-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
Telomerase, the enzyme that maintains telomeres, preferentially lengthens short telomeres. The baker’s yeast Pif1 DNA helicase inhibits both telomerase-mediated lengthening of existing telomeres and the formation of new telomeres at double strand breaks. By virtue of its ATPase activity, Pif1 reduces the level of telomerase binding to telomeres. Here, we report that the association of the telomerase subunits Est2 and Est1 at a DNA break was increased in the absence of Pif1, suggesting that Pif1 affects telomere length and new telomere formation by similar mechanisms. In cells lacking Pif1, Est2 and Est1 no longer bound preferentially to short telomeres, a larger fraction of telomeres was lengthened and the amount of telomeric DNA added per telomere was increased compared to wild type cells. Furthermore, by two different assays, Pif1 bound preferentially to long telomeres in vivo. Thus, preferential lengthening of short telomeres is achieved in part by targeting Pif1, a negative regulator of telomerase, to long telomeres.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005186
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005186Summary
Telomerase, the enzyme that maintains telomeres, preferentially lengthens short telomeres. The baker’s yeast Pif1 DNA helicase inhibits both telomerase-mediated lengthening of existing telomeres and the formation of new telomeres at double strand breaks. By virtue of its ATPase activity, Pif1 reduces the level of telomerase binding to telomeres. Here, we report that the association of the telomerase subunits Est2 and Est1 at a DNA break was increased in the absence of Pif1, suggesting that Pif1 affects telomere length and new telomere formation by similar mechanisms. In cells lacking Pif1, Est2 and Est1 no longer bound preferentially to short telomeres, a larger fraction of telomeres was lengthened and the amount of telomeric DNA added per telomere was increased compared to wild type cells. Furthermore, by two different assays, Pif1 bound preferentially to long telomeres in vivo. Thus, preferential lengthening of short telomeres is achieved in part by targeting Pif1, a negative regulator of telomerase, to long telomeres.
Introduction
Telomerase is a specialized reverse transcriptase that extends the G-strand of telomeric DNA using its RNA subunit as a template. In Saccharomyces cerevisiae, telomerase consists minimally of Est2, the catalytic reverse transcriptase, TLC1, the templating telomerase RNA, and Est1 and Est3, two telomerase accessory subunits that are both essential for telomerase action in vivo. In addition, yeast telomerase requires Cdc13, the sequence specific TG1-3-binding subunit of the CST (Cdc13-Stn1-Ten1) complex that has dual roles in protecting telomeres from degradation and recruiting telomerase to DNA ends (reviewed in [1]).
Yeast telomerase is regulated by both the cell cycle and telomere length. Telomerase-mediated telomere lengthening occurs only in late S/G2 phase, even though telomerase activity is present throughout the cell cycle [2, 3]. Although Est2 and TLC1 are telomere associated even in G1 phase when telomerase is not active, Est1 [4] and Est3 [5] come to the telomere primarily in late S/G2 phase. Short telomeres are preferentially lengthened by telomerase [6, 7], a pattern that can be explained by higher levels of telomerase binding to short telomeres late in the cell cycle [8, 9]. Both the Tel1 checkpoint kinase [8] and Tbf1 [10], a telomere structural protein that binds to the sub-telomeric DNA of some telomeres, target telomerase to short telomeres. Tel1 itself binds preferentially to short telomeres [8, 11], as does the Mre11-Rad50-Xrs2 (MRX) complex [12], which recruits Tel1 to telomeres [8]. Short telomeres have reduced levels of Rif2 [8, 12], a telomere structural protein that negatively regulates telomerase [13, 14]. Because Rif2 and the MRX complex compete with each other for telomeric DNA binding [15], in the absence of Rif2, Tel1 no longer binds preferentially to short telomeres [12].
The S. cerevisiae Pif1 is the founding member of a helicase family that exists in virtually all eukaryotes (reviewed in [16]). Pif1 was first identified because of its important role in maintaining mitochondrial DNA [17]. However, there are two forms of Pif1 depending on whether the first or second AUG is used to start translation, one destined for mitochondria and one localized to nuclei [18, 19]. Nuclear Pif1 inhibits telomerase at both telomeres and double strand breaks (DSBs) [18–20]. Thus, telomeres are longer and the rate of de novo telomere addition to DSBs is greatly elevated in pif1 mutant cells. Checkpoint-mediated phosphorylation of Pif1 is required for Pif1 inhibition of telomerase at DSBs but not at telomeres [21]. In vivo and in vitro, Pif1 uses its ATPase activity to displace telomerase from DNA ends [22]. Pif1 also has more general roles in chromosome maintenance: it facilitates replication and suppresses DNA damage at G-quadruplex motifs, cooperates with Dna2 to process long Okazaki fragments, is needed for stability of mitochondrial DNA, and promotes break-induced replication (BIR) (reviewed in [16]; also, [23–25]).
Here, we show that Pif1 acts similarly at DSBs and telomeres in that its presence was associated with lower levels of telomerase binding at DSBs (as it is at telomeres [22]). Using an assay that monitors telomeric DNA addition at individual telomeres [7], we find that Pif1 reduced both the frequency and processivity of telomere addition. Moreover, telomerase was no longer bound preferentially to short telomeres in Pif1-deficient cells. By two assays, Pif1 bound preferentially to long telomeres. Together, these data can be explained if Pif1 is more likely to remove telomerase from those telomeres that are least in need of lengthening.
Results
Pif1 reduces telomerase association at DSBs
Pif1 uses its ATPase activity to evict telomerase from telomeres [22], which can explain how it suppresses telomere lengthening. Pif1 also inhibits telomere addition to DSBs [19, 20]. To determine if Pif1 affects telomerase binding to DSBs, we used chromatin immuno-precipitation (ChIP) to monitor the association of Est1 and Est2 to an induced DSB in the presence and absence of Pif1 (Fig 1). These experiments were carried out in a strain with a galactose-inducible HO endonuclease and an HO recognition site ~13 kb from the end of chromosome VII-L, the only accessible HO site in the strain (Fig 1A) [26]. We used a strain with an 80-bp tract of TG1-3 telomeric DNA (TG80, Fig 1A grey box) adjacent to the HO site to increase the rate of de novo telomere addition [26]. Cells also expressed a Myc-tagged protein (Est2, Est1, or Cdc13). Experiments were carried out in PIF1 and pif1-m2 versions of the strain, where pif1-m2 cells express wild type (WT) levels of mitochondrial Pif1 and reduced nuclear Pif1 [18]. Although pif1-m2 retains some nuclear function, we used it because pif1-m2 cells progress through the cell cycle similarly to WT cells, unlike pif1Δ cells which progress more slowly owing to their reduced mitochondrial function [19]. In addition, pif1Δ cells are very hard to synchronize. The efficiency of cleavage at the HO site, which was monitored by Southern blotting, was not affected by Pif1 levels (~65–80% cutting in both WT and pif1-m2 cells; S1 Fig).
Fig. 1. Telomerase, but not Cdc13, association is increased at a double strand break in pif1-m2 cells. 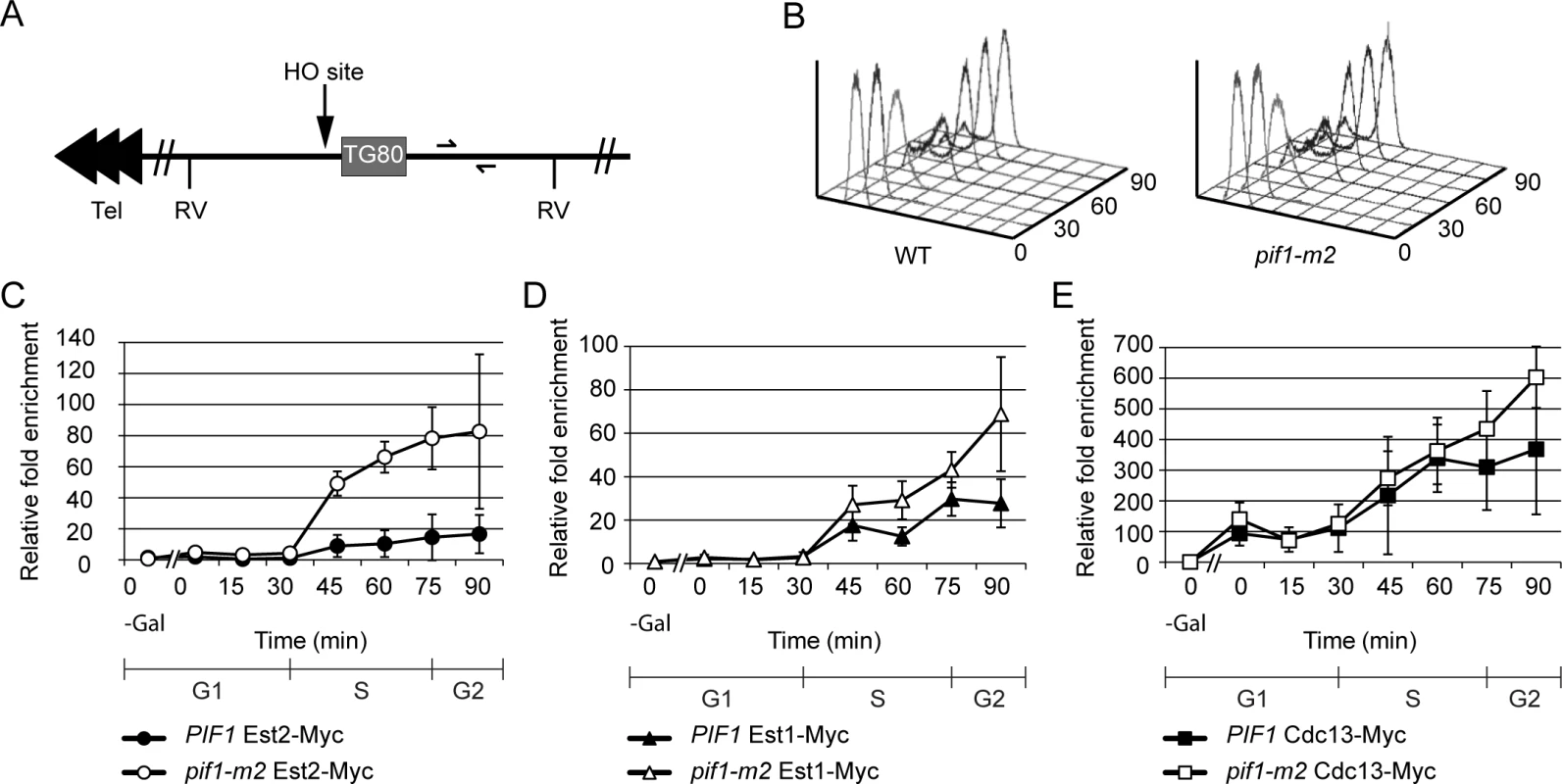
(A) Chromosome VII-L was modified by inserting an HO endonuclease recognition site and an adjacent 80 bp tract of TG1-3 DNA ~13 kb from the left telomere [26]. Half arrows indicate unique sequences used for real-time PCR amplification. Each arrowhead represents ~100 bps C1-3A/TG1-3 DNA. RV, EcoRV recognition site. (B) Cell cycle progression was monitored with flow cytometry. (C) Results were quantitated using real-time PCR. Est2 binding was significantly increased in pif1-m2 (open circles) compared to WT cells (closed circles) at 45, 60, and 75 min (p = 0.0018 to 0.011; two-tailed unpaired t-tests). (D) A modest but reproducible increase in Est1 binding in pif1-m2 cells during and after S phase was observed in four independent synchronies: at 60, 75, and 90 min, Est1 binding in pif1-m2 (open triangles) was 1.1–3.6x (average 2.3x), 1.2–2.3x (average 1.5x), and 2.1–3.4x (average 2.5x) increased over that in WT cells (closed triangles), respectively. This increase was significant at 60 and 90 min (p = 0.014, 0.028). Overlapping standard deviations at the 75 min time point (p = 0.054) and all large standard deviations are due to slight differences in HO cutting efficiency in four independent experiments. (E) Cdc13 binding in WT (closed squares; as shown previously in [12]) and pif1-m2 (open squares) cells was statistically indistinguishable. HO cleavage was induced in WT or pif1-m2 cells that were first arrested in late G1 phase with Δ-factor. After HO action, cells were released from the G1 phase arrest. In both strains, ChIP samples were taken in G1 arrested cells both before (“0—gal” time points) and after HO induction and throughout the synchronous cell cycle that occurred upon removal from Δ-factor (0–90 min time points; 24°C).
Samples from each time point were also assessed by FACS to determine cell cycle position, which demonstrated that pif1-m2 and WT cells moved similarly through the cell cycle (Fig 1B). ChIP samples were quantified using real-time PCR and normalized to input DNA. In this and other ChIP experiments, results are presented as fold enrichment of binding to the DSB compared to binding to a control site (ARO1). We examined binding of Est2 (Fig 1C), Est1 (Fig 1D) and Cdc13 (Fig 1E) to the HO break site in WT and pif1-m2 cells.
None of the three proteins was associated with the HO recognition site before HO induction (Fig 1C–1E, 0—gal time point). In both strains, Est2 binding to TG80-HO was at background levels in G1 and early S phase (Fig 1C, WT closed squares; pif1-m2, open squares, 0–30 min time points). In both strains, high levels of Est2 binding to the DSB were detected from mid-S phase through the end of the cell cycle (45–90 min time points). However, average Est2 binding was over four times higher in pif1-m2 compared to WT cells (Fig 1C, open circles). Est1 showed a similar pattern of binding to the DSB, occurring at background levels in G1 and early S phase with strong binding from mid-S phase to the end of the cell cycle (Fig 1D). Although Est1 binding was significantly higher in pif1-m2 versus WT cells from mid-S to the end of the cell cycle, the difference was fairly modest (~1.5–2.5-fold over WT levels; see Fig 1 legend for p values). As reported previously [12], Cdc13 showed strong association with TG80-HO after HO induction. Cdc13 enrichment at TG80-HO was ~100 to 150-fold over background during G1 phase and steadily increased to ~400 - to 600-fold over background by the end of the cell cycle. However, unlike Est2 and Est1, Cdc13 binding to TG80-HO was not affected significantly by reduced Pif1 (Fig 1E, open squares).
We conclude that Pif1 affects telomere addition to DSBs by reducing telomerase binding to breaks, as it does at telomeres. This effect is specific for telomerase, as binding of Cdc13 was unaffected by Pif1 at either telomeres [22] or DSBs (Fig 1E).
Pif1 reduces the fraction of elongated telomeres and amount of telomerase-mediated telomere elongation
Pif1-mediated removal of telomerase could regulate telomere length by affecting the frequency of telomere addition or telomerase processivity (or both). To distinguish among these possibilities, we used the Single Telomere Extension (STEX) assay [7], which analyzes lengthening at individual telomeres at nucleotide resolution over a single cell cycle in diploid PIF1 and pif1Δ cells (Fig 2). Freshly dissected pif1Δ spore clones were used quickly after dissection from a heterozygous diploid to minimize the negative effects of mitochondrial DNA loss on growth rate.
Fig. 2. Absence of Pif1 results in increased frequency and extent of telomerase lengthening. 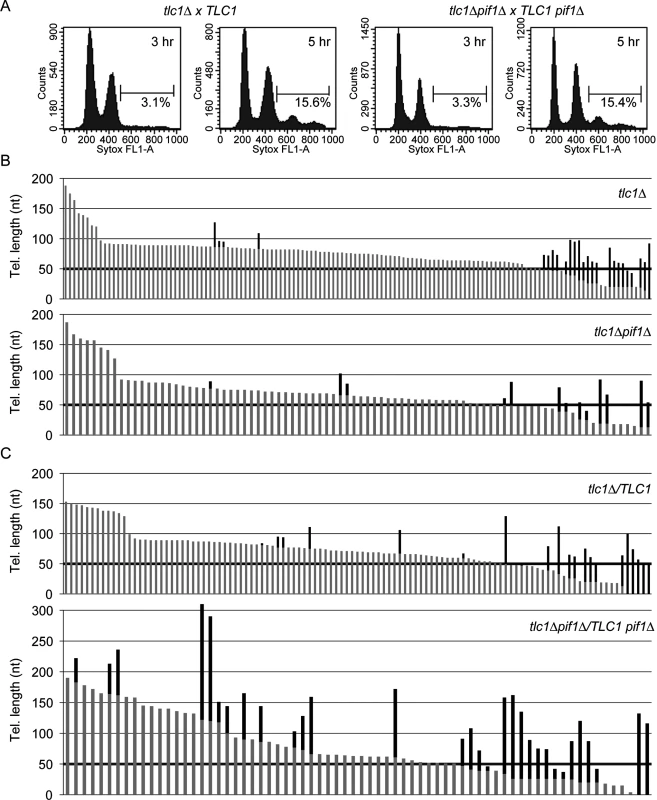
(A) Flow cytometry was used to measure DNA content of PIF1 tlc1Δ and pif1Δ tlc1Δ recipient samples (after mating with PIF1 TLC1 donor or pif1Δ TLC1 donor cells, respectively) at 3 and 5 h post-mixing. Percentages indicate cells with a 4C DNA content, which corresponds to cells that have gone through one S phase after mating. ~93% of the recipient cells mated in both crosses, and replication of the diploid genomes occurred by 5 h post-mixing (15.5% out of an expected 16.7% of cells had 4C DNA content at this time). Thus, the null status of PIF1 did not affect growth rates or mating efficiency during the small number of generations required to obtain sufficient cells for this experiment. (B) Telomere length is plotted; individual telomeres are shown as vertical lines (gray, parental telomeric sequence; black, telomere lengthening events). Telomere VII-L was sequenced from telomerase-deficient tlc1Δ (top; n = 134 telomeres) and tlc1Δ pif1Δ (bottom; n = 86 telomeres) haploid cells after approximately 30 doublings following dissection from JP192 and JP223, respectively. Telomere elongation frequency (p = 0.712; Fisher’s exact test) and length of telomeric sequence added (p = 0.543; Mann-Whitney U test) are indistinguishable between the two strains. (C) Telomere VII-L was sequenced after the first post-zygotic S phase from tlc1Δ /TLC1 (top; n = 111 telomeres) and tlc1Δ pif1Δ /TLC1 pif1Δ (bottom; n = 70 telomeres) diploid cells 5 h after reintroduction of telomerase by mating to telomerase-deficient strains. The frequency of telomere elongation in tlc1Δ pif1Δ /TLC1 pif1Δ cells is significantly increased over tlc1Δ /TLC1 cells by ~2-fold (p = 0.000180; Fisher’s exact test). Additionally, the average length of added telomeric sequence is significantly increased (p = 0.0265; Mann-Whitney U test) in tlc1Δ pif1Δ/TLC1 pif1Δ cells (72 nt) compared with tlc1Δ/TLC1 cells (43 nt). For each cross, telomere sequencing data are compiled results from two independent experiments. Due to high amount of telomerase independent elongation events at very short telomeres, we excluded telomeres of 50 bps or shorter in determining both the frequency and extent of elongation (threshold is indicated by bold horizontal lines). Telomerase-deficient tlc1Δ PIF1 or tlc1Δ pif1Δ spore clones (recipient cells) were mated to telomerase proficient TLC1 PIF1 or TLC1 pif1Δ cells (donor cells), respectively, to restore telomerase activity in the resulting diploids. To distinguish recipient from donor telomeres, URA3 was integrated adjacent to the left telomere of chromosome VII in the recipient strain. Mating efficiencies and cell cycle progression, which were determined by flow cytometry (Fig 2A), were similar in the two crosses (Fig 2A). ~93% of the recipient cells mated in both crosses (Fig 2A) and replication of the diploid genomes occurred by 5 h post-mixing (15.5% out of an expected 16.7% of cells had 4C DNA content at this time; see Fig 2A legend). Thus, the null status of PIF1 did not affect growth rates or mating efficiency during the small number of generations required to obtain sufficient cells for this experiment. The URA3-marked VII-L telomeres derived from recipient cells were amplified, cloned, and sequenced. Because yeast telomerase adds irregular TG1-3 repeats [27], pre-existing telomeric DNA can be distinguished from the newly added sequence [7, 28].
Prior to mating, we measured telomere lengthening in the parental haploid tlc1Δ PIF1 (Fig 2B, top) and tlc1Δ pif1Δ (Fig 2B, bottom) spore clones to obtain an estimate of the frequency of lengthening in the absence of telomerase, which likely occurs via recombination. In both strains, many of the short telomeres (≤50 bps) were lengthened in the absence of telomerase. Among longer telomeres (≥50 bps), the fraction of telomeres lengthened (3.7% and 4.8%) by telomerase-independent events and the average amount of telomeric DNA added (39 and 35 nt) were similar in, respectively, tlc1Δ and tlc1Δ pif1Δ haploid cells. To limit analysis to telomeres lengthened by telomerase, we considered only telomeres longer than 50 bps for further analysis.
Next, we prepared DNA from the diploid cells at the end of the first post-zygotic S phase in both WT (Fig 2C, top) and pif1Δ (Fig 2C, bottom) cells. The elongation frequency for telomere VII-L in tlc1Δ pif1Δ/TLC1 pif1Δ was 28% for telomeres over 50 bp. In contrast, only 7.2% of the telomeres were lengthened in WT cells, a significant difference (see Fig 2 legend for p values). Moreover, the average length of telomeric sequence added to individual telomeres in tlc1Δ pif1Δ/TLC1 pif1Δ cells was 72 nt, a value significantly greater than 45 nt that was added in PIF1 cells (see Fig 2 legend for p values). The value obtained in PIF1 WT (tlc1Δ/TLC1) cells in our experiments was the same as reported previously for STEX in tlc1Δ/TLC1 cells [7]. Taken together, these data suggest that Pif1 reduces both the frequency and processivity of telomerase action.
Pif1 is required for preferential binding of telomerase to short telomeres
In earlier work, we did not see a difference in Est2 binding to bulk telomeres in asynchronous cells [12]. To explore the effects of Pif1 on telomerase binding, we combined an inducible short telomere system [6] with ChIP in cells going synchronously through S phase (see [8] and Fig 3). The experimental strains used in these experiments had a modified chromosome VII-L in which a cassette containing telomeric repeats flanked by recognition sites (FRT) for the Flp1 site-specific recombinase was positioned directly adjacent to the telomere (Fig 3A left, experimental strain), as well as an integrated copy of a galactose-inducible FLP1. The control strains were isogenic to the experimental strains except that the cassette at modified chromosome VII-L had no telomeric DNA between the two FRT sites [6] (Fig 3A, right, control strain). In both strains, FLP1 expression was induced by adding galactose, which caused recombination between the two FRT sites. In the experimental strain, FLP action resulted in loss of the telomeric DNA between the sites, thus generating a short ~100 bp telomere at chromosome VII-L that is preferentially lengthened by telomerase for multiple cell cycles [6]. The telomere adjacent to the control cassette is maintained at the strain characteristic length (~300 bp in WT cells) regardless of whether or not it is acted upon by Flp1 (Fig 3A). Both the experimental and control strains expressed a Myc-tagged protein (Est1, Est2 or Yku80). These experiments were carried out in parallel in WT and pif1-m2 versions of both the experimental and control strains. Cells were synchronized, and ChIP samples were prepared and quantified as described for the DSB experiments.
Fig. 3. Preferential S-phase Est2 binding at short telomeres is decreased in pif1-m2 cells. 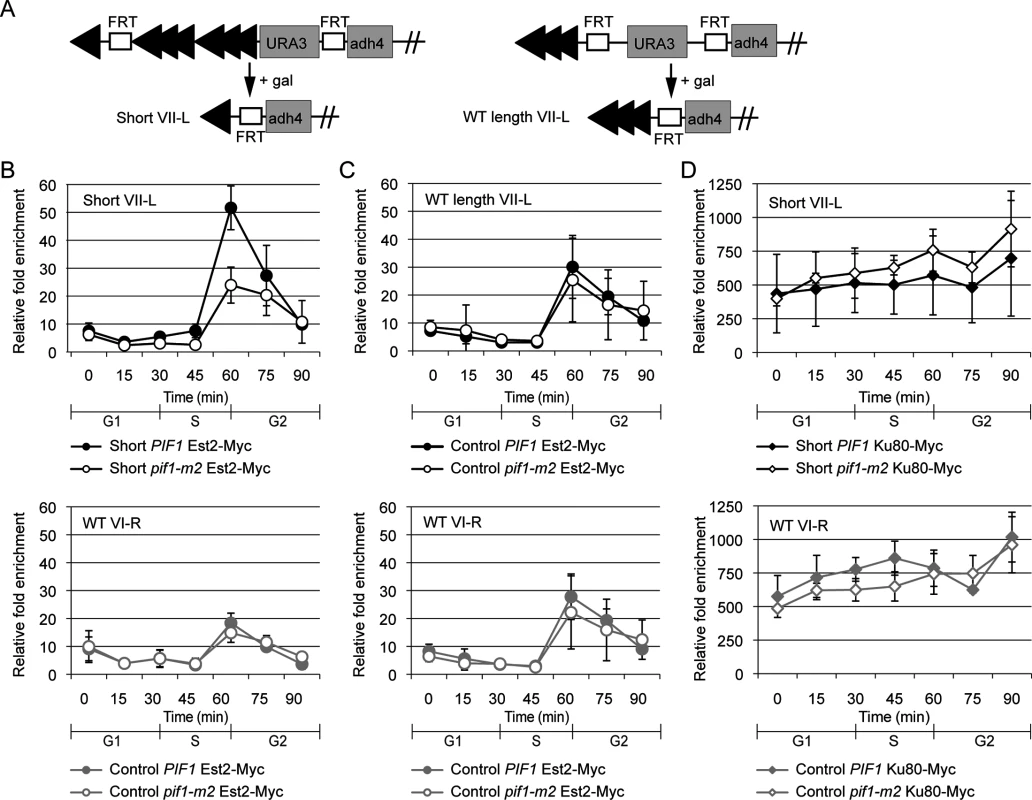
(A) Schematic of modified chromosome VII-L inducible short telomere constructs. In these strains, Flp1 causes recombination between the two FRT sites. Each arrowhead represents ~100 bp C1-3A/TG1-3 DNA. The 34 bp FRT recognition sites are labeled. Flp1 action generates a ~90 bp telomere (“Short VII-L”) in a WT strain [8]. A control strain (“WT length VII-L”) in which Flp1 action generates a ~300 bp telomere was also used [6]. (B) The lengths and identities of the telomeres being studied are indicated in the upper left corner of each graph. Binding of Est2-Myc at short telomere VII-L (top) was significantly decreased in pif1-m2 (open circles) compared with WT cells (closed circles) during S phase (45 min, p = 0.025; 60 min, p = 0.0092; two-tailed unpaired t-tests). Binding of Est2 at WT length telomere VI-R (bottom) in the same cells was statistically indistinguishable in pif1-m2 and WT cells. Although Est2 binds telomeres in G1 and early S phase, this binding is not evident in these experiments because it occurs mainly in the subtelomeric region that is deleted by FLP action, rather than at the very end of the chromosome [8]. (C) Binding of Est2 at WT length telomere VII-L (top) was statistically indistinguishable between pif1-m2 (open circles) and WT (closed circles) cells. Binding of Est2 at WT length telomere VI-R (bottom) in the same cells was unaffected by the pif1-m2 mutation. (D) Binding of Ku80-Myc at short telomere VII-L (top) was statistically indistinguishable in pif1-m2 (open diamonds) compared with WT cells (closed diamonds). Ku80 binding at WT length telomere VI-R (bottom) in the same cells was statistically indistinguishable in pif1-m2 and WT cells. Galactose was added to G1-arrested cells to induce expression of Flp1, which caused recombination at the VII-L telomeric region in all strains, resulting in either a short VII-L telomere (experimental strain) or a VII-L telomere that was the same length as the other telomeres in the cell (control strain). The efficiency of recombination was similar in all strains (S2A Fig). After Flp1 action, cells were released from G1 arrest (time 0), and samples were removed for analysis throughout the first synchronous cell cycle (15 to 90 min). The efficiency of recombination, which was monitored in each experiment (S2A Fig), was equivalent (~75%) in WT and pif1-m2 cells for both the experimental and control strains.
Using the same system, previous studies found that Est2 and Est1 binding was approximately two times higher on the short VII-L telomere versus the WT length VI-R telomere in the same cells or the WT length VII-L or VI-R telomeres in the control strain [8, 9]. For this study, we recapitulate the preferential binding of Est2 and Est1 to the short VII-L telomere in WT cells (Figs 3B and S3). Notably, preferential Est2 binding to the shortened VII-L telomere was lost in pif1-m2 cells (Fig 3B, upper panel, open circles), even though the amount of Est2 binding to the unaltered VI-R telomere in the same cells was unchanged (Fig 3B lower panel, see legend for p values). In addition, levels of Est2 binding were the same in pif1-m2 and WT versions of the control strain at the VII-L telomere (Fig 3C, upper panel). Similar results were obtained for Est1 (S3 Fig).
To determine if Pif1 acts preferentially on telomerase binding or alternatively affects all telomere-binding proteins, we examined Myc-tagged Ku80 telomere association as a function of Pif1 (Fig 3D). Consistent with earlier results [8], Yku80 bound robustly and equally well to short (Fig 3D, top panel, filled diamonds) and WT length (Fig 3D, bottom panel, filled diamonds) telomeres in WT cells. In contrast to Est2 and Est1, levels of Yku80 telomere binding in pif1-m2 cells were not significantly different from WT binding at either the short (Fig 3D, top panel, open diamonds) or WT length telomeres (Fig 3D, lower panel, open diamonds). Thus, higher telomerase binding to short telomeres is Pif1 dependent. This result is unlikely to be an artifact of telomeres being longer in pif1-m2 than in WT cells, as the level of binding of telomerase to the VI-R telomere in both the control and experimental strains and to the VII-L telomere in the control strain was similar in WT and pif1-m2 cells (Fig 3). Likewise, levels of Yku80 binding were not affected by the presence of Pif1 (Fig 3D).
Pif1 binds preferentially to long telomeres
In WT cells, telomerase preferentially binds [8, 9] and lengthens [6, 7] short telomeres. However, this binding preference was not detectable in pif1 cells (Figs 3B and S3). Because Pif1 uses its ATPase activity to remove telomerase from DNA ends [22], these results could be explained if Pif1 preferentially removes telomerase from WT length and/or long telomeres. This model predicts that the average length of telomeres that are Pif1-associated will be longer than the average for bulk telomeres.
To test this possibility, we determined the average size of telomeric DNA in an anti-Pif1 immuno-precipitate (Fig 4). We epitope-tagged a mutant version of Pif1, Pif1-K264A, in which the invariant lysine in the Walker A box is mutated to alanine. Although Pif1-K264A is helicase dead in vivo and in vitro [18], it binds single-stranded DNA as well as WT Pif1 [22]. We used Pif1-K264A because it gives a stronger signal in ChIP than WT Pif1, probably because it is trapped at its binding sites [29]. However, pif1-K264A cells have long telomeres, and the experiment to determine the lengths of Pif1-bound telomeres must be done in cells with WT telomere length. Because the effects of pif1-K264A on telomere length are recessive [18], we did the experiment in a heterozygous diploid (pif1-K264A-Myc/PIF1). As a control, we monitored the lengths of telomeres in an anti-Yku80 immuno-precipitate in a pif1-K264A/PIF1 diploid background.
Fig. 4. Pif1 preferentially binds to long telomeres. 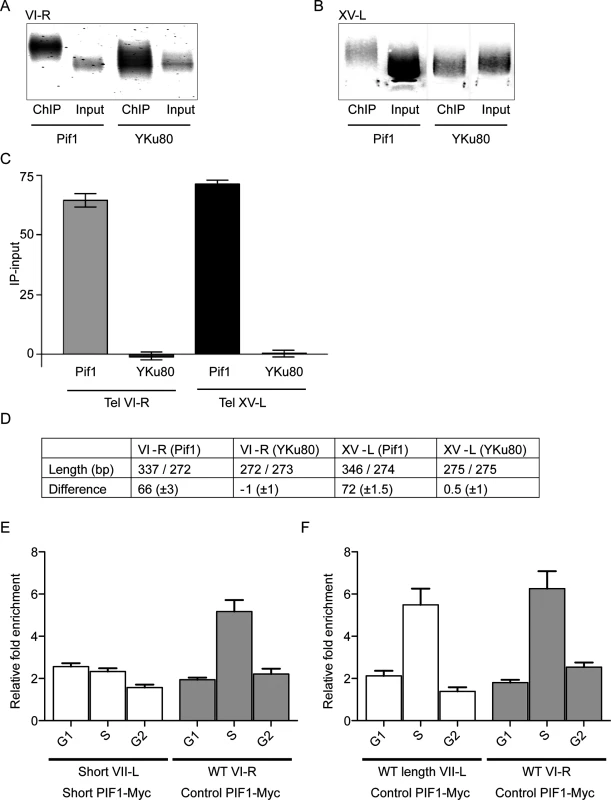
Panels A-D show results from ChIP experiments on two diploid strains that had WT length telomeres but were heterozygous at the PIF1 locus (PIF1/pif1-K264A). The only difference between the two strains is that one expressed Pif1-K264A-Myc and the other expressed Yku80-Myc. DNA was either prepared directly from the two strains (Input) or after immuno-precipitation with anti-Myc antibody (ChIP). Both input and ChIP samples were C-tailed and amplified by PCR using primers for telomere VI-R or XV-L and then separated on 1.8% agarose gels. Input and ChIP amplified DNA after gel purification from telomere VI-R (A) or XV-L (B). (C) Graphical presentation of the average difference in bps in lengths of DNA in ChIP and Input samples from the two strains (Pif1 and YKu80) and at both tested telomeres (VI-R and XV-L). Error bars represent one standard deviation from the average for four independent experiments. Using a student’s t-test, the differences between the average telomere lengths of the Input versus ChIP samples were significant for cells expressing Pif1-K264A-Myc (p<0.0001 for both VI-R and XV-L) but not for cells expressing Yku80-Myc (p = 0.9 for VI-R; p = 0.8 for XV-L). (D) The first row shows the average length of telomeres in the two samples (ChIP sample/input sample) for both telomeres in the two strains. The second row shows the average difference in telomere length (length in ChIP minus length in input samples) for the two strains and telomeres. (E and F) Pif1 binding to WT and short telomeres in the inducible VII-L short telomere (panel E) and control WT length VII-L (panel F) strains. Both strains express Pif1-Myc. Both panels show averages ± SD from three experiments. Data are presented as average binding in G1 phase (time points 0, 15 and 30 min; see Fig 3), S phase (time points 45 and 60), and G2 phase (time points 75 and 90). In the experimental strain (panel E), Pif1 binding in S phase was twice as high to the WT VI-R telomere than to the short VII-L in the same cells (p = 0.007; two-tailed unpaired t-tests). In the control strain (panel F), Pif1 bound equally well in S phase to WT length VII-L and WT VI-R telomeres (p = 0.5; two-tailed unpaired t-tests). In both strains, Pif1 binding to WT length telomeres was significantly higher in S phase than in G1 or G2 (p <0.009; two-tailed unpaired t-tests). Chromatin was prepared from both diploid strains and processed for PCR amplification either before (input samples) or after (ChIP samples) immuno-precipitation. After C-tailing the purified DNA, PCR primers were used to amplify either the VI-R (Fig 4A) or XV-L (Fig 4B) telomeres. The PCR-amplified DNA in the input and immuno-precipitated samples was gel separated (Fig 4A and 4B), and the average sizes of the DNA in these samples were determined using an AlphaImager 3400 Molecular Weight Analysis program (Fig 4A, VI-R; B, XV-L).
For both the VI-R and XV-L telomeres, the average size of telomeric DNA in the anti-Pif1 immuno-precipitate was significantly longer in the ChIP samples compared to input DNA (the average size in bps ± SD of telomeric DNA in the ChIP samples was 337.1± 2.0 for VI-R and 346.2 ± 3.4 for XV-L; the average size of input telomeric DNA was 272.7 ± 1.1 for VI-R and 274.0 ± 2.0 for XV-L; Fig 4C and 4D; see Fig 4 legend for p values). As expected for the strain expressing Yku80-Myc [12], the average sizes in bps of telomeric DNA in the ChIP and input samples were indistinguishable (averages ± SD for ChIP samples were 273.3 ± 0.8 and 275.1 ± 1.3, respectively, for telomeres VI-R and XV-L). Thus, Pif1 binds preferentially to long telomeres. This effect is specific for Pif1 because Yku80 binding showed no length preference in the same assay (Fig 4A–4D).
To extend the finding that Pif1 binds preferentially to longer telomeres, we monitored Pif1-Myc binding to short and WT length telomeres using the inducible short telomere system. In both the experimental and control strains, Pif1 telomere binding occurred in S phase. However, in different experiments, the peak of Pif1 binding to a given telomere occurred at different times in S phase. Therefore, for each telomere in both strains, the ChIP binding is presented as average binding from three independent experiments in G1 phase (pooling 0, 15 and 30 min time points), S phase (pooling 45 and 60 min time points), and G2 phase (pooling 75 and 90 min time points) (Fig 4E and 4F). In both, the experimental and control strains, Pif1 binding to all telomeres was low in G1 and G2 phase. In the experimental strain, Pif1 binding was similarly low in S phase to short VII-L. In contrast, binding of Pif1 to the WT length VI-R telomere in both the control and experimental strains and to the WT VII-L telomere in the control strain was three times higher in S phase (Fig 4E and 4F). Thus, using a very different assay, Pif1 binds more robustly to longer telomeres.
Discussion
In S. cerevisiae and mammals, short telomeres are preferentially elongated by telomerase [6, 7, 30]. This preference is explained in part by proteins that bind preferentially to short telomeres, such as the MRX complex [12] and Tel1[8, 9, 11]. The presence of these (and other) proteins in combination with low levels of Rif2 result in the preferential binding of telomerase to short telomeres [8, 12, 15]. Here, we show that telomere binding of Pif1, a negative regulator of telomerase, is also length dependent with longer telomeres having higher binding (Fig 4).
In vivo and in vitro, Pif1 removes telomerase from DNA ends without affecting binding levels of telomere structural proteins [22]. Here we show that Pif1 acts similarly at DSBs (Fig 1) because in the absence of Pif1, Est1 (Fig 1D) and especially Est2 (Fig 1C) bound at higher levels to an induced DSB. In contrast, Cdc13 binding to the break (Fig 1E) was not Pif1-sensitive. Thus, the ability of Pif1 to inhibit telomere addition to spontaneous DSBs [19, 20] can be explained by Pif1 removing telomerase from these breaks.
Pif1-mediated removal of telomerase could affect the frequency of telomerase action, its processivity, or its preference for short telomeres. We used two different methods to determine the impact of Pif1 on these events. STEX experiments indicate that telomerase is more processive in vivo in the absence of Pif1, as telomerase added an average of 72 nt (pif1Δ) versus 45 nt (WT) to the VII-L telomere in a single cell cycle (Fig 2). Consistent with this finding, Pif1 reduces telomerase processivity in vitro [22], and telomeres are longer in pif1-m2 and pif1Δ cells [19].
STEX also revealed that the fraction of telomeres acted upon by telomerase in vivo was almost four times higher in pif1Δ (28%) versus WT (7.2%) cells (Fig 2). In contrast, in vitro, Pif1-dependent release of Est2 from telomeric oligonucleotides increases the fraction of elongated oligonucleotides by freeing Est2 from its original substrate [22] to which it is otherwise tightly bound [31]. The fact that the fraction of telomeres lengthened in the presence of Pif1 is lower in vivo and higher in vitro is likely explained by the high concentration of telomeric oligonucleotides in vitro versus the small number of telomeres in cells. In addition, as Est1 and Est3 binding to telomeres is limited to a short period late in S phase [4, 5], telomerase action occurs only during a narrow window of the cell cycle [2, 3] while the standard in vitro system is Est1 and Est3-independent [32].
To determine if Pif1 affects the preferential lengthening of short telomeres, we used an assay in which a single short telomere is induced in cells with otherwise WT length telomeres [6]. This assay was used previously to show that in WT cells, Est2 and Est1 bind preferentially to short telomeres [8, 9]. However, in pif1-m2 cells, we see similar levels of Est2 and Est1 at short and WT length telomeres (Figs 3B and S3). This loss of preferential binding of Est2 and Est1 to the short VII-L telomere in pif1-m2 cells is unlikely to be a consequence of all telomeres being longer in pif1-m2 cells [18, 19]. Even though the average length of the “short” VII-L telomere (±SD) (196 ± 11.7 bp) was longer in pif1-m2 versus WT (124.8 ± 7.2 bps) cells (S2B Fig), 196 bp is still short enough to be preferentially lengthened by telomerase in WT cells [7]. Moreover, levels of Est2 binding to the control VI-R telomere were not affected by Pif1 (nor was Yku telomere binding) (Fig 3C and 3D). Thus, our results cannot be explained by its being more difficult to ChIP Est2 to longer telomeres. Finally, in the control strains the difference in the average post-recombination lengths of the VII-L telomeres in WT versus pif1-m2 cells was even larger (71 bp difference in experimental versus 78 bp in control strains) (S2B Fig), yet the levels of Est2 association in the control strains were not PIF1-sensitive (Fig 3C and 3D). Together with the STEX results, our data demonstrate that Pif1 contributes to the preferential targeting of telomerase to short telomeres.
If Pif1 binds more readily to long telomeres, it could explain how Pif1 contributes to preferential telomerase activity at short telomeres. To test this possibility, we determined the average length of Pif1-associated telomeres. For both, the VI-R and XV-L telomeres, Pif1-associated telomeres were about 70 bps longer than bulk telomeres (Fig 4D). In contrast, using the same assay, Yku80-associated telomeres were the same length as bulk telomeres in the two strains. In addition, in S phase, Pif1 bound better to WT length telomeres than to a very short telomere (Fig 4E and 4F). These data argue that Pif1 contributes to the selective lengthening of short telomeres by binding to and removing telomerase preferentially from longer telomeres. This finding is interesting in light of an in vitro study that used single molecule analyses to show that Pif1 was better able to displace telomerase from substrates with long TG1-3 single-strand tails [33]. If longer telomeres have longer G-tails in vivo, the two observations may be linked. Rif2, another negative regulator of yeast telomerase, also binds to a greater extent at wild type than to short telomeres [12]. Thus, preferential lengthening of short telomeres is achieved by proteins like Pif1 and Rif2 that act preferentially at longer telomeres and proteins like MRX, Tel1, and Tbf1 that act preferentially at shorter telomeres. It is tempting to speculate that Rif2 recruits Pif1 preferentially to long telomeres.
Pif1 efficiently binds to and unwinds G4 structures in vitro and suppresses DNA damage at G4 motifs in vivo (reviewed in [16]; also, [23–25]). Thus, a unifying model for Pif1 action is that it inhibits telomerase by unwinding telomeric G4 structures. However, intra-molecular G4 structures inhibit telomerase [34]. Thus, it is difficult to attribute the inhibitory effects of Pif1 on telomerase to its G4-unwinding activity. However, the presence of G4 structures might stimulate Pif1’s ability to displace telomerase as it does Pif1 unwinding of duplex DNA [35].
Another possibility is that Pif1 uses its ATPase activity to displace the protein subunits of telomerase from DNA ends, the type of protein eviction activity attributed to the closely related S. cerevisiae Rrm3 and the S. pombe Pfh1 helicases during chromosome replication [36, 37]. Although we can not rule out this model, it seems unlikely as Pif1 does not affect Cdc13 [22] or Yku (Fig 3D) telomere binding. Rather we favor a model where Pif1 removes telomerase from telomeres by disrupting the telomerase RNA-telomeric DNA intermediate as Pif1 unwinds RNA/DNA hybrids very efficiently [38, 39].
In summary, the Pif1 helicase affects multiple aspects of the telomerase reaction: it reduces telomerase processivity, the frequency of telomere elongation, and the preference of telomerase for short telomeres. All of these effects could be a result of Pif1 binding preferentially to (and hence telomerase removal from) telomeres that are longer than average in length. Because yeast telomerase is not abundant, with fewer telomerase complexes than telomeres [5, 40, 41], this regulation is important to ensure that those telomeres most in need of lengthening receive telomerase.
Materials and Methods
Yeast strains and growth conditions
Strains and primers are presented in, respectively, Tables 1 and 2. Experiments were carried out in RAD5+ versions of W303, unless otherwise indicated. Deletions eliminated entire ORFs. The pif1-m2 and pif1-K264A strains were made as in [19]. Epitope tagging to generate Est1-G8-Myc9, Est2-G8-Myc18, Cdc13-Myc9, Yku80-G8-Myc18, Pif1-Myc13 and Pif1-K264A-Myc13 was carried out as described [8, 29, 42, 43]. Each Myc-tagged protein was expressed from its endogenous locus and promoter. Except for the experiments using Myc-Pif1-K264A, the tagged protein was the only form of the protein in cells. STEX strains contained URA3 adjacent to the VII-L telomere [44]. The strains used to analyze telomerase binding as a function of telomere length contained a galactose-inducible FLP1 and a modified chromosome VII-L for excision of telomere-adjacent DNA [6].
Tab. 1. Strains used in this study. 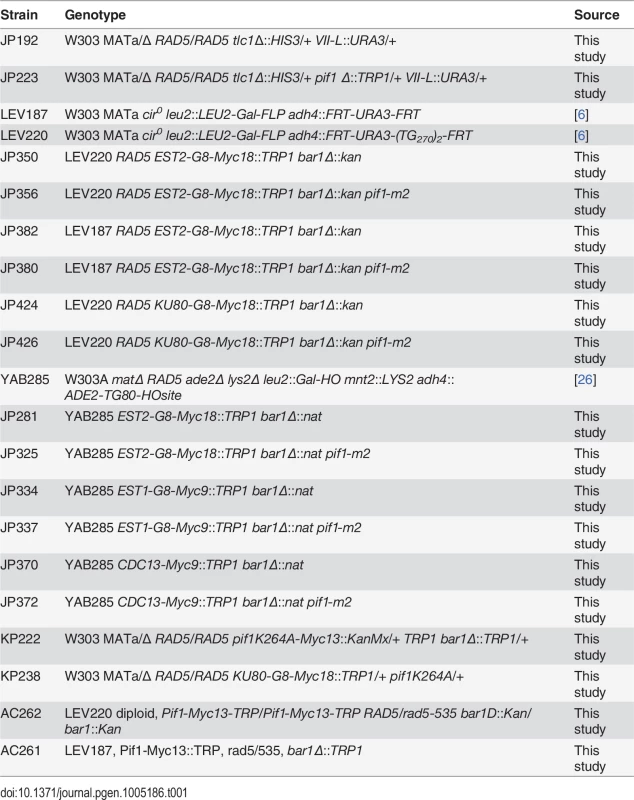
Tab. 2. Primers used in this study. 
* [26]; Strains for DSB experiments had a galactose-inducible HO gene and a modified chromosome VII-L with the HO endonuclease recognition site between ADH4 and MNT2 [26]. A heterozygous (pif1-K264A/PIF1) diploid strain in which either Pif1-K264A or Yku80 was epitope tagged was used to determine the lengths of Pif1-associated telomeres. For experiments using galactose, cells were grown in 3% raffinose prior to induction in 1% galactose. For HO experiments, cells were maintained on media lacking lysine to prevent leaky expression from the HO gene.
Single telomere extension (STEX) assays
Recipient cells were prepared from freshly dissected spore clones from JP192 (tlc1Δ::HIS3 PIF1) or JP223 (tlc1Δ::HIS3 pif1Δ::TRP1) and grown to OD660 0.2. Next, 6 x 107 recipient cells were mated with 1 x 107 of either WT or pif1Δ::TRP1 donor cells freshly dissected from JP192 or JP223. Mating mixtures were filtered onto four membranes (Microfil S, Millipore), placed onto pre-warmed YEPD plates, and incubated at 30°C for 3 h [7]. Cells were resuspended in 30 ml synthetic media lacking histidine (YC-his) and then added to 90 ml of fresh YC-his. Fifty ml resuspended mating cells were taken immediately for analysis; the remaining cells were incubated at 30°C with shaking at 160 rpm. After 2 h (5 h after initial mixing), an additional 50 ml were taken for analysis. Mating efficiency was monitored by flow cytometry by determining the proportion of cells containing >2N DNA content after correcting for the ratio of recipient to donor cells in the mating mixture (i.e., 100% mating of recipient cells results in 16.7% of total cells with >2N DNA content). Reported experiments had >85% mating efficiency. DNA was isolated using Masterpure Yeast DNA Purification Kits (Epicentre). Telomere PCR was performed using 400 ng of DNA as described [7], except that primers TEL7L and PolyG18, specific to the URA3-marked telomere VII-L, were used for amplification (Table 2). PCR products were ligated into the pDRIVE vector (Qiagen) as per the manufacturer’s instructions. Plasmid inserts were sequenced (Genewiz, South Plainfield, NJ) using the SP6 primer. Sequences were aligned by eye and analyzed using the contig assembler of Vector NTI (Invitrogen). Where indicated, Fisher’s Exact tests (which test potential relationships between categorical variables) and Mann-Whitney U tests (non-parametric tests of significance for two independent sample groups) were used to determine statistical significance.
Cell synchrony and induction of telomere shortening or cutting at HO site
Strains used for analysis of protein association at either a short telomere or a double strand break were grown overnight at 30°C in rich media plus 3% raffinose to OD660 0.15. Synthetic Δ-factor was added (final concentration 160 μM), and cells were incubated at 30°C for ~2 h or until > 90% of cells were unbudded (G1 phase). Samples were then taken for flow cytometry, Southern blot analysis, and ChIP (“0-gal” time point). Dry galactose (Sigma) was added to the rest of the culture (final concentration 1%) to induce either the FLP recombinase or the HO endonuclease; cells were incubated at 30°C for 3 h [8]. Cells were filtered and then resuspended in YEPD with a final concentration of 160 μM Δ-factor and incubated for 15 min at 24°C. The cells were filtered again and resuspended in rich medium containing glucose (no Δ-factor) and 170 μg/ml P6911 Protease (Sigma) and released into the cell cycle at 24°C. Samples were taken every 15 min for one cell cycle and analyzed by flow cytometry, Southern blot, and ChIP [45].
Chromatin immunoprecipitation (ChIP)
ChIP was performed as described [46], except that cells were lysed by adding 10 μl 5 mg/ml Zymolyase 100T (MP Biomedicals) to thawed samples, which were then incubated at 37°C for ~10 min or until visual inspection showed >90% lysis. Myc-tagged proteins were immunoprecipitated using an Δ-Myc monoclonal antibody (Clontech). Immunoprecipitated DNA was quantitated by real-time PCR on an iCycler iQ Real-Time PCR Detection System (Bio-Rad Laboratories) as in [45], except that primers were used to amplify either a region adjacent to a short telomere on chromosome VII-L (primers FRT+ and FRT - [8]) and WT telomere VI-R (primers VI-R+ and VI-R-) or a region adjacent to an HO recognition site (primers FTM7 and RTM7 [26]). ChIP samples were normalized to inputs; data are presented as fold enrichment relative to a non-telomeric control site on chromosome IV (ARO1, using primers RT-ARO+ and RT-ARO). Each synchrony was repeated a minimum of three times to obtain an average enrichment value. Error bars represent the standard deviation from three or more independent experiments. A two-tailed students t-test was used to determine statistical significance (p≤0.05).
Determining lengths of Pif1-associated telomeres
After ChIP from asynchronously growing diploid strains, telomere PCR was performed with minor modification of the methods in [12]. Briefly, ChIP samples were C-tailed according to manufactures instructions using T4 polynucleotide kinase (Invitrogen). PCR conditions were 58°C annealing for 30 s and 72°C extension for 45 s. The PCR products were separated on a 1.8% (w/v) MetaPhor (Lonza) agarose gel. A subset of the DNA was cloned and sequenced to establish that it was indeed telomeric DNA: of the 90 sequenced clones, 89% were telomeric DNA while the remainder had little or no insert DNA. The AlphaImager 3400 Molecular Weight Analysis program was used to determine the average telomere length in each sample. Experiments were performed in three biological replicates; telomere length was determined at two different telomeres (VI-R and XV-L).
Supporting Information
Zdroje
1. Lue NF. Plasticity of telomere maintenance mechanisms in yeast. Trends Biochem Sci. 2010;35 : 8–17. PubMed Central PMCID: PMC2818170. doi: 10.1016/j.tibs.2009.08.006 19846312
2. Marcand S, Brevet V, Mann C, Gilson E. Cell cycle restriction of telomere elongation. Curr Biol. 2000;10 : 487–90. 10801419
3. Diede SJ, Gottschling DE. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases alpha and delta. Cell. 1999;99 : 723–33. 10619426
4. Taggart AKP, Teng S-C, Zakian VA. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science. 2002;297 : 1023–6. 12169735
5. Tuzon CT, Wu Y, Chan A, Zakian VA. The Saccharomyces cerevisiae telomerase subunit Est3 binds telomeres in a cell cycle - and Est1-dependent manner and interacts directly with Est1 in vitro. PLoS Genet. 2011;7:e1002060. PubMed Central PMCID: PMCPMC3088721. doi: 10.1371/journal.pgen.1002060 21573139
6. Marcand S, Brevet V, Gilson E. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 1999;18 : 3509–19. 10369690
7. Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase - extendible and-nonextendible states. Cell. 2004;117 : 323–35. 15109493
8. Sabourin M, Tuzon C, VA Z. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol Cell. 2007;27 : 550–61. PubMed Central PMCID: PMCPMC2650483. 17656141
9. Bianchi A, Shore D. Increased association of telomerase with short telomeres in yeast. Genes Dev. 2007;21 : 1726–30. PubMed 17639079.
10. Arneric M, Lingner J. Tel1 kinase and subtelomere-bound Tbf1 mediate preferential elongation of short telomeres by telomerase in yeast. EMBO Rep. 2007;8 : 1080–5. PubMed 17917674.
11. Hector R, Shtofman R, Ray A, Chen B, Nyun T, Berkner K, et al. Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol Cell. 2007;27 : 851–8. 17803948
12. McGee J, Phillips J, Chan A, Sabourin M, Paeschke K, Zakian VA. Reduced Rif2 and lack of Mec1 target short telomeres for elongation rather than double-strand break repair. Nature Struct Molec Biol 2010;17 : 1438–45. PubMed Central PMCID: PMCPMC3058685. doi: 10.1038/nsmb.1947 21057524
13. Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev 1997;11 : 748–60. 9087429
14. Teng S-C, Chang J, McCowan B, Zakian VA. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol Cell. 2000;6 : 947–52. 11090632
15. Hirano Y, Fukunaga K, Sugimoto K. Rif1 and rif2 inhibit localization of tel1 to DNA ends. Mol Cell. 2009;33 : 312–22. Epub 2009/02/17. doi: S1097-2765(09)00024-0 [pii] doi: 10.1016/j.molcel.2008.12.027 PubMed 19217405.
16. Bochman ML, Sabouri N, Zakian VA. Unwinding the functions of the Pif1 family helicases. DNA Repair (Amst). 2010;9 : 237–49. PubMed Central PMCID: PMCPMC2853725. doi: 10.1016/j.dnarep.2010.01.008 20097624
17. Foury F, Lahaye A. Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J. 1987;6 : 1441–9. 3038524
18. Zhou J-Q, Monson EM, Teng S - C, Schulz VP, Zakian VA. The Pif1p helicase, a catalytic inhibitor of telomerase lengthening of yeast telomeres. Science. 2000;289 : 771–4. PubMed Central PMCID: PMCPMID:10926538. 10926538
19. Schulz VP, Zakian VA. The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell. 1994;76 : 145–55. PubMed Central PMCID: PMCPMID:8287473. 8287473
20. Myung K, Chen C, Kolodner RD. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature. 2001;411 : 1073–6. PubMed Central PMCID: PMCPMID:11429610. 11429610
21. Makovets S, Blackburn EH. DNA damage signalling prevents deleterious telomere addition at DNA breaks. Nat Cell Biol. 2009;11 : 1383–6. Epub 2009/10/20. doi: ncb1985 [pii] doi: 10.1038/ncb1985 PubMed 19838171.
22. Boule J, Vega L, Zakian V. The Yeast Pif1p helicase removes telomerase from DNA. Nature. 2005;438 : 57–61. PubMed Central PMCID: PMCPMID:16121131. 16121131
23. Paeschke K, Bochman ML, Garcia PD, Cejka P, Friedman KL, Kowalczykowski SC, et al. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature. 2013;497 : 458–62. PubMed Central PMCID: PMCPMC3680789. doi: 10.1038/nature12149 23657261
24. Saini N RS, Elango R, Ayyar S, Zhang Y, Deem A, Ira G, Haber JE, Lobachev KS, Malkova A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature. 2013;502(7471):389–92. doi: 10.1038/nature12584 PubMed Central PMCID: PMCPMC3804423. 24025772
25. Wilson MA KY, Xu Y, Chung WH, Chi P, Niu H, Mayle R, Chen X, Malkova A, Sung P, Ira G. Pif1 helicase and Polδ promote recombination-coupled DNA synthesis via bubble migration. Nature. 2013;502(7471):393–6. doi: 10.1038/nature12585 PubMed Central PMCID: PMCPMC3915060. 24025768
26. Bianchi A, Negrini S, Shore D. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol Cell. 2004;16 : 139–46. PubMed 15469829.
27. Forstemann K, Lingner J. Molecular basis for telomere repeat divergence in budding yeast. Mol Cell Biol. 2001;21 : 7277–86. PubMed 11585910.
28. Wang S-S, Zakian VA. Sequencing of Saccharomyces telomeres cloned using T4 DNA polymerase reveals two domains. Mol Cell Biol. 1990;10 : 4415–19. PubMed Central PMCID: PMCPMC361005. 2196453
29. Paeschke K, Capra JA, Zakian VA. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell. 2011;145 : 678–91. PubMed Central PMCID: PMCPMC3129610. doi: 10.1016/j.cell.2011.04.015 21620135
30. Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107 : 67–77. 11595186
31. Prescott J, Blackburn E. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 1997;11 : 2790–800. 9353249
32. Lingner J, Cech TR, Hughes TR, Lundblad V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci USA. 1997;94 : 11190–5. 9326584
33. Li JR YT, Chien IC, Lu CY, Lin JJ, Li HW. Pif1 regulates telomere length by preferentially removing telomerase from long telomere ends. Nucleic Acids Res. 2014;42(13):8527–36. doi: 10.1093/nar/gku541 PubMed Central PMCID: PMCPMC4117769. 24981509
34. Zahler AM, Williamson JR, Cech TR, Prescott DM. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991;350 : 718–20. 2023635
35. Duan XL LN, Yang YT, Li HH, Li M, Dou SX, Xi XG. G-Quadruplexes Significantly Stimulate Pif1 Helicase-catalyzed Duplex DNA Unwinding. The Journal of Biological Chemistry. 2015. PubMed Central PMCID: PMCPMID: 25627683.
36. Ivessa AS, Lenzmeier BA, Bessler JB, Goudsouzian LK, Schnakenberg SL, Zakian VA. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol Cell. 2003;12 : 1525–36. PubMed Central PMCID: PMCPMID:14690605. 14690605
37. Sabouri N, McDonald KR, Webb CJ, Cristea IM, Zakian VA. DNA replication through hard-to-replicate sites, including both highly transcribed RNA Pol II and Pol III genes, requires the S. pombe Pfh1 helicase. Genes Dev. 2012;26 : 581–93. PubMed Central PMCID: PMCPMC3315119. doi: 10.1101/gad.184697.111 22426534
38. Boule JB, Zakian VA. The yeast Pif1p DNA helicase preferentially unwinds RNA DNA substrates. Nucleic Acids Res. 2007;35 : 5809–18. PubMed Central PMCID: PMCPMC2034482. 17720711
39. Zhou R ZJ, Bochman ML, Zakian VA, Ha T. Periodic DNA patrolling underlies diverse functions of Pif1 on R-loops and G-rich DNA. Elife. 2014;3. doi: 10.7554/eLife.02190 PubMed Central PMCID: PMCPMC3999857
40. Mozdy AD, Cech TR. Low abundance of telomerase in yeast: implications for telomerase haploinsufficiency. RNA. 2006;12 : 1721–37. PubMed 16894218.
41. Wu Y, Zakian VA. The telomeric Cdc13 protein interacts directly with the telomerase subunit Est1 to bring it to telomeric DNA ends in vitro. PNAS 2011;108 : 20362–9. PubMed Central PMCID: PMCPMC3251085. doi: 10.1073/pnas.1100281108 21969561
42. Tsukamoto Y, Taggart AKP, Zakian VA. The role of the Mre11-Rad50-Xrs2 complex in telomerase-mediated lengthening of Saccharomyces cerevisiae telomeres. Curr Biol. 2001;11 : 1328–35. 11553325
43. Sabourin M, Tuzon C, Fisher T, Zakian V. A flexible protein linker improves the function of epitope-tagged proteins in Saccharomyces cerevisiae. Yeast. 2007;24 : 39–45. 17192851
44. Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63 : 751–62. PubMed Central PMCID: PMCPMID:2225075. 2225075
45. Goudsouzian L, Tuzon C, Zakian VA. S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol Cell. 2006;24 : 603–10. 17188035
46. Fisher T, Taggart A, Zakian V. Cell cycle-dependent regulation of yeast telomerase by Ku. Nat Struct Mol Biol. 2004;11 : 1198–205. 15531893
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



