-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
Establishing neural circuits during brain development relies on the ability for neurons to find their appropriate targets, a task that depends on recognition molecules whose expression must be tightly controlled. Very little is known of the factors that control the expression of these molecules. Here we have used the genetically amenable fly retina to address this issue. We identify Orthodenticle, a protein involved in mammalian brain development, as a main component of a new genetic cascade controlling the expression of recognition molecules that govern the wiring of the fly retina. Our work raises the issue that Orthodenticle might play a similar role in mammals. It also sheds new light on the genetic basis for wiring a model neural circuit during development.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005303
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005303Summary
Establishing neural circuits during brain development relies on the ability for neurons to find their appropriate targets, a task that depends on recognition molecules whose expression must be tightly controlled. Very little is known of the factors that control the expression of these molecules. Here we have used the genetically amenable fly retina to address this issue. We identify Orthodenticle, a protein involved in mammalian brain development, as a main component of a new genetic cascade controlling the expression of recognition molecules that govern the wiring of the fly retina. Our work raises the issue that Orthodenticle might play a similar role in mammals. It also sheds new light on the genetic basis for wiring a model neural circuit during development.
Introduction
The Drosophila compound eye has long served as a powerful system to dissect the genetic and molecular basis for establishing neural circuits during development. Each of the 750 facet lenses (i.e., ommatidia) that form the fly eye contains six outer photoreceptors (R1-R6) and two inner photoreceptors (R7 and R8). R1-R6 are involved in motion detection and express the broad-spectrum Rhodopsin1 (Rh1) [1]. These photoreceptors project axons and form synapses in the lamina, a ganglion that lies directly below the retina. Neural wiring of the outer photoreceptors in the lamina follows the principle of neural superposition, where six neighbouring ommatidia each project one outer photoreceptor terminal to one given synaptic-column (Fig 1) [2,3]. R7 and R8 are involved in chromatic discrimination and express different rhodopsin genes. R7 photoreceptors express either the UV-rhodopsin rh3 or rh4 [4], whereas R8 photoreceptors express either the blue rh5 or green-rh6 [5,6]. Within each ommatidium, the light-gathering organelle of R7 shares its optical axis with that of the underlying R8 photoreceptor. Therefore, these two neurons can compare the chromatic composition of the incident light. In this context, each pair of R7/R8 axons is found within one column but their respective growth cones establish synaptic connections in distinct synaptic-layers (Fig 1B). R8 axons terminate within the superficial M3 layer and R7 axons terminate deeper in the medulla, in the M6 layer.
Fig. 1. Schematic representation of the Drosophila visual system. 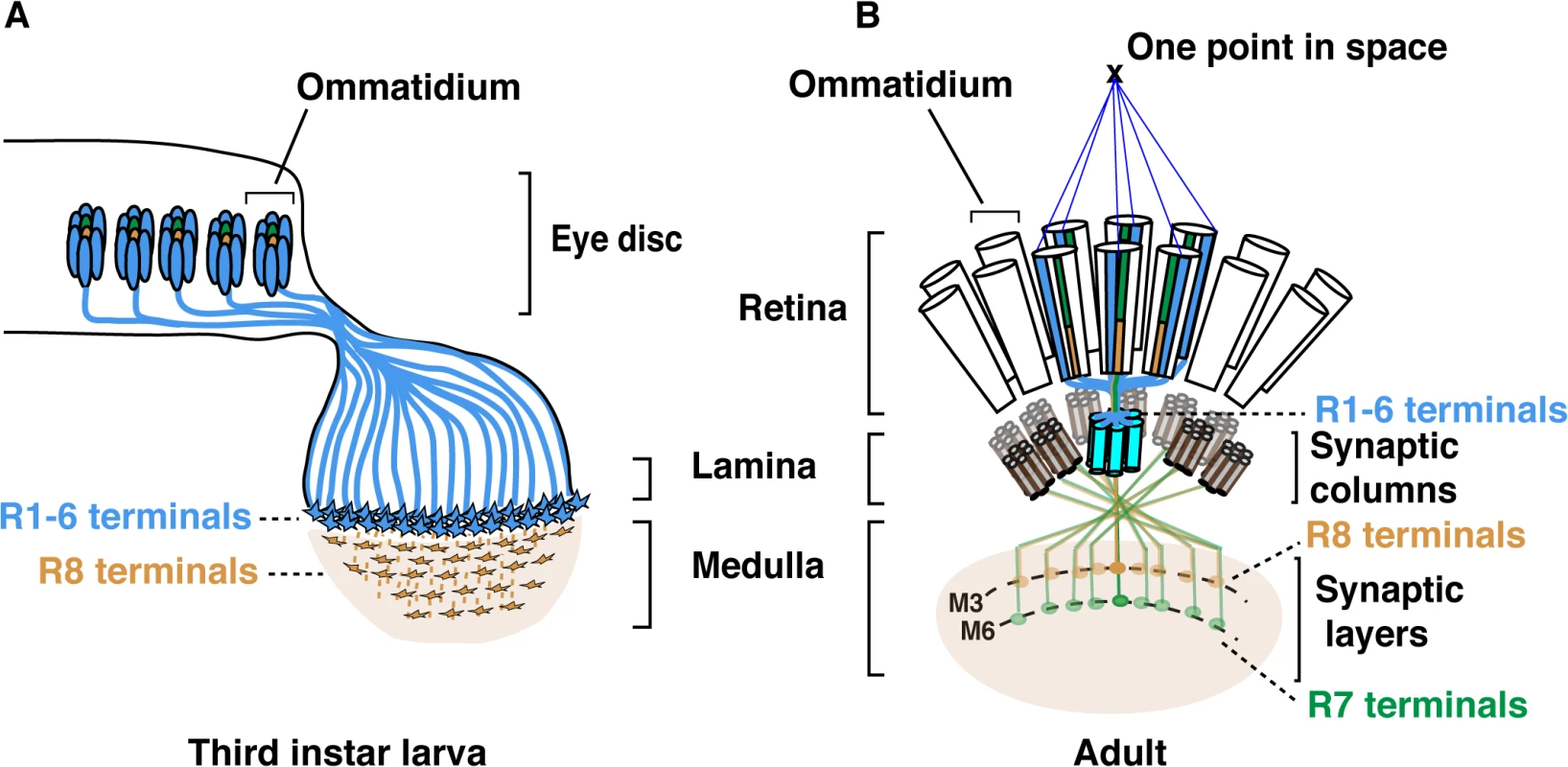
(A) Schematic representation of Drosophila axonal photoreceptor projections in the third instar larva optic lobe. The outer R1-R6 (indicated in light blue) from each ommatidium in the eye disc project their axons into the lamina part of the brain. At this early developmental stage, the inner photoreceptor R8s (yellow) project through the lamina and establish a regular retinotopic array of terminals in the medulla. (B) Schematic representation of the adult Drosophila visual system. R-cell axons are organized into synaptic-columns and layers. Six different photoreceptors (indicated in light blue) from six neighbouring ommatidia share the same optical axis and pool their axons in the same synaptic-column in the lamina [2]. R8 (orange) and R7 (green) photoreceptor axons pass through the lamina and terminate in distinct synaptic-layers M3 (R8) and M6 (R7). The layer-specific targeting of R7 and R8 photoreceptors is a dynamic process that follows a precise temporal sequence over the late third instar larval and pupal developmental stages (Fig 1 and S1 Fig). Within each ommatidium, R8 is the first photoreceptor to differentiate and to extend its axon toward the medulla. Approximately 24 h later, the R7 axons follow [7–9]. Later during pupal development, R8 and R7 growth cones are positioned in temporary layers, where they remain until the midpupal stage (S1 Fig). At this developmental stage, the growth cones of R7 and R8 regain motility and progress synchronously to their respective final synaptic-layers.
Several cell adhesion molecules (CAMs) and recognition molecules govern synaptic-column and-layer targeting of R1-6, R7 and R8 [10,11]. fmi [12], gogo [13], NCadherin (NCad) [14] and the LAR receptor tyrosine phosphatase [15] are required for R1-6 axons to select appropriate synaptic-columns in the lamina. Furthermore, fmi and gogo regulate proper spacing of the R8 axons and their targeting to the superficial medulla layer M1 [7],[16]. Later in development, these two factors cooperate to direct the R8 terminals to their final destination, the M3 synaptic-layer [17]. In addition, the CAM leucine-rich-repeat molecule Caps also contributes to the layer targeting of R8, presumably by promoting adhesion between the R8 growth cones and medulla neurons in the recipient M3 layer [8]. In the case of R7, NCad [14] together with LAR [15] and the Insulin Receptor (InR) [18] are required to promote targeting to the M6 layer. The expression of these various recognition molecules and CAMs must be tightly regulated to achieve photoreceptor subtype-specific axon terminal targeting. However, little is known about the transcriptional regulatory gene networks that lie upstream of these CAMs and recognition molecules.
A few transcription factors are known to regulate synaptic-column and layer targeting in the fly visual system. Amongst these, the zinc-finger protein Sequoia (Seq) promotes layer-specific targeting of R7 and R8 early during larval development. This is achieved by regulating the temporal expression of NCad in these photoreceptors [19]. In addition, the R8-specific transcription factor Sens has been shown to couple R8-rhodopsin expression with synaptic-layer targeting [20]. In the case of R7, axon projection to the M6 layer requires the repression of sens by the NFY-C transcription factor [20]. In the absence of NFY-C, sens is ectopically expressed in R7 and in turn, promotes the expression of caps and probably that of other recognition molecules [20]. This results in R7 photoreceptors projecting their terminals to the M3 layer, where R8 axons normally terminate. This phenotype is, however, not seen in all NFY-C mutant photoreceptors, indicating that at least one other pathway might regulate layer-specific targeting in R8.
Here, we have identified the homeobox-containing protein Otd, a transcription factor known to govern photoreceptor morphogenesis and rhodopsin expression [21–24], as a regulator of synaptic-column and-layer targeting in the fly retina. Otd is the founding member of the mammalian OTX1/OTX2/CRX family of transcription factors. These factors regulate brain patterning and retinal morphogenesis across phyla. In the visual cortex, OTX1 regulates the connectivity of cortical neurons with subcortical projections [25]. Moreover, in mice, CRX-mutant photoreceptors are unable to initiate appropriate synaptogenesis in the outer plexiform layer [26]. These findings indicate a potential role for this gene family during neural circuit development, but how these factors might achieve such a function is not understood. We show that otd function is required in R1-6 to promote synaptic-column specific innervation of the lamina. We find that this new function for otd is in part mediated by the recognition molecules Fmi and Gogo. In addition, our work demonstrates that a similar genetic network employing otd, fmi, gogo and the CAM caps, regulates the layer targeting of the R8 photoreceptor subtype. In this photoreceptor subtype, we find that layer-specific targeting is regulated by both otd and sens, which function as independent transcriptional inputs regulating a partially overlapping set of CAMs and recognition molecules.
Results
otd is required for synaptic-column targeting of R1-6 in the lamina
otd is expressed in all differentiating photoreceptors, but all indications are that it is not required for cell fate specification [21,22]. This is supported by the fact that otd mutant ommatidia contain the full complement of photoreceptors including R1-6 and both R7 and R8 (Fig 2A and 2B).
Fig. 2. The early pattern of photoreceptor axon projections is affected in otd mutants. 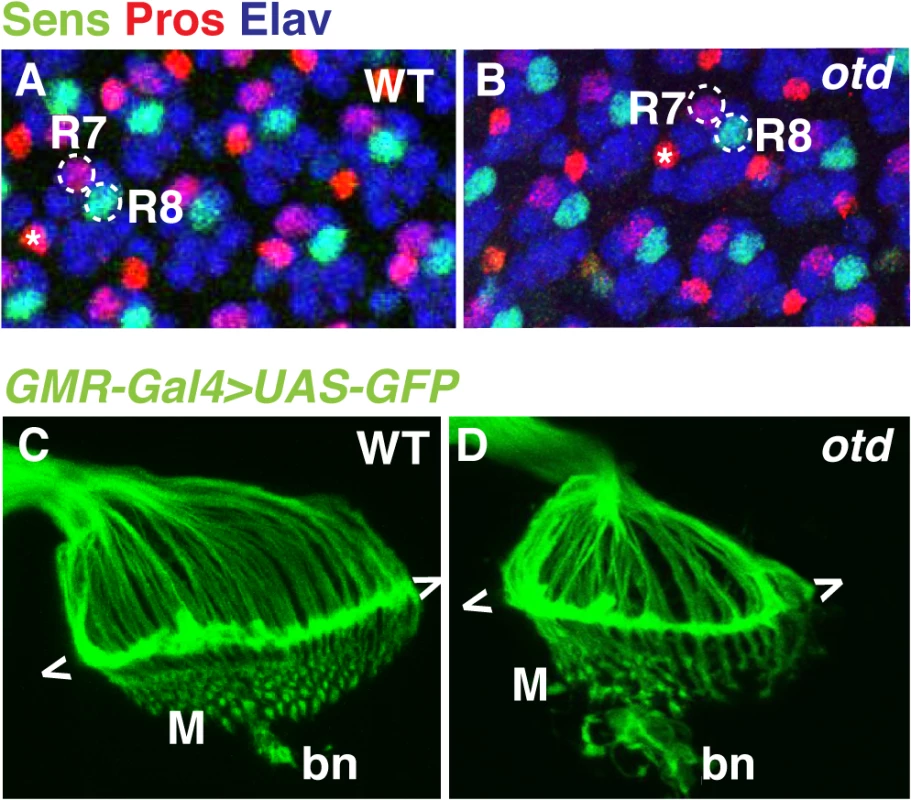
Wild-type (A) and otduvi mutant (B) eye imaginal discs from third instar larvae stained for the neural marker Elav (blue) and for the R7- and R8-specific markers Pros (red) and Sens (green) respectively. Asterisks indicate the position of the bristle cell. Wild-type (C) and otduvi (D) photoreceptor axon projections in third-instar larva were visualized by expressing UAS-mCD8::GFP under the control of the GMR-Gal4 driver [43]. The growth cones of R1-R6 form a neural plexus (chevrons) at the lamina (L). R8 axons project to the medulla neuropile (M). bn stands for the Bolwig’s nerve. To examine the early pattern of connectivity of otd mutant photoreceptors, we labelled the axons of all photoreceptors in third instar larvae by expressing UAS-mCD8::GFP under control of the GMR-Gal4 driver. In wild-type larvae, the R1-R6 axons terminate in the lamina plexus forming a continuous line of fluorescent signal, while R8 neurons project through the lamina and form a regular array in the medulla neuropile (Fig 1A, Fig 2C). In otd mutant retinae, the axon projections of R1-6 terminate in the lamina but tend to form bundles (Fig 2D). In addition, the medulla, which at this early developmental stage predominantly contains R8 axons, appears disorganized (Fig 2D). These observations prompted us to further investigate the role of otd during photoreceptor axon targeting.
We first examined the lamina column targeting of otd mutant R1-6 photoreceptors in more detail. To this end, we used electron microscopy to quantify the number of afferent R1-6 photoreceptor neurons that target each lamina column (also referred to as lamina cartridge). In the main part of the retina, outside of the midline of wild-type eyes, six R1-6 afferent axons converge on one synaptic column in the lamina (Fig 1B and 3A). In contrast, we find that in the case of retinae mutant for otd, the number of axon terminals that innervate the lamina columns varies, ranging from 2 and 10 (Fig 3B and 3G). This quantification reveals a failure of the R1-6 axons to innervate their appropriate synaptic-columns, demonstrating that otd is required for axonal targeting of R1-6 in the lamina.
Fig. 3. otd is required for synaptic-column targeting of R1-6 in the lamina. 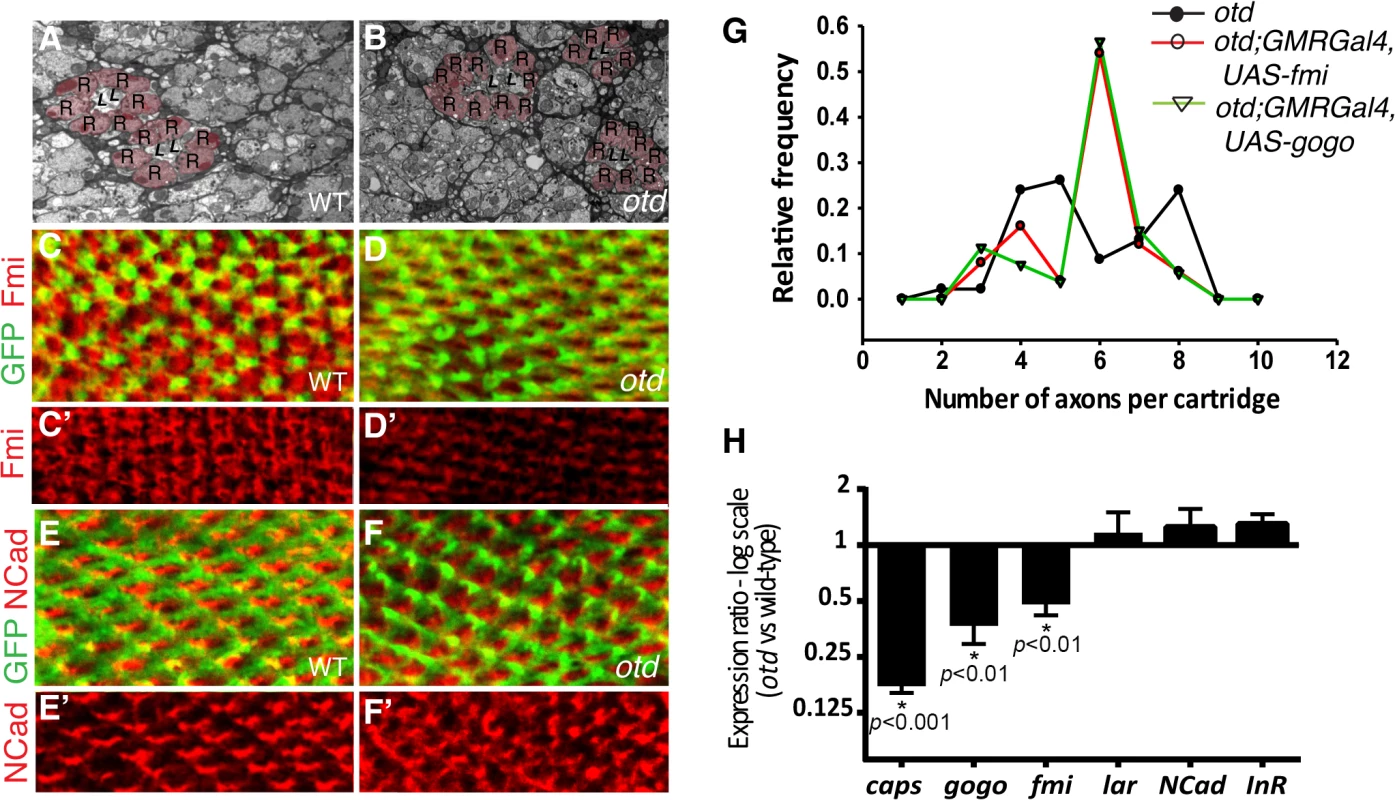
Electron micrographs of lamina cross-sections showing the normal organization of synaptic-columns in a wild-type lamina (A) and the defects in lamina column innervation observed in the case of otd mutant retina (B). Photoreceptor terminals are colored in pink (R), central lamina neurons are labeled ‘L’. Confocal sections of wild-type (C-C’,E,E’) and otd mutant (D,D’,F,F’) lamina at 40% after puparium formation. R1-6 axons are labeled by mCD8::GFP (green in C,D and E,F) and stained for Fmi (C-D’) (red) or NCad (E-F’) (red). (G) Frequency distribution polygon showing the numbers of R1-6 axons terminals innervating each synaptic column in otduvi mutant lamina (black line) and in otduvi mutants where either fmi (red line) or gogo (green line) are expressed using the GMR-Gal4 driver line. Data were gathered from EM micrographs. A Levene's test for the equality of variances, was applied. The variance in the number of axon terminals per cartridge is significantly lower in the otduvi; GMR-Gal4; UAS-fmi and otduvi; GMR-Gal4; UAS-gogo lamina (1.66 and 1.70 respectively) compared to otduv mutant flies (2.83, p<0.05 in both cases). (H) Real-time PCR quantification of caps, gogo, fmi, lar, NCad and InR mRNA normalized to GAPDH mRNA levels comparing wild-type and otd mutant retina at 40% after puparium formation. n = at least three independent mRNA extracts from wild-type and otd-mutant retinas. Error bars represent SEM. fmi and gogo function downstream of otd in R1-6
The photoreceptor targeting phenotype observed in the larval optic lobe and in the adult lamina in the absence of otd is reminiscent of that described in photoreceptors mutant for the recognition molecules and CAMs fmi, gogo or NCad [12,14,16]. During synaptic-column targeting in the lamina, these proteins promote interactions between R1-6 growth cones within each fascicle. This drives each growth cone to separate from the fascicle and extend laterally, to innervate the appropriate synaptic - column target [27,28]. Consistent with a function for otd in regulating appropriate R1-6 targeting in the lamina, we find that, when compared to wild-type (Fig 3C and 3C’), the protein expression of Fmi is greatly diminished in the growth cones of otd mutant R1-6 (Fig 3D and 3D’). In addition, a significant reduction of both fmi and gogo mRNA levels can be measured in developing retina mutant for otd (Fig 3H). Whereas the mRNA and protein levels of NCad (Fig 3E, 3F’ and 3H), as well as the mRNA levels of LAR (Fig 3H) which both regulate column targeting of R1-6 [14,15], remain unchanged.
Our data suggest that otd is required for sorting the R1-6 axons into their respective lamina columns via promoting the expression of fmi and gogo. Relative differences in the expression levels of Fmi between R1-6 growth cones within the same fascicle regulate neural superposition establishment [29]. Overexpressing fmi in R1-6, using the GMR-Gal4 leads to high levels of Fmi in R1-6 growth cones (S2 Fig) and is accompanied by defects in neural cartridges morphology including variable numbers of R1-6 terminal inputs (S2 Fig). However, we also observe numerous wild-type neural cartridges, indicating that the GMR-Gal4 driver line might be suitable for attempting rescue experiments using the otduvi mutant and the UAS-fmi strains.
Therefore, to test the hypothesis that fmi and gogo mediates part of otd function during neural superposition, we used electron microscopy to quantify the number of afferent otd-mutant R1-6 axons in lamina columns in which either fmi or gogo was re-introduced using the GMR-Gal4 driver line. Our quantification revealed that re-introducing fmi or gogo in otd-mutant photoreceptors partially restores the distribution of R1-6 axon projections towards the wild-type situation of six afferent terminals per synaptic-column (Fig 3G). We note that while the overall levels of Fmi are higher in otduvi mutant R1-6 when re-introducing Fmi using the GMR-Gal4 driver, R1-6 growth cones still exhibit differences in Fmi levels (S2 Fig). Taken together, our data demonstrate that otd-dependent transcriptional regulation of the recognition molecules fmi and gogo is required for the proper synaptic-column targeting of R1-R6 in the developing lamina.
otd is required for synaptic-layer specific targeting of R8
Processing of chromatic information relies on R7 and R8 establishing their synapses in two distinct layers of the medulla (Fig 1B and S1 Fig). In R8, layer-specific targeting relies on the expression of fmi, gogo and caps, amongst other adhesion and recognition molecules. Interestingly, similar to fmi and gogo, we find that the mRNA levels for caps are greatly reduced in otd mutant retina (Fig 3H). This reduction is also observed in R8 using an in vivo reporter gene for the caps locus, capsGal4-UASGFP [8] (Fig 4A and 4B). In addition, the Caps protein is no longer detected in the otd mutant R8 axons, leading to gaps in expression at the M3 layer, where the R8 axons normally terminate (Fig 4D and 4E’). Therefore, otd is required for the expression of key recognition molecules and CAMs that are known to govern the layer-specific targeting of R8.
Fig. 4. Caps expression is downregulated in otduvi mutant R8 photoreceptors. 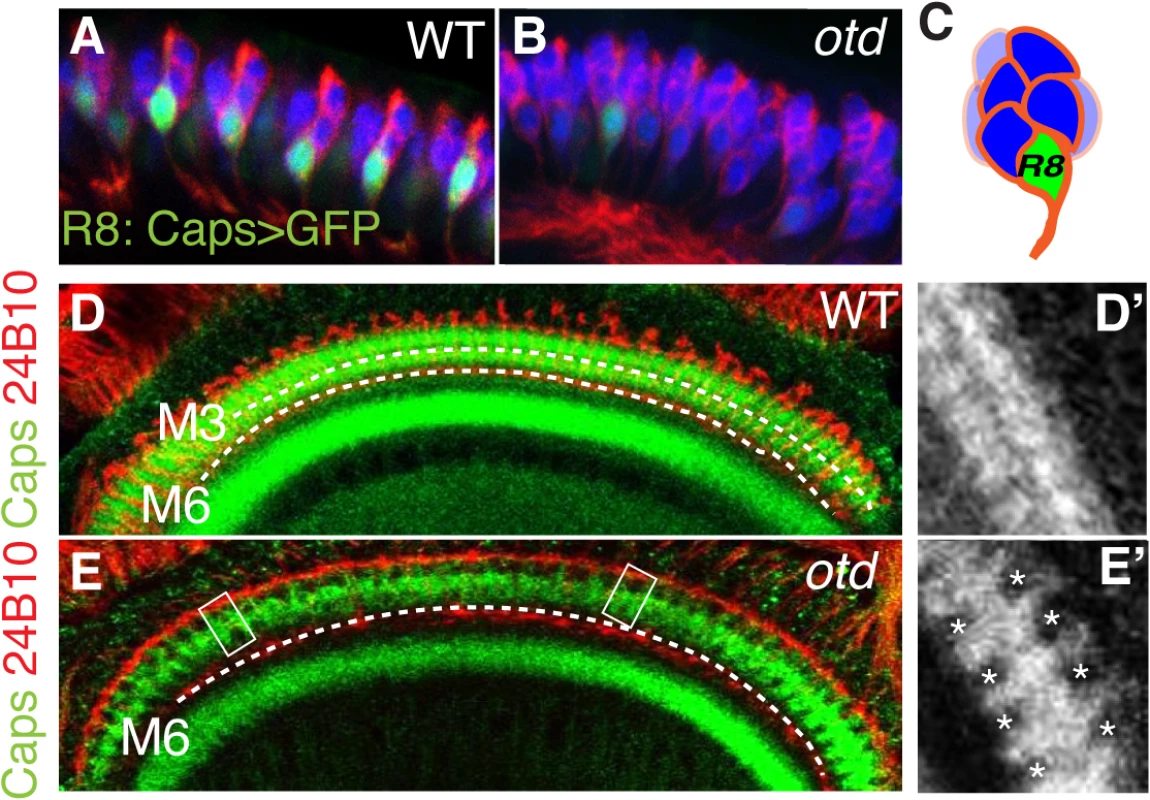
Side view of wild-type (A) and otduvi mutant (B) third instar larva eye disc revealing that caps expression is strongly reduced in otd mutant R8. The expression of mCD8-GFP is under the control of caps-Gal4 [8]. Elav (blue) and 24B10 (red) stain the full set of photoreceptors. (C) Representation of an ommatidium showing the basal localization of the R8 photoreceptor in green. Expression of Caps (green) in wild-type (D, D’) and otduvi mutant (E, E’) optic lobes (60% after puparium formation). Photoreceptor-cell-axons are stained with the 24B10 antibody. The R8 (M3) and R7 (M6) recipient layers are indicated by dashed lines in this and the following figures. When compared to wild-type, otduvi mutant retina show a reproducible gap pattern detected in the R8 layer (M3) (D’, E’). These gaps correspond to a loss of Caps expression in the afferent R8 axons (boxed in E and magnified in E’). These experiments suggest that in the absence of otd, layer-specific targeting of R8 should be affected. To test this hypothesis, we examined the projection pattern of R8 terminals in otduvi animals, using the R8 specific Rh6-lacZ reporter transgene. When examining adult medulla stained for Rh6-lacZ, we found that 59% (n = 503 of 854) of mature R8 terminals fail to stop in the M3 layer and instead terminate in the M6 layer, where R7 terminals are normally found (Fig 5A, 5B’ and 5H). This phenotype can also be detected using the null allele otdJA101 [30] combined to the MARCM system [31] (S3 Fig). In this case, as for otduvi, not all R8 axons project ectopically in the M6 layer, suggesting that during R8 synaptic layer-specific projection, another redundant pathway might function in parallel of otd.
Fig. 5. otd is required for R8 synaptic-layer targeting. 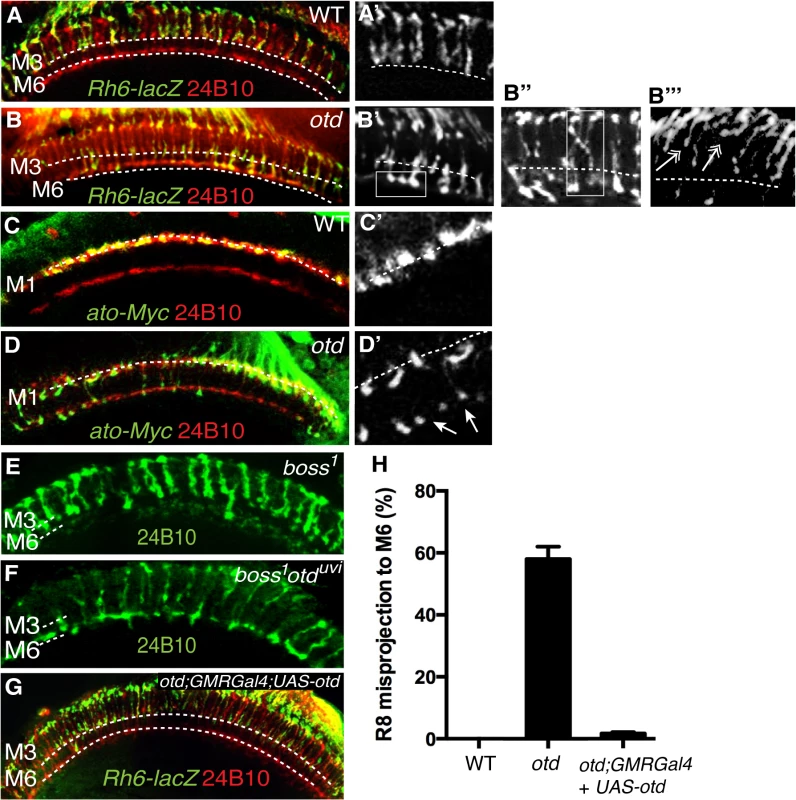
Wild-type (A,A’) and otduvi mutant (B,B’) adult optic lobes expressing the R8 specific marker Rh6-lacZ stained with anti-β-galactosidase (green). In wild-type (A,A’) Rh6-lacZ-positive R8 axons terminate in the M3 layer. otduvi mutants (B-B”) display a strong R8 axon misprojection phenotype. R8 axons specifically overshoot to the M6 layer (boxed in B’ and quantified in Fig 5H) while some R8 axons invade neighbouring columns cross-laterally and terminate in abnormal positions (boxed in B”). Several R8 axons terminals can also be seen to stall at more superficial layers and fail to innervate the medulla (double arrows in b”‘). All panels (C-F) show photoreceptor axon projections stained with the 24B10 antibody (red). R8 axons in wild-type (c,c’) and otduvi mutant (D,D’) optic lobes (40% after puparium formation) are visualized using the ato-τ-myc transgene stained with anti-Myc antibody (green). At this early stage of development, in otduvi mutant retina, R8 growth cones fail to stop in the M1 layer and extend specifically to the R7 temporary layer (n = 438 misprojecting axons of 798 R8 axons quantified in the two temporary layers). The staining for ato-τ-Myc is magnified in (C’,D’) and arrows in (D’) indicate misprojecting R8 axons. boss1 mutant (E) and otduvi/boss1 double-mutant (F) retina. Adult optic lobes stained with 24B10 antibody. In (E), residual 24B10 staining in the R7 M6 layer is derived from medulla neurons [44]. (E) boss1 mutant R8 terminate in the R8 recipient layer M3, with only a few R8 targeting to the M6 layer. (F) Most of the otduvi/boss1 double-mutants display a strong R8 mis-projection phenotype in the R7 layer, M6. (G) otduvi mutant flies where Otd expression has been restored specifically in the photoreceptors using the GMRGal4 driver. In this context, staining of the R8 specific marker Rh6-lacZ (green) demonstrates that normal R8 photoreceptor targeting is almost completely restored. (H) Quantification of the misprojections of Rh6-lacZ-positive R8 axons in wild-type, otduvi mutant and otduvi mutant flies where Otd expression has been restored using the GMR-Gal4 driver. In addition to the ectopic projection of R8 terminals to the M6 layer, we also detect various defects that include crossing of R8 terminals into neighbouring columns and column thickening (Fig 5B”). These phenotypes are reminiscent of those originally reported for caps mutants [8]. In addition, several R8 terminals are stalled in a more superficial layer, at the periphery of the medulla. This phenotype is difficult to quantify but is readily visible in medulla stained for the R8 reporter Rh6-LacZ (Fig 5B”‘). This stalling phenotype is similar to that described for the fmi and gogo mutant R8s [17]. Thus, taken together, our data are consistent with otd being required for the expression of fmi, gogo and caps in R8.
Interestingly, when we tested for axon projection defects at early developmental stages, before R7 and R8 have reached their final recipient layers (M6 and M3 respectively), we found that up to 55% (n = 438 of 798) of otd mutant R8 terminals already misproject in the R7 temporary layer (Fig 5C and 5D’). This early phenotype cannot be explained solely by the loss caps, as the caps mutant phenotype has been shown to be very mild at comparable early developmental stages [8]. Given the specific early redirection of the R8 terminals to the temporary R7 layer phenotype, we conclude that in addition to being required in R8 for the expression of R8 CAMs, otd must also have a role in preventing R8s from becoming competent to project to the R7 layer.
In principle, the early mistargeting of R8 axons could result from the conversion of R8 photoreceptors to an R7 cell fate. To address this issue, we stained otd-mutant eye discs for the R7 - and R8-specific markers Prospero (Pros) and Sens respectively and could confirm that no R8 to R7 transformations take place in the absence of otd (Fig 2A and 2B). Moreover, the expression of sens is maintained in R8 during pupal development (S4 Fig). This finding excludes the possibility that otd indirectly affects R8 axon targeting by regulating cell fate specification. Alternatively, as the majority of otd-mutant R8 axons specifically innervate the R7 layer, this raises the possibility that they might be unable to defasciculate from their R7 partner. To investigate this possibility, we tested whether R7 neurons contribute to the otd mutant R8 mistargeting phenotype by looking at boss1 mutant ommatidia, which lack the R7 photoreceptor. In otd/boss 1 double mutant flies, we found that the otd mutant R8 mistargeting to the M6 layer is maintained (60% n = 149 of 251). This indicates that the otd phenotype does not depend on the presence of the R7 axon (Fig 5E and 5F).
Finally, we sought to test if the otd mutant R8 photoreceptors that misproject to the M6 layer are able to form synapses in this layer. To this end, we used an antibody raised against Bruchpilot, a protein localized to the active zone of synapses of the optic neuropile [32,33]. We found that Bruchpilot staining co-localized with the terminals of R8 photoreceptors that project ectopically in the M6 layer (S5 Fig). Given the crowded nature of the medulla, in which many different cell types must specifically interconnect at every layer, some nonspecific co-localization of antibody staining might occur. To rule out this possibility, we used an R8-Gal4 line to specifically express a GFP-labeled version of Bruchpilot (UAS-BRP-GFP) in R8 neurons, thereby visualizing only those synapses made by R8 axons (S5 Fig). The co-localization between the GFP signal and R8 terminals indicates that otd R8 axons that misproject to the M6 layer are likely to establish synaptic connections, within this layer. Therefore, while otd is required for the expression of fmi, gogo and caps in R8, it seems to be dispensable for proper synaptogenesis.
otd functions in the R8 photoreceptor
Most of the experiments described thus far were carried out using an eye specific otd allele, otduvi. This allele consists of a partial deletion of an eye-specific enhancer and results in a strong reduction in Otd protein expression in photoreceptors [21]. To ensure that the otd phenotype we report here is due to a function of otd in photoreceptors and not in the developing optic lobe, we used three distinct approaches. First, to evaluate the possibility of a reduction in otd expression in otduvi brain, we assessed otd mRNA levels in the optic lobes of wild-type and otduvi flies. We found no difference in otd transcript levels between wild-type and otduvi mutants in the optic lobe, whereas, in the retina of otduvi flies, otd expression is downregulated by approximately 50-fold compared to wild-type (S4 Fig). Second, we induced eye-specific RNAi-mediated knockdown of otd expression using two different UAS-otd RNAi lines combined with the eye specific GMR-Gal4 driver line. These two lines are effective at knocking down otd expression in the photoreceptors (S4 Fig). The specific R8 mistargeting phenotype characteristic of otd was seen with both of these RNAi lines (S4 Fig). The misprojection phenotype was less severe in the otd RNAi knockdown lines (24% n = 115 of 479) than in otduvi. This correlates with the residual expression levels seen in knockdown lines (S4 Fig) and suggests that the penetrance of the otd mutant phenotype in R8 depends on the transcript level. Third, we performed photoreceptor-specific rescue experiments using the GMR-Gal4 driver and available UAS-otd transgene lines to re-introduce otd specifically in the retina in otduvi mutant flies. With this approach, the R8 synaptic-layer targeting defects quantified in otduvi mutant retinae can be almost completely rescued. R8 misprojections in the rescued flies were found to be 2% (n = 9 of 451), compared to the 59% misprojection seen in the otduvi mutant (Fig 5G and 5H). In these retinas, the caps mRNA is restored to levels that are comparable to those measured in wild-type retina (S6 Fig). In addition, the levels of fmi and gogo mRNA are also significantly increased and thus closer to wild-type when compared to otd-mutant retina (S6 Fig). Importantly, overexpressing otd in an otherwise wild-type retina does not affect the pattern of projection of R7 and R8 (S7 Fig). Taken together, our data demonstrate that the requirement of otd for the expression of optimal levels of caps, fmi and gogo is autonomous to the R8 photoreceptor.
otd and sens provide independent transcriptional inputs during R8 layer targeting
The otd mutant defects in R8 layer targeting described here are also observed in R8s that lack the gene encoding the transcription factor sens [20]. Thus, our data raise the possibility that sens might be downregulated in the absence of otd. When we tested this hypothesis, we found this not to be the case, as sens is expressed normally in the absence of otd (Fig 2B). Likewise, otd expression is unaffected in photoreceptors in which sens expression has been reduced (Fig 6B). Therefore, otd must function in parallel to sens during layer targeting of R8 photoreceptors.
Fig. 6. otd and sens are required for R8 synaptic layer targeting. 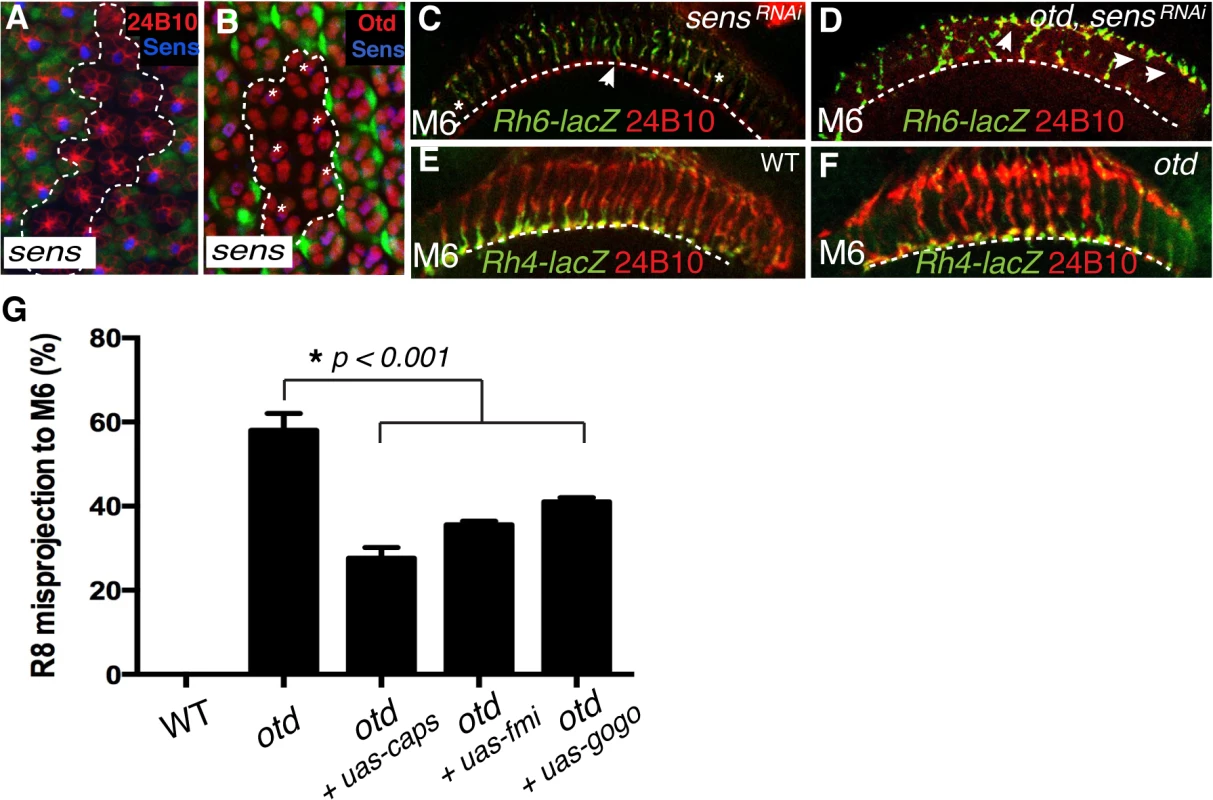
All panels (A-F) show photoreceptor cell projections stained with 24B10 (red). (A) Expression of UAS-sensRNAi (GFP-negative ommatidia, encircled by a dotted line) in wild-type tissue (GFP positive) 48 h after clone induction using the tub>GFP>Gal4 system. UAS-sensRNAi expressing cells show a clear reduction in Sens protein levels (blue). (B) Expression of UAS-sensRNAi transgene (GFP-negative ommatidia, encircled by a dotted line) in wild-type tissue (GFP positive) 48 h after clone induction using the tub>GFP>Gal4 system. Otd expression (red) is unaffected in UAS-sensRNAi expressing R8 cells (indicated by asterisks). (C) Rh6-lacZ-positive R8 axons in sensRNAi (R8-specific driver-Gal4109–68;UAS-sensRNAi) and in otduvi mutant combined with sensRNAi retina (D), stained with anti-β-galactosidase (green). sens knockdown in the retina leads to a few defects in R8 axon projection, however the combination of the sens knockdown and otduvi mutant leads to a complete failure of R8 layer-specific targeting, indicating that sens and otd act in parallel. (E,F) Layer-specific targeting of the R7 photoreceptors is assessed using the R7 specific transgene Rh4-lacZ. As in wild-type (E), Rh4-lacZ positive R7 terminals (green) correctly target to the M6 layer in otduvi mutants flies (F). (G) Quantification of the misprojections of Rh6-lacZ-positive R8 axons in wild-type, otduvi mutant and otduvi mutant flies where either caps, fmi or gogo expression has been restored. Since Caps is present only in R8, UAS-caps is expressed under the control of an R8-specific Gal4 driver, while UAS-gogo and UAS-fmi are expressed using the pan-photoreceptor GMR-Gal4 driver. Restoring caps expression in otd-mutant R8 photoreceptors partially rescues the otduvi mutant R8 mis-targeting phenotype (from 59% R8 misprojection in otduvi to 25% in otduvi + UAS-caps). Similarly, expression of fmi and gogo in otduvi mutant photoreceptors partially suppress the R8 misprojection phenotype (36% R8 axons misprojecting in otduvi + UAS-fmi, n = 374 of 1039; 40% R8 axons misprojecting in otduvi + UAS-gogo, n = 436 of 1090). Percentages indicate quantitative assessment of R8 axons as detected in M3 and M6 layers only. In wild-type, all R8 neurons target to the M3 layer, thus the mistargeting percentage is zero. If this model is correct, attenuating the expression of both otd and sens should lead to a complete failure for R8 terminals to reach the M6 layer. To test this model we generated otd mutant photoreceptors in which the sens transcript was reduced using a specific RNAi strain (tested in Fig 6A), thus still allowing for normal R8 specification [34]. Consistent with our model, we observed a complete failure of R8 layer targeting in the medulla in the double mutant otduvi/sensRNAi flies with a majority of R8 terminals either stalling at a more superficial layer or overshooting specifically to the M6 layer (Fig 6D). This demonstrates that otd and sens provide independent transcriptional inputs during synaptic-layer targeting of the R8 photoreceptor.
caps, fmi and gogo function downstream of otd in R8
Next, in order to test if caps, fmi and gogo function downstream of otd during R8 layer targeting, we asked if re-introducing these genes in an otduvi mutant retina suppresses the R8 mis-targeting phenotype. To this end, we used both a pan-photoreceptor and an R8 specific Gal4 strain combined with either UAS-fmi [35], UAS-gogo [16] or UAS-caps [8]. The Rh6-LacZ transgene was used to score the R8 axon terminals in the medulla. Re-introducing caps leads to a partial rescue of the R8 mis-targeting phenotype (from 59% R8 mis-targeting in otduvi to 25% in otduvi + UAS-caps; n = 225 of 903). fmi and gogo cooperate in a non-redundant manner during R8 layer targeting [17]. The expression of these two recognition molecules is not completely abolished in otduvi mutant retinae and is instead likely to be sub-optimal (Fig 3D’ and 3H). In this context, re-introducing fmi or gogo gives a partial rescue of the R8 mis-targeting phenotype (36% of R8 axons mis-target in otduvi + UAS-fmi, n = 374 of 1039; 40% of R8 axons mis-target in otduvi + UAS-gogo, n = 436 of 1090) (Fig 6G). We attribute this partial rescue to the suppression of the stalling phenotype of R8 terminals (Fig 5B”‘) thereby resulting in a greater proportion of otd mutant R8 axons projecting correctly to the R8 layer. Altogether, these experiments demonstrate that otd is required for the synaptic-layer targeting of R8, at least in part by promoting the expression of fmi, gogo and caps.
otd is not required for synaptic-layer targeting of R7
As otd is expressed in all photoreceptors, it might also be required for the projection of R7 photoreceptors. To address this question, we examined the layer-specific targeting of the R7 photoreceptors in vivo using the R7 Rh4-lacZ reporter. We found that all otd mutant R7 axons stop correctly in the M6 layer (Fig 6E and 6F). Thus otd function during column - and layer-specific targeting is restricted to the R1-6 and R8 photoreceptor subtypes. Consistent with a lack of phenotype during R7 layer-specific targeting, we found that the expression levels of the main recognition molecules that govern this process (i.e., NCad, LAR and InR) are not affected in the absence of otd (Fig 3H).
Discussion
Little is known about the transcriptional regulation that controls the establishment of genetically hardwired patterns of synaptic connections during development. The factors and molecular pathways that govern the establishment of the typical pattern of R1-6 connectivity in the lamina have yet to be fully characterized. During neural superposition in the main part of the retina, each lamina column is innervated by axons coming from at least six neighbouring facets (i.e., one axon per facet is contributed to one synaptic-column) (Fig 1B). Although the CAMs and recognition molecules NCad, Fmi, Gogo and LAR have been shown to regulate this process, very little is known about the upstream transcriptional regulatory gene networks that control the expression of these molecules. Here we identify otd as a transcription factor required for synaptic-column targeting of R1-6 in the fly visual system (Fig 7). We show that in the developing retina, this phenotype correlates with a significant downregulation of fmi and gogo, and that re-introducing these genes in otd mutant R1-6 can ameliorate the lamina column innervation defects observed in otduvi mutant retinae. This indicates that otd function is required in R1-6 to promote the expression of optimal levels of these key factors to control lamina synaptic-column targeting. Our finding that the expression of NCad or LAR are not affected by the loss of otd suggests that additional independent transcriptional inputs are likely to operate in R1-6 during synaptic-column innervation.
Fig. 7. Transcriptional inputs required for proper photoreceptor targeting in the Drosophila visual system. 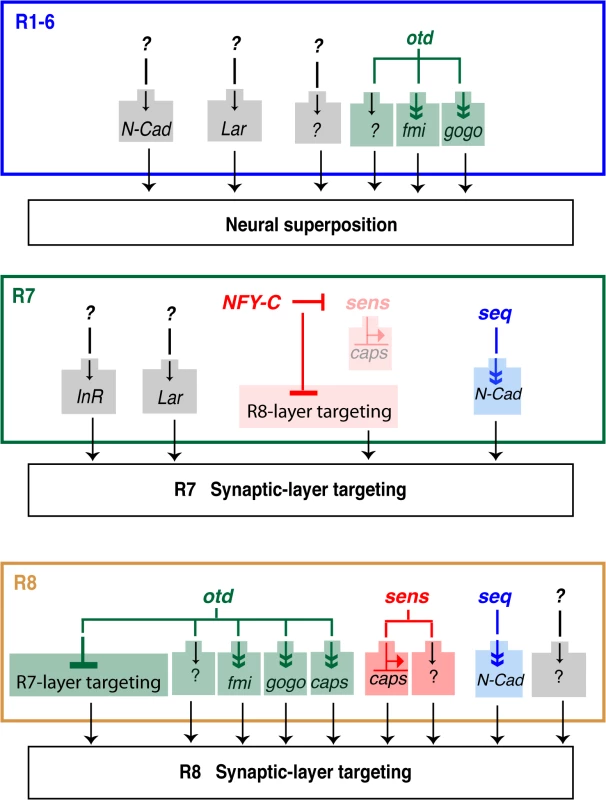
Summary of the transcription factors that regulate column- and layer-specific targeting of the R1-6 and R7 and R8 photoreceptors. Predicted indirect regulation is shown with a double arrowhead (Sens binds directly on the caps promoter). Although all of the currently known transcriptional regulators (i.e., seq, sens and otd) are presented, for clarity, only those CAMs and adhesion molecules most relevant to our findings are shown here. Within each axon bundle, relative differences in Fmi expression promotes the lateral extension of the R1-6 growth cones so that they project toward different neural cartridges [12,29]. In this context, Fmi and N-Cad act redundantly [36]. Interestingly, it is not clear how fmi expression is differentially regulated in R1-6. One possibility is that expression of fmi is solely regulated at the transcriptional level. Another possibility is that differential expression of Fmi between R1-6 growth cones involves both transcriptional and posttranscriptional regulations. Alternatively, as photoreceptors are recruited sequentially during retina morphogenesis, differences between R1-6 expression levels might also be due to the onset of transcription at the fmi locus. Our ability to ameliorate the otduvi phenotype in the lamina by re-introducing fmi (or gogo), suggests that post-transcriptional regulation of these molecules might operate during neural superposition. As expected, when we express fmi using the GMR-Gal4 driver line in otherwise otduvi mutant animals, we observe an overall increase in the level of the Fmi protein in the corresponding lamina. However, we note that the Fmi staining remains relatively heterogeneous, and reminiscent of the Fmi staining seen in wild type lamina. While this suggests a level of post-transcriptional regulation it might equally be explained by the presence of another factor, present at different levels in each R1-6 cells, and that might limit the concentration Fmi at the growth cone. It is also possible that the temporal pattern of expression of the GMR-Gal4 driver line is compatible with the endogenous pattern of fmi expression. In favour of this notion, although overexpressing fmi with this driver line in an otherwise wild-type retina leads to defects in neural superposition, we also detect many wild-type lamina cartridges, despite the fact that in these experiments the overall levels of Fmi are higher than in wild-type. Further work will be required to understand how exactly the expression of Fmi is regulated at the level of the R1-6 growth cones during neural superposition.
In addition to being required for proper synaptic-column targeting of R1-6, we demonstrate that otd is specifically required for layer-specific targeting of the R8 photoreceptor subtype (Fig 7). During early phases of visual system development, dynamic expression of seq in R7/R8 regulates the expression of NCad to ensure the separation of the R7 and R8 terminals in their respective temporary layers [19]. Later in development, sens has been shown to coordinate the expression of rhodopsins [20,37] and layer-specific targeting in R8. One of the CAMs implicated downstream of sens during R8 synaptic-layer targeting is caps [20]. In sens-mutant R8, a fraction of R8 terminals project ectopically to the M6 layer (where R7 terminals normally project). Interestingly, in NFY-C mutant R7 photoreceptors, where sens and caps are ectopically expressed, not all terminals misproject in the M3 layer (where R8 terminals are normally found). Taken together, this indicates that at least one other pathway must be involved in regulating R8 layer-specific targeting. Our work indicates that otd regulates this process independently of sens and thus is part of a new gene regulatory network that cooperates with sens during R8 layer targeting. Our finding that the expression of otd and sens is not interdependent suggests a model in which these two transcription factors act in parallel pathways in R8. Because sens/otd double mutant R8s show an additive phenotype when compared to the respective single mutants, we conclude that otd and sens provide convergent transcriptional inputs during R8 synaptic-layer targeting.
In R8, we find that otd function correlates with a transcriptional downregulation of fmi, gogo, and caps. fmi or gogo mutant R8 are characterized by strong axon targeting defects. R8 axons form bundles and often stall at the surface of the medulla in the temporary M1 layer [7,12,16]. This is thought to be due to a function of these two factors in promoting the release of the R8 growth cones from the M1 temporary layer, thus allowing them to innervate their final destination, the M3 synaptic-layer. caps function in R8 is a little more controversial. Early work indicates that it is important for the proper stabilization of R8 terminals in the M3 layer in the medulla [8]. However, recent work indicates that caps function in R8 might be much more limited than previously thought and that perhaps other members of the leucine-rich-repeat gene family might act redundantly with caps during R8 layer targeting [38]. Nevertheless, ectopic caps expression in R7 is sufficient to re-direct their axon terminals to the M3 layer where R8 normally terminates [8].
The range of R8 projection phenotypes that we observe in otd mutant retinae is compatible with fmi, gogo and caps mediating at least part of otd function during R8 synaptic-layer targeting. However, our work demonstrates that even early in visual system development (i.e. 40% APF), a large proportion of the otd mutant R8 axons project their terminals specifically to the R7 temporary layer. This early misprojection phenotype for the R8 axon terminals is not seen in the fmi or gogo single mutants. In addition, this phenotype is only detected at a very low frequency in caps mutants [8]. Therefore, the phenotype caused by loss of otd function in R8 cannot be fully explained by the measured decrease in caps, fmi and gogo expression. One possible explanation for the early misprojection of R8 terminals to the R7 temporary layer is that otd might be required in R8 to suppress at least part of the R7-specific genetic program that governs axon terminal projection to the R7 synaptic layer. This scenario would be analogous to the situation found in the R7 subtype, where NFY-C represses part of the R8-targeting genetic network [20]. The genetic program that governs axon projection in R7 is not fully understood. Further work will therefore be required to test the hypothesis that otd is required in R8 to prevent these neurons from becoming competent to project to the M6 synaptic layer where R7s normally project.
Nevertheless, we present evidence that fmi, gogo and caps act downstream of otd in R8. Given that ectopic Caps expression in R7 is sufficient to redirect R7 terminals to the R8 M3 layer, our ability to partially rescue the otd-mutant R8 misprojection phenotype by re-expressing caps is expected. The mild rescue we observe when re-introducing fmi or gogo is perhaps more surprising, given that these molecules function together in R8. However, although reduced, the expression of fmi and gogo is not completely lost in the absence of otd. Therefore, basal levels of fmi and gogo are available to support the partial rescues we obtain with fmi or gogo. Importantly, the disorganization of the R8 pattern of axon projections that we observe in the third instar larvae, together with the many instances of R8 terminals stalling at the surface of the medulla later during development, is also observed in the case of fmi and gogo single mutant R8s [16,17]. We therefore attribute the mild rescue of the otd mutant phenotype by fmi and gogo to a rescue of a fraction of the stalled R8 growth cones.
While our work demonstrates that otd is part of a new gene regulatory network required for synaptic-column and layer targeting in the fly visual system, the relationship between this transcription factor and the downstream effectors fmi, gogo and caps is not clear. In this context, it is interesting to note that Sens can bind to a putative Sens-binding domain in the caps promoter and, together with Otd, can also bind to the rh5 and rh6 promoters [20,37]. This raises the issue that Otd might bind and cooperate with Sens directly on the caps promoter and perhaps that of fmi and gogo. The promoter and potential regulatory elements of caps, fmi and gogo are relatively large and not well defined, making it difficult to identify potential otd binding sites, a tandem repeat of (TAATCC) [23]. No clear Otd binding site can be found within the caps promoter and in particular in the immediate vicinity of the putative Sens binding site [20]. As for the caps locus, examining the fmi and gogo promoters does not reveal any clear Otd binding consensus sites. In addition, cloning of a 1.5 Kb caps promoter that contains the putative Sens binding site in front of the LacZ reporter gene does not lead to any detectable expression in vivo. Overall, the available evidence does not support a simple and direct binding of otd on the fmi, gogo or caps promoters. Although it is not possible to rule out that Otd might bind to more distal regions of these promoters or in introns, we favor a model in which Otd regulates the expression of these recognition molecules through an indirect mechanism and/or through a complex interaction with partner transcription factors.
Taken together, our data suggest that Otd is part of a novel gene regulatory network that is required for the optimal expression of a set of conserved CAMs and recognition molecules in the developing fly visual system. Further work will be required in order to identify all of the components of this gene regulatory network. It will also be interesting to test to what extent OTX1 and OTX2, the mammalian orthologs of Otd, regulate column - and layer-specific targeting of subsets of neurons during vertebrate brain development. During brain development in mice, otx2 has been shown to be required for the expression of the adhesion molecule RCadherin and Ephri-A2 [39]. In the chick, otx2 is co-expressed with the caps ortholog leucine-rich repeat neuronal 1 (Lrrn1) at the posterior midbrain border [40]. These observations suggest that Otd might be closely associated with the expression of adhesion and recognition molecules in developing neurons across phyla.
Materials and Methods
Drosophila genetics
The following fly strains were used: wild-type (Canton-S). w, otduvi, w, otduvi; GMR-Gal4;Rh6-lacZ, GMR-Gal4;Rh6-lacZ, otduvi;GMR-Gal4;Rh4-lacZ, GMR-Gal4;Rh4-lacZ, UAS-mCD8::GFP, UAS-sensRNAi (line 27287, Bloomington Stock Center), UAS-otdRNAi (lines 29342 and 34327, Bloomington Stock Center), otduvi;capsGal4, capsGal4, otduvi;GMRGal4 combined to UAS-fmi or UAS-gogo or UAS-otd, the R8-specific driver Gal4109–68 combined with UAS-caps-Ia5, ato-τ-myc, otduvi; ato-τ-myc, boss1 Sb/boss1TM6B (gift from H. Kramer, University of Texas Southwestern Medical Center, Dallas, TX), w, otduvi; boss1 Sb/boss1TM6B; UAS-bruchpilotGFP (lines 36291, 36292, Bloomington Stock Center), otdJA101 FRT19/FM7, ey3.5-FLP, tubPGAL80, FRT19; act-GAL4,UAS-lacZ/Cyo.
Immunohistochemistry
Fly brains were dissected in PBS and fixed for 1 h in PLP (2% paraformaldehyde, 75 mM Lysine, 37 mM Sodium phosphate buffer, pH 7.4). Eye imaginal discs and pupal retinas were dissected in PBS and fixed in PLP for 20 min. For synapses stained with antibodies against Bruchpilot, fly brains were processed as in [41]. Antibodies and dilutions used were as follows: rabbit anti-βGal (1 : 5,000 Cappel) (Biogenesis); rat anti-Elav (1 : 40) (from Developmental Studies Hybridoma Bank [DSHB], clone Rat-Elav-7E8A10); mouse anti-prospero (DSHB clone mouse Pros-MR1A); mouse anti-Bruchpilot (1 : 50, nc82, DSHB), guinea pig anti-senseless (1 : 1000, H.J. Bellen), mouse mAb24B10 (1 : 1000; DSHB), mouse anti-flamingo (1 : 20; DSHB), rabbit anti-Myc (1 : 250; Santa Cruz Biotechnology), rabbit anti-capricious (1 : 2000, A. Nose) and rat anti-otd (1 : 500, T. Cook). Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories: goat anti-mouse, anti-rabbit, anti-rat, and anti-guinea pig F(ab)2 fragments coupled to FITC, Cy3, or Cy5 (1 : 200 for FITC and Cy5, and 1 : 400 for Cy3). Brains, pupal and adult retinas were mounted in Vectashield (Vector Laboratories).
Quantification of the mistargeting R8 axon terminals
For each brain analysed, the total number of labelled R8 axon terminals that reached the M3 and M6 layers was counted and the percentage of R8 axons mistargeting to M6, the layer in which wild-type R7 axons terminate, was calculated. At least 14 brains for each genotype were analysed. The mean and standard error of the percentage of mutant R8 neurons mistargeting to M6 was calculated. R8 axon terminals that stalled at the more superficial layers were not included in the quantification.
RT-PCR
RNA extraction on approximately 20 Canton S and 20 otduvi staged retinae was carried out in triplicates for each genotype (40 h APF) were used for each RT-PCR. Real-time PCR was performed using SYBR MESA BLUE Kit (Eurogentec). Oligonucleotides used are listed in S1 Table. Statistical significance was calculated using an unpaired t-test with significance at P<0.05.
Electron microscopy
Electron microscopy was performed as in [42] with a Tecnai G2 Spirit transmission electron microscope (FEI).
Supporting Information
Zdroje
1. O'Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, et al. (1985) The Drosophila ninaE gene encodes an opsin. Cell 40 : 839–850. 2985266
2. Meinertzhagen IA, Hanson, T.E. (1993) The development ofthe optic lobe: Bate, Martinez Arias.
3. Clandinin TR, Zipursky SL (2000) Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron 28 : 427–436. 11144353
4. Montell C, Jones K, Zuker C, Rubin G (1987) A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. The Journal of neuroscience: the official journal of the Society for Neuroscience 7 : 1558–1566.
5. Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, et al. (1996) Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron 17 : 1101–1115. 8982159
6. Papatsenko D, Sheng G, Desplan C (1997) A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development 124 : 1665–1673. 9165115
7. Senti KA, Usui T, Boucke K, Greber U, Uemura T, et al. (2003) Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Current biology: CB 13 : 828–832. 12747830
8. Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A (2006) Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron 49 : 205–213. 16423695
9. Ting CY, Yonekura S, Chung P, Hsu SN, Robertson HM, et al. (2005) Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development 132 : 953–963. 15673571
10. Hadjieconomou D, Timofeev K, Salecker I (2011) A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol 21 : 76–84. doi: 10.1016/j.conb.2010.07.012 20800474
11. Astigarraga S, Hofmeyer K, Treisman JE (2010) Missed connections: photoreceptor axon seeks target neuron for synaptogenesis. Curr Opin Genet Dev 20 : 400–407. doi: 10.1016/j.gde.2010.04.001 20434326
12. Lee RC, Clandinin TR, Lee CH, Chen PL, Meinertzhagen IA, et al. (2003) The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nature neuroscience 6 : 557–563. 12754514
13. Hein I, Suzuki T, Grunwald Kadow IC (2013) Gogo receptor contributes to retinotopic map formation and prevents R1-6 photoreceptor axon bundling. PloS one 8: e66868. doi: 10.1371/journal.pone.0066868 23826162
14. Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL (2001) N-cadherin regulates target specificity in the Drosophila visual system. Neuron 30 : 437–450. 11395005
15. Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, et al. (2001) Drosophila LAR regulates R1-R6 and R7 target specificity in the visual system. Neuron 32 : 237–248. 11683994
16. Tomasi T, Hakeda-Suzuki S, Ohler S, Schleiffer A, Suzuki T (2008) The transmembrane protein Golden goal regulates R8 photoreceptor axon-axon and axon-target interactions. Neuron 57 : 691–704. doi: 10.1016/j.neuron.2008.01.012 18341990
17. Hakeda-Suzuki S, Berger-Muller S, Tomasi T, Usui T, Horiuchi SY, et al. (2011) Golden Goal collaborates with Flamingo in conferring synaptic-layer specificity in the visual system. Nature neuroscience 14 : 314–323. doi: 10.1038/nn.2756 21317905
18. Song J, Wu L, Chen Z, Kohanski RA, Pick L (2003) Axons guided by insulin receptor in Drosophila visual system. Science 300 : 502–505. 12702880
19. Petrovic M, Hummel T (2008) Temporal identity in axonal target layer recognition. Nature 456 : 800–803. doi: 10.1038/nature07407 18978776
20. Morey M, Yee SK, Herman T, Nern A, Blanco E, et al. (2008) Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature 456 : 795–799. doi: 10.1038/nature07419 18978774
21. Vandendries ER, Johnson D, Reinke R (1996) orthodenticle is required for photoreceptor cell development in the Drosophila eye. Developmental biology 173 : 243–255. 8575625
22. Fichelson P, Brigui A, Pichaud F (2012) Orthodenticle and Kruppel homolog 1 regulate Drosophila photoreceptor maturation. Proceedings of the National Academy of Sciences of the United States of America 109 : 7893–7898. doi: 10.1073/pnas.1120276109 22547825
23. Tahayato A, Sonneville R, Pichaud F, Wernet MF, Papatsenko D, et al. (2003) Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Developmental cell 5 : 391–402. 12967559
24. McDonald EC, Xie B, Workman M, Charlton-Perkins M, Terrell DA, et al. (2010) Separable transcriptional regulatory domains within Otd control photoreceptor terminal differentiation events. Developmental biology 347 : 122–132. doi: 10.1016/j.ydbio.2010.08.016 20732315
25. Weimann JM, Zhang YA, Levin ME, Devine WP, Brulet P, et al. (1999) Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron 24 : 819–831. 10624946
26. Morrow EM, Furukawa T, Raviola E, Cepko CL (2005) Synaptogenesis and outer segment formation are perturbed in the neural retina of Crx mutant mice. BMC neuroscience 6 : 5. 15676071
27. Velez MM, Gohl D, Clandinin TR, Wernet MF (2014) Differences in neural circuitry guiding behavioral responses to polarized light presented to either the dorsal or ventral retina in Drosophila. Journal of neurogenetics: 1–30.
28. Schwabe T, Borycz JA, Meinertzhagen IA, Clandinin TR (2014) Differential adhesion determines the organization of synaptic fascicles in the Drosophila visual system. Current biology: CB 24 : 1304–1313. doi: 10.1016/j.cub.2014.04.047 24881879
29. Chen PL, Clandinin TR (2008) The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron 58 : 26–33. doi: 10.1016/j.neuron.2008.01.007 18400160
30. Finkelstein R, Smouse D, Capaci TM, Spradling AC, Perrimon N (1990) The orthodenticle gene encodes a novel homeo domain protein involved in the development of the Drosophila nervous system and ocellar visual structures. Genes & development 4 : 1516–1527.
31. Wu JS, Luo L (2006) A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nature protocols 1 : 2583–2589. 17406512
32. Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, et al. (2006) Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312 : 1051–1054. 16614170
33. Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, et al. (2006) Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49 : 833–844. 16543132
34. Frankfort BJ, Mardon G (2002) R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development 129 : 1295–1306. 11880339
35. Kimura H, Usui T, Tsubouchi A, Uemura T (2006) Potential dual molecular interaction of the Drosophila 7-pass transmembrane cadherin Flamingo in dendritic morphogenesis. Journal of cell science 119 : 1118–1129. 16507587
36. Schwabe T, Neuert H, Clandinin TR (2013) A network of cadherin-mediated interactions polarizes growth cones to determine targeting specificity. Cell 154 : 351–364. doi: 10.1016/j.cell.2013.06.011 23870124
37. Xie B, Charlton-Perkins M, McDonald E, Gebelein B, Cook T (2007) Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development 134 : 4243–4253. 17978002
38. Berger-Muller S, Sugie A, Takahashi F, Tavosanis G, Hakeda-Suzuki S, et al. (2013) Assessing the role of cell-surface molecules in central synaptogenesis in the Drosophila visual system. PloS one 8: e83732. doi: 10.1371/journal.pone.0083732 24386266
39. Rhinn M, Brand M (2001) The midbrain—hindbrain boundary organizer. Current opinion in neurobiology 11 : 34–42. 11179870
40. Tossell K, Andreae LC, Cudmore C, Lang E, Muthukrishnan U, et al. (2011) Lrrn1 is required for formation of the midbrain-hindbrain boundary and organiser through regulation of affinity differences between midbrain and hindbrain cells in chick. Developmental biology 352 : 341–352. doi: 10.1016/j.ydbio.2011.02.002 21315708
41. Edwards TN, Meinertzhagen IA (2009) Photoreceptor neurons find new synaptic targets when misdirected by overexpressing runt in Drosophila. The Journal of neuroscience: the official journal of the Society for Neuroscience 29 : 828–841.
42. Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, et al. (2006) Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Current biology: CB 16 : 140–149. 16431366
43. Freeman M (1996) Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87 : 651–660. 8929534
44. Miller AC, Seymour H, King C, Herman TG (2008) Loss of seven-up from Drosophila R1/R6 photoreceptors reveals a stochastic fate choice that is normally biased by Notch. Development 135 : 707–715. doi: 10.1242/dev.016386 18199577
45. Pichaud F, Desplan C (2001) A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development 128 : 815–826. 11222137
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



