-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
ATP-dependent nucleosome remodelers influence genetic processes by altering nucleosome occupancy, positioning, and composition. In vitro, Saccharomyces cerevisiae ISWI and CHD remodelers require ∼30–85 bp of extranucleosomal DNA to reposition nucleosomes, but linker DNA in S. cerevisiae averages <20 bp. To address this discrepancy between in vitro and in vivo observations, we have mapped the genomic distributions of the yeast Isw1, Isw2, and Chd1 remodelers at base-pair resolution on native chromatin. Although these remodelers act in gene bodies, we find that they are also highly enriched at nucleosome-depleted regions (NDRs), where they bind to extended regions of DNA adjacent to particular transcription factors. Surprisingly, catalytically inactive remodelers show similar binding patterns. We find that remodeler occupancy at NDRs and gene bodies is associated with nucleosome turnover and transcriptional elongation rate, suggesting that remodelers act on regions of transient nucleosome unwrapping or depletion within gene bodies subsequent to transcriptional elongation.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003317
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003317Summary
ATP-dependent nucleosome remodelers influence genetic processes by altering nucleosome occupancy, positioning, and composition. In vitro, Saccharomyces cerevisiae ISWI and CHD remodelers require ∼30–85 bp of extranucleosomal DNA to reposition nucleosomes, but linker DNA in S. cerevisiae averages <20 bp. To address this discrepancy between in vitro and in vivo observations, we have mapped the genomic distributions of the yeast Isw1, Isw2, and Chd1 remodelers at base-pair resolution on native chromatin. Although these remodelers act in gene bodies, we find that they are also highly enriched at nucleosome-depleted regions (NDRs), where they bind to extended regions of DNA adjacent to particular transcription factors. Surprisingly, catalytically inactive remodelers show similar binding patterns. We find that remodeler occupancy at NDRs and gene bodies is associated with nucleosome turnover and transcriptional elongation rate, suggesting that remodelers act on regions of transient nucleosome unwrapping or depletion within gene bodies subsequent to transcriptional elongation.
Introduction
Nucleosome remodelers use the energy of ATP hydrolysis to alter histone-DNA contacts, slide nucleosomes, and exchange or remove histones and entire nucleosomes. They are ubiquitous throughout eukaryotic evolution [1], and alterations in their expression are found in human congenital anomaly syndromes and cancers [2]–[5], highlighting their central role in cellular life.
Yeast Isw1, Isw2, and Chd1 remodeler complexes bind ∼30–85 bp of extranucleosomal DNA, which is required for efficient nucleosome repositioning in vitro [6]–[10], and histone H3 depletion stimulates Isw2 nucleosome sliding in vivo [11], presumably by creating regions of accessible DNA. Additionally, the human ISWI remodeler SNF2h functions in vitro as a dimeric sensor of linker length, with one ATPase molecule contacting extranucleosomal DNA on each side of a nucleosome, and the efficiency of its nucleosome repositioning activity is decreased with decreasing linker length [12]–[14]. Similarly, the human CHD7 remodeler, mutated in the developmental disorder CHARGE syndrome [5], requires ≥40 bp of extranucleosomal DNA for remodeling in vitro [15]. Yeast Isw1, Isw2, and Chd1 act at various points in gene bodies [16]–[21], which contain arrays of regularly spaced nucleosomes [22]. However, the average nucleosomal repeat length in S. cerevisiae is 165 bp [22]. Given that 147 bp of DNA is wrapped around the histone octamer, only ∼18 bp of DNA is extranucleosomal. Thus, much more DNA on either side of a nucleosome is required for ISWI and CHD proteins to act than is available between nucleosomes. This paradox is even more severe in S. pombe, where the Chd1-like remodelers hrp1 and hrp3 act within gene bodies [23]–[25], but nucleosomal repeats average 154 bp and linkers are therefore only ∼7 bp [26].
To address this paradox, we mapped the genomic distributions of yeast Isw1, Isw2, and Chd1 on native chromatin. We show that yeast ISWI and CHD remodelers are highly enriched at nucleosome-depleted regions (NDRs) flanking transcription units, where they bind to extended stretches of linker DNA flanking transcription factor binding sites (TFBSs). We find that remodeler binding is positively associated with nucleosome turnover and transcriptional elongation rate, suggesting that ISWI and CHD remodelers first associate with naked DNA within NDRs and subsequently relocate to gene bodies following nucleosome disruption by RNA Polymerase II transit, upon which there is ample linker DNA to promote their efficient remodeling activity.
Results/Discussion
Genome-wide mapping of remodeler binding on native chromatin
We performed immunoprecipitation of uncrosslinked (native) chromatin digested with micrococcal nuclease (MNase) combined with high-throughput paired-end sequencing (N-ChIP-seq) for S. cerevisiae Isw1, Isw2, and Chd1. N-ChIP does not rely on formaldehyde fixation, which crosslinks primary amines such as those in lysine-rich histones [27]. Thus, we circumvent crosslinking of remodelers solely to their nearest nucleosome [17] and are able to directly map the interaction of remodelers with DNA. We and others have previously mapped transcription factors (TFs) on native chromatin from yeast and human cells [28]–[30], and we have shown similar recovery of MNase-protected DNA fragments from both total nuclei and solubilized chromatin from yeast without crosslinking [30]. Thus, it appears that formaldehyde crosslinking may be dispensable for the detection of protein-DNA interactions.
ChIP and input samples were prepared for sequencing using a modified protocol that recovers fragments as small as ∼25 bp, enabling base-pair resolution mapping of remodeler occupancy [30]. Samples treated with MNase for 2.5′ displayed a nucleosomal peak centered at ∼170 bp, which was shifted to ∼160 bp in the 10′ samples (Figure 1A). Examination of these size distributions indicated that ChIP samples were enriched for supernucleosomal DNA fragments relative to nucleosomes. To assess this observation systematically, we determined the area under the curve for nucleosomal (141–250 bp) and supernucleosomal (251–428 bp) fragments from each sample. ChIP samples, regardless of factor, displayed supernucleosomal/nucleosomal ratios 1.74–3.87 (2.5′ MNase, P = 0.009) and 2.26–2.76 (10′ MNase, P = 0.002) times greater than that seen in input samples (Table S2), suggesting that Isw1, Isw2, and Chd1 participate in the protection of large stretches of DNA.
Fig. 1. N-ChIP-seq localizes ISWI and CHD remodelers throughout the genome. 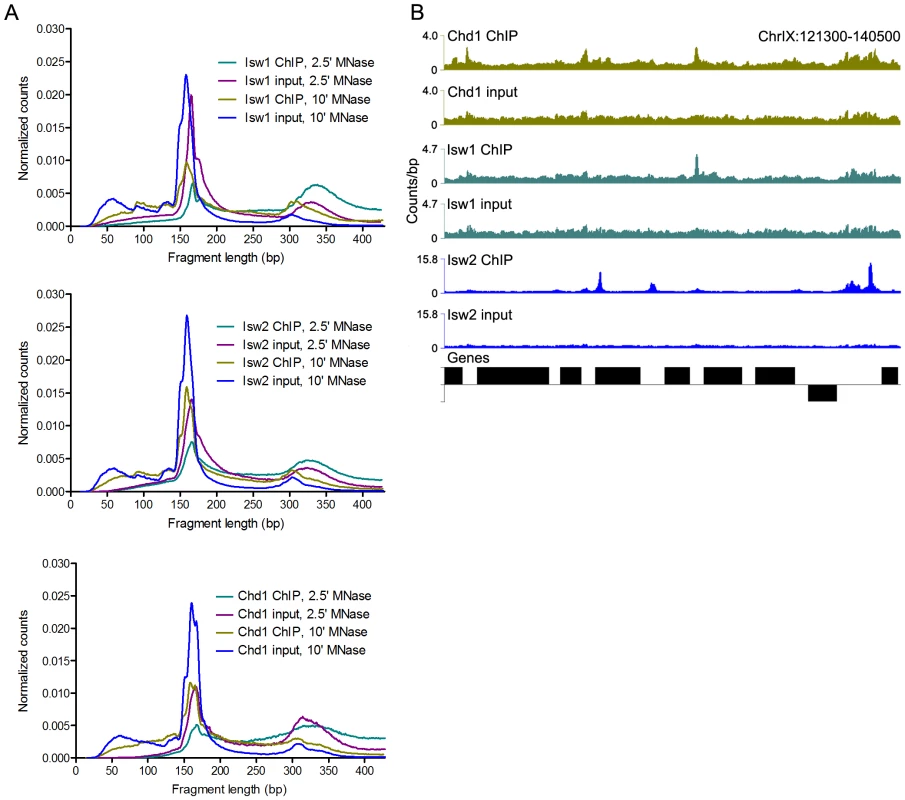
(A) Size distributions of mapped paired-end 2.5′ and 10′ MNase-digested wild-type Isw1, Isw2, and Chd1 ChIP and input fragments. Slight variations in the amount of supernucleosomal (>251 bp) fragments are attributable to minor variation in the degree of MNase digestion for each sample, as evidenced by slight differences in the nucleosomal maxima for each sample. (B) Binding profiles for 10′ MNase-digested Isw1, Isw2, and Chd1 samples across a representative region of the genome. The number of paired-end reads overlapping each genomic position (counts/bp) is indicated on the Y-axis. See Figure S1 for additional remodeler binding profiles. N-ChIP-seq revealed specific sites of enrichment for all three remodelers throughout the yeast genome (Figure 1B and Figure S1). We then tested if we could recapitulate known features of remodeler-genome association by N-ChIP-seq. We focused on Isw2, which has previously been characterized by genome-wide ChIP-chip [20], [31] and the recently developed ChIP-exo method [17]. ChIP-exo is a modification of the standard ChIP-seq protocol employing exonuclease digestion to improve the resolution of crosslinking ChIP-seq. Our N-ChIP-seq methodology is distinct from these techniques in that it does not rely on formaldehyde fixation, which also crosslinks primary amines to fix protein-protein interactions. We first obtained lists of Isw2 peaks determined by ChIP-chip [20] and ChIP-exo [17] as well as sites with altered nucleosome positioning in an isw2Δ strain [20]. We then determined the input-normalized N-ChIP-seq signal for each base pair in a 2-kb window centered on each ChIP or remodeling site and averaged the signal for each base pair within the window for each class of sites (Isw2 X-ChIP-chip, Isw2 ChIP-exo, and Isw2 remodeling) to generate average N-ChIP-seq profiles. We detected enrichment of Isw2 by N-ChIP-seq at sites bound by Isw2 in both X-ChIP-chip and ChIP-exo and sites of chromatin remodeling by Isw2 but not around randomly selected nucleosomes (Figure 2A). The two peaks on either side of the midpoint represent robust enrichment of Isw2 on either side of transcription factor binding sites. We note that the enrichment of Isw2 at these sites does not imply an effect on expression of nearby genes, only that our technique is able to replicate Isw2 binding sites previously determined by X-ChIP methodologies.
Fig. 2. Comparison of Isw2 N-ChIP and X-ChIP data. 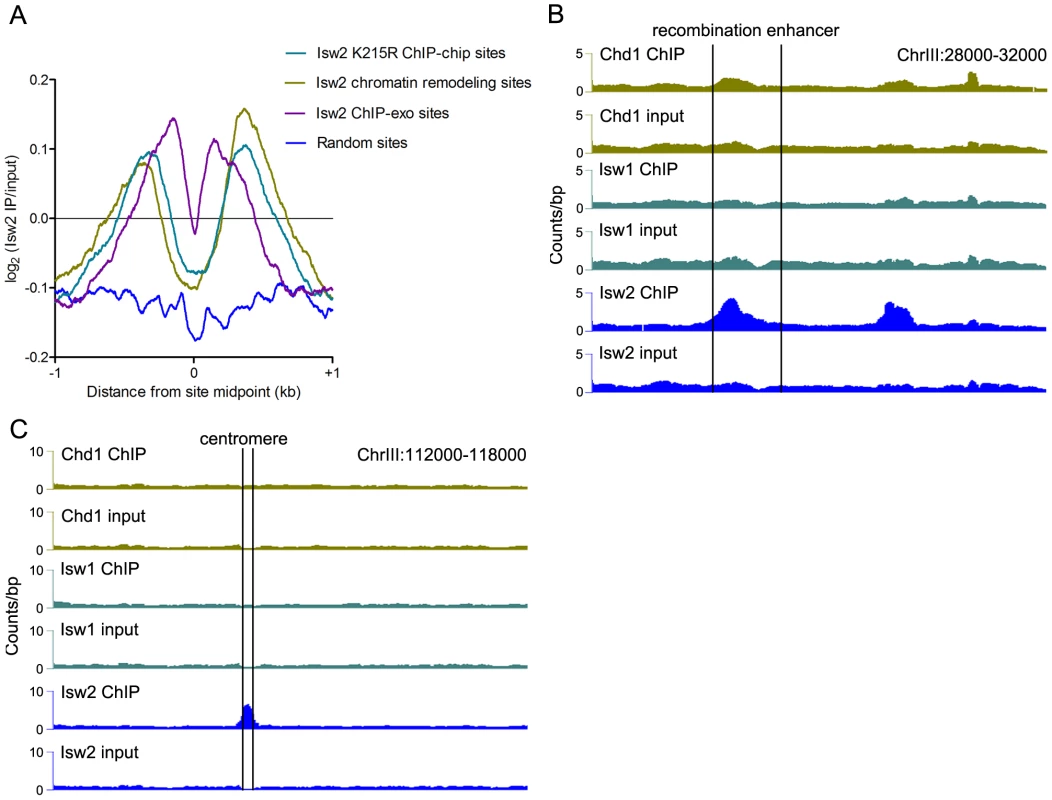
(A) Aggregate profiles of log2(Isw2 IP/input) at sites bound by catalytically inactive (K227R) Isw2 in ChIP-chip experiments (2128 sites), sites with altered nucleosome positioning in an isw2Δ strain (1399 sites), sites bound by wild-type Isw2 in ChIP-exo experiments (1251 sites), and around random nucleosomes (1399 sites).Also shown are profiles of remodeler binding at the (B) chrIII recombination enhancer and (C) chrIII centromere (marked by vertical lines). Sites of Isw2 action display high A+T content, which stiffens DNA and disfavors nucleosome formation [32]. We analyzed the bendability of DNA at sites occupied solely by Isw2 or by Isw1 and/or Chd1 without Isw2. Isw2-only N-ChIP-seq sites showed a reduction of ∼0.375 in average bendability compared to Isw1/Chd1 sites (P = 5.3×10−41). We also detected strong enrichment of Isw2 but little or no binding of Isw1 or Chd1 at the A+T rich yeast recombination enhancer (Figure 2B), as previously demonstrated [32]. We further noted enrichment of Isw2 at the chromosome III centromere, a ∼90% A+T sequence (Figure 2C). These results indicate that our N-ChIP-seq protocol faithfully captures known features of Isw2 genomic association.
Isw2 preferentially binds centromeres
The enrichment of Isw2 at the chromosome III centromere led us to investigate Isw2 centromeric association in more detail. Aggregate analysis of remodeler signal at all 16 yeast centromeres revealed robust enrichment of Isw2, but not Isw1 or Chd1 (Figure 3A). To precisely delineate the regions of the centromere bound by Isw2, we employed V-plotting [30]. In a V-plot, the midpoint of each paired-end fragment is assigned a dot in two-dimensional space, wherein the X-axis value is the distance of the fragment midpoint from a defined genomic feature and the Y-axis value is the length of the fragment. V-plotting of Isw2 ChIP and input data revealed striking enrichment of Isw2 to the right of centromere midpoints, over the CDEII and CDEIII regions (Figure 3B). Analysis of centromeric sequence composition confirmed the A+T rich nature of yeast centromeres, further supporting the preference of Isw2 for A+T rich DNA (Figure 3C). The association of Isw2 with centromeres is consistent with previous X-ChIP results showing association of Isw2 with two yeast centromeres [33] as well as studies demonstrating a role for Isw2 in pericentromeric nucleosome dynamics [34] and centromeric association of the human ISWI-containing RSF complex [35].
Fig. 3. Isw2 associates with centromeres. 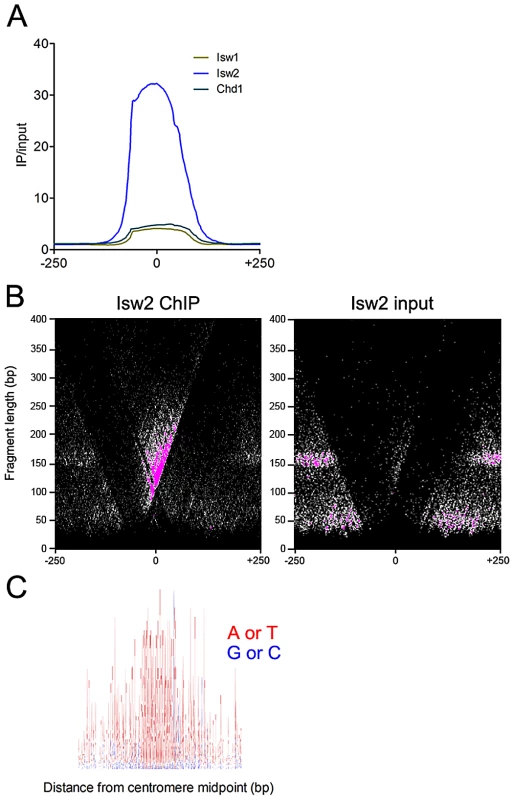
(A) Aggregate plot of Isw1, Isw2, and Chd1 ChIP/input signal at all 16 yeast centromeres. (B) V-plot of Isw2 ChIP data for all 16 yeast centromeres showing enrichment of Isw2 to the CDEIII side of centromeres. (C) Sequence logo of all 16 yeast centromeres spanning 400 bp centered on the centromere midpoint. A+T are represented as red and G+C are represented as blue, demonstrating the high A+T content of centromeres. The binding of Isw2 to centromeres is thus consistent with its preference for association with regions of high A+T content. The sequence logo was generated with WebLogo (http://weblogo.berkeley.edu). ISWI and CHD remodelers occupy extended linker DNA at transcription factor binding sites
Previous work has demonstrated that sequence-specific TFs induce nucleosome depletion upon binding [36]–[41], presumably by exposing stretches of linker DNA. We therefore hypothesized that the enrichment of supernucleosomal DNA fragments in ChIP samples might reflect remodeler association with the extended linker DNA flanking binding sites for nucleosome-phasing TFs. We assessed transcription factor binding site (TFBS) occupancy of Isw1, Isw2, and Chd1 using V-plotting. We first analyzed remodeler occupancy at binding sites for the Abf1 TF. Consistent with our previous data [30], well-phased flanking nucleosomes were observed at Abf1 sites as discrete dot clusters to either side of the TFBS (Figure 4A and Figure S2A).
Fig. 4. ISWI and CHD remodelers associate with TFBSs. 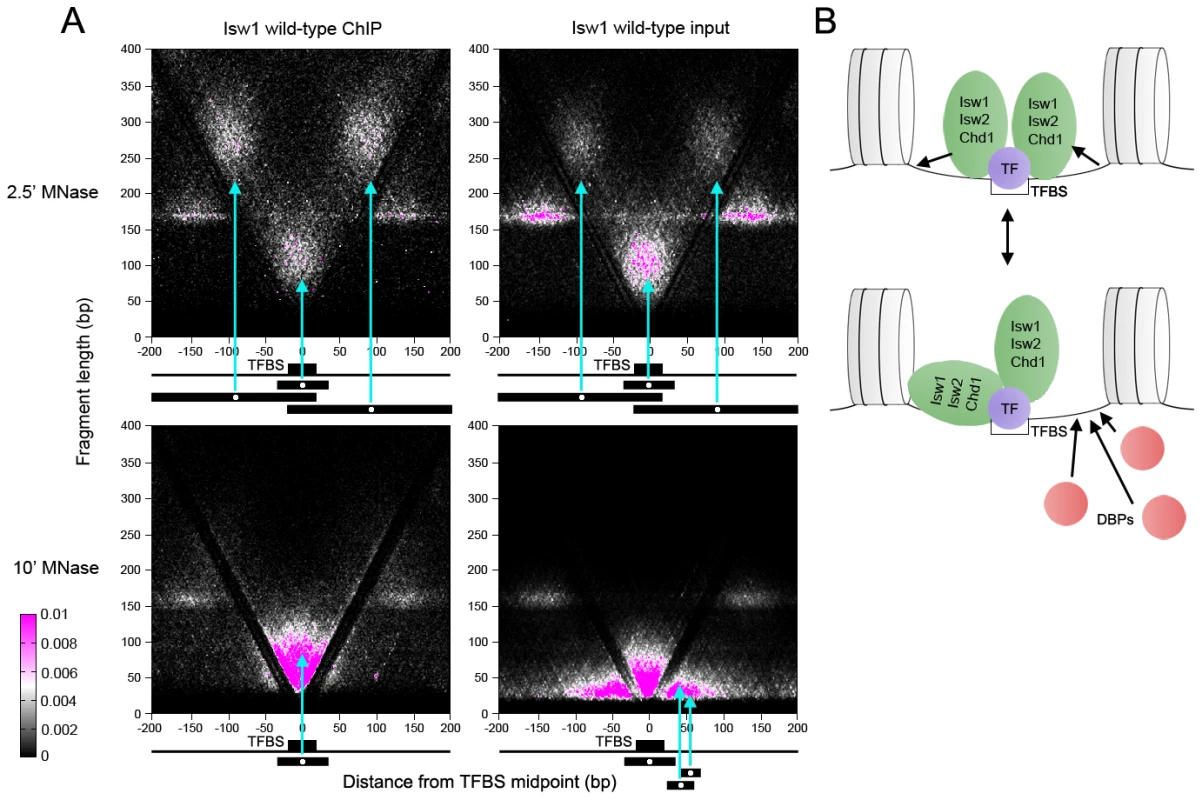
(A) V-plots of wild-type Isw1 ChIP and soluble input chromatin at binding sites for the Abf1 TF after 2.5′ and 10′ MNase digestion. Flanking nucleosomes are visualized as well-defined dot clusters on either side of the TFBS. Example fragments contributing to the generation of various V-plot features are shown schematically below each plot. Cyan arrows point to the position of each fragment midpoint within the V-plot. Similar results were seen for wild-type Isw2 and Chd1 at Abf1 sites and for Isw1, Isw2, and Chd1 at Cbf1, Mbp1, and Reb1 binding sites (Figures S3 and S4). (B) Interpretive schematic of V-plot results. DBPs; DNA-binding proteins. In samples treated with MNase for 2.5′, we noted robust enrichment of supernucleosomal fragments spanning ∼250–300 bp and centered between the TFBS and each flanking nucleosome in ChIP, and to a lesser extent, input samples. These supernucleosomal fragments represent continuous protection of DNA from the flanking nucleosome to the opposite side of the TFBS, with remodeler complexes occupying the linker DNA spanning the nucleosome and TFBS. Additionally, supernucleosomal fragments were depleted from the more heavily digested 10′ samples, while smaller fragments flanking both sides of the TFBS were seen (Figure 4A and Figure S2A), suggesting that remodeler-linker DNA associations display varying degrees of stability.
As observed previously, clusters of subnucleosomal particles flank TFBSs in our input chromatin samples [30]. These particles were not observed in remodeler ChIP V-plots (Figure 4A and Figure S2A), indicating that they are not remodelers but rather small DNA-binding proteins. Fragment sizes in the central protected region of the ChIP V-plots extended ∼30 bp higher than in the input V-plots, perhaps indicating stable association of remodelers closer to the TFBS (Figure 4A and Figure S2A). We also generated V-plots of 2.5′ and 10′ MNase ChIP and input data using Cbf1, Mbp1, and Reb1 binding sites, which, like Abf1 sites, display well-phased flanking nucleosomes (Figure S2B–S2D). In each case, similar V-plots were seen for Isw1, Isw2, and Chd1.
These V-plot observations suggest that remodelers bind to regions of extended linker DNA created by TF-induced nucleosome depletion at TFBSs and protect variable amounts of flanking DNA (Figure 4B, top). A remodeler molecule then occupies an extended region up to the flanking nucleosome to generate the large supernucleosomal fragment clusters observed after 2.5′ MNase digestion. Unprotected linker DNA on the non-remodeler protected side of the TF could then be occupied by DNA-binding proteins (Figure 4B, bottom). Depletion of supernucleosomal fragments in the 10′ MNase-digested samples suggests that remodelers dynamically protect linker DNA on one or the other side of the TFBS. This association of Isw1, Isw2, and Chd1 with substantial extranucleosomal DNA is consistent with in vitro studies in yeast, Drosophila, and humans [6]–[10], [42], [43].
Remodeler association with transcription factor binding sites is ATP-independent
We next asked whether remodeler inactivation would alter binding around TFBSs. Surprisingly, elimination of remodeler catalytic activity via a lysine-to-arginine substitution in the conserved GXGKT ATP-binding motif [21], [44] did not substantially affect the size distribution of mapped fragments (Figure 5A and Figure S4) or the genomic binding profiles of remodelers (Figure 5B). We assessed the effect of remodeler catalytic inactivation by V-plotting wild-type and catalytically inactive (K227R) ChIP and input data at sites for the Reb1 TF generated by ChIP-exo [45], which also allowed us to assess V-plot patterns using an independent set of binding sites. V-plot patterns generated with ChIP-exo Reb1 sites were nearly indistinguishable from those created using Reb1 ChIP-chip sites (Figure 5C, Figure S3, Figure S5). Catalytic inactivation of remodelers also had no noticeable effect on V-plot patterns at TFBSs (Figure 5C, Figure S3, and Figure S5), implying that remodeler association with TFBSs is ATP-independent.
Fig. 5. ISWI and CHD remodeler association with TFBSs is ATP-independent. 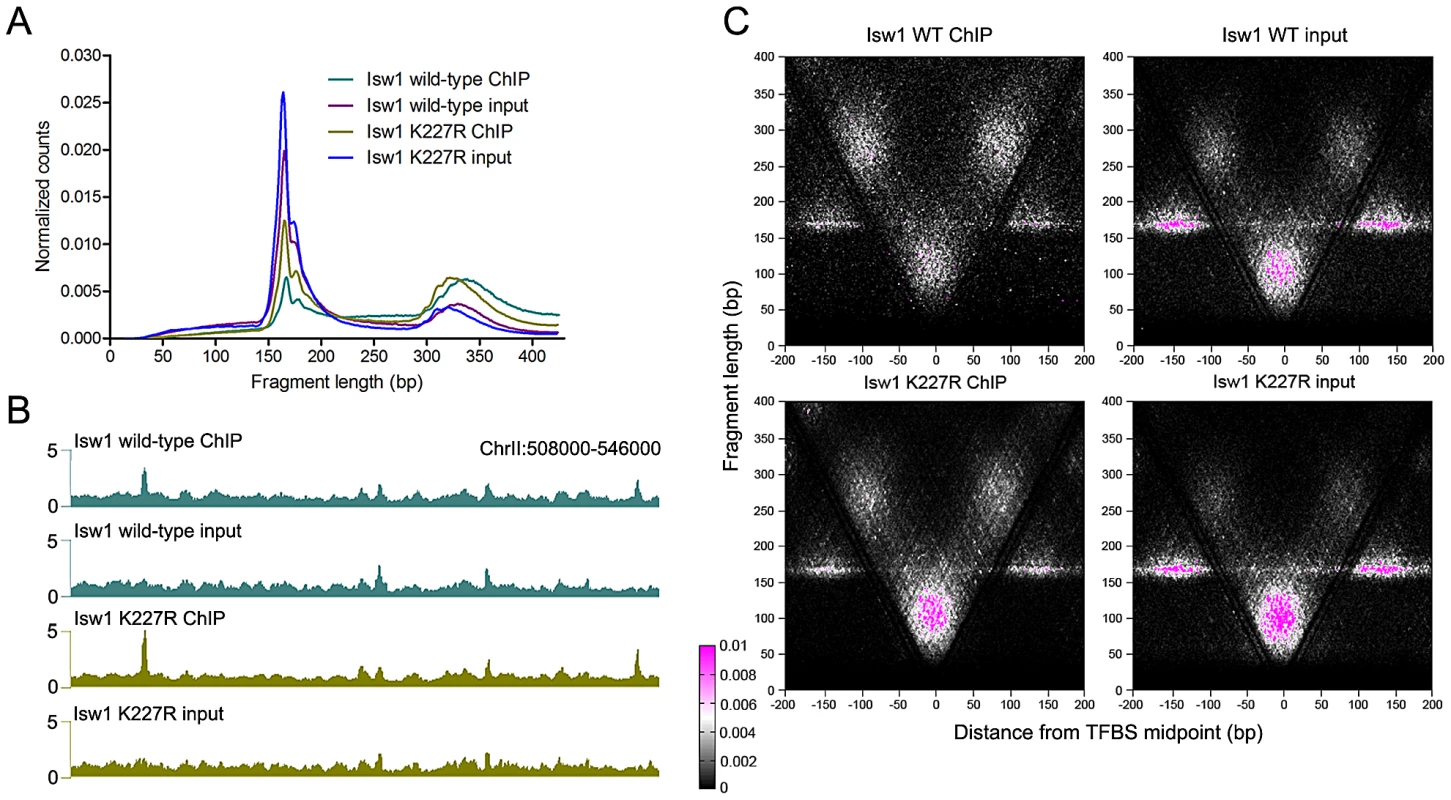
(A) Size distributions of mapped paired-end 2.5′ MNase-digested wild-type and catalytically inactive K227R Isw1 ChIP and input fragments. Similar profiles were seen for Isw2 K215R and Chd1 K407R (Figure S4). (B) Profiles of wild-type and K227R Isw1 binding along a representative segment of the genome. (C) V-plots of wild-type and K227R Isw1 ChIP and soluble input chromatin at binding sites for the Reb1 TF, determined by ChIP-exo, after 2.5′ MNase digestion. The overall fragment size in the Isw1 K227R ChIP and input samples is slightly reduced when compared to wild-type, indicative of technical variation in MNase digestion. Similar results were seen for K227R Isw1 and catalytically inactive Isw2 (K215R) and Chd1 (K407R) at Abf1 and other TFBSs (Figures S4 and S6). ISWI and CHD remodelers preferentially bind NDRs
The sharp patterns of phased nucleosomes on either side of binding sites for Abf1, Reb1 and other TFs is consistent with their known role in creating NDRs that lead to transcriptional activation [46]. Isw1and Chd1 position nucleosomes within gene bodies in vivo, while Isw2 generally positions nucleosomes flanking NDRs, preventing directional nucleosome shifting within gene bodies [16]–[21]. We therefore asked whether there is a relationship between remodeler occupancy and dynamics around individual TFBSs and features of adjacent genes. Consistent with their known functions, we observed enrichment of Chd1 and Isw1 within gene bodies (Figure 6A–6B), while Isw2 showed robust enrichment at NDRs, where TFBSs are generally located in yeast, with little gene body binding (Figure 6C). Strikingly, equal or slightly greater enrichment than that seen in gene bodies for Chd1 and Isw1 was observed at NDRs at the 5′ and 3′ ends of verified ORFs (Figure 6A–6B). We also detected Isw1 association with 5′ NDRs after adding a formaldehyde crosslinking step to our N-ChIP-seq protocol (Figure S6), indicating that remodeler-NDR association is not due to opportunistic, nonspecific interactions of remodelers with free DNA during chromatin preparation and immunoprecipitation. We assessed the association of remodeler binding with NDR size, reasoning that if remodeler-NDR interactions were simply due to the presence of large regions of naked DNA in the chromatin preparation, larger NDRs would display greater remodeler association. No such correlations were observed (Isw1 R2 = 0.0339; Isw2 R2 = 0.0397; Chd1 R2 = 0.0519), further arguing against nonspecific association of remodelers with free DNA during our experiments.
Fig. 6. ISWI and CHD remodelers bind NDRs. 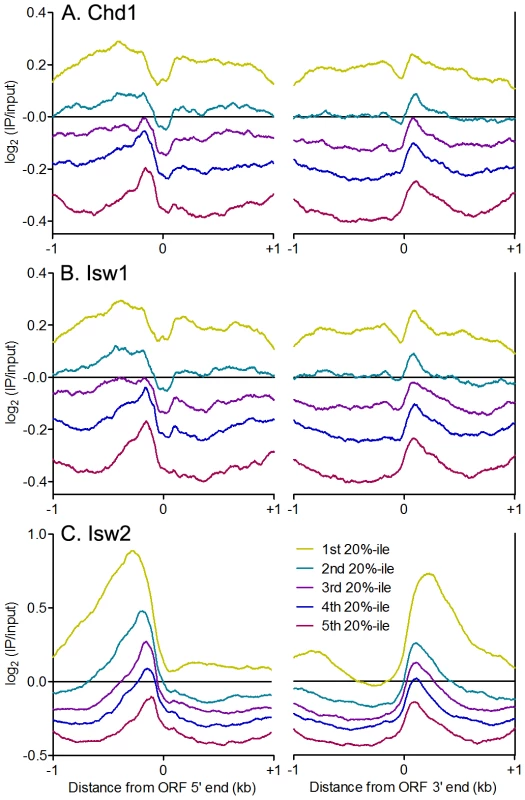
Aggregate plots of log2(IP/input) signal ±1 kb of verified ORF 5′ and 3′ ends for (A) Chd1, (B) Isw1, and (C) Isw2 ranked separated into quintiles by average remodeler signal. Our Isw2 N-ChIP-seq results are somewhat different from those generated using ChIP-exo. We found Isw2 to be enriched upstream of ORF 5′ ends to a distance of nearly 1 kb in some cases (Figure 6C), whereas ChIP-exo showed very discrete localization of Isw2 to the TSS, immediately adjacent to the +1 nucleosome [17]. The differences between our N-ChIP-seq results and those of the ChIP-exo study may be explained by the crosslinking of Isw2 to the +1 nucleosome in ChIP-exo. Isw2 that is not adjacent to a nucleosome is less likely to be crosslinked and more likely to be lost during subsequent solubilization of chromatin. Our results indicate that there is at least some transient interaction between Isw2 and the +1 nucleosome, and we posit that this is the fraction of Isw2 previously mapped by ChIP-exo.
ISWI and CHD remodeler association with gene bodies is associated with transcription-coupled nucleosome turnover
Our results demonstrate that yeast ISWI and CHD remodelers associate not only with gene bodies, where their effects are seen, but also with regions of extended linker DNA flanking TFBSs within NDRs. In the case of Isw2, robust binding to linker DNA within NDRs is consistent both with its in vitro preference for substantial extranucleosomal DNA and its action positioning nucleosomes adjacent to NDRs [7]–[9], [20]. However, while the binding of Chd1 and Isw1 to linker DNA within NDRs is in line with their in vitro association with significant amounts of linker DNA [6], [10], it appears at odds with their function within gene bodies [16]–[19], [21]. To reconcile the robust occupancy of Isw1 and Chd1 around NDRs with their function within gene bodies, we suggest that TFs generate regions of nucleosome depletion, which expose extended linker DNA and are thus favorable for remodeler-chromatin association. Transcriptional elongation then disrupts or evicts nucleosomes, extending regions of linker DNA within gene bodies and enabling efficient remodeling of the remaining gene-body nucleosomes and/or newly deposited nucleosomes by Isw1 and Chd1, while Isw2 simultaneously positions NDR-flanking nucleosomes to prevent directional shifting of gene-body nucleosomes, which could lead to harmful cryptic transcription [20].
This model predicts that Chd1 and Isw1 association with gene bodies depends on transcription rate. As such, we would not expect to observe an association between remodeler binding and steady-state expression levels, as infrequently transcribed genes may yield mRNAs with long half-lives and vice versa [47]. Indeed, we observed no correlation between remodeler occupancy and steady-state gene expression (Figure 7A). Given that transcription is associated with histone turnover [48]–[52], we hypothesized that there might be a relationship between remodeler association and turnover. High turnover within ORF 5′ ends was postulated to reflect a requirement to maintain nucleosome depletion at promoters [51], which is achieved, at least in part, by TFs such as Abf1 and Reb1 and the SWI/SNF-family remodeler RSC [39], [41]. Comparison of histone turnover data with remodeler N-ChIP-seq data revealed a positive association between binding of remodelers to ORF 5′ ends and gene bodies and nucleosome turnover (Figure 7B). We also compared remodeler binding with transcription rate and found that highly transcribed genes were more highly bound by Isw1, Isw2, and Chd1 (Figure 7C). Taken together, these data suggest that Isw1 and Chd1 binding within gene bodies displaying high nucleosome turnover is a consequence of transcriptional elongation. While Isw2 generally does not bind gene bodies, its loss affects gene body nucleosomes, which shift to their thermodynamically favored positions in its absence [20]. The increased binding of Isw2 to the 5′ NDRs of highly transcribed genes may therefore reflect the increased nucleosome disruption caused by transcriptional elongation and, consequently, the increased requirement for the positioning activity of Isw2.
Fig. 7. ISWI and CHD remodeler binding is positively associated with histone turnover and transcription rate. 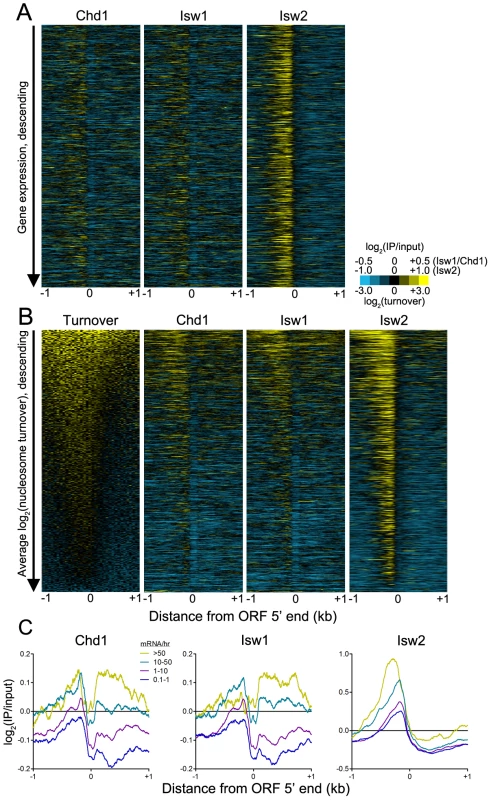
(A) Heat maps of log2(IP/input) signal for Chd1, Isw1, and Isw2 ±1 kb of verified ORF 5′ ranked descending by gene expression. (B) Heat maps of log2(nucleosome turnover) and log2(IP/input) signal for Chd1, Isw1, and Isw2 ±1 kb of verified ORF 5′ ranked descending by average nucleosome turnover across the entire 2-kb window. (C) Aggregate plots of Chd1, Isw1, and Isw2 log2(IP/input) signal ±1 kb of verified ORF 5′ ends, separated by transcription rate in mRNA/hr [47]. What is the relationship of ISWI and CHD remodelers to transcription? Previous work has demonstrated that simultaneous deletion of Isw1, Isw2, and Chd1 in yeast does not alter promoter nucleosome occupancy or positioning [16], indicating that these remodelers are unlikely to influence RNA polymerase II binding to promoters. Strikingly, this same work showed that loss of Isw1, Isw2, and Chd1 had relatively little effect on gene transcription, despite causing massive disorganization of gene body chromatin structure. These findings suggested that the major role of ISWI and CHD remodelers, and gene body chromatin organization in general, is to prevent cryptic transcription within gene bodies [20], [53]. In the case of Chd1 deletion, cryptic transcription is linked to increased gene body histone turnover, indicating a role for Chd1 in suppressing histone turnover [53]. This result is also consistent with our finding that Chd1 binding is associated with nucleosome turnover, as regions displaying high turnover would both provide exposed DNA for Chd1 to bind and require Chd1 to suppress excessive histone turnover and subsequent cryptic transcription. Furthermore, loss of Chd1 impairs post-elongation nucleosome reassembly [54]. In light of our data and these previous results, we propose the following model for the relationship of Chd1 to transcription. A round of transcriptional initiation and elongation occurs, displacing or evicting nucleosomes. Chd1, either by interacting with the elongation machinery or following in the wake of RNA polymerase II, can then scan for regions of exposed linker DNA to which it can bind and suppress turnover and, by extension, cryptic transcription. In support of this possibility, Chd1 has been shown to genetically and physically interact with the transcription machinery in several species [55]–[58], and Isw1 and Isw2 genetically interact with elongation factors in yeast [59].
It is estimated that there is sufficient Isw2 for a molecule be bound every 2–5 kb of DNA, with a similar abundance for Chd1 and twice that amount for Isw1 (T.T., unpublished). Additionally, high salt is required for remodeler extraction from cells, indicating that they are bound to chromatin [44], [60]. Therefore, as much as 5–10% of the yeast genome, perhaps most NDRs, may be occupied by Isw1, Isw2, and Chd1. Drosophila ISWI is similarly abundant [61] and multiple Chd1-related remodelers are highly abundant and active in many higher eukaryotes [62]–[64], suggesting that the binding of ISWI and CHD remodelers at NDRs and their subsequent action downstream is an ancestral mechanism for chromatin structure maintenance.
Materials and Methods
Yeast methods
Yeast cells were grown in YPD and harvested at OD600 = 0.6–0.8. Mutation of Chd1 K407 was carried out as follows. Primers F-5′ - GGTACCCTTGGGAGAATGCCACAGAT -3′, containing a KpnI site (underlined) and R-5′ - ATCGATCTTCGTCAACGGCCATAAAT -3′, containing a ClaI site (underlined) were used to amplify nt 961–1543 of CHD1. The amplified fragment was TOPO-cloned into pCR2.1. pCR2.1-CHD1-961–1543 was digested with KpnI and ClaI and the CHD1 fragment was ligated into pRS406 and mutagenized with the QuikChange kit (Agilent). Mutation was confirmed by sequencing and introduced a strain harboring a Chd1-3xFLAG allele via the pop-in/pop-out method [65]. Yeast strains used in this study are given in Table S1.
Nuclear isolation and chromatin preparation
Nuclei were isolated from yeast cells and stored at −80°C in 5 ml sorbitol-PIPES-calcium (SPC) buffer as described [66]. For X-ChIP, cells were fixed with 1/10 culture volume buffered formaldehyde solution (50 mM HEPES-KOH, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 11% formaldehyde) for 15 min at RT with gentle rotation and quenched with 18 ml/100 ml culture volume 2.5 M glycine prior to nuclear isolation. Upon thawing, SPC buffer was supplemented with 1 mM phenylmethanesulfonylfluoride (PMSF), 10 µg/ml LPC [66], and 2 mM CaCl2. Chromatin was digested with MNase for 2.5 or 10 min and digestion was stopped by the addition of EDTA to 10 mM. MNase-treated nuclei were then passed 4 times through a 20-gauge needle and 4 times through a 26-gauge needle [30], [67] and centrifuged at 10,000 RPM for 10 min. The supernatant (S1) was held on ice. The pellet was resuspended in 5 ml extraction buffer (10 mM phosphate buffer pH 7.4, 70 mM NaCl, 0.75 mM EDTA, 0.1% Triton X-100) supplemented with 1 mM PMSF and 10 µg/ml LPC and rocked on a nutator for 4 hours at 4°C. Samples were centrifuged at 13,000 RPM for 15 min at 4°C and the supernatant (S2) was saved. Triton X-100 was added to S1 to a final concentration of 0.1%, S1 and S2 were combined, and the salt concentration of the combined 10 ml chromatin solution was adjusted to 80 mM with NaCl.
ChIP
100 µl of each chromatin sample was saved as input. 100 µl of FLAG M2 magnetic beads (Sigma M8823, lot #041M6135) were used for all remodeler-FLAG immunoprecipitations (IPs). Beads were washed 3 times with PBS/0.5% BSA and added to the chromatin samples. IPs were incubated overnight at 4°C with end-over-end rotation. Beads were washed 3 times with IP buffer (10 mM phosphate buffer, pH 7.4, 0.75 mM EDTA, 70 mM NaCl) supplemented with 10 µg/ml LPC and 1 mM PMSF and resuspended in 400 µl IP buffer. 1/50th volumes of 5 M NaCl and 0.5 mM EDTA were added to IP and input samples and RNA was digested with 1 µg RNase A for 10 min at 37°C. SDS was added to IP and input samples to a concentration of 0.5% and protein was digested with 80 µg Proteinase K for 20 min at 65°C. 20 µg glycogen was added to each IP and input sample followed by extraction with 1 volume phenol/chloroform/isoamyl alcohol. DNA was precipitated with 2 volumes 100% ethanol for 30 min at −80°C and washed twice with 500 µl 100% ethanol. Precipitated DNA was resuspended in 20 µl 0.1X TE buffer, pH 8.0 and quantified by PicoGreen [30].
X-ChIP was performed as above with the following modifications. In addition to washing in IP buffer, IPs were washed twice with TSE I (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris pH 8.0, 150 mM NaCl) and twice with TSE II (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris pH 8.0, 500 mM NaCl). Elution and crosslink reversal was performed overnight at 65°C in elution buffer (50 mM Tris pH 8.0, 10 mM EDTA, 1% SDS). Following elution, 1 volume of TE buffer pH 8.0 was added to each sample to dilute the SDS to 0.5% for RNase A and proteinase K treatment as above.
Sequencing library preparation
Sequencing libraries were prepared according to our previously described modification of the standard Illumina protocol [30]. Libraries were sequenced for 25 cycles in paired-end mode on the Illumina HiSeq 2000 platform at the Fred Hutchinson Cancer Research Center Genomics Shared Resource.
Data analysis
Paired-end sequencing data were processed and aligned to the V64/SacCer3 genome build with Novoalign (Novocraft; http://www.novocraft.com) and processed as described [30], [68]. Numbers of paired-end fragments mapped to the genome for each sample are given in Table S2. Size distributions were normalized such that the area under the curve for each sample was equal to 1. V-plots were generated with gnuplot (http://www.gnuplot.info). The V-plot scale represents the percentage of fragments used in constructing each V-plot contained in each bin (pixel) of the plot (160,400 bins total).
Isw2 K215R ChIP-chip binding sites, sites of chromatin remodeling by Isw2, and random sites were obtained from Whitehouse et al. [20] and Isw2 ChIP-exo binding sites were obtained from Yen et al. [17]. The log2(IP/input) N-ChIP-seq signal for each base pair ±1 kb of the midpoint of each Isw2 K215R-bound region, site of Isw2 remodeling, Isw2 ChIP-exo site, and random site was determined as above and averaged to generate aggregate plots.
For DNA bendability analysis, 29 100 bp regions bound by Isw2 without Isw1 and/or Chd1 and 29 100 bp regions bound by Isw1 and/or Chd1 without Isw2 were obtained. Regions were analyzed using the bend.it server [69] with a window size of 39 bp. Bendability values for each position within the window were averaged and the two classes of sites were compared by t-test.
The SGD verified ORFs list was used to determine transcription unit length and ORF 5′ and 3′ end coordinates. For heatmaps, the log2(IP/input) for each base pair ±1 kb of each ORF 5′ or 3′ coordinate was determined using a custom Perl script [70]. Heatmaps were visualized with Java TreeView [71]. All heatmaps were generated using 10′ MNase data and are oriented such that the direction of transcription for all genes is to the right. Expression data were obtained from GEO (GSM552681) [72]. Nucleosome turnover data were obtained from Dion et al. [51]. Nucleosome turnover signal was obtained as above, but using 40 bp windows due to the lower resolution of these data. Transcription rates were obtained from Holstege et al. [47]. Correlations between remodeler signal and NDR size were generated using a previously annotated list of NDRs [73] and the maximum log2(IP/input) N-ChIP-seq signal within each NDR.
Data availability
Sequencing data generated in this publication have been deposited with GEO (GSE39331).
Supporting Information
Zdroje
1. FlausA, MartinDMA, BartonGJ, Owen-HughesT (2006) Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res 34 : 2887–2905.
2. WilsonBG, RobertsCWM (2011) SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 11 : 481–492.
3. RyanRJH, BernsteinBE (2012) Genetic Events That Shape the Cancer Epigenome. Science 336 : 1513–1514.
4. BoerkoelCF, TakashimaH, JohnJ, YanJ, StankiewiczP, et al. (2002) Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet 30 : 215–220.
5. ZentnerGE, LaymanWS, MartinDM, ScacheriPC (2010) Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A 152A: 674–686.
6. GangarajuVK, BartholomewB (2007) Dependency of ISW1a Chromatin Remodeling on Extranucleosomal DNA. Mol Cell Biol 27 : 3217–3225.
7. ZofallM, PersingerJ, BartholomewB (2004) Functional Role of Extranucleosomal DNA and the Entry Site of the Nucleosome in Chromatin Remodeling by ISW2. Mol Cell Biol 24 : 10047–10057.
8. DangW, KagalwalaMN, BartholomewB (2007) The Dpb4 Subunit of ISW2 Is Anchored to Extranucleosomal DNA. J Biol Chem 282 : 19418–19425.
9. KagalwalaMN, GlausBJ, DangW, ZofallM, BartholomewB (2004) Topography of the ISW2-nucleosome complex: insights into nucleosome spacing and chromatin remodeling. EMBO J 23 : 2092–2104.
10. McKnightJN, JenkinsKR, NodelmanIM, EscobarT, BowmanGD (2011) Extranucleosomal DNA Binding Directs Nucleosome Sliding by Chd1. Mol Cell Biol 31 : 4746–4759.
11. GossettAJ, LiebJD (2012) In Vivo Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in Saccharomyces cerevisiae. PLoS Genet 8: e1002771 doi:10.1371/journal.pgen.1002771
12. RackiLR, YangJG, NaberN, PartenskyPD, AcevedoA, et al. (2009) The chromatin remodeller ACF acts as a dimeric motor to space nucleosomes. Nature 462 : 1016–1021.
13. YangJG, MadridTS, SevastopoulosE, NarlikarGJ (2006) The chromatin-remodeling enzyme ACF is an ATP-dependent DNA length sensor that regulates nucleosome spacing. Nat Struct Mol Biol 13 : 1078–1083.
14. BlosserTR, YangJG, StoneMD, NarlikarGJ, ZhuangX (2009) Dynamics of nucleosome remodelling by individual ACF complexes. Nature 462 : 1022–1027.
15. BouazouneK, KingstonRE (2012) Chromatin remodeling by the CHD7 protein is impaired by mutations that cause human developmental disorders. Proc Natl Acad Sci U S A
16. GkikopoulosT, SchofieldP, SinghV, PinskayaM, MellorJ, et al. (2011) A Role for Snf2-Related Nucleosome-Spacing Enzymes in Genome-Wide Nucleosome Organization. Science 333 : 1758–1760.
17. YenK, VinayachandranV, BattaK, KoerberRT, PughBF (2012) Genome-wide Nucleosome Specificity and Directionality of Chromatin Remodelers. Cell 149 : 1461–1473.
18. XellaB, GodingC, AgricolaE, Di MauroE, CasertaM (2006) The ISWI and CHD1 chromatin remodelling activities influence ADH2 expression and chromatin organization. Mol Microbiol 59 : 1531–1541.
19. TiroshI, SigalN, BarkaiN (2010) Widespread remodeling of mid-coding sequence nucleosomes by Isw1. Genome Biol 11: R49.
20. WhitehouseI, RandoOJ, DelrowJ, TsukiyamaT (2007) Chromatin remodelling at promoters suppresses antisense transcription. Nature 450 : 1031–1035.
21. SimicR, LindstromDL, TranHG, RoinickKL, CostaPJ, et al. (2003) Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J 22 : 1846–1856.
22. AlbertI, MavrichTN, TomshoLP, QiJ, ZantonSJ, et al. (2007) Translational and rotational settings of H2A.Z nucleosomes across the Saccharomycescerevisiae genome. Nature 446 : 572–576.
23. ShimYS, ChoiY, KangK, ChoK, OhS, et al. (2012) Hrp3 controls nucleosome positioning to suppress non-coding transcription in eu - and heterochromatin. EMBO J 31 : 4375–4387.
24. PointnerJ, PerssonJ, PrasadP, Norman-AxelssonU, StralforsA, et al. (2012) CHD1 remodelers regulate nucleosome spacing in vitro and align nucleosomal arrays over gene coding regions in S. pombe. EMBO J 31 : 4388–4403.
25. HennigBP, BendrinK, ZhouY, FischerT (2012) Chd1 chromatin remodelers maintain nucleosome organization and repress cryptic transcription. EMBO Rep 13 : 997–1003.
26. LantermannAB, StraubT, StralforsA, YuanG-C, EkwallK, et al. (2010) Schizosaccharomyces pombe genome-wide nucleosome mapping reveals positioning mechanisms distinct from those of Saccharomyces cerevisiae. Nat Struct Mol Biol 17 : 251–257.
27. O'NeillLP, TurnerBM (2003) Immunoprecipitation of native chromatin: NChIP. Methods 31 : 76–82.
28. RocaH, FranceschiRT (2008) Analysis of transcription factor interactions in osteoblasts using competitive chromatin immunoprecipitation. Nucleic Acids Res 36 : 1723–1730.
29. KentNA, AdamsS, MoorhouseA, PaszkiewiczK (2011) Chromatin particle spectrum analysis: a method for comparative chromatin structure analysis using paired-end mode next-generation DNA sequencing. Nucleic Acids Res 39: e26.
30. HenikoffJG, BelskyJA, KrassovskyK, MacAlpineDM, HenikoffS (2011) Epigenome characterization at single base-pair resolution. Proc Natl Acad Sci U S A 108 : 18318–18323.
31. GelbartME, BachmanN, DelrowJ, BoekeJD, TsukiyamaT (2005) Genome-wide identification of Isw2 chromatin-remodeling targets by localization of a catalytically inactive mutant. Genes Dev 19 : 942–954.
32. WhitehouseI, TsukiyamaT (2006) Antagonistic forces that position nucleosomes in vivo. Nat Struct Mol Biol 13 : 633–640.
33. ZhangZ, ReeseJC (2004) Ssn6-Tup1 requires the ISW2 complex to position nucleosomes in Saccharomyces cerevisiae. EMBO J 23 : 2246.
34. VerdaasdonkJS, GardnerR, StephensAD, YehE, BloomK (2012) Tension-dependent nucleosome remodeling at the pericentromere in yeast. Mol Biol Cell 23 : 2560–2570.
35. PerpelescuM, NozakiN, ObuseC, YangH, YodaK (2009) Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol 185 : 397–407.
36. TsukiyamaT, BeckerPB, WuC (1994) ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature 367 : 525–532.
37. BernsteinB, LiuC, HumphreyE, PerlsteinE, SchreiberS (2004) Global nucleosome occupancy in yeast. Genome Biol 5: R62.
38. GanapathiM, PalumboMJ, AnsariSA, HeQ, TsuiK, et al. (2011) Extensive role of the general regulatory factors, Abf1 and Rap1, in determining genome-wide chromatin structure in budding yeast. Nucleic Acids Res 39 : 2032–2044.
39. BaiL, OndrackaA, Cross FrederickR (2011) Multiple Sequence-Specific Factors Generate the Nucleosome-Depleted Region on CLN2 Promoter. Mol Cell 42 : 465–476.
40. LickwarCR, MuellerF, HanlonSE, McNallyJG, LiebJD (2012) Genome-wide protein-DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature 484 : 251–255.
41. HartleyPD, MadhaniHD (2009) Mechanisms that Specify Promoter Nucleosome Location and Identity. Cell 137 : 445–458.
42. BrehmA, LangstG, KehleJ, ClapierCR, ImhofA, et al. (2000) dMi-2 and ISWI chromatin remodelling factors have distinct nucleosome binding and mobilization properties. EMBO J 19 : 4332–4341.
43. YangJG, MadridTS, SevastopoulosE, NarlikarGJ (2006) The chromatin-remodeling enzyme ACF is an ATP-dependent DNA length sensor that regulates nucleosome spacing. Nat Struct Mol Biol 13 : 1078–1083.
44. TsukiyamaT, PalmerJ, LandelCC, ShiloachJ, WuC (1999) Characterization of the Imitation Switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes Dev 13 : 686–697.
45. RheeHS, PughBF (2011) Comprehensive Genome-wide Protein-DNA Interactions Detected at Single-Nucleotide Resolution. Cell 147 : 1408–1419.
46. IyerVR (2012) Nucleosome positioning: bringing order to the eukaryotic genome. Trends Cell Biol 22 : 250–256.
47. HolstegeFCP, JenningsEG, WyrickJJ, LeeTI, HengartnerCJ, et al. (1998) Dissecting the Regulatory Circuitry of a Eukaryotic Genome. Cell 95 : 717–728.
48. SchwabishMA, StruhlK (2004) Evidence for Eviction and Rapid Deposition of Histones upon Transcriptional Elongation by RNA Polymerase II. Mol Cell Biol 24 : 10111–10117.
49. KristjuhanA, SvejstrupJQ (2004) Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J 23 : 4243–4252.
50. WorkmanJL (2006) Nucleosome displacement in transcription. Genes Dev 20 : 2009–2017.
51. DionMF, KaplanT, KimM, BuratowskiS, FriedmanN, et al. (2007) Dynamics of Replication-Independent Histone Turnover in Budding Yeast. Science 315 : 1405–1408.
52. MitoY, HenikoffJG, HenikoffS (2005) Genome-scale profiling of histone H3.3 replacement patterns. Nat Genet 37 : 1090–1097.
53. SmolleM, VenkateshS, GogolMM, LiH, ZhangY, et al. (2012) Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat Struct Mol Biol 19 : 884–892.
54. LeeJ-S, GarrettAS, YenK, TakahashiY-H, HuD, et al. (2012) Codependency of H2B monoubiquitination and nucleosome reassembly on Chd1. Genes Dev 26 : 914–919.
55. KelleyDE, StokesDG, PerryRP (1999) CHD1 interacts with SSRP1 and depends on both its chromodomain and its ATPase/helicase-like domain for proper association with chromatin. Chromosoma 108 : 10–25.
56. SimicR, LindstromDL, TranHG, RoinickKL, CostaPJ, et al. (2003) Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J 22 : 1846–1856.
57. QuanTK, HartzogGA (2010) Histone H3K4 and K36 Methylation, Chd1 and Rpd3S Oppose the Functions of Saccharomyces cerevisiae Spt4–Spt5 in Transcription. Genetics 184 : 321–334.
58. LinJJ, LehmannLW, BonoraG, SridharanR, VashishtAA, et al. (2011) Mediator coordinates PIC assembly with recruitment of CHD1. Genes Dev 25 : 2198–2209.
59. CollinsSR, MillerKM, MaasNL, RoguevA, FillinghamJ, et al. (2007) Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446 : 806–810.
60. TranHG, StegerDJ, IyerVR, JohnsonAD (2000) The chromo domain protein Chd1p from budding yeast is an ATP-dependent chromatin-modifying factor. EMBO J 19 : 2323–2331.
61. TsukiyamaT, DanielC, TamkunJ, WuC (1995) ISWI, a member of the SWl2/SNF2 ATPase family, encodes the 140 kDa subunit of the nucleosome remodeling factor. Cell 83 : 1021–1026.
62. WangM, WeissM, SimonovicM, HaertingerG, SchrimpfSP, et al. (2012) PaxDb, a database of protein abundance averages across all three domains of life. Mol Cell Proteomics
63. HallJA, GeorgelPT (2007) CHD proteins: a diverse family with strong ties. Biochem Cell Biol 85 : 463–476.
64. MarfellaCGA, ImbalzanoAN (2007) The Chd family of chromatin remodelers. Mutat Res 618 : 30–40.
65. SchererS, DavisR (1979) Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A 76 : 4951–4955.
66. FuruyamaS, BigginsS (2007) Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc Natl Acad Sci U S A 104 : 14706–14711.
67. JinC, FelsenfeldG (2007) Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev 21 : 1519–1529.
68. KrassovskyK, HenikoffJG, HenikoffS (2012) Tripartite organization of centromeric chromatin in budding yeast. Proc Natl Acad Sci U S A 109 : 243–248.
69. VlahovičekK, KajánL, PongorS (2003) DNA analysis servers: plot.it, bend.it, model.it and IS. Nucleic Acids Res 31 : 3686–3687.
70. ZentnerGE, SaiakhovaA, ManaenkovP, AdamsMD, ScacheriPC (2011) Integrative genomic analysis of human ribosomal DNA. Nucleic Acids Res 39 : 4949–4960.
71. SaldanhaAJ (2004) Java Treeview—extensible visualization of microarray data. Bioinformatics 20 : 3246–3248.
72. TsankovAM, ThompsonDA, SochaA, RegevA, RandoOJ (2010) The Role of Nucleosome Positioning in the Evolution of Gene Regulation. PLoS Biol 8: e1000414 doi:10.1371/journal.pbio.1000414
73. YadonAN, Van de MarkD, BasomR, DelrowJ, WhitehouseI, et al. (2010) Chromatin Remodeling around Nucleosome-Free Regions Leads to Repression of Noncoding RNA Transcription. Mol Cell Biol 30 : 5110–5122.
74. ZhaoX, MullerEGD, RothsteinR (1998) A Suppressor of Two Essential Checkpoint Genes Identifies a Novel Protein that Negatively Affects dNTP Pools. Mol Cell 2 : 329–340.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



