-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
The CsrRS (or CovRS) two component system controls expression of up to 15% of the genome of group A Streptococcus (GAS). While some studies have suggested that the sensor histidine kinase CsrS responds to membrane perturbations as a result of various environmental stresses, other data have implicated the human antimicrobial peptide LL-37 and extracellular Mg2+ as specific signals. We now report that Mg2+ and LL-37 have opposite effects on expression of multiple genes that are activated or repressed by the transcriptional regulator CsrR. Using a GAS isolate representative of the recently emerged and widely disseminated M1T1 clone implicated in severe invasive disease, we found marked up-regulation by CsrRS of multiple virulence factors including pyrogenic exotoxin A, DNase Sda1, streptolysin O, and the hyaluronic acid capsular polysaccharide, among others. Topology and surface protein labeling studies indicated that CsrS is associated with the bacterial cell membrane and has a surface-exposed extracellular domain accessible to environmental ligands. Replacement of a cluster of three acidic amino acids with uncharged residues in the extracellular domain of CsrS abrogated LL-37 signaling and conferred a hyporesponsive phenotype consistent with tonic activation of CsrS autokinase activity, an effect that could be overridden by mutation of the CsrS active site histidine. Both loss - and gain-of-function mutations of a conserved site in the receiver domain of CsrR established an essential role for lysine 102 in CsrS-to-CsrR signal transduction. These results provide strong evidence that Mg2+ and LL-37 are specific signals that function by altering CsrS autokinase activity and downstream phosphotransfer to CsrR to modulate its activity as a transcriptional regulator. The representation of multiple antiphagocytic and cytotoxic factors in the CsrRS regulon together with results of in vitro phagocytic killing assays support the hypothesis that CsrRS mediates conversion of GAS from a colonizing to an invasive phenotype in response to signaling by host LL-37.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002361
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002361Summary
The CsrRS (or CovRS) two component system controls expression of up to 15% of the genome of group A Streptococcus (GAS). While some studies have suggested that the sensor histidine kinase CsrS responds to membrane perturbations as a result of various environmental stresses, other data have implicated the human antimicrobial peptide LL-37 and extracellular Mg2+ as specific signals. We now report that Mg2+ and LL-37 have opposite effects on expression of multiple genes that are activated or repressed by the transcriptional regulator CsrR. Using a GAS isolate representative of the recently emerged and widely disseminated M1T1 clone implicated in severe invasive disease, we found marked up-regulation by CsrRS of multiple virulence factors including pyrogenic exotoxin A, DNase Sda1, streptolysin O, and the hyaluronic acid capsular polysaccharide, among others. Topology and surface protein labeling studies indicated that CsrS is associated with the bacterial cell membrane and has a surface-exposed extracellular domain accessible to environmental ligands. Replacement of a cluster of three acidic amino acids with uncharged residues in the extracellular domain of CsrS abrogated LL-37 signaling and conferred a hyporesponsive phenotype consistent with tonic activation of CsrS autokinase activity, an effect that could be overridden by mutation of the CsrS active site histidine. Both loss - and gain-of-function mutations of a conserved site in the receiver domain of CsrR established an essential role for lysine 102 in CsrS-to-CsrR signal transduction. These results provide strong evidence that Mg2+ and LL-37 are specific signals that function by altering CsrS autokinase activity and downstream phosphotransfer to CsrR to modulate its activity as a transcriptional regulator. The representation of multiple antiphagocytic and cytotoxic factors in the CsrRS regulon together with results of in vitro phagocytic killing assays support the hypothesis that CsrRS mediates conversion of GAS from a colonizing to an invasive phenotype in response to signaling by host LL-37.
Introduction
Human beings are thought to be the principal if not exclusive host for group A Streptococcus (S. pyogenes, GAS). The organism's primary environmental niche is the human pharynx where GAS can colonize the epithelium without evoking any clinical symptoms, or it can produce local inflammation and symptomatic streptococcal pharyngitis [1], [2]. GAS also causes impetigo, a superficial skin infection, and, less commonly, severe invasive infections such as necrotizing fasciitis, bacteremia, and streptococcal toxic shock [3], [4]. The regulated expression of a variety of gene products enhances GAS survival in the human host through a dynamic process of adaptation to stresses that may change depending on the precise anatomic location of the bacteria in the body, environmental factors, and engagement of host defense mechanisms [5], [6].
Two component regulatory systems (TCS) play an important role in such dynamic adaptation of many bacteria to changing environmental conditions [7], [8]. CsrRS (also called CovRS) is the most extensively characterized TCS in GAS. First identified as a regulator of the has operon that encodes the enzymes required for synthesis of the hyaluronic acid capsular polysaccharide, CsrRS has since been shown to affect expression of as much as 15% of the GAS genome including genes encoding many virulence factors [9]–[12]. Genetic evidence and similarity to TCS in other species have suggested that CsrS is a sensor histidine kinase whose phosphorylation state is influenced by environmental signals, while CsrR is a transcriptional regulator whose activity at target promoters is controlled by phosphorylation. It is presumed, but not proven, that phosphorylation of CsrR results from phosphotransfer from CsrS. It has also been proposed that CsrS has a phosphatase activity and can dephosphorylate CsrR [13]. Transcriptional profiling of CsrR - or CsrRS-mutants has indicated that CsrR acts primarily, although not exclusively, as a repressor of gene expression, as mutants exhibit increased expression of most CsrRS-regulated genes, and phosphorylation of CsrR in vitro enhances its binding to regulated promoters [9], [10], [14], [15].
While it is clear that CsrRS influences expression of many important GAS products, a unifying explanation of the adaptive role of the CsrRS system is still unproven. One proposal is that CsrRS represents a system to detect and respond to a variety of environmental stresses, such as elevated temperature, acidic pH, and high osmolarity, all of which might result in alterations in physical properties of the bacterial cell membrane and consequent signaling through CsrS [13]. An alternative model is that CsrS recognizes specific ligands, and that interaction of these ligands with its extracellular domain (ECD) results in changes in CsrS autokinase activity and/or phosphatase activity for CsrR. The latter model is based on the findings that increased concentrations of extracellular Mg2+ result in widespread down-regulation of CsrR-repressed genes, an effect dependent on a functional CsrS and not reproduced by other cations [11], [16]. Thus, Mg2+ may serve as a specific stimulus for activation of CsrS kinase activity with downstream phosphorylation of CsrR. The human antimicrobial peptide LL-37 has been shown to have effects on CsrRS signaling opposite to those of elevated Mg2+. Concentrations of LL-37 far below those that inhibit GAS growth were shown to stimulate increased expression of the has operon and three other CsrR-repressed genes in a CsrS-dependent fashion [17]. While these two models are not necessarily mutually exclusive, it is difficult to reconcile LL-37 signaling with a model of non-specific membrane perturbation since the effects of LL-37 on gene expression were not reproduced by a broad range of doses of other antimicrobial peptides, including other cathelicidins, of similar or greater antibacterial potency [17].
The highly specific effect of LL-37 to stimulate up-regulation of CsrR-repressed genes suggests that CsrRS functions to detect and counteract host immune effectors that mediate bacterial clearance from the infected host. Circumstantial evidence for such a role comes from isolation of spontaneous CsrRS mutants in the setting of invasive GAS infection, both in patients with severe invasive GAS infection and in experimental animals [18]–[21]. Exposure of wild type GAS to LL-37 or inactivation of CsrRS by mutation results in increased expression of factors that dramatically enhance GAS resistance to opsonophagocytic killing [12], [17]. These observations suggest that a physiologic role of CsrRS is to detect relatively low concentrations of LL-37 as a signal of mobilization of host defenses including the recruitment of phagocytic leukocytes and to trigger a global transcriptional response that enhances GAS resistance to phagocytosis.
We now report the results of further investigation that provides strong support for this hypothesis. LL-37 not only activates expression of the four previously identified loci, but also stimulates either activation or repression of multiple CsrRS-regulated genes. Signaling by LL-37 is dependent on CsrS, which is shown to have a surface-exposed domain on the bacterial cell. Transduction of the LL-37 signal requires specific domains of both CsrS and its cognate regulator CsrR to induce changes in gene expression. A critical consequence of LL-37-mediated CsrRS-signaling is enhanced resistance to phagocytic killing by human blood leukocytes, a bacterial phenotype that is central to both persistence of GAS in the human host and pathogenesis of invasive infection.
Results
LL-37 and Mg2+ have opposite effects on expression of multiple CsrRS-regulated genes
Earlier work by Gryllos et al. found that exposure of GAS to subinhibitory concentrations of LL-37 up-regulated expression of hasB, spyCEP/scpC/prtS, mac/IdeS, and SPy0170, genes that were shown previously to be down-regulated by extracellular Mg2+ in a CsrRS-dependent manner [11], [17]. Furthermore, the stimulatory effect of LL-37 on CsrRS-regulated gene expression could be blocked by high concentrations of Mg2+. These findings suggested the hypothesis that Mg2+ and LL-37 act as opposing extracellular signals for the CsrS sensor histidine kinase. To test if other CsrRS-regulated genes also respond to both stimuli, we investigated ten additional genes for their responsiveness to LL-37 and Mg2+ in GAS strain 854. This strain was chosen for further analyses because initial experimentation showed marked up-regulation of the four previously characterized CsrRS target genes by LL-37, signaling that was completely abrogated in an isogenic csrS deficient mutant [17]. Furthermore, strain 854 is representative of the widely disseminated M1T1 clone associated with invasive GAS infections over the past three decades [22]–[25].
In the present study, we found that exposure of strain 854 to 100 nM LL-37 resulted in up-regulation of speA (pyrogenic exotoxin A), sda1 (DNase), ska (streptokinase), slo (streptolysin O), nga (NAD-glycohydrolase), and SPy0136 (hypothetical protein; N.B., throughout this paper, unnamed open reading frames are designated by SPy numbers according to the SF370 or MGAS315 genome sequences [26], [27]), as assessed by quantitative RT-PCR (qRT-PCR) analysis of RNA samples from LL-37-treated and untreated bacteria (Figure 1). Culture of strain 854 in 15 mM Mg2+ had the opposite effect from that evoked by LL-37. That is, Mg2+ exposure resulted in down-regulation of these genes relative to their expression at baseline in unsupplemented medium (Figure 1). Conversely, expression of several genes in strain 854 was repressed by LL-37 and up-regulated by Mg2+. Genes in the latter category included metB (putative cystathionine beta-lyase), SPy1414 (putative cation (potassium) transport protein), grab (protein G-related α2-macroglobulin-binding protein), and speB (cysteine protease) (Figure 1).
Fig. 1. LL-37 and Mg2+ have opposite effects on expression of CsrRS-regulated genes. 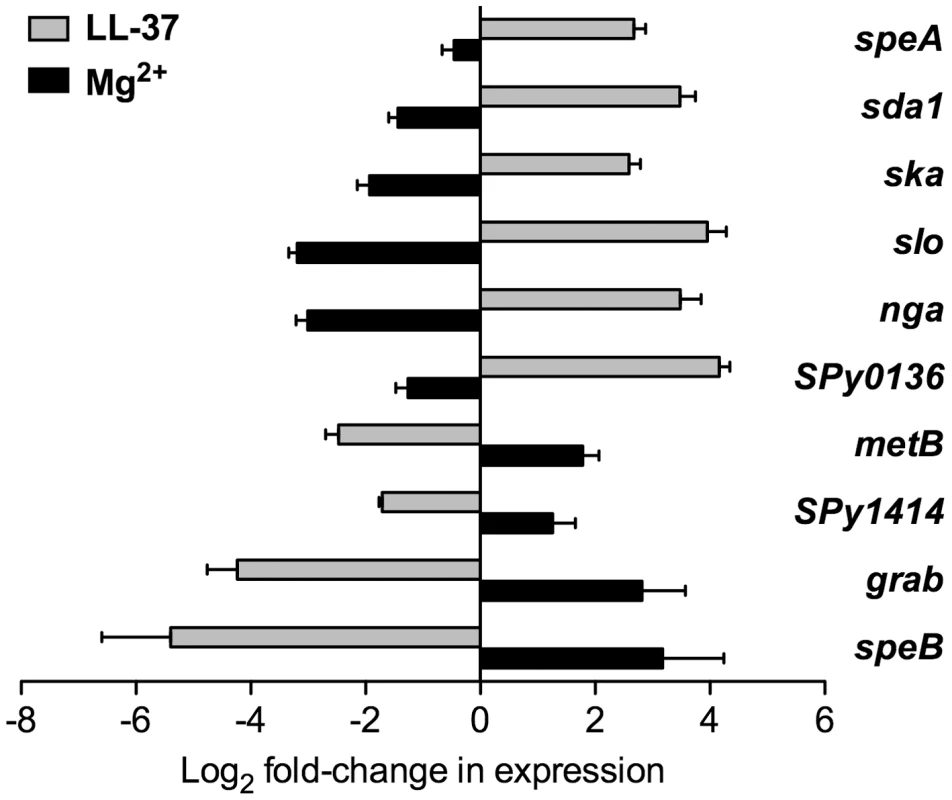
Expression of ten CsrRS-regulated genes in response to LL-37 or Mg2+ was quantified by qRT-PCR. Data represent mean fold-change ± SEM of gene expression in cultures grown in the presence of 100 nM LL-37 (grey bars) or 15 mM Mg2+ (black bars) relative to that in control cultures grown in unsupplemented medium (n = 3 – 5). Significant differences in response to LL-37 were found for all tested genes (P<0.008 for speA, sda1, ska, SPy0136, metB, SPy1414, grab; P<0.05 for slo, nga and speB). Significant differences in response to Mg2+ were found for all tested genes (P<0.02) except for speA and SPy1414. To verify that the changes in gene expression observed in response to LL-37 resulted in corresponding changes in production of the encoded proteins, we assayed four representative virulence determinants from this group of CsrRS-regulated genes. Growth of strain 854 in the presence of LL-37 resulted in marked increases in SLO and NADase and repression of SpeB, as assessed by western blot, and increased DNase activity (Figure S1). DNase activity associated with invasive M1T1 isolates such as strain 854 has been shown to be due predominantly to the enzyme encoded by the prophage-associated sda1 gene (also called sdaD2), a member of the CsrRS regulon [28]. These results corroborate the qRT-PCR data and, together, they extend earlier findings that LL-37 can up-regulate gene expression to include several additional CsrRS-controlled genes. Moreover, they show that expression of certain CsrRS-regulated genes is repressed, rather than stimulated, by LL-37. For both categories of genes, the effect of Mg2+ is opposite to that of LL-37, an observation that supports the hypothesis that the two molecules act as functionally antagonistic stimuli for signaling through CsrRS.
CsrS is associated with the cell membrane and includes a surface-exposed domain
The predicted histidine kinase CsrS is thought to represent a cell-surface sensor component of the CsrRS TCS that detects and responds to environmental signals. According to secondary structure and membrane protein model predictions, CsrS contains two membrane-spanning domains near the N-terminus that flank a predicted ECD of 151 amino acids [16]. To test these model predictions, membrane and cytoplasmic fractions of wild type GAS 854 and control csrS-deficient strain 854csrSΩ were isolated from whole cell lysates, fractionated by SDS-PAGE, and analyzed by western blot with anti-CsrS serum. CsrS was found exclusively in membranes of wild type bacteria and, as expected, was absent from csrS mutant preparations (Figure 2A). Like CsrS, the unrelated membrane protein OpuABC [11] was also in wild type 854 membranes, but not in the cytoplasmic fractions (Figure 2A). Consistent with its predicted cytosolic localization, the CsrR protein was mainly detected in the cytoplasmic fraction. These results localized CsrS to the GAS cell membrane.
Fig. 2. CsrS is associated with the cell membrane and contains a surface-exposed domain. 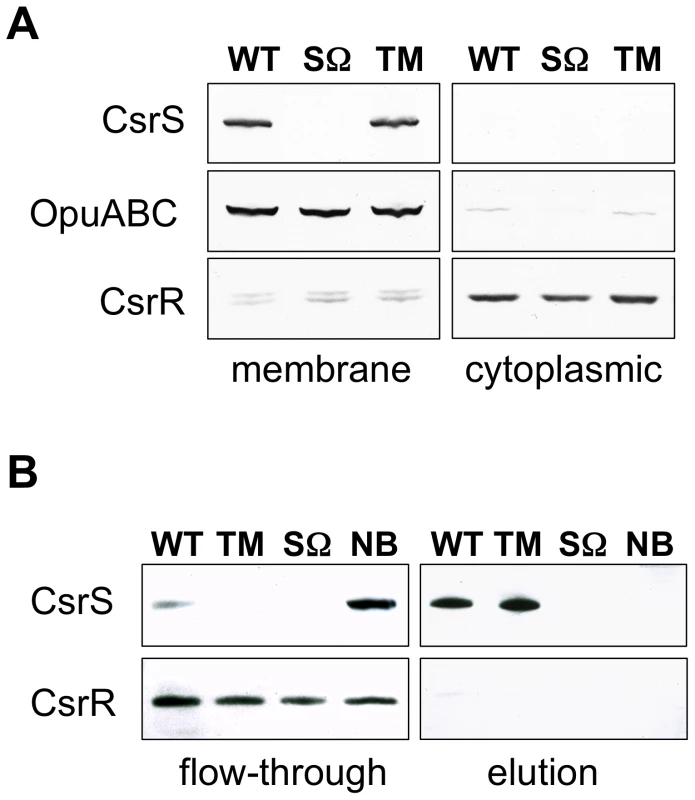
A) Western blot analysis of membrane and cytoplasmic fractions isolated from whole cell lysates of GAS wild type strain 854 (WT), isogenic csrS deficient mutant strain 854csrSΩ (SΩ), and 854csrSTM (TM) that expresses CsrS with 3 point mutations in the predicted extracellular domain. Specific antisera against CsrS, an unrelated membrane protein OpuABC, and CsrR were used to detect the respective proteins in both fractions. B) Biotin labeling via a disulfide linker of surface-exposed proteins in whole cells of wild type strain 854 (WT), 854csrSΩ (SΩ) and 854csrSTM (TM). After lysis of labeled cells, biotinylated proteins were captured on a NeutrAvidin column and then eluted by reducing the disulfide linker. Specific antisera detected CsrS in the eluted fraction, as expected for a surface-exposed protein, and CsrR in the flow-through, as expected for a cytoplasmic protein. As a control, wild type 854 cells were treated similarly, but without biotin labeling (NB). Results shown in both panels are representative of at least two independent experiments. In order to test whether CsrS is accessible to signaling molecules in the extracellular environment, we labeled proteins exposed on the bacterial surface with biotin via a disulfide linker. Biotinylated proteins were isolated from bacterial cell lysates using NeutrAvidin resin affinity chromatography. Resin-bound proteins were released by reduction of the disulfide bond linking biotin to the GAS surface proteins, fractionated by SDS-PAGE, and analyzed by western blot with CsrS antiserum. CsrS was detected predominantly in this eluted fraction (Figure 2B), a result that indicates CsrS was accessible to biotinylation, i.e., that a portion of the protein is exposed to the extracellular environment. CsrR, used here as a control cytosolic protein, did not react with biotin, and was detected only in the unbound flow-through fraction (Figure 2B). These data demonstrate that CsrS is a membrane-associated protein and includes a surface-exposed domain, conclusions consistent with our hypothesis that the ECD of CsrS functions as the sensor domain for environmental signals.
A cluster of acidic amino acid residues in the CsrS ECD is critical to LL-37 signaling
We noted previously that the predicted ECD of CsrS includes a cluster of negatively charged amino acids that corresponds to a similar cluster in PhoQ of S. typhimurium and E. coli implicated in binding of cationic ligands [17], [29], [30]. Preliminary experiments using wild type or mutant forms of csrS to complement in trans a csrS mutant of M-type 3 GAS strain DLS003 suggested that three charged residues in the ECD were required for LL-37 signaling. However, these experiments were not definitive as the level of CsrS protein expressed from the mutant construct was higher than that observed in the wild type strain [17]. To examine more thoroughly the role of the predicted CsrS ECD in LL-37 sensing by CsrS, we introduced point mutations into the chromosomal csrS locus of GAS strain 854 by allelic replacement. Four independent mutant strains were constructed in which one or all three negatively charged amino acids localized in a small cluster of acidic residues (148DHIED152, Figure 3A) were substituted with similar uncharged residues (D148N, E151Q or D152N). Mutation of these three amino acids did not affect expression levels or surface localization of mutant CsrS, as similar quantities of CsrS were detected in western blots of membrane fractions obtained from the csrS triple point mutant strain 854csrSTM and wild type 854 (Figure 2A), and similar amounts of CsrS were labeled by biotinylation on the mutant strain cell surface (Figure 2B). The four resulting isogenic csrS mutants 854csrSD148N, 854csrSE151Q, 854csrSD152N, and 854csrSTM were tested for LL-37-mediated up-regulation of hasB, spyCEP, mac, and SPy0170 expression. Wild type strain 854 and each of the mutant strains were grown to early exponential phase in the presence or absence of 100 nM LL-37, and gene expression was assessed by qRT-PCR. In contrast to wild type, the isogenic csrS triple mutant showed little or no change in gene expression in response to LL-37 (Figure 3B). The csrS mutants with single amino acid substitutions (D148N, E151Q or D152N) all showed moderate LL-37-mediated up-regulation of the four target genes, but less than that observed in wild type (Figure 3B). Mutation of this region of the CsrS ECD also abrogated or severely blunted the effect of Mg2+ to repress, or in the case of grab, to activate, CsrRS-regulated gene expression (Figure S2).
Fig. 3. LL-37 and Mg2+ signaling of CsrRS-regulated genes involves a cluster of negatively charged amino acid residues located in the CsrS extracellular domain. 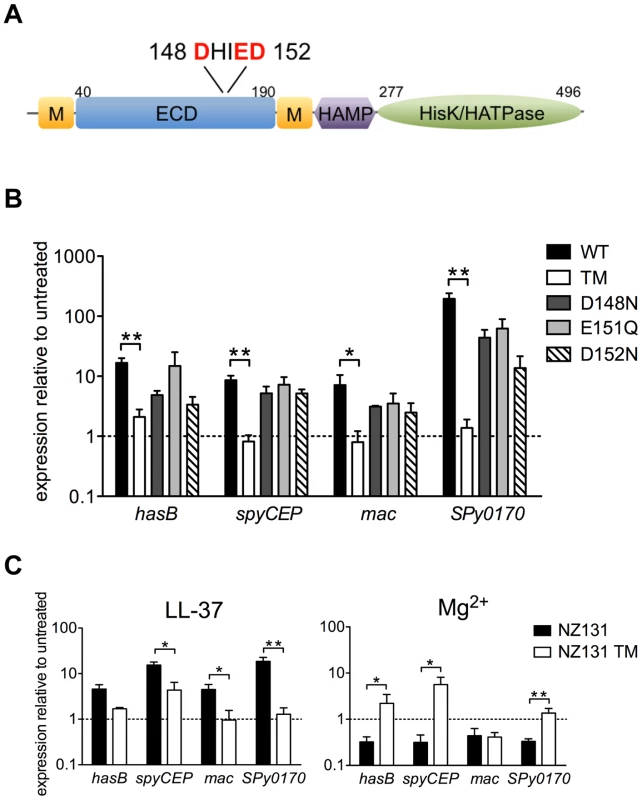
A) Schematic representation of predicted CsrS protein domains. CsrS consists of two membrane-spanning domains (M), an extracellular domain (ECD), a cytosolic HAMP domain, a histidine kinase domain (HisK) and ATPase domain (HATPase). Acidic amino acids in the ECD replaced by uncharged residues in isogenic mutant strains are indicated in red. B) LL-37 stimulation of gene expression in wild type strain 854 (WT), isogenic csrS triple point mutant 854csrSTM (TM), and isogenic csrS single point mutants 854csrSD148N (D148N), 854csrSE151Q (E151Q), and 854csrSD152N (D152N). Expression of hasB, spyCEP, mac, and SPy0170 was measured by qRT-PCR. C) LL-37 and Mg2+ responses in M-type 49 strain NZ131 and its isogenic csrS triple point mutant NZ131TM (NZ131 TM). For panels B and C, data represent mean ratios ± SEM of gene expression in strains grown in the presence of 100 nM LL-37 (panel B; panel C, left) or 15 mM Mg2+ (panel C, right) compared to control cultures of the same strain grown in unsupplemented THY medium (n = 3 – 5). A broken line denotes a ratio of 1, which indicates no change in expression relative to that in unsupplemented medium. Asterisks denote significant differences between wild type and csrS triple point mutant (* P<0.05, ** P<0.006). The results above provide evidence that the mutated cluster of acidic residues in the predicted ECD is critical for LL-37 and Mg2+ signaling through CsrS in strain 854. To confirm these findings and to test their generality for other GAS strains, we constructed an analogous csrS triple point mutant of M-type 49 strain NZ131 and examined its response to LL-37 by qRT-PCR. Similar to the results in the 854 background, we observed almost complete loss of LL-37-stimulated up-regulation of hasB, mac, and SPy0170 in NZ131csrSTM, and a marked reduction in spyCEP up-regulation (Figure 3C, left panel). In wild type NZ131, expression of these four genes was repressed during growth in 15 mM Mg2+, but no such repression was observed for hasB, spyCEP, or Spy0170 in the NZ131csrSTM (Figure 3C, right panel). Thus, similar findings in two independent strain backgrounds highlight the importance of a small cluster of negatively charged amino acids in the predicted CsrS ECD in LL-37 and Mg2+ signaling through CsrRS.
The pattern of gene regulation in 854csrSTM is consistent with constitutive activation of CsrS autokinase activity
During characterization of the csrS triple mutant, we noted that mutant bacteria formed compact, glossy colonies similar to wild type 854, but distinctly different from the mucoid colony appearance of 854csrSΩ lacking CsrS. To verify that the distinctive colony morphology reflected a difference in capsule gene expression, we compared relative expression of hasB (from the hyaluronic acid capsule biosynthetic operon) in the three strains. As expected, in the absence of supplemental Mg2+ or LL-37, expression of hasB was increased more than 50-fold in strain 854csrSΩ relative to that in wild type 854, whereas hasB expression in the csrS triple point mutant was actually reduced by 40% compared to wild type (Table 1). This finding of reduced capsule gene expression suggested that the ECD mutations in the triple mutant resulted not only in refractoriness to regulation by LL-37, but also in increased activity of the CsrR response regulator, presumably by enhancing its phosphorylation in the absence of signaling from an external ligand. Such an effect could result from increased autokinase activity of CsrS or reduced phosphatase activity of CsrS for phospho-CsrR. To test this hypothesis, we compared expression of additional CsrRS-regulated genes in the csrS triple mutant relative to wild type 854. As observed for hasB, in the absence of supplemental Mg2+ or LL-37, expression of spyCEP, mac, and SPy0170 was down-regulated in the triple mutant compared to wild type, whereas expression of each of these genes was significantly up-regulated in 854csrSΩ relative to wild type expression levels (Table 1). Furthermore, expression of grab was increased in the triple mutant relative to wild type levels (data not shown). As grab is activated by supplemental Mg2+ and repressed by LL-37 (Figure 1), this result is also consistent with the proposed model of increased CsrR activity in the triple mutant.
Tab. 1. Effect of csrS and csrR mutations on expression of CsrRS-regulated genes in GAS strain 854 under standard growth conditions. 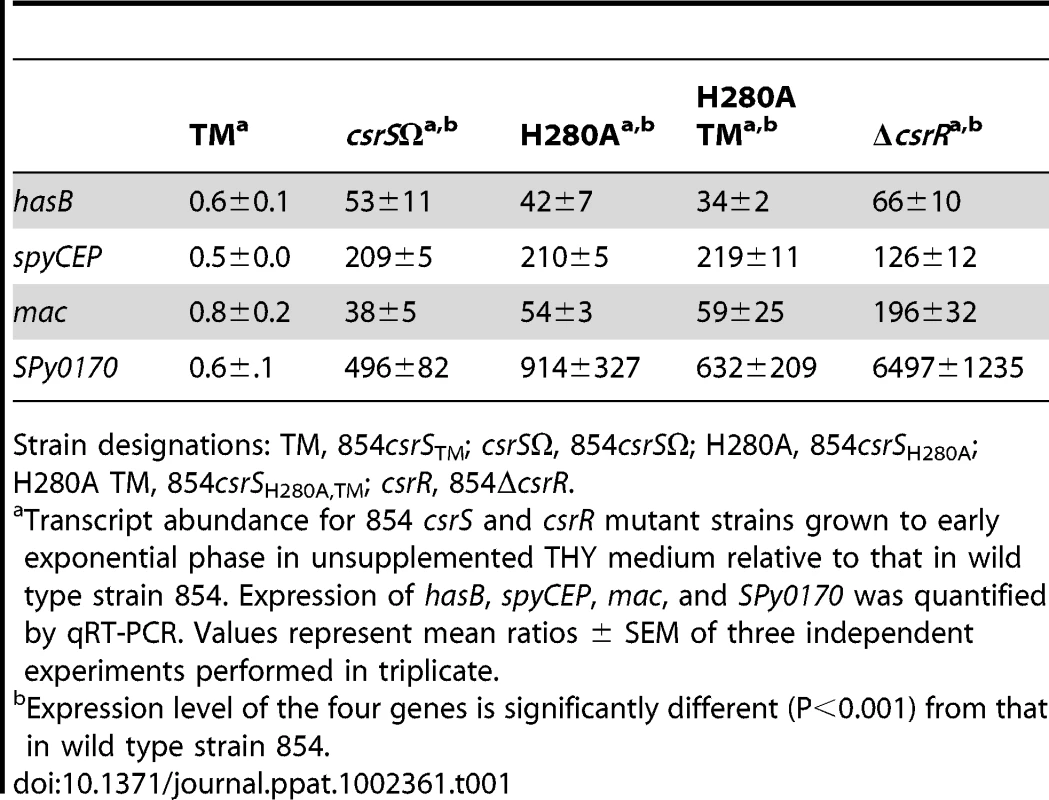
Strain designations: TM, 854csrSTM; csrSΩ, 854csrSΩ; H280A, 854csrSH280A; H280A TM, 854csrSH280A,TM; csrR, 854ΔcsrR. To test directly whether the altered ECD of the triple mutant changed gene regulation by affecting autokinase activity of CsrS, we inactivated the kinase by replacing the active site histidine residue with alanine (H280A). As expected, when introduced in strain 854, this mutation resulted in a mucoid colony morphology, and the mutant strain 854csrSH280A displayed marked up-regulation of CsrRS-repressed genes in a pattern very similar to that observed in 854csrSΩ (Table 1). Similarly, introduction of the H280A mutation into the CsrS triple mutant resulted in mucoid colonies and a comparable derepression of CsrRS-repressed genes as in 854csrSΩ and in 854csrSH280A (Table 1). Since mutation of the active site histidine of CsrS abrogated the suppressive effect of the ECD triple mutant, the most parsimonious model is that these mutations in the ECD affect gene expression by altering the autokinase activity of CsrS. While an effect on phosphatase activity is not excluded by these experiments, the results suggest strongly that the ECD triple point mutant expresses a constitutively active CsrS histidine kinase that is relatively refractory to signaling induced by external stimuli.
Signaling through CsrRS modulates GAS resistance to opsonophagocytic killing, a key feature of invasive disease isolates
CsrRS regulates the expression of several genes that encode products implicated in GAS resistance to opsonophagocytic killing and cytotoxicity: hasABC, slo, nga, spyCEP, sda1, mac, and speB. Upregulation of antiphagocytic factors by host LL-37 is expected to enhance virulence in vivo; however, testing this hypothesis directly in an animal model is not possible, currently, since cathelicidins of other mammalian species do not share the CsrRS-signaling activity of human LL-37 [17]. Because in vitro resistance to phagocytic killing by human blood leukocytes correlates with GAS virulence in vivo [31], we used an in vitro assay to assess the effect of LL-37 on phagocytic resistance as a proxy for effects on in vivo virulence. As would be predicted by the effects of LL-37 on regulation of antiphagocytic factors, exposure of four unrelated wild type GAS strains to LL-37 increased resistance of all four strains to phagocytic killing in vitro [17]. Inactivation of CsrR in the M-type 3 strain DLS003 also resulted in increased resistance to phagocytic killing by human peripheral blood leukocytes, consistent with the marked up-regulation of CsrRS-regulated antiphagocytic factors in the mutant strain [12].
Because deletion of CsrS results in a similar, although less marked, up-regulation of CsrRS-controlled genes, we expected that deletion of CsrS or inactivation of its histidine kinase activity would also lead to increased resistance to phagocytic killing. In vitro opsonophagocytic assays of 854csrSΩ and 854csrSH280A confirmed these predictions: both mutant strains were highly resistant to phagocytic killing by human blood leukocytes in vitro similar to a ΔcsrR mutant (Figure 4). In marked contrast, the csrS triple mutant was as susceptible to killing as wild type 854 in the absence of LL-37, but did not show any increase in phagocytic resistance in response to LL-37 unlike wild type 854 (Figure 4). These observations further support the proposed model that the csrS triple mutant exhibits constitutive activation of CsrS autokinase activity and tonic phosphorylation of CsrR. An important consequence is down-regulation of CsrRS-controlled antiphagocytic factors and hyporesponsiveness to the stimulatory effect of LL-37.
Fig. 4. LL-37 signaling depends on a functional CsrS to induce GAS resistance to opsonophagocytic killing. 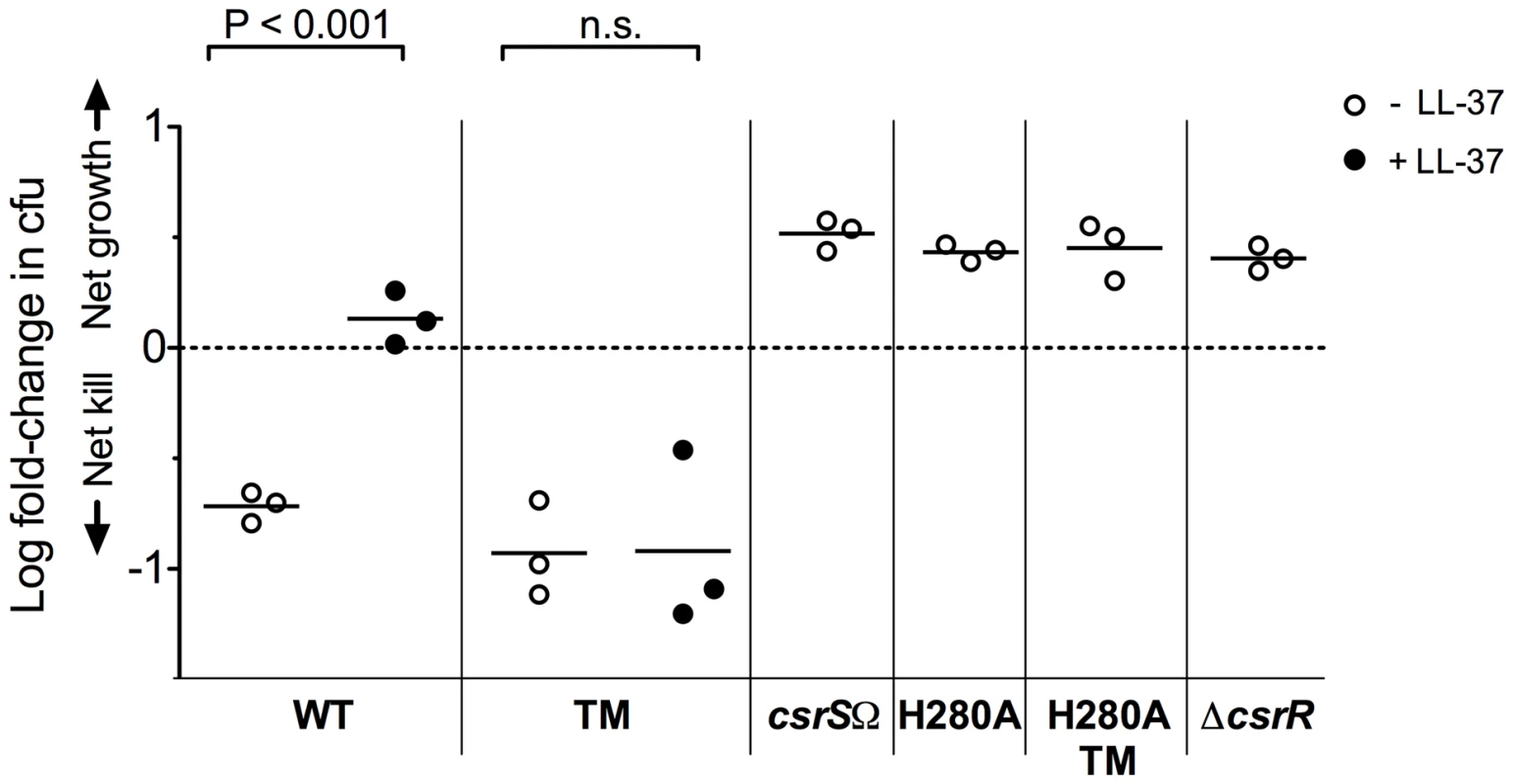
Wild type strain 854 (WT), isogenic csrS mutants 854csrSTM (TM), 854csrSΩ (csrSΩ), 854H280A (H280A), and 854H280A,TM (H280A TM), and isogenic csrR deletion mutant 854ΔcsrR (ΔcsrR) were grown in the absence (open symbols) or presence (filled symbols) of 100 nM LL-37. Bacteria were then mixed with human peripheral blood leukocytes for 1 h in the presence of 10% human serum as complement source. Values represent the log of mean fold-change in cfu. Each symbol represents a single experiment performed in duplicate. When exposed to LL-37, wild type 854 showed a significant increase in resistance to phagocytic killing compared to untreated bacteria (P<0.001), whereas the isogenic csrS triple mutant (TM) did not (n.s. = not significant). Mutant strains 854csrSΩ, 854H280A, 854H280A,TM, and 854ΔcsrR were highly resistant to phagocytic killing in the absence of supplemental LL-37. A conserved residue in the CsrR receiver domain is required for LL-37 and Mg2+ signaling
The current working model for the CsrRS TCS is that of a classical sensor histidine kinase linked by phosphotransfer to a response regulator whose activity is controlled by its phosphorylation state. The experiments described above provide new evidence to support the surface location and stimulus-regulated histidine kinase activity of CsrS. We investigated also the role of CsrR in this model by further characterizing strain 950771, a GAS M-type 3 strain that exhibits a high level of capsular polysaccharide production that does not increase upon exposure to LL-37. Sequencing the csrRS locus in 950771 revealed a point mutation in csrR (K102R) in the highly conserved CsrR receiver domain [17]. The lysine residue that is mutated in 950771 is conserved not only in the csrR locus of all sequenced GAS strains, but also in response regulators of many TCS in a wide variety of bacterial species where it occupies a location near the conserved aspartic acid residue that is the site of phosphorylation [32]. In E. coli CheY, substitution of arginine for lysine at the corresponding site (K109R) did not prevent phosphorylation, but abrogated induction of tumbling motility that normally results from CheY phosphorylation, a finding interpreted to mean that the conserved lysine is required for phosphorylation to produce the active conformation of the response regulator [33]. To test whether K102 is required for GAS CsrR to transmit a signal from CsrS, we replaced R102 in strain 950771 with the consensus lysine residue (R102K). Whereas isolate 950771 (R102) showed no response to LL-37 or to Mg2+, strain 950771csrRR102K restored both up-regulation of hasB, spyCEP, mac, and SPy0170 in response to LL-37 and down-regulation in response to 15 mM Mg2+ (Figure 5A). In addition, correction of CsrR to the consensus K102 sequence markedly reduced expression of all four genes during growth in unsupplemented medium (Figure 5B), a result that implies that K102 is necessary for transduction of the signal mediated by tonic phosphorylation of CsrR by CsrS under standard laboratory growth conditions.
Fig. 5. A lysine residue at position 102 is essential for CsrR to transduce LL-37 signaling. 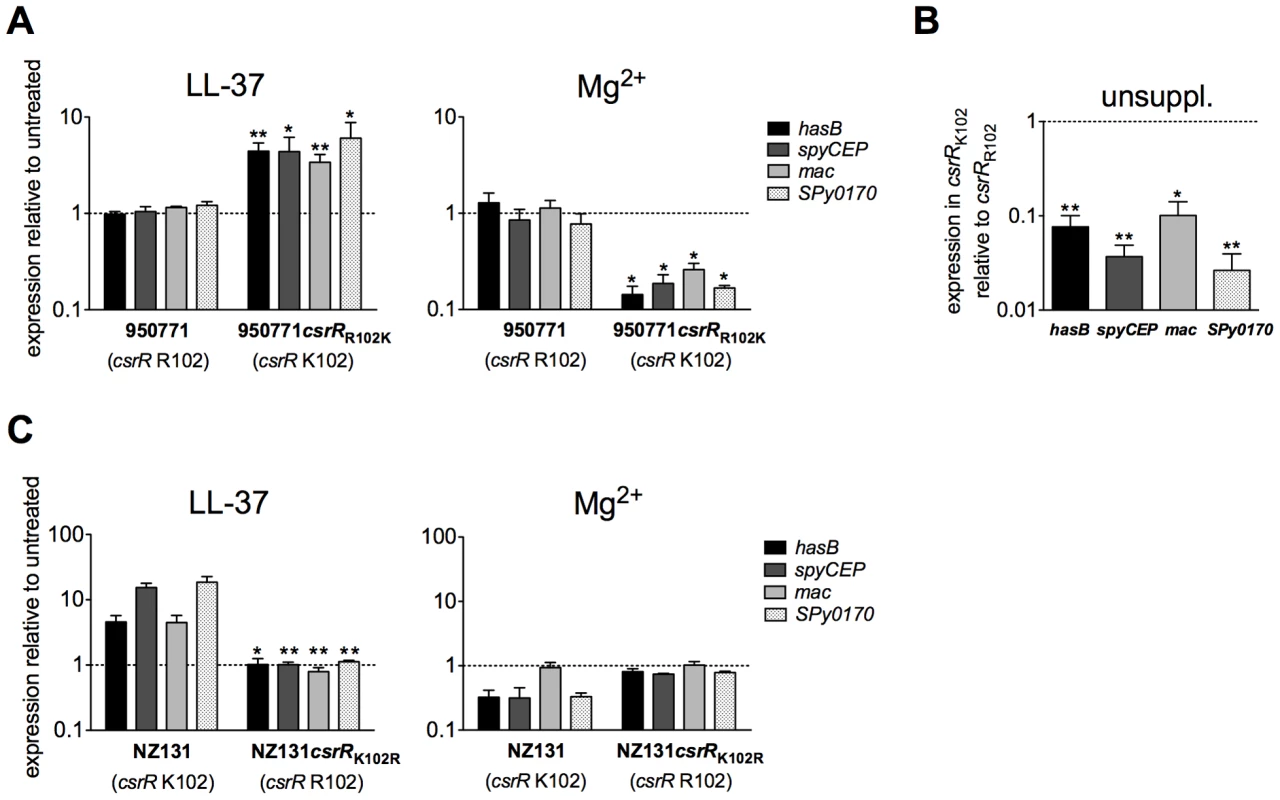
A) Regulation of CsrRS-dependent genes in 950771 and isogenic mutant strain 950771csrRR102K in response to LL-37 and Mg2+. Gene expression was measured in strain 950771 with non-consensus CsrR (csrR R102) and in its isogenic mutant strain 950771csrRR102K in which csrR residue 102 was changed from arginine to the consensus lysine (csrR K102); strains were grown in the presence of 100 nM LL-37 (left panel) or 15 mM Mg2+ (right panel). B) Comparison of baseline expression of hasB, spyCEP, mac, and SPy0170 in isogenic mutant strain 950771csrRR102K (consensus CsrR) with that in strain 950771 (non-consensus CsrR, csrR R102). Cultures were grown to early exponential phase in unsupplemented THY medium (unsuppl.). C) Mutation of csrR K102 in GAS wild type strain NZ131 prevents LL-37 and Mg2+ signaling. Gene expression was measured in NZ131 with consensus CsrR (csrR K102) and in its isogenic mutant strain NZ131csrRK102R (csrR R102) grown in the presence of 100 nM LL-37 (left panel) or 15 mM Mg2+ (right panel). For all panels, data represent mean ratios ± SEM of at least three independent experiments done in triplicate. A broken line denotes a ratio of 1, which indicates no change in expression relative to control. Asterisks denote significant differences in expression between parental strain and respective mutant strains shown in each panel (* P<0.05, ** P<0.008). To further test whether the K102R CsrR mutation is sufficient to prevent CsrRS-mediated modulation of target gene expression, we also introduced the K102R mutation in wild type strain NZ131. In contrast to wild type NZ131 that exhibited a 2 - to 25-fold increase in expression of hasB, spyCEP, mac, and SPy0170 in response to 100 nM LL-37, mutant strain NZ131csrRK102R showed no response (Figure 5C, left panel). Moreover, the mutant failed to repress expression of these genes in response to 15 mM Mg2+ (Figure 5C, right panel). Together, these results indicate that the conserved lysine residue at position 102 in CsrR is required for signal transduction from CsrS to modulate target gene expression in response to extracellular LL-37 or Mg2+.
Discussion
Previous studies have demonstrated that CsrRS regulates expression of more than 100 GAS genes including those encoding many important virulence determinants [9]-[11]. In addition, evidence has been presented that elevated levels of extracellular Mg2+ result in down-regulated expression of CsrR-repressed genes, whereas exposure of GAS to subinhibitory concentrations of LL-37 has the opposite effect [11], [16], [17]. Results of the present study demonstrate the same reciprocal pattern of regulation by LL-37 and Mg2+ for an expanded repertoire of GAS genes. While the predominant pattern of regulation is one of up-regulation of gene expression by LL-37 and down-regulation by Mg2+, we report several instances of the opposite pattern, that is, repression of gene expression by LL-37 and activation by Mg2+. The current investigation provides new experimental evidence that supports a model of CsrRS as a classical TCS that responds to these environmental signals through modulation of CsrS autokinase activity, with downstream signaling that depends on phosphotransfer from CsrS to the CsrR transcriptional regulator. A critical consequence of CsrRS signaling by LL-37 is the coordinated modulation of expression of multiple genes in a fashion that dramatically increases GAS resistance to killing by phagocytes, a bacterial phenotype that enhances virulence and promotes invasive infection in vivo.
In addition to the previously demonstrated up-regulation by LL-37 of the hyaluronic acid capsule synthesis operon, mac/IdeS (Mac/IgG protease), and spyCEP (IL-8 protease), we found that LL-37 activated and Mg2+ repressed expression of genes encoding several important virulence factors including ska (streptokinase), slo (streptolysin O), and nga (NAD-glycohydrolase), as well as speA (pyrogenic exotoxin A) and sda1 (DNase), two virulence determinants encoded by prophages associated with the invasive M1T1 GAS clonal group [34]. The opposite pattern of regulation was observed for speB (cysteine protease) and grab (protein G-related α2-macroglobulin-binding protein). Repressed expression of speB in response to LL-37 may also contribute to an invasive phenotype, as the speB-encoded cysteine protease has been proposed to degrade the anti-phagocytic M1 protein and to inactivate the sda1 gene product, a DNase that itself enhances GAS virulence by degrading neutrophil extracellular traps (NETs) [21], [25], [35], [36].
Protein modeling of CsrS indicates the presence of two membrane-spanning regions that flank a domain predicted to form an extracellular loop that represents a potential site for interaction with environmental stimuli [12], [16]. In cell-fractionation experiments, we found that CsrS is physically associated with the bacterial cell membrane, as predicted by this model. Furthermore, CsrS on intact bacterial cells was accessible to biotin-labeling, a result that implies that a domain of the protein lies in the extracellular space. We also investigated the role in CsrS-mediated signal transduction of a small cluster of negatively charged amino acids in the CsrS ECD. Because a similar cluster of acidic residues has been implicated in binding of cationic ligands to E. coli and S. typhimurium PhoQ, we tested in GAS the effect of substituting uncharged amino acids for these three residues. While our intent had been to disrupt binding of Mg2+ and/or LL-37 to the CsrS ECD, we discovered that this relatively small alteration in the ECD not only abrogated ligand signaling, but also resulted in a global effect on the CsrRS regulon consistent with tonic activation of CsrS autokinase activity. Support for this hypothesis also came from the observation that the effects of the csrS ECD mutations were overridden by mutating the active site histidine of the CsrS kinase domain, a result that implies that the effects of the former mutations on target gene regulation are mediated through CsrS kinase activity. Thus, in the absence of increased extracellular Mg2+ or exposure to LL-37, expression of CsrR-repressed genes was reduced in the csrS triple mutant. The finding that neutralizing the charge of three amino acids in the CsrS ECD leads to an apparent activation of CsrS kinase activity and hyporesponsiveness to ligand signaling suggests that the mutations result in a conformational change in the cytoplasmic domain of CsrS that mimics that induced by binding of Mg2+ to the ECD. It is tempting to speculate that binding of Mg2+ and/or LL-37 to the same region of the ECD also modulates kinase activity by this mechanism, although attempts to demonstrate specific binding of either ligand to the ECD have, so far, been unsuccessful. The data summarized above suggest strongly that LL-37 signaling depends on direct interaction of the peptide and/or Mg2+ with the extracellular domain of CsrS. However, we cannot exclude an alternative signaling mechanism such as membrane disruption by LL-37 that secondarily results in altered CsrS autokinase activity.
We found that deletion of CsrS or inactivation of its kinase activity produced a similar pattern of altered gene expression as deletion of CsrR, although the magnitude of change in gene expression was somewhat smaller for some genes. These observations imply that, under laboratory growth conditions, CsrS activates CsrR, presumably by phosphorylation, increasing its activity as a transcriptional regulator. Activation of CsrR by CsrS can be increased by exposure to elevated extracellular Mg2+ or reduced by exposure to LL-37. The results discussed above support a model in which expression of the CsrRS regulon depends on the equilibrium between the phosphorylated and unphosphorylated states of CsrR. Increased extracellular Mg2+ or mutation of critical residues in the CsrS ECD increases CsrS phosphorylation and enhances phosphotransfer to CsrR, shifting the equilibrium toward phospho-CsrR with consequent repression of CsrR-repressed genes and activation of CsrR-activated genes. Conversely, exposure to LL-37 or deletion of CsrS shifts the equilibrium toward unphosphorylated CsrR, which is less active in regulating target promoters.
Transduction of these modulating signals to altered transcriptional regulation depends also on the presence of a conserved lysine residue at position 102 in the receiver domain of CsrR. A natural mutant with a conservative arginine substitution at this position was refractory to signaling by extracellular Mg2+ or exposure to LL-37 and exhibited a pattern of gene expression similar to that of a CsrR deletion mutant. These phenotypes were confirmed by repairing the natural mutant to the consensus K102 and by introducing the K102R mutation into an unrelated wild type strain. On the basis of these findings and work by others on the role of the corresponding lysine residue in bacterial TCS, we conclude that CsrR K102 is critical to transducing the signal of CsrR phosphorylation and to modulation of CsrR-mediated transcriptional regulation at target promoter sequences.
Several studies have documented the emergence of GAS strains with spontaneous inactivating mutations in CsrS or CsrR in the setting of invasive infection [18]–[20]. Because such mutants have a gene expression profile that results in a multifactorial enhancement of resistance to clearance by host phagocytes, these mutant variants have a strong selective advantage for survival in microenvironments such as the bloodstream or deep tissue sites where they are exposed to attack by host phagocytes. However, analysis of a collection of GAS pharyngeal isolates indicated that CsrRS mutants are distinctly rare in this setting, in marked contrast to isolates from patients with severe systemic infection [19]. The predominance of strains with a functional CsrRS system in the pharynx implies that CsrRS-mediated dynamic regulation of gene expression in response to environmental cues contributes to adaptation of GAS to its preferred environmental niche. During initial colonization, the low concentration of LL-37 on the resting pharyngeal epithelium is predicted to result in an intermediate level of CsrRS activation and a corresponding moderate expression of CsrRS-regulated virulence factors. This “colonizing” phenotype, however, can change quickly in response to increased local concentrations of LL-37. The striking up-regulation of an antiphagocytic phenotype upon exposure to LL-37 enables the organism to maintain the capacity to arm itself against host effectors and thus resist clearance. The coordinated program of altered gene expression induced by LL-37 signaling can tip the balance of pathogen-host interaction from one of asymptomatic colonization to uncontrolled invasive infection. Paradoxically, secretion of LL-37 from injured epithelial cells or from degranulation of recruited neutrophils as part of the host innate immune response may trigger local or systemic invasion by GAS as a result of CsrRS-mediated virulence factor expression.
Methods
Ethics statement
The human subjects aspects of this study were approved by the institutional review board of Children's Hospital Boston. Written informed consent was provided by study participants.
Bacterial strains and growth conditions
Wild type GAS strains used in this study and isogenic mutants derived from them are described in Table 2. GAS M-type 1 strain 854 is a clinical isolate from a patient with a retroperitoneal abscess [17]. GAS M-type 49 strain NZ131 is a skin isolate from a patient with glomerulonephritis [37]. GAS strain 950771 is an M-type 3 clinical isolate from a child with necrotizing fasciitis and sepsis [38]. GAS strains were grown at 37°C in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract (THY) or on THY agar or trypticase-soy agar (BD Bioscience) supplemented with 5% defibrinated sheep blood. Escherichia coli (E. coli) strains DH5α (New England Biolabs) and StrataClone (Stratagene) were used for cloning. Recombinant protein overexpression for antisera production was carried out using E. coli strain BL21(DE3) (Novagen). Antibiotics were used when necessary at the following concentrations: for GAS, erythromycin 1 µg/ml; for E. coli, erythromycin 200 µg/ml, kanamycin 50 µg/ml, carbenicillin 100 µg/ml, and ampicillin 100 µg/ml.
Tab. 2. GAS strains used in this study. 
RNA isolation and qRT-PCR
GAS cultures were grown in THY broth supplemented with or without 100 nM LL-37 or 15 mM MgCl2, and cells were harvested either at early exponential (A600 nm 0.25), mid-exponential (A600 nm 0.5), late exponential (A600 nm 0.8) or early stationary (A600 nm ∼1) growth phase. Total RNA extraction from bacterial cells was performed as described [11] during the growth phase at which target gene expression was maximal. RNA concentration and purity were determined using a NanoDrop spectrophotometer ND-1000 (Thermo Fisher Scientific). Quantitative RT-PCR was performed on an ABI PRISM 7300 Real-Time PCR system (Applied Biosystems) using the QuantiTect SYBR Green RT-PCR kit (Qiagen). Primers used are listed in Table S1. Expression level of each target gene was normalized to recA (spyM3_1800/SPy2116) and analyzed using the ΔΔCt method as described [11]. Replicate experiments were performed from at least three independent RNA preparations in triplicate. Statistical analysis was performed using the paired Student's t-test for expression level comparison under different growth conditions in a single strain and the unpaired t-test for testing differences between strains.
LL-37 synthesis
The human cathelicidin LL-37 (a gift of Robert I. Lehrer, UCLA, CA, USA) was synthesized as described previously and its purity was confirmed by high-performance liquid chromatography and mass spectrometry [39].
Construction of recombinant plasmids for GAS mutagenesis
To introduce single point and triple point mutations in the CsrS ECD region, vector pORIcsrS containing the wild type csrS sequence [11] served as template to amplify the entire plasmid by PCR with primer pair HTW 13/14 for csrS(D148N) substitution, HTW 15/16 for csrS(E151Q) substitution, HTW 17/18 for csrS(D152N) substitution, or csrS418-F(muNHIQN)/csrS480-R(muNHIQN) for csrS triple point substitution (D148N,E151Q,D152N) as described in the Quikchange site-directed mutagenesis protocol (Stratagene). From the resulting plasmids, csrS fragments used for allelic replacement were PCR-amplified with Phusion high-fidelity DNA polymerase (Finnzymes, New England Biolabs) by using primers csrS-F(PshAI) and rt0245-R and cloned into vector pSC-B (StrataClone blunt PCR cloning kit, Stratagene).
To introduce a csrS H280A mutation into GAS strain 854 and into the isogenic triple mutant strain 854csrSTM, a csrS fragment was amplified by PCR from wild type 854 chromosomal DNA with primer pair 5005_149F/5005_1204R and cloned into pGEM-T (Promega). The resulting plasmid was used for Quikchange site-directed mutagenesis to csrS H280A with primer pair H280A-F/H280A-R.
For generating a csrR deletion mutant in strain 854 (854ΔcsrR), an overlap PCR using Phusion DNA polymerase was performed of the region upstream of csrR with primer pair Reg1P-F/HTW52 and the region downstream of csrR including about 500 bp of csrS with primer pair HTW 53/54. The hybridized strands of the two resulting PCR products were used as a template for the second PCR amplifying a 1 kb fragment encompassing a CsrR deletion of amino acids 3–223. The final product was ligated into vector pSC-B.
Subsequently, the csrS or ΔcsrR fragments described above were released from pSC-B or pGEM-T by SalI/BamHI digestion and were subcloned into the temperature-sensitive shuttle vector pJRS233 [40].
To introduce the consensus CsrR (csrR(K102)) into GAS strain 950771 and non-consensus CsrR (csrR(R102)) into GAS strain NZ131, csrR R102 was amplified from 950771 chromosomal DNA was amplified by using Platinum Taq high fidelity DNA polymerase (Invitrogen) and primers CsrP-F and csrS176-R. The PCR product was cloned into pGEM-T and then subcloned into pJRS233 using PstI and XbaI restriction sites to generate pJRS-csrR(R102). Resulting plasmid pJRS-csrR(R102) was then used for Quikchange site-directed mutagenesis to convert csrR(R102) to csrR(K102) by using primer pair HTW 71/72, creating plasmid pJRS-csrR(K102). All primers are described in Table S1.
Allelic exchange mutagenesis in GAS
Recombinant pJRS233 shuttle plasmids were electroporated into GAS strains 854, 854csrSTM, NZ131, or 950771 and then subjected to allelic gene replacement as described [38]. To confirm the genotype of mutant strains, csrR and csrS loci were PCR-amplified with Easy-A high fidelity DNA polymerase (Stratagene) from chromosomal DNA and the sequences confirmed by DNA sequencing (DNA Sequencing Core, Brigham and Women's Hospital, Boston, MA, USA).
Preparation of GAS cell membrane and cytoplasmic fractions
Cultures of 854, 854csrSΩ, and 854csrSTM were grown in THY at 37°C to an A600 nm of ∼0.4, cells were collected (1250 × g, 8 min) and washed once with 10 mM Tris-HCl, pH 8.0, and resuspended in 360 µl hypotonic TEG buffer (10 mM Tris-HCl, 1 mM EDTA, 20% glucose, pH 8.0) supplemented with protease inhibitor cocktail III (Calbiochem). For peptidoglycan degradation, mutanolysin (∼500 units, Sigma) and lysozyme (∼17,700 units, Sigma) were added and samples were shaken at 1000 rpm at 37°C for 1 h in an Eppendorf thermomixer. Cells were washed once in 500 µl TEG buffer and resuspended in 500 µl TE buffer (10 mM Tris-HCl, 5 mM EDTA, pH 8.0) supplemented with protease inhibitor cocktail (Roche). Cells were lysed by ultrasonication (5×3 sec bursts on level 5, Sonic Dismembrator model 60, Fisher Scientific) on ice followed by centrifugation (Eppendorf 5417C 10,000 × g) for 20 min at 4°C to remove cell debris. Membranes were separated from the cytoplasmic fraction by ultracentrifugation of supernatants (Beckman Coulter Ultima, TLA-100.3 rotor) for 1 h at 90,000 × g at 4°C. Membranes and cytoplasmic fractions were resuspended in SDS-PAGE sample buffer and heated to boiling.
Preparation of culture supernatant samples
GAS strain 854 was grown in liquid culture to A600nm 0.7 (late exponential phase) or A600nm 1.2 (stationary phase) in the absence or presence of 100 nM LL-37. Bacteria were removed by centrifugation (21,000 × g, 5 min). Cell-free supernatants were used for assays of DNase activity or mixed with sample buffer and heated to boiling before SDS-PAGE and western blot analysis.
Western blotting
Samples were fractionated on 10% (membrane and cytoplasmic fractions) or 4–12% gradient (supernatant proteins) NuPAGE Novex Bis-Tris gels and then transferred to nitrocellulose membranes for western blotting as previously described [11]. Blots were incubated with specific rabbit antiserum against GAS CsrS ECD [11], CsrR, SLO [41], NADase [41], or SpeB (Toxin Technology, Sarasota, FL) at a 1∶1000 dilution, or with mouse antiserum against GAS membrane protein OpuABC (courtesy of Giuliano Bensi, Novartis Vaccines) at a 1∶3000 dilution, each followed by horseradish-peroxidase-linked secondary antibody [11]. Signal development was carried out using the SuperSignal West Pico chemiluminescence substrate (Thermo Scientific Pierce).
Biotinylation of surface-exposed proteins in GAS
For surface biotinylation the Cell Surface Protein Isolation Kit (Pierce) was used according to the manufacturer's protocol with the following modifications. GAS were grown in 30 ml THY at 37°C to A600 nm ∼ 0.3, cells were harvested (Centra CL3, Thermo IEC, 1250 × g, 8 min), washed twice in 1.5 ml PBS (Eppendorf 5417C, 9800 × g, 1 min), and resuspended in 1.5 ml Sulfo-NHS-SS-Biotin labeling solution. The biotinylation is reversible by cleavage of the disulfide bond in Sulfo-NHS-SS-Biotin. As a negative control, wild type 854 cells were incubated with PBS instead of biotin labeling solution. After 30 min agitation at 4°C, 100 µl quenching solution was added to treated cells and the cells were centrifuged at 6800 × g for 4 min. Cells were washed twice in 1.5 ml TBS (25 mM Tris-HCl, 0.15 M NaCl, pH 7.2) and frozen at −20°C. Frozen cells were resuspended in 250 µl lysis buffer supplemented with 2.5 µl protease inhibitor cocktail III (Calbiochem) and lysed by two rounds of ultrasonication (5×1 s bursts, level 1) with an incubation on ice in between. Lysates were centrifuged at 20,800 × g for 4 min at 4°C. Supernatants were incubated with 250 µl immobilized NeutrAvidin resin for 60 min in spin-columns with end-over-end rotation. Flow-through samples were retained and mixed with SDS-PAGE sample buffer. Resin was washed four times with 500 µl wash buffer supplemented with protease inhibitor and incubated with 200 µl SDS-PAGE sample buffer with 50 mM DTT for 60 min with end-over-end rotation at RT. Eluates were collected by brief centrifugation of uncapped spin-columns. Samples were heated at 95°C for 5 min and stored at −20°C until needed for western blot analysis. A 1∶3 mixture of antiserum to CsrS ECD and antiserum to N-terminal truncated CsrS was used to detect CsrS protein on the blots.
Expression of recombinant Csr proteins components and development of antisera
Full-length CsrR and N-terminal truncated CsrS (CsrSΔ1-231) and were fused separately to a N-terminal His6 tag by cloning into overexpression vector pET-28a (Novagen) PCR-amplified DNA fragments obtained with primer pairs JL-48/JL-49 and HTW 37/46, respectively. Following overexpression by IPTG induction, recombinant proteins were affinity purified using Ni2+-NTA resin (Qiagen) under native conditions (His6-CsrR) or under denaturing conditions (His6-CsrSΔ1-231) according to the manufacturer's protocol. Purified proteins were used to immunize rabbits (LAMPIRE Biological Laboratories, Inc., Pipersville, Pennsylvania, USA). Reactivity of immune sera against CsrS or CsrR was evaluated by western blotting of GAS lysates.
DNase activity assay
DNase activity in GAS culture supernatants was assayed as described by Aziz et al. with modifications [42]. Supernatants were diluted 1∶125 in sterile deionized water, and 10 µL samples were mixed with 1 µg plasmid DNA in 100 mM Tris, pH 7.5, supplemented with 1 mM CaCl2 and 1 mM MgCl2 in a 15 µL total reaction volume. Samples were incubated at 37°C for 20 min and then were stopped by the addition of 20 mM EDTA. Samples were analyzed on 1% agarose gels and DNA was visualized with SYBR Safe DNA stain (Invitrogen).
Opsonophagocytosis assays
GAS resistance to phagocytic killing was evaluated by an in vitro assay as described [43]. In brief, GAS strains grown to early exponential phase with or without 100 nM LL-37 were mixed with freshly isolated human peripheral blood leukocytes at a multiplicity of infection of 3 – 4 in the presence of 10% human serum as complement source. Aliquots were withdrawn for quantitative culture immediately after mixing and after 1 h end-over-end rotation at 37°C. Results were reported on a log scale as the fold-change in cfu defined as the total cfu after incubation divided by the total starting cfu. Statistical significance of differences in the capacity of GAS strains to resist opsonophagocytic killing were evaluated by one-way ANOVA with Bonferroni's post-test analysis.
Supporting Information
Zdroje
1. TanzRRShulmanST 1998 Streptococcal pharyngitis: the carrier state, definition, and management. Pediatr Ann 27 281 285
2. WesselsMR 2011 Clinical practice. Streptococcal pharyngitis. N Engl J Med 364 648 655
3. ErikssonBKAnderssonJHolmSENorgrenM 1998 Epidemiological and clinical aspects of invasive group A streptococcal infections and the streptococcal toxic shock syndrome. Clin Infect Dis 27 1428 1436
4. StevensDL 2000 Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Ann Rev Med 51 271 288
5. CunninghamMW 2000 Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13 470 511
6. KreikemeyerBMcIverKSPodbielskiA 2003 Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol 11 224 232
7. BeierDGrossR 2006 Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol 9 143 152
8. LaubMTGoulianM 2007 Specificity in two-component signal transduction pathways. Ann Rev Genetics 41 121 145
9. DaltonTLCollinsJTBarnettTCScottJR 2006 RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. J Bacteriol 188 77 85
10. GrahamMRSmootLMMigliaccioCAVirtanevaKSturdevantDE 2002 Virulence control in group A Streptococcus by a two-component gene regulatory system: Global expression profiling and in vivo infection modeling. Proc Natl Acad Sci U S A 99 13855 13860
11. GryllosIGrifantiniRColapricoAJiangSDeforceE 2007 Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol Microbiol 65 671 683
12. LevinJCWesselsMR 1998 Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol 30 209 219
13. DaltonTLScottJR 2004 CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J Bacteriol 186 3928 3937
14. ChurchwardG 2007 The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol Microbiol 64 34 41
15. GusaAAGaoJStringerVChurchwardGScottJR 2006 Phosphorylation of the group A Streptococcal CovR response regulator causes dimerization and promoter-specific recruitment by RNA polymerase. J Bacteriol 188 4620 4626
16. GryllosILevinJCWesselsMR 2003 The CsrR/CsrS two-component system of group A Streptococcus responds to environmental Mg2+. Proc Natl Acad Sci U S A 100 4227 4232
17. GryllosITran-WinklerHJChengMFChungHBolcomeR3rd 2008 Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc Natl Acad Sci U S A 105 16755 16760
18. EnglebergNCHeathAMillerARiveraCDiRitaVJ 2001 Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J Infect Dis 183 1043 1054
19. IkebeTAtoMMatsumuraTHasegawaHSataT 2010 Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates. PLoS Pathog 6 e1000832
20. SumbyPWhitneyARGravissEADeLeoFRMusserJM 2006 Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog 2 e5
21. WalkerMJHollandsASanderson-SmithMLColeJNKirkJK 2007 DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med 13 981 985
22. AzizRKKotbM 2008 Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis 14 1511 1517
23. ClearyPPKaplanELHandleyJPWlazloAKimMH 1992 Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339 518 521
24. MusserJMKapurVSzetoJXPSwansonDMartinD 1995 Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect Immun 63 994 1003
25. SumbyPPorcellaSFMadrigalAGBarbianKDVirtanevaK 2005 Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis 192 771 782
26. FerrettiJJMcShanWMAjdicDSavicDJSavicG 2001 Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci U S A 98 4658 4663
27. BeresSBSylvaGLBarbianKDLeiBHoffJS 2002 Genome sequence of a serotype M3 strain of group A Streptococcus: phage - encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci U S A 99 10078 10083
28. SumbyPBarbianKDGardnerDJWhitneyARWeltyDM 2005 Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci U S A 102 1679 1684
29. GroismanEA 2001 The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 183 1835 1842
30. WaldburgerCDSauerRT 1996 Signal detection by the PhoQ sensor-transmitter. Characterization of the sensor domain and a response-impaired mutant that identifies ligand - binding determinants. J Biol Chem 271 26630 26636
31. LancefieldRC 1962 Current knowledge of the type specific M antigens of group A streptococci. J Immunol 89 307 313
32. VarugheseKI 2002 Molecular recognition of bacterial phosphorelay proteins. Curr Opin Microbiol 5 142 148
33. LukatGSLeeBHMottonenJMStockAMStockJB 1991 Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J Biol Chem 266 8348 8354
34. AzizRKEdwardsRATaylorWWLowDEMcGeerA 2005 Mosaic prophages with horizontally acquired genes account for the emergence and diversification of the globally disseminated M1T1 clone of Streptococcus pyogenes. J Bacteriol 187 3311 3318
35. BuchananJTSimpsonAJAzizRKLiuGYKristianSA 2006 DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol 16 396 400
36. RaederRWoischnikMPodbielskiABoyleMD 1998 A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin binding properties. Res Microbiol 149 539 548
37. SimonDFerrettiJJ 1991 Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol Lett 66 219 224
38. AshbaughCDWarrenHBCareyVJWesselsMR 1998 Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Invest 102 550 560
39. SawaiMVWaringAJKearneyWRMcCrayPBJrForsythWR 2002 Impact of single-residue mutations on the structure and function of ovispirin/novispirin antimicrobial peptides. Protein Eng 15 225 232
40. Perez-CasalJPriceJAMaguinEScottJR 1993 An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol 8 809 819
41. MichosAGryllosIHakanssonASrivastavaAKokkotouE 2006 Enhancement of streptolysin O activity and intrinsic cytotoxic effects of the group A streptococcal toxin, NAD-glycohydrolase. J Biol Chem 281 8216 8223
42. AzizRKIsmailSAParkHWKotbM 2004 Post-proteomic identification of a novel phage-encoded streptodornase, Sda1, in invasive M1T1 Streptococcus pyogenes. Mol Micobiol 54 184 197
43. GryllosIGrifantiniRColapricoACaryMEHakanssonA 2008 PerR confers phagocytic killing resistance and allows pharyngeal colonization by group A Streptococcus. PLoS Pathog 4 e1000145
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



