-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Speciation and Introgression between and
While speciation is often depicted as a simple population split, in many cases it is likely more complex. Recently, whole genome sequencing and computational methods to interpret patterns of genomic variation have facilitated the inference of complex speciation histories. We present and analyze genomic data to infer the speciation history of an ecological and evolutionary model species pair - Mimulus guttatus/M. nasutus. We infer that M. nasutus split from a central Californian M. guttatus population approximately 200–500 kya, roughly corresponding to M. nasutus’ shift to self-fertilization. We document ongoing gene flow between these species where they co-occur. Finally, we present patterns genomic divergence suggesting that natural selection disfavors introgression of M. nasutus ancestry in M. guttatus.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004410
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004410Summary
While speciation is often depicted as a simple population split, in many cases it is likely more complex. Recently, whole genome sequencing and computational methods to interpret patterns of genomic variation have facilitated the inference of complex speciation histories. We present and analyze genomic data to infer the speciation history of an ecological and evolutionary model species pair - Mimulus guttatus/M. nasutus. We infer that M. nasutus split from a central Californian M. guttatus population approximately 200–500 kya, roughly corresponding to M. nasutus’ shift to self-fertilization. We document ongoing gene flow between these species where they co-occur. Finally, we present patterns genomic divergence suggesting that natural selection disfavors introgression of M. nasutus ancestry in M. guttatus.
Introduction
While speciation is often depicted as a simple event in which a single species splits into two, there is increasing evidence that this process is often more complex. In particular, speciation reflects a tension among divergence, the assortment of ancestral variation, ecological interactions and in some cases introgression that play out across the environment of the incipient species. Historically, a population genetic view of the process of speciation has been limited to few loci, where stochasticity in ancestral processes can prevent strong inferences about isolation and gene flow. By contrast, whole genome resequencing (even of only a few individuals) reveals many genealogical histories across contiguous genomic regions to provide well-resolved views of population history, divergence and introgression [1]–[5]. Here, we present a population genomic investigation of the speciation history of two closely related species of yellow monkeyflowers, the primarily outcrossing Mimulus guttatus, and the self-pollinating M. nasutus – an evolutionary model system for which the genetic and ecological basis of reproductive isolation is reasonably well characterized [6].
In flowering plants, speciation often involves a shift in pollinator (e.g., [7]–[9]) or mating system (e.g., [7], [10]–[12]), with concomitant divergence in key floral traits causing reproductive isolation between lineages. The evolutionary transition from outcrossing to self-fertilization, as occurred in M. nasutus, is of particular interest because the expected reduction in both the effective population size and effective recombination rate [13], [14] can dramatically alter population genetic processes and patterns of genomic variation [15], [16]. Recent evidence for elevated levels of putatively deleterious alleles in selfing taxa [17]–[19] is consistent with the idea that inbreeding reduces the effectiveness of purifying selection (due to a lowered effective population size). However, we still have few examples of the effects of self-fertilization on patterns of diversity across the genome, particularly in the context of recently diverged and potentially hybridizing species. Genomic datasets from young selfing species can uniquely inform the process of mating system divergence by allowing us to compare regions of the genome that share a common ancestor before or after the origin of self-fertilization and thus understand the assortment of ancestral variation [20].
The M. guttatus – M. nasutus species pair is an excellent model for investigating the causes and consequences of mating system evolution and species divergence. M. guttatus is primarily outcrossing (although the outcrossing rate varies across populations [21]–[23]) with large, bee-pollinated flowers and occupies diverse ecological habitats throughout western North America. M. nasutus is highly selfing with reduced, mostly closed flowers. Although these species are often found in different microhabitats, the range of M. nasutus is broadly nested within that of M. guttatus and the two species do co-occur. In sympatry, M. nasutus and M. guttatus are partially reproductively isolated by differences in floral morphology, flowering phenology, and pollen-pistil interactions [24]–[26]. Although early-generation hybrids occur in nature [24], [27], numerous intrinsic hybrid incompatibilities decrease hybrid fitness [28]–[30]. Based on the most detailed population genetic analyses of Mimulus to date (two and six nuclear loci, respectively [30], [31]), M. nasutus exhibits reduced diversity compared to M. guttatus, and some M. guttatus sequences are nearly identical to M. nasutus, suggestive of historical introgression. However, this limited view of the genome cannot resolve the timing and genomic consequences of divergence between Mimulus species, nor can it inform the extent or consequences of introgression between them.
We present the first population genomic analysis of M. guttatus and M. nasutus, spanning diverse ecotypes collected from throughout the species' ranges. We use these dense and contiguous population genomic data to estimate the population-split time, quantify rapid loss of ancestral variation accompanying the transition to selfing in M. nasutus, and identify ongoing, bidirectional introgression. Additionally, we observe a negative correlation between the recombination rate and interspecific divergence between M. nasutus and sympatric M. guttatus, a result best explained by selection against introgressed M. nasutus ancestry in M. guttatus. Our approach provides a detailed view of differentiation and introgression in a tractable ecological, genetic, and evolutionary model system.
Results
We present and analyze a population genomic dataset of nineteen lab and/or naturally inbred (see Table S1) Mimulus samples – thirteen M. guttatus, five M. nasutus, and one of M. dentilobus, an outgroup. We generated sequence data for five of these samples (four M. nasutus and one M. guttatus), and accessed data for the other 14 samples from previously existing resources (see Materials and Methods for sequence sources and processing details). Collections spanned the ecological and geographic ranges of each species (Figure 1A and Table S1). Many of our analyses focus on four M. guttatus and four M. nasutus collections sequenced to relatively high depth (13.8×–24.7×) and with identical read lengths (100 bp, paired-end reads). Pairwise comparisons of nucleotide diversity among all nineteen samples are presented in Tables S2A and S3. Individual heterozygosity in the eight focal samples was relatively low (see METHODS) and was not clustered in regions of residual heterozygosity (contra the observation in naturally inbred Capsella rubella [20]) suggesting that natural and lab inbreeding has resulted in near total homozygosity by (recent) descent. Throughout this manuscript, we present results from samples aligned with bwa [32]. In Table S2B, we show that qualitative patterns of relative differentiation in focal samples are consistent when analyzed with a different alignment program, Stampy [33], demonstrating that our results are robust to the choice of bioinformatic pipeline.
Fig. 1. Relationships among Mimulus samples. 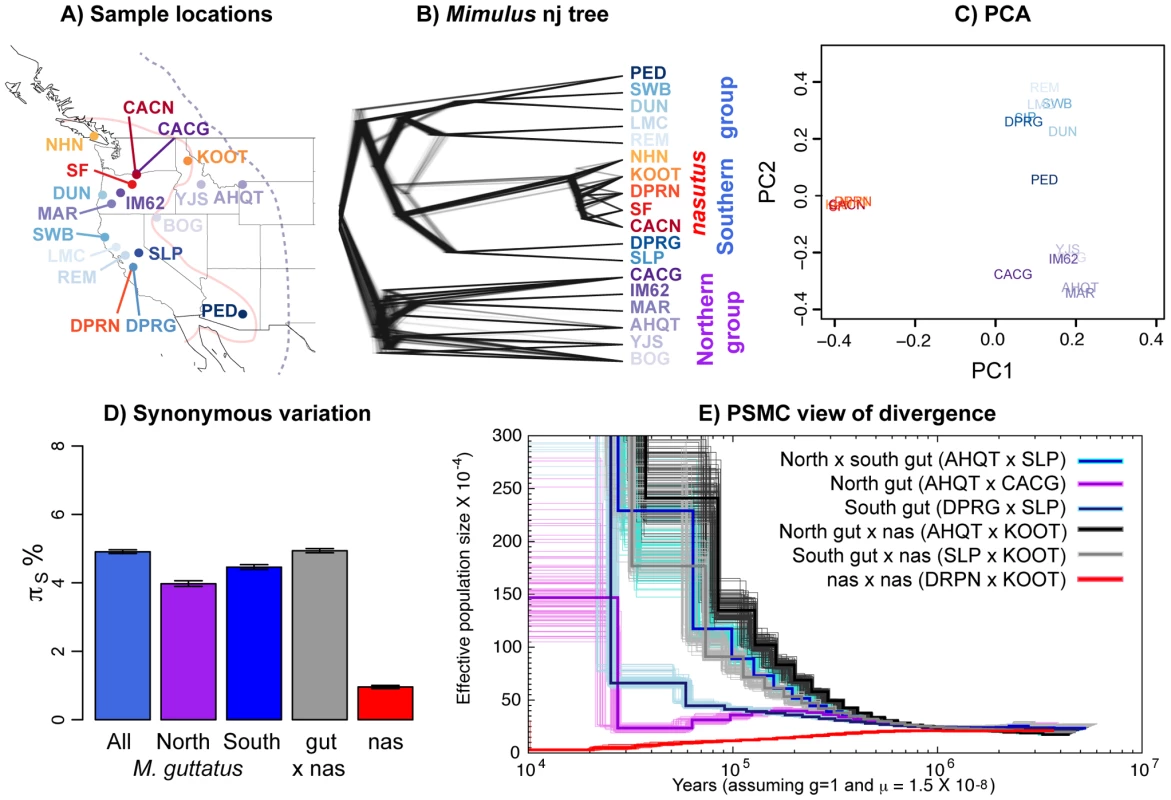
A) A map of all samples with identity and species denoted by population code and color, respectively. M. guttatus individuals are colored in blues and purples, and M. nasutus is colored in oranges and reds. Approximate species distribution boundaries are shown for M. guttatus with the purple dashed line and for M. nasutus with the solid pink line. Sympatric samples are marked by lines originating in the same location. B) A neighbor-joining tree for all samples rooted by M. dentilobous. Sample and species colors are identical to those in 1A. The consensus tree was constructed from the pairwise distance matrix described in the main text with the nj function in the R package, ape, and smoothed with the function, chronopl, with λ = 1, an implementation of Sanderson's nonparametric rate smoothing program, r8s [102]. The distribution of trees is plotted using densitree [103] as implemented in the R package, phangorn [103]. Each of the one thousand trees is a resampling of the 14,000 SNPs with replacement. All of the 14,000 trees support a strong split between northern and southern groups. The one exception is LMC, a California sample that clusters with southern and northern M. guttatus in 542 and 458 of the 1,000 bootstrapped trees, respectively. C) A principle component analysis of these data, excluding M. dentilobus. D) The mean number of pairwise sequence differences per fourfold degenerate site (πS) within and among Mimulus species and populations, including uncertainty via a block bootstrap. E) Demographic history as inferred by the PSMC. Inferred population size through time is shown for pairwise combinations of haploid genomes of M. guttatus and/or M. nasutus individuals. Black/gray = interspecific comparisons with allopatric M. guttatus. Blue and violet = intraspecific M. guttatus comparisons. Red = intraspecific M. nasutus. For each pair-wise comparison, the thick dark line represents the point inference and each lighter-colored, thin line represents 1 of 100 bootstraps (see Text S1). Of our focal M. guttatus samples, CACG and DPRG are narrowly sympatric with M. nasutus populations from the northern and southern portion of both species' ranges, respectively. Our focal northern allopatric M. guttatus collection, AHQT, is well outside the geographic range of M. nasutus. By contrast, the southern allopatric collection (SLP) is geographically close to M. nasutus populations. Our focal M. nasutus collections also include sympatric and allopatric samples from the north and south (Table S1).
Speciation history
Overall patterns of genomic differentiation show deep population structure in M. guttatus, with M. nasutus diverging from a central Californian M. guttatus population approximately 200 kya.
To visualize pairwise relatedness, we constructed a rate-smoothed neighbor-joining (nj) tree (see METHODS for a discussion of the nj approach in population genetics). This tree clearly displays a deep phylogeographic split within M. guttatus, roughly corresponding to northern and southern parts of its range; however, geography is an imperfect predictor of genetic structure within M. guttatus (e.g., DUN is from a northern latitude yet clusters with our southern M. guttatus samples). The tree places all M. nasutus samples as a node within the southern M. guttatus cluster (Figure 1B). The fact that M. guttatus is paraphyletic suggests that M. nasutus budded from within a structured ancestral M. guttatus population. A principle component analysis (PCA, Figure 1C) also reveals the genetic structure within M. guttatus – PC2 differentiates northern and southern M. guttatus groups. Consistent with the single origin of M. nasutus, PC1 separates M. guttatus from the strongly clustered M. nasutus, presumably as a consequence of a shared history of genetic drift among these M. nasutus samples. Down-sampling to any one M. nasutus sample controls for this shared drift, and places M. nasutus within southern M. guttatus (Figure S1).
To support these qualitative inferences we generated a quantitative description of genetic structure within M. guttatus, focusing on our high-coverage (focal) samples. Pairwise sequence diversity at synonymous sites within northern (πS AHQT×CACG = 3.97% [3.89%–4.06%]) and southern (πS DPR×SLP = 4.45% [4.39%–4.52%]) M. guttatus samples is significantly lower than that within M. guttatus overall (πS = 4.91% [4.85%–4.96%]), and between the north and south (πS = 5.26% [5.20%–5.30%], Figure 1D). Diversity within the northern and southern clades is consistent with a very large effective population size (Ne) of approximately one and a half million chromosomes for both groups (assuming the per generation per base mutation rate, μ = 1.5×10−8 [following Koch et al. 2001]). As a simple estimate of the population split time (τ generations), ignoring possible introgression, we assume that the divergence between populations is the sum of pairwise diversity (π) within an ancestral population and the product of the per-generation mutation rate, μ, and two times the split time [34]. Using this relationship, and representing ancestral diversity by the southern M. guttatus samples, we set τ = (πS NorthGut×SouthGut−πS SouthGut)/2μ and estimate a split between northern and southern Mimulus populations more than a quarter of a million years ago (265 ky [251 ky–280 ky], assuming an annual life history). As above, this estimate assumes μ = 1.5×10−8/bp/generation but can be linearly rescaled by alternative estimates of μ. For example, readers can multiply divergence time estimates by a factor of two if they prefer the estimate of μ = 7×10−9/bp/generation [35].
Interspecific divergence between M. guttatus and M. nasutus (dS = 4.94% [4.88%–5.00%]) is comparable to overall M. guttatus diversity, and exceeds diversity within northern or southern M. guttatus collections (Figure 1D). We derive a simple estimate of split time between M. guttatus and M. nasutus as we did above to estimate the split between focal northern and southern M. guttatus samples. Using the difference between divergence of M. nasutus from the southern, allopatric M. guttatus sample (to minimize the influence of recent introgression and historical divergence between M. guttatus’ genetic clusters) and a proxy for diversity in an ancestral population (southern M. guttatus), we estimate that M. nasutus and M. guttatus split approximately 200 ky ago, τ = (πS Nas×AlloSouthGut−πS SouthGut)/2μ = 0.5875%/2μ = 196 ky [181 ky–212 ky].
As a complementary inference of historical patterns of divergence within M. guttatus and between species, we applied Li and Durbin's implementation of the pairwise sequentially Markovian coalescent (PSMC) [36] to pairwise combinations of focal haploid genomes (Figures 1E and S2, S3, S4, S5, S6). The PSMC analysis infers large population sizes within both northern (CACG×AHQT) and southern (SLP×DPRG) samples, with an apparent bout of strong recent population growth. However, as we have sampled from a structured population the inferred larger recent population sizes likely represent reduced coalescent rates caused by population structure, rather than dramatic recent increases in Ne. Likewise we infer a very low rate of coalescence (a very large effective population size) in the recent past between northern and southern M. guttatus (SLP×AHQT) compared to within these groups (SLP×DPRG and CACG×AHQT, Figures 1E and S2) likely reflecting the strong genetic structure within range-wide M. guttatus.
We also use this PSMC analysis for an additional estimate of the approximate split time, by assessing when the inferred coalescent rate between species decreases (i.e., the population size estimate increases) relative to the rate within M. guttatus [see 36]. In doing so, we focus on the southern M. guttatus samples that fall closest to M. nasutus in our nj tree. The inferred coalescent rate between M. nasutus and southern M. guttatus (SLP×KOOT, gray line) decreases relative to the rate within southern M. guttatus (SLP×DPRG, dark blue/navy line), i.e., the lines diverge, from ∼500 to ∼300 kya, suggesting either a gradual split between species over that time span, or a hard split sometime within that range (Figures 1E and S3). This result, which represents an upper bound on time since speciation, is qualitatively similar to our lower estimate based on synonymous nucleotide variation among these samples. We note that for both analyses, historical introgression of M. nasutus into SLP would make this split seem more recent than it actually was.
Genomic consequences of the transition to selfing
Patterns of genomic variation within M. nasutus reflect the genomic consequences of a recent transition to selfing. Synonymous diversity within M. nasutus (πS = 1.09% [1.03%–1.14%], Figure 1D) is one fifth that observed within M. guttatus, consistent with a high rate of genetic drift since M. nasutus’ origin. Moreover, most ancestral variation in M. nasutus has been homogenized: of the fixed differences between M. nasutus and M. guttatus, 90% are derived in M. nasutus and 10% are derived in M. guttatus (when polarizing by M. dentilobus). Although M. nasutus has lost much of its ancestral variation, shared variants still constitute a much higher proportion of its polymorphism (50%) relative to an equally sized sample of M. guttatus (10%). This pattern reflects both the paraphyly of M. guttatus and the incomplete sorting of variation present in M. nasutus’ founders.
Consistent with this reduction in nucleotide diversity and incomplete sorting of ancestral variation, PSMC analyses infer a dramatic decline in M. nasutus’ effective population size after it split from M. guttatus (compare red and black-gray lines in Figure 1E, see also Figure S4), suggesting that the evolution of selfing roughly coincided with M. nasutus’ split from M. guttatus. We caution, however that interpretation of PSMC's estimated population size in M. nasutus is not straightforward. This is because the transition to selfing reduces the population recombination rate more than the population mutation rate [14]; however, Li and Durbin's [36] implementation of the PSMC assumes that both these values change proportionally with the historical effective population size.
Relative to expectations under selective neutrality and demographic equilibrium, M. nasutus contains an excess of high-frequency derived synonymous alleles (Figure S7). We interpret this observation as a reflection of a recent population contraction. This interpretation is in agreement with the decreased synonymous diversity in M. nasutus relative to M. guttatus and our PSMC-based inference of a reduction in Ne. However, population structure within M. nasutus may also contribute to this excess of high frequency derived alleles [37], [38]. By contrast, in M. guttatus we observe slightly more rare synonymous alleles than expected under a neutral equilibrium model, reflecting recent growth, population structure, and/or weak selection against unpreferred codons.
The distribution of synonymous diversity across the genome (overlapping 5 kb windows with a 1 kb slide, Figure 2A) bolsters the view that M. nasutus’ genomic diversity is a mixture of closely related genomic regions that rapidly coalesce in the small M. nasutus population, and distantly related regions that do not coalesce until joining a large M. guttatus-like ancestral population. In pairwise comparisons of sequence diversity within M. nasutus, half of the genomic windows are differentiated by πS<0.5% (corresponding to ∼170 thousand years of divergence), reflecting recent common ancestry since the species split. On the other hand, one third of such windows are differentiated by πS>2.0%, reflecting deep ancestry in a large ancestral population (Figure 2A, see Figure S8 for different window sizes).
Fig. 2. Genomic consequences of the transition to selfing. 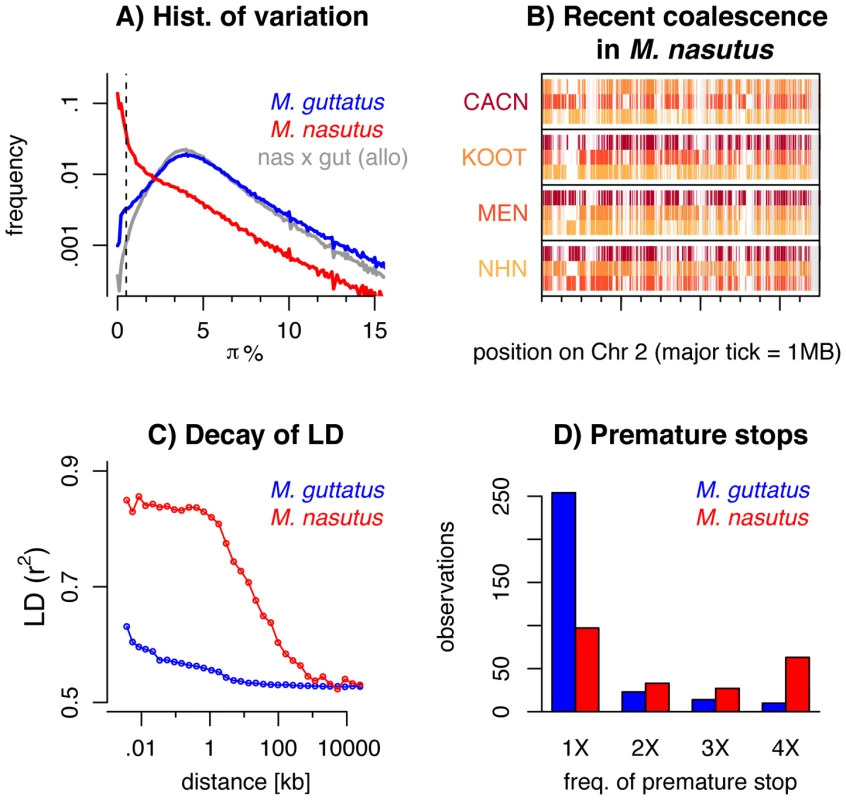
A) A histogram of pairwise sequence diversity (π) within and between species in overlapping 5 kb windows. For interspecific comparisons we focus only on allopatric M. guttatus populations. The dotted line denotes πS<0.5% or 170 ky of divergence. B) Moving along a part of chromosome two, for all M. nasutus samples, we color genomic regions in which the focal individual (y-label) and another M. nasutus sample, indicated by color, recently coalesce (πS≤0.5%). White regions coalesce more distantly in the past (πS>0.5%) and gray regions indicate insufficient density of informative sites. Major tick-marks on the x-axis indicate 1 megabase. C) Linkage disequilibrium (measured as r2) within M. nasutus (red) and M. guttatus (blue), as a function of physical distance. D) The number of premature stop codons observed in one, two, three, or four M. nasutus (red) and M. guttatus (blue) samples. These findings contrast sharply with comparisons within M. guttatus, as well as between M. nasutus and allopatric M. guttatus samples, for which recent common ancestry since the species split is rare (πS<0.5% for less than 1.5% of 5 kb windows) and deep coalescence is the norm (mode πS = 4%, Figure 2A, a result roughly consistent across window sizes, Figure S8). Under the neutral coalescent, a pair of lineages will fail to find a common ancestor with each other by generation t with probability e−t/Ne*, where Ne* is the (constant) effective number of chromosomes. Therefore, the observation that half of our M. nasutus windows share a common ancestor in the past ∼170 ky, by an admittedly crude calculation, predicts a population size between 150k and 250k effective chromosomes (compared to the estimated Ne* of 1.5 million in M. guttatus from synonymous diversity, above). This ten-fold reduction in effective population size as compared to M. guttatus far exceeds both the two-fold decrease in Ne expected to accompany the evolution of selfing and the four-fold decrease calculated by the difference in intraspecific variation.
Across the genome, the mosaic nature of ancestry within M. nasutus is apparent as long contiguous regions of recent common ancestry (colored windows in Figures 2B and S9) interrupted by regions of deep ancestry, due to incomplete lineage sorting and/or historical introgression (white windows in Figure 2B and S9). This block-like ancestry structure results in extensive linkage disequilibrium (LD) in M. nasutus. In contrast to M. guttatus, for which the sample pairwise LD drops halfway towards its minimum values within only 15–20 base-pairs, LD in M. nasutus decays much more slowly, not dropping halfway towards its minimum values until 22 kb (Figure 2C). This represents a thousand-fold difference in the decay of LD, as compared to a more modest ten-fold reduction in the effective population size between M. nasutus and M. guttatus. This dramatic difference in the scale of LD between Mimulus species is likely due to a major reduction in the effective recombination rate within the selfing M. nasutus. We use this difference to derive a simple estimate of M. nasutus’ selfing rate using Eq. 1 of Nordborg [14]. Nordborg showed that the ratio of the population-scaled recombination to mutation rate in selfers is reduced by a factor of 1-F compared to the same population if it was outcrossing, where F is the inbreeding coefficient. Substituting in the hundred fold difference in the ratios of effective population size and decay of LD between M. nasutus and M. guttatus, we arrive at 1−F = 0.01. Assuming a constant selfing rate s, F = s/(2−s), M. nasutus’ selfing rate is approximately 99%.
Patterns of sequence variation suggest a reduced efficacy of purifying selection in M. nasutus, a result consistent with extensive genetic drift and/or linked selection within M. nasutus. All M. nasutus samples contain more premature stop codons than any M. guttatus sample (M. nasutus: mean 124.0, range = 121–126, M. guttatus: mean 95.5, range = 86–102), and a large proportion of these premature stops are at high frequency in M. nasutus (Figure 2D). For 27 of the 29 fixed differences for a premature stop codon, M. nasutus carries the premature stop and M. guttatus carries the intact allele. We acknowledge that errors in annotation could underlie some of the excess of premature stop codons inferred in M. nasutus. However, M. nasutus and the focal southern M. guttatus samples are equally diverged from the reference and yet our southern M. guttatus samples do not show this excess. As such, annotation error is likely not a strong contributor to the large and consistent interspecific differences in premature stop codons observed.
Additionally, after standardizing by synonymous variation, we observe an excess of putatively deleterious, non-synonymous variation in M. nasutus relative to M. guttatus (πN/πS = 0.197 [0.192–0.203] and 0.157 [0.155–0.160], respectively). However, this difference is not yet reflected in divergence between the species (dN/dS = 0.156 [0.154–0.159]), presumably because interspecific sequence differences largely reflect variation that predates the origin of selfing in M. nasutus rather than the relatively few mutations accrued within the past ∼170 ky. This pattern of elevatedπN/πS in selfing species but only modest dN/dS between selfers and their close relatives is common [15], even in genome-wide analyses (e.g., [20]). We note that elevated πN/πS in selfing species may reflect the faster rate at which nonsynonymous diversity approaches equilibrium after a reduction in diversity compared to synonymous mutations [39], [40], rather than a consequence of a reduced efficacy of purifying selection. However, this interpretation cannot explain the absolute excess of premature stop codons in M. nasutus.
Ongoing gene flow and its consequences
Inferring the history of introgression from M. nasutus into M. guttatus
A surprising result from our analyses of genomic divergence is that although the sympatric northern M. guttatus sample (CACG) is far from M. nasutus in both PCA space and position in the neighbor joining tree (Figures 1B and 1C, respectively), CACG is least diverged from M. nasutus according to the expected number of synonymous pairwise differences (see Table S2A, a result that holds across alignment pipelines, Table S2B). To test whether these seemingly contradictory observations are due to introgression, we used Treemix to construct a population tree featuring admixture events [41]. Treemix finds a clear signal of admixture from M. nasutus into the population ancestral to CACG (Figure 3A). This result holds across a range of different sample subsets (Figure S10). Some Treemix analyses also suggest introgression from M. nasutus into the population ancestral to our southern, sympatric M. guttatus (DPRG); however, the manifestation of this second signal varies across sample subsets (Figure S10, see Text S1). A D-test of introgression based on 4 populations [42] provides additional evidence for historical introgression of M. nasutus ancestry into both sympatric M. guttatus samples. Specifically we find that D(CACG, AHQT; M. nasutus, SLP) and D(DPRH, SLP; M. nasutus, AHQT) are both significantly greater than zero (D estimates equal 0.408 and 0.068, and standard errors of 0.074 and 0.013, respectively, see METHODS), further supporting M. nasutus introgression into CACG and DPRG.
Fig. 3. Introgression of M. nasutus material into M. guttatus. 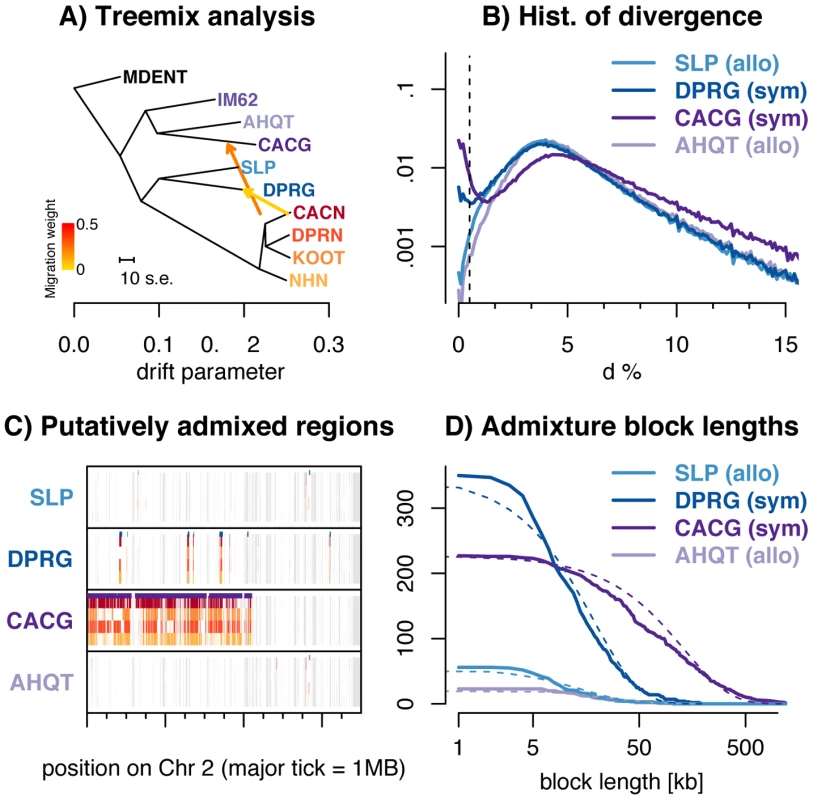
A) Treemix suggests introgression from M. nasutus in to sympatric M. guttatus samples. B) A histogram of interspecific pairwise sequence divergence in 5 kb windows for each M. guttatus sample. C) Introgression across a chromosome - Moving along a part of chromosome two for all M. guttatus samples, we color genomic regions in which the focal individual (y-label) and a M. nasutus sample, indicated by color, recently coalesce (πS≤0.5%). White regions coalesce more distantly in the past (πS>0.5%) and gray regions indicate insufficient density of informative sites. Major tick marks on the x-axis indicate 1 megabase. Purple bars above each focal individual denote greater than a 95% posterior probability of M. nasutus ancestry as inferred from our HMM. D) Admixture block length distribution - The number of admixed blocks, as inferred by a greater than 95% posterior probability of M. nasutus ancestry from our HMM, longer than x. The expected exponential distribution is marked with a dotted line. The bimodal distribution of divergence between M. nasutus and CACG in overlapping 5 kb genomic windows (dark purple lines in Figures 3B, see other window sizes in S11) provides further evidence for introgression. For most windows, CACG shows deep divergence with M. nasutus (half of windows have more than 5% sequence divergence), but approximately one tenth of windows show nearly no divergence (π<0.5%) and were likely recently introduced by introgression. We find a similar, but more subtle, bimodal distribution of divergence between the sympatric, southern M. guttatus sample, DPRG, and M. nasutus (2.5% of 5 kb windows in this comparison exhibit less than 0.5% sequence divergence – dark blue lines in Figure 3B). This signal is apparent across a range of window sizes, with increasing window size dampening this effect as short admixture blocks are averaged into the genomic background (see Figure S11). We do not observe this binomial distribution of divergence times between M. nasutus and allopatric M. guttatus – less than 0.5% of genomic windows in SLP and AHQT are less than 0.5% diverged from M. nasutus (Figures 2A, 3B, S8, and S11). The PSMC analysis also reflects ongoing introgression in sympatry, as it highlights recent common ancestry between sympatric M. guttatus samples (CACG and DPRG) and M. nasutus (Figures S5 and S6). Moreover, genomic regions of low interspecific divergence are spatially clustered (Figures 3C and S9), consistent with recent and ongoing introgression that is slowly broken down over generations of recombination. We point readers towards the recent work of Wilkinson-Herbots [43] for parameter inference from distributions like those in Figures 2B and 3B.
We utilize the spatial distribution of windows of low interspecific divergence to probabilistically infer regions of recent M. nasutus or M. guttatus ancestry across the genomes of focal M. guttatus samples (Figures 3C and S9) using a Hidden Markov Model (‘HMM’ see METHODS). We estimate M. nasutus ancestry proportions of 15.1% and 5.7% in our sympatric northern (CACG) and southern (DPRG) M. guttatus samples, respectively, using output from the HMM. We find much lower proportions of M. nasutus ancestry in the allopatric M. guttatus genomes samples, 1.1% in the south (SLP) and 0.6% in the north (AHQT). This low level of admixture inferred in allopatric samples may reflect low levels of gene flow into allopatric M. guttatus and/or misassigned regions of incomplete lineage sorting.
To learn about the timing of admixture, we find contiguous regions of individual M. guttatus genomes with a greater than 95% posterior probability of M. nasutus ancestry and display the length distribution of these blocks in Figure 3D. The block length distribution can be used to disentangle complex histories of recent introgression [44], [45]. The mean admixture block lengths are 132 kb (∼0.74 cM, n = 227 blocks) and 18.6 kb (∼0.10 cM, n = 350 blocks) for northern (CACG) and southern (DPRG) sympatric M. guttatus samples, respectively. Because our HMM occasionally breaks up what seem to be contiguous blocks of admixed ancestry, we instituted strategies to fuse admixture blocks, none of which greatly influenced our block length distributions (see Text S1 and Figure S12). Under a single temporal pulse of gene flow, admixture block lengths are exponentially distributed with a mean length (in Morgans) that is the reciprocal of the number of generations since admixture. With this model, we estimate 135 and 962 generations since admixture for CACG and DPRG samples, respectively (Table S4). Because these estimates are much more recent than the split time of ∼200 kya, this pattern cannot be explained by incomplete lineage sorting.
Although this pulse model provides an intuitive summary of time back until an admixed region in M. guttatus coalesces with M. nasutus, our data are inconsistent with a model of a single admixture pulse. In comparison to the exponential decay expected from a single admixture pulse, there are fewer blocks of M. nasutus ancestry of intermediate length in admixed M. guttatus genomes (Figure 3D and Table S4). Thus, these estimates should be viewed as average times back to an admixture event, rather than an estimated date of one admixture event. Furthermore, many of these blocks come from far enough back in the past that they are likely derived from a very large number of historical introgression events (Table S4, see also Methods and Text S1 for quantitative support for these points).
Detecting introgression from M. guttatus into the selfer M. nasutus
We also detected a signal of introgression from M. guttatus into M. nasutus. Identifying this signal is difficult, compared to identifying introgression into M. guttatus, due to the short scale of LD in M. guttatus and the similarity of interspecific divergence and M. guttatus diversity. Both the incomplete sorting of ancestral variation and recent introgression from M. guttatus can cause one M. nasutus sample to differ from all others in some genomic regions. However, introgressed regions differ from incompletely sorted variation, in that admixed regions should (1) persist for a long physical distance and (2) more closely resemble the putative source of admixture – a geographically close M. guttatus – than other M. nasutus samples. To test whether introgression from M. guttatus contributes to variation in M. nasutus, we focus on long genomic regions (>20 kb) where one “outlier” M. nasutus sample differs from all other M. nasutus samples (as inferred by pairwise πS>1%, alternative thresholds explored in Text S1). We then ask whether these outlier sequences are less diverged from allopatric but geographically close M. guttatus samples than the non-outliers. For example, are “outlier” genomic regions in a northern M. nasutus sample less diverged from allopatric northern M. guttatus than are the three non-outlier M. nasutus samples in these regions? By excluding sympatric M. guttatus samples, we avoid conflating introgression into M. nasutus with the extensive signature of introgression from M. nasutus into M. guttatus.
Pooling across northern M. nasutus samples (NHN, KOOT, CACN), outlier windows are more often genetically closer to the northern M. guttatus sample (AHQT) than are the non-outlier windows (272 of 490, one sided binomial test against the null expectation of 50% P = 0.008. Table S5 and Text S1 show that this result is robust to designation of outlier windows). However, this result is individually significant only for our most geographically remote M. nasutus sample, NHN (141 of NHN's 249 outlier windows are closer to northern M. guttatus than are the non-outlier M. nasutus). Additionally, the southern M. nasutus sample, DPRN, contained the smallest proportion of outlier windows resembling northern M. guttatus. By contrast, no samples have a disproportionate share of outliers genetically close to southern M. guttatus, SLP (Table S5), consistent with little or no introgression into DPRN (note, however, that this comparison is likely underpowered because of the genetic proximity of southern M. guttatus to the population that founded M. nasutus).
Divergence between M. nasutus and sympatric M. guttatus samples increases with decreasing local recombination rate
We observed a genome-wide negative relationship between absolute synonymous divergence from M. nasutus (i.e., the mean number of pairwise sequence differences at synonymous sites) and the local recombination rate in both sympatric M. guttatus samples. (DPRG×M. nasutus, Spearman's ρ = −0.080, P = 0.0008; CACG×M. nasutus, Spearman's ρ = −0.0718, P = 0.0027). These sympatric M. guttatus samples display approximately a 5% increase in synonymous nucleotide divergence to M. nasutus in genomic regions with below average recombination rates. This signal is substantially weaker in the allopatric southern M. guttatus sample (SLP×M. nasutus: Spearman's ρ = −0.0521, P = 0.0297), which is nested within the range of M. nasutus, and seems to be absent in comparisons with the allopatric northern M. guttatus sample, which occurs well outside of M. nasutus’ range (AHQT×Nas: Spearman's ρ = −0.0261, P = 0.2768). This result holds after accounting for the potential confounding effects of sequencing depth and divergence from the M. dentilobus, a proxy for mutation rate variation (see Methods, Text S1 and Table S6).
This negative relationship between divergence and the recombination rate in sympatry is consistent with selection against M. nasutus ancestry reducing effective gene flow at linked sites (see [46], for related theory and tentative evidence to date), and inconsistent with alternative scenarios. Specifically, neutral processes cannot generate this correlation because mean neutral coalescent times are independent of recombination rates [47]. Additionally, although selective sweeps and background selection can influence relative measures of divergence, such as FST [46], [48], [49], these models of linked selection do not influence the expected substitution rates at neutral sites [50].
We observe no relationship between diversity and the recombination rate within M. guttatus (Spearman's ρ = −0.0275, P = 0.244) or M. nasutus (Spearman's ρ = 0.0291, P = 0.218). Therefore, it seems unlikely that linked selection processes within species (background selection and selective sweeps) could explain this result. Note that this lack of signal within populations is interesting in its own right, as it suggests that linked selection may not strongly influence species-wide diversity in M. guttatus. Furthermore a lack of a correlation within M. nasutus suggests that we do not have any clear evidence of linked selection being the cause of the rapid loss of diversity in M. nasutus.
Discussion
Speciation history and the origin of M. nasutus
Genetically, M. nasutus clusters with central Californian M. guttatus samples, suggesting that speciation post-dated the differentiation of some M. guttatus populations. Thus, speciation in this pair is best described as a ‘budding’ of M. nasutus from M. guttatus, rather than a split of an ancestral species into two. We observe an approximate coincidence between the timing of divergence and the decline in population size in M. nasutus (as inferred from our PSMC analysis). This observation could be a result of the transition to selfing being linked to speciation (see [11] for phylogenetic evidence of this link in the Solanaceae and [51], [52] for a likely case in Capsella). However, given the misspecification of the PSMC model for the transition to selfing (see above), this observation should be treated with caution. More work is needed to develop methods to test whether split times and changes in selfing rate occur concurrently to see if this is indeed a general pattern.
Future genomic analyses across the M. guttatus complex and other species groups will facilitate an in-depth view of the causes and consequences of speciation by the budding of selfing and/or endemic populations from widespread parental species. We note that recent phylogenetic analyses of species' ranges suggest that this mode of speciation is common in Mimulus [53] and other flowering plants [54].
We estimate that M. nasutus split from a M. guttatus population within the last two hundred to five hundred thousand years (with our estimate of ∼200 ky, inferred from differences in synonymous sequence differences within and between species, and the estimate of ∼500 ky corresponding to conservative estimates of population splits from the PSMC). This lies between the ∼50 ky separating selfing Capsella rubella from outcrossing C. grandiflora [20], [55] and Arabidopsis thaliana which has potentially been selfing for over a million years ([56], having split from A. lyrata ∼3–9 Mya [57]). Although 200 ky represents a relatively short time evolutionarily, it implies that M. nasutus managed to survive numerous dramatic bioclimatic fluctuations.
The transition to selfing and its genomic consequences
The transition from outcrossing to self-fertilization in M. nasutus has had clear consequences on patterns of genomic variation. In M. nasutus, linkage disequilibrium exceeds that in M. guttatus by three orders of magnitude. This result suggests a high selfing rate in M. nasutus (estimated above at 99%), consistent with direct estimates from field studies [24]. We observe a four-fold drop in diversity and infer a ten-fold reduction in the recent effective population size in M. nasutus compared to M. guttatus, values far exceeding the two-fold decrease in Ne expected as a direct consequence of selfing [58], [59]. This more than two-fold reduction in Ne of selfing populations relative to their outcrossing relatives has been identified in other plant [17], [55] and animal [60]–[62] species pairs, and may be partially due to extreme founding bottlenecks, frequent colonization events and/or demographic stochasticity that further increase the rate of genetic drift [13], as well as a heightened influence of linked selection in selfing taxa [60], [63]–[65].
Selfing populations are expected to experience a reduced efficacy of purifying selection accompanying the drop in effective population size and recombination rates [15], [65], [66]. Consistent with these predictions, M. nasutus has accumulated numerous putatively deleterious mutations, including nonsynonymous variants and premature stop codons. Presumably, this elevation in radical genetic variants reflects a reduction in the efficacy of purifying selection due to a high rate of genetic drift and linked selection, as well as perhaps the escape of some genes (e.g., loci involved in pollinator attraction) from the selective constraints they faced in an outcrossing population (e.g., [17]).
Selfing as a reproductive barrier and its significance for ongoing gene flow
Despite multiple reproductive isolating barriers, including mating system differences, we find ongoing, bidirectional introgression between M. guttatus and M. nasutus.
Evidence of ongoing introgression from the selfer, M. nasutus, into the outcrosser, M. guttatus, is particularly stark. There are numerous evolutionary implications of introgression from selfers to outcrossers. Introgression of deleterious mutations accumulated in selfers may introduce a genetic load to outcrossers. This burden would result in selection against genetic material from selfers in hybridizing outcrossing populations, and could ultimately favor reinforcement of reproductive isolation. Alternatively, such introgression could provide a multi-locus suite of variation facilitating self-fertilization, and other correlated traits (e.g., drought resistance and rapid development), in favorable environments, as appears to be the case in introgression between wild and domestic beets (Beta vulgaris, [67]).
Evidence of introgression from M. guttatus into M. nasutus is subtler, but is potentially critically important. Even relatively low levels of introgression into a selfer may rescue the population from a build up of deleterious alleles, and reintroduce adaptive variation, and so may lower its chances of extinction, a fate considered likely for most selfing lineages [68], [69]. However, before potentially rescuing a selfing population from extinction, genomic regions introduced from outcrossing species must themselves survive a purging of deleterious recessive alleles.
Higher rates of introgression from M. nasutus to M. guttatus would be consistent with the prediction that backcrosses should be asymmetric – because bees preferentially visit plants with larger flowers [70], [71] and/or larger floral displays [72], [73], both features of M. guttatus, visits to M. nasutus and F1 hybrids are likely preceded and followed by visits to M. guttatus [24], [30]. Consistent with this prediction, direct estimates of hybridization in the DPR sympatric population reveal that F1 hybrids are the product of M. nasutus maternal and M. guttatus paternal parents, respectively [24]. However, we caution that it is considerably more challenging to identify introgression into M. nasutus than into M. guttatus, as the similarity between interspecific divergence and diversity in M. guttatus makes historical admixture difficult to separate from the incomplete sorting of M. nasutus’ ancestral variation. We further note that, although asymmetrical introgression from selfers to outcrossers has been detected in other systems (Pitcairnia [74], and potentially in Geum [75], [76]), the relative contribution of selfing vs. other isolating barriers and/or selection is unclear. Dense sampling of sympatric and allopatric populations of outcrossing species experiencing ongoing gene flow with selfing relatives will allow for tests of these hypotheses. Importantly, the number, location and length-distribution of admixture blocks identified from genomic analyses provide information about the longer-term consequences and pace of introgression between selfers and outcrossers.
Selection against hybrids and implications for species maintenance
The numerous short blocks (in addition to long blocks) of M. nasutus ancestry observed in M. guttatus suggest that M. nasutus ancestry can potentially persist in an M. guttatus background for many generations. Despite this, M. guttatus and M. nasutus are still ecologically and genetically distinct.
We identified a genome-wide signature of selection against introgression of M. nasutus ancestry in M. guttatus, in the form of a negative relationship between the local recombination rate and absolute divergence. This relationship was highly significant in both sympatric comparisons, but only weakly significant in parapatry, and insignificant in allopatry. Additionally, we did not find a relationship between recombination and diversity within either species. Moreover, unlike a negative relationship between the recombination rate and relative measures of differentiation, such as FST or the number of fixed differences [e.g.], [ 77], [78,79], this finding cannot also be explained by a high rate of hitchhiking or background selection within populations since the species split [46], [48], [49]. Instead, it seems more consistent with M. nasutus ancestry being selected against more strongly in regions of low recombination due to linkage with maladaptive alleles that introgression would introduce. This suggests that the genome has potentially congealed as a barrier to gene flow in low recombination regions. We note that this ‘congealing’ (sensu Barton [80], [81]) requires a threshold density of locally adaptive mutations, measured in recombination distance, and does not require a complex model of multi-locus coadaptation. Previous reports of absolute divergence near the breakpoints of inversions (e.g., [82], [83]), or in centromeres relative to telomeres [84], suggested this result; however, genome-wide evidence for this basic prediction is scarce.
Further work, including experiments measuring selection on genetic variants in the wild, and larger sample sizes from both allopatric and sympatric populations, is needed to pinpoint which (if any) genomic regions are particularly strongly selected against in hybrids. Genetically mapped loci for adaptive interspecific differences [85] and hybrid inviability and sterility [29] are promising candidates. Indeed, recent analyses of the distribution of Neanderthal haplotype blocks in ∼1000 human genomes has identified genomic candidates for adaptive introgression from Neanderthals to humans and an apparent paucity of introgression at loci putatively influencing male fertility [86].
Summary and future prospects
Our analyses of whole genomes from the M. nasutus - M. guttatus species pair provide a broad view of both the historical divergence in this group and the ongoing processes by which they remain distinct. Less than a half million years ago, a semi-isolated M. guttatus population evolved self-pollination and ultimately transformed into modern day M. nasutus. In the intervening time, this population experienced a contraction in effective population size, and accumulated deleterious mutations while spreading geographically across western North America. More broadly, our work demonstrates that much can be learned about population history from resequencing relatively few samples in a group with an annotated genome and an integrated physical-genetic map.
Despite numerous reproductive isolating barriers [24], [25], [27]–[29], sympatric populations of M. guttatus and M. nasutus are still exchanging genes. The low diversity and extensive linkage disequilibrium in M. nasutus facilitates straightforward identification of M. nasutus-like ancestry in M. guttatus, and we use the length-distribution of these blocks to parameterize the recent history of introgression. The many short M. nasutus ancestry blocks suggest that its ancestry can persist in M. guttatus beyond early generation hybrids, and the length distribution of this ancestry is consistent with more than one pulse of introgression. The genomic distribution of introgression is non-random – in sympatry, absolute interspecific divergence is greater in regions of reduced recombination, suggesting selection against long blocks of M. nasutus ancestry in M. guttatus. Additional sequencing of individuals in sympatry will help better parameterize the dynamics and extent of introgression from M. guttatus to M. nasutus and clarify the action of selection for or against admixed ancestry across the genome.
Materials and Methods
Mimulus sampling and whole genome sequencing
We utilized a combination of existing [downloaded from the NCBI SRA, sequenced by 87] and newly generated whole genome sequence data from 19 different lab and/or naturally inbred Mimulus accessions, including 13 M. guttatus, 5 M. nasutus, and 1 M. dentilobus individual as an outgroup (Table S1). Samples varied in their geography and life history. Mean sequencing depths range from 2× to 25×, and read lengths include 36, 76, and 100 base pair paired end reads. We present SRA accession numbers as well as depth, read length and additional sample information in Table S1, and note that we obtained the DPRG sequence data directly from the U.S. Department of Energy Joint Genome Institute. Our analysis included newly generated whole genome sequences from five lines (CACG, CACN, DPRN, NHN, and KOOT), and we present details of sequence generation in Text S1.
Genome sequence alignment, SNP identification and annotation
We aligned paired end reads to the M. guttatus v2.0 reference genome [87] using Burrows-Wheeler Aligner (bwa [32]) with a minimum alignment quality threshold of Q29 (filtering done using SAMtools [88]). Alignment-processing details can be found in Text S1. We produced a high quality set of invariant sites and SNPs simultaneously for all lines using the GATK Unified Genotyper, with a site quality threshold of Q40 [89], [90]. For all analyses described below, we exclusively used genotype calls from reference scaffolds 1–14, corresponding to the 14 chromosomes in the Mimulus genome. For all analyses (except PSMC, which requires a consistently high density of data, see below), we set also set a strict minimum depth cutoff of 10 reads per site. To assign genotypes at heterozygous sites, we randomly selected one of two alternate alleles. Such heterozygous sites are not concentrated in long genomic regions and account for approximately 1% and 2% of synonymous SNPs in average focal M. nasutus and M. guttatus samples, respectively. This translates to individual synonymous heterozygosity of approximately 0.2% and 0.5% in M. nasutus and M. guttatus, respectively, even before additional filtering to remove misaligned sites (see below). Because sequence diversity is relatively high in our sample, we also analyzed patterns of pairwise sequence diversity using reads aligned with Stampy [33] (expected divergence set to 5%) and an otherwise identical pipeline to that described above. We describe the number of reads mapped with bwa and Stampy for our focal, high coverage lines in Table S7.
To minimize misclassifying mismapped paralogs as SNPs, we then removed triallelic sites and censured genotypes at sites where individual depth was two standard deviations away from mean depth. After these filtering steps, we classified remaining genic loci as zero, two, three, or fourfold degenerate using the Mimulus guttatus v2.0 gene annotations provided by phytozome [87]. Compared to bwa, the Stampy pipeline generated quantitatively larger estimates of sequence diversity, but qualitatively similar results (i.e., rank order of pairwise πS, Table S2B). Because Stampy doubled individual heterozygosity at synonymous sites, and increased πN/πS, we believe that it may have mismapped a greater proportion of our reads. Therefore, although Stampy aligned a greater number of reads to the reference genome than bwa (Table S7), we conservatively focus on our bwa alignments for our major analyses. As noted above, none of our qualitative conclusions depend on the read alignment pipeline used.
Data analysis
In addition to descriptions of our analyses, below and in Text S1, we recreate many analyses, including our PCA, and HMM in a file submitted to Dryad. These analyses can be run on the processed genotypic data for all samples at SNPs used in nj and PCA analyses as well as comparisons between focal samples in 1 kb windows across the genome, all available from doi:10.5061/dryad.vp645.
NJ tree and PCA
We first analyzed broad patterns of genomic differentiation captured by principle component analysis and a neighbor-joining (nj) tree for all M. guttatus and M. nasutus samples. For both these analyses (and the Treemix analyses) we downsampled to 1,000 synonymous SNPs per chromosome with more than one copy of the minor allele (14,000 informative sites in total). In this downsampling we insisted that no focal samples have missing genotypic data and then prioritized by the number of individuals with sequence data (see Text S1). After this downsampling, all individuals had genotypic data at more than 60% of SNPS, and 97% of these SNPs had genotypic data for at least 16 of our 19 individuals.
We construct the nj tree with the nj function in the R [91] package ape [92], and root it by the outgroup, M. dentilobus. An nj tree is an empirical description of a distance matrix, rather than a phylogenetic model. As such, they have a long history of use in population genetics [93]–[96], where complex population structure and migration history yields a complex distribution of genealogies across the genome. Indeed, in cases of recent speciation or ongoing gene flow, we expect the specific assumption of a phylogenetic model - that the evolutionary relationships can be described by a strictly bifurcating directed acyclic graph - to be explicitly violated. Perhaps due to the widespread application of nj trees in population genetics, the behavior of admixed populations in nj trees is known (see [97]), and readers can evaluate the nj tree with this knowledge.
We construct our PCA with customized R scripts accounting for missing genotype data described in Text S1. Because all five M. nasutus samples are very closely related, they have a large effect on PCA space, as is clear from the observation that PC1 cleanly separates M. nasutus and M. guttatus. Downsampling to any one M. nasutus sample removes this distortion and places M. nasutus firmly within southern M. guttatus samples (Figure S1).
Nucleotide diversity and divergence
Preliminary analyses of nucleotide variation showed a strong influence of sequence features such as read depth and length (Table S3) on diversity estimates. In general, samples with low depth and short reads are less different from all other samples than are samples with high depth and long reads (Tables S3), a likely consequence of difficulties in aligning short reads when they differ substantially from the reference. We therefore focus all following analyses on eight samples with high and consistent mean read depths (13×–24×) and read lengths (all 100 bp paired end reads).
Divergence and diversity. We quantified patterns of synonymous and nonsynonymous sequence variation at four-fold and zero-fold degenerate sites, respectively. For each pairwise comparison, we count the number of pairwise sequence differences and number of sites for which both samples have data above our quality and depth thresholds. To generate confidence intervals for our point estimates of diversity and divergence that acknowledge the non-independence of sequence variants due to linkage disequilibrium, we resample 100 kb windows with replacement. We also compared pairwise synonymous differences before and after removing putatively introgressed regions (see Text S1 and Table S8 for more details). Notably, after removing regions of putative introgression, the two southern M. nasutus samples were equally diverged from M. nasutus, and were both genetically closer to M. nasutus than the introgression-censured northern M. guttatus samples.
Allele Frequency Spectrum (AFS). To polarize the AFS, we examined all sites passing our depth and quality thresholds for M. dentilobus as well as all focal samples. We labeled the M. dentilobus allele as ancestral, and the alternate allele as derived.
Premature stop codon identification. We searched for premature stop codons in each Mimulus accession using the Mimulus guttatus v2.0 gene annotations. We defined a mutant stop codon to be premature only if all three nucleotide sites were available for the codon, if it occurred in a gene for which at least 25% of the codons were available in the sample, and if the codon did not occur in the last 5% of all codons on the 3′ end of the gene.
Correlations between recombination rate and diversity (or divergence). We estimated genetic distances in centiMorgans (cM) using information from three existing Mimulus genetic linkage maps: one intra-population M. guttatus composite map, IMxIM (integrated from three different F2 maps between individuals originating from the IM population [98]), and two inter-specific F2 maps between IM62 M. guttatus (the reference line) and SF M. nasutus, IMxSF_2006 [85] and IMxSF_2009 (C. Wu and J. Willis unpublished). All three linkage maps are available at www.mimulusevolution.org. See Text S1 for more details.
With this integrated map, we estimated a local recombination rate for every 100 kb window, smoothed by the mean rate in the surrounding 500 kb. In each window we calculated mean pairwise sequence diversity at synonymous sites and used a non-parametric spearman rank sign test to evaluate the relationship between synonymous sequence diversity and the local recombination rate. We excluded windows with fewer than 100 pairwise comparisons, and regions without recombination estimates. The final number of 100 kb windows included in each pairwise comparison ranged from 1,773–2,023 (∼177.3–202.3 Mb), or 60.5–69.0% of the reference genome. Moreover, the set of windows in each analysis largely overlapped – 1,756 windows were common to all analyses meaning that for a given test 87% to 99% of the windows were used in all other analyses. In Text S1 and Table S6, we show that divergence to M. dentilobus and local depth do not influence our qualitative conclusions.
In interpreting our negative relationship between the recombination rate and interspecific divergence across the genome of hybridizing M. guttatus, we note that genomic regions with the lowest recombination rates have the highest densities of centromeric and TE-like repeats, and in contrast, high-recombination regions have the highest local gene-densities per physical distance (data not shown). However, there is no obvious mechanistic link between this observation and the consistent negative correlation between divergence and recombination between M. nasutus and sympatric, but not allopatric, M. guttatus.
PSMC. As a complementary inference of historical demography and differentiation, we applied Li and Durbin's implementation of the pairwise sequentially Markovian coalescent (PSMC) [36] to pairwise combinations of focal haploid genomes. We phased the genome of each focal sample by randomly choosing an allele at each heterozygous site; residual heterozygosity was extremely low (see above) therefore we did not expect this to significantly affect our results (see Text S1 for additional details). Due to high diversity in our dataset, we used a window size of 10 bp for PSMC analysis. For all comparisons, we ran PSMC for 20 iterations and used the following input settings: recombination/mutation ratio (r) = 1.25, Tmax = 10, number of intervals (n) = 60, number of population size parameters = 24, parameter distribution pattern = ‘1*4+1*4+1*3+18*2+1*3+1*4+1*6’. We represented time using a generation time of 1 year and a mutation rate of 1.5×10−8. We note that choosing a fixed value for the recombination/mutation ratio is appropriate for comparisons between species and within M. guttatus; however, this does not capture the change in this ratio accompanying the transition to selfing in M. nasutus. Therefore quantitative estimates of population decline through time within M. nasutus are best viewed as very rough approximations. To generate a measure of variability in the PSMC estimates, we ran 100 bootstrap analyses for each pairwise comparison (see Text S1 for additional details). Finally, we note that applying PSMC to our Stampy-aligned dataset yielded consistent biological conclusions (see Text S1 and Figures S13, S14, S15, S16) to our bwa dataset.
Introgression analyses
Treemix. As a test for recent admixture between M. guttatus and M. nasutus, we used Pickrell and Pritchard's (2012) Treemix method to model the evolutionary history of a group as a series of splits and gene flow events. This method has been previously shown to perform well, even when single individuals represent a group (see Section SI 11 of [99]). For these analyses, we used the same subsample of 14,000 SNPs used for the nj tree and PCA described above. We specified a Treemix block size of 50 SNPs and estimated the evolutionary history including 1, 2, 3 or 4 admixture events. We also ran analyses with and without the reference line IM62 included, and with all nineteen Mimulus samples (see Text S1 and results, as well as Figure S10).
Four population D test for admixture. Following the notation of Patterson et al. [100], we denote sample allele frequencies from populations Y, Z, W and X in lowercase. Here, Y is a sympatric M. guttatus population; Z is an allopatric M. guttatus population geographically close to Y; W is M. nasutus, and X is an allopatric M. guttatus population geographically distant from Y. Summing across i loci
We evaluate significance with a block jackknife, dropping one chromosome in each run [100].HMM. We implement the forward-backward algorithm and posterior decoding as described by Durbin et al. [101] in a customized R script controlling for underflow (available upon request) to calculate posterior probabilities of M. nasutus or M. guttatus ancestry across all four focal M. guttatus samples.
We take as our emissions the minimum pairwise π between our focal M. guttatus sample and all M. nasutus samples in non-overlapping 1 kb windows (πclose_nas). We compared πclose_nas to the genome-wide distribution of π between a M. nasutus sample and its genetically closest M. nasutus sample and π between M. guttatus samples and the genetically closest M. nasutus sample, to calculate the emission probability of admixed or pure M. guttatus ancestry, respectively. In doing so we accounted for both the heterogeneity in the number of informative sites across the genome and the fact that we compare each M. nasutus sample to three others, while we compare each M. guttatus sample to all four M. nasutus samples (see Text S1).
We calculate ti,j, the probability of transitioning from ancestry of species i to ancestry from species j from α, the admixture proportion, and r, the product of the recombination rate per 1 kb multiplied by a point estimate of the number of generations since admixture. We set ti,j to tgut,gut = (1−r)+r (1−α), tgut,nas = r α, tnas,gut = r (1−α), and tnas,nas = (1−r)+r α, and optimize these parameters with the Nelder-Mead algorithm implemented in the R function, optim, calculating the likelihood of our data given these parameters from the forward algorithm. α estimated in this way is similar to estimates from the proportion of low divergence windows presented in the text, suggesting that our data provide information about both α and r. We assume that r is constant across windows, ignoring the influence of the recombination rate on the transition rate.
Inference of introgression history. The number of admixture blocks and our point estimate of admixture timing are strong evidence that admixture is not the result of a single chance M. nasutus ancestor in the history of these samples. To see this, consider that our current day genome is expected to be broken into X chunks v generations ago, where X is the number of recombination events (i.e., the map length in Morgans which is ∼14.7 in Mimulus, times the number of generations, v), plus the number of chromosomes (14 in Mimulus). So, for example, the CACG genome is broken into X = 2,219 chunks at our point estimate of its admixture time, v = 150 generations ago (150 generations times 14.7 Morgans plus 14 chromosomes). These X chunks are drawn from 2v genealogical ancestors spread across many ancestors, meaning that CACG is expected to inherit 2,219/2150∼1/2139 chromosomal segments from a typical genealogical ancestor, and therefore under a point model of admixture the odds of CACG inheriting two ancestry blocks from the same ancestor is vanishingly small. Therefore, each of the 227 M. nasutus ancestry blocks observed in CACG descends from a different admixture event, implying a vast number of admixed ancestors in the history of the sympatric populations M. guttatus. The case is starker for DPRG, for which we infer a much older point estimate of the admixture time.
Because recombination is a Poisson process, under a single admixture time, v, we expect the variance in admixture block length to equal the square of the mean block length. However the variance in admixture block lengths is inconsistent with this expectation for both southern (DPRG, mean = 0.0010 M, σ = 0.0013 M, Bootstrap P<0.001), and northern (CACG, mean = 0.0074 M, σ = 0.0102M, Bootstrap P<0.001) sympatric M. guttatus samples. This argues against a point model of admixture and suggests ongoing and sustained gene flow into M. guttatus where it is sympatric with M. nasutus. We acknowledge that these calculations are somewhat crude, especially because we assume a constant recombination rate genome-wide. Nonetheless, the extreme variation in admixture block length and the large number of visually obvious blocks in Figure S9 supports this qualitative result.
We note that since our inference of the extreme improbability that two admixture blocks are derived from the same introgression event relied on a point model, we must soften this conclusion. It is plausible that a few young ancestry blocks in CACG are descended from the same admixture event, however, the rejection of a point admixture model strengthens our major conclusion that gene flow is ongoing and that our samples do not represent single admixture events.
Inference of introgression from M. guttatus to M. nasutus
To test for introgression from M. guttatus into M. nasutus, we took advantage of the structure of genetic variation in M. nasutus – for most of the genome all individuals are remarkably similar, and when this is not the case, one individual often differs sharply from all others. The genetic variation in such genomic regions has either been maintained from the stock of ancestral variation, or it has been reintroduced by introgression upon secondary contact. The extreme genetic variation and miniscule extent of linkage disequilibrium within M. guttatus makes these two alternative hypotheses nearly indistinguishable in any given region; however, by collating information across all such regions we can test the introgression hypothesis.
To do so, we find long regions (20 kb) of the M. nasutus genome for which one individual is a genetic outlier, as described above. In these regions, we ask whether the outlier is more closely related to a specific M. guttatus sample than are the non-outliers. Under incomplete lineage sorting, there is a 50% probability that this is the case. However, under an admixture model this probability is greater than 50% if the M. guttatus sample is more closely related to the potential admixture source than it is to the population that founded M. nasutus. See Text S1 for more details, and Table S5 for the robustness of this inference to our specific rules for identifying outlier windows.
We perform this test for all M. nasutus samples individually against two focal allopatric M. guttatus samples from the northern (AHQT) and southern (SLP) groups. In addition to testing the one sided hypothesis of whether the outlier sample is more often like a given M. guttatus sample, we also pool our northern M. nasutus samples to amplify any signal.
Supporting Information
Zdroje
1. CahillJA, GreenRE, FultonTL, StillerM, JayF, et al. (2013) Genomic evidence for island population conversion resolves conflicting theories of polar bear evolution. PLoS Genet 9: e1003345.
2. FrantzLA, SchraiberJG, MadsenO, MegensHJ, BosseM, et al. (2013) Genome sequencing reveals fine scale diversification and reticulation history during speciation in Sus. Genome Biol 14: R107.
3. GreenRE, KrauseJ, BriggsAW, MaricicT, StenzelU, et al. (2010) A draft sequence of the Neandertal genome. Science 328 : 710–722.
4. MillerW, SchusterSC, WelchAJ, RatanA, Bedoya-ReinaOC, et al. (2012) Polar and brown bear genomes reveal ancient admixture and demographic footprints of past climate change. Proc Natl Acad Sci U S A 109: E2382–2390.
5. Heliconius GenomeC (2012) Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487 : 94–98.
6. WuCA, LowryDB, CooleyAM, WrightKM, LeeYW, et al. (2008) Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity (Edinb) 100 : 220–230.
7. Grant V (1971) Plant Speciation. New York: Columbia University Press.
8. KayKM, SchemskeDW (2003) Pollinator assemblages and visitation rates for 11 species of neotropical Costus (Costaceae). Biotropica 35 : 198–207.
9. SchemskeDW, BradshawHDJr (1999) Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc Natl Acad Sci U S A 96 : 11910–11915.
10. BakerHG (1959) Reproductive methods as factors in speciation. Cold Spring Harbor Symposium on Quantitative Biology 24 : 177–191.
11. GoldbergEE, IgicB (2012) Tempo and mode in plant breeding system evolution. Evolution 66 : 3701–3709.
12. WrightSI, KaliszS, SlotteT (2013) Evolutionary consequences of self-fertilization in plants. Proc Biol Sci 280 : 20130133.
13. CharlesworthD, WrightSI (2001) Breeding systems and genome evolution. Curr Opin Genet Dev 11 : 685–690.
14. NordborgM (2000) Linkage disequilibrium, gene trees and selfing: an ancestral recombination graph with partial self-fertilization. Genetics 154 : 923–929.
15. GleminS, BazinE, CharlesworthD (2006) Impact of mating systems on patterns of sequence polymorphism in flowering plants. Proc Biol Sci 273 : 3011–3019.
16. LefflerEM, BullaugheyK, MatuteDR, MeyerWK, SegurelL, et al. (2012) Revisiting an old riddle: what determines genetic diversity levels within species? PLoS Biol 10: e1001388.
17. HazzouriKM, EscobarJS, NessRW, Killian NewmanL, RandleAM, et al. (2013) Comparative population genomics in Collinsia sister species reveals evidence for reduced effective population size, relaxed selection, and evolution of biased gene conversion with an ongoing mating system shift. Evolution 67 : 1263–1278.
18. NessRW, SiolM, BarrettSC (2012) Genomic consequences of transitions from cross - to self-fertilization on the efficacy of selection in three independently derived selfing plants. BMC Genomics 13 : 611.
19. QiuS, ZengK, SlotteT, WrightS, CharlesworthD (2011) Reduced efficacy of natural selection on codon usage bias in selfing Arabidopsis and Capsella species. Genome Biol Evol 3 : 868–880.
20. BrandvainY, SlotteT, HazzouriKM, WrightSI, CoopG (2013) Genomic identification of founding haplotypes reveals the history of the selfing species Capsella rubella. PLoS Genet 9: e1003754.
21. RitlandK (1990) Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44 : 1230–1241.
22. AwadallaP, RitlandK (1997) Microsatellite variation and evolution in the Mimulus guttatus species complex with contrasting mating systems. Mol Biol Evol 14 : 1023–1034.
23. WillisJH (1993) Partial self-fertilization and inbreeding depression in 2 populations of Mimulus guttatus. Heredity (Edinb) 71 : 145–154.
24. MartinNH, WillisJH (2007) Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61 : 68–82.
25. KiangYT, HamrickJL (1978) Ecological adaptation and reproductive isolation in the Mimulus guttatus - M. nasutus complex. American Midland Naturalist 100 : 269–276.
26. DiazA, MacNairMR (1999) Pollen tube competition as a mechanism of prezygotic reproductive isolation between Mimulus nasutus. American Journal of Botany 144 : 471–478.
27. VickeryRK (1964) Barriers to gene exchange between members of the Mimulus guttatus complex (Scrophulariaceae). Evolution 18 : 52–69.
28. CaseAL, WillisJH (2008) Hybrid male sterility in Mimulus (Phrymaceae) is associated with a geographically restricted mitochondrial rearrangement. Evolution 62 : 1026–1039.
29. SweigartAL, MasonAR, WillisJH (2007) Natural variation for a hybrid incompatibility between two species of Mimulus. Evolution 61 : 141–151.
30. SweigartAL, WillisJH (2003) Patterns of nucleotide diversity in two species of Mimulus are affected by mating system and asymmetric introgression. Evolution 57 : 2490–2506.
31. ModliszewskiJL, WillisJH (2012) Allotetraploid Mimulus sookensis are highly interfertile despite independent origins. Mol Ecol 21 : 5280–5298.
32. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
33. LunterG, GoodsonM (2011) Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res 21 : 936–939.
34. HudsonRR, KreitmanM, AguadeM (1987) A test of neutral molecular evolution based on nucleotide data. Genetics 116 : 153–159.
35. OssowskiS, SchneebergerK, Lucas-LledoJI, WarthmannN, ClarkRM, et al. (2010) The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 327 : 92–94.
36. LiH, DurbinR (2011) Inference of human population history from individual whole-genome sequences. Nature 475 : 493–496.
37. PtakSE, PrzeworskiM (2002) Evidence for population growth in humans is confounded by fine-scale population structure. Trends Genet 18 : 559–563.
38. StadlerT, HauboldB, MerinoC, StephanW, PfaffelhuberP (2009) The impact of sampling schemes on the site frequency spectrum in nonequilibrium subdivided populations. Genetics 182 : 205–216.
39. PenningsPS, KryazhimskiyS, WakeleyJ (2014) Loss and recovery of genetic diversity in adapting populations of HIV. PLoS Genet 10: e1004000.
40. SongYS, SteinruckenM (2012) A simple method for finding explicit analytic transition densities of diffusion processes with general diploid selection. Genetics 190 : 1117–1129.
41. PickrellJK, PritchardJK (2012) Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet 8: e1002967.
42. DurandEY, PattersonN, ReichD, SlatkinM (2011) Testing for ancient admixture between closely related populations. Mol Biol Evol 28 : 2239–2252.
43. Wilkinson-HerbotsHM (2012) The distribution of the coalescence time and the number of pairwise nucleotide differences in a model of population divergence or speciation with an initial period of gene flow. Theor Popul Biol 82 : 92–108.
44. HellenthalG, BusbyGBJ, BandG, WilsonJF, CapelliC, et al. (2014) A genetic atlas of human admixture history. Science 343 : 747–751.
45. GravelS (2012) Population genetics models of local ancestry. Genetics 191 : 607–619.
46. NachmanMW, PayseurBA (2012) Recombination rate variation and speciation: theoretical predictions and empirical results from rabbits and mice. Philos Trans R Soc Lond B Biol Sci 367 : 409–421.
47. HudsonRR (1991) Gene genealogies and the coalescent process. Oxford Surveys in Evolutionary Biology 7 : 1–44.
48. CharlesworthB (1998) Measures of divergence between populations and the effect of forces that reduce variability. Mol Biol Evol 15 : 538–543.
49. NoorMA, BennettSM (2009) Islands of speciation or mirages in the desert? Examining the role of restricted recombination in maintaining species. Heredity (Edinb) 103 : 439–444.
50. BirkyCWJr, WalshJB (1988) Effects of linkage on rates of molecular evolution. Proc Natl Acad Sci U S A 85 : 6414–6418.
51. GuoYL, BechsgaardJS, SlotteT, NeufferB, LascouxM, et al. (2009) Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proc Natl Acad Sci U S A 106 : 5246–5251.
52. FoxeJP, SlotteT, StahlEA, NeufferB, HurkaH, et al. (2009) Recent speciation associated with the evolution of selfing in Capsella. Proc Natl Acad Sci U S A 106 : 5241–5245.
53. GrossenbacherDL, VelozSD, SextonJP (2014) Niche and range size patterns suggest that tpeciation begins in small, ecologically diverged populations in North American Monkeyflowers (Mimulus spp.). Evolution 68 : 1270–1280 DOI: 10.1111/evo.12355
54. AnackerBL, StraussSY (2014) The geography and ecology of plant speciation: range overlap and niche divergence in sister species. Proc Biol Sci 281 : 20132980.
55. SlotteT, HazzouriKM, AgrenJA, KoenigD, MaumusF, et al. (2013) The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat Genet 45 : 831–835.
56. TangC, ToomajianC, Sherman-BroylesS, PlagnolV, GuoYL, et al. (2007) The evolution of selfing in Arabidopsis thaliana. Science 317 : 1070–1072.
57. KochM, HauboldB, Mitchell-OldsT (2001) Molecular systematics of the Brassicaceae: evidence from coding plastidic matK and nuclear Chs sequences. Am J Bot 88 : 534–544.
58. PollakE (1987) On the theory of partially inbreeding finite populations. I. Partial selfing. Genetics 117 : 353–360.
59. NordborgM, DonnellyP (1997) The coalescent process with selfing. Genetics 146 : 1185–1195.
60. CutterAD, PayseurBA (2013) Genomic signatures of selection at linked sites: unifying the disparity among species. Nat Rev Genet 14 : 262–274.
61. CutterAD, PayseurBA (2003) Selection at linked sites in the partial selfer Caenorhabditis elegans. Mol Biol Evol 20 : 665–673.
62. AndersenEC, GerkeJP, ShapiroJA, CrissmanJR, GhoshR, et al. (2012) Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat Genet 44 : 285–290.
63. McVeanGA, CharlesworthB (2000) The effects of Hill-Robertson interference between weakly selected mutations on patterns of molecular evolution and variation. Genetics 155 : 929–944.
64. KaplanNL, HudsonRR, LangleyCH (1989) The “hitchhiking effect” revisited. Genetics 123 : 887–899.
65. CharlesworthB, MorganMT, CharlesworthD (1993) The effect of deleterious mutations on neutral molecular variation. Genetics 134 : 1289–1303.
66. LynchM, ConeryJ, BürgerR (1995) Mutation accumulation and the extinction of small populations. American Naturalist 146 : 489–518.
67. ArnaudJ, FénartS, CordellierM, CuguenJ (2010) Populations of weedy crop–wild hybrid beets show contrasting variation in mating system and population genetic structure. Evolutionary applications 2 : 305–318.
68. GoldbergEE, KohnJR, LandeR, RobertsonKA, SmithSA, et al. (2010) Species selection maintains self-incompatibility. Science 330 : 493–495.
69. TakebayashiN, MorrellPL (2001) Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. American Journal of Botany 88 : 1143–1150.
70. MartinNH (2004) Flower size preferences of the honeybee (Apis mellifera) foraging on Mimulus guttatus (Scrophulariaceae). Evolutionary Ecology Research 6 : 777–782.
71. MollerAP (1995) Bumblebee preference for symmetrical flowers. Proc Natl Acad Sci U S A 92 : 2288–2292.
72. RobertsonAW, MacnairMR (1995) The effects of floral display size on pollinator service to individual flowers of Myosotis and Mimulus. Oikos 72 : 106–114.
73. MakinoTT, OhashiK, SakaiS (2007) How do floral display size and the density of surrounding flowers influence the likelihood of bumble bee revisitation to a plant? Functional Ecology 21 : 87–95.
74. Palma-SilvaC, WendtT, PinheiroF, BarbaraT, FayMF, et al. (2011) Sympatric bromeliad species (Pitcairnia spp.) facilitate tests of mechanisms involved in species cohesion and reproductive isolation in Neotropical inselbergs. Mol Ecol 20 : 3185–3201.
75. RuhsamM, HollingsworthPM, EnnosRA (2011) Early evolution in a hybrid swarm between outcrossing and selfing lineages in Geum. Heredity (Edinb) 107 : 246–255.
76. RuhsamM, HollingsworthPM, EnnosRA (2013) Patterns of mating, generation of diversity, and fitness of offspring in a Geum hybrid swarm. Evolution 67 : 2728–2740.
77. ViaS (2009) Natural selection in action during speciation. Proc Natl Acad Sci U S A 106 : 9939–9946.
78. TurnerTL, HahnMW, NuzhdinSV (2005) Genomic islands of speciation in Anopheles gambiae. PLoS Biol 3 : 1572–1578.
79. GeraldesA, BassetP, SmithK, NachmanMW (2011) Higher differentiation among subspecies of the house mouse (Mus musculus) in genomic regions with low recombination. Molecular Ecology 10 : 4722–4736.
80. BartonN (1983) Multilocus clines. Evolution 37 : 454–471.
81. KruukLE, BairdSJ, GaleKS, BartonNH (1999) A comparison of multilocus clines maintained by environmental adaptation or by selection against hybrids. Genetics 153 : 1959–1971.
82. StevisonLS, HoehnKB, NoorMA (2011) Effects of inversions on within - and between-species recombination and divergence. Genome Biol Evol 3 : 830–841.
83. McGaughSE, NoorMA (2012) Genomic impacts of chromosomal inversions in parapatric Drosophila species. Philos Trans R Soc Lond B Biol Sci 367 : 422–429.
84. CarneiroM, FerrandN, NachmanMW (2009) Recombination and speciation: loci near centromeres are more differentiated than loci near telomeres between subspecies of the European rabbit (Oryctolagus cuniculus). Genetics 181 : 593–606.
85. FishmanL, KellyAJ, WillisJH (2002) Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution 56 : 2138–2155.
86. SankararamanS, MallickS, DannemannM, PruferK, KelsoJ, et al. (2014) The genomic landscape of Neanderthal ancestry in present-day humans. Nature 507 : 354–357.
87. Institute DJG Mimulus Genome Project. http://www.phytozome.net/
88. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
89. DePristoMA, BanksE, PoplinR, GarimellaKV, MaguireJR, et al. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43 : 491–498.
90. McKennaA, HannaM, BanksE, SivachenkoA, CibulskisK, et al. (2010) The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20 : 1297–1303.
91. Team RC (2013) R: A language and environment for statistical computing. Vienna, Austria.
92. ParadisE, ClaudeJ, StrimmerK (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20 : 289–290.
93. JakobssonM, ScholzSW, ScheetP, GibbsJR, VanLiereJM, et al. (2008) Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451 : 998–1003.
94. BowcockAM, Ruiz-LinaresA, TomfohrdeJ, MinchE, KiddJR, et al. (1994) High resolution of human evolutionary trees with polymorphic microsatellites. Nature 368 : 455–457.
95. ReichD, PattersonN, CampbellD, TandonA, MazieresS, et al. (2012) Reconstructing Native American population history. Nature 488 : 370–374.
96. ReichD, GreenRE, KircherM, KrauseJ, PattersonN, et al. (2010) Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468 : 1053–1060.
97. KopelmanNM, StoneL, GascuelO, RosenbergNA (2013) The behavior of admixed populations in neighbor-joining inference of population trees. Pac Symp Biocomput 273–284.
98. Lee YW (2009) Genetic analysis of standing variation for floral morphology and fitness components in a natural population of Mimulus guttatus (common monkeyflower). PhD, Duke University.
99. RaghavanM, SkoglundP, GrafKE, MetspaluM, AlbrechtsenA, et al. (2014) Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505 : 87–91.
100. PattersonN, MoorjaniP, LuoY, MallickS, RohlandN, et al. (2012) Ancient admixture in human history. Genetics 192 : 1065–1093.
101. Durbin R (1998) Biological sequence analysis : probabalistic models of proteins and nucleic acids. Cambridge, UK New York: Cambridge University Press. xi, 356 p. p.
102. SandersonMJ (2002) Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Molecular Biology and Evolution 19 : 101–109.
103. BouckaertRR (2010) DensiTree: making sense of sets of phylogenetic trees. Bioinformatics 26 : 1372–1373.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Pánevní endometrióza spojená s volnou tekutinou v peritoneální dutině snižuje úspěšnost otěhotnění po intrauterinní inseminaci
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




