-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
Two-cysteine peroxiredoxins are ubiquitous peroxidases that play various functions in cells. In Leishmania and related trypanosomatids, which lack catalase and selenium-glutathione peroxidases, the discovery of this family of enzymes provided the molecular basis for peroxide removal in these organisms. In this report the functional relevance of one of such enzymes, the mitochondrial 2-Cys peroxiredoxin (mTXNPx), was investigated along the Leishmania infantum life cycle. mTXNPx null mutants (mtxnpx−) produced by a gene replacement strategy, while indistinguishable from wild type promastigotes, were found unable to thrive in a murine model of infection. Unexpectedly, however, the avirulent phenotype of mtxnpx− was not due to lack of the peroxidase activity of mTXNPx as these behaved like controls when exposed to oxidants added exogenously or generated by macrophages during phagocytosis ex vivo. In line with this, mtxnpx− were also avirulent when inoculated into murine hosts unable to mount an effective oxidative phagocyte response (B6.p47phox−/− and B6.RAG2−/− IFN-γ−/− mice). Definitive conclusion that the peroxidase activity of mTXNPx is not required for parasite survival in mice was obtained by showing that a peroxidase-inactive version of this protein was competent in rescuing the non-infective phenotype of mtxnpx−. A novel function is thus proposed for mTXNPx, that of a molecular chaperone, which may explain the impaired infectivity of the null mutants. This premise is based on the observation that the enzyme is able to suppress the thermal aggregation of citrate synthase in vitro. Also, mtxnpx− were more sensitive than controls to a temperature shift from 25°C to 37°C, a phenotype reminiscent of organisms lacking specific chaperone genes. Collectively, the findings reported here change the paradigm which regards all trypanosomatid 2-Cys peroxiredoxins as peroxide-eliminating devices. Moreover, they demonstrate, for the first time, that these 2-Cys peroxiredoxins can be determinant for pathogenicity independently of their peroxidase activity.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002325
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002325Summary
Two-cysteine peroxiredoxins are ubiquitous peroxidases that play various functions in cells. In Leishmania and related trypanosomatids, which lack catalase and selenium-glutathione peroxidases, the discovery of this family of enzymes provided the molecular basis for peroxide removal in these organisms. In this report the functional relevance of one of such enzymes, the mitochondrial 2-Cys peroxiredoxin (mTXNPx), was investigated along the Leishmania infantum life cycle. mTXNPx null mutants (mtxnpx−) produced by a gene replacement strategy, while indistinguishable from wild type promastigotes, were found unable to thrive in a murine model of infection. Unexpectedly, however, the avirulent phenotype of mtxnpx− was not due to lack of the peroxidase activity of mTXNPx as these behaved like controls when exposed to oxidants added exogenously or generated by macrophages during phagocytosis ex vivo. In line with this, mtxnpx− were also avirulent when inoculated into murine hosts unable to mount an effective oxidative phagocyte response (B6.p47phox−/− and B6.RAG2−/− IFN-γ−/− mice). Definitive conclusion that the peroxidase activity of mTXNPx is not required for parasite survival in mice was obtained by showing that a peroxidase-inactive version of this protein was competent in rescuing the non-infective phenotype of mtxnpx−. A novel function is thus proposed for mTXNPx, that of a molecular chaperone, which may explain the impaired infectivity of the null mutants. This premise is based on the observation that the enzyme is able to suppress the thermal aggregation of citrate synthase in vitro. Also, mtxnpx− were more sensitive than controls to a temperature shift from 25°C to 37°C, a phenotype reminiscent of organisms lacking specific chaperone genes. Collectively, the findings reported here change the paradigm which regards all trypanosomatid 2-Cys peroxiredoxins as peroxide-eliminating devices. Moreover, they demonstrate, for the first time, that these 2-Cys peroxiredoxins can be determinant for pathogenicity independently of their peroxidase activity.
Introduction
The last 15 years have contributed decisively towards the dissection of the enzymatic pathways that lead to peroxide elimination in trypanosomatids, a group of organisms that includes Leishmania spp., Trypanosoma brucei and Trypanosoma cruzi, the causative agents of the different manifestations of leishmaniasis, African sleeping sickness and Chagas' disease, respectively. In these protozoan parasites, which lack the highly efficient enzymes catalase and selenium-containing glutathione peroxidases (GPXs), members of the peroxiredoxin family are regarded as key elements of the peroxide-reduction machinery [1].
Peroxiredoxins (PRXs) are ubiquitous enzymes that use a redox active cysteine residue (peroxidatic Cys) to reduce a broad spectrum of substrates, namely H2O2, organic hydroperoxides and peroxynitrite (ONOO−). Upon reduction of the peroxide, the peroxidatic Cys-SH is oxidized to sulfenic acid (Cys-SOH). In PRXs harboring two active cysteines (known as 2-Cys PRXs), the sulfenic acid is reduced by another Cys residue (resolving Cys) to form a disulfide. According to the location of the resolving Cys, 2-Cys PRXs can be classified as typical or atypical [2]. In trypanosomatids all PRXs characterized to date fall in the category of typical 2-Cys [3]. Peroxiredoxins return to their reduced state upon reduction of the disulfide by an appropriate electron donor. In trypanosomatids such reductant is a unique oxidoreductase of the thioredoxin superfamily, known as tryparedoxin [4], which itself is reduced by these organisms' specific thiol trypanothione [N1,N8-bis(glutathionyl)spermidine; 1,3]. For that reason trypanosomatid PRXs are commonly referred to as tryparedoxin peroxidases or TXNPxs. Apart from participating in antioxidant defense, members of the 2-Cys PRX subfamily are increasingly recognized as playing a more subtle and sophisticated role as regulators of peroxide-mediated cell signaling [2]. More recently, a function as molecular chaperones has also been proposed for some of these enzymes [5], [6].
In trypanosomatids 2-Cys PRXs are present in the parasites' cytosol and single mitochondrion [1]. Cytosolic 2-Cys PRXs (or cTXNPxs) are believed to work as general antioxidant devices that minimize the oxidative insult generated by the parasites' host. In agreement with this, overexpression of cTXNPx in L. infantum and T. cruzi was found to confer resistance to H2O2 and peroxynitrite of exogenous origin [7]–[12], while down-regulation of the T. brucei counterpart enhanced sensitivity to bolus H2O2 [13]. This peroxidase activity should be particularly relevant for Leishmania and T. cruzi, which can invade and proliferate in phagocytes. Not surprisingly, cTXNPxs of these organisms were pointed out as important virulence factors [12], [14]–[17].
Mitochondrial TXNPxs (or mTXNPxs) are, due to their location, favorably positioned to eliminate peroxides produced endogenously, namely those formed as by-products of oxidative phosphorylation, and this was argued to be their main function [7], [8], [11]. However, the observation that overexpression of mTXNPx confers protection towards exogenously added and macrophage-derived H2O2 and peroxynitrite [7], [8], [10], [11], suggested that these enzymes might also contribute to shield trypanosomatids from the oxidative challenge induced by their hosts. This assumption was strengthened by the observation that increased mTXNPx expression is associated with virulent T. cruzi phenotypes [17]. Apart from functioning as general antioxidant devices, the Leishmania donovani mTXNPx was reported to prevent H2O2-induced programmed cell death [18]. One additional, peroxidase-related role suggested for mTXNPxs was regulation of kinetoplast DNA (kDNA) replication. The kDNA is a network of catenated maxi and mini DNA circles that compose the mitochondrial DNA of trypanosomatids and whose replication is initiated when the universal minicircle sequence binding protein (UMSBP) binds specific sequences on minicircle DNA [19]. UMSBP binds to the DNA when it is reduced and is released when oxidized. According to the proposed model, mTXNPx would oxidize UMSBP [20].
The present study aimed at dissecting the functional relevance of mTXNPx in Leishmania infantum. Leishmania have a digenic life cycle that includes two morphologically and physiologically distinct stages: the promastigote (an extracellular form residing in the insect vector) and the amastigote (an intracellular form living inside the mammalian host). Using a homozygous knockout L. infantum line unable to express mTXNPx it was found that, while redundant in promastigotes, this mitochondrial 2-Cys PRX is essential for the establishment of a successful infection in mammals. This result establishes mTXNPx as factor determinant for Leishmania pathogenicity. Importantly, the data gathered here indicate that the essential role played by mTXNPx is not related to its peroxidase activity. Rather, the decreased infectivity of the mutants may be explained by the ability of mTXNPx to function as a chaperone, an activity disclosed here.
Results
Generation of mTXNPx mutants
To study the relevance of mTXNPx during the L. infantum life cycle, a mutant parasite line unable to express this enzyme was produced by homologous recombination. Since Leishmania has a diploid genome, two successive rounds of gene targeting were required to obtain homozygous knockout mutants. The first mTXNPx allele was replaced by a NEO integration cassette, while the second was disrupted with a HYG construct (Figure 1A). As confirmed by Southern blot (SB) of the double transfectants, both mTXNPx alleles were successfully targeted, thus resulting in the generation of a Δmtxnpx::NEO/Δmtxnpx::HYG mutant (hereafter referred to as mtxnpx−). The mtxnpx− mutant was further manipulated in order to obtain a parasite line with restored mTXNPx expression. This was achieved by integrating the mTXNPx ORF into the small sub-unit rRNA locus using the pSSU-PHLEO-infantum vector (Figure 1A), which is a modified version of pSSU-NEO-infantum [21]. The rescued knockout mutants, designated mtxnpx−/+mTXNPx (Δmtxnpx::NEO/Δmtxnpx::HYG [pSSU-PHLEO-infantum-mTXNPx]), were confirmed by PCR to have the mTXNPx ORF and the PHLEO cassette correctly integrated into the ribosomal locus (Figure 1C). Western blot and indirect immunofluorescence analysis showed that mtxnpx− mutants lack mTXNPx expression and that the rescued mtxnpx−/+mTXNPx parasites express mTXNPx in its correct subcellular compartment, the mitochondrion (Figures 1D and E).
Fig. 1. Generation of mTXNPx mutants. 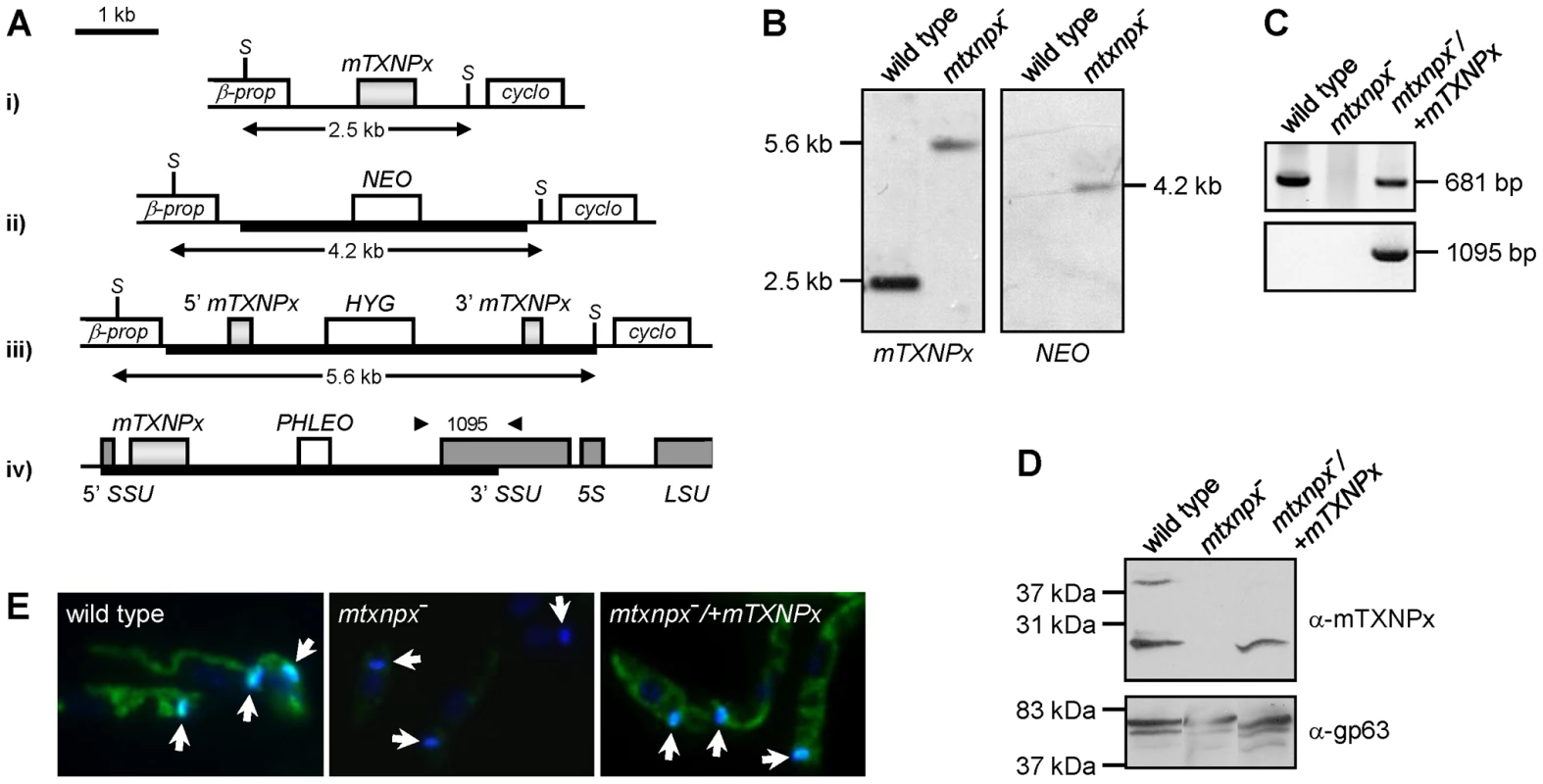
A. Genomic organization of the mTXNPx locus with its flanking β-propeller (β-prop) and cyclophilin (cyclo) genes in i) wild type and in ii) NEO- and iii) HYG- targeted alleles. The HYG disruption construct eliminated 144 nt of the mTXNPx coding sequence, leaving intact the first 292 and the last 245 nt (light grey boxes). SacI (S) restriction sites are indicated. Scheme iv) represents the small subunit 18S rRNA (SSU) locus of mtxnpx−/+mTXNPx mutants with the integrated PHLEO construct harboring the mTXNPx coding sequence. Dark grey boxes represent the 5′ and 3′ coding regions of SSU, of 5S rRNA and of the large subunit 28S rRNA (LSU). Thick lines represent the integrated DNA constructs. Arrowheads show the location of primers used in PCR analysis (in C.) to diagnose for the correct integration of the PHLEO cassette into the ribosomal locus. The number in between arrowheads refers to the expected size of the corresponding PCR product. B. Southern blot analysis of SacI-digested genomic DNA of wild type and mtxnpx− mutants, hybridized with mTXNPx and NEO ORFs. In mtxnpx−, the 5.6 kb band revealed by the mTXNPx probe corresponds to the gene disrupted by the HYG cassette. C. PCR analysis of genomic DNA from wild type, mtxnpx− and mtxnpx−/+mTXNPx parasites, using specific primers to amplify mTXNPx ORF (681 bp) or to diagnose for the correct integration of the PHLEO cassette into the ribosomal locus (1095 bp; primer location represented in A.). D. Western blot analysis of wild type, mtxnpx− and mtxnpx−/+mTXNPx promastigotes, incubated with the anti-mTXNPx antibody and, upon stripping, with anti-gp63 antibody (control for loading). The band above 37 kDa in the upper panel is dimeric mTXNPx. E. Indirect immunofluorescence of parasites lines as above, incubated with anti-mTXNPx antibody (green), merged with DAPI (blue). 1000× magnification. The kDNA is indicated by arrows. mTXNPx is redundant in the insect stage of L. infantum
The mtxnpx− parasite line was generated in the promastigote stage and when grown under standard culture conditions, i.e. in serum supplemented RPMI medium at 25°C, it appeared morphologically normal (data not shown) and displayed growth rates similar to that of wild type promastigotes. Most notably, no defects in kinetoplast morphology and division were noticed (Figure 1E). These observations argue against the involvement of mTXNPx in kDNA replication, a function previously attributed to mitochondrial TXNPxs [20], [22]. Even though these results indicate that mTXNPx is redundant in the insect form of L. infantum, they do not discard a relevant role for this enzyme during the amastigote stage.
mTXNPx is crucial for the long term survival of L. infantum in the mammalian host
The consequence of mTXNPx depletion on the survival of L. infantum amastigotes was assessed by inoculating BALB/c mice, an animal model for visceral leishmaniasis (VL), with equal numbers of stationary phase mtxnpx−, wild type or mtxnpx−/+mTXNPx promastigotes. At defined time points after infection, parasite loads in the liver and spleen (the organs preferentially infected by VL strains) were analyzed by the limiting dilution assay (LDA). Results in Figure 2 show that elimination of mTXNPx had a remarkable effect on the outcome of infection and that this aggravated over time. Indeed, whilst 2 weeks after infection mtxnpx− could still be recovered from both infected organs, at 4 and 8 weeks the mtxnpx− infection indexes were much lower than those of wild type parasites, being below the detection limit of the assay (2.7 log units) at 14 weeks. The impaired virulence of mtxnpx− was recovered in knockout parasites with restored mTXNPx expression (mtxnpx−/+mTXNPx), confirming that it specifically results from depletion of this enzyme (Figure 2). Of notice, the parasitemia levels of mice infected with mtxnpx−/+mTXNPx were consistently below those produced by wild type parasites, a phenomenon that is frequently observed in complemented knockout mutants [23]–[25]. This is likely due to the fact that in the rescued mutants mTXNPx expression is not under the control of its own untranslated regions (as occurs in wild type parasites), but rather it is regulated by the rRNA promoter of the small sub-unit rRNA and by the intergenic region of the cysteine proteinase B 2.8 gene cluster [26].
Fig. 2. Depletion of mTXNPx impairs Leishmania virulence. 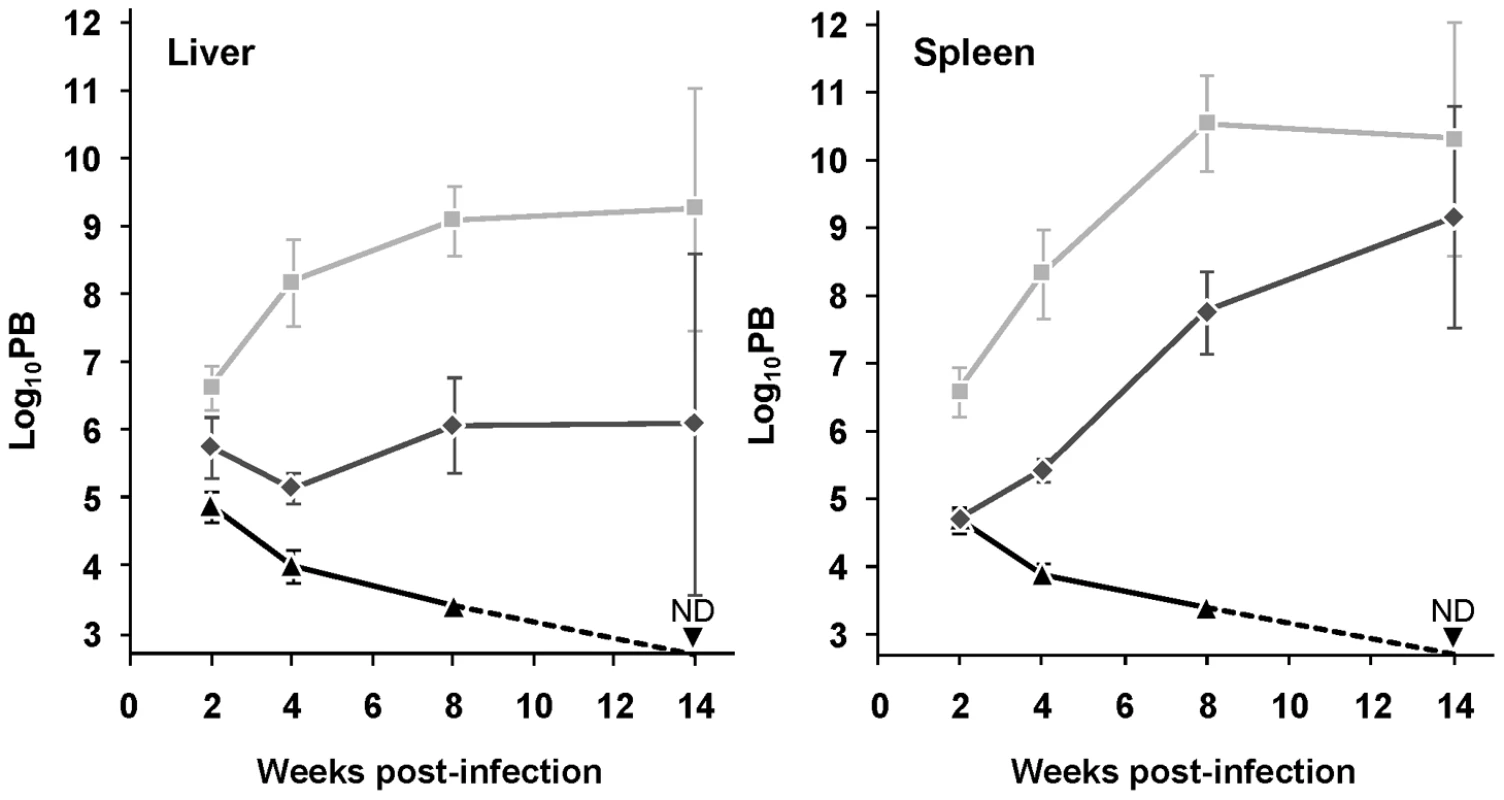
Wild type (light grey squares), mtxnpx− (black triangles) and mtxnpx−/+mTXNPx (dark grey diamonds) promastigotes were inoculated intraperitoneally into BALB/c mice. At different time points after infection, parasite burden (PB) was determined by the limiting dilution assay, as the number of parasites per gram of liver (left) or spleen (right). The Y axis indicates log10PB. Data represent mean and standard error of the mean of 6 independent experiments (involving a total of 85 animals infected with mtxnpx−), except for mtxnpx−/+mTXNPx-infected mice, which refer only to 4 experiments. ND, not detected. From week 4 onward, differences between mtxnpx− and controls are statistically significant. Statistical analysis of these results is in TextS1 and Table S2. In short, these results show that mTXNPx is crucial for the long term survival of L. infantum amastigotes in the mammalian host. The impaired capacity of mTXNPx-depleted parasites to thrive in mice is not due to their failure to invade host cells (the macrophages) or to differentiate into amastigotes, as inferred from microscopic observation of monolayers of peritoneal macrophages from C57/BL6 mice infected with mtxnpx− parasites (Figure S1). The next question is which function, disturbed in mutant parasites, is leading to such defect in infectivity.
Depletion of mTXNPx has no impact on the parasite antioxidant capacity
The L. infantum mTXNPx is an efficient reductase of hydroperoxides [27] as well as of peroxynitrite (the apparent second order rate for peroxynitrite reduction by mTXNPx is 1.6×106 M−1 s−1, at pH 7.4 and 37°C; Romao S, Radi R and Tomás AM, unpublished results). As suggested previously [7], [8], [11], the most likely function for mTXNPxs is the elimination of peroxides generated as a consequence of the parasite's aerobic metabolism. Accordingly, mtxnpx− mutants were assessed for their sensitivity to antimycin A (AA), an inhibitor of the mitochondrial electron transport chain that leads to the local production of reactive oxygen species [28]. However, the observation that AA had the same growth inhibitory effect in mtxnpx− and wild type promastigotes (Figure 3A) suggests that mTXNPx is not critical for the elimination of peroxides generated within the mitochondrion. A different explanation for the impaired infectivity of mtxnpx− could be their inability to deal with host-derived oxidants [11]. However, this premise found no support in the observation that susceptibility of mtxnpx− promastigotes to H2O2 (added as bolus) or to the peroxynitrite donor 3-morpholinosydnonimine hydrochloride (SIN-1) was similar to that of wild type parasites (Figures 3B and C). This hypothesis was explored further by exposing mtxnpx− to host-derived oxidants. As shown in the NBT assay in Figure 3D (inset), phagocytosis of L. infantum promastigotes by murine peritoneal macrophages triggers the generation of superoxide anion (O2.−), a precursor of H2O2. The absence of mTXNPx did not render promastigotes more sensitive to such oxidative burst, as deduced from the comparison of the infection indexes of macrophages inoculated with mtxnpx− and control parasites (wild type and mtxnpx−/+mTXNPx) (Figure 3D). Additional evidence that mTXNPx is not implicated in Leishmania protection against host-derived oxidants was obtained in an in vivo infection experiment using mutant mice with impaired pro-oxidant capacity, namely B6.p47phox−/− and B6.RAG2−/− IFN-γ−/− mice. B6.p47phox−/− mice carry a targeted disruption of the p47 subunit of the phagocyte NADPH oxidase (phox) complex that is responsible for phagocytosis-induced production of O2.−. B6.RAG2−/− IFN-γ−/− mice lack B and T cells and do not express interferon gamma (IFN-γ), i.e. a pro-inflammatory cytokine predominantly secreted by type-1 T cells that activates the inducible nitric oxide synthase (iNOS) and up-regulates phox, thus allowing for peroxynitrite generation in macrophages. Mutants and control mice of the same genetic background (C57BL/6) were infected with equal numbers of mtxnpx− and of control parasites (wild type and mtxnpx−/+mTXNPx) and 5 weeks later the parasite burden was analyzed. The results, plotted in Figure 3E, show that the number of mtxnpx− parasites recovered from the organs of infected mice was either consistently below that of control parasites or undetectable irrespective of the mouse strain serving as host. In other words, even when inoculated in hosts that are unable to mount an efficient oxidative response, mtxnpx− do not recover infectivity. This observation reinforces the idea that the essential function of mTXNPx in amastigotes is not to provide protection against peroxides of exogenous origin. Additionally, it indicates that the avirulent phenotype of mtxnpx− is not due to any microbicidal component induced by the host adaptive immune system, namely by the T lymphocytes. Rather, the inability of mtxnpx− to thrive in mammalian hosts appears to be a consequence of factors inherent to the parasite.
Fig. 3. Depletion of mTXNPx has no impact on the antioxidant capacity of promastigotes. 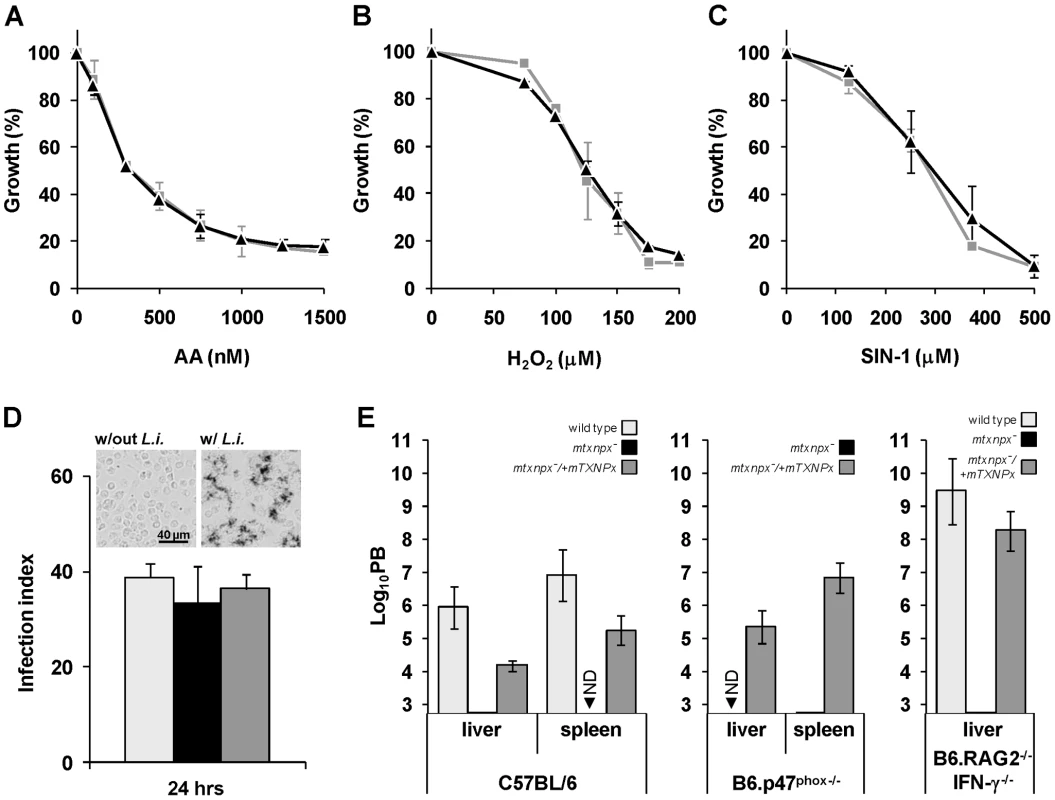
A. B. C. Wild type (light grey squares) and mtxnpx− (black triangles) promastigotes were cultured in the presence of increasing concentrations of (A) antimycin A (AA), (B) H2O2 or (C) the peroxynitrite donor 3-morpholinosydnonimine hydrochloride (SIN-1). Four days later cell densities were measured in a spectrophotometer at 600 nm. The data is expressed as the percentage of promastigote replication relative to control cultures without any exogenous agent. Graphs represent means and standard deviation of three or more experiments (each performed in duplicate). D. Monolayers of peritoneal macrophages from C57BL/6 mice were infected with stationary phase wild type (light grey bars), mtxnpx− (black bars) or mtxnpx−/+mTXNPx (dark grey bars) promastigotes and 24 hrs later the infection indexes determined. The inset shows light microscopy photographs of macrophages incubated with NBT in the absence (“w/out L.i.”) or presence (“w/L.i.”) of parasites. Deposits of dark blue insoluble formazan resulting from NBT reaction with superoxide anion were visible in macrophages 30 min after contact with L. infantum promastigotes (right panel), but not in macrophages to which no parasites were added (left panel). Images were acquired at 100× magnification. E. Mice from different strains (C57BL/6, B6.p47phox−/− and B6.RAG2−/− IFN-γ−/−) were inoculated intraperitoneally with wild type (light grey bars), mtxnpx− (black bars) or mtxnpx−/+mTXNPx (dark grey bars) stationary phase promastigotes. Five weeks after infection parasite burden (PB) was determined in the livers and spleens of mice by the limiting dilution assay. The Y axis represents log10PB. Data represent mean and standard error of the mean of 3 independent experiments involving a total of 72 animals. ND, not detected. Differences between mtxnpx− and controls are statistically significant. Statistical analysis of these results is in TextS1 and Table S3. The critical function of mTXNPx is independent of its peroxidase activity
Results in the previous section suggested that the crucial function played by mTXNPx during infection is not that of an antioxidant device. To definitively conclude about the contribution of mTXNPx peroxidase activity to Leishmania infectivity, a peroxidase-inactive variant of the enzyme (mTXNPxC81S) was tested for its ability to rescue the avirulent phenotype of the knockouts. Cys81 is the peroxidatic Cys of mTXNPx and its replacement by a serine should abolish peroxidase activity, as observed previously [29], [30]. Loss of peroxidase activity of the C81S variant was confirmed in vitro using a recombinant enzyme lacking the first 26 amino acids that compose the mitochondrial targeting peptide (ΔmTXNPxC81S) to mimic the mature protein in the parasite's mitochondrion [8]. As expected from previous mutational analysis [29], [30], when assayed for TXNPx activity, the ΔmTXNPxC81S mutein was unable to catalyze H2O2 reduction, even when present at a 10-fold higher concentration than the control wild type ΔmTXNPx enzyme (Figure 4A). Of notice, on the basis of circular dichroism spectra, substitution of the peroxidatic Cys to a Ser did not alter the secondary structure of the mutein relative to the wild type enzyme, the estimation of α-helix and β-strand contents for both enzymes being of 35% and 29%, respectively (data not shown).
Fig. 4. The peroxidase activity of mTXNPx is not a critical determinant of L. infantum virulence. 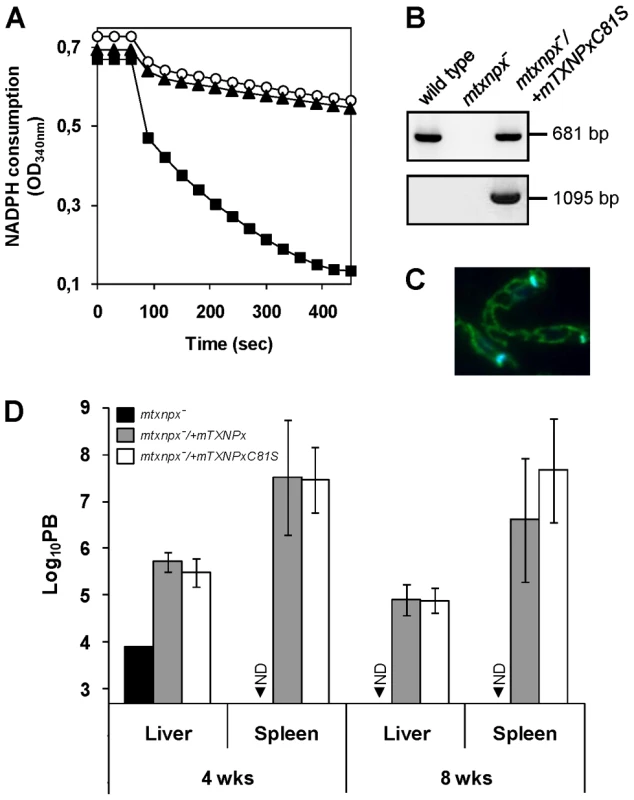
A. Classical assay for TXNPx enzymatic activity. Reaction mixtures contained 200 µM NADPH, 0.5 U ml−1 LiTR, 50 µM TS2, 2.5 µM LiTXN2 and recombinant TXNPx. ΔmTXNPx was added to a final concentration of 0.5 µM (filled squares) and ΔmTXNPxC81S to 5 µM (filled triangles). Reactions were started by addition of 70 µM H2O2 at 60 sec and peroxidase activity followed by monitoring NADPH consumption at 340 nm. The negative control contains no TXNPx (open circles). B. PCR analysis of genomic DNA from mtxnpx−/+mTXNPxC81S parasites using specific primers to amplify the mTXNPx ORF (681 bp) or to diagnose for the correct integration of the PHLEO cassette into the ribosomal locus (1095 bp; location of primers in Figure 1A). Controls, performed with genomic DNA from wild type and mtxnpx− parasites, are also included. C. Indirect immunofluorescence of mtxnpx−/+mTXNPxC81S parasites incubated with the anti-mTXNPx antibody (green labeling), merged with DAPI (blue labeling). Parasites were photographed at 1000× magnification. D. Parasite burden (PB) in liver and spleen of BALB/c mice, determined by the limiting dilution assay, 4 and 8 weeks after infection with mtxnpx− (black bars), mtxnpx−/+mTXNPx (dark grey bars) or mtxnpx−/+mTXNPxC81S (open bars) stationary phase promastigotes. The Y axis represents log10PB. Data indicate mean and standard error of the mean of 2 independent experiments involving a total of 54 animals. ND, not detected. Differences between mtxnpx− and controls are statistically significant. Statistical analysis of these results is in TextS1 and Table S4. To test whether expression of mTXNPxC81S could restore infectivity of mTXNPx knockouts, mtxnpx−/+mTXNPxC81S mutants were generated by transfecting mtxnpx− with pSSU-PHLEO-infantum-mTXNPxC81S. Confirmation for the correct integration of the construct was obtained by PCR (Figure 4B) and the exact subcellular location verified by indirect immunofluorescence analysis (Figure 4C). The mtxnpx−/+mTXNPxC81S transfectants were then inoculated into BALB/c mice and the parasite burden in livers and spleens evaluated at 4 and 8 weeks post infection. The results, depicted in Figure 4D, show that virulence was recovered in mtxnpx− complemented with mTXNPxC81S and that the parasite load produced by this line was similar to that of mtxnpx−/+mTXNPx. These observations thus provide solid evidence that the essential role played by mTXNPx during the infective stage of Leishmania is independent of its peroxidase activity.
Is mTXNPx functioning in vivo as a molecular chaperone?
In addition to acting as peroxidases, 2-Cys PRXs may in some cases exhibit chaperone activity [5], [6], an attribute that, to date, has never been described for any TXNPx. The possibility of mTXNPx acting as a chaperone was therefore investigated as an attempt to provide an alternative function for this enzyme in Leishmania amastigotes.
The chaperone activity of purified recombinant ΔmTXNPx was assessed in vitro by testing its ability to suppress the aggregation of thermally denatured citrate synthase (CS). When incubated at 43°C citrate synthase unfolds, leading to significant aggregation that can be monitored by measuring light scattering in a spectrofluorometer [31]. Addition of ΔmTXNPx to the reaction at a 10-fold molar excess completely suppressed the thermal aggregation of CS (Figure 5A). This effect was much less pronounced when ΔmTXNPx was added at 5-fold molar excess. Importantly, the ΔmTXNPxC81S mutein was also capable of preventing CS aggregation, exhibiting the same behavior as the wild type enzyme (Figure 5A).
Fig. 5. Evidences for the chaperone activity of mTXNPx. 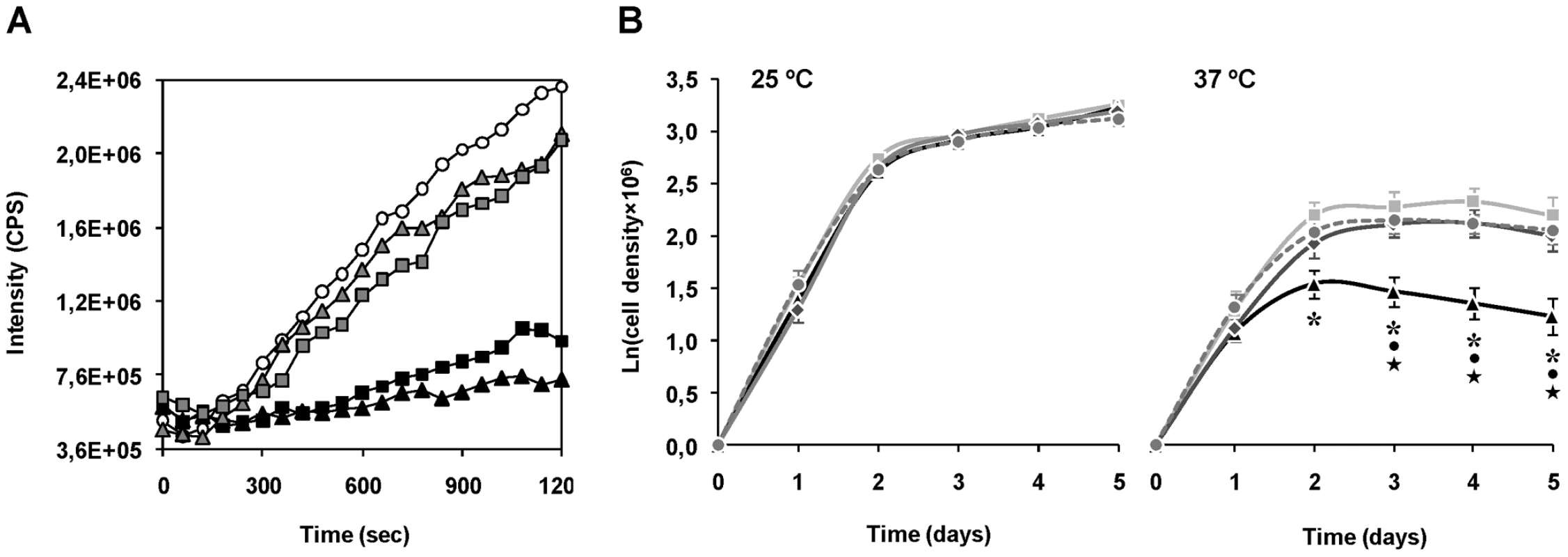
A. Chaperone activity of purified recombinant ΔmTXNPx and ΔmTXNPxC81S was analyzed in vitro by assessing the enzymes' ability to suppress the thermal aggregation of citrate synthase (CS). Aggregation of CS was induced at 43°C and monitored by measuring light scattering at 500 nm in the absence (open circles) or presence of 5∶1 (grey-filled squares) or 10∶1 (black squares) ratios of ΔmTXNPx monomers to CS monomers. Alternatively, the mutein ΔTXNPxC81S was added to the reaction at 5∶1 (grey-filled triangles) or 10∶1 (black triangles) ratios. B. Wild type (light grey squares), mtxnpx− (black triangles) mtxnpx−/+mTXNPx (dark grey diamonds) and mtxnpx−/+mTXNPxC81S (dark grey circles and dashed line) L. infantum promastigotes were seeded at 106 ml−1 in complete RPMI medium and incubated at 25°C (left) and 37°C (right). The Y axis represents ln (or loge) of cell densities recorded throughout 5 days. Values represent mean and standard error of the mean of 4 to 8 independent curves. Also indicated are the statistically significant differences (i.e. p<0.05) between mtxnpx− and each of the control parasite lines, i.e. wild type (*), mtxnpx−/+mTXNPx (•) and mtxnpx−/+mTXNPxC81S (★). To gain insight into the physiological significance of the chaperone activity of mTXNPx a thermotolerance assay was conducted. The experiment consisted in monitoring cell growth of mtxnpx− and control parasites (wild type, mtxnpx−/+mTXNPx, and mtxnpx−/+mTXNPxC81S) at both 25°C and 37°C, which are the temperatures encountered by the parasite in the insect vector and in the mammalian host, respectively. The cell growth curves depicted in Figure 5B show that, while at 25°C the proliferation rates of all parasite lines were indistinguishable, at 37°C the growth rate of mtxnpx− was significantly lower than that of wild type parasites. The thermo-sensitive phenotype observed for mtxnpx− was recovered in the rescued mtxnpx−/+mTXNPx and mtxnpx−/+mTXNPxC81S mutants. These observations show that expression of mTXNPx renders promastigotes more permissive to 37°C, i.e. this enzyme confers thermotolerance to L. infantum irrespective of its peroxidase activity. It is thus reasonable to speculate that the crucial role played by mTXNPx during amastigote development in the mammalian host is that of a molecular chaperone.
Discussion
In parasites of the family Trypanosomatidae the peroxidase activity of 2-Cys PRXs (which in these organisms are designated TXNPxs) is accepted as the basis of their physiologic functions [1], [3]. Data presented in this report demonstrate that this activity is not crucial in the case of mTXNPx, even though the protein itself is essential for L. infantum amastigote survival. Instead, experimental evidence is provided suggesting that it may be the activity of mTXNPx as a molecular chaperone, unraveled here, that is determinant for parasite viability.
The finding that a peroxidase-inactive version of mTXNPx is competent to ensure amastigote survival excludes per se a crucial role for the peroxidase-related functions previously attributed to mTXNPxs, namely elimination of peroxides and regulation of H2O2-induced apoptosis [7], [8], [11], [18]. Accordingly, these activities must be taken over by other means. Detoxification of exogenously-derived peroxides (such as those generated by the host immune response) might be efficiently fulfilled by cytosolic 2-Cys PRXs and non-selenium glutathione peroxidase-like enzymes (nsGPX) [7], [8], [11], [32]–[34]. As for reduction of peroxides of mitochondrial origin, different scenarios can be envisaged. One possibility is that mitochondrial nsGPX carries out this antioxidant function. This hypothesis requires that the substrate specificities of nsGPX and mTXNPx overlap [33]–[35], and implies the existence of an efficient nsGPX reductant in the mitochondrion, which is still to be found [36]. Of notice, in the particular case of H2O2, this could diffuse and be reduced by cytosolic peroxidases. In a different perspective, elimination of peroxides may occur via non-catalytic pathways. In this situation, low molecular weight thiols, such as trypanothione (the trypanosomatids' specific thiol), glutathione, ovothiol, free cysteines and even thiol groups exposed on protein surfaces would directly reduce peroxides [37]–[39]. In either circumstance, an ascorbate peroxidase, located in the intermembrane space of the Leishmania mitochondrion [40] (gene LinJ.34.0070 in the L. infantum GeneDB), may also contribute to peroxide elimination in this organelle.
A different role previously attributed to mTXNPxs is the regulation of kDNA replication. As proposed by Sela et al. [20], mTXNPx would affect kDNA replication by oxidizing UMSBP and, consequently, turning “off” the binding of this protein to the minicircle origin of replication. However, in this report no evidence could be found linking mTXNPx depletion to defects in replication rate or in kDNA morphology of L. infantum promastigotes. Even though these analyses could not be extended to amastigotes, kDNA replication is highly conserved in trypanosomatids [19], making it difficult to accept that mTXNPx would regulate kDNA replication only in this life stage. Moreover, the proposed model for kDNA replication established a fundamental role for a mitochondrial TXN as reductant of UMSBP and it was shown previously that such enzyme is not essential in Leishmania and does not exist in trypanosomes [36]. The mechanism underlying regulation of kDNA replication involving mTXNPxs (and a mitochondrial TXN) must, therefore, be re-evaluated.
This work has also, for the first time, uncovered that trypanosomatid 2-Cys PRXs can function as molecular chaperones and that this activity might be relevant for parasite survival in vivo. Several pieces of evidence point into this direction. i) Purified recombinant mTXNPx was shown to prevent the thermal aggregation of CS, a well-known chaperone substrate [31]. This activity does not depend on the peroxidatic capacity of mTXNPx, as the mTXNPxC81S mutein was also active in this assay, thus providing a rationale for the recovery of virulence observed for mtxnpx−/+mTXNPxC81S. This is in line with observations reported by Jang et al. [5]. ii) The chaperone activity of 2-Cys PRXs is known to depend on their quaternary structure and, as shown before, mTXNPx is capable of associating into decameric ring-like structures [27], which are the minimal oligomeric arrangements required for such function [41]. iii) Gain of chaperone function and loss of peroxidase activity of 2-Cys PRXs are, in most organisms, accompanied with the overoxidized form of the protein [2] and mTXNPx undergoes overoxidation when promastigotes are exposed to 37°C (Figure S2). Since Leishmania do not possess sulfiredoxins to enzymatically regenerate overoxidized 2-Cys PRXs [1] as occurs in higher eukaryotes, such peroxidase-inactive form of mTXNPx is likely to persist until the protein is synthesized de novo. iv) Additional support consubstantiating a role for mTXNPx as a chaperone came from the observation that lack of mTXNPx expression rendered promastigotes more sensitive to 37°C, the temperature encountered in the mammalian host. Such thermo-sensitive phenotype is reminiscent of some chaperone-deficient mutants [42]–[44]. Accepting that mTXNPx is a molecular chaperone in vivo, one can only speculate how this function could impact on parasite survival under the conditions met in the host. In mitochondria, molecular chaperones are associated with a multitude of functions. They participate in the biogenesis of new mitochondrial peptides by assisting their trans-membrane transport and their refolding to the native conformation inside the matrix. In addition, they are part of the mitochondrial protein quality control system that repairs damaged or misfolded proteins or mediates their removal by proteolysis [45]. Under situations of cellular stress (including elevated temperatures), the activity of mitochondrial chaperones becomes even more imperative for protein homeostasis [45]. Accordingly, it is reasonable to assume that mTXNPx functions as a molecular chaperone that ensures integrity of mitochondrial functions, its activity being particularly relevant when parasites reside in vertebrate host.
Another important outcome of this report is the identification of mTXNPx as a new factor critical for Leishmania infectivity. This finding may have impact on the development of new strategies to control leishmaniasis as the mtxnpx− parasite line produced here can be regarded as the basis of a live attenuated vaccine. In this context, the observation that mtxnpx− cannot give rise to a productive infection even in immunocompromised mice, suggests that this strategy would not pose major safety issues.
In conclusion, the work presented in this manuscript sheds lights on the functional relevance of mTXNPx by showing that, even though this molecule is crucial for L. infantum survival during infection of the vertebrate host, its peroxidase activity is superfluous. A novel peroxidase-unrelated function, as a molecular chaperone, is proposed for this enzyme, which could be critical for mitochondrial functionality under the conditions encountered by the parasite in the mammalian host. This report thus constitutes a turning point into the current state of knowledge regarding the physiologic role of peroxiredoxins in trypanosomatids.
Materials and Methods
Ethics statement
The experimental animal procedures were approved by the Local Animal Ethics Committee of Institute for Molecular and Cell Biology, University of Porto, Portugal and licensed by DGV (General Directory of Veterinary, Ministry of Agriculture, Rural Development and Fishing, Govt. of Portugal), in May 18, 2006 with reference 520/000/000/2006. All animals were handled in strict accordance with good animal practice as defined by national authorities (DGV, Law nu1005/92 from 23rd October) and European legislation EEC/86/609.
Parasite cultures
Leishmania infantum promastigostes (MHOM MA67ITMAP263) were cultured at 25°C in RPMI 1640 Glutamax medium, supplemented with 10% inactivated fetal bovine serum (FBSi), 50 U ml−1 penicillin, 50 µg ml−1 streptomycin (all from Gibco) and 25 mM hepes sodium salt pH 7.4 (Sigma).
Generation of knockout and rescue constructs
Primers used to generate the constructs are summarized in Table S1. The accuracy of all assembled constructs was verified by sequencing. To produce the NEO replacement construct, sections of the 5′ and 3′ non-coding sequences flanking the mTXNPx ORF were PCR-amplified from a cosmid clone [8] using primers P1/P2 and P3/P4, respectively. Following digestion with the appropriate restriction enzymes, PCR products were cloned into the BamHI-XhoI and KpnI sites of the pTEX-NEO plasmid [46], on both sides of the neomycin phosphotransferase (NEO) gene. To assemble the HYG disruption construct, fragments containing part of the 5′ and 3′ untranslated regions and of the mTXNPx coding sequence were amplified by PCR from genomic DNA of the NEO-targeted mutants, using primers P5/P6 and P7/P8. Upon digestion with the appropriate restriction enzymes, PCR products were cloned into BamHI-EcoRV and KpnI sites of the pTEX-HYG plasmid, a version of pTEX-NEO wherein the NEO gene had been replaced by the hygromycin phosphotransferase (HYG) open reading frame. To generate the rescue construct, the mTXNPx ORF was introduced into the XmaI and the Klenow-treated BamHI restriction sites of the pSSU-NEO-infantum plasmid [21]. This plasmid is a modified version of the pSSU-int [26], wherein the HYG gene had been replaced by NEO and the 5′ flanking sequence of the small sub-unit rRNA (18S rRNA) gene of Leishmania mexicana substituted by the homologous sequence of L. infantum. Upon cloning of the mTXNPx coding sequence into pSSU-NEO-infantum, the NEO ORF was replaced by the bleomycin hydrolase (PHLEO) gene. One additional rescue construct (pSSU-NEO-infantum-mTXNPxC81S), containing a mutated version of mTXNPx, was generated by site directed mutagenesis (see below). Before transfection of L. infantum promastigotes, the NEO, HYG and PHLEO constructs were linearized by digestion with HincII, BamHI-SacI, and NdeI-PmeI, respectively, and purified from agarose gels by electroelution.
Production of the mTXNPxC81S mutein
A site directed mutagenesis strategy was employed to introduce the Cys81 to Ser (C81S) mutation in the mTXNPx ORF cloned into pSSU-PHLEO-infantum (detailed above) and pET28c (detailed below). For this, the full-length plasmids were PCR-amplified with sense and antisense mutated primers (P9 and P10 in Table S1) using Pfu polymerase (Stratagene). Upon digestion of parental (wild type) DNA with 10 units of DpnI (New England Biolabs) for 1 h at 37°C, the reaction (4 µl) was used directly to transform Escherichia coli DH5α strain. Mutant colonies were confirmed to carry the site-directed mutation by sequencing both strands.
Transfection of L. infantum and isolation of mutants
Promastigotes in the logatithmic phase of growth were electroporated at 450 V and 350–400 µF with 1 to 5 µg of DNA as described elsewhere [47]. Parasites were allowed to recover in 10 ml of culture medium without selective drugs for 24 hours. Drugs were then added to 5 ml of the liquid culture and the remaining 5 ml were pelleted and plated onto agar plates containing the same drug(s). Geneticin (G418; Sigma) was used at 15 µg ml−1, hygromycin (Invitrogen) at 10 µg ml−1 and bleomycin (Sigma) at 17.5 µg ml−1. Upon 2 to 3 weeks of growth on agar, colonies were picked up and transferred into liquid medium.
Indirect immunofluorescence assay (IFAT)
Immunofluorescence assays were performed according to Castro et al. [8]. Briefly, parasites were fixed with 4% paraformaldehyde (w/v) in PBS (0.1 M sodium phosphate buffer pH 7.2, 0.15 M NaCl), spotted onto polylysine-coated microscope slides, permeabilized with 0.1% (v/v) Triton X-100 and incubated with the polyclonal anti-mTXNPx antibody [8] and 4′,6-diamidino-2-phenylindole (DAPI). The secondary antibody was Alexa Fluor 488 anti-rabbit IgG (Molecular Probes). Slides were mounted in Vectashield (Vector Laboratories) and examined with an AxioImager Z1 microscope (Carl Zeiss, Germany).
Drug sensitivity assays
L. infantum promastigotes in the logarithmic phase of growth were seeded at 106 cells ml−1 in 24-well plates containing increasing concentrations (in duplicate) of either antimycin A (AA), H2O2 or 3-morpholinosydnonimine hydrochloride (SIN-1) (all from Sigma). Parasites were allowed to grow for 3–4 days and cell densities were measured in a spectrophotometer at 600 nm.
Animals and ethics statement
BALB/c, C57BL/6 and National Marine Research Institute (NMRI) mice were purchased from Charles River (Madrid, Spain). Mice with a targeted disruption of the p47 subunit of the NADPH-oxidase complex on a C57BL/6 background (B6.p47phox−/−) were purchased from Taconic (Lille Skensved, Denmark). Until the day of infection with L. infantum, and as prophylatic treatment against bacterial infection, trimethoprim-sulfamethoxazole (Bactrim; 600 mg l−1) was administered in the drinking water to B6.p47phox−/− mice. Mice double deficient in gamma interferon (IFN-γ) and in recombinase activating gene-2 (RAG-2) were obtained by crossing single knock out strains on C57BL/6 background. All mice were raised in specific pathogen-free conditions. Euthanasia was performed in a 20% isofluorane atmosphere and all efforts were made to minimize suffering.
Infection of monolayers of murine macrophages with promastigotes
Macrophages obtained by peritoneal lavage of C57/BL6 mice were seeded at 4×105 cells per well in DMEM Glutamax complemented with 10% FBSi, 50 U ml−1 penicillin and 50 µg ml−1 streptomycin (all from Gibco), and allowed to adhere for 2 hrs at 37°C, 5% CO2. Upon washing with Hanks balanced salt solution (HBSS, Gibco), macrophages were incubated with opsonized promastigotes (in the stationary phase of growth) at a parasite:macrophage ratio of 10∶1. For detection of superoxide anion (O2·−), a freshly prepared solution of 1 mg ml−1 nitroblue tetrazolium (NBT), prepared in Dulbecco's PBS was added to macrophages. After 30 min of incubation at 37°C, cells were washed with HBSS, fixed with methanol and deposition of formazan observed with an optical microscope Olympus CX31. For determination of infection indexes, infection with promastigotes was allowed to proceed for 3 hrs, after which non-internalized parasites were removed with HBSS. Cells were cultured for additional 24 hrs and then washed, fixed with 4% paraformaldehyde (w/v) in PBS, permeabilized with 0.1% (v/v) Triton X-100, and incubated with the anti-cTXNPx1 [8] antibody and propidium idodide (PI). Secondary antibody was Alexa Fluor 488 anti-rabbit IgG (Molecular Probes). Slides were mounted in Vectashield (Vector Laboratories). Images were acquired with an Axiocam MR ver.3.0 camera (Carl Zeiss, Germany) coupled to an AxioImager Z1 microscope (Carl Zeiss, Germany). A minimum of 3000 macrophages was counted in each experiment using specifically designed software (unpublished data) and the infection index was obtained by multiplying the percentage of infected macrophages by the average number of intracellular amastigotes per macrophage.
Determination of parasite burden by the limiting dilution assay
Parasites of all lines were passaged through NMRI mice at least twice prior to infection experiments. Next, 108 L. infantum stationary phase promastigotes, were inoculated intraperitoneally into 6 - to 9-weeks old male BALB/c mice. At defined time points, mice were sacrificed and their livers and spleens excised, weighed and homogenized in Schneider's medium (Sigma) supplemented with 10% FBSi, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin (all from Gibco), 5 mM hepes sodium salt pH 7.4 (Sigma), 5 µg ml−1 phenol-red (Sigma) and 2% sterile human urine. Homogenates were then diluted to 10 mg ml−1 and these cell suspensions titrated in quadruplicate across a 96-well plate in serial four-fold dilutions (four titrations per organ). After two weeks of growth at 25°C, the last dilution containing promastigotes was recorded and the number of parasites per gram of organ (parasite burden) calculated as described by Buffet et al. [48]. The detection limit of this method is 500 parasites g−1 (i.e. 2.7 log units).
Heterologous expression and purification of ΔmTXNPx and ΔmTXNPxC81S
To mimic the mature mitochondrial protein, truncated wild type mTXNPx (ΔmTXNPx), i.e. lacking the first 26 amino acids that compose the mitochondrial targeting peptide [8], was produced in the E. coli strain BL21 (DE3) Tuner upon transformation a pET28c (Novagen) construct containing the ΔmTXNPx gene fragment (PCR-amplified with primers P11/P12 in Table S1). To produce the mutant ΔmTXNPxC81S protein, the pET28c-ΔmTXNPxC81S was generated by site directed mutagenesis (detailed above) and used to transform the same E. coli strain. Both enzymes were expressed in bacteria as fusion proteins carrying an N-terminal six histidine tag. Upon induction for 3 hrs at 30°C in the presence of 50 µg ml−1 kanamycin and 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG), bacteria were pelleted, suspended in 500 mM NaCl, 20 mM Tris-HCl pH 7.6, disrupted by sonication and centrifuged at 30,000×g for 30 min at 4°C. The supernatant was applied to a His Bind resin (Novagen) column and the recombinant protein eluted with an imidazole gradient (5 to 1000 mM) at a flow rate of 1 ml min−1. Fractions confirmed to contain the protein by SDS-PAGE were pooled, applied to PD-10 columns (Amersham) and eluted in 50 mM sodium phosphate buffer pH 8.0. For removal of the His tag, enzymes were first digested with biotinylated thrombin (Novagen), and then incubated with immobilized streptavidin for removal of the protease. Proteins were concentrated by ultrafiltration, recovered in 40 mM hepes pH 7.5 and quantified by the bicinchoninic acid (BCA) protein assay (Pierce), using bovine serum albumin (BSA) as standard.
Determination of peroxidase activity
Routine determination of TXNPx activity was performed according to Nogoceke et al. [4]. Briefly, reaction mixtures were prepared in a total volume of 300 µl of 50 mM Tris-HCl, 1 mM EDTA, pH 8.0, containing 200 µM NADPH, 0.5 U ml−1 L. infantum TR, 50 µM trypanothione disulfide (TS2, Bachem), 2.5 µM L. infantum TXN2 [49] and varying concentrations of ΔmTXNPx or ΔmTXNPxC81S. Reactions were started by addition of 70 µM hydrogen peroxide (H2O2, Sigma) and NADPH consumption was followed at 340 nm. All reactions were performed at 25°C and monitored with a Shimadzu UV-2401 PC spectrophotometer (Shimadzu Corporation).
Determination of chaperone activity
The chaperone activity of ΔmTXNPx and ΔmTXNPxC81S was measured using citrate synthase (CS) from porcine heart (Sigma) as substrate, as described before [31]. Each enzyme was diluted in 40 mM hepes sodium salt pH 7.5 to reach final concentrations of 0.75 or 1.5 µM. A cocktail of protease inhibitors and 100 µM DTT were added to the reaction mixture to prevent degradation of CS and disulfide cross-linking between the peroxiredoxin and CS molecules, respectively, both of which could result in an apparent chaperone-like activity. Samples were pre-incubated at 43°C for 5 min and the reaction started by addition of CS to a final concentration of 0.15 µM. Light scattering due to CS aggregation at 43°C was monitored using a FluoroMax-4 spectrofluorometer, with excitation and emission wavelengths set to 500 nm and excitation and emission slits set to 2 nm. Data were recorded for 20 min.
Thermotolerance assays
L. infantum promastigotes of all lines, previously synchronized by 4–5 daily passages of 5×105 cells ml−1, were seeded at 106 ml−1 and allowed to grow for 4 days at either 25°C or 37°C. Every 24 hours cell densities were determined with a Neubauer-counting chamber for growth curve determination.
Statistical analysis
Comparisons between the parasite lines were carried out using analysis of variance. When normality or homogeneity of variances was not observed the Kruskal-Wallis non parametric test was used. In this case, multiple comparisons were carried using the Mann-Whitney test, with the Bonferroni correction. In order to investigate whether the distribution of the negative versus positive organs (in the LDA) was independent of the parasite line, the Chi-square and the Fisher's exact tests were employed (see Text S1). Statistical significance was assessed for p<0.05. The analysis was carried out using IBM SPSS Statistics 19.
Supporting Information
Zdroje
1. CastroHTomásAM 2008 Peroxidases of Trypanosomatids. Antioxid Redox Signal 10 1593 1606
2. RheeSGWooHA 2011 Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H2O2, and Protein Chaperones. Antioxid Redox Signal 15 781 794
3. Krauth-SiegelRLCominiMASchleckerT 2007 The trypanothione system. FlohéLHarrisJR Peroxiredoxin Systems New York Springer 231 251
4. NogocekeEGommelDUKiessMKaliszHMFlohéL 1997 A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem 378 827 836
5. JangHHLeeKOChiYHJungBGParkSK 2004 Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117 625 635
6. MoonJCHahYSKimWYJungBGJangHH 2005 Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J Biol Chem 280 28775 28784
7. WilkinsonSRTempertonNJMondragonAKellyJM 2000 Distinct mitochondrial and cytosolic enzymes mediate trypanothione-dependent peroxide metabolism in Trypanosoma cruzi. J Biol Chem 275 8220 8225
8. CastroHSousaCSantosMCordeiro-da-SilvaAFlohéL 2002 Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic Biol Med 33 1552 1562
9. BarrSDGedamuL 2003 Role of peroxidoxins in Leishmania chagasi survival. Evidence of an enzymatic defense against nitrosative stress. J Biol Chem 278 10816 10823
10. LinYCHsuJYChiangSCLeeST 2005 Distinct overexpression of cytosolic and mitochondrial tryparedoxin peroxidases results in preferential detoxification of different oxidants in arsenite-resistant Leishmania amazonensis with and without DNA amplification. Mol Biochem Parasitol 142 66 75
11. PiacenzaLPeluffoGAlvarezMNKellyJMWilkinsonSR 2008 Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage - and endogenously-derived peroxynitrite. Biochem J 410 359 368
12. IyerJPKaprakkadenAChoudharyMLShahaC 2008 Crucial role of cytosolic tryparedoxin peroxidase in Leishmania donovani survival, drug response and virulence. Mol Microbiol 68 372 391
13. WilkinsonSRHornDPrathalingamSRKellyJM 2003 RNA interference identifies two hydroperoxide metabolizing enzymes that are essential to the bloodstream form of the african trypanosome. J Biol Chem 278 31640 31646
14. AcestorNMasinaSIvesAWalkerJSaraviaNG 2006 Resistance to oxidative stress is associated with metastasis in mucocutaneous leishmaniasis. J Infect Dis 194 1160 1167
15. WalkerJAcestorNGongoraRQuadroniMSeguraI 2006 Comparative protein profiling identifies elongation factor-1beta and tryparedoxin peroxidase as factors associated with metastasis in Leishmania guyanensis. Mol Biochem Parasitol 145 254 264
16. PiacenzaLAlvarezMNPeluffoGRadiR 2009 Fighting the oxidative assault: the Trypanosoma cruzi journey to infection. Curr Opin Microbiol 12 415 421
17. PiacenzaLZagoMPPeluffoGAlvarezMNBasombrioM 2009 Enzymes of the antioxidant network as novel determiners of Trypanosoma cruzi virulence. Int J Parasitol 39 1455 1464
18. HarderSBenteMIsermannKBruchhausI 2006 Expression of a mitochondrial peroxiredoxin prevents programmed cell death in Leishmania donovani. Eukaryot Cell 5 861 870
19. LiuBLiuYMotykaSAAgboEEEnglundPT 2005 Fellowship of the rings: the replication of kinetoplast DNA. Trends Parasitol 21 363 369
20. SelaDYaffeNShlomaiJ 2008 Enzymatic mechanism controls redox-mediated protein-DNA interactions at the replication origin of kinetoplast DNA minicircles. J Biol Chem 283 32034 32044
21. BeattieLPeltanAMaroofAKirbyABrownN 2010 Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog 6 e1000805
22. MotykaSADrewMEYildirirGEnglundPT 2006 Overexpression of a cytochrome b5 reductase-like protein causes kinetoplast DNA loss in Trypanosoma brucei. J Biol Chem 281 18499 18506
23. WieseM 1998 A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. Embo J 17 2619 2628
24. HuynhCSacksDLAndrewsNW 2006 A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med 203 2363 2375
25. SpäthGFEpsteinLLeaderBSingerSMAvilaHA 2000 Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A 97 9258 9263
26. MisslitzAMottramJCOverathPAebischerT 2000 Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol 107 251 261
27. CastroHBuddeHFlohéLHofmannBLünsdorfH 2002 Specificity and kinetics of a mitochondrial peroxiredoxin of Leishmania infantum. Free Radic Biol Med 33 1563 1574
28. LoschenGFlohéLChanceB 1971 Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett 18 261 264
29. MontemartiniMKaliszHMHechtHJSteinertPFlohéL 1999 Activation of active-site cysteine residues in the peroxiredoxin-type tryparedoxin peroxidase of Crithidia fasciculata. Eur J Biochem 264 516 524
30. FlohéLBuddeHBrunsKCastroHClosJ 2002 Tryparedoxin peroxidase of Leishmania donovani: molecular cloning, heterologous expression, specificity, and catalytic mechanism. Arch Biochem Biophys 397 324 335
31. BuchnerJGrallertHJakobU 1998 Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol 290 323 338
32. WilkinsonSRMeyerDJKellyJM 2000 Biochemical characterization of a trypanosome enzyme with glutathione-dependent peroxidase activity. Biochem J 352 Pt 3 755 761
33. SchleckerTSchmidtADirdjajaNVonckenFClaytonC 2005 Substrate specificity, localization, and essential role of the glutathione peroxidase-type tryparedoxin peroxidases in Trypanosoma brucei. J Biol Chem 280 14385 14394
34. KönigJFairlambAH 2007 A comparative study of type I and type II tryparedoxin peroxidases in Leishmania major. FEBS J 274 5643 5658
35. DiechtierowMKrauth-SiegelRL 2011 A tryparedoxin-dependent peroxidase protects African trypanosomes from membrane damage. Free Radic Biol Med 51 856 868
36. CastroHRomaoSCarvalhoSTeixeiraFSousaC 2010 Mitochondrial redox metabolism in trypanosomatids is independent of tryparedoxin activity. PLoS One 5 e12607
37. HillebrandHSchmidtAKrauth-SiegelRL 2003 A second class of peroxidases linked to the trypanothione metabolism. J Biol Chem 278 6809 6815
38. TrujilloMBuddeHPiñeyroMDStehrMRobelloC 2004 Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J Biol Chem 279 34175 34182
39. RequejoRHurdTRCostaNJMurphyMP 2010 Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J 277 1465 1480
40. DolaiSYadavRKPalSAdakS 2008 Leishmania major ascorbate peroxidase overexpression protects cells against reactive oxygen species-mediated cardiolipin oxidation. Free Radic Biol Med 45 1520 1529
41. ParkJWPiszczekGRheeSGChockPB 2011 Glutathionylation of peroxiredoxin I induces decamer to dimers dissociation with concomitant loss of chaperone activity. Biochemistry 50 3204 3210
42. SanchezYLindquistSL 1990 HSP104 required for induced thermotolerance. Science 248 1112 1115
43. HübelAKrobitschSHöraufAClosJ 1997 Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol Cell Biol 17 5987 5995
44. QueitschCHongSWVierlingELindquistS 2000 Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12 479 492
45. VoosW 2009 Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol 160 718 725
46. KellyJMWardHMMilesMAKendallG 1992 A shuttle vector which facilitates the expression of transfected genes in Trypanosoma cruzi and Leishmania. Nucleic Acids Res 20 3963 3969
47. RomaoSCastroHSousaCCarvalhoSTomásAM 2009 The cytosolic tryparedoxin of Leishmania infantum is essential for parasite survival. Int J Parasitol 39 703 711
48. BuffetPASulahianAGarinYJNassarNDerouinF 1995 Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother 39 2167 2168
49. CastroHSousaCNovaisMSantosMBuddeH 2004 Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Mol Biochem Parasitol 136 137 147
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



