-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
The vascular tissues in the shoot of Arabidopsis thaliana (Arabidopsis) plants are organized in vascular bundles, disposed in a conserved periodic radial pattern. It is known that this pattern emerges due to the accumulation of the phytohormone auxin, which is actively transported by the so-called efflux and the influx carriers. Efflux carriers facilitate polar transport of auxin from inside the cell to the extracellular space, while influx carriers pump auxin from outside the cell to its interior in a non-polar manner. Although a role for auxin efflux carriers in the emergence of this pattern has been recognized, the role of auxin influx carriers has remained hitherto neglected. In this study, we combine theoretical and experimental approaches to unravel the role of the auxin influx carriers in the formation of plant vasculature. Our analysis uncovers primary roles for the auxin influx carriers in vascular patterning, revealing that auxin influx carriers modulate both patterning and the differentiation of the water transporting vascular cells, known as xylem cells.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005183
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005183Summary
The vascular tissues in the shoot of Arabidopsis thaliana (Arabidopsis) plants are organized in vascular bundles, disposed in a conserved periodic radial pattern. It is known that this pattern emerges due to the accumulation of the phytohormone auxin, which is actively transported by the so-called efflux and the influx carriers. Efflux carriers facilitate polar transport of auxin from inside the cell to the extracellular space, while influx carriers pump auxin from outside the cell to its interior in a non-polar manner. Although a role for auxin efflux carriers in the emergence of this pattern has been recognized, the role of auxin influx carriers has remained hitherto neglected. In this study, we combine theoretical and experimental approaches to unravel the role of the auxin influx carriers in the formation of plant vasculature. Our analysis uncovers primary roles for the auxin influx carriers in vascular patterning, revealing that auxin influx carriers modulate both patterning and the differentiation of the water transporting vascular cells, known as xylem cells.
Introduction
Auxin is an essential phytohormone for the control of plant growth and development. Its transport and distribution throughout the plant create numerous organized patterns in plant tissues, such as leaf venation [1], the wide variety of phyllotactic patterns [2–5], and the periodic shoot vascular patterning [6,7]. Auxin is also involved in the emergence of new organ primordia [4,8], root apical meristem maintenance [9,10], root gravitropism [11–13], lateral root development [8,14], and xylem differentiation [15,16] amongst other developmental processes.
A proportion of auxin is synthesized in the shoot apex and polarly transported in a cell-to-cell manner to the root and to other plant tissues [17]. The chemiosmotic model explains how auxin is polarly transported throughout the plant [18,19]. According to this model, once auxin enters the cell where the pH is less acidic (cytosol pH≈7) than in the apoplast (pH≈5.5), it becomes deprotonated; this hydrophilic form remains then trapped inside the cell. In order to exit the cell, auxin needs active protein transporters that can pump it out. The asymmetric localization within the cell membrane of a subset of these transporters or auxin efflux carriers named PIN (PIN-FORMED) [6] results into one of the main characteristics of auxin transport: its polarity. Depending on the positioning of the PINs, directional fluxes and auxin gradients are created, driving the accumulation of auxin maxima in specific groups of cells [3–5]. Disruption of auxin polar transport significantly alters auxin maxima distribution, resulting in aberrant development [2,6–8,12]. In addition to the PIN efflux carriers, the PGP (P-glycoprotein) ABC-like transporters also export auxin from the cytoplasm to the apoplast [20]. PGP transporters do not localize asymmetrically, but they have been proposed to interact with PINs, subsequently affecting auxin polar transport [21–23].
Unlike auxin efflux from cells, auxin enters the cells either by passive diffusion or by the action of auxin influx carriers. These comprise a multi-gene family in Arabidopsis containing four highly conserved genes: AUX1 and the AUX1-like genes LAX1, LAX2, and LAX3 [24–26]. In contrast with efflux carriers, influx carriers do not show a polar distribution within most cells with exception of the root protophloem cells [27,28]. The absence of strong phenotypes in influx mutants, especially under long day conditions, as well as their non-polar distribution has prevented extended studies on the role of influx carrier mutants on patterning in the past. So far, all the AUX1/LAX family members have been associated with changes in vascular transport [29], leaf positioning [30,31] and root stem cell patterning [32]. Despite the reported redundancy, AUX1/LAX family members not only have distinct tissue-specific expression patterns, but also exert different functions, suggesting that these genes underwent sub-functionalization and likely provided additional mechanisms of regulation to the plant [25,31]. For instance, within Arabidopsis primary root, AUX1 has been reported to localize in the columella, the lateral root cap, the epidermis and the stele [25,33,34], LAX1 is localized within the mature vascular tissue, with weak expression in the root tip immature vasculature [25], LAX2 is localized within the root stem cell niche, the provascular cells and the stele [25] and LAX3 is localized in the columella and the stele cells [25,35]. In addition, AUX1, LAX1 and LAX2 are differently localized at multiple developmental stages in the lateral root primordia whereas LAX3 is localized in the outer tissues in front of the primordia [25,35,36]. AUX1, the most studied influx carrier, has been attributed roles in root gravitropism, petal initiation and lateral root development [36–38]. Furthermore, LAX2 influx carrier has been recently reported to confer continuity to the vascular strands in cotyledons [25]. In addition, LAX3 has been shown to promote lateral root emergence and apical hook development [35,39]. Theoretical studies have proposed a stabilizing role for influx carriers on periodic patterning rather than major roles in pattern emergence [40–42]. Experimentally, the analysis of aux1lax mutants confirmed the stabilizing role in shoot phyllotactic patterning [31]. Phenotypes were visible under short day conditions, suggesting that AUX1/LAX transporters may be particularly relevant under certain environmental conditions. Nevertheless, the functional relevance of AUX1/LAX proteins in the periodic vascular patterning of the shoot remains unknown.
Here we provide theoretical and experimental evidence for a yet uncharacterized role of auxin influx carriers controlling periodic vascular patterning and the differentiation of xylem cells in plants. The vascular tissues in the shoot of Arabidopsis plants are organized in vascular bundles (VB), disposed in periodic repetitions along a circular vascular ring. Each VB is composed of meristematic procambial cells and of differentiated vascular cells termed xylem and phloem (S1 Fig, shown as grey, blue and green cells, respectively), which arise each from centripetal and centrifugal divisions of the procambial cells [43,44]. In between the VBs, interfascicular fibers (IF) differentiate, supporting the inflorescence stem [43,45] (S1 Fig, light blue cells). The analysis of quadruple knockout mutants of AUX1/LAX transporters disclosed fewer and more spaced VBs in the shoot of Arabidopsis. This phenotype is in agreement with our mathematical and computational modeling predictions, which show that auxin influx carriers facilitate periodic patterning and increase its periodicity. Furthermore, a reduced differentiation of the vascular cells is observed in both shoot and root tissues. Our data support that influx carriers promote cytoplasmic auxin signaling, which has been previously shown to drive xylem differentiation [15,16,46,47]. In addition, our modeling analysis predicts a novel role for apoplastic auxin as inhibitor of xylem differentiation.
Results
Auxin influx carriers increase the number of auxin maxima and facilitate periodic auxin patterning
We have previously shown that periodic auxin distribution is relevant for VB pattern formation [7]. In order to investigate the role of influx carriers on periodic distributions of auxin, we first performed a theoretical and computational analysis (Materials and Methods). A minimal modeling approach was selected by assuming that auxin polar transport sets auxin maxima along a ring of provascular cells, which ultimately drive VB emergence [7]. Albeit this approach with its simplified geometry cannot drive quantitative predictions, it is expected to provide key features underlying the role of influx carriers for periodic auxin patterns (S2 Fig). A previous model on auxin polar transport known to drive periodic auxin maxima [3,41] was considered and further elaborated by including auxin apoplastic diffusion [7] (Materials and Methods, S2 Fig). In the model, auxin uptake into the cells occurs actively through influx carriers as well as passively, while auxin exits the cells through polarly localized efflux carriers as described in [3,41]. The model also takes into account that the synthesis of both types of carriers, as well as the polar localization of efflux carriers, depends on auxin concentration [3,14,35,41,48,49]. Parameter values for auxin polar transport were chosen according to the literature (S1 Table) [34,50–54]. Both theoretical and computational analyses were performed through linear stability analysis of the homogeneous state and numerical integration of the dynamics, respectively (Material and Methods and S1 Text). The model drives periodic maxima of auxin concentration as expected [3,4,55] with influx and efflux carriers being more abundant in those cells harboring auxin maxima (S3 Fig). This localization of carriers arises from the auxin-induced synthesis of influx and efflux carriers, which was set in the model to embrace experimental evidences on the auxin-induced expression of carriers [14,35,48,49]. Yet, according to the modeling of auxin dynamics, the auxin-induced synthesis of carriers is not essential for pattern formation [3,4,55] (S4 Fig). We defined I as intensity of the active influx transport (S1 Text and S1 Table), which is proportional to the maximal amount of influx carriers a cell in the ring array can have. For simplicity, hereafter we use the term 'amount of influx carriers' for I.
Our analysis predicts that the amount of influx carriers can control the periodicity of the pattern, driving changes in the number of auxin maxima (Fig 1A and S1 Video). When the amount of influx carriers is decreased, less auxin maxima arise in a ring with a fixed number of cells (Fig 1A right panel). Hence, influx carriers promote auxin maxima to be closer together in terms of number of cells, up to a limit (Fig 1B and 1C). While pattern periodicity modulation was previously associated only to efflux carriers [3,4], our modeling results unveil a novel role for influx carriers in this process. Auxin entrance into the cells is essential for periodic pattern formation, by enabling the polar transport of auxin to take place. We confirmed that passive entrance into the cells, independently from influx carriers, can be enough to sustain periodic patterning, as expected (Fig 1C). Yet, we found that influx carriers become essential for patterning in high apoplastic diffusion conditions, in which passive entrance of auxin into the cell is not enough to enable the periodic patterning (Fig 1C and S1 Text). Therefore, our results show that influx carriers promote pattern formation as well.
Fig. 1. Theoretical and simulation results predict that auxin influx carriers facilitate periodic patterning and promote auxin maxima. 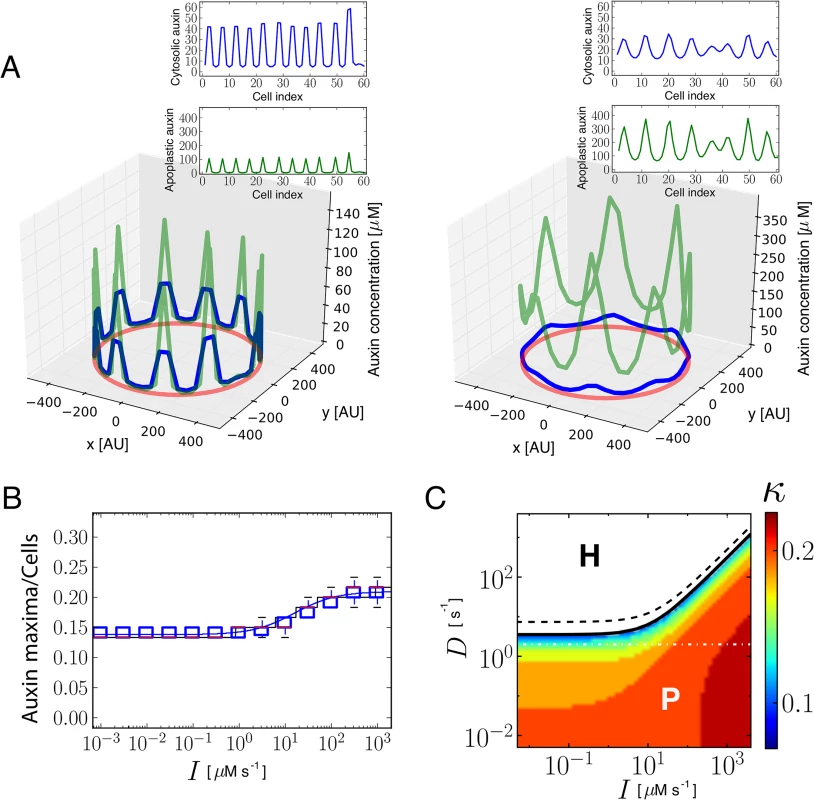
(A) Snapshots of simulation results showing periodic distribution of auxin inside and outside cells for higher (left, I = 100 μM s-1) and lower (right, I = 0.001 μM s-1) influx carriers levels along a ring of vascular tissue composed of 60 cells surrounded by the apoplast. Cytosolic (blue) and apoplastic (green) auxin concentrations at time t = 17.5 are shown. The red circular line represents the ring of cells in the tissue. Insets depict the same results projected into a 2D plane. Space is represented in arbitrary units [AU]. Influx and efflux carriers distributions are described in S1 Text. (B) Simulation (boxplot) and theoretical estimation (κ, depicted by solid lines; see S1 Text) results of the inverse value of the number of cells between cytosolic auxin maxima at different influx carriers levels (I). Each boxplot depicts the results for 30 simulations with different initial auxin distributions (Materials and Methods). Simulations were done for rings of 60 cells. The bottom and the top of the boxes represent the first and third quartile, enclosing the 25%-75% data range, the red line within the box stands for the median, and the low and high whiskers enclose the 1.5×(25%-75%) data range. The theoretical estimation is performed through linear stability analysis for a ring of 60 and 1200 cells (black and blue lines, respectively). Theory and simulations show that influx impairment enlarges the periodicity of the pattern, increasing the distance between consecutive auxin maxima. (C) Phase diagram obtained from theoretical linear stability analysis on a ring of 60 cells on the parameters space of amount of influx carriers (I) and apoplastic diffusion coefficient (D). The solid line divides the space in two regions (Methods): in the H region (white, above the solid line) the homogeneous state is linearly stable and no periodic pattern can be formed from small perturbations of it. In the P region (colored, below the solid line) the homogeneous state is linearly unstable and a periodic pattern can arise. The dashed black line corresponds to an analytical approximation to the solid black line (S1 Text, Eq S34). The color scale shows the theoretical estimation of the inverse value of the average number of cells between cytosolic auxin maxima (κ). The horizontal dashed-dotted white line depicts the line along which simulations are presented in panels A and B. For low apoplastic diffusion D, periodic patterning can still occur at low influx parameter values I, while high influx values are necessary for patterning at higher apoplastic diffusion coefficients. Main parameter values: E = 105 μM s-1, D = 2 s-1, Dca = 15 s-1 and auxin threshold for transporters activation θI = θP = 10 μM. Other parameter values can be found in S1 Text. Auxin influx carrier mutants have fewer vascular bundles in the shoot
To study the role of auxin influx carriers in the shoot vascular patterning, we first evaluated the expression pattern of the influx carriers’ proteins in the shoot. Radial sections at the basal region of the shoot inflorescence stem revealed that AUX1, LAX1, LAX2 and LAX3 fluorescent protein fusions show expression in the shoot vascular tissues (S5 Fig). This expression is localized at the VB (S5 Fig), where both auxin response and efflux carriers expression are known to occur [6,7,56] in agreement with the distribution predicted by modeling (S3 Fig).
We then analyzed radial sections at the basal region of the shoot inflorescence stem of mutants for auxin influx carriers [31] grown in short days. The depletion of all auxin influx carriers resulted in a significant reduction in the number of VBs as compared to the WT (Fig 2A–2D). Single aux1 mutant showed no VB number phenotype whereas aux1lax1lax2 triple mutant exhibited a similar phenotype than the quadruple (S6 and S7 Figs). These results are consistent with all AUX1/LAX family members being expressed in the shoot vasculature where they could play redundant functions. As previously reported in the context of phyllotaxis [31], the quadruple mutant exhibited higher phenotypic variability than the wild type (S8 Fig), supporting a stabilizing role for auxin influx carriers.
Fig. 2. Auxin influx carrier quadruple mutants show fewer vascular bundles in the inflorescence stem due to an increased spacing of vascular bundles and to a decreased number of cells in the provascular ring. 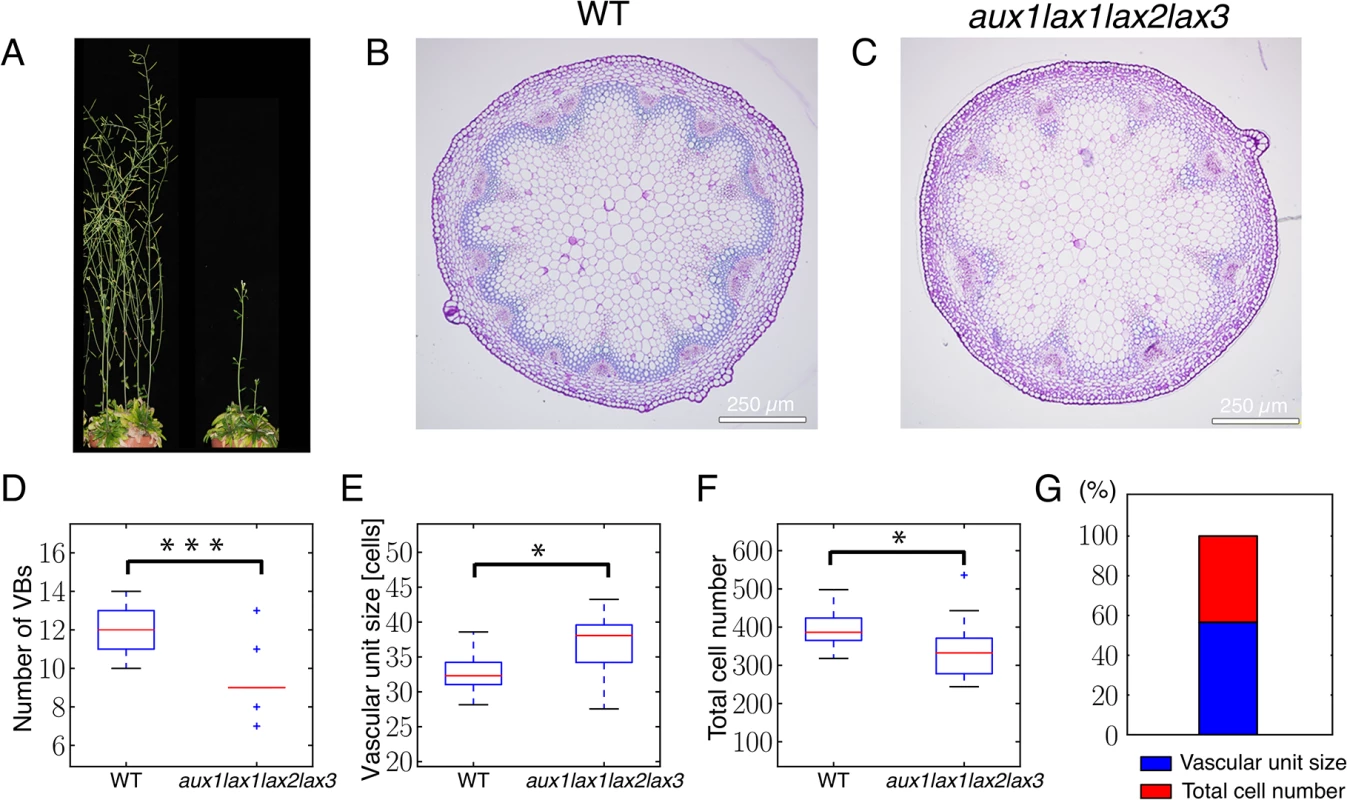
(A) WT 14-week-old plant (left) and aux1lax1lax2lax3 quadruple mutant 14-week-old plant. (B) Basal shoot cross section of WT shoot inflorescence stem. (C) Basal shoot cross section of aux1lax1lax2lax3 quadruple mutant shoot inflorescence stem. (D-F) Boxplots of VB number (D), vascular unit size (E), and total cell number across the provascular ring (F) for WT and aux1lax1lax2lax3 mutant vascular rings. For the total cell number quantification along the shoot stem section, the ring of cells formed by the interfascicular fiber cells and the procambial cells within the vascular bundle were taken into account. The vascular unit size measures the spacing of VBs position and was defined as the number of procambial cells along the ring within a VB plus the number of interfascicular fiber cells up to the next VB in this ring. Note that the vascular unit size is enlarged in influx mutant plants, being consistent with the theoretical predictions shown in Fig 1. (G) Percentage of expected contribution of VB spacing (red) and total cell number (blue) to the change in VB number in the aux1lax1lax2lax3 mutant, computed by using Eq 2. All plants were grown under short day conditions. In panels D-G, n = 24 for Col-0 and n = 18 for quadruple mutant plants. Scale bars: 250 μm. *: p-value≤ 0.01; ***: p-value≤ 0.0001. We next evaluated the number of cells involved in this pattern. To this end, we decomposed the vascular pattern of shoot cross-sections into vascular units (S1 Fig), each one of them constituted by the cells along a cell file in a VB and by the cells within the immediately adjacent interfascicular fibers [7]. The spacing of VBs was defined as the number of cells in the vascular unit, i.e. the vascular unit size. Our results show a significant decrease in the number of VBs and the total cell number accompanied by a larger spacing of VBs in terms of number of cells (Fig 2E and 2F). This enlargement of the spacing is in agreement with the role of influx carriers predicted by the mathematical model (Fig 1).
Since the reduction of VB number could be influenced by both the increase in VB spacing and the reduction of provascular cell number, we quantified the expected contribution of each of these two elements to this phenotype (Materials and Methods). Our results show that the increase in VB spacing in aux1lax1lax2lax3 mutants can account for 57% of the change in VB number, while the decrease in total cell number explains the remaining 43% of the change in VB number (Fig 2G). In the triple aux1lax1lax2 mutant, a similar trend is found (S7 Fig).
Auxin influx mutants show impaired xylem differentiation
Besides the phenotype in the periodic patterning, the vascular differentiation was also impaired in the influx mutants. Compared to the WT, aux1lax1lax2lax3 and aux1lax1lax2 mutants clearly show a reduced differentiation of both the interfascicular fiber cells and of the xylem cells within the shoot VB (Fig 3A–3F). This impairment was accompanied by a significant increase and a higher variability in the number of undifferentiated cell layers within the VB of aux1lax1lax2lax3 mutants, when compared to the WT (Fig 3A–3G). Together, these results uncover a role for auxin influx carriers in promoting xylem differentiation in the plant shoot.
Fig. 3. Influx carrier mutants show impaired xylem differentiation in Arabidopsis shoot stem. 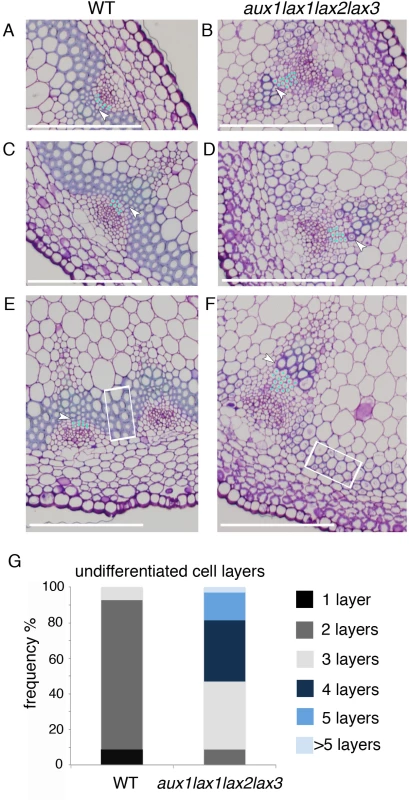
(A-B) VB magnification of a shoot basal cross section for WT (A, C and E) and aux1lax1lax2lax3 quadruple mutant (B, D and F) 14-weeks old plants grown in short day conditions. Light blue dots indicate undifferentiated cell layers in procambium tissue between phloem and xylem differentiated cells. First differentiated xylem cell is indicated by white arrow. The undifferentiated cell layers comprise both the procambial cells and the meristematic xylem cells (round cells with undifferentiated walls between the procambium and the xylem). Note that above the procambial cells appear some undifferentiated cells with different shape than the procambial cells. This round shape is more characteristic from xylem cells while the cell walls are not differentiated. Therefore, we quantify them as undifferentiated cells, which can comprise both, procambial and meristematic xylem cells. White squares highlight interfascicular fiber cells. Scale bar: 100 μm. (G) Frequency distribution of the number of undifferentiated cell layers, for WT and aux1lax1lax2lax3 mutants (n = 24 VB). To address whether the roles of influx carriers in periodic patterning and differentiation are independent, we investigated the vascular differentiation phenotype in tissues where vascular patterning is not periodic, such as the primary root. Histological analysis on the primary roots of aux1lax1lax2lax3 mutants showed impaired xylem vessel differentiation (S9 Fig), and AUX1/LAX-VENUS lines revealed root cambium/xylem-specific expression (see Materials and Methods and S10 Fig), confirming that AUX1/LAX promote xylem differentiation independently of modulating periodic patterning.
Next, the vascular phenotype in the shoot stem of influx mutants grown in long day was analyzed, since in these light conditions no apparent vascular bundle number phenotype is seen in aux1lax1lax2lax3 mutants (Figs 4A, 4B, 4D and 4E and S11), in agreement with the phyllotactic phenotypes described previously for these mutants in these conditions [31]. We found that the vascular differentiation was impaired in the quadruple mutants, albeit the phenotype was milder than in short day conditions (Figs 4G, 4H, 4J and 4K and S11). These results support that the role of AUX1/LAX in vascular differentiation is more prevalent than their role on modulating the vascular bundle number.
Fig. 4. Vascular pattern and xylem differentiation phenotypes in long day and short day conditions for influx aux1lax1lax2lax3 and efflux pin1pin2 mutants. 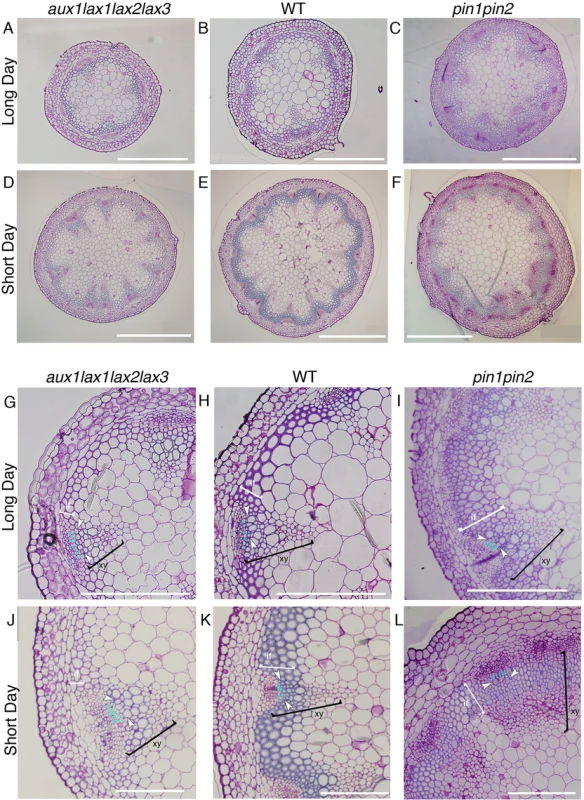
Basal shoot cross section of aux1lax1lax2lax3 (A, D), WT (B, E) and pin1pin2 (C, F) plants grown in long day conditions (A-C) and in short day conditions (D-E). WT and the aux1lax1lax2lax3 plants grown in long day conditions showed no statistical significant differences in number of VBs, total cell number, nor average vascular unit size (S11 Fig, n = 18 for WT, n = 15 for aux1lax1lax2lax3). Scale bars: 500μm. VB detail of aux1lax1lax2lax3 (G, J), WT (H, K) and pin1pin2 (I, L) shoot inflorescence stem grown in long day conditions (G-I) and in short day conditions (J-L). Light blue dots indicate undifferentiated cell layers in procambium tissue between phloem and xylem differentiated cells. First differentiated xylem cell is indicated by white arrow. White brackets highlight the interfascicular fiber cells (if). Black brackets highlight the xylem cells (xy). Scale bars: 200 μm. Frequency distribution of the number of undifferentiated cell layers in long day conditions for the three genotypes is shown in S11 Fig. The role uncovered herein for the auxin influx carriers on xylem differentiation is opposed to that already described for efflux carriers [6] (Fig 4G–4L). In agreement, the efflux carrier mutant pin1pin2 grown in short days shows increased xylem and interfascicular fiber differentiation similarly to what was shown in long days [7] (Fig 4C, 4F, 4I and 4L). Therefore, our results disclose that influx and efflux carriers have opposite roles in xylem cell differentiation.
The effect of auxin influx carriers on extracellular auxin accumulation emulates the xylem differentiation phenotype
Since auxin signaling mediates vascular differentiation processes [16,46,57–59], we reasoned that alterations of auxin concentration mediated by influx carriers could result into impairment of auxin signaling, which in turn would drive defects in xylem differentiation and vascular patterning. To evaluate whether auxin signaling is impaired in influx mutants we analyzed the expression of the auxin response reporter DR5:GFP [60] in the aux1lax1lax2 mutant backgrounds. The triple influx carrier mutant DR5:GFP aux1lax1lax2 exhibited diminished auxin response, specifically at the VB, when compared to the DR5:GFP WT (S7 Fig). Since DR5:GFP reports the level of TIR1/AFB-mediated auxin signaling [61], these results point at a reduction of cytoplasmic auxin in the absence of influx carriers. We then turned into our modeling approach to unveil which of the multiple effects of influx carriers on auxin transport and distribution could underlie the control of xylem differentiation. To this end, we searched for those effects of influx carriers on auxin distribution that satisfy the restrictions we find in the xylem differentiation phenotype: the effect of influx carriers on xylem differentiation is (i) independent of the role on modulating the periodic pattern, (ii) is more pervasive than the modulation of the periodic pattern and (iii) is opposed to that of efflux carriers. Note that our mathematical analysis revealed that influx and efflux carriers do not necessarily drive antagonistic effects. For instance, both influx and efflux carriers promote periodic pattern formation (Figs 1 and S12) [2,3], faster patterning processes (S1 Video) [7], and stabilization of the pattern [40–42]. Therefore, the third condition (iii) also imposes a restriction on which effect of influx carriers controls xylem differentiation.
The analysis showed that influx carriers increase cytosolic auxin in those cells harboring the auxin maxima (Figs 1A and S13), in agreement with the reduced response exhibited by DR5:GFP in the auxin maxima of the triple aux1lax1lax2 mutant background (S7 Fig). In addition, influx carriers tend to reduce spatial differences in the concentration of apoplastic auxin (what we call pattern amplitude of the apoplastic auxin) as well as to diminish, as expected [51], the apoplastic auxin concentration (S13 Fig). Importantly, this role influx carriers have on apoplastic auxin concentration satisfies the three conditions described above (i-iii). This role is more pervasive than that on modulating the pattern periodicity (Figs 5 and S14). Moreover, it is independent of the modulation of the periodicity, since changes of the periodicity of the pattern do not require changes in the concentration of extracellular auxin (S15 Fig). In addition, it is opposed to the effect driven by efflux carriers, which tend to increase the differences of apoplastic auxin across the ring of cells and to increase the apoplastic auxin concentration (Figs 5 and S13 and S14, Materials and Methods). While extracellular auxin is dependent on influx carriers such that the three conditions (i-iii) above hold, cytosolic auxin is not. Cytosolic auxin is promoted both by influx and efflux carriers at the cells harboring auxin maxima (Figs 5 and S13 and S14). Taken together, our computational analysis supports that influx carriers promote cytoplasmic auxin signaling and thereby xylem differentiation and proposes that auxin signaling may respond as well to changes in extracellular apoplastic auxin concentration driven by influx carriers to control vascular differentiation (Fig 6).
Fig. 5. Modeling shows that influx carriers diminish differences in the concentration of auxin in the apoplast. 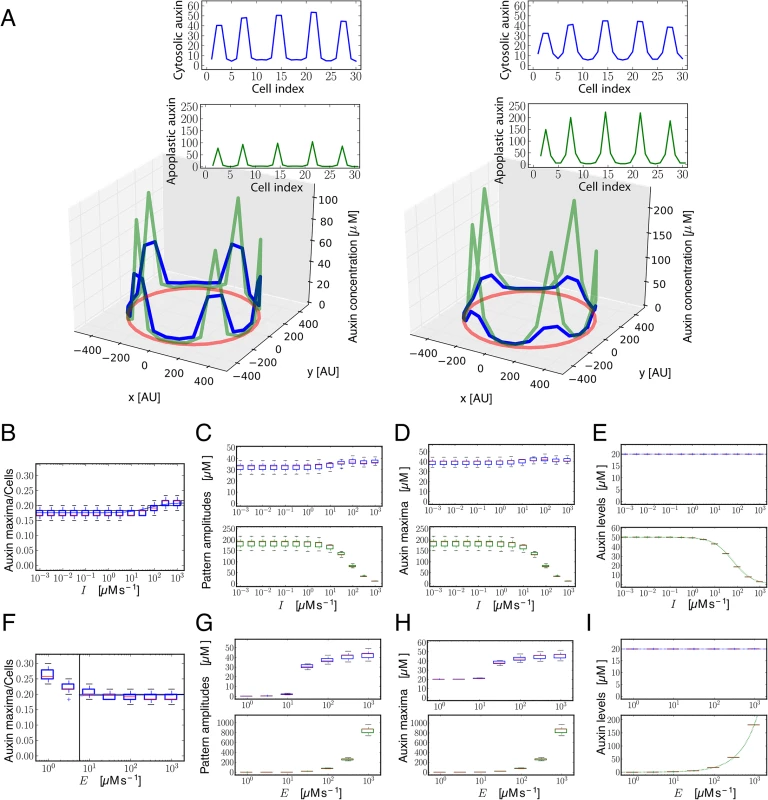
(A) Snapshots of simulation results showing periodic distribution of auxin inside and outside cells for higher (left, I = 100 μM s-1) and lower (right, I = 0.001 μM s-1) influx carriers levels along a ring of vascular tissue composed of 30 cells surrounded by the apoplast. Cytosolic (blue) and apoplastic (green) auxin concentrations at time t = 17.5 are shown. The red circular line represents the ring of cells in the tissue. Insets depict the same results projected into a 2D plane. Space is represented in arbitrary units [AU]. The number of auxin maxima is the same in both cases. (B-E, F-I) Simulation results showing the number of cytosolic auxin maxima over the total number of cells (B,F), the amplitude of the pattern of auxin (C,G), the averaged auxin maxima levels (D,H) and the averaged auxin values along the vascular ring (E,I) in the cytosol (top panels, blue boxplots) and in the apoplast (bottom panels, green boxplots) as a function of the amount of influx carriers I (B-E) and of efflux carriers E (F-I). Each boxplot depicts the results for 30 simulations with different initial auxin distributions (Methods). Simulations in B-I were done for rings of 60 cells until time t = 17.5. Depicted boxplot components are the same as in Fig 1B. Other details of panels (B, F) are the same as in Fig 1B. Vertical line in (F) indicates the critical parameter value (in this case, the efflux carriers levels E) above which the pattern can not emerge, derived from linear stability analysis. Dotted lines in (E,I) panels correspond to the theoretical auxin homogeneous steady states given by Eqs S9 and S35. See Materials and Methods and S13 Fig for more details on the computation of the amplitude and average levels. All parameter values as in Fig 1 except for passive influx Dca = 50 s-1. E = 105 μM s-1 for A-E panels, while I = 100 μM s-1 for F-I panels. Fig. 6. A model of apoplastic and cytoplasmic auxin control of xylem differentiation. 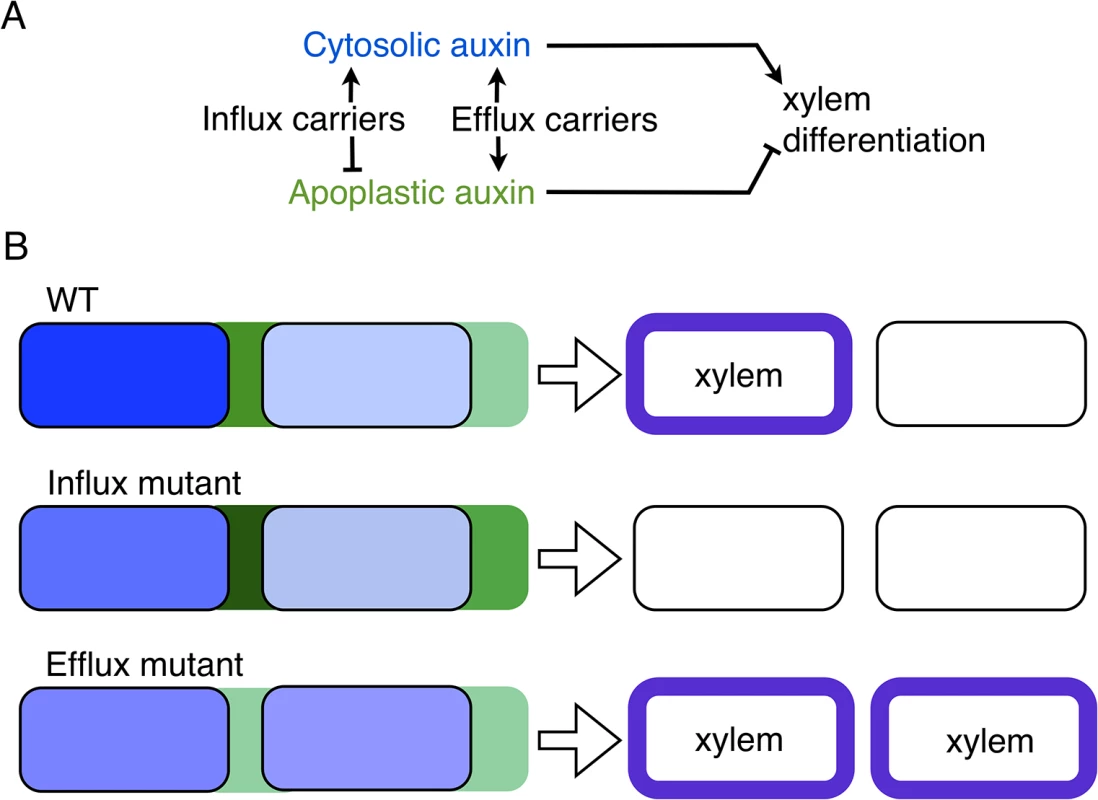
(A) Auxin cytoplasmic signaling is required for xylem differentiation [16,46,57–59]. Our computational analysis predicts apoplastic (extracellular) auxin as an inhibitor of this differentiation. Both efflux and influx carriers increase cytoplasmic auxin concentration at auxin maxima, while they antagonistically regulate apoplastic auxin concentration. Arrows stand for activation, while blunt arrows for inhibition. (B) Cytoplasmic (blue) and apoplastic (green) auxin concentrations in two cells (rectangles with rounded corners). Lighter blue and green account for decreased cytoplasmic and apoplastic concentrations respectively. The predicted differentiation phenotypes of WT, influx and efflux mutants are depicted on the right. Xylem differentiated cells are depicted with blue borders. Discussion
Mathematical modeling has recently emerged as an effective discipline to characterize auxin patterns and the processes driven by them, like vascular patterning in the root [1–4,9,10,40,42,55,62]. From these studies it is known that auxin-dependent polarization of efflux carriers can drive periodic patterning [3,4,55]. Our mathematical model predicts that auxin influx carriers, despite not being polarized in our model, can modulate the periodicity of the auxin pattern. The observed changes in the vascular pattern periodicity in the shoots of influx mutants are in agreement with the model prediction. Moreover, our model shows that influx carriers can facilitate periodic patterning. Intuitively, the roles of auxin influx carriers in patterning disclosed in this study can be understood from the competition between polar transport and apoplastic diffusion. Polar transport is at its most effective mode when the auxin expelled from the cytosol is able to reach only the adjacent cells and not cells located further away [51]. Efficient uptake of auxin by influx carriers facilitates that this happens.
Early mathematical models proposed that influx carriers stabilize phyllotactic patterning [40–42]. Recently, it has been shown that influx carriers can have additional roles in patterning, such as setting which root cells have high levels of auxin [63]. The role of influx carriers on promoting auxin maxima and facilitating periodic patterning may have previously remained unnoticed in theoretical analyses because either apoplastic diffusion and/or auxin-induced synthesis of carriers were not taken into account. These novel roles are uncovered through modeling only when either one or both elements are included (S1 Text). The inclusion of apoplastic diffusion in the model revealed another interesting aspect of auxin periodic patterning: efflux carriers do not modulate the periodicity of patterns as influx carriers do (S12 Fig). Therefore, this suggests that the distorted vascular bundle phenotypes in efflux mutants are not because of modulation of the periodicity, and may arise from the strong slowing down of the auxin transport dynamics as previously proposed [7]. In addition, future 3D modeling approaches that take into account the connectivity of the vascular strands with the phyllotactic pattern [64] will help in a better understanding of the phyllotactic and vascular pattern formation.
Based on our modeling results, it is tempting to speculate what in auxin transport is distinct between short day and long day conditions that can explain the differences we found in phenotypes (Fig 4), namely, that quadruple influx carrier mutants in long day only show reduced xylem differentiation and not a vascular bundle phenotype. Assuming the xylem differentiation scheme of Fig 6, the model indicates that long day conditions could be mimicked by (1) the absence of auxin-induced synthesis of carriers together with lower auxin apoplastic diffusion coefficients than in short day (S14 Fig), or by (2) an increase of the ratio of passive influx transport across the cell membrane over the active one when compared to short day conditions (Fig 5). We found that carriers are localized at auxin maxima in long day conditions (S16 Fig), supporting auxin-induced synthesis for this photoperiodic condition and discarding the first scenario. Regarding the second scenario, the model shows that the concentration of apoplastic auxin is lower when the passive influx transport across cell membranes increases (Figs 5 and S13 and S1 Text). This predicts that the influx carriers mutant plants should display a milder differentiation phenotype in long days than in short days. This prediction is in agreement with the phenotypes exhibited by aux1lax1lax2lax3 mutant shoots inflorescence stems (Figs 3 and 4 and S11). It is worth stressing that predicting these differences between the differentiation phenotypes in long day and short day conditions of influx carriers mutants is restrictive. For instance, no difference of differentiation phenotype is expected if long day conditions corresponded just to lower active influx transport than in short days, while the opposite difference is predicted by a photoperiod-dependent change of apoplastic auxin diffusion (S14 Fig). Based on this analysis, we may hypothesize that the photoperiod could change the balance between passive and active influx transport across the cell membrane, being passive influx more relevant at long day conditions than at short days. Yet, in both conditions, active influx transport is expected to be more important than passive entrance into the cells. Passive auxin entrance into the cells could increase in long days by increasing the cell membrane permeability, for instance. In addition, the amount of active influx carriers may decrease in long days as well. Potentially, active influx transport could be modified by the photoperiod through light-modulation of intracellular trafficking [65,66].
In summary, by combining experimental and theoretical approaches, we propose novel roles for auxin influx carriers in vascular patterning and differentiation during plant development. By assuming that auxin maxima position VBs [7], we evaluated the role of auxin influx carriers in the periodic patterning of auxin maxima in the Arabidopsis shoot inflorescence stem. auxlax1lax2lax3 mutants showed a reduction in VB number in the shoot stem involving both an increase in the spacing of the pattern and a reduction in the total number of cells along the provascular ring. This increase in the spacing can be explained by the role of influx carriers predicted by our modeling approach. Moreover, the quadruple auxlax1lax2lax3 mutants also depicted decreased xylem differentiation. Analysis of shoot and root phenotypes indicates a pervasive role of auxin influx carriers in promoting differentiation of xylem cells that is independent on their role on periodic vascular patterning. Our data support the established idea that TIR1/AFB-mediated auxin signaling, operating in cytoplasm and nucleus, is required for xylem differentiation [15,16,46,47]. In addition, our computational analysis predicts that extracellular auxin can be sensed from the apoplastic space and inhibit xylem cell differentiation (Fig 6). While no direct empirical evidence for apoplastic auxin signalling controlling xylem differentiation is described, it has been reported that apoplastic auxin can be sensed by the cell surface receptor ABP1-TMK and drive the downstream auxin signaling [67]. Furthermore, the potential connection between apoplastic and cytosolic auxin signaling pathways has been also shown [67]. However, very recent evidence has challenged the role of ABP1 as auxin signalling component [68]. Further work will contribute to unravel the molecular mechanism that drives these phenotypes as well as to understand the impact of environmental conditions on auxin-driven patterning.
Materials and Methods
Mathematical modeling
We used the mathematical model of polar auxin transport by [3] in the form it is presented in [41] with the inclusion of apoplastic auxin transport as in [7] (S2 Fig). Given that auxin diffuses fast in the cytosol [69], we considered for simplification auxin concentration homogeneously distributed inside the cell, what would be consistent with instantaneous diffusion of auxin across the cytosol. In the model, auxin is pumped into the cell through the influx carriers, which are homogeneously distributed in the cell membrane. Moreover, auxin is pumped out of the cell to the apoplast through the efflux carriers, which can be polarly distributed in the cells. We considered efflux carriers’ localization to depend on the concentration of auxin in neighboring cells and, for simplicity, to be at equilibrium, as done in [3,40,41]. The model takes into account that a constant fraction of the auxin (set by the pH condition) is protonated and is passively transported into the cells as in [3]. Auxin production and degradation is set to occur inside cells. In the model, we refer to cytosolic and apoplastic auxin as the auxin inside and outside the cell, respectively.
The dimensional model equations for cytosolic auxin concentration in cell i and apoplastic auxin concentration in the apoplastic compartment i (see scheme in S2 Fig) read
with τ being time, Dw the apoplastic diffusion coefficient, σc and vc the auxin production and degradation rates, Vcell the cell volume, Vap the apoplast volume, Wij the ratio between the contact area of cell i with apoplast j and the cell volume. ▽i2 is the discrete Laplacian. Jij stands for the auxin flux from the cell i to the apoplast j and contains the protonated passive auxin transport, and both active transports due to the influx and efflux carriers as described in [41] (see S1 Text for details). Active influx transport was simplified to drive only entrance of anionic auxin into the cell. Analogously, active efflux transport was set to mediate only the exit of anionic auxin from the cell. For simplicity and with the aim of focusing in the linear regime of the dynamics, we considered linear auxin fluxes for both passive and active transports (S1 Text).For convenience, we analyzed the model in nondimensionalized time units (S1 Text). The resulting model parameters can be related to physico-chemical magnitudes that have been measured or can be estimated, even though we expect having robust behaviors that are not very dependent on parameter values. In our study, we chose to mainly vary the following effective dimensional parameters: the influx parameter I, the apoplastic diffusion coefficient D and the efflux parameter E. Our simplified geometry corresponds to a line of cells with periodic boundary conditions and no cell division was included.
Simulation details
We integrated the dynamical model for auxin transport (Eqs S7-S8) through a Runge-Kutta method of 4th order [70] with time step dt = 0.0001 being t the non-dimensional time. Most of the parameter values were set according to already published work, some of it based on experimental data (S1 Table). Unless otherwise stated, we set as initial conditions the homogeneous solution with small variability. We integrated the dynamics until a fixed time point (t = 17.5). This time point was chosen such that a periodic pattern of auxin maxima was established for the parameter values of the normal conditions (Fig 1A) but was still incipient at efflux mutant conditions, yielding distorted patterns for this mutant (S12 Fig and S1 Text).
For each pattern at time t = 17.5, we quantified the ratio of the number of cytosolic auxin maxima over the number N of cells in the simulated provascular ring. For a periodic pattern, this provides a measurement of the characteristic wavenumber. Note that the inverse of this quantity is the average number of cells between two auxin maxima (i.e. the characteristic wavelength or spacing). In the simulations, we observed very incipient maxima much smaller than the rest. We omitted such incipient maxima for the quantification of maxima spacing, the cytosolic and apoplastic auxin average maxima and pattern amplitudes. We determined that a cytosolic (or apoplastic) auxin maxima was incipient at the end of any given simulation when the difference of its cytosolic (or apoplastic) auxin value with the average cytosolic (or apoplastic) minima in such single simulation was less than 0.15 times the cytosolic (or apoplastic) auxin pattern amplitude in such simulation. By omitting these maxima, which arise especially at high influx levels (see incipient maxima near cell 60 in left panel of Fig 1A), we have seen that the numerical simulations are in better agreement with the theoretical prediction for the periodicity of the pattern, especially at higher influx levels.
Simulations were performed with custom-made programs written in Fortran77 and in C++. We provide a code written in Mathematica [version 9.0, Wolfram Research; code provided in pdf and Mathematical notebook format (.nb)] that can be used by the reader to test how influx carriers and other model parameters affect to the pattern formation process (see S1 Code).
Theoretical prediction of the emerging pattern
In pattern formation studies, linear stability analysis enables the prediction of the characteristic wavelength of the emerging patterns [71], and it has already been used for mathematical auxin transport models (see for instance [3,40,41]). We performed linear stability analysis over the stationary homogeneous state [71] (details are found in S1 Text). This analysis provides theoretical predictions on which parameter values can drive periodic pattern formation and on how the periodic pattern that starts to be formed depends on the parameter values. Accordingly, through this analysis we extracted predictions on how the characteristic wavenumber (κ) (i.e. the number of periodic maxima over the total number of cells) depends on the model parameters (see S1 Text for details).
Plant material and growth conditions
All the mutant plants analyzed here were in Arabidopsis thaliana Columbia-0 (Col-0) ecotype background. Seeds for DR5:GFP in aux1lax1lax2 and Col-0 WT backgrounds and auxlax1lax2lax3 were described elsewhere [31]. Seeds were surface-sterilized in 35% sodium hypochlorite, vernalized at 4°C for 48h, and germinated on plates containing 1x Murashige and Skoog (MS) medium. Seedlings were grown for 10 days on plates under short day photoperiod (8h light / 16h dark; 8470 lux; 20–23°C) and then transplanted to soil and grown under the same conditions for 14 weeks. The main shoot inflorescence stem was cut at approximately 1 cm above the rosette for sectioning and further histological analysis. Short day conditions can occasionally drive the emergence of aerial rosettes in both WT and aux1lax1lax2lax3 adult plants. Plants that showed these aerial rosettes development were not considered for our analysis.
The VENUS fluorescent protein [72] fusions of AUX1/LAX proteins were generated by a recombineering approach [73] and have been described before for AUX1 [63]; LAX1 and LAX2 [25]. In brief, VENUS was fused in frame after the codon 116 for AUX1; 122 for LAX1; 110 for LAX2 and 114 for LAX3 to create respective AUX1/LAX fluorescent protein constructs (ProAUX1:AUX1-VENUS; ProLAX1:LAX1-VENUS; ProLAX2:LAX2-VENUS and ProLAX3:LAX3-VENUS). Transformation of Agrobacterium (C58) and Arabidopsis was done as described before [74]. Transgene-specific cDNA sequences of these lines were PCR-amplified and sequenced to ensure against rearrangements of the transgenes.
Histology and microscopy
Inflorescence stem sections from both WT and mutant Arabidopsis plants were fixed at 4°C overnight in 1.25% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Samples were dehydrated through a graded series of ethanol (30%, 50%, 70%, 90% and 100%; 45 min each one) and then infiltrated in 1 : 1 Historesin-I (Technkovit):ethanol for 30 min at room temperature, followed by 100% Historesin-I 100% at 4°C overnight. Blocks were prepared by placing samples into plastic molds, which were filled with 100% Historesin-II (Technkovit). Each mold was covered with parafilm and kept overnight at 4°C to accelerate their solidification. Historesin-I and II were prepared following the manufacturer’s instructions. Transverse stem sections (3 μm) were obtained with a Leica Microtome (Microtome RM2265, Leica). Sections were stained with 0.1% Toluidine blue in 0.1M NaPO4 pH7.0, rinsed and mounted in water for microscopical visualization in an Axiophot Microscope (Zeiss). GFP-fluorescence was observed in hand-made sections from the same part of the stem in a stereomicroscope (SZX16, Olympus). VENUS fluorescence lines (same part of the stem as described above) grown in short day conditions were incubated for 1–2 hours in 4% para-formaldehyde in PBS under vacuum conditions. After three washes with PBS, the samples were mounted in a hand-made block of 4% agarose and 0.01% Triton X-100 in PBS (pH 7.2–7.4). 150 μm sections were cut in a Micron HM650V vibratome and analyzed in a Leica TCS SP5II HCS A confocal microscope (Leica). Kr/Ar 488 laser was used with an excitation wavelength of 514 nm and detected an emission window of 525–569 nm for the VENUS/YFP. An excitation wavelength of 405 nm and an emission window of 434–483 nm were used for the blue autofluorescence of the xylem.
For the root histological studies, seedlings were grown on soil for 5 weeks on long day photoperiods (16h light/ 8h dark; 23°C). The root samples were embedded in Historesin (Leica) as described in [75], and 5–10 μm sections were cut approximately 5 mm below the hypocotyl. The sections were stained with toluidine blue and only the vessel elements in the primary phase of secondary xylem development were quantified with ImageJ. Vessels formed during the secondary phase were not quantified, since fibers with thick cell walls are formed then [76], thus making it difficult to distinguish the vessel elements from fibers.
Quantitative vascular analysis
Quantification of all the vascular parameters (stem diameters, number of cells, interfascicular fiber length and number of undifferentiated cell layers) was performed manually or using ImageJ software (http://rsb.info.nih.gov/ij/). Vascular bundles and cells were manually counted from microscope images. To determine whether the WT samples were statistically significant with respect to mutant samples, we performed the Wilcoxon rank sum test with Matlab. When we performed the test on the vascular unit sizes of WT samples against those of mutant samples, we chose the average vascular unit size per plant as the tested variable, but we have confirmed that the test results were very similar if we took the median of the vascular unit size per plants. Plots were performed with Python 2.7 by means of the Matplotlib package and with Excel. Quantification of the contribution of VB spacing (characteristic wavelength, λ) and total cell number (N) to the change in VB number (V) was computed through
where ΔX = Xmutan t−XWT with X being the median value found for each variable. This relation stems from V = N / λ. The percentage of contribution of VB spacing is then 100ΔλVWT / (ΔV λWT).Supporting Information
Zdroje
1. Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes & development 20 : 1015–1027.
2. Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, et al. (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426 : 255–260. 14628043
3. Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E (2006) An auxin-driven polarized transport model for phyllotaxis. Proceedings of the National Academy of Sciences of the United States of America 103 : 1633–1638. 16415160
4. Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, et al. (2006) A plausible model of phyllotaxis. Proceedings of the National Academy of Sciences of the United States of America 103 : 1301–1306. 16432192
5. Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, et al. (2009) Integration of transport-based models for phyllotaxis and midvein formation. Genes & development 23 : 373–384.
6. Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, et al. (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 : 2226–2230. 9856939
7. Ibañes M, Fàbregas N, Chory J, Caño-Delgado AI (2009) Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proceedings of the National Academy of Sciences of the United States of America 106 : 13630–13635. doi: 10.1073/pnas.0906416106 19666540
8. Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, et al. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 : 591–602. 14651850
9. Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, et al. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433 : 39–44. 15635403
10. Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449 : 1008–1013. 17960234
11. Muller A, Guan C, Galweiler L, Tanzler P, Huijser P, et al. (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. The EMBO journal 17 : 6903–6911. 9843496
12. Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415 : 806–809. 11845211
13. Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, et al. (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nature cell biology 8 : 249–256. 16489343
14. Peret B, Middleton AM, French AP, Larrieu A, Bishopp A, et al. (2013) Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Molecular systems biology 9 : 699. doi: 10.1038/msb.2013.43 24150423
15. Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, et al. (2011) A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Current biology: CB 21 : 917–926. doi: 10.1016/j.cub.2011.04.017 21620702
16. Fukuda H, Komamine A (1980) Establishment of an Experimental System for the Study of Tracheary Element Differentiation from Single Cells Isolated from the Mesophyll of Zinnia elegans. Plant physiology 65 : 57–60. 16661142
17. Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant journal: for cell and molecular biology 28 : 465–474. 11737783
18. Rubery PH, Sheldrake AR (1974) Carrier-mediated auxin transport. Planta 118 : 101–121. doi: 10.1007/BF00388387 24442257
19. Raven JA (1975) Transport of indoleacetic acid in plant cells in relation to ph and electrical potential gradients, and its significance for polar iaa transport. New Phytologist 74 : 163–172.
20. Geisler M, Murphy AS (2006) The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS letters 580 : 1094–1102. 16359667
21. Bandyopadhyay A, Blakeslee JJ, Lee OR, Mravec J, Sauer M, et al. (2007) Interactions of PIN and PGP auxin transport mechanisms. Biochemical Society transactions 35 : 137–141. 17233620
22. Mravec J, Kubes M, Bielach A, Gaykova V, Petrasek J, et al. (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135 : 3345–3354. doi: 10.1242/dev.021071 18787070
23. Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, et al. (2009) ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. The Plant journal: for cell and molecular biology 57 : 27–44. doi: 10.1111/j.1365-313X.2008.03668.x 18774968
24. Bennett MJ, Marchant A, Green HG, May ST, Ward SP, et al. (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273 : 948–950. 8688077
25. Peret B, Swarup K, Ferguson A, Seth M, Yang Y, et al. (2012) AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. The Plant cell 24 : 2874–2885. doi: 10.1105/tpc.112.097766 22773749
26. Swarup R, Peret B (2012) AUX/LAX family of auxin influx carriers-an overview. Frontiers in plant science 3 : 225. doi: 10.3389/fpls.2012.00225 23087694
27. Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J (2006) Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. The Plant cell 18 : 3171–3181. 17114355
28. Smith RS, Bayer EM (2009) Auxin transport-feedback models of patterning in plants. Plant, cell & environment 32 : 1258–1271.
29. Novoselova ES, Mironova VV, Omelyanchuk NA, Kazantsev FV, Likhoshvai VA (2013) Mathematical modeling of auxin transport in protoxylem and protophloem of Arabidopsis thaliana root tips. Journal of bioinformatics and computational biology 11 : 1340010. doi: 10.1142/S0219720013400106 23427992
30. Stieger PA, Reinhardt D, Kuhlemeier C (2002) The auxin influx carrier is essential for correct leaf positioning. The Plant journal: for cell and molecular biology 32 : 509–517. 12445122
31. Bainbridge K, Guyomarc'h S, Bayer E, Swarup R, Bennett M, et al. (2008) Auxin influx carriers stabilize phyllotactic patterning. Genes & development 22 : 810–823.
32. Ugartechea-Chirino Y, Swarup R, Swarup K, Peret B, Whitworth M, et al. (2010) The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Annals of botany 105 : 277–289. doi: 10.1093/aob/mcp287 19952011
33. Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, et al. (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes & development 15 : 2648–2653.
34. Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, et al. (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nature cell biology 7 : 1057–1065. 16244669
35. Swarup K, Benkova E, Swarup R, Casimiro I, Peret B, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nature cell biology 10 : 946–954. doi: 10.1038/ncb1754 18622388
36. Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, et al. (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant cell 14 : 589–597. 11910006
37. Marchant A, Kargul J, May ST, Muller P, Delbarre A, et al. (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. The EMBO journal 18 : 2066–2073. 10205161
38. Lampugnani ER, Kilinc A, Smyth DR (2013) Auxin controls petal initiation in Arabidopsis. Development 140 : 185–194. doi: 10.1242/dev.084582 23175631
39. Vandenbussche F, Petrasek J, Zadnikova P, Hoyerova K, Pesek B, et al. (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137 : 597–606. doi: 10.1242/dev.040790 20110325
40. Heisler MG, Jönsson H (2006) Modeling Auxin Transport and Plant Development. J Plant Growth Regul 25 : 302–312.
41. Sahlin P, Soderberg B, Jönsson H (2009) Regulated transport as a mechanism for pattern generation: capabilities for phyllotaxis and beyond. Journal of theoretical biology 258 : 60–70. doi: 10.1016/j.jtbi.2009.01.019 19490869
42. Wabnik K, Kleine-Vehn J, Balla J, Sauer M, Naramoto S, et al. (2010) Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Molecular systems biology 6 : 447. doi: 10.1038/msb.2010.103 21179019
43. Essau K (1977) Anatomy of Seed Plants. Wiley, New York 2nd Edition.
44. Jurgens G (2001) Apical-basal pattern formation in Arabidopsis embryogenesis. The EMBO journal 20 : 3609–3616. 11447101
45. Essau K (1965) Plant Anatomy. Wiley, New York.
46. Fukuda H (2004) Signals that control plant vascular cell differentiation. Nature reviews Molecular cell biology 5 : 379–391. 15122351
47. Mahonen AP, ten Tusscher K, Siligato R, Smetana O, Diaz-Trivino S, et al. (2014) PLETHORA gradient formation mechanism separates auxin responses. Nature 515 : 125–129. doi: 10.1038/nature13663 25156253
48. Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, et al. (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current biology: CB 15 : 1899–1911. 16271866
49. Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, et al. (2010) ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143 : 111–121. doi: 10.1016/j.cell.2010.09.027 20887896
50. Goldsmith MH, Goldsmith TH, Martin MH (1981) Mathematical analysis of the chemosmotic polar diffusion of auxin through plant tissues. Proceedings of the National Academy of Sciences of the United States of America 78 : 976–980. 16592983
51. Kramer EM (2004) PIN and AUX/LAX proteins: their role in auxin accumulation. Trends in plant science 9 : 578–582. 15564124
52. Kramer EM, Frazer NL, Baskin TI (2007) Measurement of diffusion within the cell wall in living roots of Arabidopsis thaliana. Journal of experimental botany 58 : 3005–3015. 17728296
53. Kramer EM (2008) Computer models of auxin transport: a review and commentary. Journal of experimental botany 59 : 45–53. 17431022
54. Alim K, Frey E (2010) Quantitative predictions on auxin-induced polar distribution of PIN proteins during vein formation in leaves. The European physical journal E, Soft matter 33 : 165–173. doi: 10.1140/epje/i2010-10604-5 20571847
55. van Berkel K, de Boer RJ, Scheres B, ten Tusscher K (2013) Polar auxin transport: models and mechanisms. Development 140 : 2253–2268. doi: 10.1242/dev.079111 23674599
56. Zourelidou M, Absmanner B, Weller B, Barbosa IC, Willige BC, et al. (2014) Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. eLife 3.
57. Fosket DE, Torrey JG (1969) Hormonal control of cell proliferation and xylem differentiation in cultured tissues of Glycine max var. Biloxi. Plant physiology 44 : 871–880. 5816361
58. Sachs T (1981) The Control of the Patterned Differentiation of Vascular Tissues: Academic Press.
59. Perrot-Rechenmann C (2010) Cellular responses to auxin: division versus expansion. Cold Spring Harbor perspectives in biology 2: a001446. doi: 10.1101/cshperspect.a001446 20452959
60. Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant cell 9 : 1963–1971. 9401121
61. Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, et al. (2005) Plant Development Is Regulated by a Family of Auxin Receptor F Box Proteins. Developmental Cell 9 : 109–119. 15992545
62. Muraro D, Mellor N, Pound MP, Help H, Lucas M, et al. (2014) Integration of hormonal signaling networks and mobile microRNAs is required for vascular patterning in Arabidopsis roots. Proceedings of the National Academy of Sciences of the United States of America 111 : 857–862. doi: 10.1073/pnas.1221766111 24381155
63. Band LR, Wells DM, Fozard JA, Ghetiu T, French AP, et al. (2014) Systems analysis of auxin transport in the Arabidopsis root apex. The Plant cell 26 : 862–875. doi: 10.1105/tpc.113.119495 24632533
64. Kang J, Tang J, Donnelly P, Dengler N (2003) Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytologist 158 : 443–454.
65. Laxmi A, Pan J, Morsy M, Chen R (2008) Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PloS one 3: e1510. doi: 10.1371/journal.pone.0001510 18231596
66. Ding Z, Galvan-Ampudia CS, Demarsy E, Langowski L, Kleine-Vehn J, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nature cell biology 13 : 447–452. doi: 10.1038/ncb2208 21394084
67. Xu T, Dai N, Chen J, Nagawa S, Cao M, et al. (2014) Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343 : 1025–1028. doi: 10.1126/science.1245125 24578577
68. Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, et al. (2015) Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proceedings of the National Academy of Sciences of the United States of America 112 : 2275–2280. doi: 10.1073/pnas.1500365112 25646447
69. Rutschow HL, Baskin TI, Kramer EM (2011) Regulation of solute flux through plasmodesmata in the root meristem. Plant physiology 155 : 1817–1826. doi: 10.1104/pp.110.168187 21325566
70. Press WH, Teukolsky SA, Vetterling WT, Flannery BP (1993) Numerical Recipes in FORTRAN; The Art of Scientific Computing: Cambridge University Press.
71. Cross M, Greenside H (2009) Pattern formation and dynamics in nonequilibrium systems. Cambridge University Press New York.
72. Tursun B, Cochella L, Carrera I, Hobert O (2009) A toolkit and robust pipeline for the generation of fosmid-based reporter genes in C. elegans. PloS one 4: e4625. doi: 10.1371/journal.pone.0004625 19259264
73. Zhou R, Benavente LM, Stepanova AN, Alonso JM (2011) A recombineering-based gene tagging system for Arabidopsis. The Plant journal: for cell and molecular biology 66 : 712–723. doi: 10.1111/j.1365-313X.2011.04524.x 21294796
74. Peret B, Swarup R, Jansen L, Devos G, Auguy F, et al. (2007) Auxin influx activity is associated with Frankia infection during actinorhizal nodule formation in Casuarina glauca. Plant physiology 144 : 1852–1862. 17556507
75. Mahonen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, et al. (2000) A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes & development 14 : 2938–2943.
76. Chaffey N, Cholewa E, Regan S, Sundberg B (2002) Secondary xylem development in Arabidopsis: a model for wood formation. Physiologia plantarum 114 : 594–600. 11975734
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





