-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
A subset of patients with stable asthma has prominent neutrophilic and reduced eosinophilic inflammation, which is associated with attenuated airways hyper-responsiveness (AHR). Haemophilus influenzae has been isolated from the airways of neutrophilic asthmatics; however, the nature of the association between infection and the development of neutrophilic asthma is not understood. Our aim was to investigate the effects of H. influenzae respiratory infection on the development of hallmark features of asthma in a mouse model of allergic airways disease (AAD). BALB/c mice were intraperitoneally sensitized to ovalbumin (OVA) and intranasally challenged with OVA 12–15 days later to induce AAD. Mice were infected with non-typeable H. influenzae during or 10 days after sensitization, and the effects of infection on the development of key features of AAD were assessed on day 16. T-helper 17 cells were enumerated by fluorescent-activated cell sorting and depleted with anti-IL-17 neutralizing antibody. We show that infection in AAD significantly reduced eosinophilic inflammation, OVA-induced IL-5, IL-13 and IFN-γ responses and AHR; however, infection increased airway neutrophil influx in response to OVA challenge. Augmented neutrophilic inflammation correlated with increased IL-17 responses and IL-17 expressing macrophages and neutrophils (early, innate) and T lymphocytes (late, adaptive) in the lung. Significantly, depletion of IL-17 completely abrogated infection-induced neutrophilic inflammation during AAD. In conclusion, H. influenzae infection synergizes with AAD to induce Th17 immune responses that drive the development of neutrophilic and suppress eosinophilic inflammation during AAD. This results in a phenotype that is similar to neutrophilic asthma. Infection-induced neutrophilic inflammation in AAD is mediated by IL-17 responses.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002244
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002244Summary
A subset of patients with stable asthma has prominent neutrophilic and reduced eosinophilic inflammation, which is associated with attenuated airways hyper-responsiveness (AHR). Haemophilus influenzae has been isolated from the airways of neutrophilic asthmatics; however, the nature of the association between infection and the development of neutrophilic asthma is not understood. Our aim was to investigate the effects of H. influenzae respiratory infection on the development of hallmark features of asthma in a mouse model of allergic airways disease (AAD). BALB/c mice were intraperitoneally sensitized to ovalbumin (OVA) and intranasally challenged with OVA 12–15 days later to induce AAD. Mice were infected with non-typeable H. influenzae during or 10 days after sensitization, and the effects of infection on the development of key features of AAD were assessed on day 16. T-helper 17 cells were enumerated by fluorescent-activated cell sorting and depleted with anti-IL-17 neutralizing antibody. We show that infection in AAD significantly reduced eosinophilic inflammation, OVA-induced IL-5, IL-13 and IFN-γ responses and AHR; however, infection increased airway neutrophil influx in response to OVA challenge. Augmented neutrophilic inflammation correlated with increased IL-17 responses and IL-17 expressing macrophages and neutrophils (early, innate) and T lymphocytes (late, adaptive) in the lung. Significantly, depletion of IL-17 completely abrogated infection-induced neutrophilic inflammation during AAD. In conclusion, H. influenzae infection synergizes with AAD to induce Th17 immune responses that drive the development of neutrophilic and suppress eosinophilic inflammation during AAD. This results in a phenotype that is similar to neutrophilic asthma. Infection-induced neutrophilic inflammation in AAD is mediated by IL-17 responses.
Introduction
Asthma is a complex disease of the airways that is generally characterized by symptoms of wheeze, cough, breathlessness and airway inflammation. While eosinophilic inflammation has been considered to be the hallmark of airway inflammation in asthma [1], [2], it is present in only 50% of asthmatics [3]. Non-eosinophilic asthma has now been described in persistent [4], [5] and severe asthma, [6] as well as in steroid naïve asthma [7]. Further investigation of the non-eosinophilic subtype has identified a subgroup with an intense neutrophilic bronchitis [5], [8] with increased interleukin (IL)-8 [4]. Compared to eosinophilic asthmatics, neutrophilic asthmatics have reduced eosinophilic inflammation and AHR. Furthermore, they are frequently resistant to corticosteroid treatment, which results in a significant proportion of asthma-related health care costs [5], [8], [9], [10], [11], [12]. IL-17 is also elevated in asthma and other obstructive airway diseases that are characterized by increased neutrophils [13], [14], [15], [16].
IL-8 and IL-17 are important mediators of neutrophilic inflammation during infection and in disease states [4], [12], [13], [17], [18] and their elevated expression in neutrophilic asthma correlates with increased levels of neutrophils in sputum [15]. IL-8 is a potent neutrophil chemoattractant, produced by macrophages, lymphocytes, epithelial cells and neutrophils [19], [20]. IL-17 is produced by several cells including Th17 cells [21], [22], [23], γδ T cells [24], [25], neutrophils [26], and macrophages [27], [28]. IL-17 has critical roles in host defence against bacterial infections [29], [30], [31], [32], suggesting a potential role in the pathogenesis of bacterial-induced neutrophilic asthma.
Chronic bacterial colonization is evident in the airways of patients with neutrophilic asthma [12] and is also associated with an intense neutrophilic bronchitis in asthma [33]. H. influenzae is a common bacterium of the respiratory tract, is one of the bacteria most frequently isolated from the airways of neutrophilic asthmatics [12], [33], and often causes recurrent respiratory disease [34], [35], [36] in those with compromised airways. Nevertheless, how H. influenzae is associated with the pathogenesis of neutrophilic asthma is unknown. Specifically, whether infection promotes the pathogenesis of neutrophilic asthma, or if neutrophilic asthmatics are predisposed to infection is not known.
In this study we used murine models of H. influenzae infection and OVA-induced allergic airways disease, which mimics hallmark features of human asthma, to elucidate the potential association between infection and the development of neutrophilic asthma.
Results
Non-typeable Haemophilus influenzae infection
In order to investigate the association between H. influenzae lung infection and asthma we first established and characterized a murine model of NTHi lung infection alone. Inoculation intratracheally (i.t.) with 5x105 CFU of NTHi resulted in a mild respiratory infection that induced inflammatory responses but did not significantly affect lung function (Figure 1).
Fig. 1. Characterization of NTHi infection. 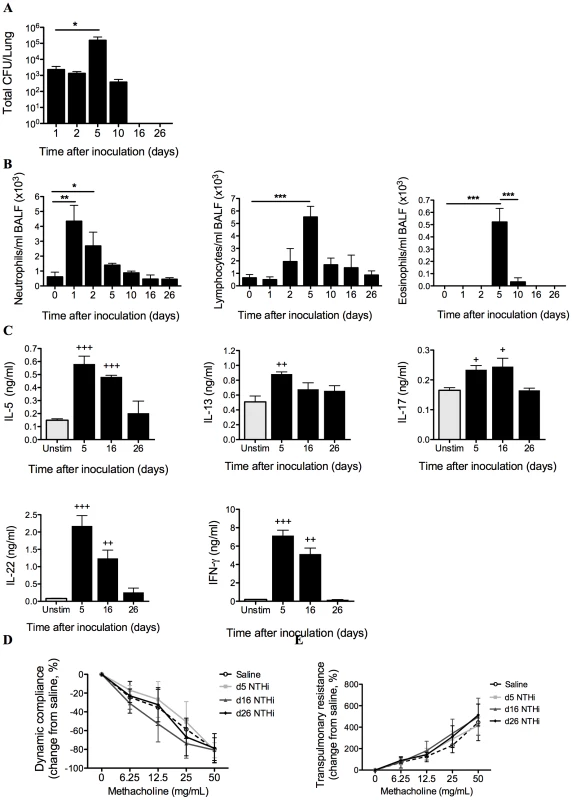
The profile of infection was assessed in mice that only received NTHi (i.e. not OVA), by performing a time-course of bacterial recovery from BALF and lung homogenates (A), and airway inflammation represented by BALF neutrophils, lymphocytes and eosinophils (B). IL-5, IL-13, IL-17, IL-22 and IFN-γ (C) release from MLN T cells stimulated with killed NTHi compared to unstimulated cells was also determined. Lung function in terms of AHR (dynamic compliance (D) and transpulmonary resistance (E)) in response to increasing doses of methacholine was assessed 5, 16 and 26 days after inoculation. N.B. P values for compliance and resistance were determined for the entire dose response curve, *** p<0.001, ** p<0.01, * p<0.05 compared to indicated time points, +++ p<0.001, ++ p<0.01, + p<0.05 compared to unstimulated controls. Bacterial numbers in bronchoalveolar lavage fluid (BALF) and lung homogenates peaked at 5 days and were cleared 16 days after inoculation (Figure 1A). NTHi infection induced airway inflammation. Neutrophil influx into the airways peaked at 24 hours post-infection while lymphocytes and eosinophils were significantly increased at 5 days. Neutrophil numbers returned to baseline after 5 days, while lymphocyte and eosinophil numbers returned to baseline after 10 days post-infection (Figure 1B). Infection also induced significant but low level increases in NTHi-induced IL-5, IL-13, IL-17 and IL-22, and higher levels of IFN-γ release from mediastinal lymph nodes (MLN) cultures after 5 days, which returned to baseline levels after 26 days (Figure 1C). Infection did not affect lung function, with no changes in AHR (dynamic compliance or transpulmonary resistance in response to increasing doses of methacholine) compared to sham infected (Saline) controls 5, 16 and 26 days after inoculation (Figure 1D–E).
NTHi infection suppresses key features of Th2-driven eosinophilic AAD
To investigate the effect of infection on AAD in sensitized animals, groups were infected during (d0 NTHi+OVA) or 10 days after (d10 NTHi+OVA) OVA sensitization (Figure 2A), and AAD assessed on day 16. Infection suppressed OVA-induced T cell cytokine responses, inflammatory cell influx and AHR in AAD (Figure 2).
Fig. 2. NTHi infection suppressed key features of Th2-driven eosinophilic AAD. 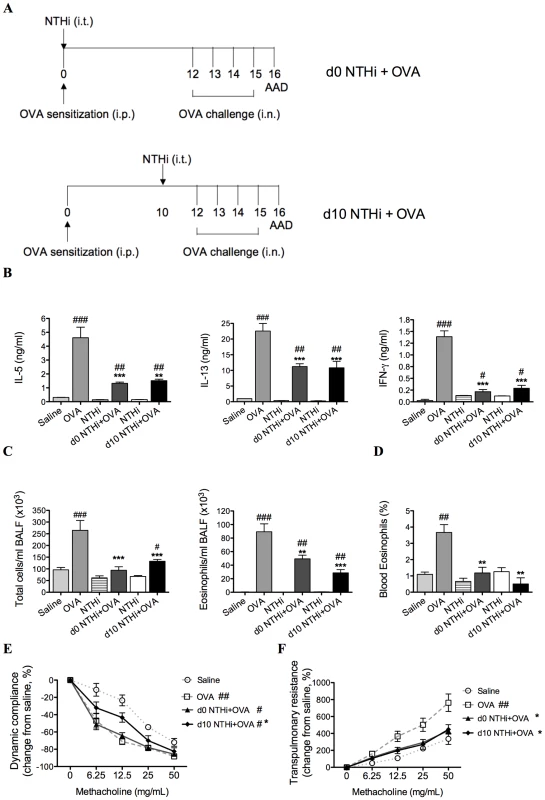
Groups of mice were infected during (d0 NTHi+OVA) or 10 days after (d10 NTHi+OVA) OVA sensitization (A), and AAD was analyzed (on day 16). The effects of infection on IL-5, IL-13, and IFN-γ (B) release from MLN T cells stimulated with OVA, BALF total cell and eosinophil counts (C) and the percentage of blood eosinophils (D) were assessed. AHR in terms of dynamic compliance (E) and transpulmonary resistance (F) was determined (see figure 1D-E for infection only AHR results). Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. N.B. P values for compliance and resistance were determined for the entire dose response curve, ### p<0.001, ## p<0.01, # p<0.05 compared to Saline controls, *** p<0.001, ** p<0.01, * p<0.05 compared to OVA controls. The development of AAD (OVA groups) resulted in increased OVA-induced release of IL-5, IL-13 and IFN-γ from MLN and splenic T cells, eosinophilic inflammation and AHR (decreased compliance and increased resistance in response to methacholine), compared to uninfected, nonallergic (Saline) controls (Figure 2B–F and Figure 3). Infection during (d0 NTHi+OVA) or after (d10 NTHi+OVA) sensitization resulted in significant reductions in OVA-induced IL-5, IL-13 and IFN-γ release from MLN T cells (Figure 2B), compared to uninfected, allergic (OVA) controls. Infection also significantly reduced the numbers of total cells and eosinophils in the airways and blood (Figure 2C-D). The reduction in eosinophils correlated with the reduced release of IL-5 from MLN T cells. Infection significantly suppressed, but did not abolish AHR in AAD. Infection during sensitization (d0 NTHi+OVA) had no effect on compliance, but significantly reduced resistance of the lungs. However, infection after sensitization (d10 NTHi+OVA), significantly suppressed both compliance and resistance (Figure 2E–F). Notably, compliance remained decreased in infected, allergic (NTHi+OVA) groups compared to uninfected, nonallergic (Saline) controls.
Fig. 3. NTHi infection suppressed systemic IL-13 and IFN-γ responses in AAD. 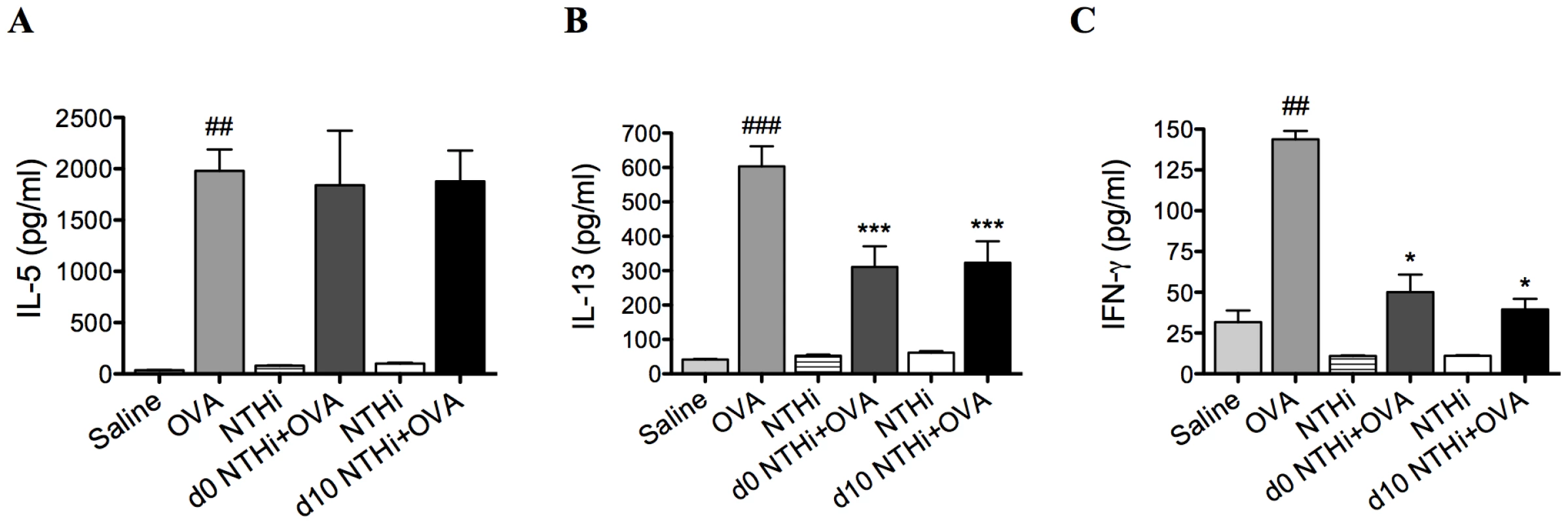
Groups of mice were infected during (d0 NTHi+OVA) or 10 days after (d10 NTHi+OVA) OVA sensitization, and AAD was analyzed (on day 16). The effect of infection on IL-5 (A), IL-13 (B), and IFN-γ (C) release from splenocytes stimulated with OVA was assessed. Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ### p<0.001, ## p<0.01 compared to Saline controls, *** p<0.001, * p<0.05 compared to OVA controls. NTHi infection suppresses systemic responses in AAD
Infection during (d0 NTHi+OVA) or after (d10 NTHi+OVA) sensitization had no effect on systemic IL-5 (Figure 3A) but significantly reduced systemic IL-13 and IFN-γ release from splenocytes (Figure 3B–C), compared to uninfected, allergic (OVA) controls.
T regulatory cells (Tregs) are not involved in the suppression of AAD
To determine if Tregs were involved in the suppression of AAD, Tregs, TGF-β and IL-10 were quantified on day 16 of the model. TGF-β and IL-10 are critical immunosuppressive factors that are produced by Tregs. NTHi infection during and after sensitization did not alter the numbers of Tregs (Figure 4A) in the lung, compared to uninfected allergic controls. Notably, infection decreased the expression of TGF-β (Figure 4B) and IL-10 (Figure 4C) in lung tissue in infected, allergic groups compared to uninfected, allergic controls.
Fig. 4. NTHi infection does not affect T regulatory cells in the suppression of AAD. 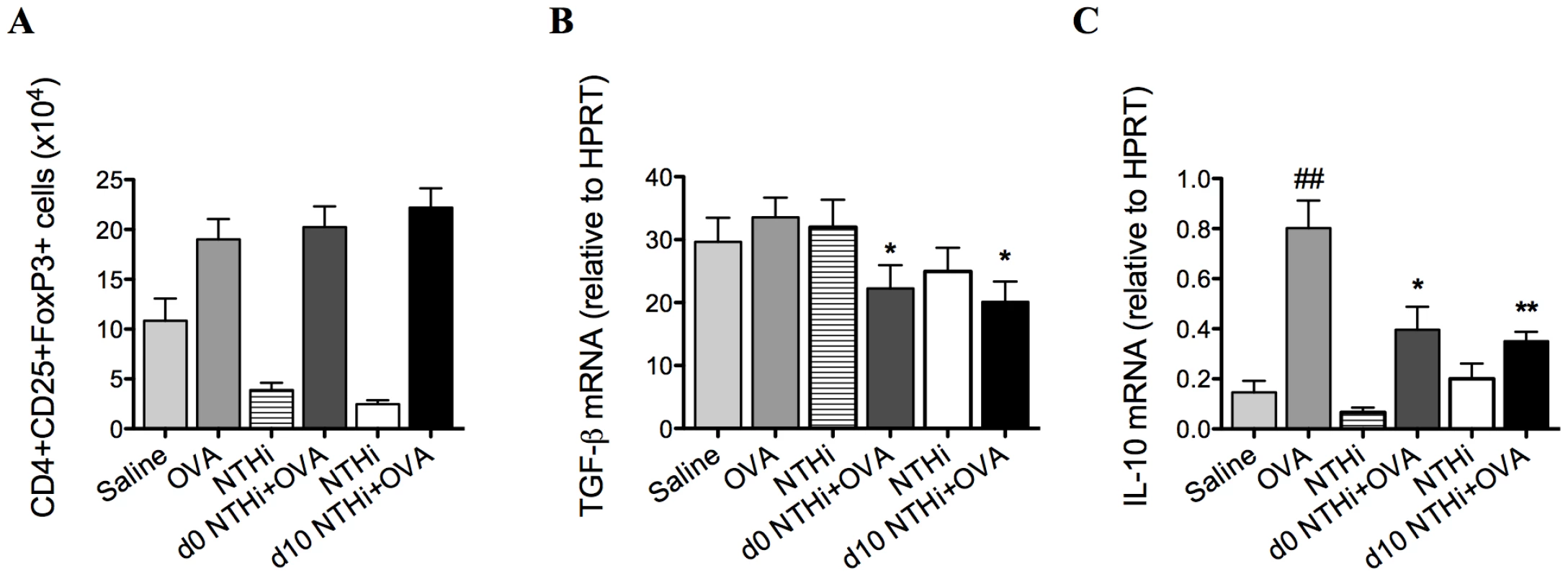
Treg numbers in the lung (A), TGF-β (B) and IL-10 (C) mRNA expression in lung tissue were assessed (on day 16). Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ## p<0.01, compared to Saline controls, ** p<0.01, * p<0.05 compared to OVA controls. Infection reduced markers of antigen presentation and co-stimulation in the suppression of AAD
To determine if alterations in antigen-presenting cells were involved in the suppression of AAD, the effect of infection on MHCII and CD86 expressing DCs was also investigated (on day 16). The development of AAD resulted in increases in the numbers and proportions of MHCII expressing plasmacytoid DCs (pDCs) and myeloid DCs (mDCs), and CD86 expressing MHCII+ pDCs and mDCs in MLNs and lungs (Figure 5A–H), compared to uninfected, nonallergic controls. Infection during or after sensitization resulted in significant decreases in MHCII and CD86 expressing pDCs and mDCs compared to uninfected, allergic controls (Figure 5A–H).
Fig. 5. NTHi infection reduced markers of antigen presentation and activation in AAD. 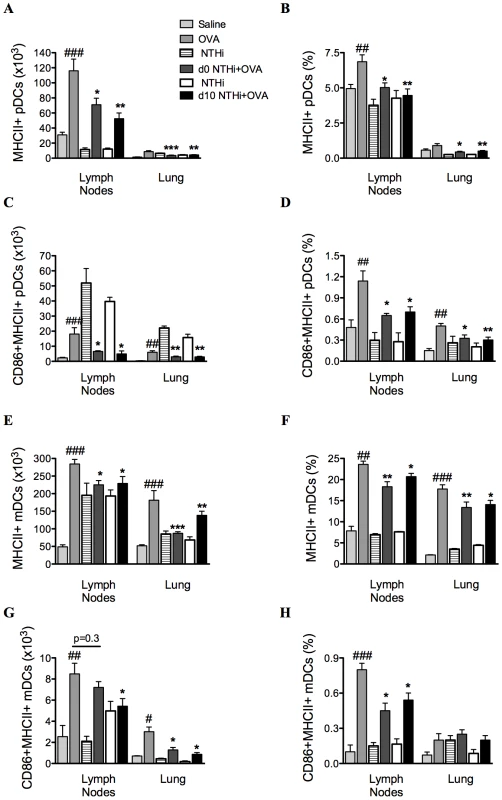
The numbers and proportions of MHCII and CD86 expressing pDCs (A–D) and mDCs (E–H) in MLNs and lungs were determined. Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ###p<0.001, ## p<0.01, # p<0.05 compared to Saline controls, ***p<0.001, ** p<0.01, * p<0.05 compared to OVA controls. Infection enhances neutrophilic inflammation in AAD
We then assessed the effects of infection on other features of AAD (on day 16). Significantly, whilst eosinophilic inflammatory responses were suppressed by infection during AAD, NTHi infection induced AAD with an enhanced neutrophilic inflammatory profile.
The development of AAD resulted in an increase in neutrophil influx into the airways (Figure 6). Infection during or after sensitization resulted in a two-fold increase in neutrophil recruitment in BALF compared to uninfected, allergic controls. Moreover, infected allergic groups had a four-fold increase in neutrophil recruitment compared to groups with infection alone (i.e. infected, nonallergic groups) at the same time point after infection (i.e. 16d and 5d after infection, Figure 6). These results demonstrate that the combination of infection with AAD results in enhanced neutrophilic inflammation. Collectively, our results show that NTHi infection in AAD may induce a phenotype of neutrophilic AAD that resembles neutrophilic asthma in humans.
Fig. 6. NTHi infection induces neutrophilic inflammation in AAD. 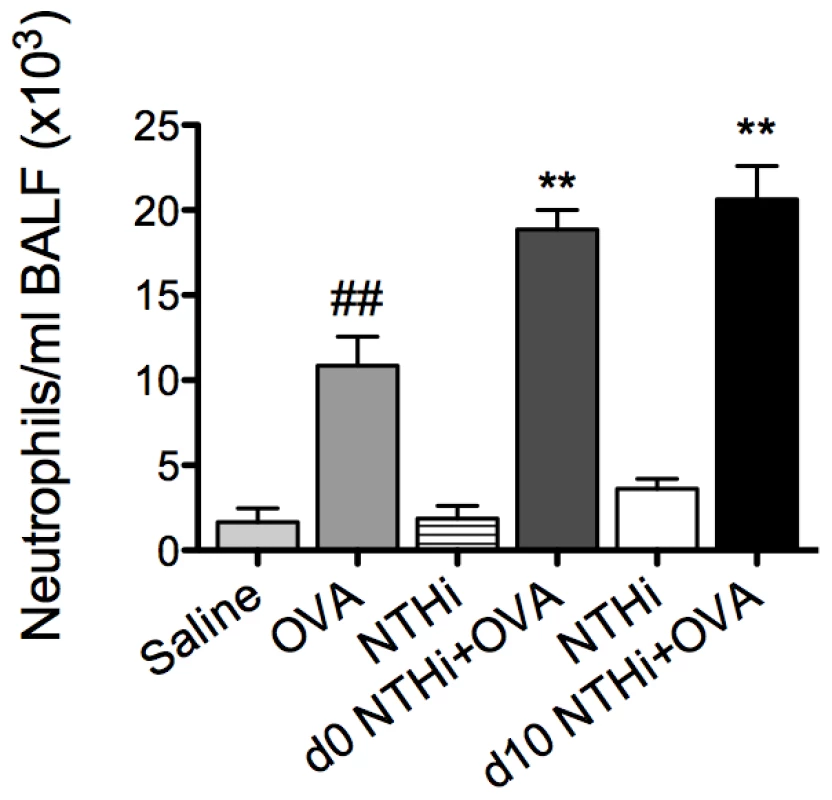
The effect of NTHi infection on BALF neutrophil numbers in AAD was determined (on day 16) and compared to groups with AAD without infection or infection alone without AAD. Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ## p<0.01 compared to Saline controls, ** p<0.01 compared to OVA controls. Infection-induced neutrophilic inflammation is associated with increases in IL-17 responses
Neutrophilic inflammation in asthma has been linked with increased IL-17 expression and IL-17 has been shown to be involved in neutrophil recruitment in response to bacterial infection. Therefore, the effects of infection on IL-17 responses during infection-induced neutrophilic AAD were further investigated. The experiments described hereafter were performed with infection during (d0 NTHi+OVA) OVA sensitization.
The profile of neutrophil influx into the airways and IL-17 production was determined in lung tissue and MLNs during the development of infection-induced neutrophilic AAD (1, 5, 12 and 16d, Figure 7A). The development of AAD resulted in increases in neutrophilic influx into the airways on day 12 and 16, and had minimal effects on IL-17 responses. Significantly greater numbers of neutrophils were recruited into the airways during infection and OVA sensitization (1d) and after OVA challenge (16d) in infected, allergic compared to uninfected, allergic groups (Figure 7B).
Fig. 7. NTHi infection induces increased IL-17 responses that correlate with neutrophil influx in neutrophilic AAD. 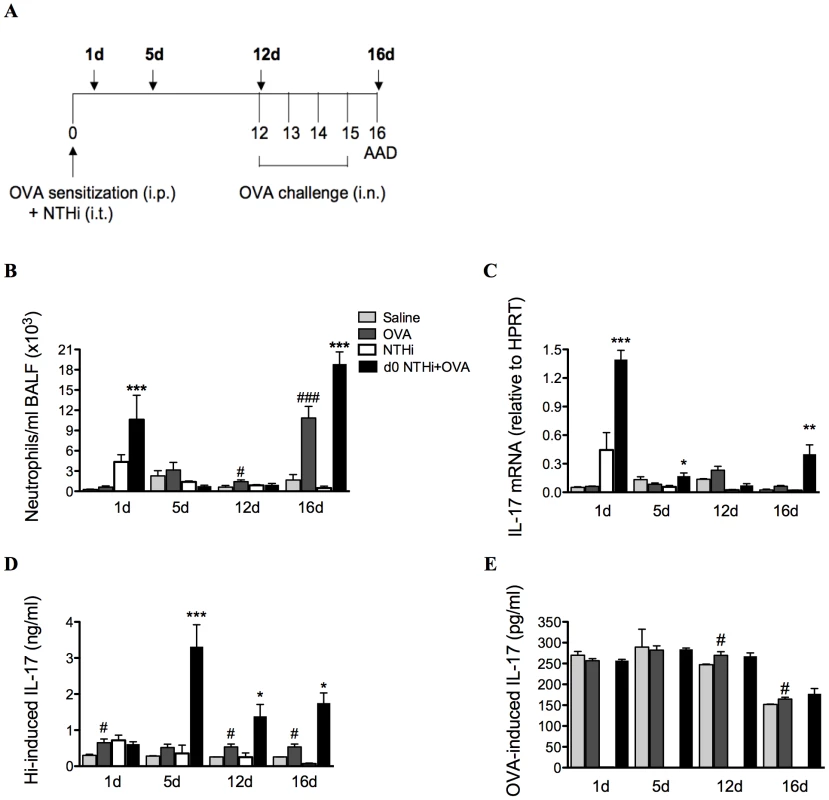
To investigate the association between neutrophil influx and IL-17 responses, a time-course (A), of neutrophils in BALF (B), IL-17 mRNA expression in lung tissue (C), and IL-17 release from MLN T cells stimulated with killed NTHi (D), and OVA (E) was assessed. Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ### p<0.001, # p<0.05 compared to Saline controls, *** p<0.001, ** p<0.01, * p<0.05 compared to OVA controls. Importantly, increases in the infection-induced neutrophil influx were accompanied by significant increases in IL-17 responses in pulmonary tissue and MLN T cells. The expression of IL-17 mRNA in lung tissue was significantly elevated 1 day after infection in infected, allergic groups (Figure 7C) and returned to baseline levels by day 12, immediately prior to OVA challenge. Expression again significantly increased on day 16, after OVA challenges.
NTHi-induced IL-17 release from MLN T cells was also increased from days 5 to 16 in infected, allergic compared to uninfected, allergic groups (Figure 7D). Interestingly, infection did not affect OVA-induced IL-17 release (Figure 7E). Taken together, these data demonstrate that infection induces increased IL-17 responses in lung tissue and MLNs that correlate with elevated airway neutrophil numbers in infection-induced neutrophilic AAD.
Infection-induced early influx of neutrophils is associated with early increases in neutrophil chemokine responses
IL-17 can induce neutrophilic inflammation by enhancing the expression of the chemotactic factor IL-8. Therefore, the mRNA expression and protein levels of KC and MIP2, the mouse orthologs of IL-8, in lung tissue were also investigated. KC and MIP2 mRNA and protein were elevated 1 day after infection and OVA sensitization in the lungs of infected, allergic, compared to uninfected, allergic groups, (Figure 8A–B). There were no differences in mRNA expression between groups at later time points. Therefore, the early induction of neutrophil influx into the lung during the development of infection-induced neutrophilic AAD is associated with early increases in neutrophil chemokine responses during infection.
Fig. 8. Early neutrophil influx is associated with enhanced neutrophil chemokine expression. 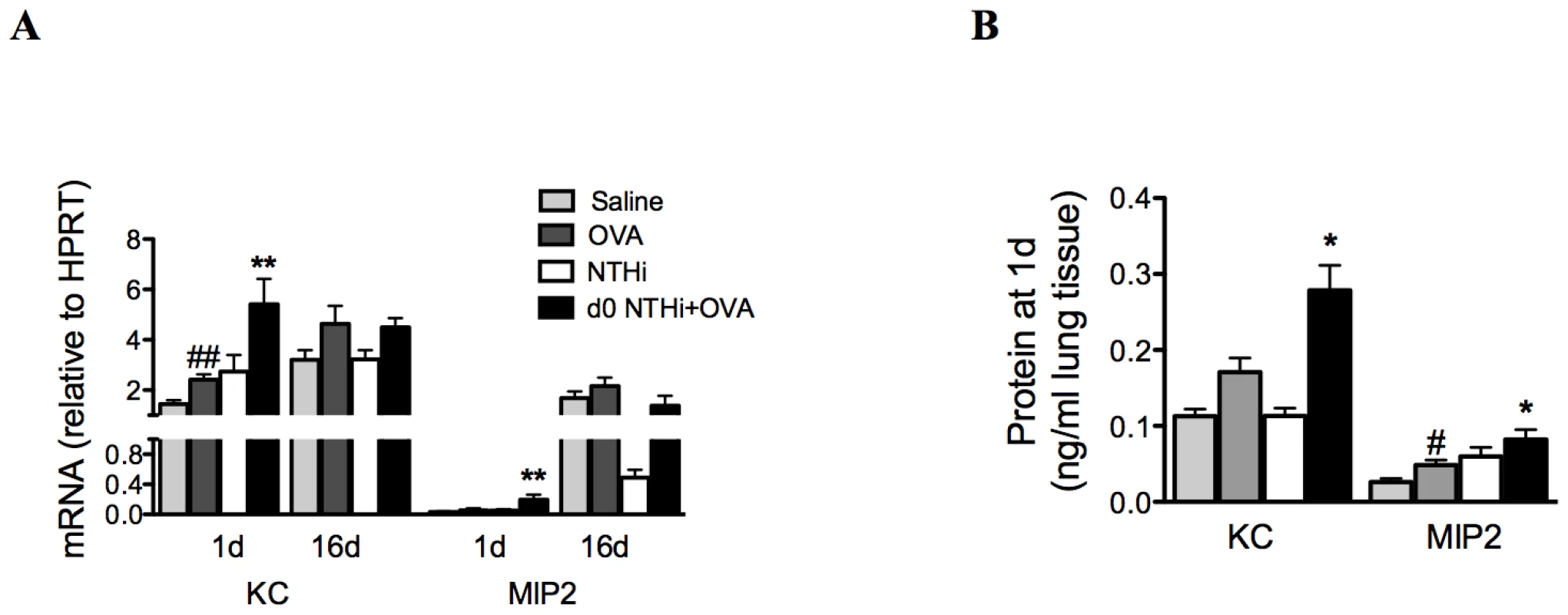
To investigate the association between early neutrophil influx and neutrophil chemokine responses, KC and MIP2 mRNA expression (on day 1 and 16, A) and protein levels (day 1 only, B) in lung tissue were assessed. Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ## p<0.01, # p<0.05 compared to Saline controls, ** p<0.01, * p<0.05 compared to OVA controls. Infection induces Th17 cell differentiation and IL-17 production from Th17 cells
To investigate the mechanisms that underpin infection-induced neutrophilic AAD, we assessed the potential cellular sources of IL-17 and the role of adaptive and innate immune cells in its release.
The development of AAD resulted in modest increases in IL-17 factors and responses (Figure 9A–E). ROR-γt, the Th17 differentiation factor was assessed, and expression was significantly elevated after 12 and 16 days in the lungs of infected, allergic, compared to uninfected, allergic groups (Figure 9A), suggesting that there was enhanced Th17 polarization. The numbers and proportions of T cells that were Th17 cells in lung tissue and MLNs were then determined by flow cytometry. CD3+CD4+IL-17+ (Th17) cells were significantly increased in the lungs after 12 and 16 days in infected, allergic groups (Figure 9B–C), but in the MLNs were increased only at day 5 (Figure 9D–E). These results indicate that Th17 cells in the lungs and MLNs may be the potential adaptive immune source of IL-17 after day 5.
Fig. 9. NTHi infection induces Th17 cell differentiation and IL-17 production from Th17 cells in neutrophilic AAD. 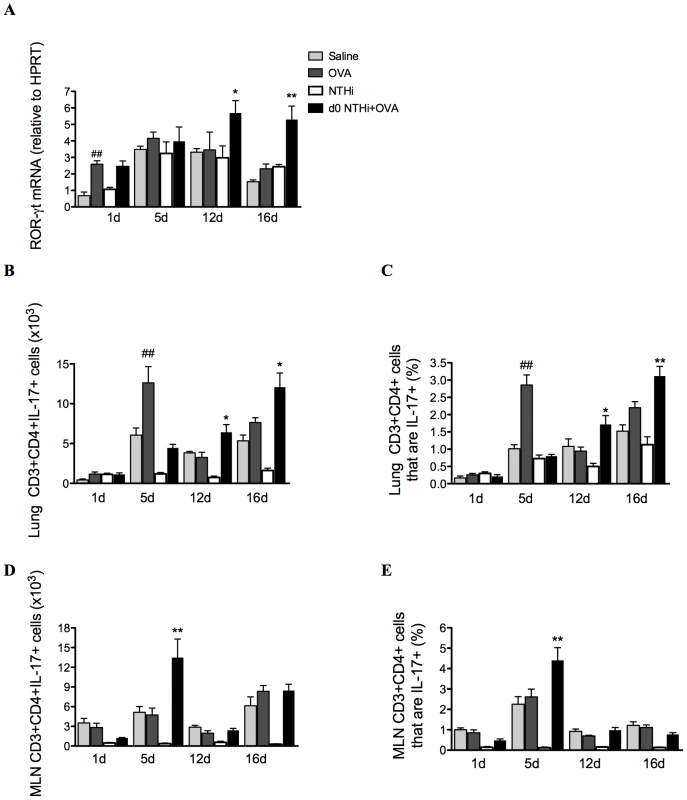
Th17 cell differentiation in lung tissues was examined by determination of the expression of the transcription factor ROR-γt (A). Th17 cell numbers and proportions were quantified in lungs (B–C) and lymph nodes (D–E). Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ## p<0.01 compared to Saline controls, ** p<0.01, * p<0.05 compared to OVA controls. Infection induces IL-17 production from macrophages and neutrophils
We then assessed which cells were the early innate sources of IL-17 on day 1. Increased numbers and proportions of pulmonary macrophages (Figure 10A–B) and to a lesser extent neutrophils (Figure 10C–D) produced increased amounts of IL-17 at early but not other time-points in infected, allergic groups compared to uninfected, allergic controls. Lung macrophages and neutrophils isolated on day 1 also had increased levels of IL-17 mRNA transcripts (Figure 10E–F).
Fig. 10. NTHi infection induces early IL-17 production from lung macrophages and neutrophils in neutrophilic AAD. 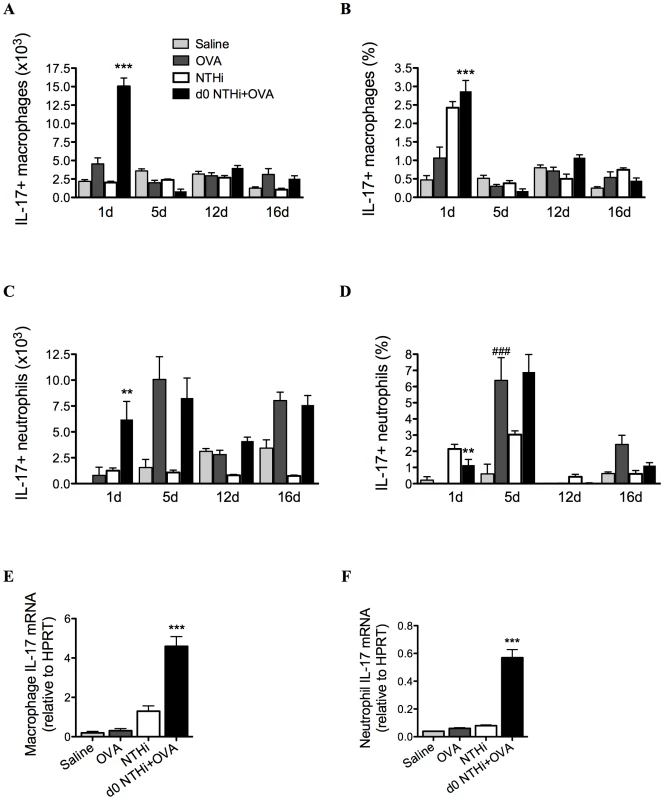
To determine early cellular sources of IL-17, IL-17 producing macrophage (A–B) and neutrophil (C–D) numbers and proportions in lungs were assessed. IL-17 mRNA was also assessed in isolated lung macrophages (E) and neutrophils (F). Data for the corresponding NTHi control groups were obtained at the same time point after infection as data from the NTHi+OVA groups. ### p<0.001 compared to Saline controls, *** p<0.001, ** p<0.01 compared to OVA controls. Taken together these results demonstrate that infection induces early IL-17 responses from lung macrophages and neutrophils and later responses from Th17 cells in lungs and MLNs that are associated with neutrophil influx into the airways.
NTHi-induced neutrophilic AAD is dependent on IL-17
We have shown that neutrophilic inflammation in infection-induced neutrophilic AAD correlates with increased expression of IL-17 during OVA challenge. To determine whether infection-induced neutrophilic inflammation is mediated by IL-17, IL-17 was depleted in infected, allergic groups during AAD, by administration of anti-IL-17 monoclonal antibody during OVA challenge on days 11 and 13 (Figure 11A), and AAD assessed (on day 16). This approach has previously been shown to deplete IL-17 in vivo [21]. Importantly, IL-17 depletion significantly reduced the numbers of neutrophils in the BALF compared to isotype treatment of infected, allergic groups (Figure 11B). Significantly, neutrophil numbers were not different to those observed in uninfected, allergic groups, which were unaffected by treatment. IL-17 depletion also significantly reduced KC mRNA expression levels in the lung, but had no effect on MIP2 mRNA (Figure 11C). Anti-IL-17 treatment of infected, allergic groups also partially restored IL-5 (3.198±0.679 ng/ml in anti-IL-17 compared to 1.576±0.238 ng/ml in isotype-treated infected allergic groups p<0.01) and IL-13 (18.240±1.533 ng/ml in anti-IL-17 compared to 11.988±0.938 ng/ml in isotype-treated infected allergic groups p<0.01) responses, but had no affect on IFN-γ release. These results demonstrate that infection-induced IL-17 release is responsible for neutrophil influx into the airways and the induction of neutrophilic AAD.
Fig. 11. NTHi infection-induced IL-17 is required for the induction of neutrophilic AAD and is partially responsible for the effects of infection on T cell responses. 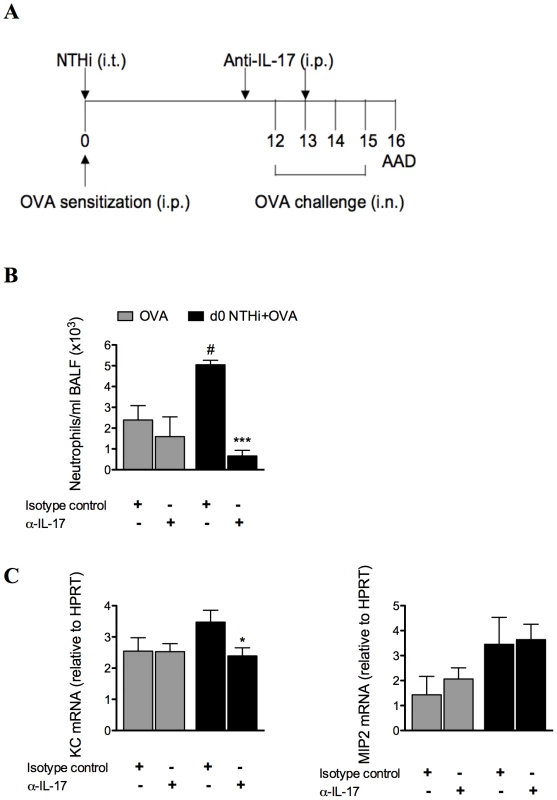
To confirm the role of IL-17, anti-IL-17 monoclonal antibody was administered i.p. on days 11 and 13 of infection-induced neutrophilic AAD, and AAD was analyzed (on day 16) (A). The effects on BALF neutrophil influx (B), and KC and MIP2 mRNA expression in lung tissue (C) were assessed. # p<0.05 compared to OVA controls, *** p<0.001, * p<0.05 compared to isotype controls. Discussion
In this study we have demonstrated for the first time that H. influenzae respiratory infection drives IL-17-mediated development of neutrophilic AAD. NTHi infection suppressed pulmonary and systemic eosinophilic inflammation and reduced Th2 cytokine responses and AHR in AAD. However, infection induced neutrophilic inflammation during AAD, by promoting early (innate) and late (adaptive) IL-17 responses from pulmonary macrophages and Th17 cells, respectively. This indicates that H. influenzae infection may modulate immune responses in asthmatics that promote the development of neutrophilic asthma.
NTHi is commonly isolated from the nasopharynx of healthy individuals, but is also associated with chronic airway diseases such as bronchiectasis [37], COPD [38], and chronic bronchitis [39]. NTHi is the bacterium most commonly isolated during COPD exacerbations, and NTHi strains isolated during these exacerbations induce higher levels of IL-8, and subsequent neutrophil recruitment to the airways, than colonizing strains [40]. Simpson and colleagues have recently demonstrated that a large proportion of neutrophilic asthmatics are colonized with H. influenzae, have increased innate immune activation, and 6-8 fold higher endotoxin levels compared to other asthma subtypes and healthy controls [12]. A more recent study has demonstrated that 41% of neutrophilic asthmatics assessed had a significant load of potentially pathogenic bacteria, and H. influenzae was identified in 60% of patients that tested positive for these bacteria [33]. We have extended these studies to show that H. influenzae may promote neutrophilic asthma by suppressing Th2-mediated responses that are associated with alterations in antigen-presenting cells, and by inducing potent neutrophilic inflammation that is driven by Th17 responses.
We show that infection during and after sensitization inhibits characteristic features of eosinophilic asthma. Irrespective of the time of inoculation, infection significantly reduced both local and systemic allergen-induced cytokine release from MLNs and splenocytes, as well as airway and blood eosinophil recruitment. All of these effects may lead to the suppression of AHR. Tregs are an important cell involved in immune tolerance and the suppression of inflammation [41]. We show that Treg numbers were not changed by infection, and TGF-β and IL-10 expression in the lung, which are involved in the suppression of inflammation by Tregs, were reduced by infection in AAD. These results suggest that Tregs are not involved in the suppression of cytokines or cellular inflammation. The role of infection on DC function was investigated as DCs play an integral role in the uptake and presentation of antigen to naïve T cells, and as a result direct immune responses [42]. Infection significantly decreased markers associated with antigen presentation and co-stimulation of DCs. Therefore, infection is able to alter the phenotype of antigen-presenting cells, which may affect the interaction between APCs and T cells, and result in attenuated adaptive responses to allergen.
By contrast, NTHi infection induced potent neutrophilic inflammation in the airways. Persistent airway neutrophilia is also a feature common to chronic airway diseases, such as COPD [38], chronic bronchitis [39] and bronchiectasis [43], where recurrent infection is known to play an important role in pathogenesis. Neutrophilic inflammation is often associated with acute asthma exacerbations, and in particular infection-mediated exacerbations. Indeed, several studies have shown increased neutrophilic inflammation in both viral and bacterial infection-induced exacerbations [44], [45], [46]. Here we show that NTHi induces strong neutrophilic inflammatory responses, and may be involved in the development of neutrophilic asthma through the induction of neutrophilic inflammation. We demonstrate that immune responses that lead to the development of NTHi-induced neutrophilic AAD occur in two phases. The first involves innate immune activation during infection that is likely to result in neutrophil chemoattraction to the airways. NTHi infection during OVA sensitization resulted in a significant neutrophil influx to the airways 1 day after infection, a two-fold increase compared to NTHi infection alone. This correlated with the early production of KC, MIP2 and IL-17 in the lungs. These neutrophils and to a greater extent macrophages were able to produce significantly more IL-17 than those from infected, nonallergic and uninfected allergic controls. Therefore these cells, particularly macrophages, may be sources of early (innate) IL-17 release. This observation may have important implications for other diseases where the innate source of IL-17 has not yet been identified.
The second phase involves adaptive immune responses during allergen challenge resulting in increased infection-mediated Th17 responses. During the challenge phase, days 12-15, there was a significant upregulation of ROR-γt and IL-17 mRNA in the lungs of infected allergic groups compared to infected, nonallergic and uninfected allergic controls. These results directly correlated with increases in Th17 cells in the lungs of infected, allergic groups. The increased production of IL-17 from T cells in conjunction with increases in ROR-γt expression suggest that infection drives Th17 responses that preferentially induce IL-17 production and neutrophilic inflammation during subsequent allergen challenge.
Collectively, our data suggest that infection induces early responses, involving neutrophils, KC, MIP2 and IL-17 expression that may prime the host for enhanced Th17-mediated neutrophilic responses upon later allergen challenge, which subsequently induces neutrophilic AAD. Our findings are consistent with data from a recent study by Bullens et al., which showed that increased IL-17 responses in asthmatics correlate with increased neutrophil numbers in sputum [15]. Hellings et al., [21] demonstrated that IL-17 is important in lung neutrophil recruitment in response to an allergen, while Ye et al., [29] showed that IL-17 responses and signalling through the IL-17R is vital for neutrophil recruitment and host defence against Klebsiella pneumoniae infection. Here we extend these findings by demonstrating that H. influenzae infection-induced IL-17 responses in AAD may play a role in driving neutrophilic inflammation in asthma. We recently demonstrated that chlamydial respiratory infection is also able to drive neutrophilic asthma [47]. Chlamydial infection suppressed Th2-mediated eosinophilic inflammation and promoted neutrophilic inflammation and AAD. Neutrophilic asthmatics are resistant to corticosteroid treatment, which is the mainstay of asthma therapy [10], and with evidence that asthmatics with infection are more resistant to steroids than asthmatics with no infection [48], alternative therapies are needed for infection-induced neutrophilic asthma.
It is possible that either infection alone or the synergistic effects of infection and AAD are required for the induction of the neutrophilic AAD phenotype. NTHi infection alone did increase neutrophil influx into the airways (p<0.01), IL-17 mRNA expression in the lung (p<0.05) and the percentages of lung macrophages and neutrophils producing IL-17 (both p<0.001) 1 day after infection, compared to uninfected, nonallergic (Saline) controls. These effects would be expected as they are normal responses to infection; however, they may contribute to the establishment of a pro-neutrophilic environment and the subsequent development of neutrophilic AAD upon allergen challenge. However, NTHi infection does not persist past 10 days, and yet is able to modify AAD after 16 days. It is, therefore, likely that the major affect of the infection is to induce persistent immune changes, that continue even after the clearance of the infection, that synergize with allergen exposure to drive neutrophilic AAD. This mechanism has recently been proposed in humans [49].
OVA sensitization at the time of, or prior to, infection, and subsequent OVA challenge does not affect NTHi load. When infection occurs during sensitization, bacteria are still cleared by day 16, and; when infection occurs 10 days after sensitization, bacterial recovery at the end of the protocol (i.e. 5 days later), is the same as that in the infected, non-allergic group (data not shown).
It is likely that the induction of a neutrophilic phenotype is specific to a subset of infectious agents, particularly NTHi and Chlamydia [12], [33], [50]. In other studies we have investigated the impact on AAD of an unrelated respiratory pathogen and commensal bacterium, Streptococcus pneumoniae, which is not associated with neutrophilic asthma. We show that S. pneumoniae infection or components do not induce changes in IL-17 responses or increase neutrophilic inflammation with infection or component administration either before, during or after the induction of AAD [51], [52]. Both NTHi - and Chlamydia-induced neutrophilic AAD, may potentially be driven by conserved pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) or CpGs. Numerous studies have investigated the effects of LPS and CPG in AAD, and the interactions are complex. LPS is highly variable and different types and levels of LPS have different effects in AAD. Low dose LPS administration during sensitization promotes Th2 responses and is a risk factor for severe asthma [53], [54], while high doses decrease Th2 responses and induce non-eosinophilic inflammation [55], [56], [57], [58]. Chlamydia-derived LPS is atypical and is 1,000 fold less immunogenic compared to other bacterial-derived LPS [59], and therefore, is unlikely to be the cause of Chlamydia-induced neutrophilic AAD. CpGs when given together with antigen in established disease induce Th1 and regulatory T cell responses that suppress the features of AAD including Th2 responses and AHR [60], [61], [62]. We have shown that depleting neutrophils during a Chlamydia infection inhibits the development of neutrophilic AAD, by contrast IL-17 responses drive NTHi-induced neutrophilic AAD. Therefore, we suggest that it is specific immune responses to these, as well as potentially other infections, which are driven by as yet unidentified factors in the infections, that are the mechanisms that drive neutrophilic AAD [47]. Our studies do not rule out LPS, CpGs, or other PAMPs as drivers of the neutrophilic phenotype and further research is required to elucidate this possibility.
Importantly we have shown that infection-induced neutrophilic AAD and the suppression of Th2 responses are dependent upon IL-17. Depletion of IL-17 with anti-IL-17 monoclonal antibody during AAD prevented the development of infection-induced neutrophilic AAD. This suggests that IL-17 is critical in the recruitment of neutrophils and may suppress Th2 responses in infection-induced neutrophilic inflammation and neutrophilic asthma. Wakashin et al., demonstrated that adoptive transfer of antigen-specific Th17 cells induced airway neutrophil recruitment, which supports our data [63]. Little is known about how Th17 and Th2 cells interact with or regulate each other. We demonstrate that infection inhibits cytokine release compared to uninfected allergic controls, and that anti-IL-17 treatment partially restored these effects, while having no effect on eosinophil recruitment (data not shown). This suggests that other mechanisms are also involved in the suppression of Th2 responses by NTHi infection, which requires further investigation. Several recent studies have investigated the relationship between Th17 and Th2 cells. Schnyder-Candrian et al., showed that the administration of rIL-17 in a murine model of AAD significantly reduced allergen-induced eotaxin, thymus and activation-regulated chemokine (TARC) and IL-5, thereby reducing eosinophilic inflammation [64], while another study showed that inhibiting IL-17 in AAD also reduced airway eosinophils, neutrophils, AHR, and Th2 cytokines [65]. These data suggest that IL-17 may suppress or promote eosinophilic inflammation, but the mechanisms that drive these different effects remain unknown. Our data is in agreement with Schnyder-Candrian et al., who suggest that IL-17 interferes with DC activation and antigen uptake, which prevents T cell activation and reduced IL-4, -5 and -13 production, leading to suppressed allergic responses. Interestingly, a recent study has shown a CD4+ T cell subtype that expresses both Th17 and Th2 cytokines, including IL-4, IL-5, IL-13, IL-17 and IL-22, and this subset is increased in asthmatics compared to healthy controls [66]. However, these cells have thus far only been found in the periphery, and confirmation of their presence is needed in BAL, sputum and/or bronchial biopsies.
In conclusion, we show that H. influenzae infection may be involved in the development of neutrophilic asthma. Infection suppressed features of Th2-mediated eosinophilic AAD, while inducing features of neutrophilic asthma that are mediated by infection-induced IL-17. Therefore, infection-induced IL-17 responses may play a major role in the pathogenesis of neutrophilic asthma. Our studies indicate the important role of infection in driving neutrophilic asthma-like disease, and identify new areas of investigation that may enhance the understanding of disease progression. Developing new treatments targeting infection may lead to better management of individuals with this disease phenotype.
Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the NSW Animal Research Regulation 2005, and the Australian Code of Practice for the care and use of animals for scientific purposes (National Health and Medical Research Council). All protocols were approved by the Animal Care and Ethics Committee of the University of Newcastle (permit number 987/0111). All surgery was performed under sodium pentobarbital anaesthesia, and all efforts made to minimize pain and suffering.
AAD model
Six to eight week old female BALB/c mice were used. Mice were sensitized by intraperitoneal (i.p.) injection, with OVA (50 µg, Sigma-Aldrich, Castle Hill, NSW, Australia) with the Th2-inducing adjuvant Rehydrogel (1mg, in 200 µl sterile saline, Reheis, Berkeley Heights, USA). On days 12 to 15 mice were challenged intranasally (i.n.) with OVA (10 µg, 50 µl) and AAD was assessed on day 16 [67]. Controls were sham sensitized to saline.
NTHi infection model
NTHi (NTHi-289) glycerol stocks were plated onto chocolate agar plates (Oxoid, SA, Australia), grown overnight (37°C, 5% CO2), then washed off the plate and suspended in sterile PBS. To determine the effects of infection, mice were inoculated i.t. with 5x105 CFU NTHi (in 30 µl PBS) during (Day 0) or after (Day 10) OVA sensitization. Controls were infected but not exposed to OVA. In preliminary studies we determined that this inoculum induced an infection from which the mice recovered and could be used to study the effects of infection on AAD.
Cellular inflammation
BALF was collected and processed as previously described [68]. Briefly, the left lung was tied off and the right lung was washed twice with Hank's buffered salt solution (700 µl, HBSS; Trace Scientific, Noble Park, Vic, Australia). Cells were pelleted and resuspended in red blood cell lysis buffer, washed and resuspended in HBSS, then cytocentrifuged (300g, 5 min, ThermoFisher Scientific, Scoresby, Vic, Australia) onto microscope slides. Blood smears were prepared from a drop of whole blood. BALF and blood cells were stained with May-Grunwald-Giemsa, and differential leukocyte counts were enumerated using light microscopy [68].
Bacterial recovery
Right lobes of lungs, from which BALF had been obtained, were aseptically removed and homogenized in 1ml of sterile PBS. Serial dilutions of BALF and lung homogenates were prepared in sterile PBS, plated onto chocolate agar plates and incubated overnight (37°C, 5% CO2). Colonies were enumerated and bacterial numbers per right lung calculated.
Lung function
AHR was measured in response to increasing doses of aerosolized methacholine, by whole body invasive plethysmography as previously described [47]. Briefly, mice were anaesthetized and tracheas were cannulated and attached to a ventilator. Peak dynamic compliance and transpulmonary resistance were assessed by analysis of pressure and flow waveforms following challenge with increasing doses of aerosolized methacholine (Sigma-Aldrich).
T cell cytokines
Supernatants from lung draining MLN T cells were restimulated with OVA (200 µg/ml) or ethanol-killed NTHi (2×107 CFU/ml) and cultured for six days (5% CO2, 37°C, 1x106 cells per well). After culture supernatants containing soluble factors were recovered and analyzed for IL-5, IFN-γ, (BD Biosciences, North Ride, NSW, Australia), IL-13, IL-17A and IL-22 (R&D Systems, Minneapolis, MN, USA) by ELISA, according to manufacturer's instructions [69].
Lung protein cytokines
Whole lungs were homogenized in RIPA buffer (1ml, Sigma-Aldrich) and incubated on ice for 5 mins. Cells were pelleted and the supernatant recovered and analyzed for KC and MIP2 by ELISA, (R&D Systems), according to manufacturer's instructions.
Cytokine expression in lungs
RNA was TRIZOL extracted from whole lung homogenates according to manufacturer's instructions (Invitrogen, Mount Waverly, Vic, Australia). Target gene expression was determined relative to the reference gene hypoxanthine-guanine phosoribosyltransferase (HPRT) [69]. Primers used were IL-17, Fwd 5′-aaacatgagtccagggagagcttt-3′, Rev 5′-actgagcttcccagatcacagagg-3′; ROR-γt, Fwd 5′ - ccgctgagagggcttcac-3′, Rev 5′ - tgcaggagtaggccacattaca-3′; MIP2, Fwd 5′ - ctagctgcctgcctcattctac-3′, Rev 5′ - caacagtgtacyyacgcagacg-3′; KC Fwd 5′ - cttggggacaccttttagca-3′, Rev 5′ - gctgggattcacctcaagaa-3′; TGF-β Fwd 5′ - cccgaagcggactactatgctaaa-3′, Rev 5′ - ggtaacgccaggaattgttgctat-3′; IL-10, Fwd 5′-catttgaattccctgggtgagaag-3′, Rev 5′ - gccttgtagacaccttggtcttgg-3′; and HPRT Fwd 5′ - aggccagactttgttggatttgaa-3′, Rev 5′ - caacttgcgctcatcttaggcttt-3′.
Flow cytometry
Single cell suspensions of MLNs and collagenase-D digested lungs were prepared. IL-17 producing cells were identified by stimulation with phorbol 12-myristate 13-acetate (PMA, 0.1 µg/ml) and ionomycin (1 µg/ml, Sigma-Aldrich) in the presence of Brefeldin A (8 µg/ml, Sigma-Aldrich) for 4 hours [70]. Cells were incubated with Fc block for 15mins, then stained for surface markers CD4, CD3, CD11b, Gr-1 (BD Bioscience), or F4/80 (eBioscience, San Diego, CA, USA), fixed with 4% paraformaldehyde (PFA), permeabilized with 0.1% saponin, and stained for intracellular IL-17 (or isotype control rat IgG2a, eBioscience). Tregs were identified using surface markers CD4, CD25 and a staining kit for intracellular FoxP3 (or IgG2a isotype control) according to manufacturer's instructions (eBioscience). pDCs were characterized as CD11clowCD11b-B220+, and mDCs characterized as CD11c+CD11b+B220− (BD Bioscience); using MHCII and CD86 (R&D Systems) for activation and co-stimulation status. All cells were analyzed using a FACS Canto (BD Bioscience) [47].
Lung macrophage and neutrophil separation and mRNA isolation
Single cell suspensions of collagenase-D digested lung tissue were prepared, and resuspended in red blood cell lysis buffer. Resuspended cells were either incubated overnight (5% CO2, 37°C, 1x106 cells per well) and macrophages isolated by adherence to culture plates; or were put through a mouse neutrophil enrichment kit (Stemcell Technologies, Melbourne, Vic, Australia) and neutrophils isolated by negative selection following manufacturer's instructions. mRNA was purified from these macrophages and neutrophils using a PureLink RNA mini kit (Invitrogen) according to manufacturer's instructions.
Depletion of IL-17 during infection-induced neutrophilic AAD
Monoclonal anti-IL-17A neutralizing antibody (clone 50104, rat IgG2a) was administered by i.p. injection (100 µg/mouse, eBioscience) on days 11 and 13, and features of AAD were assessed on day 16. Control groups were uninfected and treated with anti-IL-17 or treated with IgG2a isotype control antibody [47].
Statistics
Results are presented as mean ± standard error of the mean (SEM) from 6–8 mice, in duplicate. Significance was determined by one-way ANOVA or Student t-test (GraphPad Software, CA, USA).
Zdroje
1. UmetsuDTMcIntireJJAkbariOMacaubasCDeKruyffRH 2002 Asthma: an epidemic of dysregulated immunity. Nat Immunol 3 715 720
2. BusseWWLemanskeRF 2001 Asthma. New Engl J Med 344 350 362
3. DouwesJGibsonPPekkanenJPearceN 2002 Non-eosinophilic asthma: importance and possible mechanisms. Thorax 57 643 648
4. GibsonPGSimpsonJLSaltosN 2001 Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 119 1329 1336
5. PavordIDBrightlingCEWoltmannGWardlawAJ 1999 Non-eosinophilic corticosteroid unresponsive asthma. Lancet 353 2213 2214
6. WenzelSESchwartzLBLangmackELHallidayJLTrudeauJB 1999 Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Resp Crit Care 160 1001 1008
7. GodonPBouletLPMaloJLCartierALemiereC 2002 Assessment and evaluation of symptomatic steroid-naive asthmatics without sputum eosinophilia and their response to inhaled corticosteroids. Eur Respir J 20 1364 1369
8. SimpsonJLScottRBoyleMJGibsonPG 2006 Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology 11 54 61
9. AdcockIMItoK 2004 Steroid resistance in asthma: a major problem requiring novel solutions or a non-issue? Curr Opin Pharmacol 4 257 262
10. GreenRHBrightlingCEWoltmannGParkerDWardlawAJ 2002 Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 57 875 879
11. BerryMMorganAShawDEParkerDGreenR 2007 Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 62 1043 1049
12. SimpsonJLGrissellTVDouwesJScottRJBoyleMJ 2007 Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax 62 211 218
13. MoletSHamidQDavoinebFNutkuETahaaR 2001 IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 108 430 438
14. StefanoADCaramoriGGnemmiIContoliMVicariC 2009 T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 157 316 324
15. BullensDTruyenECoteurLDilissenEHellingsP 2006 IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 7 135 143
16. HansbroPMKaikoGEFosterPS 2011 Cytokine/anti-cytokine therapy - novel treatments for asthma? Brit J Pharmacol 163 81 95
17. LeipeJGrunkeMDechantCReindlCKerzendorfU 2010 Th17 cells in autoimmune arthritis. Ann Rheum Dis 69 A69 A70
18. JiYYiweiCXueYDiGLubingZ 2009 Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 60 1472 1483
19. HaradaASekidoNAkahoshiTWadaTMukaidaN 1994 Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukocyte Biol 56 559 564
20. PellmeSMorgelinMTapperHMellqvistU-HDahlgrenC 2006 Localization of human neutrophil interleukin-8 (CXCL-8) to organelle(s) distinct from the classical granules and secretory vesicles. J Leukocyte Biol 79 564 573
21. HellingsPWKasranALiuZVandekerckhovePWuytsA 2003 Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Resp Cell Mol 28 42 50
22. LangrishCLChenYBlumenscheinWMMattsonJBashamB 2005 IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201 233 240
23. LiangSCLongAJBennettFWhittersMJKarimR 2007 An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol 179 7791 7799
24. LockhartEGreenAMFlynnJL 2006 IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177 4662 4669
25. RoarkCLSimonianPLFontenotAPBornWKO'BrienRL 2008 γδ T cells: an important source of IL-17. Curr Opin Immunol 20 353 357
26. FerrettiSBonneauODuboisGRJonesCETrifilieffA 2003 IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol 170 2106 2112
27. SongCLuoLLeiZLiBLiangZ 2008 IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol 181 6117 6124
28. ZhouQDestaTFentonMGravesDTAmarS 2005 Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA Protein. Infect Immun 73 935 943
29. YePRodriguezFHKanalySStockingKLSchurrJ 2001 Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194 519 528
30. FeinenBGaffenSLJerseAERussellMW 2009 IL-17 elicits a neutrophil-attractant response to Neisseria gonorrhoeae infection. J Immunol 182 38.25
31. ScurlockAMO'ConnellCMAndrewsCWFooteIPDarvilleTM 2009 Mucosal T-helper 17 responses to Chlamydia genital tract infection. J Allergy Clin Immun 123 S138 S138
32. WuQMartinRJRinoJGBreedRTorresRM 2007 IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect 9 78 86
33. WoodLGSimpsonJLHansbroPMGibsonPG 2010 Potentially pathogenic bacteria cultured from the sputum of stable asthmatics are associated with increased 8-isoprostane and airway neutrophilia. Free Radical Res 44 146 154
34. ErwinALSmithAL 2007 Nontypeable Haemophilus influenzae: understanding virulence and commensal behaviour. Trends Microbiol 15 355 362
35. MurphyTF 2003 Respiratory infections caused by non-typeable Haemophilus influenzae. Curr Opin Infect Dis 16 129 134
36. MurphyTFBrauerALSchiffmacherATSethiS 2004 Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Resp Crit Care 170 266 272
37. AngrillJAgustiCde CelisRRanoAGonzalezJ 2002 Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 57 15 19
38. BarnesPJ 2002 New treatments for COPD. Nat Rev Drug Discov 1 437 446
39. WilsonR 2000 Evidence of bacterial infection in acute exacerbations of chronic bronchitis. Semin Respir Infect 15 208 215
40. LookDCChinCLManzelLJLehmanEEHumlicekAL 2006 Modulation of airway inflammation by Haemophilus influenzae isolates associated with chronic obstructive pulmonary disease exacerbation. Proc Am Thorac Soc 3 482 483
41. SeroogyCMGernJE 2005 The role of T regulatory cells in asthma. J Allergy Clin Immun 116 996 999
42. JakubzickCTackeFLlodraJvan RooijenNRandolphGJ 2006 Modulation of dendritic cell trafficking to and from the airways. J Immunol 176 3578 3584
43. BoytonRJ 2008 Bronchiectasis. Medicine 36 315 320
44. WarkPABJohnstonSLMoricISimpsonJLHensleyMJ 2001 Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 19 68 75
45. SamuelLFWilliamWB 2005 The role of rhinovirus in asthma exacerbations. J Allergy Clin Immun 116 267 273
46. FahyJVKimKWLiuJBousheyHA 1995 Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immun 95 843 852
47. HorvatJCStarkeyMRKimRYBeagleyKWPrestonJA 2010 Chlamydial respiratory infection during allergen sensitization drives neutrophilic allergic airways disease. J Immunol 184 4159 4169
48. ChoYSKimTBLeeTHMoonKALeeJ 2005 Chlamydia pneumoniae infection enhances cellular proliferation and reduces steroid responsiveness of human peripheral blood mononuclear cells via a tumor necrosis factor-alpha-dependent pathway. Clin Exp Allergy 35 1625 1631
49. WangFHeXYBainesKJGunawardhanaLPSimpsonJL 2011 Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J 38 567 574
50. PatelKKVicencioAGDuZTsirilakisKSalvaPS 2010 Infectious Chlamydia pneumoniae is associated with elevated interleukin-8 and airway neutrophilia in children with refractory asthma. Pediatr Infect Dis J 29 1093 1098
51. PrestonJAThorburnANStarkeyMRBeckettELHorvatJC 2010 Streptococcus pneumoniae infection suppresses allergic airways disease by inducing regulatory T cells. Eur Respir J 37 1 12
52. ThorburnANO'SullivanBJRanjenyTKumarRKFosterPS 2010 Pneumococcal conjugate vaccine-induced regulatory T cells suppress the development of allergic airways disease. Thorax 65 1053 1060
53. DongLLiHWangSLiY 2009 Different doses of lipopolysaccharides regulate the lung inflammation of asthmatic mice via TLR4 pathway in alveolar macrophages. J Asthma 46 229 233
54. EisenbarthSCPiggottDAHuleattJWVisintinIHerrickCA 2002 Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 196 1645 1651
55. Delayre-OrthezCBeckerJde BlayFFrossardNPonsF 2005 Exposure to endotoxins during sensitization prevents further endotoxin-induced exacerbation of airway inflammation in a mouse model of allergic asthma. Int Arch Allergy Imm 138 298 304
56. RodriguezDKellerACFaquim-MauroELde MacedoMSCunhaFQ 2003 Bacterial lipopolysaccharide signaling through toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol 171 1001 1008
57. KimY-KOhS-YJeonSGParkH-WLeeS-Y 2007 Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol 178 5375 5382
58. LiJJWangWBainesKJBowdenNAHansbroPM 2010 IL-27/IFN-γ induce MyD88-dependent steroid-resistant airway hyperresponsiveness by inhibiting glucocorticoid signaling in macrophages. J Immunol 185 4401 4409
59. IngallsRRicePQureshiNTakayamaKLinJ 1995 The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun 63 3125 3130
60. KlineJNKitagakiKBusingaTRJainVV 2002 Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol 283 L170 L179
61. FonsecaDEKlineJN 2009 Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Delivery Rev 61 256 262
62. SerebriskyDTeperAAHuangC-KLeeS-YZhangT-F 2000 CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol 165 5906 5912
63. WakashinHHiroseKMaezawaYKagamiS-JSutoA 2008 IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Resp Crit Care 178 1023 1032
64. Schnyder-CandrianSTogbeDCouillinIMercierIBrombacherF 2006 Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med 203 2715 2725
65. ParkSJLeeKSKimSRMinKHChoeYH 2009 Peroxisome proliferator-activated receptor γ agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J Immunol 183 3259 3267
66. CosmiLMaggiLSantarlasciVCaponeMCardilicchiaE 2010 Identification of a novel subset of human circulating memory CD4+ T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol 125 222 230
67. HorvatJCBeagleyKWWadeMAPrestonJAHansbroNG 2007 Neonatal Chlamydial infection induces mixed T-cell responses that drive allergic airway disease. Am J Resp Crit Care 176 556 564
68. PrestonJAEssilfieA-THorvatJCWadeMABeagleyKW 2007 Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine 25 8154 8162
69. HorvatJCStarkeyMRKimRYPhippsSGibsonPG 2010 Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol 125 617 625.e616
70. TakatoriHKannoYWatfordWTTatoCMWeissG 2009 Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206 35 41
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



