-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
Daily nutrition, in addition to being a source of energy, contains micronutrients, a class of nutrients including vitamins which are essential for life and which act by orchestrating a vast number of developmental and physiological processes. During metabolism, micronutrients are frequently transformed into their bioactive forms. Nuclear hormone receptors are a family of proteins functioning as ligand-regulated transcription factors which can sense such bioactive molecules and translate those signals into transcriptional, adaptive responses. Retinoid X receptors occupy a central place in this signaling as they directly interact, and thereby control, activities of several nuclear hormone receptors. We report here the identification of a novel bioactive form of vitamin A, which is the first endogenous form of this vitamin capable to bind and activate retinoid X receptors. Accordingly, we show that this single molecule displays biological activity similar to synthetic agonists of retinoid X receptors and coordinates transcriptional activities of several nuclear receptor signaling pathways. Those findings may have immediate biomedical implications, as retinoid X receptors are implicated in the control of a number of physiological functions and their pathology.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005213
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005213Summary
Daily nutrition, in addition to being a source of energy, contains micronutrients, a class of nutrients including vitamins which are essential for life and which act by orchestrating a vast number of developmental and physiological processes. During metabolism, micronutrients are frequently transformed into their bioactive forms. Nuclear hormone receptors are a family of proteins functioning as ligand-regulated transcription factors which can sense such bioactive molecules and translate those signals into transcriptional, adaptive responses. Retinoid X receptors occupy a central place in this signaling as they directly interact, and thereby control, activities of several nuclear hormone receptors. We report here the identification of a novel bioactive form of vitamin A, which is the first endogenous form of this vitamin capable to bind and activate retinoid X receptors. Accordingly, we show that this single molecule displays biological activity similar to synthetic agonists of retinoid X receptors and coordinates transcriptional activities of several nuclear receptor signaling pathways. Those findings may have immediate biomedical implications, as retinoid X receptors are implicated in the control of a number of physiological functions and their pathology.
Introduction
Micronutrients such as vitamin A and polyunsaturated fatty acids are essential ingredients of mammalian diet and can act as bioactive molecules. Nuclear hormone receptors sense such molecular signals and accordingly regulate gene expression, thus functioning as ligand-controlled transcription factors. Retinoid X receptors (RXRs) occupy a central place in nuclear receptor signaling as obligatory heterodimerization partners for several of those receptors. RXR ligands can regulate the activity of only some heterodimers including for example LXR-RXR or PPAR-RXR, collectively called permissive heterodimers in opposition to non-permissive heterodimers, like RAR-RXR, which cannot be activated by RXR ligands alone [1,2]. Ligand-dependent modulation might be particularly relevant for the control of a wide range of physiological events. For example, among complex functions, working memory was shown sensitive to RXR ligand activities in mice [3], whereas at the cellular and molecular level, differentiation of monocyte-derived dendritic cells is one of the well characterized experimental models used to study the activities of RXR ligands [4,5]. Such ligands are also known to act as powerful inducers of apoptosis in cancer cells [6,7] or as modulators of lipid and glucose metabolism, which has stimulated their clinical development for the treatment of cancer and metabolic diseases [8]. Recent studies on antidepressant or neuro-regenerative activities of RXR specific agonists suggest also their utility for the treatment of some neuropsychiatric or neurodegenerative disorders [3,9,10].
In parallel to development and use of synthetic RXR ligands, several endogenous agonists have been proposed [11,12,13], but their physiological relevance remains questionable for different reasons. For example, 9-cis-retinoic acid (9CRA), an isomer of all-trans-retinoic acid (ATRA), was proposed and largely accepted as an endogenous RXR ligand [14,15]. However, although 9CRA can bind and activate RXRs at low concentrations, it was either undetectable [16,17,18,19,20,21] or was not present in sufficient concentrations [22] to enable RXR-mediated signaling in mammalian organisms. Docosahexaenoic acid (DHA), an alternative RXR ligand, was shown to bind and transactivate RXRs under pharmacological conditions [11,23,24], but in the physiological setting it can be detected in the brain mainly in esterified form contributing to e.g. structural components of the cell, while the pool of this fatty acid available for RXR activation remains too low [25,26]. Finally, phytanic acid [27,28] also suggested to bind RXR was not conclusively proven to be physiologically relevant.
In this study, we addressed the nature of known and novel endogenous retinoids and their role in RXR signaling in vivo by chemical, molecular and functional studies in distinct models.
Results
In order to search for endogenous retinoids which may act as RXR ligand(s), we first employed behavioral and pharmacological analyses sensitive to RXR signaling as a tool to identify animal models with reduced RXR signaling. In particular, we focused on spatial working memory previously reported as dependent on RXR and not RAR functions and more importantly also dependent on RXR ligand activities [3]. Using delayed non-match to place (DNMTP) task, we found that mice carrying a null mutation of cellular retinol binding protein I (RBP1), known for its role in retinoid metabolism [29], display memory deficits which phenocopy the effect of the loss of function of Rxrγ, a functionally predominant RXR in control of working memory (Fig 1A and ref. [3]). In particular, Rbp1-/- and Rxrγ -/- mice performed significantly worse when compared to wild type (WT) mice at 3 or 6 min inter-trial intervals (ITI) in DNMTP task, attaining chance level (complete forgetting) already at 6 min, whereas WT mice performed at chance level only at 12 or 18 min depending on individual (Fig 1A, see grey part of the left panel). These data suggest that RXR signaling is compromised in Rbp1-/- mice.
Fig. 1. Compromised RXR signaling in Rbp1-/- mice. 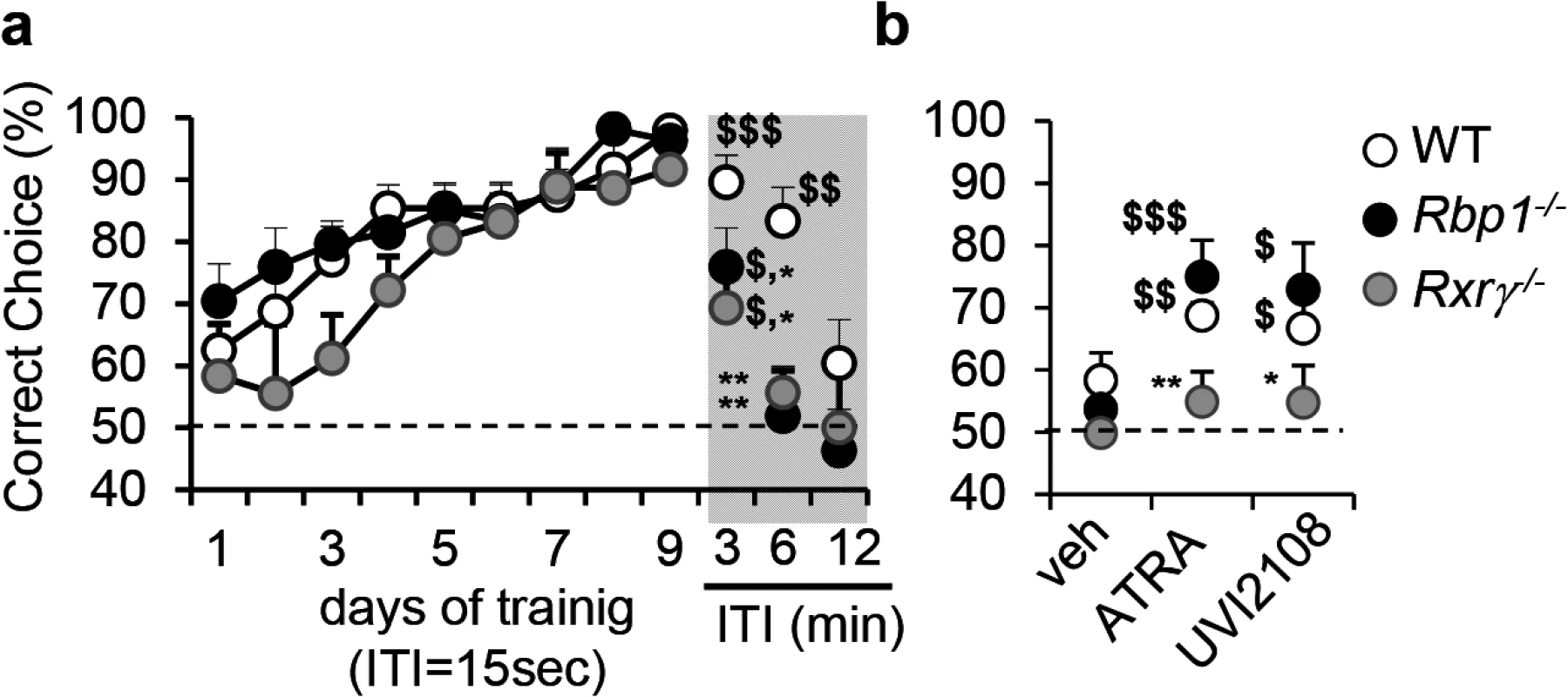
(a) Rbp1-/- (n = 8) and Rxrγ-/- (n = 7) mice acquired working memory DNMTP task similarly to WT (n = 8) mice (F[8,160] = 14, p<0.001; ANOVA on repeated measures) when trained with 15 sec inter-trial intervals (ITI), but showed forgetting when tested at 3, and 6 min ITIs, which was more rapid than 12 or 18 min in WT mice (indicated in the graph as 12 min ITI). (b) RXR agonist or ATRA improved working memory performance 6hrs after treatment in Rbp1-/- mice (n = 8) tested at 6 min ITI or WT mice (n = 8) at 12 or 18 min ITIs, but was inactive in Rxrγ-/- mice (n = 7) at ITI of 6 min. Compromised RXR signalling in Rbp1-/- was further supported in spontaneous alternation task, a distinct test for working memory (S1 Fig). Statistical differences identified with PLSD Fischer test were indicated as: *, p<0.05; **, p<0.01 as compared to WT controls in respective groups; $, p<0.05; $$, p<0.01; $$$, p<0.001 in comparison with 50% of chance level. To challenge this hypothesis functionally, we took advantage of the sensitivity of working memory performance in delayed task to treatment with RXR agonists [3]. Activation of RXR signaling by the synthetic RXR agonist, UVI2108 (also known as SR11217 or BMS649) or by ATRA, which under pharmacological conditions is rapidly transformed in vivo to RXR agonist 9CRA[12,30], reversed memory deficits in Rbp1-/- mice, but remained ineffective in mice lacking RXRγ (Fig 1B). Working memory deficits were also observed in a distinct, rodent specific memory test of spontaneous alternation in the Y-maze (S1 Fig). Treatments with ATRA, UVI2108 and other RXR agonists, including DHA and methoprene acid, but not pan-RAR agonist TTNPB led to pro-mnemonic effects in Rbp1-/-, but not Rxrγ -/- mice (S1 Fig), supporting the possibility of compromised RXR signaling due to reduced availability of RXR ligand(s) in Rbp1-/- mice. Accordingly, reduced expression of RXRγ could not explain behavioral deficits in Rbp1-/- mice. On the contrary, expression of RXRγ was clearly increased in Rbp1-/- striatum attaining level of 3.2 ± 0.6 in Rbp1-/- mice as compared to 1.2 ± 0.3 arbitrary RNA units (qRT-PCR).
To evaluate RXR ligand availability in Rbp1-/- mice, we first addressed concentration of 9CRA in mouse brain and serum. Using a sensitive method of retinoic acid detection based on HPLC separation followed by highly specific DAD detection and destructive MS-MS [20], we clearly identified ATRA in serum (0.3 ± 0.1 ng/ml) and brain (0.6 ± 0.1 ng/g) samples from WT mice, whereas in the range of 9CRA elution no conclusive peak was identified indicating that 9CRA is absent or its levels were under our detection limit of 0.1 ng/g and thereby too low for sufficient RXR-activation in WT (Fig 2A) and Rbp1-/- animals. We then focused on dihydroretinoids described as novel endogenous retinoids [31,32]. Using stereo - and enantiocontrolled organic synthesis we obtained a series of dihydroretinoids, including all-trans-13,14-dihydroretinoic acid (ATDHRA) and its stereoisomer 9-cis-13,14-dihydroretinoic acid (9CDHRA; see Materials and methods for details of its synthesis), which we next used as reference molecules in HPLC-MS-MS analyses. Such analyses were focused on liver, as major site of Rbp1 expression, serum, through which retinoids are distributed to target organs, and brain, with discrete areas expressing Rbp1 [33]. We identified two major peaks, which co-eluted with standards of ATDHRA and 9CDHRA at UV specific absorption of 290 nm (Fig 2B, left panel). Such co-elution was also observed at dihydroretinoid-specific MS-MS settings (Fig 2B, right panel). Concentrations of 9CDHRA were high in serum samples attaining 118 ± 15 ng/ml (corresponding to ~4 x 10-7M), 135 ± 12 ng/g in mouse liver (corresponding to a concentration of ~7x10-7M) and relatively low (7 ± 1 ng/g, corresponding to ~2 x 10-8M) in brain. A direct comparison of these retinol metabolites in WT and littermate Rbp1-/- mice (Fig 2C) showed comparable concentration of ATDHRA in contrast to significantly decreased 9CDHRA levels in serum, liver and brain of Rbp1-/- mice. Importantly, whereas such decrease in serum may be at the origin of systemic reduction of RXR signaling, almost complete loss of 9CDHRA availability in brains of Rbp1-/- mice suggest more significant reduction of local RXR signalling in this organ. Furthermore, whole brain measures reflect most probably more dramatic changes of 9CDHRA levels in discrete brain areas expressing Rbp1 [33]. Unfortunately it is technically impossible to identify retinoid concentrations in these small areas, which can be only prompted by whole brain measures.
Fig. 2. 9CDHRA is present in mouse serum, brain and liver. 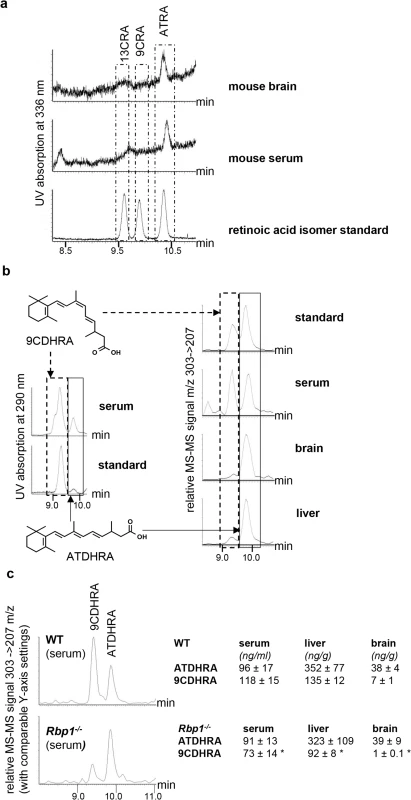
(a) Elution profiles of WT mouse serum (n = 7) and brain (n = 3) samples in comparison with three retinoic acid standards (all-trans [ATRA], 13-cis [13CRA] and 9-cis retinoic acid [9CRA] with retention times 10.4, 9.6, and 9.9 min, respectively) allow identification of ATRA but not 9CRA. Boxes represent areas with comparable retention times to identify co-eluting peaks. (b) 9-cis-13,14-dihydroretinoic acid (9CDHRA; retention time 9.4 min highlighted by dotted-line box) is present in mouse serum (n = 7), brain (n = 3) and liver (n = 7) samples as determined by co-elution with a mixture of 9CDHRA and all-trans-13,14-dihydroretinoic acid (ATDHRA; retention time 9.9 min, highlighted by continuous-line box) standard solution and confirmed by MS-MS (303->207 m/z) and DAD (290 nm) detection. (c) Significant reduction of 9CDHRA, but not ATDHRA levels in serum, brain and liver of Rbp1-/- animals (n = 8, n = 3 for brain) as compared to WT mice (n = 8, n = 3 for brain). All the error bars represent S.E.M. Direct evidence for 9CDHRA binding to RXR was given by electrospray ionisation mass spectrometry (ESI-MS) performed in non-denaturing conditions with purified RXR ligand binding domains (LBD). In order to evaluate relative affinities of R - and S-enantiomers and 9CDHRA for RXR LBD, titration experiments were monitored by ESI-MS. As shown in Fig 3A and 3B, all retinoids bind to hRXRα LBD used in these studies as model RXR LBD due to high conservation of LBD structure among all RXRs. Analyses of peak amplitude revealed that R-9CDHRA has approximately 30% lower affinity than 9CRA, but about 65% higher affinity than S-9CDHRA. Quantitative binding affinities to RXRa LBD obtained by fluorescence quenching assay (S2 Fig) are equal to 90 ± 20 nM for R-9CDHRA and 20 ± 10 nM for 9CRA, and fall in the range of published Kd [34] indicating that 9CDHRA binds RXRs with high affinity at concentrations which are physiologically relevant. Since 9CRA can bind to RARs we also tested affinity of 9CDHRA for all three RAR isotypes. ESI-MS experiments performed with hRARα, β and γ LBDs (S3A and S3B Fig) revealed that 9CDHRA (R and S) bind to all RAR LBD isotypes.
Fig. 3. 9CDHRA binds and transactivates RXR in vitro, and displays RXR agonist-like activities in vivo. 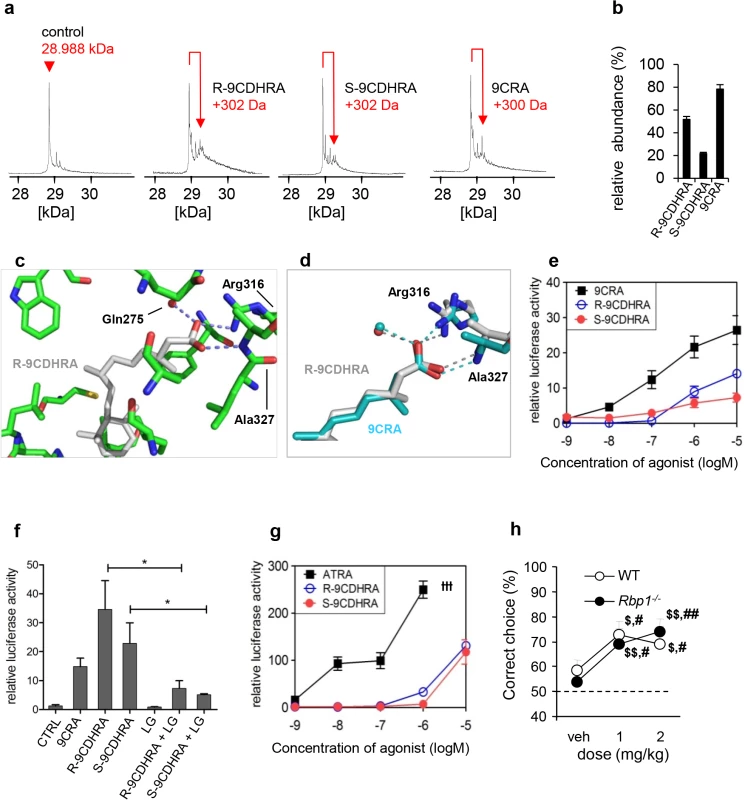
(a) ESI mass spectra of hRXRα LBD protein after incubation with a 5-fold molar excess of 9CRA, R-9CDHRA and S-9CDHRA. (b) Distribution plot of the percentage of bound hRXRα. (c) Close-up view showing the ligand-binding pocket of RXRα bound to R-9CDHRA (in grey). Residues in close contacts (<3.5 Å) are labelled. Dashed lines indicate hydrogen bonds and the red sphere a water molecule. (d) Superposition of the RXRalpha ligand-binding pocket bound to R-9CDHRA (grey) and 9CRA (cyan, PDB code: 1XDK). Superposition was made on the protein. Hydrogen bonds with R-9CDHRA or 9CRA are shown by grey and cyan dashed lines, respectively. Water molecules are shown by spheres (red for RXR-R-9CDHRA and cyan for RXR-9CRA). (e) Transcriptional activation of RXRα by R-9CDHRA and S-9CDHRA in comparison to 9CRA (10–5–10-9M) in RXR-reporter COS1 cells. (f) 9CRA (10-7M) and both 9CDHRA enantiomers (10-5M) induced RXRα-mediated signaling. RXR-antagonist LG101208 (LG; 10-6M) diminished activity of both 9CDHRA enantiomers (10-5M). (g) Transcriptional activation of RAR-RXR heterodimers by R- and S-9CDHRA in comparison to ATRA in RARα-RXRα-reporter COS1 cells. (h) Increasing doses of R-9CDHRA reversed working memory deficits in Rbp1-/- mice and showed pro-mnemonic activity in WT mice (n = 8/group) in DNMTP task when tested at minimal ITI, at which mice performed at chance level (50%) and which was 6min for the Rbp1-/- or 12min for WT mice. ttt: ATRA at concentration 10–5 M was cytotoxic. *, p<0.05. #, p<0.05; ##, p<0.01 as compared to vehicle treatment in the same group; $, p<0.05; $$, p<0.01; one group student t-test for comparison with performance at chance level of 50%. All the error bars represent S.E.M. To provide structural evidence of the binding of R-9CDHRA to RXR, the hRXRα LBD was crystallized in complex with R-9CDHRA and a 13-residue peptide comprising the nuclear receptor-binding surface NR2 of NCoA2. Note that the residues lining the ligand binding pocket are strictly conserved between RXRα and RXRγ and that the conclusions drawn for RXRα will also be valid for RXRγ. The structure refined at 1.8 Å resolution (S1 Table) revealed the canonical active agonist conformation common to all previously reported agonist-bound nuclear receptor LBDs with 12 or 13 α-helices organized in a three-layered sandwich (S3C and S3D Fig). R-9CDHRA adopts a similar binding mode as 9CRA [35,36] including interactions of the carboxylate group of the ligand with Arg316 (H5), and hydrogen bonds with the amide group of Ala327 in the beta turn (Fig 3C and 3D). The number of contacts is similar between the two ligands although some interactions are weaker in the case of R-9CDHRA compared to 9CRA as for example the interactions with Leu436 (4.0 Å instead of 3.6 Å for 9CRA), Arg316 (2.7 Å instead of 2.3 Å) or Trp305 (4.3 Å instead of 3.5 Å) that account for the weaker binding of R-9CDHRA. In silico comparison of S - and R-9CDHRA binding mode in RXRα LBP revealed that the opposite configuration at C13 leading to slightly different side chain conformation of S-9CDHRA may underlay its lower affinity to RXR (S3E Fig), further supported by the lower relative binding affinity measured by ESI-MS.
The relevance of R-9CDHRA and S-9CDHRA receptor binding for transcriptional activities of RXRs was tested in COS1 reporter cell lines transfected with a RXRα expression vector (Fig 3E–3G). In agreement with previous reports, 9CRA induced transcription of reporter gene at concentrations starting from 10-9M, whereas R-9CDHRA or S-9CDHRA displayed similar activity to 9CRA, although at concentrations higher than 10-7M. Importantly, the activities of R - and S-9CDHRA at 10-5M were prevented by co-treatment with an RXR-antagonist LG101208 at 10-6M (Fig 3F). 9CDHRAs also activated RAR-RXR signaling in COS1 model reporter cells (transfected with RARα and RXRα expression vectors) starting at 10-6M (Fig 3G). Considering that all RXR isotypes share the same structure of their ligand binding pockets, the present data obtained with the RXRα isotype indicate that 9CDHRA may efficiently bind to all RXRs and induce their transcriptional activities at concentrations found in physiological conditions.
Behavioral analyses revealed that the 9CDHRA modulation of RXR functions is also relevant in vivo. Accordingly, acute treatment with R-9CDHRA improved memory performance of Rbp1-/- mice as compared to vehicle treatment or chance level of 50% when tested in DNMTP task at ITI of 6 min (Fig 3H). R-9CDHRA treatments also raised performance of WT mice when tested at long ITIs of 12 or 18 min, at which time the corresponding WT mice treated with vehicle performed at chance level. Such treatment did not improve performance of Rxrγ-/- mice (57 ± 7% of correct choices) indicating RXR specificity of 9CDHRA effects, which is further supported by similar effects of 9CDHRA and UVI2108 treatments (compare Fig 3H and 3L).
In order to identify 9CDHRA specificity for induction of RXR-dependent transcriptional activity at the transcriptomic level and its capacity to activate permissive heterodimers, we took advantage of human differentiating monocyte-derived dendritic cell cultures, a well characterized in vitro model for studies of signaling through RXR and its heterodimers [4,5]. The gene expression changes induced by R-9CDHRA, S-9CDHRA, other RXR ligands or ligands for RXR partners, revealed that R-9CDHRA and 9CRA regulate approximately the same number of transcripts (518 and 450, respectively; Fig 4). Importantly, 384 transcripts were similarly regulated by both agonists (Fig 4A and 4B), which corresponded to 85% of all transcripts regulated by 9CRA. Within this set, a group of 61 transcripts was also regulated by LG268, a synthetic RXR specific ligand used in our analysis as a reference in previous studies of this model [4,5]. Remarkably, none of the transcripts were regulated solely by 9CRA and LG268, and not by 9CDHRA, indicating that 9CDHRA induces similar gene expression changes as 9CRA.
Fig. 4. Molecular evidence for 9CDHRA selective activation of RXRs. 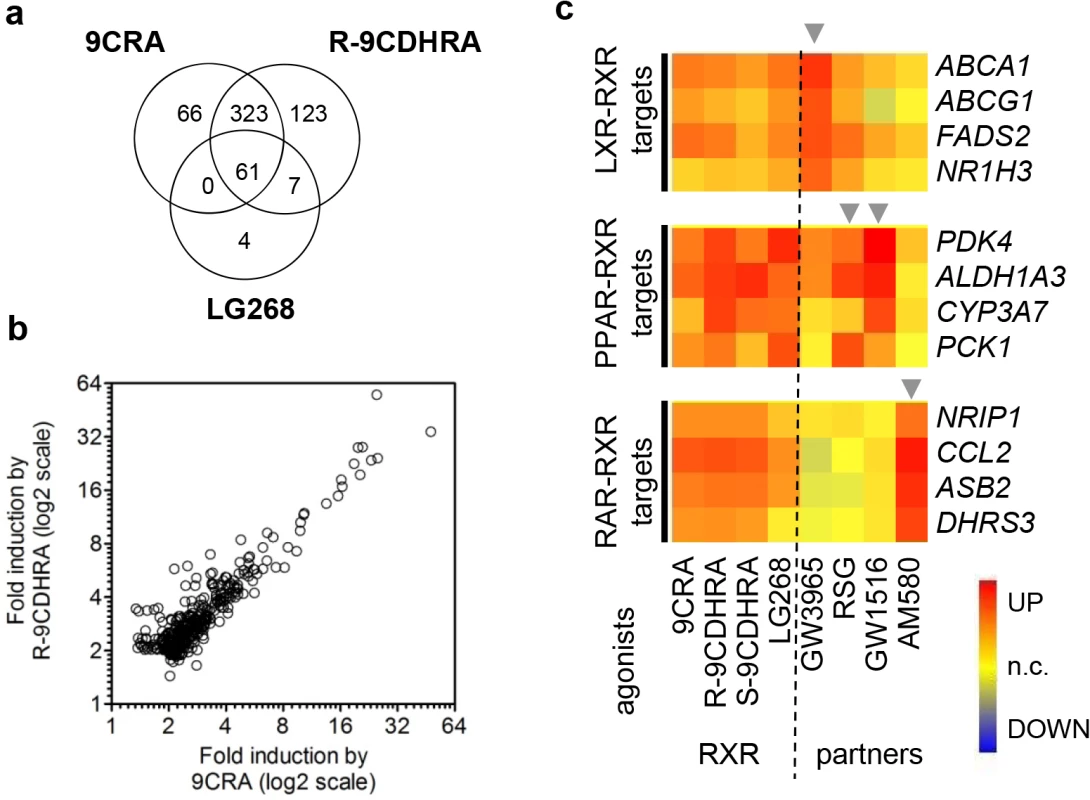
(a) Significant overlap between global transcriptional changes induced by R-9CDHRA (10-5M), 9CRA (10-6M) or a synthetic RXR agonist (LG268; 10-7M) revealed by DNA microarray analyses in differentiating monocyte-derived human dendritic cells. (b) Scatter plot comparison of fold-changes for transcripts altered by 9CRA and R-9CDHRA treatments. (c) S- and R-9CDHRA, similarly to 9CRA and/or LG268 (see “RXR” columns), induced the expression of genes (see rows) identified previously as direct transcriptional targets of LXR-RXR, PPAR-RXR and RAR-RXR. Corresponding transcripts were also induced by agonists of respective RXR-heterodimer partners (see “partners” column and arrowheads): GW3965 (LXRα/β; 10-6M), RSG (PPARγ; 10-6M), GW1516 (PPARδ; 10-6M) and AM580 (RAR; 10-7M). We also investigated the capacity of 9CDHRA for activating permissive heterodimers, e.g. LXRα/γ-RXR, PPARγ -RXR, and PPARδ-RXR. As expected, we found that R-9CDHRA, similarly to 9CRA and LG268, could induce the expression of many genes, which are known as direct targets of RXR permissive heterodimers. Accordingly these genes were also regulated by LXR or PPAR specific ligands (Fig 4C). To address whether 9CDHRA also activates RAR-RXR target genes we compared the effect of RXR ligands and AM580, a synthetic RARα selective ligand. Typically genes induced by AM580 were not induced by any other agonist of permissive partners (Fig 4C), but were also induced by 9CDHRA or 9CRA. Collectively, gene expression profiling indicated that R - and S-9CDHRAs display RXR agonist activity, but can also activate RARs, acting thus with similar selectivity to 9CRA.
Discussion
Although RXRs occupy central position in signaling of several nuclear hormone receptors acting as their heterodimerisation partner, endogenous ligand(s) of RXRs, its metabolic pathway and physiological functions were not conclusively determined. We found that Rbp1-/- displayed a phenotype suggestive of reduced RXR signaling, which could not be attributed to the reduced levels of RXR expression or of 9CRA, the potential endogenous RXR ligand which we and others failed to detect [16,17,18,19,20] in wild type animals. Using chemical approaches of HPLC-MS and organic synthesis we identified 9CDHRA, a novel endogenous retinoid, the concentrations of which were significantly reduced in serum, liver and brain of Rbp1-/- mice. Several lines of evidence indicate that 9CDHRA treatment activates RXRs in vitro and in vivo at physiologically relevant concentrations, suggesting that it acts as an endogenous RXR agonist.
We report herein that RBP1 modulates animal behavior by control of the availability of an RXR ligand. Accordingly, mice carrying null mutation of RBP1 display working memory deficits, the hallmark of deficient signalling through RXRγ, a functionally predominant RXR in the control of working memory in adult mice [3,37]. Reduced expression of RXRγ do not account for these changes, suggesting compromised availability of RXR ligand. This unique model enabled us to search for the endogenous RXR ligand(s). As our initial analyses failed to detect 9CRA in wild type and Rbp1-/- mice, we turned our attention to dihydroretinoids proposed recently as a novel group of bioactive, endogenous retinoids [31]. Using HPLC-MS-MS conditions specific for detection of dihydroretinoic acids, including 13,14-dihydroretinoic acids, and aided by organic synthesis we detected the presence of ATDHRA and 9CDHRA in mouse serum, liver and brain in WT mice and Rbp1-/- mice. Serum and liver concentration of 9CDHRA were particularly high, ranging from 4 to 7x10-7M, and much lower in whole-brain extracts in WT animals. Nevertheless they were significantly reduced in all corresponding samples of Rbp1-/- mice.
Reduced serum availability of 9CDHRA in Rbp1-/- mice may result from reduced synthesis of 9CDHRA in the liver, the main site of RBP1 expression [29]. Because levels of ATDHRA were comparable in the serum, liver and brain of WT and Rbp1-/- mice, reduced levels of 9CDHRA in Rbp1-/- mice indicate that RBP1 plays an important role specifically in generation of different forms of 9-cis-retinoids as previously suggested [38]. Thus, 9CDHRA, similarly to ATRA and 1,25-dihydroxy-vitamin D3 could act in endocrine and paracrine manner as a lipid hormone of nutritional origin being distributed in the serum but also synthetized locally in specific organs [39,40]. In consequence, reduced systemic levels of 9CDHRA may synergize with local reduction of its synthesis in specific brain areas of Rbp1-/- mice leading to compromised RXR activities and mnemonic deficits. In favor of this hypothesis, systemic administration of R-9CDHRA, a 9CDHRA enantiomer obtained by stereoselective chemical synthesis, normalized working memory deficits in Rbp1-/- mice. That such effects are mediated by RXRs may be suggested by absence of promnemonic effects of 9CDHRA and other RXR ligands in mice carrying null mutation of RXRγ, a functionally predominant RXR in the control of these brain functions [3].
Direct evidence of 9CDHRA binding to RXRs is provided by electrospray ionisation mass spectrometry (ESI-MS) performed in non-denaturing conditions using purified RXR LBD and fluorescence quenching assay. In particular, R-9CDHRA binds RXR LBD with affinity close to that of 9CRA as indicated by respective Kd values of 90 ± 20 nM for 9CDHRA and 20 ± 10 nM for 9CRA. Such data are supported by the crystal structure of the complex of R-9CDHRA with RXR LBD, in which R-9CDHRA adopts the canonical active agonist conformation and the carboxylate interacts with Arg316. Importantly, the R-9CDHRA enantiomer efficiently induces RXR transcriptional activity in reporter cell assays at physiologically relevant concentrations below 10-6M, which can be prevented by co-administration of RXR pan-antagonist LG101208. Although S-9CDHRA displays lower affinity to bind RXR LBD in ESI-MS, most probably due to the inverse positioning of the C20-carbon atom, it is also active in the transactivation of RXR in vitro with threshold concentration between 10–7 and 10-6M.
Further relevance of 9CDHRA for the activation of RXRs in vitro was indicated by the regulation of transcriptional targets of LXR-RXR or PPAR-RXR permissive heterodimers, which are known to be sensitive to pharmacological activation of RXR as well as its nuclear receptor partners. Such activation was demonstrated in human dendritic cells cultured in vitro, a well-established model for analyses of RXR signalling [4,5]. Importantly, almost all (68 out of 72) transcripts regulated by LG268 (RXR-specific agonist) were also regulated by 9CDHRA. Such a result obtained in a course of transcriptomics study was very similar to the data obtained for 9CRA, which controlled 61 out 72 LG268 transcriptional targets, indicating the extremely high capacity of 9CDHRA and 9CRA to control RXR transcriptional targets. Such genes correspond most probably to permissive heterodimers, and indirect targets of liganded RXR and their regulation provide evidence that 9CDHRA can control RXR signalling also in human cells. High degree of overlap between transcriptional activities of 9CRA and 9CDHRA, which goes beyond the activation of RXR-specific transcripts, reflects their capacity to bind and transactivate also RARs. That 9CRA and 9CDHRA act as a mixed RXR and RAR agonists is supported by about 80% overlap in transcriptional changes induced by R-9CDHRA (or S-9CDHRA) and 9CRA.
Whereas 13,14-dihydroretinol was detected by Moise and colleagues [31] as a hepatic retinol saturase (RETSAT) metabolite in the mammalian organism, other dihydroretinoids or their precursors were also identified in other vertebrates [41,42] and also non-vertebrates [43]. Besides RETSAT-mediated retinol metabolism as the major potential pathway for endogenous 9CDHRA synthesis, also apo-carotenoids and carotenoids may serve as substrates for dehydrogenation via RETSAT or other saturases, followed by the synthesis of dihydroretinoic acids [44,45].
This metabolic pathway may be phylogenetically ancient, as RXR orthologs from several non-vertebrate species including mollusk [46], primitive chordate like amphioxus [47] and some primitive insects like Tribolium [48] or Locusta migratoria [49,50] have also a potential to bind RXR ligands. In addition, Locusta migratora ultraspiracle (a fly RXR ortholog), displayed higher affinity to bind 9CRA than human RXR [49], raising the possibility that 9CDHRA could also activate the USP pathway and be an ancestral RXR ligand. Based on our data, it is tempting to suggest that in addition to the active ligands originating from vitamin A1 and vitamin A2, 9CDHRA and its nutritional precursors may represent a third novel distinct pathway of vitamin A metabolism and signaling, which evolved specifically to control RXR activity.
In summary we have characterized 9CDHRA as the first endogenous and physiologically relevant retinoid that acts as RXR ligand in mammals. Reduced memory functions due to compromised RXR-mediated signaling in Rbp1-/- mice result from lower brain levels of 9CDHRA, reflecting reduced serum transport and local brain synthesis of 9CDHRA in Rbp1-/- mice. Future determination of the metabolic pathways involved in 9CDHRA synthesis and signaling and its tissue specificity will be important for further understanding of the functional relevance of 9CDHRA to animal physiology, pathology and evolution.
Materials and Methods
Animals
Rbp1-/- and Rxrγ -/- mutants as well as wild type (WT) control mice were raised on a mixed genetic background (50% C57BL/6J and 50% 129SvEms/j; bred for more than 10 generations) from heterozygous crosses as described [29,51], and tested at the age of 3 months for chemical analyses and 3–6 months for behavioral analyses. All mice were housed in groups of 4–5 mice per cage in a 7am-7pm light/dark cycle in individually ventilated cages (Techniplast, Italy). Food (standard chow diet, D04 from SAFE, France) and water were freely available. All experiments were carried out in accordance with the European Community Council Directives of 24 November 1986 (86/609/EEC) and in compliance with the guidelines of CNRS and the French Agricultural and Forestry Ministry (decree 87848).
Behavioral procedures
All behavioral tests were carried out in the Institute Clinique de la Souris (http://www.ics-mci.fr/) according to standard operating procedures. To study working memory, behaviorally naïve groups of mice were tested in the DNMTP in the T-maze according to a protocol previously described [52] with modifications to facilitate pharmacological tests [3]. Spontaneous alternation was evaluated in the Y-maze apparatus according to the protocol described in detail in the S1 Text.
Analytical procedures
Serum and tissue samples for chemical analyses were collected during the light phase of the light/dark cycle between 3-4pm, around 9–10 hours after the last major food intake, which in our lighting conditions takes place around 6-7am. High performance liquid chromatography mass spectrometry–mass spectrometry (HPLC-MS-MS) analyses were performed under dark yellow/amber light using previously validated protocol [20]. For the detection of 13,14-dihydroretinoic acid MS-MS settings were 303 -> 207 m/z using the same dwell time and collision energy comparable to the MS-MS specific settings of retinoic acids. Quantification was performed as previously described [20]. For details of sample preparation see S1 Text.
Reporter cell lines
COS1 cells were maintained in DMEM medium with 10% FBS, 5% L-glutamine, 1% penicillin streptomycin in 24-well plates and transfections were carried out in triplicates. Cells were transfected with equal amounts of relevant plasmids including Gal-RXRα-LBD for RXR-reporter line or Gal-RARα-LBD and Gal-RXRα-LBD for RAR-RXR reporter line, a reporter plasmid (luciferase MH100-TKLuc reporter construct with GAL-binding site [53] and beta-galactosidase (for transfection efficiency calculation). For details of transfection and measurements see the S1 Text.
Binding assays
cDNAs encoding hRXRα LBD (223–462), hRARα LBD (153–421), hRARβ LBD (169–414) and hRARγ LBD (178–423) were cloned into the pET28b vector to generate N-terminal His-tag fusion proteins. Purification was carried out as previously described [54,55], including a metal affinity chromatography and a gel filtration. For details of sample preparation and ESI-MS and fluorescence quenching analyses see the S1 Text.
Structure analysis of RXR-LBD in complex with R-9CDHRA
Details on crystallization, X-ray data collection and structure determination can be found in the S1 Text, S3 Fig and S1 Table. The coordinates and structure factors are deposited in the Protein Data Bank under the accession codes 4ZSH.
Animal treatments
R-9CDHRA, UVI2108, ATRA (Sigma), DHA (Sigma), MA (Sigma) and TTNPB (Sigma), were dissolved in ethanol and DMSO, and then mixed with sunflower oil, so that the final solution contained 3% ethanol and 3% DMSO. Vehicle treatments consisted of 3% ethanol and 3% DMSO solution in sunflower oil. Treatments were administered by intraperitoneal injections at volume/weight ratio 3 ml/kg between 8-10am and 5–6 h before the test as previously validated [3].
Human dendritic cell (DC) generation and DNA microarray analysis
The generation and transcriptional analysis of differentiating DCs were performed as described previously [5] and are detailed in the S1 Text. Microarray data were deposited into the Gene Expression Omnibus database under accession no. GSE48573.
Statistical analysis
The comparisons of behavioral performance in Rbp1-/ - and Rxrγ-/ - mice were carried out using the protected least significant difference (PLSD) Fischer test. The pharmacological data for the treatments in WT and Rbp1-/ - or Rxrγ-/ - mice were analysed using 2-way analysis of variance (ANOVA)—with treatment and genotype as two independent factors and behavioral responses as dependent variables. The evolution of learning curves in WT, Rbp1-/ - and Rxrγ-/ - mice were done using ANOVA on repeated measures. Global and post-hoc statistical analyses were performed using student t-test for two-group comparisons. Significant differences are indicated in the corresponding figures.
Chemical synthesis—Synthesis of dihydroretinoids
To confirm whether relative MS-MS signal detected at 303>207 m/z corresponds to 9CDHRA, the stereoselective synthesis of both enantiomers of 9-cis-13,14-dihydroretinoic acid was carried out following the previously described strategy based on a palladium-catalyzed Csp2-Csp2 Suzuki coupling [56]. Details of the stereocontrolled synthesis, purification and characterization of the (R) - and (S)-enantiomers of 9-cis-13,14-dihydroretinoic acid are provided in the S1 Text.
Supporting Information
Zdroje
1. Perez E, Bourguet W, Gronemeyer H, de Lera AR (2012) Modulation of RXR function through ligand design. Biochim Biophys Acta 1821 : 57–69. doi: 10.1016/j.bbalip.2011.04.003 21515403
2. Shulman AI, Larson C, Mangelsdorf DJ, Ranganathan R (2004) Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 116 : 417–429. 15016376
3. Wietrzych-Schindler M, Szyszka-Niagolov M, Ohta K, Endo Y, Perez E, et al. (2011) Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. Biol Psychiatry 69 : 788–794. doi: 10.1016/j.biopsych.2010.12.017 21334601
4. Szatmari I, Torocsik D, Agostini M, Nagy T, Gurnell M, et al. (2007) PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood 110 : 3271–3280. 17664351
5. Szeles L, Poliska S, Nagy G, Szatmari I, Szanto A, et al. (2010) Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol Endocrinol 24 : 2218–2231. doi: 10.1210/me.2010-0215 20861222
6. Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, et al. (1995) Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem 38 : 3146–3155. 7636877
7. Shankaranarayanan P, Rossin A, Khanwalkar H, Alvarez S, Alvarez R, et al. (2009) Growth factor-antagonized rexinoid apoptosis involves permissive PPARgamma/RXR heterodimers to activate the intrinsic death pathway by NO. Cancer Cell 16 : 220–231. doi: 10.1016/j.ccr.2009.07.029 19732722
8. Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H (2007) RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6 : 793–810. 17906642
9. Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, et al. (2011) Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci 14 : 45–53. doi: 10.1038/nn.2702 21131950
10. Lerner V, Miodownik C, Gibel A, Kovalyonok E, Shleifer T, et al. (2008) Bexarotene as add-on to antipsychotic treatment in schizophrenia patients: a pilot open-label trial. Clin Neuropharmacol 31 : 25–33. doi: 10.1097/WNF.0b013e31806450da 18303488
11. de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, et al. (2000) Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290 : 2140–2144. 11118147
12. Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, et al. (1992) 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68 : 397–406. 1310260
13. Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, et al. (1992) 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature 355 : 359–361. 1309942
14. Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, et al. (1993) Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A 90 : 30–34. 8380496
15. Evans RM, Mangelsdorf DJ (2014) Nuclear Receptors, RXR, and the Big Bang. Cell 157 : 255–266. doi: 10.1016/j.cell.2014.03.012 24679540
16. Sakhi AK, Gundersen TE, Ulven SM, Blomhoff R, Lundanes E (1998) Quantitative determination of endogenous retinoids in mouse embryos by high-performance liquid chromatography with on-line solid-phase extraction, column switching and electrochemical detection. J Chromatogr A 828 : 451–460. 9916324
17. Ulven SM, Gundersen TE, Sakhi AK, Glover JC, Blomhoff R (2001) Quantitative axial profiles of retinoic acid in the embryonic mouse spinal cord: 9-cis retinoic acid only detected after all-trans-retinoic acid levels are super-elevated experimentally. Dev Dyn 222 : 341–353. 11747070
18. Kane MA, Chen N, Sparks S, Napoli JL (2005) Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J 388 : 363–369. 15628969
19. Gundersen TE, Bastani NE, Blomhoff R (2007) Quantitative high-throughput determination of endogenous retinoids in human plasma using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21 : 1176–1186. 17330217
20. Rühl R (2006) Method to determine 4-oxo-retinoic acids, retinoic acids and retinol in serum and cell extracts by liquid chromatography/diode-array detection atmospheric pressure chemical ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom 20 : 2497–2504. 16862622
21. Wolf G (2006) Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev 64 : 532–538. 17274495
22. Arnold SL, Amory JK, Walsh TJ, Isoherranen N (2012) A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J Lipid Res 53 : 587–598. doi: 10.1194/jlr.D019745 22192917
23. Fan YY, Spencer TE, Wang N, Moyer MP, Chapkin RS (2003) Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis 24 : 1541–1548. 12844485
24. Egea PF, Mitschler A, Moras D (2002) Molecular recognition of agonist ligands by RXRs. Mol Endocrinol 16 : 987–997. 11981034
25. Elabdeen HR, Mustafa M, Szklenar M, Rühl R, Ali R, et al. (2013) Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS One 8: e70838. doi: 10.1371/journal.pone.0070838 23951021
26. Szklenar M, Kalkowski J, Stangl V, Lorenz M, Rühl R (2013) Eicosanoids and docosanoids in plasma and aorta of healthy and atherosclerotic rabbits. J Vasc Res 50 : 372–382. doi: 10.1159/000350865 23969947
27. Kitareewan S, Burka LT, Tomer KB, Parker CE, Deterding LJ, et al. (1996) Phytol metabolites are circulating dietary factors that activate the nuclear receptor RXR. Mol Biol Cell 7 : 1153–1166. 8856661
28. Lemotte PK, Keidel S, Apfel CM (1996) Phytanic acid is a retinoid X receptor ligand. Eur J Biochem 236 : 328–333. 8617282
29. Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, et al. (1999) Cellular retinol-binding protein I is essential for vitamin A homeostasis. Embo J 18 : 4903–4914. 10487743
30. Duell EA, Kang S, Voorhees JJ (1996) Retinoic acid isomers applied to human skin in vivo each induce a 4-hydroxylase that inactivates only trans retinoic acid. J Invest Dermatol 106 : 316–320. 8601734
31. Moise AR, Kuksa V, Imanishi Y, Palczewski K (2004) Identification of all-trans-retinol:all-trans-13,14-dihydroretinol saturase. J Biol Chem 279 : 50230–50242. 15358783
32. Shirley MA, Bennani YL, Boehm MF, Breau AP, Pathirana C, et al. (1996) Oxidative and reductive metabolism of 9-cis-retinoic acid in the rat. Identification of 13,14-dihydro-9-cis-retinoic acid and its taurine conjugate. Drug Metab Dispos 24 : 293–302. 8820419
33. Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445 : 168–176. 17151600
34. Chen ZP, Shemshedini L, Durand B, Noy N, Chambon P, et al. (1994) Pure and functionally homogeneous recombinant retinoid X receptor. J Biol Chem 269 : 25770–25776. 7929281
35. Egea PF, Mitschler A, Rochel N, Ruff M, Chambon P, et al. (2000) Crystal structure of the human RXRalpha ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. Embo J 19 : 2592–2601. 10835357
36. Pogenberg V, Guichou JF, Vivat-Hannah V, Kammerer S, Perez E, et al. (2005) Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J Biol Chem 280 : 1625–1633. 15528208
37. Krzyzosiak A, Szyszka-Niagolov M, Wietrzych M, Gobaille S, Muramatsu S, et al. (2010) Retinoid x receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron 66 : 908–920. doi: 10.1016/j.neuron.2010.05.004 20620876
38. Kane MA, Bright FV, Napoli JL (2011) Binding affinities of CRBPI and CRBPII for 9-cis-retinoids. Biochim Biophys Acta 1810 : 514–518. doi: 10.1016/j.bbagen.2011.02.009 21382444
39. Prosser DE, Jones G (2004) Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29 : 664–673. 15544953
40. Rühl R, Bub A, Watzl B (2008) Modulation of plasma all-trans retinoic acid concentrations by the consumption of carotenoid-rich vegetables. Nutrition 24 : 1224–1226. doi: 10.1016/j.nut.2008.05.022 18653315
41. Aydemir G, Kasiri Y, Birta E, Beke G, Garcia AL, et al. (2013) Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene's anti-cancer potential. Mol Nutr Food Res 57 : 739–747. doi: 10.1002/mnfr.201200548 23378045
42. Moise AR, Isken A, Dominguez M, de Lera AR, von Lintig J, et al. (2007) Specificity of zebrafish retinol saturase: formation of all-trans-13,14-dihydroretinol and all-trans-7,8 - dihydroretinol. Biochemistry 46 : 1811–1820. 17253779
43. Foster JM, Pennock JF, Marshall I, Rees HH (1993) Biosynthesis of isoprenoid compounds in Schistosoma mansoni. Mol Biochem Parasitol 61 : 275–284. 8264731
44. Gouranton E, Aydemir G, Reynaud E, Marcotorchino J, Malezet C, et al. (2011) Apo-10'-lycopenoic acid impacts adipose tissue biology via the retinoic acid receptors. Biochim Biophys Acta 1811 : 1105–1114. doi: 10.1016/j.bbalip.2011.09.002 21963687
45. Sicilia T, Bub A, Rechkemmer G, Kraemer K, Hoppe PP, et al. (2005) Novel lycopene metabolites are detectable in plasma of preruminant calves after lycopene supplementation. J Nutr 135 : 2616–2621. 16251620
46. de Groot A, de Rosny E, Juillan-Binard C, Ferrer JL, Laudet V, et al. (2005) Crystal structure of a novel tetrameric complex of agonist-bound ligand-binding domain of Biomphalaria glabrata retinoid X receptor. J Mol Biol 354 : 841–853. 16274693
47. Tocchini-Valentini GD, Rochel N, Escriva H, Germain P, Peluso-Iltis C, et al. (2009) Structural and functional insights into the ligand-binding domain of a nonduplicated retinoid X nuclear receptor from the invertebrate chordate amphioxus. J Biol Chem 284 : 1938–1948. doi: 10.1074/jbc.M805692200 18986992
48. Iwema T, Billas IM, Beck Y, Bonneton F, Nierengarten H, et al. (2007) Structural and functional characterization of a novel type of ligand-independent RXR-USP receptor. Embo J 26 : 3770–3782. 17673910
49. Nowickyj SM, Chithalen JV, Cameron D, Tyshenko MG, Petkovich M, et al. (2008) Locust retinoid X receptors: 9-Cis-retinoic acid in embryos from a primitive insect. Proc Natl Acad Sci U S A 105 : 9540–9545. doi: 10.1073/pnas.0712132105 18606996
50. Palli SR, Kapitskaya MZ, Potter DW (2005) The influence of heterodimer partner ultraspiracle/retinoid X receptor on the function of ecdysone receptor. FEBS J 272 : 5979–5990. 16302963
51. Krezel W, Dupe V, Mark M, Dierich A, Kastner P, et al. (1996) RXR gamma null mice are apparently normal and compound RXR alpha +/-/RXR beta-/-/RXR gamma-/ - mutant mice are viable. Proc Natl Acad Sci U S A 93 : 9010–9014. 8799145
52. Wietrzych M, Meziane H, Sutter A, Ghyselinck N, Chapman PF, et al. (2005) Working memory deficits in retinoid X receptor gamma-deficient mice. Learn Mem 12 : 318–326. 15897255
53. Nagy L, Kao HY, Love JD, Li C, Banayo E, et al. (1999) Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev 13 : 3209–3216. 10617570
54. Lippert WP, Burschka C, Gotz K, Kaupp M, Ivanova D, et al. (2009) Silicon analogues of the RXR-selective retinoid agonist SR11237 (BMS649): chemistry and biology. ChemMedChem 4 : 1143–1152. doi: 10.1002/cmdc.200900090 19496083
55. Osz J, Brelivet Y, Peluso-Iltis C, Cura V, Eiler S, et al. (2012) Structural basis for a molecular allosteric control mechanism of cofactor binding to nuclear receptors. Proc Natl Acad Sci U S A 109: E588–594. doi: 10.1073/pnas.1118192109 22355136
56. Pazos Y, Iglesias B, de Lera AR (2001) The Suzuki coupling reaction in the stereocontrolled synthesis of 9-cis-retinoic acid and its ring-demethylated analogues. J Org Chem 66 : 8483–8489. 11735529
57. Brunger AT (1992) Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355 : 472–475. 18481394
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



