on chromosome 10p12 and schizophrenia in the Irish study of high density schizophrenia families (ISHDSF) and the Irish case-control study of schizophrenia (ICCSS). Am J Med Genet B Neuropsychiatr Genet 153B: 323–331.
51. WenningMR, MelloMP, AndradeTG, LanaroC, AlbuquerqueDM, et al. (2009) PIP4KIIA and beta-globin: transcripts differentially expressed in reticulocytes and associated with high levels of Hb H in two siblings with Hb H disease. Eur J Haematol 83 : 490–493.
52. MoskovitzJ (2005) Roles of methionine suldfoxide reductases in antioxidant defense, protein regulation and survival. Curr Pharm Des 11 : 1451–1457.
53. GabbitaSP, AksenovMY, LovellMA, MarkesberyWR (1999) Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J Neurochem 73 : 1660–1666.
54. GlaserCB, YaminG, UverskyVN, FinkAL (2005) Methionine oxidation, alpha-synuclein and Parkinson's disease. Biochim Biophys Acta 1703 : 157–169.
55. SembaK, ArakiK, LiZ, MatsumotoK, SuzukiM, et al. (2006) A novel murine gene, Sickle tail, linked to the Danforth's short tail locus, is required for normal development of the intervertebral disc. Genetics 172 : 445–456.
56. ZachgoJ, KornR, GosslerA (1998) Genetic interactions suggest that Danforth's short tail (Sd) is a gain-of-function mutation. Dev Genet 23 : 86–96.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Pánevní endometrióza spojená s volnou tekutinou v peritoneální dutině snižuje úspěšnost otěhotnění po intrauterinní inseminaci
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



