-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
Malaria remains one of the most serious health problems globally, but our understanding of the biology of the Plasmodium parasite and the pathogenesis of severe disease is still limited. Human cerebral malaria (HCM), a severe neurological complication characterized by rapid progression from headache to convulsions and unrousable coma, causes the death of hundreds of thousands of children in Africa annually. To better understand the pathogenesis of cerebral malaria, we imaged immune cells in brain microvessels of mice with experimental cerebral malaria (ECM) versus mice with malarial hyperparasitemia, which lack neurological impairment. Death from ECM closely correlated with plasma leakage, platelet marginalization, and the recruitment of significantly more leukocytes to postcapillary venules compared to hyperparasitemia. Leukocyte arrest in postcapillary venules caused a severe restriction in the venous blood flow and the immunomodulatory drug FTY720 prevents this recruitment and death from ECM. We propose a model for ECM in which leukocyte arrest, analogous to the sequestration of P. falciparum infected red blood cells in HCM, severely restricts the venous blood flow, which exacerbates edema and swelling of the brain at the agonal comatose stage of the infection, leading to intracranial hypertension and death.
Published in the journal: . PLoS Pathog 10(12): e32767. doi:10.1371/journal.ppat.1004528
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004528Summary
Malaria remains one of the most serious health problems globally, but our understanding of the biology of the Plasmodium parasite and the pathogenesis of severe disease is still limited. Human cerebral malaria (HCM), a severe neurological complication characterized by rapid progression from headache to convulsions and unrousable coma, causes the death of hundreds of thousands of children in Africa annually. To better understand the pathogenesis of cerebral malaria, we imaged immune cells in brain microvessels of mice with experimental cerebral malaria (ECM) versus mice with malarial hyperparasitemia, which lack neurological impairment. Death from ECM closely correlated with plasma leakage, platelet marginalization, and the recruitment of significantly more leukocytes to postcapillary venules compared to hyperparasitemia. Leukocyte arrest in postcapillary venules caused a severe restriction in the venous blood flow and the immunomodulatory drug FTY720 prevents this recruitment and death from ECM. We propose a model for ECM in which leukocyte arrest, analogous to the sequestration of P. falciparum infected red blood cells in HCM, severely restricts the venous blood flow, which exacerbates edema and swelling of the brain at the agonal comatose stage of the infection, leading to intracranial hypertension and death.
Introduction
Plasmodium falciparum is responsible for an estimated 600,000 deaths annually, principally in children under the age of five [1]. Clinical symptoms range from intermittent fevers and chills to potentially fatal complications including severe anemia and cerebral malaria [2]. The mortality rate in comatose pediatric patients, most frequently due to respiratory arrest, is 15–20% despite optimal medical care [3], but the underlying pathology is unclear.
Molecular and cellular mechanisms involved in the pathogenesis of human cerebral malaria (HCM) include a predominantly pro-inflammatory cytokine profile, endothelial activation via the NF-κB pathway with upregulation of adhesion molecules, glia cell activation, and sequestration of infected red blood cells (iRBC), monocytes, and platelets within brain capillaries [3]–[6]. However, the cellular mechanisms associated with HCM cannot be directly observed in the human brain. Ophthalmological examination of the retinal pathology generally correlates with course and etiology of malarial encephalopathy [2], [7], but despite significant recent improvements [8], this technique lacks the resolution to observe the dynamic behavior of individual iRBC, leukocytes, and platelets, their exact location within the microvasculature, mechanisms of vascular leakage or possibly occlusion, and the sequence of these events. Elucidation of CM pathogenesis therefore requires the use of a robust small animal model that closely reflects clinical symptoms, histopathology, and immune mechanisms associated with the pathophysiology of HCM.
P. berghei ANKA (PbA) infected CBA, Swiss Webster, or CB57Bl/6 mice represent a well-characterized and widely used model for experimental cerebral malaria (ECM) that shares a number of similarities with P. falciparum HCM [5], [6], [9]–[12]. Both ECM and HCM are characterized by severe vasculopathy, i.e. endothelial activation and dysfunction with increased expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin, upregulation of inflammatory cytokines, reduced blood flow, vascular leakage, acute edema of both vasogenic and cytotoxic origin, and microhemorrhages leading to neurological impairment [12]–[17]. Platelet activation, dysregulation of the coagulation cascade, thrombocytopenia, and platelet accumulation in the brain are also found in both HCM and ECM [18]–[20]. We have previously shown by intravital microscopy (IVM) that platelet marginalization and blood brain barrier (BBB) disruption are central to ECM pathophysiology [21]. Platelets are thought to impair vascular repair and increase BBB permeability by potentiating the iRBC-induced endothelial damage in the early stages of HCM development [22]–[24]. Circulating platelet-derived microparticles are increased in severe P. falciparum malaria and serve as a biomarker for neurological involvement [13], [25].
The murine PbA model has also provided ample evidence for a contribution of CD8+ and CD4+ T cells to the late stages of ECM development [26]–[30]. Both CD8+ T cells, generally considered the terminal effector cells, and CD4+ T cells must accumulate in the cerebral microvasculature for ECM to occur [11], [27], [28], [31]–[35] and may also be responsible for the ECM-associated leukocyte infiltration [36]. While ECM development was thought to involve CD8+ T cell-induced endothelial apoptosis via perforin - and granzyme B-mediated cytotoxicity resulting in BBB disruption [32], [33], [37], we recently showed by IVM that ECM closely correlates with widespread opening of the BBB and that this occurs in the absence of significant endothelial death [21]. The BBB at the level of postcapillary venules encompasses two layers, the vascular endothelium with its basement membrane and the glia limitans with associated basement membranes and astrocyte endfeet, which are separated by the perivascular space [38]. This section of the BBB is functionally distinct from other areas of the BBB, for example that at the capillary level, which consists of a single layer composed of endothelia, gliovascular membrane, and astrocyte endfeet [38]. IVM also revealed that ECM correlates with platelet deposition, leukocyte arrest, and de novo expression of the pattern recognition receptor CD14 on the endothelial surface from postcapillary venules, but not from capillaries or arterioles [21]. Strikingly, inhibition of platelet deposition and leukocyte recruitment by blockage of LFA-1 mediated cellular interactions prevented ECM and disruption of the BBB in PbA-infected mice [21]. Thus, it appears that the ultimate cause of coma and death in ECM is a universal breakdown of the BBB at the level of postcapillary venules [21].
In the PbA-infected CBA/CaJ mouse model, vascular leakage, neurological signs, and death from ECM can be prevented by treatment with the endothelial barrier-stabilizing sphingosine 1 analog FTY720 (fingolimod) [21], [39], an immunomodulatory FDA-approved drug for oral treatment of relapsing multiple sclerosis (MS) [40] that acts as an agonist for sphingosine 1-phosphate (S1P) receptors [41]. In experimental autoimmune encephalomyelitis (EAE), FTY720 prevents T cell recruitment to the brain by down-modulating the expression of S1P1 receptors on the T cell surface. This favors the CCR7-mediated retention of naïve and central memory T cells within secondary lymphatic tissues [42], leading to a reduction in the numbers of naïve and central memory T cells, but not effector memory T cells, in the blood [43]. FTY720 may also prevent stimulation of vascular endothelia or activation of CD8+ effector T cells in the spleen by decreasing CD11c+ DC migration and function and by destabilizing DC/T cell interactions thus preventing the formation of an immunological synapse [44], [45]. In addition to its involvement in T cell activation and targeting to the brain, FTY720 is also thought to have a directly stabilizing effect on endothelial junctions at the BBB [46]–[49]. However, the exact mechanism by which FTY720 prevents BBB opening remains unclear to date.
Here, we show that ECM is associated with the accumulation of numerous leukocytes within postcapillary and larger venules and that the resulting microrheological alterations severely restrict the venous blood flow. Treatment with FTY720 significantly reduced the recruitment of these leukocytes indicating their involvement in the pathogenesis of ECM [21], [39]. Leukocyte arrest likely increases the intracranial pressure, similarly to P. falciparum iRBC sequestration in pediatric HCM, which is typically associated with a poor clinical outcome [50].
Results
Three week-old CBA/CaJ mice were infected with PbA-GFP or P. yoelii 17XL (PyXL)-RFP and monitored throughout the course of development of ECM or hyperparasitemia [21]. In this study, a total of 78 PbA-infected mice were subjected to IVM at the time of ECM (day 6–8), 18 PbA-infected mouse before ECM (day 5), 15 PbA-infected mice that failed to develop ECM (day 9), and 25 uninfected control mice (Table S1). We also examined 30 PbA-infected mice that were treated with FTY720 and failed to develop ECM (day 8–9) and 8 PbA-infected mice that developed ECM on day 8 despite treatment with FTY720. As controls for parasitemia, 62 PyXL-infected mice with >50% parasitemia (day 5) were analyzed. Throughout our IVM experiments, we examined deep microvessels, i.e. branches of penetrating arterioles and venules [51], [52], which were in direct continuation with the cortical capillary bed. Imaging of CX3CR1GFP/+ mice confirmed that the postcapillary venules, capillaries, and arterioles used for analysis are embedded in fluorescent microglia and thus clearly located in the cerebral cortex, i.e. underneath the pial microvasculature (Figure S1) [21].
ECM correlates with microrheological alterations in postcapillary venules
IVM revealed that the venous blood flow in postcapillary venules from PbA-infected mice with neurological signs (day 6) was strikingly altered. Vascular labeling with Evans blue revealed that postcapillary venules from mice with ECM exhibited a marginal zone devoid of RBCs (Figure 1A, Video S1). Instead, this zone contained variable numbers of leukocytes that were either rolling along the endothelium, crawling, or firmly attached (Figure S2A and S2C, Video S2). Minimal projections of time sequences emphasize the boundary between the functional lumen in the center of the postcapillary venules and the RBC-free marginal zone and suggest that the functional lumen available for the blood flow is significantly restricted during ECM (Figure S2B and S2D). This phenomenon was even more pronounced in larger venules. Neither arterioles from mice with ECM (Figure 1B, Video S3) nor postcapillary venules or arterioles from mice with hyperparasitemia (Figure 1C and 1D, Video S4) showed any significant functional vascular restriction, i.e. narrowing of the passageway available for the blood flow, compared to uninfected control mice (Figure 1E and 1F, Video S5), a finding we attribute to the absence of steric hindrance generated by adherent leukocytes in these vessels. Multiple measurements of the total vascular diameter (from endothelium to endothelium) and the functional diameter (used by the blood flow) of 50 randomly chosen postcapillary venules from 4 mice with ECM revealed a mean functional diameter of 70.5±13.7% compared to data from 3 uninfected control mice, corresponding to a functional vascular cross-section of 55.8±19.1% (Figure 1G). Notably, complete vascular occlusion, whether in postcapillary venules or other microvessels, was not observed during ECM. In 50 randomly chosen postcapillary venules from 3 PyXL-infected mice with hyperparasitemia, the functional postcapillary venule diameter and cross-section was 95.5±3.4% and 92.7±5.1%, respectively. No microrheological alterations were found in postcapillary venules or arterioles from PbA-infected mice prior to ECM (day 5), in PbA-infected mice that failed to develop ECM (day 9), or in uninfected control mice. As reported previously [21], PbA-infected mice that did develop ECM despite treatment FTY720 exhibited vascular leakage suggesting that the venous blood flow restriction was similar to untreated PbA-infected mice with ECM.
Fig. 1. ECM correlates with microrheological alterations in postcapillary venules. 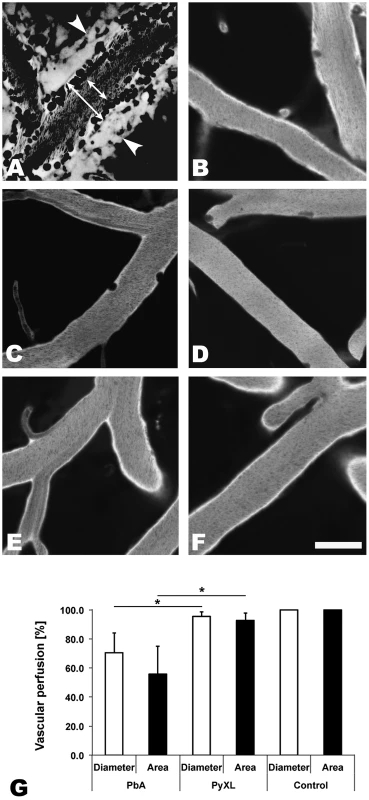
CBA/CaJ mice were infected with PbA, PyXL, or no parasites. To assess the blood flow within the cortical microvasculature, time sequences were converted to minimal projections. A) In mice with ECM, the functional postcapillary venule diameter (short arrow), i.e. the perfused portion of the vessel, is considerably reduced compared to the entire vessel diameter (long arrow). Visualization of the vascular lumen with Evans blue reveals a zone along the endothelium of postcapillary venules (A) that contains adherent leukocytes (dark circles or ovals), but is devoid of RBC (dark streaks in the center). Note that migrating leukocytes are represented multiple times in minimal projections. Leakage of Evans blue into the perivascular space and brain parenchyma is apparent on either side of the postcapillary venule (arrowheads). B) Arterioles from mice with symptomatic ECM do not exhibit any restriction in diameter. C) The small number of adherent leukocytes (dark circles) in mice with hyperparasitemia does not cause any significant restriction in the functional postcapillary venule lumen. Neither arterioles from mice with hyperparasitemia (D) nor postcapillary venules or arterioles from uninfected control mice (E and F) exhibit any microrheological alterations. Scale bars = 20 µm. See Videos S1–S5 for the corresponding dynamic data. G) Measurement of the total and functional vascular diameters and cross-sections reveals that the blood flow in postcapillary venules from mice with ECM, but not from mice with hyperparasitemia, is severely restricted. Postcapillary venules from uninfected control mice exhibit no luminal restriction. Thus, ECM correlates with a significant functional constraint, but not complete blockage, of the passageway available for the venous blood flow. This is significant, because any restriction in the venous efflux from the brain likely exacerbates edema formation, a hallmark of both ECM and HCM [16], [17], [53]. A venous efflux problem would also explain the increased intracranial pressure, which is frequently observed in pediatric CM in Africa [50]. Indeed, MRI imaging has identified increased intracranial pressure as the strongest predictor of death [54], [55].
Leukocyte recruitment during ECM and hyperparasitemia
Quantitative offline IVM analysis of the various cell densities revealed that leukocytes are recruited to the cortical microvasculature not only in response to ECM, but also hyperparasitemia, albeit at a significantly lower density (Figure 2). Specifically, more CD8+ T cells, neutrophils, and macrophages were recruited in ECM compared to hyperparasitemia, while the density of all other cell types analyzed did not differ between these two infections (Figure 3A). The cortical microvasculature of uninfected control mice exhibited virtually no arrested leukocytes suggesting that in the absence of an inflammatory stimulus, innate immune cells do not monitor the BBB.
Fig. 2. Leukocytes are recruited to cortical postcapillary venules during both ECM and hyperparasitemia. 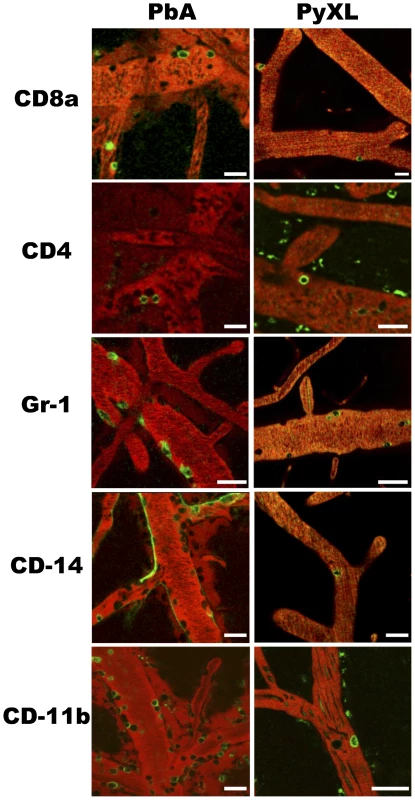
CBA/CaJ mice were infected with PbA, PyXL, or no parasites, subjected to craniotomy at the time of neurological signs or the parasitemia exceeding 50%, and prepared for intravital microscopy. CD8+ T cells, CD4+ T cells, neutrophils, monocytes, and macrophages were labeled by intravenous inoculation of fluorochrome-conjugated mAb (see Materials and Methods for details) and appear as open green circles. The vascular lumen was visualized with Evans blue (red). Representative images from intravital microscopy movies showing arrested green CD8+ T cells (CD8a), CD4+ T cells (CD4), neutrophils (GR-1), monocytes (CD14), and macrophages (CD11b) in postcapillary venules from PbA-infected mice with ECM and PyXL-infected mice with hyperparasitemia. Note that anti-CD14 labels the endothelium (green outline) during ECM in addition to monocytes (open green circles). Scale bars = 20 µm. See Videos S6–S16 for dynamic information. Fig. 3. Leukocyte recruitment to cortical postcapillary venules during ECM and hyperparasitemia. 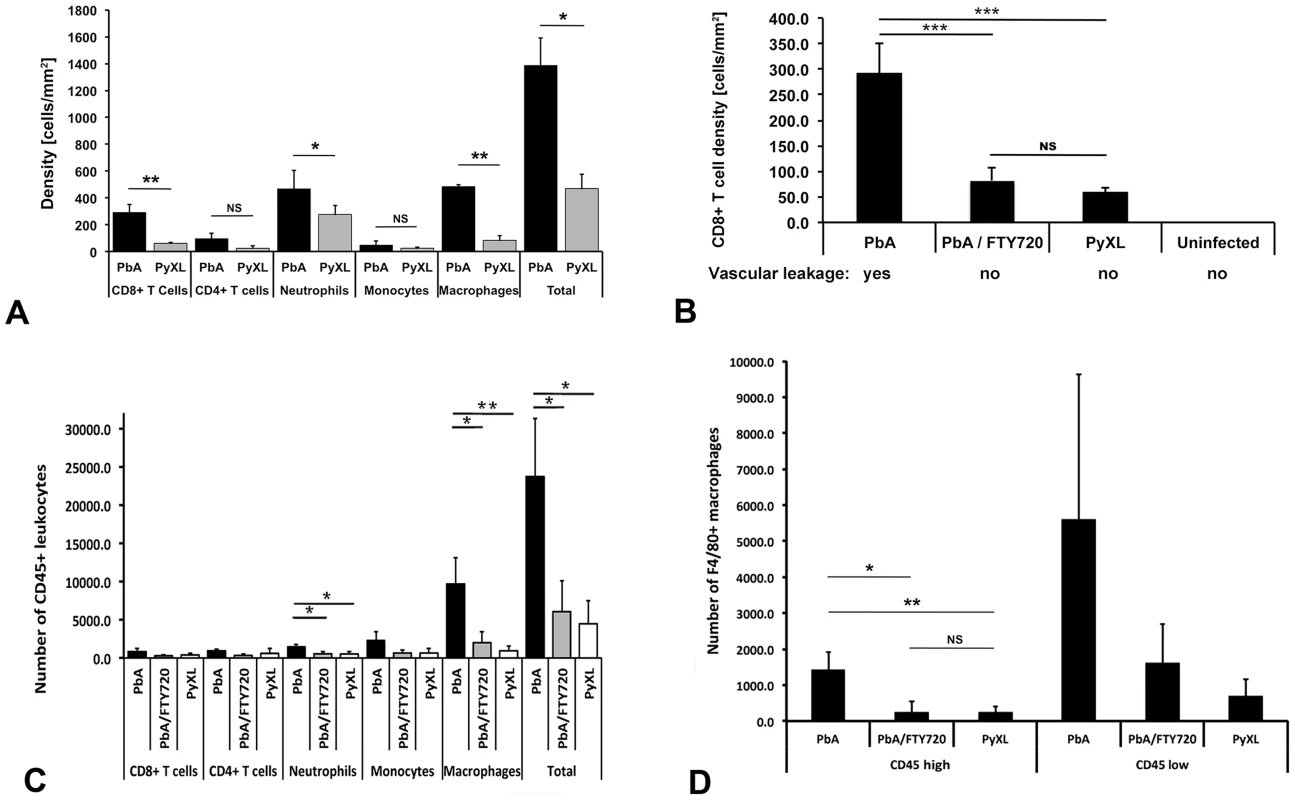
A) CBA/CaJ mice were infected with PbA, PyXL, or no parasites, subjected to craniotomy at the time of neurological signs or the parasitemia exceeding 50%, and prepared for intravital microscopy. CD8+ T cells, CD4+ T cells, Gr-1+ neutrophils, CD14+ monocytes, and CD11b+ macrophages were labeled by intravenous inoculation of mAb against CD8+a, CD4+, GR-1, CD14, and CD11b, respectively. Significantly larger numbers of CD8+ T cells, neutrophils and macrophages were found in postcapillary venules from mice with ECM compared to hyperparasitemia (P<0.05). The density of CD4+ T cells and monocytes was not significantly different. Most of the arrested leukocytes are macrophages followed by neutrophils, CD8+ T cells, CD4+ T cells, and monocytes. The data represent the mean cell density/mm2 ± STD. Significance (*, P<0.05; **, P<0.001) was determined with 1-way ANOVA. See Tables S2–S6 for details. B) Compared to mice with ECM (PbA), significantly less CD8+ T cells are recruited to postcapillary venules from mice with hyperparasitemia (PyXL). Treatment with FTY720 reduces the density of CD8+ T cells to levels similar to those found in PyXL infected mice. No arrested CD8+ T cells were found in the cortical microvasculature from uninfected mice. Vascular leakage was observed in mice with ECM (day 6–8; 76 measurements from 5 mice), but not in FTY720-treated PbA-infected mice (day 8; 19 measurements from 4 mice), PyXL infected mice with hyperparasitemia (day 5; 20 measurements from 6 mice), or uninfected control mice (>10 mice). C) Leukocytes were isolated from the brains of PbA-infected (day 6–8), PbA-infected/FTY720-treated (day 9), or PyXL-infected mice (day 5) and analyzed by flow cytometry. Most of the ECM-associated leukocytes are F4/80+ macrophages followed by Ly-6C+ monocytes, Ly-6G+ neutrophils, CD8+ T cells, and CD4+ T cells. Significance (*, P<0.05; **, P<0.01) was determined with 1-way ANOVA followed by Tukey's test for multiple comparisons. See Table S7 for details. D) FTY720 treatment of PbA-infected mice reduces the number of CD45hi ( = blood-derived) macrophages significantly to a level similar to that found in PyXL-infected mice with hyperparasitemia. No significant differences were found for CD45lo ( = parenchymal) macrophages. See Table S7 for details. CD8+ and CD4+ T cells
On the day of neurological signs, the density of CD8+ T cells arrested in postcapillary venules from PbA-infected mice was 292.1±58.9 cells/mm2 (Figure 3A, Table S2). While 92.7% of these T cells were intravascular, 7.3% had extravasated into the perivascular space. No CD8+ T cells were found on the day prior to ECM or in mice that survived the critical period of ECM development. Interestingly, CD8+ T cells were also found in postcapillary venules from PyXL-infected mice with hyperparasitemia and severe anemia. However, with 61.0±8.1 cells/mm2, the density was significantly lower (t-test: T(4) = 8.69; P<0.01) and no T cells were found in the perivascular space of these mice. CD8+ T cells were absent from the cortical microvasculature of uninfected control mice. Examination of the cellular dynamics by IVM revealed that CD8+ T cells crawled along postcapillary venule endothelia at similar velocities during both ECM and hyperparasitemia (2.98±2.28 µm/min and 3.03±1.62 µm/min, respectively) (Figure S3, Videos S6 and S7). No extravascular CD8+ T cells were observed during hyperparasitemia. In agreement with previous reports, no differences were found in CD4+ T cells and densities between PbA and PyXL infections [26] (Figure 3, Table S3, Video S8 and S9). Together, these findings suggest that neurological signs and CD8+ T cell recruitment are correlated and occur rapidly.
Neutrophils
Significantly higher numbers of neutrophils were found in PCV and whole brain during ECM compared to HP. The density of GR-1 (Ly6G) positive neutrophils in postcapillary venules from PbA-infected mice with ECM on day 6–8 was 467.6±137.6 cells/mm2. No neutrophils were detected prior to ECM or in mice that survived the critical period without ECM development (Figure 3A, Table S4). PyXL-infected mice also exhibited neutrophils in postcapillary venules, albeit at the significantly lower density of 276.3±65.9 cells/mm2 at the time of hyperparasitemia (t-test: T(5) = 2.80; P<0.05). Uninfected control mice did not exhibit arrested neutrophils. GR-1+ neutrophils did not extravasate into the perivascular space, neither during ECM nor during hyperparasitemia (Videos S10 and S11). Further, FTY720 treatment of PbA-infected mice reduced the number of neutrophils significantly so that levels similar to those found during HP were reached (Figure 3C, Table S7), which supports the previously suggested involvement of neutrophils in the ECM-associated vasculopathy and edema formation [26].
Monocytes and macrophages
Monocytes were present at similar densities in mice with ECM and HP (Figure 3, Table S5), which is consistent with earlier reports showing that monocytes are not involved in iRBC accumulation in the brain at the time of ECM [26]. The density of CD11b+ macrophages, however, was significantly higher during ECM (484.1±13.1 cells/mm2) compared to during hyperparasitemia (110.2±17.4 cells/mm2) (t-test: T(2) = 16.56; P<0.01; Figure 3A, Table S6). In contrast to monocytes, which were exclusively found to be intravascular (Figure 2, Videos S12 and S13), CD11b+ macrophages were occasionally observed in the perivascular space (Figure 2, Videos S14 and S15). While macrophages could be tissue or blood derived, intravenous labeling suggests that the fluorescent extravascular cells are of the hematopoietic origin. However, we cannot exclude that the cells represent resident perivascular macrophages that were labeled by fluorescent marker leaking into the perivascular space.
Role of S1P receptors
We reported that the immunomodulatory drug FTY720 reduces the overall number of arrested leukocytes in the cortical microvasculature of PbA-infected mice [21], [39]. To determine the effect of FTY720 on CD8+ T cell arrest, mice were treated starting on day 1 prior to infection and on days 1, 3, and 5 after infection. FTY720 treatment allowed 73% of the mice to survive the critical period of ECM development (day 6–8) without exhibiting neurological signs until they were analyzed on day 9. Only ECM-negative mice were examined. While postcapillary venules from untreated mice contained 292.1±58.9 CD8+ T cells/mm2 at the time of neurological signs (Table S2), FTY720 treatment significantly reduced the cell density to 81.9±25.9 cells/mm2 (ANOVA; F(2) = 59.41; P<0.001) (Figure 3B, Table S2). Despite the presence of these CD8+ T cells in cortical postcapillary venules, FTY720 treated mice failed to develop ECM. Interestingly, PyXL-infected mice with hyperparasitemia exhibited a similar density of CD8+ T cells, 61.0±8.1 cells/mm2 (Table S2), within postcapillary venules (Tukey's test: T = 1.59; ns). No CD8+ T cells were detected in the cortical microvasculature of uninfected control mice. Of note, neither the CD8+ T cells remaining after FTY720 treatment of PbA-infected mice nor those found in PyXL-infected mice with hyperparasitemia had any effect on the integrity of the BBB (Figure 3B). Thus, it appears that FTY720 treatment prevented a subpopulation of CD8+ T cells from traveling to the brain and that this coincided with the absence of vascular leakage and neurological signs.
Leukocyte accumulation in the entire brain
To elucidate the composition of specific cellular subtypes involved in pathology we quantified leukocytes by flow cytometry in perfused whole brains. In contrast to IVM, no significant difference in CD8+ T cell recruitment was observed between ECM and HP suggesting that flow cytometry may lack the sensitivity to distinguish important focal variations in cellular composition as arrested leukocytes were not observed in other vessels such as capillaries or arterioles.
Overall, significantly more CD45+ leukocytes were found in the brains during ECM compared to hyperparasitemia (23729.3±7573.8 vs. 4483.0±2971.6; ANOVA: F(2) = 12.42; P<0.001, Tukey's test: T = −4.49; P<0.05) and PbA/FTY720 mice (6059.7±4070.7; Tukey's test: T = −4.12; P<0.05) (Figure 3C, Table S7). Confirming the IVM data, ECM was associated with the recruitment of significantly higher numbers of Ly6G+ neutrophils than in hyperparasitemia (1470.7±325.5 vs. 518.0±317.2; ANOVA: F(2) = 9.33; P<0.05), while there was no significant difference in the number of Ly6C+ monocytes.
The largest increase in cell numbers was observed for F4/80+ macrophages during ECM compared to hyperparasitemia (9648.0±3432.1 vs. 938.0±645.9; ANOVA: F(2) = 14.35; P<0.01). Equivalent results were obtained for CD11b+ macrophages (ANOVA: F(2) = 6.50; P<0.01). Because total brain leukocytes necessarily contain a large proportion of parenchymal macrophages, we distinguished these from blood-derived macrophages by their low level of CD45 expression [36], [56]. When the CD45lo parenchymal macrophages (microglia) were excluded, the number of the remaining mostly intravascular CD45hi F4/80+ macrophages was significantly higher during ECM compared to PyXL-infected mice (1750.0±285.0 vs. 243.3±171.5; ANOVA: F(2) = 30.19; P<0.01) (Figure 3D).
FTY720 treatment of PbA-infected mice significantly reduced the number of Ly-6G+ neutrophils (Tukey's Test: T = −3.69; P<0.05), and both total and CD45hi F4/80+ macrophages (Tukey's test: T = −4.31; P<0.05) in PbA-infected mice so that no significant difference was found for any of the cell types between PbA/FTY720 mice on day 9 and PyXL-infected mice with hyperparasitemia on day 5 (Figure 3B-D, Table S7). Equivalent results were obtained for CD11b+ macrophages (Table S7). Because neither FTY720-treated PbA-infected mice nor PyXL-infected mice with hyperparasitemia exhibit vascular leakage or neurological signs, it appears that FTY720 prevents BBB opening and the associated leukocyte recruitment, although we cannot exclude that FTY720 affects the brain directly.
ECM-associated changes in leukocyte phenotype
Flow cytometry revealed two distinct leukocyte subsets, namely CD45hi and CD45lo (Figure 4A), both of which were significantly more numerous in the brains of PbA-infected mice (ANOVA: F(2) = 10.38; P<0.05 and ANOVA: F(2) = 27.21; P<0.01, respectively) compared to PyXL-infected mice (Tukey's: T = −4.45; P<0.05 and Tukey's test: T = −7.37; P<0.01, respectively) (Table S8). FTY720 treatment significantly reduced the number of both CD45hi (Tukey's test: T = −3.08; P<0.05) and CD45lo (Tukey's test: T = −3.99; P<0.05) leukocytes. While the number of CD45hi cells after FTY720 treatment was not statistically different from PyXL infection, the number of CD45lo cells, although significantly decreased compared to PbA infection, remained significantly higher compared to PyXL-infected mice with hyperparasitemia (Tukey's test: T = −3.38; P<0.05) (Table S8). Significantly more ICAM-1+ (Table S9) and CD69+ leukocytes (Table S10) were present in the CD45hi and the CD45lo leukocyte subsets from PbA-infected mice compared to PyXL-infected mice (Figure 4B–D). FTY720 treatment significantly reduced the number of ICAM-1+, CD69+, and GrB+ leukocytes compared to PbA-infected mice with ECM (Table S9, S10, and S11). Further, the CD45hi subset from PbA-infected mouse brains contained consistently higher numbers of ICAM-1+, CD69+, and GrB+ CD8+ T cells compared to PbA/FTY720 mice, although this difference was not statistically significant (Figure S4A, Table S12). Furthermore, the median expression levels of ICAM-1, CD69 and GrB in these CD45hi CD8+ T cells were similar amongst PbA-infected, PbA/FTY720, and PyXL-infected mice (Figure S4B-D). Likely, the ECM-associated vasculopathy is caused by the high density of activated leukocytes in the postcapillary venules.
Fig. 4. ECM is associated with brain recruitment of CD45+ leukocytes. 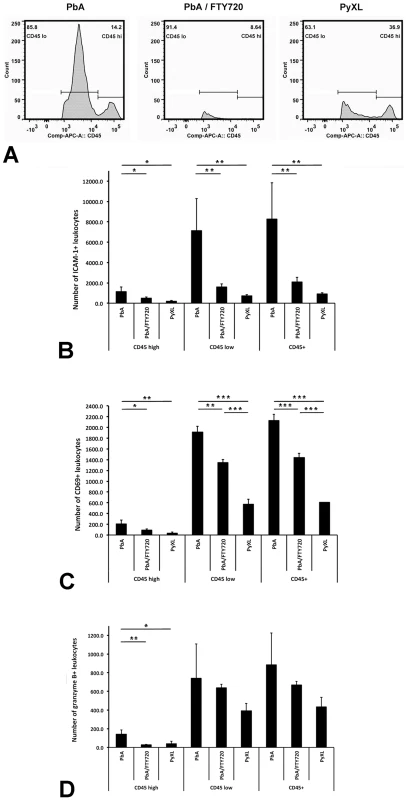
A) PbA-infected mice exhibit significantly more CD45hi and CD45lo leukocytes in the brain compared to PyXL-infected mice. FTY720 treatment of PbA-infected mice reduces the number of CD45hi and CD45lo leukocytes to levels below those observed in PyXL-infected mice. ECM is associated with larger numbers of both CD45hi and CD45lo ICAM-1+ (B), CD69+ (C), and GrB+ (D) leukocytes compared to PyXL-infected mice. FTY720 treatment reduces the number of these leukocytes significantly, but not to the level found in PyXL-infected mice. Effect of FTY720 treatment reduces the number of GrB+ CD45hi leukocytes. The data are based on groups of at least 3 mice per experimental condition. Significance (*, P<0.05; **, P<0.01) was determined with 1-way ANOVA followed by Tukey's test for multiple comparisons. See Tables S8–S11 for details. Endothelial activation and expression of adhesion molecules
CD14 and PECAM-1 expression
Next, we explored molecular interactions that could potentially mediate leukocyte arrest to the microvascular endothelium. Various fluorescent markers were intravenously inoculated, which results in immunolabeling of molecules expressed on or bound to the luminal endothelial surface, while intracellular and extravascular binding sites remain undetected. Previous work indicated that postcapillary venules differ from similarly sized arterioles by differential expression of CD14 and CD31 [21]. PECAM-1 (CD31), generally considered a universal marker of vascular endothelia, was predominantly expressed in arterioles and somewhat less in capillaries. While PECAM-1 was constitutively low in postcapillary venules from infected and uninfected mice, independently of the experimental conditions, CD14 appeared at the time of ECM (day 6–8) on the endothelial surface of postcapillary venules, while capillaries and arterioles were negative [21]. Here we show that surprisingly, CD14 expression in postcapillary venules was not abrogated by FTY720 treatment of PbA-infected mice (Figure S5, Videos S12 and S16). In control mice, i.e. PyXL-infected mice with hyperparasitemia (day 5) and uninfected mice, the entire microvasculature including postcapillary venules was consistently CD14-negative (Figure S5, Video S13).
ICAM-1 expression on endothelia
Endothelial ICAM-1 plays a role in in leukocyte traversal across the BBB [57] and has been implicated in the pathogenesis of both HCM and ECM [58]–[62]. Intravenous labeling revealed that ICAM-1 was significantly upregulated (GLM: F(3) = 154.14; P<0.001) in postcapillary venules from PbA-infected mice with ECM (Figure 5A and 5E, Table S13, Video S17) compared to uninfected control mice (Tukey's Test: T = 19.97; P<0.001) (Figure 5C and 5E, Table S12). Interestingly, ICAM-1 upregulation coincided with a labeling pattern that outlined the endothelial junctions. In agreement with earlier reports [63]–[65], ICAM-1 was also significantly upregulated in postcapillary venules from PyXL-infected mice with hyperparasitemia (Figure 5B and 5E, Video S18) compared to uninfected control mice (Tukey's Test: T = 18.79, P<0.001; Figure 5C, Video S19). No difference in endothelial ICAM-1 expression was found between PbA-infected mice with ECM and PyXL-infected mice with hyperparasitemia (Tukey's Test: T = −2.05; P = 0.324; Table S13), which is in agreement with the recruitment of leukocytes to postcapillary venules from both mice with ECM and hyperparasitemia [21]. Surprisingly, however, ICAM-1 was also significantly upregulated in arterioles, both in mice with ECM (Tukey's Test: T = 8.28; P<0.001) and hyperparasitemia (Tukey's Test: T = 4.73; P<0.001) compared to uninfected controls (Figure 5E, Table S13), although leukocyte arrest was not observed in this section of the microvascular tree. Further, ICAM-1 was also expressed in postcapillary venules from PbA-infected mice that had been treated with FTY720 (Figure 5D). Taken together, these findings do not support a role for endothelial ICAM-1 in ECM pathogenesis.
Fig. 5. Both ECM and hyperparasitemia are associated with upregulation of endothelial ICAM-1. 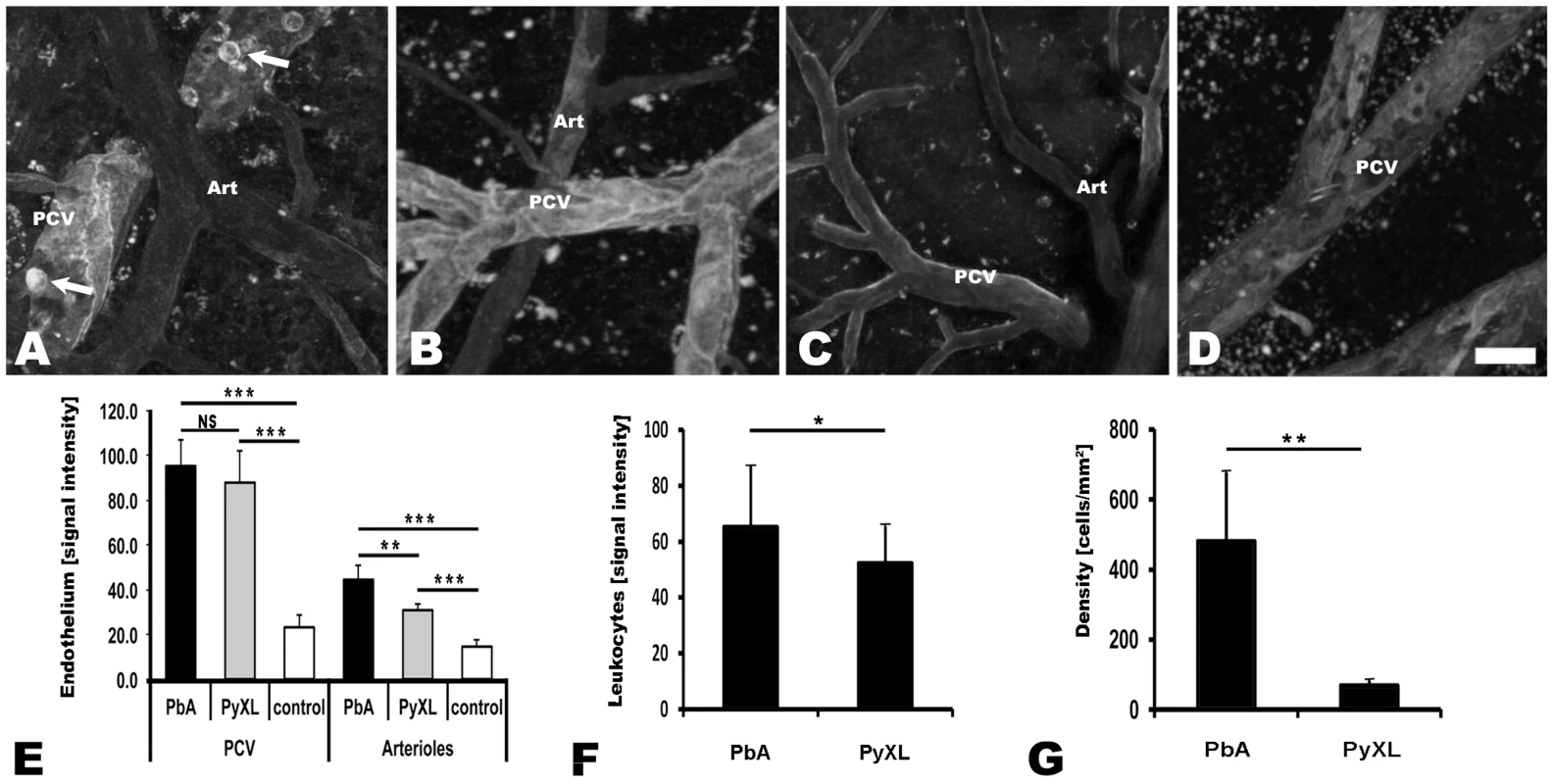
CBA/CaJ mice were infected with PbA, PyXL, or no parasites (control) and inoculated with PE-conjugated anti-ICAM-1 and Evans blue. A–D) Maximum projections of representative intravital microscopy movies showing endothelial ICAM-1 expression in postcapillary venules, and less so in arterioles (Art), in mice infected with PbA and PyXL compared to uninfected control mice. Note the pronounced ICAM-1 label along the endothelial junctions. A) PbA-infected mouse with ECM (day 6), B) PyXL-infected mouse with hyperparasitemia (day 5), C) uninfected control mouse. Note that ECM, but not hyperparasitemia, is associated with the expression of ICAM-1 on the surface of arrested leukocytes (light open circles, arrows). D) Endothelial ICAM-1 is upregulated, while ICAM-1 expressing leukocytes are absent, after FTY720 treatment of PbA-infected mice (day 9, no neurological signs). Scale bar = 20 µm. E) The endothelial ICAM-1 signal is similarly increased in cortical postcapillary venules from mice infected with PbA and PyXL compared to uninfected control mice. ICAM-1 is significantly upregulated in cortical arterioles during both ECM and hyperparasitemia compared to uninfected control mice. Compared to postcapillary venules, however, the overall level of ICAM-1 expression in arterioles is significantly lower. F) ICAM-1 fluorescence emission of individual leukocytes (N = 6) is higher during ECM compared to hyperparasitemia. G) The density of ICAM-1 expressing leukocytes in postcapillary venules from mice with ECM is significantly higher compared to mice with hyperparasitemia. Significance (*, P<0.05; **, P<0.01) was determined with 1-way ANOVA followed by Tukey's test for multiple comparisons. See Table S13 for details and Videos S17–S19 for the corresponding dynamic data. ICAM-1 expression on leukocytes
In contrast, IVM showed that ECM correlates with ICAM-1 upregulation on leukocytes (Figure 5A). The level of ICAM-1 expression per leukocyte was significantly higher (t-test: T(10) = 2.58; P<0.05) during ECM (69.2±19.7; day 6–8, 6 mice) compared to hyperparasitemia (48.0±8.9; day 5, 6 mice) (Figure 5F). More strikingly, the density of ICAM-1 positive leukocytes in postcapillary venules was significantly higher (t-test: T(8) = 4.30, P<0.01) during ECM compared to hyperparasitemia (Figure 5G). Thus, ECM is associated with the arrest of large numbers of ICAM-1+ leukocytes, consistent with a recent report on the contribution of leukocyte ICAM-1 to ECM development [66].
Flow cytometry identified the majority of these ICAM-1+ leukocytes as macrophages (Figure 6A). Macrophages constituted 65.5% and 54.5% of the ICAM-1+ leukocytes in the PbA and the PbA/FTY720 groups, respectively, but only 32.1% in the PyXL mice. Significantly higher numbers of ICAM-1+ CD45+ macrophages were present during ECM (ANOVA: F(2) = 13.31; P<0.01) compared to PbA/FTY720 mice (9306.0±3552.2 vs. 1834.3±1414.9; Tukey's test: T = −4.76, P<0.01) (Figure 6B, Table S14). Interestingly, FTY720 treatment of PbA-infected mice reduced the number of ICAM-1+ F4/80+ macrophages to levels comparable to those found in PyXL-infected mice (656.7±504.1; Tukey's test: T = −0.65, P = 0.8; Figure 6B, Table S14). Similarly, there was a significant difference in the CD45hi subset of ICAM-1+ F4/80+ macrophages (ANOVA: F(2) = 30.10; P<0.01) between PbA-infected and PbA/FTY720 mice (1713.3±285.3 vs. 365.0±298.2; Tukey's test: T = −6.45; P<0.01; Table S14), i.e. the subset that is comprised mostly of blood-derived macrophages [36], [56]. FTY720 treatment of PbA-infected mice reduced the number of ICAM-1+ F4/80+ macrophages in the CD45hi subset to levels similar to those found in PyXL-infected mice (219.0±161.6) so that again, similar numbers were found in the PbA/FTY720 and PyXL-infected groups (Tukey's test: T = −0.70, P = 0.773; Table S14). Equivalent results were obtained for CD11b+ macrophages (Table S7). Thus, ECM is associated with 1) the recruitment of large numbers of blood-derived ICAM-1+ macrophages to postcapillary venules and 2) a dramatic increase in the number of ICAM-1+ cells in the parenchyma of the brain, most likely microglia [36], [56]. As FTY720 treatment prevented this increase in ICAM-1+ macrophages, these data support the idea that the ECM-associated edema has both a vasogenic and a cytotoxic component [17], [36].
Fig. 6. ECM is associated with the recruitment of ICAM-1+ macrophages. 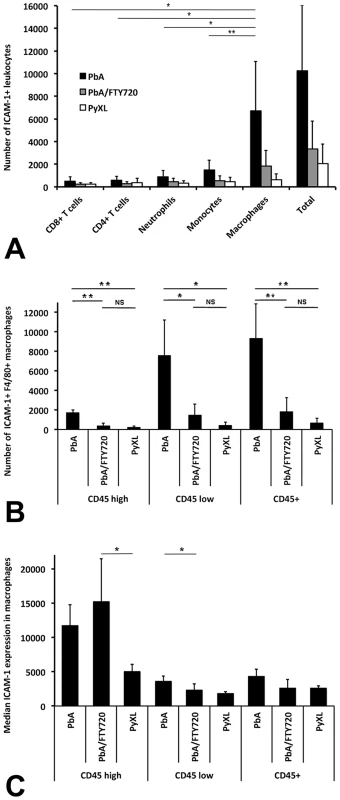
Leukocytes were isolated from PbA-infected, PbA-infected/FTY720-treated, or PyXL-infected mice and subjected to flow cytometric analysis. A) The vast majority of ICAM-1+ leukocytes are macrophages, which were identified with mAb F4/80. B) FTY720 treatment reduces the number of ICAM-1 macrophages to levels similar to those found in PyXL-infected mice. C) No significant difference was found for the median ICAM-1 expression levels in macrophages from PbA-infected versus PbA-infected/FTY720-treated mice. Significance (*, P<0.05; **, P<0.01) was determined with 1-way ANOVA followed by Tukey's test for multiple comparisons. See Table S14 and S15 for details. In agreement with the reported upregulation of ICAM-1 on macrophages under various other inflammatory conditions [67]–[70], we found higher, albeit statistically not significantly different, levels of ICAM-1 expression on F4/80+ macrophages in PbA-infected mice compared to PyXL-infected mice (4332.6±1007.0 vs. 2560.0±357.7; ANOVA: F(2) = 4.47; P = 0.05) (Figure 6C, Table S15). Interestingly, FTY720 treatment of PbA-infected mice reduced the level of ICAM-1 expression in F4/80+ macrophages (2606.3±1258.1) resulting in levels similar to those found in PyXL-infected mice (2560.0±357.7). ICAM-1 expression on F4/80+ CD45lo macrophages was significantly higher in ECM compared to hyperparasitemia (ANOVA: F(2) = 5.57; P<0.05), but no difference in expression level was found between PbA/FTY720 with either ECM or hyperparasitemia infections (Tukey's test: T = −2.35.24, P = 0.122 and T = −0.79, P = 0.720, respectively). While ICAM-1 expression on F4/80+ CD45hi macrophages was similar between ECM and hyperparasitemia, a significant difference was observed was between PbA/FTY720 and PyXL-infected mice (ANOVA: F(2) = 5.40; P<0.05). Thus, ECM is associated with a greater number of ICAM-1+ macrophages (Figure 6B) and FTY720 treatment prevented this effect. It appears, therefore, that ICAM-1+ macrophages, not endothelia (Figure 5), are involved in the ECM-associated vasculopathy.
ECM correlates with platelet arrest and P-selectin deposition in postcapillary venules
P-selectin release from platelet α-granules or endothelial Weibel-Palade bodies promotes the binding of platelets, leukocytes, and plasma proteins to the vascular wall [71]–[73]. Because both platelet marginalization and P-selectin expression have been implicated in the pathogenesis of both HCM and ECM [18], [20]–[22], [74]–[78], we determined the distribution of this adhesion molecule with respect to arrested platelets in the cortical microvasculature. Upon manifestation of neurological signs, PbA-infected CBA/CaJ mice were inoculated with a PE-conjugated mAb against P-selectin (CD62P), eFluor 450-conjugated anti-CD41 to detect platelets and Evans blue to visualize the vascular lumen [21]. IVM revealed small clusters of marginalized platelets that colocalized with patches of P-selectin on cortical postcapillary venule endothelia (Figure 7, Video S20). Occasionally, we observed strings of platelets that appeared to be attached to clusters of platelets (Video S21) as has been suggested to occur in HCM based on in vitro experiments [79]. In contrast, PyXL-infected mice with hyperparasitemia showed no evidence for P-selectin expression or platelet arrest (Figure 7, Video S22). Unlike postcapillary venules, arterioles were consistently negative for P-selectin or arrested platelets, both during ECM and hyperparasitemia. Thus, ECM, but not hyperparasitemia, is associated with marginalization of small numbers of platelets along postcapillary venule endothelia and P-selectin release, either from platelets or endothelia. However, the highly focal nature of both platelet arrest and P-selectin release contrasts with the uniform endothelial activation as evidenced by CD14 expression, ICAM-1 upregulation, and vascular leakage observed during ECM. Thus, leukocyte arrest is not limited to the P-selectin positive portions of the postcapillary venule endothelia.
Fig. 7. ECM correlates with the arrest of P-selectin expressing platelets in postcapillary venules. 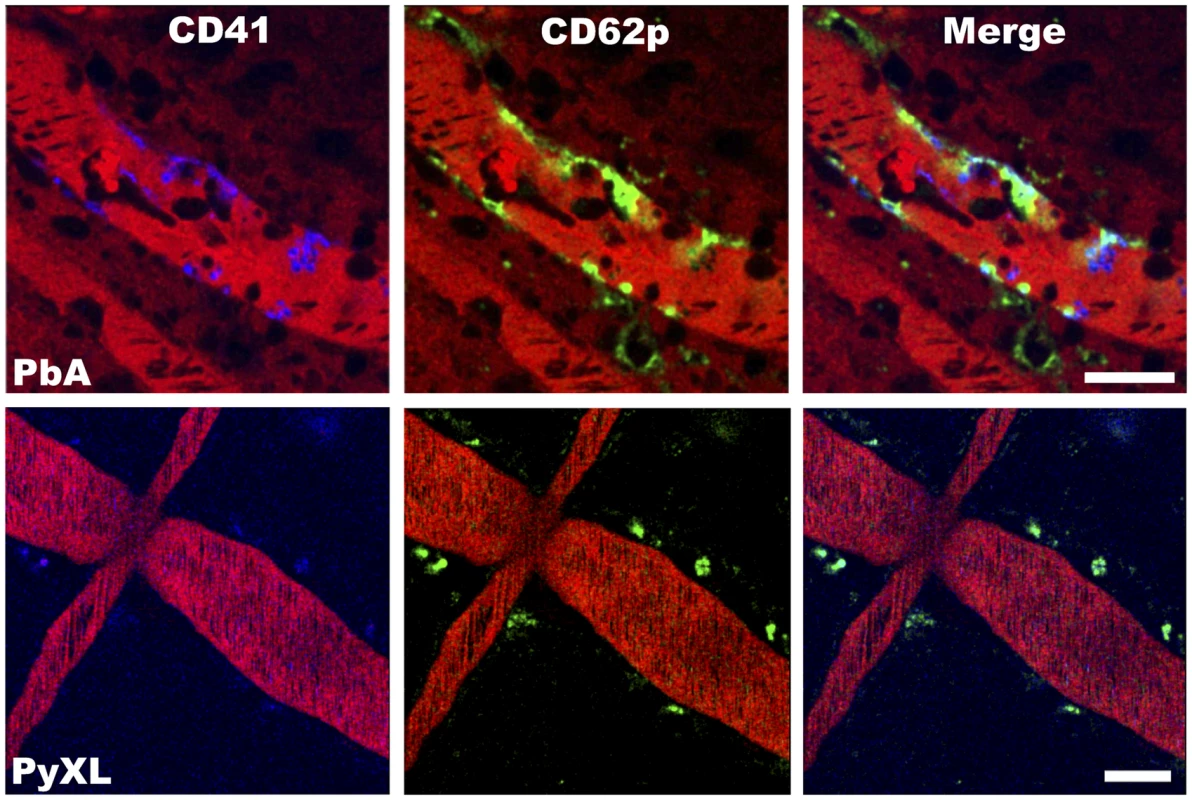
PbA-infected mice exhibit small clusters of CD41+ platelets (blue) and patches of P-selectin (green) along the wall of postcapillary venules at the time of ECM. Platelets remained in circulation and P-selectin was not detected in postcapillary venules from PyXL-infected mice with hyperparasitemia. The vascular lumen is visualized with Evans blue (bright red). Note that Evans blue has leaked into the brain parenchyma of the PbA-infected mouse (dark red shade on either side of the postcapillary venule). Scale bars = 50 µm. See Video S20 and 22 for the corresponding dynamic data. Endothelial junction protein expression is unaltered during ECM
FTY720 was previously shown to prevent vascular leakage, neurological signs, and death from ECM [21], [39]. To evaluate whether FTY720 protects the BBB by preserving the integrity of endothelial junctions [48], [80], [81], we determined the expression level of the tight junction (TJ) proteins claudin-5, occludin, and ZO-1 in the cerebral cortex and the cerebellum of 4 PbA-infected mice with ECM (day 6–8), 3 FTY720-treated PbA-infected mice that did not exhibit any neurological signs (day 8 or 9), and 3 PyXL-infected mice with hyperparasitemia (day 5) (Figure S6 and S7). Quantification of the fluorescence emission of specific antibodies on 3–4 immunolabeled cryostat sections per experimental condition yielded no significant reduction in protein expression under the different infection and treatment conditions compared to 3 uninfected control mice (Table S16). This finding suggests that the TJs remained morphologically intact and supports the hypothesis that the ECM-associated vascular leakage is based on a regulated, potentially reversible, mechanism of BBB opening [21], [82].
Thus, comparison of two Plasmodium infection models revealed: 1) The venous blood flow impairment during ECM is caused by the arrest of significantly higher numbers of CD8+ T cells, neutrophils, and in particular macrophages in cortical postcapillary venules compared to hyperparasitemia. While a small number of CD8+ T cells and macrophages extravasated into the perivascular space, most of the recruited leukocytes remained intravascular. 2) FTY720 treatment of PbA-infected mice reduced, but did not completely prevent leukocyte accumulation in postcapillary venules, which is consistent with the finding that low numbers of arrested leukocytes are present in PyXL-infected mice with hyperparasitemia without causing vascular leakage or neurological signs. 3) ECM closely correlates with the recruitment of large numbers of ICAM-1 expressing F4/80+ macrophages to the brain. As FTY720 treatment did not reduce the ICAM-1 expression level, the high density of these macrophages in postcapillary venules likely enhances the ECM-associated vascular pathology. 4) Leukocyte recruitment coincides with the onset of neurological signs, but follows BBB opening, as vascular leakage can be observed 1 day prior to symptomatic ECM [21].
Discussion
In this study, we identify a novel key determinant of ECM pathogenesis, namely that leukocyte arrest along the wall of postcapillary venules causes microrheological alterations that severely impair the venous blood flow. Based on our findings, we hypothesize that infection with PbA opens the BBB, which leads to the recruitment of numerous activated CD8+ T cells, ICAM-1+ macrophages, and neutrophils (Figure 8). The resulting steric hindrance of the blood flow in postcapillary and larger venules impairs, but does not block, the venous efflux from the brain, which exacerbates the vasogenic edema and causes death as a consequence of intracranial hypertension.
Fig. 8. Model for the ECM-associated venous blood flow restriction. 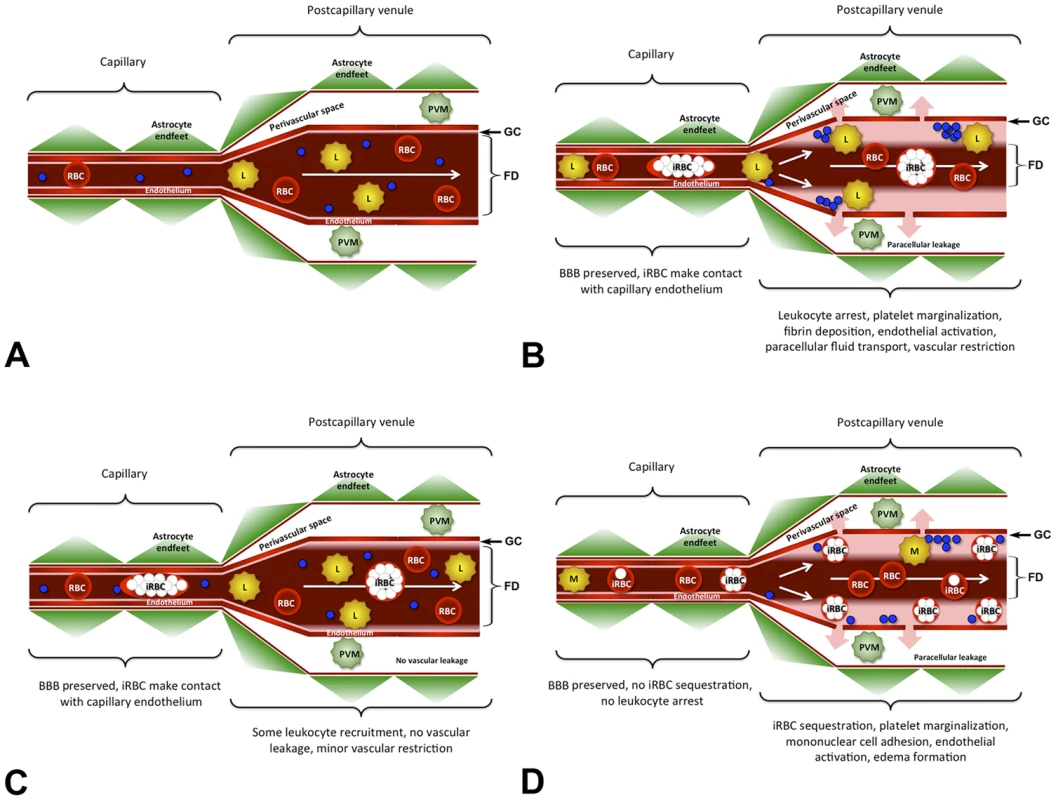
A) Under normal conditions, blood passes through the cerebral microvasculature without leukocytes arresting in postcapillary venules. Except for the narrow glycocalix (GC) lining the vascular endothelium, the entire vascular diameter is used for the bloodstream, the functional diameter (FD) is not restricted, and the BBB is intact. B) Infection with PbA causes endothelial junction opening at the BBB in the absence of junction protein degradation or endothelial death. During ECM, arrested leukocytes form steric obstacles that reduce the functional diameter (FD) of postcapillary venules resulting in a severe restriction of the venous blood flow. As a consequence, the paracellular leakage of plasma into the PVS (pink arrows) is exacerbated. Like uninfected RBC, iRBC travel with the bloodstream and do not arrest. C) During hyperparasitemia, significantly fewer leukocytes and no platelets adhere to the postcapillary venule endothelium compared to ECM. Consequently, the restriction in the venous blood flow is minor and there is no vascular leakage. D) Hypothetical model for pediatric P. falciparum HCM. While leukocytes, RBC, and all iRBC pass through capillaries without adhering to the endothelium, late-stage iRBC, mononuclear cells, and platelets sequester on the wall of postcapillary venules thus restricting the venous blood flow and exacerbating the leakage of plasma into the perivascular space. Because of the significant reduction in the functional diameter (FD) of the postcapillary venule, ring-stage iRBC, uninfected RBC, and most leukocytes must flow through the center of the vessel. The arterial blood flow remained unaffected under all experimental conditions. PVM = perivascular macrophage, iRBC = infected red blood cell, L = leukocyte, M = mononuclear cell, blue circles = platelets. Under physiological conditions, the luminal surface of vascular endothelia is covered with a glycocalix, a 0.5 to >1 µm layer of membrane-bound proteoglycans and glycoproteins that repels RBCs and is critically involved in inflammatory responses, blood coagulation, and blood flow regulation [83]–[86]. IVM visualizes this glycocalix as a thin red layer, covering arteriolar endothelia from infected and uninfected mice and postcapillary venule endothelia from uninfected control mice. During ECM, the thickness of the RBC-free layer in postcapillary and larger venules was drastically increased. Because the glycocalix typically degrades under inflammatory conditions, leading to exposure of adhesion molecules, leukocyte adhesion, and impairment of endothelial barrier function [85], [86], the restriction in the venous blood flow during ECM is likely not caused by components of the glycocalix, but by increasing numbers of arrested leukocytes that prevent RBC from approaching the endothelium. Although the functional cross-section of postcapillary venules was occasionally reduced by more than 80%, complete vascular obstruction was not observed. These findings argue in favor of a combined vascular sequestration and immuno-pathological etiology of ECM [87]–[89].
The reduction in the venous blood flow must be expected to have major consequences for the physiology of the brain. First, the overall hypoperfusion of the brain, enhanced by inadequate contact between RBCs and the endothelium, likely contributes to the drastically reduced O2 delivery to the cerebral parenchyma observed in ECM-susceptible C57BL/6 mice [90]. In addition, by increasing the wall shear stress, leukocyte adhesion is expected to reduce the blood volume flow in postcapillary venules dramatically [91]. Finally, a reduction of the venous efflux from the skull, caused by a generalized narrowing of the lumen of venous microvessels, necessarily increases the intracranial pressure. The finding that brains from mice with ECM, but not hyperparasitemia, are swollen and spongy and bulge out of the skull, if the Dura mater is accidentally damaged during craniotomy clearly documents the dramatically increased intracranial pressure during the agonal phase of the disease. The reduced venous efflux from the brain may exacerbate vascular leakage, brain edema, and hemorrhages - cerebral alterations that are also associated with HCM [92]. Brain swelling and edema is extremely common in adult HCM on CT scan [93], [94]. Increased intracranial pressure has long been associated with poor prognosis and neurological sequelae in severe pediatric HCM [50], [95]–[98]. In fact, recent longitudinal MRI observations in Malawian children have identified intracranial hypertension as the single most important MRI finding associated with HCM development and the most reliable predictor of death [54], [99].
PbA-infected mice also exhibit arteriolar vasospasms during the final stage of ECM [100] and it has been suggested that the reduction in the cerebral blood flow observed by MRI [101] is due to increased production of vasoconstrictive factors or inhibition of vasodilating mediators [102]. Subsequent work [103], [104] revealed that endothelin-1 (ET-1), a potent vasoactive peptide with inflammatory and platelet-activating properties [105]–[108], is upregulated during both ECM and HCM [109]–[112]. Indeed, the arteriolar vasoconstrictive effect of ET-1 could be responsible for ECM induction in the PbA-infected C57BL/6 mouse model [108], because injection of exogenous ET-1 induces neurological signs in PbNK65-infected mice, which normally do not develop ECM [113], and because blockage of the ET-1 receptor A prevents ECM development in PbA-infected mice [110]. However, ET-1 has a plasma half-life of well under one minute in rodents [114] so that vasoconstriction alone cannot explain the increased intracranial pressure observed during ECM. Because ET-1 also stimulates endothelial activation with upregulation of adhesion molecules, promotes leukocyte adhesion, and increases vascular permeability [105], [106], there is a possibility that ET-1 induces ECM by restricting the venous blood flow. Similarly, administration of nitric oxide (NO), a key messenger involved in regulation of platelet adhesion and inflammatory and immune responses [115], decreased both leukocyte accumulation and vascular resistance in larger venules of PbA-infected mice [78], [116]–[121]. We conclude that the ultimate cause of death from ECM is a combination of arteriolar vasoconstriction and severe reduction in the blood efflux from the brain due to leukocyte adhesion in the venous microvasculature.
Compared to PyXL-infected mice with hyperparasitemia, PbA infection triggered the recruitment of significantly more CD8+ T cells to postcapillary venules at the time of, but not prior to, ECM development. Together with the finding that CD8+ T cells were absent in mice that survived the critical time for ECM development, these data suggest that neurological signs and T cell recruitment are correlated and occur rapidly. CD8+ T cells from C57BL/6 mice immunized with PyXNL can confer protection against lethal PyXL infection [122], whereas CD8+ T cell accumulation in the brain of PbA-infected C57BL/6 mice was abolished and the mice were completely protected from ECM when co-infected with P. yoelii [123], [124]. However, similar numbers of CD8+ T cells accumulated in the brains of PbA-infected C57BL/6 mice with ECM and PbNK65-infected mice without neurological signs [34], further supporting the notion that ECM-eliciting parasites such as PbA induce the recruitment of a qualitatively different CD8+ T cell population to the brain.
FTY720 treatment decreased the number of CD8+ T cells to levels similar to those found in PyXL-infected mice. Despite the presence of the remaining CD8+ T cells, neither FTY720-treated PbA-infected mice nor PyXL-infected mice developed neurological signs. A small percentage of CD8+ T cells entered the perivascular space during ECM, but not hyperparasitemia. This could be explained by upregulation of the leukocyte common antigen CD45, because CD45 expression is typically enhanced in response to stress signals, leading to increased leukocyte motility [125] and brain infiltration, for example after seizure [126]. ECM coincided with larger numbers of CD8+ T cells expressing CD69, one of the earliest lymphocyte activation markers [127], [128], and FTY720 treatment reduced the number of CD69+ CD8+ T cells to levels similar to those found during hyperparasitemia. Previous work supports a correlation between ECM and CD69+ CD8+ T cells: 1) Recruitment and activation of CD8+ T cells and CD69 expression were reduced in ECM-resistant mice [129]. 2) Peripheral CD8+ T cells were predominantly CD69+ during ECM and expressed the phenotype of memory T cells [130]. 3) The ECM-associated upregulation of CD69 was reversed and disease was prevented by interference with the angiotensin I pathway [131]. 4) Ghanaian P. falciparum infected pediatric patients with clinical HCM or severe anemia showed similar T cell activation profiles with a significantly increased frequency of CD69+ cells compared to asymptomatic children [132]. The median expression levels per cell of CD69 and GrB did not differ between the experimental groups suggesting that ECM pathogenesis correlates with a high density of CD69+ and GrB+ CD45hi CD8+ T cells in postcapillary venules. FTY720 treatment of PbA-infected mice reduced the number of CD4+ T cells to levels similar to those observed for PyXL-infected mice. Together, these data suggest that FTY720 treatment prevents ECM by inhibiting the trafficking of activated CD8+ and CD4+ T cells to the brain.
FTY720 treatment of PbA-infected mice also reduced the number of macrophages and neutrophils to levels similar to those found in the brains of PyXL-infected mice, supporting the notion that these leukocytes exacerbate edema formation during ECM [26], [27]. Of particular importance, FTY720 treatment prevented the recruitment of large numbers of ICAM-1+ macrophages to the brains of PbA-infected mice. This finding sheds light on an unexpected new role of ICAM-1 in the pathogenesis of ECM. While FTY720 may preserve the integrity of the BBB primarily by preventing leukocyte recruitment to the brain, activated platelets release phosphorylated FTY720 [133], [134], which acts as a full agonist on the endothelial S1P receptor S1PR4; therefore, FTY720 may prevent the ECM-associated vascular leakage by strengthening the endothelial actin cytoskeleton [135], [136]. Further, FTY720 may regulate endothelial barrier function by directly modulating endothelial junction tightness, because the increased vascular leakage observed in mice deficient in plasma S1P can be reversed by restoring plasma S1P levels [47], [48], [80], [81]. This finding may explain why FTY720 administration must be started prior to the onset of vascular leakage [21], [39] and why attempts to rescue mice with symptomatic ECM, when leukocyte recruitment was already in progress, were unsuccessful (A. Movila and U. Frevert, unpublished observations). Further, FTY720 can cross the BBB and may thus be able to directly modulate parenchymal cells by interacting with S1P receptors in the CNS. The resulting feedback from the CNS on the activation status of the BBB may in turn alter the interaction with the immune cells. Future testing of FTY720 or related compounds in the ECM model is expected to reveal more detail on the exact mechanism of BBB opening and vascular inflammation in ECM. The ECM model may also improve our understanding of the pathogenesis of HCM. Ugandan, Malawian, and Central Indian children with HCM exhibit decreased S1P plasma levels compared to those with uncomplicated malaria and a low angiotensin-1 to angiotensin-2 plasma ratio discriminates HCM and severe non-cerebral from uncomplicated malaria and also predicts mortality from HCM [39], [137]–[140]. As these reports strongly suggest the involvement of the S1P pathway HCM, screening for novel immunomodulatory drugs and exploration of their endothelial barrier-promoting effects is warranted.
We observed significantly more neutrophils in postcapillary venules and whole brain during ECM compared to hyperparasitemia. Considering that the role of neutrophils in the pathogenesis of CM is understudied to date, this finding is of particular interest. FTY720 treatment of PbA-infected mice reduced the number of neutrophils significantly so that levels similar to those found during hyperparasitemia were reached, which supports the previously suggested involvement of neutrophils in the ECM-associated vasculopathy and edema formation [26], [141].
The number of arrested monocytes was increased similarly in PbA - and PyXL-infected mice compared to uninfected control mice suggesting that monocyte recruitment correlates with Plasmodium infection in general, not ECM in particular. This finding is in agreement with earlier reports showing that monocytes are not involved in iRBC accumulation in the brain at the time of ECM [26], [27]. Intravenous injection of fluorescent anti-CD14 revealed that monocytes were generally confined to the vascular lumen, although we occasionally detected labeled monocytes in the Virchow-Robin space. Similarly, intravenous injection of the macrophage marker anti-CD11b resulted in labeling of PVM. Because part of the PVM population derives from blood monocytes [53], these fluorescent monocytes may have been labeled before extravasating to replenish the pool of PVM in the perivascular space.
Flow cytometric measurement of leukocyte recruitment to the brain essentially corroborated our IVM findings suggesting that the cortical microvasculature reflects the ECM-associated pathological events in the entire brain. A few conceptual differences between IVM and flow cytometry are noteworthy. First, IVM confirmed the notion that the healthy murine brain has an extremely low level of immune surveillance with the almost complete absence of T cells, neutrophils, monocytes, and B cells [21], [142]. Expression of adhesion molecules is low on the healthy human postcapillary venule endothelium, and upregulation of P-selectin, E-selectin, ICAM-1, and VCAM-1 contributes to the pathogenesis of HCM [53], [58], [143]. Second, IVM provides information on the location of recruited leukocytes and their dynamics with respect to the complex and heterogeneous microvascular network of the brain. Third, intravenous labeling generally visualizes only intravascular leukocytes including those that extravasate between marker injection and imaging. However, the BBB at the level of postcapillary venules is naturally leaky, i.e. it allows passage of macromolecules including immunoglobulins into the Virchow-Robin space [38]. During ECM, this barrier becomes even more permeable, which makes distinction of recently extravasated from resident perivascular cells difficult. While CD8+ T cells do not patrol the cerebral microvasculature of uninfected mice and are therefore unlikely to extravasate into the perivascular space of naïve mice, the fluorescent CD11b+ macrophages we found in the perivascular space of mice with ECM could either have been labeled intravascularly and then extravasated or they could have been labeled after entering the perivascular space due to diffusion of the cell-specific markers across the BBB. Thus, while the majority of leukocytes remain confined to the vascular lumen during ECM, a small number of CD8+ T cells and possibly CD11b+ macrophages extravasate into the perivascular space. Fourth, flow cytometric measurement of the expression level of CD45 allowed distinction between blood-derived and parenchymal macrophages [56]. Using this approach, we found that ECM is associated not only with significantly higher numbers of ICAM-1+ CD45hi ( = blood-derived) macrophages, but also ICAM-1+ CD45lo ( = parenchymal) macrophages compared to mice with hyperparasitemia and FTY720-treated PbA-infected mice. Together with the ECM-associated activation of microglia [144] and the enhanced expression of CCR5 on non-hematopoietic cells [145], ICAM-1 upregulation on both blood-derived and parenchymal macrophages supports the notion of a mixed vasogenic and cytotoxic nature of the edema in ECM [17], [146]. Finally, IVM was critical to visualize endothelial activation markers within defined parts of the microvascular tree. Thus, IVM and flow cytometry provided highly complementary results.
BBB opening in the young CBA/CaJ mice used here begins at least one day prior to the onset of neurological signs, while leukocyte recruitment to the brain occurs only afterwards [21]. In 129,B6 mice, neither antibody-mediated neutrophil depletion nor clodronate-mediated macrophage depletion, done 1 or 2 days prior to ECM development, respectively, prevented neurological signs [26], [145]. While this was interpreted as neutrophils and macrophages not being involved in ECM development, an alternative explanation is that the BBB was already leaky at this late time point and the resulting vasogenic edema had already caused microglial activation. Clodronate liposomes do not cross the BBB [147]–[149] so that the microglia was likely unaffected and the resulting cytotoxic edema not prevented in these studies. Further, depletion of macrophages and granulocytes by administration of AP20187 to MAFIA mice on days 5, 6, and 7 after infection resulted in a >80% reduction of these cells in the blood [27], [150]. Again, as AP20187 does not cross the BBB [151], ECM development likely manifested because the activated microglia was not eliminated. Importantly, CD8+ T cells alone failed to induce any of the typical signs of ECM including convulsions and death without the direct neurotoxic effect of intravenously administered folic acid [26], [145]. Thus, it appears that both hematogenic and parenchymal macrophages play a role of in the pathogenesis of ECM. A more recent study in C57BL/6 mice emphasizes the crucial role of monocytes/macrophages in lymphocyte recruitment to the brain. Clodronate treatment 2 days prior to manifestation of neurological signs reduced the recruitment of CD8+ T cells, CD4+ T cells, and NK cells to the brain 2.8-fold, 1.8-fold, and 4.6-fold, respectively, and failed to alter the course of disease development [152]. Clodronate treatment 2 days prior to infection with PbA, on the other hand, prevented ECM development [152]. Thus, the exact mechanism by which blood-derived macrophages contribute to disease development needs further and more specific investigation. Taken together, these data suggest the following scenario: PbA infection induces BBB opening, which results in vasogenic edema and microglial activation. The ensuing cytotoxic edema then leads to endothelial activation with recruitment of leukocytes that in concert restrict the venous blood flow, which further weakens the already impaired BBB. Eventually, these events culminate in the typical irreversible damage associated with fatal malarial encephalopathy. However, the differential progression of the vasogenic versus the cytotoxic edema and their relative contribution to brainstem compression and death clearly require further experimental attention.
The critical role of ICAM-1 in the pathogenesis of severe P. falciparum malaria and the binding of P. falciparum iRBC to the endothelium is generally accepted [59], [61], [153]–[159]. However, comparison of the endothelial ICAM-1 expression levels in the brains of P. falciparum versus P. vivax infected individuals is necessary to determine whether or not HCM correlates with upregulation of ICAM-1 in postcapillary venules. ICAM-1 upregulation has also been implicated in the pathogenesis of ECM [63], [77], [78], [160]–[162]. While the cellular origin of ICAM-1 was not determined, treatment of PbA-infected mice with NO reduced ICAM-1 upregulation in the brain along with leukocyte sequestration and vascular leakage [78]. We show that upregulation of endothelial ICAM-1 correlates with Plasmodium infection in general, not with ECM in particular. Because ICAM-1 upregulation was not observed in mice deficient in RAG-1 or IFN-γ on day 6 after infection with PbA [163], the Th1 type cytokines IFN-γ and lymphotoxin, in synergy with TNF from macrophages [163], [164], may induce ICAM-1 expression throughout the brain microvasculature of ECM-susceptible mice, both during ECM and hyperparasitemia. Together with the finding that FTY720 treatment of PbA-infected mice does not prevent ICAM-1 upregulation in postcapillary venules, these data argue against a role of this endothelial adhesion molecule in the pathogenesis of ECM.
CD14 appeared on the surface of postcapillary venule endothelia selectively at the time of neurological signs (day 6–8), which is in agreement with the well-known involvement of CD14 in endothelial activation, neuroinflammation, and leukocyte recruitment to the cerebral microvasculature [165]–[168] and the finding that CD14-deficient mice are protected against ECM [169]. Further, because CD14 plays an important role in the non-phlogistic clearance of apoptotic cells [170], its expression on postcapillary venule endothelia may reflect the well-documented increase in apoptosis during ECM [171]–[173]. Interestingly, malaria-associated microparticles carry phosphatidylserine on their surface [174]. Because the plasma levels of endothelial, platelet, and erythrocytic microparticles increase at the onset of the ECM-associated neurological signs [175], CD14 may be involved in the recruitment of microparticles to postcapillary venule endothelia. Platelet-derived microparticles are known to promote macrophage differentiation [176] and may contribute to the observed upregulation of ICAM-1 on the macrophage surface.
We show that ECM occurs without apparent physical degradation of endothelial TJs, measurable loss of claudin-5, occludin, and ZO-1 and in the absence of endothelial death [21]. Together, these findings argue against widespread and irreversible endothelial injury, for example due to cytotoxic T cell-mediated apoptosis or long-lasting vascular occlusion [177], as a major pathogenetic mechanism leading to malarial coma and death [82], [88], [178], [179]. Instead, and in agreement with the observation that ECM-susceptible mice can be rescued by anti-LFA-1 treatment even minutes before imminent death [180], [181], the preserved TJ integrity suggests that BBB opening during ECM is under the control of a regulated mechanism [21], [82], [182]. In support of this notion, fast-acting anti-malarial drugs can prevent ECM one day before the expected onset of neurological signs, although CD8+ T cells still accumulate in the brain [32], [34].
Platelets play a crucial role in the early stages of the pathogenetic cascade of ECM [9], [183], [184]. Blockage of platelet activation or shedding of platelet-derived microparticles confers resistance to ECM [13], [22], [160], [184]. At the time of ECM, platelets arrest focally and in small numbers in the cortical microvasculature [21] where they closely associate with P-selectin positive areas on postcapillary venule endothelia, confirming the reported involvement of both platelets [18], [20]–[22], [74]–[76] and P-selectin [77], [78] in the pathogenesis of HCM and ECM. Although platelets produce many factors including VEGF, ROS, and thrombin that are able to directly impair endothelial barrier function [185], the highly focal nature of platelet arrest [20], [21] is difficult to reconcile with the homogenous vascular leakage throughout the entire venous microvasculature at the time of ECM [21]. In agreement with their crucial role early in the pathogenesis of ECM, platelets are more likely to accomplish their vascular permeability-augmenting effect indirectly, namely by 1) facilitating leukocyte arrest in postcapillary venules [22], [57], [186], [187], 2) boosting ROS formation in leukocytes [185], and 3) enhancing cytokine secretion and cytotoxic capacity of effector T cells [188]. Thus, platelets adhering to small foci of endothelial P-selectin may serve as early nucleation points for the gradually spreading, cytokine-induced vasculopathy observed in postcapillary venules from ECM-susceptible mice.
In conclusion, we find that steric hindrance, mediated by large numbers of arrested CD8+ T cells, macrophages, and neutrophils in postcapillary venules, causes a severe restriction in the venous blood flow. Based on this observation, we hypothesize that completely different pathogenetic mechanisms - cytoadherence of P. falciparum iRBC in HCM and sequestration of leukocytes in ECM - can result in the same pathophysiological outcome: a severe reduction in the blood efflux from the brain. If the resulting increase in intracranial pressure intensifies the cerebral edema to the point of brainstem herniation, then compression of the respiration centers in pons and medulla could cause death by respiratory arrest.
Intracranial hypertension is a known risk factor for poor outcome in pediatric HCM [189], [190], but how this leads to neural injury is unknown [191], [192]. As discussed in a recent review [193], intracranial hypertension eliminates the need to explain any selective recognition mechanism Plasmodium might use to target multiple sensitive sites in the brain [194]. Intracranial hypertension leading to general swelling and hypoperfusion of the brain can also explain all of the neurological sequelae in HCM survivors [194]. Reports associating loss of smell, deafness, and blindness with both HCM and ECM support the notion that sequestration of iRBC as well as leukocytes can cause intracranial hypertension [192], [195]–[200]. Further, leukocyte sequestration is likely also involved in intracranial hypertension during P. falciparum HCM, as artesunate treatment was more efficacious in Asian adults compared to African children with more mononuclear cell accumulation [157], [201], [202] and also failed to rescue HCM patients with a low parasite biomass in the brain [203]. Thus, HCM and ECM induce very similar neurological symptoms and sequelae.
Thus, despite fundamental differences in parasite biology, the non-cytoadherent rodent parasite PbA could be used as a model to better understand how rheological alterations might lead to the annual death from HCM of over half a million people [204]. Perhaps more importantly, venous efflux disturbances due to leukocyte sequestration could also explain the cerebral complications that are increasingly reported for severe infections with P. vivax, another knobless (essentially non-cytoadherent) Plasmodium species [205]–[214]. The marked proinflammatory responses and reversible microvascular dysfunction associated with P. vivax infections [215], [216], together with the shared propensity for reticulocyte invasion [217]–[220], may render the PbA-infected mouse model suitable for study of the pathogenesis of severe P. vivax malaria. Hence, this model promises to shed light on the ultimate cause(s) of death from cerebral malaria.
Materials and Methods
Ethics statement
This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee, NYU School of Medicine (Protocol number 101201-01). All surgery was performed under ketamine-xylazine-acepromazine anesthesia, and all efforts were made to minimize suffering.
Parasites
P. berghei and P. yoelii parasites were maintained by passage through female Anopheles stephensi mosquitoes [221]. The green fluorescent P. berghei ANKA strain (PbA-GFP) was a kind gift from Dr. Andy Waters, University of Glasgow, UK [222]. The lethal P. yoelii strain 17XL, originally derived from the non-lethal 17X strain [223], was kindly provided by Dr. James Burns (Drexel University College of Medicine) [224], [225]. WT PyXL were transfected to express RedStar, an improved version of the red fluorescent protein drFP583/DsRed/RFP [226], under the control of the elongation factor 1α promoter using a novel replacement strategy [21], [227], [228] and termed PyXL-RFP [21]. Both PbA-GFP and PyXL-RFP emit fluorescence throughout the entire life cycle.
Mice and infection
Mice were CBA/CaJ (Jackson Laboratory, Bar Harbor, ME). Animals were maintained and used in accordance with recommendations in the guide for the Care and Use of Laboratory Animals. CBA/CaJ mice were infected at the age of 3 weeks (body weight of 12–15 g) by intraperitoneal injection of 0.5–10×106 iRBC as described [21]. In our hands, 3 week-old CBA/CaJ mice responded to PbA infection with neurological signs and died from ECM comparable to adult mice used by others [3], [6], [18], [22], [23], [76], [141], [169], [229]–[244]. The parasitemia was monitored daily using Giemsa stained blood smears and mice were sacrificed upon development of ECM or hyperparasitemia.
Assessment of neurological signs
PbA-infected mice were considered ECM positive when two or more parameters clearly indicated behavioral alteration including body position, spontaneous activity, startle response, tremor, gait, touch escape, and righting reflex [245]. Quantitative assessment of ECM-associated neurological signs was performed using the Rapid Murine Coma and Behavior Scale (RMCBS) with values of 3–7 defined here as severe, 8–16 as mild, and 17–20 as no ECM [246]. PyXL-infected mice exhibited a hunched position, pale skin color, and an increased respiration rate when parasitemia levels reached levels around 80%, but failed to present typical neurological manifestations.
FTY720 treatment
For IVM, groups of 15 PbA-infected CBA/CaJ mice received one daily oral dose of 0.3 mg/kg FTY720 starting one day before infection or no treatment as described [21], [39]. At the onset of neurological signs, mice were injected with Evans blue and examined by IVM. Mice surviving the critical period of ECM development were inoculated with Evans blue and PE-conjugated species anti-species CD8a and imaged on day 9. Groups of 5 PyXL-infected or uninfected mice were inoculated with the same markers and imaged on day 5 for comparison. For flow cytometry, PbA-infected mice, FTY720-treated PbA-infected mice, and PyXL-infected mice were analyzed on day 6–8, day 8, and day 5, respectively.
Anesthesia, craniotomy, and intravital microscopy
Mice were anesthetized by intraperitoneal injection of a cocktail of 50 mg/kg ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IO), 10 mg/kg xylazine (Rompun, Bayer, Shawnee Mission, KS), and 1.7 mg/kg acepromazine (Boehringer Ingelheim Vetmedica, St. Joseph, MO) (KXA mix) and surgically prepared for intravital imaging of the brain as described [21], [247], [248]. CBA/CaJ mice were infected with PbA and subjected to brain IVM 1) on day 5 prior to the appearance of neurological signs, 2) on day 6–8 upon ECM development, or 3) on day 9 after the window of ECM development had passed. PyXL-infected mice were imaged upon the parasitemia exceeding 50% on day 5. Uninfected mice were used as controls. Prior to imaging, mice were inoculated with Evans blue and matching combinations of fluorescent markers. Despite severe illness of the animals, optimization of anesthesia, craniotomy, and injection of fluorescent markers allowed us to obtain good recordings from approximately 70% of the mice with ECM and 50% of the mice with hyperparasitemia.
Monitoring cell density and dynamics in the cortical microvasculature
iRBC were identified by fluorescent protein expression in the parasites or reflection of hemozoin [21]. The vascular lumen was visualized by intravenous injection of 100 µl of a 1% solution of Evans blue. Vascular endothelia were labeled intravenously with Alexa 488 or eFluor 450-conjugated rat anti-mouse PECAM-1 (CD31; clone MEC13.3, BioLegend, San Diego, CA) and phycoerythrin (PE) - or Alexa 647-conjugated rat anti-mouse CD14 (clone Sa2-8, eBioscience). CD8+ T cells, CD4+ T cells, monocytes, macrophages, neutrophils, or platelets were labeled by intravenous injection of 3–5 µg of the following fluorochrome-conjugated monoclonal antibodies using appropriate color-matching combinations: PE-conjugated rat anti-mouse CD8a (clone 53-6.7; eBioscience, San Diego, CA), eFluor 450 or PE-conjugated rat anti-mouse CD4+ (clone GK 1.5, eBioscience), Pacific blue-conjugated rat anti-mouse CD11b (clone M1/70, BioLegend), eFluor 450-, PE - or Alexa 647-conjugated rat anti-mouse GR-1 (Ly-6G/6c; clone RB6-8C5, eBioscience and BioLegend), and eFluor 450-conjugated rat anti-mouse CD41 (clone MWReg30, eBioscience), respectively. ICAM-1 expression was visualized with intravenously inoculated PE-conjugated rat anti-mouse CD54 (clone YN 1/1.7.4, Biolegend). P-selectin was detected with PE-conjugated rat anti-human/mouse CD62p (KO2.3, eBioscience).
Quantification of leukocyte arrest and ICAM-1 expression
Multiple time sequences and 3D stacks were recorded for quantification of the number of arrested leukocytes in the vascular lumen. Depending on the experimental conditions, 20–45 postcapillary venules and arterioles were analyzed per mouse. Arrested leukocytes were defined in each vessel segment as cells that did not detach from the endothelial lining within the observation period. CD8+ T cells, CD4+ T cells, neutrophils, monocytes, and macrophages were quantified by counting the number of cells per square millimeter of vessel surface [249]. The relative density of the various leukocyte populations was determined in multiple fields of view per experimental condition and expressed as the mean ± SEM of arrested cells as well as the percentage of the total cell number. Velocities were measured with Imaris Track as described [250].
To quantify endothelial ICAM-1 expression, confocal 3D stacks of postcapillary venules or similarly sized arterioles were acquired from 2 mice each infected with PbA, PyXL, or no parasites. The fluorescence signal intensity across 10 (PbA) or 12 (PyXL and control) vessel volumes was collected from 3D data and quantified in ImageJ. To quantify leukocyte ICAM-1 expression, the relative fluorescence emission from individual leukocytes was measured from mice infected with PbA or PyXL. As for endothelial ICAM-1 expression, confocal 3D stacks were collected and projections were created in AutoDeblur. A total of 6 stacks from 2 mice per experimental condition were analyzed.
Brain leukocyte harvesting and flow cytometry
Leukocytes were prepared from the brain (cerebrum and cerebellum) using established procedures [251]–[253]. Briefly, mice were perfused intracardially with Mg2+ and Ca2+-free PBS to dislodge nonadherent leukocytes. Next, the brain was removed and gently minced through a mesh strainer (mesh size: 100 µm; Fisher Scientific) using a syringe plunger. The homogenate was suspended in 10 ml HBSS containing 0.05% collagenase D (Roche Diagnostics, Indianapolis, IN), 0.1 µg/ml of the trypsin inhibitor TLCK (Sigma), 10 µg/ml DNase I (Sigma), and 10 mM Hepes buffer, pH 7.4. The tissue slurry was gently rocked at RT for 60 min and then allowed to settle at 1 g for 30 min. The supernatant was collected and 5 ml of the suspension was layered onto 10 ml of a density separation medium containing 7.5 ml of RPMI medium containing 10% FBS, 10 mM HEPES, and 2.5 ml Ficoll Paque (GE HealthCare) in a 50 ml conical centrifuge tube and centrifuged at 400 g for 30 min. The overlying media and tissue debris were removed, the entire gradient medium was diluted ten-fold with HBSS and centrifuged at 300 g for 10 min. Isolated leukocytes were washed twice with Mg2+ and Ca2+-free PBS before phenotyping. A total of 10 PbA-infected mice with ECM, 6 PbA-infected/FTY720-treated, and 6 PyXL-infected mice with hyperparasitemia were subjected to flow cytometric analysis. Uninfected control mice were not included in the analysis, because the cerebral microvasculature of these animals does not exhibit arrested leukocytes.
The following antibodies were used for leukocyte phenotyping: Total leukocytes were detected with PE-Cy7-conjugated rat anti-mouse CD45 (clone 30-F11; eBioscience, San Diego, CA), T cells with APC-Cy7-conjugated Armenian hamster anti-mouse CD3 (clone 145-2C11; BD Biosciences, San Jose, CA), CD8+ T cells with eFluor 450 or Alexa 700-conjugated rat anti-mouse CD8a (clone 53-6.7; eBioscience or Biolegend, San Diego, CA), CD4+ T cells with PE - Texas Red-conjugated rat anti-mouse CD4+ (clone RM4-5; Life Technologies, Carlsbad, CA, neutrophils with FITC-conjugated rat anti-mouse Ly6G (clone 1A8; Biolegend), monocytes with Alexa 700-conjugated rat anti-mouse Ly6C (clone HK 1.4; Biolegend), and macrophages with anti-mouse CD11b (clone M1/70; eBioscience) or PE - or eFluor 450-conjugated anti-mouse F4/80 (clone BM8; eBioscience). CCR5 (CD195) was detected with PE-conjugated rat anti-mouse CD195 (clone HM-CCR5; Biolegend), CD69 with Pacific Blue-conjugated rat anti-mouse CD69 (clone H1.2F3; Biolegend), ICAM-1 with APC-conjugated rat anti-mouse CD54 (clone YN1/1.7.4; eBioscience), and granzyme B with FITC-conjugated anti-mouse granzyme B (clone GB11; Biolegend). Data were acquired with a 5-laser, 17-color LSR II Analytic Flow Cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Treestar, Ashland, OR).
Microrheology
Time series showing the blood flow in postcapillary venules or similarly sized arterioles were acquired from mice infected with PbA, PyXL, or no parasites and converted to minimal projections to visualize the portion of the vascular lumen used for blood flow. The vascular lumen was visualized by IV inoculation of Evans blue [21]. IVM movies were converted to minimal projections to visualize the perfused center of the vessel. Multiple measurements were taken for each vessel to determine the entire vascular diameter (distance between endothelia) versus the perfused part of the vessel (dark center) (Figures 1 and S2). Vascular restriction is expressed as percent reduction in vessel diameter or cross-section.
Intravital confocal microscopy and image processing
The cortical microvasculature was imaged with an inverted Leica TCS SP2 AOBS confocal system as described [21]. Time series and 3D data sets were acquired with Leica Confocal Software [248], [250], [254]. Imaris 7.4 (Bitplane, Saint Paul, MN), Image-Pro Plus (Media Cybernetics, Bethesda, MD), AutoDeBlur (Media Cybernetics, Bethesda, MD), and NIH ImageJ were used for further image analysis, deconvolution, and 3D reconstruction.
Endothelial junction integrity
Blood was removed by perfusion with PBS via the left ventricle [251]. Brains were snap-frozen for preparation of cryostat sections. To determine the expression level of tight junction proteins, sections were fixed in 95% ice-cold ethanol for 30 minutes and then permeabilized in acetone for 1 min at RT. After blocking in 50% goat serum for 45 min, sections were labeled with rabbit polyclonal antibody against claudin 5 (34-1600), rabbit polyclonal anti-occludin (40-4700), mouse monoclonal anti-ZO1 (33-9100), all from Invitrogen. Rat monoclonal antibody anti-CD31 (553370) was from BD Biosciences. Sections were then incubated for 2 h at RT with secondary Alexa antibodies (Life Technologies) and mounted in Vectashield with DAPI (H1200, Vector Labs, Burlingame, CA). Depending on the experimental condition, 9–16 images from various regions of the cerebrum and cerebellum were analyzed. Data were acquired at the same magnification with Zeiss Axiovison software using an Axio Imager-D2 Zeiss fluorescent microscope and imported into Image J for quantification of vascular leakage and tight junction protein expression. For tight junction protein expression, the fluorescence threshold was set with the “triangle” setting of Image J to limit the measurement of the brightest signal only, corresponding to the tight junctions. Ratios were then calculated by comparing the experimental groups with the control group using Excel. Significance was determined by t-test.
Statistical analysis
Statistical analysis was performed using Minitab® 17 (Minitab, State College, PA). Data sets were tested for normality and equal variances and when necessary data was Log10 transformed to obtain a normal distribution. Data were analyzed either by t-test, one-way ANOVA, or General Linear Model as appropriate. Where transformation was not successful, the non-parametric Mann-Whitney U test was used.
Supporting Information
Zdroje
1. WHO (2013) World Malaria Report: 2013. Geneva, Switzerland: World Health Organization.
2. MilnerDAJr (2010) Rethinking cerebral malaria pathology. Curr Op Infect Dis 23 : 456–463.
3. HaldarK, MurphySC, MilnerDA, TaylorTE (2007) Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol 2 : 217–249.
4. ClarkIA, AwburnMM, WhittenRO, HarperCG, LiombaNG, et al. (2003) Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malaria J 2 : 6.
5. HuntNH, GrauGE, EngwerdaC, BarnumSR, van der HeydeH, et al. (2010) Murine cerebral malaria: the whole story. Trends Parasitol 26 : 272–274.
6. de SouzaJB, HafallaJCR, RileyEM, CouperKN (2010) Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137 : 755–772.
7. WhiteVA, LewallenS, BeareNA, MolyneuxME, TaylorTE (2009) Retinal pathology of pediatric cerebral malaria in Malawi. PLoS ONE 4: e4317.
8. BeareNA, LewallenS, TaylorTE, MolyneuxME (2011) Redefining cerebral malaria by including malaria retinopathy. Future Microbiol 6 : 349–355.
9. LouJ, LucasR, GrauGE (2001) Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin Microbiol Rev 14 : 810–820.
10. LambTJ, BrownDE, PotocnikAJ, LanghorneJ (2006) Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med 8 : 1–22.
11. EngwerdaC, BelnoueE, GrunerAC, ReniaL (2005) Experimental models of cerebral malaria. Curr Top Microbiol Immunol 297 : 103–143.
12. ReniaL, PotterSM, MauduitM, RosaDS, KayibandaM, et al. (2006) Pathogenic T cells in cerebral malaria. Int J Parasitol 36 : 547–554.
13. CombesV, ColtelN, FailleD, WassmerSC, GrauGE (2006) Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier. Int J Parasitol 36 : 541–546.
14. GrauGE, PiguetPF, VassalliP, LambertPH (1989) Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev 112 : 49–70.
15. LanghorneJ, QuinSJ, SanniLA (2002) Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chem Immunol 80 : 204–228.
16. SanniLA (2001) The role of cerebral oedema in the pathogenesis of cerebral malaria. Redox Rep 6 : 137–142.
17. PenetM-F, ViolaA, Confort-GounyS, Le FurY, DuhamelG, et al. (2005) Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci 25 : 7352–7358.
18. CoxD, McConkeyS (2010) The role of platelets in the pathogenesis of cerebral malaria. Cell Mol Life Sci 67 : 557–568.
19. MoxonCA, HeydermanRS, WassmerSC (2009) Dysregulation of coagulation in cerebral malaria. Mol Biochem Parasitol 166 : 99–108.
20. CombesV, RosenkranzAR, RedardM, PizzolatoG, LepidiH, et al. (2004) Pathogenic role of P-selectin in experimental cerebral malaria: importance of the endothelial compartment. Am J Pathol 164 : 781–786.
21. NacerA, MovilaA, BaerK, MikolajczakSA, KappeSH, et al. (2012) Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog 8: e1002982.
22. FailleD, El-AssaadF, AlessiMC, FusaiT, CombesV, et al. (2009) Platelet-endothelial cell interactions in cerebral malaria: the end of a cordial understanding. Thromb Haemost 102 : 1093–1102.
23. FailleD, CombesV, MitchellAJ, FontaineA, Juhan-VagueI, et al. (2009) Platelet microparticles: a new player in malaria parasite cytoadherence to human brain endothelium. FASEB J 23 : 3449–3458.
24. BarbierM, FailleD, LoriodB, TextorisJ, CamusC, et al. (2011) Platelets alter gene expression profile in human brain endothelial cells in an in vitro model of cerebral malaria. PLoS ONE 6: e19651.
25. MfonkeuJB, GouadoI, KuateHF, ZambouO, CombesV, et al. (2010) Biochemical markers of nutritional status and childhood malaria severity in Cameroon. Br J Nutr 104 : 886–892.
26. BelnoueE, KayibandaM, VigarioAM, DescheminJC, van RooijenN, et al. (2002) On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol 169 : 6369–6375.
27. ClaserC, MalleretB, GunSY, WongAY, ChangZW, et al. (2011) CD8 T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS ONE 6: e18720.
28. McQuillanJA, MitchellAJ, HoYF, CombesV, BallHJ, et al. (2011) Coincident parasite and CD8 T cell sequestration is required for development of experimental cerebral malaria. Int J Parasitol 41 : 155–163.
29. HermsenC, van de WielT, MommersE, SauerweinR, ElingW (1997) Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology 114 (Pt 1) 7–12.
30. AmanteFH, HaqueA, StanleyAC, Rivera FdeL, RandallLM, et al. (2010) Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol 185 : 3632–3642.
31. Franke-FayardB, JanseCJ, Cunha-RodriguesM, RamesarJ, BuscherP, et al. (2005) Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci U S A 102 : 11468–11473.
32. HaqueA, BestSE, UnossonK, AmanteFH, de LabastidaF, et al. (2011) Granzyme B expression by CD8+ T cells is required for the development of experimental cerebral malaria. J Immunol 186 : 6148–6156.
33. NitcheuJ, BonduelleO, CombadiereC, TefitM, SeilheanD, et al. (2003) Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol 170 : 2221–2228.
34. BaptistaFG, PamplonaA, PenaAC, MotaMM, PiedS, et al. (2010) Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect Immun 78 : 4033–4039.
35. AmanteFH, HaqueA, StanleyAC, RiveraFdL, RandallLM, et al. (2010) Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol 185 : 3632–3642.
36. PaisTF, ChatterjeeS (2005) Brain macrophage activation in murine cerebral malaria precedes accumulation of leukocytes and CD8+ T cell proliferation. J Neuroimmunol 163 : 73–83.
37. PotterS, Chan-LingT, BallHJ, MansourH, MitchellA, et al. (2006) Perforin mediated apoptosis of cerebral microvascular endothelial cells during experimental cerebral malaria. Int J Parasitol 36 : 485–496.
38. OwensT, BechmannI, EngelhardtB (2008) Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 67 : 1113–1121.
39. FinneyCA, HawkesCA, KainDC, DhabangiA, MusokeC, et al. (2011) S1P is associated with protection in human and experimental cerebral malaria. Mol Med 17 : 717–725.
40. KippM, AmorS (2012) FTY720 on the way from the base camp to the summit of the mountain: relevance for remyelination. Mult Scler 18 : 258–263.
41. HlaT, BrinkmannV (2011) Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 76: S3–8.
42. BrinkmannV, BillichA, BaumrukerT, HeiningP, SchmouderR, et al. (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9 : 883–897.
43. MetzlerB, GfellerP, WieczorekG, LiJ, Nuesslein-HildesheimB, et al. (2008) Modulation of T cell homeostasis and alloreactivity under continuous FTY720 exposure. Int Immunol 20 : 633–644.
44. TaylorPA, EhrhardtMJ, LeesCJ, TolarJ, WeigelBJ, et al. (2007) Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD). Blood 110 : 3480–3488.
45. IdzkoM, HammadH, van NimwegenM, KoolM, MullerT, et al. (2006) Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest 116 : 2935–2944.
46. BrinkmannV, CysterJG, HlaT (2004) FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant 4 : 1019–1025.
47. SanchezT, Estrada-HernandezT, PaikJH, WuMT, VenkataramanK, et al. (2003) Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 278 : 47281–47290.
48. LeeMJ, ThangadaS, ClaffeyKP, AncellinN, LiuCH, et al. (1999) Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99 : 301–312.
49. DevKK, MullershausenF, MattesH, KuhnRR, BilbeG, et al. (2008) Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther 117 : 77–93.
50. NewtonCR, KirkhamFJ, WinstanleyPA, PasvolG, PeshuN, et al. (1991) Intracranial pressure in African children with cerebral malaria. Lancet 337 : 573–576.
51. ShihAY, BlinderP, TsaiPS, FriedmanB, StanleyG, et al. (2013) The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16 : 55–63.
52. RisserL, PlouraboueF, SteyerA, CloetensP, Le DucG, et al. (2007) From homogeneous to fractal normal and tumorous microvascular networks in the brain. J Cereb Blood Flow Metab 27 : 293–303.
53. MedanaIM, TurnerGD (2006) Human cerebral malaria and the blood-brain barrier. Int J Parasitol 36 : 555–568.
54. KampondeniSD, PotchenMJ, BeareNA, SeydelKB, GloverSJ, et al. (2013) MRI findings in a cohort of brain injured survivors of pediatric cerebral malaria. Am J Trop Med Hyg 88 : 542–546.
55. Seydel K, Kampondeni S, Potchen M, Birbeck G, Molyneux M, et al.. (2011) Clinical Correlates of Magnetic Resonance Imaging in Pediatric Cerebral Malaria. ASTMH Annual Meeting Philadelphia, PA: American Society of Tropical Medicine and Hygiene.
56. FordAL, GoodsallAL, HickeyWF, SedgwickJD (1995) Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol 154 : 4309–4321.
57. DietrichJB (2002) The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol 128 : 58–68.
58. KimH, HigginsS, LilesWC, KainKC (2011) Endothelial activation and dysregulation in malaria: a potential target for novel therapeutics. Curr Opin Hematol 18 : 177–185.
59. ChakravortySJ, CraigA (2005) The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol 84 : 15–27.
60. ShermanIW, EdaS, WinogradE (2003) Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect/Institut Pasteur 5 : 897–909.
61. HoM, WhiteNJ (1999) Molecular mechanisms of cytoadherence in malaria. Am J Physiol 276: C1231–1242.
62. AmaniV, VigarioAM, BelnoueE, MarussigM, FonsecaL, et al. (2000) Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur J Immunol 30 : 1646–1655.
63. KaulDK, LiuXD, NagelRL, ShearHL (1998) Microvascular hemodynamics and in vivo evidence for the role of intercellular adhesion molecule-1 in the sequestration of infected red blood cells in a mouse model of lethal malaria. Am J Trop Med Hyg 58 : 240–247.
64. ShearHL, MarinoMW, WanidworanunC, BermanJW, NagelRL (1998) Correlation of increased expression of intercellular adhesion molecule-1, but not high levels of tumor necrosis factor-alpha, with lethality of Plasmodium yoelii 17XL, a rodent model of cerebral malaria. Am J Trop Med Hyg 59 : 852–858.
65. HaqueA, EchchannaouiH, SeguinR, SchwartzmanJ, KasperLH, et al. (2001) Cerebral malaria in mice: interleukin-2 treatment induces accumulation of gammadelta T cells in the brain and alters resistant mice to susceptible-like phenotype. Am J Pathol 158 : 163–172.
66. RamosTN, BullardDC, DarleyMM, McDonaldK, CrawfordDF, et al. (2013) Experimental cerebral malaria develops independently of endothelial expression of intercellular adhesion molecule-1 (icam-1). J Biol Chem 288 : 10962–10966.
67. GoebelerM, RothJ, KunzM, SorgC (1993) Expression of intercellular adhesion molecule-1 by murine macrophages is up-regulated during differentiation and inflammatory activation. Immunobiology 188 : 159–171.
68. RuettenH, ThiemermannC, PerrettiM (1999) Upregulation of ICAM-1 expression on J774.2 macrophages by endotoxin involves activation of NF-kappaB but not protein tyrosine kinase: comparison to induction of iNOS. Mediators Inflamm 8 : 77–84.
69. NovosadJ, HolickaM, NovosadovaM, KrejsekJ, KrcmovaI (2011) Rapid onset of ICAM-1 expression is a marker of effective macrophages activation during infection of Francisella tularensis LVS in vitro. Folia Microbiol (Praha) 56 : 149–154.
70. Fattal-GermanM, Le Roy LadurieF, LecerfF, Berrih-AkninS (1996) Expression of ICAM-1 and TNF alpha in human alveolar macrophages from lung-transplant recipients. Ann N Y Acad Sci 796 : 138–148.
71. JenneCN, KubesP (2013) Immune surveillance by the liver. Nat Immunol 14 : 996–1006.
72. OchoaCD, WuS, StevensT (2010) New developments in lung endothelial heterogeneity: Von Willebrand factor, P-selectin, and the Weibel-Palade body. Semin Thromb Hemost 36 : 301–308.
73. LowensteinCJ, MorrellCN, YamakuchiM (2005) Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 15 : 302–308.
74. WassmerSC, CombesV, CandalFJ, Juhan-VagueI, GrauGE (2006) Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infection and Immunity 74 : 645–653.
75. WassmerSC, LepolardC, TraoreB, PouvelleB, GysinJ, et al. (2004) Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J Infect Dis 189 : 180–189.
76. GrauGE, MackenzieCD, CarrRA, RedardM, PizzolatoG, et al. (2003) Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis 187 : 461–466.
77. LinaresM, Marin-GarciaP, Perez-BenaventeS, Sanchez-NogueiroJ, PuyetA, et al. (2012) Brain-derived neurotrophic factor and the course of experimental cerebral malaria. Brain Res 1490 : 210–224.
78. ZaniniGM, CabralesP, BarkhoW, FrangosJA, CarvalhoLJ (2011) Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice. J Neuroinflammation 8 : 66.
79. BridgesDJ, BunnJ, van MourikJA, GrauG, PrestonRJ, et al. (2010) Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 115 : 1472–1474.
80. CamererE, RegardJB, CornelissenI, SrinivasanY, DuongDN, et al. (2009) Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119 : 1871–1879.
81. OoML, ChangSH, ThangadaS, WuMT, RezaulK, et al. (2011) Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice. J Clin Invest 121 : 2290–2300.
82. FrevertU, NacerA (2013) Immunobiology of Plasmodium in liver and brain. Parasite Immunol 35 : 267–282.
83. PriesAR, SecombTW, GaehtgensP (2000) The endothelial surface layer. Pflugers Arch 440 : 653–666.
84. ReitsmaS, SlaafDW, VinkH, van ZandvoortMA, oude EgbrinkMG (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454 : 345–359.
85. FelsJ, JeggleP, LiashkovichI, PetersW, OberleithnerH (2014) Nanomechanics of vascular endothelium. Cell Tissue Res 355 : 727–737.
86. AlphonsusCS, RodsethRN (2014) The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 69 : 777–784.
87. BerendtAR, TumerGD, NewboldCI (1994) Cerebral malaria: the sequestration hypothesis. Parasitol Today 10 : 412–414.
88. ClarkIA, RockettKA (1994) The cytokine theory of human cerebral malaria. Parasitol Today 10 : 410–412.
89. GrauGE, de KossodoS (1994) Cerebral malaria: mediators, mechanical obstruction or more? Parasitol Today 10 : 408–409.
90. CabralesP, MartinsYC, OngPK, ZaniniGM, FrangosJA, et al. (2013) Cerebral tissue oxygenation impairment during experimental cerebral malaria. Virulence 4 : 686–697.
91. KoutsiarisAG, TachmitziSV, BatisN, KotoulaMG, KarabatsasCH, et al. (2007) Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 44 : 375–386.
92. AmpawongS, CombesV, HuntNH, RadfordJ, Chan-LingT, et al. (2011) Quantitation of brain edema and localisation of aquaporin 4 expression in relation to susceptibility to experimental cerebral malaria. Int J Clin Exp Pathol 4 : 566–574.
93. MohantyS, MishraSK, PatnaikR, DuttAK, PradhanS, et al. (2011) Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis 53 : 349–355.
94. LooareesuwanS, WilairatanaP, KrishnaS, KendallB, VannaphanS, et al. (1995) Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis 21 : 300–309.
95. NewtonCR, CrawleyJ, SowumniA, WaruiruC, MwangiI, et al. (1997) Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child 76 : 219–226.
96. WallerD, CrawleyJ, NostenF, ChapmanD, KrishnaS, et al. (1991) Intracranial pressure in childhood cerebral malaria. Trans R Soc Trop Med Hyg 85 : 362–364.
97. MurphyS, Cserti-GazdewichC, DhabangiA, MusokeC, Nabukeera-BarungiN, et al. (2011) Ultrasound findings in Plasmodium falciparum malaria: a pilot study. Pediatr Crit Care Med 12: e58–63.
98. NewtonCR, MarshK, PeshuN, KirkhamFJ (1996) Perturbations of cerebral hemodynamics in Kenyans with cerebral malaria. Pediatr Neurol 15 : 41–49.
99. PotchenMJ, KampondeniSD, SeydelKB, BirbeckGL, HammondCA, et al. (2012) Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol 33 : 1740–1746.
100. CabralesP, ZaniniGM, MeaysD, FrangosJA, CarvalhoLJM (2010) Murine cerebral malaria is associated with a vasospasm-like microcirculatory dysfunction, and survival upon rescue treatment is markedly increased by nimodipine. Am J Pathol 176 : 1306–1315.
101. KennanRP, MachadoFS, LeeSC, DesruisseauxMS, WittnerM, et al. (2005) Reduced cerebral blood flow and N-acetyl aspartate in a murine model of cerebral malaria. Parasitol Res 96 : 302–307.
102. MartinsYC, Daniel-RibeiroCT (2013) A new hypothesis on the manifestation of cerebral malaria: The secret is in the liver. Med Hypotheses 81 : 777–783.
103. HoltmaatA, BonhoefferT, ChowDK, ChuckowreeJ, De PaolaV, et al. (2009) Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protocols 4 : 1128–1144.
104. MostanyR, Portera-CailliauC (2008) A craniotomy surgery procedure for chronic brain imaging. J Vis Exp e680 610.3791/3680 DOI: 3710.3791/3680
105. King-VanVlackCE, MewburnJD, ChaplerCK, MacDonaldPH (2003) Hemodynamic and proinflammatory actions of endothelin-1 in guinea pig small intestine submucosal microcirculation. Am J Physiol Gastrointest Liver Physiol 284: G940–948.
106. CalleraGE, TouyzRM, TeixeiraSA, MuscaraMN, CarvalhoMH, et al. (2003) ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension 42 : 811–817.
107. McCarronRM, WangL, StanimirovicDB, SpatzM (1993) Endothelin induction of adhesion molecule expression on human brain microvascular endothelial cells. Neurosci Lett 156 : 31–34.
108. FreemanBD, MachadoFS, TanowitzHB, DesruisseauxMS (2014) Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. E-pub ahead of print doi:10.1016/j.lfs.2014.04.021
109. MachadoFS, DesruisseauxMS, Nagajyothi, KennanRP, HetheringtonHP, et al. (2006) Endothelin in a murine model of cerebral malaria. Exp Biol Med (Maywood) 231 : 1176–1181.
110. DaiM, FreemanB, BrunoFP, ShikaniHJ, TanowitzHB, et al. (2012) The novel ETA receptor antagonist HJP-272 prevents cerebral microvascular hemorrhage in cerebral malaria and synergistically improves survival in combination with an artemisinin derivative. Life Sci 91 : 687–692.
111. BasilicoN, SpecialeL, ParapiniS, FerranteP, TaramelliD (2002) Endothelin-1 production by a microvascular endothelial cell line treated with Plasmodium falciparum parasitized red blood cells. Clin Sci (Lond) 103 Suppl 48 : 464S–466S.
112. WenischC, WenischH, WilairatanaP, LooareesuwanS, VannaphanS, et al. (1996) Big endothelin in patients with complicated Plasmodium falciparum malaria. J Infect Dis 173 : 1281–1284.
113. Martins C, Tanowitz H, Weiss L, Desruisseaux MS (2014) Endothelin-1 treatment induces experimental cerebral malaria in Plasmodium berghei NK65 infected mice. Malaria 2014: Advances in Pathophysiology, Biology and Drug Development New York, NY: New York Academy of Sciences.
114. SirvioML, MetsarinneK, SaijonmaaO, FyhrquistF (1990) Tissue distribution and half-life of 125I-endothelin in the rat: importance of pulmonary clearance. Biochem Biophys Res Commun 167 : 1191–1195.
115. WillenborgDO, StaykovaM, FordhamS, O'BrienN, LinaresD (2007) The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol 191 : 16–25.
116. OngPK, MelchiorB, MartinsYC, HoferA, Orjuela-SanchezP, et al. (2013) Nitric oxide synthase dysfunction contributes to impaired cerebroarteriolar reactivity in experimental cerebral malaria. PLoS Pathog 9: e1003444.
117. Orjuela-SanchezP, OngPK, ZaniniGM, MelchiorB, MartinsYC, et al. (2013) Transdermal glyceryl trinitrate as an effective adjunctive treatment with artemether for late-stage experimental cerebral malaria. Antimicrob Agents Chemother 57 : 5462–5471.
118. ZaniniGM, MartinsYC, CabralesP, FrangosJA, CarvalhoLJ (2012) S-nitrosoglutathione prevents experimental cerebral malaria. J Neuroimmune Pharmacol 7 : 477–487.
119. CabralesP, ZaniniGM, MeaysD, FrangosJA, CarvalhoLJ (2011) Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis 203 : 1454–1463.
120. SerghidesL, KimH, LuZ, KainDC, MillerC, et al. (2011) Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One 6: e27714.
121. HawkesM, OpokaRO, NamasopoS, MillerC, ConroyAL, et al. (2011) Nitric oxide for the adjunctive treatment of severe malaria: hypothesis and rationale. Med Hypotheses 77 : 437–444.
122. ImaiT, ShenJ, ChouB, DuanX, TuL, et al. (2010) Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur J Immunol 40 : 1053–1061.
123. VozaT, VigarioAM, BelnoueE, GrunerAC, DescheminJ-C, et al. (2005) Species-specific inhibition of cerebral malaria in mice coinfected with Plasmodium spp. Infect Immun 73 : 4777–4786.
124. ClarkCJ, PhillipsRS (2011) Cerebral malaria protection in mice by species-specific Plasmodium coinfection is associated with reduced CC chemokine levels in the brain. Parasite Immunol 33 : 637–641.
125. ShivtielS, KolletO, LapidK, SchajnovitzA, GoichbergP, et al. (2008) CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med 205 : 2381–2395.
126. SilverbergJ, GinsburgD, OrmanR, AmassianV, DurkinHG, et al. (2010) Lymphocyte infiltration of neocortex and hippocampus after a single brief seizure in mice. Brain Behav Immun 24 : 263–272.
127. CambiaggiC, ScupoliMT, CestariT, GerosaF, CarraG, et al. (1992) Constitutive expression of CD69 in interspecies T-cell hybrids and locus assignment to human chromosome 12. Immunogenetics 36 : 117–120.
128. ToughDF, SunS, ZhangX, SprentJ (1999) Stimulation of naive and memory T cells by cytokines. Immunol Rev 170 : 39–47.
129. FauconnierM, PalomoJ, BourigaultML, MemeS, SzeremetaF, et al. (2012) IL-12Rbeta2 is essential for the development of experimental cerebral malaria. J Immunol 188 : 1905–1914.
130. BoubouMI, ColletteA, VoegtleD, MazierD, CazenavePA, et al. (1999) T cell response in malaria pathogenesis: selective increase in T cells carrying the TCR V(beta)8 during experimental cerebral malaria. Int Immunol 11 : 1553–1562.
131. Silva-FilhoJL, SouzaMC, Ferreira-DasilvaCT, SilvaLS, CostaMF, et al. (2013) Angiotensin II is a new component involved in splenic T lymphocyte responses during Plasmodium berghei ANKA infection. PLoS One 8: e62999.
132. BoeufPS, LoizonS, AwandareGA, TettehJK, AddaeMM, et al. (2012) Insights into deregulated TNF and IL-10 production in malaria: implications for understanding severe malarial anaemia. Malar J 11 : 253.
133. AnadaY, IgarashiY, KiharaA (2007) The immunomodulator FTY720 is phosphorylated and released from platelets. Eur J Pharmacol 568 : 106–111.
134. KiharaA, IgarashiY (2008) Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim Biophys Acta 1781 : 496–502.
135. GarciaJG, LiuF, VerinAD, BirukovaA, DechertMA, et al. (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108 : 689–701.
136. SchaphorstKL, ChiangE, JacobsKN, ZaimanA, NatarajanV, et al. (2003) Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol 285: L258–267.
137. ConroyAL, GloverSJ, HawkesM, ErdmanLK, SeydelKB, et al. (2012) Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study. Crit Care Med 40 : 952–959.
138. ConroyAL, LaffertyEI, LovegroveFE, KrudsoodS, TangpukdeeN, et al. (2009) Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J 8 : 295.
139. LovegroveFE, TangpukdeeN, OpokaRO, LaffertyEI, RajwansN, et al. (2009) Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE 4: e4912.
140. JainV, LucchiNW, WilsonNO, BlackstockAJ, NagpalAC, et al. (2011) Plasma levels of angiopoietin-1 and -2 predict cerebral malaria outcome in Central India. Malar J 10 : 383.
141. SenaldiG, VesinC, ChangR, GrauGE, PiguetPF (1994) Role of polymorphonuclear neutrophil leukocytes and their integrin CD11a (LFA-1) in the pathogenesis of severe murine malaria. Infect Immun 62 : 1144–1149.
142. RossiB, AngiariS, ZenaroE, BuduiSL, ConstantinG (2011) Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J Leukoc Biol 89 : 539–556.
143. HuntNH, GolenserJ, Chan-LingT, ParekhS, RaeC, et al. (2006) Immunopathogenesis of cerebral malaria. Int J Parasitol 36 : 569–582.
144. MedanaIM, HuntNH, Chan-LingT (1997) Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia 19 : 91–103.
145. BelnoueE, KayibandaM, DescheminJC, ViguierM, MackM, et al. (2003) CCR5 deficiency decreases susceptibility to experimental cerebral malaria. Blood 101 : 4253–4259.
146. PenetMF, KoberF, Confort-GounyS, Le FurY, DalmassoC, et al. (2007) Magnetic resonance spectroscopy reveals an impaired brain metabolic profile in mice resistant to cerebral malaria infected with Plasmodium berghei ANKA. J Biol Chem 282 : 14505–14514.
147. GaleaI, FeltonLM, WatersS, van RooijenN, PerryVH, et al. (2008) Immune-to-brain signalling: the role of cerebral CD163-positive macrophages. Neurosci Lett 448 : 41–46.
148. GaleaI, PalinK, NewmanTA, Van RooijenN, PerryVH, et al. (2005) Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia 49 : 375–384.
149. PolflietMM, ZwijnenburgPJ, van FurthAM, van der PollT, DoppEA, et al. (2001) Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol 167 : 4644–4650.
150. HowlandSW, PohCM, GunSY, ClaserC, MalleretB, et al. (2013) Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med 5 : 916–931.
151. WangNK, LaiCC, LiuCH, YehLK, ChouCL, et al. (2013) Origin of fundus hyperautofluorescent spots and their role in retinal degeneration in a mouse model of Goldmann-Favre syndrome. Dis Model Mech 6 : 1113–1122.
152. PaiS, QinJ, CavanaghL, MitchellA, El-AssaadF, et al. (2014) Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathog 10: e1004236.
153. Fernandez-ReyesD, CraigAG, KyesSA, PeshuN, SnowRW, et al. (1997) A high frequency African coding polymorphism in the N-terminal domain of ICAM-1 predisposing to cerebral malaria in Kenya. Hum Mol Genet 6 : 1357–1360.
154. OckenhouseCF, TegoshiT, MaenoY, BenjaminC, HoM, et al. (1992) Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med 176 : 1183–1189.
155. OcholaLB, SiddondoBR, OchollaH, NkyaS, KimaniEN, et al. (2011) Specific receptor usage in Plasmodium falciparum cytoadherence is associated with disease outcome. PLoS ONE 6: e14741.
156. CraigA, Fernandez-ReyesD, MesriM, McDowallA, AltieriDC, et al. (2000) A functional analysis of a natural variant of intercellular adhesion molecule-1 (ICAM-1Kilifi). Hum Mol Genet 9 : 525–530.
157. SilamutK, PhuNH, WhittyC, TurnerGD, LouwrierK, et al. (1999) A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol 155 : 395–410.
158. NewboldC, WarnP, BlackG, BerendtA, CraigA, et al. (1997) Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg 57 : 389–398.
159. PinoP, TaoufiqZ, NitcheuJ, VouldoukisI, MazierD (2005) Blood-brain barrier breakdown during cerebral malaria: suicide or murder? Thromb Haemost 94 : 336–340.
160. GrauGE, Tacchini-CottierF, VesinC, MilonG, LouJN, et al. (1993) TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cytokine Netw 4 : 415–419.
161. FavreN, Da LaperousazC, RyffelB, WeissNA, ImhofBA, et al. (1999) Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect 1 : 961–968.
162. LiJ, ChangWL, SunG, ChenHL, SpecianRD, et al. (2003) Intercellular adhesion molecule 1 is important for the development of severe experimental malaria but is not required for leukocyte adhesion in the brain. J Investig Med 51 : 128–140.
163. BauerPR, Van Der HeydeHC, SunG, SpecianRD, GrangerDN (2002) Regulation of endothelial cell adhesion molecule expression in an experimental model of cerebral malaria. Microcirculation 9 : 463–470.
164. WeiserS, MiuJ, BallHJ, HuntNH (2007) Interferon-gamma synergises with tumour necrosis factor and lymphotoxin-alpha to enhance the mRNA and protein expression of adhesion molecules in mouse brain endothelial cells. Cytokine 37 : 84–91.
165. ZhouH, AndoneguiG, WongCH, KubesP (2009) Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J Immunol 183 : 5244–5250.
166. ZhouH, LapointeBM, ClarkSR, ZbytnuikL, KubesP (2006) A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol 177 : 8103–8110.
167. GriffithsM, NealJW, GasqueP (2007) Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int Rev Neurobiol 82 : 29–55.
168. LloydKL, KubesP (2006) GPI-linked endothelial CD14 contributes to the detection of LPS. Am J Physiol Heart Circ Physiol 291: H473–481.
169. OakleyMS, MajamV, MahajanB, GeraldN, AnantharamanV, et al. (2009) Pathogenic roles of CD14, galectin-3, and OX40 during experimental cerebral malaria in mice. PLoS ONE 4: e6793.
170. DevittA, MoffattOD, RaykundaliaC, CapraJD, SimmonsDL, et al. (1998) Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392 : 505–509.
171. WieseL, HempelC, PenkowaM, KirkbyN, KurtzhalsJA (2008) Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J 7 : 3.
172. WieseL, KurtzhalsJA, PenkowaM (2006) Neuronal apoptosis, metallothionein expression and proinflammatory responses during cerebral malaria in mice. Exp Neurol 200 : 216–226.
173. LacknerP, BurgerC, PfallerK, HeusslerV, HelbokR, et al. (2007) Apoptosis in experimental cerebral malaria: spatial profile of cleaved caspase-3 and ultrastructural alterations in different disease stages. Neuropathol Appl Neurobiol 33 : 560–571.
174. LingZL, CombesV, GrauGE, KingNJ (2011) Microparticles as immune regulators in infectious disease - an opinion. Front Immunol 2 : 67.
175. El-AssaadF, WhewayJ, HuntNH, GrauGE, CombesV (2014) Production, fate and pathogenicity of plasma microparticles in murine cerebral malaria. PLoS Pathog 10: e1003839.
176. VasinaEM, CauwenberghsS, FeijgeMA, HeemskerkJW, WeberC, et al. (2011) Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis 2: e211.
177. MacPhersonGG, WarrellMJ, WhiteNJ, LooareesuwanS, WarrellDA (1985) Human cerebral malaria a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. ajp 119 : 385–401.
178. ClarkIA, BuddAC, AllevaLM, CowdenWB (2006) Human malarial disease: a consequence of inflammatory cytokine release. Malar J 5 : 85.
179. ClarkIA, SchofieldL (2000) Pathogenesis of malaria. Parasitol Today 16 : 451–454.
180. GrauGE, PointaireP, PiguetPF, VesinC, RosenH, et al. (1991) Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol 21 : 2265–2267.
181. FalangaPB, ButcherEC (1991) Late treatment with anti-LFA-1 (CD11a) antibody prevents cerebral malaria in a mouse model. Eur J Immunol 21 : 2259–2263.
182. FrevertU, NacerA, CabreraM, MovilaA, LeberlM (2014) Imaging Plasmodium immunobiology in the liver, brain, and lung. Parasitol Int 63 : 171–186.
183. van der HeydeHC, GramagliaI, SunG, WoodsC (2005) Platelet depletion by anti-CD41 (alphaIIb) mAb injection early but not late in the course of disease protects against Plasmodium berghei pathogenesis by altering the levels of pathogenic cytokines. Blood 105 : 1956–1963.
184. SunG, ChangWL, LiJ, BerneySM, KimpelD, et al. (2003) Inhibition of platelet adherence to brain microvasculature protects against severe Plasmodium berghei malaria. Infect Immun 71 : 6553–6561.
185. Granger DN, Senchenkova E (2010) Endothelial Barrier Dysfunction. Inflammation and the Microcirculation. San Rafael (CA): Morgan& Claypool Lifesciences.
186. LawsonC, WolfS (2009) ICAM-1 signaling in endothelial cells. Pharmacol Rep 61 : 22–32.
187. SumaginR, LomakinaE, SareliusIH (2008) Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol 295: H969–H977.
188. LiN (2008) Platelet-lymphocyte cross-talk. J Leukoc Biol 83 : 1069–1078.
189. IdroR, CarterJA, FeganG, NevilleBG, NewtonCR (2006) Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child 91 : 142–148.
190. van HensbroekMB, PalmerA, JaffarS, SchneiderG, KwiatkowskiD (1997) Residual neurologic sequelae after childhood cerebral malaria. J Pediatr 131 : 125–129.
191. BeareNA, GloverSJ, LewallenS, TaylorTE, HardingSP, et al. (2012) Prevalence of raised intracranial pressure in cerebral malaria detected by optic nerve sheath ultrasound. Am J Trop Med Hyg 87 : 985–988.
192. IdroR, Kakooza-MwesigeA, BalyejjussaS, MirembeG, MugashaC, et al. (2010) Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes 3 : 104.
193. FrevertU, NacerA (2014) Fatal cerebral malaria: a venous efflux problem. Front Cell Infect Microbiol 4 : 155.
194. IdroR, MarshK, JohnCC, NewtonCR (2010) Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 68 : 267–274.
195. ZhaoH, AoshiT, KawaiS, MoriY, KonishiA, et al. (2014) Olfactory plays a key role in spatiotemporal pathogenesis of cerebral malaria. Cell Host Microbe 15 : 551–563.
196. SchmutzhardJ, KositzCH, LacknerP, PritzC, GlueckertR, et al. (2011) Murine cerebral malaria: histopathology and ICAM 1 immunohistochemistry of the inner ear. Trop Med Int Health 16 : 914–922.
197. SchmutzhardJ, KositzCH, LacknerP, DietmannA, FischerM, et al. (2010) Murine malaria is associated with significant hearing impairment. Malar J 9 : 159.
198. ZhaoSZ, MackenzieIJ (2011) Deafness: malaria as a forgotten cause. Ann Trop Paediatr 31 : 1–10.
199. SagguR, FailleD, GrauGE, CozzonePJ, ViolaA (2011) In the Eye of Experimental Cerebral Malaria. Am J Pathol 179 : 1104–1109.
200. BrewsterDR, KwiatkowskiD, WhiteNJ (1990) Neurological sequelae of cerebral malaria in children. Lancet 336 : 1039–1043.
201. Dorovini-ZisK, SchmidtK, HuynhH, FuW, WhittenRO, et al. (2011) The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol 178 : 2146–2158.
202. MilnerDAJr, ValimC, CarrRA, ChandakPB, FosikoNG, et al. (2013) A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar J 12 : 191.
203. WhiteNJ, TurnerGD, DayNP, DondorpAM (2013) Lethal malaria: Marchiafava and Bignami were right. J Infect Dis 208 : 192–198.
204. WHO (2011) World Malaria Report: 2011. Geneva, Switzerland: World Health Organization.
205. MohanA, SharmaSK, BollineniS (2008) Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis 45 : 179–193.
206. AnsteyNM, JacupsSP, CainT, PearsonT, ZiesingPJ, et al. (2002) Pulmonary manifestations of uncomplicated falciparum and vivax malaria: cough, small airways obstruction, impaired gas transfer, and increased pulmonary phagocytic activity. J Infect Dis 185 : 1326–1334.
207. MaguireGP, HandojoT, PainMC, KenangalemE, PriceRN, et al. (2005) Lung injury in uncomplicated and severe falciparum malaria: a longitudinal study in papua, Indonesia. J Infect Dis 192 : 1966–1974.
208. SarkarD, RayS, SahaM, ChakrabortyA, TalukdarA (2013) Clinico-laboratory profile of severe Plasmodium vivax malaria in a tertiary care centre in Kolkata. Trop Parasitol 3 : 53–57.
209. SarkarS, BhattacharyaP (2008) Cerebral malaria caused by Plasmodium vivax in adult subjects. Indian J Crit Care Med 12 : 204–205.
210. BhattacharjeeP, DubeyS, GuptaVK, AgarwalP, MahatoMP (2013) The clinicopathologic manifestations of Plasmodium vivax malaria in children: a growing menace. J Clin Diagn Res 7 : 861–867.
211. AbdallahTM, AbdeenMT, AhmedIS, HamdanHZ, MagzoubM, et al. (2013) Severe Plasmodium falciparum and Plasmodium vivax malaria among adults at Kassala Hospital, eastern Sudan. Malar J 12 : 148.
212. GehlawatVK, AryaV, KaushikJS, GathwalaG (2013) Clinical spectrum and treatment outcome of severe malaria caused by Plasmodium vivax in 18 children from northern India. Pathog Glob Health 107 : 210–214.
213. PinzonMA, PinedaJC, RossoF, ShinchiM, Bonilla-AbadiaF (2013) Plasmodium vivax cerebral malaria complicated with venous sinus thrombosis in Colombia. Asian Pac J Trop Med 6 : 413–415.
214. BegMA, KhanR, BaigSM, GulzarZ, HussainR, et al. (2002) Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg 67 : 230–232.
215. ThapaR, PatraV, KunduR (2007) Plasmodium vivax cerebral malaria. Indian Pediatr 44 : 433–434.
216. AndradeBB, Reis-FilhoA, Souza-NetoSM, ClarencioJ, CamargoLM, et al. (2010) Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J 9 : 13.
217. CromerD, EvansKJ, SchofieldL, DavenportMP (2006) Preferential invasion of reticulocytes during late-stage Plasmodium berghei infection accounts for reduced circulating reticulocyte levels. Int J Parasitol 36 : 1389–1397.
218. McNallyJ, O'DonovanSM, DaltonJP (1992) Plasmodium berghei and Plasmodium chabaudi chabaudi: development of simple in vitro erythrocyte invasion assays. Parasitology 105 (Pt 3) 355–362.
219. RussellB, SuwanaruskR, BorlonC, CostaFT, ChuCS, et al. (2011) A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 118: e74–81.
220. PanichakulT, SattabongkotJ, ChotivanichK, SirichaisinthopJ, CuiL, et al. (2007) Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol 37 : 1551–1557.
221. Vanderberg JP, Gwadz R (1980) The transmission by mosquitoes of Plasmodia in the laboratory. In: Kreier J, editor. Malaria: pathology, vector studies and culture. New York, N.Y.: Academic Press. pp. 154–218.
222. Franke-FayardB, TruemanH, RamesarJ, MendozaJ, van der KeurM, et al. (2004) A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol 137 : 23–33.
223. YoeliM, HargreavesBJ (1974) Brain capillary blockage produced by a virulent strain of rodent malaria. Science 184 : 572–573.
224. WeissL (1989) Mechanisms of splenic control of murine malaria: cellular reactions of the spleen in lethal (strain 17XL) Plasmodium yoelii malaria in BALB/c mice, and the consequences of pre-infective splenectomy. Am J Trop Med Hyg 41 : 144–160.
225. YoeliM, HargreavesB, CarterR, WallikerD (1975) Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann Trop Med Parasitol 69 : 173–178.
226. KnopM, BarrF, RiedelCG, HeckelT, ReichelC (2002) Improved version of the red fluorescent protein (drFP583/DsRed/RFP). Biotechniques 33 : 592–602.
227. MikolajczakSA, AlyAS, DumpitRF, VaughanAM, KappeSH (2008) An efficient strategy for gene targeting and phenotypic assessment in the Plasmodium yoelii rodent malaria model. Mol Biochem Parasitol 158 : 213–216.
228. TarunAS, BaerK, DumpitRF, GrayS, LejarceguiN, et al. (2006) Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int J Parasitol 36 : 1283–1293.
229. NieCQ, BernardNJ, NormanMU, AmanteFH, LundieRJ, et al. (2009) IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog 5: e1000369.
230. RestJR (1982) Cerebral malaria in inbred mice. I. A new model and its pathology. Trans Royal Soc Trop Med Hyg 76 : 410–415.
231. Van den SteenPE, DeroostK, Van AelstI, GeurtsN, MartensE, et al. (2008) CXCR3 determines strain susceptibility to murine cerebral malaria by mediating T lymphocyte migration toward IFN-gamma-induced chemokines. Eur J Immunol 38 : 1082–1095.
232. AmanteFH, StanleyAC, RandallLM, ZhouY, HaqueA, et al. (2007) A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am J Pathol 171 : 548–559.
233. GrauGE, HeremansH, PiguetPF, PointaireP, LambertPH, et al. (1989) Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA 86 : 5572–5574.
234. GrauGE, PiguetPF, EngersHD, LouisJA, VassalliP, et al. (1986) L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol 137 : 2348–2354.
235. HearnJ, RaymentN, LandonDN, KatzDR, de SouzaJB (2000) Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect Immun 68 : 5364–5376.
236. JenningsVM, ActorJK, LalAA, HunterRL (1997) Cytokine profile suggesting that murine cerebral malaria is an encephalitis. Infect Immun 65 : 4883–4887.
237. JenningsVM, LalAA, HunterRL (1998) Evidence for multiple pathologic and protective mechanisms of murine cerebral malaria. Infect Immun 66 : 5972–5979.
238. KamiyamaT, TatsumiM, MatsubaraJ, YamamotoK, RubioZ, et al. (1987) Manifestation of cerebral malaria-like symptoms in the WM/Ms rat infected with Plasmodium berghei strain NK65. J Parasitol 73 : 1138–1145.
239. Lacerda-QueirozN, RodriguesDH, VilelaMC, MirandaASd, AmaralDCG, et al. (2010) Inflammatory changes in the central nervous system are associated with behavioral impairment in Plasmodium berghei (strain ANKA)-infected mice. Exp Parasitol 125 : 271–278.
240. MackeyLJ, HochmannA, JuneCH, ContrerasCE, LambertPH (1980) Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin Exp Immunol 42 : 412–420.
241. PatelSN, BerghoutJ, LovegroveFE, AyiK, ConroyA, et al. (2008) C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J Exp Med 205 : 1133–1143.
242. YanezDM, ManningDD, CooleyAJ, WeidanzWP, van der HeydeHC (1996) Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol 157 : 1620–1624.
243. PolderTW, JerusalemCR, ElingWMC (1991) Morphological characteristics of intracerebral arterioles in clinical (Plasmodium falciparum) and experimental (Plasmodium berghei) cerebral malaria. J Neurol Sci 101 : 35–46.
244. McElroyPD, BeierJC, OsterCN, OnyangoFK, OlooAJ, et al. (1997) Dose - and time-dependent relations between infective Anopheles inoculation and outcomes of Plasmodium falciparum parasitemia among children in western Kenya. Am J Epidemiol 145 : 945–956.
245. HatcherJP, JonesDN, RogersDC, HatcherPD, ReavillC, et al. (2001) Development of SHIRPA to characterise the phenotype of gene-targeted mice. Behav Brain Res 125 : 43–47.
246. CarrollRW, WainwrightMS, KimKY, KidambiT, GomezND, et al. (2010) A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS ONE 5 e13124.
247. FrevertU, EngelmannS, ZougbédéS, StangeJ, NgB, et al. (2005) Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol 3: e192.
248. BaerK, KlotzC, KappeSH, SchniederT, FrevertU (2007) Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog 3: e171.
249. BeckerMD, NobilingR, PlanckSR, RosenbaumJT (2000) Digital video-imaging of leukocyte migration in the iris: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J Immunol Methods 240 : 23–37.
250. CabreraM, PeweLL, HartyJT, FrevertU (2013) In vivo CD8+ T cell dynamics in the liver of Plasmodium yoelii immunized and infected mice. PLoS One 8: e70842.
251. GundraUM, MishraBB, WongK, TealeJM (2011) Increased disease severity of parasite-infected TLR2-/ - mice is correlated with decreased central nervous system inflammation and reduced numbers of cells with alternatively activated macrophage phenotypes in a murine model of neurocysticercosis. Infect Immun 79 : 2586–2596.
252. IraniDN, GriffinDE (1991) Isolation of brain parenchymal lymphocytes for flow cytometric analysis. Application to acute viral encephalitis. J Immunol Methods 139 : 223–231.
253. MishraBB, GundraUM, WongK, TealeJM (2009) MyD88-deficient mice exhibit decreased parasite-induced immune responses but reduced disease severity in a murine model of neurocysticercosis. Infect Immun 77 : 5369–5379.
254. CabreraM, FrevertU (2012) Novel in vivo imaging techniques for the liver microvasculature. IntraVital 1 : 107–114.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus InfectionČlánek P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Microbial Programming of Systemic Innate Immunity and Resistance to Infection
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
- Measles Immune Suppression: Functional Impairment or Numbers Game?
- Cellular Mechanisms of Alpha Herpesvirus Egress: Live Cell Fluorescence Microscopy of Pseudorabies Virus Exocytosis
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection
- Loss of Dynamin-Related Protein 2B Reveals Separation of Innate Immune Signaling Pathways
- Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Infection
- Unveiling the Intracellular Survival Gene Kit of Trypanosomatid Parasites
- Extreme Divergence of Tropism for the Stem-Cell-Niche in the Testis
- HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4 T Cells
- P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
- Hypercytotoxicity and Rapid Loss of NKp44 Innate Lymphoid Cells during Acute SIV Infection
- Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
- Crystal Structure of Calcium Binding Protein-5 from and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
- Chronic Parasitic Infection Maintains High Frequencies of Short-Lived Ly6CCD4 Effector T Cells That Are Required for Protection against Re-infection
- Specific Dysregulation of IFNγ Production by Natural Killer Cells Confers Susceptibility to Viral Infection
- HSV-2-Driven Increase in the Expression of αβ Correlates with Increased Susceptibility to Vaginal SHIV Infection
- Murine Anti-vaccinia Virus D8 Antibodies Target Different Epitopes and Differ in Their Ability to Block D8 Binding to CS-E
- Brothers in Arms: Th17 and Treg Responses in Immunity
- Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Typhimurium Colitis
- A Negative Feedback Modulator of Antigen Processing Evolved from a Frameshift in the Cowpox Virus Genome
- Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms
- The Non-receptor Tyrosine Kinase Tec Controls Assembly and Activity of the Noncanonical Caspase-8 Inflammasome
- Targeted Changes of the Cell Wall Proteome Influence Ability to Form Single- and Multi-strain Biofilms
- Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
- The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
- Anti-α4 Antibody Treatment Blocks Virus Traffic to the Brain and Gut Early, and Stabilizes CNS Injury Late in Infection
- Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
- Microbial Urease in Health and Disease
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against
- IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
- A Natural Genetic Variant of Granzyme B Confers Lethality to a Common Viral Infection
- Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression
- Differential PfEMP1 Expression Is Associated with Cerebral Malaria Pathology
- The Role of the NADPH Oxidase NOX2 in Prion Pathogenesis
- Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions
- The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
- Expression of Suppressor of Cytokine Signaling 1 (SOCS1) Impairs Viral Clearance and Exacerbates Lung Injury during Influenza Infection
- Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells
- SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in
- Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication
- Oral Streptococci Utilize a Siglec-Like Domain of Serine-Rich Repeat Adhesins to Preferentially Target Platelet Sialoglycans in Human Blood
- SV40 Utilizes ATM Kinase Activity to Prevent Non-homologous End Joining of Broken Viral DNA Replication Products
- Amphipathic α-Helices in Apolipoproteins Are Crucial to the Formation of Infectious Hepatitis C Virus Particles
- Proteomic Analysis of the Acidocalcisome, an Organelle Conserved from Bacteria to Human Cells
- Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



