-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
Current knowledge does not provide a clear, definite view of central mechanisms controlling energy balance upon cold-activated thermogenesis. Here we show that upon cold exposure lean mice maintain body composition but increase food intake to fuel thermogenesis, whereas cold-exposed mice with DIO utilize endogenous fat stores and then transition to increased food intake as body composition approaches that of the lean controls. Using knockout mice with leptin and Ucp1 gene deficiency our study indicates that the relative energy utilization from food intake and endogenous energy reserves to maintain body temperature during cold exposure is independent of both leptin action and brown fat-linked thermogenesis. Using a combination of genetic and biological approaches, we demonstrate that Npvf gene expression in the hypothalamus is regulated by changes in ambient temperature in a manner independent of the nutritional status of the mouse.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005287
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005287Summary
Current knowledge does not provide a clear, definite view of central mechanisms controlling energy balance upon cold-activated thermogenesis. Here we show that upon cold exposure lean mice maintain body composition but increase food intake to fuel thermogenesis, whereas cold-exposed mice with DIO utilize endogenous fat stores and then transition to increased food intake as body composition approaches that of the lean controls. Using knockout mice with leptin and Ucp1 gene deficiency our study indicates that the relative energy utilization from food intake and endogenous energy reserves to maintain body temperature during cold exposure is independent of both leptin action and brown fat-linked thermogenesis. Using a combination of genetic and biological approaches, we demonstrate that Npvf gene expression in the hypothalamus is regulated by changes in ambient temperature in a manner independent of the nutritional status of the mouse.
Introduction
Reduced ambient temperature will increase thermogenesis and reduce obesity. However its long-term effectiveness as a strategy to reduce obesity has been questioned because of the expectation that increased energy expenditure for the cold environment will increase food intake, thereby neutralizing the weight reducing effects of the cool environment [1], a skepticism also associated with the effectiveness of physical activity as an anti-obesity strategy [2]. This skepticism emerges from the adipostat hypothesis itself, which predicts that reductions in fat mass by cold stimulation will be compensated by increased food intake to maintain its adiposity index [3]. On the other hand, studies on loss of fat mass by increasing thermogenesis with the chemical uncoupler dinitrophenol (DNP) showed that increased food intake does not necessarily occur [4]. Therefore compensation as predicted by the adipostat model may also not occur in association with BAT thermogenesis. Since chemical uncoupling by DNP, or even activation of thermogenesis by adrenergic receptor agonists [5], are unregulated inductions of thermogenesis, compared to normal physiological mechanisms regulating body temperature, the problem of predicting the effectiveness of achieving energy homeostasis from food intake and endogenous energy reserves during cold exposure remains. Specifically, when an individual is exposed to a cold environment how the physiological decision is made to use endogenous energy reserves or to increase food intake and how this decision is influenced by the obese state of the individual is unknown.
Although significant recent research progress has enhanced our understanding of the central control of BAT thermogenesis and energy expenditure in cold-exposed mammals, some areas are yet not well understood. In cold-exposed animals increased thermogenesis is associated with increased feeding, but is not accompanied by a gain of weight [6]. Coordinated increases in thermogenesis and food intake during cold exposure are controlled by signaling events in hypothalamus that are undefined. Within the hypothalamus, only a few genes are known to be differentially regulated in response to reduced ambient temperature [7–12], but one cannot identify a clear pattern of neuropeptide expression characteristic for the hypothalamic response to the cold. The contribution of the selective neuro-hormone systems such as NPY or TRH in the regulation of cold-activated thermogenesis and feeding behavior has been extensively studied using pharmacologic approaches [13,14] or animal knockout models [15–17]. However, neither of these approaches identifies a critical molecule or describes signaling events that account for central mechanisms controlling energy availability and utilization under cold conditions.
In this study, using wild-type B6 and brown fat deficient Ucp1-/- mice with DIO and genetically obese (Lep-/-) mice, we first determined that cold-induced thermogenesis is preferentially fueled by oxidation of fat reserves in individuals with environmental obesity and by food intake in lean individuals. We then analyzed global gene expression in the hypothalamus of cold-exposed mice and found that suppression of Npvf neuropeptide precursor mRNA levels occurred in the three models of obesity. To our knowledge Npvf is the only transcriptional target in hypothalamus known to be selectively regulated by changes in ambient temperature.
Results
Experiment 1: Energy expenditure during cold exposure of mice with DIO
A wide range of body weight in genetically identical B6 mice results from their high natural variation in susceptibility to DIO [18]. We utilized this variation together with feeding mice a HFD for different lengths of time to generate mice with a range of adiposity. After 8 weeks or 1 week of feeding a HFD a cohort of mice was produced in which body weight ranged between 32.4 and 43.8g (greater obese mice) and between 24.4 and 32.7g (lesser obese mice) (Fig 1A). Reducing the ambient temperature from 24 to 4°C resulted in an immediate lowering in body weight that was highest on day one and gradually diminished during the succeeding days (Figs 1B and S1A). Although lesser and greater (range of body weight) obese mice showed the same response, the weight loss was larger in the greater obese group than the lesser obese group (Figs 1B, 1C, S1A and S1B). Fat mass was the major endogenous substrate fueling thermogenesis (Figs 1D and S1B). In the greater obese group, after 4 days at 4°C 97.5 kJ of energy came from fat mass and 33.8 kJ from fat free mass. For the lesser obese group, 30 kJ came from fat mass and 22.9 kJ from fat-free mass. Thus, 4 days of cold exposure resulted in total use of endogenous energy that equaled 131.3 kJ for the greater obese mice and only 52.9 kJ for the lesser obese mice (Fig 1D and 1E). After one day at 4°C both groups of mice experienced a slight decline in body temperature (1–2°C), however, by the 2nd day at 4°C all mice were able to thermoregulate and maintain their body temperature at the level at which they started (36 ± 1°C).
Fig. 1. Changes in endogenous substrate utilization and food intake associated with cold-induced thermogenesis in mice with variable levels of DIO. 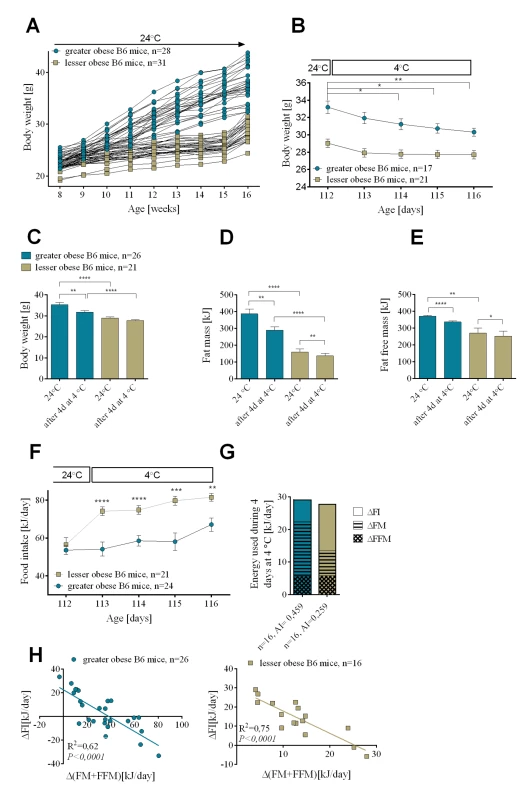
Diet-induced increase in body weight (A). Reduction in body weight during cold exposure (B). Changes in body weight (C), fat mass (D), and fat free mass (E) before and after 4 days at 4°C. Daily changes in food intake during 4 consecutive days at 4°C (F). Comparison of energy utilization from endogenous reserves and food intake in greater obese and lesser obese B6 mice (G). Correlations between daily increase in food consumed and internal body reserves mobilized per day during 4 days in the cold (H). Data are expressed as mean ± SEM. *, significant differences (t test, *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001). FI, food intake; FM, fat mass; FFM, fat free mass. If the lesser obese group utilized less of their endogenous energy reserves during cold exposure than the greater obese, then where did the energy for thermogenesis come from? For this we measured food intake. After 16 weeks on the dietary regime at 24°C, as described in the Methods, food intake was 56.5 ± 3.64 kJ/day for the lesser obese and 53.6 ± 2.29 kJ/day for the greater obese (Fig 1F). When mice were transferred to 4°C, food intake immediately increased in the lesser obese mice to 75 kJ/day (35% increase) and to 84 kJ/day (50% increase) after 1 and 4 days, respectively; the increase in food intake was smaller in the greater obese mice going to 55 kJ/day (2% increase) and 67 kJ/day (25% increase), respectively, after 1 and 4 days in the cold. With increasing time at 4°C the difference in food intake between the lesser and greater obese groups was reduced (S1C Fig), consistent with the diminishing difference in fat mass. After the first day of cold exposure the difference in food consumption between mice from 2 cohorts equaled 20.06 ± 4.53 kJ, after 4 days at 4°C it was 14.44 ± 4.23 kJ (Fig 1F) and only 9.53 ± 2.87 kJ after 7 days at 4°C (S1C Fig). Cold-induced thermogenesis is associated with increased consumption of fuel reserves and, as evident in Figs 1F and S1C, mice with lower endogenous fuel reserves compensate by increasing food intake, a process that apparently increases with time as endogenous fuel reserves become depleted. After 4 days at 4°C, regardless of the level of obesity present in the animals before cold exposure, cumulative energy coming from feeding and mobilized endogenous energy stores was comparable in the greater and lesser obese mice (Fig 1G).
For both lesser and greater obese animals linear regression analysis revealed a strong negative correlation between energy reserves (fat and fat free mass) mobilized per day and daily energy consumed during time spent in the cold (R2 = 0.62 for greater obese mice and R2 = 0.75 for lesser obese after 4 days in the cold) (Fig 1H). An equally strong negative relationship was observed when values of adiposity index calculated for each mouse before cold exposure were plotted against daily food intake during 4 days at 4°C (R2 = 0.74 for both greater and lesser obese mice) (S1D Fig).
An important observation is that mice with robust DIO after 8 weeks on a high fat diet at 24°C will concurrently increase food intake and reduce body weight when transferred to an ambient temperature of 6°C (S2A and S2B Fig). They will stabilize both body weight and food intake to a new state of energy balance to maintain body temperature. When they are returned to 24°C food intake returns to the level observed before the cold exposure and they resume the increase in adiposity characteristic of B6 mice.
Leptin status and the cold challenge
At 24°C there were no differences in the level of plasma free fatty acids (FFAs) and insulin between the groups (Fig 2A and 2B). After 4 days at 4°C, greater obese mice had significantly elevated levels of circulating FFAs in comparison to lesser obese, consistent with increased fat mobilization in the greater obese animals. Substantial fat mass loss after 7 days of cold exposure resulted in reduced plasma FFAs in both groups of mice. Similarly, after 7 days at 4°C, circulating insulin was decreased in greater and lesser obese mice compared to 24°C (Fig 2B). At 24°C leptin levels were positively correlated with adiposity (Fig 2C). Leptin levels did not drop during the first 4 days at 4°C, only after 7 days in the cold did highly significant reductions in leptin levels occur (Fig 2D).
Fig. 2. Insulin and leptin resistance in wild-type B6 DIO mice. 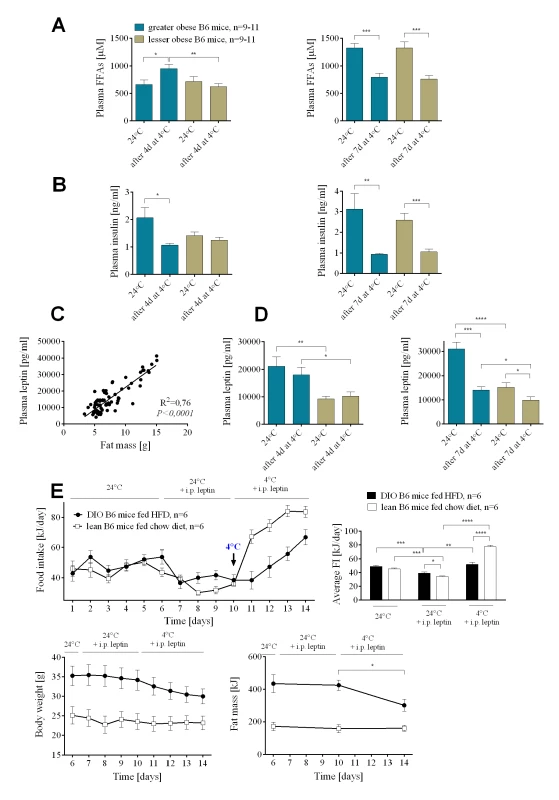
Changes in plasma free fatty acids (A) and insulin (B) before and after 4 and 7 days at 4°C in greater and lesser obese B6 mice. Correlation between fat mass and plasma leptin levels in DIO mice (C). Changes in plasma leptin (D) before and after 4 and 7 days at 4°C in DIO mice. Changes in food intake and body weight and composition (E) in mice administered with leptin at 24 and 4°C. Data are expressed as mean ± SEM. *, significant differences (t test, *, P < 0.05; **, P < 0.01; ***, P < 0.005). We evaluated the effects of leptin administration to DIO B6 mice fed HFD or lean B6 mice fed chow diet on the food intake and utilization of endogenous energy substrates before and after cold challenge. Leptin administration at 24°C decreased average daily food intake from 49.12±1.68 to 39.15±1.69 kJ in DIO mice and from 45.40±1.55 to 34.30±1.21 kJ, in chow fed lean mice (Fig 2E). There was no effect of leptin administration on either body weight or body composition of mice at 24°C (Fig 2E). When the ambient temperature was reduced from 24 to 4°C lean mice receiving leptin immediately increased food intake, whereas their body weight and fat mass did not change. On the other hand, cold-exposed and leptin-administered DIO mice immediately utilized endogenous reserves, then as these reserves diminished, they increased food intake (Fig 2E). These results on food intake and fat utilization with leptin administration are not different from the phenotypes in the absence of exogenous leptin (Fig 1B and 1F).
Experiment 2 Part (a): The effects of leptin deficiency on cold-induced energy expenditure
Mice deficient in either leptin or the leptin receptor are cold intolerant when acutely exposed to 4°C; however, they are able to adapt to a lower temperature if the exposure is gradual [19–21], thereby enabling an analysis of energy utilization during a cold challenge. Although there were large differences in body mass and composition between Lep+/? and Lep-/- mice fed a low fat chow diet at 24°C, after 9 days in the cold neither genotype showed significant changes in body weight mass nor composition (Fig 3A–3C).
Fig. 3. The effects of leptin deficiency on the relative utilization of food intake and endogenous energy stores during cold-induced energy expenditure. 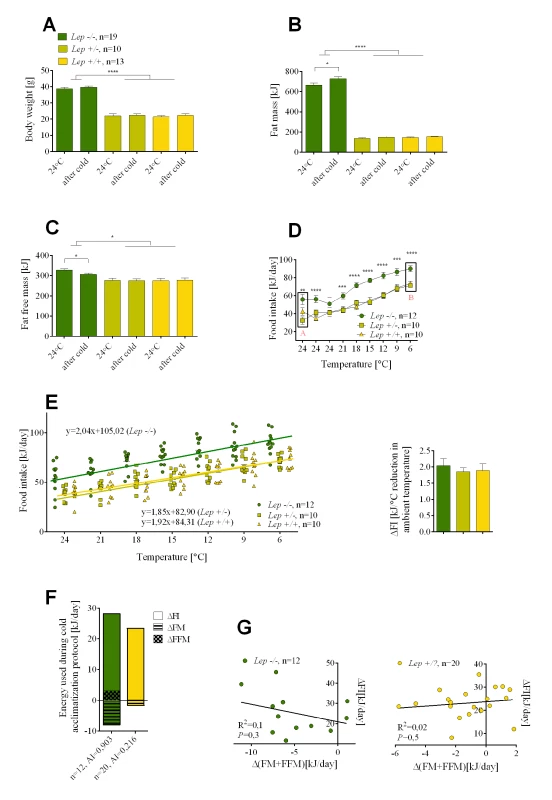
Changes in body weight (A), fat mass (B), fat free mass (C), and food intake (D) determined before and after cold adaptation protocol in Lep-/-, Lep+/- and wild-type Lep+/+ mice. The rate of an increase in food intake per degree Celsius reduction in ambient temperature in mutant Lep-/- and control mice (E). Comparison of energy utilization from endogenous reserves and food intake in mutant Lep-/- and Lep+/? controls (F). Correlations between daily increase in food consumed and internal body reserves mobilized per day in the cold (G). Data are expressed as mean ± SEM. *, significant differences between mice (t test, *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001). FI, food intake; FM, fat mass; FFM, fat free mass. With no reduction in endogenous energy reserves we looked to an increase in food intake. At 24°C average daily food intake was about 40% higher in leptin-deficient than in the Lep+/? control mice, as previously observed by Coleman [20] (Fig 3D). One would anticipate that this source of energy would be used to fuel thermogenesis, however, reducing the ambient temperature by 3°C per day resulted in an increase in food intake in both control and Lep-/- mice. This food intake curve is displaced upward by an amount corresponding to the difference in food intake between control Lep+/? and Lep-/- mice at 24°C (Fig 3D). Therefore, the rate of increase in food intake per degree Celsius reduction in ambient temperature by the control mice and Lep-/- mice was essentially indistinguishable (Fig 3E). The most striking observation was that Lep-/- mice, already hyperphagic at 24°C, further increased their food intake under a cold challenge. After correcting for the slight changes in body composition that occurred in mice upon cold exposure, the total energy used for cold-induced thermogenesis was equal in leptin-deficient and control mice (Fig 3F). Accordingly, there were no significant correlations either for control lean Lep+/? or for obese Lep-/- mice between the daily increase in food intake and endogenous body fuel reserves mobilized per day in the cold (Fig 3G), in contrast to the significant correlations in DIO mice (Fig 1H).
Experiment 2 Part (b): The effects of UCP1 deficiency on cold-induced energy expenditure
It is assumed that non-shivering thermogenesis of brown fat is essential for providing the heat to protect the animal from the cold. Indeed Ucp1-/- newborn mice on either the B6 and 129 genetic backgrounds cannot survive the first days of birth in a breeding room maintained at ~23°C and Ucp1-/- adult mice acutely exposed to the cold at 4°C will succumb within 5 hours [22,23]. However, similar to Lep-/- mice, Ucp1-/- mice can adapt to the cold [24]. Ucp1-/- and Ucp1+/? mice were exposed to the cold using the same protocol as that used for Lep-/- mice, except that DIO was first induced at 24°C as with the greater and lesser obese mice (Fig 1A). The level of obesity for the Ucp1+/? resembled that of the greater obese B6.+/+ mice, whereas the Ucp1-/- mice resembled the lesser obese mice (Fig 4A–4C), even though they were fed the HFD for the full 8 weeks. This is expected, since at 24°C Ucp1-/- mice are resistant to DIO [23]. At 24°C food intake was similar for mutant and control mice, whereas the daily energy intake during cold adaptation was higher for Ucp1-/- mice (Fig 4D). Similar to the results of the initial experiment with wild type B6 mice, Ucp1+/? mice which had the greater obese phenotype preferentially lost fat mass during cold adaptation, whereas the Ucp1-/- mice which had the lesser obese phenotype preferentially increased food intake (Fig 4E). Thus, energy balance and substrate utilization in DIO Ucp1-/- mice during cold exposure resembles that of lesser obese wild-type mice.
Fig. 4. Changes in endogenous substrate utilization and food intake in cold-exposed Ucp1-/- and Ucp1+/? mice with DIO. 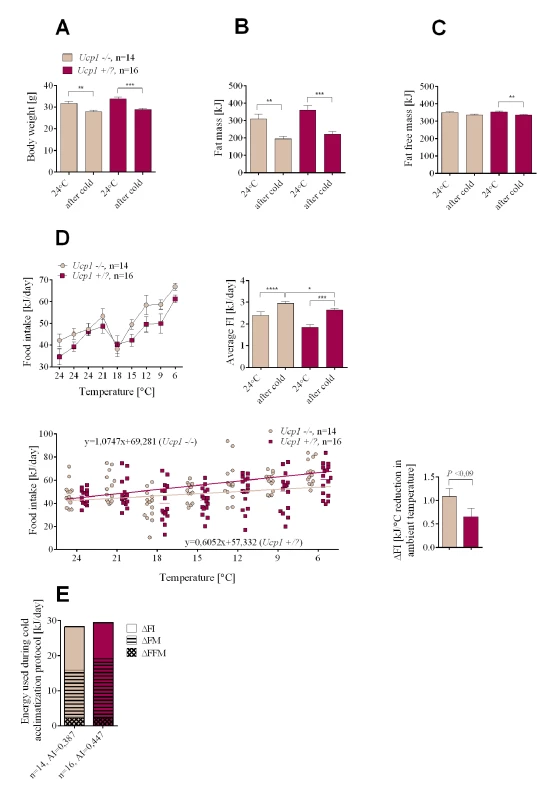
Changes in body weight (A), fat mass (B), fat free mass (C), and food intake (D) measured at normal ambient temperature (24°C) and during the cold adaptation protocol. Comparison of energy utilization from endogenous reserves and food intake in Ucp1-/- and Ucp1+/? controls (E). Data are expressed as mean ± SEM. *, significant differences between mice (t test, *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001). FI, food intake; FM, fat mass; FFM, fat free mass. In summary, UCP1-dependent brown fat thermogenesis is not required to derive the weight reducing benefits of adapting to the cold and there is no mechanism associated with thermogenesis that will increase food intake of the greater obese to preserve the obese state. There is a mechanism, however, to preserve a minimal adiposity index typified by young adult C57BL/6J mice fed a low fat chow diet. Total energy consumption as shown by 6 experimental groups (Figs 1G, 3F and 4E) indicates that energy expenditure during cold exposure is generally similar, except that Ucp1-/- mice are metabolically inefficient and have higher O2 consumption per mouse [25]. The difference among groups describes source of energy for the induction of thermogenesis, endogenous reserves vs food intake, and it is this difference which is the focus of this study.
A molecular pathway associated with cold activated thermogenesis
At 24°C Lep-/- mice are hyperphagic compared to the Lep+/+ or Lep+/- mice (Fig 3D). Reducing the ambient temperature from 24 to 6°C was accompanied by a graded parallel increase in food intake, corresponding to approximately 50 kJ of energy for both control and mutant mice (Fig 3D). Consequently, the same leptin-independent increase in food intake was observed during the transition from 24 to 6°C in both Lep+/+ and Lep-/-. Since the energy content of Lep+/+ and Lep-/- mice was unchanged during cold exposure, thermogenesis is fueled solely by food intake. Accordingly, we predicted that the same changes in gene expression associated with the central regulation of thermogenesis by the hypothalamus must occur in both Lep+/+ and Lep-/- mice during the transition from 24 to 6°C. Microarray analysis of gene expression was performed on hypothalamic tissue dissected from Lep-/- and Lep+/+ mice kept at different temperature conditions, that is, in mice maintained at 24°C (point A, Fig 3D) and in mice in which the ambient temperature had been reduced to 6°C (point B, Fig 3D). We identified a small subset of genes in Lep-/- in common with Lep+/+ mice during the transition from 24 to 6°C (Fig 5A). Among these genes, neuropeptide VF precursor (Npvf), showed a robust down-regulated expression of 4.0 and 3.5 fold in the hypothalamus of cold-exposed Lep-/- and Lep+/+, respectively. A group of genes encoding for G protein-coupled receptors (GPCRs) including the dopamine receptor D1 (Drd1a), adenosine receptor 2A (Adora2a), GABA(A) receptor subunit delta (Gabdr) and Gpr88 as well as some of their downstream targets including cAMP-regulated phosphprotein 21 (Arpp21) and protein phosphatase 1 regulatory subunit 1B (Ppp1r1b) were up-regulated 1.4 to 3 fold in both Lep+/+ and Lep-/- mice following cold exposure. Cold exposure also increased the expression of antidiuretic hormone arginine vasopressin (Avp) gene in both mutant and wild-type animals by 1.8 and 1.4 fold, respectively. Each of the genes expressed in parallel in Lep+/+ and Lep-/- mice were validated by qRT-PCR (Fig 5B).
Fig. 5. Npvf gene is a hypothalamic biomarker of cold-activated thermogenesis. 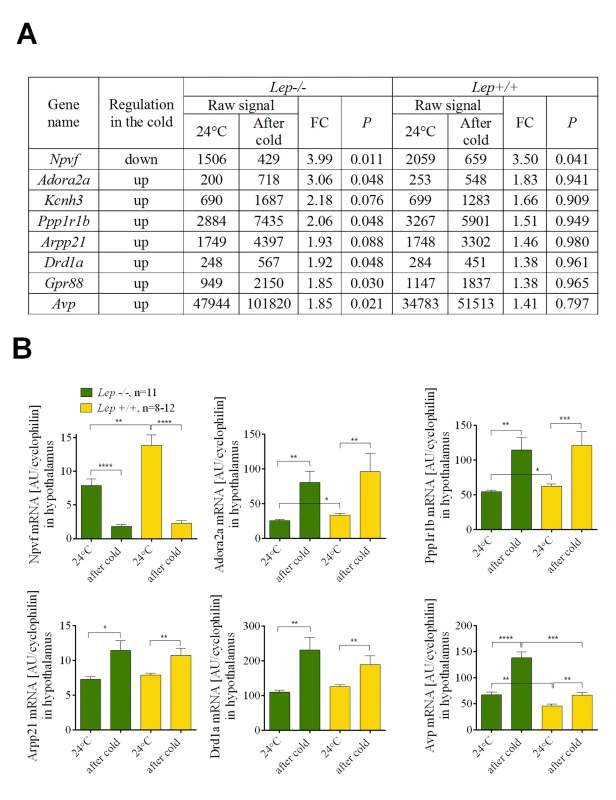
List of the genes that were similarly regulated upon cold exposure in Lep-/- and wild-type Lep+/+ mice (A). Verification of microarray data using qRT-PCR in Lep-/- and wild-type Lep+/+ mice (B). Data are expressed as mean ± SEM. *, significant differences between mice (t test, *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001). Fold change (FC) was calculated based on normalized signal values. Experiment 3: Regulation of Npvf expression in mouse hypothalamus under variable thermogenic conditions
To further investigate a potential role for Npvf in food intake as a function of cold, we determined its expression in the hypothalamus of mice with different levels of dietary-induced obesity following cold exposure (Figs 1A and S1A). Similar to the experiment with Lep+/+ and Lep-/- mice, Npvf expression was suppressed in both greater and lesser obese mice after the temperature shift from 24 to 4°C, but its expression was not associated with either adiposity or food intake (Fig 6A). Increasing the duration of cold exposure at 4°C from 1 to 7 days gradually amplifies the reduction in Npvf mRNA levels. In an independent experiment DIO mice that were maintained at 4°C for 14 days and then returned to 24°C for 25 days restored their levels of Npvf mRNA to that initially observed at 24°C (Fig 6A). Npvf mRNA expression in hypothalamic tissue showed a positive correlation with ambient temperature. Mice kept for 14 days at thermoneutrality (29°C) had higher expression of Npvf mRNA in hypothalamus than mice maintained at 24°C. Similarly, 2 weeks at 17°C resulted in a reduction of mRNA expression to levels below that observed at 24°C (Fig 6B).
Fig. 6. The expression of Npvf in the hypothalamus responds to reduced ambient temperature and time of exposure to the cold, but is not associated with the level of non-shivering thermogenesis in iBAT. 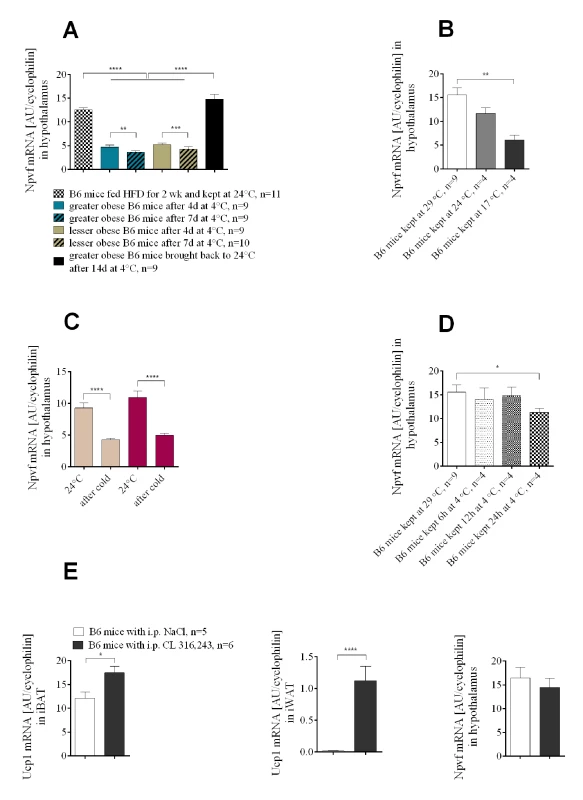
Cold-induced changes in Npvf mRNA expression in greater obese and lesser obese B6 mice (A). Ambient temperature-dependent expression of Npvf mRNA in hypothalamus of chow-fed wild-type B6 mice (B). Cold-induced changes in Npvf mRNA expression in mutant Ucp1-/- and normal control Ucp1+/? mice (C). Time-course of changes in the level of Npvf mRNA in hypothalamus under low temperature conditions (D). Changes in the expression of Ucp1 mRNA in brown and white adipose tissue and Npvf mRNA in hypothalamus (E) in response to 7 days of either CL 316,243 or saline administration at 29°C in B6 mice. Data are expressed as mean ± SEM. *, significant differences between mice (t test, *, P < 0.05; **, P < 0.01; ****, P < 0.001). Although modulation of Npvf precursor mRNA occurs during cold-stimulated thermogenesis, an involvement of Npvf in the regulation of non-shivering thermogenesis in brown fat is unlikely. Down-regulation of Npvf mRNA was not influenced by the absence of UCP1 protein (Fig 6C). Acute exposure to the cold requires an immediate response for heat generation and leads to immediate UCP1 production in BAT and WAT. A separate experiment performed to illustrate time-course of changes in the expression of Npvf under low temperature conditions showed that significant suppression in the amount of Npvf mRNA does not occur before 12h at 4°C; a significant decrease in the accumulation of Npvf mRNA in hypothalamus is found after 24h at 4°C compared to 29°C (Fig 6D). Moreover, one week administration of β3-adrenergic agonist CL 316,243 (1mg/kg of body weight) did not result in the suppression of Npvf mRNA in hypothalamus compared to saline-treated control mice (Fig 6E), providing evidence that changes in expression of the Npvf gene are not linked to heat production or brown adipocyte induction in peripheral β3-AR-expressing tissue targets.
Expression of CNS and peripheral genes associated with energy metabolism
Genes associated with food intake in the hypothalamus, thermogenesis in the adipose tissue, and lipid metabolism in the liver and adipose tissues were analyzed by qRT-PCR. No patterns in gene expression could illuminate mechanisms associated with the phenotypes described above (see Supplement; S3A and S3B Fig for thermogenic genes in iBAT and iWAT, S4A and S4B Fig for neuropepetides of feeding behavior, and S5A Fig for genes of fatty acid metabolism in the liver, S5B Fig in iBAT and S5C Fig in iWAT).
Discussion
We show that the total energy expended by a mouse from food intake and endogenous energy reserves to sustain thermogenesis during cold exposure is independent of the degree of obesity in the animals. This is true in genetically obese Lep-/- mice, chow-fed wild-type mice (Fig 3F) and in wild-type mice and B6.Ucp1-/- with variable levels of DIO (Figs 1G and 4E, respectively). However, in chow-fed mice the energy that is necessary to sustain a thermogenic program to maintain body temperature in the cold comes exclusively from feeding, as observed by others [20,26]; whereas in a wild-type mouse with diet-induced obesity induced by a high-fat diet, the fuel to support thermogenesis is obtained from endogenous energy reserves (mostly fat) and food intake. In DIO mice the source of energy required to maintain body temperature during cold exposure is determined by the degree of obesity. In DIO mice the energy reserves in fat mass are not privileged or restricted as those in a normal wild-type mouse maintained on a low-fat chow diet, rather they are utilized in proportion to their absolute levels. DIO mice with the highest levels of stored fat immediately mobilize fat, subsequently as these reserves become depleted, food intake becomes progressively a larger contributor to the fuel mix. In contrast, those mice that are at the other end of the DIO spectrum, the lesser obese mice, will preferentially increase food intake and use less of their endogenous fuel reserves to support thermogenesis. An important finding is that wild type mice with high levels of adiposity behave in response to cold exposure by the utilization of available energy sources in a manner that is independent of hormonal status, i.e. leptin and insulin. Serum leptin levels measured before cold exposure indicated that leptin resistance should have been higher in the greater obese mice than in the lesser obese, predicting a defense of adipose stores and higher food intake in greater obese mice. However, from the very beginning of cold exposure a defense of the fat status in mice with leptin levels predictive of leptin resistance was not observed. In fact the opposite was observed, with food intake reduced and fat utilization increased in the greater obese mice compared to the lesser obese.
Interestingly, our observations on body composition-dependent differential fuel selection occurring during cold exposure in DIO mice parallels findings in exercising human subjects (32). Moderate to intense physical activity performed regularly and on a long-term basis by lean individuals is compensated for by a corresponding change in food intake while body mass is maintained. On the other hand, obese individuals with excess fat storage do not significantly increase food intake and loss of body fat occurs as a consequence [27]. A return of mice fed a HFD from 6 to 24°C leads to a decrease in food intake and increase in adiposity characteristic of their phenotype on a high fat diet (S2A and S2B Fig). We previously observed the same response of mice fed a high fat diet when energy balance was interrupted with food restriction [18]. Accordingly, mice do not assume increased levels of food intake transiently acquired when they are in the cold, rather food intake is set by the requirements for heat production as originally hypothesized by Brobeck [28]. The long-term defense of body weight in humans and mice has been described and discussed as a consequence of under - and over-feeding [29]; however, mechanisms associated with a negative energy balance resulting from reduced energy intake during dieting may be different from increased energy expenditure in response to cold-induced energy expenditure, since the latter condition is supported by increased food intake and the neutralization of insulin and leptin resistance [2,30].
Wild-type B6 (Lep+/ - or Lep+/+) mice fed a low fat chow diet exhibited almost no change in endogenous energy reserves, that is, lean mass or fat mass when the ambient temperature was reduced from 24 to 6°C, but they increased food intake. This observation fits with the thermostatic theory proposed by John Brobeck in the late 1940s, which relates the regulation of body temperature to the control of feeding behavior [28]. Brobeck summed up his theory by saying: “…animals eat to keep warm and stop eating to prevent hyperthermia”. In the present study, the wild-type B6 mouse maintained energy balance and body composition on a normal diet, when exposed to the cold, by increasing calorie intake. Importantly, the Lep-/- mouse behaved in the same manner, it adapted to the cold by increasing food intake in a manner quantitatively indistinguishable from the normal B6 mouse and it preserved its endogenous energy reserves. The β-oxidation of fat stores of Lep-/- mice is not an option for fuel to maintain body temperature [31] and this is a major factor in cold intolerance of leptin-deficient mice during acute exposure [32]. Lep-/- mice sensed that existing fat stores were unavailable and compensated by increasing food intake in a leptin-independent manner. This feeding behavior in the cold underscores the inability of mice with leptin-deficiency to utilize endogenous fat reserves; furthermore it also shows that in the face of a cold challenge fuel for thermogenesis must come from food intake. On the other hand, the wild-type mouse on a low fat chow diet can access its energy reserves in an acute situation, but quickly turns to increased food intake to maintain energy balance. This similarity in the metabolic response to the cold environment between normal and leptin-deficient mouse suggests that leptin is not important for the acute thermogenic phenotype in the Lep-/- mouse, nor for the regulation of food intake during cold exposure by normal wild-type mice fed a chow diet.
Mice with mutations to leptin and the leptin receptor have a thermogenic phenotype in which body temperature drops about 10°C in about 4 hours at an ambient temperature of 4°C [21]; however, as illustrated in Fig 3D they can adapt to the cold when it is gradually reduced. A key feature of cold-induced thermogenesis in normal animals is the increase in food intake that occurs over and above the increase in food intake necessary to support nutrition [33–36]; as exemplified by the remarkable boost in food intake that occurs in lactating females exposed to the cold [26]. This suggests that central mechanisms controlling food intake, as related to nutrition, growth and body composition, may be independent of those associated with cold-induced thermogenesis. A similar idea has been put forth by Speakman and Krol [37], but with a necessary role for leptin in the cold-induced food intake, which we did not see, nor was a role for leptin proposed by Melnyk and Himms-Hagen [6]. We had observed previously, as did Coleman [20], that Lep-/- mice exposed to the cold further increased food intake above that normally occurring in these mice [19]. This preliminary observation has been extended in this study to show that this hyperphagia, which is above that normally occurring in Lep-/- mice fed a chow diet, is very similar in magnitude and kinetics to that occurring in Lep+/+ mice. Accordingly, mechanisms controlling cold-associated food intake in Lep-/- mice are independent of leptin-based regulation of food intake. We tested further the role of leptin in regulating thermogenesis during cold exposure in wild-type DIO mice. Plasma leptin and insulin levels in DIO mice of this study are remarkably similar to mice described in previous studies that were leptin resistant [38]. If the mobilization of fuels for cold-induced thermogenesis in DIO mice is controlled by the leptin resistance at the time of cold exposure, then one would predict that food intake would be high and mobilization of endogenous fat stores would be low. However, within one day of exposure to the cold the opposite phenotype was observed in DIO mice: food intake was low and fat mobilization was high. Even 4 days after cold exposure plasma levels of leptin were not significantly different from those at 24°C; only after 7 days in the cold were the levels of leptin significantly reduced (Fig 2D). Additional leptin administered intraperitoneally to DIO and lean mice did not affect the observed pattern of energy substrate utilization in the cold (Fig 2E). This data additionally suggests that the mechanism controlling food intake during acute cold exposure is independent of leptin signaling. Chronic cold adaptation may involve leptin by another mechanism [19].
The primary motive driving this study was to explore the feasibility of using cold exposure as an anti-obesity strategy. Human studies on brown fat show dramatic inter-individual differences in brown adipocyte content and BAT activity [39,40]. Thus, it is important to assess how the cold-stimulated effect of body weight reduction is influenced when the capacity for thermogenesis in brown fat is variable. The extent to which BAT-mediated adaptive thermogenesis could account for variability in substrate utilization in the reduced ambient temperature is also not known. For these reasons we evaluated the phenotype of DIO Ucp1-/- mice lacking functional brown fat. Ucp1-/- mice are sensitive to the cold; however, they can adapt to the cold if the ambient temperature is gradually reduced [24,41].Therefore, if UCP1 is essential to the thermogenic process, then in its absence the capacity for heat production from brown fat would be severely suppressed and we could expect effects on food intake and or the utilization of endogenous fuels that would differ from the wild-type mouse. As expected, when the ambient temperature was reduced average food intake was higher in the Ucp1-/- mice than in control mice, because these mice are less obese when fed a high-fat diet and they burned less of their endogenous reserves compared to normal Ucp1+/? mice with the greater obese phenotype (Fig 4B and 4D). Thus, there does not seem to be any difference in the pattern of utilization of endogenous food reserves or food intake between UCP1-deficient and wild type mice, provided that these mice have similar adiposity phenotypes as occurs with the lesser and greater obese mice.
QRT-PCR analysis of the expression of several genes in the hypothalamus encoding neuropeptides implicated with food intake did not provide evidence for the involvement of any of the neuropeptides associated with food intake with the possible exception of CART and POMC which have expression reduced by 30% and 50% in Lep+/+ only. However, a microarray analysis of gene expression in Lep-/- and Lep+/+ mice at 24 and 6°C showed that the expression of neuropeptide VF precursor was decreased 4-fold during cold exposure, and a similar level of down-regulation for this gene was observed for all three of the genetic models we have studied. In rodent brain, the sequence of the Npvf precursor gene predicts two–RFamide peptides: RFRP-1 and RFRP-3, also named NPSF and NPVF [42,43]. There is an expanding body of evidence for a role of various–RFamide peptides in the modulation of nociception, hormone secretion, reproduction or blood pressure [44–46]. Finally, although little is known of the functional significance of this particular biological effect, various–RFamide peptides were able to illicit a transient 10–300% induction or suppression of food intake in chicks, rats or mice after i.c.v. injection [44,47–50]. Moreover, food restriction or deprivation, both stimulating hunger and food hoarding, have been shown to be positively correlated with activation of RFRP-3 cells in the DMH of Syrian hamsters [51]. Effects on thermogenesis are unknown. From a functional viewpoint, specific expression of Npvf mRNA in the rodent central nervous system is restricted to a population of neurons localized between dorsomedial hypothalamic (DMH) and ventromedial hypothalamic (VMH) nucleus [42,43,52,53], which is consistent with a putative role in feeding or thermogenic processes [54]. Our observations on lack of association between leptin status and regulation of Npvf mRNA in the cold demonstrate that Npvf system in hypothalamus is unlikely to be leptin responsive, which is in agreement with a recent study, where no evidence for leptin signaling after leptin injection or detection of leptin receptors in RFRP3 expressing neurons was found in mice hypothalamus [55]. Thus, the reduction in Npvf expression at lower temperature when a higher level of energy expenditure (EE) is required suggests that reduction of Npvf releases a brake on EE. Although increased food intake provides the fuel for the increase in EE in lesser obese mice, endogenous fat provides the fuel in greater obese mice. Since Npvf is similarly suppressed in both lesser and greater obese mice, neither endogenous substrate or food intake per se are the signals associated with Npvf expression levels. Lack of association between body energy reserves and hypothalamic Npvf expression was also shown in the recent study in which no significant difference in Npvf mRNA was detected between mice fed high-fat and low-fat diet for 20 weeks [55].
A motive for conducting our experiment was to establish in a mouse model the effects of cold on substrate utilization and long-term effects of cold exposure on food intake after a return to ambient temperature. This study clearly showed that upon cold exposure obese mice fuel their increase in energy expenditure with endogenous fat supplies, whereas lean mice increase food intake. An analysis of three mouse models of obesity suggests that reduced ambient temperature is effective in reducing diet-induced obesity without long-term compensatory increases in food intake. Whether humans will behave in a similar manner needs to be determined. The second part of our study uncovered evidence for a new hypothalamic signaling pathway, involving the Npvf gene, that is regulated in cold-activated thermogenesis. We will work towards determining whether a similar signaling pathway is present in humans.
Materials and Methods
Animals
Breeding pairs of C57BL/6J.+/+, C57BL/6J.Ucp1+/- and C57BL/6J.Lep+/- mice were obtained through the generosity of Dr. Martin Klingenspor of the Technical University of Munich, Germany. All procedures concerned with breeding, housing, maintenance and experimental treatment of the mice were approved by the Local Animal Care and Use Committee for University of Warmia and Mazury, Olsztyn. Guidelines for animal experiments followed EU Directive 2010/63/EU.
Experiment 1: Energy expenditure during cold exposure of mice with DIO
The goal of this protocol was to generate a series of mice with a range of adiposities by use of a high-fat diet to determine the effects of cold exposure on changes in food intake and endogenous energy stores. Breeding pairs of C57BL/6J+/+ mice were housed at standard temperature (24±1°C) and maintained in ventilated rooms under a standard-day photoperiod (12 : 12-h light-dark period, lights on from 0700 to 1900 h) with free access to low-fat diet (PicoLab Rodent Diet 20, LabDiet 5053, 11.9 kcal % fat) and water. At 21 days of age male progeny were weaned and housed in groups of 3–5 in plastic cages with fresh sawdust bedding. Body weight and body composition by NMR (Bruker, BioSpin, Germany) were monitored until mice were 8 weeks of age, at which time mice were individually housed and divided into two nutritional groups matched for similar mean body mass and body fat content to form the lesser and greater obese groups. By 8 weeks of age, when mice were still on a low-fat diet, their body weights ranged from 19.2–25.5g (Fig 1A). Based on the NMR analysis, the distribution in body weights was mainly caused by differences in fat mass, with only a small contribution from fat free mass The greater obese group was fed a high-fat diet (AIN-76A with 33% hydrogenated coconut oil, 58 kcal % fat) from the 8th to 16th week to establish a range of mice with a higher adiposity index. The lesser obese would continue to be fed the low-fat diet (PicoLab Rodent Diet 20, LabDiet 5053, 11.9 kcal % fat) for 7 weeks and then the high fat diet for the 16th week. Mice with body weights and fat mass ranging from 24.4 to 43.8g and 3.7 to 18.8g, respectively, were formed (Fig 1A–1D). The aim of such a dietary intervention was to establish as broad a range in adiposity as possible between two groups of mice and at the same time to induce metabolic adaptations associated with high-fat feeding in the lesser obese mice at the time of cold exposure. In addition to having mice with a variation in adiposity, food intake of the lesser and greater obese mice was measured for the 16th week, just prior to being exposed to the cold. Food intake, initially varied in the lesser obese mice when presented with a highly palatable high-fat diet for the first time; however, on the last day before cold exposure, there was no significant difference in food intake between the lesser and greater obese mice (S1E Fig).
In order to determine the relative contribution of food intake and endogenous energy reserves to fuel cold-induced thermogenesis, individually housed mice in the lesser and greater obese groups of mice were transferred to a cold room at 4°C for either 4 or 7 days. Food intake (high-fat; AIN-76A) and body weight were measured daily and body composition was analyzed by NMR at the end of the cold exposure. To calculate daily energy expenditure in the cold coming from endogenous and exogenous energy sources, fat mass and fat free mass measured after cold exposure were subtracted from fat mass and fat free mass measured before cold exposure and divided by number of days spent in the cold; average daily food intake measured at 24°C was subtracted from average daily food intake measured at 4°C. Energy values in kJ for g of fat mass or fat free mass were calculated as follows: 4.18 kJ/kcal × (9 or 4 kcal/g, respectively). Energy values in kJ for g of low-fat chow diet or high-fat diet were calculated as follows: 4.18 kJ/kcal × (3.07 or 5.44 kcal/g, respectively).
To observe the effects of cold-induced hyperphagia on DIO mice that were returned to an ambient temperature of 24°C, adult C57BL6/J+/+ mice were fed a high-fat diet (AIN-76A) from 8 to 16 weeks of age then transferred to 4°C until food intake stabilized over a course of 16 days. Mice were returned to an ambient temperature of 24°C and the suppression of food intake was monitored for an additional month.
To assess the effects of leptin treatment (1μg/g BW twice a day) on utilization of energy fuel coming from endogenous reserves or food intake, we measured daily changes in food intake, body weight and composition in 8 week-old lean B6 male mice fed chow diet and 16 week-old DIO B6 male mice fed HFD. Leptin was administered for 4 days at 24°C and for an additional 4 days at 4°C.
Experiment 2: This experiment had parts (a) and (b)
Part (a): The effects of leptin deficiency on cold-induced energy expenditure
Eight week-old wild-type C57BL/6J.Lep+/+, (n = 13), heterozygous C57BL/6J.Lep+/-, (n = 10) and homozygous null C57BL/6J.Lep-/-, (n = 19) mice of both sexes were individually housed in an environmentally controlled chamber (EHRET GmbH, Emmendingen, Germany) and fed standard rodent chow pellets (LabDiet 5053, 11.9 kcal % fat) during the whole experiment. Housing conditions (photoperiod, air changes) were the same as in experiment 1. After 3 days of habituation to the chamber environment (24°C), food intake at 24°C was recorded during 3 consecutive days using high-precision food weighting sensors (PhenoMaster System, TSE Systems GmbH, Bad Homburg, Germany), then, the temperature in the chamber was reduced by 3°C/day to 6°C at which time mice were kept at 6°C for additional 2 days. Since Lep-/- mice cannot tolerate an acute reduction in ambient temperature to 6°C, this gradual reduction in ambient temperature was implemented to adapt the mice to the cold [20,21]. Body composition was analyzed by NMR before and after the cold test.
Part (b): The effects of UCP1 deficiency on cold-induced energy expenditure
Both backcross (C57BL/6J.Ucp1-/- x C57BL/6J.Ucp1+/-) and intercross (C57BL/6J.Ucp1+/- x C57BL/6J.Ucp1+/-) matings were used to generate C57BL/6J.Ucp1-/- mice together with heterozygous and homozygous normal (wild-type) controls [23]. Male mice were fed chow diet (LabDiet 5053, 11.9 kcal % fat) until 8 weeks of age. Obesity was induced in mice by feeding them a high-fat diet (AIN-76A, 58 kcal % fat) from 8 to 16 weeks of age. At 16 weeks of age mice were transferred to the temperature-controlled chamber (EHRET GmbH). Housing conditions, the protocol for exposing the mice to the cold and phenotyping of adiposity and food intake was the same as that described for part (a).
Energy expenditure was calculated as described for experiment 1.
Experiment 3: Regulation of Npvf expression in mouse hypothalamus under variable thermogenic conditions
All mice used in the following studies were adult wild-type C57BL/6J+/+ mice. Mice were placed in individual cages with free access to food (LabDiet 5053) and water. At the end of each experiment mice were sacrificed and hypothalamus was dissected in order to measure the level of Npvf mRNA expression. To establish the influence of different ambient temperature on Npvf expression mice were kept in climate-controlled rodent incubators set to 29 and 17°C for the period of weeks prior to sacrifice. Additional experiment was performed to observe the kinetics of changes in the level of Npvf mRNA in the cold. All mice used in this study were first allowed to acclimate to 29°C for 2 weeks before cold challenge. Temperature of the housing unit was then transitioned from 29 to 6°C and mice were cold-challenged for 6, 12 or 24h. To evaluate the effects of the β3 adrenergic receptor agonist on Ucp1 and Npvf thermoneutrally acclimated mice were injected subcutaneously with 1 mg/kg BW/day CL 316,243 or saline for 7 days.
Metabolic and molecular assays
Mice were anesthetized by the solution of ketamine, xylopan and chlorpromazine (26.6 mg/ml, 1.67 mg/ml and 0.53 mg/ml, respectively, 40μl/10g body weight) and the blood was collected through heart puncture to EDTA coated tubes. After decapitation, interscapular brown adipose tissue depot (iBAT), inguinal white adipose tissue depot (iWAT) and the liver were removed, rapidly frozen in liquid nitrogen and stored at -80°C for subsequent preparation of total RNA. To isolate the whole hypothalamus, the brain was removed and placed on an ice-cooled glass plate with the cortex facing down. The hypothalamus was dissected along the following boundaries: laterally 2 mm either side of the third ventricle, 2 mm dorsally from the base of the brain and rostrocaudally from the optic chiasm to the posterior border of the mammillary bodies. The dissected hypothalami were stored at -80°C until further analysis. The blood was centrifuged for 10 min at 3,000 g, 4°C. Plasma was removed and stored at -80°C until assayed.
Plasma hormones and metabolites
Plasma insulin and leptin were measured by enzyme-linked immunosorbent assay with commercial kits (Wide range mouse insulin immunoassay kit, Biorbyt Ltd., Cambridge, UK; Mouse/rat leptin ELISA kit, Phoenix Pharmacuticals, Inc., Burlingame, CA, United States, respectively). Assessment of FFA in plasma was performed with plasma non-esterified free fatty acid detection kit (Zenbio, Inc., Research Triangle Park, NC, United States).
Quantitative real-time PCR
Total RNA was isolated from adipose tissue, liver and hypothalamus using TRI Reagent and BCP phase separation reagent (Molecular Research Center Inc. Cincinnati, OH, United States). RNA was further purified by using the RNAeasy minikit (QIAGEN, Valencia, CA, United States) and stored at -80°C in RNase-free H2O with addition of SUPERase-In (Ambion, Austin, TX, United States) for RNase protection. Quality and quantity of RNA was determined using UV spectrophotometry (Nanodrop) and agarose gel visualization of intact RNA. Quantitative RT-PCR using TaqMan probes and primers (Applied Biosystems, Foster City, CA, United States) was performed with standard curves generated using pooled RNA isolated from corresponding tissues collected from eight 8 week old C57BL/6J.+/+ mice. Probe and primer sequences used to perform the analyses are available upon request. All the gene expression data were normalized to the level of cyclophilin b.
Microarray analysis of gene expression in the hypothalamus of Lep-/- and Lep+/+ mice
Total RNA was isolated from the hypothalamus of 8 Lep-/- and 8 Lep+/+ mice maintained at 24 and 6°C, as described above. RNA with RNA Integrity number higher than 8.5 (Agilent 2100 Bioanalyzer, Agilent Technologies, Santa Clara, CA) was used for microarray analysis of each individual mouse. RNA was amplified, labeled and hybridized onto chips containing over 56,000 probes of mouse genes (Agilent Single Color SurePrint G3 Mouse GE 8x60K Microarray Kit, G4852A, Agilent Technologies) according to manufacturer’s guidelines. Agilent Feature Extraction software was used for array image analysis. Absolute and comparative analyses were performed using the GeneSpring GX 10 (Agilent Technologies). Quality control filtering after quantile normalization resulted in approximately 33,000 probes. Probes that were not above microarray background signal or whose sequences could not be mapped to Ensembl transcripts were discarded. Fold change of gene expression was calculated based on the normalized signal values. Genes were considered significantly down-regulated or up-regulated if the fold-change was less than -1.4 or greater than 1.4, respectively, and the FDR-corrected P-value was less than 0.05. To validate the reliability of the results obtained from the microarray analysis, we performed qRT-PCR for all genes of interest.
Statistical analysis
Graphs were created with the GraphPad Prism Software (Version 6.0, GraphPad Software, Inc.; La Jolla, USA). All data sets were analyzed using Student’s test for groups (GraphPad Prism Software). Data are presented as means ± SEM. Differences between the means for all tests were considered statistically significant if P < 0.05.
Supporting Information
Zdroje
1. Seale P, Lazar MA (2009) Brown fat in humans: turning up the heat on obesity. Diabetes 58 : 1482–1484. doi: 10.2337/db09-0622 19564460
2. Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, et al. (2011) The influence of physical activity on appetite control: an experimental system to understand the relationship between exercise-induced energy expenditure and energy intake. Proc Nutr Soc 70 : 171–180. doi: 10.1017/S0029665110004751 21226975
3. Kennedy GC (1953) The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 140 : 578–596. 13027283
4. Cannon B, Nedergaard J (2009) Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc 68 : 401–407. doi: 10.1017/S0029665109990255 19775494
5. Arch JR (2008) The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol 378 : 225–240. doi: 10.1007/s00210-008-0271-1 18612674
6. Melnyk A, Himms-Hagen J (1998) Temperature-dependent feeding: lack of role for leptin and defect in brown adipose tissue-ablated obese mice. Am J Physiol 274: R1131–1135. 9575979
7. Perello M, Stuart RC, Vaslet CA, Nillni EA (2007) Cold exposure increases the biosynthesis and proteolytic processing of prothyrotropin-releasing hormone in the hypothalamic paraventricular nucleus via beta-adrenoreceptors. Endocrinology 148 : 4952–4964. 17584968
8. Park JJ, Lee HK, Shin MW, Kim SJ, Noh SY, et al. (2007) Short-term cold exposure may cause a local decrease of neuropeptide Y in the rat hypothalamus. Mol Cells 23 : 88–93. 17464216
9. Cabral A, Valdivia S, Reynaldo M, Cyr NE, Nillni EA, et al. (2012) Short-term cold exposure activates TRH neurons exclusively in the hypothalamic paraventricular nucleus and raphe pallidus. Neurosci Lett 518 : 86–91. doi: 10.1016/j.neulet.2012.04.059 22580206
10. Pereira-da-Silva M, Torsoni MA, Nourani HV, Augusto VD, Souza CT, et al. (2003) Hypothalamic melanin-concentrating hormone is induced by cold exposure and participates in the control of energy expenditure in rats. Endocrinology 144 : 4831–4840. 12960043
11. Sanchez E, Fekete C, Lechan RM, Joseph-Bravo P (2007) Cocaine - and amphetamine-regulated transcript (CART) expression is differentially regulated in the hypothalamic paraventricular nucleus of lactating rats exposed to suckling or cold stimulation. Brain Res 1132 : 120–128. 17174283
12. McCarthy HD, Kilpatrick AP, Trayhurn P, Williams G (1993) Widespread increases in regional hypothalamic neuropeptide Y levels in acute cold-exposed rats. Neuroscience 54 : 127–132. 8515838
13. Egawa M, Yoshimatsu H, Bray GA (1991) Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol 260: R328–334. 1996720
14. Small CJ, Liu YL, Stanley SA, Connoley IP, Kennedy A, et al. (2003) Chronic CNS administration of Agouti-related protein (Agrp) reduces energy expenditure. Int J Obes Relat Metab Disord 27 : 530–533. 12664087
15. Chao PT, Yang L, Aja S, Moran TH, Bi S (2011) Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab 13 : 573–583. doi: 10.1016/j.cmet.2011.02.019 21531339
16. Dimitrov EL, Kim YY, Usdin TB (2011) Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J Neurosci 31 : 18166–18179. doi: 10.1523/JNEUROSCI.2619-11.2011 22159128
17. Nillni EA, Xie W, Mulcahy L, Sanchez VC, Wetsel WC (2002) Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in Cpefat/fat mice. J Biol Chem 277 : 48587–48595. 12270926
18. Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, et al. (2006) Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet 2: e81. 16733553
19. Ukropec J, Anunciado RV, Ravussin Y, Kozak LP (2006) Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 147 : 2468–2480. 16469807
20. Coleman DL (1982) Thermogenesis in diabetes-obesity syndromes in mutant mice. Diabetologia 22 : 205–211. 7075918
21. Trayhurn P, James WP (1978) Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch 373 : 189–193. 565045
22. Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, et al. (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387 : 90–94. 9169872
23. Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, et al. (2003) Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111 : 399–407. 12569166
24. Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, et al. (2001) Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J 15 : 2048–2050. 11511509
25. Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP (2006) UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/ - mice. J Biol Chem 281 : 31894–31908. 16914547
26. Johnson MS, Speakman JR (2001) Limits to sustained energy intake. V. Effect of cold-exposure during lactation in Mus musculus. J Exp Biol 204 : 1967–1977. 11441038
27. Melzer K, Kayser B, Saris WH, Pichard C (2005) Effects of physical activity on food intake. Clin Nutr 24 : 885–895. 16039759
28. Brobeck JR (1948) Food intake as a mechanism of temperature regulation. Yale J Biol Med 20 : 545–552. 18872321
29. Leibel RL, Rosenbaum M, Hirsch J (1995) Changes in energy expenditure resulting from altered body weight. N Engl J Med 332 : 621–628. 7632212
30. Bukowiecki LJ (1989) Energy balance and diabetes. The effects of cold exposure, exercise training, and diet composition on glucose tolerance and glucose metabolism in rat peripheral tissues. Can J Physiol Pharmacol 67 : 382–393. 2667731
31. Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, et al. (2002) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415 : 339–343. 11797013
32. Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, et al. (1998) Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest 102 : 1724–1731. 9802886
33. Harris RB, Mitchell TD, Kelso EW, Flatt WP (2007) Changes in environmental temperature influence leptin responsiveness in low - and high-fat-fed mice. Am J Physiol Regul Integr Comp Physiol 293: R106–115. 17442784
34. Bing C, Frankish HM, Pickavance L, Wang Q, Hopkins DF, et al. (1998) Hyperphagia in cold-exposed rats is accompanied by decreased plasma leptin but unchanged hypothalamic NPY. Am J Physiol 274: R62–68. 9458899
35. Zhao ZJ (2011) Serum leptin, energy budget, and thermogenesis in striped hamsters exposed to consecutive decreases in ambient temperatures. Physiol Biochem Zool 84 : 560–572. doi: 10.1086/662553 22030849
36. Krol E, Speakman JR (2003) Limits to sustained energy intake. VI. Energetics of lactation in laboratory mice at thermoneutrality. J Exp Biol 206 : 4255–4266. 14581596
37. Speakman JR, Krol E (2011) Limits to sustained energy intake. XIII. Recent progress and future perspectives. J Exp Biol 214 : 230–241. doi: 10.1242/jeb.048603 21177943
38. El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS (2000) Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105 : 1827–1832. 10862798
39. Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, et al. (2011) Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 19 : 1755–1760. doi: 10.1038/oby.2011.125 21566561
40. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, et al. (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360 : 1509–1517. doi: 10.1056/NEJMoa0810780 19357406
41. Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP (2001) Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem 276 : 12460–12465. 11279075
42. Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, et al. (2001) Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem 276 : 36961–36969. 11481330
43. Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, et al. (2000) New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2 : 703–708. 11025660
44. Johnson MA, Tsutsui K, Fraley GS (2007) Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51 : 171–180. 17113584
45. Yang HY, Fratta W, Majane EA, Costa E (1985) Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci U S A 82 : 7757–7761. 3865193
46. Jhamandas JH, Goncharuk V (2013) Role of neuropeptide FF in central cardiovascular and neuroendocrine regulation. Front Endocrinol (Lausanne) 4 : 8.
47. Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, et al. (2005) Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res 1050 : 94–100. 15979587
48. Cline MA, Bowden CN, Calchary WA, Layne JE (2008) Short-term anorexigenic effects of central neuropeptide VF are associated with hypothalamic changes in chicks. J Neuroendocrinol 20 : 971–977. doi: 10.1111/j.1365-2826.2008.01749.x 18540998
49. Chartrel N, Dujardin C, Anouar Y, Leprince J, Decker A, et al. (2003) Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc Natl Acad Sci U S A 100 : 15247–15252. 14657341
50. Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, et al. (2008) Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 199 : 105–112. doi: 10.1677/JOE-08-0197 18653621
51. Klingerman CM, Williams WP 3rd, Simberlund J, Brahme N, Prasad A, et al. (2011) Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells are Associated with Changes in Sexual Motivation and Food Hoarding, but not Sexual Performance and Food Intake. Front Endocrinol (Lausanne) 2 : 101. doi: 10.3389/fendo.2011.00101 22649396
52. Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, et al. (2003) Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res 982 : 156–167. 12915251
53. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, et al. (2006) Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 103 : 2410–2415. 16467147
54. Morrison SF, Madden CJ, Tupone D (2014) Central Neural Regulation of Brown Adipose Tissue Thermogenesis and Energy Expenditure. Cell Metab.
55. Rizwan MZ, Harbid AA, Inglis MA, Quennell JH, Anderson GM (2014) Evidence that hypothalamic RFamide related peptide-3 neurones are not leptin-responsive in mice and rats. J Neuroendocrinol 26 : 247–257. doi: 10.1111/jne.12140 24612072
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



