-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
A central question in bacterial physiology is how bacteria sense and respond to their environment. The archetype of bacterial signaling systems is the two-component signaling system composed of a sensor protein histidine kinase that activates a transcription factor response regulator in response to a specific signal. In addition, bacteria also have signaling systems composed of eukaryotic-like Ser/Thr kinases and phosphatases. Even though these systems do not have dedicated transcription factors, they are capable of affecting gene expression. Here we show that a eukaryotic-like Ser/Thr kinase conserved in all sequenced Gram-positive bacteria converges with an essential two-component signaling system to regulate gene expression in the model organism Bacillus subtilis. We show that this eukaryotic-like Ser/Thr kinase phosphorylates the response regulator of a highly conserved and essential two-component signaling system, thereby increasing its activity. This phosphorylation results in the regulation of genes involved in the essential process of cell wall metabolism. Given that bacterial cell wall metabolism is the target of many known antibiotics, and mutations in both of these signaling systems change the antibiotic sensitivity of a number of important Gram-positive pathogens, we expect that our analysis will suggest novel insight into the emergence of antibiotic resistance.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005275
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005275Summary
A central question in bacterial physiology is how bacteria sense and respond to their environment. The archetype of bacterial signaling systems is the two-component signaling system composed of a sensor protein histidine kinase that activates a transcription factor response regulator in response to a specific signal. In addition, bacteria also have signaling systems composed of eukaryotic-like Ser/Thr kinases and phosphatases. Even though these systems do not have dedicated transcription factors, they are capable of affecting gene expression. Here we show that a eukaryotic-like Ser/Thr kinase conserved in all sequenced Gram-positive bacteria converges with an essential two-component signaling system to regulate gene expression in the model organism Bacillus subtilis. We show that this eukaryotic-like Ser/Thr kinase phosphorylates the response regulator of a highly conserved and essential two-component signaling system, thereby increasing its activity. This phosphorylation results in the regulation of genes involved in the essential process of cell wall metabolism. Given that bacterial cell wall metabolism is the target of many known antibiotics, and mutations in both of these signaling systems change the antibiotic sensitivity of a number of important Gram-positive pathogens, we expect that our analysis will suggest novel insight into the emergence of antibiotic resistance.
Introduction
Regulatory Ser/Thr phosphorylation has been assumed to be largely absent from prokaryotes. However, recently, phosphoproteomic analysis has identified numerous (~50) proteins phosphorylated on Ser or Thr residues in both Gram-positive and Gram-negative bacteria [1]. Likely candidates for the enzymes responsible for these modifications are bacterial proteins with structural and mechanistic similarities to eukaryotic Ser/Thr kinases (eSTKs) and phosphatases (eSTPs) [2,3]. These enzymes have been identified in phylogenetically diverse bacteria, and eSTKs may be directly responsible for a number of the observed Ser/Thr phosphorylations. Thus, how these kinases regulate cellular physiology remains unclear, but their conservation across the majority of sequenced bacterial species suggests that they play an important role.
Regulatory phosphorylation in bacteria has largely been studied in the context of two-component systems (TCS) that govern the expression of a regulon in response to a physiological signal. These signaling systems include a sensor histidine kinase, which when activated transphosphorylates a cognate response regulator, typically a DNA-binding protein, on a conserved aspartate residue. Once phosphorylated, the response regulator dimerizes and binds specific sequences in the promoters of the genes in its regulon. Hints that eSTKs might similarly be involved in transcriptional regulation come from studies demonstrating that mutations in eSTKs cause large-scale changes in gene expression [4–6]. However, in contrast to TCS systems which typically use response regulator phosphorylation to regulate gene expression, eSTKs and the eSTPs generally lack identified DNA binding motifs, suggesting they likely mediate changes in gene expression indirectly. Although eSTKs and eSTPs are also not generally associated with a specific partner transcription factor, at least some of the observed transcriptional changes may be due to direct phosphorylation of a transcription factor. As the regulons governed by some TCSs have been extensively studied, overlap between transcriptional changes caused by mutations in eSTKs and eSTPs and certain TCS systems has been observed [7,8]. A plausible mechanism for these effects is that an eSTK phosphorylates components of a TCS system and thereby affecting gene expression. Consistently, eSTKs from a number of organisms have been shown to phosphorylate TCS proteins, and these modifications affect expression of genes in the respective TCS regulon [7,8].
All sequenced Gram-positive bacteria contain a single membrane-bound eSTK comprised of an extracellular domain composed of three or four PASTA repeats and a cytoplasmic domain with extensive homology to eukaryotic Ser/Thr kinases [3,9]. The PASTA (Peptidoglycan binding and Ser/Thr kinase associated) motif was originally identified in penicillin-binding proteins that are responsible for peptidoglycan polymerization, suggesting that it mediates peptidoglycan recognition [10]. Consistent with this hypothesis, isolated PASTA domains interact in vitro with a peptide corresponding to the peptidoglycan stem peptide [11–13]. In the Gram-positive bacterium B. subtilis, the PASTA-containing eSTK is PrkC, which is required for spore germination [14] and for regulation of the production of a secreted muramidase [15] in response to muropeptides. PrkC is transcribed during all phases of growth, and is co-transcribed with the eSTP PrpC [16]. Thus, PrkC could play a role in the regulation of peptidoglycan metabolism in B. subtilis.
A signaling system that is known to be important for peptidoglycan metabolism is the WalRK TCS, a well conserved, essential TCS found in low-GC-Gram-positive bacteria including B. subtilis [17–19]. Mutations in the response regulator WalR that alter its function significantly result in the generation of B. subtilis L-forms, variants that lack cell wall [20]. The essentiality of WalR likely derives from its regulation of at least one WalRK regulon member, although the identity of that gene, or combination of genes, is not known for most WalRK-containing species (see S. pneumoniae for an exception [21]). This essentiality is unusual; WalRK is the only essential TCS (out of 34) in B. subtilis [17]. The core WalRK regulon conserved in different species comprises ~10 genes involved in cell wall metabolism, including several peptidoglycan hydrolases [18]. In B. subtilis, ~20 WalRK regulon genes have been identified through several approaches including the use of a hybrid regulator [22], an IPTG-inducible WalRK operon [23,24], and ChIP [25]. Differential regulation of WalRK regulated genes is observed upon treatment with various cell wall-targeting antibiotics, suggesting that the WalK activating ligand is a molecule derived from the cell wall [18].
As the eSTK PrkC and the TCS WalRK have functional overlap, it is plausible that these two types of signaling systems converge in Gram-positive bacteria. Consistent with this hypothesis, deletion of the PrkC homologs in S. pneumoniae (StkP; [5]), S. mutans (PknB; [6]), S. pyogenes (Stk; [26]) and S. aureus (PknB; [4]) resulted in the down-regulation of genes known to be under control of either WalR or its homolog VicR. In the model Gram-positive B. subtilis, yocH, a well-characterized member of the WalRK regulon encoding a cell wall hydrolase, is regulated by both WalR and PrkC. WalR directly binds the yocH promoter in vitro [22], and yocH transcription is activated by WalR expression in vivo [27]. Intriguingly, yocH also demonstrates PrkC-dependent activation in response to cell wall-derived muropeptides [15]. Thus, these data suggest a functional interaction between PrkC and the WalRK TCS. Here we use in vitro and in vivo approaches to demonstrate that, consistent with this hypothesis, PrkC regulates the WalRK TCS through phosphorylation of WalR, resulting in the regulation of genes involved in cell wall metabolism by an eSTK.
Results
PrkC regulates yocH transcription in stationary phase
Prior work demonstrated that expression of the WalR regulon gene yocH is controlled both by WalR binding to PyocH [22,27] and by PrkC through an unknown mechanism [15]. These observations suggest two possible mechanisms of PrkC dependent yocH activation: 1) PrkC acts on the WalRK TCS to regulate yocH and consequently other members of the WalR regulon, or 2) PrkC acts through another (unknown) mechanism on yocH (Fig 1A). The first hypothesis predicts that changes in PrkC activity resulting in the activation of yocH would also result in transcriptional changes across the WalR regulon, namely increased activation of genes activated by WalR, as well as increased repression of genes repressed by WalR. The second hypothesis predicts that changes in PrkC activity that activate yocH may have little, or no consistent, effect on the expression of other members of the WalR regulon.
Fig. 1. The kinase PrkC and the phosphatase PrpC regulate the WalRK regulon gene yocH. 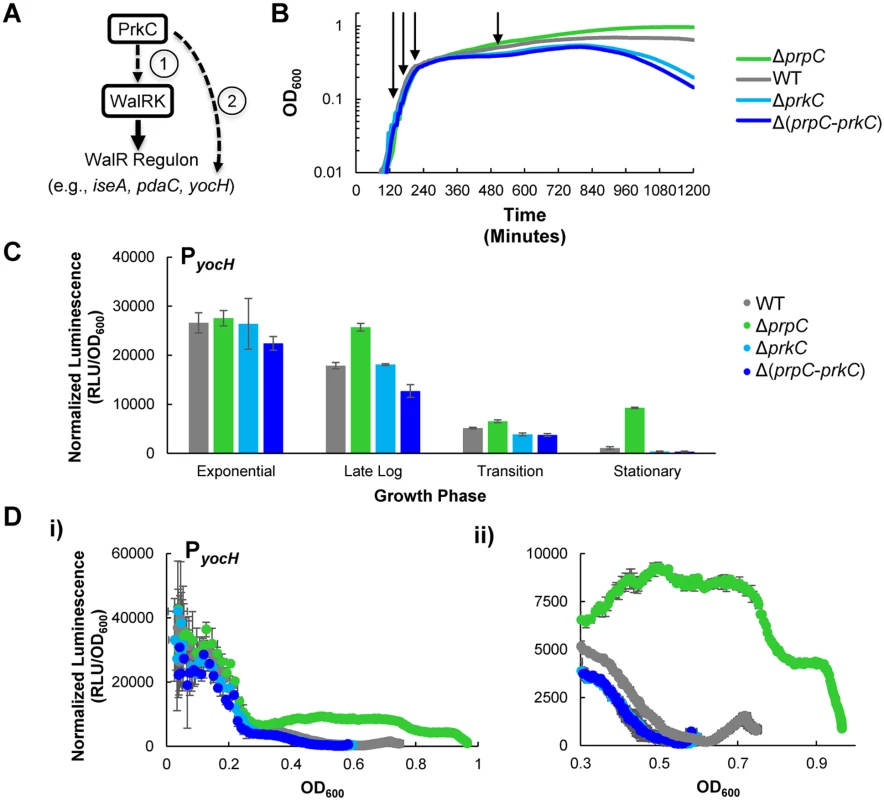
A) Models of PrkC activation of the WalRK regulon gene yocH. (1) PrkC acts via the WalRK system on yocH, thereby also affecting other WalRK regulon genes. (2) Alternately, PrkC acts through another mechanism on yocH. B) Growth curves of WT, ΔprpC, ΔprkC, and Δ(prpC-prkC) strains in LB. 150 μl cultures were grown in 96-well plates and OD600 was measured every 5 minutes. Under these conditions, strains lacking the kinase (‘ΔprkC’), or both the kinase and phosphatase (‘Δ(prpC-prkC)’), exhibit reduced survival in late stationary phase. Strains lacking only the phosphatase (‘ΔprpC’) have increased survival. No difference between any of the strains is observed in log phase. Shown are typical growth curves, plotted as mean OD600 as a function of time post-inoculation. Two shades of blue are used to group by color both strains lacking the kinase to highlight their similarity. C) Activation of yocH by PrkC in stationary phase. Transcriptional changes in yocH expression during exponential, late log, transition, and stationary phases in WT, ΔprpC, ΔprkC, and Δ(prpC-prkC) backgrounds. Relative luminescence (RLU) of a PyocH-lux transcriptional reporter was measured at each growth phase indicated in panel (B) and normalized by OD600 to give normalized luminescence. In stationary phase, yocH is >20 fold activated in a ΔprpC background (green) relative to Δ(prpC-prkC) (dark blue). A ~3.5 fold difference is observed between WT (gray) and Δ(prpC-prkC) (dark blue) strains (shown in detail in S1 Fig). Growth phases picked for comparison of gene expression correspond to OD600~0.1 (‘Exponential’), 0.2 (‘Late Log’), 0.3 (‘Transition’), and 0.5 (‘Stationary’) phase prior to the onset of lysis) as indicated by arrows in (B). D) PrkC-dependent yocH activation begins at the exit from exponential phase. i) Normalized luminescence of PyocH-lux plotted as a function of OD600 values from exponential phase through stationary phase in WT, ΔprpC, ΔprkC, and Δ(prpC-prkC) backgrounds. Points are plotted to the maximum OD600 observed for each strain. ii) Detail of plot in i), from OD600~0.3 through the max OD600 reached for each strain. To test these alternate hypotheses, we first determined when PrkC activity has the strongest effect on yocH expression. We constructed a transcriptional fusion of the yocH promoter region to the luxABCDE operon, originally from P. luminescens, optimized for expression in Gram-positive bacteria [28] and integrated it into an ectopic locus in the chromosome. As the luminescence signal from the luxABCDE operon is relatively unstable, it can be used to measure both increases and decreases in gene expression [29]. Therefore, we used the PyocH-luxABCDE transcriptional reporter (hereafter referred to as PyocH-lux) to continuously monitor changes in yocH expression throughout growth in rich media (LB) in a plate reader. Under these conditions, the doubling time for the WT strain harboring the PyocH-lux reporter is roughly 22 minutes in exponential phase, comparable to the optimal growth rate of B. subtilis in LB at 37°C. We measured yocH expression as well as the corresponding growth curves (OD600) in the plate reader every 5 minutes in four genetic backgrounds: wild type (‘WT’), ΔprpC (no phosphatase), ΔprkC (no kinase), and Δ(prpC-prkC) (neither phosphatase nor kinase). Under these conditions, the growth of these four strains is consistent with the previously reported stationary phase survival defect of ΔprkC strains and increased longevity of ΔprpC strains (Fig 1B) [30]. To highlight the consistent similarity between the ΔprkC and Δ(prpC-prkC) strains, indicating that the observed ΔprpC phenotypes are epistatic to prkC, ΔprkC mutants are grouped by color (Fig 1, shades of blue). Furthermore, these experiments also demonstrated that while there is no growth phenotype for ΔprkC and ΔprpC mutant strains during log phase growth, mild differences begin to appear during the transition to stationary phase and become more pronounced later in stationary phase prior to the onset of lysis (Fig 1B). Since transcriptional changes near the onset of lysis may be complicated to interpret, we chose to examine PrkC-dependent changes in gene expression at four characteristic growth points, as determined by position on the growth curve and corresponding measured OD600 (arrows indicate points for WT, Fig 1B): 1) exponential phase (OD600~0.1), 2) late log (OD600~0.2), 3) transition phase (OD600~0.3), and 4) stationary phase (OD600~0.5). We picked OD600~0.5 as a representative point for stationary phase measurements, as we observe comparable growth rates for all genetic backgrounds near this OD600, and it is prior to the onset of lysis. Note that OD600 values measured by the plate reader differ from those measured by a standard laboratory spectrophotometer. See materials and methods for more details.
To characterize PrkC-dependent yocH expression at each of these characteristic growth phases, we compared the normalized luminescence (relative luminescence, RLU, normalized by OD600) of the PyocH-lux reporter in each genetic background at each characteristic growth phase (Fig 1C). Interestingly, although prkC and prpC are expressed throughout all growth phases [16], mutants in prkC and prpC did not exhibit strong changes in yocH expression until stationary phase (Fig 1C). To determine more precisely when in the growth cycle changes in yocH expression begin to appear, we plotted normalized luminescence as a function of OD600 for data acquired at 5 minute intervals for each genetic background (Fig 1D), yielding a synchronized picture of gene expression as a function of growth state. For clarity, these graphs show only the pre-lysis data (to maximum OD600) for each strain. In WT cells, yocH expression is high in exponential phase and falls in stationary phase (Fig 1C and 1D, gray), consistent with prior work [31]. Interestingly, this trend was reversed in a ΔprpC background beginning at the exit from log phase (OD600~0.3, Fig 1D, ii). This effect is PrkC dependent as both ΔprkC and Δ(prpC-prkC) strains show very similar expression profiles (compare shades of blue, Fig 1D). The WT expression profile (gray) lies between ΔprpC and ΔprkC, and is strongly biased towards ΔprkC. This suggests that under these conditions, PrkC exerts a relatively small average effect on yocH expression in WT cells, ~3.5 fold (S1 Fig), when compared to the total possible PrkC-dependent effect observed in a ΔprpC background. The difference in expression between ΔprpC and ΔprkC backgrounds suggests that PrkC is capable of exerting a >20 fold change on yocH expression in stationary phase.
PrkC regulates the WalR regulon genes iseA and pdaC
If PrkC regulates yocH expression through a WalR-dependent mechanism (Fig 1A), we would expect it to control expression of other genes in the WalR regulon. Two genes repressed by WalR were found to have the highest average WalR occupancy of their promoters in a ChIP study [25]: iseA (yoeB), encoding an inhibitor of autolysin activity [32], and pdaC (yjeA), a peptidoglycan deacetylase [33]. To determine if PrkC regulates iseA and pdaC under the same conditions where we observed PrkC-dependent regulation of yocH, we constructed PiseA-luxABCDE and PpdaC-luxABCDE transcriptional reporters. Using these reporters and the same growth conditions and techniques used to monitor yocH expression, we measured iseA and pdaC expression using normalized luminescence as a function of OD600 (Fig 2A). Strikingly, we observed that both iseA and pdaC exhibit strong PrkC-dependent repression at the same OD600 and growth phases where yocH exhibited PrkC-dependent activation. That is, beginning at the exit from log phase (OD600~0.3) and becoming most significant in stationary phase, both iseA and pdaC show increased repression in a ΔprpC background that is relieved in both ΔprkC and Δ(prpC-prkC) backgrounds (Fig 2A). We examined this growth phase dependence by extracting the normalized luminescence at four specific optical densities from the continuous data (Fig 2B). Similarly to what we observed for yocH, the WT strains have expression profiles in stationary phase between the ΔprpC and ΔprkC backgrounds, consistent with opposing regulation. In strains carrying a ΔprpC mutation, PiseA-lux in stationary phase is expressed ~4x less and PpdaC-lux is expressed >10x less than in strains carrying Δ(prpC-prkC) mutations (Fig 2B). As yocH is activated by WalR and both iseA and pdaC are repressed by WalR, these data strongly support a model (Fig 1A) where PrkC acts through the WalRK system to transcriptionally regulate yocH, iseA, pdaC, and other members of the WalR regulon.
Fig. 2. The PrkC kinase and the PrpC phosphatase regulate the WalR regulon genes iseA and pdaC. 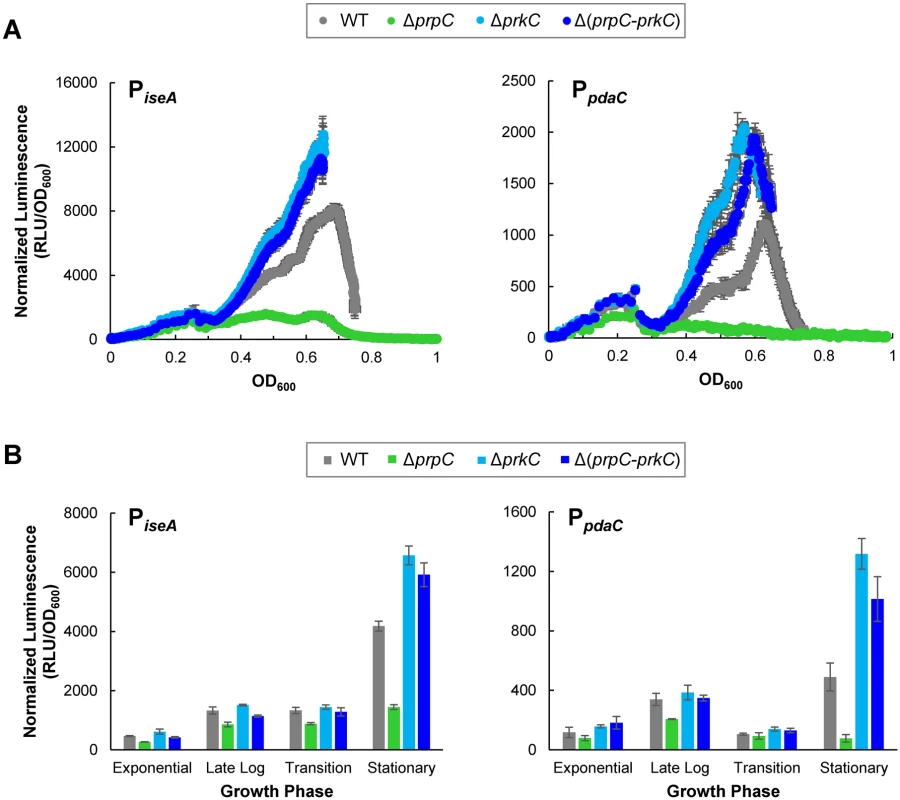
A) PrkC-dependent repression of iseA and pdaC is observed starting at the exit from exponential phase through stationary phase. Normalized luminescence as a function of OD600 throughout growth from exponential phase through stationary phase for PiseA-lux (left) and PpdaC-lux (right). Points are plotted to the maximum OD600 observed for each strain. B) Comparison of PrkC-dependent changes in iseA (left) and pdaC (right) expression during exponential, late log, transition, and stationary phases. In stationary phase, iseA is ~4x repressed in a ΔprpC (green) background compared to a Δ(prpC-prkC) background (dark blue), and pdaC is ~13x repressed. PrkC expression complements ΔprkC phenotypes
We tested whether inducible heterologous PrkC expression complements a ΔprkC mutation specifically in stationary phase by comparing the expression of the WalR reporters in strains carrying an inducible copy of prkC (Pspac-prkC) to their expression in WT and ΔprkC backgrounds (Fig 3). To confirm that these results represent systematic changes in expression from transition phase through early stationary phase (OD600~0.3–0.5), we also compared the normalized luminescence as a function of OD600 for each strain and IPTG concentration (S2 Fig). Without the addition of the inducer IPTG, the strain to test complementation, ΔprkC Pspac-prkC, appears similar to ΔprkC for all three promoters (compare two lightest shades of blue, Figs 3 and S2). Under these conditions, the small amount of “leaky” expression without inducer is sufficient to complement expression of PyocH, and nearly complement PiseA (compare gray and lightest shade of blue, Figs 3 and S2). At 10 μM IPTG, Pspac-prkC complements the ΔprkC deletion for the expression of PpdaC, and PyocH and PiseA also display similar expression to the wild type (compare gray and blue, Figs 3 and S2). For each promoter, the effect of increasing inducer concentration was consistent with increasing WalR activity specifically in stationary phase: increased repression of pdaC and iseA, and increased activation of yocH. Although ΔprkC Pspac-prkC was grown in the continuous presence of inducer, changes in gene expression for pdaC, iseA, and yocH did not appear until early stationary phase, consistent with the previous observation that the PrkC-dependent effect on the WalR regulon is growth phase specific (Figs 1 and 2). Thus, a low level of prkC expression in trans is sufficient to complement expression of pdaC, iseA, and yocH in stationary phase.
Fig. 3. Inducible PrkC expression complements ΔprkC effects on expression of WalR regulon genes during stationary phase. 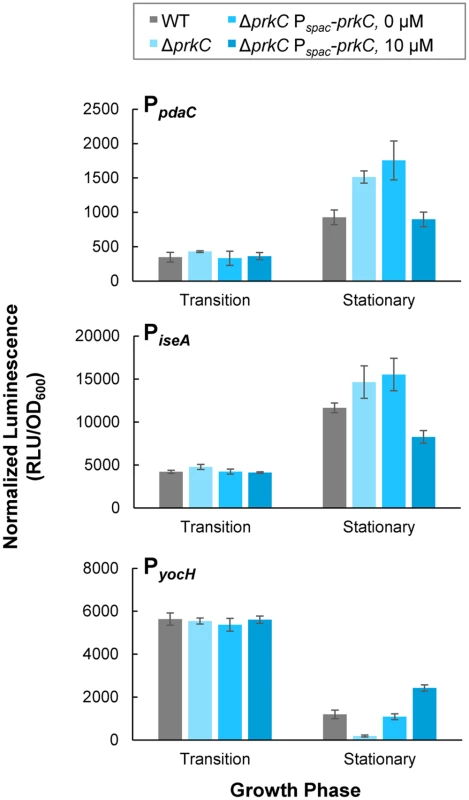
Normalized luminescence was measured in PpdaC-lux (top), PiseA-lux (middle), and PyocH-lux (bottom) reporters in a ΔprkC Pspac-prkC background grown in the presence of 0 and 10 μM IPTG. Expression in transition and stationary phases was compared to WT (gray), and ΔprkC (light blue) backgrounds. PrkC overexpression resembles ΔprpC phenotypes
The eSTK prkC is co-transcribed with its cognate eSTP prpC. Previous work suggested that the kinase activity of PrkC and the phosphatase activity of PrpC have opposing modes of action on B. subtilis physiology. Specifically, mutants in prkC and prpC have opposing stationary phase lysis phenotypes ([30] and Fig 1B) and overexpression of PrpC was shown to prevent PrkC mediated induction of yocH [15]. Consistent with these observations, prkC and prpC mutations have opposing effects on the expression of the WalR regulon genes yocH, iseA, and pdaC in stationary phase (Figs 1 and 2). We therefore sought to determine if the expression phenotypes for WalR regulon genes in a ΔprpC mutant background reflect high net PrkC kinase activity. To test this, we overexpressed prkC from Pspac-prkC (in a strain lacking endogenous prkC) using higher levels of IPTG induction than were necessary to complement the ΔprkC mutation (Fig 3). Higher levels of induction (100 μM or 1 mM IPTG) of Pspac-prkC caused expression of PyocH to increase and expression of PiseA and PpdaC to decrease (Fig 4), consistent with further increases in WalR activity. These effects are similar to those observed in the presence of a ΔprpC mutation (green) suggesting that kinase overexpression overwhelms the phosphatase activity under these conditions (Fig 4). This further suggests that the ΔprpC expression phenotypes observed for yocH, iseA, and pdaC in stationary phase (Figs 1 and 2) are caused by high levels of PrkC-dependent WalR phosphorylation, generated by the absence of phosphatase activity. With 100 μM or 1 mM IPTG induction (shades of dark blue), the reporters in the ΔprpC mutant (green) background all appear similar (Fig 4), suggesting that the reporters in the ΔprpC background reflect the maximal kinase activity for that condition.
Fig. 4. PrkC overexpression phenocopies ΔprpC effects on expression of WalR regulon genes. 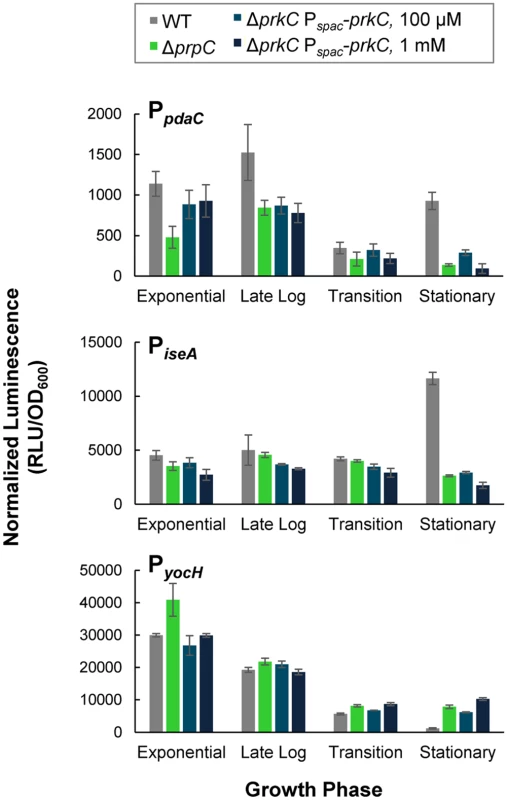
Normalized luminescence at characteristic growth phases of PpdaC-lux (top), PiseA-lux (middle), and PyocH-lux (bottom) was measured in WT (gray), and ΔprpC (green) backgrounds and in a ΔprkC Pspac-prkC background in the presence of 100 μM (medium blue) and 1 mM IPTG (dark blue). Although the prpC-prkC transcript is detected from exponential through stationary phase in LB [16], the heterologous overexpression of PrkC (Fig 4) produces only mild expression phenotypes prior to stationary phase. This observation, combined with the data indicating that PrkC-dependent transcriptional changes in the WalR regulon occur primarily in stationary phase (Figs 1 and 2), suggests that these effects are not simply caused by changes in prkC expression.
The eSTK PrkC phosphorylates WalR in vitro
As PrkC is membrane-bound and does not contain any predicted DNA binding domains, the observed co-regulation of three separate genes in the WalR regulon is likely to be indirect. One potential mechanism is a direct interaction of PrkC with WalR resulting in the phosphorylation of WalR on Ser or Thr residue(s). We examined this possibility by taking advantage of the previous observation that the cytoplasmic domain of PrkC autophosphorylates in vitro [34] and observed that PrkC robustly transphosphorylated WalR (Fig 5A). Mass spectrometry analysis of a PrkC-WalR kinase reaction performed with cold ATP suggested that WalR Thr101 was the only phosphorylated residue. To verify this interpretation, we generated mutant WalR proteins containing either a T101S or a T101A mutation and repeated the in vitro kinase assays. Strikingly, PrkC did not transphosphorylate either WalR T101S or WalR T101A mutant proteins, suggesting PrkC has a high degree of specificity for Thr101 (Fig 5A). WalR Thr101 is located at the dimerization interface of the receiver domain (Fig 5B, red).
Fig. 5. PrkC phosphorylates WalR Thr101 in vitro. 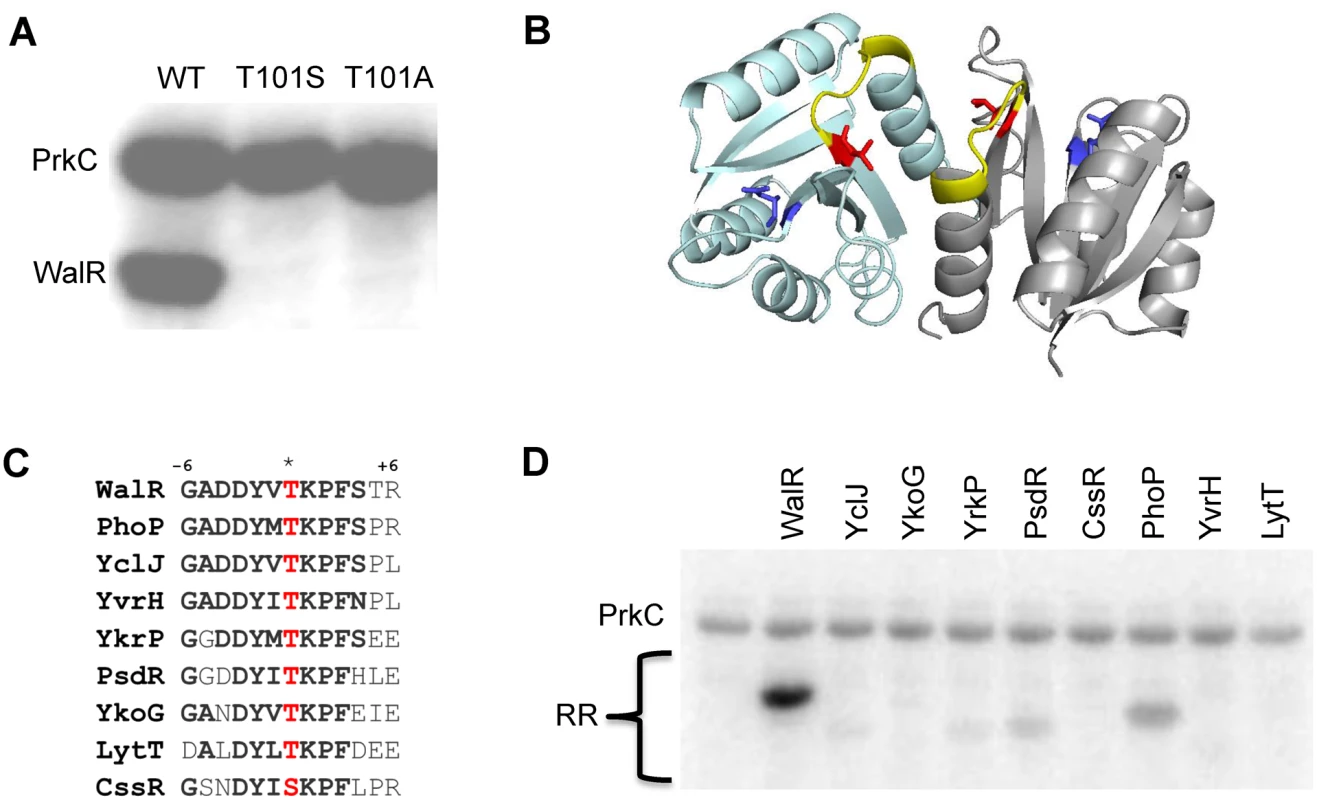
A) PrkC transphosphorylates WalR but not a WalR T101S or a WalR T101A mutant protein in an in vitro kinase assay. 1 μM PrkC and 4 μM WalR, WalR T101S, or WalR T101A were incubated with [γ-32P]-ATP for 30 minutes at 37°C. B) WalR phosphosites highlighted on the WalR receiver domain dimer crystal structure (PDB ID: 2ZWM) with individual protomers shown in cyan and gray. Asp53, the site of WalK phosphorylation, is shown in blue and Thr101, the site of PrkC phosphorylation, is shown in red. Surface exposed residues from Lys102 through Arg107 (+6 position) are highlighted in yellow. C) Amino acid sequence conservation in the β5-α5 region of the receiver domain surrounding the conserved threonine or serine residue (*, red) for B. subtilis response regulators with a conserved threonine or serine residue at the homologous position to WalR Thr101. Response regulators are WalR, YclJ, YkoG, YrkP, PsdR, CssR, PhoP, YvrH, and LytT. D) PrkC has specificity for WalR in vitro. 1 μM PrkC and 4 μM of each response regulator in (C) was incubated at 37°C with [γ-32P]-ATP for 10 minutes or 30 minutes (S3 Fig). PrkC has specificity for WalR in vitro
Work with M. tuberculosis eSTKs revealed specificity for particular phosphoacceptors based on sequences surrounding the phosphosite [35]. A ClustalO alignment of the 34 B. subtilis response regulators identified eight response regulators with a Thr or Ser residue at the position homologous to WalR Thr101 (PhoP, YvrH, PsdR, YkoG, YclJ, YrkP, LytT, and CssR; Fig 5C). In all of these response regulators, the Ser or Thr residue is surrounded by well-conserved residues (Fig 5C, bold), including those located on the surface exposed loop from the +1 to +6 position (Fig 5B, yellow). However, despite this strong conservation, when we purified the eight response regulators and performed in vitro kinase assays with PrkC, we observed that PrkC much more robustly phosphorylated WalR than the other response regulators following 10’ incubation (Fig 5D). Increasing the incubation time of the reactions to 30’ did not increase the phosphorylation of any other substrates (S3A Fig). We verified that similar amounts of total protein were loaded for each response regulator (S3B Fig), and that none of the response regulators exhibited a background signal in the absence of PrkC (S3C Fig).
WalR is phosphorylated on Thr101 in vivo
The ability of PrkC to affect the expression of WalR-dependent genes and to phosphorylate WalR in vitro on Thr101 suggests that this modification occurs in vivo. To detect WalR Thr phosphorylation in vivo, we immunoprecipitated FLAG-tagged WalR from OD600 matched stationary phase LB cultures of wild type (WT), ΔprpC, and ΔprkC strains. As a control for non-specific immunoprecipitation of proteins, strains lacking a FLAG-tagged protein were also used. Each sample was run on a gel cast with Phos-tag acrylamide, allowing for the separation of phosphorylated proteins by SDS-PAGE (Fig 6, top). To determine if any shifted bands observed were dependent on Phos-tag, and therefore indicative of phosphorylation, a control gel without Phos-tag was run in parallel (Fig 6, bottom). Since Thr phosphorylations are relatively heat stable compared to Asp phosphorylations, samples were boiled prior to gel loading. Visualized by subsequent Western blotting for the FLAG-tagged protein, a Phos-tag-dependent second band was observed in the WT sample, was significantly enhanced in the ΔprpC sample, but did not appear in the ΔprkC sample (Fig 6, top). This result indicated the presence of a heat stable PrkC-dependent WalR phosphorylation in vivo.
Fig. 6. PrkC-dependent WalR phosphorylation is observed in vivo. 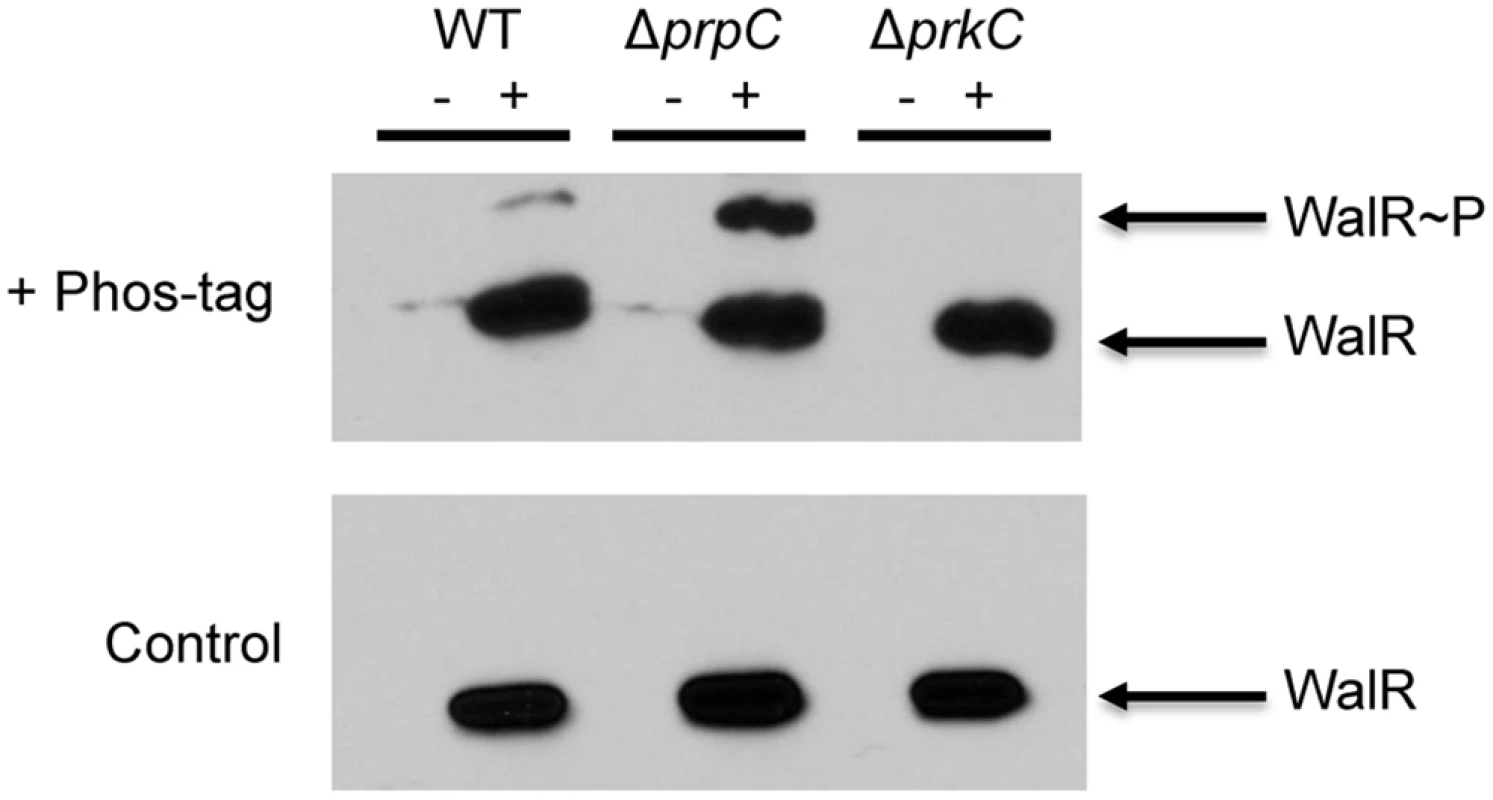
Anti-FLAG western blots of WalR immunoprecipitated from WT, ΔprpC, and ΔprkC backgrounds expressing WalR-FLAG (‘+’ lanes). WT, ΔprpC, and ΔprkC strains lacking WalR-FLAG were used as controls (‘-‘ lanes). Top: SDS-PAGE supplemented with 37.5 μM Phos-tag results in a second band in WT and ΔprpC (Δphosphatase) backgrounds, but not in a ΔprkC (Δkinase). Bottom: In a control gel lacking Phos-tag, samples were identically loaded and run in parallel. To remove any residual heat labile Asp phosphorylation, samples were boiled prior to gel loading. To verify that this PrkC-dependent WalR phosphorylation was on the same residue observed in vitro, mass spectrometry was performed on WalR-FLAG immunoprecipitated from ΔprpC cells. In two separate experiments, phospho-peptides containing Thr101 were found by mass spectrometry, providing further evidence that this modification occurs in vivo (S4 Fig). To confirm that the experimentally observed phospho-peptides and unmodified peptides had masses that are consistent with the WalR Thr101 region, the experimentally determined masses were compared to both the theoretically predicted masses and the masses observed from synthetic peptides (S4 Fig).
WalR Thr101 is required for the PrkC-dependent effect on yocH expression
PrkC-dependent WalR Thr101 phosphorylation was observed in vivo, suggesting that WalR Thr101 phosphorylation was responsible for the PrkC-dependent gene expression phenotypes observed for yocH (Fig 1). Therefore, we generated a strain containing a walR T101A mutation at the endogenous locus. Since the WalR T101A mutant may exhibit PrkC independent changes in expression compared to wild type WalR, direct comparison of yocH expression between waRT101A and walRWT backgrounds cannot be performed. Therefore, we generated PyocH reporter strains in the walRT101A background in otherwise WT, ΔprpC, and Δ(prpC-prkC) backgrounds to test for PrkC dependence. In parallel, we measured the expression of yocH using normalized luminescence as a function of OD600 in both walRT101A and walRWT backgrounds (Fig 7A). Log phase yocH expression was comparable between walRWT and walRT101A, indicating that the walRT101A mutant is functional at the yocH promoter in vivo (Fig 7A, compare OD600<0.3, left and right). Strikingly, in the walRT101A background, PrkC-dependent yocH activation in stationary phase was lost, with the WT, ΔprpC and Δ(prpC-prkC) backgrounds all showing similar expression profiles (Fig 7A, left). This is in contrast to the walRWT background, particularly in stationary phase (OD600>0.4), where the strongest PrkC-dependent activation is observed in a walRWT background (Fig 7A, right inset). At the same stationary phase OD600 used in Figs 1–4, we found that in the walRWT background, a >20 fold difference is observed between the ΔprpC and Δ(prpC-prkC) backgrounds, whereas no significant difference is observed in the walRT101A mutant background (Fig 7B). In the 40 min prior to OD600~0.5 (‘Stationary Phase’) we observed comparable growth rates for WT, ΔprpC, ΔprkC, Δ(prpC-prkC) in both walRWT (S5 Fig, top) and walRT101A (S5 Fig, bottom) backgrounds.
Fig. 7. yocH does not show PrkC-dependent activation in a WalR T101A mutant. 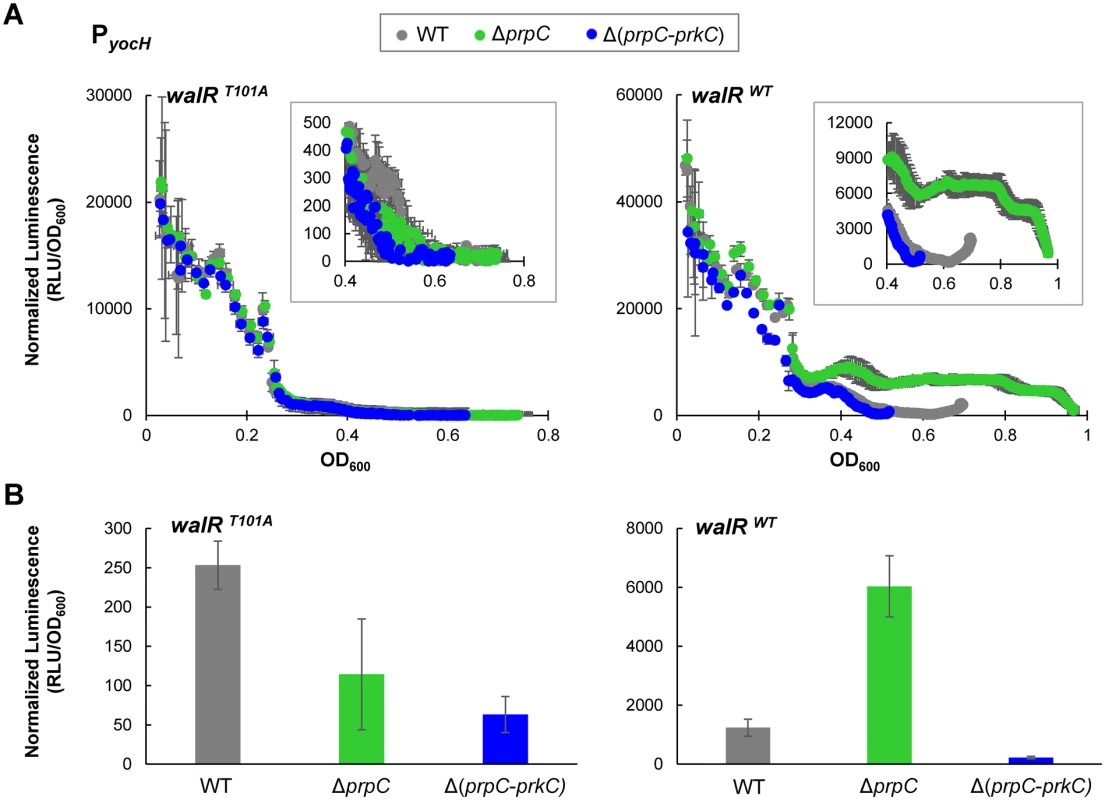
A) Parallel experiments measuring PrkC-dependent yocH activation in walRT101A point mutant (left) and walRWT strains (right). Normalized luminescence from a PyocH-lux transcriptional reporter is plotted as a function of OD600 from exponential phase through the maximum OD600 reached for each strain. In both walRT101A and walRWT backgrounds, yocH is highly expressed in log phase. Insets: Detail of stationary phase expression of yocH in WT, ΔprpC and Δ(prpC-prkC) in walRT101A (left) and walRWT (right) backgrounds. B) Normalized luminescence of PyocH-lux reporters in stationary phase (OD600~0.5) for walRT101A (left) and walRWT strains (right). In a walRT101A background, no significant difference is observed between ΔprpC and Δ(prpC-prkC) in stationary phase, whereas a >20 fold change is observed between ΔprpC and Δ(prpC-prkC) in a walRWT background (right). PrkC-dependent repression of iseA and pdaC is reduced in a WalR T101A mutant
To confirm that WalR Thr101 is also required for the PrkC-dependent repression of iseA and pdaC, we performed experiments similar to those described above for yocH. Log phase expression of iseA and pdaC in walRT101A and walRWT backgrounds was comparable, suggesting that WalR T101A is also functional at these promoters as a repressor (S6 Fig). Whereas both PiseA and PpdaC show strong PrkC-dependent repression in the walRWT background in stationary phase, in the walRT101A background, the repression is at least ~10 fold relieved between ΔprpC and ΔprkC (Fig 8). Consistent with a loss of PrkC-dependent activity at these promoters in stationary phase, the loss of repression in the walRT101A mutant is primarily due to the reduction of repression in the ΔprpC background, not to an increase in expression in a ΔprkC background. The residual-PrkC dependent effect on these promoters may be due to additional PrkC activity not dependent on WalR Thr101 phosphorylation that is sensed by WalK (S6 Fig). As iseA and pdaC were identified as the two sites in the B. subtilis chromosome with the highest WalR occupancy [25], it is possible that they are more sensitive than yocH to WalRK activity.
Fig. 8. iseA and pdaC show reduced PrkC-dependent repression in a walRT101A mutant background. 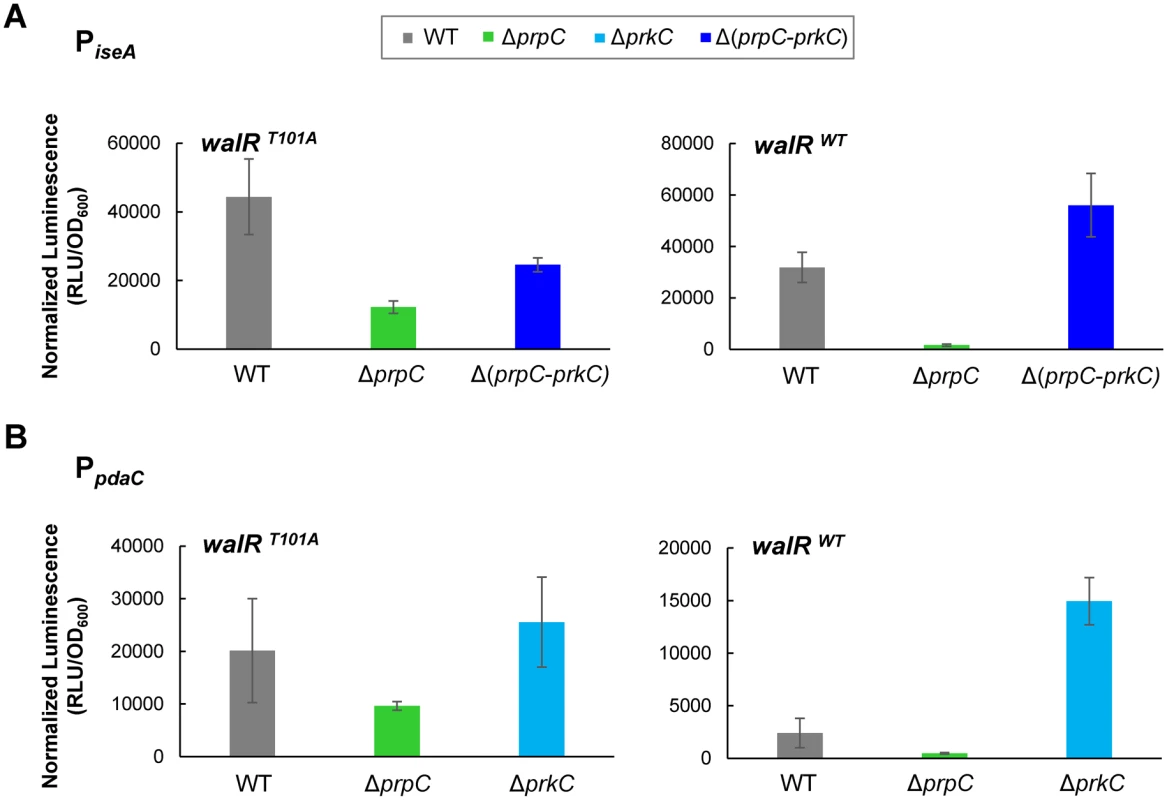
Normalized luminescence of PiseA-lux (A) and PpdaC-lux (B) reporters in stationary phase (OD600~0.5) in walRT101A (left) and walRWT strains (right). For both iseA and pdaC, repression was reduced >10 fold between ΔprpC and ΔprkC in the walRT101A mutant compared to the walRWT background. Discussion
Here, we provide the first known mechanism for PrkC-dependent gene regulation in B. subtilis by demonstrating that PrkC phosphorylates the response regulator WalR on Thr101 both in vitro and in vivo. Overall, this work supports a model (Fig 9) where PrkC, a PASTA-domain-containing eSTK, is activated and phosphorylates WalR at Thr101. This is supported by the in vitro data demonstrating direct phosphorylation of WalR Thr101 by PrkC (Fig 5), as well as the presence of PrkC-dependent phosphorylation observed at Thr101 in vivo (Figs 6 and S4). Transcriptional data from the WalR-dependent genes yocH, iseA, and pdaC (Figs 1,2, 7 and 8) demonstrates that this secondary phosphorylation increases both the activation and repression of WalR regulon genes (Fig 9, bottom). In ΔprpC strains, increases in WalR Thr101 phosphorylation are observed in vivo, and changes in expression of WalR-dependent genes is consistent with high levels of PrkC activity (Figs 4 and 6), demonstrating that PrpC and PrkC have opposing modes of action.
Fig. 9. Model of PrkC dependent WalR phosphorylation. 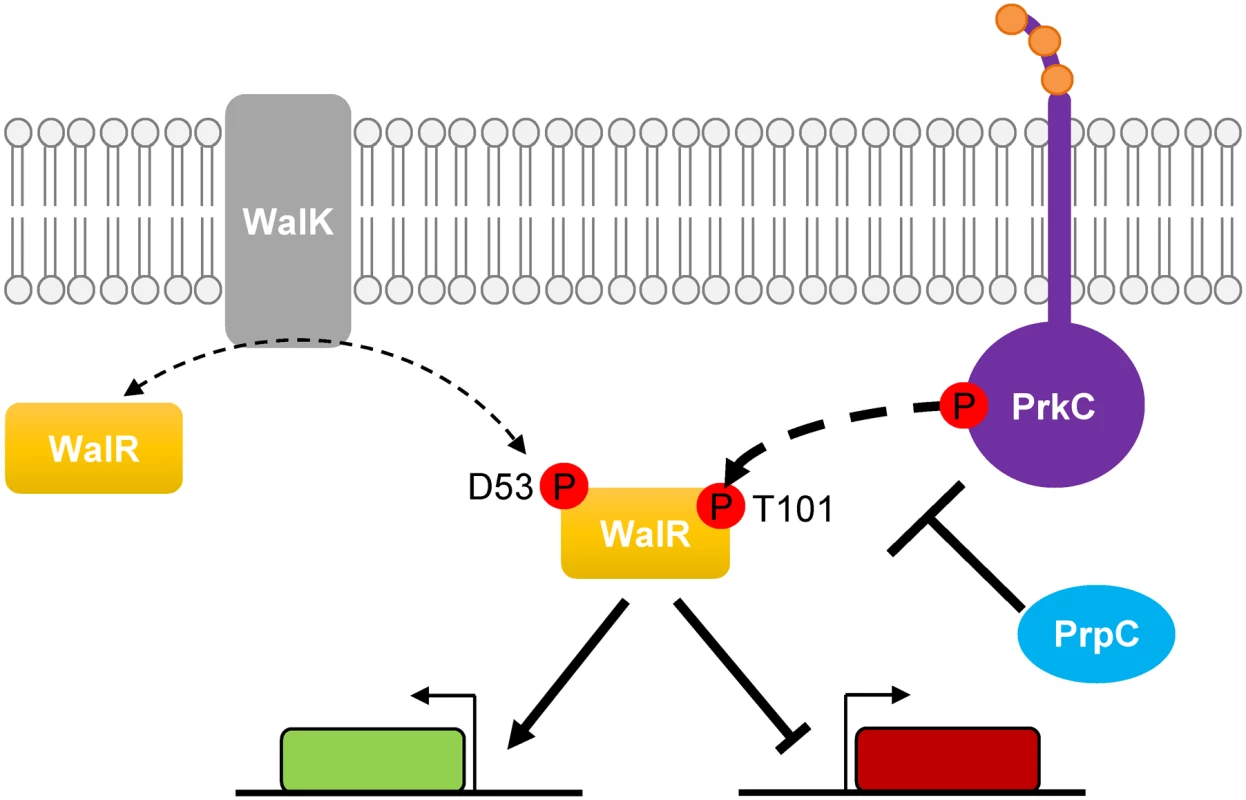
The response regulator WalR is phosphorylated on Asp53 by the histidine kinase WalK, leading to activation and repression of genes in the WalR regulon. WalR is also phosphorylated by the PASTA-domain-containing eSTK PrkC on Thr101 (bold dashed arrow) leading to enhanced activation or repression of genes in the WalR regulon. The eSTP PrpC decreases the amount of WalR Thr101 phosphorylation. The effect of PrkC on the expression of the WalR regulon is most significant as growth slows in stationary phase (Figs 1 and 2). As continuous expression of prkC in trans does not cause changes in gene expression during rapid growth, the mild effect of PrkC on the WalR regulon in log phase is likely not due to lack of expression. Rather, it suggests that PrkC regulation of the WalRK system becomes significant as the activity of the WalRK system begins to decrease in stationary phase [31]. Thus, by acting as a second sensor for the system, PrkC can provide an input to increase WalR activity even as WalK activity decreases. Since both PrkC and WalK are hypothesized to sense cell wall-related processes, and both are highly conserved signaling systems in Gram-positive bacteria, this may suggest that they have separate sensory inputs in vivo, such as sensing different aspects or different steps in cell wall growth and metabolism. The PASTA domain of PrkC interacts in vitro with muropeptides [13], suggesting that it may directly sense some aspect of the cell wall. WalK localizes to septal regions, and it has been hypothesized that some aspect of cell wall synthesis during active division is required to keep WalK activity high [18,36–39]. Taken together, separate sensory inputs working through PrkC and WalK may regulate the expression of genes responsible for cell wall metabolism by phosphorylation of WalR on two different sites.
WalR Thr101, the site of PrkC phosphorylation both in vitro and in vivo, is in the receiver domain at the α4-β5-α5 dimerization interface (Fig 5B). This region is important for both activity and regulation of protein-protein interactions in OmpR family response regulators such as WalR [40,41]. In response regulators of this type, this region has been shown to undergo important conformational changes when Asp phosphorylation occurs [42]. Therefore WalR Thr101 phosphorylation may impact the structural conformation of WalR in complex ways, including by altering the mechanism by which WalK-mediated Asp phosphorylation activates WalR. However, the gene expression data presented here suggests that the net effect of WalR Thr101 phosphorylation is consistent with an increase in WalR activity.
The high degree of sequence conservation between B. subtilis response regulators with conserved Ser or Thr residues in the receiver domain (Fig 5C) suggests that the specificity of PrkC for WalR is mediated by a region which is not conserved. Previous work dissecting the in vitro specificity of six different eSTKs in M. tuberculosis suggested that phosphorylation is enhanced with highly hydrophobic residues at the +3 and +5 positions surrounding the phosphosite [35]. Given that this region is surface exposed and the +3 site is conserved among the B. subtilis response regulators, the residue at the +5 position may govern specificity. This possibility is consistent with data that residues not directly adjacent to the phosphorylation site can affect the specific phosphorylation of response regulators by histidine kinases [43,44].
Other PrkC substrates including EF-Tu, EF-G, CpgA, YezB, YkwC, YvcK, and several metabolic enzymes [14,45–49] have been reported. However, evidence suggesting that PrkC-dependent phosphorylation of a transcription factor is important for gene regulation is relatively scarce. Recently, the transcription factor AbrB was found to be phosphorylated in vitro on S86 by PrkC and the two other B. subtilis eSTKs YabT and PrkD, resulting in a decrease in AbrB DNA binding activity [50]. However, AbrB phosphorylation in vivo was dependent on the presence of all three eSTKs, making the precise signal sensed and the dominant kinase responsible for physiologically relevant phosphorylation unclear.
Numerous examples of eSTK-dependent phosphorylation of transcription factors, including response regulators, have been reported in other Gram-positive bacteria (for recent reviews, see [2] and [8]). Although most of these examples are derived from in vitro observations, in vivo phosphorylation has been observed for both S. pneumoniae RR06 [51] and S. pyogenes CovR [52]. Examples in which specific in vitro phosphosites have been identified include S. aureus GraR [53] and VraR [54], M. tuberculosis DosR and Rv2175c [55–57], S. pneumoniae RitR [58] and RR06 [51], and Group A and Group B Streptococci CovR [52,59]. In some of these examples (e.g., RitR [58], GraR [53] and VraR [54]), the eSTK phosphorylates the response regulator in the DNA binding domain, suggesting that the effects on gene expression are caused by changing the affinity of the response regulator for its DNA binding sites. Alternatively, response regulators can be phosphorylated in the receiver domain (e.g., CovR [52,59], DosR [55], and VraR [54]). Of these, the most similar example to the WalR Thr101 phosphorylation we observed here is S. aureus VraR, which is phosphorylated in vitro on four residues including Thr106 located at the dimerization interface.
The essentiality of WalRK and its homologues in many low G+C Gram-positive bacteria, combined with its effect on virulence [60] and antibiotic resistance [61,62], has made mutations in the WalRK system in clinical pathogens of particular interest. Of note, single nucleotide substitutions within walR cause an increase in vancomycin resistance [63]. Whole genome sequencing of clinical isolates of VISA (vancomycin intermediate S. aureus) has identified WalR mutations (walR T101S and walR T101R) at the homologous Thr to the PrkC-WalR Thr phosphosite reported here [64]. This observation suggests that WalR Thr101 plays an important role in antibiotic resistance in S. aureus.
In conclusion, our characterization of PrkC-dependent phosphorylation of WalR helps to clarify the role of PrkC in stationary phase B. subtilis physiology and gene regulation. The intersection of the eSTK-eSTP pair PrkC-PrpC with the essential WalRK TCS system provides two sensory inputs to regulate the essential process of cell wall metabolism in B. subtilis. Parallels with closely related systems in Gram-positive pathogens suggest that this mechanism of regulation may be conserved and relevant to further understanding the role of eSTK phosphorylation of response regulators in Gram-positive physiology in the future.
Materials and Methods
Strains and strain construction
Bacillus subtilis strains were constructed by transformation into B. subtilis 168 trpC2 (PB2) obtained from Chet Price [30] and its derivatives unless otherwise noted. Strains used in each figure are listed in S1 Table, and details of strain and plasmid construction are in S2, S3 and S4 Tables. B. subtilis strains were transformed with 5–10 μl of plasmid DNA or 1–2 μl of genomic DNA using the two-step method [65]. Genomic DNA was prepared using the Wizard Genomic DNA Purification Kit (Promega) according to the manufacturer’s instructions. Cultures were grown in LB (Lennox).
Luminescence plate reader assays for growth curves and gene expression
Early log phase cultures were grown in LB inoculated from single colonies obtained from plates grown overnight at 37°C. The cultures were diluted 1 : 30 (150 μl final volume) into fresh LB in 96-well plates and grown at 37°C with continuous shaking for >20 h in a Tecan Infinite 200 plate reader. Measurements of luminescence and OD600 were taken at 5 min intervals. Media only and lux - controls were used for background subtraction for OD600 and luminescence respectively. All cultures were grown in triplicate. Note that OD600 values measured in the Tecan plate reader differ from the spectrophotometer by both a scale factor and an offset, making OD600~0.1 measurement in the plate reader correspond to OD600~0.4 in a standard laboratory spectrophotometer.
Immunoprecipitation of WalR-FLAG
WalR-FLAG was immunoprecipitated from 25 ml (Phos-tag experiment) or 600 ml (mass spectrometry experiment) cultures at OD600~0.4 using anti-FLAG M2 magnetic beads (Sigma M8823) according to the manufacturer’s instructions with the following modifications. Initial lysis to break down the cell wall was performed in 50 mM Tris pH 7.5, 150 mM NaCl, 0.1% NP-40, 5 μg/ml DNaseI, 3 mM MgCl2, 1x Halt phosphatase inhibitor cocktail (Pierce), 1 mM PMSF, and 1 mg/ml lysozyme. After 20 min of lysis on ice with periodic vortexing, cell pellets were spun down and resuspended in lysis buffer without lysozyme and applied to 0.1 mm silica beads (BioSpec, 11079101z) in pre-chilled screw cap tubes. Cells were lysed using a FastPrep-24 5G instrument (MP Biomedicals) using 4 runs of 6.5 m/s for 40 seconds with samples placed on ice for 3 min between each run. Lysates were cleared by centrifugation at 19,000 x g for 20 min at 4°C. Elutions from the anti-FLAG M2 magnetic beads were performed competitively by addition of the 3x-FLAG peptide (Sigma F4799).
Separation and visualization of phosphorylated WalR by Phos-tag acrylamide
10% resolving gels for SDS-PAGE were cast with ±37.5 μM Phos-tag acrylamide (Wako, AAL-107, 304–93521), and 5% stacking gels were prepared as per the manufacturer’s instructions. Prior to gel loading, 35 μL of each sample was boiled at 100°C for 15 seconds to remove any residual heat labile Asp phosphorylation. Approximately 30 ng of WalR-FLAG was loaded per lane. Gels were run and transferred as recommended by the manufacturer with the following modifications: SDS-PAGE gels were loaded and run in parallel on a Bio-Rad mini-protean gel apparatus at constant voltage (150 V) for approximately 100 min (an additional ~20 minutes after the dye front ran off the gel) at 4°C. Gels were fixed twice for 15 min in transfer buffer supplemented with 5 mM EDTA to remove Mn2+ from the gel, followed by a 10 min incubation in transfer buffer. Transfer to PVDF membranes was performed using a wet tank method (Bio-Rad Mini Trans-Blot) at a constant 350 mA for 80 min. Subsequent Western blotting was performed using standard protocols with an anti-FLAG-Peroxidase antibody (Sigma-Aldrich s#SAB4200119).
In vitro kinase assays with PrkC
PrkC kinase reactions were performed in kinase buffer (50 mM Tris-HCl pH 7.5, 50 mM KCl, 10mM MgCl2, and 0.5 mM DTT). All reactions were performed in the presence of 400 μM cold ATP and 2 μCi (~ 20 nM) of γ-32P-ATP. The eSTK was used at a final concentration of 1 μM and substrates were added at a final concentration of 4 μM. Reactions were incubated at 37°C for 30 min unless otherwise indicated. 5X SDS-PAGE buffer was added to the reactions and subsequently boiled at 95°C for 5 min. Samples were run on a 12% SDS-PAGE gel and dried for 30 min at 80°C in a gel dryer. Dried gels were exposed to a phosphoscreen and visualized using a Typhoon Scanner (GE Healthcare).
Additional Materials and Methods, including protein purification and mass spectrometry for the detection of WalR phosphorylation both in vitro and in vivo, can be found in S1 Text.
Supporting Information
Zdroje
1. Mijakovic I, Macek B (2012) Impact of phosphoproteomics on studies of bacterial physiology. FEMS Microbiol Rev 36 : 877–892. doi: 10.1111/j.1574-6976.2011.00314.x 22091997
2. Dworkin J (2015) Ser/Thr phosphorylation as a regulatory mechanism in bacteria. Curr Opin Microbiol 24C: 47–52.
3. Pereira SF, Goss L, Dworkin J (2011) Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiology and molecular biology reviews: MMBR 75 : 192–212. doi: 10.1128/MMBR.00042-10 21372323
4. Donat S, Streker K, Schirmeister T, Rakette S, Stehle T, et al. (2009) Transcriptome and functional analysis of the eukaryotic-type ser/thr kinase PknB in Staphylococcus aureus. J Bacteriol 191 : 4056–4069. doi: 10.1128/JB.00117-09 19376851
5. Saskova L, Novakova L, Basler M, Branny P (2007) Eukaryotic-type serine/threonine protein kinase StkP is a global regulator of gene expression in Streptococcus pneumoniae. J Bacteriol 189 : 4168–4179. 17416671
6. Banu LD, Conrads G, Rehrauer H, Hussain H, Allan E, et al. (2010) The Streptococcus mutans serine/threonine kinase, PknB, regulates competence development, bacteriocin production, and cell wall metabolism. Infect Immun 78 : 2209–2220. doi: 10.1128/IAI.01167-09 20231406
7. Burnside K, Rajagopal L (2012) Regulation of prokaryotic gene expression by eukaryotic-like enzymes. Curr Opin Microbiol 15 : 125–131. doi: 10.1016/j.mib.2011.12.006 22221896
8. Wright DP, Ulijasz AT (2014) Regulation of transcription by eukaryotic-like serine-threonine kinases and phosphatases in Gram-positive bacterial pathogens. Virulence 5 : 863–885. doi: 10.4161/21505594.2014.983404 25603430
9. Jones G, Dyson P (2006) Evolution of transmembrane protein kinases implicated in coordinating remodeling of gram-positive peptidoglycan: inside versus outside. J Bacteriol 188 : 7470–7476. 16936012
10. Yeats C, Finn RD, Bateman A (2002) The PASTA domain: a beta-lactam-binding domain. Trends Biochem Sci 27 : 438. 12217513
11. Maestro B, Novakova L, Hesek D, Lee M, Leyva E, et al. (2011) Recognition of peptidoglycan and beta-lactam antibiotics by the extracellular domain of the Ser/Thr protein kinase StkP from Streptococcus pneumoniae. FEBS Lett 585 : 357–363. doi: 10.1016/j.febslet.2010.12.016 21167155
12. Mir M, Asong J, Li X, Cardot J, Boons GJ, et al. (2011) The extracytoplasmic domain of the Mycobacterium tuberculosis Ser/Thr kinase PknB binds specific muropeptides and is required for PknB localization. PLoS pathogens 7: e1002182. doi: 10.1371/journal.ppat.1002182 21829358
13. Squeglia F, Marchetti R, Ruggiero A, Lanzetta R, Marasco D, et al. (2011) Chemical Basis of Peptidoglycan Discrimination by PrkC, a Key Kinase Involved in Bacterial Resuscitation from Dormancy. Journal of the American Chemical Society 133 : 20676–20679. doi: 10.1021/ja208080r 22111897
14. Shah IM, Laaberki MH, Popham DL, Dworkin J (2008) A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135 : 486–496. doi: 10.1016/j.cell.2008.08.039 18984160
15. Shah IM, Dworkin J (2010) Induction and regulation of a secreted peptidoglycan hydrolase by a membrane Ser/Thr kinase that detects muropeptides. Mol Microbiol 75 : 1232–1245. doi: 10.1111/j.1365-2958.2010.07046.x 20070526
16. Nicolas P, Mader U, Dervyn E, Rochat T, Leduc A, et al. (2012) Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335 : 1103–1106. doi: 10.1126/science.1206848 22383849
17. Winkler ME, Hoch JA (2008) Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J Bacteriol 190 : 2645–2648. doi: 10.1128/JB.01682-07 18245295
18. Dubrac S, Bisicchia P, Devine KM, Msadek T (2008) A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol 70 : 1307–1322. doi: 10.1111/j.1365-2958.2008.06483.x 19019149
19. Fabret C, Hoch JA (1998) A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J Bacteriol 180 : 6375–6383. 9829949
20. Dominguez-Cuevas P, Mercier R, Leaver M, Kawai Y, Errington J (2012) The rod to L-form transition of Bacillus subtilis is limited by a requirement for the protoplast to escape from the cell wall sacculus. Molecular microbiology 83 : 52–66. doi: 10.1111/j.1365-2958.2011.07920.x 22122227
21. Ng WL, Robertson GT, Kazmierczak KM, Zhao J, Gilmour R, et al. (2003) Constitutive expression of PcsB suppresses the requirement for the essential VicR (YycF) response regulator in Streptococcus pneumoniae R6. Molecular microbiology 50 : 1647–1663. 14651645
22. Howell A, Dubrac S, Andersen KK, Noone D, Fert J, et al. (2003) Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol Microbiol 49 : 1639–1655. 12950927
23. Bisicchia P, Noone D, Lioliou E, Howell A, Quigley S, et al. (2007) The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis. Mol Microbiol 65 : 180–200. 17581128
24. Dubrac S, Boneca IG, Poupel O, Msadek T (2007) New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. Journal of bacteriology 189 : 8257–8269. 17827301
25. Salzberg LI, Powell L, Hokamp K, Botella E, Noone D, et al. (2013) The WalRK (YycFG) and sigma(I) RsgI regulators cooperate to control CwlO and LytE expression in exponentially growing and stressed Bacillus subtilis cells. Mol Microbiol 87 : 180–195. doi: 10.1111/mmi.12092 23199363
26. Bugrysheva J, Froehlich BJ, Freiberg JA, Scott JR (2011) Serine/threonine protein kinase Stk is required for virulence, stress response, and penicillin tolerance in Streptococcus pyogenes. Infection and Immunity 79 : 4201–4209. doi: 10.1128/IAI.05360-11 21788381
27. Szurmant H, Nelson K, Kim EJ, Perego M, Hoch JA (2005) YycH regulates the activity of the essential YycFG two-component system in Bacillus subtilis. J Bacteriol 187 : 5419–5426. 16030236
28. Qazi SN, Counil E, Morrissey J, Rees CE, Cockayne A, et al. (2001) agr expression precedes escape of internalized Staphylococcus aureus from the host endosome. Infect Immun 69 : 7074–7082. 11598083
29. Radeck J, Kraft K, Bartels J, Cikovic T, Durr F, et al. (2013) The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J Biol Eng 7 : 29. doi: 10.1186/1754-1611-7-29 24295448
30. Gaidenko TA, Kim TJ, Price CW (2002) The PrpC serine-threonine phosphatase and PrkC kinase have opposing physiological roles in stationary-phase Bacillus subtilis cells. J Bacteriol 184 : 6109–6114. 12399479
31. Botella E, Hubner S, Hokamp K, Hansen A, Bisicchia P, et al. (2011) Cell envelope gene expression in phosphate-limited Bacillus subtilis cells. Microbiology 157 : 2470–2484. doi: 10.1099/mic.0.049205-0 21636651
32. Yamamoto H, Hashimoto M, Higashitsuji Y, Harada H, Hariyama N, et al. (2008) Post-translational control of vegetative cell separation enzymes through a direct interaction with specific inhibitor IseA in Bacillus subtilis. Mol Microbiol 70 : 168–182. doi: 10.1111/j.1365-2958.2008.06398.x 18761694
33. Kobayashi K, Sudiarta IP, Kodama T, Fukushima T, Ara K, et al. (2012) Identification and characterization of a novel polysaccharide deacetylase C (PdaC) from Bacillus subtilis. J Biol Chem 287 : 9765–9776. doi: 10.1074/jbc.M111.329490 22277649
34. Madec E, Stensballe A, Kjellstrom S, Cladiere L, Obuchowski M, et al. (2003) Mass spectrometry and site-directed mutagenesis identify several autophosphorylated residues required for the activity of PrkC, a Ser/Thr kinase from Bacillus subtilis. J Mol Biol 330 : 459–472. 12842463
35. Prisic S, Dankwa S, Schwartz D, Chou MF, Locasale JW, et al. (2010) Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc Natl Acad Sci U S A 107 : 7521–7526. doi: 10.1073/pnas.0913482107 20368441
36. Fukushima T, Szurmant H, Kim EJ, Perego M, Hoch JA (2008) A sensor histidine kinase co-ordinates cell wall architecture with cell division in Bacillus subtilis. Molecular microbiology 69 : 621–632. doi: 10.1111/j.1365-2958.2008.06308.x 18573169
37. Fukushima T, Furihata I, Emmins R, Daniel RA, Hoch JA, et al. (2011) A role for the essential YycG sensor histidine kinase in sensing cell division. Mol Microbiol 79 : 503–522. doi: 10.1111/j.1365-2958.2010.07464.x 21219466
38. Szurmant H, Mohan MA, Imus PM, Hoch JA (2007) YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis. J Bacteriol 189 : 3280–3289. 17307850
39. Szurmant H, Bu L, Brooks CL 3rd, Hoch JA (2008) An essential sensor histidine kinase controlled by transmembrane helix interactions with its auxiliary proteins. Proc Natl Acad Sci U S A 105 : 5891–5896. doi: 10.1073/pnas.0800247105 18408157
40. Toro-Roman A, Mack TR, Stock AM (2005) Structural analysis and solution studies of the activated regulatory domain of the response regulator ArcA: a symmetric dimer mediated by the alpha4-beta5-alpha5 face. J Mol Biol 349 : 11–26. 15876365
41. Bourret RB (2010) Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol 13 : 142–149. doi: 10.1016/j.mib.2010.01.015 20211578
42. Kern D, Volkman BF, Luginbuhl P, Nohaile MJ, Kustu S, et al. (1999) Structure of a transiently phosphorylated switch in bacterial signal transduction. Nature 402 : 894–898. 10622255
43. Casino P, Rubio V, Marina A (2009) Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 139 : 325–336. doi: 10.1016/j.cell.2009.08.032 19800110
44. Capra EJ, Perchuk BS, Lubin EA, Ashenberg O, Skerker JM, et al. (2010) Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet 6: e1001220. doi: 10.1371/journal.pgen.1001220 21124821
45. Absalon C, Obuchowski M, Madec E, Delattre D, Holland IB, et al. (2009) CpgA, EF-Tu and the stressosome protein YezB are substrates of the Ser/Thr kinase/phosphatase couple, PrkC/PrpC, in Bacillus subtilis. Microbiology 155 : 932–943. doi: 10.1099/mic.0.022475-0 19246764
46. Pompeo F, Freton C, Wicker-Planquart C, Grangeasse C, Jault JM, et al. (2012) Phosphorylation of CpgA protein enhances both its GTPase activity and its affinity for ribosome and is crucial for Bacillus subtilis growth and morphology. J Biol Chem 287 : 20830–20838. doi: 10.1074/jbc.M112.340331 22544754
47. Ravikumar V, Shi L, Krug K, Derouiche A, Jers C, et al. (2014) Quantitative phosphoproteome analysis of bacillus subtilis reveals novel substrates of the kinase PrkC and phosphatase PrpC. Mol Cell Proteomics 13 : 1965–1978. doi: 10.1074/mcp.M113.035949 24390483
48. Foulquier E, Pompeo F, Freton C, Cordier B, Grangeasse C, et al. (2014) PrkC-mediated Phosphorylation of Overexpressed YvcK Protein Regulates PBP1 Protein Localization in Bacillus subtilis mreB Mutant Cells. J Biol Chem 289 : 23662–23669. doi: 10.1074/jbc.M114.562496 25012659
49. Pietack N, Becher D, Schmidl SR, Saier MH, Hecker M, et al. (2010) In vitro Phosphorylation of Key Metabolic Enzymes from Bacillus subtilis: PrkC Phosphorylates Enzymes from Different Branches of Basic Metabolism. J Mol Microbiol Biotechnol 18 : 129–140. doi: 10.1159/000308512 20389117
50. Kobir A, Poncet S, Bidnenko V, Delumeau O, Jers C, et al. (2014) Phosphorylation of Bacillus subtilis gene regulator AbrB modulates its DNA-binding properties. Mol Microbiol 92 : 1129–1141. doi: 10.1111/mmi.12617 24731262
51. Agarwal S, Agarwal S, Pancholi P, Pancholi V (2012) Strain-specific regulatory role of eukaryote-like serine/threonine phosphatase in pneumococcal adherence. Infect Immun 80 : 1361–1372. doi: 10.1128/IAI.06311-11 22311926
52. Horstmann N, Saldana M, Sahasrabhojane P, Yao H, Su X, et al. (2014) Dual-site phosphorylation of the control of virulence regulator impacts group a streptococcal global gene expression and pathogenesis. PLoS Pathog 10: e1004088. doi: 10.1371/journal.ppat.1004088 24788524
53. Fridman M, Williams GD, Muzamal U, Hunter H, Siu KW, et al. (2013) Two unique phosphorylation-driven signaling pathways crosstalk in Staphylococcus aureus to modulate the cell-wall charge: Stk1/Stp1 meets GraSR. Biochemistry 52 : 7975–7986. doi: 10.1021/bi401177n 24102310
54. Canova MJ, Baronian G, Brelle S, Cohen-Gonsaud M, Bischoff M, et al. (2014) A novel mode of regulation of the Staphylococcus aureus Vancomycin-resistance-associated response regulator VraR mediated by Stk1 protein phosphorylation. Biochem Biophys Res Commun 447 : 165–171. doi: 10.1016/j.bbrc.2014.03.128 24704444
55. Chao JD, Papavinasasundaram KG, Zheng X, Chavez-Steenbock A, Wang X, et al. (2010) Convergence of Ser/Thr and two-component signaling to coordinate expression of the dormancy regulon in Mycobacterium tuberculosis. J Biol Chem 285 : 29239–29246. doi: 10.1074/jbc.M110.132894 20630871
56. Canova MJ, Veyron-Churlet R, Zanella-Cleon I, Cohen-Gonsaud M, Cozzone AJ, et al. (2008) The Mycobacterium tuberculosis serine/threonine kinase PknL phosphorylates Rv2175c: mass spectrometric profiling of the activation loop phosphorylation sites and their role in the recruitment of Rv2175c. Proteomics 8 : 521–533. doi: 10.1002/pmic.200700442 18175374
57. Cohen-Gonsaud M, Barthe P, Canova MJ, Stagier-Simon C, Kremer L, et al. (2009) The Mycobacterium tuberculosis Ser/Thr kinase substrate Rv2175c is a DNA-binding protein regulated by phosphorylation. J Biol Chem 284 : 19290–19300. doi: 10.1074/jbc.M109.019653 19457863
58. Ulijasz AT, Falk SP, Weisblum B (2009) Phosphorylation of the RitR DNA-binding domain by a Ser-Thr phosphokinase: implications for global gene regulation in the streptococci. Mol Microbiol 71 : 382–390. doi: 10.1111/j.1365-2958.2008.06532.x 19040630
59. Lin WJ, Walthers D, Connelly JE, Burnside K, Jewell KA, et al. (2009) Threonine phosphorylation prevents promoter DNA binding of the Group B Streptococcus response regulator CovR. Molecular microbiology 71 : 1477–1495. doi: 10.1111/j.1365-2958.2009.06616.x 19170889
60. Delaune A, Dubrac S, Blanchet C, Poupel O, Mader U, et al. (2012) The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect Immun 80 : 3438–3453. doi: 10.1128/IAI.00195-12 22825451
61. Friedman L, Alder JD, Silverman JA (2006) Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother 50 : 2137–2145. 16723576
62. Jansen A, Turck M, Szekat C, Nagel M, Clever I, et al. (2007) Role of insertion elements and yycFG in the development of decreased susceptibility to vancomycin in Staphylococcus aureus. Int J Med Microbiol 297 : 205–215. 17418637
63. Howden BP, McEvoy CR, Allen DL, Chua K, Gao W, et al. (2011) Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog 7: e1002359. doi: 10.1371/journal.ppat.1002359 22102812
64. Hafer C, Lin Y, Kornblum J, Lowy FD, Uhlemann AC (2012) Contribution of selected gene mutations to resistance in clinical isolates of vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 56 : 5845–5851. doi: 10.1128/AAC.01139-12 22948864
65. Harwood CR, Cutting SM, editors (1990) Molecular biological methods for Bacillus New York: Wiley.
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



