-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
The tailing of RNAs with non-templated uridines, known as uridylation, is often associated with RNA degradation. We previously identified HESO1 as a nucleotidyl transferase that uridylates microRNAs (miRNAs) to lead to their degradation in Arabidopsis. But HESO1 cannot account for all the miRNA uridylation activity in vivo. Here, we have uncovered UTP:RNA URIDYLYLTRANSFERASE 1 (URT1) as another nucleotidyl transferase that uridylates miRNAs. HESO1 and URT1 have different substrate preferences and act cooperatively to tail miRNAs. We show that both enzymes are able to act on ARGONAUTE1 (AGO1)-bound miRNAs and that the tailed miRNAs stay bound by AGO1. We show that URT1-mediated tailing affects the activities of miR165/6 and miR171a differently. This study reveals intricate miRNA uridylation processes as well as functional outcomes of miRNA uridylation.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005119
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005119Summary
The tailing of RNAs with non-templated uridines, known as uridylation, is often associated with RNA degradation. We previously identified HESO1 as a nucleotidyl transferase that uridylates microRNAs (miRNAs) to lead to their degradation in Arabidopsis. But HESO1 cannot account for all the miRNA uridylation activity in vivo. Here, we have uncovered UTP:RNA URIDYLYLTRANSFERASE 1 (URT1) as another nucleotidyl transferase that uridylates miRNAs. HESO1 and URT1 have different substrate preferences and act cooperatively to tail miRNAs. We show that both enzymes are able to act on ARGONAUTE1 (AGO1)-bound miRNAs and that the tailed miRNAs stay bound by AGO1. We show that URT1-mediated tailing affects the activities of miR165/6 and miR171a differently. This study reveals intricate miRNA uridylation processes as well as functional outcomes of miRNA uridylation.
Introduction
The three major types of small RNAs in eukaryotes, microRNAs (miRNAs), small interfering RNAs (siRNAs) and piwi-interacting RNAs (piRNAs), impact many biological processes such as development, self/non-self recognition, genome stability, and adaption to environment. Given the widespread and indispensible functions of small RNAs, it is crucial to understand their biogenesis and turnover. A common step in the biogenesis of miRNAs and siRNAs in plants, as well as piRNAs and certain endogenous siRNAs in animals, is 2’-O-methylation on the 3’ terminal ribose by the small RNA methyltransferase HUA ENHANCER1 (HEN1) [1,2,3,4,5,6,7]. In Arabidopsis hen1 mutants, miRNAs and siRNAs become 3’ truncated, 3’ uridylated, and reduced in abundance [8]. Similarly, in animal hen1 mutants, piRNAs and/or siRNAs become 3’ truncated and 3’ uridylated [2,3,4,6]. This indicates that 2’-O-methylation protects small RNAs from 3’ truncation by an exonuclease(s) and 3’ tailing by a nucleotidyl transferase(s).
In a previous study, we identified Arabidopsis HEN1 SUPPRESSOR1 (HESO1) as a nucleotidyl transferase responsible for miRNA uridylation in vivo [9,10]. Loss of function in HESO1 results in reduced 3’ tailing and increased abundance for most miRNAs in hen1 backgrounds [9,10], indicating that tailing leads to miRNA degradation. Small RNA profiling in hen1-8, a partial loss-of-function hen1 mutant [11], and the hen1-8 heso1-1 double mutant reveals that 3’ truncation happens independently of 3’ uridylation, and that 3’ uridylation occurs on both full-length and 3’ truncated species [10]. Among the ten genes encoding potential nucleotidyl transferases, HESO1 is the only one whose loss of function partially suppresses the morphological defects of hen1 mutants [9,10,12], suggesting that HESO1 is the major miRNA uridylation enzyme. However, despite heso1-1 being a null allele [10], considerable levels of miRNA uridylation were detected by small RNA profiling in the hen1-8 heso1-1 double mutant [10], indicating that (an)other nucleotidyl transferases could tail unmethylated miRNAs.
In vivo, a miRNA exists in miRISC (miRNA-induced silencing complex) in which the miRNA is bound by an ARGONAUTE (AGO) protein. In Arabidopsis, AGO1 is the effector for almost all miRNAs [13,14]. Molecular genetic evidence suggests that uridylation occurs on AGO1-bound miRNAs in vivo. First, 3’ truncated and tailed miRNAs in hen1 mutants are bound by AGO1 in vivo [12,15]. Second, a partial loss-of-function ago1 mutation, ago1-11, suppresses the 3’ truncation and tailing of miRNAs in the weak hen1-2 mutant [15]. Moreover, HESO1 was shown to uridylate an in vitro reconstituted miRISC [16].
Given that uridylated miRNAs are associated with AGO1, uridylation may also alter the activities of miRNAs in addition to its role in miRNA degradation. Some Arabidopsis miRNAs efficiently trigger the production of phased secondary siRNAs (phasiRNAs) from their target transcripts, and some of the phasiRNAs can also regulate genes in trans and are named trans-acting siRNAs (ta-siRNAs) [17,18,19]. A common feature of the trigger miRNAs—their 22 nt length—endows them with the ability to generate phasiRNAs [20,21]. For example, miR173 is predominantly 22 nt in vivo and initiates ta-siRNA biogenesis from two noncoding transcripts TAS1 and TAS2 [17,18,19]. In a recent study [15], it was found that miR171a becomes predominantly 22 nt in hen1 mutants and initiates the biogenesis of phasiRNAs from its targets At2g45160 and At3g60630, suggesting that mono-uridylation of miR171a allows it to trigger phasiRNA biogenesis. But the enzyme that mono-uridylates miR171a in hen1 mutants is not HESO1 and remains unknown [15]. In hen1 mutants, despite various degrees of tailing of many miRNAs, only miR171 family members acquire the ability to trigger phasiRNA biogenesis [15]. The effects of tailing on the activities of other miRNAs remain unknown.
Most of the nine HESO1 paralogs, which we refer to as NUCLEOTIDYL TRANSFERASE PROTEIN (NTP) genes unless they have been previously named otherwise (S1 Fig), remain uncharacterized. One gene (At2g45620) was shown to encode a nucleotidyl transferase and named UTP:RNA URIDYLYLTRANSFERASE 1 (URT1) [22]. URT1 was found to uridylate messenger RNAs that have an oligoadenylate tail [22].
In this study, we identified URT1 as the nucleotidyl transferase with the second highest impact on miRNA uridylation among ten nucleotidyl transferases in Arabidopsis. URT1 and HESO1 prefer miRNA substrates with different 3’ nucleotides in vitro, and act on different size variants of the same miRNAs in vivo. We found that URT1 and HESO1 act sequentially on some miRNAs, with URT1 mono-uridylating the miRNAs followed by their further uridylation by HESO1. Both HESO1 and URT1 are able to tail AGO1-bound miRNAs and the uridylated species stay associated with AGO1. The tailing of AGO1-bound miR165/6 reduces its slicing activity while the monouridylation of miR171a endows an ability to trigger the biogenesis of phasiRNAs. Thus, miRNA tailing affects the activities of miRNAs in addition to causing miRNA degradation.
Results
URT1 is responsible for the uridylation of some miRNAs in hen1-8
The presence of substantial levels of miRNA uridylation in hen1-8 heso1-1 implied that (an)other nucleotidyl transferases can also tail miRNAs. To identify the nucleotidyl transferase(s), we sought to determine the effects of mutations in each of the nine NTP genes (S1 Table and S1A Fig) on miRNA uridylation. A mutation in each of the nine NTP genes was combined with hen1-8 and small RNA libraries were constructed from the nine double mutants as well as hen1-8 (S2 Table). Levels of tailing were calculated as the ratio of the number of reads with tails over the number of total reads for a particular miRNA.
The mutation in URT1, urt1-1, was found to reduce the tailing of a few miRNAs. Among 107 miRNAs that were detected at 30 reads per million or greater abundance in both hen1-8 and hen1-8 urt1-1 libraries, ten showed reduced tailing in hen1-8 urt1-1 (Fig 1A). The effects of urt1-1 on the tailing of a small number of miRNAs contrasted the widespread effects of heso1 mutations [9,10]. This suggested that HESO1 could act on most miRNAs in hen1-8 urt1-1 except for a few miRNAs. Indeed, for the miRNAs showing reduced tailing in hen1-8 urt1-1, the heso1-1 mutation had no or little effect on them whereas it had a strong effect on other miRNAs (Fig 1B; [10]). When we separately quantified tailing on full-length and 3’ truncated species, we found that urt1-1 and heso1-1 had distinct effects on the different forms of the same miRNAs (Fig 1C and 1D). For example, urt1-1 strongly reduced 3’ tailing of full-length miR171a and 3’ truncated miR158a, but heso1-1 affected the tailing of 3’ truncated miR171a and full-length miR158a (Fig 1C and 1D). Therefore, the two proteins appeared to prefer different miRNAs or different forms of the same miRNAs in vivo. Unlike heso1-1, which partially rescues the hen1-8 morphological phenotype [10], urt1-1 does not rescue the hen1-8 morphological phenotype (S2 Fig).
Fig. 1. Quantification of miRNA tailing by small RNA high throughput sequencing. 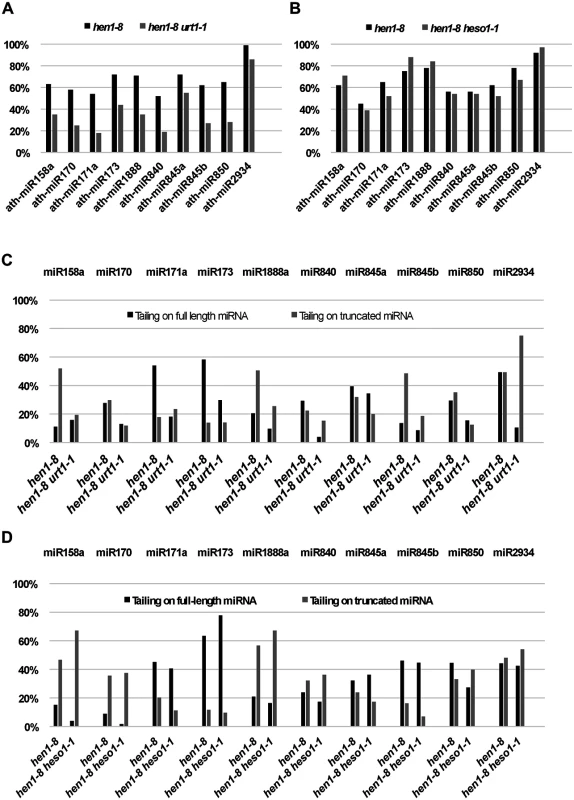
(A) The proportions of tailed reads for ten miRNAs in hen1-8 and hen1-8 urt1-1. (B) The proportions of tailed reads for the same ten miRNAs in hen1-8 and hen1-8 heso1-1. The proportions of tailed species were calculated as %(sum of tailed-only and truncated-and-tailed reads divided by total read number). (C-D) The levels of tailing on full-length and 3’ truncated species from ten miRNAs in hen1-8 and hen1-8 urt1-1 (C), and hen1-8 and hen1-8 heso1-1 (D). The levels of tailing on full-length miRNAs were calculated as a percentage (number of tailed-only reads divided by sum of tailed and non-tailed full-length reads). The levels of tailing on truncated miRNAs were calculated as a percentage (number of truncated-and-tailed reads divided by sum of truncated-and-tailed and truncated-only reads). In contrast to mutations in HESO1 or URT1, mutations in the other eight NTP genes did not cause any discernable effects on miRNA tailing (S3 Fig). As an example, miR158 showed reduced tailing in hen1-8 urt1-1 as compared to hen1-8 (arrow in Fig 2A). Patterns of miR158 3’ truncation and tailing in the other eight hen1-8 ntp double mutants were identical to each other and to hen1-8 (Fig 2A and S4A Fig). The nearly identical patterns of hen1-8 and seven hen1-8 ntp genotypes in Fig 2A also reflected the reproducibility of the high throughput sequencing-based quantification of miRNA 3’ truncation and tailing.
Fig. 2. Distinct and coordinated tailing of miR158 by URT1 and HESO1. 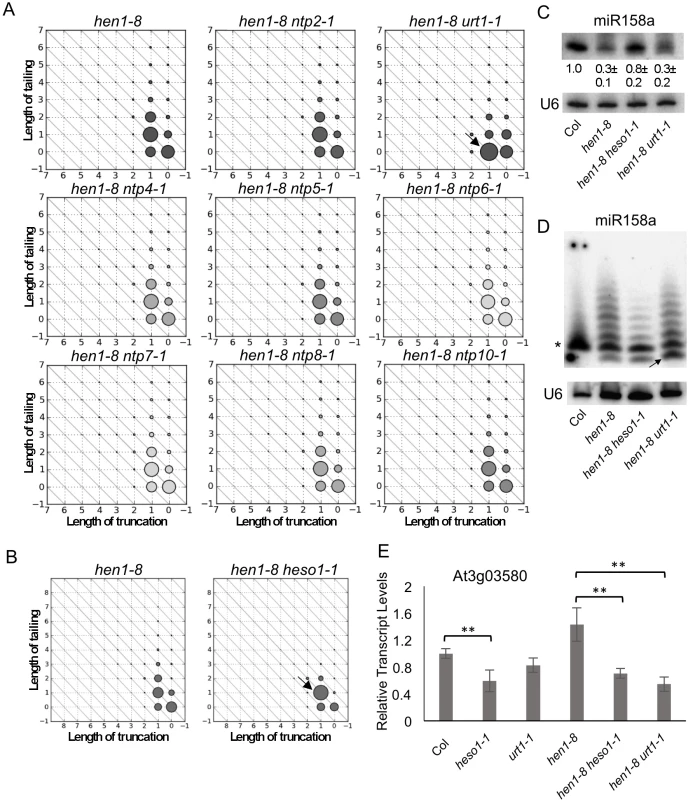
(A-B) Diagrams that represent the status of 3’ truncation and 3’ tailing of miR158a in various genotypes in vivo as determined by small RNA sequencing. In the diagrams, the X and Y axes represent the number of nucleotides truncated from, and tailed onto, the miRNA 3’ end, respectively. The sizes of the circles indicate the relative abundance of the miR158 variants. Nine and two genotypes were examined in (A) and (B), respectively, as indicated. In each figure, samples from the different genotypes were processed at the same time for small RNA library construction. Altogether in (A) and (B), mutations in nine NUCLEOTIDYL TRANSFERASE PROTEIN (NTP) genes, including URT1 and HESO1, were examined. Refer to S3 Fig for results on hen1-8 mee44. A miR158 species with a 1-nt truncation (marked by an arrow in (A)) accumulates in hen1-8 urt1-1. The miR158 species at the (1,1) position (marked by an arrow in (B)) accumulates in hen1-8 heso1-1. (C-D) The accumulation of miR158 in hen1-8, hen1-8 heso1-1, hen1-8 urt1-1 as determined by northern blotting. Total RNAs (5 μg for Col and 50 μg for all hen1 genotypes) were resolved in a short (C) and a long (D) polyacrylamide gel, the latter allowing the separation of miR158 species that differ by 1 nt. U6 serves as a loading control. In (C), the relative abundance of miR158, shown as mean +/- SD below the miR158 gel image, was calculated from three biological replicates. In (D), the arrow marks the 1-nt truncated species, whereas the asterisk indicates the 20-nt band. This band is deduced to represent 20-nt RNAs as it is the size of miR158 in wild type (Col), and miR158 is known to be 20-nt long in wild type. (E) Relative transcript levels of At3g03580, a target of miR158, as determined by real-time RT-PCR. ACTIN1 was used as an internal control. Error bars representing SD were calculated from three biological replicates. Significant differences are indicated by ** (P value < 0.01) for the pairs of genotypes that were compared. URT1 exhibits nucleotidyl transferase activity on unmethylated miRNA in vitro
URT1 was previously shown to have RNA nucleotidyl transferase activity in vitro and uridylate oligoadenylated RNAs in vivo [22]. To confirm this activity and to examine its substrate preferences, we expressed and purified recombinant, 6XHis-tagged URT1 from E. coli (S1C Fig). The recombinant protein was incubated with synthetic, unmethylated miR173 in the presence of different ribonucleotide triphosphates. URT1 was able to add multiple ribonucleotides to miR173 (Fig 3, lanes 4–7), confirming that URT1 is a nucleotidyl transferase. The lengths of the products were longest when UTP was in the reaction (Fig 3, lanes 4–7), indicating that URT1, like HESO1 [9,10], prefers UTP. To rule out the possibility that the nucleotidyl transferase activity was due to a contaminating protein from E. coli, two conserved aspartate residues in the nucleotidyl transferase domain were mutated to alanine (S1B Fig). The mutant protein, 6XHis-URT1m was expressed in E. coli and purified as was the wild-type protein (S1C Fig). His-URT1m failed to tail miR173 (Fig 3, lanes 11–12).
Fig. 3. Nucleotidyl transferase assays for URT1. 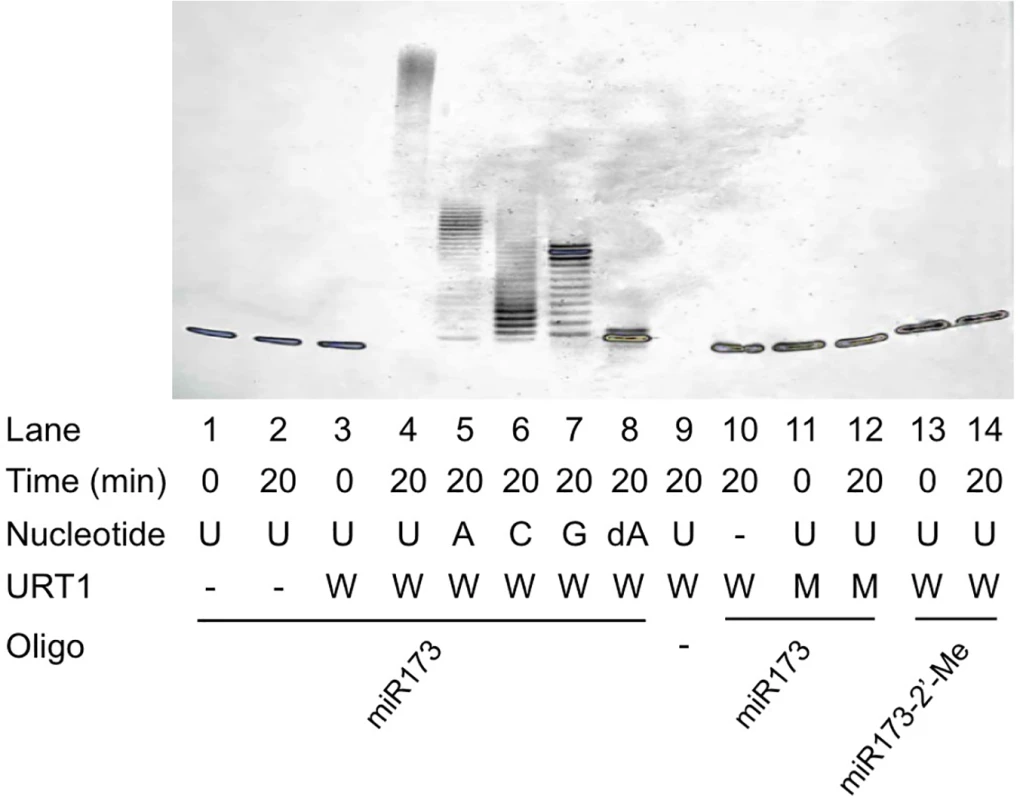
Recombinant His-tagged wild type URT1 or a catalytic mutant was included in the reactions. The wild type and mutant proteins are indicated by “W” and “M”, respectively. The proteins were incubated with an RNA oligonucleotide (oligo) in the presence of a nucleotide triphosphate, and the products were resolved on 15% denaturing polyacrylamide gels. miR173 and miR173-2’-Me are un-methylated and methylated, respectively, on the 2’ OH of the 3’ terminal ribose. Nucleotidyl transferase activity is represented by the presence of higher molecular weight bands relative to the input miRNA. Note that in lane 4, the substrate RNA was completely converted to higher molecular weight products at the top of the gel. The ‘‘-” signs indicate the absence of the nucleotide triphosphate, protein, or RNA oligonucleotide. We next investigated URT1’s requirement for the 2’ OH of the 3’ terminal ribose in the substrate miRNA. When deoxyadenosine triphosphate (dATP) was included as the only nucleotide in the reaction, URT1 added a single deoxyadenylate to miR173, but further nucleotide addition was inefficient (Fig 3, lane 8). This indicated that the 2’ OH on the 3’ terminal ribose is a feature of the substrate recognized by URT1. When 2’-O-methylated miR173 was incubated with URT1 in the presence of UTP, no tailing was observed (Fig 3, lanes 13–14). This indicated that URT1 activity, like that of HESO1 [10], is completely inhibited by 2’-O-methylation on its substrate RNA.
URT1 and HESO1 act cooperatively on miR158 by tailing different forms
Small RNA profiling in hen1-8 urt1-1 and hen1-8 heso1-1 revealed that URT1 and HESO1 preferred different forms of miR158 (Fig 2A and 2B). Note that the various miR158 species will be referred to by their X and Y coordinates as shown in Fig 2A and 2B. In hen1-8, miR158 existed in full-length and 1-nt truncated forms (miR158 (0,0) and miR158 (1,0), respectively) and both underwent various degrees of tailing (Fig 2A). In hen1-8 heso1-1, tailing of the full-length form was greatly reduced, indicating that HESO1 was responsible for the tailing of full-length miR158 (Fig 2B). In hen1-8 urt1-1, full-length miR158 species with tails were unaffected, indicating that URT1 did not tail full-length miR158 (Fig 2A). In hen1-8 heso1-1, there was an increase in miR158 (1,1), a miR158 species with 1-nt truncation and a 1-nt tail (Fig 2B; arrow). Correspondingly, there was a decrease in the abundance of miR158 (1,1+), i.e., species of miR158 (1,1) with longer tails (Fig 2B). Therefore, HESO1 was responsible for tailing miR158 (1,1) but not miR158 (1,0). On the other hand, the abundance of miR158 (1,0) was increased with a corresponding decrease in miR158 (1,0+) species in hen1-8 urt1-1 (Fig 2A). Taken together, these results suggested that 1) HESO1 but not URT1 tails full-length miR158; and 2) URT1 adds a 1-nt tail to the 1-nt truncated miR158 to result in miR158 (1,1), which is further tailed by HESO1.
To verify these results, northern blots were carried out on wild type, hen1-8, hen1-8 urt1-1, and hen1-8 heso1-1. When RNAs were separated with a 1-nt resolution, it was clear that the 20-nt miR158 band was the predominant band in hen1-8 heso1-1 (Fig 2D). This 20-nt band corresponded to both miR158 (0,0) and miR158 (1,1) (Fig 2B), and the northern blot results were consistent with the small RNA profiling data. On the other hand, in hen1-8 urt1-1, the 1-nt truncated form of miR158 accumulated to a higher level relative to hen1-8 (Fig 2D; arrow), consistent with the small RNA profiling data (Fig 2A; arrow). We also quantified the levels of miR158 in the four genotypes by not resolving the miR158 size variants in northern blots. Consistent with previous results, the amount of miR158 was higher in hen1-8 heso1-1 as compared to hen1-8 (Fig 2C). urt1-1 did not lead to an increase in miR158 abundance (Fig 2C).
miR158 is only partially methylated in wild type and is subjected to uridylation by both HESO1 and URT1 even in wild type background ([15]; S4B Fig). As in the hen1-8 background, HESO1 and URT1 act on full-length and 1-nt truncated miR158, respectively, in wild type (S4B Fig).
Next, we tested whether URT1 or HESO1 impacts the activity of miR158 by examining the expression of the miR158 target gene At3g03580. The heso1-1 mutation caused a reduction in At3g03580 transcript levels as compared to wild type, but the urt1-1 mutation did not have a significant effect (Fig 2E). The levels of the At3g03580 transcript were reduced in hen1-8 heso1-1 relative to hen1-8 (Fig 2E), consistent with higher miR158 levels in this genotype (Fig 2C). Interestingly, the expression of At3g03580 was decreased also in hen1-8 urt1-1 (Fig 2E), despite the fact that miR158 levels were similar between hen1-8 urt1-1 and hen1-8 (Fig 2C). This prompted us to test whether tailing affects the slicer activity of miRNAs (see below).
URT1 and HESO1 sequentially tail miR173 and reduce the biogenesis of ta-siRNAs
Small RNA sequencing revealed that URT1 and HESO1 acted sequentially to tail miR173. miR173 is largely non-tailed in wild type (S4C Fig). In hen1-8, full-length, 22-nt miR173 was tailed to various larger sizes (Fig 4A and 4B). Both heso1-1 and urt1-1 affected miR173 tailing but in different ways. In hen1-8 heso1-1, the mono-uridylated, 23-nt form of miR173 accumulated predominantly (Fig 4B), indicating that HESO1 preferentially used this 23-nt form as the substrate for uridylation in vivo. The enzyme that performed the mono-uridylation of miR173 to produce the 23-nt form was URT1, as the 22-nt form of miR173 was the predominant species in hen1-8 urt1-1 (Fig 4A). These observations from small RNA sequencing were confirmed by northern blotting. The 23-nt and larger species were predominant in hen1-8 heso1-1, whereas the 22-nt species was predominant in hen1-8 urt1-1 (Fig 4C). Taken together, these results indicate that URT1 performs mono-uridylation of miR173; subsequently, HESO1 preferentially uridylates the 23-nt miR173. Moreover, consistent with uridylation leading to miRNA degradation, miR173 levels were increased in both hen1-8 heso1-1 and hen1-8 urt1-1 relative to hen1-8 (Fig 4D). When a URT1-GFP transgene driven by the URT1 promoter was introduced into hen1-8 urt1-1, the increase in miR173 levels was rescued in two independent transgenic lines (S5A Fig). In a HEN1 background, neither heso1-1 nor urt1-1 affected the levels of miR173 (S5B Fig).
Fig. 4. Sequential tailing of miR173 in hen1 by URT1 and HESO1 and its impacts on ta-siRNA biogenesis. 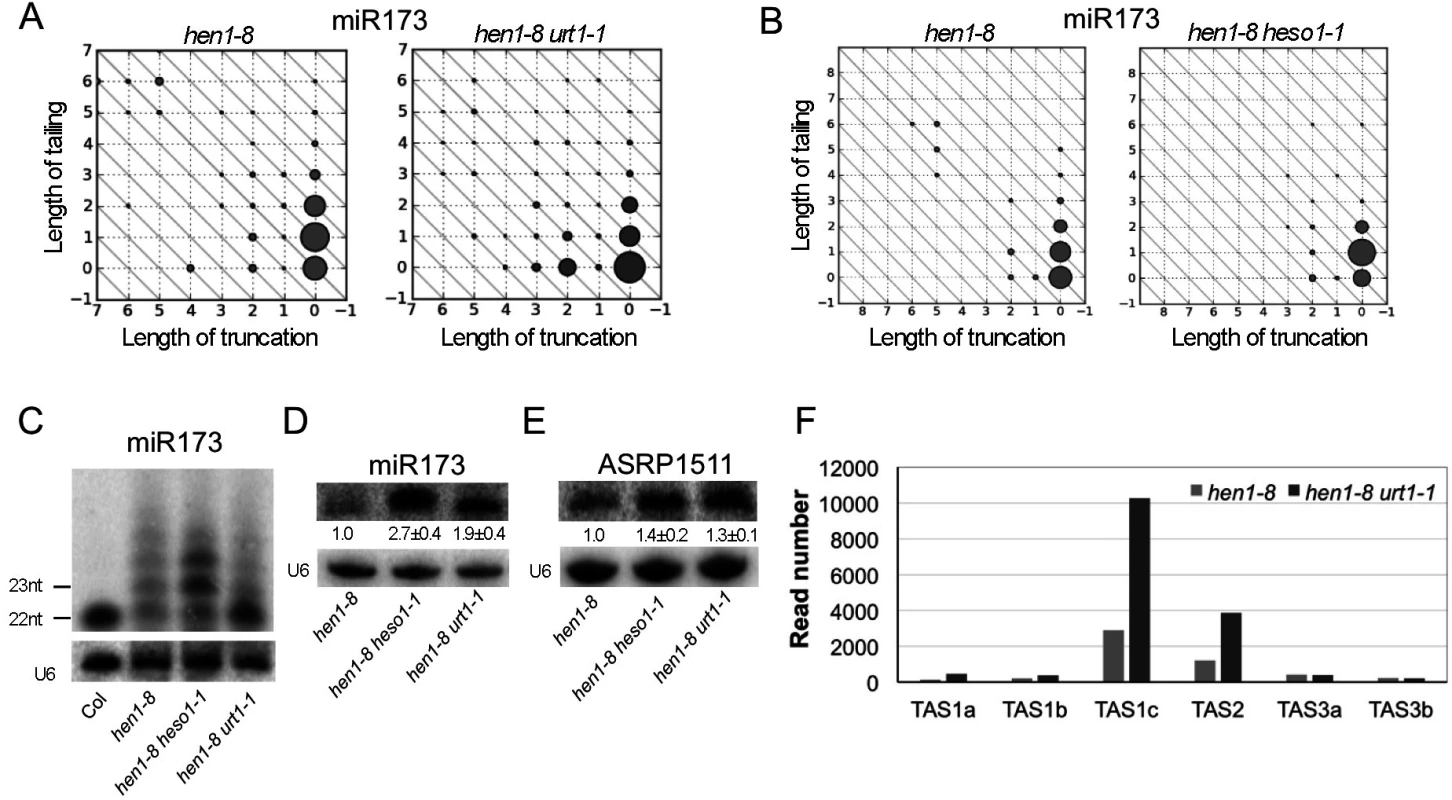
(A-B) The accumulation of various miR173 species as determined by small RNA sequencing. Refer to the Fig 3 legend for the description of the diagrams. The two samples in (A) or (B) were processed in the same experiment, but (A) and (B) were from two different experiments. In hen1-8 urt1-1, the full-length miR173 species at position (0,0) accumulates to higher levels in comparison to hen1-8. In hen1-8 heso1-1, the miR173 species with a 1-nt tail at position (0,1) accumulates to higher levels as compared to hen1-8. (C) The accumulation of miR173 variants of different sizes in various genotypes was determined by northern blotting. 5 μg and 50 μg of total RNAs were used for Col (wild type) and hen1-8 genotypes, respectively. (D) The amount of miR173 in hen1-8, hen1-8 heso1-1 and hen1-8 urt1-1 was determined by northern blotting. RNAs were resolved in a short gel so as not to separate the size variants. The numbers below the miR173 gel image represent the relative abundance of miR173 in various genotypes. The error bars represent SD and were calculated from three biological replicates. (E) The accumulation of ASRP1511, a ta-siRNA from the TAS2 locus, as determined by northern blotting. The error bars represent SD and were calculated from three biological replicates. (F) The abundance of ta-siRNAs from various TAS loci in hen1-8 and hen1-8 urt1-1 as determined by small RNA sequencing. The Y axis represents relative read number of the ta-siRNAs in the two genotypes normalized to total small RNAs. As a 22-nt miRNA, miR173 triggers the biogenesis of ta-siRNAs from TAS1 and TAS2 loci [17,18,19,20,21]. We next examined whether URT1 impacted ta-siRNAs whose biogenesis requires miR173. Northern blots were performed to detect ASRP1511, a ta-siRNA from the TAS2 locus [17]. ASRP1511 levels were higher in hen1-8 urt1-1 or hen1-8 heso1-1 than in hen1-8 (Fig 4E). The increase in TAS2 ta-siRNA levels caused by urt1-1 and heso1-1 mutations could be multifactorial—an increase in miR173 levels in both hen1-8 urt1-1 and hen1-8 heso1-1 (Fig 4D), an increase in the 22-nt form of miR173 in hen1-8 urt1-1 (Fig 4C), and compromised uridylation of the ta-siRNAs themselves in hen1-8 heso1-1 [10].
We also examined the abundance of TAS1, TAS2 and TAS3 ta-siRNAs in small RNA libraries. TAS3 ta-siRNAs were included as a control, as their trigger, miR390 [23], was not affected by the urt1-1 mutation in terms of tailing (S3 Fig). When normalized to total small RNAs, the abundance of TAS1 and TAS2 ta-siRNAs was much higher in hen1-8 urt1-1 than in hen1-8, while that of TAS3 ta-siRNAs was not affected (Fig 4F). hen1-8 heso1-1 was not included in the analysis as the global increase in miRNA and ta-siRNA levels prevented effective normalization.
URT1 and HESO1 have different substrate specificities in vitro
The above small RNA sequencing results described above revealed differential preferences for miR158 and miR173 forms by HESO1 and URT1. For example, in vivo, full-length miR158 is mainly tailed by HESO1 while 1-nt truncated miR158 (miR158-1) is mainly tailed by URT1. To determine whether this reflected different substrate preferences for the two enzymes, we examined HESO1 and URT1 activities on miR158 and miR158-1 in vitro. As miR158-1 differs from miR158 both in length and in the nature of the 3’ nucleotide (miR158-1 ends in C while miR158 ends in A), we also included three full-length miR158 forms ending in C, G, or U (referred to as miR158A-C, miR158A-G, and miR158A-U, respectively) in comparison to the natural miR158 ending in A. As the two enzymes required different buffer conditions for optimal activities, it was not reasonable to compare the activities of the two enzymes on the same substrate. But it was possible to compare the activities of an enzyme on different substrates. When comparing nucleotidyl transferase activities on different substrates, the disappearance of the substrate over a time course was considered as the criteria for preference for the substrate.
Among the four full-length miR158 forms (Fig 5A, lanes 1, 3–4), HESO1 had a clear preference for miR158A-U, as reflected by the disappearance of this substrate but not others at the earliest time point (Fig 5A, lanes 6, 9–10). With longer time points, it was apparent that miR158A-G was the second most preferred substrate. At 4 min, less non-tailed miR158A-G was present compared to non-tailed miR158 or miR158A-C (Fig 5A, compare lane 14 to lane 11 or 13). At 10 min, while substantial amount of non-tailed miR158 or miR158A-C was present, almost all miR158A-G was tailed (Fig 5A, compare lane 19 to lane 16 or 18).
Fig. 5. Nucleotidyl transferase assays to evaluate the substrate preferences by HESO1 and URT1. 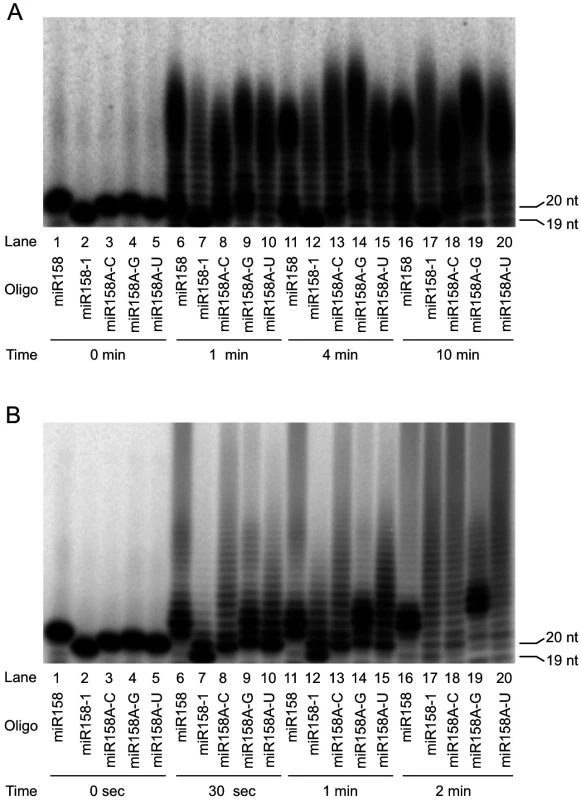
Reactions were conducted with HESO1 (A) or URT1 (B) and five RNA oligonucleotides (oligo) in a time course as indicated. The sizes of the input miRNAs are indicated (19 nt for miR158-1 and 20 nt for the full-length forms of miR158). The RNAs were 5’ 32P-labeled and gel purified prior to the reactions. The substrate/enzyme ratio was 1:2 for HESO1 and 1:8 for URT1. The sizes of the RNA substrates are indicated. URT1 showed a strong preference for miR158 ending in A, as the substrate was depleted at the earliest time point while the other miR158 forms still remained (Fig 5B, compare lane 6 to lanes 8–10). The preference for A-ending substrates is consistent with the finding that URT1 uridylates mRNAs with oligoadenylate tails in vivo [22]. The other three forms of miR158 (miR158A-C, miR158A-G, and miR158A-U) were similarly used by URT1 in the reaction time course (Fig 5B, lanes 8–10, 13–15, and 18–20).
miR158-1 was the least favored substrate among the five miR158 forms tested for both HESO1 and URT1 (Fig 5A and 5B). But miR158-1 was better tolerated by URT1 than by HESO1, as it was tailed only slightly more slowly than miR158A-C, miR158A-G, or miR158A-U by URT1 (Fig 5B, compare lane 12 to lanes 13–14, and lane 17 to lanes 18–20). But it was barely tailed by HESO1 when the full-length miR158 forms were substantially tailed (Fig 5A, compare lane 12 to lanes 13–14, and lane 17 to lanes 18–20). This difference of the two enzymes could potentially explain the in vivo tailing of miR158-1 by URT1 but not HESO1.
We next tested whether tailing of miR158 or miR158-1 was more efficient with both HESO1 and URT1 acting together. In order to compare the activities of different enzymes, we had to use a single buffer, and we chose the URT1 buffer for all three reactions, URT1 alone, HESO1 alone, and URT1 and HESO1 together. Indeed, for both miR158 and miR158-1, the two enzymes together tailed the miRNAs better than each enzyme alone (S6 Fig).
URT1 and HESO1 act on AGO1-bound miRNAs
We previously showed that HESO1 is able to tail miR166 in a reconstituted AGO1-miR166 RISC [16]. We sought to determine whether URT1 had the capability to act on AGO1-bound miRNAs and to confirm the ability of HESO1 to tail AGO1-bound miRNAs in native miRISCs. To obtain native, AGO1-bound, but unmethylated miRNAs for use as substrates, we took advantage of the fact that miRNAs lack methylation and show drastically reduced uridylation in the hen1-2 heso1-2 urt1-3 background (see Wang et al., companion manuscript). AGO1 was immunoprecipated from this genetic background, and the immunoprecipate (IP) was subjected to western blotting to detect AGO1 and northern blotting to detect miR165/6 and miR172. Both AGO1 and the two miRNAs were found in the IP (Fig 6 and S7 Fig). In comparison to the miRNA species in wild-type plants, the miRNAs in the AGO1 IP from hen1-2 heso1-2 urt1-3 included both full-length and truncated species (Fig 6).
Fig. 6. URT1 and HESO1 can tail AGO1-bound miRNAs. 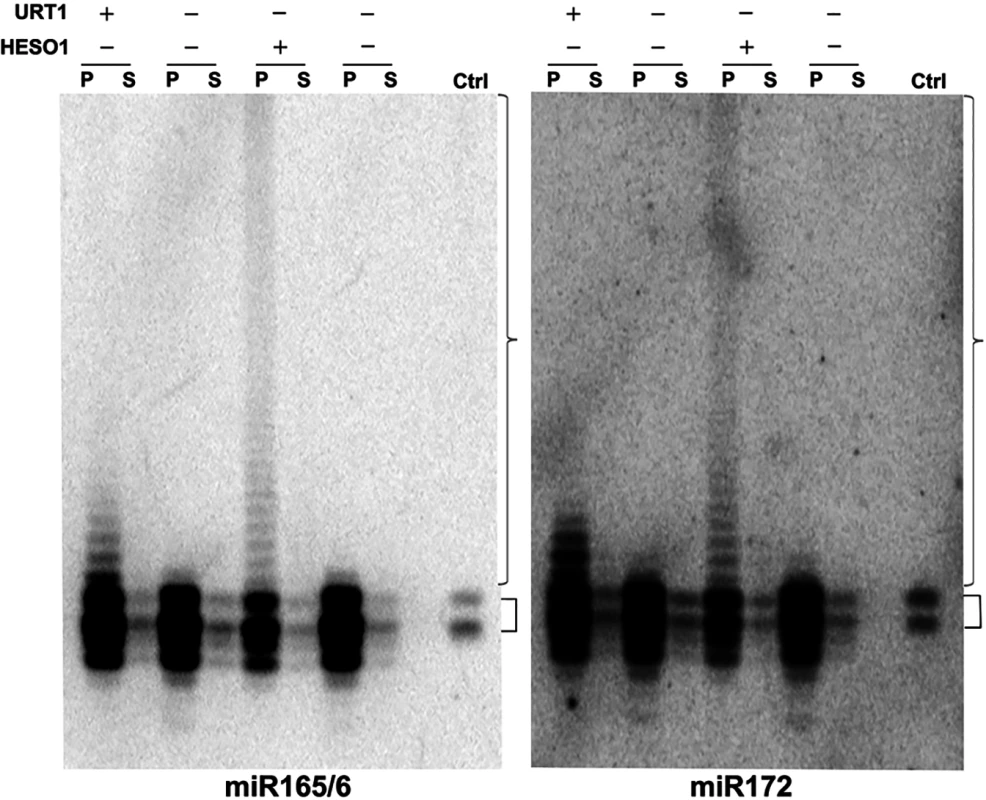
Nucleotidyl transferase assays were conducted with recombinant URT1, HESO1, or no enzyme as indicated, AGO1 immunoprecipitate (IP) as the substrate, and cold UTP. AGO1 IP was prepared from hen1-2 heso1-2 urt1-3 so that the associated miRNAs lack 2’-O-methylation or tailing. After the reactions, the reaction mixes were separated into the precipitate (P) and supernatant (S) fractions and the RNAs were isolated separately from the two fractions and subjected to northern blotting to detect miR165/6 and miR172. The lanes “ctrl” were total RNA from wild type included during gel electrophoresis. The smaller brackets indicate full-length miRNAs from wild type. Both full-length and 3’ truncated miRNAs were present in the AGO1 IP from hen1-2 heso1-2 urt1-3. The larger brackets indicate tailed miRNAs. The AGO1 IP was used as a substrate in reactions with URT1 or HESO1 and cold UTP. After the reactions, AGO1 was collected in the precipitate, and RNAs in both the precipitate (associated with AGO1) and the supernatant (released from AGO1) were detected by northern blotting using probes against miR165/6 or miR172. HESO1 was able to tail the two miRNAs in native miRISCs (Fig 6). In fact, the lengths of tails added by HESO1 exceeded 100 nucleotides. Despite such long tails, the tailed miRNAs were still bound by AGO1, as they were present in the precipitate rather than the supernatant (Fig 6). URT1 was also able to tail AGO1-bound miR165/6 and miR172 (Fig 6), but the lengths of tails introduced by URT1 were much shorter than those generated by HESO1. The miRNAs tailed by URT1 were also associated with AGO1 (Fig 6).
URT1 tails miR171a to 22 nt in hen1 to initiate phasiRNA biogenesis
Given that both HESO1 and URT1 can tail miRNAs in native miRISCs and that tailed miRNAs in hen1 mutants are associated with AGO1 in vivo [12,15], we asked whether tailed miRNAs could be functional. We previously observed that miR171a, which is 21-nt long (S4D Fig) and unable to trigger the biogenesis of secondary phasiRNAs from its target genes At2g45160 and At3g60630 in wild type, acquired this ability in hen1 mutants [15]. According to small RNA sequencing, 22-nt and 21-nt forms of miR171a were the two most abundant forms in hen1-1 and hen1-8 mutants [15] (Fig 7A and 7B), and we hypothesized that the tailing of 21-nt miR171a to 22 nt endowed the ability to trigger phasiRNA biogenesis. But this hypothesis was not tested as the enzyme that tailed miR171a to 22 nt was unknown. It was not HESO1, as in hen1-8 heso1-1, the 22-nt form showed an increase in abundance, probably because further tailing of this form was reduced ([15]; Fig 7B). We found that this enzyme was URT1 since the 21-nt form became the most abundant form in hen1-8 urt1-1 (Fig 7A). In fact, the over-accumulation of miR171a (0,0) and miR171a (0,1) in hen1-8 urt1-1 and hen1-8 heso1-1, respectively (Fig 7A and 7B), indicates that URT1 tails full-length miR171a by one nucleotide in vivo and the 1-nt-tailed form is further tailed by HESO1.
Fig. 7. Tailing of miR171a in hen1 by URT1 triggers phasiRNA biogenesis. 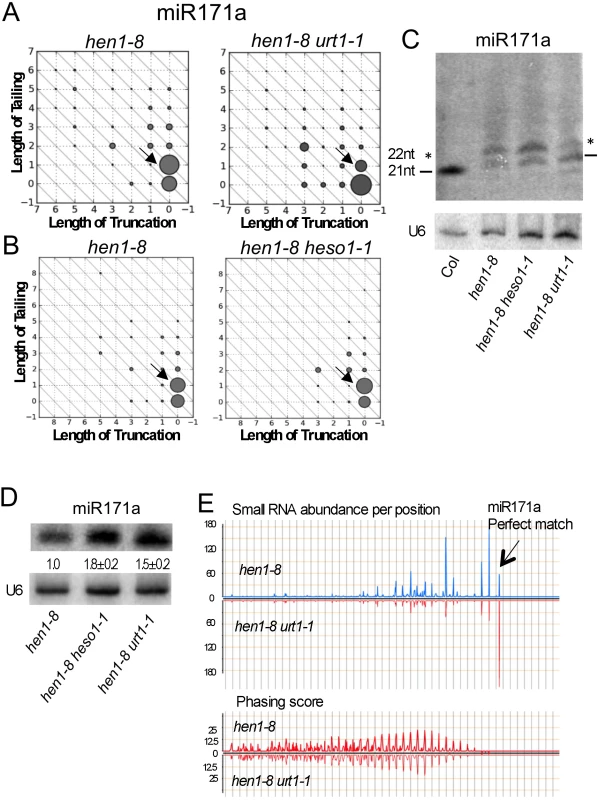
(A-B) The accumulation of various miR171a species as determined by small RNA sequencing. Refer to the Fig 2 legend for the description of the diagrams. The two samples in (A) or (B) were processed in the same experiment, but (A) and (B) were from two different experiments. The 22-nt miR171a form is marked by an arrow in each of the diagrams. This form is reduced in hen1-8 urt1-1 but not hen1-8 heso1-1. (C) The accumulation of miR171a variants of different sizes in various genotypes was determined by northern blotting. 5 μg and 50 μg of total RNAs were used for Col (wild type) and hen1-8 genotypes, respectively. The 21-nt bands are marked by the two dashes; the 22-nt bands are marked by the two asterisks. (D) The amount of miR171a in hen1-8, hen1-8 heso1-1 and hen1-8 urt1-1 was determined by northern blotting. RNAs were resolved in a short gel so as not to separate the size variants. The numbers below the miR171a gel image represent the relative abundance of miR171a in various genotypes. The error bars represent SD and were calculated from three biological replicates. (E) The impact of the urt1-1 mutation on phasiRNA production from the miR171a target gene At2g45160. The X axes in the two panels represent the transcript of At2g45160 in the 3’ to 5’ orientation from left to right. In the top panel, the relative abundance of secondary siRNAs from At2g45160 was plotted. The arrow marks the position of the miR171a-binding site in the transcript, which is 5’ to all the secondary siRNAs. In the bottom panel, the phasing scores of the secondary siRNAs were plotted, showing that the secondary siRNAs are phasiRNAs in both genotypes. To verify these results from small RNA sequencing, we performed northern blotting of miR171a at a single nucleotide resolution. In both hen1-8 and hen1-8 heso1-1, the abundance of the 22-nt form was higher than that of the 21-nt form (Fig 7C). In hen1-8 urt1-1, the opposite was observed (Fig 7C). Therefore, URT1 was responsible for the production of 22-nt miR171a by tailing miR171a by one nucleotide. Moreover, consistent with the cooperative tailing of miR171a by both URT1 and HESO1, miR171a levels were higher in hen1-8 urt1-1 and hen1-8 heso1-1 as compared to hen1-8 (Fig 7D).
Next, we examined whether the urt1-1 mutation, which reduced the levels of 22-nt miR171a, affected the production of phasiRNAs triggered by miR171a in hen1-8. From hen1-8 and hen1-8 urt1-1 small RNA libraries, secondary siRNAs mapping to At2g45160 (a target of miR171a) were normalized to total small RNAs and plotted along the At2g45160 gene (Fig 7E, top panel). It was clear that these secondary siRNAs were reduced in abundance in hen1-8 urt1-1. When the secondary siRNAs were examined for their phasing status, i.e., whether they occurred in 21-nt intervals from one another, no difference was observed in the phasing scores in hen1-8 and hen1-8 urt1-1 (Fig 7E, bottom panel). This indicated that phasiRNAs were produced from At2g45160 in both genotypes, but their abundance was much lower in hen1-8 urt1-1. Therefore, the tailing of miR171a to 22 nt in hen1 by URT1 promotes miR171a-triggered phasiRNA biogenesis.
Tailing of miR165/6 by URT1 reduces its slicing activity
Many miRNAs are normally 21-nt in length and they are tailed to various sizes including 22 nt in hen1 mutants. But miR171 is the only miRNA that acquires the ability to generate phasiRNAs in hen1 mutants [15], suggesting that the 22-nt forms of most miRNAs are not functional. In addition, the expression of At3g03580, a target of miR158, was repressed more effectively in hen1 urt1 than in hen1 (Fig 2E), despite the fact that miR158 was similar in abundance in the two genotypes. This suggested that tailed forms of miR158 are not as effective in target repression.
We decided to examine the effects of tailing by URT1 on the activities of miRNAs in vitro using the slicer assay as the functional output of miRNAs. We immunoprecipitated AGO1 from hen1-2 heso1-2 urt1-3, and used the IP as the substrate for the tailing reaction with URT1. Following the tailing reaction, AGO1 was collected in the precipitate, washed, and incubated with a fragment of the PHB transcript (a target of miR165/6) to assay the slicer activity of AGO1-miR165/6 (a scheme of the procedure is shown in Fig 8A). While the AGO1 IP was able to cleave the PHB RNA, the AGO1 IP after the tailing reaction with URT1 failed to cleave the PHB RNA (Fig 8B). miR156/6 and AGO1 were present after the URT1 reaction, as shown by northern blotting and western blotting, respectively (Fig 8C and 8D). Tailing of miR165/6 by URT1 was also visible by northern blotting (Fig 8C). To confirm that the loss of cleavage activity was due to miR165/6 tailing, we conducted the tailing reaction with heat-inactivated URT1 or the URT1 catalytic mutant. Neither enzyme affected the cleavage activity of miR165/6 (Fig 8B–8D). This result was initially unexpected, as we did not expect 100% tailing of miR165/6 by URT1. But upon closer examination of the profiles of miR165/6 in northern blotting (Fig 8C), it seemed that most of the miR165/6 species were tailed by a small number of nucleotides, as the profiles looked different when functional URT1 was used.
Fig. 8. Tailing of AGO1-bound miR165/6 by URT1 drastically reduces its slicer activity. 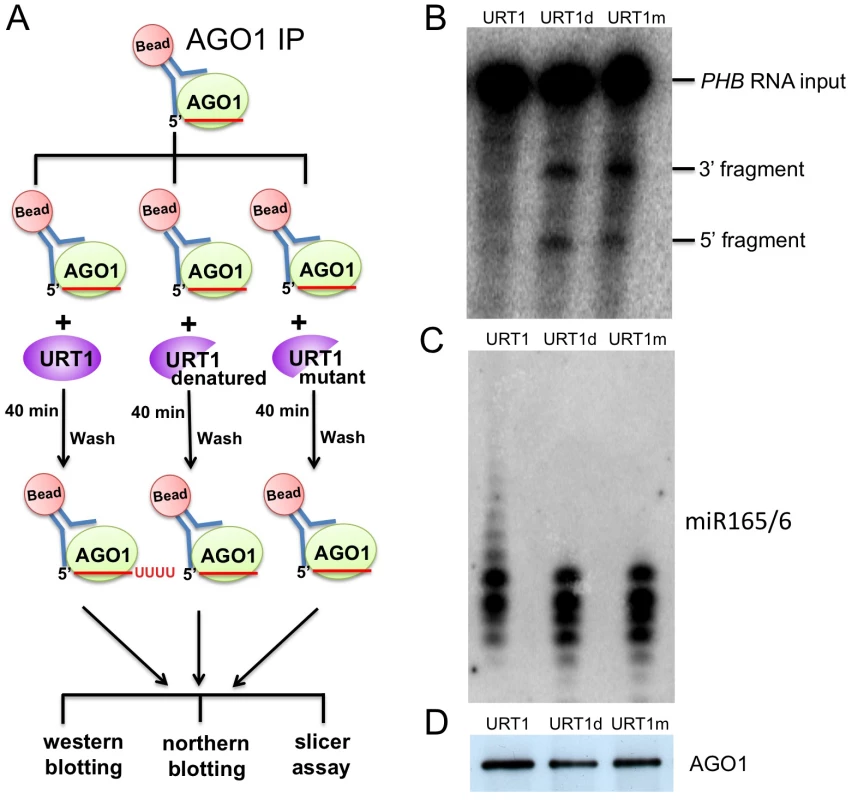
(A) An experimental scheme. AGO1 was immunoprecipitated from hen1 heso1-2 urt1-3. The IP was used as the substrate for tailing reactions by one of three enzymes. Each tailing reaction was then split into three equal portions for western blotting to quantify AGO1, northern blotting to determine the status of tailing of AGO1-bound miR165/6, and slicer assay. (B) Slicer assay following the tailing reactions. A portion of the PHB RNA containing the miR165/6 binding site was body-labeled with 32P and used as the input RNA. (C) Northern blotting to detect miR165/6 from the AGO1-bound fraction after the tailing reactions. (D) Western blotting to detect AGO1 after the tailing reactions. Discussion
URT1 and HESO1 are nucleotidyl transferases with similar and distinct properties
In this study, we identify URT1 as a nucleotidyl transferase that tails unmethylated miRNAs and show that URT1 acts with the previously identified nucleotidyl transferase HESO1 in parallel or sequentially to tail various forms of the same miRNA in vivo. As nucleotidyl transferases, URT1 and HESO1 are similar in that both prefer UTP over the other three nucleotides and both are completely inhibited by 2’-O-methylation in their substrate RNA. These biochemical properties are consistent with molecular genetic observations that uridylated miRNAs are prevalent only in hen1 mutants [8].
URT1 and HESO1 also exhibit distinct substrate preferences, especially with regard to the identity of the 3’ nucleotide in the substrate RNA. Our biochemical studies suggest that HESO1 has the strongest preference for RNA ending in U whereas URT1 has the strongest preference for RNA ending in A. This preference, together with the tendency to use UTP instead of other nucleotides for both enzymes, could explain the predominant role of HESO1 in miRNA tailing in vivo. Once HESO1 adds a U to the end of a miRNA, regardless of the nature of its 3’ nucleotide, the resulting tailed miRNA ends in a U and is likely a preferred substrate for HESO1. Therefore, HESO1 is likely able to hold on to the product of its reaction and use it as a substrate, i.e., HESO1 could be a processive enzyme. URT1, on the other hand, prefers A-ending miRNAs, but once it adds a U, the resulting miRNA is not a good substrate. In fact, this can be seen in the activity assay in Fig 5B (lanes 6, 11, 16), where the species with a few additional nucleotides persist. This suggests that URT1 is probably not a processive enzyme. Small RNA profiling in hen1-8 and hen1-8 urt1-1 in this study is consistent with this conclusion. We found that that URT1 mono-uridylates miR158, miR171a, and miR173 in vivo. After mono-uridylation of miR171a and miR173 by URT1, HESO1 further uridylates the mono-uridylated, U-ending miRNAs in vivo.
When both enzymes are active in vivo, one would expect HESO1 to out-compete URT1 in miRNA tailing. When one enzyme is knocked out, such as in hen1-8 heso1-1 or hen1-8 urt1-1, the remaining enzyme should in theory have access to all miRNAs. In hen1-8 heso1-1, miRNA tailing is drastically reduced with monouridylation being the predominant forms left [10]. This is consistent with the conclusion that URT1 is not a highly processive enzyme. In hen1-8 urt1-1, most miRNAs are still fully tailed, suggesting that HESO1 can act on most miRNAs. The ten miRNAs that show reduced tailing in hen1-8 urt1-1 must be refractory to HESO1 activity. In fact, most of the miRNAs end in C, A or G (S8 Fig), which are non-preferred 3’ nucleotides for HESO1. The exceptions are miR845a and b, for which the affected forms in hen1-8 urt1-1 end in U (S6 Fig). It is unclear why HESO1 does not act effectively on these miRNAs in vivo.
It should be noted that the preferences for the 3’ ending nucleotide cannot fully account for the substrate preferences of the enzymes, nor their processivity. For example, for both URT1 and HESO1, miR158-1, which ends in C, is not as good a substrate as miR158A-C, in which the last nucleotide of miR158 is mutated to C (Fig 5). The sequence of the 3’ region of the miRNA substrate probably also matters.
There are ten potential nucleotidyl transferases in the genome. Two of them are now shown to tail unmethylated miRNAs. HESO1 and URT1 also act on mRNAs or mRNA fragments [16,22]. Both HESO1 and URT1 are completely inhibited by 2’-O-methylation in their miRNA substrates, it would be interesting to know whether any of the eight proteins can act on methylated miRNAs. It would also be interesting to know what RNA substrates these enzymes act on, such as siRNAs, other noncoding RNAs, or mRNAs.
Tailing of AGO1-bound miRNAs and the fate of the tailed miRNAs
An important discovery of this study is that both HESO1 and URT1 can act on native miRISCs to tail miRNAs and that the tailed miRNAs remain bound by AGO1 in vitro. It is surprising that HESO1 is able to add more than 100 nucleotides to AGO1-bound miRNAs in vitro, as miRNAs in hen1 mutants have much shorter tails (usually less than eight nucleotides [10,15]). As tailed miRNAs in hen1 mutants are bound by AGO1 in vivo [12,15], it is likely that only miRNAs with short tails are found in vivo because only these species can be stably accommodated by AGO1, i.e., with the 5’ and 3’ ends of the miRNAs in the respective binding pockets in AGO1. miRNAs with long tails are unlikely to have their 3’ end protected by the PAZ domain of AGO1 and are likely susceptible to degradation or 3’ trimming to generate miRNAs with shorter tails.
Given that HESO1 and URT1 can act on AGO1-bound miRNAs and miRNAs with short tails can be accommodated by AGO1, degradation may not be the only outcome of miRNA tailing. In this study, we show that tailing of AGO1-bound miR165/6 by URT1 nearly abolishes its target RNA cleavage activity. Most of the AGO1-bound miR165/6 species had short tails after the URT1 reaction and were bound by AGO1, yet they lost their activity. This suggests that the tailed species are not properly positioned in AGO1 for optimal slicer activity. Our results on the expression of At3g03580, a target of miR158, are also consistent with tailed miR158 species not being functional (Fig 2E). We suspect that miRNA tailing leads to reduced miRNA activity in general, but an exception is miR171a. Mono-uridylation of 21-nt miR171a by URT1 in hen1 mutants triggers the production of phasiRNAs from a miR171a target gene. This demonstrates that mono-uridylated miR171a is functional in vivo and that 3’ tailing alters the activities of this miRNA.
Materials and Methods
Plant materials
All Arabidopsis strains are in the Columbia background except for hen1-2 heso1-2 urt1-3 (generated in the companion manuscript), which is in the Landsberg background. Seeds of nine ntp mutants were obtained from the Gabi-Kat collection or ARBC collection (see S1 Table for details). hen1-8 ntp double mutants were made by crossing hen1-8 with ntp mutants and genotyping F2 populations for plants homozygous for both hen1-8 and ntp mutations. Plants were grown under long day (16 h light/ 8 h darkness) conditions at 22°C.
Plasmid construction
Towards the construction of the pURT1:URT1-GFP plasmid, a 1.4 kb fragment upstream of the URT1 coding region was amplified by PCR with primers 3-Kpn1-1610F(PF) and URT1-pst1-PR as the promoter, and cloned into TSK108, a pENTRY-D-topo-based Gateway entry vector, at KpnI and PstI sites. Subsequently, the cDNA (without the stop codon) was amplified by PCR with primers URT1-pst1-CDSF and URT1-SPE1-CDSR and inserted next to the promoter at PstI and SpeI sites. Then the promoter-cDNA fragment was moved into pMDC107 [24] by LR reaction. The plasmid was used to transform hen1-8 urt1-1 plants by Agrobacterium (GV3101)-mediated floral dip transformation.
To construct the pET32a-URT1_WT plasmid for the expression of wild-type URT1 in E. coli, the coding sequence of URT1 was amplified using primers URT1-sac1-F and URT1-Xho1-R and cloned into pET-32a. The orientation of the insertion was confirmed by sequencing.
To construct the pET32a-URT1_M plasmid for the expression of the catalytic mutant of URT1 in E. coli, mutagenesis of URT1 was first performed by PCR with primers that incorporated the D491A and D493A mutations in the URT1 coding sequence. Primers URT1-Sac1-F and URT1-DADA-R were used to generate the 5’ URT1 fragment; primers URT1-DADA-F and URT1-Xho1-R were used to generate the 3’ URT1 fragment. The two fragments were annealed and used as the template to amplify the full-length URT1m using URT1-Sac1-F and URT1-Xho1-R as primers. This full-length fragment was cloned into pET-32a. The orientation of the insertion was confirmed by sequencing. See S3 Table for sequences of primers.
Quantitative RT-PCR analysis
Reverse transcription and real-time PCR were performed as described [25]. Total RNAs were prepared from inflorescences, and converted to cDNAs using Superscript III reverse transcriptase (Invitrogen) and oligo-dT. The cDNAs were then used as templates for real-time PCR with gene-specific primers. Real-time PCR was performed in triplicates using on a Biorad IQcycler apparatus with the Quantitech SYBR green kit (BioRad). The ACTIN8 gene was used as the internal control. Primers used are listed in S3 Table.
Northern blotting
RNA isolation and northern blotting to detect small RNAs were performed as described [26]. Total RNAs were extracted from inflorescences or AGO1 immunoprecipitate using the TRIzol reagent (Invitrogen). 5′-end-labeled (32P) antisense DNA oligonucleotides were used to detect miRNAs. Sequences of probes are shown in S3 Table.
Phylogenetic analysis
A phylogenetic analysis was performed for ten putative Arabidopsis nucleotidyl transferases, MUT68 from Chlamydomonas reinhardtii, TUT4 from Homo sapiens, and Cid1 from Schizosaccharomyces pombe. Sequences corresponding to the NT_PAP_TUTase (cd05402) domain [27] from these proteins were aligned using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/) with default parameters [28]. The phylogenetic tree was generated using MEGA5 [29].
Small RNA library construction and sequencing
Cloning of small RNAs was carried out as described [30]. 50 μg total RNAs were resolved in a 15% polyacrylamide gel and 15–40 nt small RNAs were eluted from an excised gel piece. The small RNAs were ligated sequentially with the 3' and 5' adapters using the Small RNA Sample Preparation Kit (Illumina). Sufficient amounts of products were obtained by performing a reverse transcription reaction followed by a low-cycle PCR amplification. The libraries were barcoded and sequenced in one lane on an Illumina HiSeq2000.
Analysis of miRNA 3’ truncation and 3’ tailing
Small RNA reads that passed Illumina’s quality control were separated into different genotypes according to the indexes. These high-quality small RNA reads were then mapped to the TAIR10 Arabidopsis genome using Bowtie [31]. The reads that matched to annotated tRNAs/rRNAs/snRNAs/snoRNAs were removed. The total numbers of reads that passed the quality and tRNA/rRNA filters for the various genotypes and replicates are listed in S2 Table.
To analyze miRNA 3’ tailing, a previously-developed bioinformatics pipeline was employed [15]. Briefly, any small RNA read that could not be perfectly mapped back to the genome was trimmed one nucleotide at a time from the 3’ end until the remaining sequence was perfectly mapped to the genome. The trimmed 3’ sequence was designated as the “tail” whereas the longest 5’ genome-mapped component (the “head”) was compared to all annotated miRNAs in miRBase to ascertain from which miRNAs they were derived.
To quantify the extent of tailing, Arabidopsis miRNAs annotated in miRBase v17 [32] were examined. Small RNA reads with the 5’ head perfectly aligning to each one of the annotated miRNAs were identified, and the amount of tailing was calculated as the ratio of the number of reads with tails to that of total reads of variants derived from a particular miRNA.
Small RNA phasing analysis was conducted as previously described [33]. Small RNA abundances from the antisense strand were combined with those of the sense strand, based on an anticipated 2 nt overhang at their 3’ ends, which is a typical feature of Dicer-produced small RNA duplexes. Phasing scores and combined abundances of small RNAs were graphed using a customized Perl script.
Protein purification and enzymatic assays
The pET32a-URT1_WT and pET32a-URT1 _M plasmids were transformed into the E. coli strain BL21 Star™(DE3) for protein expression. The transgenic E. coli strains were cultured at 30°C until the OD reached 0.5. IPTG was added to a final concentration of 0.1 mM and the culture was incubated at 16°C overnight. The recombinant proteins were purified using Ni-NTA agarose (Invitrogen) under native conditions following the manufacturer's instructions. After the extract containing a recombinant protein was loaded onto the column, the column was washed four times with the following wash buffers: wash buffer 1 (200 mM NaCl, 50 mM Tris-HCl pH 8.0, 40 mM imidazole), wash buffer 2 (200 mM NaCl, 50 mM Tris-HCl pH 8.0, 60 mM imidazole), wash buffer 3 (200 mM NaCl, 50 mM Tris-HCl pH 8.0, 100 mM imidazole), and wash buffer 4 (200 mM NaCl, 50 mM Tris-HCl pH 8.0, 120 mM imidazole). Then the recombinant protein was eluted with elution buffer (200 mM NaCl, 50 mM Tris-HCl pH 8.0, 500 mM imidazole).
The URT1 enzymatic activity assays in Fig 3 were conducted in 10 μl reaction mixtures containing 4.8 pmole recombinant wild-type or mutant URT1, 1 μl of 40 mM miR173 and 1 mM of different nucleotide triphosphates in the URT1 reaction buffer (20 mM Tris-HCl pH 8.0, 50 mM NaCl, 0.7 mM MnCl2, 10 mM MgCl2, 0.5 mM DTT, and 100 μg/mL BSA). Reactions were conducted at room temperature for 0–20 min, and stopped by the addition of formamide dissolved in RNA loading dye. The reaction mixtures were denatured at 95°C for 5 min, incubated on ice for 5 min, and loaded on a 15% polyacrylamide urea gel. After gel electrophoresis, the gel was stained by ethidium bromide and imaged with a Gel Doc™ XRS+ imaging system (BIO-RAD).
The URT1 enzymatic activity assays in Fig 5 were conducted as described above except that the RNA oligonucleotide substrates were 5’ 32P-labeled and the products were visualized by autoradiography. 1 pmole of RNA oligonucleotides and 8 pmole of URT1 were present in the reactions. The HESO1 enzymatic assays in Fig 5 were performed as described [10]. 1 pmole of RNA oligonucleotides and 2 pmole of HESO1 were present in the reactions. For the 5’ labeling of RNA oligonucleotides, a 50 μl reaction mixture containing 100 μM oligonucleotide, 20 U T4 Polynucleotide Kinase (New England Biolabs), 1X T4 PNK buffer and 4 μl ATP [γ-32P] (3000Ci/mmol 10mCi/ml from PerkinElmer) was incubated at 37°C for 1 hour. The RNA was then purified with Illustra MicroSpin™ G-25 Columns (GE Healthcare) according to the manufacturer’s instructions. The RNA oligonucleotides were resolved in a 15% denaturing polyacrylamide gel, and gel pieces containing the full-length RNAs were excised, and the RNAs were eluted and used in the URT1 or HESO1 reactions.
The URT1 and HESO1 enzymatic activity assays in S6 Fig were conducted in 10 μl reaction mixtures containing 1 pmole of 5’ 32P-labeled miR158 or miR158-1, enzymes (see below), and 1 mM UTP in the URT1 reaction buffer (described above). For the reactions with miR158, 4 pmole URT1 alone, 4 pmole HESO1 alone, or 4 pmole URT1 and 4 pmole HESO1 together were used. The reaction mixtures were incubated for 1min at room temperature. For reactions with miR158-1, 8 pmole URT1 alone, 2 pmole HESO1 alone or 8 pmole URT1 and 2 pmole HESO1 together were used. The reaction mixtures were incubated at room temperature for 30 s.
Immunoprecipitation of AGO1 and associated small RNAs
0.4–1.0g of seedling tissue from hen1-2 heso1-2 urt1-3 plants was collected and ground to a fine powder in liquid nitrogen. 1 ml of IP buffer (50 mM Tris pH 7.5, 150 mM NaCl, 10% Glycerol, 0.1% NP-40, 4mM MgCl2, 5mM DTT and 1x protease inhibitor cocktail (Roche)) was added to the powder. The suspension was incubated at 4°C for 30 min and centrifuged at 16,000g for 15 min at 4°C. The supernatant was collected, filtered through 2 lays of Miracloth, and centrifuged as before. The supernatant was transferred into a new tube and pre-cleared with Dynabeads-Protein-A (Life Technologies) for 1 h at 4°C. The supernatant was separated from the beads using a magnetic stand and transferred into a new tube. The extract was then mixed with 2 μl of AGO1 antibody (Agrisera) and the mixture was incubated for 2 h with gentle shaking at 4°C. 20 μl pre-cleared Dynabeads-Protein-A was added and incubation was continued for another hour. The beads were washed four times with 1 ml wash IP buffer. Finally, the beads (i.e., AGO1 immunoprecipitate) were collected with a magnetic stand.
Nucleotidyl transferase assays on AGO1-bound miRNAs
AGO1 IP was first performed as described above. The beads (AGO1 immunoprecipitate) were equally split into four portions, two for URT1 reactions and two for HESO1 reactions. The beads were washed three times with the URT1 or the HESO1 reaction buffer. After the washes, the beads were fully resuspended and equally split into two tubes (one for enzyme reaction and one for “no enzyme” control). Subsequently, the beads were collected and added to a 20 μl reaction mixture containing 0.7 pmole 6XHis-HESO1 or 38.5 pmole 6HisXURT1, reaction buffer (described above or in [10]) and cold UTP. The reactions were incubated for 40 min, the beads were separated from the supernatant with a magnetic stand, and RNA extraction was performed from the beads and the supernatant. Finally RNAs were resolved by gel electrophoresis and subjected to northern blotting to detect miR165/6 and miR172.
Slicer assay on AGO1-bound miRNAs after URT1-mediated tailing
AGO1 IP was first performed as described above using 0.5g of hen1-2 heso1-2 urt1-3 seedlings. The beads (AGO1 immunoprecipitate) were washed three times with the URT1 reaction buffer, and split into 3 equal portions, to be used for reactions with URT1, denatured URT1 (denaturing by boiling for 5 min), and the URT1 catalytic mutant (URT1m), respectively. Subsequently, each portion of beads was collected and added to a 60 μl reaction mixture containing 19 pmole 6XHis-URT1 (or denatured URT1 or URT1m), reaction buffer (described above) and cold UTP. The reactions were carried out at room temperature for 40 min; afterwards the beads were collected and washed 3 times with the IP buffer. The beads in each tube were further split into three equal portions, with one portion to be used for western blotting to quantify AGO1, another portion to be used for northern blotting to quantify AGO1-bound miR165/166, and the third portion to be used for the slicer assay. The beads in the third portion were collected and added to a 20 μl reaction mixture containing 15μl IP buffer, 4μl 5 X cleavage buffer and 1μl uniformly radiolabeled PHB (see the in vitro transcription section below), a target of miR165/6. The reactions were incubated for 1h, and RNA extraction was performed. Finally. RNAs were resolved by gel electrophoresis and visualized by autoradiography. 5 X cleavage buffer: 5 mM ATP, 1 mM GTP, 6 mM MgCl2, 125 mM creatine phosphate, 150 mg/mL creatine kinase and 2 unit/mL RNasin RNase Inhibitor.
In vitro transcription
The template for in vitro transcription is generated by PCR using primers specific for the miR165/166 target gene PHB. The PCR-amplified PHB fragment is about 300 bp that contains the miR165/166 target region. T7 promoter sequence is added to the 5’end of the forward primer. The Riboprobe T7 kit (Promega) was used to generate transcripts from this PHB fragment. In a 20 μl reaction, 1μl DNA template and 3μl UTP [α-32P] (3000Ci/mmol 10mCi/ml from PerkinElmer) was added. The reaction was incubated at 37°C for 1 hour, and then stopped by the addition of formamide dissolved in RNA loading dye. The reaction mixtures were denatured at 95°C for 5 min, incubated on ice for 5 min, and loaded on a 5% polyacrylamide urea gel. The band of the expected size was excised and the RNAs were eluted and used in the cleavage assay.
Accession numbers
The small RNA sequencing data in this study have been deposited at the GEO repository under the ID# GSE61362.
Supporting Information
Zdroje
1. Billi AC, Alessi AF, Khivansara V, Han T, Freeberg M, et al. (2012) The Caenorhabditis elegans HEN1 ortholog, HENN-1, methylates and stabilizes select subclasses of germline small RNAs. PLoS genetics 8: e1002617. doi: 10.1371/journal.pgen.1002617 22548001
2. Horwich MD, Li C, Matranga C, Vagin V, Farley G, et al. (2007) The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol 17 : 1265–1272. 17604629
3. Kamminga LM, Luteijn MJ, den Broeder MJ, Redl S, Kaaij LJ, et al. (2010) Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J 29 : 3688–3700. doi: 10.1038/emboj.2010.233 20859253
4. Kamminga LM, van Wolfswinkel JC, Luteijn MJ, Kaaij LJ, Bagijn MP, et al. (2012) Differential impact of the HEN1 homolog HENN-1 on 21U and 26G RNAs in the germline of Caenorhabditis elegans. PLoS genetics 8: e1002702. doi: 10.1371/journal.pgen.1002702 22829772
5. Kirino Y, Mourelatos Z (2007) Mouse Piwi-interacting RNAs are 2'-O-methylated at their 3' termini. Nature structural & molecular biology 14 : 347–348.
6. Kurth HM, Mochizuki K (2009) 2'-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA 15 : 675–685. doi: 10.1261/rna.1455509 19240163
7. Montgomery TA, Rim YS, Zhang C, Dowen RH, Phillips CM, et al. (2012) PIWI associated siRNAs and piRNAs specifically require the Caenorhabditis elegans HEN1 ortholog henn-1. PLoS genetics 8: e1002616. doi: 10.1371/journal.pgen.1002616 22536158
8. Li J, Yang Z, Yu B, Liu J, Chen X (2005) Methylation protects miRNAs and siRNAs from a 3'-end uridylation activity in Arabidopsis. Current biology: CB 15 : 1501–1507. 16111943
9. Ren G, Chen X, Yu B (2012) Uridylation of miRNAs by hen1 suppressor1 in Arabidopsis. Current biology: CB 22 : 695–700. doi: 10.1016/j.cub.2012.02.052 22464191
10. Zhao Y, Yu Y, Zhai J, Ramachandran V, Dinh TT, et al. (2012) The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Current biology: CB 22 : 689–694. doi: 10.1016/j.cub.2012.02.051 22464194
11. Yu B, Bi L, Zhai J, Agarwal M, Li S, et al. (2010) siRNAs compete with miRNAs for methylation by HEN1 in Arabidopsis. Nucleic acids research 38 : 5844–5850. doi: 10.1093/nar/gkq348 20448024
12. Zhao Y, Mo B, Chen X (2012) Mechanisms that impact microRNA stability in plants. RNA biology 9 : 1218–1223. doi: 10.4161/rna.22034 22995833
13. Baumberger N, Baulcombe DC (2005) Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proceedings of the National Academy of Sciences of the United States of America 102 : 11928–11933. 16081530
14. Qi Y, Denli AM, Hannon GJ (2005) Biochemical specialization within Arabidopsis RNA silencing pathways. Molecular cell 19 : 421–428. 16061187
15. Zhai J, Zhao Y, Simon SA, Huang S, Petsch K, et al. (2013) Plant MicroRNAs Display Differential 3' Truncation and Tailing Modifications That Are ARGONAUTE1 Dependent and Conserved Across Species. The Plant cell 25 : 2417–2428. doi: 10.1105/tpc.113.114603 23839787
16. Ren G, Xie M, Zhang S, Vinovskis C, Chen X, et al. (2014) Methylation protects microRNAs from an AGO1-associated activity that uridylates 5' RNA fragments generated by AGO1 cleavage. Proceedings of the National Academy of Sciences of the United States of America 111 : 6365–6370. doi: 10.1073/pnas.1405083111 24733911
17. Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121 : 207–221. 15851028
18. Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS (2004) SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes & development 18 : 2368–2379.
19. Vazquez F, Vaucheret H, Rajagopalan R, Lepers C, Gasciolli V, et al. (2004) Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Molecular cell 16 : 69–79. 15469823
20. Chen HM, Chen LT, Patel K, Li YH, Baulcombe DC, et al. (2010) 22-Nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proceedings of the National Academy of Sciences of the United States of America 107 : 15269–15274. doi: 10.1073/pnas.1001738107 20643946
21. Cuperus JT, Carbonell A, Fahlgren N, Garcia-Ruiz H, Burke RT, et al. (2010) Unique functionality of 22-nt miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in Arabidopsis. Nature structural & molecular biology 17 : 997–1003.
22. Sement FM, Ferrier E, Zuber H, Merret R, Alioua M, et al. (2013) Uridylation prevents 3' trimming of oligoadenylated mRNAs. Nucleic acids research 41 : 7115–7127. doi: 10.1093/nar/gkt465 23748567
23. Montgomery TA, Howell MD, Cuperus JT, Li D, Hansen JE, et al. (2008) Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133 : 128–141. doi: 10.1016/j.cell.2008.02.033 18342362
24. Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant physiology 133 : 462–469. 14555774
25. Kim YJ, Zheng B, Yu Y, Won SY, Mo B, et al. (2011) The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J 30 : 814–822. doi: 10.1038/emboj.2011.3 21252857
26. Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Current biology: CB 12 : 1484–1495. 12225663
27. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, et al. (2011) CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic acids research 39: D225–229. doi: 10.1093/nar/gkq1189 21109532
28. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 : 2947–2948. 17846036
29. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular biology and evolution 24 : 1596–1599. 17488738
30. Zhao Y, Yu Y, Zhai J, Ramachandran V, Dinh TT, et al. (2012) The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Curr Biol 22 : 689–694. doi: 10.1016/j.cub.2012.02.051 22464194
31. Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology 10: R25. doi: 10.1186/gb-2009-10-3-r25 19261174
32. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic acids research 36: D154–158. 17991681
33. Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, et al. (2011) MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes & development 25 : 2540–2553.
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Akutní intermitentní porfyrie
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



