-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
article has not abstract
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005229
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1005229Summary
article has not abstract
For a long time, bacterial populations were regarded as being uniform with respect to the physiology and morphology of the individual cells. However, a variety of different sub-populations may coexist in seemingly uniform populations. This was first observed for the formation of genetically competent cells of the Gram-positive model organism Bacillus subtilis. Genetic competence is the ability to take up and incorporate free DNA from the environment into a cell. This allows the cell to acquire new properties, but comes at the risk that the new DNA may also cause harm to the receiving cell. Therefore, it is not surprising that, even under conditions that trigger the development of competence, only about 10% of a population enters the competent state. The phenomenon that underlies this phenotypic heterogeneity at the level of gene expression is referred to as bistability or bistable gene expression [1,2]. The molecular triggers that cause the separation into distinct subpopulations are not yet fully explored. Importantly, a major factor in bistability is the existence of positive or negative feedback loops [1,3,4]. In this issue of PLOS Genetics, Gamba et al. [5] have identified yet another feedback loop that controls bistable expression of competence genes in B. subtilis.
The best-studied examples of bistable gene expression are: the entry to sporulation, the development of genetic competence, and the choice between motility and biofilm formation; all in B. subtilis. These examples also helped to uncover basic principles in the design of the feedback loops resulting in heterogeneity, and of the determining molecular events. At the mechanistic level, bistability is caused by a signal (a protein or a molecule) that is normally present at low concentration, but which is strongly amplified once a certain threshold has been exceeded. This threshold can be reached in individual cells due to the intrinsic stochastic variability in gene expression, also referred to as noise [6]. As the primary event in bistability, the concentration or activity of a key regulator of the network must be subject to stochastic regulation. This regulation may occur by controlling the cellular concentration of the protein by proteolysis, by regulatory protein-protein interactions or by covalent modification. Indeed, all these mechanisms have been demonstrated as being decisive for bistable gene expression in B. subtilis (see Table 1) [7].
Tab. 1. Molecular mechanisms behind bistable gene expression in <i>B</i>. <i>subtilis</i>. 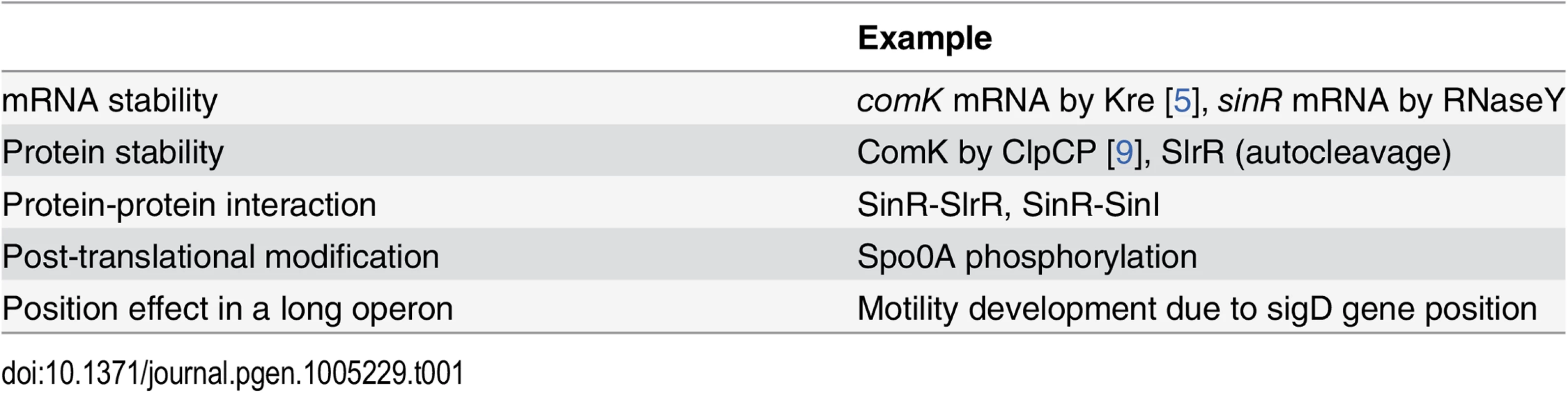
Gamba et al. [5] have now used transposon mutagenesis to identify a novel player in bistable expression of competence genes. Importantly, they also unravel a novel level of control of bistability—by controlling the stability of the mRNA of a key transcription factor. The analysis of bistable gene expression is often hampered by the complexity of the involved regulatory elements. This is also the case for the control of the genes required for the uptake and integration of foreign DNA in B. subtilis, collectively called the competence genes. The expression of these genes is activated by the transcription factor ComK. The expression of the comK gene is regulated by at least six different transcription regulators, including ComK itself (see http://subtiwiki.uni-goettingen.de/wiki/index.php/ComK for an overview [8]). Moreover, the ComK protein is degraded by the ClpCP protease at low cell densities [9]. This complex control results in bistable expression of ComK, i.e., one portion of a population growing in competence medium expresses ComK, whereas the other portion does not. Gamba and colleagues have now designed a clever screening system to uncover novel factors that are involved in the control of bistable expression of ComK. For this purpose, they expressed the comK gene under the control of a promoter (comG) that is exclusively controlled by ComK in a strain unable to degrade the ComK protein. Interestingly, even this minimal expression system for ComK still allows bistable expression of ComK and of genetic competence [10]. Transposon mutagenesis of this strain and a screen for mutants with high competence promoter activity and expression of competence genes in the majority of cells resulted in the identification of Kre (ComK repressor), a novel factor in the control of bistable gene expression.
In a set of elegant experiments, the authors demonstrate that the loss of Kre results in overexpression of ComK, uniform expression of competence genes, and increased genetic competence. In contrast, overexpression of Kre resulted in the opposite phenotypes. The function of Kre could be attributed to the control of ComK mRNA stability, i.e., in a kre mutant, the half-life of the comK mRNA increases. Thus, Kre is a novel factor that affects mRNA stability, and therefore an addition to the network of proteins implicated in RNA degradation in B. subtilis. It is tempting to speculate that Kre might modify the activity of one of the endo - and exoribonucleases that interact to achieve coordinated RNA degradation and processing in B. subtilis [11]. Strikingly, the relation between Kre and ComK is not limited to the control of comK mRNA stability but also involves the repression of kre transcription by ComK in competent cells (see Fig 1). Thus, Kre and ComK form a double negative feedback loop that is characteristic for the control of bistable gene expression. The functional significance of the link between Kre and ComK is further supported by the observation that the Kre protein is present only in bacteria that also contain ComK.
Fig. 1. Feedback loops that control the bistable gene expression of genetic competence in B. subtilis. 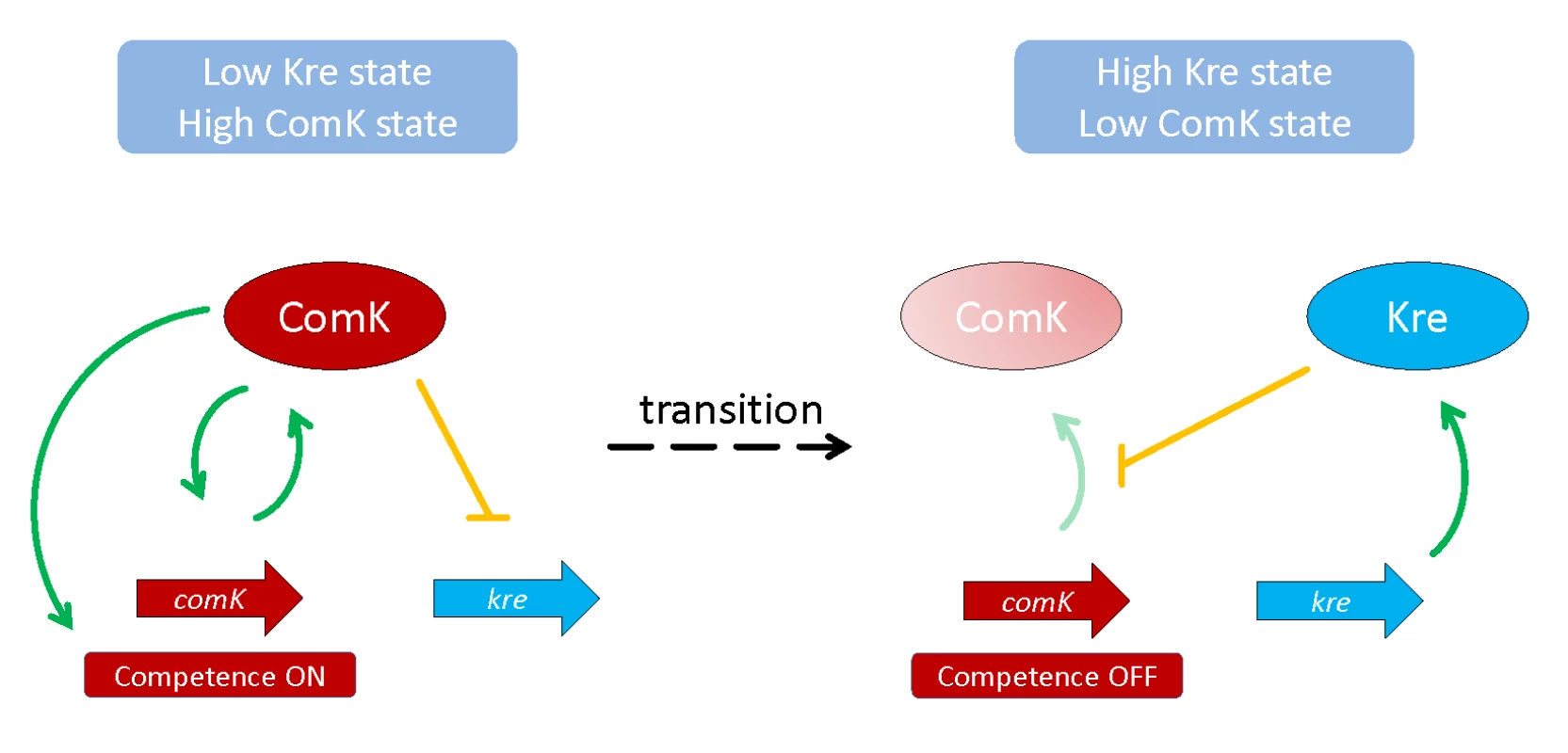
If Kre is low, the comK mRNA and, thus, ComK protein can accumulate, resulting in the expression of competence genes. In contrast, high activity of Kre results in degradation of comK mRNA, and the cells do not enter the competence state. The fact that a key regulator of a bistable expression system is controlled at the level of mRNA stability is not unprecedented: recently, it has been demonstrated that the mRNA for the master regulator of biofilm formation, SinR, is subject to control by RNase Y [12]. Moreover, RNA polymerase processivity or mRNA turnover of the very long B. subtilis operon encoding motility genes including the sigma factor gene sigD were also shown to be the decisive factors for stochastic expression of motility genes at the level of single cells [13]. The study of Gamba et al. [5] reinforces the implication of mRNA stability for bistable gene expression. This work opens a window to novel mechanisms of the control of bistable gene expression and paves the way to their discovery.
Zdroje
1. Smits WK, Kuipers OP, Veening JW (2006) Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol 4 : 259–271. 16541134
2. Dubnau D, Losick R (2006) Bistability in bacteria. Mol Microbiol 61 : 564–572. 16879639
3. Tiwari A, Ray JC, Narula J, Igoshin OA (2011) Bistable responses in bacterial genetic networks: designs and dynamical consequences. Math Biosci 231 : 76–89. doi:10.1016/j.mbs.2011.03.004 21385588
4. Casadesus J, Low DA (2013) Programmed heterogeneity: epigenetic mechanisms in bacteria. J Biol Chem 288 : 13929–13935. doi: 10.1074/jbc.R113.472274 23592777
5. Gamba P, Jonker MJ, Hamoen LW (2015) A novel feedback loop that controls bimodal expression of genetic competence. PLoS Genet 11(6): e1005047. doi: 10.1371/journal.pgen.1005047
6. Qian H (2012) Cooperativity in cellular biochemical processes: noise-enhanced sensitivity, fluctuating enzyme, bistability with nonlinear feedback, and other mechanisms for sigmoidal response. Annu Rev Biophys 41 : 179–204. doi: 10.1146/annurev-biophys-050511-102240 22404682
7. Veening JW, Smits WK, Kuipers OP (2008) Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62 : 193–210. doi: 10.1146/annurev.micro.62.081307.163002 18537474
8. Michna RH, Commichau FM, Tödter D, Zschiedrich C, Stülke J (2014) SubtiWiki—a database for the model organism Bacillus subtilis that links pathway, interaction and expression information. Nucleic Acids Res 42: D692–D698. doi: 10.1093/nar/gkt1002 24178028
9. Turgay K, Hahn J, Burghoorn J, Dubnau D (1998) Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J 17 : 6730–6738. 9890793
10. Smits WK, Eschevins CC, Susanna KA, Bron S, Kuipers OP, Hamoen LW (2005) Stripping Bacillus: ComK auto-stimulation is responsible for the bistable response in competence development. Mol Microbiol 56 : 604–614. 15819618
11. Lehnik-Habrink M, Mäder U, Lewis RJ, Stülke J (2012) RNA degradation in Bacillus subtilis: an interplay of essential endo - and exoribonucleases. Mol Microbiol 84 : 1005–1017. doi: 10.1111/j.1365-2958.2012.08072.x 22568516
12. Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J (2011) RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol 81 : 1459–1473. doi: 10.1111/j.1365-2958.2011.07777.x 21815947
13. Cozy LM, Cairns DB (2010) Gene position in a long operon governs motility development in Bacillus subtilis. Mol Microbiol 76 : 273–285. doi: 10.1111/j.1365-2958.2010.07112.x 20233303
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



