-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
Plant-parasitic nematodes have a major impact on global food security, as they reduce the annual yield of food crops by approximately 10 percent. For decades, the application of non-selective toxic chemicals to infested soils controlled outbreaks of plant-parasitic nematodes. The recent bans on most of these chemicals has redirected attention towards a wider use of basal, broad-spectrum immunity to nematodes in crop cultivars. However, it is currently not known if this most ancient layer of immunity affects host invasion by plant-parasitic nematodes at all. Basal immunity in plants relies on the detection of molecular patterns uniquely associated with infections in the apoplast by surface-localized receptors. Here, we demonstrate that venom allergen-like proteins in secretions of soil-borne cyst nematodes suppress immune responses mediated by surface-localized pattern recognition receptors. Migratory stages of cyst nematodes most likely deliver venom allergen-like proteins together with a range of plant cell wall-degrading enzymes into the apoplast of host cells. We therefore conclude that these nematodes most likely secrete venom allergen-like proteins to modulate host responses triggered by the release of immunogenic fragments of damaged plant cell walls.
Published in the journal: . PLoS Pathog 10(12): e32767. doi:10.1371/journal.ppat.1004569
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004569Summary
Plant-parasitic nematodes have a major impact on global food security, as they reduce the annual yield of food crops by approximately 10 percent. For decades, the application of non-selective toxic chemicals to infested soils controlled outbreaks of plant-parasitic nematodes. The recent bans on most of these chemicals has redirected attention towards a wider use of basal, broad-spectrum immunity to nematodes in crop cultivars. However, it is currently not known if this most ancient layer of immunity affects host invasion by plant-parasitic nematodes at all. Basal immunity in plants relies on the detection of molecular patterns uniquely associated with infections in the apoplast by surface-localized receptors. Here, we demonstrate that venom allergen-like proteins in secretions of soil-borne cyst nematodes suppress immune responses mediated by surface-localized pattern recognition receptors. Migratory stages of cyst nematodes most likely deliver venom allergen-like proteins together with a range of plant cell wall-degrading enzymes into the apoplast of host cells. We therefore conclude that these nematodes most likely secrete venom allergen-like proteins to modulate host responses triggered by the release of immunogenic fragments of damaged plant cell walls.
Introduction
Soil-borne plant-parasitic nematodes are major constraints on global food security, as they reduce the annual yield of food crops by approximately 10 percent [1], [2]. This figure is a global average and may therefore be somewhat misleading. In areas where people depend on local cultivation of staple crops the effect of these microscopic roundworms can be devastating. The impact of plant-parasitic nematodes on food production provides plant breeders with a strong incentive to better exploit genetic variation in resistance to nematodes in crop cultivars. However, this requires knowledge of the mechanisms underlying the activation and suppression of plant innate immunity by plant-parasitic nematodes, an area which is currently underexplored [3], [4].
Plants utilize pattern recognition receptors belonging to the receptor-like kinase (RLK)/Pelle superfamily to detect extracellular microbes or their actions in the apoplast (i.e. the extracellular matrix; [5], [6]). The recognition of immunogenic microbe - and damage-associated molecular patterns by receptor-like kinases activates intracellular immune signaling pathways, resulting in a wide range of structural and chemical defenses [7], [8]. Several members of the RLK/Pelle superfamily in plants lack a cytoplasmic kinase domain, while they are nonetheless able to activate immune responses to pathogens (e.g. Cf-proteins in tomato; [9]–[11]). The activity of these so-called receptor-like proteins requires mediation by other transmembrane proteins, or cytoplasmic membrane-associated kinases, that function as co-factors within multimeric receptor complexes [12], [13]. At present, little evidence is available on the role of surface-localized pattern recognition receptors in immunity to parasitic nematodes in plants.
Recently, we showed that the receptor-like protein Cf-2 in tomato mediates dual disease resistance by guarding a common virulence target of a nematode and a fungus [14]. Perturbations of the apoplastic papain-like cysteine protease Rcr3pim by two unrelated effectors from the leaf mold fungus Cladosporium fulvum and from the root parasitic nematode Globodera rostochiensis activate Cf-2-mediated disease resistance. The function of Rcr3pim, or any of its close homologs in tomato, has not yet been resolved [15]–[17]. Tomato plants harboring the Rcr3pim allele, but not the receptor Cf-2, are far more susceptible to infections by G. rostochiensis than tomato plants lacking Rcr3pim [14]. Apoplastic Rcr3pim is a molecular target of the venom allergen-like protein Gr-VAP1 of G. rostochiensis, which is secreted by infective juveniles during the onset of parasitism. However, the role of this venom allergen-like protein (VAP), or its interaction with Rcr3pim, in nematode virulence is not clear.
Venom allergen-like proteins constitute a monophyletic clade of cysteine-rich secretory proteins within the Sperm Coating Protein/Tpx-1/Ag-5/Pr-1/Sc-7 (SCP/TAPS) superfamily ([18], [19]). Members of this clade show similarity to venom allergen 5 from vespid wasps, pathogenesis-related protein PR-1 from plants, brain tumor specific proteins in humans, and a wide range of other secreted proteins (reviewed in [18]). Venom allergen-like proteins have been identified in all animal - and plant-parasitic nematodes studied to date [19], [20]. They are even the most abundantly secreted proteins during the onset of parasitism of some animal-parasitic nematodes [19], [21]–[24]. In spite of their conservation, abundance, and strong association with the onset of parasitism, little is currently known of the function of venom allergen-like protein in nematode infections in plants and in animals [19].
Sedentary plant-parasitic nematodes, such as cyst nematodes (genera Globodera and Heterodera) and root-knot nematodes (genus Meloidogyne), deliver effectors into the apoplast and cytoplasm of host cells to induce the formation of a permanent feeding structure [25]–[27]. The permanent feeding structure is the sole source of plant nutrients for sedentary nematodes throughout their life [28]. Besides altering host cell metabolism and function, sedentary nematodes also use effectors to modulate host immunity [3], [27]. Specific immunity to nematodes in host plants often involves a programmed cell death in or around permanent feeding structures, resulting in the developmental arrest of feeding juveniles [3]. The most advanced sedentary nematodes deliver effectors into the cytoplasm of host cells to suppress the defense-related programmed cell death mediated by intracellular immune receptors [29], [30]. However, sedentary nematodes are extracellular parasites and their prolonged contact with surrounding host cells makes them also vulnerable to detection by surface-localized pattern recognition receptors [14]. Recent discoveries with the root-knot nematode M. incognita suggest that sedentary plant-parasitic nematodes may have adapted to this by evolving a separate set of apoplastic effectors to further control host immunity [31], [32].
The unique conservation of venom allergen-like proteins in secretions of animal - and plant-parasitic nematodes might point to a common activity of these effector proteins in the extracellular matrix of animal and plant cells. However, the composition, structure, and function of the extracellular matrix of animal and plant cells are fundamentally different [33], [34]. A possible exception might be that both in animals and plants nematodes encounter an innate immune system that relies on the surveillance of the extracellular matrix by surface-localized pattern recognition receptors [35], [36]. Earlier work has demonstrated that a secreted venom allergen-like protein from the animal-parasitic nematode Necator americanus acts in vitro as an antagonistic ligand of the integrin complement receptor 3, a pattern recognition receptor on the surface of human neutrophils [37]–[40]. This observation led us to investigate if venom allergen-like proteins of plant-parasitic nematodes similarly interfere with the functioning of surface-localized immune receptors in plants. To address this question, we first analyzed if venom allergen-like proteins are important for the onset of parasitism by silencing the expression of Gr-VAP1 in infective juveniles of G. rostochiensis. Next, we analyzed the effect of ectopic venom allergen-like proteins in transgenic plants on susceptibility to nematodes, and diverse plant pathogenic fungi, oomycetes, and bacteria. Based on the response of these plants to the immunogenic epitope flg22 from bacterial flagellin [41], we concluded that the venom allergen-like proteins suppress basal plant defenses to biotic stresses. We further provide evidence that the breakdown of basal immunity by ectopic venom allergen-like proteins involves a plant cell wall-associated subtilisin-like serine protease, not previously linked to defense regulation in plants. Remarkably, our data also suggest that cyst nematodes exploit a trade-off mechanism between resistance to biotic and abiotic stress to ward off host defense responses.
Results
The venom allergen-like protein Gr-VAP1 is required for the onset of parasitism by G. rostochiensis
To investigate whether venom allergen-like proteins are required for the onset of parasitism by sedentary plant-parasitic nematodes, we soaked infective juveniles of G. rostochiensis in double-stranded RNA, matching 820 base pairs of the Gr-VAP1 transcript sequence. Reverse transcription PCR on nematodes, soaked in Gr-VAP1-specific dsRNA, showed a significant reduction in Gr-VAP1 transcript levels, whereas the control treatment with dsRNA matching the NAU gene from Drosophila melanogaster did not alter Gr-VAP1 expression (Fig. 1A). Next, susceptible tomato plants (Solanum lycopersicum, cultivar Moneymaker) were challenged with the dsRNA-treated infective juveniles, and monitored for nematode infections for 7 days post inoculation. Treatment with Gr-VAP1-specific dsRNA significantly reduced the number of nematodes inside tomato roots compared to the treatment with NAU-specific dsRNA (Fig. 1B). We therefore concluded that the apoplastic venom allergen-like protein Gr-VAP1 is required for the establishment of successful infections by G. rostochiensis during the onset of parasitism.
Fig. 1. The venom allergen-like protein Gr-VAP1 is required for the onset of parasitism in host plants. 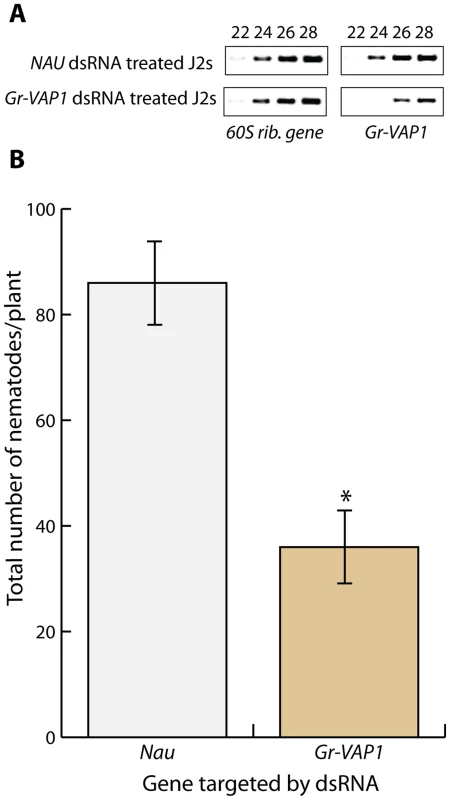
(A) RNA interference specifically knocked down Gr-VAP1 expression in pre-parasitic second stage juveniles of G. rostochiensis. Semi-quantitative reverse transcription-PCR of Gr-VAP1 and a reference gene (60S rib. gene) in pre-parasitic second juveniles in double stranded RNA either matching the Gr-VAP1 sequence or the sequence of the NAU gene of Drosophila melanogaster as control. Numbers indicate the cycles in the PCR. (B) The knockdown of Gr-VAP1 expression significantly reduces the number of infective juveniles of G. rostochiensis inside roots of tomato plants (S. lycopersicum). Pre-parasitic second juveniles were either treated with double stranded RNA matching the Gr-VAP1 or the Nau sequence. Bars represent standard error of mean of number of nematodes per plant at 7 days after inoculation over 10 replicates. Asterisk marks significance in a Student's t-test (with P-value <0.05). Gr-VAP1 expression coincides with nematode migration inside host plants
Both infective second-stage juveniles and adult males of G. rostochiensis migrate through host tissues, which causes significant damage to host cells. By contrast, intermediate juvenile stages and adult females are immobile, and thus induce little damage. To determine whether the expression of Gr-VAP1 in G. rostochiensis coincides with either migration or sedentarism in host plants, we used semi-quantitative reverse transcription PCR on nematodes isolated from infected potato roots at different time points prior to and post host invasion (Fig. 2). Gr-VAP1 was highly expressed in infective second stage juveniles during the onset of parasitism. Thereafter, the level of Gr-VAP1 expression declined in successive sedentary juvenile stages inside host roots to total absence in sedentary adult females. However, the expression of Gr-VAP1 was raised again in migratory adult males. We therefore concluded that the temporal expression of Gr-VAP1 in G. rostochiensis coincides with nematode migration inside host plants.
Fig. 2. The expression of Gr-VAP1 coincides with host invasion and migration of Globodera rostochiensis. 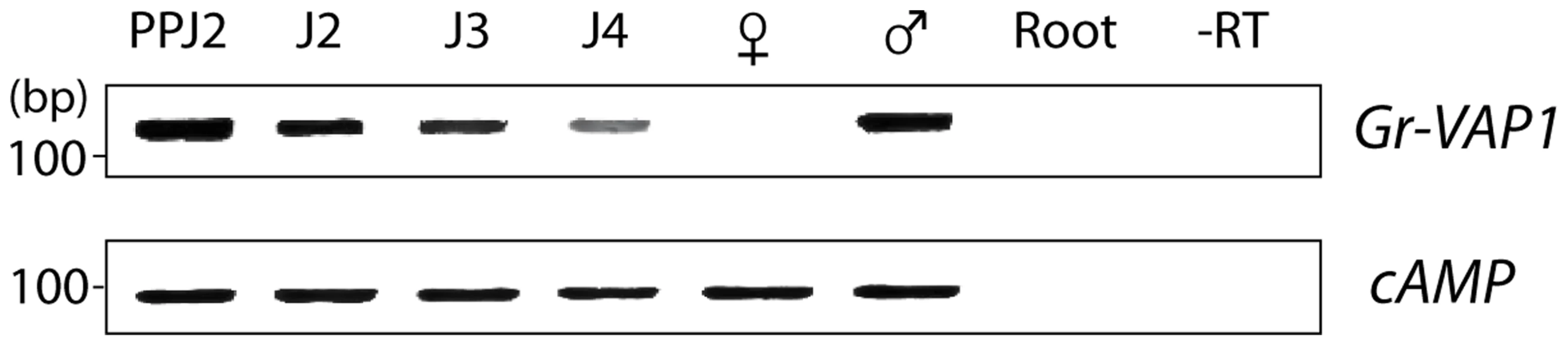
The expression of Gr-VAP1, as shown by semi-quantitative reverse transcription PCR, is highly up-regulated in the migratory stages of G. rostochiensis (ppJ2, J2, and males (♂)), while it declines after initiation of the permanent feeding site in the sedentary juvenile stages (J3 and J4, and adult females (♀). Changes in expression of Gr-VAP1 were assessed using the constitutively expressed cAMP-dependent protein kinase (cAMP) gene in G. rostochiensis as reference. Reactions using uninfected tomato roots as template (Root) and without reverse transcriptase (-RT) were included as controls. Ectopic Gr-VAP1 increases susceptibility of potato plants to G. rostochiensis
To examine whether Gr-VAP1 affects the susceptibility of host plants to G. rostochiensis, we generated transgenic potato plants ectopically overexpressing Gr-VAP1. Two randomly selected independent transgenic lines without any visible anomalies in shoots and roots were challenged with infective juveniles of G. rostochiensis. Six weeks after inoculation the number of adult females in plants expressing Gr-VAP1 was significantly higher than in the corresponding empty vector control plants (Fig. 3A). To confirm that the altered nematode susceptibility correlates with Gr-VAP1 expression, we used a real-time quantitative reverse transcription PCR on the two potato lines expressing Gr-VAP1. Transgenic line Gr-VAP1-A, that showed the highest nematode susceptibility, had a 7.9-fold higher expression of Gr-VAP1 than transgenic line Gr-VAP1-B. We therefore concluded that ectopic Gr-VAP1 enhances the susceptibility of potato plants to G. rostochiensis.
Fig. 3. Apoplastic Gr-VAP1 suppresses immunity of potato plants to G. rostochiensis. 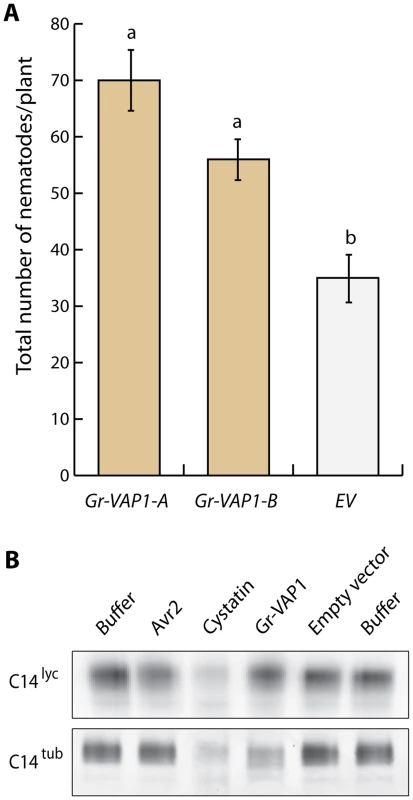
(A) Transgenic potato plants stably overexpressing Gr-VAP1 in the apoplast show enhanced susceptibility to G. rostochiensis. The number of nematodes per plant was compared at 6 weeks post inoculation for two independent transgenic potato lines harboring either Gr-VAP1 (Gr-VAP1-A and Gr-VAP1-B) or the corresponding T-DNA insert of the empty binary expression vector (EV). The expression constructs included native signal peptide for secretion of Gr-VAP1. Bars represent standard errors of the means. Different letters indicate statistically significant differences between plant genotypes as determined with ANOVA (with P-values <0.05). (B) Apoplastic Gr-VAP1 perturbs the active site of the extracellular defense-related papain-like cysteine protease C14tub of potato (S. tuberosum). Image shows binding of the fluorescent activity-based probe DCG-04 to the active site of C14tub and C14lyc of tomato (S. lycopersicum) following treatment with Gr-VAP1 isolated from apoplastic fluids of agroinfiltrated leaves. Treatments with the Avr2, egg white cystatin, and apoplastic fluids from agroinfiltrations with the empty binary expression vector (Empty vector), and with buffer alone (Buffer) were included as controls. Like the effector Avr2 from C. fulvum [42], we expected Gr-VAP1 to interact with other extracellular papain-like cysteine proteases in different host plant species of G. rostochiensis. We used DCG-04 activity profiling to demonstrate that Gr-VAP1 also perturbs the active site of the apoplastic papain-like cysteine protease C14tub from potato (S. tuberosum) (Fig. 3B). By contrast, Gr-VAP1 does not interfere with the binding of fluorescent DCG-04 to apoplastic C14lyc from tomato (S. lycopersicum). For this experiment, Gr-VAP1 and C14tub/lyc were separately produced in the apoplast of agroinfiltrated leaves of Nicotiana benthamiana. Protease activity was subsequently determined by the binding of fluorescent DCG-04 to C14tub and C14lyc in the presence of Gr-VAP1 on gels of mixtures of isolated apoplastic fluids. The experiment was repeated three times, and each attempt resulted in significantly less binding of the fluorescent probe to C14tub, but not to C14lyc (S1 Figure). Only C14tub from potato is under strong diversifying selection, because of which it is thought to be involved defenses [43]. We therefore concluded that the enhanced susceptibility by ectopic Gr-VAP1 in potato most likely involves the perturbation of C14tub and perhaps other apoplastic papain-like cysteine proteases.
Ectopic venom allergen-like proteins enhance the susceptibility of Arabidopsis to multiple unrelated plant pathogens
Arabidopsis thaliana is a far better model to study the molecular changes induced by venom allergen-like proteins in plants than either potato or tomato. However, G. rostochiensis is not able to establish infections in A. thaliana. We therefore continued our investigations with two homologous venom allergen-like proteins from the beet cyst nematode Heterodera schachtii, which is a parasite of A. thaliana. These two venom allergen-like proteins are formally designated as Nem-Hsc-SCP/TAPS-1A and Nem-Hsc-SCP/TAPS-2A [18], but for the remainder of this paper they are referred to as Hs-VAP1 and Hs-VAP2. Hs-VAP1 is 81.4 percent identical to Gr-VAP1, while Hs-VAP2 shows only 34.8 percent sequence identity to Gr-VAP1. In comparison, a previously reported venom allergen-like protein from the root-knot nematode Meloidogyne incognita (hereafter named Mi-VAP1; [44]) shows about 28.6% identity to Gr-VAP1 and Hs-VAP1, while it is for 33.9% of its sequence identical to Hs-VAP2 (S2 Figure).
To investigate if ectopic venom allergen-like proteins from H. schachtii and G. rostochiensis alter the susceptibility of A. thaliana to cyst nematodes, we generated transgenic plants overexpressing Gr-VAP1, Hs-VAP1, and Hs-VAP2, including their native signal peptides for secretion. We challenged two independent single insertion lines, without visible anomalies in shoots and roots, of each construct with infective juveniles of H. schachtii. Twenty-eight days after inoculation the number of females per plant in plants expressing Gr-VAP1, Hs-VAP1, and Hs-VAP2 was significantly higher than in the corresponding transgenic empty vector line or in the wild type Col-0 plants (Fig. 4A). We therefore concluded that venom allergen-like proteins from two unrelated cyst nematodes commonly enhance the susceptibility of different plant species to nematode infections.
Fig. 4. Ectopic venom allergen-like proteins suppress basal immunity in Arabidopsis thaliana. 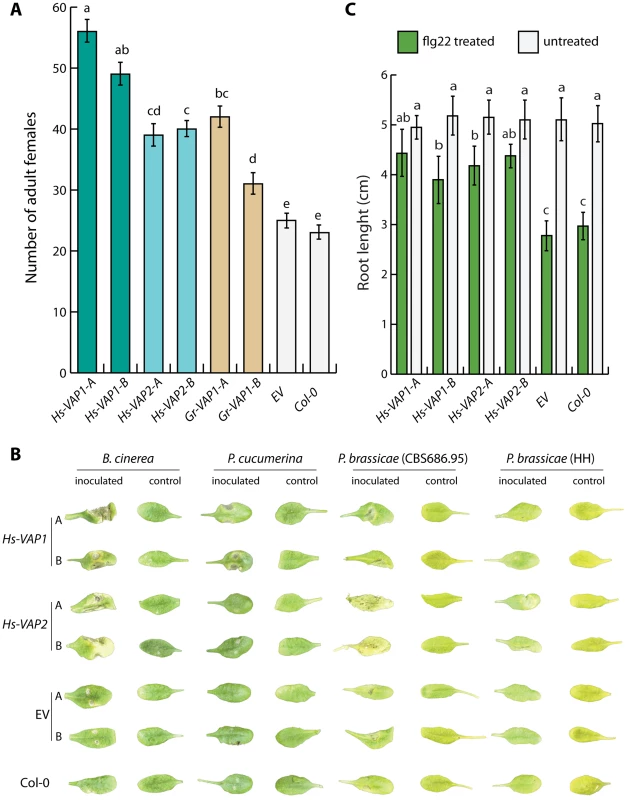
(A) Heterologous expression of the venom allergen-like protein Gr-VAP1 from G. rostochiensis, and Hs-VAP1 and Hs-VAP2 from Heterodera schachtii in the apoplast of transgenic Arabidopsis lines enhances their susceptibility to H. schachtii. Two independent transgenic lines per construct (-A and -B) were compared with corresponding transgenic line harboring the T-DNA of the empty vector (EV) and wild type A. thaliana (Col-0). Bars represent mean number of nematodes per plants with standard errors of the means. Letters indicate statistical significance when using P-value <0.05 as threshold. (B) Ectopic Hs-VAP1 and Hs-VAP2 enhance development of disease symptoms of fungal and oomycete pathogens in leaves of transgenic Arabidopsis lines. Pictures show symptoms on leaves inoculated with Botrytis cinerea, Plectosphaerella cucumeria, and two isolates of Phytophthora brassicae, or mock inoculated. (C) Ectopic Hs-VAP1 and Hs-VAP2 suppress seedling growth response of Arabidopsis to the immunogenic peptide flg22. Bars represent mean root length of transgenic lines with standard error of mean after 10 days in the presence or absence of 10 µM flg22. Next, we reasoned that if the VAP-enhanced susceptibility of the transgenic Arabidopsis lines to cyst nematodes involves modulation of the innate immunity, these lines might also be more susceptible to entirely unrelated plant pathogens. To test this, we analyzed the transgenic Arabidopsis lines overexpressing Hs-VAP1 and Hs-VAP2 for their susceptibility towards Botrytis cinerea, Plectosphaerella cucumerina, a virulent and a non-virulent isolate of Phytophthora brassicae, Alternaria brassicicola, Verticillium dahliae, and Pseudomonas syringae pv. tomato (Fig. 4B, and S3 Figure). The overexpression of both Hs-VAP1 and Hs-VAP2 significantly increased the severity of the grey mold symptoms caused by B. cinerea in the transgenic Arabidopsis plants (S4A Figure). Similarly, both Hs-VAP1 and Hs-VAP2 significantly increased susceptibility of Arabidopsis plants to infections by P. syringae pv. tomato (S3A Figure). Only Arabidopsis plants overexpressing Hs-VAP1 showed larger necrotic lesions following the inoculation with the fungus P. cucumerina (S4B Figure). By contrast, the oomycete P. brassicae (isolate CBS686.95) only caused faster developing and larger lesions on transgenic Arabidopsis expressing Hs-VAP2 (S4C Figure). Surprisingly, the P. brassicae isolate HH, which is not virulent on wild type A. thaliana Col-0, was able to colonize transgenic A. thaliana lines expressing Hs-VAP2 (S4D Figure). However, neither Hs-VAP1 nor Hs-VAP2 altered the susceptibility of A. thaliana towards the fungal pathogens A. brassicicola or V. dahliae, both of which do not cause expanding lesions in Arabidopsis ecotype Col-0. Altogether, our data suggests that ectopic Hs-VAP1 and Hs-VAP2, albeit differently, modulate basal innate immunity of A. thaliana toward multiple, but not all, plant pathogens. Furthermore, ectopic VAPs specifically altered the susceptibility of Arabidopsis to pathogenic microbes that typically cause expanding lesions in their necrotrophic phase (i.e. B. cinerea, P. cucumerina, P. brassicae, and P. syringae pv. tomato).
Ectopic venom allergen-like proteins abrogate the response of Arabidopsis to the immunogenic peptide flg22
The flagella of P. syringae pv. tomato harbor an immunogenic epitope of twenty two amino acids (flg22) that is recognized as a pathogen-associated molecular pattern in Arabidopsis [45]. Prolonged exposure to flg22 elicits a persistent basal immune response in seedlings of Arabidopsis Col-0 plants, which occurs at the expense of plant growth [45]. We used this phenotype to confirm that ectopic venom allergen-like proteins undermine basal immunity in our transgenic Arabidopsis lines. As expected, treatment with flg22 significantly inhibited seedling growth in both wild-type Arabidopsis and in our transgenic lines harboring the empty expression vector (Fig. 4C). By contrast, the expression of Hs-VAP1 and Hs-VAP2 in Arabidopsis seedlings largely abrogated this growth inhibition by flg22 (Fig 4C; S5 Figure). Remarkably, the leaves of the transgenic plants overexpressing Hs-VAP1 and Hs-VAP2 also remained much greener as compared to the leaves of wild type Col-0 and empty vector control plants following the treatment with flg22. As the perception of flg22 in Arabidopsis is mediated by the extracellular pattern recognition receptor FLS2 [45], we concluded that ectopic venom allergen-like proteins most likely modulate the activation of basal immunity by surface-localized immune receptors.
Extracellular papain-like cysteine proteases regulate immunity to cyst nematodes in Arabidopsis
The apoplastic effectors Avr2 of C. fulvum and Gr-VAP1 of G. rostochiensis commonly inhibit the extracellular papain-like protease Rcr3pim in tomato [14]. Although C. fulvum is not a pathogen of Arabidopsis either, ectopic Avr2 has been shown to interact with multiple extracellular papain-like cysteine proteases of Arabidopsis [42]. To investigate if heterologous expression of Avr2 through its interactions with extracellular papain-like cysteine proteases also affects susceptibility of Arabidopsis to nematode infections, we challenged transgenic Arabidopsis plants stably overexpressing Avr2 with H. schachtii. Four weeks post inoculation the number of adult females of H. schachtii per root was significantly higher in plants overexpressing Avr2 than in the corresponding wild type Arabidopsis plants (Fig. 5A). This data shows that the inhibition of extracellular papain-like cysteine proteases by promiscuous effectors from different non-adapted plant attackers (i.e. Gr-VAP1 and Avr2) undermines basal immunity in Arabidopsis.
Fig. 5. Defense-related extracellular papain-like cysteine proteases regulate immunity to cyst nematodes in Arabidopsis. 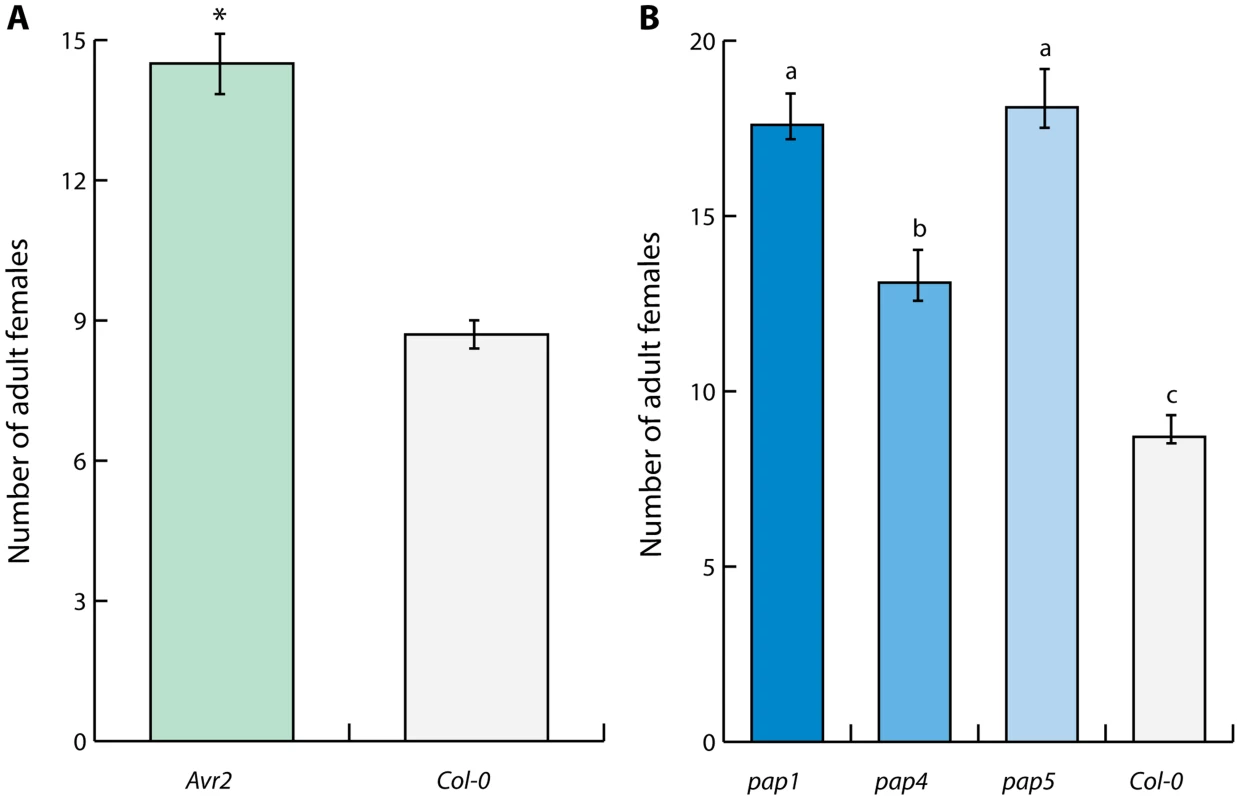
(A) Inhibition of papain-like cysteine proteases by heterologous expression of the apoplastic effector Avr2 from Cladosporium fulvum in transgenic Arabidopsis lines suppresses immunity to H. schachtii. The bars represent mean number of nematodes per plant with standard error at four weeks post inoculation in transgenic line harboring ectopic Avr2 in the apoplast (Avr2) and the corresponding wild type Arabidopsis. Asterisk indicates statistical significance when using P-value <0.05 as threshold (Student's t-test). (B) Members of the extracellular papain-like cysteine protease family AtPAP in Arabidopsis are required for immunity to H. schachtii. Bars represent mean number of nematodes per plant with standard error of mean. Different letters indicate statistically significant differences between homozygous knockout mutants pap1, pap4, pap5 and corresponding wild type Arabidopsis at four weeks after inoculation determined with ANOVA (with P-values <0.05). To further confirm the importance of extracellular papain-like cysteine proteases in basal immunity to nematode infections, we challenged the homozygous knockout mutants pap-1, pap-4, and pap-5 of Arabidopsis with H. schachtii. Members of the pap gene family are the closest homologs of Rcr3pim in Arabidopsis [14]. The loss of functional pap genes in all three mutant Arabidopsis lines resulted in significantly enhanced susceptibility to H. schachtii (Fig. 5B). We therefore conclude that conserved extracellular protease-based immune signaling networks most likely regulate basal immunity to plant-parasitic nematodes in multiple unrelated plants.
Ectopic venom allergen-like proteins regulate apoplastic and chloroplastic immune signaling in Arabidopsis
To better understand the molecular basis of the suppression of basal immunity by venom allergen-like proteins, we analyzed the transcriptomes of Arabidopsis lines expressing Hs-VAP1, Hs-VAP2, and the corresponding transgenic empty vector control plants. In total, the expression of 1294 genes was significantly down-regulated, while 535 genes were significantly up-regulated in the Arabidopsis lines overexpressing either Hs-VAP1 or Hs-VAP2 (False Discovery Rate <0.05) (S6A Figure and S6B Figure). More than sixty percent of the Arabidopsis genes strongly down-regulated by ectopic Hs-VAP1 and Hs-VAP2 encode a protein that is either predicted to be extracellular or localized to the plasma membrane (S4C Figure; S1 Table and S2 Table). By contrast, the predicted subcellular location of the products of the genes strongly up-regulated by either Hs-VAP1 or Hs-VAP2 are more evenly distributed over different cellular compartments (S3 Table and S4 Figure).
To resolve specific pathways particularly affected by the overexpression of Hs-VAP1 and Hs-VAP2 in A. thaliana, we subjected all differentially expressed genes to a KEGG pathway gene set enrichment analysis [46], [47]. The KEGG pathway most significantly altered by both Hs-VAP1 and Hs-VAP2 in Arabidopsis is named ‘Plant-pathogen interactions' (KO04626; S5 Table; FDR <10-12). The vast majority of these Arabidopsis genes currently assigned to this pathway have been associated with innate immunity to plant pathogens [48]. We therefore concluded that the overexpression of venom allergen-like proteins in Arabidopsis particularly affects molecular components in immune signaling pathways.
To further investigate the expression of specific genes associated with the loss of immunity in the transgenic Arabidopsis plants overexpressing Hs-VAP1 and Hs-VAP2, we first focused on the most down-regulated genes (Table 1). The annotations of many of the most down-regulated genes point to an involvement of plant cell wall-associated processes such as modification (e.g. plant invertase/pectin methylesterase inhibitor family, and glycosyl hydrolases), signaling (e.g. proline-rich extension-like receptor kinases), and protein processing (e.g. subtilisin-like serine proteases).
Tab. 1. Ten most down-regulated transcripts by ectopic venom allergen-like proteins in Arabidopsis. 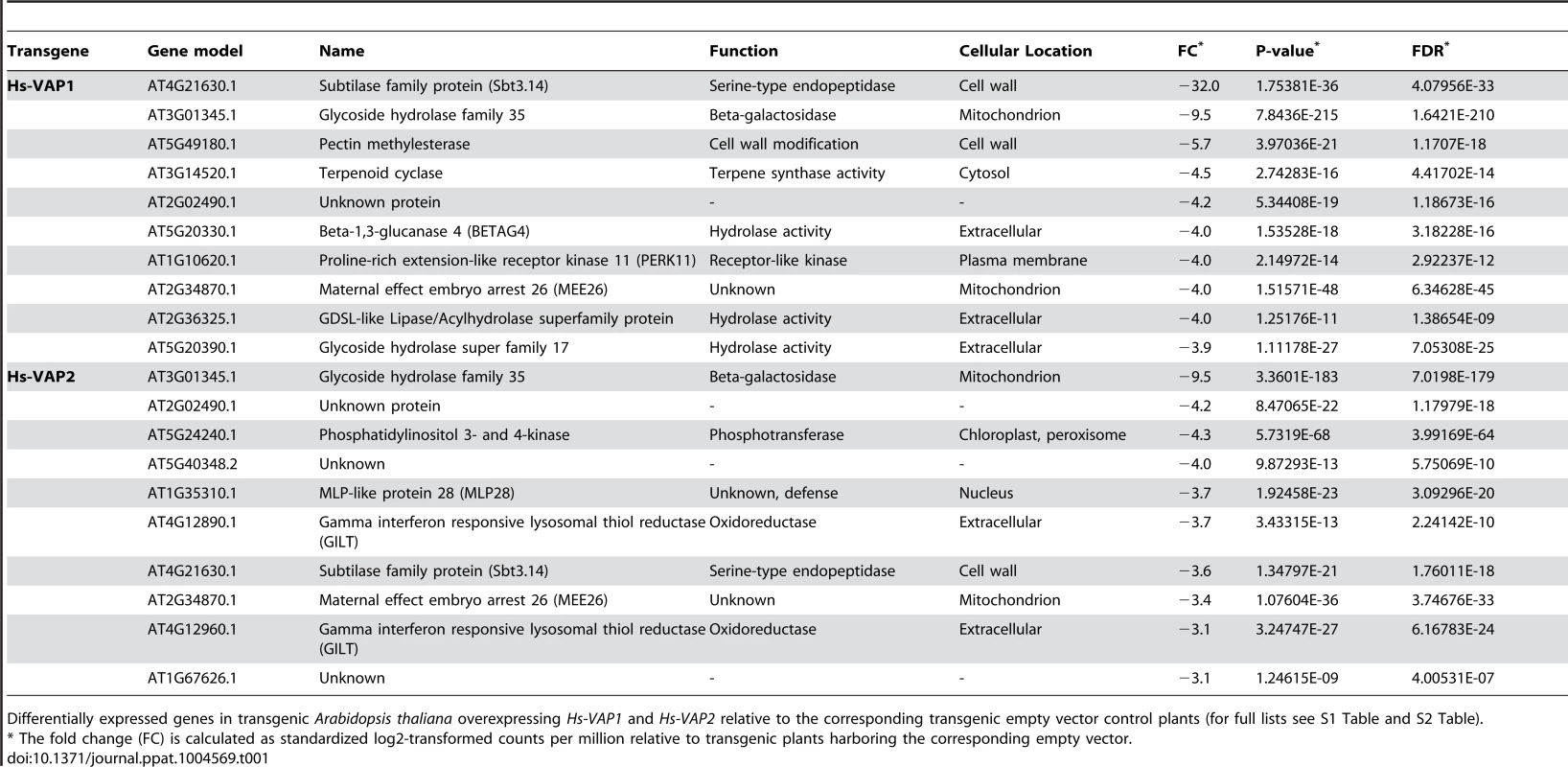
Differentially expressed genes in transgenic Arabidopsis thaliana overexpressing Hs-VAP1 and Hs-VAP2 relative to the corresponding transgenic empty vector control plants (for full lists see S1 Table and S2 Table). An exceptionally strong down-regulation was observed for gene locus AT4G21630 in Hs-VAP1-overexpressing Arabidopsis plants (Log2 fold change = −32.0). Albeit less, AT4G21630 was also strongly down-regulated in the transgenic Arabidopsis lines overexpressing Hs-VAP2. AT4G21630 encodes a putative plant cell wall-associated subtilase-like serine protease (i.e. AtSBT3.14; [49]). The role of AtSBT3.14 in Arabidopsis is not known, but a closely related homolog from the same subtilase subfamily, named AtSBT3.3, acts as an extracellular molecular switch in priming of defense responses [50]. To investigate if AtSTB3.14 is required for basal immunity of Arabidopsis to the cyst nematodes, we challenged a homozygous knockout mutant line with H. schachtii. Four weeks post inoculation the number of adult females was almost twice as high in the Atsbt3.14 knock-mutant, as compared to the corresponding Col-0 wild type Arabidopsis plants (Fig. 6). We therefore concluded that the strong down-regulation of AtSBT3.14 most likely contributes to the loss of basal immunity in Arabidopsis plants overexpressing Hs-VAP1 and Hs-VAP2.
Fig. 6. A plant cell wall-associated subtilase and non-photochemical quenching in chloroplasts regulate immunity to plant-parasitic nematodes. 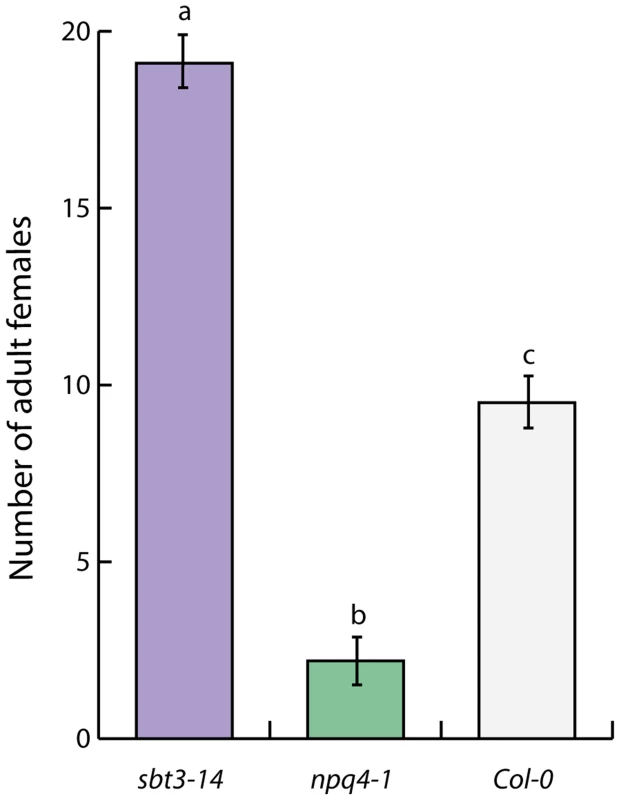
The lack of the subtilisin-like serine protease AtSBT3.13 and the chlorophyll-associated Photosystem II subunit S protein in homozygous Arabidopsis mutants (sbt3.14 and npq4-1, respectively) significantly alters their susceptibility to H. schachtii. Bars represent mean number of nematodes per plant with standard error of mean. Different letters indicate statistically significant differences between homozygous knockout mutants and corresponding wild type Arabidopsis at four weeks after inoculation (determined with ANOVA (with P-values <0.05). Next, we focused on four of the most up-regulated transcripts in the transgenic Arabidopsis plant overexpressing Hs-VAP1 and Hs-VAP2 (Log2 fold change>30.9; Table 2). Two of these transcripts derive from gene loci encoding unknown proteins (i.e. AT1G44608 and AT1G44542). However, the two other transcripts are splice variants from the same and most up-regulated gene in both Hs-VAP1 and Hs-VAP2 overexpressing plants (i.e. AT1G44575, or NPQ4). NPQ4 encodes the chlorophyll-associated Photosystem II subunit S protein (PsbS), which is involved in non-photochemical quenching of excess excitation energy [51].
Tab. 2. Ten most up-regulated transcripts by ectopic venom allergen-like proteins in Arabidopsis. 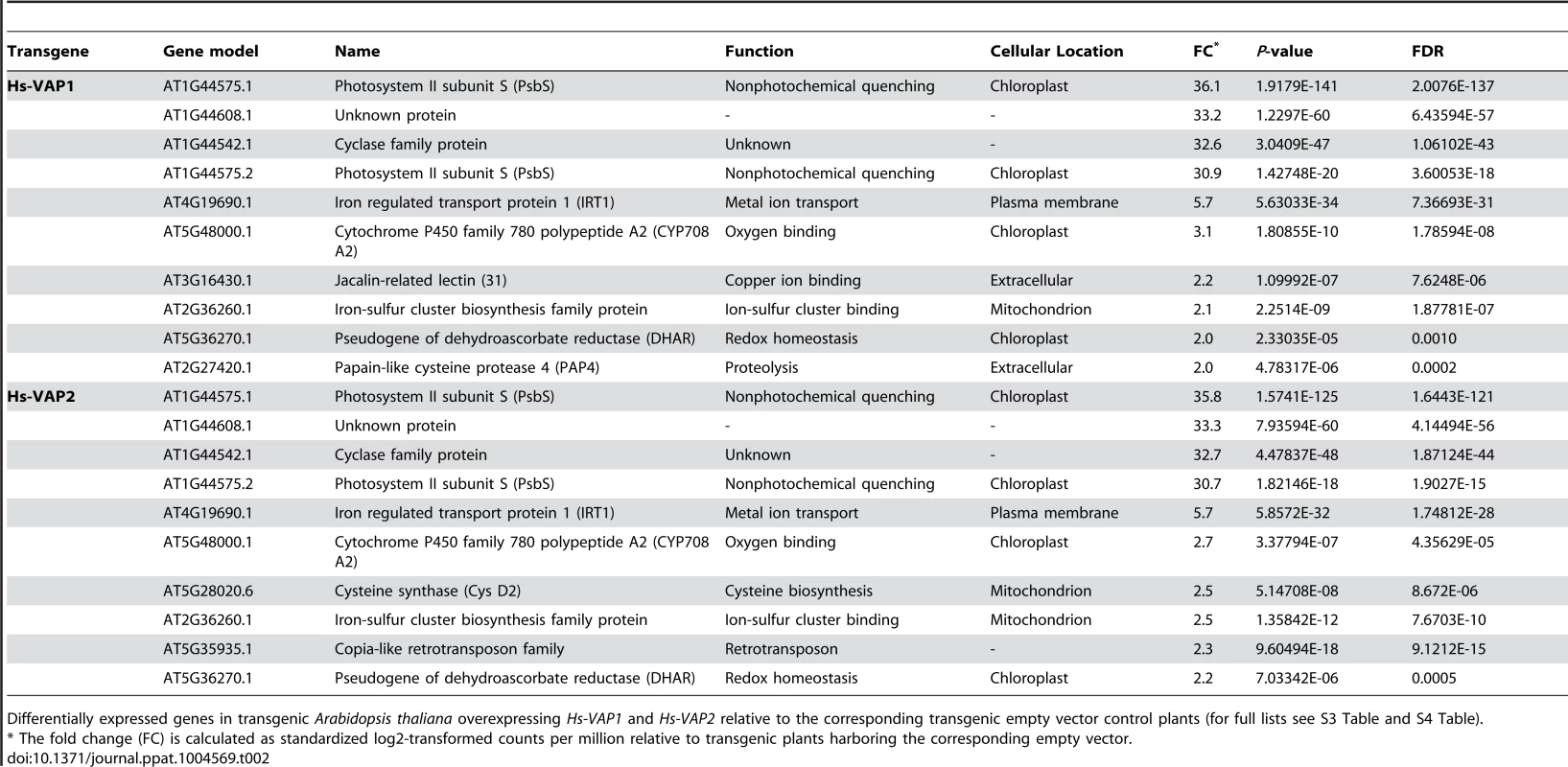
Differentially expressed genes in transgenic Arabidopsis thaliana overexpressing Hs-VAP1 and Hs-VAP2 relative to the corresponding transgenic empty vector control plants (for full lists see S3 Table and S4 Table). Recently, it was shown that the Arabidopsis knockout mutant npq4-1 lacking PsbS displays an enhanced response to flg22 [52]. The npq4-1 knockout mutant is also less attractive to herbivorous insects than the corresponding wild type Arabidopsis plants [53]. We used the npq4-1 knockout mutant to demonstrate that the lack of PsbS enhances immunity of Arabidopsis to H. schachtii (Fig. 6). We therefore conclude that the constitutively enhanced expression of NPQ4 by ectopic Hs-VAP1 and Hs-VAP2 in transgenic Arabidopsis most likely undermines their ability to mount an adequate immune response.
Apoplastic venom allergen-like proteins suppress defense-related programmed cell death activated by cell surface receptors
The increase of non-photochemical quenching capacity may block the activation of singlet oxygen-dependent programmed cell death [54], [55]. To investigate if the venom allergen-like proteins are able to suppress programmed cell death, we transiently co-expressed several inducers of cell death and nematode VAPs in leaves of Nicotiana benthamiana (S6 Table; Fig. 7). Because N. benthamiana is not a host of G. rostochiensis or H. schachtii, we also included the venom allergen-like protein Mi-VAP1 from the polyphagous M. incognita in these cell death suppression assays. Both Mi-VAP1 and Hs-VAP1 consistently suppressed the cell death induced by the Phytophthora infestans elicitin INF1 (Fig. 7A). Both Mi-VAP1 and Hs-VAP1 also suppressed the cell death induced by extracellular receptor protein Cf-4 from tomato and its cognate elicitor Avr4 from C. fulvum (Fig. 7B). All tested VAPs similarly suppressed the cell death induced by the extracellular receptor-like protein Cf-9 from tomato and its cognate elicitor Avr9 from C. fulvum (Fig. 7C). Surprisingly, none of the venom allergen-like proteins suppressed the cell death responses induced by several cytoplasmic immune receptors and their cognate elicitors (e.g. Rx1, Gpa2, R3a, Blb2). To confirm that the ectopic VAPs harboring their native signal peptide for secretion are indeed secreted to the apoplast in planta, we analyzed apoplastic fluids isolated from agroinfiltrated leaf of N. benthamiana on western blots using antiserum towards an additional carboxyl terminal FLAG affinity tag on the proteins (S7 Figure). Taken together, we conclude that apoplastic venom allergen-like proteins selectively suppress the activation of the programmed cell death by surface-localized immune receptors.
Fig. 7. Ectopic venom allergen-like proteins from cyst and root-knot nematodes selectively suppress defense-related programmed cell death. 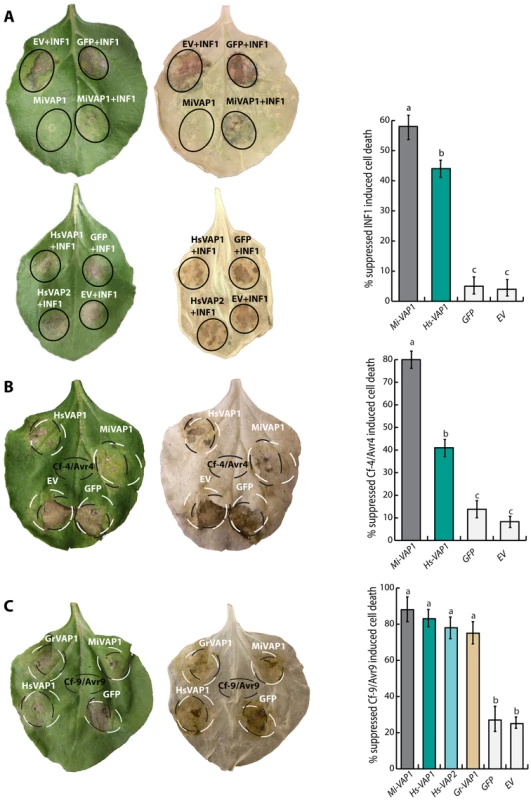
(A) Agroinfiltration assays in Nicotiana benthamiana showing the transient co-expression in the apoplast of cell death inducing elicitin INF1 of Phytophthora infestans and venom allergen-like proteins from G. rostochiensis (Gr-VAP1), H. schachtii (Hs-VAP1 and Hs-VAP2), and Meloidogyne incognita (Mi-VAP1). Co-expressions with the corresponding empty binary vector (EV) and green fluorescent protein (GFP) were included as controls. (B and C) Transient co-expression of receptor-like proteins Cf-4 and Cf-9 from tomato and their cognate elicitors Avr4 and Avr9 from C. fulvum with venom allergen-like proteins and controls as described above. Photographs were taken 4 days post infiltration for INF1, and 7 days post infiltration for Cf-4/Avr4 and Cf9/Avr9. The bars represent the mean number of events in which cell death suppression was observed for a total of 60 inoculation spots over 5 biological replicates (with standard error of mean). Different letters indicate a significant difference when using P-value <0.05 (in an ANOVA). Discussion
Since their first identification in the canine hookworm Ancylostoma caninum (Ac-ASP; [21]) and the root-knot nematode M. incognita (Mi-MSP1/VAP1; [44]) venom allergen-like proteins are thought to be crucial for the onset of parasitism of nematodes in animals and plants. However, in spite of their conservation, relative abundance in nematode secretions, and strong association with the onset of parasitism, the role of venom allergen-like proteins in host-parasite interactions has so far remained elusive [18], [19]. To date, the only available functional data on the role of a venom allergen-like protein in secretions of parasitic nematodes point to a perturbation of a complement receptor on human immune cells [37]–[40]. Here, we demonstrate that plant-parasitic nematodes most likely deliver venom allergen-like proteins into the apoplast of host cells to suppress basal immunity mediated by surface-localized immune receptors.
Several lines of evidence in our data suggest that plant-parasitic nematodes may use venom allergen-like proteins to modulate immune responses activated by tissue damage caused by migratory nematodes inside host plants. First, the expression of Gr-VAP1 in G. rostochiensis coincides with host invasion by infective juveniles and intracellular migration by adult males inside plants (Fig. 2). Both host invasion and migration by parasitic nematodes involves the enzymatic breakdown of plant cell walls, resulting in extensive damage to host tissue [56]. More importantly, Gr-VAP1 is secreted at the same time and from the same pharyngeal glands in G. rostochiensis as an elaborate set of plant cell wall-degrading enzymes [14]. The transient knockdown of these plant cell wall-degrading enzymes in infective juveniles of G. rostochiensis inhibits the onset of parasitism [56]. Similarly, the transient knockdown of Gr-VAP1 expression during host invasion significantly reduced the number of infective juveniles inside susceptible tomato plants (Fig. 1), showing that this venom allergen-like protein is also a critical factor during the onset of parasitism.
Although we have not formally shown that plant-parasitic nematodes deliver VAPs into the apoplast of host cells in planta, our previous work demonstrates that this is nonetheless most likely the case [14]. More specifically, we have shown that Gr-VAP1 associates with and perturbs apoplastic Rcr3pim in tomato, and that this perturbation specifically activates nematode resistance and programmed cell death mediated by the extracellular receptor-like protein Cf-2. Furthermore, tomato plants that harbor apoplastic Rcr3pim, but not Cf-2, are almost twice as susceptible to nematode infections than tomato plants with allelic variants of apoplastic Rcr3 to which Gr-VAP1 is unable to bind or tomato plants that have no functional Rcr3 at all. Altogether, we conclude that venom allergen-like proteins may specifically function as apoplastic suppressors of immune responses triggered by plant cell wall fragments released by the enzymatic breakdown of plant cell walls during nematode migration inside host plants [57], [58].
Little work has been done on the importance of surface-localized pattern recognition receptors mediating damage-triggered immunity in nematode-plant interactions. Transcriptome analyses of nematode-infected roots suggest that plant cell wall-associated legume-like lectin receptor kinases might be involved in basal immunity to H. schachtii in Arabidopsis [59], but further research is needed to corroborate this. Interestingly, both Hs-VAP1 and Hs-VAP2 significantly down-regulate the expression of five proline-rich extensin-like receptor kinases (i.e. At4G34440, AtPERK5; At3G18810, AtPERK6; At1G49270, AtPERK7; At1G10620, AtPERK11; S1 Table and S2 Table)). These PERKs belong to a family of fifteen predicted transmembrane receptor-like kinases in Arabidopsis. The extracellular domain in PERKs shares similarity with plant cell wall-associated extensin proteins [60], but the biological function of most members of the AtPERK family is unknown. However, the expression of BnPERK1 from Brassica napus is rapidly induced following wounding, because of which it is thought to mediate early events in defense responses to cell wall damage by invading plant pathogens [61].
The modulation of basal immunity by venom allergen-like proteins in plants most likely involves at least two different classes of extracellular proteases. The first class of extracellular proteases regulating basal immunity to nematode infections in plants concerns the papain-like cysteine proteases. The inhibition of the extracellular papain-like cysteine protease Rcr3pim from S. pimpinellifolium by Gr-VAP1 results in enhanced susceptibility of tomato plants to G. rostochiensis [14]. Here, we showed that Gr-VAP1 also perturbs the extracellular papain-like cysteine protease C14tub from potato (S. tuberosum; Fig. 3B), while ectopic Gr-VAP1 significantly increased the susceptibility of potato plants to G. rostochiensis (Fig. 3A). Remarkably, ectopic Gr-VAP1 also suppressed basal immunity of Arabidopsis, even though this plant species is not a host of G. rostochiensis. However, a similar phenomenon has been observed with the apoplastic effector Avr2 of the C. fulvum, which acts as an inhibitor of Rcr3pim and several other extracellular papain-like cysteine proteases in tomato [15], [16], [42], [62]. Although A. thaliana is not a host of C. fulvum either, ectopic Avr2 nonetheless interacts with multiple papain-like cysteine proteases required for basal defense in Arabidopsis [42]. The inhibition of apoplastic papain-like cysteine proteases by ectopic Avr2 also suppresses immunity of Arabidopsis to H. schachtii (Fig. 5A). Similarly, the lack of three papain-like cysteine proteases most related to Rcr3pim in Arabidopsis mutants (i.e. pap1, pap4, and pap5) suppresses immunity to H. schachtii (Fig. 5B) Altogether, these findings position the inhibition of extracellular papain-like cysteine proteases by apoplastic effectors as an important regulatory process in plant innate immunity to cyst nematodes.
The second class of extracellular proteases most likely involved in the suppression of basal immunity by venom allergen-like proteins concerns subtilisin-like serine proteases. The transcript most down-regulated by ectopic Hs-VAP1 in Arabidopsis encodes the plant cell wall-associated subtilisin-like serine protease AtSBT3.14 (S1 Table; AT4G21630; [49], [63]). AtSBT3.14 is also down-regulated by ectopic Hs-VAP2, albeit to a lesser extent. The Arabidopsis SBT family comprises 56 members, most of which are still uncharacterized. AtSBT3.14 is specifically expressed in roots, siliques, and dry seed of A. thaliana, but its function is not known [63]. AtSBT3.14 belongs to the same subfamily as AtSBT3.3, which functions as an extracellular molecular switch in the priming of defense responses in Arabidopsis [50]. T-DNA insertion knockout mutations in the AtSBT3.3 gene compromise innate immunity of Arabidopsis. The loss of immunity of Arabidopsis mutants lacking a functional AtSBT3.14 gene to H. schachtii suggests that this plant cell wall-associated subtilase may also act as an extracellular regulator of basal innate immunity (Fig. 6).
Most of the genes differentially regulated by the overexpression of venom allergen-like proteins in Arabidopsis are typically associated with innate immunity and plant cell wall-associated processes. A notable exception to this is NPQ4, which was the most up-regulated gene in both Hs-VAP1 and Hs-VAP2 overexpressing plants. The PsbS protein encoded by NPQ4 is involved in non-photochemical fluorescence quenching in the thylakoid membranes of chloroplasts [51], [64]. PsbS functions as photo protectant by mediating the thermal dissipation of excess excitation energy of singlet chlorophyll. Saturation of the electron transport chain in the photosystem II by excess light can lead to the accumulation of excited chlorophyll, which when insufficiently quenched by PsbS transfers its energy to oxygen to form highly reactive singlet oxygen [65]. The non-photochemical quenching capacity in chloroplasts thus regulates the generation of singlet oxygen [54]. Singlet oxygen can be the cause of oxidative damage, but on the other hand it is also involved in the peroxidation of lipids into oxylipin hormones (e.g. jasmonic acid; [54]) and in the onset of programmed cell death [55], [66]. It is for this duality that PsbS is thought to play a key role in the trade-off between the ability to protect against abiotic photo-oxidative stress and the ability to mount effective redox-dependent immune responses to biotic invaders [54].
Our data shows that ectopic Hs-VAP1 and Hs-VAP2 suppress innate immune responses in Arabidopsis, at least partly, through their regulation of PsbS. As PsbS is a rate-limiting factor in non-photochemical quenching of excited singlet chlorophyll [67], the more than 30-fold increase in the expression of NPQ4 by ectopic venom allergen-like proteins in the transgenic Arabidopsis most likely reduces the formation of singlet oxygen under biotic stress [54]. As a consequence, the constitutive augmentation of the non-photochemical quenching capacity by elevated levels of PsbS will probably also affect the production of oxylipin hormones (i.e. jasmonic acid, and its precursors) and the signaling of programmed cell death in response to biotic stress [54]. By contrast, PsbS-deficient npq4-1 mutant Arabidopsis plants show an enhanced production of jasmonic acid in response to herbivory by feeding insects [68]. We used the same Arabidopsis mutant line to demonstrate that PsbS-deficient plants are immune to infections by H. schachtii (Fig. 6). This finding shows that a functional PsbS protein is required for virulence of H. schachtii in Arabidopsis, possibly for down-regulating oxylipin hormone signaling or other singlet oxygen-dependent immune responses.
While a PsbS-centered model may offer a plausible explanation for the suppression of immune responses by ectopic venom allergen-like proteins in leafs, the biological relevance of PsbS as regulator immunity to parasitic nematodes in roots is more puzzling. PsbS is localized in chloroplasts, which mainly occur in aerial plant parts that are exposed to light but not in roots. However, the Arabidopsis plants used in nematode infection assays are routinely cultured in vitro on translucent media in a light/dark cycle to monitor the infection over time. It has been shown before that permanent feeding structures formed by H. schachtii under these circumstances harbor chloroplasts [69]. Importantly, it has also been shown that NPQ4 is strongly up-regulated in permanent feeding structures of H. schachtii as compared to other root cells in the elongation zone of Arabidopsis plants kept in a light/dark cycle [70]. One could argue that the occurrence of chloroplasts and the expression of NPQ4 in nematode-induced feeding structures in roots are artifacts caused by the unnatural exposure of the roots to light. However, others have shown that permanent feeding structures of H. schachtii in Arabidopsis roots that are kept in the dark also harbor plastids with similar fluorescence spectra as chloroplasts [70]. More research is therefore needed to further investigate the nature and functions of PsbS in plastids of nematode-infected roots. Taken together, we conclude that non-photochemical quenching capacity is at least partly responsible for regulating innate immunity of roots to infection by H. schachtii under our experimental conditions.
In conclusion, plants monitor the integrity of their cell walls with specific surface-localized pattern recognition receptors [71], [72]. The recognition of fragments of plant cell walls can elicit strong basal defense responses that counteract further invasion by microbial invaders [73], [74]. As plant-parasitic nematodes cause significant damage to plant cell walls during their migration inside host plants, they could evidently benefit from effectors that suppress immunity triggered by fragments from damaged plant cell walls. Our data allows for a model in which apoplastic venom allergen-like proteins of plant-parasitic nematodes suppress host defenses activated by the detection of fragments of plant cell walls released by migrating nematodes. This model could also explain why ectopic VAPs particularly affect the susceptibility of Arabidopsis to diverse unrelated lesion-forming plant pathogens, the symptoms of which also involve significant plant cell wall modifications.
So far, we have identified three components of the molecular mechanisms that are most likely underlying the suppression of plant innate immunity by apoplastic venom allergen-like proteins (i.e. extracellular papain-like cysteine proteases, cell wall-associated subtilisin-like serine protease, and the chlorophyll-associated Photosystem II subunit S protein). As our mutant analyses showed, each of these components separately has a major impact on immunity to cyst nematodes in Arabidopsis. An important question that needs further research is if all three components are part of a single signaling pathway that spans different subcellular compartments. This might not be the case, because promiscuous effectors like Gr-VAP and Avr2 can interact with multiple apoplastic papain-like cysteine proteases, each of which may feed into different signaling pathways.
Materials and Methods
Knockdown of Gr-VAP1 expression by RNA interference
Gr-VAP1 expression in preparasitic second stage juveniles (ppJ2s) of G. rostochiensis was knocked-down by soaking nematodes in double-stranded (ds) RNA matching 820 base pairs of the Gr-VAP1 coding sequence as described by Chen et al [75] and Rehman et al [56]. Briefly, a cDNA fragment was PCR-amplified with the primers Gr-VAP1-RNAiFW and Gr-VAP1-RNAiR (S7 Table) using Gr-VAP1 cDNA as template. The amplified cDNA fragment of Gr-VAP1 was subsequently used as template for generating dsRNA in vitro using the Megascript RNAi kit (Ambion, Cambridgeshire, UK). Double-stranded RNA matching the sequence of the Nautilus gene from Drosophila melanogaster (Genbank accession number M68897) was used as control treatment. RNA interference was induced in nematodes by soaking approximately 15,000 freshly hatched ppJ2s of G. rostochiensis Ro1 Mierenbos in a 1 mg/ml dsRNA solution, including 50 mM octopamine, 3 mM spermidine, and 0.05% gelatin. The treatments were done in duplo so that 15,000 juveniles could be processed further for infectivity assay on tomato seedlings and 15,000 juveniles could be used for semi-quantitative reverse transcription (RT)-PCR.
To test the effect of RNA interference on infectivity of G. rostochiensis, we inoculated plates with five two-week old tomato seedlings (cultivar Moneymaker) on Gamborg B5 medium with 400 dsRNA-treated ppJ2s [56]. For each treatment a total number of 10 plates was inoculated with dsRNA-treated ppJ2s. The plants were grown at 24°C and light/dark cycles of 16 h/8 h. Seven days post inoculation, the roots were stained with acid fuchsin, destained using acidified glycerol, and the number of nematode per root was determined using a dissection microscope. The means of numbers of nematodes per plant were tested for significant differences in a one-way ANOVA.
To analyze Gr-VAP1 expression after dsRNA treatment, total RNA was extracted from dsRNA-treated ppJ2s using the RNeasy Mini kit (Qiagen, Hilden, Germany). RT-PCR was done following the protocol of the SuperScript™ III One-Step RT-PCR System (Invitrogen) using the primer Gr-VAP1-sRTFw and Gr-VAP1-sRTRv (S7 Table), which were designed outside the region targeted by the dsRNA. The expression of the 60S acidic ribosomal protein-encoding gene (Genbank accession number BM354715.1) was analyzed with primers 60S-RTFw and 60S-RTRv (S7 Table) as a reference for constitutive expression. Aliquots of the PCR solutions were visualized on ethidium bromide stained 1% agarose gel after 28 cycles.
Fluorescent protease activity profiling
Fluorescent activity based protease profiling was used to test whether Gr-VAP1 perturbs the active site of cysteine proteases. The cysteine proteases C14tub of S. tuberosum and C14lyc of S. lycopersicum were transiently overexpressed in apoplastic fluids of N. benthamiana leaves following agroinfiltration [62]. Twenty-five to fifty microliters of apoplastic fluid was incubated with either 100 nM of P. pastoris produced Avr2, 100 nM cystatin from chicken egg-white (Sigma-Aldrich), or 300 nM of Gr-VAP1 isolated from apoplastic fluids of agroinfiltrated N. benthamiana leaves (see below) in 50 mM sodium acetate (pH 5.5) and 100 µM DTT. To label the available active sites in these cysteine proteases, the proteins were subsequently incubated for 5 h with 1 µM of fluorescent DCG-04-TMR [76]. Fluorescent labeled proteins were separated in 12% Bis-Tris gels (Invitrogen), which were subsequently analyzed using a fluorescent imager scanner (Molecular Imager FX, Bio-Rad, Hercules, CA, USA). Labelling densities were quantified in triplicates using the computer software Quantity One 4.6.9 (Bio-Rad Laboratories).
Temporal expression of Gr-VAP1 in G. rostochiensis
To study the expression of Gr-VAP1 at different time points before and after inoculation, we used semi-quantitative RT-PCR as described above. Messenger RNA extraction and cDNA synthesis was conducted on parasitic second, third, and fourth stage juveniles and the adult males and females isolated from roots of susceptible potato (cultivar Bintje) at 13, 19, 23, 27, and 34 days post inoculation respectively. Gr-VAP1 expression in these developmental stages was examined by targeting a gene specific fragment of 146 base pairs of Gr-VAP1 with primers Gr-VAP1-RTFw and Gr-VAP1-RTRv (S7 Table). The constitutively expressed cAMP-dependent protein kinase (Gr-cAMP; GenBank accession number BM343563) was PCR amplified with the primers cAMP-RTFw and cAMP-RTRv (S7 Table) as a reference. We included reactions without reverse transcriptase to test for contaminating genomic DNA of the nematodes, while non-infected potato roots were included to check for non-specific amplification of host-derived cDNA.
Generating transgenic potato plants
Transgenic potato plants (Line V; genotype 6487-9) overexpressing Gr-VAP1 in the apoplast were generated as described by Postma et al [30]. Briefly, potato stem pieces were incubated for 10 minutes with a suspension of Agrobacterium tumefaciens strain AGL1 carrying the Gr-VAP1 cDNA sequence, including its native signal peptide for secretion, in pMDC32 [14], [77]. Transformant callus was selected on ZCVK medium (MS20 medium, 8 g/l plant agar, 1 mg/l zeatin, 100 mg/l kanamycin, 200 mg/l cefotaxim, 200 mg/l vancomycin; pH 5.8). The introgression of Gr-VAP1 insert was checked by PCR on genomic DNA extracted from plant leaves using the DNeasy Plant Mini Kit (Qiagen). The expression of Gr-VAP1 was checked by quantitative PCR (qPCR) using the primers qGrVAP1-Fw and qGrVAP1-Rv (S7 Table) on RNA extracted from leaves using the RNeasy Plant Mini Kit (Qiagen). The constitutively expressed actin was amplified with the primers StActinF and StActinR (S7 Table) as a reference. qPCR was performed using Absolute QPCR SYBR Green Mix (Thermo Fisher Scientific) with amplification of 15 min at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 63°C and 30 s at 72°C.
Infection assays on potato plants
Dried cysts of G. rostochiensis pathotype Ro1-Mierenbos were soaked in potato root diffusate on a 100-µm sieve to collect ppJ2s [78]. Remnants of roots and other debris were removed from suspensions of freshly hatched ppJ2s using centrifugation in sucrose gradient. Prior to inoculation potato plants the ppJ2s were surface sterilized, and resuspended in sterile 0.7% (w/v) solution of Gelrite (Duchefa) as previously described [30]. Approximately, 200 ppJ2s were inoculated onto 3-week-old in vitro-grown plants potato plant. Adult females per plant were counted 6 to 8 weeks after inoculation. Two independently transformed potato lines were used in these experiments. The infection assays were repeated at least 3 times.
Identification and cloning of venom allergen-like protein from H. schachtii
To identify and clone venom allergen-like proteins from the beet cyst nematode H. schachtii, we first queried the expressed sequence tag database at Genbank using the sequence of Gr-VAP1 as query. Four cDNA library clones, from which matching expressed sequence tags derived, were acquired from “The Washington University Nematode EST Project”[79]. Re-sequencing of library insert in these clones, with the primers M13Fw and M13Rv (S7 Table), resulted in the identification of two full-length cDNA sequences encoding novel venom allergen-like proteins named Hs-VAP1 and Hs-VAP2. The cDNA sequences encoding the complete open reading frames of Hs-VAP1 and Hs-VAP2, including native signal peptides for secretion, were PCR - amplified with gene specific primers Hs-VAP1-GWFw, HsVAP1-GwRv, HsVAP2-GWFw, and HsVAP2-GwRv (S7 Table) and cloned into the entry vector pENT/D-TOPO (Invitrogen). The inserts in these entry vectors were subcloned into the binary plasmids pMDC32 for stable plant transformation [77] or pGWB411 [80] for transient expression, using Gateway technology (Invitrogen).
Generation of transgenic Arabidopsis
To generate transgenic Arabidopsis lines constitutively overexpressing venom allergen-like proteins in apoplast, we transformed A. thaliana Columbia 0 with A. tumefaciens strain GV3101 carrying constructs of Gr-VAP1, Hs-VAP1, and Hs-VAP2 in pMDC32, and pMDC32 without insert, using the floral dip method [81]. Primary transformants were selected on agar with 50 µg/ml kanamycin after which the plants were transferred to soil to produce seeds. Several independent homozygous single insertion lines were selected, and T3 and T4 generations were used for infection assays (see below). The introgression of Hs-VAP1 and Hs-VAP2 was checked by PCR on genomic DNA extracted from seedlings using the DNeasy Plant Mini Kit (Qiagen). The expression of the transgenes was checked by qPCR using the primers qHsVAP1-F, qHsVAP1-R, qHsVAP2-F, and qHsVAP2-R (S7 Table) on RNA extracted from seedlings using the RNeasy Plant Mini Kit (Qiagen). The clathrin adaptor complex medium subunit family protein (At5g46630) was amplified with the primers AtClathrinF and AtClathrinR (S7 Table) as a reference. qPCR was performed using Absolute QPCR SYBR Green Mix (Thermo Fisher Scientific) with amplification of 15 min at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 60°C and 30 s at 72°C.
Seeds of the homozygous transgenic T-DNA insertion mutants of the cysteine proteases PAP1 (At2g34080), PAP4 (At2g27420), PAP5 (At3g49340), the serine protease SBT3.13 (At4g21630), and the chlorophyll-associated Photosystem II subunit S (At1g44575) were obtained from the SALK homozygote T-DNA collection. The mutant plants were propagated under standard greenhouse conditions of a 16-h/8-h light/dark regime and 60% relative humidity.
Infection assays on Arabidopsis plants
Seeds from transgenic Arabidopsis and wild-type A. thaliana Col-0 were vapor sterilized and planted in 12-well cell culture plates (Greiner bio-one) containing modified Knop's medium [82]. Plants were grown at 24°C under 16-h-light/8-h-dark conditions. Two-week-old seedlings were inoculated with ∼250 surface-sterilized ppJ2s of H. schachtii [83]. Two and four weeks after inoculation, the number of female J4s of H. schachtii was counted by visual inspection. The statistical significance of the pairwise differences between plant genotypes and the empty pMDC32 vector control and the wild type Arabidopsis was assessed with a one-way ANOVA.
The susceptibility of the Arabidopsis plants to infections by B. cinerea, P. cucumerina, A. brassicicola, and P. brassicae was determined on 4-week-old soil-grown plants [42], [84], [85]. Briefly, for B. cinerea, P. cucumerina, A. brassicicola, plants were drop inoculated by placing two 4-µl drops of conidial suspension (5×105 conidia/ml) on each leaf. Plants were incubated at 20°C, 100% relative humidity, and a 16-h/8-h light/dark regime. Arabidopsis was inoculated with P. brassicae by placing 5-mm-diameter mycelial plugs of a 2-week-old P. brassicae agar plate culture onto leaves. Subsequently, the plants were incubated at 16°C, 100% relative humidity, and a 16-h/8-h light/dark regime. After two days the mycelial plugs were removed from the leaves. Disease progression for these pathogens was scored at regular intervals, and representative pictures were taken at 4 days after inoculation. The statistical significance of the pairwise differences between plant genotypes and the empty pMDC32 vector control was assessed with a one-way ANOVA.
For inoculation of Arabidopsis with V. dahliae, 2-week-old soil-grown plants were uprooted and inoculated by dipping the roots for 2 min in a conidial suspension (106 conidia/ml). After replanting in soil, plants were incubated at standard greenhouse conditions of a 16-h/8-h light/dark regime and 60% relative humidity. Disease progression was monitored until 25 days after inoculation. The statistical significance of the pairwise differences between plant genotypes and the empty pMDC32 vector control was assessed with a one-way ANOVA. All infection assays were performed at least 2 times.
The susceptibility of the Arabidopsis plants to infections by P. syringae pv. tomato DC3000 was determined on 2-week-old Arabidopsis seedlings as previously described [86]. Briefly, three 2-µl drops of a cell suspension of Pst at 109 CFU/ml, in 10 mM MgSO4 supplemented with 0.01% (v/v) Silwet L77, was inoculated on the two most expanded and in the center of the leaf rosette. Inoculated plants were subsequently incubated at 21°C, 100% relative humidity, and a 16-h/8-h light/dark regime. Disease severity was scored 3 days after challenge inoculation. Colonization levels of the bacteria were determined with the method described by Pieterse et al. [87]. The statistical significance of the pairwise differences between plant genotypes and the empty pMDC32 vector control was assessed with a one-way ANOVA.
Growth inhibition assays
Arabidopsis growth inhibition assays were performed as described elsewhere [88]. Briefly, seedlings were grown for 5 days on MS agar plates, supplemented with 1% w/v sucrose and 0.8% agar. Subsequently, seedlings were transferred to liquid MS medium supplied with 10 µM of the flg22 (QRLSTGSRINSAKDDAAGLQIA) synthetic peptide. One seedling was placed on 400 µl of medium in wells of 24-well-plates. The effect of treatment with the flg22 peptide on the growth of transgenic and wild type Arabidopsis (Col-0) seedlings was analyzed after 7 days by measuring root length. Statistical significance of the difference between plant genotypes was assessed with a one-way ANOVA.
RNA-seq on Arabidopsis plants
Two-weeks-old transgenic Arabidopsis plants, grown under the same conditions as for the infection with H. schachtii, were collected, flash-frozen in liquid nitrogen and total RNA was extracted with the Maxwell® 16 LEV simplyRNA purification kit (Promega). cDNA synthesis, library preparation (200-bp inserts), and Illumina sequencing (90-bp paired-end reads) was performed at BGI (Hong-Kong). Reads were mapped to the Arabidopsis genome (tair10) using TopHat and transformed into a count per gene per sample by using the BEDTools suite (function coverageBed). The edgeR [89] method was used to analyze differentially expressed genes (DEGs) between groups. DEGs were mapped to Gene Ontology (GO) terms in the database (http://www.geneontology.org/), and gene numbers were calculated for every term using an ultra-geometric test to find significantly enriched GO terms in DEGs. Calculated p-value went through a Bonferroni Correction, taking corrected p-value ≤0.05 as a threshold. KEGG pathway enrichment analysis was used to identify significantly enriched metabolic pathways or signal transduction pathways in DEGs comparing with the whole genome background. Subcellular localization was determined for all DEGs using the SUBcellular localization database for Arabidopsis proteins [90].
Suppression of defense-related programmed cell death in Nicotiana benthamiana
The suppression of programmed cell death in leaves of N. benthamiana was assessed by using Gr-VAP1, Hs-VAP1, Hs-VAP2, and Mi-VAP1 (including their native signal peptide for secretion) subcloned into pGWB411 [80]. The Mi-VAP1 construct was synthetized at GeneArt (Life Technologies) based on the sequence in Genbank (accession AAD01511.1). All constructs were transferred to Agrobacterium tumefaciens GV3101, and used for agroinfiltration in leaves of the N. benthamiana. Empty pGWB411 and plasmids carrying GFP in pGWB411 [80] were used as controls to assess the non-specific suppression of programmed cell death by agroinfiltration. The transient co-expression by agroinfiltration of several pairs of resistance genes and cognate elicitors was used to induce programmed cell death in leaves of N. benthamiana (S6 Table; [30]). A. tumefaciens harboring individual binary vectors was grown at 28°C in liquid yeast extract peptone medium with appropriate antibiotics for 16 h. The bacteria were spun down and resuspended in infiltration medium to an optical density at 600 nm (OD600) of 0.1 [30]. Agroinfiltration was done the abaxial side of the leaves of N. benthamiana using a 1 ml syringe. Co-infiltration of different constructs was performed by mixing equal volumes of the bacterial suspensions to a final optical density of 0.3. Agroinfiltrated leaves were monitored for up to 7 d for cell death symptoms.
To assess whether the VAPs harboring their native signal peptide for secretion (in pGWB411) were indeed secreted to the apoplast of agroinfiltrated leaves of N. benthamiana, we isolated apoplastic fluids by vacuum-infiltrating ice-cold extraction buffer (50 mM phosphate-buffered saline pH = 7.4, 100 mM NaCl, and 0.1% v/v Tween-20) for 10 min. Infiltrated leaves were surface dried and placed in a 10-ml syringe hanging in a 50 ml tube and centrifuged at 2000 g for 10 min at 4°C. Apoplastic fluids were subsequently separated under reducing conditions by SDS-PAGE on a 12% Bis-Tris gel and transferred to an Invitrolon™ PVDF membrane (Life Technologies). For visualization of VAPs on western blots, we used a mouse monoclonal ANTI-FLAG® M2-Peroxidase (HRP) antibody to detect the FLAG tag at the carboxyl terminus of the recombinant proteins. Pictures were taken using the G:BOX Chemi System device (SynGene).
Supporting Information
Zdroje
1. Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, et al. (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol: 946–961.
2. McCarter JP (2009) Molecular approaches toward resistance to plant-parasitic nematodes. In: Berg RH, Taylor CG, editors. Cell biology of plant nematode parasitism.Berlin Heidelberg: Springer Verlag. pp.239–267.
3. Goverse A, Smant G (2014) The Activation and Suppression of Plant Innate Immunity by Parasitic Nematodes. Annu Rev Phytopathol 52: in press.
4. WilliamsonVM, KumarA (2006) Nematode resistance in plants: the battle underground. Trends Genet 22 : 396–403.
5. GishLA, ClarkSE (2011) The RLK/Pelle family of kinases. Plant J 66 : 117–127.
6. BollerT, FelixG (2009) A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60 : 379–407.
7. SchwessingerB, ZipfelC (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11 : 389–395.
8. ChisholmST, CoakerG, DayB, StaskawiczBJ (2006) Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 124 : 803–814.
9. KruijtM, De KockMJD, De WitPJGM (2005) Receptor-like proteins involved in plant disease resistance - Review. Mol Plant Pathol 6 : 85–97.
10. DixonMS, JonesDA, KeddieJS, ThomasCM, HarrisonK, et al. (1996) The Tomato Cf-2 Disease Resistance Locus Comprises Two Functional Genes Encoding Leucine-Rich Repeat Proteins. Cell 84 : 451–459.
11. WangG, FiersM, EllendorffU, WangZ, de WitPJGM, et al. (2010) The diverse roles of extracellular leucine-rich repeat-containing receptor-like proteins in plants. Crit Rev Plant Sci 29 : 285–299.
12. LiebrandTWH, van den BurgHA, JoostenMHAJ (2014) Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci 19 : 123–132.
13. MonaghanJ, ZipfelC (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol 15 : 349–357.
14. Lozano-TorresJL, WilbersRHP, GawronskiP, BoshovenJC, Finkers-TomczakA, et al. (2012) Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc Natl Acad Sci U S A 109 : 10119–10124.
15. KrügerJ, ThomasCM, GolsteinC, DixonMS, SmokerM, et al. (2002) A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296 : 744–747.
16. RooneyHCE, Van't KloosterJW, Van Der HoornRAL, JoostenMHAJ, JonesJDG, et al. (2005) Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science 308 : 1783–1786.
17. SongJ, WinJ, TianM, SchornackS, KaschaniF, et al. (2009) Apoplastic effectors secreted by two unrelated eukaryotic plant pathogens target the tomato defense protease Rcr3. Proc Natl Acad Sci U S A 106 : 1654–1659.
18. CantacessiC, CampbellBE, VisserA, GeldhofP, NolanMJ, et al. (2009) A portrait of the "SCP/TAPS" proteins of eukaryotes - Developing a framework for fundamental research and biotechnological outcomes. Biotechnol Adv 27 : 376–388.
19. CantacessiC, GasserRB (2012) SCP/TAPS proteins in helminths - where to from now? Mol Cell Probes 26 : 54–59.
20. JasmerDP, GoverseA, SmantG (2003) Parasitic Nematode Interactions with Mammals and Plants. Annu Rev Phytopathol 41 : 245–270.
21. HawdonJM, JonesBF, HoffmanDR, HotezPJ (1996) Cloning and characterization of Ancylostoma-secreted protein: A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem 271 : 6672–6678.
22. HawdonJM, NarasimhanS, HotezPJ (1999) Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol Biochem Parasitol 99 : 149–165.
23. DatuBJD, GasserRB, NagarajSH, OngEK, O'DonoghueP, et al. (2008) Transcriptional changes in the hookworm, Ancylostoma caninum, during the transition from a free-living to a parasitic larva. PLoS Negl Trop Dis 2: e130.
24. MulvennaJ, HamiltonB, NagarajSH, SmythD, LoukasA, et al. (2009) Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum. Mol Cell Proteomics 8 : 109–121.
25. HaegemanA, MantelinS, JonesJT, GheysenG (2012) Functional roles of effectors of plant-parasitic nematodes. Gene 492 : 19–31.
26. HeweziT, BaumTJ (2013) Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant Microbe Interact 26 : 9–16.
27. MitchumMG, HusseyRS, BaumTJ, WangX, EllingAA, et al. (2013) Nematode effector proteins: An emerging paradigm of parasitism. New Phytol 199 : 879–894.
28. Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J (2013) Nematode feeding sites: unique organs in plant roots. Planta: 1–12.
29. ChronisD, ChenSY, LuSW, HeweziT, CarpenterSCD, et al. (2013) A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J 74 : 185–196.
30. PostmaWJ, SlootwegEJ, RehmanS, Finkers-TomczakA, TytgatTOG, et al. (2012) The effector SPRYSEC-19 of Globodera rostochiensis suppresses CC-NB-LRR-mediated disease resistance in plants. Plant Physiol 160 : 944–954.
31. JaouannetM, MaglianoM, ArguelMJ, GourguesM, EvangelistiE, et al. (2013) The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant Microbe Interact 26 : 97–105.
32. JaubertS, MilacAL, PetrescuAJ, De Almeida-EnglerJ, AbadP, et al. (2005) In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol Plant Microbe Interact 18 : 1277–1284.
33. CarpitaN, McCannM, GriffingLR (1996) The plant extracellular matrix: News from the cell's frontier. Plant Cell 8 : 1451–1463.
34. AumailleyM, GayraudB (1998) Structure and biological activity of the extracellular matrix. J Mol Med 76 : 253–265.
35. NurnbergerT, BrunnerF, KemmerlingB, PiaterL (2004) Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol Rev 198 : 249–266.
36. AusubelFM (2005) Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6 : 973–979.
37. MoyleM, FosterDL, McGrathDE, BrownSM, LarocheY, et al. (1994) A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J Biol Chem 269 : 10008–10015.
38. BowerMA, ConstantSL, MendezS (2008) Necator americanus: The Na-ASP-2 protein secreted by the infective larvae induces neutrophil recruitment in vivo and in vitro. Exp Parasitol 118 : 569–575.
39. AsojoOA, GoudG, DharK, LoukasA, ZhanB, et al. (2005) X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J Mol Biol 346 : 801–814.
40. Del ValleA, JonesBF, HarrisonLM, ChadderdonRC, CappelloM (2003) Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol Biochem Parasitol 129 : 167–177.
41. ChinchillaD, BauerZ, RegenassM, BollerT, FelixG (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18 : 465–476.
42. Van EsseHP, Van't KloosterJW, BoltonMD, YadetaKA, Van BaarlenP, et al. (2008) The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 20 : 1948–1963.
43. KaschaniF, ShababM, BozkurtT, ShindoT, SchornackS, et al. (2010) An effector-targeted protease contributes to defense against Phytophthora infestans and is under diversifying selection in natural hosts. Plant Physiol 154 : 1794–1804.
44. DingX, ShieldsJ, AllenR, HusseyRS (2000) Molecular cloning and characterisation of a venom allergen AG5-like cDNA from Meloidogyne incognita. Int J Parasitol 30 : 77–81.
45. Gómez-GómezL, BollerT (2000) FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5 : 1003–1011.
46. KanehisaM, GotoS (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28 : 27–30.
47. Kanehisa M (2013) Molecular network analysis of diseases and drugs in KEGG. In: Mamitsuka H, Kanehisa M, DeLisi C, editors. pp.263–275.
48. KanehisaM, GotoS, HattoriM, Aoki-KinoshitaKF, ItohM, et al. (2006) From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res 34: D354–357.
49. SchallerA, StintziA, GraffL (2012) Subtilases - versatile tools for protein turnover, plant development, and interactions with the environment. Physiol Plant 145 : 52–66.
50. RamírezV, LópezA, Mauch-ManiB, GilMJ, VeraP (2013) An Extracellular Subtilase Switch for Immune Priming in Arabidopsis. PLoS Pathog 9: e1603445.
51. LiXP, BjörkmanO, ShihC, GrossmanAR, RosenquistM, et al. (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403 : 391–395.
52. GöhreV, JonesAME, SklenárJ, RobatzekS, WeberAPM (2012) Molecular crosstalk between PAMP-triggered immunity and photosynthesis. Mol Plant Microbe Interact 25 : 1083–1092.
53. JankanpaaHJ, FrenkelM, ZulfugarovI, ReicheltM, Krieger-LiszkayA, et al. (2013) Non-Photochemical Quenching Capacity in Arabidopsis thaliana Affects Herbivore Behaviour. PLoS One 8: e53232.
54. Demmig-AdamsB, CohuCM, AmiardV, ZadelhoffG, VeldinkGA, et al. (2013) Emerging trade-offs - impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins as regulators of development and defense. New Phytol 197 : 720–729.
55. DanonA, MierschO, FelixG, den CampRGLO, ApelK (2005) Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J 41 : 68–80.
56. RehmanS, ButterbachP, PopeijusH, OvermarsH, DavisEL, et al. (2009) Identification and characterization of the most abundant cellulases in stylet secretions from Globodera rostochiensis. Phytopathol 99 : 194–202.
57. HeilM (2012) Damaged-self recognition as a general strategy for injury detection. Plant Signaling and Behavior 7 : 576–580.
58. SinhaD, GuptaMK, PatelHK, RanjanA, SontiRV (2013) Cell Wall Degrading Enzyme Induced Rice Innate Immune Responses Are Suppressed by the Type 3 Secretion System Effectors XopN, XopQ, XopX and XopZ of Xanthomonas oryzae pv. oryzae. PLoS One 8: e75867.
59. BouwmeesterK, GoversF (2009) Arabidopsis L-type lectin receptor kinases: Phylogeny, classification, and expression profiles. J Exp Bot 60 : 4383–4396.
60. NakhamchikA, ZhaoZ, ProvartNJ, ShiuSH, KeatleySK, et al. (2004) A comprehensive expression analysis of the Arabidopsis proline-rich extensin-like receptor kinase gene family using bioinformatic and experimental approaches. Plant Cell Physiol 45 : 1875–1881.
61. SilvaNF, GoringDR (2002) The proline-rich, extensin-like receptor kinase-1 (PERK1) gene is rapidly induced by wounding. Plant Mol Biol 50 : 667–685.
62. ShababM, ShindoT, GuC, KaschaniF, PansuriyaT, et al. (2008) Fungal effector protein AVR2 targets diversifying defense-related cys proteases of tomato. Plant Cell 20 : 1169–1183.
63. RautengartenC, SteinhauserD, BussisD, StintziA, SchallerA, et al. (2005) Inferring hypotheses on functional relationships of genes: Analysis of the Arabidopsis thaliana subtilase gene family. PLoS Comput Biol 1 : 297–312.
64. LiXP, GilmoreAM, CaffarriS, BassiR, GolanT, et al. (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279 : 22866–22874.
65. RoachT, Krieger-LiszkayA (2012) The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. BBA Bioenergetics 1817 : 2158–2165.
66. TriantaphylidesC, HavauxM (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14 : 219–228.
67. LiXP, Muller-MouleP, GilmoreAM, NiyogiKK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci U S A 99 : 15222–15227.
68. FrenkelM, KulheimC, JankanpaaaHJ, SkogstromO, Dall'OstoL, et al. (2009) Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol 9.
69. GolinowskiW, GrundlerFMW, SobczakM (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 194 : 103–116.
70. SzakasitsD, HeinenP, WieczorekK, HofmannJ, WagnerF, et al. (2009) The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J 57 : 771–784.
71. DecreuxA, MessiaenJ (2005) Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol 46 : 268–278.
72. BrutusA, SiciliaF, MaconeA, CervoneF, De LorenzoG (2010) A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci U S A 107 : 9452–9457.
73. D'OvidioR, MatteiB, RobertiS, BellincampiD (2004) Polygalacturonases, polygalacturonase-inhibiting proteins and pectic oligomers in plant-pathogen interactions. BBA Proteins Proteom 1696 : 237–244.
74. MoerschbacherBM, MierauM, GraeßnerB, NollU, MortAJ (1999) Small oligomers of galacturonic acid are endogenous suppressors of disease resistance reactions in wheat leaves. J Exp Bot 50 : 605–612.
75. ChenQ, RehmanS, SmantG, JonesJT (2005) Functional analysis of pathogenicity proteins of the potato cyst nematode Globodera rostochiensis using RNAi. Mol Plant Microbe Interact 18 : 621.
76. GreenbaumD, MedzihradszkyKF, BurlingameA, BogyoM (2000) Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem Biol 7 : 569–581.
77. CurtisMD, GrossniklausU (2003) A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol 133 : 462–469.
78. De BoerJM, OvermarsHA, BakkerJ, GommersFJ (1992) Analysis of two-dimensional protein patterns from developmental stages of the potato cyst-nematode, Globodera rostochiensis. Parasitology 105 : 461–474.
79. MartinJ, AbubuckerS, HeizerE, TaylorCM, MitrevaM (2012) Nematode.net update 2011: Addition of data sets and tools featuring next-generation sequencing data. Nucleic Acids Res 40: D720–D728.
80. NakagawaT, SuzukiT, MurataS, NakamuraS, HinoT, et al. (2007) Improved gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotech Biochem 71 : 2095–2100.
81. CloughSJ, BentAF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 : 735–743.
82. SijmonsPC, GrundlerFMW, Von MendeN, BurrowsPR, WyssU (1991) Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J 1 : 245–254.
83. BaumTJ, WubbenMTEIi, HardyKA, SuH, RodermelSR (2000) A screen for Arabidopsis thaliana mutants with altered susceptibility to Heterodera schachtii. J Nematol 32 : 166–173.
84. Van EsseHP, BoltonMD, StergiopoulosI, De WitPJGM, ThommaBPHJ (2007) The chitin-binding Cladosporium fulvum effector protein Avr4 is a virulence factor. Mol Plant Microbe Interact 20 : 1092–1101.
85. BouwmeesterK, de SainM, WeideR, GougetA, KlamerS, et al. (2011) The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathog 7: e1001327.
86. van de MortelJE, de VosRCH, DekkersE, PinedaA, GuillodL, et al. (2012) Metabolic and transcriptomic changes induced in arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol 160 : 2173–2188.
87. PieterseCMJ, Van WeesSCM, HofflandE, Van PeltJA, Van LoonLC (1996) Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8 : 1225–1237.
88. Gómez-GómezL, FelixG, BollerT (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18 : 277–284.
89. RobinsonMD, McCarthyDJ, SmythGK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 : 139–140.
90. TanzSK, CastledenI, HooperCM, VacherM, SmallI, et al. (2013) SUBA3: A database for integrating experimentation and prediction to define the SUBcellular location of proteins in Arabidopsis. Nucleic Acids Res 41: D1185–D1191.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus InfectionČlánek P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to InfectionČlánek Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Microbial Programming of Systemic Innate Immunity and Resistance to Infection
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
- Measles Immune Suppression: Functional Impairment or Numbers Game?
- Cellular Mechanisms of Alpha Herpesvirus Egress: Live Cell Fluorescence Microscopy of Pseudorabies Virus Exocytosis
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection
- Loss of Dynamin-Related Protein 2B Reveals Separation of Innate Immune Signaling Pathways
- Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Infection
- Unveiling the Intracellular Survival Gene Kit of Trypanosomatid Parasites
- Extreme Divergence of Tropism for the Stem-Cell-Niche in the Testis
- HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4 T Cells
- P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
- Hypercytotoxicity and Rapid Loss of NKp44 Innate Lymphoid Cells during Acute SIV Infection
- Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
- Crystal Structure of Calcium Binding Protein-5 from and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
- Chronic Parasitic Infection Maintains High Frequencies of Short-Lived Ly6CCD4 Effector T Cells That Are Required for Protection against Re-infection
- Specific Dysregulation of IFNγ Production by Natural Killer Cells Confers Susceptibility to Viral Infection
- HSV-2-Driven Increase in the Expression of αβ Correlates with Increased Susceptibility to Vaginal SHIV Infection
- Murine Anti-vaccinia Virus D8 Antibodies Target Different Epitopes and Differ in Their Ability to Block D8 Binding to CS-E
- Brothers in Arms: Th17 and Treg Responses in Immunity
- Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Typhimurium Colitis
- A Negative Feedback Modulator of Antigen Processing Evolved from a Frameshift in the Cowpox Virus Genome
- Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms
- The Non-receptor Tyrosine Kinase Tec Controls Assembly and Activity of the Noncanonical Caspase-8 Inflammasome
- Targeted Changes of the Cell Wall Proteome Influence Ability to Form Single- and Multi-strain Biofilms
- Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
- The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
- Anti-α4 Antibody Treatment Blocks Virus Traffic to the Brain and Gut Early, and Stabilizes CNS Injury Late in Infection
- Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
- Microbial Urease in Health and Disease
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against
- IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
- A Natural Genetic Variant of Granzyme B Confers Lethality to a Common Viral Infection
- Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression
- Differential PfEMP1 Expression Is Associated with Cerebral Malaria Pathology
- The Role of the NADPH Oxidase NOX2 in Prion Pathogenesis
- Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions
- The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
- Expression of Suppressor of Cytokine Signaling 1 (SOCS1) Impairs Viral Clearance and Exacerbates Lung Injury during Influenza Infection
- Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells
- SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in
- Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication
- Oral Streptococci Utilize a Siglec-Like Domain of Serine-Rich Repeat Adhesins to Preferentially Target Platelet Sialoglycans in Human Blood
- SV40 Utilizes ATM Kinase Activity to Prevent Non-homologous End Joining of Broken Viral DNA Replication Products
- Amphipathic α-Helices in Apolipoproteins Are Crucial to the Formation of Infectious Hepatitis C Virus Particles
- Proteomic Analysis of the Acidocalcisome, an Organelle Conserved from Bacteria to Human Cells
- Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



