-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
The blood stage of malaria parasites is responsible for all the morbidity and mortality associated with malaria. During the blood stage, malaria parasites invade and multiply within host erythrocytes. The process of erythrocyte invasion requires specific interactions between host receptors and parasite ligands. Many of the key parasite proteins that bind host receptors are localized in apical organelles called micronemes. Here, we demonstrate that cAMP serves as a key regulator that controls the timely secretion of microneme proteins during invasion. We show that exposure of merozoites to a low K+ environment, as found in blood plasma, leads to a rise in cytosolic cAMP levels due to activation of the cytoplasmic, bicarbonate-sensitive adenylyl cyclase β (PfACβ). A rise in cAMP activates protein kinase A (PKA), which regulates microneme secretion. In addition, cAMP triggers a rise in cytosolic Ca2+ levels through the Epac pathway. Increases in both cAMP and Ca2+ levels are essential for triggering microneme secretion. Identification of the different elements in the cAMP-dependent signaling pathways that regulate microneme secretion during invasion provides novel targets to block erythrocyte invasion, inhibit blood stage parasite growth and prevent malaria.
Published in the journal: . PLoS Pathog 10(12): e32767. doi:10.1371/journal.ppat.1004520
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004520Summary
The blood stage of malaria parasites is responsible for all the morbidity and mortality associated with malaria. During the blood stage, malaria parasites invade and multiply within host erythrocytes. The process of erythrocyte invasion requires specific interactions between host receptors and parasite ligands. Many of the key parasite proteins that bind host receptors are localized in apical organelles called micronemes. Here, we demonstrate that cAMP serves as a key regulator that controls the timely secretion of microneme proteins during invasion. We show that exposure of merozoites to a low K+ environment, as found in blood plasma, leads to a rise in cytosolic cAMP levels due to activation of the cytoplasmic, bicarbonate-sensitive adenylyl cyclase β (PfACβ). A rise in cAMP activates protein kinase A (PKA), which regulates microneme secretion. In addition, cAMP triggers a rise in cytosolic Ca2+ levels through the Epac pathway. Increases in both cAMP and Ca2+ levels are essential for triggering microneme secretion. Identification of the different elements in the cAMP-dependent signaling pathways that regulate microneme secretion during invasion provides novel targets to block erythrocyte invasion, inhibit blood stage parasite growth and prevent malaria.
Introduction
All the clinical symptoms of Plasmodium falciparum malaria are attributed to the blood stage of the parasite life cycle. The intra-erythrocytic stage of the life cycle is initiated when liberated P. falciparum merozoites invade and multiply within host red blood cells. Following the development of mature schizonts, next generation merozoites egress from infected erythrocytes and invade uninfected erythrocytes to start a new cycle of infection. Invasion of erythrocytes by P. falciparum merozoites is a complex multi-step process that is mediated by specific molecular interactions between red cell surface receptors and parasite protein ligands [1], [2]. A number of parasite ligands that mediate receptor binding during invasion reside in apical membrane-bound organelles known as micronemes and rhoptries [1], [2]. Timely secretion of these parasite ligands to the merozoite surface is critical for successful invasion [3], [4].
Microneme and rhoptry proteins are secreted from free P. falciparum merozoites in a two-step process [5]. First, exposure of extracellular merozoites to a low [K+] environment typical of blood plasma leads to a rise in cytosolic Ca2+ via a phospholipase C (PLC)-dependent pathway, which triggers translocation of microneme proteins such as 175 kD erythrocyte binding antigen (EBA175) and apical merozoite antigen-1 (PfAMA1) to the merozoite surface [5]. Subsequently, binding of EBA175 and its homologs to their erythrocyte receptors triggers secretion of rhoptry proteins such as PfRH2b, Clag3.1 and PfTRAMP [5], [6].
The pathways by which exposure of P. falciparum merozoites to a low K+ environment triggers a rise in cytosolic Ca2+ and microneme secretion are not understood. Here, we demonstrate that another ubiquitous second messenger, namely, 3'-5' cyclic adenosine monophosphate (cAMP), plays a central role in regulating cytosolic Ca2+ levels and microneme secretion during merozoite invasion of red blood cells. We demonstrate that exposure of merozoites to a low K+ environment as found in blood plasma activates the bicarbonate-sensitive cytoplasmic adenylyl cyclase β (PfACβ) leading to a rise in cytosolic cAMP levels and activation of protein kinase A (PKA), which regulates microneme secretion. In mammalian cells, the cAMP responsive PKA, which regulates diverse cellular processes in response to a rise in cytosolic cAMP levels, is composed of two catalytic subunits and two regulatory subunits [7]. Unlike mammalian cells, P. falciparum has a single inhibitory regulatory subunit (PfPKAr) and a single catalytic subunit (PfPKAc) [8]–[12]. As the PfPKAr subunit is not predicted to dimerize, the holoenzyme is likely to be composed of a one-to-one ratio of PfPKAr∶PfPKAc [12]. The PfPKAr subunit is predicted to have 2 cyclic nucleotide binding domains. When cAMP binds to one or both of these it provokes a conformational change that engenders the dissociation of the PfPKAr∶PfPKAc complex and activation of the released PfPKAc subunit that phosphorylates its specific substrates [8]–[12]. Like Plasmodium, Toxoplasma also encodes cAMP-dependent PKA and its inhibition leads to a block in tachyzoite growth [13]. Increase in cytosolic cAMP levels that would activate Toxoplasma PKA also mediates the tachyzoite to bradyzoite developmental switch [14]–[16]. In addition to activating PKA, we demonstrate that cAMP activates the Epac pathway [17] in P. falciparum merozoites, which triggers a rise in cytosolic Ca2+ leading to microneme release. cAMP thus plays a central role in regulating microneme secretion during red blood cell invasion by P. falciparum merozoites.
Results
cAMP production by adenylyl cyclase β (PfACβ) in P. falciparum merozoites
cAMP is produced in eukaryotic cells either by a transmembrane adenylyl cyclase (tmAC), which has a putative K+ channel at the N-terminus, or by a cytoplasmic soluble adenylyl cyclase (sAC). Homologs of both the tmAC (PF3D7_1404600) and sAC (PF3D7_0802600) (www.plasmodb.org) are encoded in P. falciparum genome [18], [19]. However, the gene encoding the P. berghei transmembrane AC (PbACα) has been knocked out with no deleterious effect on growth of blood stage parasites although sporozoites are impaired in hepatocyte infectivity [20]. Given that PbACα does not appear to have an essential role in blood stage growth we focused our attention on the soluble AC in P. falciparum (PfACβ).
Mouse antiserum raised against a synthetic peptide derived from PfACβ detects a protein of expected size (>250 kD) in schizont and merozoite lysates by Western blotting (S1 Figure). Detection of PfACβ by immunofluorescence assay (IFA) demonstrates that it co-localizes in late stage schizonts and merozoites with the cytoplasmic protein PfNapL [21] (Fig. 1). PfACβ is thus expressed in P. falciparum merozoites and is localized in the cytoplasm.
Fig. 1. Production of cAMP in P. falciparum merozoites by adenylyl cyclase β (PfACβ) and microneme exocytosis. 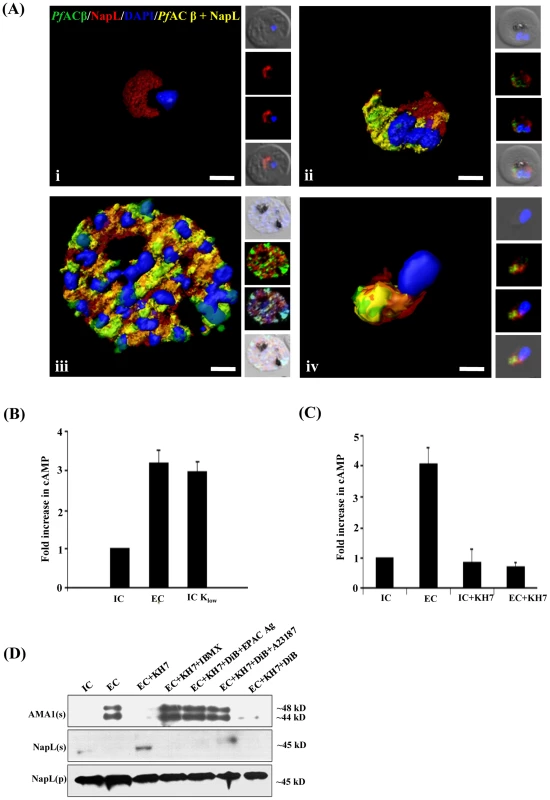
A) Expression of PfACβ in P. falciparum blood stages. Immunofluorescence assays (IFA) were used to detect PfACβ (green) in P. falciparum rings (i), trophozoites (ii), schizonts (iii) and merozoites (iv) using mouse antisera against a peptide derived from PfACβ. Nuclear DNA was stained with DAPI (blue). Rabbit antiserum against P. falciparum cytoplasmic protein PfNAPL (red) was used for co-localization. Yellow indicates overlap of PfACβ and PfNAPL. B) Exposure of P. falciparum merozoites to low K+ triggers production of cAMP. P. falciparum merozoites were isolated in buffer mimicking intracellular ionic environment (IC buffer – 140 mM KCl, 5 mM NaCl, 1 mM MgCl2, 5.6 mM glucose, 25 mM HEPES, pH 7.2) and transferred to buffer mimicking extracellular ionic environment (EC buffer – 5 mM KCl, 140 mM NaCl, 1 mM MgCl2, 2 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2) or IC-Klow buffer (IC-Klow buffer – 5 mM NaCl, 5 mM KCl, 135 mM choline-Cl, 1 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2). Levels of cytosolic cAMP in merozoite lysates were measured using a colorimetric cAMP Direct Immunoassay Kit (Calbiochem) as described in Materials & Methods. Total protein content in each merozoite sample was determined using Pierce Protein Assay Kit (Pierce). Amount of cAMP per µg of protein was determined for each merozoite sample and used to calculate fold increase compared to cAMP per µg of protein in control merozoites in IC buffer. Graphs represent mean fold change (± SD) in cAMP levels per µg of protein in merozoites under different conditions with respect to cAMP per µg of protein in merozoites in IC buffer from three independent experiments. C) Mammalian soluble AC inhibitor KH7 blocks rise in cytosolic cAMP levels following transfer of P. falciparum merozoites to low K+ buffer. P. falciparum merozoites were isolated in IC buffer and transferred to EC buffer with or without treatment with KH7. Levels of cAMP were measured in merozoite lysates as described above. Graphs represent mean fold change (± SD) in cAMP levels per µg of protein in merozoites under different conditions with respect to cAMP per µg of protein in merozoites in IC buffer from three independent experiments. Treatment with KH7 inhibits increase in cytosolic cAMP levels when merozoites are transferred from IC to EC buffer. D) Inhibition of microneme secretion by KH7. P. falciparum merozoites were transferred from IC to EC buffer with or without treatment with KH7. Secretion of PfAMA1 in merozoite supernatants (AMA(s)) was detected by Western blotting. Presence of cytoplasmic protein PfNapL was detected in P. falciparum merozoite supernatants (NapL(s)) and lysates of merozoite pellets (NapL(p)) under different conditions by Western blotting to control for merozoite lysis and number of merozoites used in the different conditions, respectively. Treatment of merozoites with KH7 inhibits secretion of microneme protein PfAMA1 following transfer from IC to EC buffer. Treatment of merozoites with KH7+IBMX, KH7+DiB+Epac Agonist and KH7+DiB+A23187 restores microneme secretion. However, treatment with KH7+DiB does not restore microneme secretion. Exposure of P. falciparum merozoites to an ionic environment with low K+ as found in blood plasma triggers a rise in cytosolic Ca2+ and secretion of microneme proteins [5]. To determine if exposure of free merozoites to a low K+ environment also leads to a rise in cytosolic cAMP, we measured cAMP levels in purified merozoites suspended in a buffer that mimics the intracellular ionic environment (IC buffer – 140 mM KCl, 5 mM NaCl, 1 mM MgCl2, 5.6 mM glucose, 25 mM HEPES, pH 7.2) and following transfer to a buffer that mimics extracellular ionic environment with low K+ levels (EC buffer – 5 mM KCl, 140 mM NaCl, 1 mM MgCl2, 2 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2). cAMP levels were also measured before and after merozoites were transferred from IC to IC-Klow buffer (IC-Klow buffer – 5 mM NaCl, 5 mM KCl, 135 mM choline-Cl, 1 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2) to determine if change in environmental K+ level alone can trigger changes in cAMP levels. There is a distinct rise in cAMP when merozoites are transferred from IC to EC buffer, or from IC to IC-Klow buffer (Fig. 1B). Importantly, transfer of free merozoites to a low K+ buffer in the presence of KH7, which inhibits mammalian sAC [19], blocks the rise in cytosolic cAMP (Fig. 1C).
Regulation of microneme secretion by cAMP
We monitored the secretion of microneme protein PfAMA1 following the transfer of free P. falciparum merozoites from IC to EC buffer with or without prior treatment with KH7. PfAMA1 is proteolytically cleaved into 48 kD and 44 kD fragments that are released into the supernatant following translocation to the merozoite surface [22]. PfAMA1 was detected in merozoite supernatants following transfer from IC to EC buffer by Western blotting using anti-PfAMA1 sera (Fig. 1D). Transfer of extracellular merozoites from IC to EC buffer in the presence of KH7 blocks the secretion of PfAMA1 (Fig. 1D). Treatment of merozoites with KH7 in the presence of 3-isobutyl-1-methylxanthine (IBMX), which inhibits 3’,5’-cAMP phosphodiesterase (PDE) and maintains high cAMP levels in cells [23], reverses the block in microneme secretion (Fig. 1D). Secretion of another microneme protein, EBA175, is also inhibited if merozoites are treated with KH7 prior to transfer from IC to EC buffer (S2 Figure).
cAMP production in P. falciparum merozoites upon exposure to a low K+ environment
Production of cAMP by soluble adenylyl cyclases is regulated by HCO3− ions in diverse organisms [24]–[26]. Incubation of P. falciparum merozoite lysates with ATP and different concentrations of HCO3− ions showed that increasing amounts of cAMP are produced in presence of increasing concentrations of HCO3− ions suggesting the presence of a HCO3− sensitive adenylyl cyclase activity (Fig. 2A). Moreover, cAMP production is inhibited by KH7 (Fig. 2A). These observations confirm the presence of a HCO3−-sensitive ACβ in free P. falciparum merozoites.
Fig. 2. Production of cAMP by HCO3− sensitive PfACβ and regulation of microneme secretion. 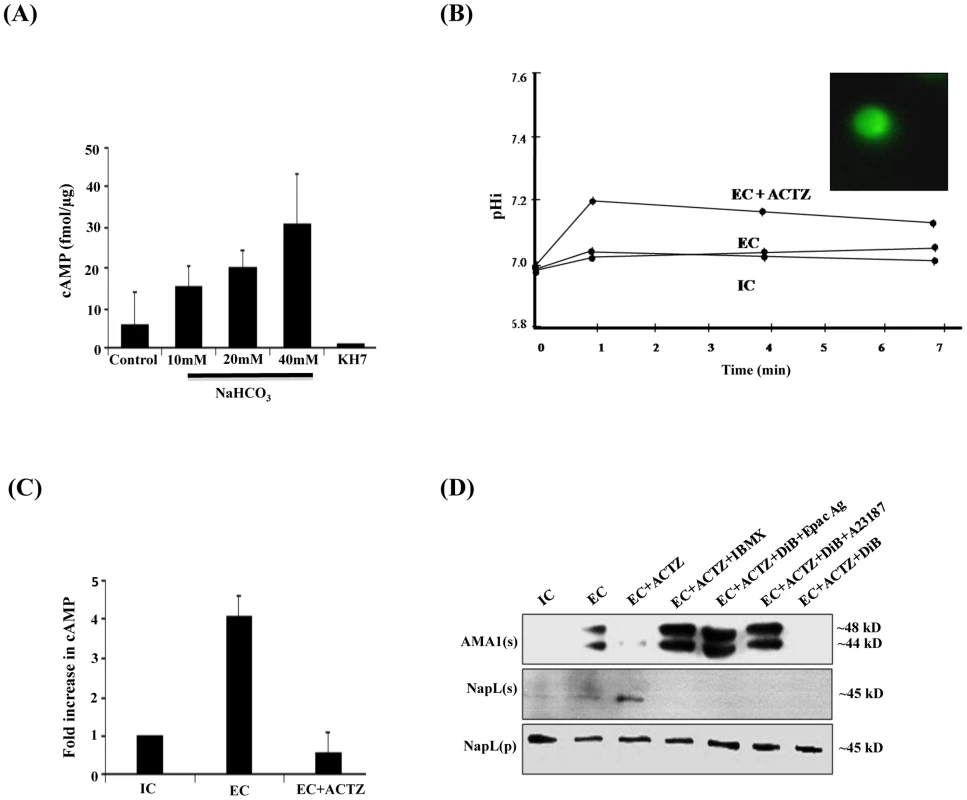
A) HCO−3 sensitive adenylyl cyclase activity in P. falciparum merozoite lysates. P. falciparum merozoite lysates were incubated with increasing concentrations of NaHCO3 (10 mM, 20 mM and 40 mM) and ATP for 30 min at 30°C and production of cAMP was measured. Levels of cAMP are reported (mean + SD from three independent experiments) as femtomoles per µg of merozoite protein. Merozoite lysates without exogenous ATP were used as negative controls. Production of cAMP increased with increasing concentrations of NaHCO3. Addition of KH7 in presence of 40 mM NaHCO3 inhibited cAMP production. B) Intracellular pH (pHi) of P. falciparum merozoites in different ionic environments with and without treatment with carbonic anhydrase (CA) inhibitor acetazolamide (ACTZ). P. falciparum merozoites loaded with the pH-sensitive fluorescent dye BCECF-AM (inset) were transferred from IC to EC buffer with or without prior treatment with ACTZ. Fluorescence signal from BCECF was measured and used to determine pHi using a standard curve (S6 Figure) as described in Materials and Methods. The pHi of P. falciparum merozoites remains unchanged following transfer from IC to EC buffer. Pre-treatment of P. falciparum merozoites with ACTZ results in a rise in pHi following transfer from IC to EC buffer (EC+ACTZ). C) ACTZ blocks rise in intracellular cAMP following transfer of P. falciparum merozoites from IC to EC buffer. P. falciparum merozoites were transferred from IC to EC buffer with or without treatment with ACTZ. Fold change (mean ± SD) in cAMP levels is reported from 3 independent experiments. Pre-treatment with ACTZ inhibits rise in cAMP in merozoites following transfer from IC to EC buffer. D) ACTZ blocks microneme secretion following transfer of P. falciparum merozoites from IC to EC buffer. P. falciparum merozoites were transferred form IC to EC buffer with or without prior treatment with CA inhibitor, ACTZ. Secretion of PfAMA1 in merozoite supernatants (AMA1(s)) was detected by Western blotting. Presence of cytoplasmic protein PfNapL was detected in P. falciparum merozoite supernatants (NapL(s)) and lysates of merozoite pellets (NapL(p)) under different conditions by Western blotting to control for merozoite lysis and number of merozoites used in the different conditions, respectively. Treatment of merozoites with ACTZ prior to transfer from IC to EC buffer inhibits secretion of microneme protein PfAMA1. Treatment of merozoites with ACTZ+IBMX, ACTZ+DiB+Epac Agonist and ACTZ+DiB+A23187 restores microneme secretion. However, treatment with ACTZ+DiB does not restore microneme secretion. Cytosolic pH (pHi) is controlled in eukaryotic cells by carbonic anhydrase (CA), which catalyzes the reversible hydration of CO2 to produce H+ and HCO3− (CO2+H2O↔HCO3−+H+) [27]. Acetazolamide (ACTZ), a specific inhibitor of CA, can disrupt intracellular pH homeostasis [28], [29]. We tested whether the transfer of free merozoites from IC to EC buffer in presence of ACTZ affects pHi. P. falciparum merozoites collected in IC buffer were loaded with the membrane permeable pH-sensitive fluorescent dye BCECF-AM [30] and transferred to EC buffer in the presence or absence of ACTZ. pHi does not change when free merozoites are transferred from IC to EC buffer. In contrast, there is a rapid rise in pHi when free merozoites are transferred from IC to EC buffer in presence of ACTZ (Fig. 2B). These observations indicate that PfCA activity is necessary for maintenance of pHi when merozoites are transferred to a low K+ environment.
The observation that pHi rises when merozoites are transferred to EC in presence of ACTZ is consistent with a model in which PfCA maintains pHi by producing H+ and HCO3−. To test whether the generation of HCO3− in free merozoites following transfer from IC to EC buffer leads to activation of PfACβ, we measured levels of cAMP in merozoites with or without treatment with ACTZ. Treatment of merozoites with ACTZ prior to transfer from IC to EC buffer inhibits rise in cAMP (Fig. 2C) as well as secretion of PfAMA1 (Fig. 2D). Treatment of P. falciparum merozoites with ACTZ in presence of IBMX reverses the block in microneme secretion (Fig. 2D). These results suggest that when free merozoites are exposed to an extracellular-like low K+ environment, the production of HCO3− by PfCA to maintain pHi leads to activation of PfACβ and generation of cAMP, which triggers microneme secretion. Exposure of merozoites in IC buffer to increasing concentrations of HCO3− also leads to a dose-dependent increase in microneme secretion (S3 Figure) confirming that HCO3− plays a key role in regulating microneme secretion.
Potential role of cAMP-responsive protein kinase A (PKA) in regulation of microneme secretion
PKA commonly plays a role as a cAMP-responsive effector in signaling pathways [7]. To investigate the potential role of PfPKA in microneme discharge, we measured phosphorylation of kemptide, a specific substrate of PKA, by lysates of P. falciparum merozoites in IC and EC buffers with and without addition of KH7. There is a distinct increase in kemptide phosphorylation with lysates made from merozoites in EC buffer compared to IC buffer (Fig. 3A). Pre-treatment with KH7 inhibits this increase in kemptide phosphorylation by merozoite lysates in EC buffer suggesting that a cAMP-responsive kinase is activated when merozoites are transferred to EC buffer (Fig. 3A).
Fig. 3. cAMP responsive kinase activity in P. falciparum merozoites under different ionic conditions and microneme secretion. 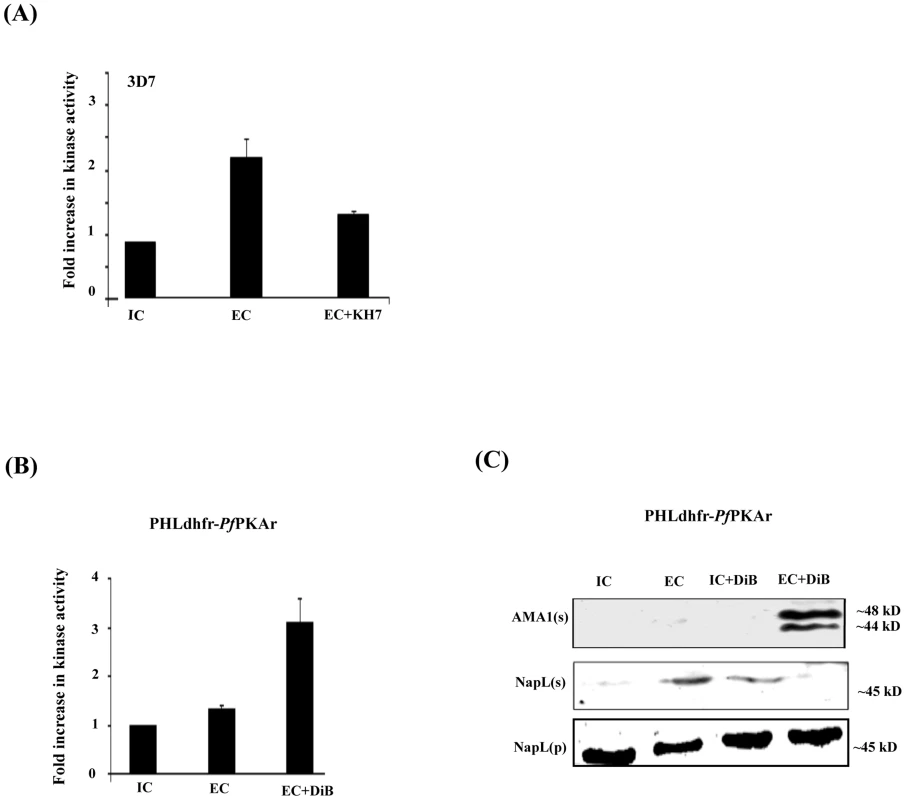
A) Kemptide phosphorylation by P. falciparum 3D7 merozoite lysates under different ionic environments. P. falciparum 3D7 merozoites were transferred from IC buffer to EC buffer with or without treatment with ACβ inhibitor, KH7. Merozoite lysates made under different conditions were incubated with kemptide, a protein kinase A (PKA) substrate, and γ-32P-ATP. Fold change (mean ± SD) in phosphorylation of kemptide under different ionic conditions is reported relative to phosphorylation in IC buffer from 3 independent experiments. B) Kemptide phosphorylation by lysates of transgenic P. falciparum PHL dhfr-PfPKAr merozoites under different ionic environments. P. falciparum PHL dhfr-PfPKAr merozoites were isolated in IC buffer and transferred to EC buffer with or without treatment with dibutryl-cAMP (DiB). Phosphorylation of kemptide by merozoite lysates made under different conditions was measured in presence of γ-32P-ATP. Fold change in kemptide phosphorylation (mean ± SD) by merozoite lysates under different conditions is reported relative to that in IC buffer from 3 independent experiments. C) Microneme secretion in P. falciparum PHL dhfr-PfPKAr merozoites. P. falciparum PHL dhfr-PfPKAr merozoites were transferred from IC to EC buffer, IC buffer+DiB, or EC buffer+DiB and secretion of PfAMA1 into merozoite supernatants (AMA1(s)) was detected by Western blotting. Presence of cytoplasmic protein PfNapL was detected in P. falciparum merozoite supernatants (NapL(s)) and lysates of merozoite pellets (NapL(p)) under different conditions by Western blotting to control for merozoite lysis and number of merozoites used in the different conditions, respectively. PfAMA1 was not secreted when P. falciparum PHL dhfr-PfPKAr merozoites were transferred from IC to IC+DiB, or from IC to EC buffer. PfAMA1 was secreted when P. falciparum PHL dhfr-PfPKAr merozoites were transferred from IC to EC+DiB. We used a molecular genetic approach to explore the regulatory role of PfPKA in microneme secretion. The P. falciparum transgenic line PHL dhfr-PfPKAr over-expresses the regulatory subunit (PfPKAr) (11, S4 Figure). Overexpression of PfPKAr results in reduced PKA activity and a parasite growth defect, which is restored by addition of the non-hydrolyzable cAMP analog dibutryl-cAMP (DiB) [11]. In contrast to wild type P. falciparum merozoites (Fig. 3A), lysates of P. falciparum PHL dhfr-PfPKAr merozoites in EC buffer do not phosphorylate kemptide at higher levels compared to lysates made in IC buffer (Fig. 3B). Treatment of P. falciparum PHL dhfr-PfPKAr merozoite lysates with DiB increases kemptide phosphorylation (Fig. 3B). These observations indicate that kemptide phosphorylation is primarily due to PfPKA and PfPKA activity increases when merozoites are transferred from IC to EC buffer.
Next, we followed secretion of PfAMA1 upon transfer of P. falciparum PHL dhfr-PfPKAr merozoites from IC to EC buffer with and without DiB treatment (Fig. 3C). PfAMA1 is not secreted when P. falciparum PHL dhfr-PfPKAr merozoites are transferred from IC to EC buffer (Fig. 3C). However, PfAMA-1 secretion is restored when P. falciparum PHL dhfr-PfPKAr merozoites are treated with DiB in EC buffer presumably due to the activation of PfPKA (Fig. 3C). These observations suggest that PfPKA plays a role in regulation of microneme secretion. Impairment of microneme secretion due to PfPKAr overexpression may be one of the reasons for poor growth of P. falciparum PHL dhfr-PfPKAr parasites.
Crosstalk between cAMP and Ca2+ signaling in P. falciparum merozoites and regulation of microneme secretion
Changes in the levels of cytosolic Ca2+ and cAMP control a variety of functions in diverse cell types [31]. We examined the crosstalk between these two ubiquitous second messengers in extracellular P. falciparum merozoites. We found that treatment of merozoites with the Ca2+ chelator, BAPTA-AM, or PLC inhibitor, U73122, does not affect the increase in cAMP levels when merozoites are transferred from IC to EC buffer (Fig. 4A). In contrast, treatment of merozoites with the ACβ inhibitor KH7 inhibits the increase in cytosolic Ca2+ levels when merozoites are transferred from IC to EC buffer (Fig. 4B) indicating that cAMP plays a key role in regulating cytosolic Ca2+ levels in P. falciparum merozoites.
Fig. 4. Crosstalk between cAMP and Ca2+ in P. falciparum merozoites. 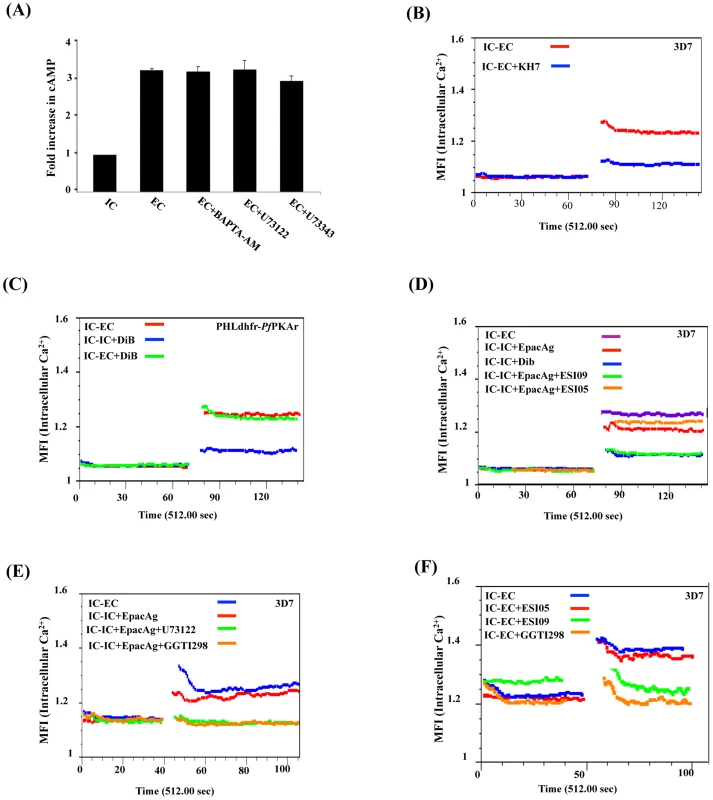
A) Ca2+ does not regulate cytosolic cAMP levels. P. falciparum merozoites were transferred from IC to EC buffer with or without treatment with BAPTA-AM or U73122. Levels of cytosolic cAMP were measured in merozoite lysates before and after transfer to EC buffer. Fold changes in cAMP levels per µg of merozoite protein (mean ± SD from 3 independent experiments) in different conditions relative to cAMP levels in IC buffer (mean ± SD) from 3 independent experiments are reported. Treatment of merozoites with BAPTA-AM or U73122 does not have any effect on rise in intracellular cAMP levels following transfer from IC to EC buffer. B) Rise in cytosolic Ca2+ is inhibited by ACβ inhibitor KH7. P. falciparum merozoites were loaded with Fluo-4AM and transferred from IC to EC buffer with or without treatment with KH7. Cytosolic Ca2+ levels in P. falciparum merozoites were measured before and after transfer by flow cytometry. Treatment with KH7 inhibits the rise in cytosolic Ca2+ following transfer to EC buffer. C) PKA does not regulate cytosolic Ca2+. P. falciparum PHL dhfr-PfPKAr merozoites were loaded with Fluo-4AM and transferred from IC to EC buffer or from IC to IC+DiB or from IC to EC+DiB. Cytosolic Ca2+ levels rise normally following transfer of P. falciparum merozoites from IC to EC buffer. Cytosolic Ca2+ levels do not rise when P. falciparum PHL dhfr-PfPKAr merozoites in IC buffer are treated with DiB indicating that PKA does not play a role in regulating Ca2+ levels in merozoites. D) Regulation of cytosolic Ca2+ levels in P. falciparum 3D7 merozoites by Epac. P falciparum merozoites loaded with Fluo-4AM were transferred from IC to EC buffer or from IC to IC buffer containing Epac agonist 8-Pcpt-2’-O-Me-cAMP (IC+Epac agonist), DiB (IC+DiB), or Epac agonist and Epac inhibitors (IC+Epac agonist+ESI-09 or IC+Epac agonist+ESI-05). Cytosolic Ca2+ levels rise when merozoites are transferred from IC to EC buffer, or IC buffer to IC+Epac agonist, but not when they are transferred from IC buffer to IC+DiB. EPAC1 antagonist ESI-09 inhibits rise in Ca2+ stimulated by Epac agonist. E) Regulation of cytosolic Ca2+ levels in P. falciparum 3D7 merozoites by Epac agonist, PLC inhibitor and Rap1 inhibitor. P falciparum merozoites loaded with Fluo-4AM were transferred from IC to EC buffer, or from IC to IC buffer containing Epac agonist (IC+Epac agonist), or IC buffer containing Epac agonist and PLC inhibitor (IC+Epac agonist+U73122), or IC buffer containing Epac agonist and Rap1 inhibitor GGTI298 (IC+Epac agonist+GGTI298). Cytosolic Ca2+ levels rise when merozoites are transferred from IC to EC buffer, or from IC to IC+Epac agonist. PLC inhibitor U73122 and Rap1 inhibitor GGTI298 inhibit rise in cytosolic Ca2+ stimulated by Epac agonist. F) Cytosolic Ca2+ levels in P. falciparum 3D7 merozoites following transfer to EC buffer in presence of Epac and Rap1 inhibitors. P falciparum merozoites loaded with Fluo-4AM were transferred from IC to EC buffer containing Epac inhibitors (EC+ESI-09 or EC+ESI-05), or EC buffer containing Rap1 inhibitor (EC+GGTI298). Cytosolic Ca2+ rises when merozoites are transferred from IC to EC buffer. Presence of Epac inhibitor ESI-09 and Rap1 inhibitor GGTI298 inhibits rise in cytosolic Ca2+. Next, we found that Ca2+ levels increase in transgenic P. falciparum PHL dhfr-PKAr merozoites following their transfer from IC to EC buffer (Fig. 4B). Moreover, treatment of transgenic merozoites with DiB in IC buffer, which activates PfPKA, does not lead to further increase in cytosolic Ca2+ levels (Fig. 4C). These observations indicate that PfPKA does not play a role in generation of free cytosolic Ca2+ in P. falciparum merozoites following transfer from IC to EC buffer.
Alternative cAMP-responsive effectors, Epac 1 and Epac 2, have been shown to activate phospholipase C (PLC) in presence of cAMP leading to rise in cytosolic Ca2+ in mammalian cells [17], [32], [33]. P. falciparum codes for a single putative parasite PfEpac (PF3D7_1417400). Treatment of merozoites in IC buffer with the Epac agonist, 8-pCPT-2’-O-Me-cAMP [34], leads to a rise in cytosolic Ca2+ levels (Fig. 4D). This increase in Ca2+ is blocked if merozoites are treated with ESI-09, which inhibits both mammalian Epac 1 and Epac 2 [35] (Fig. 4D). ESI-05, which only inhibits mammalian Epac 2 [36], does not block increase in cytosolic Ca2+ following treatment with the Epac agonist (Fig. 4D). In mammalian cells, Epac activated by cAMP catalyzes the transfer of GTP to Rap1 [17]. Rap1-GTP activates PLC and triggers a rise in cytosolic Ca2+ through the PLC pathway [17]. Stimulation of merozoites with the Epac agonist in presence of GGTI298 [37], [38], which disrupts Rap1 activity, or the PLC inhibitor, U73122 [5], [39], inhibits rise in cytosolic Ca2+ (Fig. 4E). Moreover, treatment of merozoites with either ESI-09 or GGTI298 prior to transfer from IC to EC buffer also blocks rise in cytosolic Ca2+ (Fig. 4F) and inhibits PfAMA1 secretion (Fig. 5A, 5B). In contrast, ESI-05, which does not inhibit increase in cytosolic Ca2+ levels (Fig. 4F), does not block secretion of PfAMA1 (Fig. 5A). Treatment of merozoites with GGT1298 together with the Ca2+ ionophore A23187 restores microneme secretion (Fig. 5B). Thus, a cAMP-responsive Epac-Rap1 pathway is likely to be involved in regulating Ca2+ levels in P. falciparum merozoites.
Fig. 5. Role of Epac and PKA in regulation of microneme secretion and erythrocyte invasion by P. falciparum merozoites. 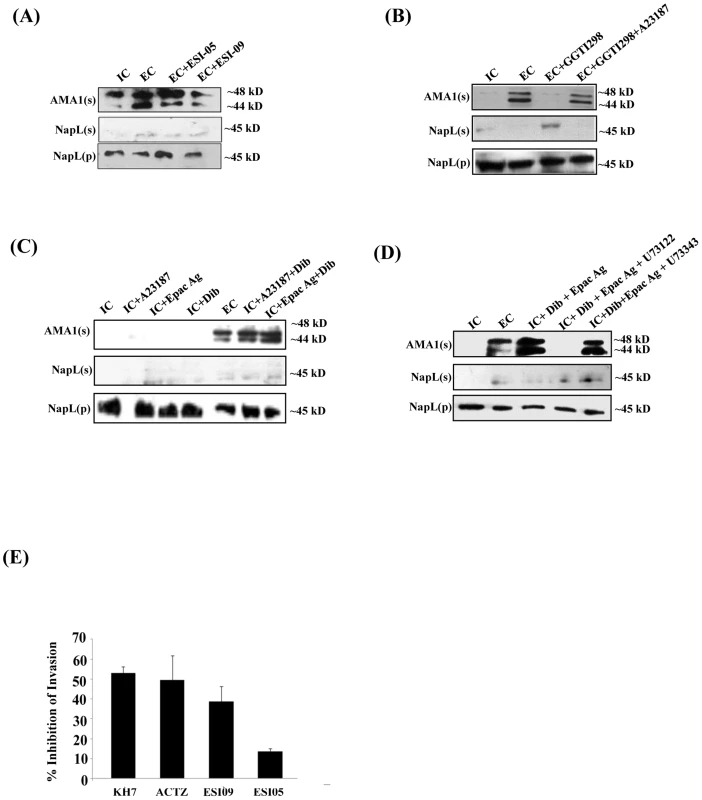
P. falciparum merozoites were transferred from IC to EC buffer with or without treatment with various inhibitors. Secretion of PfAMA1 into merozoite supernatants (AMA1(s)) was detected by Western blotting. Cytoplasmic protein PfNapL was detected in P. falciparum merozoite supernatants (NapL(s)) and pellets (NapL(p)) by Western blotting under different conditions to control for merozoite lysis and number of merozoites used, respectively. A) Epac inhibitor ESI-09 blocks microneme secretion. Treatment of merozoites with 25 µM ESI-09 inhibits secretion of microneme protein PfAMA1 following transfer from IC to EC buffer. B) Rap1 inhibitor GGTI298 blocks microneme secretion. Treatment of merozoites with GGTI298 inhibits secretion of PfAMA1 following transfer from IC to EC buffer. Treatment of merozoites with GGTI298 in presence of A23187 does not block PfAMA1 secretion. C) Inhibition of microneme secretion by Epac and PKA pathway blockade. P. falciparum merozoites in IC buffer were treated with Ca2+ ionophore A23187 (IC+A23187), Epac agonist 8-Pcpt-2’-O-Me-cAMP (IC+Epac Ag) or dibutryl-cAMP (IC+DiB), or with various combinations (IC+A23187+DiB or IC+Epac Ag+DiB). Treatment of merozoites in IC buffer with Epac Ag and DiB (IC+Epac Ag+DiB) or A23187 and DiB (IC+A23187+DiB) triggers microneme secretion. D) P. falciparum merozoites in IC buffer were treated with dibutryl-cAMP and Epac agonist (IC+Dib+Epac agonist) or DiB+Epac Ag+PLC inhibitor U73122 or its inactive analog U73343. Treatment with U73122 inhibits microneme secretion induced by treatment with Dib and Epac Ag. E) Effect of various inhibitors of cAMP and Ca2+ signaling pathways on erythrocyte invasion by P. falciparum merozoites. P. falciparum 3D7 merozoites were isolated and allowed to invade erythrocytes in the presence of KH7 (50 µM), ACTZ (100 µM), ESI-09 (25 µM) and ESI-05 (25 µM). Newly invaded trophozoites were stained with ethidium bromide and scored by flow cytometry. Merozoites were allowed to invade erythrocytes in the absence of inhibitors as control. Percent invasion inhibition rates in presence of inhibitors are reported. Data represent mean (± SD) from three independent experiments with SD. To confirm the role of cAMP-responsive effectors PfPKA and PfEpac in microneme secretion, we treated merozoites in IC buffer with the Epac agonist, the Ca2+ ionophore A23187 and DiB, either individually or in combination. Treatment of merozoites in IC buffer with Epac agonist, A23187 or DiB individually does not trigger PfAMA1 secretion (Fig. 5C). However, treatment of merozoites in IC buffer with A23187 and DiB, or Epac-agonist and DiB triggers PfAMA1 secretion (Fig. 5C). The PLC inhibitor U73122 blocks PfAMA1 secretion induced by treatment of merozoites in IC buffer with Epac-agonist and DiB (Fig. 5D). However, the inactive analog, U73343 has no inhibitory effect. These observations suggest that Epac-agonist triggers rise in Ca2+ through the PLC pathway. Moreover, both Ca2+ and cAMP surges are required to trigger the secretion of microneme proteins in free merozoites.
Blockade of cAMP - & Ca2+-signaling and inhibition of erythrocyte invasion by P. falciparum merozoites
Erythrocyte invasion assays were performed with purified extracellular merozoites in the presence of inhibitors of cAMP - and Ca2+-mediated signaling pathways. Merozoites were treated with ACβ inhibitor KH7, the CA inhibitor ACTZ, or Epac inhibitors ESI-09 and ESI-05, and allowed to invade human erythrocytes. Successful invasion events were scored by flow cytometry using DNA intercalating dye ethidium bromide to identify infected erythrocytes. KH7, ACTZ and ESI-09 inhibit invasion (Fig. 5E) adding to the evidence that both cAMP and Ca2+-dependent signaling pathways play critical roles in the process of red cell invasion by P. falciparum merozoites.
Discussion
We have previously demonstrated that exposure of merozoites to a low K+ environment, as found in blood plasma, triggers a rise in cytosolic Ca2+ through a PLC mediated pathway leading to microneme secretion [5]. Although it is clear that a rise in cytosolic Ca2+ plays a key role in regulating microneme release, the signal transduction mechanisms by which exposure of extra-erythrocytic merozoites to a low K+ environment leads to a rise in cytosolic Ca2+ were not known.
Here, we demonstrate the role of cAMP in regulating levels of cytosolic Ca2+ and microneme secretion in response to exposure of merozoites to a low K+ environment. The transmembrane channel linked expressed in Paramecium, which contains a putative K+ channel-like transmembrane domain fused to the adenylyl cyclase catalytic domain, produces cAMP when the organism is exposed to a low K+ environment [18]. A homolog of tmAC, referred to as ACα is expressed in blood stage Plasmodium parasites. However, deletion of the gene encoding P. berghei ACα does not have any deleterious effect on growth of blood stage parasites [20]. In contrast, deletion of the gene encoding P. berghei ACα impairs hepatocyte invasion by P. berghei sporozoites [20]. These observations suggest that the soluble adenylyl cyclase, ACβ may play a more significant role in regulation of cAMP levels in Plasmodium merozoites.
Here, we have used a series of pharmacological inhibitors to explore the role of PfACβ as well as other players involved in signaling pathways that control microneme secretion. Based on the observations made here, we propose the following model for the regulation of microneme secretion in response to environmental signals (Fig. 6). We propose that when merozoites are exposed to a low K+ environment, PfCA produces HCO3− and H+ ions to maintain pHi homeostasis (Fig. 6). Given the lack of information about the nature of ion channels present on the surface of P. falciparum merozoites, it is difficult to develop a molecular model to explain the activation of PfCA for maintenance of pHi leading to production of HCO3− when merozoites are exposed to a low K+ environment. Production of HCO3− by PfCA activates PfACβ leading to a surge in cAMP levels in free merozoites (Fig. 6). Treatment of merozoites with CA inhibitor, ACTZ, or PfACβ inhibitor, KH7, blocks production of cAMP and inhibits microneme secretion. cAMP thus serves as a key second messenger that regulates microneme secretion in free merozoites during red cell invasion (Fig. 6).
Fig. 6. Model for cAMP and Ca2+ mediated signaling pathways that regulate microneme secretion in P falciparum merozoites. 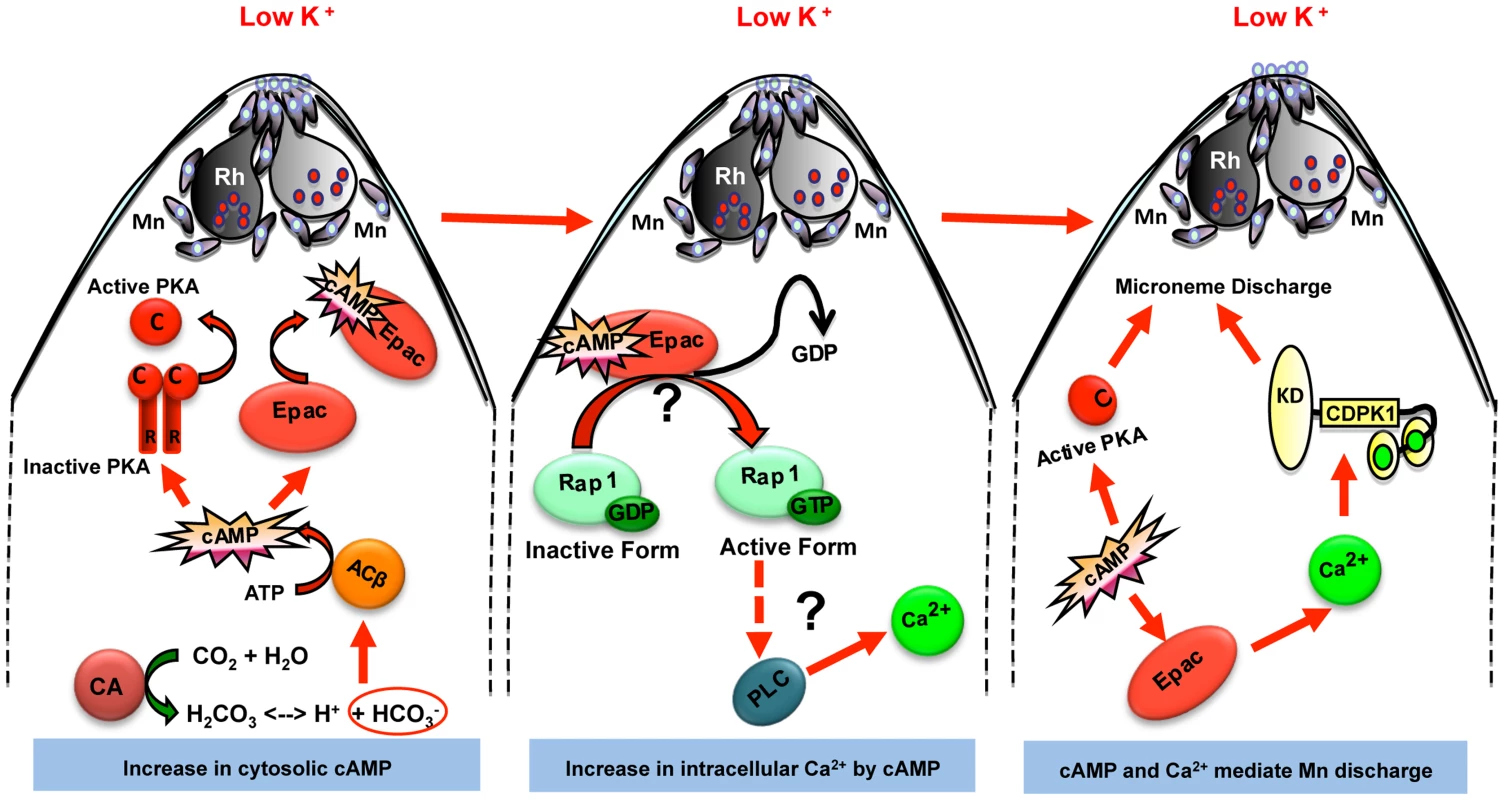
Exposure of P. falciparum merozoites to low K+ environment as present in blood plasma leads to production of H+ and HCO3− ions by carbonic anhydrase (CA) to maintain pH. HCO3− ions activate cytoplasmic adenylyl cyclase (ACβ) leading to rise in cytosolic levels of cAMP. Elevation of cAMP activates its downstream effectors PKA and Epac. Epac activates Rap1 by transferring GTP. Rap1-GTP activates PLC leading to rise in cytosolic Ca2+ levels, which leads to activation of calcium dependent protein kinase 1 (CDPK1) and calcium dependent phosphatase, calcineurin (CN), both of which directly play roles in microneme secretion. Mn, micronemes; Rh, rhoptries. Next, we demonstrated that production of cAMP in merozoites leads to activation of the cAMP-responsive kinase, PfPKA. We confirmed using a genetic approach that PfPKA plays a role in microneme secretion. Over-expression of the regulatory subunit, PfPKAr, inhibits PfPKA activity and blocks microneme secretion implicating PfPKA in regulation of microneme secretion. PfPKA has been shown to phosphorylate the cytoplasmic domain of PfAMA1 during invasion [40], although the precise functional role in the invasion process is not yet known. A global study of changes in protein phosphorylation in merozoites following their transfer to a low K+ environment is needed to identify the signaling pathways involved in microneme secretion. Determination of changes in protein phosphorylation in the presence and absence of PfPKA inhibition will identify substrates of PfPKA that may be involved in microneme secretion and invasion.
Given the essential roles that both Ca2+ and cAMP play in regulating microneme secretion in free merozoites, we examined the crosstalk between these key second messengers. Inhibiting rise in cytosolic Ca2+ by treatment of merozoites with BAPTA-AM or PLC inhibitor U73122 does not affect rise in cAMP when merozoites are exposed to a low K+ environment. In contrast, blocking a rise in cAMP with KH7 does inhibit increase in cytosolic Ca2+ levels. Thus, a rise in cAMP levels precedes and is necessary for an increase in cytosolic Ca2+ when merozoites are exposed to a low K+ environment.
We explored the mechanism by which cAMP regulates cytosolic Ca2+ levels in merozoites and found that PfEpac, rather than PfPKA, is responsible. We present pharmacological evidence to indicate that merozoites possess functional homologs of Epac and Rap1 that activate PLC to trigger a rise in cytosolic Ca2+ in response to increase in cAMP levels. A potential PfEpac is encoded by PF3D7_1417400 (www.plasmodb.org). A search for Rap1 homologs identifies a number of P. falciparum genes predicted to encode small GTPases. It remains to be seen which one these is the functional homolog of Rap1 that activates PLC in response to rise in cAMP in P. falciparum merozoites.
Previous studies with mature schizonts demonstrated that activation of a cGMP-dependent protein kinase, PfPKG, triggers release of proteins from micronemes and exonemes in merozoites leading to egress [41]. Secretion of exoneme proteins such as the protease PfSUB1 plays a critical role in merozoite egress from infected erythrocytes [42], [43]. The external, or internal signals that trigger a rise in cGMP and activate PfPKG leading to egress are not known. A sharp rise in cytosolic Ca2+ in intra-erythrocytic merozoites is also observed just prior to release of microneme and exoneme proteins and parasite egress [5], [43], [44]. The pathways for crosstalk between cGMP and Ca2+ in P. falciparum merozoites during egress have not been defined and whether cGMP-dependent PfPKG plays a role in microneme secretion in free merozoites after egress is not known. It will be important to understand the crosstalk between cGMP, cAMP and Ca2+ in merozoites leading to apical organelle exocytosis both before and after egress.
One possible mechanism for such crosstalk may be through phosphorylation of the PfPKAr subunit by PfPKG that would relieve inhibition of the catalytic PfPKAc subunit. PfPKAr displays characteristics of both mammalian RI and RII subunits [12]. Mammalian RI and RII subunits have distinct inhibitor sites. PfPKAr has a serine in the P-site typical of RII subunits and is phosphorylated by PfPKA in vitro [12]. However, PfPKAr also has a serine in the P+2 site typical of RI subunits. In mammalian cells this site is phosphorylated by PKG [45]. Like the P-site, the P+2 site in PfPKAr is also phosphorylated in vivo in P. falciparum schizonts [46]. Moreover, PfPKG can phosphorylate PfPKAr in vitro (Baker D. and Langsley G., unpublished data). Thus, PfPKG phosphorylation of PfPKAr could provide a mechanism for crosstalk between cGMP - and cAMP-mediated signaling.
A recent study describes a different mechanism for elevation of cytosolic Ca2+ in merozoites leading to microneme release [47]. This study suggests that interaction of the rhoptry neck protein PfRH1 with its erythrocyte receptor can trigger a surge in cytosolic Ca2+ levels in merozoites leading to release of microneme proteins such as EBA175 [47]. It remains to be seen if cAMP levels also rise and play a role in regulating Ca2+ levels following receptor-engagement by PfRH1. Moreover, the observation that PfRH1 knock-out parasites invade erythrocytes efficiently and display normal blood stage growth [48] suggests that P. falciparum merozoites must have alternative signaling mechanisms to trigger microneme release. Whether other members of the PfRH family such as PfRH2a/b can also mediate rise in cytosolic Ca2+ and microneme release remains to be determined. Alternatively, as described here, exposure to low K+ as found in blood plasma may provide such an alternative mechanism to trigger rise in cytosolic Ca2+ and microneme release.
Our study demonstrates that cAMP plays a central role in regulating microneme secretion during invasion (Fig. 6). Our data provides evidence to suggest that cAMP not only regulates microneme secretion by activating PfPKA, but it also regulates cytosolic Ca2+ levels by activation of PfEpac. Our definition of the signal transduction pathways leading to rise in cAMP and Ca2+ levels in response to external signals and the identification of cAMP and Ca2+ responsive effectors provides multiple points for therapeutic intervention. For example, inhibition of PfCA, PfACβ and PfEpac blocks microneme secretion and red blood cell invasion by P. falciparum merozoites. Small molecule inhibitors that specifically target these key-signaling molecules may provide valuable leads for the development of novel anti-malarial drugs that block blood stage growth of malaria parasites.
Materials and Methods
In vitro parasite culture
P. falciparum 3D7 was cultured in complete RPMI (RPMI 1640 (Invitrogen, USA), 27.2 mg/L hypoxanthine (Sigma, USA), 0.5 gms/L Albumax I (Gibco, USA) and 2 gm/L sodium bicarbonate (Sigma, USA) using O+ human erythrocytes under mixed gas (5% O2, 5% CO2 and 90% N2) as described previously [49]. P. falciparum PHL dhfr-PfPKAr was cultured in complete RPMI according to the protocol described previously [11].
Generation of PfACβ specific antisera
A peptide (1916–1930 aa) derived from PfACβ (PlasmoDB ID PF3D7_0802600) was synthesized, conjugated to keyhole limpet hemocyanin (KLH) and used to immunize mice to obtain anti-PfACβ specific antibodies.
Generation of PfPKAr and PfPKAc specific antisera
The coding sequence of PfPKAr was amplified by PCR from P. falciparum cDNA with primers containing BamHI and XhoI restriction sites. The fragment was inserted into pCR2.1-TOPO and verified by sequencing. After digestion by XhoI and BamHI the insert was cloned into pGEX6P-1 (GE Healthcare) and transformed into E. coli BL21 that were grown in 50 mL of LB medium containing ampicillin (100 µg/mL, Euromedex). Recombinant protein expression was induced at 30°C for 4 h by addition of 0.2 mM IPTG (Euromedex). Cells were collected by centrifugation and lysed by sonication in lysis buffer (1× PBS-1 mM salt, 1% Triton, EDTA, protease inhibitors). The lysate was centrifuged (15000 rpm, 4°C, 60 min) and the soluble fraction incubated with glutathione sepharose beads (GE Healthcare) with stirring at 4°C for 90 min. Beads were washed with buffer (1× PBS, 1% Triton, 1 mM EDTA), the GST-tagged recombinant protein was eluted using increasing concentrations of glutathione. Purified recombinant antigen was used to immunize two rats to obtain anti-PfPKAr specific antibodies.
PfPKAc was cloned in the same way as PfPKAr. To increase stability of the recombinant catalytic subunit, we co-expressed PfPKAc-His with PfPKAr-GST in E. coli BL21. Transformed bacteria were grown in 50 mL of LB medium containing ampicillin (100 µg/mL, Euromedex). Recombinant protein expression was induced at 37°C for 4 h by addition of 0.5 mM IPTG (Euromedex). The bacterial pellet was washed once with 4 volumes of PBS and lysed using 4 volumes of Tris 20 mM at 4°C. After sonication, the lysate was centrifuged (6000 g, 4°C, 15 min) and the pellet resuspended in 3 volumes of inclusion body wash solution (2 M Urea, 500 mM NaCl, 2% NP40, 20 mM Tris-HCl, pH 8.0). The inclusion bodies were solubilised (5 mM Imidazole, 1 mM β-mercaptoethanol, 6 M Guanidine-HCl 6M, 20 mM Tris-HCl pH 8.0) and recombinant protein purified by metal affinity chromatography. His-tagged PfPKAc was eluted (8 M Urea to 0 M Urea, 20 mM imidazole to 500 mM imidazole, 500 mM NaCl, 1 mM β-mercaptoethanol, 20 mM Tris-Hcl pH 8.0) and used to immunize two rabbits to obtain anti-PfPKAc specific antibodies.
Isolation of P. falciparum merozoites
P. falciparum blood stage cultures were synchronized with 5% sorbitol for at least two successive cycles. Synchronized P. falciparum cultures were used to isolate merozoites as described previously [5], [43]. Briefly, when majority of infected erythrocytes reached mature schizont stage with prominent segmentation of merozoites, the culture was resuspended in IC buffer (IC buffer – 140 mM KCl, 5 mM NaCl, 1 mM MgCl2, 5.6 mM glucose, 25 mM HEPES, pH 7.2) and schizonts were allowed to rupture to release merozoites over one hour at 37°C. Unruptured scizonts and uninfected erythrocytes were separated by centrifugation at 500 g for 5 min. The supernatant containing free merozoites was centrifuged at 3300 g for 5 min to collect merozoites. The merozoites were resuspended in IC buffer. The purity of merozoite preparations was confirmed by Giemsa staining (S5 Figure). Viability of merozoite preparations was estimated by labeling merozoites with dihydroethidine. Fluorescent merozoites were scored by flow cytometry and viability of merozoite preparations was estimated to be ∼65%.
Drug treatments
P. falciparum merozoites were isolated in IC buffer and treated for 15 mins with the following at concentrations shown: 100 µM IBMX (3-isobutyl-1-methylxanthine, Calbiochem, USA), 50 µM KH7 (Calbiochem, USA), 100 µM ACTZ (acetazolamide, Sigma, USA), 20 µM DiB-cAMP (adenosine 3,5 cyclic monophosphate, N6, O2 - dibutyryl sodium salt, Calbiochem, USA), 10 µM Ca2+ ionophore A23187 (Calbiochem, USA), 50 µM BAPTA-AM (Calbiochem, USA), 10 µM U73122 (Calbiochem, USA), 10 µM U73343 (Calbiochem, USA), 200 µM Epac agonist (8-pCPT-2’-O-Me-cAMP, Sigma, USA), 25 µM Epac antagonist ESI-05 (Biolog, Germany), 25 µM Epac antagonist ESI-09 (Biolog, Germany) and 50 µM geranyl geranyl transferase inhibitor, GGTI 298, which disrupts Rap1 function. Merozoites were then transferred to EC buffer (5 mM KCl, 140 mM NaCl, 1 mM MgCl2, 2 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2) followed by either measurement of cytosolic cAMP and Ca2+ levels in merozoites or microneme secretion.
Microneme secretion assay
P. falciparum merozoites were isolated in IC buffer (140 mM KCl, 5 mM NaCl, 1 mM MgCl2, 5.6 mM glucose, 25 mM HEPES, pH 7.2), incubated for 15 min at 37°C with or without various pharmacological inhibitors or agonists as described above and transferred to EC buffer (5 mM KCl, 140 mM NaCl, 1 mM MgCl2, 2 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2) or IC-Klow buffer (5 mM NaCl, 5 mM KCl, 135 mM choline-Cl, 1 mM EGTA, 5.6 mM glucose, 25 mM HEPES, pH 7.2) with or without pharmacological inhibitors or agonists for 15 min at 37°C. Merozoites were separated by centrifugation as described above. Merozoite supernatants and merozoite pellets were separated by SDS-PAGE on a 12% gel under reducing conditions. Microneme proteins, PfAMA1 and EBA175, were detected in merozoite supernatants by Western blotting using anti-PfAMA1 and anti-EBA175 rabbit sera, respectively. Rabbit sera against cytoplasmic protein PfNapL were used to detect PfNapL in supernatants and merozoite pellets to estimate cell lysis and confirm that similar amounts of merozoites were used for each condition, respectively. Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG goat antibodies (Sigma, USA) were used as secondary antibodies and detected by enhanced chemi-luminescence using ECL-Prime Western Blotting Detection reagent (GE Healthcare).
Measurement of intracellular cAMP levels
P. falciparum merozoites were lysed with 0.1 N HCl and clarified by centrifugation at 6000 g. Supernatants were used for measuring the cytosolic levels of cAMP using cAMP direct immunoassay kit (Calbiochem, USA) as per manufacturer’s protocol. The kit uses a polyclonal antibody to bind cAMP present in the sample in a competitive manner. A standard curve was generated using known amounts of cAMP standards provided in the kit. Quantitative measurement of cAMP was obtained from the equation derived from the standard curve. The total protein content in each merozoite lysate sample was determined using Pierce BCA Protein Assay Kit (Pierce, USA) based on bicinchoninic acid (BCA). The amount of cAMP per µg of protein in each merozoite lysate sample was determined and used to calculate fold increase in cAMP per µg of merozoite protein material in each sample compared to control (merozoites in IC buffer).
Adenylyl cyclase activity assay
P falciparum merozoites were isolated in RPMI 1640 as described, washed twice with phosphate buffered saline (PBS) and resuspended in 50 mM Tris pH 7.5, 150 mM NaCl. Merozoites were lysed by repeated freeze-thaw cycles and the lysate was clarified by centrifugation at 6000 g for 1 min at 4°C. The supernatant was incubated with different concentrations of NaHCO3 (0 mM, 10 mM, 20 mM and 40 mM) in the presence of 10 mM MgCl2, 10 mM CaCl2, 5 mM ATP and 500 µM IBMX for 30 min at 30°C. The soluble adenylyl cyclase inhibitor KH7 (50 µM) was used as negative control. The reactions were stopped by the addition of 0.1 N HCl and the level of cAMP produced was measured using the cAMP direct immunoassay kit as described above.
Protein kinase A activity assay
P. falciparum merozoites were isolated as described above and incubated with or without KH7 (50 µM) in IC buffer for 15 min prior to transfer to EC buffer. Merozoite pellets were collected by centrifugation at 3000 g and used immediately to measure cAMP-dependent protein kinase A (PKA) activity. Merozoites were resuspended in ice-cold TNET buffer (50 mM Tris 7.4, 150 mM NaCl, 1 mM EDTA, and 1% Triton, protease inhibitor cocktail (Pierce, USA), 50 mM Na3VO4, 50 mM NaF) and lysed by repeated freeze-thaw cycles (4 cycles). The lysates were cleared by centrifugation at 6000 g for 15 min at 4°C. Total amount of protein in the supernatant was determined by (bicinchoninic acid assay (BCA) (Pierce, USA) using known amounts of bovine serum albumin (BSA) as standard. PKA activity was measured by quantitating incorporation of 32P in kemptide, a known peptide substrate of PKA (L-R-R-A-S-L-G, Kemptide PKA peptide substrate, Promega, USA) as described previously [9].
Measurement of intracellular pH in P. falciparum merozoites
Intracellular pH (pHi) of P. falciparum merozoites was measured using the pH-sensitive fluorescent dye BCECF (2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein) [30], [50]. P. falciparum merozoites were isolated in RPMI 1640 and loaded with BCECF-AM (acetoxymethylester of BCECF, Invitrogen, USA) for 20 min at 37°C in IC buffer. The merozoites were washed to remove excess dye and incubated with or without ACTZ in IC buffer at 37°C for 10 min. Merozoites were collected by centrifugation and resuspended in IC or EC buffer in the presence or absence of ACTZ and transferred to a 96 well microtiter plate (200 µL/well). Samples were excited monochromatically with λ1 = 440 nm and λ2 = 492 nm in succession for 0–10 minutes and emission was recorded at 535 nm on a fluorimeter (Perkin-Elmer Victor, USA). A quantitative measure of the pHi is provided by the ratio of the fluorescence measured at 535 nm following excitation at 492 nm and 440 nm. For each experiment, a pH calibration curve was prepared by resuspending merozoites in buffer containing 130 mm KCl, 25 mM HEPES, 20 mM glucose and 1 mM MgCl2 with a pH range of 6.6 to 8.0 followed by the addition of 10 µm nigericin (Sigma). Nigericin equilibrates the intracellular and extracellular pH. The equation derived from the linear regression curve obtained by plotting the fluorescence ratio at different pH values (S6 Figure) was used to calculate pHi of merozoites in different conditions.
Measurement of cytosolic Ca2+ levels in P. falciparum merozoites
P. falciparum merozoites were isolated as described above, loaded with 10 µM Fluo-4-AM for 20 min at 37°C, washed, resuspended in IC buffer and used for experiments within 5 min, as described earlier [5], [51]. Fluo-4-AM loaded P. falciparum merozoites were treated with cAMP or Ca2+ modulating agents and transferred from IC to EC buffer. Fluorescence signal from Fluo-4 in merozoites under different conditions was measured by flow cytometry using FACSCalibur (Becton Dickinson, USA) and analyzed using CellQuest software. Briefly, Fluo-4-AM loaded merozoites were excited at 488 nm and fluorescence signal was detected with a 430 nm/30 nm band pass filter for periods of 2–3 mins. Merozoites were gated on the basis of their forward scatter and side scatter. The mean fluorescence intensity (MFI), which reflects cytosolic Ca2+ levels in merozoites, was plotted against time using FlowJo software.
Erythrocyte invasion assay by P. falciparum merozoites
P. falciparum merozoites isolated as described above were either mock-treated or treated with 50 µm KH7 (Calbiochem, USA), 100 µm ACTZ (Sigma, USA), 25 µM ESI-05 (Biolog, Germany) or 25 µM ESI-09 (Biolog, Germany) for 15 min at 37°C, washed with IC buffer, resuspended in EC buffer and incubated with erythrocytes in EC buffer at 37°C under mixed gas environment for 2 h to allow invasion. EC buffer was then replaced with complete RPMI. After 18–20 h of incubation in complete RPMI under mixed gas environment to allow development of ring stages, the percentage of infected erythrocytes was scored by flow cytometry to determine invasion rates, as described earlier [52].
Immunofluorescence microscopy
P. falciparum schizonts and merozoites were smeared on glass slides, dried and fixed with pre-chilled methanol. Smears were blocked with 3% BSA in 1× PBS for 2 h at room temperature (RT) and probed with anti-PfACβ mouse sera diluted 1∶100, followed by Alexa-Fluor 488 conjugated goat anti-mouse IgG antibody diluted 1∶200. For co-immuno-staining, smears were also probed with rabbit sera (1∶100 dilution) against the cytoplasmic protein PfNAPL [20]. After washing, smears were incubated with Alexa-Fluor 488-conjugated goat anti-mouse IgG (1∶200, Molecular Probes, USA) and Alexa-Fluor 594-conjugated goat anti-rabbit IgG (1∶200 dilution, Molecular Probes, USA), for 1 hr at RT. The slides were washed, mounted with 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI, Molecular Probes, USA) and antifade mounting media (Molecular Probes, USA) and analyzed using a Nikon A1-R confocal microscope.
Supporting Information
Zdroje
1. CowmanAF, CrabbBS (2006) Invasion of red blood cells by malaria parasites. Cell 124 : 755–766.
2. GaurD, ChitnisCE (2011) Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites. Curr Opin Microbiol 14 : 422–428.
3. SharmaP, ChitnisCE (2013) Key molecular events during host cell invasion by Apicomplexan pathogens. Curr Opin Microbiol 16 : 432–437.
4. BaumJ (2013) A complete molecular understanding of malaria parasite invasion of the human erythrocyte: are we there yet? Pathog Glob Health 107 : 107–110.
5. SinghS, AlamMM, Pal-BhowmikI, BrzostowskiJA, ChitnisCE (2010) Distinct external signal trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog 6: e1000746.
6. SiddiquiFA, DhawanS, SinghS, SinghB, GuptaP, et al. (2013) A thrombospondin structural repeat containing rhoptry protein from Plasmodium falciparum mediates erythrocyte invasion. Cell Microbiol 15 : 1341–56.
7. KimC, XuongNH, TaylorSS (2005) Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science 307 : 690–696.
8. ReadLK, MikkelsenRB (1990) Cyclic AMP - and Ca2+ -dependent protein kinases in Plasmodium falciparum. Exp Parasitol 71 : 39–48.
9. SyinC, ParzyD, TraincardF, BoccaccioI, JoshiMB, et al. (2001) The H89 cAMP-dependent protein kinase inhibitor blocks Plasmodium falciparum development in infected erythrocytes. Eur J Biochem 268 : 4842–4849.
10. LiJ, CoxLS (2000) Isolation and characterisation of a cAMP-dependent protein kinase catalytic subunit gene from Plasmodium falciparum. Mol Biochem Parasitol 109 : 157–163.
11. MerckxA, NivezMP, BouyerG, AlanoP, LangsleyG, et al. (2008) Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS Pathogens 4: e19.
12. HasteNM, TalabaniH, DooA, MerckxA, LangsleyG, et al. (2012) Exploring the Plasmodium falciparum cyclic-adenosine monophosphate (cAMP)-dependent protein kinase (Pf PKA) as a therapeutic target. Microbes and Infection 14 : 838–850.
13. KurokawaH, KatoK, IwanagaT, SugiT, SudoA, et al. (2011) Identification of Toxoplasma gondii cAMP dependent protein kinase and its role in tachyzoite growth. PLoS One 2011 6(7): e22492 doi: 10.1371/journal.pone.0022492
14. KirkmanLA, WeissLM, KimK (2001) Cyclic nucleotide signaling in Toxoplasma gondii bradyzoite differentiation. Infect Immun 69(1): 148–53.
15. EatonMS, WeissLM, KimK (2006) Cyclic nucleotide kinases and tachyzoite-bradyzoite transition in Toxoplasma gondii. 2013. Int J Parasitol 36(1): 107–14.
16. HartmannA, Arroyo-OlarteRD, ImkellerK, HegemannP, LuciusR, et al. (2013) Optogenetic modulation of an adenylate cyclase in Toxoplasma gondii demonstrates a requirement of the parasite cAMP for host-cell invasion and stage differentiation. J Biol Chem 288(19): 13705–17 doi: 10.1074/jbc.M113.465583
17. GloerichM, BosJL (2010) Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50 : 355–375.
18. WeberJH, VishnyakovA, HambachK, SchultzA, SchiltzJE, et al. (2004) Adenylyl cyclases from Plasmodium, Paramecium and Tetrahymena are novel ion channel/enzyme fusion proteins. Cell Signal 16 : 115–125.
19. SalazarE, BankEM, RamseyN, HessKC, DeitschDW, et al. (2012) Characterization of Plasmodium falciparum adenylyl cyclase β and its role in erythrocytic stage parasites. PLoS One 7: e39769.
20. OnoT, Cabrita-SantosL, LeitaoR, BettiolE, PurcellLA, et al. (2008) Adenylyl cyclase α and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathog 4(2): e1000008.
21. ChandraBR, OlivieriA, SilvestriniF, AlanoP, SharmaA (2005) Biochemical characterization of the two nucleosome assembly proteins from Plasmodium falciparum. Mol Biochem Parasitol 142 : 237–47.
22. HowellSA, Withers-MartinezC, KockenCH, ThomasAW, BlackmanMJ (2001) Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J Biol Chem 276(33): 31311–20.
23. BeavoJA, RogersNL, CroffordOB, HardmanJG, SutherlandEW, NewmanEV (1970) Effects of xanthine derivatives on lipolysis and on adenosine 3’,5’-monophosphate phosphodiesterase activity. Mol Pharmacol 6 : 597–603.
24. ChenY, CannMJ, LitvinTN, IourgenkoV, SinclairML, et al. (2000) Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289 : 625–628.
25. CannMJ, HammerA, ZhouJ, KanacherT (2003) A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J Biol Chem 278 : 35033–35038.
26. KobayashiM, BuckJ, LevinLR (2004) Conservation of functional domain structure in bicarbonate-regulated “soluble” adenylyl cyclases in bacteria and eukaryotes. Dev Genes Evol 214 : 503–509.
27. LindskogS (1997) Structure and mechanism of carbonic anhydrase. Pharmcol Ther 74 : 1–20.
28. KrungkraiJ, KrungkraiSR, SupuranCT (2008) Carbonic anhydrase inhibitor: Inhibition of Plasmodium falciparun carbonic anhydrase with aromatic/heterocyclic sulfonamides - in vivo and in vitro studies. Bioorg Med Chem Lett 18 : 5466–5474.
29. KrungkraiJ, SupuranCT (2008) The alpha carbonic anhydrase from malaria parasite and its inhibition. Curr Pharm Des 14 : 631–640.
30. RinkTJ, TsienRY, PozzanT (1982) Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol 95 : 189–96.
31. BorodinskyLN, SpitzerNC (2006) Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE pe22
32. HoqueKM, WoodwordOM, RossumDB, ZachosNC, ChenL, et al. (2009) Epac1 mediates protein kinase A-independent mechanism of forskolin activated intestinal chloride secretion. J Gen Physiol 135 : 43–58.
33. PurvesGI, KamishimaT, DaviesLM, QuayleJM, DartC (2009) Exchange protein activated by AMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J Physiol 587 : 3639–3650.
34. EnserinkJM, ChristensenAE, de RooijJ, van TriestM, SchwedeF, et al. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4(11): 901–6.
35. AlmahariqM, TsalkovaT, MeiFC, ChenH, ZhouJ, et al. (2013) A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83(1): 122–8.
36. TsalkovaT, MeiFC, LiS, ChepurnyOG, LeechCA, et al. (2012) Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci U S A 109 : 18613–8.
37. VogtA, QianY, McGuireTF, HamiltonAD, SebtiSM (1996) Protein geranylgeranylation, not farnesylation, is required for the G1 to S phase transition in mouse fibroblasts. 1996. Oncogene 13(9): 1991–9.
38. ChakrabartiD, Da SilvaT, BargerJ, PaquetteS, PatelH, PattersonS, AllenCM, et al. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J Biol Chem 277 : 42066–73.
39. YuleDI, WilliamsJA (1992) U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbochol but not to JMV-180 in rat pancreatic acinar cells. J Biol Chem 267 : 13830–13835.
40. LeykaufK, TreeckM, GilsonPR, NeblT, BraulkeT, et al. (2010) Protein kinase A dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite. PLoS Pathog 6: e1000941.
41. CollinsCR, HackettF, StrathM, PenzoM, Withers-MartinezC, et al. (2013) Malaria parasite cGMP-dependent protein kinase regulates blood stage merozoite secretory organelle discharge and egress. PLoS Pathog 9: e1003344.
42. YeohS, O'DonnellRA, KoussisK, DluzewskiAR, AnsellKH, et al. (2007) Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 131 : 1072–1083.
43. AgarwalS, SinghMK, GargS, ChitnisCE, SinghS (2012) Ca(2+) -mediated exocytosis of subtilisin-like protease 1: a key step in egress of Plasmodium falciparum merozoites. Cell Microbiol 15 : 910–921.
44. GlushakovaS, LizunovV, BlankPS, MelikovK, HumphreyG, et al. (2013) Cytoplasmic free Ca2+ is essential for multiple steps in malaria parasite egress from infected erythrocytes. Malar J 30 : 12–41.
45. GeahlenRL, KrebsEG (1980) Regulatory subunit of the type I cAMP-dependent protein kinase as an inhibitor and substrate of the cGMP-dependent protein kinase. J Biol Chem 255 : 1164–1169.
46. LasonderE, TreeckM, AlamM, TobinAB (2012) Insights into the Plasmodium falciparum schizont phospho-proteome. Microbes Infect 14 : 811–819.
47. GaoX, GunalanK, YapSSL, PreiserP (2013) Triggers of key calcium signals during erythrocyte invasion by Plasmodium falciparum. Nature Commun 4 : 2862 doi: 10.1038
48. TrigliaT, DuraisinghMT, GoodRT, CowmanAF (2005) Reticulocyte-binding protein homologue 1 is required for sialic acid dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol 55 : 162–174.
49. TragerW, JensenJB (1976) Human malaria parasites in continuous culture. Science 193 : 673–675.
50. Van der HeydenN, BenaimG, DocampoR (1996) The role of a H+-ATPase in the regulation of cytoplasmic pH in Trypanosoma cruzi epimastigotes. Biochem J 318 : 103–109.
51. SinghS, ChitnisCE (2013) Flow cytometry-based methods for measurement of cytosolic calcium and surface proteins expression in Plasmodium falciparum merozoites. Methods Mol Biol 923 : 281–290.
52. SaharT, ReddyKS, BharadwajM, PandeyAK, SinghS, et al. (2011) Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS One 6: e17102.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus InfectionČlánek P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to InfectionČlánek Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Microbial Programming of Systemic Innate Immunity and Resistance to Infection
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
- Measles Immune Suppression: Functional Impairment or Numbers Game?
- Cellular Mechanisms of Alpha Herpesvirus Egress: Live Cell Fluorescence Microscopy of Pseudorabies Virus Exocytosis
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection
- Loss of Dynamin-Related Protein 2B Reveals Separation of Innate Immune Signaling Pathways
- Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Infection
- Unveiling the Intracellular Survival Gene Kit of Trypanosomatid Parasites
- Extreme Divergence of Tropism for the Stem-Cell-Niche in the Testis
- HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4 T Cells
- P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
- Hypercytotoxicity and Rapid Loss of NKp44 Innate Lymphoid Cells during Acute SIV Infection
- Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
- Crystal Structure of Calcium Binding Protein-5 from and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
- Chronic Parasitic Infection Maintains High Frequencies of Short-Lived Ly6CCD4 Effector T Cells That Are Required for Protection against Re-infection
- Specific Dysregulation of IFNγ Production by Natural Killer Cells Confers Susceptibility to Viral Infection
- HSV-2-Driven Increase in the Expression of αβ Correlates with Increased Susceptibility to Vaginal SHIV Infection
- Murine Anti-vaccinia Virus D8 Antibodies Target Different Epitopes and Differ in Their Ability to Block D8 Binding to CS-E
- Brothers in Arms: Th17 and Treg Responses in Immunity
- Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Typhimurium Colitis
- A Negative Feedback Modulator of Antigen Processing Evolved from a Frameshift in the Cowpox Virus Genome
- Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms
- The Non-receptor Tyrosine Kinase Tec Controls Assembly and Activity of the Noncanonical Caspase-8 Inflammasome
- Targeted Changes of the Cell Wall Proteome Influence Ability to Form Single- and Multi-strain Biofilms
- Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
- The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
- Anti-α4 Antibody Treatment Blocks Virus Traffic to the Brain and Gut Early, and Stabilizes CNS Injury Late in Infection
- Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
- Microbial Urease in Health and Disease
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against
- IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
- A Natural Genetic Variant of Granzyme B Confers Lethality to a Common Viral Infection
- Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression
- Differential PfEMP1 Expression Is Associated with Cerebral Malaria Pathology
- The Role of the NADPH Oxidase NOX2 in Prion Pathogenesis
- Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions
- The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
- Expression of Suppressor of Cytokine Signaling 1 (SOCS1) Impairs Viral Clearance and Exacerbates Lung Injury during Influenza Infection
- Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells
- SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in
- Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication
- Oral Streptococci Utilize a Siglec-Like Domain of Serine-Rich Repeat Adhesins to Preferentially Target Platelet Sialoglycans in Human Blood
- SV40 Utilizes ATM Kinase Activity to Prevent Non-homologous End Joining of Broken Viral DNA Replication Products
- Amphipathic α-Helices in Apolipoproteins Are Crucial to the Formation of Infectious Hepatitis C Virus Particles
- Proteomic Analysis of the Acidocalcisome, an Organelle Conserved from Bacteria to Human Cells
- Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



