-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
Cell-to-cell movement of plant viruses occurs via plasmodesmata (PD), organelles that evolved to facilitate intercellular communications. Viral movement proteins (MP) modify PD to allow passage of the virus particles or nucleoproteins. This passage occurs via several distinct mechanisms one of which is MP-dependent formation of the tubules that traverse PD and provide a conduit for virion translocation. The MP of tubule-forming viruses including Grapevine fanleaf virus (GFLV) recruit the plant PD receptors called Plasmodesmata Located Proteins (PDLP) to mediate tubule assembly and virus movement. Here we show that PDLP1 is transported to PD through a specific route within the secretory pathway in a myosin-dependent manner. This transport relies primarily on the class XI myosins XI-K and XI-2. Inactivation of these myosins using dominant negative inhibition results in mislocalization of PDLP and MP and suppression of GFLV movement. We also found that the proper targeting of specific markers of the Golgi apparatus, the plasma membrane, PD, lipid raft subdomains within the plasma membrane, and the tonoplast was not affected by myosin XI-K inhibition. However, the normal tonoplast dynamics required myosin XI-K activity. These results reveal a new pathway of the myosin-dependent protein trafficking to PD that is hijacked by GFLV to promote tubule-guided transport of this virus between plant cells.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002327
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002327Summary
Cell-to-cell movement of plant viruses occurs via plasmodesmata (PD), organelles that evolved to facilitate intercellular communications. Viral movement proteins (MP) modify PD to allow passage of the virus particles or nucleoproteins. This passage occurs via several distinct mechanisms one of which is MP-dependent formation of the tubules that traverse PD and provide a conduit for virion translocation. The MP of tubule-forming viruses including Grapevine fanleaf virus (GFLV) recruit the plant PD receptors called Plasmodesmata Located Proteins (PDLP) to mediate tubule assembly and virus movement. Here we show that PDLP1 is transported to PD through a specific route within the secretory pathway in a myosin-dependent manner. This transport relies primarily on the class XI myosins XI-K and XI-2. Inactivation of these myosins using dominant negative inhibition results in mislocalization of PDLP and MP and suppression of GFLV movement. We also found that the proper targeting of specific markers of the Golgi apparatus, the plasma membrane, PD, lipid raft subdomains within the plasma membrane, and the tonoplast was not affected by myosin XI-K inhibition. However, the normal tonoplast dynamics required myosin XI-K activity. These results reveal a new pathway of the myosin-dependent protein trafficking to PD that is hijacked by GFLV to promote tubule-guided transport of this virus between plant cells.
Introduction
Plant viruses are intracellular parasites that recruit numerous host factors for their replication and movement within plants. Virus cell-to-cell movement involves transport from replication factories to the cell periphery, passage through plasmodesmata (PD) interconnecting adjacent cells, and long-distance transport via the phloem vasculature [1]. All plant viruses encode one or more specialized movement proteins (MP) facilitating virus transport. The structurally and mechanistically diverse MP employ at least three different movement strategies. The first movement strategy is represented by Tobacco mosaic virus (TMV) MP that directly binds and chaperones viral RNA genome via modified PD [2]–[4]. The second movement strategy involves MP that heavily modify PD structure by forming tubules through which the assembled virions traverse PD [5], [6]. The third type of movement strategies is used primarily by the filamentous viruses, which usually require more than one MP and capsid protein for efficient intercellular transport [7]. The longest known filamentous viruses, closteroviruses, have evolved the most complex machinery that includes a virion-associated movement device and a membrane-targeted MP [8].
Although a number of cellular factors that interact with MPs and/or are localized to PD have been identified, their functional relevance in intercellular transport processes remained largely hypothetical [9]. A new family of PD-resident proteins, Plasmodesmata Located Proteins (PDLPs), was recently characterized in Arabidopsis thaliana [10]. PDLPs are type-I membrane proteins that traffic along the secretory pathway to reach the plasma membrane (PM) lining the PD interior. We have recently demonstrated functional significance of PDLP isoforms for movement of tubule-forming viruses including Grapevine fanleaf virus (GFLV), an RNA nepovirus causing severe grapevine disease [11]. We showed that PDLPs act as receptors required for assembly of the PD-traversing tubules by the GFLV MP 2B. Inactivation of PDLPs resulted in defective tubule formation and GFLV transport. PDLPs appear to represent essential host components for the tubule-forming movement machinery, because the cell-to-cell movement of the evolutionary dissimilar pararetrovirus, Cauliflower mosaic virus (CaMV), was also affected by PDLP down-regulation [11].
One of the central problems in virus transport research is the physical nature of virus translocation within and between cells. Two principal possibilities include diffusion through compartmentalized cytosol and/or endomembrane system and active transport involving cytoskeletal motility. A cytoskeleton-dependent transport route was described in several animal virus models [12] including microtubular motor-driven transport of Human immunodeficiency virus (HIV) [13] and actin tail-propelled transport of Vaccinia virus [14]. The transport mechanisms of plant viruses remain to be a matter of debate, ironically so for the first virus ever discovered, TMV. For the PD targeting of TMV ribonucleoprotein complexes, evidence has been provided for microtubule-dependent [15], [16] and actomyosin-dependent [17], [18] transport, as well as for diffusion in the endoplasmic reticulum (ER) network [19]. Although these mechanisms are not necessarily mutually exclusive, it seems that the growing number of plant viruses are reported to recruit actomyosin for moving their genomes, virions, or MPs to or through PD [20].
The actomyosin motility in plants, from algae to angiosperms, is driven by two classes of myosin motors, VIII and XI, which are evolutionary related to class V myosins present in protists, fungi, and animals [21]. The model plant Arabidopsis thaliana encodes 13 class XI and four class VIII myosins [22]. Class XI myosins function in the trafficking of Golgi stacks, peroxisomes, mitochondria, and ER streaming [23]–[25]. Because inactivation of Arabidopsis class XI myosins affects cell growth and plant development [26], [27], these molecular motors are likely to transport the secretory vesicles required for cell expansion. Although myosins VIII were proposed to associate with PD, ER, plasma membrane, and endosomes [28]–[30], in the absence of genetic evidence, their functional significance remains a mystery.
The first experimental support for actomyosin-dependent PD targeting of a viral protein was provided for a closteroviral Hsp70 (Heat shock protein 70) homolog, a virion component required for viral movement [31], [32]. It was also shown that Hsp70 localization to PD specifically relies on class VIII myosins [33]. Very recently, it was found that MP of a dissimilar tenuivirus also relies on myosins VIII for PD targeting [34]. In contrast, myosins XI were recently implicated in TMV movement [18].
In this study, we investigate the role of the actomyosin motility in PD-targeting of PDLP, and consequently, in tubule-guided cell-to-cell movement of GFLV. We demonstrate that myosins XI, but not VIII, mediate intracellular trafficking and PD targeting of the GFLV MP receptor PDLP. We show that inactivation of certain class XI myosins affects GFLV cell-to-cell movement. Furthermore, we explore the roles of myosins XI in the subcellular targeting of several compartment-specific fluorescent reporters. Taken together, our data delineate a specific, myosin XI-dependent, endomembrane transport pathway for PD-localised plant proteins that contributes to GFLV transport between the cells.
Results
GFLV cell-to-cell movement is actomyosin-dependent
To determine if a functional actin cytoskeleton is required for cell-to-cell movement of GFLV, we applied the actin microfilament depolymerising agent Latrunculin B (LatB) [35] to Nicotiana benthamiana leaves before infection. GFLV cell-to-cell movement was assessed 3 days post inoculation (dpi) by measuring the size of infection foci of a recombinant GFLV encoding red fluorescent protein-fused reporter (GFLV-RFP) [11]. Box plot was used as statistical method to study the range of infection foci diameters in the different treatments. Figure 1A shows a ∼2.5-fold reduction in mean infection focus area in the LatB treated leaves compared to the control, indicating that GFLV spread requires an intact actomyosin motility system.
Fig. 1. Effects of LatB treatment and myosin tail expression on cell-to-cell movement of GFLV. 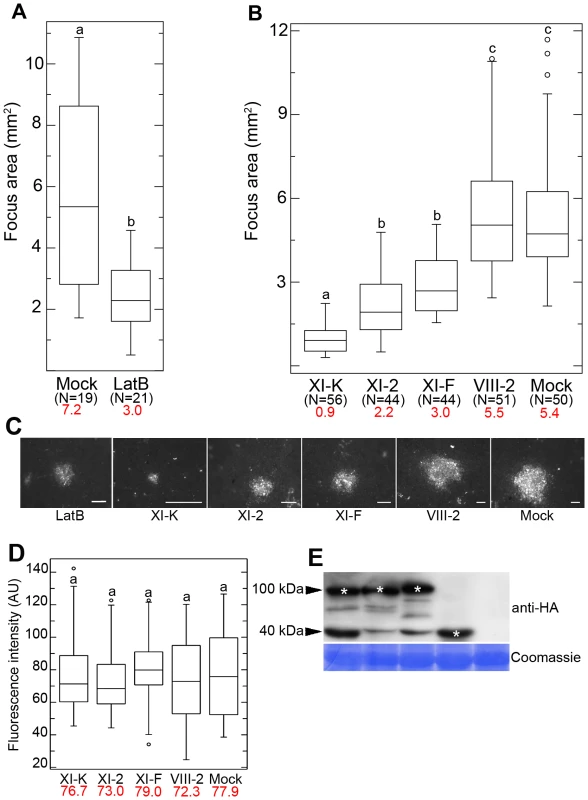
(A) Mean size of GFLV-RFP infection foci at 3 dpi is reduced in 10 µM LatB treated leaves compared to control leaves. (B) Transient expression of the myosin XI-K tail strongly inhibits GFLV-RFP cell-to-cell movement with the myoisn XI-2 and XI-F tails having significant, but less strong effects, and myosin VIII-2 tail being indistinguishable from the mock-infiltrated control. (C) Representative images of the GFLV-RFP infection foci formed in the leaves expressing myosin tails as indicated. Scale bars, 1 mm. (D) Fluorescence intensity (AU, arbitrary units) of the same foci shown in (B) was not significantly different among all experimental conditions. (E) Immunoblot analysis using HA-specific antibodies showed similar expression levels for the HA-tagged myosin tails in the inoculated leaves used in (B) and (C). Bands corresponding to the class XI (approximately 100 kDa) and class VIII (approximately 40 kDa) myosin tails are marked by asterisks. Coomassie blue staining (bottom panel) shows equal loading. Each box plot depicts measurements from the 25th to 75th percentile. The error bars correspond to the 10th and 90th percentiles. The horizontal bar in each box represents the median value. The circles indicate outliers. Different letters (a, b and c) above the box plots indicate statistically significant differences between the different treatments determined by t-test (P<0.001) (A) and ANOVA (P<0.05) (B and D). N, total number of the foci analysed in 3 independent experiments. Mean values are given in red. Because the GFLV MP or small icosahedral virions were unlikely to induce actin tail formation similar to large poxviruses [14], we assumed that the myosin motors were involved in virus intercellular movement. To address this possibility, we used dominant negative inhibition of myosin function via transient overexpression of the headless myosins that possess C-terminal globular tail domains. Because these domains are specifically involved in myosin cargo binding and motor domain activation [36], [37], their ectopic expression suppresses activity of the endogenous myosins. This approach was successfully used for the interference with the functions of myosins VIII and XI in N. benthamiana and Arabidopsis [23], [24], [33]. It is important that we expressed N. benthamiana myosin tails in this same plant species, because heterologous myosin expression often results in mislocalization (Peremyslov VV and VV Dolja, unpublished data).
The myosin tail-expressing and control leaves were inoculated with GFLV-RFP, and the resulting infection foci were measured at 3 dpi. We found that the inhibition of the myosins XI affected the size of the infection foci (Figures 1B and C). The boxes for all myosin XI tails treatments are compressed in comparison with VIII-2 and control (Figure 1B) indicating less distribution around the median showing lower median. These results indicate an affect of the expression of myosin XI tails on virus cell-to-cell movement. Expression of the myosin XI-K tail had the most dramatic effect reducing virus cell-to-cell movement by factor 6 compared to the control. Similar, albeit milder effects were observed upon expression of the myosin XI-2 and XI-F tails (Figures 1B and C). By contrast, virus movement was not significantly different from the control when myosin VIII-2 tails were expressed (Figures 1B and C). The expression of the hemagglutinin epitope (HA)-tagged myosin VIII-2, XI-K, XI-2, and XI-F tails [23] was detected using immunoblot analysis and anti-HA antibodies. This analysis confirmed that the recombinant proteins had the expected sizes (myosins VIII possess much shorter tails than those of myosins XI) and similar levels of accumulation (Figure 1E).
The reduction in the size of infection foci could be attributed either to a defect in virus transport between cells, or to a reduction in virus replication in response to myosin tail expression. To address the latter possibility, we quantified GFLV-RFP fluorescence intensity normalised to the area in a large number of infection foci (N ≥44 for each experimental variant). This analysis unequivocally demonstrated that there were no significant differences between the control and each of the myosin tail-expressing variants (Figure 1D). We therefore concluded that the dominant negative inhibition of the myosin XI-K, and to a lesser extent, of myosins XI-2 and XI-F, specifically affected the cell-to-cell movement of GFLV.
Class XI myosins facilitate tubule formation in virus-infected cells
Because GFLV cell-to-cell movement occurs via tubules assembled by 2B MP [11], [38], we were interested to determine if tubule formation is impaired upon myosin XI tail expression. To this end, we used transient co-expression of the myosin tails and the GFP:2B that is able to form tubules [38] and assessed tubule formation using confocal laser scanning microscopy.
As expected, no effect on tubule assembly was observed when GFP:2B was transiently co-expressed with the tail of myosin VIII-2 (Figure 2A). Ectopic expression of the myosin XI-2 tail resulted in fewer as well as shorter tubules (Figure 2B, Compare insets in figure 2A and B). The most conspicuous effect on tubule formation was observed upon expression of the myosin XI-K tail. As shown in Figure 2C, no discernible tubules were observed in this case. Instead, GFP:2B was distributed diffusely in the cortical cytoplasm and nucleus, attesting to a major disruption of not only the tubule assembly, but also GFP:2B localization at PD (Figure 2C). Immunoblot analysis using 2B - and HA-specific antibodies confirmed co-expression of GFP:2B and each of the myosin tails (Figure 2D). These data clearly indicated that functional myosins XI in general, and myosin XI-K in particular, are required for proper subcellular targeting of the GFLV MP, and subsequent formation of the PD-traversing tubules by this protein.
Fig. 2. Transient expression of the myosin XI-K tail disrupts formation of the PD-associated tubules by the GFLV movement protein GFP:2B. 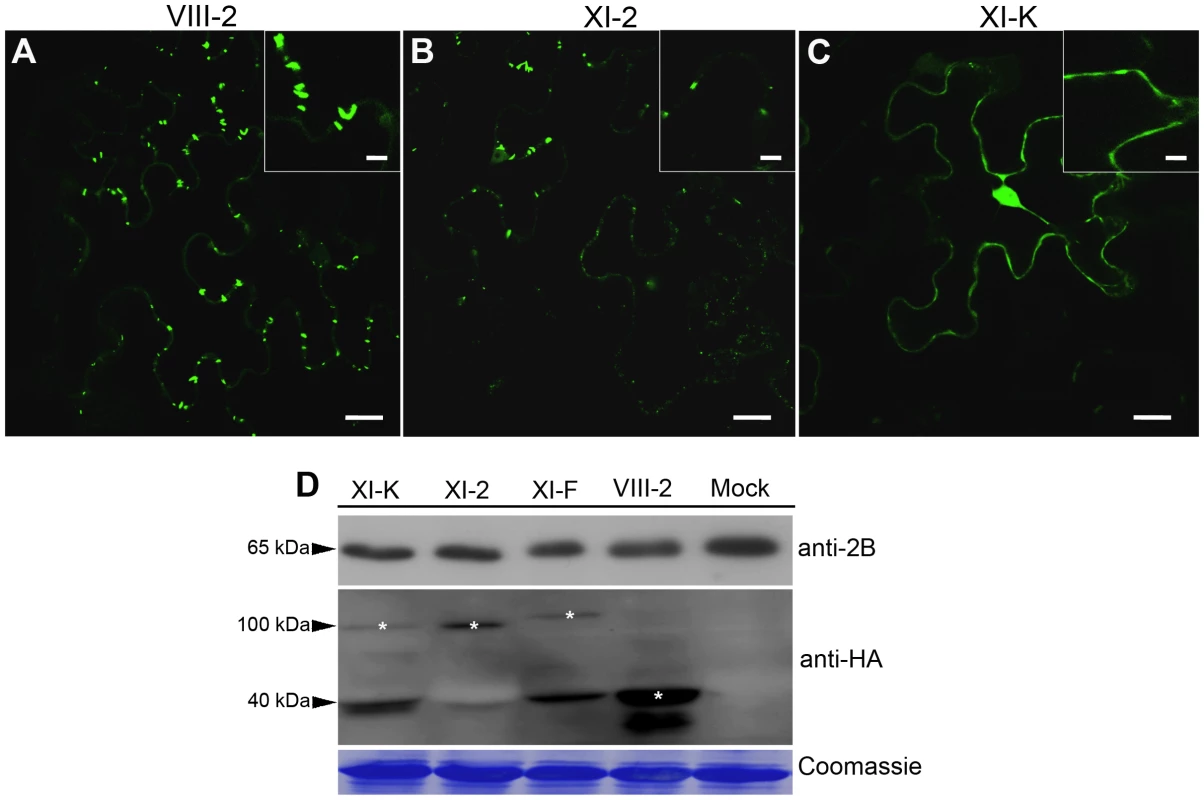
(A) Representative confocal image showing normal, PD-localized tubules formed by GFP:2B in control leaves co-infiltrated with empty vector-transformed Agrobacterium, myosin VIII-2 or XI-F tails. (B) Co-expression of the myosin XI-2 tail reduces tubules formation by GFP:2B. (C) Co-expression of the myosin XI-K tail results in the nucleo-cytoplasmic redistribution of GFP:2B; no tubules are formed under these conditions. Insets are shown in higher magnification. (D) Immunoblot analysis using 2B- (top panel) or HA-specific (middle panel) antibodies revealed similar expression levels for GFP:2B, as well as somewhat variable levels of the HA-tagged myosin tails. Bands corresponding to the class XI (100 kDa) and class VIII (40 kDa) myosin tails are marked by asterisks. Coomassie blue staining (bottom panel) shows equal loading. Scale bars, 20 µm; inside insets, 10 µm. PDLP1 trafficking is driven by the actomyosin motility system
We demonstrated previously that accumulation of PDLP isoforms at PD is crucial for tubule formation by GFLV MP and virus cell-to-cell movement [11]. To determine if PDLP trafficking along the ER-to-Golgi-to-PD pathway [10] requires actomyosin motility, we co-expressed GFP-tagged PDLP1 (PDLP1:GFP) and the spectrally distinct Golgi marker Man1:RFP [39]. Man1:RFP served as an internal control for Golgi motility in experiments using LatB to test whether PDLP1 movement is actomyosin-dependent. Additionally, the ATPase inhibitor 2,3 butanedione monoxime (BDM) reported to inhibit myosin activity [39], [40] was applied to assess the role of myosins in PDLP1 trafficking to PD. As shown in Figure 3, translocation of Man1:RFP (Figure 3A) and PDLP1:GFP-labelled bodies (Figures 3B and video S1) under control conditions occurs along considerably overlapping tracks (compare Figures 3A and B) and with similar velocities of 1.64 µm/sec ± 0.18 and 2.01 µm/sec ± 0.32, respectively (Figure 3G). Strikingly, LatB treatment nearly abolished trafficking of both Man1:RFP (Figure 3C) and PDLP1:GFP (Figure 3D). The resulting measured mean velocities of less than 0.11 µm/sec (Figure 3G) are attributed most likely to Brownian motion-dependent wobbling, cytosol dynamics due to the activity of microtubule-associated motors, and/or the drift of the entire specimen. Very similar results were obtained upon BDM treatment (Figures 3E and G). Statistical analyses revealed highly significant velocity differences between control (mock) and LatB or BDM treatments (t-test, p<0.01, Figure 3G). Taken together, these results suggest that trafficking of PDLP1 bodies occurs via a route similar to that of Man1:RFP-labelled Golgi stacks, and is actomyosin-dependent.
Fig. 3. Effects of the microfilament inhibitor LatB and myosin inhibitor BDM on trafficking velocity of Golgi stacks marked by Man1:RFP or PDLP1:GFP labelled bodies. 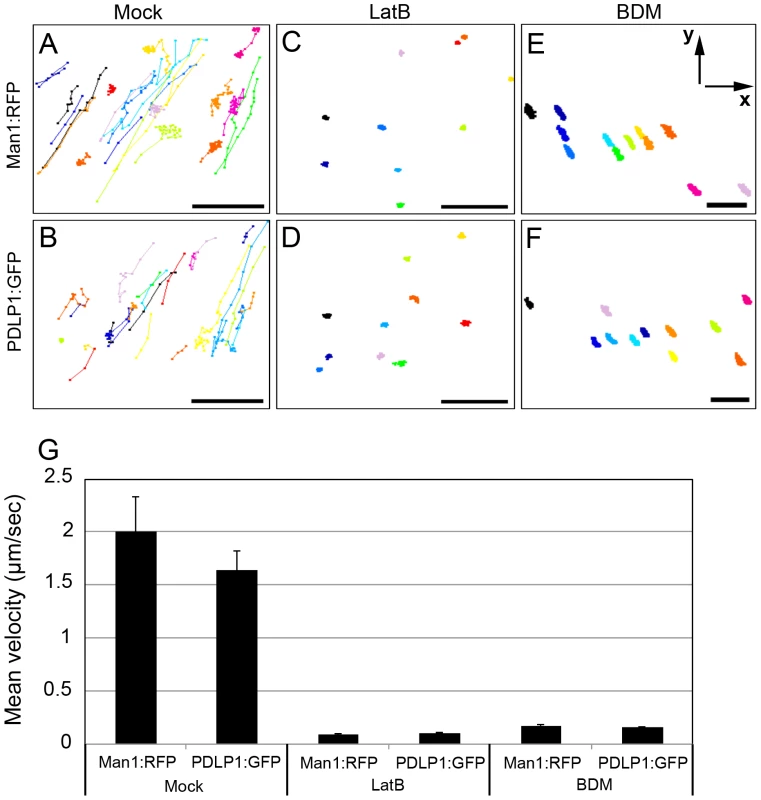
(A–F) Tracks, represented by different colours, of the individual Man1:RFP fluorescent Golgi stacks (A, C, E) or PDLP1:GFP-tagged bodies (B, D, F) were imaged for 2 min in the presence of 0.1% DMSO (Mock; A, B), 10 µM LatB (C, D), or 10 mM BDM (E, F) and depicted via connected dots. (G) Mean of Man1:RFP (Golgi marker) and PDLP1:GFP velocity were calculated using images used in A–F. Number of investigated Golgi stacks and PDLP1:GFP, ≥27 (mock); ≥10 (Lat B, BDM). Axes are given in panel E. Scale bars, 10 µm. PDLP1 localization to PD specifically requires class XI myosins
To investigate the potential myosin contributions to PDLP1 transport to PD, we co-expressed PDLP1:GFP with the tails of class VIII and XI myosins including VIII-1, VIII-2, VIII-B, XI-K, XI-2, and XI-F. Figure 4A to C shows representative images of this analysis. The normal pattern of PDLP1:GFP localization to PD was observed in empty vector control and in the leaves expressing each of the three class VIII myosin tails (Figure 4A), or the myosin XI-F tail (not shown). In a sharp contrast, expression of the myosin XI-2 (Figure 4B) or myosin XI-K (Figure 4C) tails resulted not only in disruption of the specific PD targeting as seen by the absence of typical punctate labelling in the cell wall, but also in formation of multiple abnormal PDLP1:GFP aggregates in the cytosol (Figures 4B and C). Three independent experiments revealed that approximately 60% of the epidermal cells expressing myosin XI-2 or XI-K tails presented such aggregates, whereas no PDLP1 aggregates were detected in any other experimental variants (Figure 4D).
Fig. 4. Transient co-expression of PDLP1:GFP and myosin XI-K or XI-2 tails leads to the PDLP1:GFP mislocalization and aggregation. 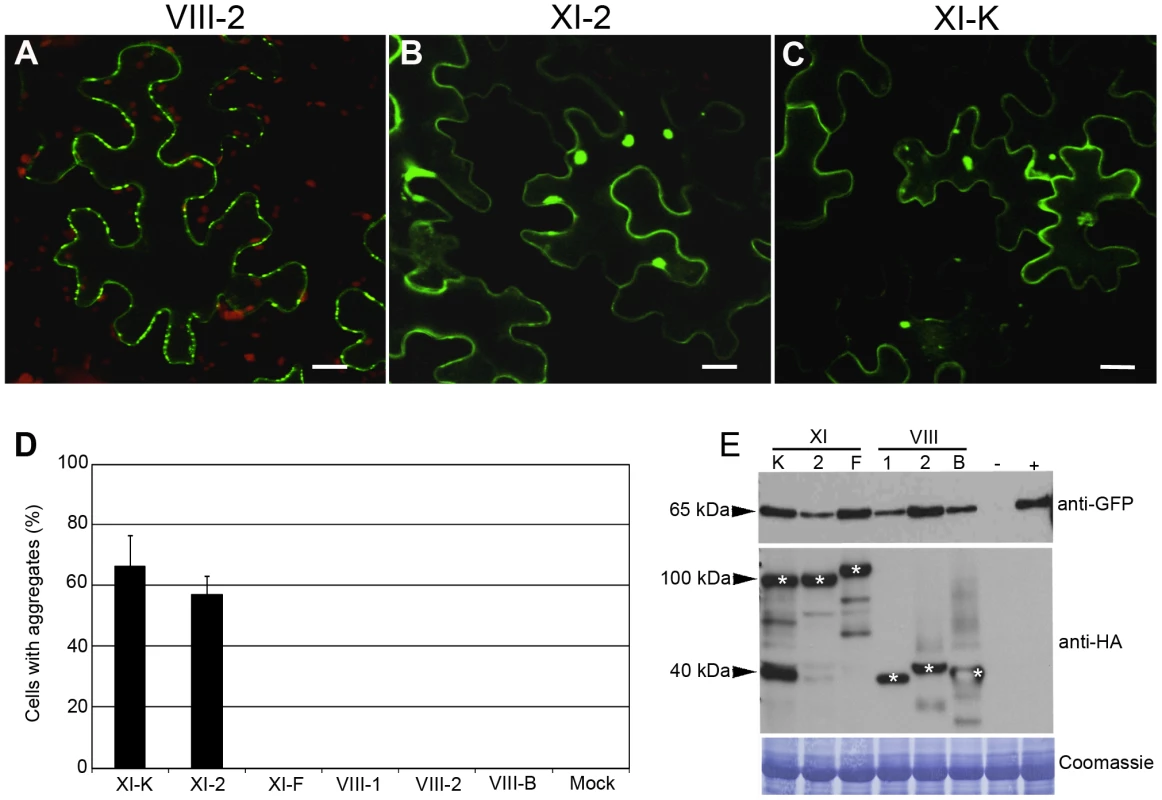
(A) PDLP1:GFP co-expressed with empty vector displays normal PD localization. Similar localization is observed in the presence of the myosin VIII-1, VIII-2, VIII-B, or XI-F tails (not shown). (B and C) Co-expression of PDLP1:GFP with the myosin XI-2 tail (B) or myosin XI-K tail (C) results in the distribution of PDLP1:GFP to the cell periphery and to formation of large cytosolic aggregates. Scale bars, 20 µm. (D) Percentage of cells showing PDLP1:GFP aggregates upon co-expression with the different myosin tail variants. Error bars indicate standard error of the mean of 3 independent experiments; between 200 and 300 cells were analysed for all experimental variant. (E) Immunoblot analysis of leaves infiltrated with PDLP1:GFP (approximately 65 kDa; top panel) and myosin tails (middle panel; marked with asterisks) as indicated. Mock-infiltrated and PDLP1:GFP controls are marked (−) and (+), respectively. The coomassie-blue stained PVDF membrane to validate equal loading is shown in bottom panel. To determine if PDLP1:GFP mislocalization and/or aggregation was due to excessive protein accumulation, we analyzed the steady-state levels of PDLP1:GFP and myosin tails using immunoblotting and GFP - or HA-specific antibodies, respectively. As expected, GFP-specific antibodies revealed a 62-kDa PDLP1:GFP-specific product in all samples except for the mock-infiltrated control [lane (-) in Figure 4E, top panel]. Although protein levels varied slightly between the different experimental conditions, no correlation between high levels of PDLP1:GFP expression and aggregate formation was observed. Indeed, highest PDLP1:GFP accumulation levels were seen with samples expressing myosin XI-F and VIII-2 tails (Figure 4E), where no aggregates were formed (Figure 4D). Conversely, samples expressing myosin XI-2 tails exhibited the lowest PDLP1:GFP accumulation, and nearly 60% of the corresponding cells showed PDLP1:GFP aggregates (Figures 4D and E). In regard to the myosin tail expression, accumulation levels were very similar both for class XI myosin tails (approximately 100 kDa) and VIII (approximately 40 kDa) (Figure 4E, asterisks). We concluded that, among the 6 tested myosins, only expression of the myosin XI-2 and XI-K tails specifically induced mislocalization and abolished PD targeting of PDLP1:GFP.
The myosins XI-K and XI-2 are the principal drivers of cell dynamics including organelle trafficking and F-actin organization, as well as diffuse and polarized cell growth [25]–[27]. Therefore, we were interested to determine if the contributions of these same myosins to PDLP1 localization were general or affected a specific targeting pathway. Firstly, we addressed a potential role of myosins in protein targeting to the plasma membrane (PM) using the PM marker TM23:GFP [41]. As shown in Figure 5A and 5B, the distribution pattern of TM23:GFP was not affected by the expression of myosin VIII-2 or XI-K tails; in both cases the marker was localized throughout the PM. Co-expression of the marker and myosin tails in all experimental conditions were validated by immunoblot analysis (Figure 5C).
Fig. 5. Effects of myosin XI-K tail expression on the localization of different cellular markers in N. benthamiana. 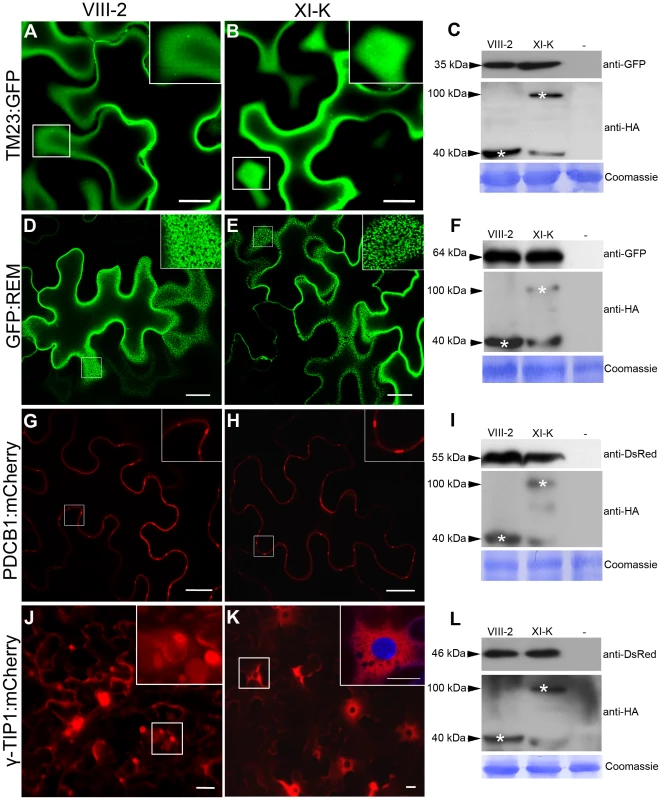
(A–B) The subcellular localization of the TM23:GFP marker at the PM is not affected by expression of myosin XI-K tails. (D–E) No effect on the lipid raft/PM localization of GFP:REM was observed upon coexpression with myosin XI-K tails. (G–H) The normal localization to PD necks of the PDCB1:mCherry was not affected by expression of myosin XI-K tails. (J–K) Co-expression of the myosin XI-K tail (K) with the tonoplast marker γ-TIP1:mCherry inhibits formation of the tonoplast-derived bulbs (J, inset) and leads to uniform distribution of the marker within the tonoplast and in the perinuclear compartment (K, inset. DAPI staining shows nucleus). Scale bars, 20 µm. (C, F, I, L) The expression of the cellular markers (top panels) and myosin tails (middle panels) in each experimental variant was validated using immunoblot analysis and antibodies as indicated. Bottom panels show equal loading verified by Coomassie-staining. Secondly, we investigated localization of the green fluorescent protein-tagged remorin (GFP:REM), a membrane microdomain marker localized in the PM and in PD [42]. Contrarily to PDLP1, remorin down regulate virus cell-to-cell movement by interacting directly with, MP of a potexvirus. As described [42], GFP:REM clustered in discrete PM domains; this localization pattern was not altered upon the transient expression of myosin tails VIII-2 (Figure 5D) or XI-K (Figure 5E). Immunoblot analysis confirmed expression of GFP:REM and myosin tails in each experimental variant (Figure 5F).
Thirdly, we examined the targeting of the plasmodesmata callose binding 1 (PDCB1) protein fused to mCherry (PDCB1:mCherry), which is localized to the PD neck region [43]. Once again, overexpression of the tails of myosin VIII-2 (Figure 5G) or XI-K (Figure 5H) had no observable effect on PDCB1:mCherry targeting to PD-enriched areas at the cell periphery (see Figure 5I for the PDCB1:mCherry and myosin tail expression). Collectively, these results show that in contrast to PDLP1:GFP or GFP:2B the transport or retention of the three tested protein markers targeted to the PM and/or PD was not affected by overexpression of the myosin VIII-2 or XI-K tails, indicating a distinct PD-transport route for PDLP1:GFP.
Finally, we were interested to determine if the transport along the secretory pathway directed to the vacuole membrane (tonoplast) rather than to the PM is myosin-dependent. This question was addressed using the tonoplast-specific marker γ-TIP1 fused to mCherry (γ-TIP1:mCherry; [44]). The vacuoles in fully expanded plant cells usually account for the most of cell volume [45]. The tonoplast surrounding these gigantic organelles is constantly reshaped via formation of the transvacuolar strands and spherical tonoplast invaginations often called bulbs [46]. The tonoplast and bulbs were readily visualized in the γ-TIP1:mCherry-expressing control cells, as well as in myosin VIII-2-expressing cells (Figure 5J). Interestingly, expression of the myosin XI-K tails abolished the bulb formation and led to enrichment of γ-TIP1:mCherry in the perinuclear tonoplast domain (Figure 5K). As in the previous experiments, similar levels of the marker and myosin tail accumulation were confirmed using immunoblot analysis (Figure 5L). We concluded that although the transport of tonoplast-targeted protein was unaffected upon myosin tail expression, the normal tonoplast dynamics required myosin XI-K activity. In general, our observations using dominant negative expression of myosin tails suggest that specific inhibition of myosin XI-K activity affected a specific trafficking pathway of PDLP1 to PD rather than caused an indiscriminate suppression of the endomembrane transport.
Discussion
The role of cytoskeletal motility in viral infection is a rapidly progressing albeit relatively young field of research at the frontiers of virology and cell biology. The microtubule-dependent transport of retroviruses to nucleus [47] and Herpesvirus to axon endings [48], actin-dependent formation of the virological synapses through which HIV moves between cells [49], and an actin-tail propelled transport of poxviruses [50] are a few illuminating discoveries in this field. Animal and plant viruses share multiple replication mechanisms that rely on conserved features of eukaryotic cells [51], [52]. In contrast, virus cell-to-cell movement in plants occurs via the plant-specific PD, channel-like organelles providing symplasitc continuity between adjacent cells [53]. To accomplish movement through PD, plant viruses have evolved dedicated MPs that target and modify PD to mediate virus passage. One of the principal mechanisms of MP action is a tubule-guided PD transport used by a wide variety of the RNA and retroid DNA viruses [1] whereby MP modifies PD by assembly into multimeric tubules through which virion movement occurs.
Most of the previous work on plant virus-cytoskeleton relationships involved chemical inhibitors [20]. Although useful for an initial insight, this approach is not unlike a sledgehammer because global disruption of microtubules or microfilaments causes dramatic changes in cell physiology that are difficult to associate with specific mechanisms of virus replication or transport. Even in the cases like TMV, where genetic and other more subtle approaches were used [16], [18], [19], [54], the picture is less than clear. In a large part, difficulties in reconciling work from different labs stem from the incomplete understanding of the cellular partners required for the MP function. Our recent discovery of PDLPs as host receptors [10], [11] that mediate PD targeting of the tubule-forming MPs of the nepovirus GFLV and the caulimovirus CaMV provided a unique opportunity to address the role of actomyosin motility in virus transport using both the chemical and the more specific dominant negative inhibition of myosins [23], [33].
Combining these approaches, we revealed critical contributions of the myosin motors in the GFLV transport between the cells. We identified myosin XI-K as a principal driver of this process with additional contributions provided by other class XI, but not class VIII myosins. Furthermore, we obtained important new insight into myosin-driven endomembrane transport in plants by showing that myosin XI-K acts in a specific pathway within a general ER-to-Golgi-to-PM transport network.
Because GFLV transport is tubule-dependent, it was important to determine if myosin inactivation interfered with tubule formation or PD localization. Our previous work using suspension cell culture has shown that tubule assembly requires ER-to-Golgi pathway, whereas cytoskeletal systems appeared to contribute to tubule targeting [38]. Here, we found that the inhibition of myosin XI-K resulted in a conspicuous nucleo-cytosolic redistribution of the GFP:2B with no detectable PD-associated tubules. Thus, tubule formation was specifically affected by myosin inactivation.
As was demonstrated recently, 2B assembles tubules at PD via interaction with the host PDLP receptors [11] that, in turn, are transported to PD along the ER-to-Golgi pathway [10]. Therefore, both GFLV movement and tubule formation at PD require proper PDLP targeting. To determine if PDLP targeting was actomyosin dependent, we investigated PDLP1:GFP transport pathway using cytoskeletal inhibitors and dominant negative inhibition of the individual myosins. We found that PDLP1:GFP was present in mobile bodies whose rapid trafficking was abolished by application of LatB or BDM similarly to Golgi stacks whose transport in plants relies entirely on myosins XI [27].
Furthermore, we showed that the myosins XI-K and XI-2, but not XI-F, VIII-1, VIII-2, and VIII-B are required for PDLP1 delivery to PD. Inactivation of the two former myosins resulted in PDLP1:GFP redistribution in the cortical cytoplasm and inclusion bodies that were never observed in the cells where other myosins were inhibited. Given the strong correlation between disruption of PDLP targeting and GFLV movement by interference with myosins XI-K and XI-2 (Figures 1B and 4B-D), we propose that the primary contribution of these myosins to virus transport is the delivery of PDLP-receptors to PD. It is important to stress that this result is also the first indication of myosin XI function in the trafficking of secretory vesicles to the PM/PD compartment.
The next question to ask was if PDLP transport occurred along a common post-Golgi secretory pathway, or represented a specialized route within this pathway driven primarily by myosins XI-K and XI-2. To address this question, we assessed a role of myosin XI-K in the targeting of markers differentially localized to: i) entire PM; ii) lipid raft subdomains within PM and PD; iii) PD neck or iv) vacuolar membrane (tonoplast). We found that proper targeting of the former three markers was not affected by myosin XI-K inhibition suggesting that the myosin XI-K-dependent PDLP targeting represents a specific route within a broad endomembrane transport network. In addition, we found that myosin XI-K is required for the normal tonoplast reshaping via transient invaginations.
It was previously demonstrated that PD targeting of the closteroviral Hsp70 homolog requires myosins VIII [33], although significance of this process for virus movement was not addressed. It was also found that myosin XI-2 knockdown reduced TMV movement [18], but this effect was not linked to a specific mechanism. Together with our previous work [10], [11], [38], this study provides a basis for an advanced mechanistic model of myosin-dependent virus movement.
According to this model (Figure 6), the GFLV MP and its host receptor, PDLP, traffic to the cell periphery along distinct pathways. 2B reaches PD by diffusion or by association with microtubules [38]. The transport route employed by PDLP is dependent on the myosins XI with XI-K playing the principal role. At PD, MP binds PDLP for anchorage and tubule assembly. Because transient inhibition of PDLP traffic to PD reduces virus movement (Figure 1), it seems that steady-state supply of this receptor is required for the formation of tubules that restructure PD. Finally, assembled GFLV virions enter tubules and translocate into adjacent cells. It remains to be determined if virion transport to and through tubules involves cytoskeleton-dependent motility.
Fig. 6. Model for PDLP and GFLV MP 2B targeting to PD and tubule formation. 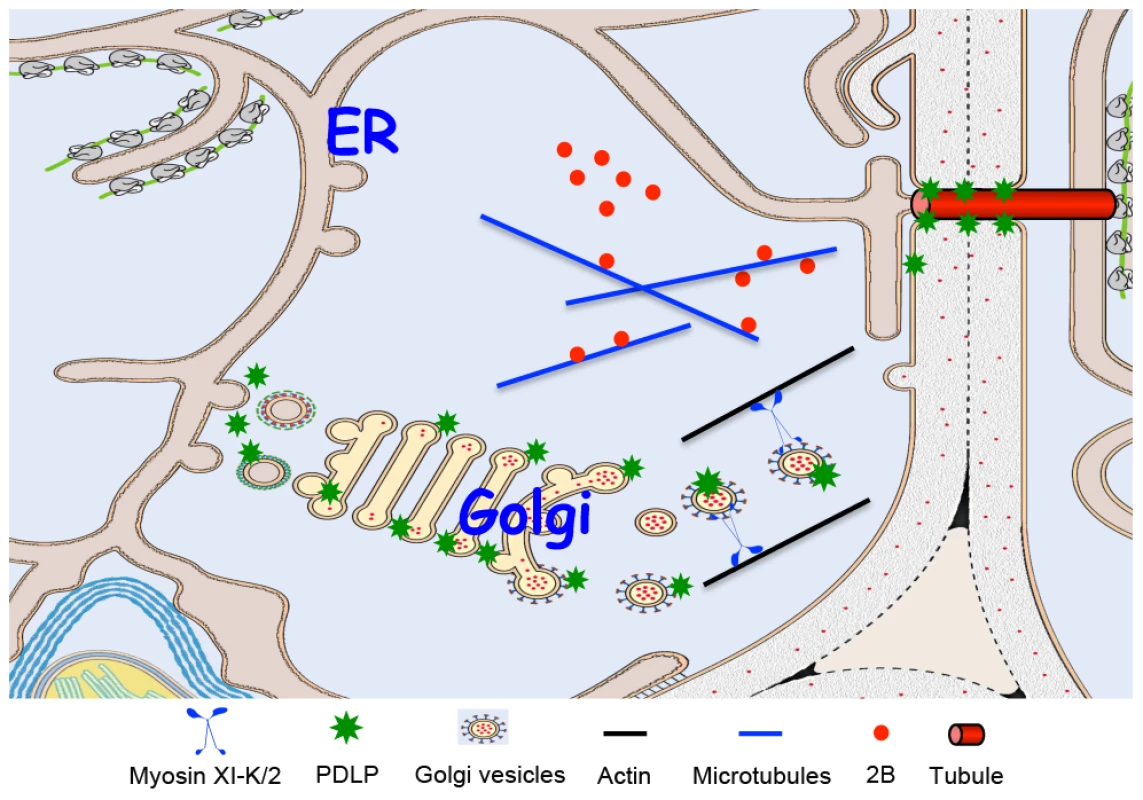
The GFLV MP (2B) reaches PD by diffusion or by microtubule – mediated transport. PDLP traffics along the secretory pathway and its post-Golgi delivery to the plasmamembrane and/or PD relies on myosin XI-K and myosin XI-2. Within PD, interaction between 2B and PDLP promotes tubule formation to allow GFLV virion cell-to-cell movement. The emerging picture of the plant-virus interactions with myosin motors is complex and nuanced. It appears that closteroviral Hsp70 homolog directly recruits myosins VIII for virion delivery to PD [33], whereas tenuiviral MP uses myosin VIII-assisted vesicular transport for the same task [34]. Currently, the PD-directed transport of these viral proteins remains the only experimentally supported function of the class VIII myosins. On the other hand, TMV MP targeting to PD does not require myosins [34], whereas myosin XI-2 facilitates TMV movement likely via delivering the ER-associated viral replication complexes to PD [18], [20], [55], [56]. This latter hypothesis resonates well with the role of myosins XI-2 and XI-K in ER transport [25]. In the case of GFLV presented here, the virus relies on the myosins XI-K and XI-2 for the trafficking of the host MP receptor PDLP to PD.
In addition to important insight into virus-cytoskeleton interactions, our work suggests novel functions of the myosins XI-K and XI-2 in vesicle trafficking and vacuole remodelling. These myosins were previously shown to drive the trafficking of Golgi stacks, peroxisomes, and mitochondria [26], [27], as well as the ER flow [25]. Here we show that these same myosins are also involved in PDLP delivery to PD via a specific endomembrane transport pathway, as well as in remodelling of the vacuolar membrane. Further inquiries into the mechanisms of myosin-dependent transport are certain to deepen our understanding of the cell interior dynamics and the importance of these processes for virus movement.
Materials and Methods
Plant material and virus inoculation
All experiments were performed using N. benthamiana, an experimental GFLV host that supports the complete systemic infection cycle. The plants were grown in growth chambers under 16/8h light/dark cycles, 24/20°C day/night temperatures and approximately 70% humidity. Agroinfiltrated and/or virus-infected leaves were of the same age and size and were maintained at the same conditions. Approximately 300 ng of purified GFLV-RFP virions was mechanically inoculated into N. benthamiana leaves.
Transient protein expression
The binary vectors designed to express HA-epitope tagged N. benthamiana myosin tails VIII-1, VIII-2, VIII-B, XI-K, XI-F, and XI-2 were described earlier [33]. The fluorescent reporter proteins used to visualize subcellular compartments were as follows: GFP:2B, the GFLV MP forming tubules at PD of virus-infected cells [11]; PDLP1:GFP localized in the PM lining PD channel [10]; PDCB1:mCherry targeted to the PD neck [43]; GFP:REM localized to lipid rafts within PM and PD [42]; TM23:GFP labelling the entire PM [41]; Man1:RFP associated with Golgi-stacks [39]; tonoplast-specific γ-TIP1:mCherry [44]. All plasmids were transformed into Agrobacterium tumefaciens (strain LBA4404) that was used for agroinfiltration at a final optical density (OD 600 nm) of 0.3 [11]. Leaf samples were processed for imaging or immunoblot analysis at 48 hours post infiltration.
Drug treatments
To analyze the effect of the actin microfilament disassembly drug LatB on GFLV infection, N. benthamiana leaves were infiltrated with 10 µM LatB in 0.1% DMSO 6 hours prior to inoculation with GFLV-RFP. In addition, 10 µM LatB or 10 mM 2,3 butanedione monoxime in water solution (BDM; an ATPase inhibitor that disrupts myosin function) were vacuum infiltrated into N. benthamiana leaf disks 36 hours after agroinfiltration to examine the effects of these inhibitors on the trafficking and localization of PDLP1:GFP and Man1:RFP. Leaf disks were kept in a moisture chamber and were observed at 12 hours after the treatment. Control infiltrations were performed either with 0.1% DMSO or with water.
Immunoblot analysis
Total protein extracts were obtained by grinding N. benthamiana leaf disks in Laemmli buffer, separated by SDS-PAGE, and transferred by electroblotting to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). To detect myosin tails, membranes were probed with anti-HA-peroxidase antibodies (Sigma-Aldrich) at 1∶5,000 dilution. For the GFLV movement protein 2B, affinity purified GFLV 2B-specific rabbit antibody [57] was used in 1∶10,000 dilution. The expression of all other GFP-tagged proteins was assayed using, the monoclonal anti-GFP antibodies (Clontech) diluted to 1∶5,000. The expression of mCherry-fused γ-TIP1 and PDCB1 was detected using polyclonal anti-DsRed antibodies as recommended by manufacturer (Clontech).
Confocal laser scanning microscopy and image processing
Cells expressing fluorescent proteins were imaged using a Zeiss LSM510 laser scanning confocal microscope with a C-Apo-chromat (63X/1.2 W Korr) water objective lens under multitrack mode. Excitation/emission wavelengths were 488 nm/505 to 545 nm for GFP and 543/long pass 560 nm for RFP. Confocal images were processed using LSM510 software version 2.8 (Zeiss).
GFLV-RFP infection foci were examined under a Leica MacroFluo epifluorescent microscope equipped with the apochromatically corrected zoom system Z16 APO, a 5x objective and a DFC 360FX camera. All imaging was conducted under identical illumination and exposure conditions to allow comparisons. Following acquisition, images were processed using ImageJ (1.38u), and Adobe Photoshop (v7.0) software.
Statistical analyses
Statistical evaluations were made using ANOVA R software or Student's t-test where appropriate.
Supporting Information
Zdroje
1. Benitez-AlfonsoYFaulknerCRitzenthalerCMauleAJ 2010 Plasmodesmata: gateways to local and systemic virus infection. Mol Plant Microbe Interact 23 1403 1412
2. TzfiraTRheeYChenMHKunikTCitovskyV 2000 Nucleic acid transport in plant-microbe interactions: the molecules that walk through the walls. Annu Rev Microbiol 54 187 219
3. BoevinkPOparkaKJ 2005 Virus-host interactions during movement processes. Plant Physiol 138 1815 1821
4. HeinleinM 2002 Plasmodesmata: dynamic regulation and role in macromolecular cell-to-cell signaling. Curr Opin Plant Biol 5 543 552
5. RitzenthalerCHofmannC 2007 Tubule-guided movement of plant viruses. WaigmannEHeinleinM Plant Cell Monogr 7 Berlin-Heidelberg Springer-Verlag 63 83
6. NiehlAHeinleinM 2010 Cellular pathways for viral transport through plasmodesmata. Protoplasma 248 75 99
7. Verchot-LubiczJTorranceLSolovyevAGMorozovSYJacksonAO 2010 Varied movement strategies employed by triple gene block-encoding viruses. Mol Plant Microbe Interact 23 1231 1247
8. DoljaVVKreuzeJFValkonenJP 2006 Comparative and functional genomics of closteroviruses. Virus Res 117 38 51
9. OparkaKJ 2004 Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci 9 33 41
10. ThomasCLBayerERitzenthalerCFernandez-CalvinoLMauleAJ 2008 Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 6 e7
11. AmariKBoutantEHofmannCSchmitt-KeichingerCFernandez - CalvinoL 2010 A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog 6 e1001119
12. SattentauQ 2008 Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol 6 815 826
13. FacklerOTKräusslichHG 2006 Interactions of human retroviruses with the host cell cytoskeleton. Curr Opin Microbiol 9 409 415
14. GreberUFWayM 2006 A superhighway to virus infection. Cell 124 741 54
15. BoykoVFerralliJHeinleinM 2000 Cell-to-cell movement of TMV RNA is temperature-dependent and corresponds to the association of movement protein with microtubules. Plant J 22 315 325
16. BoykoVHuQSeemanpillaiMAshbyJHeinleinM 2007 Validation of microtubule-associated Tobacco mosaic virus RNA movement and involvement of microtubule-aligned particle trafficking. Plant J 51 589 603
17. WrightKMWoodNTRobertsAGChapmanSBoevinkP 2007 Targeting of TMV movement protein to plasmodesmata requires the actin/ER network: Evidence from FRAP. Traffic 8 21 31
18. HarriesPAParkJWSasakiNBallardKDMauleAJ 2009 Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proc Natl Acad Sci U S A 106 17594 17599
19. Guenoune-GelbartDElbaumMSagiGLevyAEpelBL 2008 Tobacco mosaic virus (TMV) replicase and movement proteinfunction synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol Plant-Microbe Interact 21 335 345
20. HarriesPASchoelzJENelsonRS 2010 Intracellular transport of viruses and their components: utilizing the cytoskeleton and membrane highways. Mol Plant Microbe Interact 23 1381 1393
21. PeremyslovVVMocklerTCFilichkinSAFoxSEJaiswalP 2011 Expression, splicing, and evolution of the myosin gene family in plants. Plant Phys 155 1191 1204
22. ReddyASDayIS 2001 Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol 2 1 17
23. AvisarDProkhnevskyAIMakarovaKSKooninEVDoljaVV 2008a Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol 146 1098 1108
24. PeremyslovVVProkhnevskyAIAvisarDDoljaVV 2008 Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146 1109 1116
25. UedaHYokotaEKutsunaNShimadaTTamuraK 2010 Myosin - dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci U S A 107 6894 6899
26. ProkhnevskyAIPeremyslovVVDoljaVV 2008 Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci U S A 105 19744 19749
27. PeremyslovVVProkhnevskyAIDoljaVV 2010 Class XI myosins are required for development, cell expansion, and F-actin organization in Arabidopsis. Plant Cell 22 1883 1897
28. ReicheltSKnightAEHodgeTPBaluskaFSamajJ 1999 Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J 19 555 567
29. GolombLAbu-AbiedMBelausovESadotE 2008 Different subcellular localizations and functions of Arabidopsis myosin VIII. BMC Plant Biol 8 3
30. SattarzadehAFranzenRSchmelzerE 2008 The Arabidopsis class VIII myosin ATM2 is involved in endocytosis. Cell Motil Cytoskeleton 65 457 468
31. AlzhanovaDVNapuliAJCreamerRDoljaVV 2001 Cell-to-cell movement and assembly of a plant closterovirus: Roles for the capsid proteins and Hsp70 homolog. EMBO J 20 6997 7007
32. ProkhnevskyAIPeremyslovVVDoljaVV 2005 Actin cytoskeleton is involved in targeting of a viral Hsp70 homolog to the cell periphery. J Virol 79 14421 14428
33. AvisarDProkhnevskyAIDoljaVV 2008b Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog. J Virol 82 2836 2843
34. YuanZChenHChenQOmuraTXieL 2011 The early secretory pathway and an actin-myosin VIII motility system are required for plasmodesmatal localization of the NSvc4 protein of Rice stripe virus. Virus Res 159 62 68
35. MortonWMAyscoughKRMcLaughlinPJ 2000 Latrunculin alters the actin monomer subunit interface to prevent polymerization. Nat Cell Biol 2 376 378
36. KrementsovDNKrementsovaEBTrybusKM 2004 Myosin V: regulation by calcium, calmodulin, and the tail domain. J Cell Biol 164 877 886
37. PashkovaNJinYRamaswamySWeismanLS 2006 Structural basis for myosin V discrimination between distinct cargoes. EMBO J 25 693 700
38. LaporteCVetterGLoudesAMRobinsonDGHillmerS 2003 Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY-2 cells. Plant Cell 15 2058 2075
39. NebenführAGallagherLADunahayTGFrohlickJAMazurkiewiczAM 1999 Stop-and-go movements of plant Golgi stacks are mediated by the actomyosin system. Plant Physiol 121 1127 1142
40. TominagaMYokotaESonobeSShimmenT 2000 Mechanism of inhibition of cytoplasmic streaming by a myosin inhibitor, 2,3-butanedione monoxime. Protoplasma 213 46 54
41. BrandizziFFrangneNMarc-MartinSHawesCNeuhausJM 2002 The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14 1077 92
42. RaffaeleSBayerELafargeDCluzetSGerman RetanaS 2009 Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs Potato virus X movement. Plant Cell 21 1541 1555
43. SimpsonCThomasCFindlayKBayerEMauleAJ 2009 An arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 21 581 594
44. NelsonBKCaiXNebenführA 2007 A multi-color set of in vivo organelle markers for colocalization studies in Arabidopsis and other plants. Plant J 51 1126 1136
45. MartyF 1999 Plant Vacuoles. Plant Cell 11 587 599
46. SaitoCUedaTAbeHWadaYKuroiwaT 2002 A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J 29 245 255
47. SuzukiYCraigieR 2007 The road to chromatin - nuclear entry of retroviruses. Nature Rev Microbiol 5 187 196
48. LymanMGEnquistLW 2009 Herpesvirus interactions with the host cytoskeleton. J Virol 83 2058 2066
49. HallerCFacklerOT 2008 HIV-1 at the immunological and T-lymphocytic virological synapse. Biol Chem 389 1253 1260
50. DoddingMPWayM 2009 Nck - and N-WASP-dependent actin-based motility is conserved in divergent vertebrate poxviruses. Cell Host Microbe 6 536 550
51. den BoonJADiazAAhlquistP 2010 Cytoplasmic viral replication complexes. Cell Host Microbe 8 77 85
52. NagyPDWangRYPoganyJHafrenAMakinenK 2011 Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 411 374 82
53. XuXMJacksonD 2010 Lights at the end of the tunnel: new views of plasmodesmal structure and function. Curr Opin Plant Biol 13 684 692
54. GillespieTBoevinkPHauptSRobertsAGTothR 2002 Functional analysis of a DNA shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of Tobacco mosaic virus. Plant Cell 14 1207 1222
55. KawakamiSWatanabeYBeachyRN 2004 Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci U S A 101 6291 6296
56. LiuJ-ZBlancaflorEBNelsonRS 2005 The Tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol 138 1853 1865
57. RitzenthalerCPinckMPinckL 1995 Grapevine fanleaf nepovirus P38 putative movement protein is not transiently expressed and is a stable final maturation product in vivo. J Gen Virol 76 907 915
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



