-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
article has not abstract
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002165
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002165Summary
article has not abstract
Introduction
Classic studies over the last two decades have made virus-induced activation of the mammalian interferon-β (ifnβ) gene a prototype of eukaryotic gene regulation [1]–[6]. Indeed, the compact ∼50 base-pair enhancer region upstream of the ifnβ transcription start site is amongst the best-studied stretches of mammalian DNA, and its function in regulation of ifnβ expression is considered a paradigm of stimulus-activated mammalian gene regulation.
In a widely accepted model, RNA virus infection of most cell types triggers the activation of three classes of transcription factor—interferon regulatory factors (IRFs)-3/7, NF-κB, and ATF-2/c-Jun—downstream of the RIG-I-like receptor (RLR) family of viral RNA sensors [7]–[9]. These transcription factors bind well-defined adjacent sites in the ifnβ enhancer to nucleate formation of an “enhanceosome”. The nascent enhanceosome then recruits chromatin-modifying enzymes and general transcription factors to initiate transcription of ifnβ and launch the type I IFN antiviral innate immune response [1], [2], [10]. Implicit in the inherently cooperative nature of enhanceosome formation is the supposition that IRFs-3/7, NF-κB, and ATF-2/c-Jun are all perhaps equally necessary for virus-driven ifnβ expression. Recent findings from our laboratories and other groups, however, suggest an alternate view of NF-κB function in antivirus responses: that NF-κB is indeed required for ifnβ expression, but only before (and very early after) infection. As the infection unfolds, NF-κB is no longer necessary for ifnβ induction, and instead takes on a more general role in the expression of non-IFN innate immune and pro-inflammatory genes; meanwhile, IRFs-3/7 inherit ifnβ expression to propel the type I IFN antiviral system. In this article, we update the enhanceosome paradigm by proposing temporally distinct functions for NF-κB in the RLR-triggered innate immune response.
Unexpected Results from NF-κB Gene-Targeted Mice
Given that IRFs-3/7, NF-κB, and ATF-2/c-Jun assemble on the ifnβ enhancer, it was expected that all three factors would be critical for virus-triggered induction of ifnβ. In line with this expectation, studies using mice deficient in IRF-3 and/or IRF-7 have convincingly shown essential roles for these IRFs in production of IFN-β and other type I IFNs [11]–[13]. We were therefore surprised to discover that cells from mice genetically deficient in key NF-κB subunits (such as RelA, c-Rel, or p50) were mostly normal in their ability to activate ifnβ expression after virus infection [14]. Indeed, cells lacking virtually all detectable RLR-triggered NF-κB activity continued to support robust virus-induced ifnβ expression [14], [15]. Thus, while NF-κB is activated by virus infection and does associate with the ifnβ enhancer, it does not appear to be required for subsequent transcription of ifnβ. These findings raise two key questions: (1) what is the function of the NF-κB site in the ifnβ promoter, and (2) what is the function of NF-κB in virus-triggered innate immune responses, if not to activate ifnβ?
Function of NF-κB before Infection: Maintenance of Basal ifnβ Activity
Recent work has begun to provide answers to both these questions. Using an in silico approach to analyze cells deficient in RelA (the primary transactivating component of virus-induced NF-κB), we have found that NF-κB controls expression of several IFN-dependent innate immune pathways by, unexpectedly, maintaining constitutive expression of ifnβ in uninfected cells [16].
It has long been known that constitutive low-level expression of ifnβ is necessary for maintenance of an IFN-β autocrine signal that keeps the uninfected cell in a primed state of antiviral readiness [17], [18]. Since the type I IFN antiviral system is dependent on feed-forward signal amplification, even small differences in basal gene expression translate into major downstream deficiencies. We have found that in the absence of RelA, basal expression of ifnβ is reduced, and autocrine IFN-β signaling is compromised. Consequently, there is a delay in the induction of ifnβ after infection, and, later, severe defects in the activation of the type I IFN response [14], [16], [19]. This tardiness in type I IFN feed-forward signaling has negative consequences for host antiviral immunity: RelA-deficient embryo fibroblasts are very susceptible to interferon-sensitive RNA viruses such as vesicular stomatitis virus (Rhabdoviridae), Newcastle disease virus, and Sendai virus (both Paramyxoviridae), despite producing copious amounts of IFN-β later during the course of infection [16], [19]. In these cells, diminished IFN-β expression prior to infection (and early after infection, see below) allows the virus a head start, and even though IFN-β production eventually catches up to (and even exceeds) wild-type levels, the temporal advantage conferred to the actively replicating RNA viruses during an acute infection ultimately proves insurmountable [16], [19]. These findings highlight the importance of timely IFN-β production (rather than the maximal amount produced) in innate immunity to an acute RNA virus infection.
The precise mechanism that generates constitutive NF-κB activity is currently not known. We have found that NF-κB cycles robustly through the nuclei of uninfected primary cells in an IKK-β-dependent manner, and IKK-β-deficient cells are also defective in autocrine IFN-β-mediated basal interferon-stimulated gene expression [16]. Our preliminary findings suggest that neither tumor necrosis factor-α nor Toll-like receptors (TLRs) lie upstream of IKK-β as a source of constitutive NF-κB [16].
Function of NF-κB Early in Infection: Role in ifnβ Induction
In addition to controlling constitutive ifnβ expression, NF-κB is also the earliest-arriving virus-activated enhanceosome component, appearing on the ifnβ enhancer within 2 hours of virus infection (and approximately 2 and 4 hours ahead of ATF-2 and IRF-3, respectively) [20]. Recent elegant experiments from the Thanos laboratory show that NF-κB, despite being found in rate-limiting amounts in the cell, manages to gain such rapid access to the ifnβ enhancer via a novel process of inter-chromosomal transfer from putative NF-κB “receptor centers” [21]. In their model, specialized genomic loci containing readily accessible NF-κB binding sites serve as temporary receptors for incoming nuclear NF-κB, following which NF-κB is shuttled to either of two ifnβ loci to initiate monoallelic ifnβ expression. Later in an infection, feed-forward production of IRF-7 drives bi-allelic ifnβ expression to accelerate the type I IFN response [21].
Consistent with this model, we have also found that NF-κB has a key role in early virus-induced ifnβ expression [19]. This early requirement for NF-κB may stem from how the co-activator CBP/p300 is recruited to the ifnβ locus: an ∼30 amino-acid region within the NF-κB RelA subunit (termed the “synergism domain”) has been demonstrated to be essential for the initial capture and stabilization of CBP/p300 at the enhanceosome [22]. Although IRFs and c-Jun can independently associate with CBP/p300, the ability to synergize with other enhanceosome components to anchor CBP/p300 and bridge the enhanceosome to the RNA polymerase II transcriptional machinery appears to be unique to the NF-κB RelA subunit [22]–[24]. Once CBP/p300 is at the ifnβ enhancer (3–4 hours post infection [20]), IRFs are already robustly activated and capable of binding CBP/p300 to drive ifnβ transcription without further requirement for NF-κB. Indeed, IRF-3 can form a stable complex with CBP/p300 in the absence of other enhanceosome components [25], [26], and data suggest that IRF-3′s transcriptional activity can almost entirely be accounted for by its ability to capture CBP/p300 [27]. Collectively, these findings allow us to propose a model in which, early in infection, low levels of individual enhanceosome components cooperate to tether CBP/p300 to the ifnβ locus in a manner crucially dependent on NF-κB RelA. Later in infection (when activated IRF-3 dimers are found in larger amounts) IRF-3 can perform this function by itself, and the requirement for NF-κB is obviated. It is very likely that a similar IRF-3-dependent mechanism also accounts for ifnβ expression in the complete absence of NF-κB RelA [14], [19].
Function of NF-κB Later in Infection: Regulating Pro-Inflammatory and Anti-Necroptotic Gene Expression
Once IRFs have been activated, NF-κB appears to be unnecessary for ifnβ expression, and instead switches to regulating a distinct set of genes that comprise roughly 25% of all RLR targets [16]. The NF-κB-dependent subset of the RLR transcriptome is especially enriched for genes encoding (1) chemokines, chemokine signaling, and adhesion molecules, (2) matrix metalloproteinases and allied proteases involved in remodeling the extracellular matrix, and (3) proteins involved in antigen processing and presentation, including a large number of classical and non-classical major histocompatibility class I molecules. In addition, RelA is also weakly activated by IFN-β itself [16], [28], and is required for induction of a small subset (<5%) of interferon-stimulated genes (most notably those encoding chemokines CxCl11 and Ccl3) [16]. Finally, RelA-deficient cells treated with the virus mimetic poly(I:C) are very susceptible to a novel form of cell death termed necroptosis [29], [30], indicating that RelA might also transcriptionally control a cell survival program to prolong pro-inflammatory gene expression from the infected cell [16], [31]. Collectively, these findings show that the NF-κB arm of the type I IFN antiviral response is focused primarily on generating pro-inflammatory and pro-survival signals, rather than on activating cell-intrinsic antiviral effectors (or on feed-forward amplification of IFN signaling itself).
Conclusions
We propose here an updated view of NF-κB's overall function in the innate antivirus response, in which NF-κB has a crucial constitutive (and early) role in ifnβ expression followed by an equally important and potentially more general later role in regulating expression of genes involved in recruitment and activation of the adaptive immune response. Interestingly, other groups have demonstrated that c-Jun also participates in maintenance of autocrine IFN-β, while IRF-3 and IRF-7 may not [32], [33]. Taken together, these findings support the idea that NF-κB and c-Jun sustain basal/early ifnβ expression, while IRF-3 and IRF-7 instead dominate IFN-β production following virus infection (Figure 1). Important areas for future investigation include: (1) the source of constitutive NF-κB activity; (2) the role of other IRFs (for example, IRF-1) in constitutive ifnβ expression; and (3) evaluation of cell type-specific roles for different NF-κB subunits in anti-virus responses in vivo. For example, the key type I IFN producing plasmacytoid dendritic cells utilize TLRs, rather than RLRs, to activate ifnβ [34]. Is the requirement for—and subunit composition of—NF-κB in these cells the same as it is in cells that deploy a RLR-driven IFN response? Despite over two decades of investigation, the regulation of ifnβ expression continues to throw up surprises, and more unanticipated findings are likely forthcoming.
Fig. 1. Temporally distinct roles for NF-κB in antivirus innate immune responses. 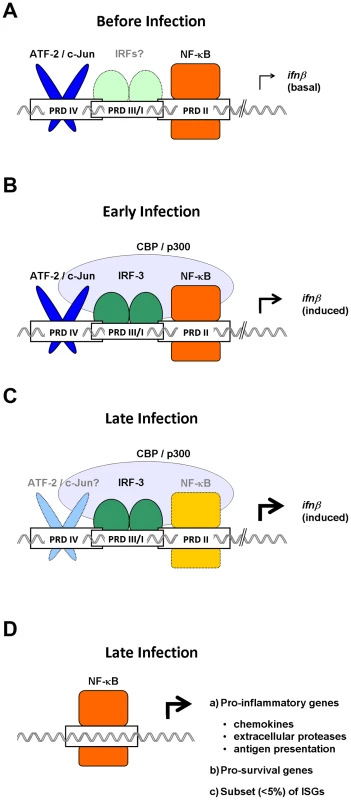
(A) In uninfected cells, NF-κB cycles robustly through the nucleus to maintain constitutive expression of basal ifnβ and sustain sutocrine IFN-β signaling. (B) Early in an infection, NF-κB cooperates with ATF-2/c-Jun and IRF-3 to recruit the transcription co-activator CBP/p300 to the ifnβ enhancer. (C) Later in an infection, IRF-3/7 powers expression of ifnβ, and NF-κB is rendered redundant in the ifnβ enhanceosome. (D) NF-κB then switches to regulating a distinct subset of non-IFN genes, including those involved in inflammation and cell survival. The relative importance of each transcription factor in driving gene expression during a particular stage of the immune response is indicated by the intensity of its color, with darker shades representing essential functions, and lighter shades indicating reduced roles.
Zdroje
1. MunshiNYieYMerikaMSengerKLomvardasS 1999 The IFN-beta enhancer: a paradigm for understanding activation and repression of inducible gene expression. Cold Spring Harb Symp Quant Biol 64 149 159
2. ManiatisTFalvoJVKimTHKimTKLinCH 1998 Structure and function of the interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol 63 609 620
3. ThanosDManiatisT 1995 Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83 1091 1100
4. PithaPMKunziMS 2007 Type I interferon: the ever unfolding story. Curr Top Microbiol Immunol 316 41 70
5. TaniguchiTTakaokaA 2002 The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol 14 111 116
6. MamaneYHeylbroeckCGeninPAlgarteMServantMJ 1999 Interferon regulatory factors: the next generation. Gene 237 1 14
7. AkiraSUematsuSTakeuchiO 2006 Pathogen recognition and innate immunity. Cell 124 783 801
8. YoneyamaMFujitaT 2009 RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev 227 54 65
9. HiscottJ 2007 Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev 18 483 490
10. FordEThanosD The transcriptional code of human IFN-beta gene expression. Biochim Biophys Acta 1799 328 336
11. HondaKYanaiHNegishiHAsagiriMSatoM 2005 IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434 772 777
12. SatoMSuemoriHHataNAsagiriMOgasawaraK 2000 Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13 539 548
13. HondaKTakaokaATaniguchiT 2006 Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25 349 360
14. WangXHussainSWangEJLiMOGarcia-SastreA 2007 Lack of essential role of NF-kappa B p50, RelA, and cRel subunits in virus-induced type 1 IFN expression. J Immunol 178 6770 6776
15. PetersKLSmithHLStarkGRSenGC 2002 IRF-3-dependent, NFkappa B - and JNK-independent activation of the 561 and IFN-beta genes in response to double-stranded RNA. Proc Natl Acad Sci U S A 99 6322 6327
16. BasagoudanavarSHThapaRJNogusaSWangJBegAA 2011 Distinct roles for the NF-kappa B RelA subunit during antiviral innate immune responses. J Virol 85 2599 2610
17. TakaokaAMitaniYSuemoriHSatoMYokochiT 2000 Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science 288 2357 2360
18. TaniguchiTTakaokaA 2001 A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol 2 378 386
19. WangJBasagoudanavarSHWangXHopewellEAlbrechtR 2010 NF-{kappa}B RelA Subunit Is Crucial for Early IFN-{beta} Expression and Resistance to RNA Virus Replication. J Immunol 185 1720 1729
20. LomvardasSThanosD 2002 Modifying gene expression programs by altering core promoter chromatin architecture. Cell 110 261 271
21. ApostolouEThanosD 2008 Virus Infection Induces NF-kappaB-dependent interchromosomal associations mediating monoallelic IFN-beta gene expression. Cell 134 85 96
22. MerikaMWilliamsAJChenGCollinsTThanosD 1998 Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol Cell 1 277 287
23. MerikaMThanosD 2001 Enhanceosomes. Curr Opin Genet Dev 11 205 208
24. SchaferSLLinRMoorePAHiscottJPithaPM 1998 Regulation of type I interferon gene expression by interferon regulatory factor-3. J Biol Chem 273 2714 2720
25. LinCHHareBJWagnerGHarrisonSCManiatisT 2001 A small domain of CBP/p300 binds diverse proteins: solution structure and functional studies. Mol Cell 8 581 590
26. WatheletMGLinCHParekhBSRoncoLVHowleyPM 1998 Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo [published erratum appears in Mol Cell 1999 Jun; 3(6): 813]. Mol Cell 1 507 518
27. YangHLinCHMaGOrrMBaffiMO 2002 Transcriptional activity of interferon regulatory factor (IRF)-3 depends on multiple protein-protein interactions. Eur J Biochem 269 6142 6151
28. YangCHMurtiAPfefferSRBasuLKimJG 2000 IFNalpha/beta promotes cell survival by activating NF-kappa B. Proc Natl Acad Sci U S A 97 13631 13636
29. BergheTVVanlangenakkerNParthoensEDeckersWDevosM 2010 Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17 922 930
30. VandenabeelePGalluzziLVanden BergheTKroemerG Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11 700 714
31. LiMShillinglawWHenzelWJBegAA 2001 The Rela(p65) subunit of NF-kappaB is essential for inhibiting double-stranded RNA-induced cytotoxicity. J Biol Chem 276 1185 1194
32. GoughDJMessinaNLHiiLGouldJASabapathyK 2010 Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol 8 e1000361 doi:10.1371/journal.pbio.1000361
33. HataNSatoMTakaokaAAsagiriMTanakaN 2001 Constitutive IFN-alpha/beta signal for efficient IFN-alpha/beta gene induction by virus. Biochem Biophys Res Commun 285 518 525
34. KawaiTAkiraS 2009 The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21 317 337
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



