-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
The nervous system is comprised of a complex network of axonal connections. This network is formed during development, when axons navigate to their target regions. Axon navigation requires multiple signaling pathways to detect and respond to extracellular guidance cues. Many of the guidance cues, receptors and signaling pathways have been identified. However, little is known about how the information encoded by different guidance cues is combined to arrive at a directional response. A key part of how this occurs is likely to involve combinatorial receptor signaling, when different guidance receptors work in combination with each other. However, the mechanisms that underlie combinatorial receptor signaling remain mostly unknown. In C. elegans, ventral axon guidance is mediated by the UNC-40 (DCC) and SAX-3 (Robo) guidance receptors. Here, we use genetic and biochemical analysis to identify a molecular mechanism that can mediate combinatorial signaling involving UNC-40, SAX-3 and the RhoGAP-like protein SYD-1.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005185
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005185Summary
The nervous system is comprised of a complex network of axonal connections. This network is formed during development, when axons navigate to their target regions. Axon navigation requires multiple signaling pathways to detect and respond to extracellular guidance cues. Many of the guidance cues, receptors and signaling pathways have been identified. However, little is known about how the information encoded by different guidance cues is combined to arrive at a directional response. A key part of how this occurs is likely to involve combinatorial receptor signaling, when different guidance receptors work in combination with each other. However, the mechanisms that underlie combinatorial receptor signaling remain mostly unknown. In C. elegans, ventral axon guidance is mediated by the UNC-40 (DCC) and SAX-3 (Robo) guidance receptors. Here, we use genetic and biochemical analysis to identify a molecular mechanism that can mediate combinatorial signaling involving UNC-40, SAX-3 and the RhoGAP-like protein SYD-1.
Introduction
Axonal migrations are guided through the developing nervous system by multiple extracellular guidance cues that activate receptors on the surface of the axonal growth cone. The axonal growth cone must integrate these multiple signals to arrive at a single directional response [1–7]. Progress has been made in characterizing individual guidance cues, receptors and their signaling pathways. However, the signaling mechanisms that allow for integration between receptor signaling pathways are largely unknown [8].
Integration of multiple guidance signals could be mediated by combinatorial receptor signaling [8]. In fact, several examples of combinatorial axon guidance receptor signaling have been identified in various systems. For example, commissural axons in the mammalian spinal cord exhibit a hierarchical interaction between the DCC (UNC-40) and Robo (SAX-3) receptors [9]. Upon reaching the midline, spinal axons are exposed to Slit, which promotes formation of a DCC/Robo heterodimer that silences the attractive response to Netrin, thereby allowing the axons to exit the midline. Variations of hierarchical interactions between the DCC and Robo receptors have also been observed in several other systems [10–13]. Moreover, hierarchical interactions between semaphorin receptors have also been observed [14,15].
Guidance signaling pathways can also exhibit synergistic interactions. For example, in C. elegans, the AVM neuron is simultaneously exposed to both the UNC-6 (Netrin) and SLT-1 (Slit) guidance cues. Both of these cues guide the AVM axon towards the ventral nerve cord. UNC-40 (DCC) can function individually as a receptor for UNC-6, whereas SAX-3 (Robo) can function individually as a receptor for SLT-1. However, UNC-40 can also function independently of UNC-6, when it binds to the SAX-3 receptor to promote the response to SLT-1 [16]. Despite these observations, the signaling mechanisms that mediate combinatorial guidance signaling remain mostly unexplored.
SYD-1 is an intracellular protein that contains a RhoGAP-like domain that has been implicated in synaptogenesis and axonal specification [17–21]. Here, we present evidence that SYD-1 can also function in axon guidance to promote UNC-40 signaling independently of its canonical ligand UNC-6. Our data suggest that UNC-40, SAX-3 and SYD-1 can function interdependently to regulate the MIG-2 GTPase. Our observations provide an example of a signaling mechanism that can mediate combinatorial guidance signaling.
Results
Interactions between the UNC-6 and SLT-1 signaling pathways
The cell body of the HSN neuron is located on the lateral body wall of C. elegans and extends an axon ventrally to the ventral nerve cord. To determine the relative contributions of guidance cues in HSN ventral axon guidance, we examined HSN axon guidance in slt-1(eh15) and unc-6(ev400) null mutants (Fig 1A–1C). We found that the unc-6 null mutants exhibited 59% guidance defects, whereas the slt-1 null mutants had 2% guidance defects. However, the unc-6 slt-1 double null mutants had 89% guidance defects. These observations suggest that the HSN axon is guided primarily by the UNC-6 and SLT-1 guidance cues. Moreover, the synergistic nature of these interactions suggest interactions between the UNC-6 and SLT-1 signaling pathways in the HSN neuron, providing a system for studying the factors that mediate interactions between these pathways.
Fig. 1. Interactions between the UNC-6 and SLT-1 signaling pathways in the HSN neuron. 
(A) Example of normal HSN axon guidance. (B) Example of defective HSN axon guidance. (C) Synergistic genetic interaction between null mutations in the genes that encode the UNC-6 and SLT-1 guidance cues. (D) The UNC-40 and SAX-3 pathways can function in parallel to each other. For the data shown in this panel, the sax-3(ky200) temperature sensitive allele was used to circumvent lethality. (E) The SAX-3 receptor can function independently of the SLT-1 ligand. A null mutation in the gene that encodes the SLT-1 guidance cue results in only rare guidance defects. However, a null mutation in the gene that encodes SAX-3, the receptor for SLT-1, results in 20% guidance defects. Guidance defects are not enhanced in sax-3 slt-1 double null mutants. (F) The UNC-40 receptor can function independently of the UNC-6 guidance cue. A null mutation in the gene that encodes the UNC-40 receptor causes HSN guidance defects at a penetrance that is similar that caused by a null mutation in the gene that encodes the UNC-6 guidance cue. However, loss of UNC-40 function enhances guidance defects in the absence of UNC-6 function, indicating that UNC-40 can function independently of UNC-6. For the data shown in panel D, we analyzed progeny of worms maintained at 20°C and shifted to 24° just prior to egg laying. For all other panels, strains were grown at 20°C. Scale bars are 10 μm. Anterior is to the right. Ventral is down. Alleles shown in all panels, except where noted in panel D, are unc-6(ev400), slt-1(eh15), sax-3(ky123), unc-40(e1430). HSN axon guidance was scored as defective if the axon failed to reach the ventral nerve cord. For the data shown in panel D, n>39. For all other data, n≥200. Brackets indicate statistically significant difference, Z test for proportions (***p<0.0001, **p<0.001). To investigate interactions between the signaling pathways that transduce the UNC-6 and SLT-1 cues, we examined guidance defects in mutants that disrupt their receptors, UNC-40 and SAX-3, respectively. We attempted to construct an unc-40; sax-3 double null mutant. However, this double null mutant was completely embryonic lethal, precluding analysis of axon guidance. As an alternative we used the sax-3(ky200) temperature sensitive mutation. The single sax-3(ky200) mutant at 24°C has guidance defects with a penetrance of 19%, suggesting that this allele behaves as a strong loss of function at 24°C (Fig 1D). We constructed an unc-40(e1430); sax-3(ky200) double mutant. Although these animals were mostly embryonic lethal at 24°C, we were able to analyze axon guidance in the survivors and found that guidance defects in unc-40(e1430); sax-3(ky200) double mutants were significantly enhanced relative to either single mutant (Fig 1D). Likewise, we also found that guidance defects in unc-6(ev400) sax-3(ky200) double mutants were significantly enhanced relative to either single mutant. These results suggest that UNC-40 and SAX-3 can function in parallel to each other and that these two receptors mediate most, if not all, of the ventral guidance signals in the HSN axon.
SAX-3 can function independently of its canonical ligand SLT-1. To investigate the relationship between SLT-1 and SAX-3, we analyzed mutants that disrupt these proteins. Although SAX-3 can function as a receptor for the SLT-1 guidance cue [22–24], we found that in the HSN, sax-3(ky123) null mutants had 20% defects, whereas slt-1(eh15) null mutants had only 2% defects (Fig 1E). The sax-3 slt-1 double mutants showed no enhancement relative to the sax-3 single mutants. These observations suggest that in addition to being a receptor for SLT-1, SAX-3 can also function independently of SLT-1. These results are consistent with several previous findings of SLT-1 independent SAX-3 function [12,23,25].
UNC-40 can function independently of its canonical ligand UNC-6. UNC-40 primarily functions as a receptor for the UNC-6 guidance cue [26–27]. However, the penetrance of HSN axon guidance defects was enhanced in unc-40(e1430); unc-6(ev400) double null mutants relative to unc-40(e1430) and unc-6(ev400) single null mutants (Fig 1F). Similar results were obtained using the unc-40(n324) null allele (S1 Fig). These observations suggest that UNC-40 can function independently of UNC-6, in the HSN neuron, consistent with previous reports of an UNC-6 independent function for UNC-40 in several different processes [16,26,28–30]. Moreover, the identification of an UNC-6 independent UNC-40 signaling activity in the HSN neuron provides a means to identify the signaling mechanisms that mediate interactions between the UNC-40 and the SAX-3 signaling pathways.
SYD-1 mediates UNC-6 independent UNC-40 signaling
To identify a signaling mechanism that can mediate UNC-6 independent UNC-40 function, we searched for candidate mutations that can enhance guidance phenotypes in unc-6 null mutants, but not in unc-40 null mutants. We found that the syd-1(ju2) null mutation could enhance guidance defects in unc-6(ev400) null mutants, but not in unc-40(e1430) null mutants (Fig 2A). We also obtained similar results using different null alleles for syd-1 and unc-40 (Figs 3B and S2). These observations suggest that SYD-1 might mediate UNC-6 independent UNC-40 signaling.
Fig. 2. SYD-1 mediates UNC-6 independent UNC-40 signaling. 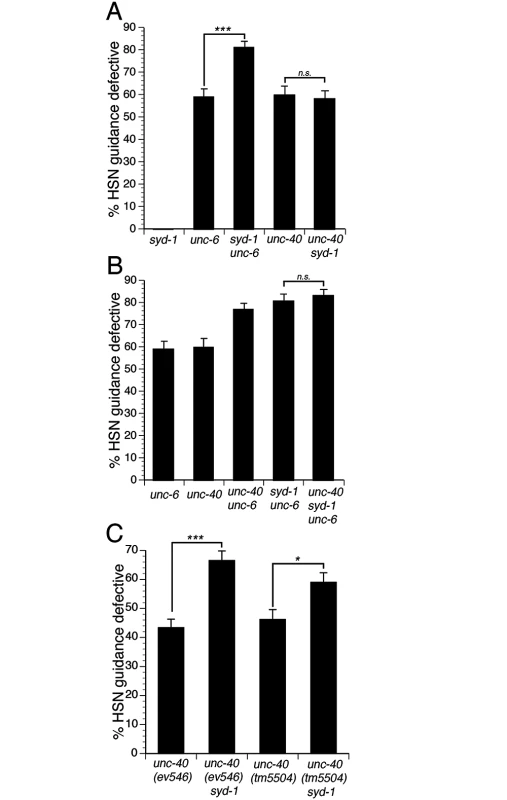
(A) SYD-1 functions in parallel to UNC-6, but not UNC-40. The syd-1(ju2) null mutation enhances HSN guidance defects in unc-6 null mutants. However, the syd-1 null mutation does not enhance guidance defects in an unc-40 null mutant. (B) SYD-1 functions with UNC-40 independently of UNC-6. Guidance defects in an unc-40; syd-1; unc-6 triple null mutant are not enhanced relative to unc-40; unc-6 or syd-1; unc-6 double null mutants. (C) The syd-1 null mutation enhances HSN guidance defects in ev546 and tm5504 hypomorphic unc-40 mutants. Alleles for panels A and B are unc-6(ev400), syd-1(ju2), unc-40(e1430). Alleles for panel C are unc-40(ev546), unc-40(tm5504) and syd-1(ju2). HSN axon guidance was scored as defective if the axon failed to reach the ventral nerve cord. For all experiments, n≥200. Brackets indicate statistically significant difference, Z test for proportions (***p<0.0001, *p<0.01). Fig. 3. The function of the syd-1 gene in axon guidance is mediated by the SYD-1C isoform. 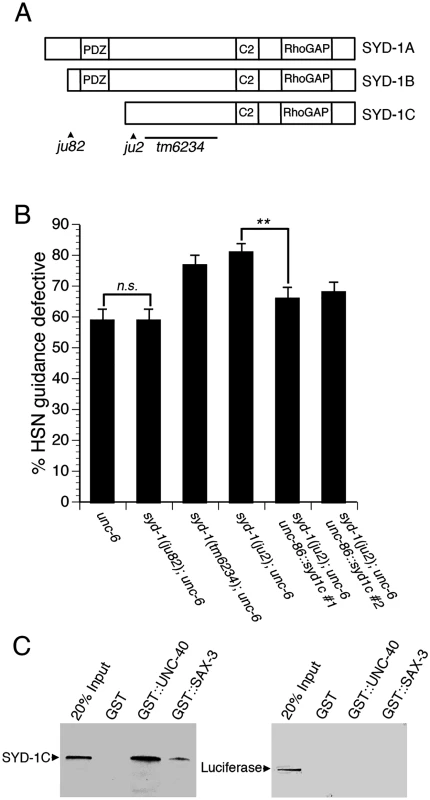
(A) Schematic of the three isoforms of SYD-1 and location of three mutations. The ju82 mutation is a nonsense mutation the affects the SYD-1A and SYD-1B isoforms. The ju2 mutation is a nonsense mutation that affects all three isoforms. The tm6234 mutation is a deletion that affects all three isoforms. (B) The SYD-1C isoform is necessary and sufficient to mediate the role of SYD-1 in axon guidance. Guidance defects in unc-6 null mutants are enhanced by the ju2 and tm6234 mutations, but not by the ju82 mutation. Guidance defects in a syd-1; unc-6 double null mutant are rescued by expressing SYD-1C in the HSN neuron with an unc-86::syd-1c transgene. (C) SYD-1C binds to the cytoplasmic domain of UNC-40 fused to GST (GST::UNC-40) and also to the cytoplasmic domain of SAX-3 fused to GST (GST::SAX-3), but not to GST alone. An unrelated protein, luciferase, does not bind to GST, GST::UNC-40, or GST::SAX-3. For reference, an amount of protein (SYD-1C or luciferase) equal to 20% of the amount of protein in the binding assay was run on the gel (20% Input). HSN axon guidance was scored as defective if the axon failed to reach the ventral nerve cord. For all experiments, n≥200. Bracket indicates statistically significant difference, Z test for proportions (**p<0.001). To determine if SYD-1 and UNC-40 function together to mediate UNC-6 independent UNC-40 function, we analyzed guidance defects in unc-40; syd-1; unc-6 triple null mutants and compared these to defects in the syd-1; unc-6 and unc-40; unc-6 double null mutants (Fig 2B). If unc-40 and syd-1 function in a genetic pathway, the triple mutant should have a penetrance of guidance defects similar to the double mutants. Indeed, we found that the penetrance of guidance defects in the unc-40; syd-1; unc-6 triple mutant was similar to the syd-1; unc-6 or the unc-40; unc-6 double null mutants. These observations are consistent with a model where UNC-40 and SYD-1 function together to mediate UNC-6 independent UNC-40 function.
To further test the idea that UNC-40 and SYD-1 function together, we analyzed double mutants between the syd-1(ju2) null allele and two different hypomorphic unc-40 alleles (Fig 2C). When two genes function in a pathway, a loss of function mutation in one gene can enhance defects associated with a hypomorphic mutation in the second gene. Indeed, we found that guidance defects associated with either of two unc-40 hypomorphic alleles could be enhanced by the syd-1(ju2) null mutation, consistent with the idea that unc-40 and syd-1 function in a genetic pathway.
Guidance function of SYD-1 is mediated by isoform C
SYD-1 is expressed as three different isoforms, known as SYD-1A, SYD-1B, and SYD-1C. SYD-1A and SYD-1B contain a PDZ domain, whereas SYD-1C does not contain the PDZ domain (Fig 3A). Loss of SYD-1A and SYD-1B disrupts axonal identity in DD and VD motor neurons [17] and also disrupts presynaptic development in the HSN neuron [20,31]. For these phenotypes, the syd-1(ju82) mutation that disrupts SYD-1A and SYD-1B, but spares SYD-1C (see Fig 3A), behaves as a null allele. These observations indicate that SYD-1C is not sufficient for axonal specification or presynaptic development.
To examine the role of each isoform of SYD-1 in axon guidance, we analyzed guidance defects in different mutant alleles of syd-1 (Fig 3B). The syd-1(ju82) allele disrupts the SYD-1A and SYD-1B isoforms, but not the SYD-1C isoform. The syd-1(ju2) and syd-1(tm6234) alleles disrupt all three isoforms. We found that the syd-1(ju82) allele, did not enhance guidance defects in unc-6 null mutants. However, both syd-1(ju2) and syd-1(tm6234) did enhance guidance defects in unc-6 null mutants. These genetic interactions suggest that the role of the syd-1 gene in guidance may be mediated by the SYD-1C isoform. To further test the role of the SYD-1C isoform, we used the unc-86 promoter to drive expression of SYD-1C in the HSN neuron. We found that expression of SYD-1C in the HSN neuron can rescue guidance defects in syd-1(ju2); unc-6(ev400) double null mutants (Fig 3B), indicating that the SYD-1C isoform is sufficient to mediate the function of the syd-1 gene in guidance. Moreover, these results also indicate that SYD-1C functions cell-autonomously in the HSN neuron. Together, these results suggest that the protein domains contained within SYD-1C are sufficient to mediate axon guidance and that the PDZ domain contained within SYD-1A and SYD-1B is not required for axon guidance. By contrast, loss of SYD-1A and SYD-1B disrupts synaptogenesis and axon specification, suggesting that the PDZ domains are required for these functions and implying that the function of SYD-1 in guidance is distinct from its role in synaptogenesis and axon specification. Although our data suggest that the SYD-1C isoform is necessary and sufficient to mediate the role of syd-1 in axon guidance, other interpretations are possible and we cannot rule out potential roles for the other isoforms of SYD-1.
The cytoplasmic domains of UNC-40 and SAX-3 bind to SYD-1C
Since genetic data suggest that UNC-40 and SYD-1 function together, we tested for a physical interaction between SYD-1C and the cytoplasmic domain of UNC-40 (Fig 3C). We found that SYD-1C binds to the cytoplasmic domain of UNC-40 fused to GST (GST::UNC-40), but not to GST alone. In addition, we found that SYD-1C can also bind to the cytoplasmic domain of SAX-3 (GST::SAX-3), but not to GST alone. By contrast, we observed no binding between an unrelated protein, luciferase, and either GST::UNC-40 or GST::SAX-3. These observations suggest that SYD-1C can bind to the cytoplasmic domains of both UNC-40 and SAX-3.
SYD-1 binds to and negatively regulates the MIG-2 GTPase
SYD-1 contains a RhoGAP-like domain, but has not been associated with any Rho GTPases. By testing candidate GTPases, we found that the GAP-like domain of SYD-1 binds to MIG-2. To confirm this interaction we performed binding assays with recombinant MIG-2 and the C-terminus of SYD-1, which contains the RhoGAP-like domain (Fig 4A). We found that MIG-2 fused to GST (GST-MIG-2) was pulled-down with the His6-tagged C-terminus of SYD-1 (His-SYD-1), while GST alone was not. Moreover, binding between MIG-2 and SYD-1 was markedly enhanced when MIG-2 was bound to GTPγS (a non-hydrolyzable analog of GTP) relative to when MIG-2 was bound to GDP. These observations indicate that the RhoGAP-like domain of SYD-1 preferentially binds to the GTP-bound active form of MIG-2.
Fig. 4. SYD-1 promotes axon guidance by negatively regulating the MIG-2 GTPase. 
(A) The RhoGAP-like domain of SYD-1 can bind to the active form of MIG-2. Binding between the C-terminal RhoGAP-like domain of SYD-1 (His-SYD-1) and GST-MIG-2 was substantially enhanced after incubation with GTPγS (GST-MIG-2 + GTPγS) relative to incubation with GDP (GST-MIG-2 + GDP). No binding was observed between His-SYD-1 and GST. (B-C) SYD-1 negatively regulates MIG-2 activity. (B) The syd-1(ju2) null mutation enhances HSN guidance defects in partially activated mig-2(gm38) mutants. However, the null mutation in syd-1 does not enhance HSN guidance defects in the fully activated mig-2(gm103) mutants. (C) Transgenic expression of SYD-1C suppresses axon guidance defects in the partially activated mig-2(gm38) mutants. HSN axon guidance was scored as defective if the axon failed to reach the ventral nerve cord. For all experiments, n≥200. Brackets indicate statistically significant difference, Z test for proportions (***p<0.0005, **p<0.005). To determine if SYD-1 can regulate MIG-2 activity, we tested for genetic interactions between the syd-1(ju2) null allele and mig-2 gain of function mutations. The mig-2(gm103) mutation encodes a fully activated MIG-2, whereas mig-2(gm38) encodes a partially activated MIG-2 [32–33]. We found that loss of syd-1 can enhance the defects associated with the partially active mig-2(gm38) allele (Fig 4B), suggesting that SYD-1 can negatively regulate MIG-2. By contrast, we observed no enhancement of the fully active mig-2(gm103) allele, consistent with the expectation that fully activated MIG-2 cannot be further activated. To further test the functional relationship between SYD-1 and MIG-2, we transgenically expressed SYD-1C in the mig-2(gm38) partially activated gain of function mutants (Fig 4C). Consistent with the idea that SYD-1 can negatively regulate MIG-2, we found that transgenic expression of SYD-1C suppresses guidance defects caused by the mig-2(gm38) gain of function mutation. To further test the interaction between SYD-1 and MIG-2, we constructed a syd-1(ju2); mig-2(ok2273) double null mutant. We found that penetrance of HSN guidance defects in these double null mutants was not enhanced relative to mig-2(ok2273) single mutants (Fig 5), further supporting the idea that SYD-1 functions with MIG-2. Together, these data are consistent with a model where SYD-1 can negatively regulate the activation state of MIG-2.
Fig. 5. MIG-2 functions with SAX-3 to regulate axon guidance. 
HSN guidance defects were observed in mig-2 null mutants and in sax-3 null mutants. HSN guidance defects were not enhanced in sax-3 mig-2 double null mutants, indicating that SAX-3 and MIG-2 function together to regulate HSN axon guidance. The mig-2 null mutant enhances HSN guidance defects in unc-40 null mutants. Alleles are syd-1 (ju2), mig-2(ok2273), sax-3(ky123), unc-40(e1430). HSN axon guidance was scored as defective if the axon failed to reach the ventral nerve cord. For all experiments, n≥200. Brackets indicate statistically significant difference, Z test for proportions (***p<0.0001). MIG-2 functions in the SAX-3 signaling pathway
To consider the role of mig-2 in HSN axon guidance, we examined the HSN axon in mig-2(ok2273) null mutants (Fig 5). We found that 14% of HSN axon migrations were defective in the mig-2 null mutants, indicating that MIG-2 is required for HSN axon guidance. Likewise, 20% of HSN axon migrations were defective in sax-3(ky123) null mutants. To determine if mig-2 functions in a genetic pathway with sax-3, we examined sax-3 mig-2 double null mutants. If two genes function in a genetic pathway, a double null mutant should have defects similar to the greatest of the single mutants. We found that the sax-3 mig-2 double mutants had a penetrance of guidance defects similar to that of sax-3 single mutants, indicating that mig-2 and sax-3 do function in a genetic pathway. Consistent with a function for mig-2 in the sax-3 pathway, we also found that the mig-2 null mutation can enhance defects in the unc-40 null mutants. Together, these genetic interactions support the conclusion that MIG-2 functions in the SAX-3 pathway.
SYD-1 function requires SAX-3
Since SYD-1 regulates MIG-2, which functions with SAX-3, we asked if SYD-1 function requires SAX-3. To address this question, we examined genetic interactions between mutations in syd-1 and sax-3. We found that the syd-1(ju2) null mutation fails to enhance axon guidance defects in sax-3(ky123) null mutants, suggesting that SYD-1 might function with SAX-3 (Fig 6A). To further determine if SYD-1 can function with SAX-3, we used the sax-3(ky200) hypomorphic allele and found that the syd-1(ju2) null mutation enhances guidance defects in sax-3(ky200) hypomorphic mutants (Fig 6B). Since, the syd-1 null mutant can enhance guidance defects in the sax-3 hypomorphic mutants, but not in sax-3 null mutants, we conclude that SYD-1 functions with SAX-3, implying that SYD-1 function requires SAX-3.
Fig. 6. SYD-1 function requires SAX-3. 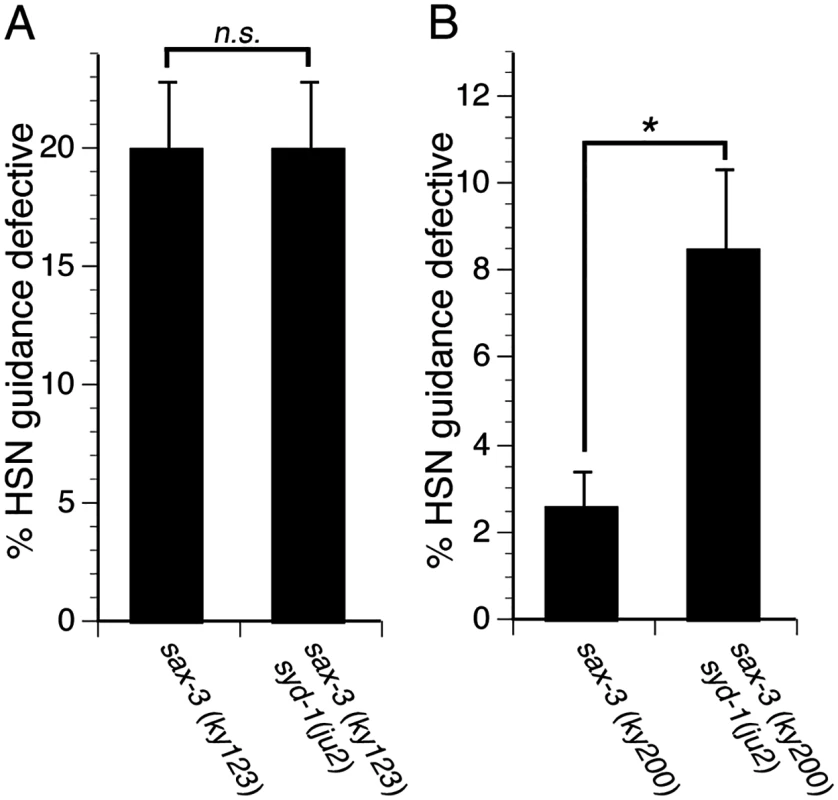
(A) The syd-1(ju2) null mutation does not enhance guidance defects in sax-3(ky123) null mutants. (B) The syd-1(ju2) null mutation enhances guidance defects in sax-3(ky200) hypomorphic mutants. For experiments with the sax-3(ky123) null mutants, we scored only severe HSN defects (the axon never reaches the ventral nerve cord). For the sax-3(ky200) hypomorphic mutants, we did not observe any severe HSN axon guidance defects, but only mild defects (the axon trajectory is defective but eventually reaches the ventral nerve cord). Therefore, for experiments with the sax-3(ky200) hypomorphic mutants, we scored mild axon guidance defects. For all experiments, n≥200. Bracket indicates statistically significant difference, Z test for proportions (*p<0.01). Discussion
The UNC-40 and SAX-3 pathways can function separately or in combination with each other
We have found that single null mutants of unc-40 and sax-3 exhibit partially penetrant guidance defects. We have also found that unc-40; sax-3 double strong loss of function mutants exhibit guidance defects that are nearly fully penetrant. Together, these results suggest that UNC-40 and SAX-3 can function in parallel to each other.
Although UNC-40 and SAX-3 can function in parallel, our genetic analysis suggests that UNC-40 and SAX-3 can also function together. This idea is consistent with previous work in the AVM neuron showing that UNC-40 and SAX-3 can function in combination with each other, whereby UNC-40 functions independently of UNC-6 to potentiate SAX-3 signaling [16]. Consistent with this genetic analysis, biochemical data indicate that both UNC-40 and SAX-3 can bind to each other [16]. Likewise, DCC and Robo, vertebrate homologs of UNC-40 and SAX-3, respectively, also exhibit functional and biochemical interactions [9–11]. Despite these findings, little is known about the signaling events that mediate combinatorial guidance receptor function.
UNC-40, SAX-3 and SYD-1 function interdependently
Our results suggest that SYD-1 function requires both UNC-40 and SAX-3, implying that UNC-40, SAX-3 and SYD-1 function interdependently. Loss of syd-1 function fails to enhance guidance defects in null mutants of either unc-40 or sax-3. However, loss of syd-1 function does enhance guidance defects in hypomorphic mutants of either unc-40 or sax-3. These observations suggest that SYD-1 function does not occur in the complete absence of UNC-40 or SAX-3, but becomes apparent when UNC-40 or SAX-3 function is reduced. Moreover, we also find that SYD-1 binds to the cytoplasmic domains of both UNC-40 and SAX-3. Together, these observations are consistent with a model whereby SYD-1 does not function in the UNC-40 individual pathway or the SAX-3 individual pathway, but rather functions in a common pathway with both UNC-40 and SAX-3. However, we note that other interpretations for these results are possible and that future investigations will be required to further define the relationships between UNC-40, SAX-3 and SYD-1.
Models for UNC-40 and SAX-3 combinatorial signaling
Our results suggest that UNC-40, SAX-3 and SYD-1 function interdependently, implying that SYD-1 can mediate combinatorial receptor signaling. We propose two models to explain how UNC-40, SAX-3 and SYD-1 could mediate combinatorial signaling (Figs S3 and S4). In the heterodimer model, UNC-40 and SAX-3 would bind to each other and function as an UNC-6 independent guidance receptor. This heterodimer receptor would bind to SYD-1 and cause negative regulation of MIG-2, which functions in the SAX-3 pathway. Alternatively, in the crosstalk model, UNC-40 and SAX-3 would not be physically associated with each other. In this crosstalk model, SYD-1 would bind to the cytoplasmic tail of the UNC-40 receptor and would negatively regulate MIG-2, which functions in the SAX-3 pathway. We favor the heterodimer model because previous results have indicated that UNC-40 and SAX-3 can bind to each other [16], our genetic analysis suggest that SYD-1 function requires both UNC-40 and SAX-3, and our biochemical analysis show that SYD-1 can bind to both UNC-40 and SAX-3. Future investigations may help differentiate between these two models, or potentially suggest other models.
SYD-1 can negatively regulate the MIG-2 GTPase
Our finding that a syd-1 null allele can enhance guidance defects in partially activated mig-2 mutants, but not in fully activated mig-2 mutants, implies that SYD-1 is involved in regulating the activation state of MIG-2. However, SYD-1 lacks two critical residues that are required for GAP activity [34–35]. Thus far, attempts to detect SYD-1 GAP activity have been unsuccessful. Since we have found that the GAP domain of SYD-1 can bind to activated MIG-2, we propose that SYD-1 functions as a scaffold that can promote negative regulation of MIG-2 activity. One possibility is that SYD-1 and MIG-2 may be part of a complex that also includes a GAP protein. Alternatively, it remains possible that SYD-1 could possess GAP activity on its own.
Axon guidance signaling requires both positive and negative regulation of Rac GTPases
We report that UNC-6 independent UNC-40 signaling is mediated by SYD-1 and that SYD-1 negatively regulates MIG-2. Thus, UNC-6 independent UNC-40 signaling can promote axon guidance by negatively regulating MIG-2. This idea is consistent with previous results showing that negative regulation of Rac promotes signaling downstream of Robo (SAX-3). In Drosophila, CrossGAP (Vilse) has been identified as a GAP that negatively regulates Rac activation to promote Robo signaling [36,37]. In mammalian neurons, srGAP1 has been identified as a GAP for Rac and Cdc42 that is required for Robo signaling [38–39]. Positive regulation of Rac has also been reported, whereby Son of Sevenless (Sos), a Guanine Nucleotide Exchange Factor (GEF), can positively regulate Rac to promote Robo signaling [40]. Thus, Robo signaling involves both positive and negative regulation of Rac. Consistent with this idea, guidance defects result from either Rac gain of function or Rac loss of function mutations [32,41]. Likewise, guidance defects are caused by loss of function or gain of function in CrossGAP, a GAP for Rac in the Drosophila [36–37]. Together, these observations indicate that axon guidance requires precise control of Rac activation state, which is achieved through both positive and negative regulation of Rac.
SYD-1C and its mammalian homolog, mSYD1A, contain predicted N-terminal Intrinsically Disordered Domains
SYD-1C resembles its mammalian homolog mSYD1A, in that both are predicted to contain an Intrinsically Disordered Domain (ID domain) at their N-terminus, rather than a PDZ domain [42]. ID domains can shift between disordered and ordered confirmations and can also engage in intramolecular interactions that affect the function of other domains [42,43]. Although SYD-1C and mSYD1A both contain predicted ID domains, the amino acid sequence of the ID domain is not conserved between SYD-1C and mSYD1A. Consistent with this different amino acid sequence, the function of SYD-1C and mSYD1A appears to be different, in that the former regulates axon guidance and the later regulates synaptic vesicle docking [42]. These different functions are likely to be mediated by different binding partners for the ID domains of SYD-1C and mSYD1A.
Evidence that the SAX-3 receptor can function independently of its canonical ligand SLT-1
Although our study has focused on UNC-6 independent UNC-40 function, we have also observed evidence for SLT-1 independent SAX-3 function. This idea is supported by the higher penetrance of HSN guidance defects in sax-3 null mutants relative to slt-1 null mutants. In fact, SLT-1 independent SAX-3 signaling has been observed in several different processes, including AVM and PVM axon guidance [12,23,25,44]. Much like in the HSN, the PVM neuron shows a higher penetrance of HSN guidance defects in sax-3 null mutants relative to slt-1 null mutants [12]. Genetic analysis has suggested that in the PVM neuron SAX-3 can inhibit UNC-40 signaling in the absence of SLT-1 [12]. This inhibitory interaction between SAX-3 and UNC-40 also occurs in the AVM neuron [12,44]. However, the AVM neuron shows a higher penetrance of guidance defects in slt-1 null mutants relative to sax-3 null mutants. The reason for the differences in slt-1 and sax-3 mutant defects in the AVM relative to the HSN and PVM are not known. However, these differences could be explained by a greater degree of redundant signaling in the HSN and PVM neurons relative to the AVM neuron.
Evidence that the UNC-6 guidance cue can function independently of its canonical receptor UNC-40
Since we have found that UNC-40 can function independently of UNC-6, one might expect unc-40 null mutants to have a greater penetrance of guidance defects relative to unc-6 null mutants. However, we have found that unc-6 and unc-40 null mutants have a similar penetrance of HSN guidance defects. One possible explanation could be that UNC-6 can also function independently of UNC-40 in the HSN. Indeed, the existence of UNC-40 independent UNC-6 activity is apparent in AVM and PVM axon guidance, where unc-6 mutants have a higher penetrance of guidance defects relative to unc-40 mutants [12]. Moreover, ENU-3 is thought to form part of an UNC-40 independent UNC-6 signaling pathway in the AVM and PVM neurons [45].
Materials and Methods
C. elegans genetics
Genetic manipulations were carried out using standard procedures. The N2 Bristol genetic background was used in all experiments and all animals were maintained at 20°C on NGM plates seeded with OP50 bacteria. The following alleles were used and are considered to be null: unc-6(ev400) [46], slt-1(eh15) [26], sax-3(ky123) [47], unc-40(e1430) [26], syd-1(ju2) [17], syd-1(tm6234), syd-1(ju82) [17], mig-2(ok2273) [48]. The following alleles are considered to be hypomorphic: sax-3(ky200) [47], unc-40(ev546) [49], unc-40(tm5504) [49]. The following alleles are considered to be gain of function: mig-2(gm38) [32], mig-2(gm103) [32]. The unc-40(ev546) allele was obtained from Joseph Culotti. The syd-1(tm6234) allele was obtained from Shohei Mitani. All other alleles were obtained from the Caenorhabditis Genetics Center (CGC).
DNA constructs
The pAGC5 plasmid includes Punc-86::syd1c::sl2::tagrfp and was created using Gibson Assembly and cloned into pCFJ910, provided by Erik Jorgensen via Addgene (Addgene Plasmid #44481). The DNA sequence encoding TAGRFP was obtained from Julie Plastino [50]. pRSET-SYD-1C (pCZGY658), which encodes His6-tagged C-terminus of SYD-1C protein (AA320-721), was generated using the Gateway cloning system (Invitrogen).
Transgenic rescue
Transgenes expressing SYD-1C in the HSN neuron were created by injecting the pAGC5 plasmid at 4 ng/μl with 50 ng/μl of odr-1::RFP and 50 ng/μl Bluescript into wildtype worms. Two independent transgenic lines were established and crossed into the syd-1(ju2); unc-6(ev400) genetic background. HSN axon guidance was analyzed as described below.
Analysis of phenotypes
Axon guidance was analyzed as described previously [51,52]. The zdIs13 transgene [53], which expresses Ptph-1::gfp, was used to visualize the HSN axon in young adult animals. Except where noted, HSN guidance defects were scored as defective if the axon failed to reach the ventral nerve cord. For experiments involving the sax-3(ky200) hypomorphic allele, only weak defects were observed, where the axon trajectory is abnormal, but the axon eventually reaches the ventral nerve cord. Therefore, in experiments involving the sax-3(ky200) hypomorphic allele, HSN axons were scored as defective if their trajectory was abnormal, but they eventually reached the ventral nerve cord.
GST binding assays with GST::MIG-2
Recombinant GST and GST-MIG-2 proteins were produced in Escherichia coli DH5α with plasmid pGEX6P and pGEX4T-MIG-2 (obtained from Hiroshi Qadota [54], respectively, by incubating for 5 h at 37°C in the presence of 0.3 mM IPTG. His6-tagged C-terminus of SYD-1C containing RhoGAP-like domain (His-SYD-1) was produced in E. coli BL21(DE3) with pRSET-SYD-1C (pCZGY658) by incubating for 16 h at 22°C in the presence of 0.3 mM IPTG. GST - and His6 - tagged proteins were extracted by sonication in PBS pH 7.4 with 0.5% Triton X-100 and protease inhibitors (Roche) and centrifuging. GST-MIG-2 was incubated with 0.1 mM of GTPγS or 1 mM of GDP (Sigma-Aldrich) in PBS pH 7.4 with 0.5% Triton X-100, 10 mM MgCl2, 10 mM EDTA, 2% glycerol and protease inhibitors for 30 min at 30°C, and then supplied additional MgCl2 to 75 mM. For pull-down analysis, His-SYD-1 was immobilized to HisPur Cobalt Resin (Thermo), incubated with GST or GST-MIG-2 proteins for 1 h at 4°C, washed with PBS pH 7.4 with 0.1% Triton X-100 and 20 mM imidazole for 4 times, eluted with 300 mM imidazole in PBS pH 7.4, 0.1% Triton X-100. The eluate and input were subjected to SDS-PAGE, and GST - and His - tagged proteins were detected by western blotting with mouse monoclonal Anti-GST antibody (Upstate Biotechnology) and Anti-His-tag mAb (Medical and biological laboratories), respectively, using ECL Advance Western Blotting Detection Kit (GE Healthcare).
Binding assays with GST::UNC-40 and GST::SAX-3
SYD-1C protein was produced with the TNT SP6 quick coupled in vitro transcription and translation system (Promega) and labeled with biotin. GST::UNC-40 was produced in bacteria and coupled to Glutathione-Sepharose. Binding assays were conducted for 16 hours at 4°C in PBS with 0.1% Triton X-100, 1% BSA and protease inhibitors. After binding, samples were washed 3 times with wash buffer (PBS and 0.1% Triton X-100). Bound material was detected by SDS-PAGE electrophoresis and western blotting with Strepavidin-Alkaline Phosphatase.
Supporting Information
Zdroje
1. Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2(5):a001941. doi: 10.1101/cshperspect.a001941 20452961
2. Blockus H, Chedotal A. The multifaceted roles of Slits and Robos in cortical circuits: from proliferation to axon guidance and neurological diseases. Curr Opin Neurobiol. 2014;27C:82–8.
3. Chedotal A, Richards LJ. Wiring the brain: the biology of neuronal guidance. Cold Spring Harb Perspect Biol. 2010;2(6):a001917. doi: 10.1101/cshperspect.a001917 20463002
4. Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2010;3(6).
5. Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–69. doi: 10.1242/dev.044529 21558366
6. O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32 : 383–412. doi: 10.1146/annurev.neuro.051508.135614 19400716
7. Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010;2(9):a001933. doi: 10.1101/cshperspect.a001933 20591992
8. Dudanova I, Klein R. Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci. 2013;36(5):295–304. doi: 10.1016/j.tins.2013.01.007 23485451
9. Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291(5510):1928–38. 11239147
10. Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, et al. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144(1):106–18. doi: 10.1016/j.cell.2010.11.053 21215373
11. Fothergill T, Donahoo AL, Douglass A, Zalucki O, Yuan J, Shu T, et al. Netrin-DCC Signaling Regulates Corpus Callosum Formation Through Attraction of Pioneering Axons and by Modulating Slit2-Mediated Repulsion. Cereb Cortex. 2013;24(5):1138–51. doi: 10.1093/cercor/bhs395 23302812
12. Fujisawa K, Wrana JL, Culotti JG. The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science. 2007;317(5846):1934–8. 17901337
13. Leyva-Diaz E, del Toro D, Menal MJ, Cambray S, Susin R, Tessier-Lavigne M, et al. FLRT3 is a Robo1-interacting protein that determines Netrin-1 attraction in developing axons. Curr Biol. 2014;24(5):494–508. doi: 10.1016/j.cub.2014.01.042 24560577
14. Ruediger T, Zimmer G, Barchmann S, Castellani V, Bagnard D, Bolz J. Integration of opposing semaphorin guidance cues in cortical axons. Cereb Cortex. 2012;23(3):604–14. doi: 10.1093/cercor/bhs044 22368082
15. Sanyas I, Bozon M, Moret F, Castellani V. Motoneuronal Sema3C is essential for setting stereotyped motor tract positioning in limb-derived chemotropic semaphorins. Development. 2012;139(19):3633–43. doi: 10.1242/dev.080051 22899844
16. Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5(11):1147–54. 12379860
17. Hallam SJ, Goncharov A, McEwen J, Baran R, Jin Y. SYD-1, a presynaptic protein with PDZ, C2 and rhoGAP-like domains, specifies axon identity in C. elegans. Nat Neurosci. 2002;5(11):1137–46. 12379863
18. Holbrook S, Finley JK, Lyons EL, Herman TG. Loss of syd-1 from R7 neurons disrupts two distinct phases of presynaptic development. J Neurosci. 2012;32(50):18101–11. doi: 10.1523/JNEUROSCI.1350-12.2012 23238725
19. Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, et al. A Syd-1 homologue regulates pre - and postsynaptic maturation in Drosophila. J Cell Biol. 2010;188(4):565–79. doi: 10.1083/jcb.200908055 20176924
20. Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, et al. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9(12):1488–98. 17115039
21. Stavoe AKH, Nelson JC, Martínez-Velázquez LA, Klein M, Samuel ADT, Colón-Ramos DA. Synaptic Vesicle Clustering Requires a Distinct MIG-10/Lamellipodin Isoform and ABI-1 downstream from Netrin. Genes and Development. 2012;26.
22. Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96(6):795–806. 10102268
23. Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, et al. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32(1):25–38. 11604136
24. Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96(6):785–94. 10102267
25. Zallen JA, Kirch SA, Bargmann CI. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999;126(16):3679–92. 10409513
26. Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87(2):187–95. 8861903
27. Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87(2):175–85. 8861902
28. Alexander M, Chan KK, Byrne AB, Selman G, Lee T, Ono J, et al. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development. 2009;136(6):911–22. doi: 10.1242/dev.030759 19211675
29. Smith CJ, Watson JD, VanHoven MK, Colon-Ramos DA, Miller DM 3rd. Netrin (UNC-6) mediates dendritic self-avoidance. Nat Neurosci. 2012;15(5):731–7. doi: 10.1038/nn.3065 22426253
30. Wang Z, Linden LM, Naegeli KM, Ziel JW, Chi Q, Hagedorn EJ, et al. UNC-6 (netrin) stabilizes oscillatory clustering of the UNC-40 (DCC) receptor to orient polarity. J Cell Biol. 2014;206(5):619–33. doi: 10.1083/jcb.201405026 25154398
31. Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, et al. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat Neurosci. 2006;9(12):1479–87. 17115037
32. Zipkin ID, Kindt RM, Kenyon CJ. Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell. 1997;90(5):883–94. 9298900
33. Feig LA, Cooper GM. Relationship among guanine nucleotide exchange, GTP hydrolysis, and transforming potential of mutated ras proteins. Mol Cell Biol. 1988;8(6):2472–8. 3043178
34. Rittinger K, Taylor WR, Smerdon SJ, Gamblin SJ. Support for shared ancestry of GAPs. Nature. 1998;392(6675):448–9. 9548249
35. Rittinger K, Walker PA, Eccleston JF, Nurmahomed K, Owen D, Laue E, et al. Crystal structure of a small G protein in complex with the GTPase-activating protein rhoGAP. Nature. 1997;388(6643):693–7. 9262406
36. Hu H, Li M, Labrador JP, McEwen J, Lai EC, Goodman CS, et al. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc Natl Acad Sci U S A. 2005;102(12):4613–8. 15755809
37. Lundstrom A, Gallio M, Englund C, Steneberg P, Hemphala J, Aspenstrom P, et al. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes Dev. 2004;18(17):2161–71. 15342493
38. Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107(2):209–21. 11672528
39. Yamazaki D, Itoh T, Miki H, Takenawa T. srGAP1 regulates lamellipodial dynamics and cell migratory behavior by modulating Rac1 activity. Mol Biol Cell. 2013;24(21):3393–405. doi: 10.1091/mbc.E13-04-0178 24006490
40. Fan X, Labrador JP, Hing H, Bashaw GJ. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40(1):113–27. 14527437
41. Lundquist EA, Reddien PW, Hartwieg E, Horvitz HR, Bargmann CI. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 2001;128(22):4475–88. 11714673
42. Wentzel C, Sommer JE, Nair R, Stiefvater A, Sibarita JB, Scheiffele P. mSYD1A, a mammalian synapse-defective-1 protein, regulates synaptogenic signaling and vesicle docking. Neuron. 2013;78(6):1012–23. doi: 10.1016/j.neuron.2013.05.010 23791195
43. Liu Y, Matthews KS, Bondos SE. Multiple intrinsically disordered sequences alter DNA binding by the homeodomain of the Drosophila hox protein ultrabithorax. J Biol Chem. 2008;283(30):20874–87. doi: 10.1074/jbc.M800375200 18508761
44. Li H, Kulkarni G, Wadsworth WG. RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J Neurosci. 2008;28(14):3595–603. doi: 10.1523/JNEUROSCI.5536-07.2008 18385318
45. Yee C, Florica R, Fillingham J, Killeen MT. ENU-3 functions in an UNC-6/netrin dependent pathway parallel to UNC-40/DCC/frazzled for outgrowth and guidance of the touch receptor neurons in C. elegans. Dev Dyn. 2014;243(3):459–67. doi: 10.1002/dvdy.24063 24123761
46. Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4(1):61–85. 2310575
47. Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92(2):217–27. 9458046
48. Alan JK, Lundquist EA. Analysis of Rho GTPase function in axon pathfinding using Caenorhabditis elegans. Methods Mol Biol. 2012;827 : 339–58. doi: 10.1007/978-1-61779-442-1_22 22144285
49. Tian C, Shi H, Xiong S, Hu F, Xiong WC, Liu J. The neogenin/DCC homolog UNC-40 promotes BMP signaling via the RGM protein DRAG-1 in C. elegans. Development. 2013;140(19):4070–80. doi: 10.1242/dev.099838 24004951
50. Batchelder EL, Hollopeter G, Campillo C, Mezanges X, Jorgensen EM, Nassoy P, et al. Membrane tension regulates motility by controlling lamellipodium organization. Proc Natl Acad Sci U S A. 2011;108(28):11429–34. doi: 10.1073/pnas.1010481108 21709265
51. Xu Y, Quinn CC. MIG-10 functions with ABI-1 to mediate the UNC-6 and SLT-1 axon guidance signaling pathways. PLoS Genet. 2012;8(11):e1003054. doi: 10.1371/journal.pgen.1003054 23209429
52. Xu Y, Ren XC, Quinn CC, Wadsworth WG. Axon response to guidance cues is stimulated by acetylcholine in Caenorhabditis elegans. Genetics. 2011;189(3):899–906. doi: 10.1534/genetics.111.133546 21868605
53. Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003;130(16):3781–94. 12835394
54. Qadota H, Miyauchi T, Nahabedian JF, Stirman JN, Lu H, Amano M, et al. PKN-1, a homologue of mammalian PKN, is involved in the regulation of muscle contraction and force transmission in C. elegans. J Mol Biol. 2011;407(2):222–31. doi: 10.1016/j.jmb.2011.01.039 21277858
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



