-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Brothers in Arms: Th17 and Treg Responses in Immunity
article has not abstract
Published in the journal: . PLoS Pathog 10(12): e32767. doi:10.1371/journal.ppat.1004456
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004456Summary
article has not abstract
Introduction
Following the discovery of T helper 17 (Th17) cells in 2005, considerable research efforts identified interleukin 17 (IL-17) and Th17 responses as essential components of immunity to the commensal fungus Candida albicans. Much less is understood about regulatory T cells (Tregs) in candidiasis. However, emerging data point towards a surprisingly complex relationship between IL-17/Th17 and Treg responses during C. albicans infections, wherein Tregs both suppress and enhance immunity. This review will discuss the role of these responses during candidiasis and the consequences for disease outcome and therapy.
IL-17/Th17 Responses Are Key Mediators of C. albicans Antifungal Immunity
IL-17-mediated immunity is crucial for protection against C. albicans infections, especially mucocutaneous infections, including oral and dermal candidiasis (reviewed in [1]). “Experiments of nature” have revealed mutations in humans that cause susceptibility to chronic mucocutaneous candidiasis (CMC), nearly all of which impact the IL-17/Th17 pathway (Table 1, reviewed in [2]). For example, individuals with mutations in IL17RA, IL17RC, IL-17F, or the IL-17 family–specific signaling molecule ACT1 suffer from CMC [3], [4] (Casanova and Puel, personal communication; see Acknowledgments). CMC can be defined as a heterogeneous group of disorders characterized by persistent or recurrent Candida infection of mucosal membranes, skin, and nails. To date, there is no animal model that fully recapitulates the complex phenotype of CMC. However, models of oral and dermal candidiasis are in agreement with human data. IL-23-/-, IL-17RA-/-, IL-17RC-/-, and Act1-/- mice are susceptible to oropharyngeal candidiasis (OPC) [5]–[7]. Similarly, IL-23-/- and IL-17A-/- mice display susceptibility to dermal candidiasis [8]. Somewhat surprisingly, IL-17RA-/- and IL-23-/- mice are not susceptible to vaginal candidiasis [9]. Although one study demonstrated that pharmacological blockade of Th17 responses increased vaginal fungal burdens, that study did not measure markers of symptomatic infection [10]. Therefore, IL-17-mediated immunity in candidiasis appears to be site dependent, though the underlying basis for this tissue specificity is enigmatic.
Tab. 1. Human genetic defects associated with susceptibility to Candida infections. 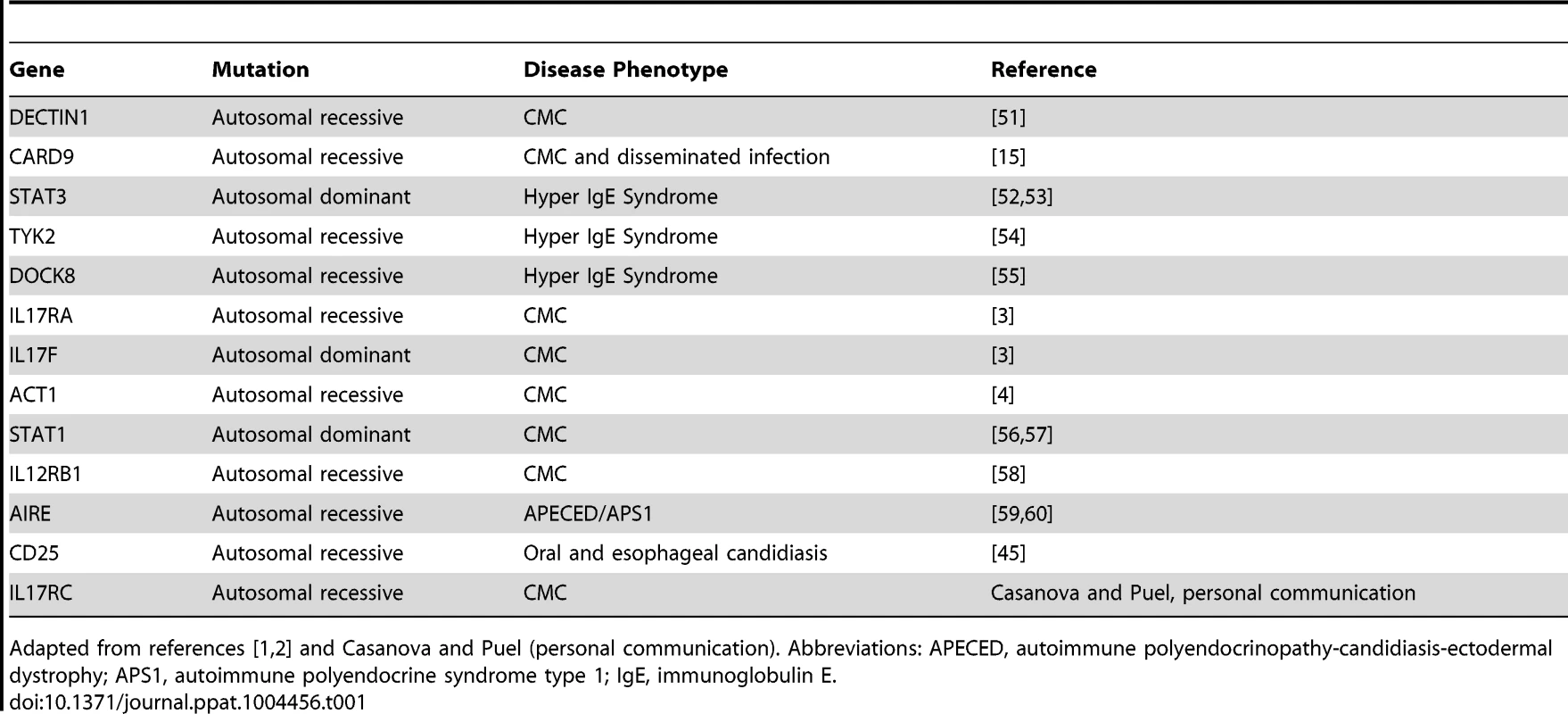
Adapted from references [1], [2] and Casanova and Puel (personal communication). Abbreviations: APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy; APS1, autoimmune polyendocrine syndrome type 1; IgE, immunoglobulin E. C. albicans also causes disseminated infections, associated with mortality rates of 50% or higher [11]. IL-17RA-/- and IL-17A-/- mice show elevated susceptibility to disseminated candidiasis [12]–[14]. However, humans with mutations in the IL-17 pathway typically do not develop disseminated disease. One exception is patients with CARD9 mutations, who display susceptibility to both CMC and disseminated infection [15]. Why other IL-17 pathway gene mutations do not predispose patients to heightened susceptibility to disseminated candidiasis is unknown, although the number of patients identified with such mutations is limited. It is possible that under predisposing conditions (antibiotic treatment, intravenous catheter use, or abdominal surgery), individuals with impairments in the IL-17 pathway may be at increased risk for disseminated candidiasis, an issue that will need to be monitored, particularly considering the impending use of anti-IL-17 biologic therapy for autoimmunity [16].
IL-17 Function and Sources
IL-17 exerts protective effects principally through the recruitment and activation of neutrophils. IL-17 primarily acts upon nonhematopoietic cells by stimulating the production of cytokines and chemokines, such as granulocyte-colony stimulating factor (G-CSF), interleukin 8 (IL-8) (humans), CXCL1, and CXCL5, which serve to expand and recruit neutrophils [1]. Depletion of neutrophils renders mice susceptible to OPC [17] and disseminated candidiasis [18]. Additionally, IL-17 signaling promotes anti-Candida killing mechanisms such as production of antimicrobial peptides (e.g., salivary histatins, β-defensins, and S100A8/9) [5], [9], [19].
CD4+ T cells are traditionally considered to be the primary cellular source of IL-17 during mucosal C. albicans infections [5], [20]. This assumption is based on the observation that patients with HIV/AIDS exhibit dramatically heightened susceptibility to OPC [21]. Moreover, most Candida-specific memory T cells in humans are Th17 cells. Similarly, in models of adaptive immunity, Th17 and not Th1 cells are induced by Candida and are protective against oral infections [20], [22].
IL-17 is produced by both conventional Th17 cells and by innate cells [23]. One recent report proposed a role for innate lymphoid cell (ILC) production of IL-17 in host defense against OPC [24]. However, IL-17 production by ILCs was not directly demonstrated. Notably, Rag1-/- mice, which lack T cells but have enriched numbers of ILCs, are highly susceptible to OPC [20], [25], raising questions about the relevance of ILCs in oral candidiasis. Our recent data show that following immediate exposure to C. albicans, oral IL-17 is produced not by ILCs but by γδ-T cells and a subset of CD4+TCRβ+ innate-like cells known as “natural” Th17 cells [26]. Whether one or all of these IL-17+ subsets are necessary for host defense in humans remains to be determined.
Treg Cells: Regulators of Infectious Disease
Tregs are a distinct subset of CD4+ T cell whose primary function is to restrict potentially pathogenic inflammatory immune responses. Tregs possess an extensive armory of suppressive mechanisms that can be cell contact dependent (acting through inhibitory receptors such as cytotoxic T-lymphocyte-associated protein 4 [CTLA-4]) or cell contact independent (acting via inhibitory cytokines and generation of suppressive metabolites) (reviewed in [27]). This suppressive tool kit makes Tregs adept at controlling cell types from both the innate and adaptive arms of the immune system. Several Treg subsets exist, including Tregs expressing CD25 and the canonical Treg transcription factor Foxp3 that will be the focus of this review. Foxp3+ Tregs can be further divided into thymus-derived Tregs, which are fully differentiated in the thymus, and peripherally derived (p)Tregs, which differentiate from naïve CD4+ T cells in the periphery following antigen stimulation. Although a detailed description of Treg biology is beyond the scope of this review, we refer the reader to several excellent reviews for further information on this subject [27], [28].
It is now appreciated that Tregs contribute to immunity against infectious pathogens. Inflammatory effector responses are critical in host defense against pathogens. However, excessive inflammatory responses can be damaging and therefore must be tightly regulated. A beneficial role for Treg-mediated restraint of immunopathology has been demonstrated in several viral and parasitic infections [29], [30]. In some settings, Tregs are also required for long-term maintenance of protective immunity, for example, in the context of Leishmania major infection [31]. Conversely, overly potent Treg suppression can inhibit protective immunity, favoring the pathogen. A detrimental role for Treg suppression has been demonstrated during Mycobacterium tuberculosis infection, in which depletion of Tregs resulted in enhanced protective responses [32]. Tregs can also promote, rather than prevent, inflammation. During mucosal herpes simplex virus infections, Tregs promoted protective effector responses via immune cell recruitment to sites of infection [33]. Therefore, Tregs can have diverse impacts, depending on the infection.
IL-17/Th17 and Treg Responses Are Intricately Linked during Candidiasis
Treg responses are elevated during C. albicans infections, suggesting a functional role. An increase in the proportion of CD4+CD25+ cells and expression of Foxp3 was detected in the mesenteric lymph nodes (LNs) and stomachs of mice intragastrically inoculated with C. albicans [34], [35]. Similarly, CD4+CD25+Foxp3+ cells expanded in mice systemically infected with C. albicans [36]. However, Treg-mediated responses to C. albicans, and indeed to other fungi, remain poorly understood.
Th17 and Treg subsets are reciprocally regulated during naïve T cell differentiation [37]. Reciprocal regulation of such responses was observed in a model of gastrointestinal candidiasis, in which increased Treg responses were associated with reduced Th17 responses and vice versa [34], [35]. Conversely, Tregs can also promote Th17 responses [37]. Accordingly, IL-17/Th17 and Treg responses are positively associated during OPC and disseminated candidiasis (Fig. 1). Treg depletion by anti-CD25 treatment results in concurrent depletion of Th17 cells during OPC. In the same model, co-transfer of CD4+CD25+ and CD4+CD25- cells into Rag1-/- mice enhanced protective Th17 responses [25]. In disseminated candidiasis, Tregs suppressed Th1 and Th2 responses while promoting Th17 responses in vitro [36]. Furthermore, Foxp3+ cell depletion in vivo was associated with reduced IL-17/Th17 responses [36]. Notably, both studies provide evidence that the mechanism of action is, at least in part, through consumption of IL-2 by Tregs through the high affinity IL-2R [25], [36]. IL-2 is essential for Treg survival but limits Th17 differentiation [38]. Therefore, Treg consumption of IL-2 reduces its local concentration, favoring Th17 development [25], [36], [39]. Whether IL-2 consumption by Tregs is a dominant mechanism for driving IL-17 responses during candidiasis remains an open question.
Fig. 1. Treg/Th17 relationship during candidiasis. 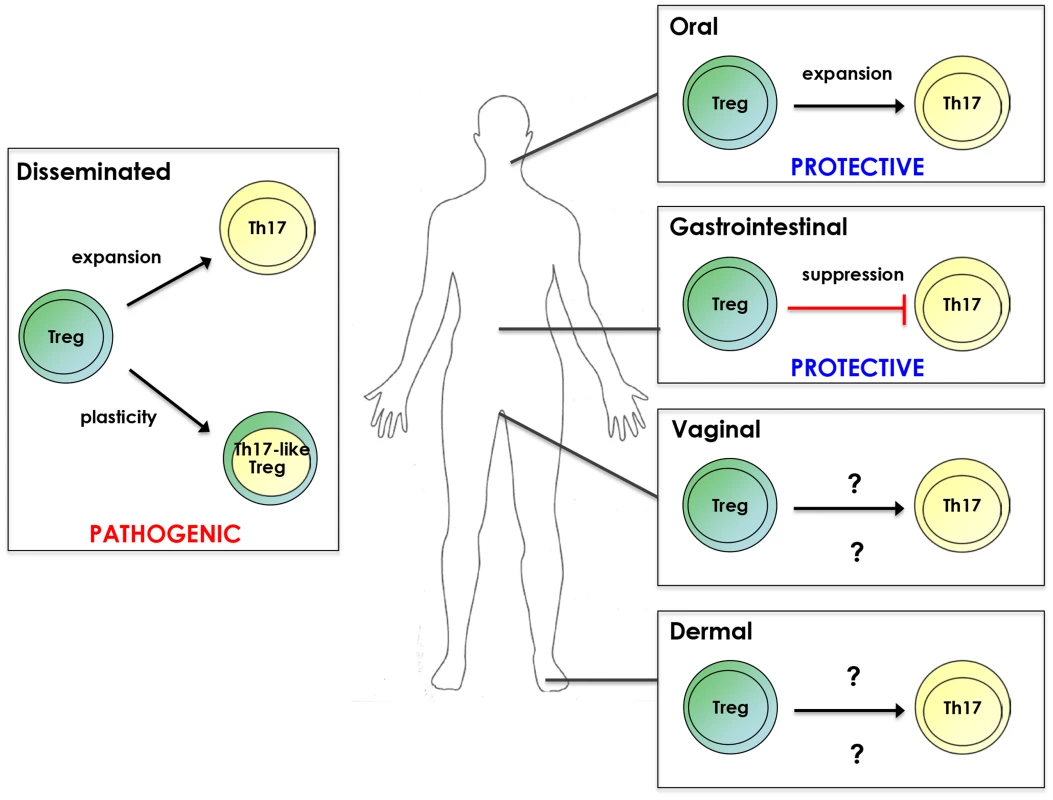
Tregs promote Th17 responses as well as acquire Th17 characteristics during disseminated infection. However, Treg/Th17 responses are associated with pathogenicity in this form of candidiasis. Conversely, Treg enhancement of Th17 responses during OPC is protective. Tregs suppress Th17 responses during gastrointestinal candidiasis, leading to decreased C. albicans colonization. Whether Tregs impact Th17 responses during vaginal and cutaneous candidiasis and the resulting outcome of disease remains to be determined. Plasticity is a phenomenon whereby CD4+ T cell subsets acquire characteristics of other populations (reviewed in [40]). For example, in some settings Tregs can express RORγt and produce IL-17A. Indeed, pTregs and Th17 cells possess an especially high degree of phenotypic flexibility [40], which has been observed in antifungal immunity. Specifically, dendritic cell recognition of β-glucans in the Candida cell wall by dectin-1 promotes conversion of Tregs to a RORγt+IL-17A+ phenotype [41]. Moreover, CD4+CD25+Foxp3+ cells isolated from systemically infected mice expressed RORγt and produced IL-17A, with the majority also expressing pTreg markers [36]. Collectively, these studies indicate that Tregs can promote IL-17/Th17 responses and acquire characteristics of Th17 cells in response to C. albicans.
Final Outcome: Location, Location, Location
Although IL-17/Th17 and Treg responses can act cooperatively during candidiasis, disease outcome is strikingly different depending on infection site. In OPC, Th17 enhancement by Tregs increased resistance to infection [25]. In contrast, Treg enhancement of Th17 responses in disseminated candidiasis was associated with reduced resistance [36]. These studies suggest that inflammatory Th17 and Treg responses are protective at mucosal surfaces but pathogenic in systemic candidiasis. Consistent with this idea, humans with defective Th17 and Treg responses are susceptible to CMC but not to disseminated candidiasis [42]–[45]. However, the concept that elevated Th17 and Treg responses are harmful in disseminated candidiasis seemingly contrasts with the apparent protective role of IL-17 in mice [12]–[14], [36]. One explanation is that these studies use knockout animals with complete genetic ablation of IL-17 components and therefore do not address the requirement of balanced immune responses. In support of a pathogenic role for unbalanced Th17 and Treg responses during candidiasis, cytokines associated with Th17 responses positively correlate with increasing disease severity in disseminated candidiasis [46]. Similarly, overzealous Th17 responses are associated with immunopathology in gastrointestinal candidiasis [47]. Furthermore, depletion of Tregs during disseminated candidiasis increases resistance to disease [48]. Ultimately, the balance between protective versus pathogenic immunity is crucial in determining disease outcome.
How immune responses are shaped depends on factors in the microenvironment. Commensal microbes ferment dietary fibers to short chain fatty acids (SCFAs) that favor tolerogenic Tregs [49]. Additionally, transforming growth factor beta (TGFβ) and retinoic acid, which are enriched at the intestinal mucosa, promote Tregs over Th17 responses [50]. Since C. albicans is a commensal of human mucosae, it is likely that these tissues have evolved tolerogenic mechanisms to live in harmony with this fungus. In contrast, internal organs are shielded from the external environment and typically lack high levels of SCFAs and retinoic acid. Therefore, inflammation induced during disseminated C. albicans infection is more likely to go unchecked compared to mucosal surfaces, resulting in collateral tissue damage. Overall, site-specific factors are pivotal in dictating the balance between protective and pathogenic Th17 and Treg responses.
Concluding Remarks
It is clear that IL-17/Th17 and Treg cells have a complex relationship, exemplified during infections with C. albicans. Although Th17 and Treg responses appear to be reciprocally regulated in certain situations (e.g., gastrointestinal candidiasis), Tregs promote Th17 activities and even acquire phenotypic characteristics of Th17 cells in other settings (e.g., oral and disseminated candidiasis). Notably, the impact of Th17 and Treg responses on disease outcome is distinct in different forms of candidiasis, highlighting the importance of microenvironment in shaping overall immunity. Elucidating the factors that determine the balance between protective versus pathogenic Th17 and Treg responses during candidiasis will be an important future avenue of research. Ultimately, it may be possible to exploit this information in order to help tune appropriate responses in the context of candidiasis.
Zdroje
1. Hernández-SantosN, GaffenSL (2012) Th17 cells in immunity to Candida albicans. Cell Host Microbe 11 : 425–435.
2. HupplerAR, BishuS, GaffenSL (2012) Mucocutaneous candidiasis: the IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther 14 : 217.
3. PuelA, CypowyjS, BustamanteJ, WrightJF, LiuL, et al. (2011) Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332 : 65–68.
4. BoissonB, WangC, PedergnanaV, WuL, CypowyjS, et al. (2013) An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39 : 676–686.
5. ContiHR, ShenF, NayyarN, StocumE, SunJN, et al. (2009) Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206 : 299–311.
6. HoAW, ShenF, ContiHR, PatelN, ChildsEE, et al. (2010) IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol 185 : 1063–1070.
7. FerreiraMC, WhibleyN, MamoAJ, SiebenlistU, ChanYR, et al. (2014) Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect Immun 82 : 1030–1035.
8. KagamiS, RizzoHL, KurtzSE, MillerLS, BlauveltA (2010) IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 185 : 5453–5462.
9. YanoJ, KollsJK, HappelKI, WormleyF, WozniakKL, et al. (2012) The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE 7: e46311.
10. PietrellaD, RachiniA, PinesM, PandeyN, MosciP, et al. (2011) Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS ONE 6: e22770.
11. PfallerMA, DiekemaDJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36 : 1–53.
12. HuangW, NaL, FidelPL, SchwarzenbergerP (2004) Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190 : 624–631.
13. SaijoS, IkedaS, YamabeK, KakutaS, IshigameH, et al. (2010) Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32 : 681–691.
14. van de VeerdonkFL, KullbergBJ, VerschuerenIC, HendriksT, van der MeerJW, et al. (2010) Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock 34 : 407–411.
15. GlockerEO, HennigsA, NabaviM, SchafferAA, WoellnerC, et al. (2009) A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361 : 1727–1735.
16. MiossecP, KollsJK (2012) Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11 : 763–776.
17. HupplerAR, ContiHR, Hernandez-SantosN, DarvilleT, BiswasPS, et al. (2014) Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol 192 : 1745–1752.
18. FulurijaA, AshmanRB, PapadimitriouJM (1996) Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 142 : 3487–3496.
19. ContiHR, BakerO, FreemanAF, JangWS, HollandSM, et al. (2011) New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol 4 : 448–455.
20. Hernandez-SantosN, HupplerAR, PetersonAC, KhaderSA, McKennaKC, et al. (2013) Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol 6 : 900–910.
21. GlockerE, GrimbacherB (2010) Chronic mucocutaneous candidiasis and congenital susceptibility to Candida. Curr Opin Allergy Clin Immunol 10 : 542–550.
22. BärE, GladiatorA, BastidasS, RoschitzkiB, Acha-OrbeaH, et al. (2012) A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol 188 : 5636–5643.
23. CuaDJ, TatoCM (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10 : 479–489.
24. GladiatorA, WanglerN, Trautwein-WeidnerK, LeibundGut-LandmannS (2013) Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 190 : 521–525.
25. PandiyanP, ContiHR, ZhengL, PetersonAC, MathernDR, et al. (2011) CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity 34 : 422–434.
26. ContiHR, PetersonAC, BraneL, HupplerAR, Hernández-SantosN, et al. (2014) Oral-resident ‘natural’ Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med In press
27. VignaliDA, CollisonLW, WorkmanCJ (2008) How regulatory T cells work. Nat Rev Immunol 8 : 523–532.
28. JosefowiczSZ, LuLF, RudenskyAY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30 : 531–564.
29. FultonRB, MeyerholzDK, VargaSM (2010) Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol 185 : 2382–2392.
30. HesseM, PiccirilloCA, BelkaidY, PruferJ, Mentink-KaneM, et al. (2004) The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 172 : 3157–3166.
31. BelkaidY, PiccirilloCA, MendezS, ShevachEM, SacksDL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 : 502–507.
32. Scott-BrowneJP, ShafianiS, Tucker-HeardG, Ishida-TsubotaK, FontenotJD, et al. (2007) Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med 204 : 2159–2169.
33. LundJM, HsingL, PhamTT, RudenskyAY (2008) Coordination of early protective immunity to viral infection by regulatory T cells. Science 320 : 1220–1224.
34. De LucaA, MontagnoliC, ZelanteT, BonifaziP, BozzaS, et al. (2007) Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol 179 : 5999–6008.
35. BonifaziP, ZelanteT, D'AngeloC, De LucaA, MorettiS, et al. (2009) Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol 2 : 362–374.
36. WhibleyN, MaccallumDM, VickersMA, ZafreenS, WaldmannH, et al. (2014) Expansion of Foxp3(+) T-cell populations by Candida albicans enhances both Th17-cell responses and fungal dissemination after intravenous challenge. Eur J Immunol 44 : 1069–1083.
37. ChenX, OppenheimJJ (2014) Th17 cells and Tregs: unlikely allies. J Leukoc Biol In press
38. BoymanO, SprentJ (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12 : 180–190.
39. ChenY, HainesCJ, GutcherI, HochwellerK, BlumenscheinWM, et al. (2011) Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity 34 : 409–421.
40. ZhouL, ChongMM, LittmanDR (2009) Plasticity of CD4+ T cell lineage differentiation. Immunity 30 : 646–655.
41. OsorioF, LeibundGut-LandmannS, LochnerM, LahlK, SparwasserT, et al. (2008) DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol 38 : 3274–3281.
42. MilnerJD, HollandSM (2013) The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol 13 : 635–648.
43. KekalainenE, TuovinenH, JoensuuJ, GyllingM, FranssilaR, et al. (2007) A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol 178 : 1208–1215.
44. SaitoM, NagasawaM, TakadaH, HaraT, TsuchiyaS, et al. (2011) Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med 208 : 235–249.
45. SharfeN, DadiHK, ShaharM, RoifmanCM (1997) Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A 94 : 3168–3171.
46. MacCallumDM, CastilloL, BrownAJ, GowNA, OddsFC (2009) Early-expressed chemokines predict kidney immunopathology in experimental disseminated Candida albicans infections. PLoS ONE 4: e6420.
47. ZelanteT, De LucaA, BonifaziP, MontagnoliC, BozzaS, et al. (2007) IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37 : 2695–2706.
48. NeteaMG, SutmullerR, HermannC, Van der GraafCA, Van der MeerJW, et al. (2004) Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 172 : 3712–3718.
49. SmithPM, HowittMR, PanikovN, MichaudM, GalliniCA, et al. (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341 : 569–573.
50. MucidaD, ParkY, KimG, TurovskayaO, ScottI, et al. (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317 : 256–260.
51. FerwerdaB, FerwerdaG, PlantingaTS, WillmentJA, van SprielAB, et al. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361 : 1760–1767.
52. MaCS, ChewGY, SimpsonN, PriyadarshiA, WongM, et al. (2008) Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 205 : 1551–1557.
53. MilnerJD, BrenchleyJM, LaurenceA, FreemanAF, HillBJ, et al. (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452 : 773–776.
54. MinegishiY, SaitoM, MorioT, WatanabeK, AgematsuK, et al. (2006) Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25 : 745–755.
55. EngelhardtKR, McGheeS, WinklerS, SassiA, WoellnerC, et al. (2009) Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 124 : 1289–1302.e1284.
56. LiuL, OkadaS, KongXF, KreinsAY, CypowyjS, et al. (2011) Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 208 : 1635–1648.
57. van de VeerdonkFL, PlantingaTS, HoischenA, SmeekensSP, JoostenLA, et al. (2011) STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 365 : 54–61.
58. de BeaucoudreyL, SamarinaA, BustamanteJ, CobatA, Boisson-DupuisS, et al. (2010) Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 89 : 381–402.
59. KisandK, Boe WolffAS, PodkrajsekKT, TserelL, LinkM, et al. (2010) Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207 : 299–308.
60. PuelA, DoffingerR, NatividadA, ChrabiehM, Barcenas-MoralesG, et al. (2010) Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207 : 291–297.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus InfectionČlánek P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to InfectionČlánek Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Microbial Programming of Systemic Innate Immunity and Resistance to Infection
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
- Measles Immune Suppression: Functional Impairment or Numbers Game?
- Cellular Mechanisms of Alpha Herpesvirus Egress: Live Cell Fluorescence Microscopy of Pseudorabies Virus Exocytosis
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection
- Loss of Dynamin-Related Protein 2B Reveals Separation of Innate Immune Signaling Pathways
- Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Infection
- Unveiling the Intracellular Survival Gene Kit of Trypanosomatid Parasites
- Extreme Divergence of Tropism for the Stem-Cell-Niche in the Testis
- HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4 T Cells
- P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
- Hypercytotoxicity and Rapid Loss of NKp44 Innate Lymphoid Cells during Acute SIV Infection
- Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
- Crystal Structure of Calcium Binding Protein-5 from and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
- Chronic Parasitic Infection Maintains High Frequencies of Short-Lived Ly6CCD4 Effector T Cells That Are Required for Protection against Re-infection
- Specific Dysregulation of IFNγ Production by Natural Killer Cells Confers Susceptibility to Viral Infection
- HSV-2-Driven Increase in the Expression of αβ Correlates with Increased Susceptibility to Vaginal SHIV Infection
- Murine Anti-vaccinia Virus D8 Antibodies Target Different Epitopes and Differ in Their Ability to Block D8 Binding to CS-E
- Brothers in Arms: Th17 and Treg Responses in Immunity
- Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Typhimurium Colitis
- A Negative Feedback Modulator of Antigen Processing Evolved from a Frameshift in the Cowpox Virus Genome
- Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms
- The Non-receptor Tyrosine Kinase Tec Controls Assembly and Activity of the Noncanonical Caspase-8 Inflammasome
- Targeted Changes of the Cell Wall Proteome Influence Ability to Form Single- and Multi-strain Biofilms
- Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
- The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
- Anti-α4 Antibody Treatment Blocks Virus Traffic to the Brain and Gut Early, and Stabilizes CNS Injury Late in Infection
- Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
- Microbial Urease in Health and Disease
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against
- IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
- A Natural Genetic Variant of Granzyme B Confers Lethality to a Common Viral Infection
- Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression
- Differential PfEMP1 Expression Is Associated with Cerebral Malaria Pathology
- The Role of the NADPH Oxidase NOX2 in Prion Pathogenesis
- Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions
- The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
- Expression of Suppressor of Cytokine Signaling 1 (SOCS1) Impairs Viral Clearance and Exacerbates Lung Injury during Influenza Infection
- Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells
- SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in
- Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication
- Oral Streptococci Utilize a Siglec-Like Domain of Serine-Rich Repeat Adhesins to Preferentially Target Platelet Sialoglycans in Human Blood
- SV40 Utilizes ATM Kinase Activity to Prevent Non-homologous End Joining of Broken Viral DNA Replication Products
- Amphipathic α-Helices in Apolipoproteins Are Crucial to the Formation of Infectious Hepatitis C Virus Particles
- Proteomic Analysis of the Acidocalcisome, an Organelle Conserved from Bacteria to Human Cells
- Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



