-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
All vector mosquito species must feed on the blood of a vertebrate host to produce eggs. Multiple cycles of blood feeding also promote frequent contacts with hosts, which enhance the risk of exposure to infectious agents and disease transmission. Blood feeding triggers the release of insulin-like peptides (ILPs) from the brain of the mosquito Aedes aegypti, which regulate blood meal digestion and egg formation. In turn, hemocytes serve as the most important constitutive defense in mosquitoes against pathogens that enter the hemocoel. Prior studies indicated that blood feeding stimulates hemocytes to increase in abundance, but how this increase in abundance is regulated is unknown. Here, we determined that phagocytic granulocytes and oenocytoids express the A. aegypti insulin receptor (AaMIR). We then showed that: 1) decapitation of mosquitoes after blood feeding inhibited hemocyte proliferation, 2) a single dose of insulin-like peptide 3 (ILP3) sufficient to stimulate egg production rescued proliferation, and 3) knockdown of the AaMIR inhibited ILP3 rescue activity. Infection studies indicated that increased hemocyte abundance enhanced clearance of the bacterium Escherichia coli at lower levels of infection. Surprisingly, however, non-blood fed females better survived intermediate and high levels of E. coli infection than blood fed females. Taken together, our results reveal a previously unrecognized role for the insulin signaling pathway in regulating hemocyte proliferation. Our results also indicate that blood feeding enhances resistance to E. coli at lower levels of infection but reduces tolerance at higher levels of infection.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002274
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002274Summary
All vector mosquito species must feed on the blood of a vertebrate host to produce eggs. Multiple cycles of blood feeding also promote frequent contacts with hosts, which enhance the risk of exposure to infectious agents and disease transmission. Blood feeding triggers the release of insulin-like peptides (ILPs) from the brain of the mosquito Aedes aegypti, which regulate blood meal digestion and egg formation. In turn, hemocytes serve as the most important constitutive defense in mosquitoes against pathogens that enter the hemocoel. Prior studies indicated that blood feeding stimulates hemocytes to increase in abundance, but how this increase in abundance is regulated is unknown. Here, we determined that phagocytic granulocytes and oenocytoids express the A. aegypti insulin receptor (AaMIR). We then showed that: 1) decapitation of mosquitoes after blood feeding inhibited hemocyte proliferation, 2) a single dose of insulin-like peptide 3 (ILP3) sufficient to stimulate egg production rescued proliferation, and 3) knockdown of the AaMIR inhibited ILP3 rescue activity. Infection studies indicated that increased hemocyte abundance enhanced clearance of the bacterium Escherichia coli at lower levels of infection. Surprisingly, however, non-blood fed females better survived intermediate and high levels of E. coli infection than blood fed females. Taken together, our results reveal a previously unrecognized role for the insulin signaling pathway in regulating hemocyte proliferation. Our results also indicate that blood feeding enhances resistance to E. coli at lower levels of infection but reduces tolerance at higher levels of infection.
Introduction
Insects primarily rely on an innate immune system for defense against pathogens and other foreign invaders. Reciprocally, a number of insects including mosquitoes can be infected by and transmit certain pathogens that cause disease in humans and other vertebrate hosts. The key feature of mosquito biology that makes them important disease vectors is that adult females must feed on the blood of their vertebrate host(s) to produce eggs. Repeated bouts of blood feeding and egg development in turn increases the risk of mosquitoes feeding on an infected host and transmitting a given pathogen from one individual to another.
At the physiological level, blood feeding by the mosquito Aedes aegypti triggers neurosecretory cells in the brain to release insulin-like peptides (ILPs) into circulation, which stimulate the ovaries to secrete ecdysteroid hormones (ECDs) [1]–[3]. ILPs from the brain together with nutrient sensing through the target of rapamycin (TOR) pathway also induce the midgut to produce enzymes that digest the protein-rich blood meal into amino acids. These amino acids are then transported from the midgut into the hemolymph where they are taken up by the fat body [4]–[6]. Amino acids, ECDs and ILPs together induce the fat body to produce vitellogenin (Vg) and other yolk proteins (YP) [7], [8], which are packaged into eggs that the female lays between 72 and 96 h post-blood meal (pbm). Pathogens in a blood meal infect mosquitoes during this same period by moving across the midgut, disseminating through the hemocoel, and invading other organs like the salivary glands or fat body.
Constitutive immune defenses in insects refer to factors like hemocytes that are always present and ready to act if an organism is infected, while inducible defenses refer to molecules like antimicrobial peptides that are produced in response to infection [9]. In most insects including mosquitoes, hemocytes serve as the most important constitutive defense element against pathogens that enter the hemocoel [10], [11]. Key functions of mosquito hemocytes include phagocytic and melanotic immune responses [12]–[17], production of soluble effector molecules with activity against bacteria and protozoa [17]–[23], and enhanced defense associated with immune priming [24]. A. aegypti produces three types of hemocytes named granulocytes, oenocytoids, and prohemocytes that are distinguished from one another by morphological, antigenic and functional characters [15]. In adult females, granulocytes account for more than 90% of the total hemocyte population and are the only cell type that is phagocytic. Oenocytoids in contrast are weakly adhesive cells that constitutively express components of the phenoloxidase cascade that regulate the formation of melanin, while prohemocytes are non-adhesive and may be putative progenitor cells.
Prior studies indicate that the number of hemocytes in adult A. aegypti progressively declines with age, which in turn correlates with age-dependent increases in susceptibility to infection by bacteria like Escherichia coli [13], [15]. In contrast, blood feeding stimulates an increase in hemocyte abundance in adult females that coincides with the timing of yolk deposition into follicles to produce eggs [15], [17]. In the current study, we hypothesized that circulatory hormones that stimulate egg development after blood feeding also mediate increases in hemocyte abundance. Our results show that loss of signaling from the mosquito brain after blood feeding inhibits the increase in hemocyte abundance that occurs after a blood meal while a single dose of insulin peptide 3 (ILP3), sufficient to stimulate egg development, rescues hemocyte proliferation.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, United States of America. The protocol was approved by The University of Georgia Institutional Animal Care and Use Committee (IACUC) (UGA Animal Use Protocol A2010-6-094), which oversees and provides veterinary care for all campus animal care facilities. IACUC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), is licensed by the US Department of Agriculture, and maintains an Assurance of Compliance with the US Public Health Service. IACUC technicians carried out all animal husbandry under strict guidelines to insure careful and consistent handling of animals.
Mosquitoes
The UGAL strain of A. aegypti was used in all experiments. All life stages were maintained under a 12 h light/dark cycle at 28°C and 70–80% humidity. Larvae were fed with fish food, and adults were maintained in cages provided with 10% sucrose ad libitum.
Endocrine reagents
ILP3 used in the study was synthesized and purified as previously described [2], while 20-hydroxyecdysone (20E) was purchased from Sigma.
Hemocyte collection
Hemocytes were collected using the high injection/recovery method of Castillo et al. [15]. In brief, adult females were placed on ice for 15 min followed by injection of 3–6 µl of collection buffer (60% Schneider's medium (Sigma), 10% fetal bovine serum (FBS) (Hyclone) and 30% citrate buffer ( = anticoagulant) (98 mM NaOH, 186 mM NaCI, 1.7 mM EDTA and 41 mM citric acid, buffer pH 4.5) (vol/vol)) between the last two abdominal sclerites using a glass needle mounted on a micromanipulator. After 20 min 25 µl of collection buffer was injected into the lateral wall of the mesothorax and while concurrently collecting the diluted hemolymph by capillary action from the original injection site in the abdomen using a second hand-held glass needle.
RT-PCR and rqRT-PCR analyses
Total RNA was extracted from hemocytes using Trizol reagent (Invitrogen). One µg of DNase-treated total RNA was used for cDNA synthesis using the Superscript III cDNA synthesis kit (Invitrogen). Hemocyte cDNA was diluted 10 fold and used 1 µl/20 µl for RT-PCR or 1 µl/10 µl for rqRT-PCR reactions with reaction mixtures containing specific primers for the AaMIR (forward 5′ CATT CCCGCTGAGCTACTTC 3′; reverse CGTCAGCACCTTCCTTCTTC 3′) or actin (control) (forward 5′ACCAACTGGGATGATATGGAG3′; reverse 5′ GTAGACAGTTTCGTGGATACCGCA3′) 0.2 mM of dNTPs and 2.5 Units Hotmaster Taq DNA polymerase (5 Prime GmbH, Hamburg, Germany) in 50-µl reaction mixtures. Cycling conditions were: 2 min of initial denaturation at 94°C, followed by 35 cycles of 20 sec denaturation step at 94°C, 30 sec annealing at 57°C, 45 sec extension at 72°C, and a final extension at 70°C for 10 min. Products were visualized on 0.5% agarose gels stained with ethidium bromide. Reactions for rqRT-PCR included iQ SYBR Green Supermix (BioRad; 5 µl/tube) and were run on a Rotor Gene Real Time-PCR cycler (Corbett) for 40 or 45 cycles under the following conditions: denaturation at 95° for 20 sec, annealing at 56° for 30 sec and extension at 72° for 30 sec. All reactions were run in quadruplicate with actin serving as the internal control (forward 5′ACCGCCG TCTACGATGCCA 3′; reverse 5′ATGGTGGTCTGCTGGTTCTT 3′). Data were analyzed by the ΔΔCt method as previously outlined [6].
In situ hybridization
A 256 bp region of the AaMIR was PCR-amplified using the primers 5′ CCCCGGTTATGAAACAGTTC3′ (forward) and 5′TCTCGATGGACAAACTTCTT3′ reverse and cloned into the TOPO (pCR4.0) TA cloning dual promoter vector (Invitrogen, Carlsbad, CA). Plasmid was then digested with NotI and KpnI (New England Biolabs), phenol/chloroform extracted and alcohol precipitated. Linearized plasmid was then used as template for synthesis of digoxigenin-labeled anti-sense and sense (negative control) AaMIR RNA probes using the DIG RNA labeling kit (SP6/T7) (Roche) following the manufacture's instructions. Probes were ethanol precipitated, resuspended in 25 µl of nuclease-free water and quantified by dot blot. In situ hybridization was then performed essentially as described by Lavine and Strand [25] using hemocytes from 1 day old females. Cells were placed in 96 well plates, allowed to settle for 30 min and fixed 4% para-formaldehyde in DEPC-treated Phosphate Buffer Saline (PBS) at 4°C for 20 minutes. After permeabilization with PBT (PBS+0.1% tween-20) for 15 min, cells were treated with Proteinase K digestion (0.1 µg/ml) for 90 sec. After refixation and washing with PBS, cells were incubated in 0.1 M Triethanolamine (TEA) buffer (pH 8.0) for 5 min, prehybridized in prehybridization buffer (2X SSC, 50% deionized formamide (Sigma), 10% dextran sulfate (Sigma) and 0.1 mg/ml Salmon sperm (Ambion)) for 1 hour at 55°C, and then hybridized for 24 h at 55° in hybridization buffer containing 100 ng/ml of probe. Following washing to high stringency for 1 h in 0.2X SSC at 55°C, cells were incubated with an anti-digoxigenin Fab fragment conjugated to alkaline phosphatase (Roche) in blocking solution (1∶5000) for 2 h at 37°C. Signal was visualized using NBT/BCIP in detection buffer followed by stoppage of reactions using TEA buffer (pH 8.0). Fixed hemocytes were also labeled with Alexa fluor 488 phalloidin and an anti-histone H1 antibody visualized using an Alexa fluor 546 secondary antibody as previously described [15]. All images were collected using a Leica inverted microscope and digital camera followed by export to Adobe Photoshop CS3 (Adobe) for assembly of figures.
RNA interference (RNAi) assays
dsMIR and dsEGFP RNAs were produced using the T7 High Yield Transcription kit (Fermentas) [3]. dsRNAs were thereafter treated with DNAse1 and RNAseA, precipitated, and resuspended in nuclease-free water at 4 µg/µl for storage at −20°C. Mosquitoes were injected with 2 µg of dsRNA at 12 to16 h post-eclosion followed by collection of hemocytes for rqRT-PCR analysis as described above. Following natural log transformation of the 2-ΔΔCT values, differences in transcript abundance value between the dsMIR and dsEGFP treatments were analyzed by t-test.
Bioassays
The effects of decapitation on hemocyte abundance were conducted by decapitating females 1–12 h pbm followed by counting the total number of hemocytes recovered from females at 24 h pbm. The effects of ILP3 or 20E on hemocyte numbers at 24 h pbm were determined by decapitating females after feeding, injecting them with 0.5–50.0 pmol of ILP3 or 0.1–1.0 µg of 20E in 1 µl of PBS. Mosquitoes injected with PBS only served as a negative control. Decapitated females heal endogenously and visibly lose no hemolymph. Hemocyte abundance per mosquito was analyzed by ANOVA followed by the Tukey-Kramer multiple comparison procedure with treatment and/or sample time serving as the independent variable.
Bromodioxyuridine (BrdU) incorporation was assessed by feeding day 2 females a sugar solution containing 1 mg BrdU per ml (Sigma). BrdU-fed females were then blood fed on day 4, and either left intact or decapitated. Hemocyte samples were then collected 24 h pbm and processed [26]. Briefly, hemocytes were placed on L-lysine coated slides and incubated in Schneider's medium for 40 min, followed by fixation with paraformaldehyde and permeabilization using PBT. Cells were incubated in 2 N HCl for 40 min and neutralized in 0.1 M sodium borate (pH 8.5) followed by incubation in blocking reagent (Roche) for 1 h and a murine anti-BrdU antibody (1∶100; Invitrogen) overnight at 4°C. After washing in PBT and incubation with an Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (1∶2000; Invitrogen) for 2 h, cells were examined and photographed as described above. To compare the percentage of BrdU-labeled hemocytes between intact and decapitated females, data were arcsin square root transformed and analyzed by ANOVA.
For infection assays, one cohort of adult females was blood fed (BF) on day 4 while a second cohort was left non-blood fed (NBF) followed 24 h later by injection of 1×103–1×105 colony forming units (CFUs) of the Migula strain of E. coli (ATCC# 25254), which are streptomycin-resistant. Third and fourth cohorts of mosquitoes were injected with dsEGFP or dsMIR RNA on day 1 followed by blood feeding on day 4 and injection with bacteria 24 h later as described above (BF-dsMIR). Mosquitoes were homogenized at 0, 3 or 24 h post-infection in 100 µl of BHI medium containing 50 µg/ml streptomycin, serially diluted and placed on BHI-streptomycin plates at 37°C for 18 h. The number of CFUs per individual was then determined (n = 5 females per treatment (BF, NBF, BF-dsEGFP, or BF-dsMIR), infection dose (1×103–1×105), and sample time (0, 3, 24 h) for a total of 180 individuals. We then compared the number of surviving bacteria per mosquito treatment and infection dose by Kruskal-Wallis tests. Other cohorts of BF and NBF mosquitoes treated as described above were maintained in cages (20 females per cage) with sugar water for 6 days post-infection. The number of mosquitoes surviving each day was then determined by counting the number of mosquitoes that died during the intervening 24 h period. The survival experiments were replicated three times using cohorts of 20 females per treatment and infection dose. To assess the effect of infection on mortality rates, we used the proportional hazards model where treatment and infection dose were the independent variables and the number of dead mosquitoes on each day was the dependent variable. All analyses were conducted using the JMP 7.0 statistical platform (SAS, Cary, NC).
Results
Granulocytes and oenocytoids express the AaMIR
A. aegypti encodes a single receptor tyrosine kinase, designated the mosquito insulin receptor (AaMIR), that is structurally homologous to the vertebrate IR [27] along with 8 ILP genes of which five (ILP1, 3, 4, 7, 8) are specifically expressed in medial neurosecretory cells in the brain [2], [28]. We previously determined that ILP3 dose-dependently stimulates yolk deposition into follicles, and that ILP3-induced egg development fully depends upon expression of the MIR in the ovaries [2], [3]. Given the similarities in timing between egg development and the increase in hemocyte abundance that occurs after blood feeding, we first assessed whether hemocytes also express the AaMIR. Using gene specific primers and total hemocyte cDNA as template, RT-PCR analysis detected AaMIR expression in hemocytes from both non-blood fed (NBF) females and blood-fed (BF) females sampled 2, 3, 9, and 24 h pbm (Fig. 1A). Granulocytes, oenocytoids, and prohemocytes are distinguished from one another in primary culture by their differences in size, biological activity, and morphology [15]. As shown in Fig. 1B and C, granulocytes rapidly attach to culture plates and extend filopodia of varying length. Oenocytoids in contrast remain spherical with a large prominent nucleus (Fig. 1D). Prohemocytes are also spherical but are distinctly smaller than oenocytoids (Fig. 1E). In situ hybridization analysis using an anti-sense AaMIR probe detected strong hybridization signals in the cytoplasm of virtually all granulocytes and oenocytoids from NBF and BF females, but detected no signal in prohemocytes (Fig. 1F–I). We also detected no signal in any hemocyte type using a sense AaMIR probe (Fig. 1J).
Fig. 1. The AaMIR is expressed in granulocytes and oenocytoids of A. aegypti. 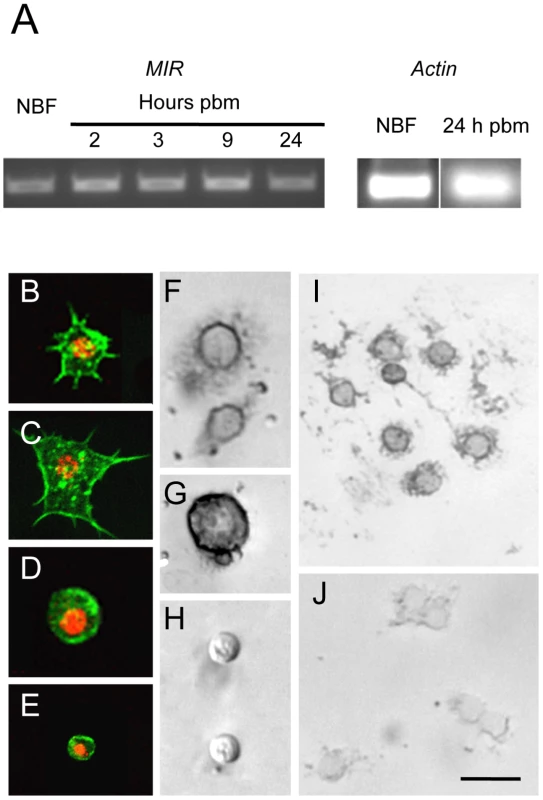
(A) Ethidium bromide stained agarose gel showing the amplified AaMIR products from RT-PCR reactions using hemocyte cDNA templates from NBF females and BF females processed at 2, 3, 9 and 24 h pbm. Amplification of A. aegypti actin from NBF females and BF females at 24 h pbm served as the loading control. (B–E) A. aegypti hemocytes whose cytoplasms are labeled with phalloidin and whose nuclei are labeled using an anti-histone H1 antibody. B and C show representative granulocytes with numerous filopodia extending from each cell that strongly adhere each cell to the surface of a culture plate. D shows a representative oenocytoid, and E shows a representative prohemocyte. (F–I) In situ hybridization images of A. aegypti hemocytes hybridized with an AaMIR antisense probe. Strong hybridization signals are detected in the cytoplasm of granulocytes (F) and oenocytoids (G) but no signal is detected in prohemocytes (H). (I) Multiple granulocytes show a positive hybridization signal. (J) In situ hybridization image of A. aegypti hemocytes hybridized with an AaMIR sense probe. Note the absence of any hybridization signal. Scale bar in J equals 50 µm with images in the other panels being at a similar magnification. Decapitation blocks the increase in hemocyte abundance that occurs after blood feeding
Since decapitation of females after blood feeding blocks yolk deposition into eggs [2], [3], we asked if decapitation also blocked the increase in hemocytes that occurs after blood feeding. Consistent with earlier studies [15], [17], we recovered significantly more hemocytes from BF females at 24 h pbm than from NBF females of the same age (Fig. 2A). This increase resulted in BF females on average containing 43% more hemocytes than NBF females. In contrast, decapitation of BF females at 2 h pbm fully blocked this increase in abundance (Fig. 2A). Decapitation of NBF females also resulted in a small but significant reduction in the total number of hemocytes recovered relative to intact (non-decapidated) NBF females at 24 h (Fig. 2A). Time course studies indicated that decapitation of BF females up to 9 h pbm prevented hemocyte numbers from increasing to the same level as non-decapitated BF females at 24 h pbm (Fig. 2B). However, females decapitated at 12 h pbm contained the same number of hemocytes as intact BF females at 24 h (Fig. 2B).
Fig. 2. Decapitation inhibits the increase in hemocyte abundance that occurs after blood feeding. 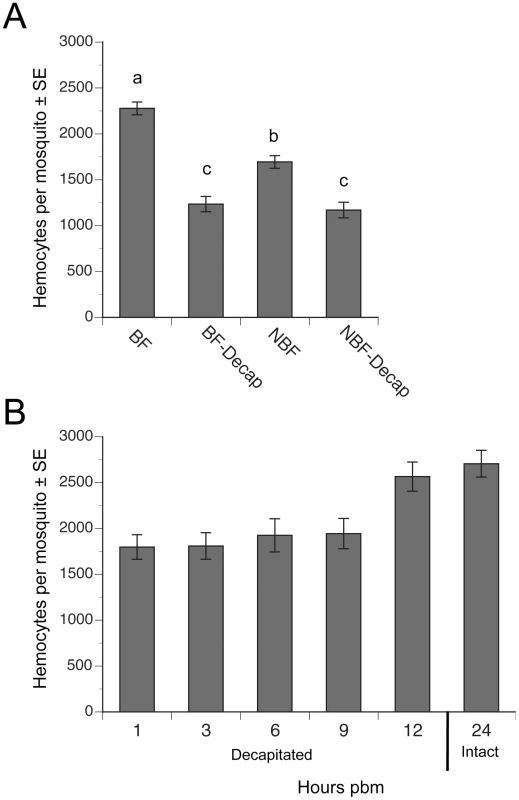
(A) Mean number (± SE) of hemocytes per mosquito in BF females, BF females decapitated 2 h pbm (BF-Decap), NBF females, and NBF-Decap females. Adult females were blood fed four days post-emergence and hemocyte numbers were determined 24 h later (day 5). NBF females were also decapitated on day four and examined on day 5. Different letters above a given bar indicate means that significantly differ (F3, 142 = 45.7; P<0.0001). (B) Mean number (± SE) of hemocytes per day 4 mosquito decapitated 1, 3, 6, 9 or 12 h pbm. All females were bled at 24 h pbm and compared to the number of hemocytes collected from intact (non-decapitated) females that were also bled at 24 h pbm (control). Females decapitated 1–9 h pbm contained significantly fewer hemocytes than non-decapitated control females, but females decapitated at 12 h pbm contained the same number of hemocytes as controls (asterisks) (F5, 72 = 6.7; P<0.0001). A higher proportion of hemocytes incorporate BrdU following blood feeding
The increase in the number of hemocytes recovered from females after blood feeding could reflect either the entry of previously sessile hemocytes into circulation, an increase in the proportion of actively cycling hemocytes, or both. If events associated with blood feeding increase the number of actively cycling hemocytes, we reasoned that the proportion of hemocytes incorporating an exogenous DNA precursor like BrdU during S phase of the cell cycle should increase. We also reasoned A. aegypti should uptake BrdU by feeding since this approach has been successfully used to BrdU label proliferating cells in other insects [29]–[31]. We therefore fed adult females sugar water containing BrdU and then compared the proportion of BrdU-labeled hemocytes in intact females 24 h pbm to females that we decapitated immediately after blood feeding. Examination of hemocytes by epifluorescence microscopy showed that feeding of BrdU resulted in strong nuclear labeling of some but not all hemocytes recovered from each female (Fig. 3A–C). As with earlier experiments, we also recovered significantly more hemocytes from intact than decapitated BF females, which indicated that BrdU feeding did not alter the increase in hemocyte abundance that normally occurs after a blood meal (Fig. 3D). However, when we assessed the percentage of hemocytes with incorporated BrdU, our results showed that significantly more hemocytes from intact, BF females were BrdU-labeled than from decapitated, BF females, which suggested that blood feeding increased the proportion of actively cycling hemocytes (Fig. 3E). Based upon morphology, we further concluded that virtually all BrdU-labeled hemocytes (>99%) were granulocytes.
Fig. 3. Blood feeding increases the proportion of hemocytes labeled by BrdU. 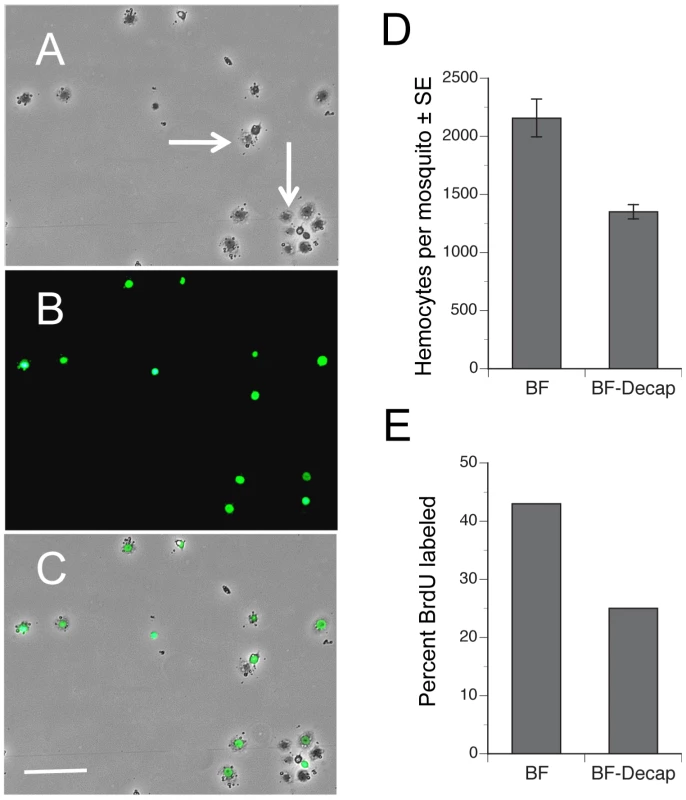
Phase-contrast (A), epiflorescent (B) and a phase-epifluorescent merged image (C) of A. aegypti hemocytes from a BF female collected 24 h pbm. The nuclei of actively cycling hemocytes that incorporated BrdU are labeled green, whereas the nuclei of representative unlabeled hemocytes exhibit no nuclear signal (arrows). Note the spread morphology of the labeled cells indicating their identity as granulocytes. Scale bar in C equals 100 µm. (D) BF intact females treated with BrdU contain more hemocytes (± SE) than females decapitated immediately after blood feeding (F1, 18 = 8.4; P<0.001). Females were pretreated with BrdU and blood fed on day 4. Hemocytes were then collected 24 h pbm. (E) Intact BF females treated with BrdU contain a higher proportion of BrdU-labeled hemocytes than females decapitated immediately after blood feeding (F1, 18 = 21.5; P<0.0002). ILP3 dose-dependently restores hemocyte abundance in blood-fed, decapitated females
Since 5 pmol or more of ILP3 rescues egg development in decapitated, BF females [2], we injected females with a single dose of ILP3 that ranged from 0.5–50 pmol. Our results showed that a 50 pmol injection fully restored hemocyte numbers in decapitated BF females to the same level found in intact BF females, while lesser amounts of ILP3 resulted in lower levels of hemocyte recovery (Fig. 4A). Decapitated, BF females injected with a range of concentrations of 20E in contrast exhibited no increases in hemocyte abundance (data not presented). To assess whether this rescue effect depended upon AaMIR expression, we injected dsMIR or dsEGFP (negative control) RNA into newly eclosed females, held them for 4 days with water, and then blood fed on day 4. rqRT-PCR analysis confirmed that dsMIR treatment reduced AaMIR transcript abundance in hemocytes relative to mosquitoes treated with dsEGFP (Fig. 4B), while bioassay results showed that dsMIR-treated females contained significantly fewer hemocytes than females pretreated with dsEGFP (Fig. 4C).
Fig. 4. ILP3 dose-dependently rescues hemocyte abundance after decapitation while dsMIR treatment reduces hemocyte abundance. 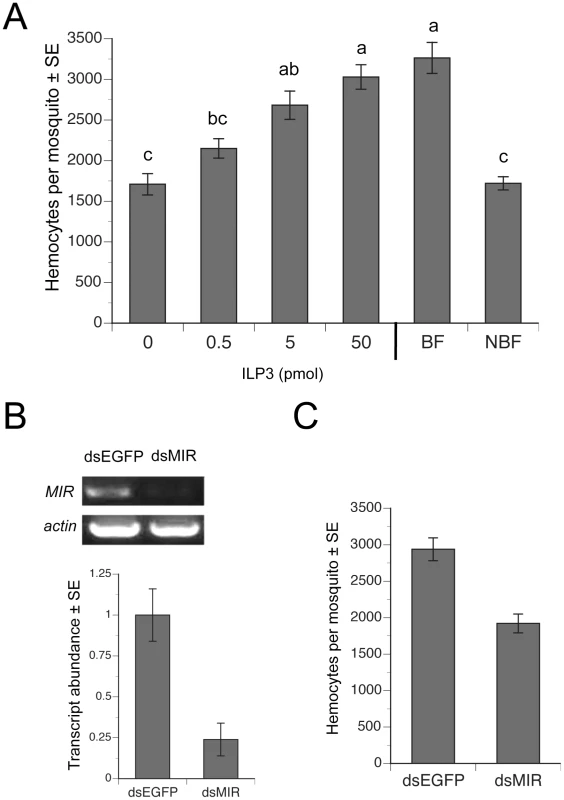
(A) Day four females were blood fed, decapitated and injected with a single dose of 0.5–50 pmol of ILP3. BF-decapitated females injected with water only (0) and intact, NBF females served as negative controls, while intact BF females (BF) served as a positive control. Mean number (± SE) of hemocytes for all treatments was determined at 24 h pbm. Different letters above a given bar indicate means that significantly differ (F5, 60 = 20.9; P<0.0001). (B) dsMIR RNA reduces AaMIR transcript abundance in hemocytes. The top of the figure shows RT-PCR products for the MIR relative to actin in hemocyte samples from mosquitoes treated with dsEGFP (control) or dsMIR RNA at 1 day post-eclosion. Hemocyte RNA pooled from 10 day 4 BF females 24 h pbm served as the source of template. The bottom of the figure shows rqRT-PCR analysis of MIR transcript abundance (± SE) in mosquito hemocytes 24 h pbm pretreated with dsEGFP or dsMIR. Transcript levels were standardized to a level of 1 for hemocytes from dsEGFP females, while transcript levels for dsMIR-treated females are expressed relative to the control. Each treatment was replicated three times using samples from 10 pooled females. dsMIR treatment significantly reduced MIR transcript abundance relative to the dsEGFP control (t-test, P<0.05). (C) dsMIR treatment reduced the mean number of hemocytes (± SE) per female relative to females treated with dsEGFP (F1, 34 = 30.7; P<0.0001). Increased hemocyte abundance enhances clearance of E. coli at lower levels of initial infection
Given previous evidence showing that 1) granulocytes phagocytize several species of bacteria including Escherichia coli [13], [15], and 2) age-dependent reductions in hemocyte abundance correlate with increased susceptibility to E. coli infection [13], we asked whether increases in hemocyte abundance following blood feeding enhances clearance of bacteria. One cohort of females was pretreated with dsMIR RNA while another was treated with dsEGFP which served as both an RNAi and wounding control. Two other cohorts were left untreated. We blood fed on day 4 the dsMIR and dsEGFP-treated cohorts and one of the untreated cohorts followed 24 h later by infection of mosquitoes in each treatment with three different amounts of streptomycin-resistant E. coli (1×103, 104 or 105 colony forming units (CFUs). This resulted in the following treatments: BF, NBF, BF-dsEGFP injected, and BF-dsMIR injected. We then determined the number of surviving bacteria at 0, 3, and 24 h post-injection by homogenizing each mosquito and culturing the supernatant on streptomycin-containing BHI plates. Overall, the number of bacteria recovered per mosquito varied significantly among treatments, infection dose, and sample time (Kruskal-Wallis test: X2 = 166.3; df = 35; P = 0.0001) (Fig. 5). Focusing on the lowest infection dose (1×103 CFUs) and 24 h sampling time, our results indicated that bacterial survival varied significantly among treatments (X2 = 11.0; df = 3; P = 0.012) (Fig. 5A–D). Closer inspection of these data showed that clearance of bacteria by BF and BF-dsEGFP females did not differ from one another (X2 = 0.01; df = 1; P = 0.9) while clearance of bacteria by NBF and BF-dsMIR females also did not differ (X2 = 0.03; df = 1; P = 0.8). In contrast, BF and BF-dsEGFP females cleared significantly more bacteria than NBF and BF-dsMIR females (X2 = 10.6; df = 1; P = 0.0012). Taken together, these results argued that increased production of hemocytes by BF and BF-dsEGFP females enhanced clearance of bacteria while pretreatment with dsMIR reduced hemocyte production and associated clearance of bacteria by BF females to similar levels as NBF females. These data also suggested that wounding at the time of dsRNA injection had no effect on the outcome of these assays. At the intermediate infection dose we tested (1×104 CFUs), the number of surviving bacteria after 24 h was also lower but no differences were detected among treatments (X2 = 0.68; df = 3; P = 0.88) (Fig. 5E–H), while at the highest dose (1×105 CFUs) the number of surviving bacteria at 24 h had similarly increased in each treatment (X2 = 3.8; df = 3; P = 0.29) (Fig. 5I–L). Inspection of hemocytes from mosquitoes across all treatments and infection doses showed that most granulocytes contained numerous internalized bacteria at both 3 and 24 h post-infection (data not presented). The data presented in Fig. 5, however, also showed that no mosquitoes fully cleared the infection by 24 h at any of the three infection doses tested.
Fig. 5. Survival of E. coli in A. aegypti varies with feeding and initial infection dose. 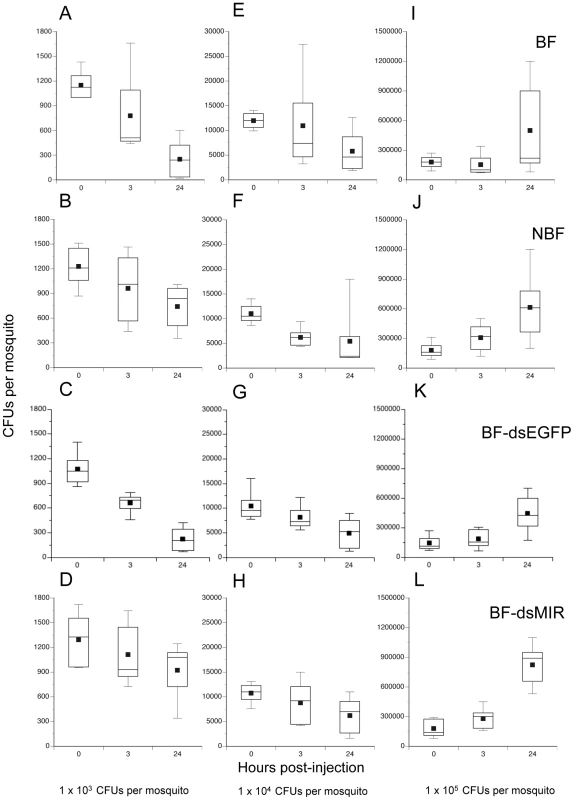
BF females (first row), NBF females (second row), BF females pretreated with dsEGFP (BF-dsEGFP) (third row), and BF females pretreated with dsMIR RNA (BF-dsMIR) (fourth row) were fed on day 4, and then infected 24 h pbm (day 5) with 1×103 (left column), 1×104 (middle column), or 1×105 (right column) CFUs of E. coli. Females from each treatment and infection dose were then processed to determine the CFUs of surviving bacteria at 0, 3 or 24 h post-infection. (A) BF females infected with 1×103 CFUs of E. coli. (B) NBF females infected with 1×103 CFUs of E. coli. (C) BF-dsEGFP females infected with 1×103 CFUs of E. coli. (D) BF-dsMIR females infected with 1×103 CFUs of E. coli. (E) BF females infected with 1×104 CFUs of E. coli. (F) NBF females infected with 1×104 CFUs of E. coli. (G) BF-dsEGFP females infected with 1×104 CFUs of E. coli. (H) BF-dsMIR females infected with 1×104 CFUs of E. coli. (I) BF-dsMIR females infected with 1×105 CFUs of E. coli. (J) NBF females treated with 1×105 CFUs of E. coli. (K) BF-dsEGFP females treated with 1×105 CFUs of E. coli. (L) BF-dsMIR females treated with 1×105 CFUs of E. coli. Each graph shows a stem and leaf plot with the lower and upper quartiles indicated by the large box. Mean survival is indicated by the small black square in the box, a horizontal bar indicates the median, and vertical lines extending from each box indicate the most extreme values. Blood fed mosquitoes are more susceptible to E. coli at higher levels of infection
We assessed the relationship between clearance of E. coli by BF and NBF females and survival by monitoring mosquito mortality for 6 days post-infection. Fig. 6 shows that nearly all BF and NBF females survived infection by 1×103 CFUs. However, analysis across all three levels of infection using the proportional hazards model revealed a strongly significant difference in the death rate of BF and NBF females (X2 = 27.4; df = 3; P<0.0001) (Fig. 6). Effect likelihood ratio tests confirmed, as expected, that death rate increased with infection dose (X2 = 19.1; df = 1; P<0.0001), but also showed that BF females died at higher rates than NBF females (X2 = 11.3; df = 1; P<0.0001) (Fig. 6). Thus, few NBF females died after 6 days when infected with 1×104 CFUs of E. coli, whereas the same dose resulted in more than 50% of BF females dying within 2 days of infection. Similarly, infection with 1×105 CFUs resulted in only 20% mortality of NBF females after 2 days and 50% mortality after 6 days. In contrast, the same dose of E. coli killed 75% of BF females within 2 days and 95% of females after 6 (Fig. 6). Since most phagocytosis of E. coli occurs within the first 24 h of infection [13] and bacterial survival after 24 h positively correlates with initial infection dose (Fig. 5), the data presented in Fig. 6 suggested that differential survival of BF and NBF females reflected an asymmetric response to surviving bacteria that hemocytes failed to clear.
Fig. 6. Survival curves (± 1 SE) for BF and NBF females fed on day 4 and infected 24 h pbm (day 5) with different doses of E. coli. 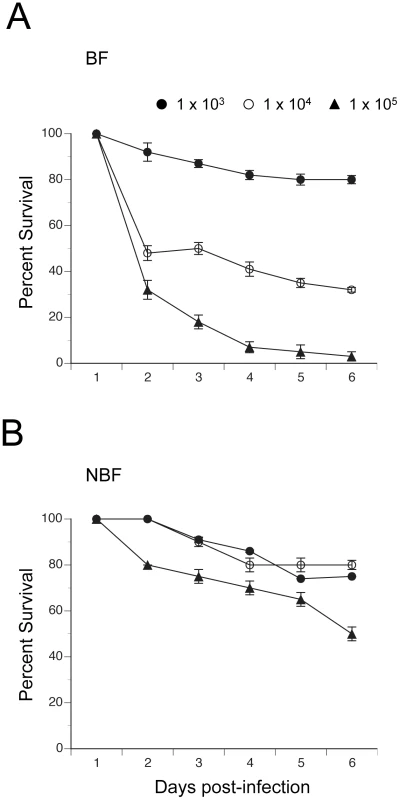
(A) BF females infected with 1×103, 1×104 or 1×105 CFUs of E. coli. (B) NBF females infected with 1×103, 1×104 or 1×105 CFUs of E. coli. Discussion
Most studies of the mosquito immune system focus on defense responses following systemic infection with bacteria or consumption of a pathogen-infected blood meal [11], [17], [32], [33]. In A. aegypti, for example, bacterial infection of the hemocoel elicits a phagocytic response by hemocytes followed by inducible expression of antimicrobial peptides [13], [21], while entry into the hemocoel from the gut by filarial nematodes activates cellular and melanotic defenses [34]. In the malaria vector Anopheles gambiae, passage of Plasmodium to the hemolymph-bathed basal side of the midgut exposes the parasite to complement-like thioester-containing proteins from hemocytes that contribute to parasite killing [18]. Plasmodium infection also damages the peritrophic matrix that surrounds the blood meal, resulting in commensal bacteria interacting with the midgut, which in turn stimulates constitutive and inducible defense responses against both bacteria and Plasmodium [24], [35]–[37].
Far less is known about how mosquitoes invest in immune defenses in the absence of infection. We reasoned that the unique requirement of vector species to blood feed also creates a cyclical pattern of increased nutrient availability and infection risk that could favor cyclical investment in constitutive defenses like hemocytes. Our results support this hypothesis by showing that the number of hemocytes recovered from females increases 40% by 24 pbm. Our results further reveal a previously unrecognized role for the insulin signaling pathway in regulating the cellular arm of the mosquito immune system and synchronizing increased production of hemocytes with reproduction.
The progressive, age-dependent decline in hemocyte abundance that occurs in the absence of blood feeding suggested to Hillyer and coworkers [13] that hemocytes do not proliferate in adult mosquitoes. However, our BrdU labeling experiments indicate that actively cycling hemocytes exist in both NBF and BF females, while the significant rise in BrdU-labeled hemocytes following a blood meal strongly suggests that increases in hemocyte abundance are due at least in part to proliferation of phagocytic granulocytes. The longer term priming experiments of Rodriguez et al. [24] suggest that granulocytes also proliferate in adult female A. gambiae. However, the average number of hemocytes per individual we and others recover from A. aegypti and A. gambiae [13], [15] is much lower than the number of hemocytes per mosquito reported by Rodriguez et al. [24]. Entry of sessile hemocytes into circulation could also contribute to the increase in hemocyte abundance after blood feeding given evidence that mobilization of sessile hemocytes can elevate the abundance of circulating hemocytes in other insects [38]. Our use of an anticoagulant to collect hemocytes, however, reduces adhesion of mosquito hemocytes and likely enhances collection of both circulating and sessile cells. Thus, the increased numbers of hemocytes recovered from BF females is likely not due to NBF females containing more sessile hemocytes that we failed to recover.
Unlike mammals, which produce both insulin and insulin-like growth factors that preferentially interact with different receptors, A. aegypti and other insects encode only a single receptor tyrosine kinase (IR) that functions as the receptor for multiple ILP family members [2], [3]. Our own studies indicate that ILP3 exhibits both metabolic (insulin-like) and growth-promoting (insulin growth factor-like) activity in regard to regulating egg development [2], [3], [6]. Our finding during the current study that granulocytes and oenocytoids express the MIR is also consistent with the suggestion that mosquito hemocytes proliferate in direct response to ILP3. The ILP bombyxin has likewise been implicated in stimulating hemocyte proliferation in the silkmoth, Bombyx mori [39], while studies with mammals implicate insulin growth factor-like signaling in proliferation of lymphocytes [40]–[42]. A recent study also implicates a cell surface protein designated crustacean hematopoietic factor (CHF) in survival of certain hemocyte types from the crayfish Pacifastacus leniusculus [43]. Interestingly, CHF contains an insulin growth factor binding protein domain, but it remains unknown whether CHF interacts with any crustacean ILP [43]. The absence of MIR expression and failure to detect any BrdU labeling of prohemocytes suggests that the increases in granulocyte abundance that occur after blood feeding are likely due to proliferation of granulocytes themselves and not the differentiation of prohemocytes into granulocytes. It remains unclear whether prohemocytes proliferate during other life stages but overall the results of the current study do not provide any evidence that the circulating cells named prohemocytes in prior studies [15], [17], [24] have a progenitor function in adult Ae. aegypti.
We recognize our in vivo experiments leave open the possibility that the effects of ILP3 on hemocyte proliferation could be indirect. That is, ILP-stimulated nutrient acquisition following blood feeding could be responsible for increased proliferation of hemocytes. Unfortunately, A. aegypti hemocytes are labile in primary culture and we currently are unable to maintain them for a long enough period to evaluate whether ILP3 directly stimulates proliferation in vitro. However, we do note that injection of ILP3 (50 pmol) into NBF females stimulates a significant increase in hemocyte abundance relative to NBF controls after 24 h (data not presented). This increase is smaller than the increase observed in decapitated BF females (Fig. 4), but these results strongly suggest that ILP3-stimulated proliferation of hemocytes is not solely due to increased availability of nutrients from digestion of the blood meal.
In vertebrates, activation of nuclear hormone receptors by their steroid hormone ligands affects both adaptive and innate immune responses [40], [44], [45]. Much less is known about the role of steroid hormones in regulating the insect immune system although 20E does up-regulate expression of a prophenoloxidase gene in an A. gambiae cell line of hemocyte origin [46] and selected antimicrobial peptide genes in the S2 and mbn-2 cell lines from Drosophila [47], [48]. 20E also stimulates proliferation of mbn-2 cells and phagocytosis by Drosophila hemocytes in vivo [49], [50]. As previously noted, release of ILP3 from the brain after blood feeding strongly upregulates the synthesis and release of 20E and other ECDs by the ovaries [2], which then interact with ILPs and TOR signaling to regulate the enzymes that digest the blood meal and yolk protein expression by the fat body [6], [7]. However, results of the current study indicate that 20E has no stimulatory effect on proliferation of hemocytes in A. aegypti.
We anticipated that blood feeding would elevate resistance to at least some potential pathogens through an increase in the number of phagocytic granulocytes. We examined this possibility using E. coli because earlier studies showed that A. aegypti granulocytes readily phagocytize this bacterium [12], [15], [17], while also showing that E. coli elicits a dose-dependent mortality response [13]. Consistent with expectations, our results indicate that BF females clear more bacteria than NBF females at the lowest level of infection (103 CFUs per female) we tested. Our results showing that pretreatment of BF females with dsMIR reduces bacterial clearance to NBF levels also supports the conclusion that enhanced clearance by BF females is due to increased numbers of granulocytes. No difference in clearance of bacteria was detected among treatments at higher levels of infection. However, this outcome was not entirely unexpected given that hemocyte abundance after blood feeding increases less than 2-fold but females were injected with 10 or 100-fold more bacteria. In effect, infection by 104 or 105 E. coli exceeded the phagocytic capacity of the granulocytes present in each treatment resulting in higher numbers of surviving bacteria after 24 h. Although Hillyer et al. [13] estimated that A. aegypti granulocytes can phagocytize more than 1000 E. coli per cell, they also detected levels of surviving bacteria 24 h and 5 days post-infection that are very similar to our results with NBF females suggesting substantial numbers of bacteria evaded phagocytosis in vivo when mosquitoes were infected with 104 or more cells.
In contrast, we did not anticipate that NBF females would better survive intermediate and high levels of E. coli infection than BF females. When viewed together, the results presented in Fig. 5 and 6 indicate that blood feeding enhances resistance at the lowest level of infection we tested as measured by reduced survival of E. coli. However, at higher levels of initial infection blood feeding reduces tolerance to E. coli which is defined in the insect immunity literature as the ability to survive infection at a given pathogen load [51]. Thus, while hemocytes clear substantial numbers of bacteria in the early stages of infection, surviving bacteria adversely affect the survival of BF females more than NBF females. Resistance and tolerance in insects have previously been shown to vary with host genotype and pathogen species [52]–[54]. Our results add to this literature by showing that resistance and tolerance to the same pathogen dramatically changes in A. aegypti as a consequence of blood meal digestion and/or mobilization of resources for reproduction.
The mechanism(s) underlying reduced tolerance to E. coli in BF females remains unclear. In Drosophila, both bacterial infection and infection-independent activation of the Toll signaling pathway suppresses insulin signaling which reduces energy stores but enhances tolerance by potentially increasing investment in inducible defenses under Toll regulation [55], [56]. While blood feeding activates insulin signaling and other processes related to yolk protein production in A. aegypti [2], [7], [8], studies in A. gambiae indicate that the presence of Vg diminishes killing of Plasmodium [57]. Thus, our finding of diminished tolerance could reflect either the effects of bacterially induced NF-kB signaling, the effects of Vg interfering with killing of bacteria, or changes in allocation of nutritional resources that adversely impact tolerance. Interestingly, activation of NF-kB signaling following Plasmodium infection also reduces Vg expression in A. gambiae [57], which in light of the aforementioned studies with Drosophila could reflect an indirect response mediated by reduced insulin signaling. A second possibility for reduced tolerance is that metabolism of vertebrate blood is both energetically expensive and generates toxic compounds like heme or ammonia that must be inactivated [58]. Recent findings by Oliveira et al. [59], for example, show that blood meal-derived heme reduces production of reactive oxygen species (ROS) in the midgut of A. aegypti, which normally regulates growth of gut microbiota but upon down regulation allows the gut microbiota to greatly increase in abundance. It is unlikely reduced ROS production in the midgut affected the systemic infection of the hemocoel we generated in our experiments. However, these results do suggest other processes related to tolerance could be adversely affected by processing the blood meal.
Zdroje
1. SieglaffDHDuncanKABrownMR 2005 Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochem Mol Biol 3 471 490
2. BrownMRClarkKDGuliaMZhaoZGarczynskiSF 2008 An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA 105 5716 5721
3. WenZGuliaMClarkKDDharaACrimJW 2010 Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol 328 47 55
4. BrandonMCPenningtonJEIsoeJZamoraJSchillingerAS 2008 TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect Biochem Mol Biol 38 916 922
5. IsoeJRascónAAJrKunzSMiesfeldRL 2009 Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 39 903 912
6. Gulia-NussMRobertsonAEBrownMRStrandMR 2011 Insulin-like peptides and the Target of Rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. Plos One 6 e20401
7. AttardoGMHansenIARaikhelAS 2005 Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol 35 661 675
8. RoySGHansenIARaikhelAS 2007 Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol 37 1317 1326
9. HaineERMoretYSiva-JothyMTRolffJ 2008 Antimicrobial defense and persistent infection in insects. Science 322 1257 1259
10. StrandMR 2008 Insect hemocytes and their role in immunity. BeckageNE Insect Immunology San Diego Academic Press 25 48
11. HillyerJF 2009 Transcription in mosquito hemocytes in response to pathogen exposure. J Biol 8 e51
12. HillyerJFSchmidtSLChristensenBM 2003 Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J Parasitol 89 62 69
13. HillyerJFSchmidtSLFuchsJFBoyleJPChristensenBM 2005 Age-associated mortality in immune challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell Microbiol 7 39 51
14. MoitaLFWang-SattlerRMichelKZimmermannTBlandinS 2005 In vivo identification of novel regulators and conserved pathways of phagocytosis in A. gambiae. Immunity 23 65 73
15. CastilloJCRobertsonAEStrandMR 2006 Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem Mol Biol 36 891 903
16. MoitaCSimõesSMoitaLFJacintoAFernandesP 2005 The cadherin superfamily in Anopheles gambiae: a comparative study with Drosophila melanogaster. Comp Funct Genomics 6 204 216
17. BatonLARobertsonAWarrEStrandMRDimopoulosG 2009 Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics 10 257
18. BlandinSShiaoSHMoitaLFJanseCJWatersAP 2004 Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 661 670
19. VlachouDSchlegelmilchTChristophidesGKKafatosFC 2005 Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Curr Biol 15 1185 1195
20. AbrahamEGPintoSBGhoshAVanlanginghamDLBuddA 2005 An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci U S A 102 16327 16332
21. BartholomayLCMayhewGFFuchsJFRocheleauTAEricksonSM 2007 Profiling infection responses in the haemocytes of the mosquito, Aedes aegypti. Insect Mol Biol 16 761 76
22. BlandinSAMaroisELevashinaEA 2008 Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host Microbe 3 364 374
23. PintoSBLombardoFKoutsosACWaterhouseRMMcKayK 2009 Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc Natl Acad Sci U S A 106 21270 21275
24. RodriguesJBraynerFAAlvesLCDixitRBarillas-MuryC 2010 Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329 1353 1355
25. LavineMDStrandMR 2003 Haemocytes from Pseudoplusia includens express multiple alpha and beta integrin subunits. Insect Mol Biol 12 441 452
26. GardinerEMStrandMR 1999 Monoclonal antibodies bind distinct classes of hemocytes in the moth Pseudoplusia includens. J Insect Physiol 45 113 126
27. GrafRLeaAOBriegelH 1998 A temporal profile of the endocrine control of trypsin synthesis in the yellow fever mosquito, Aedes aegypti. J Insect Physiol 44 451 454
28. RiehleMAFanYCaoCBrownMR 2006 Molecular characterization of insulin-like peptides in the yellow fever mosquito Aedes aegypti: expression, cellular localization, and phylogeny. Peptides 27 2547 2560
29. TrumanJWBateM 1988 Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol 125 145 157
30. GardinerEMMStrandMR 2000 Hematopoiesis in larval Pseudoplusia includens and Spodoptera frugiperda. Arch Insect Biochem Physiol 43 147 164
31. GordonSDStrandMR 2009 The polyembryonic wasp Copidosoma floridanum produces two castes by differentially parceling the germ line to daughter embryos during embryo proliferation. Dev Genes Evol 219 445 454
32. LowenbergerC 2001 Innate immune response of Aedes aegypti. Insect Biochem Mol Biol 31 219 229
33. BartholomayLCWaterhouseRMMayhewGFCampbellCLMichelK 2010 Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 330 88 90
34. ChristensenBMFortonKF 1986 Hemocyte-mediated melanization of microfilariae in Aedes aegypti. J Parasitol 72 220 225
35. HuberMCabibEMillerLH 1991 Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc Natl Acad Sci U S A 88 2807 2810
36. DongYDimopoulosG 2009 Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem 284 9835 9844
37. MeisterSAgianianBTurlureFRelógioAMorlaisI 2009 Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog 5 e1000542
38. MárkusRLaurinyeczBKuruczEHontiVBajuszI 2009 Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci USA 106 4805 4809
39. NakaharaYMatsumotoHKanamoriYKataokaHMizoguchiA 2006 Insulin signaling is involved in hematopoietic regulation in an insect hematopoietic organ. J Insect Physiol 52 105 111
40. WebsterJITonelliLSternbergEM 2002 Neuroendocrine regulation of immunity. Ann Rev Immunol 20 125 163
41. WhelanJTLudwigDLBertrandFE 2008 HoxA9 induces insulin-like growth factor-1 receptor expression in B-lineage acute lymphoblastic leukemia. Leukemia 22 1161 1169
42. NagatomoTMutaKOhgaSOchiaiMOhshimaK 2008 Insulin-like growth factor-II: a novel autocrine growth factor modulating the apoptosis and maturation of umbilical cord blood erythroid progenitors. Exp Hematol 36 401 11
43. LinXSöderhällKSöderhällI 2011 Invertebrate hematopoiesis: an astakine-dependent novel hematopoietic factor. J Immunol 186 2073 2079
44. GlassCKOgawaS 2006 Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6 44 55
45. ChowEKHRazaniBChengG 2007 Innate immune system regulation of nuclear hormone receptors in metabolic diseases. J Leukoc Biol 82 187 195
46. MüllerHMDimopoulosGBlassCKafatosFC 1999 A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem 274 11727 11735
47. SilvermanNZhouRStövenSPandeyNHultmarkD 2000 A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev 14 2461 2471
48. FlattTHeylandARusFPorpigliaESherlockC 2008 Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J Exp Biol 211 2712 24
49. DimarcqJLImlerJLLanotREzekowitzRABHoffmanJA 1997 Treatment of I(2)mbn Drosophila tumorous blood cells with the steroid hormone ecdysone amplifies the inducibility of antimicrobial peptide gene expression. Insect Biochem Mol Biol 27 877 886
50. LanotRZacharyDHolderFMeisterM 2001 Postembryonic hematopoiesis in Drosophila. Dev Biol 230 243 257
51. SchneiderDSAyresJS 2008 Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8 889 895
52. Corby-HarrisVHabelKEAliFGPromislowDE 2007 Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J Evol Biol 20 526 533
53. RåbergLSimDReadAF 2007 Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318 812 814
54. AyersJSSchneiderDS 2008 A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol 6 2764 2773
55. DionneMSPhamLNShirasu-HizaMSchneiderDS 2006 Akt and foxo dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol 16 1977 1985
56. DiAngeloJRBlandMLBambinaSCherrySBirnbaumMJ 2009 The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A 106 20853 20858
57. RonoMKWhittenMMAOulad-AbdelghaniMLevashinaEAMaroisE 2010 The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol 8 e1000434
58. ZhouGScaraffiaPYWellsMA 2005 Vector nutrition and energy metabolism. MarquardtWC Biology of disease vectors New York Elsevier 311 327
59. OliveiraJHMGoncalvesRLSLaraFADiasFAGandaraACP 2011 Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Path 7 e1001320
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



