-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
Expression quantitative trait loci (eQTLs) are genetic variants associated with gene expression phenotypes. Mapping eQTLs enables us to study the genetic basis of gene expression variation across individuals. In this study, we introduce a statistical framework for analyzing genotype-expression data collected from multiple population groups. We show that our approach is particularly effective in identifying multiple independent eQTL signals that are consistently presented across populations in the proximity of a gene. In addition, our analysis framework allows effective integration of genomic annotations into eQTL analysis, which is helpful in dissecting the functional basis of eQTLs.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005176
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005176Summary
Expression quantitative trait loci (eQTLs) are genetic variants associated with gene expression phenotypes. Mapping eQTLs enables us to study the genetic basis of gene expression variation across individuals. In this study, we introduce a statistical framework for analyzing genotype-expression data collected from multiple population groups. We show that our approach is particularly effective in identifying multiple independent eQTL signals that are consistently presented across populations in the proximity of a gene. In addition, our analysis framework allows effective integration of genomic annotations into eQTL analysis, which is helpful in dissecting the functional basis of eQTLs.
Introduction
Expression quantitative trait loci, or eQTLs, are genetic variants that are associated with gene expression levels. Mapping eQTLs can help in dissecting the molecular mechanisms by which genetic variants impact organismal phenotypes. Recent studies [1–3] have revealed that there are substantial overlaps between eQTLs and genetic variants identified from genome-wide association studies (GWAS) of disease phenotypes. In addition, eQTL mapping provides a powerful tool for investigating the regulatory machinery in different tissues [4, 5] or cellular environments [6–8].
In this paper, we jointly address three outstanding issues in eQTL mapping. First, due to the high experimental cost, most available eQTL data sets typically have limited sample sizes. To improve power of eQTL discovery, it becomes necessary to aggregate evidence across multiple data sets. Second, because a gene is typically regulated by many regulatory elements, it is highly likely that there exist multiple independent eQTLs in its proximity (i.e., cis region). In this scenario, a multi-SNP analysis is required to uncover all relevant cis acting genetic factors involved in the gene regulation process [9]. Third, the availability of extensive functional annotations [10–12] now enables integration of functional genomic information into eQTL analysis, which can be useful to dissect the functional basis of eQTLsf. Linking genomic annotations to eQTLs goes beyond genetic association analysis, and helps gain a better understanding of the underlying biological processes. Individually, some of these three issues have been discussed by previous works. For example, [3, 9, 13–16] discussed single SNP analysis of eQTLs jointly from different studies, populations or tissues. But these methods do not naturally extend to multi-SNP analysis. [17–20] examined the enrichment of selected genomic features in cis-eQTLs, mostly based on single SNP association results. To the best of our knowledge, there is no existing approach that jointly addresses all three issues in a systematic way.
In this paper, we demonstrate an integrative analysis framework to perform multi-SNP fine mapping analysis of eQTLs using cross-population samples. Our statistical methods stem from an established Bayesian framework proposed by [14, 15, 21], which has been successfully applied in mapping eQTLs from multiple tissues. We apply our statistical framework to analyze the data from the GEUVADIS project [20], where the expression-genotype data are collected from five population groups. In GWAS, trans-ethnic meta-analysis of genetic association data from diverse populations has been shown to be a powerful tool in detecting novel complex trait loci and improving resolution of fine mapping of causal variants by leveraging population heterogeneity in local patterns of linkage disequilibrium (LD) and allele frequencies [22, 23]. This approach, to the extent of our knowledge, has not been applied to eQTL analysis. Utilizing cross-population expression-genotype data, we are interested in identifying eQTL signals that show consistent effects in all populations. Furthermore, we aim to examine whether we have sufficient statistical power to identify multiple independent cis-eQTL signals with the available aggregated sample size. Finally, we set out to investigate whether the genetic variants that disrupt transcription factor (TF) binding are enriched in eQTLs, and we integrate such annotations to further improve fine mapping analysis of eQTLs. Our three main aims are also inherently inter-related and reinforce each other. With higher power through sample aggregation in mapping eQTLs, our method can identify at a high resolution potentially multiple genomic regions that harbor casual eQTLs. Consequently, these efforts improve the statistical power and precision of localization for our functional analysis.
Results
Method Overview
We start with a brief description of our statistical framework and general strategy for multi-SNP fine mapping analysis in a meta-analytic setting across multiple populations.
Statistical Model and Inference
Consider a genomic region with p SNPs that is interrogated in s different population groups. In each group i, we use a multiple linear regression model to describe the potential genetic associations between the p SNPs and the expression levels of a target gene:
where the vectors yi and ei represent the expression levels and the residual errors in population group i, and the parameters μi and σ i 2 denote the intercept and the residual error variance specific to the population group. The vector gi,j denotes the genotype of SNP j in population group i, and the regression coefficient βi,j represents its genetic effect. Across all population groups, the s linear models form a system of simultaneous linear regressions (SSLR, [15]).The problem of mapping eQTLs can be framed as identifying SNPs with non-zero βi,j values. For each SNP j, we define an indicator vector
to represent its association status in each of the s population groups. Such indicator is referred to as a “configuration” in the literature of genetic association analysis across multiple subgroups [14, 15, 24]. For each target gene, our computational procedure is designed to make joint inference on all p SNPs with respect to Γ: = {γ1, …, γp} given observed genotype data, G: = {g1,1, …, g1,p, …, gs,p}, and expression phenotype data, Y: = {y1, …, ys}.For mapping eQTLs in a cross-population meta-analytic setting, we make an important assumption that if a SNP is genuinely associated with a given expression phenotype, its underlying genetic effects are non-zero in all population groups. That is, γj = 1, if SNP j is an eQTL, and 0 otherwise. This assumption is largely motivated by the biological hypothesis that the regulatory mechanisms behind eQTLs likely remain the same across populations. Here, we acknowledge that there exists convincing evidence for population specific eQTLs [20], however, by employing the above assumption, our analysis focuses on identifying eQTLs whose effects are consistent across populations, which are the vast majority of the cases [21].
For each SNP j, we assume an independent prior for γj, which assigns most of the probability mass on γj = 0 and encourages an overall sparse structure of Γ. This is because most previous studies only identified small numbers of cis-eQTLs for any given gene. Particularly in our fine mapping analysis where p is typically in the magnitude of 103 to 104, we use
which implies that we expect a single cis-eQTL signal for the target gene a priori, as We will further justify this prior specification based on our overall strategy for fine mapping analysis, and discuss some other alternative prior specifications for enrichment analysis in the later sections.Conditional on SNP j being an eQTL, we model its genetic effects across populations, βj: = (β1,j, …, βs,j), using a flexible Bayesian prior for meta-analysis proposed in [21]. Briefly, we assume that the effect sizes of an eQTL are highly correlated while allowing some reasonable degree of heterogeneity across population groups (See the Material and Methods section for details).
Given observed data (Y, G) and a specified Γ value, a Bayes factor
can be analytically approximated with high accuracy following [15]. Based on this result, it is straightforward to compute the posterior of Γ using the Bayes rule, i.e., We implement an efficient MCMC algorithm to perform the posterior inference and summarize the fine mapping results from the posterior samples. More specifically, we compute the posterior probability for each possible Γ by the corresponding frequency in the posterior samples. We will refer to this quantity as “posterior model probability” henceforth. To evaluate the importance of each SNP, we compute a posterior inclusion probability (PIP) for each SNP by marginalizing over all posterior model probabilities. If a SNP is included in posterior models with high frequencies, its corresponding PIP tends to be large.Dealing with Genetic Data from Multiple Populations
We note that analyzing eQTL data using samples collected from multiple populations is slightly different from the standard settings in meta-analysis of genetic associations. In particular, the allele frequencies of interrogated SNPs and/or patterns of linkage disequilibrium presented in genotype data can be highly heterogeneous in different populations. The proposed multi-SNP fine mapping procedure takes advantage of the unique setting of cross-population samples, and leverages statistical power for eQTL discovery.
The allele frequency of a SNP is not directly connected to its underlying genetic effect with respect to expression levels. However, because of its impact on sampling errors, it affects the precision of estimated βi,j (denoted by β ^ i , j) in model (Eq 1). Lower allele frequency typically results in larger uncertainty (e.g., the standard error of β ^ i , j of a rare SNP is usually larger than that of a common SNP), which implies that the estimates of rare SNPs tend to be noisy and less informative. In the extreme case if a SNP is monomorphic in samples from a particular population, it should be considered completely uninformative for examining genetic associations. As shown by [15], Bayesian procedures, especially in use of Bayes factors, can precisely capture the (non-)informativeness of SNPs in each population group: e.g., including the genetic data of monomorphic SNPs yields identical inference results as discarding such SNPs from corresponding population groups. On the other hand, our adopted Bayesian meta-analysis prior enables aggregating less informative or weak association signals from low frequency SNPs, as long as they are consistent across population groups.
In pursuing consistent association signals, our fine mapping procedure takes advantage of potential varying LD patterns across multiple population groups. This is mainly because our method favors identifying eQTLs whose effects are consistent in all population groups, whereas SNPs that tag causal variants only in some populations (due to population-specific LD structures) are automatically down-weighted. As a consequence, the genomic regions that harbor causal eQTLs can be effectively narrowed down using cross-population data. This advantage becomes even more obvious when performing multiple SNP analysis, as all candidate cis-SNPs are simultaneously evaluated.
Gene-level Testing Prior to Fine Mapping cis-eQTLs
In most currently available data sets, eQTL discoveries are only confidently made in a proportion of genes. To reduce the computational cost of the fine mapping analysis, we adopt a practical procedure that first screens the genes having at least one cis-eQTL (which we will call “eGenes”).
The statistical procedure to identify eGenes across multiple subgroups has been well established in [14]. More specifically in the context of cross-population eQTL mapping, we assume a similar linear model system as (Eq 1), and perform gene-level Bayesian hypothesis testing. Namely, for each gene, we test
versus The eGenes are identified upon rejecting the corresponding null hypotheses, and we only follow up the eGenes with the multiple cis-eQTL analysis.The gene-level testing is computationally efficient, and it effectively filters out a substantial set of genes that are unlikely to be interesting for fine mapping. Furthermore, this additional procedure justifies our use of prior (Eq 2): given that a gene is identified as an eGene, expecting a single cis-eQTL prior to the fine mapping analysis seems, on average, a slightly conservative assumption.
Enrichment Analysis and Integration of Genomic Features
We further consider incorporating additional SNP-level functional annotations in our multiple cis-eQTL analysis to address the following two closely related problems:
assessing the enrichment of certain functional annotations in eQTL signals
integrating the enriched functional annotations to improve the resolution of fine mapping analysis of eQTLs
The first problem of enrichment assessment can be framed as a hypothesis test of correlations between the (eQTL) association status of all cis-SNPs (γj’s) and their functional annotations. Note, to properly quantify the level of enrichment, it is required to jointly analyze all available gene-SNP pairs instead of focusing only on eGenes. The intrinsic statistical challenge lies in the fact that the values of γj’s are not directly observed and the inference is required from the expression-genotype data. Here, we propose to test the association between the PIPs from the output of the multi-SNP cis-eQTL analysis, Pr(γj | Y, G), and the genomic annotations of interest in a logistic regression model framework. More specifically, we first carry out the proposed Bayesian multi-SNP analysis to compute PIPs for all gene-SNP pairs using the following prior specification,
In particular, we determine α0 in a similarly conservative way as (Eq 2) using the results from gene-level testing as follows: Let ge and gs denote the number of eGenes identified and the number of total gene-SNP pairs in the analysis, respectively. We set which essentially assumes that each eGene contributes only a single associated gene-SNP pair, and corresponds to α 0 = log ( κ 1 − κ ). We then fit a logistic regression model by correlating the resulting PIP with the genomic annotations of each SNP and controlling for other potential contributing factors. The key idea is that, under the described settings, the PIPs are computed assuming the null model of no enrichment of any sort. As a consequence, a strong association between the PIPs and the annotations of interest presents evidence against the null hypothesis.Upon successful enrichment assessments of genomic annotations, our Bayesian framework provides a natural way to integrate relevant annotations into the fine mapping analysis. In particular, we use a generalization of prior specification (Eq 4) for Pr(γj) to incorporate the genomic annotations of SNP j with respect to the target gene, i.e.,
where δkj denotes the k-th annotation for SNP j. The regression coefficient αk, which we refer to as “enrichment parameter”, represents the strength of association between the k-th genomic annotation and the prior probability of a SNP being an eQTL. Our inference procedure based on the Bayesian hierarchical model with prior specification (Eq 6) involves estimating both the enrichment parameters αk’s and γj’s in an iterative fashion. We give the details of this procedure and its statistical justification in the Materials and Methods section.Simulation Studies
We performed a series of simulation studies to evaluate the proposed Bayesian multi-SNP fine mapping procedures. Specifically, we simulated under the setting where eQTL data are collected from multiple population groups. This section briefly summarizes the main simulation results, and we leave the relevant details in the Materials and Methods section.
First, we examined the power of the proposed Bayesian multi-SNP analysis approach in identifying multiple independent association signals using cross-population samples. In particular, we compared its performance with the commonly applied single SNP meta-analysis procedure and a (step-wise) conditional meta-analysis procedure [25, 26], treating each population group as a participating study in a meta-analysis. To simulate the genotype-expression data in multiple population groups, we used the real genotypes of 2,500 SNPs from 100 randomly selected and distinct genomic regions (i.e., 25 consecutive SNPs per region) in the GEUVADIS data. As a consequence, there are generally modest to high levels of LD within each region, but unremarkable levels of LD between regions. (In other words, we assembled 100 relatively independent LD blocks for each gene.) It is worth noting that within each region, the variation in patterns of LD between populations is maintained. We randomly assigned one to four eQTL SNPs for each gene and simulated their expression levels according to a system of linear models (see Material and Methods section and Section S.5 of S1 Text). For evaluation, we assessed the ability of each procedure in correctly identifying the 25-SNP regions (i.e., the LD blocks) that harbor the true eQTLs. The simulation results indicate that the proposed Bayesian multi-SNP analysis approach is the most powerful in identifying independent eQTL signals among the three methods compared (Fig 1). Further investigation reveals that all three methods achieve similar power when genes contain only a single eQTL; however when a gene harbors multiple independent cis-eQTLs, the proposed Bayesian fine mapping approach becomes highly advantageous (S1 Fig). In addition, the simulation results show that the reported PIPs from the proposed Bayesian approach are well-calibrated (S2 Fig).
Fig. 1. The comparison of powers in identifying independent causal eQTL regions. 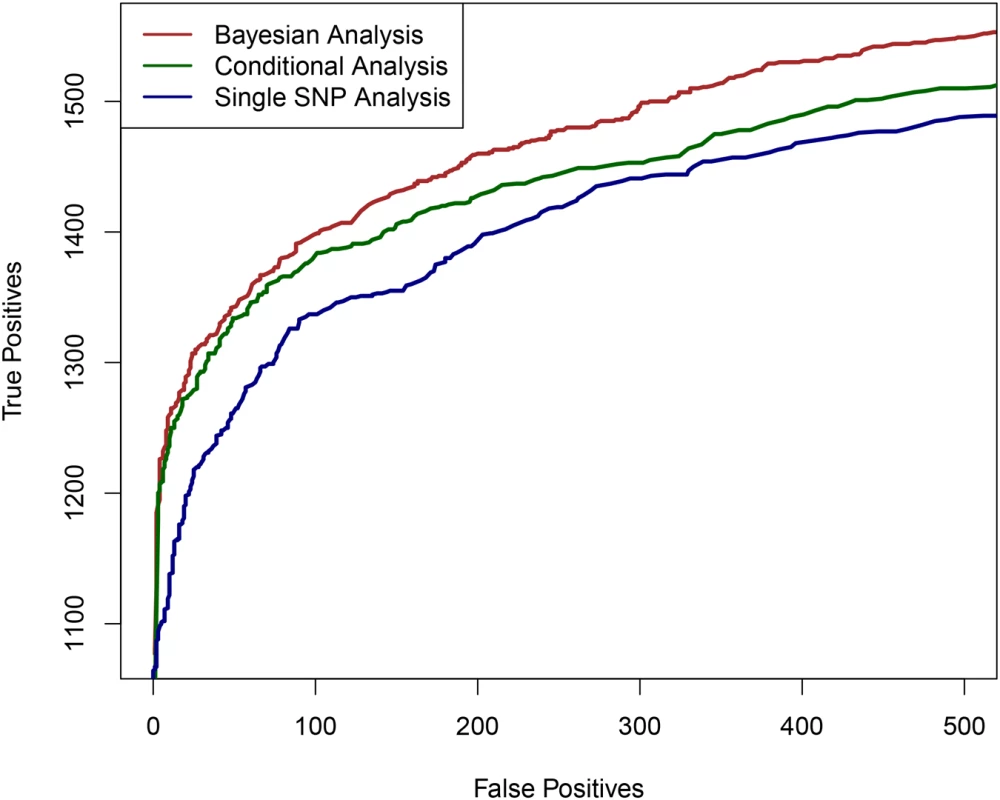
We compare the performance of the three competing methods in determining the regions harboring the true causal eQTL in a cross-population meta-analytic setting: the proposed Bayesian multi-SNP analysis method (brown line), a conditional meta-analysis approach (dark green line) and a single SNP meta-analysis approach (navy blue line). Each plotted point on the figure represents the number of true positive findings versus the number of false positive findings of a given method at a particular threshold. For any false positive value, the proposed Bayesian approach always yields the most true positive findings. We also performed additional simulations to evaluate the proposed computational procedures for enrichment testing and fine mapping analysis integrating genomic features. In brief, we found that the proposed procedures have greatly improved power in testing enrichment of genomic features when compared with standard approaches based on single SNP testing results (S2 Table). In addition, we observed that the proposed computational procedure provides accurate estimates of the enrichment parameters (S3 Fig).
Analysis of GEUVADIS Data
In this paper, we focused on analyzing the expression and genotype data collected from the GEUVADIS project [20]. More specifically, the data set consists of RNA-seq data on lymphoblastoid cell line (LCL) samples from five populations: the Yoruba (YRI), CEPH (CEU), Toscani (TSI), British (GBR) and Finns (FIN). In our analysis, we selected 420 samples which were densely genotyped in the 1000 Genomes Phase I data release [27] and 11,838 protein coding genes and lincRNAs that are deemed expressed in all five population groups. Throughout, our analysis focused on the SNPs that locate within a 200kb genomic region centered at the transcription start site (TSS) of each gene. In contrast to the original eQTL mapping approach discussed in [20], we treated each population as a single group and performed cis-eQTL analysis jointly across all five groups. We have made the full analysis results available on the website http://www-personal.umich.edu/~xwen/geuvadis/.
Power Gain in gene-level Meta-Analysis
We started our analysis of the GEUVADIS data by performing gene-level testing jointly across all five population groups.
In total, we identified 6,555 eGenes from 11,838 tested protein coding and lincRNA genes at 5% FDR level. For comparison, the numbers of eGenes identified using each population data alone are given in Table 1. The separate analysis identifies no more than 2,100 eGenes in any of the population groups. The union of the eGenes from the separate analysis yields 3,447 genes, the vast majority of which (except for 60 genes) are included in the discoveries by the joint meta-analysis. In addition, the meta-analysis identifies a total of 3,168 new eGenes.
Tab. 1. Comparison of eGene Discovery by Separate vs. Meta Analysis. 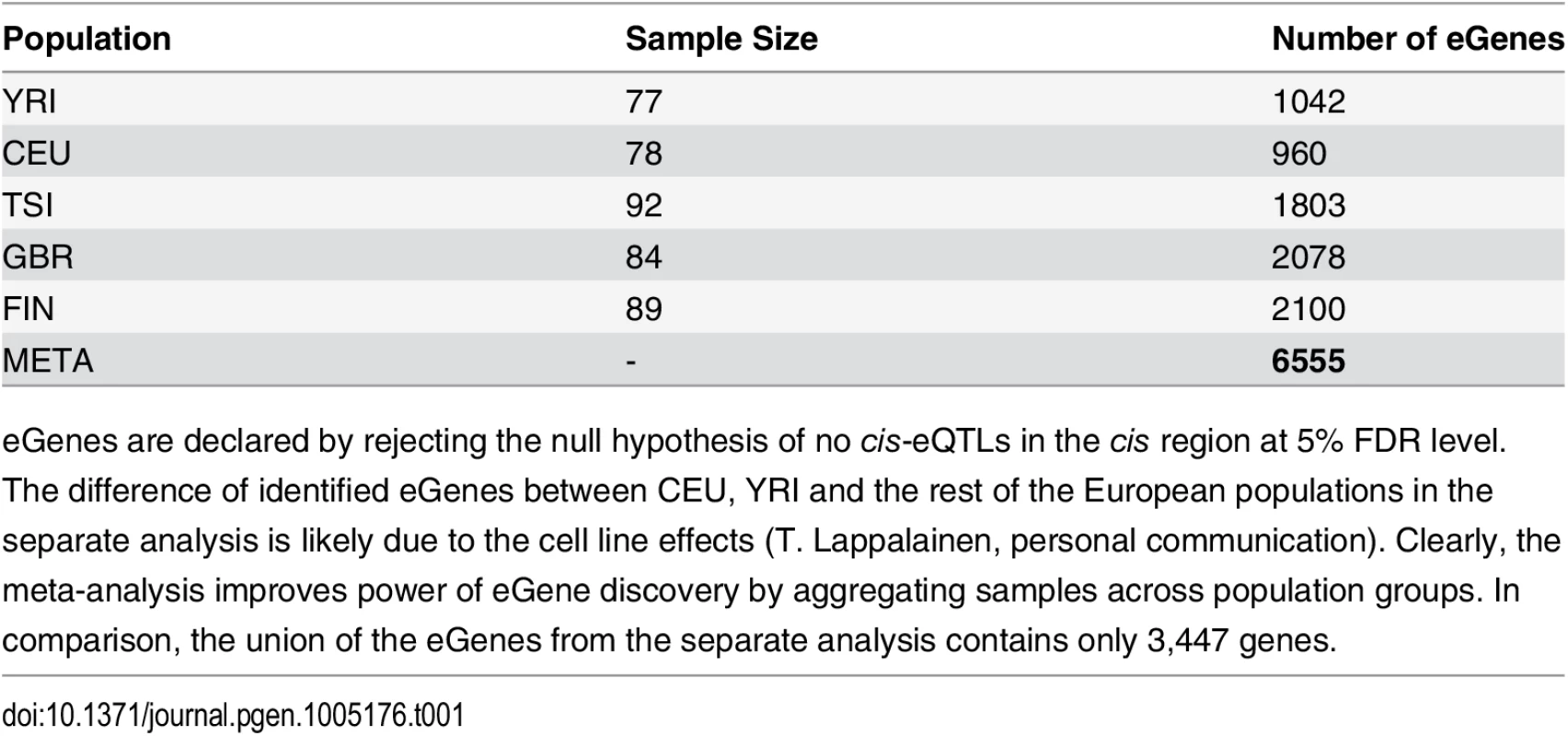
eGenes are declared by rejecting the null hypothesis of no cis-eQTLs in the cis region at 5% FDR level. Examining the set of eGenes uniquely identified in the meta-analysis, we found that they share the following common feature: when performing separate analysis within each population group, the strongest association signal in each respective cis-region only shows modest strength and does not pass the required significance threshold; however, across population groups, the same association signal tends to be highly consistent, and the overall evidence aggregated across population groups becomes quite strong. As a consequence, the joint analysis of all population groups is able to detect such signals. We demonstrate one of such examples in Fig 2 using gene NME1 (Ensembl ID: ENSG00000239672), where the gene-level Bayes factor in the joint analysis is several orders of magnitude higher than the corresponding gene-level Bayes factors in each separate population group analysis.
Fig. 2. An example of modest yet consistent eQTL signals across population groups. 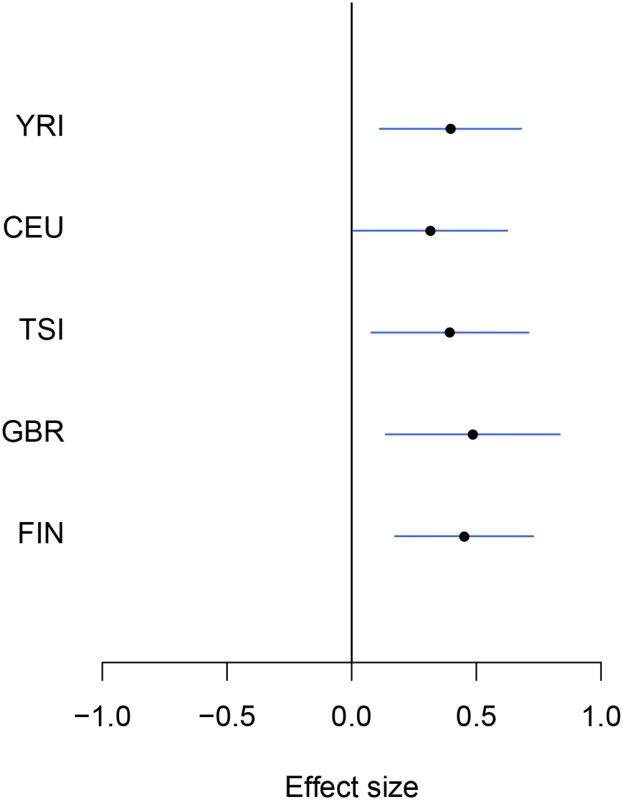
The forest plot shows the genetic effects of SNP rs7207370 with respect to the expression levels of gene NME1 (Ensembl ID: ENSG00000239672). SNP rs7207370 is one of the top associated cis-SNPs in all population groups, yet the strengths of the association signals are modest in all groups (the maximum single SNP Bayes factor, among five groups, is 18.0 in FIN, the corresponding gene-level Bayes factor is 1.8). As a consequence, the gene is not identified as an eGene in any of the separate analyses. Across populations, the SNP exhibits a strongly consistent association pattern. In the cross-population meta-analysis, the gene-level Bayes factor reaches 1.1 × 104 (single SNP Bayes factor for rs7207370 is 9.5 × 105). Multi-SNP analysis of eGenes
We followed up the gene-level analysis by performing multi-SNP fine mapping for the set of identified eGenes across all five population groups.
One of our primary aims is to identify potential multiple independent cis-eQTL signals while accounting for varying LD patterns in different populations. First, we asked how often we can identify multiple cis-eQTL signals in eGenes. To this end, for each fine mapped eGene, we computed the expected number of independent cis-eQTL signals from the corresponding posterior distributions. Fig 3 shows the histogram of posterior expected cis-eQTL signals in all 6,555 eGenes. It is clear that for the available (accumulated) sample size in the GEUVADIS data, we identified only single eQTL signals for the majority of the eGenes. Nevertheless, for a non-trivial proportion of genes, there is strong evidence that multiple cis-acting regulatory genetic variants co-exist and can be confidently identified. More specifically, there are about 14% of the eGenes (or 7% of all interrogated genes) with the posterior expected number of cis-eQTLs ≥ 2.
Fig. 3. Histogram of posterior expected number of cis-eQTLs in 6,555 identified eGenes. 
The figure indicates that we identified only single cis-eQTLs for most eGenes. However, for a non-trivial proportion of eGenes, multiple independent eQTL signals were identified. For example, in the case of gene LHPP (Ensembl ID: ENSG00000107902), four independent eQTL signals are confidently identified (Fig 4). Each eQTL signal (except one) is represented by a cluster of highly correlated SNPs in a small genomic region. Due to LD, within each cluster the correlated SNPs tend to have similar PIPs and we cannot be certain which SNP is truly driving the association signal. However, the sum of the PIPs within each cluster is very close to 1, indicating near certainty that an eQTL is located within the region. There were 134 different posterior models examined for gene LHPP in the sampling phase of the MCMC run. Interestingly, every model contains exactly 4 SNPs (which results in posterior expected number of cis-eQTLs being 4 with variance 0), each from one of the four independent clusters.
Fig. 4. An example of a gene harboring four independent cis-eQTL signals. 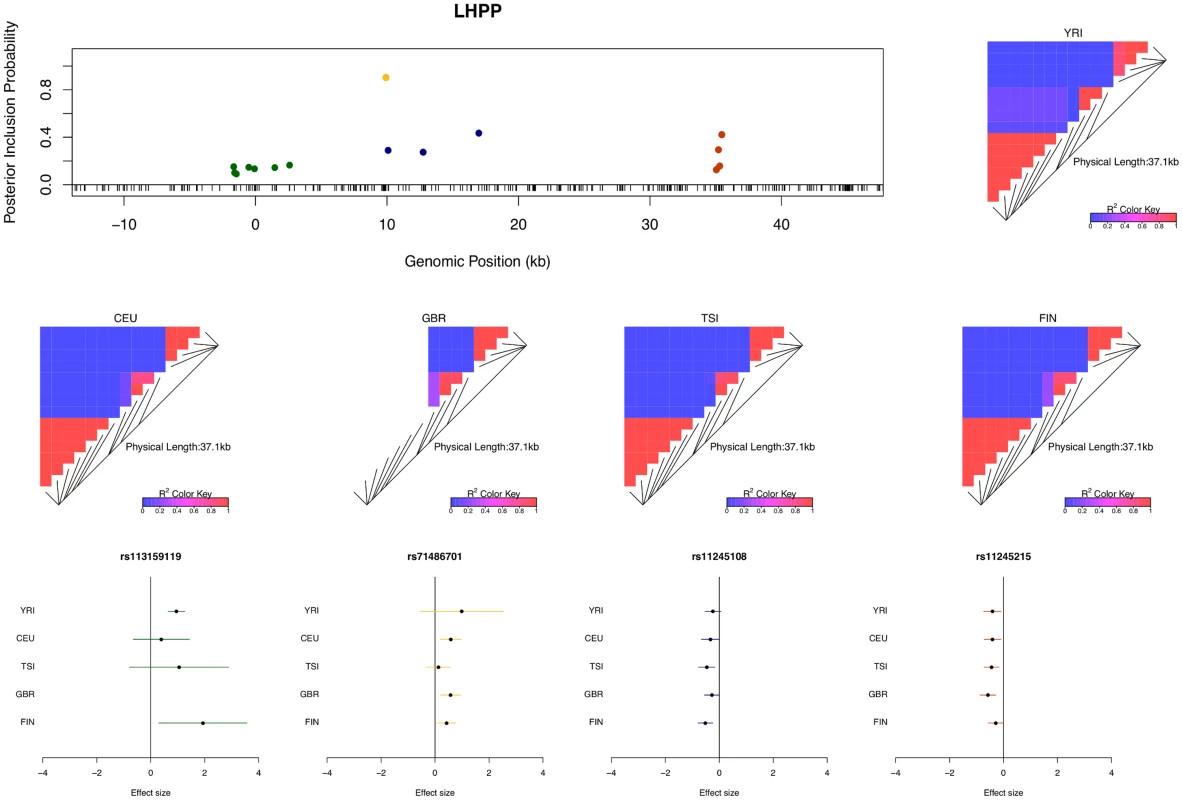
The top left panel plots the cis-SNPs with PIP ≥ 0.02. The locations of the SNPs are labeled with respect to the TSS of gene LHPP. The ticks on the x-axis indicate all interrogated cis-SNPs in the region. The SNPs with the same color are in high LD and represent the same eQTL signal. In the plot, the sums of the PIPs from the SNPs in the same colors are all ∼ 1, indicating that we are confident of the existence of each signal. The heatmaps show the LD patterns in each of the population group. They are qualitatively similar, except that the SNPs representing the first signals are monomorphic in GBR. In the bottom panel, we plot the effect sizes of eQTLs jointly estimated from one of the high posterior probability models. Each of the SNP plotted belongs to a different colored cluster in the PIP plot (as indicated by the color coding of the error bars). The effect sizes and standard errors are estimated from the multiple linear regression models (containing all four SNPs) separately fitted in each population group. All the signals show strong effect size consistency across populations. As previously discussed, even in the cases when only a single cis-eQTL signal is identified, we observed that the fine mapping procedure takes advantage of varying LD patterns across populations and narrows down the set of candidate causal variants by down-weighting SNPs that tag causal variants only in some populations. For example, in analyzing the data of gene AGO3 (Ensembl ID: ENSG00000126070) from TSI alone, we identified a strong single eQTL signal within a 144kb region. The region contains 41 SNPs that are in perfect LD and show equal strength of associations. In the other four populations, this particular region is broken into smaller LD blocks and the associations for the 41 SNPs become distinguishable. As a result, when performing multiple-SNP analysis across all populations, we narrowed down the potential causal eQTL into a 1.2kb region, with only 3 candidate SNPs (in high LD in all populations) fully explaining the observed association (Fig 5).
Fig. 5. An example of automatic LD filtering across population groups. 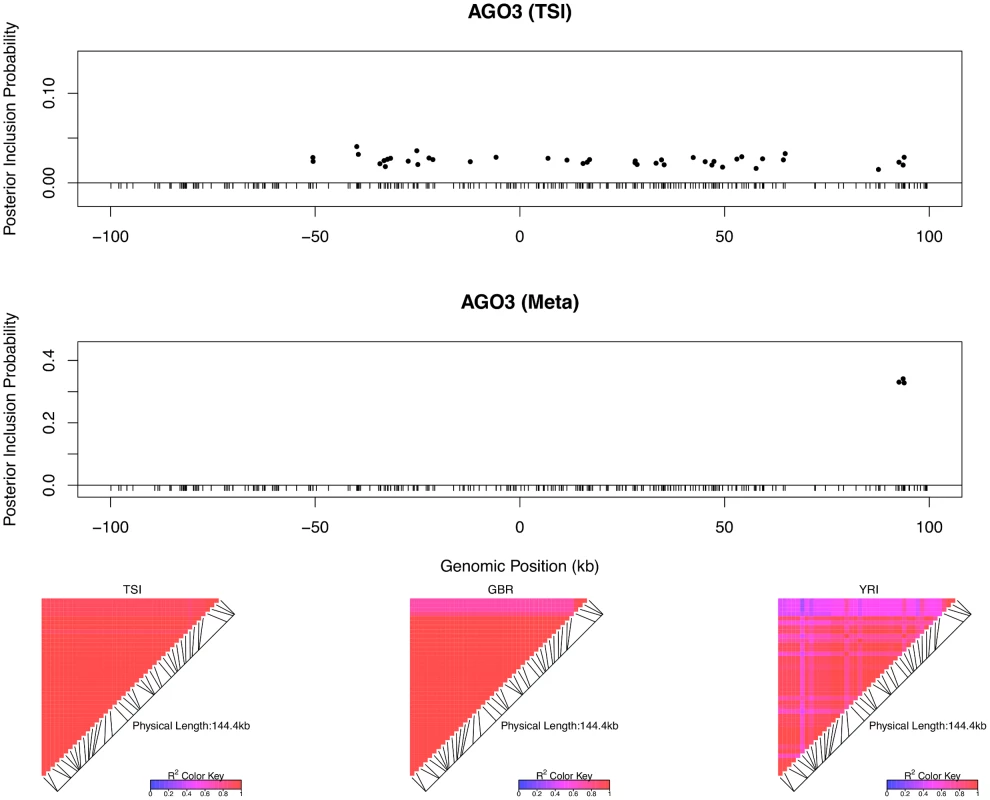
The top panel shows the result of multiple cis-eQTL analysis for gene AGO3 using only the data from TSI. The SNPs with PIPs ≥ 0.02 are plotted. All SNPs plotted are in high LD in TSI, and the sum of the PIPs across the genomic region is close to 1. The region spanned by the signals is ∼ 140 kb. We repeated the analysis jointly across all five populations, the SNPs with PIPs ≥ 0.02 are plotted in the middle panel. The genomic region harboring the eQTL is narrowed down into a 1.2 kb region enclosed by three SNPs each with PIP ∼ 0.33. The bottom panel shows the LD heatmaps between the 41 SNPs plotted in the top panel in TSI, GBR and YRI, respectively. The multiple cis-eQTL mapping method takes advantage of the varying LD patterns across populations, and automatically narrows down the region harboring the true causal cis-eQTL. To further quantify the effect of LD filtering, we selected a set of 526 eGenes that are highly likely to harbor exactly one cis-eQTL based on our multi-SNP analysis. More specifically, we selected the genes whose posterior expected number of cis-eQTLs = 1 and variance ≤ 1 × 10−4. We then ran multiple cis-eQTL analysis separately in each of the five populations for each selected gene. For every multi-SNP analysis of each gene, including the meta-analysis, we constructed a 95% credible set that contains the minimum number of SNPs with the sum of PIPs ≥ 0.95, and defined its credible region length as the distance between the right-most and left-most SNPs in the set. The histogram of reduction in credible region length by cross-population analysis is shown in Fig 6. In 486 out of 526 (or 92%) genes, we found that the cross-population analysis yields the smallest credible region length. The median ratio of credible region length by the joint analysis to the minimum credible region length by the corresponding separate analyses is 0.50 across all 526 genes, indicating that, on average, cross-population meta-analysis can effectively narrow down the genomic regions that harbor cis-eQTLs.
Fig. 6. Histogram of reduction in 95% credible region length by cross-population joint analysis. 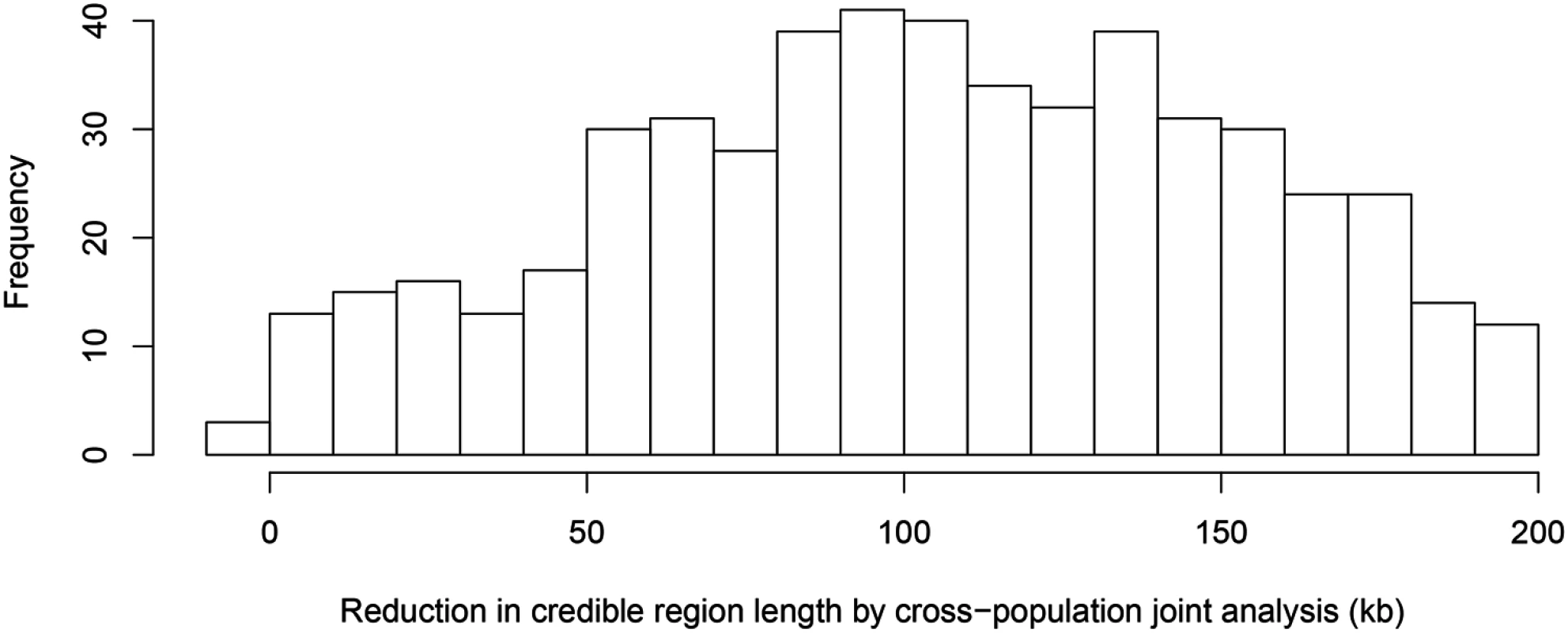
The histogram shows the differences in the credible region lengths between the average of the five separate population analyses and the joint analysis. The analysis is performed using the set of 526 eGenes that highly likely harbor exactly one cis-eQTL. Only 3 out of 526 eGenes show (slightly) increased credible region lengths (negative reduction values) in the joint analysis. Multi-SNP analysis can also be very helpful in explaining some of the extreme heterogeneity of eQTL effects across populations observed in single SNP analysis. In particular, we identified a few SNPs that, when analyzed alone, appear to show strong but opposite genetic effects on expression levels in different populations. It seemingly suggests that a particular allele of the variant increases expression levels of the target gene in one population and decreases expression levels in another population. However, going through all such individual examples, we found that none of the opposite effect association patterns is supported by our multi-SNP analysis. In mapping cis-eQTLs for gene TTC38 (Ensembl ID: ENSG00000075234), we found a set of tightly linked SNPs displaying opposite directional effects in YRI and the European populations in single SNP analysis. For example, the A allele of SNP rs6008600 shows consistently strong negative effect in the four European populations, whereas in YRI the same allele displays a highly significant positive effect (Fig 7). The multi-SNP fine mapping offers a compelling explanation for this phenomenon: two independent cis-eQTL signals were identified in the nearby regions, and interestingly, SNP rs6008600 tags one signal in YRI (r2 ≈ 0.73), whereas in the European populations, it is highly correlated (e.g., in CEU r2 ≈ 1) with the other signal (Fig 7).
Fig. 7. Multi-SNP analysis explains strong effect size heterogeneity observed in single SNP analysis. 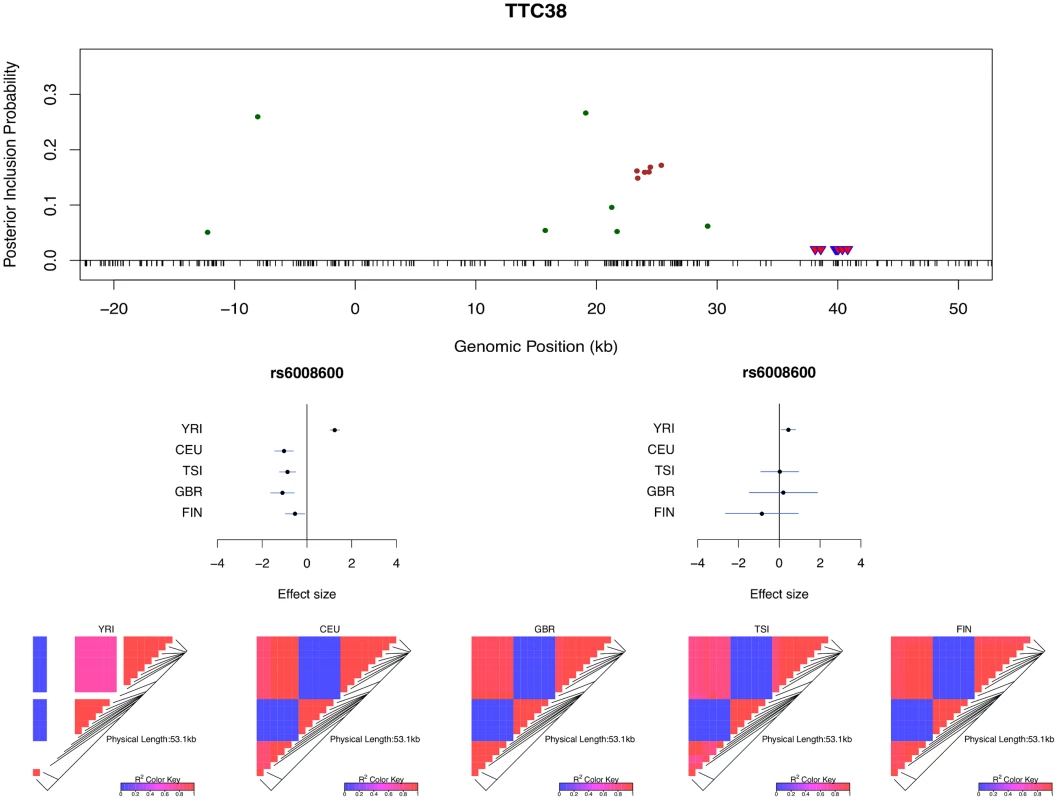
SNP rs6006800 and the SNPs in LD are labeled by the purple triangles in the top panel. They display strong but opposite effects in the European and YRI populations when analyzed alone. The middle left panel shows the effect sizes of rs6008600 separately estimated in the five populations by the single SNP analysis. The top panel shows the multiple cis-eQTL analysis result. SNPs with PIPs ≥ 0.02 are plotted. The result suggests that there are two independent signals in the region, with one represented by SNPs colored in green, and the other represented by SNPs colored in brown. The sums of the green and brown SNPs are both very close to 1. SNP rs6008600 and the SNPs in LD all have PIPs ∼ 0 in the multiple cis-eQTL analysis. The middle right panel shows the effect sizes of rs6006800 estimated from the multiple linear regression models controlling for the two independent signals in each population: the genetic association observed in the single SNP analysis is seemingly “explained away” by the two independent signals identified by the fine mapping analysis. The bottom panel shows LD heatmaps between SNPs highlighted in the top panel (green, brown SNPs and the SNPs labeled by the purple triangles) in the five populations. Some of the green SNPs are monomorphic in YRI. The opposite effects of rs6006800 are clearly explained by the varying LD patterns: rs6006800 is in high LD with the brown SNPs in YRI, whereas in European populations it tags the green SNPs. In analyzing the GEUVADIS data, we found the above phenomenon is not uncommon. To further investigate, we followed the procedure described in [21] and quantified the heterogeneity of eQTL effects for all gene-SNP pairs of the identified eGenes in a single SNP analysis. More specifically, for each SNP, we separately computed a Bayes factor (BFfix) assuming constant eQTL effects across populations (i.e., the fixed effect model) and a Bayes factor (BFmaxH) assuming completely independent eQTL effects across populations. As the most extreme opposite to the fixed effect model, the independent effect assumption corresponds to a model allowing the maximum level of effect size heterogeneity [21]. We then computed a ratio, BFmaxH/BFfix, which itself is a valid Bayes factor measuring the consistency of eQTL effects across population: a large value of the ratio (≫ 1) indicates that the data favor the maximum heterogeneity model and the eQTL effects lack of consistency across populations, whereas a small value (≪ 1) shows that the data support the fixed effect assumption. Based on this analysis, we identified 263 eGenes containing at least one SNP for which BFmaxH/BFfix ≥ 105, i.e., the evidence for inconsistent eQTL effects is overwhelming in single SNP analysis. Most of those 263 gene-SNP pairs display a pattern of seemingly population-specific effects. For example, the value of BFmaxH/BFfix for SNP rs6008600 in the above example is ∼ 1024. For those 263 most extremely heterogeneous single SNP association signals (the list is provided in S1 Dataset), we repeated the calculation of the heterogeneity measure but controlled for the consistent cis-eQTL signals identified in our multi-SNP analysis. Fig 8 compares the distributions of the BFmaxH/BFfix in the single and the multi-SNP analysis models, respectively. The result indicates a striking reduction in heterogeneity by considering multi-SNP analysis models: the median and maximum values of BFmaxH/BFfix statistics reduce to 1.1 and 103, respectively. This result seemingly suggests that a large proportion of observed heterogeneity in single SNP eQTL mapping is attributed to the varying LD patterns across populations. As a result, when the LD patterns are explicitly considered/modeled in multi-SNP eQTL analysis, we observe more consistent eQTL effects across populations.
Fig. 8. Distributions of SNP effect size heterogeneity from single and multi-SNP analyses. 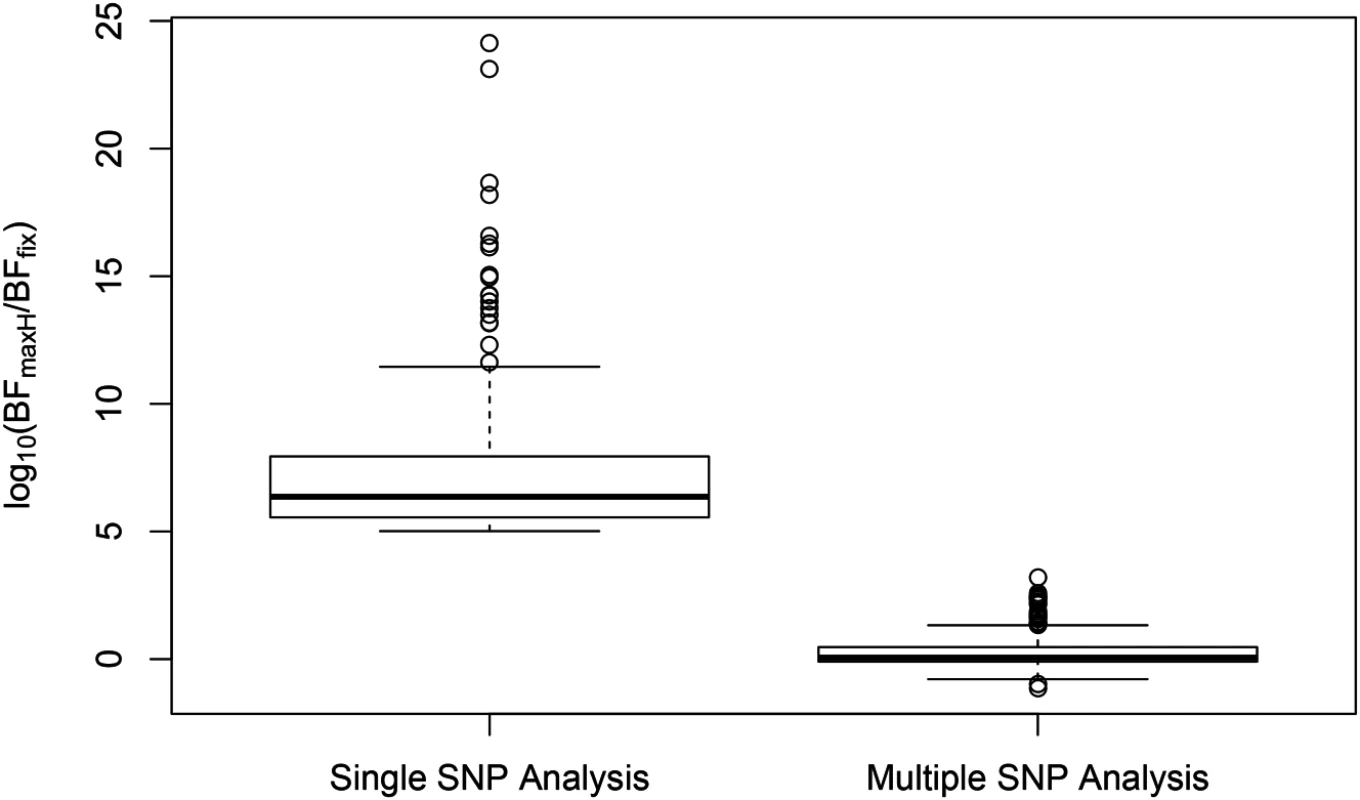
The heterogeneity of SNP effects across the five populations is measured by log10[BFmaxH/BFfix], for which large values suggest highly heterogeneous (i.e., inconsistent) genetic effects in different populations. Each point on the plot represents a SNP in a unique gene showing large effect size heterogeneity in the single SNP analysis (log10[BFmaxH/BFfix] ≥ 5). When controlling the consistent cis-eQTL signals identified by the fine mapping approach in the multi-SNP analysis, we observe a great reduction of effect size heterogeneity. Although our multi-SNP cis-eQTL mapping method confidently identifies independent association signals accounting for LD, in most examples we have examined, it usually cannot pinpoint a single causal SNP by fully resolving LD relying only on the association data. This is because highly correlated SNP genotypes are nearly “interchangeable” in our statistical association models, and therefore not identifiable. As a consequence, there are many combinations of SNPs showing equivalence or near equivalence based on observed data. Reporting a single “best” model while ignoring its intrinsic uncertainty can be highly problematic. In the previously mentioned example of gene LHPP, we found that a large proportion of the 134 reported posterior models by the MCMC algorithm exhibit very similar likelihood and posterior probabilities, and the maximum posterior model probability is only 0.02. Our Bayesian fine mapping approach summarizes the measure of uncertainties using the posterior probabilities both at model and SNP levels, which can be naturally carried over to downstream analysis.
Functional Annotations of eQTLs
Using the GEUVADIS data and integrating genomic annotations, we set out to investigate the enrichment of certain functional genomic features annotated at SNP level in cis-eQTLs. To this end, we conducted the multiple cis-eQTL analysis for all 11,858 genes using the prior (Eq 5) to obtain PIPs for all gene-SNP pairs, and then examined the correlations between the resulting PIPs and the genomic features of interest. As a side note, notwithstanding the conceptual difference between priors (Eq 2) and (Eq 5), we noted that the overall results of multiple cis-eQTL analysis for identified eGenes are markedly similar.
We first examined the relationship between the abundance of eQTL signals and the SNP distances to the TSS of their respective target genes. For this purpose, we grouped all the cis-SNPs into non-overlapping 1Kb bins according to their distances to the respective TSS. We then computed the posterior expected numbers of eQTL signals within each bin by summing over the PIPs of all the cis-SNPs falling in the bin. The results are summarized in Fig 9, which displays a strong enrichment of cis-eQTL signals nearby the TSS. It is clear that cis-eQTLs tend to cluster around the TSS, and the decay of eQTL signals away from the TSS is fast. In particular, Fig 9 suggests that 50% cis-eQTL signals are concentrated within 20kb of TSS. Although this phenomenon is well known [5, 18], our results display a much cleaner and more striking pattern comparing to the previous reports. In particular, it is worth noting that, comparing to the similar analysis based on single SNP association test results, our result indicates a much faster rate of decay in abundance of cis-eQTLs away from the TSS. This is most likely because we considered multiple cis-eQTL signals for a target gene, and our use of PIP accounted for the uncertainty of eQTL calls.
Fig. 9. Enrichment of cis-eQTLs with repect to distance to TSS. 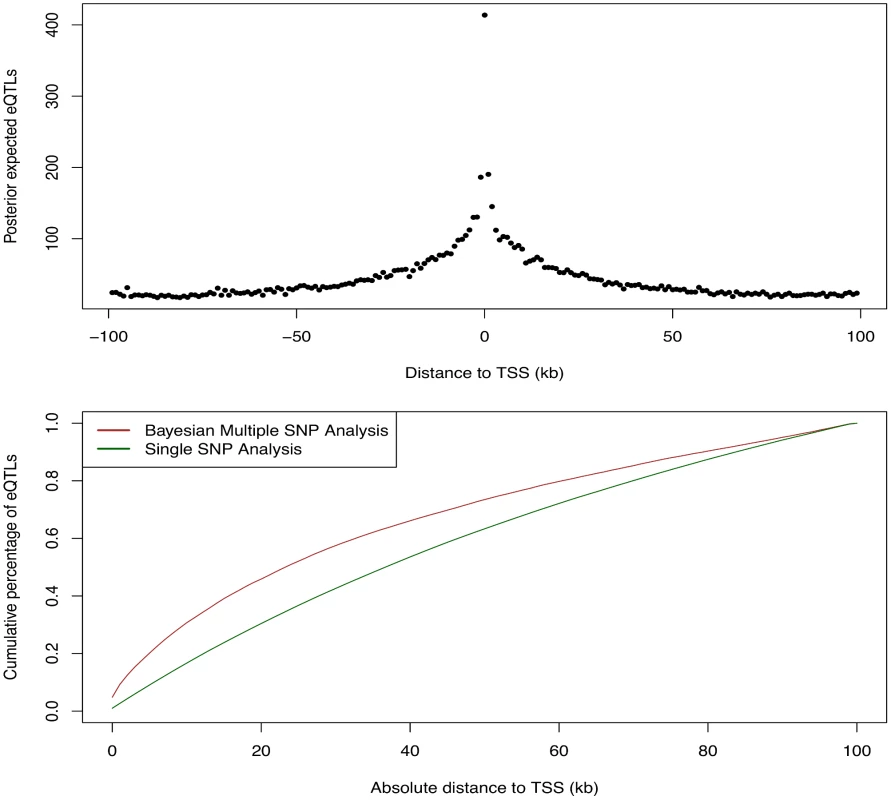
The top panel shows the distribution of posterior expected number of cis-eQTLs with respect to SNP distance to TSS. The bottom panel shows the estimated cumulative percentage of cis-eQTLs with respect to SNP disance to TSS from two different methods. The brown line represents the estimate using the PIPs from the proposed Bayesian approach. The dark green line represents the estimate from a standard method based on single SNP association results. More specifically, the dark green line is estimated by counting the number of SNPs exceeds FDR 5% threhold in each distance bin. The brown line shows a much faster decay in abundance of cis-eQTL signals away from TSS. We next asked whether eQTLs are enriched for genetic variants disrupting TF binding. Answering this question helps reveal the underlying regulatory logic linking between transcription factor binding and gene expression [17, 18, 28, 29]. To this end, we used the base-pair resolution quantitative annotation of binding variants derived from DNase-seq data for LCLs in the ENCODE project (the detailed description of this annotation and the methods used to generate it can be found in [30] and also in S1 Text). Briefly, for each TF motif a re-calibrated CENTIPEDE model [11] is built to integrate its DNase-seq footprint shape characteristics and the TF sequence preference of binding. Then, each genetic variant from the 1000 Genomes project is annotated if it overlaps with a CENTIPEDE footprint for a TF. The sequence model for the TF is also used to evaluate the prior probability for each of the two alleles to predict the impact of the genetic variant on binding. There are three mutually exclusive scenarios using this annotation:
SNPs that are not located in any DNase I footprint region, or in brief, baseline SNPs
SNPs that are in a footprint region but predicted to have little or no impact on TF binding based on the sequence model, or in brief, footprint SNPs
SNPs that are in a footprint region and predicted to strongly affect TF binding, or in brief, binding variants
We provide the complete inference results, including the estimates of the enrichment parameters for all bins defined by the distance to the TSS, in S2 Dataset. In brief, our main findings from this analysis are:
binding variants are 1.49 fold (with 95% confidence interval [1.38, 1.63]) more likely than baseline SNPs to be eQTLs, their enrichment in eQTLs is statistically highly significant (p-value = 4.93 × 10−22)
footprint SNPs are 1.15 fold (with 95% confidence interval [1.04, 1.27]) enriched in eQTLs, comparing to baseline SNPs, with the corresponding p-value = 0.0035
Finally, we returned to the fine mapping analysis and quantitatively incorporated the binding annotation information into the cis-eQTL discovery. More specifically, we plugged in the point estimates of the enrichment parameters from the association test to re-compute the Pr(γj) using the logistic model (Eq 6) for each gene-SNP pair, and re-ran the MCMC algorithm with the updated priors. As a result, the priors for binding variants are up-weighted compared with the nearby baseline and footprint SNPs. Because the computation of likelihood function is intact, the PIPs for binding variants are accordingly up-weighted. Using this approach, it becomes plausible to quantitatively distinguish SNPs in perfect LD but belonging to different annotation categories. Take Gene LY86 (Ensembl ID: ENSG00000112799) as an example (Fig 10). Before incorporating any annotation information, each cis-SNP was equally weighted a priori and the multiple cis-eQTL analysis identified three independent signals, two of which are represented by clusters of highly correlated SNPs. Utilizing the quantitative annotation information, the fine mapping procedure still confidently identifies the three original eQTL signals, however two binding variants become the top associated SNPs (according to the PIPs) in each respective cluster of SNPs that are indistinguishable from several other SNPs in the original analysis (Fig 10).
Fig. 10. Comparison of fine mapping results of gene LY86 before and after incorporating functional annotations. 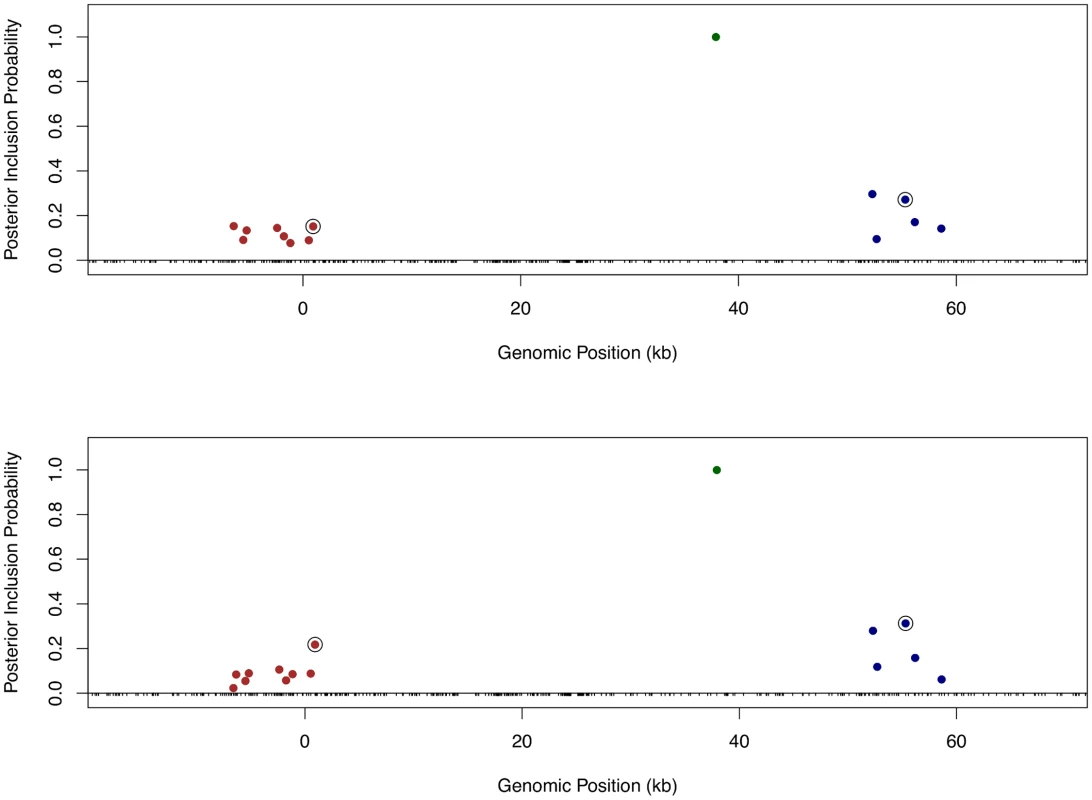
The top panel is based on the analysis using prior (Eq 5) without annotation information. The bottom panel is based on the analysis using prior (Eq 6) incorporating SNP distances to TSS and the annotations for binding variants and footprint SNPs. In both panels, SNPs with PIPs ≥ 0.02 are plotted. There are clearly three independent cis-eQTLs in the region, represented by different colors of SNPs. SNPs in smae colors are in high LD. The sums of PIPs from SNPs in same colors are all close to 1. The circled points are predicted binding variants. It is clear that binding variants are up-weighted when annotation information is incorporated into the fine mapping analysis. In the set of 526 genes that we confidently identified as harboring only one single cis-eQTL signal, when applying the equal prior without any annotation information, we found that binding variants and footprint variants are top associated SNPs in 11% and 8% of the genes (or 60 and 43 genes in number), respectively. Using the priors incorporating quantitative binding annotation and SNP distance to the TSS, the percentages of genes with binding variants and footprint SNPs as top associated SNPs increase to 16% and 11% (or 85 and 57 genes in number), respectively.
Discussion
We have presented an integrative analysis framework to perform fine mapping analysis jointly from cross-population samples while incorporating functional annotations. Our core statistical methods are built on and naturally extend the established Bayesian framework for association analysis of genetic data from heterogeneous groups [14, 15, 21]. The most notable features of our statistical framework are its ability of multi-SNP analysis and integration of SNP-level genomic features. Through simulation studies, we have demonstrated the power and efficiency of the proposed approaches. For the first time, we have applied this framework for multiple cis-eQTL fine mapping analysis in a cross-population meta-analytic setting using the GEUVADIS data.
Our analysis reveals that with an aggregated sample size of around 400, multiple independent cis-eQTL signals can be confidently identified in many genes, which makes evident the necessity of considering multiple association signals in eQTL studies. The commonly applied strategy for mapping additional association signals is to conduct conditional analysis, which can be viewed as a step-wise variable selection algorithm. Besides lack of power, one additional disadvantage of conditional analysis is that it only reports a single “best” model in the end, and completely ignores its uncertainty. From many of our examples shown in this paper, it is clear that in most cases there is typically a great deal of uncertainty in determining the truly causal SNPs due to LD, and the posterior probabilities of the “best” models are often unimpressive. Consequently, it is generally inappropriate to solely rely on the information from the “best” model in the downstream analysis (as we have illustrated in the power simulations of enrichment testing). In comparison, in addition to gaining power in identifying multiple independent cis-eQTL signals, our Bayesian approach provides much more comprehensive information that fully conveys the uncertainty of the inference result, and the quantified uncertainty information is naturally propagated in our integrative analysis of genomic features.
In this paper, we have employed a two-step procedure that first screens eGenes by performing gene-level hypothesis testing, and then carries out multi-SNP analysis for the identified eGenes. This procedure is analogous to the fine mapping procedure that is commonly used in GWAS, where interesting loci are ranked and selected by single SNP association testing before an in-depth analysis focusing on each flagged high priority locus. We find this procedure not only yields considerable computational savings, but also provides a sound argument to specify the prior inclusion probability Pr(γj).
It should be noted that our proposed analysis procedures are completely applicable for fine mapping of general QTLs in either a meta-analytic or single study setting. They can be further extended to the applications of fine mapping analysis where subgroups of eQTL data are formed by different tissues [4, 14] or cellular conditions [6, 8]. Comparing to the meta-analytic setting considered in this paper, the parameter space of {γj} in those applications is more complicated (which includes 2s potential values, where s is the number of subgroups/tissues). Nevertheless, [14] has provided a principled way to “learn” the priors on possible values that γj can take by pooling information across genes through a hierarchical model.
Our fine mapping results of eQTLs also demonstrate the benefit of utilizing cross-population samples in genetic association studies. Most importantly, the population heterogeneity of local LD patterns serves as an efficient filter that narrows down the regions harboring casual eQTLs. Nevertheless, varying LD patterns can cause some SNPs to display large degree of heterogeneity across populations in their estimated effect sizes from single SNP analysis: in the extreme cases, a SNP may appear to possess strong “population specific” effects. As we acknowledge that genuine population specific eQTLs are certainly interesting phenomena and very much likely exist, we suggest interpreting highly heterogeneous eQTL signals from single SNP analysis with caution. In our view, it may be necessary to carry out multi-SNP analysis, as we have demonstrated in this paper, to simply rule out the possibility that the seemingly population specific effects are artifacts due to varying LD patterns.
Our Bayesian inference framework naturally incorporates functional annotations in fine mapping eQTLs across population groups. This feature allows us to quantitatively assess the enrichment of certain functional features in eQTLs, and in turn to use the quantified enrichment information to prioritize annotated SNPs for fine mapping analysis. Overall, our model for integrative eQTL mapping analysis is similar to those presented in [17, 18]. However, these previous approaches make simplifying assumptions to restrict at most one cis-eQTL per gene, such that single SNP association results can be directly used. Our method relaxes this assumption and is fully integrated into our multi-SNP analysis procedure. In addition, our use of CENTIPEDE annotation to examine the relative enrichment of binding variants and footprint SNPs is also novel. Although it is largely expected that binding variants are enriched in eQTLs, it is important to note that the level of enrichment for footprint SNPs is much lower than that for binding variants. Interestingly, this finding seems concurring with the results reported by [30] where the relative enrichment of binding variants vs. footprint SNPs in other cellular and organismal phenotype QTLs is examined.
Last but not least, our multi-SNP fine mapping analysis of the GEUVADIS data has created a comprehensive resource for the community to gain better understanding of the genetic basis of gene regulation. With proper uncertainty assessments, our results enable follow-up experimental validations and functional studies of causal genetic variants that alter gene regulation. They also provide a unique and powerful resource to study population genetics of expression traits. For example, we found that the distribution of Fst of eQTL signals consistent across populations has a shorter tail than the distributions of random SNPs (Kolmogorov-Smirnov test p-value = 0.001). Nevertheless, when examining the Fst distributions between primary and secondary cis-eQTL signals (stratified by the association strength in single SNP analysis with respect to their target genes), we did not find statistical evidence to differentiate the two (Kolmogorov-Smirnov test p-value = 0.77). There are many interesting aspects to be explored with our analysis results, and we will leave the more in-depth investigations for our future work.
Materials and Methods
The computational methods used in the analysis are implemented in the software packages FM-eQTL (manuscript in preparation) and eQTLBMA [14]. They are freely available at https://github.com/timflutre/eqtlbma and https://github.com/xqwen/fmeqtl
The fine mapping results of all 6,555 identified eGenes, including the scatter plots of PIPs, forest plots of top models, and the detailed summaries from separate linear regression analysis are made available on the website http://www-personal.umich.edu/~xwen/geuvadis/
Data Pre-processing
The genotype and RNA-seq data were downloaded from the GEUVADIS project website. We selected 420 samples whose genotypes are directly measured in the 1000 Genomes project. The samples are evenly distributed in the five population groups, and the detailed breakdown of samples by population is shown in Table 1.
For the RNA-seq data, we used a slightly more stringent threshold than the original analysis [20] to select genes that are expressed in all five populations. Specifically, for each selected gene, we required > 90% individuals in each population group to have RPKM ≥ 0.1. From the 17,361 Ensembl genes that passed this filter, following the original analysis, we selected a subset of 11,838 genes consisting of annotated protein-coding genes and lincRNAs according to GENCODE [31] release 17. We log transformed the RPKM values, and used the pipeline employed in [29, 32] to remove the effect of GC content on expression measurements. We then followed the same strategy as described in [20] to remove latent confounding factors using the software package PEER [33]. However, unlike the original analysis, we ran PEER for each population group separately. In the end, we removed 15, 13, 15, 20 and 20 PEER factors for samples from YRI, CEU, TSI, GBR and FIN, respectively. Finally, the expression levels of each gene were quantile normalized across individuals separately in each population group.
For genotype data, we filtered out SNPs whose sample allele frequencies < 0.03 in the overall samples across population groups. Note, we did not apply the allele frequency filter in each population group. The SNPs passing this filter must have sample allele frequencies ≥ 0.03 in at least one population group. In general, as discussed in the Results section, rare SNPs do not impose any statistical or computational problems for our analysis. Nevertheless, removing SNPs that are not likely informative in any population group helps improve computational efficiency. Following [17, 32], we defined a 200kb cis region for each gene centered at its TSS. In total, the final data set contains 6.7 million gene-SNP pairs.
Gene-level Analysis of cis-eQTLs
For each gene, we tested the null hypothesis that asserts no cis-eQTLs. Specifically, we adopted the Bayesian hypothesis testing procedure discussed in [14]. Essentially, [14] assumes a Bayesian model that is mostly similar to (Eq 1), except for an additional simplifying assumption, “at most one cis-eQTL per gene”. Given a gene with p cis-SNPs, this additional assumption reduces the possible alternative scenarios into p single SNP association models, for which a gene-level Bayes factor can be easily computed analytically by Bayesian model averaging. In the context of eQTL mapping in multiple tissues, [14] considered all possible configurations, i.e.,γj values, for each assumed associated SNP, whereas in our analysis of the GEUVADIS data we only allowed γj ∈ {0, 1} to focus on discovering population consistent cis-eQTLs.
Briefly, for the alternative model where the j-th SNP is assumed the lone eQTL, the linear model (Eq 1) is simplified to
We modeled the correlation of genetic effects, βi,j’s across population groups through the following prior specification, Equivalently, the above prior can be represented by a multivariate normal distribution by integrating out the average effect parameter β ‾ j, i.e., where the s × s variance-covariance matrix W is given by The parameter ϕ2 + ω2 characterizes the overall genetic effects of SNP j, and ϕ2/(ϕ2 + ω2) represents the degree of heterogeneity across population groups. Following [14, 15] we considered the values of ϕ2 + ω2 from a set E = {ϕ2 + ω2 : 0.12, 0.22, 0.42, 0.82, 1.62} which covers a wide range of plausible magnitude of genetic effects. We allowed limited degree of heterogeneity by taking ϕ2/(ϕ2 + ω2) values from the set H = {ϕ2/(ϕ2 + ω2) : 0, 0.1} which reflects our prior belief that effects of genuine eQTL signals should be highly consistent across population groups. Overall, we considered a combination of |E| × |H| grid for (ϕ2, ω2) values for each alternative model. Given this model, a single SNP Bayes factor, BFj, can be analytically evaluated following [14, 15], and the corresponding gene-level Bayes factor is obtained by Bayesian model averaging asUpon obtaining the gene-level Bayes factors, we used the methods implemented in the software package eQTLBMA [14] to select eGenes at FDR 5% levels. eQTLBMA implements two types of FDR control procedures: one is a permutation based procedure which converts a gene-level Bayes factor to a corresponding p-value and control FDR using Storey’s procedure; the other procedure is based on the EBF procedure discussed in [34] which directly works with gene-level Bayes factors and avoids any permutations. We found that the latter approach is much more computationally efficient, however slightly conservative. The results reported in the Results section are based on the EBF procedure.
The power gain of the Bayesian gene-level testing procedure in identifying consistent association signals across multiple populations/subgroups and its comparison with commonly applied frequentist approaches have been fully demonstrated in [14] and [21] through simulations and real data analysis. Here, we examined a few known factors that may potentially affect the power of the gene-level analysis in the GUEVADIS data.
Using the hierarchical model proposed in [14], we performed the analysis described in [21] to estimate the effect size heterogeneity of eQTL signals across populations: our choice of the heterogeneity parameters is mostly informed by this analysis. More specially, we used a full spectrum of grid values for the heterogeneity parameter ϕ2/(ϕ2 + ω2) from a comprehensive set H = {ϕ2/(ϕ2 + ω2) : 0.0, 0.1, 0.2, …, 0.9, 1.0}. We then estimated the probability weight on each grid value by pooling information across all genes using the EM algorithm implemented in eQTLBMA. The estimates show that the majority of the probability mass is concentrated in very low heterogeneity levels (S1 Table), and the mean heterogeneity E[ϕ2/(ϕ2 + ω2)|Y, G] = 0.060, i.e., on average, the eQTL effects are very consistent across populations. This result is also highly concordant to the findings in [21] where eQTL data from microarray experiments across European, African and Asian populations were examined, and further justifies our selection of heterogeneity parameter values in the analysis.
Among other factors impacting eGene discoveries, we noted that the number of cis-SNPs is negatively correlated with the gene-level local false discovery rate (lfdr). That is, on average, genes harboring more cis-SNPs tend to exhibit stronger gene-level association signals. The Spearman’s rank correlation between the two quantities is modest (-0.20), nonetheless statistically highly significant (p-value < 2.2 × 10−16).
Multi-SNP Analysis of cis-eQTLs
In our multi-SNP analysis, we no longer assumed “one cis-eQTL per gene”, and considered the full range of alternative scenarios described by model (Eq 1). To make joint inference with respect to Γ = {γi, …, γp}, we further specified effect size distribution,
where W is constructed in the same way as in the gene-level analysis. In particular, we used the same set of grid values of (ϕ2, ω2) in the gene-level analysis. Unconditional on γj, the prior on βj is a type of “spike-and-slab”, where the “slab” is represented by a mixture of multivariate normal distribution, and the vast majority of the prior probability mass, 1 − 1 p, is assigned to the “spike” (i.e., a point mass at 0).The described linear model system including the prior specification (i.e., SSLR), is a special case of the general system considered by [15]. Given a specified Γ value, a Bayes factor, BF(Γ), contrasting to the trivial null model, Γ ≡ 0, can be analytically approximated by applying the result discussed therein. With this result, the posterior probability, Pr(Γ|Y,G), can be computed up to an unknown normalizing constant, i.e., Pr(Γ|Y, G) ∝ Pr(Γ)BF(Γ). We implemented a Metropolis-Hastings algorithm, similar to what is discussed in [15] for multivariate linear regression model (MVLR), to efficiently traverse the space of 2p possible Γ values. In particular, we designed a novel proposal distribution that utilizes marginal and conditional analysis results to prioritize SNPs with strong marginal or conditional association signals. In practice, we observed that the resulting MCMC algorithm achieves fast mixing. The details of the algorithm is provided in S1 Text (section S.1). In the end, the MCMC algorithm yields a set of Γ samples from the posterior distribution, from which we computed the PIP for each SNP by marginalization.
The posterior expected number of independent cis-eQTLs and its variance for each gene are obtained by computing the sample mean and variance of the number of non-zero γj’s in each posterior model. Equivalently, the posterior expected number of cis-eQTLs can be computed by the sum of PIPs, i.e.,
For the fine mapping analysis of the GEUVADIS data, we applied the MCMC algorithm for each identified eGene individually. We carried out 25,000 burnin steps and 50,000 repeats for each MCMC run. Taking advantage of parallel processing, we performed multiple-SNP analysis for multiple genes simultaneously in a distributed computing environment, which greatly reduced the overall computational time. The MCMC run for the eGene with the most cis-SNPs, HLA-DRB1 (Ensembl ID: ENSG00000196126, with 11,400 cis-SNPs), took 30 minutes on a computer with a single Intel Xeon 2.13GHz 8-core CPU. For average eGenes with ∼ 2,000 cis-SNPs the running time is approximately 3 to 4 minutes on the same machine. We ran our fine mapping analysis for GEUVADIS data with 8 parallel threads, the overall computation took about 30 hours.
Although the MCMC output conveys the full information of the fine mapping results, parsing the information manually to identify independent eQTL signal clusters can still be non-trivial. To tackle this problem, we designed and implemented a simple hierarchical clustering based algorithm that automatically parses the MCMC output and aides identifying independent eQTL signal clusters. The details of the algorithm are described in the S1 Text (section S.4).
Simulation Study
We performed numerical simulations to evaluate the performance of the proposed Bayesian multi-SNP analysis approach. For this purpose, we simulated the expression levels of 1,500 genes. The genotypes of cis-SNPs in each gene were assembled using the real genotype data from the GEUVADIS project. More specifically, we randomly selected 100 genomic regions each containing 25 consecutive SNPs, and directly took the observed genotypes of those 2,500 SNPs from each GEUVADIS population. Consequently, there are generally modest to high levels of LD within each region, and unremarkable levels of LD between regions. The resulting genotype data also maintain the variations in genotype frequency and local LD patterns (within each 25-SNP region) across populations. For each gene, we randomly assigned 1 to 4 population-consistent eQTL SNPs and generate the expression levels for all five populations using a system of linear models (details are described in S.5 of S1 Text). We repeated this procedure to generate the genotype-expression data for all 1,500 genes.
We analyzed the simulated data set using the following three different cis-eQTL mapping approaches:
the proposed Bayesian multi-SNP mapping method
a fixed effect single SNP meta-analysis approach
a conditional analysis approach (i.e., a forward step-wise selection procedure) built upon the single SNP meta-analysis procedure in 2
Fig 1 shows the comparison of the three approaches based on the simulated data set. It is clear that multi-SNP analysis methods (both the Bayesian and conditional analysis approaches) outperform single SNP analysis when multiple cis-eQTL signals are commonly present. More importantly, for any given fixed false positive value, the proposed Bayesian method always yields the most true positives, outperforming the conditional analysis approach. We further investigated the power gain in Bayesian multi-SNP analysis by stratifying the simulated genes by their number of cis-eQTLs. The results, shown in S1 Fig, indicate that when a gene contains only a single cis-eQTL, all three methods have very similar power. However, when multiple cis-eQTLs are harbored by a single gene, the Bayesian multi-SNP approach shows consistently superior power over the two alternative procedures.
Additionally, we examined the calibration of the reported regional PIPs using the simulated data set (S2 Fig). The results indicate that the reported regional PIPs are generally well calibrated, i.e., the reported probability values truly reflect the frequencies of the true causal regions over many independent observations. We observed that the small to modest PIP values tend to be slightly conservative, which may be explained by the use of prior model (Eq 2). This calibration property ensures a natural Bayesian FDR control [35] in determining the PIP threshold for selecting the causal regions. Fig 11 plots the comparison between estimated FDR from PIPs and the true realized FDR in the simulated data. The two quantities are in very good agreement. For example, in our simulation at the estimated 5% FDR level, we select 1432 causal regions (with PIP cutoff = 0.424) of which the realized true false discovery percentage is 4.6%. In comparison, we found it difficult to determine an appropriate p-value cutoff for conditional and single SNP analyses. The commonly applied Bonferroni correction threshold seems too stringent in our simulation setting: the single SNP analysis and the conditional analysis select only 1070 and 1113 regions (with 2 and 1 false positives), respectively.
Fig. 11. The comparison between the realized falase discovery rate and the estimated false discovery rate using the PIPs from the proposed Bayesian method in the simulation study. 
The plot indicates that the FDRs estimated by PIPs are mostly accurate and become slightly conservative for large FDR values. Enrichment Testing of Genomic Annotations
To test the enrichment of a particular genomic annotation in eQTL signals, we examine the associations between SNP level annotations of interest and the PIPs from our multi-SNP fine mapping approach computed under the null hypothesis of no enrichment.
Without loss of generality, we consider a single annotation and use δ j g to denote the annotated value of SNP j in gene g (the additional super-script for gene emphasizes the annotation is specific to each gene-SNP pair). Our testing procedure starts by computing PIPs for all gene-SNP pairs using the proposed multi-SNP cis-eQTL mapping method with the exchangeable prior specification (Eq 4). Under the null hypothesis, the resulting PIPs should be independent of the annotation information. We then fit a logistic regression model
and perform the Wald test with respect to α1.The commonly applied enrichment testing approach typically classifies the latent association status of each SNP (γ j g) into 0 or 1 depending on the single SNP association test results (usually p-values), and then test their associations with the annotations of interest. In comparison, the use of PIPs in our proposed method presents at least two obvious advantages: first, it naturally accounts for potentially multiple association signals within a single gene; and second, it fully accounts for the uncertainty in determining γ j g which is ignored by the binary classification approach. These advantages are reflected by the gain of powers in enrichment testing.
Simulation Study
We performed simulation studies to evaluate the performance of the proposed enrichment testing procedure.
We selected 2,500 genes from the GEUVADIS data. To ease computation, we only kept 50 randomly selected cis-SNPs for each gene. We randomly annotated 20% of the SNPs with a binary feature. For each SNP j in gene g, we determined its true association status by an independent Bernoulli ( p j g ) trial, where
In particular, we set α0 = −4.59, which implies about 1% of unannotated SNPs are expected to be true eQTLs. We selected values of α1 from the set {0.00, 0.25, 0.50, 0.75} for different simulations. Once the causal SNPs were determined, we simulated their effect sizes and expression levels of target genes using the same approach described in the simulation study of fine mapping analysis (section S.5 of S1 Text). For each unique α1 value, we generated 1,000 independent data sets.We analyzed each simulated data set using the proposed approach. For comparison, we also applied a standard enrichment analysis procedure principally similar to what described in [20, 36]. More specifically, we performed single SNP association tests for all gene-SNP pairs in a simulated data set, then classified the top associated SNPs of each gene as the “causal” eQTLs if their p-values pass the significance threshold at FDR 5% level. Finally, we performed a Fisher’s exact test to assess the correlation between the the classified association status and the annotation. S2 Table shows the comparison of type I errors and powers for the two approaches. The type I errors are well controlled for both methods. The proposed enrichment testing approach utilizing PIPs shows much elevated statistical powers in our simulation settings.
Integrative Fine Mapping Analysis of cis-eQTLs
To incorporate genomic annotations into the fine mapping analysis of cis-eQTLs, we specify the prior distribution of Pr(γj) by the parametric function (Eq 6), whereas the other parts of the Bayesian multi-SNP analysis model remain intact. For the g-th gene and its j-th SNP, we re-write (Eq 6) using an equivalent vector form as follows
where the enrichment parameter α is assumed to be shared across all gene-SNP pairs.Let Dg denote the collection of the annotation data for gene g. For a total of q genes, it follows from the Bayes rule that
Given a prior specification P(α) (e.g. a flat prior), the quantities on the right hand side are individually straightforward to compute analytically. It is conceptually easy to modify our MCMC algorithm for the multi-SNP analysis to jointly sample (α, Γ1, …, Γq). Nevertheless, due to the extremely high dimensionality of the target space (which is approximately the number of gene-SNP pairs, in the case of the GEUVADIS data the number is ∼ 6.7 million), the convergence of the MCMC within a reasonable time frame may be in doubt. Furthermore, the computational resources, especially the memory usage, demanded by the MCMC algorithm may be too high to afford in a practical setting.Alternatively, we consider an empirical Bayes approach which employs an EM algorithm to find the MLE of α by treating (Γ1, …, Γq) as missing data, and perform a final round of multi-SNP analysis (possibly only for eGenes) conditional on the resulting MLE α ^. The derivation of the EM algorithm is mostly straightforward, we give the relevant details in S1 Text (section S.2). Briefly at (t+1)-th iteration, in the Expectation step (E-step), we evaluate E [ γ j g | Y g , G g , α ( t ) ] for each gene-SNP pair given the current estimate α(t). It should be noted that this conditional expectation is exactly the PIP of each gene-SNP pair which can be obtained by running the multi-SNP analysis for each gene separately given the hyperparameter α(t). In the Maximization step (M-step), we find α(t+1) by fitting a logistic regression model relating PIP of each gene-SNP pair to the genomic annotations of the corresponding SNP. Overall, we describe the complete algorithm as an “MCMC-within-EM” algorithm, which is initiated at some arbitrary α(0) value, and iteratively performs multiple cis-eQTL mapping using the MCMC algorithm and maximization by fitting logistic regression models until convergence. The main computational benefit of the “MCMC-within-EM” algorithm is that the E-steps involving MCMC runs can be processed in parallel on a distributed computing system, hence the memory requirement is much relaxed.
To illustrate the procedure for estimating α, we simulated a set of eQTL data using the same scheme in the enrichment testing. In particular, we used a single binary annotation and set α1 = 1.50. We ran the MCMC-within-EM algorithm on this simulated data set with the starting point α1 = 0, and plotted the estimated α1 values in each iteration in S3 Fig. The figure indicates that the estimates of α1 quickly converge to the close neighborhood of the true value after 6 to 7 iterations.
To reduce computation, in practice we analyzed the GEUVADIS data using only a single iteration of EM where all enrichment parameters are initialized at 0. As a consequence, our reported point estimates of the enrichment parameters are likely to be lower bounds of the true values. Nevertheless, as we have shown in the analysis of the GEUVADIS data, this simplified version of the algorithm still improves the resolutions of the fine mapping analysis by up-weighting the SNPs with relevant annotations.
Supporting Information
Zdroje
1. Nica AC, Montgomery SB, Dimas AS, Stranger BE, Beazley C, et al. (2010) Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS genetics 6: e1000895. doi: 10.1371/journal.pgen.1000895 20369022
2. Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, et al. (2010) Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genetics 6: e1000888. doi: 10.1371/journal.pgen.1000888 20369019
3. Hao K, Bosse Y, Nickle DC, Pare PD, Postma DS, et al. (2012) Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS genetics 8: e1003029. doi: 10.1371/journal.pgen.1003029 23209423
4. GTEx Consortium (2013) The genotype-tissue expression (gtex) project. Nature Genetics 45 : 580–585. doi: 10.1038/ng.2653 23715323
5. Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, et al. (2009) Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325 : 1246–1250. doi: 10.1126/science.1174148 19644074
6. Maranville JC, Luca F, Richards AL, Wen X, Witonsky DB, et al. (2011) Interactions between glucocorticoid treatment and cis-regulatory polymorphisms contribute to cellular response pheno-types. PLoS genetics 7: e1002162. doi: 10.1371/journal.pgen.1002162 21750684
7. Barreiro LB, Tailleux L, Pai AA, Gicquel B, Marioni JC, et al. (2012) Deciphering the genetic architecture of variation in the immune response to mycobacterium tuberculosis infection. Proceedings of the National Academy of Sciences 109 : 1204–1209. doi: 10.1073/pnas.1115761109
8. Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, et al. (2014) Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344 : 519–523. doi: 10.1126/science.1249547 24786080
9. Brown CD, Mangravite LM, Engelhardt BE (2013) Integrative modeling of eQTLs and cis-regulatory elements suggests mechanisms underlying cell type specificity of eQTLs. PLoS Genetics 9: e1003649. doi: 10.1371/journal.pgen.1003649 23935528
10. Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the encode pilot project. Nature 447 : 799–816. doi: 10.1038/nature05874 17571346
11. Pique-Regi R, Degner JF, Pai AA, Gaffney DJ, Gilad Y, et al. (2011) Accurate inference of transcription factor binding from dna sequence and chromatin accessibility data. Genome research 21 : 447–455. doi: 10.1101/gr.112623.110 21106904
12. Hoffman MM, Ernst J, Wilder SP, Kundaje A, Harris RS, et al. (2012) Integrative annotation of chromatin elements from encode data. Nucleic acids research: gks1284.
13. Liang L, Morar N, Dixon AL, Lathrop GM, Abecasis GR, et al. (2013) A cross-platform analysis of 14,177 expression quantitative trait loci derived from lymphoblastoid cell lines. Genome research 23 : 716–726. doi: 10.1101/gr.142521.112 23345460
14. Flutre T, Wen X, Pritchard J, Stephens M (2013) A statistical framework for joint eQTL analysis in multiple tissues. PLoS Genetics 9: e1003486. doi: 10.1371/journal.pgen.1003486 23671422
15. Wen X (2014) Bayesian model selection in complex linear systems, as illustrated in genetic association studies. Biometrics 70 : 73–83. doi: 10.1111/biom.12112 24350677
16. Sul JH, Han B, Ye C, Choi T, Eskin E (2013) Effectively identifying eQTLs from multiple tissues by combining mixed model and meta-analytic approaches. PLoS genetics 9: e1003491. doi: 10.1371/journal.pgen.1003491 23785294
17. Gaffney DJ, Veyrieras JB, Degner JF, Pique-Regi R, Pai AA, et al. (2012) Dissecting the regulatory architecture of gene expression QTLs. Genome Biol 13: R7. doi: 10.1186/gb-2012-13-1-r7 22293038
18. Veyrieras JB, Kudaravalli S, Kim SY, Dermitzakis ET, Gilad Y, et al. (2008) High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS genetics 4: e1000214. doi: 10.1371/journal.pgen.1000214 18846210
19. Lee SI, Dudley AM, Drubin D, Silver PA, Krogan NJ, et al. (2009) Learning a prior on regulatory potential from eQTL data. PLoS genetics 5: e1000358. doi: 10.1371/journal.pgen.1000358 19180192
20. Lappalainen T, Sammeth M, Friedländer MR, T Hoen PA, Monlong J, et al. (2013) Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501 : 506–511. doi: 10.1038/nature12531 24037378
21. Wen X, Stephens M (2014) Bayesian methods for genetic association analysis with heterogeneous subgroups: From meta-analyses to gene-environment interactions. The Annals of Applied Statistics 8 : 176–203. doi: 10.1214/13-AOAS695
22. Morris AP (2011) Transethnic meta-analysis of genomewide association studies. Genetic epidemiology 35 : 809–822. doi: 10.1002/gepi.20630 22125221
23. Marigorta UM, Navarro A (2013) High trans-ethnic replicability of gwas results implies common causal variants. PLoS genetics 9: e1003566. doi: 10.1371/journal.pgen.1003566 23785302
24. Li G, Shabalin AA, Rusyn I, Wright FA, Nobel AB (2013) An empirical bayes approach for multiple tissue eQTL analysis. arXiv preprint arXiv:13112948.
25. Yang J, Ferreira T, Morris AP, Medland SE, Madden PA, et al. (2012) Conditional and joint multiple-snp analysis of gwas summary statistics identifies additional variants influencing complex traits. Nature Genetics 44 : 369–375. doi: 10.1038/ng.2213 22426310
26. Coviello AD, Haring R, Wellons M, Vaidya D, Lehtimäki T, et al. (2012) A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple loci implicated in sex steroid hormone regulation. PLoS genetics 8: e1002805. doi: 10.1371/journal.pgen.1002805 22829776
27. 1000 Genomes Project Consortium, et al. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491 : 56–65. doi: 10.1038/nature11632 23128226
28. Gilad Y, Rifkin SA, Pritchard JK (2008) Revealing the architecture of gene regulation: the promise of eQTL studies. Trends in Genetics 24 : 408–415. doi: 10.1016/j.tig.2008.06.001 18597885
29. Degner JF, Pai AA, Pique-Regi R, Veyrieras JB, Gaffney DJ, et al. (2012) DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482 : 390–394. doi: 10.1038/nature10808 22307276
30. Moyerbrailean GA, Harvey CT, Kalita CA, Wen X, Luca F, et al. (2014) Are all genetic variants in dnase i sensitivity regions functional? bioRxiv: 007559.
31. Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, et al. (2012) Gencode: the reference human genome annotation for the encode project. Genome research 22 : 1760–1774. doi: 10.1101/gr.135350.111 22955987
32. Pickrell JK, Marioni JC, Pai AA, Degner JF, Engelhardt BE, et al. (2010) Understanding mechanisms underlying human gene expression variation with rna sequencing. Nature 464 : 768–772. doi: 10.1038/nature08872 20220758
33. Stegle O, Parts L, Piipari M, Winn J, Durbin R (2012) Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpret ability of gene expression analyses. Nature protocols 7 : 500–507. doi: 10.1038/nprot.2011.457 22343431
34. Wen X (2013) Robust bayesian FDR control with bayes factors. arXiv preprint arXiv:13113981.
35. Newton MA, Noueiry A, Sarkar D, Ahlquist P (2004) Detecting differential gene expression with a semiparametric hierarchical mixture method. Biostatistics 5 : 155–76. doi: 10.1093/biostatistics/5.2.155 15054023
36. Fairfax BP, Humburg P, Makino S, Naranbhai V, Wong D, et al. (2014) Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science 343 : 1246949. doi: 10.1126/science.1246949 24604202
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání

























