-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMeasles Immune Suppression: Functional Impairment or Numbers Game?
article has not abstract
Published in the journal: . PLoS Pathog 10(12): e32767. doi:10.1371/journal.ppat.1004482
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1004482Summary
article has not abstract
Introduction
Measles remains a significant cause of childhood morbidity and mortality. Hallmark of the disease is a generalized immune suppression that can last for several weeks to months after resolution of measles virus (MV) infection [1]–[3], resulting in increased susceptibility to opportunistic infections [4]–[7]. At the same time, measles is associated with immune activation and induces strong MV-specific immune responses that confer lifelong immunity [8]. This contradiction is known as the “measles paradox'. Although measles-associated immune suppression has been a subject of study since the beginning of the 20th century [9], the importance of possible underlying mechanisms remains disputed.
The Immune System as “Viral Friend”
From the perspective of MV, cells of the immune system are both friend and foe. MV efficiently replicates in cells of the immune system, especially during the initial stages of the infection [10], [11]. However, the virus preferentially infects specific subsets of lymphocytes and dendritic cells (DCs). The relative susceptibility of these cells to MV infection is governed by their expression level of the cellular receptor CD150 [11]–[14]. Memory T-lymphocytes, which express CD150, are preferentially infected [13], [14]. In secondary and tertiary lymphoid tissues, the virus also replicates to high levels in follicular and marginal zone B-lymphocytes [10], [11], [13]. DCs can also be infected by MV [11], [15]–[17] and may serve as initial target cells [18], [19].
The Immune System as “Viral Foe”
In the majority of cases MV infection is self-limiting and induces strong virus-specific cellular and humoral immune responses resulting in lifelong immunity [20]. Virus neutralizing antibodies are an important correlate of protection against MV infection, but cytotoxic T-lymphocytes are crucial for clearance of infected cells [21]–[23]. Resolution of MV infection is associated with increased lymphoproliferation [8], [24] and enlargement of lymph nodes [13]. Thus, the immune system efficiently restricts MV replication and clears MV-infected cells.
Mechanisms of Measles Immune Suppression
Measles is associated with lymphopenia [25] and extensive depletion of lymphocytes from lymphoid tissues [13], [26], [27]. However, lymphocyte numbers return to normal within a week after clinical symptoms of measles have disappeared, while measles immune suppression extends for several weeks to months. Therefore, immune cell depletion was initially dismissed as a mechanism for measles immune suppression [3]. Alternative mechanisms have been proposed to explain measles-associated immune suppression, as summarized in Table 1. However, the relevance of these observations to enhanced susceptibility to opportunistic infections in vivo remains unclear.
Tab. 1. Reported mechanisms of measles immune suppression. 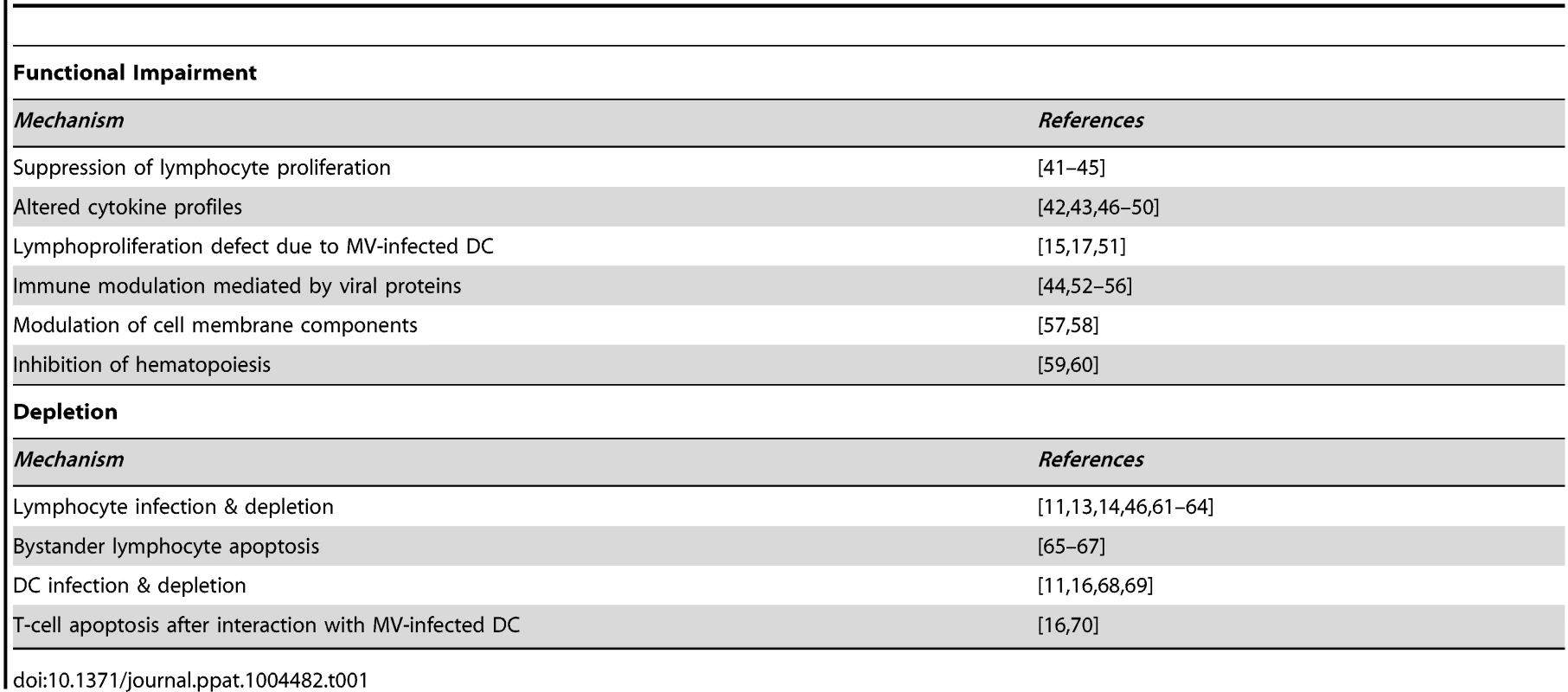
Is Suppressed Lymphoproliferation Important?
Reduced responsiveness of peripheral blood mononuclear cells to stimulation with mitogens in vitro has been considered an important mechanism underlying measles-associated immune suppression. Although the observations in these studies are not disputed, we find it difficult to reconcile this in vitro observation with the observed immune activation in vivo. Measles results in dramatic expansion of MV-specific lymphocytes followed by resolution of viremia and lymphopenia [8], [25], [28]. We recently demonstrated extensive lymphoproliferation in lymphoid tissues early after MV infection in vivo [13]. Thus, there is no evidence of suppressed lymphoproliferative responses, at least towards MV, in vivo. Rather, we believe that alterations in the composition of the peripheral lymphocyte populations before and after measles may explain these in vitro observations [13].
Do Dendritic Dells Play a Crucial Role?
DC subsets have been shown susceptible to MV infection in vitro [15]–[17] and in nonhuman primates in vivo [11], [19]. Therefore, it is likely that infection, depletion, or functional modulation of DCs contributes to measles-associated immune suppression. Nevertheless, antigen presentation does not seem to be impaired in vivo, as strong MV-specific immune responses develop during the acute stage of the disease.
Measles Damages the Respiratory Epithelium
Whereas MV targets CD150 to infect lymphoid and myeloid cells, the virus uses poliovirus receptor like 4 (also known as nectin-4) as an alternative cellular receptor to infect epithelial cells [29]–[31]. Whilst infection of epithelial cells contributes to viral transmission [32], MV also causes extensive epithelial damage in the respiratory tract [33], [34]. This epithelial injury may provide an opportunity for respiratory bacteria to adhere, replicate, and invade with increased efficiency [35].
Attenuated, Mild, Moderate, or Severe Morbillivirus Infections
MV infections display a large variability in clinical severity, ranging from vaccination with attenuated viruses, via subclinical or mild infections, to severe disease. Closely related animal morbilliviruses may even overwhelm the immune system, resulting in functional paralysis and virtual absence of virus-specific immune responses [36]–[40]. This variation is also reflected in a wide range of levels of lymphopenia, viremia, and specific immune responses (Fig. 1A–C) [13]. Natural MV infection of the naive host will normally follow the pattern of either a mild or moderate infection as displayed in Fig. 1. Whereas mild measles results in limited depletion of pre-existing CD150+ memory lymphocytes, moderate measles is associated with infection and subsequent depletion of a large fraction of those lymphocytes (Fig. 1D). Whether this depletion is mediated by necrosis, apoptosis, pyroptosis, or cytotoxic T-cells remains to be determined, but the effect is always the same: to a varying degree, measles erases immunological memory.
Fig. 1. Schematic representation of the measles paradox. 
Different levels of lymphopenia (A), systemic virus loads (B), and virus-specific immune responses (C) after subclinical (blue), mild (green), moderate (black), or severe (red) morbillivirus infections. Panel D shows a model for immune suppression caused by moderate morbillivirus infection as shown in panels A, B, and C (adapted from [13]). Future Directions: Studies in Naturally Infected Measles Patients
To improve our understanding of measles immune suppression, a transition from in vitro to in vivo studies is required. Two aspects are of crucial importance: viral tropism and depletion of immune cell subsets. We feel that it is important to characterize the phenotype of MV-infected cells during the prodromal phase of natural measles, with special emphasis on infection of DCs and memory lymphocytes. Furthermore, to address depletion of immune cell subsets, paired blood samples from children before and after measles will be required. Staining of immune cells specific for previously encountered pathogens, rather than functional assays, will allow us to distinguish between subset depletion and functional paralysis.
Conclusions
Experimental MV infections in animal models have demonstrated that percentages of infected lymphocyte subsets are higher than previously thought, especially in secondary and tertiary lymphoid tissues [11], [13]. We believe that measles immune suppression mainly results from depletion of immune cell subsets, which is masked by the rapid proliferation of MV-specific and bystander lymphocytes (Fig. 1D). This model is fully compatible with the measles paradox. Clinical studies are required to test our hypothesis that measles immune suppression is mainly a numbers game.
Zdroje
1. Schneider-SchauliesS, Schneider-SchauliesJ (2009) Measles virus-induced immunosuppression. Curr Top Microbiol Immunol 330 : 243–269.
2. HahmB (2009) Hostile communication of measles virus with host innate immunity and dendritic cells. Curr Top Microbiol Immunol 330 : 271–287.
3. GriffinDE (2010) Measles virus-induced suppression of immune responses. Immunol Rev 236 : 176–189.
4. BeckfordAP, KaschulaRO, StephenC (1985) Factors associated with fatal cases of measles. A retrospective autopsy study. S Afr Med J 68 : 858–863.
5. AkramuzzamanSM, CuttsFT, WheelerJG, HossainMJ (2000) Increased childhood morbidity after measles is short-term in urban Bangladesh. Am J Epidemiol 151 : 723–735.
6. Van den HofS, Conyn-van SpaendonckMAE, van SteenbergenJE (2002) Measles epidemic in the Netherlands, 1999–2000. J Infect Dis 186 : 1483–1486.
7. ShanksGD, LeeSE, HowardA, BrundageJF (2011) Extreme mortality after first introduction of measles virus to the polynesian island of Rotuma, 1911. Am J Epidemiol 173 : 1211–1222.
8. GriffinDE, WardBJ, JaureguiE, JohnsonRT, VaisbergA (1989) Immune activation in measles. N Engl J Med 320 : 1667–1672.
9. Von PirquetCE (1908) Das Verhalten der kutanen Tuberkulin-reaktion während der Masern. Dtsch Med Wochenschr 34 : 1297–1300.
10. McChesneyMB, MillerCJ, RotaPA, ZhuY, AntipaL, et al. (1997) Experimental measles I. Pathogenesis in the normal and the immunized host. Virology 233 : 74–84.
11. De SwartRL, LudlowM, De WitteL, YanagiY, Van AmerongenG, et al. (2007) Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog 3: e178.
12. TatsuoH, OnoN, TanakaK, YanagiY (2000) SLAM (CDw150) is a cellular receptor for measles virus. Nature 406 : 893–897.
13. De VriesRD, McQuaidS, Van AmerongenG, YükselS, VerburghRJ, et al. (2012) Measles immune suppression: lessons from the macaque model. PLoS Pathog 8: e1002885.
14. CondackC, GrivelJC, DevauxP, MargolisL, CattaneoR (2007) Measles virus vaccine attenuation: suboptimal infection of lymphatic tissue and tropism alteration. J Infect Dis 196 : 541–549.
15. GrosjeanI, CauxC, BellaC, BergerI, WildF, et al. (1997) Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med 186 : 801–812.
16. Fugier-VivierI, Servet-DelpratC, RivaillerP, RissoanMC, LiuYJ, et al. (1997) Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med 186 : 813–823.
17. SchnorrJJ, XanthakosS, KeikavoussiP, KampgenE, Ter MeulenV, et al. (1997) Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immmunosuppression. Proc Natl Acad Sci U S A 94 : 5326–5331.
18. FerreiraCS, FrenzkeM, LeonardVH, WelsteadGG, RichardsonCD, et al. (2010) Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). J Virol 84 : 3033–3042.
19. LemonK, De VriesRD, MesmanAW, McQuaidS, Van AmerongenG, et al. (2011) Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog 7: e1001263.
20. MossWJ, GriffinDE (2012) Measles. Lancet 379 : 153–164.
21. Van BinnendijkRS, PoelenMCM, KuijpersKC, OsterhausADME, UytdeHaagFGCM (1990) The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. J Immunol 144 : 2394–2399.
22. PermarSR, KlumppSA, MansfieldKG, KimWK, GorgoneDA, et al. (2003) Role of CD8+ lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol 77 : 4396–4400.
23. De VriesRD, YükselS, OsterhausADME, De SwartRL (2010) Specific CD8+ T-lymphocytes control dissemination of measles virus. Eur J Immunol 40 : 388–395.
24. MongkolsapayaJ, JayeA, CallanMFC, MagnusenAF, McMichaelAJ, et al. (1999) Antigen-specific expansion of cytotoxic T lymphocytes in acute measles virus infection. J Virol 73 : 67–71.
25. RyonJJ, MossWJ, MonzeM, GriffinDE (2002) Functional and phenotypic changes in circulating lymphocytes from hospitalized Zambian children with measles. Clin Diagn Lab Immunol 9 : 994–1003.
26. FinkeldeyW (1931) Über Riesenzellbefunde in den Gaumenmandeln, zugleich ein Beitrag zur Histopathologie der Mandelveränderungen im Maserninkubationsstadium. Virchows Arch 281 : 323–329.
27. WarthinAS (1931) Occurrence of numerous large giant cells in the tonsils and pharyngeal mucosa in the prodromal stage of measles. Arch Pathol 11 : 864–874.
28. LisseI, SambB, WhittleH, JensenH, SoumareM, et al. (1998) Acute and long-term changes in T-lymphocyte subsets in response to clinical and subclinical measles. A community study from rural Senegal. Scand J Infect Dis 30 : 17–21.
29. NoyceRS, BondreDG, HaMN, LinLT, SissonG, et al. (2011) Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog 7: e1002240.
30. MühlebachMD, MateoM, SinnPL, PruferS, UhligKM, et al. (2011) Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480 : 530–533.
31. PratakpiriyaW, SekiF, OtsukiN, SakaiK, FukuharaH, et al. (2012) Nectin4 is an epithelial cell receptor for canine distemper virus and involved in the neurovirulence. J Virol 86 : 10207–10210.
32. RacanielloV (2011) An exit strategy for measles virus. Science 334 : 1650–1651.
33. LudlowM, LemonK, De VriesRD, McQuaidS, MillarE, et al. (2013) Measles virus infection of epithelial cells in the macaque upper respiratory tract is mediated by sub-epithelial immune cells. J Virol 87 : 4033–4042.
34. LudlowM, De VriesRD, LemonK, McQuaidS, MillarE, et al. (2013) Infection of lymphoid tissues in the macaque upper respiratory tract contributes to the emergence of transmissible measles virus. J Gen Virol 94 : 1933–1944.
35. VareilleM, KieningerE, EdwardsMR, RegameyN (2011) The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev 24 : 210–229.
36. BeinekeA, PuffC, SeehusenF, BaumgärtnerW (2009) Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 127 : 1–18.
37. NguyenDT, LudlowM, Van AmerongenG, De VriesRD, YükselS, et al. (2012) Evaluation of synthetic infection-enhancing lipopeptides as adjuvants for a live-attenuated canine distemper virus vaccine administered intra-nasally to ferrets. Vaccine 30 : 5073–5080.
38. McCulloughB, KrakowkaS, KoestnerA (1974) Experimental canine distemper virus-induced lymphoid depletion. Am J Pathol 74 : 155–170.
39. Von MesslingV, SpringfeldC, DevauxP, CattaneoR (2003) A ferret model of canine distemper virus virulence and immunosuppression. J Virol 77 : 12579–12591.
40. Von MesslingV, MilosevicD, CattaneoR (2004) Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc Natl Acad Sci U S A 101 : 14216–14421.
41. HirschRL, GriffinDE, JohnsonRT, CooperSJ, Lindo de SorianoI, et al. (1984) Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin Immunol Immunopathol 31 : 1–12.
42. WardBJ, JohnsonRT, VaisbergA, JaureguiE, GriffinDE (1991) Cytokine production in vitro and the lymphoproliferative defect of natural measles virus infection. Clin Immunol Immunopathol 61 : 236–248.
43. GriffinDE, WardBJ (1993) Differential CD4 T cell activation in measles. J Infect Dis 168 : 275–281.
44. SchlenderJ, SchnorrJJ, SpielhoferP, CathomenT, CattaneoR, et al. (1996) Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci U S A 93 : 13194–13199.
45. SchnorrJJ, SeufertM, SchlenderJ, BorstJ, JohnstonICD, et al. (1997) Cell cycle arrest rather than apoptosis is associated with measles virus contact-mediated immunosuppression in vitro. J Gen Virol 78 : 3217–3226.
46. MoussallemTM, GuedesF, FernandesER, PagliariC, LancellottiCLP, et al. (2007) Lung involvement in childhood measles: severe immune dysfunction revealed by quantitative immunohistochemistry. Hum Pathol 38 : 1239–1247.
47. PolackFP, HoffmanSJ, MossWJ, GriffinDE (2002) Altered synthesis of interleukin-12 and type 1 and type 2 cytokines in rhesus macaques during measles and atypical measles. J Infect Dis 185 : 13–19.
48. AtabaniSF, ByrnesAA, JayeA, KiddIM, MagnusenAF, et al. (2001) Natural measles causes prolonged suppression of interleukin-12 production. J Infect Dis 184 : 1–9.
49. HoffmanSJ, PolackFP, HauerDA, GriffinDE (2003) Measles virus infection of rhesus macaques affects neutrophil expression of IL-12 and IL-10. Viral Immunol 16 : 369–379.
50. KarpCL, WysockaM, WahlLM, AhearnJM, CuomoPJ, et al. (1996) Mechanism of suppression of cell-mediated immunity by measles virus. Science 273 : 228–231.
51. SteineurMP, GrosjeanI, BellaC, KaiserlianD (1998) Langerhans cells are susceptible to measles virus infection and actively suppress T cell proliferation. Eur J Dermatol 8 : 413–420.
52. NiewieskS, EisenhuthI, FooksA, CleggJC, SchnorrJJ, et al. (1997) Measles virus-induced immune suppression in the cotton rat (Sigmodon hispidus) model depends on viral glycoproteins. J Virol 71 : 7214–7219.
53. AvotaE, AvotsA, NiewieskS, KaneLP, BommhardtU, et al. (2001) Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat Med 7 : 725–731.
54. MarieJC, SaltelF, EscolaJM, JurdicP, WildTF, et al. (2004) Cell surface delivery of the measles virus nucleoprotein: a viral strategy to induce immunosuppression. J Virol 78 : 11952–11961.
55. KerdilesYM, CherifB, MarieJC, TremillonN, BlanquierB, et al. (2006) Immunomodulatory properties of morbillivirus nucleoproteins. Viral Immunol 19 : 324–334.
56. KerdilesYM, SellinCI, DruelleJ, HorvatB (2006) Immunosuppression caused by measles virus: role of viral proteins. Rev Med Virol 16 : 49–63.
57. GassertE, AvotaE, HarmsH, KrohneG, GulbinsE, et al. (2009) Induction of membrane ceramides: a novel strategy to interfere with T lymphocyte cytoskeletal reorganization in viral immunosuppression. PLoS Pathog 5: e1000623.
58. AvotaE, Schneider-SchauliesS (2014) The role of sphingomyelin breakdown in measles virus immunmodulation. Cell Physiol Biochem 34 : 20–26.
59. ManchesterM, SmithKA, EtoDS, PerkinHB, TorbettBE (2002) Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J Virol 76 : 6636–6642.
60. BoussaadI, VaragnoloL, HornichV, RiegerL, KrockenbergerM, et al. (2011) Wild-type measles virus interferes with short-term engraftment of human CD34+ hematopoietic progenitor cells. J Virol 85 : 7710–7718.
61. AddaeMM, KomadaY, TaniguchiK, KamiyaT, Osei-KwasiM, et al. (1998) Surface marker patterns of T cells and expression of interleukin-2 receptor in measles infection. Acta Paediatr Jpn 40 : 7–13.
62. SullivanJL, BarryDW, LucasSJ, AlbrechtP (1975) Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med 142 : 773–784.
63. HuddlestoneJR, LampertPW, OldstoneMBA (1980) Virus-lymphocyte interactions: infection of Tg and Tm subsets by measles virus. Clin Immunol Immunopathol 15 : 502–509.
64. ItoM, WatanabeM, IharaT, KamiyaH, SakuraiM (1997) Measles virus induces apoptotic cell death in lymphocytes activated with phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore. Clin Exp Immunol 108 : 266–271.
65. OkadaH, KobuneF, SatoTA, KohamaT, TakeuchiY, et al. (2000) Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch Virol 145 : 905–920.
66. PignataC, FioreM, de FilippoS, CavalcantiM, GaetanielloL, et al. (1998) Apoptosis as a mechanism of peripheral blood mononuclear cell death after measles and varicella-zoster virus infections in children. Pediatr Res 43 : 77–83.
67. VuorinenT, PeriP, VainionpääR (2003) Measles virus induces apoptosis in uninfected bystander T cells and leads to granzyme B and caspase activation in peripheral blood mononuclear cell cultures. Eur J Clin Invest 33 : 434–442.
68. Servet-DelpratC, VidalainPO, AzocarO, Le DeistF, FischerA, et al. (2000) Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol 74 : 4387–4393.
69. Servet-DelpratC, VidalainPO, BausingerH, ManieS, Le DeistF, et al. (2000) Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol 164 : 1753–1760.
70. VidalainPO, AzocarO, LamouilleB, AstierA, Rabourdin-CombeC, et al. (2000) Measles virus induces functional TRAIL production by human dendritic cells. J Virol 74 : 556–559.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus InfectionČlánek P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to InfectionČlánek Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Microbial Programming of Systemic Innate Immunity and Resistance to Infection
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
- Measles Immune Suppression: Functional Impairment or Numbers Game?
- Cellular Mechanisms of Alpha Herpesvirus Egress: Live Cell Fluorescence Microscopy of Pseudorabies Virus Exocytosis
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection
- Loss of Dynamin-Related Protein 2B Reveals Separation of Innate Immune Signaling Pathways
- Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Infection
- Unveiling the Intracellular Survival Gene Kit of Trypanosomatid Parasites
- Extreme Divergence of Tropism for the Stem-Cell-Niche in the Testis
- HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4 T Cells
- P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
- Hypercytotoxicity and Rapid Loss of NKp44 Innate Lymphoid Cells during Acute SIV Infection
- Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
- Crystal Structure of Calcium Binding Protein-5 from and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
- Chronic Parasitic Infection Maintains High Frequencies of Short-Lived Ly6CCD4 Effector T Cells That Are Required for Protection against Re-infection
- Specific Dysregulation of IFNγ Production by Natural Killer Cells Confers Susceptibility to Viral Infection
- HSV-2-Driven Increase in the Expression of αβ Correlates with Increased Susceptibility to Vaginal SHIV Infection
- Murine Anti-vaccinia Virus D8 Antibodies Target Different Epitopes and Differ in Their Ability to Block D8 Binding to CS-E
- Brothers in Arms: Th17 and Treg Responses in Immunity
- Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Typhimurium Colitis
- A Negative Feedback Modulator of Antigen Processing Evolved from a Frameshift in the Cowpox Virus Genome
- Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms
- The Non-receptor Tyrosine Kinase Tec Controls Assembly and Activity of the Noncanonical Caspase-8 Inflammasome
- Targeted Changes of the Cell Wall Proteome Influence Ability to Form Single- and Multi-strain Biofilms
- Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
- The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
- Anti-α4 Antibody Treatment Blocks Virus Traffic to the Brain and Gut Early, and Stabilizes CNS Injury Late in Infection
- Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
- Microbial Urease in Health and Disease
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against
- IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
- A Natural Genetic Variant of Granzyme B Confers Lethality to a Common Viral Infection
- Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression
- Differential PfEMP1 Expression Is Associated with Cerebral Malaria Pathology
- The Role of the NADPH Oxidase NOX2 in Prion Pathogenesis
- Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions
- The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
- Expression of Suppressor of Cytokine Signaling 1 (SOCS1) Impairs Viral Clearance and Exacerbates Lung Injury during Influenza Infection
- Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells
- SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in
- Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication
- Oral Streptococci Utilize a Siglec-Like Domain of Serine-Rich Repeat Adhesins to Preferentially Target Platelet Sialoglycans in Human Blood
- SV40 Utilizes ATM Kinase Activity to Prevent Non-homologous End Joining of Broken Viral DNA Replication Products
- Amphipathic α-Helices in Apolipoproteins Are Crucial to the Formation of Infectious Hepatitis C Virus Particles
- Proteomic Analysis of the Acidocalcisome, an Organelle Conserved from Bacteria to Human Cells
- Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



