-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
Given the importance of gene expression changes in evolution, a better understanding of how they accumulate is desirable. However, gene regulation is a complex biochemical process and it is not a priori clear whether general trends even exist. We systematically addressed this question by testing, in C. elegans, the functions of regulatory elements of eight different genes from four other nematodes. We saw rampant variation in gene regulatory mechanisms, even between closely related species. While the differences were usually seen in a relatively small number of cells, there was a discernible trend – there were many more instances of gain, rather than loss of expression, compared to patterns directed by the C. elegans cis elements. Finally, the recurrence of ectopic expression in the same cells suggests that the paths open to evolution may be constrained by the composition of regulatory elements. We view these patterns as a reflection of general mechanisms of gene regulatory evolution and suggest that these can be refined, and others discovered, using systematic functional tests.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004435
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004435Summary
Given the importance of gene expression changes in evolution, a better understanding of how they accumulate is desirable. However, gene regulation is a complex biochemical process and it is not a priori clear whether general trends even exist. We systematically addressed this question by testing, in C. elegans, the functions of regulatory elements of eight different genes from four other nematodes. We saw rampant variation in gene regulatory mechanisms, even between closely related species. While the differences were usually seen in a relatively small number of cells, there was a discernible trend – there were many more instances of gain, rather than loss of expression, compared to patterns directed by the C. elegans cis elements. Finally, the recurrence of ectopic expression in the same cells suggests that the paths open to evolution may be constrained by the composition of regulatory elements. We view these patterns as a reflection of general mechanisms of gene regulatory evolution and suggest that these can be refined, and others discovered, using systematic functional tests.
Introduction
A complex network of molecular interactions that orchestrates gene expression provides multiple sources for regulatory variation between species [1]. Changes in transcriptional regulation can occur in two fundamentally different ways: in trans regulators [2], [3], for example through changes in protein sequences or expression patterns of transcription factors, or in cis elements via changes in identity or location of transcription factor binding sites [4], [5]. Although the importance of variation in gene regulation for evolution is well appreciated [6]–[8], many details remain to be elucidated. For example, do mutations in cis arise and go to fixation more frequently than changes in trans [9], [10]? Are regulatory mutations pleiotropic and, if so, what are their effects [11]? Our research has focused on cis-regulatory elements (CREs). These sequences consist of multiple transcription factor binding sites and a core promoter, but these motifs tend to be short, diffuse, and flexible in their locations [12]. Traditional sequence alignments may not therefore be reliable indicators of functional conservation [13]. Because cis elements integrate signals from multiple trans-acting factors in the context of an intact cell, their functions have to be assessed in vivo [14].
The study of functional evolution of cis-regulatory elements has relied on two approaches. One typically starts with the knowledge of the location of binding sites in a regulatory sequence of one species and is followed up by the functional tests of these binding sites in the original and other species [15], [16]. This approach is labor-intensive and is more difficult to scale. An alternative consists of assessing the functions of orthologous regulatory sequences, without detailed knowledge of identity and location of binding sites, from multiple species in the same trans-regulatory environment (reviewed in [17]). This approach has the advantage of being applicable to less well-studied regulatory regions and can be scaled up to multiple genes, allowing researchers to infer general rules of regulatory sequence evolution.
Because they often use different methodologies and criteria for comparisons, studies that investigate the regulatory evolution of individual genes are not easily comparable. It has therefore been difficult to generalize results and infer common features of cis-regulatory evolution. Still, several trends are evident. Multiple studies documented divergence [18]–[22] and constraint [23]–[25] in cis-regulatory mechanisms between species. While functionally equivalent enhancers in different species are often found in similar locations [26], [27], this is not always the case [28]–[30]. In some cases, differences in cis-regulatory mechanisms reflect divergence in endogenous expression patterns [30], [31]. In others, divergent regulatory mechanisms underlie overtly conserved endogenous expression patterns [32]–[34], suggesting compensatory changes in cis and in trans [17], [22], [35].
In this study, we aimed to survey the amount of functional variation that exists in gene regulatory elements of closely related species. C. elegans offers an attractive model system for this work because of its simple and invariant anatomy [36], [37], which is conserved with close relatives [38]. The ease of describing gene expression with a single-cell resolution permits more precise comparisons than those possible in other multicellular model systems. Cis-regulatory sequences are often located within 1 kb upstream of the translation start site [39]. Several species from the Caenorhabditis genus that are approximately as divergent as human and mouse [40] are routinely used for comparisons with C. elegans.
We selected eight genes from five Caenorhabditis species that have available genome sequences: C. elegans, C. briggsae, C. remanei, and C. brenneri, the latter three equidistant to C. elegans; and C. japonica, a more distantly related species. In all cases, orthologous regulatory sequences were cloned, and the expression patterns they drove were evaluated in the C. elegans trans-regulatory environment. We report several general trends of cis-regulatory divergence gleaned from these observations.
Results
Rationale and approach
The goal of this study is essentially comparative, that is, to test whether orthologous cis elements are functionally equivalent. Our work is part of a broader research program aiming to investigate functional divergence of gene regulatory systems [41]. In this study we introduced cis-regulatory sequences (fused to GFP reporters) from several Caenorhabditis species into C. elegans and compared their expression patterns to those of their C. elegans orthologs. This approach can be seen as an extension of a fruitful paradigm that analyzes gene expression in hybrid organisms [21], [42]–[44]. In our experiments the “hybrid” portions of the genome range from a few hundred to a few thousand nucleotides directing gene expression.
While it is certainly desirable to document endogenous gene expression patterns and uncover all regulatory elements required to direct them, these questions remain outside the scope of our experimental program. Instead, our goal is to assess functional conservation of cis-regulatory sequences. To do so, we need only to ascertain whether cis elements from different species direct the same or different expression patterns. To ensure comparability, only the sequences from the immediately upstream regions were considered; consequently, if some regulatory sequences are located in introns, transgenes may not recapitulate the entire endogenous expression patterns. Movements of cis elements between the upstream intergenic regions in one species and introns in another, dubbed “nomadic” enhancers [30], illustrate one type of regulatory divergence our approach can uncover. Due to the persistence of the GFP protein, we are unlikely to detect minor dynamic differences in expression patterns. Testing all cis-regulatory elements in the common trans-regulatory environment of C. elegans simplifies the interpretation of these comparative data – any difference in expression patterns, whether gain or loss, reveals functional divergence between orthologous cis-regulatory elements, regardless of the expression patterns driven by these sequences in their endogenous trans-regulatory environments.
Selection of species and genes to be tested
In addition to C. elegans, we selected for our study four species with sequenced genomes: C. briggsae, C. remanei, C brenneri, and C. japonica [45], [46]. We decided to focus on these species because, based on previous experience [19], [22], [47]–[51], we anticipated many cis-regulatory functions to be substantially conserved. Given the established phylogenetic relationships between these five species [52], our experiments interrogated the extent of functional divergence accumulated over two time scales – one between C. elegans and the equidistant C. briggsae/C. remanei/C brenneri, and another between C. elegans and a more distant C. japonica (Figure 1). Estimates suggest that the phylogenetic distance between the latter pair of species is comparable to that within the Sophophora subgenus of Drosophila [40], [52] or vertebrate classes [53]. While the phylogeny is well-resolved, the paucity of fossil Rhabditidae nematodes [54] precludes a reliable estimate of the age of species divergence.
Fig. 1. Species and genes included in this study. 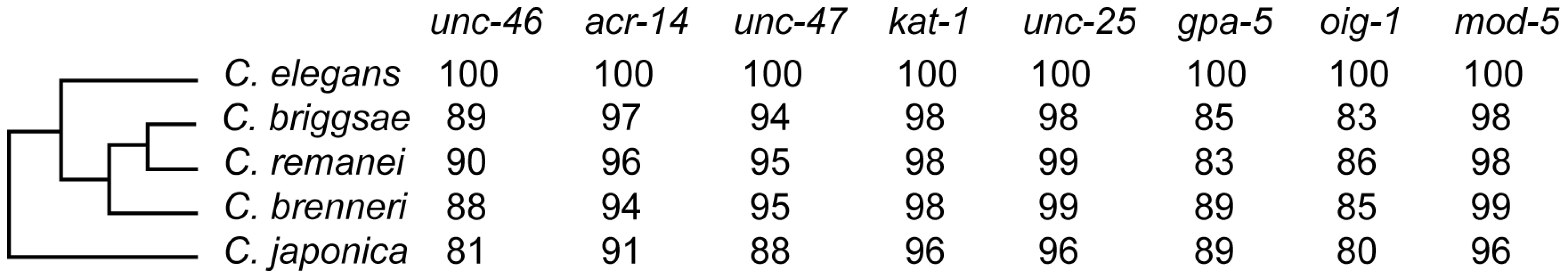
Phylogenetic relationship of the five studied species. Numbers represent relative conservation of protein sequences (compared to C. elegans) based on the BLOSUM matrix. We focused on eight genes expressed in relatively small groups of easily identifiable cells. Three genes are terminal effectors of the GABAergic fate: unc-25 [55], unc-46 [56], unc-47 [57], and are thus expressed in all GABAergic neurons. Two other genes, oig-1 and acr-14 [58], are thought to be expressed in subsets of GABAergic neurons. We chose these five coexpressed and partially coregulated [58] genes to test whether shared regulation imposes particular constraints on their evolution. To offset this bias to a particular class of neurons, we added two genes expressed in other neuronal types – one expressed in amphid (chemosensory) neurons, gpa-5 [59], and one expressed in serotonergic neurons, mod-5 [60]. The pattern of serotonergic neurons is conserved between C. elegans, C. briggsae and C. remanei [61]; the pattern of GABAergic neurons is conserved between C. elegans and C. briggsae [22], as well as with C. remanei and C. brenneri (AB & IR, unpublished data). Finally, we included one gene expressed outside the nervous system, kat-1, which encodes a conserved thiolase [62] involved in a fat storage pathway [63].
The protein-coding sequences of all eight genes are highly conserved (Figure 1). Moreover, the synteny with the immediate upstream genes is conserved among all five species (Figure S1), making us confident that all of them are single-copy, one-to-one orthologs of the C. elegans genes. We tested the entire intergenic regions containing putative cis elements to ensure that comparisons indeed included orthologous regulatory sequences.
In contrast with the high conservation of coding sequences, the noncoding upstream regions (which we assume to contain the majority of CREs [39]) are much more variable. We aligned orthologous intergenic sequences upstream of C. briggsae, C. remanei, C. brenneri, and C. japonica to their C. elegans counterparts and visualized the results using software package VISTA [64]. The CREs of unc-46, acr-14, and unc-47 showed relatively high levels of conservation, spanning ∼150 to 300 nucleotides in most or all species (Figures 2A–4A). The CREs of kat-1 and unc-25 displayed somewhat lower conservation, although some blocks of high similarity could still be clearly identified (Figures 5A, 6A). The CREs of gpa-5, oig-1, and mod-5 had little obvious evidence of conservation in the proximity of the translation start site (Figures 7A–9A), although some regions of putative conservation were present substantially upstream of these genes. Sequence comparisons within non-coding regions are notoriously challenging because we do not understand the “rules” by which these sequences evolve [1]. Therefore, we considered two additional measures of sequence divergence, namely the length of the longest contiguous sequence that is perfectly conserved between orthologs and the number of nucleotides contained within blocks of perfect conservation of 7 bp and longer. By both of these measures, cis elements of unc-46 and acr-14, and to some extent unc-47, appear to be more conserved than those of the rest of the genes included in this study (Table S1). Next we tested functional conservation of these regulatory elements. In all experiments we used sequences upstream of translation start sites, thus making translational fusion genes, to ensure that the tested regions encompass basal promoters and more distal regulatory sequences.
Fig. 2. Functional conservation and divergence of unc-46 regulation. 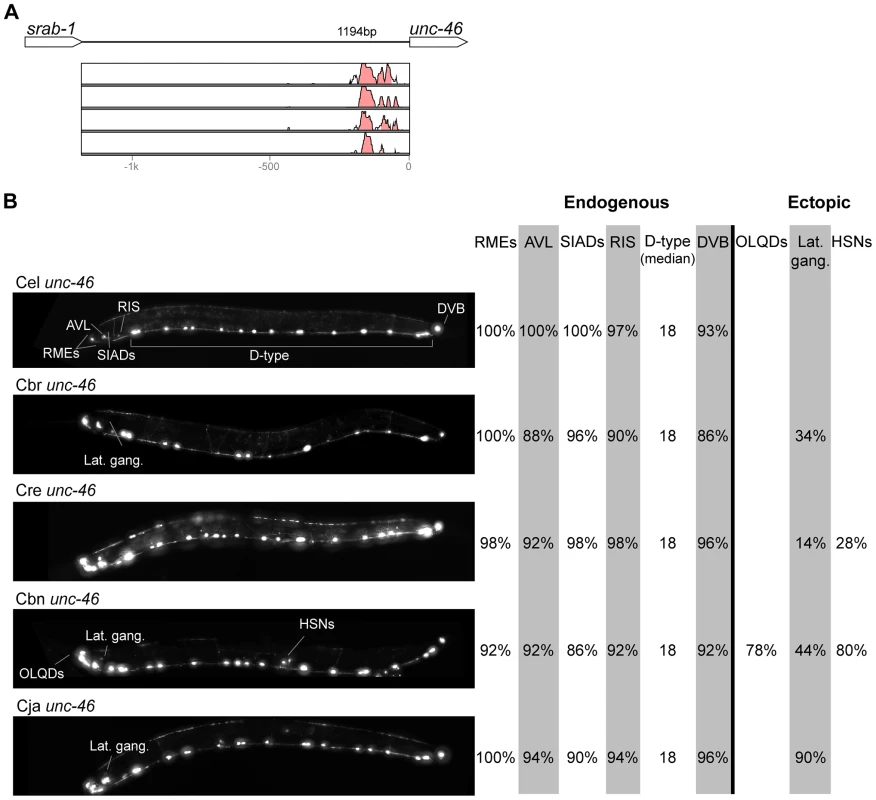
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of unc-46, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), C. brenneri (Cbn), and C. japonica (Cja) CREs of unc-46. For all cells, frequency of expression is indicated, except for D-type neurons for which the median number of expressing cells in shown. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: RMEs(RMED/V/L/R), SIADs (SIADL/R), OLQDs (OLQDL/R), Lat. gang. (unidentified pair of neurons in the lateral ganglion), HSNs (HSNL/R). Detailed data are shown in Table S3. Fig. 3. Functional conservation and divergence of acr-14 regulation. 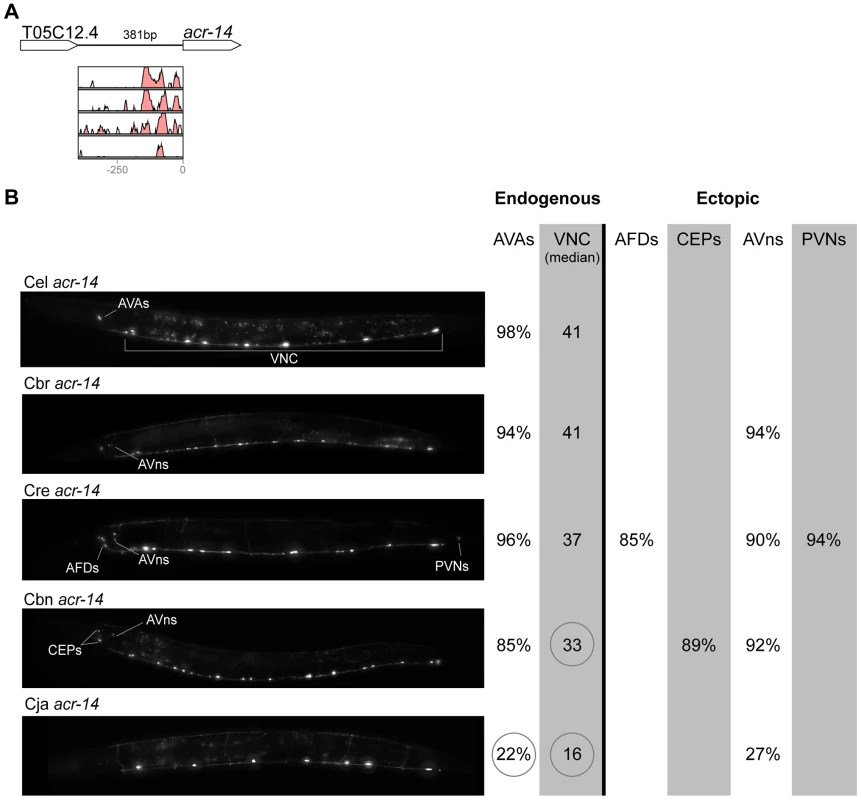
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of acr-14, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), C. brenneri (Cbn), and C. japonica (Cja) CREs of acr-14. For all cells, frequency of expression is indicated, except for the ventral nerve cord (VNC) for which the median number of expressing cells in shown. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: AVAs (AVAL/R), AFDs (AFDL/R), CEPs (CEPD/V L/R), AVns (AVHL/R or AVJL/R or AVDL/R), PVNs (PVNL/R). Reductions of expression compared to the endogenous pattern are circled. Detailed data are shown in Table S4. Fig. 4. Functional conservation and divergence of unc-47 regulation. 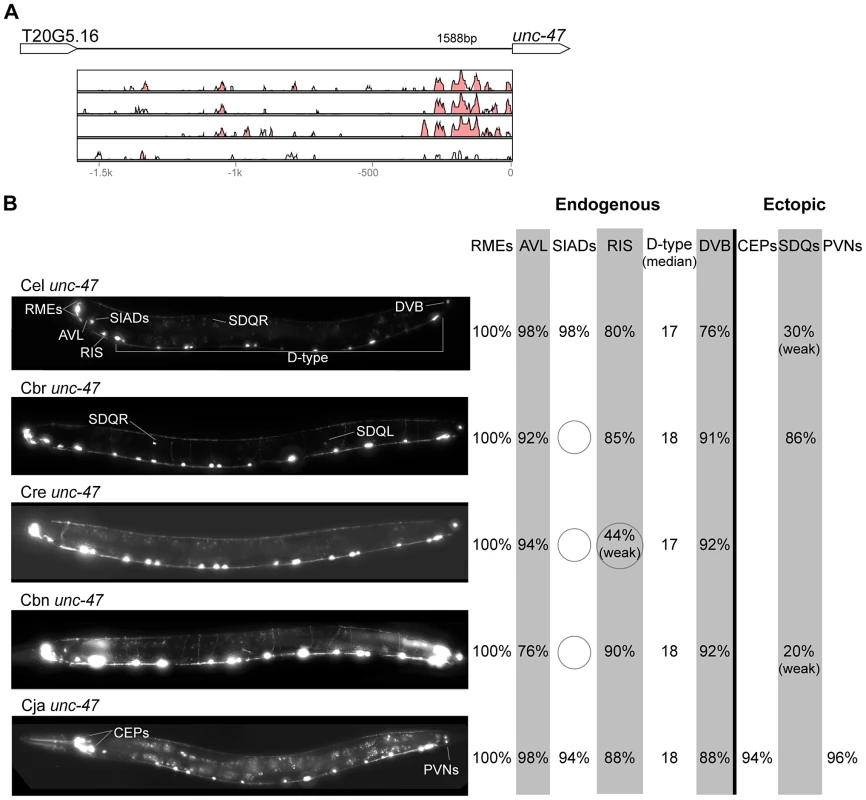
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of unc-47, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), C. brenneri (Cbn), and C. japonica (Cja) CREs of unc-47. For all cells, frequency of expression is indicated, except for D-type neurons for which the median number of expressing cells in shown. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: RMEs (RMED/V/L/R), SIADs (SIADL/R), CEPs (CEPD/V L/R), SDQs (SDQL/R), PVNs (PVNL/R). It is unclear whether expression in the SIADs is endogenous [56], [57], [68]. However, since it is consistently seen with the C. elegans CRE, we included it in the endogenous pattern. We classified the strong expression of the C. briggsae unc-47 CRE in SDQL/R as ectopic, even though weak SDQR expression was observed with the C. elegans CRE, because of the dramatic differences in the frequency and intensity of expression [22]. Reduction and losses of expression compared to the endogenous pattern are circled. Detailed data are shown in Table S5. Fig. 5. Functional conservation and divergence of kat-1 regulation. 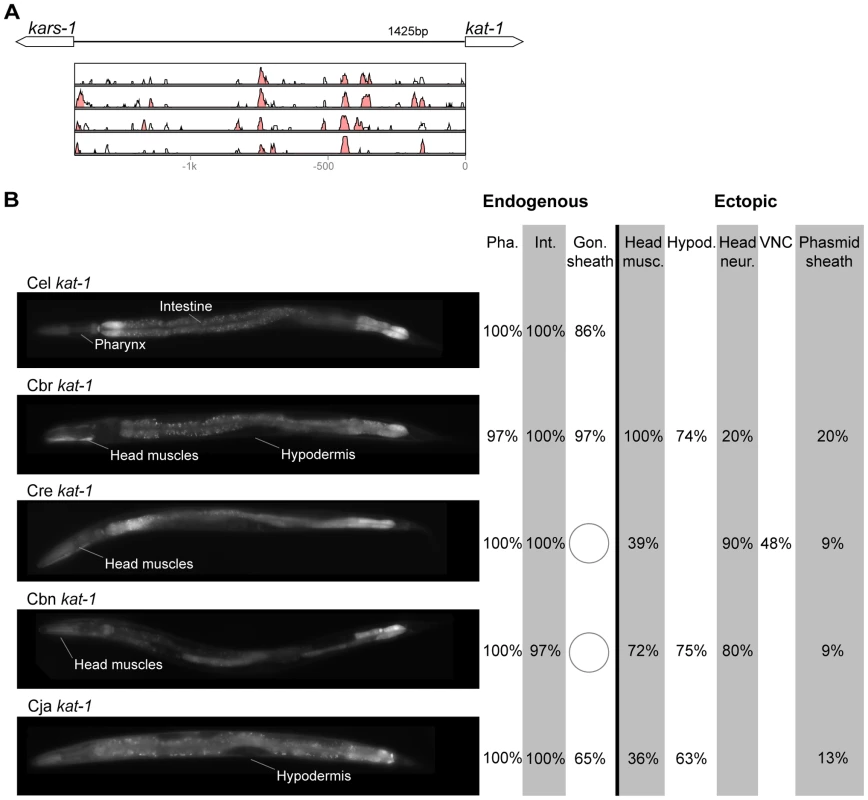
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of kat-1, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), C. brenneri (Cbn), and C. japonica (Cja) CREs of kat-1. Frequency of expression in different tissues is shown: Pha. (pharynx), Int. (intestine), Gon. sheath (gonadal sheath), Head musc. (head muscles), Hypod. (hypodermis), Head neur. (head neurons), VNC (ventral nerve cord). Losses of expression compared to the endogenous pattern are circled. Detailed data are shown in Table S6. Fig. 6. Functional conservation and divergence of unc-25 regulation. 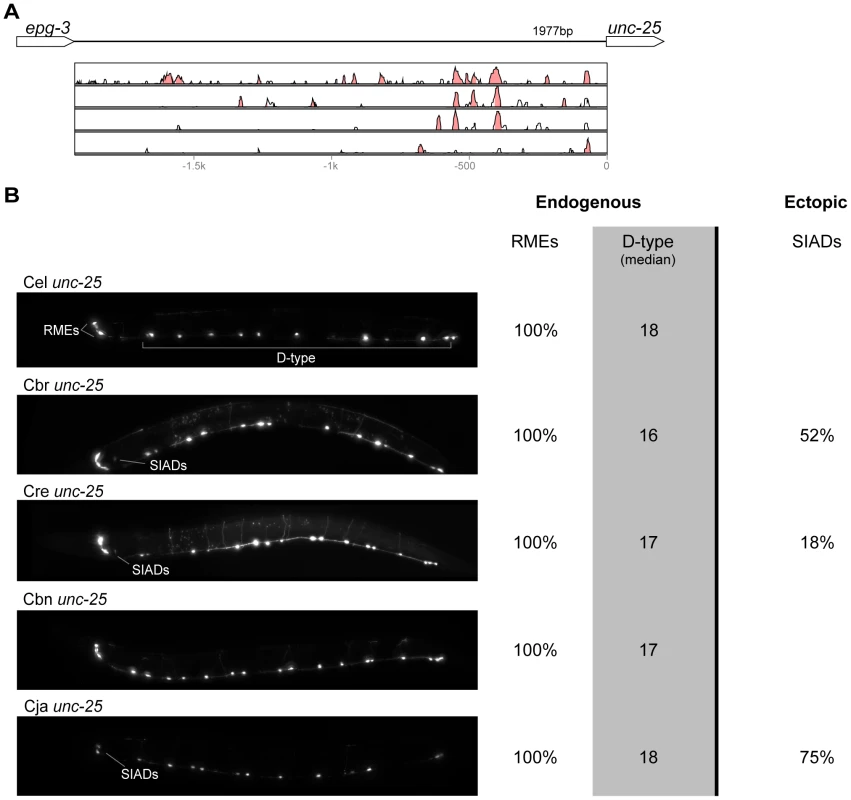
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of unc-25, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), C. brenneri (Cbn), and C. japonica (Cja) CREs of unc-25. For all cells, frequency of expression is indicated, except for D-type neurons for which the median number of expressing cells in shown. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: RMEs (RMED/V/L/R), SIADs (SIADL/R). Detailed data are shown in Table S7. Fig. 7. Functional conservation and divergence of gpa-5 regulation. 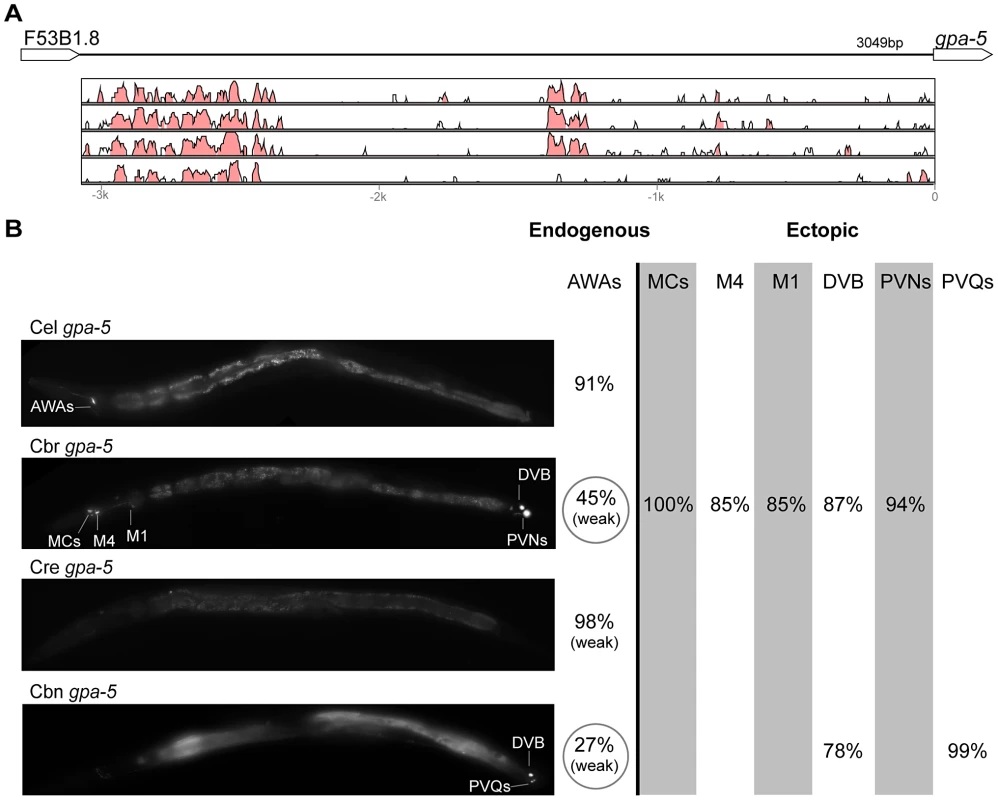
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of gpa-5, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), and C. brenneri (Cbn) CREs of gpa-5. For all cells, frequency of expression is indicated. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: AWAs (AWAL/R), MCs (MCL/R), PVNs (PVNL/R), PVQs (PVQL/R). Reductions of expression compared to the endogenous pattern are circled. Detailed data are shown in Table S8. Fig. 8. Functional conservation and divergence of oig-1 regulation. 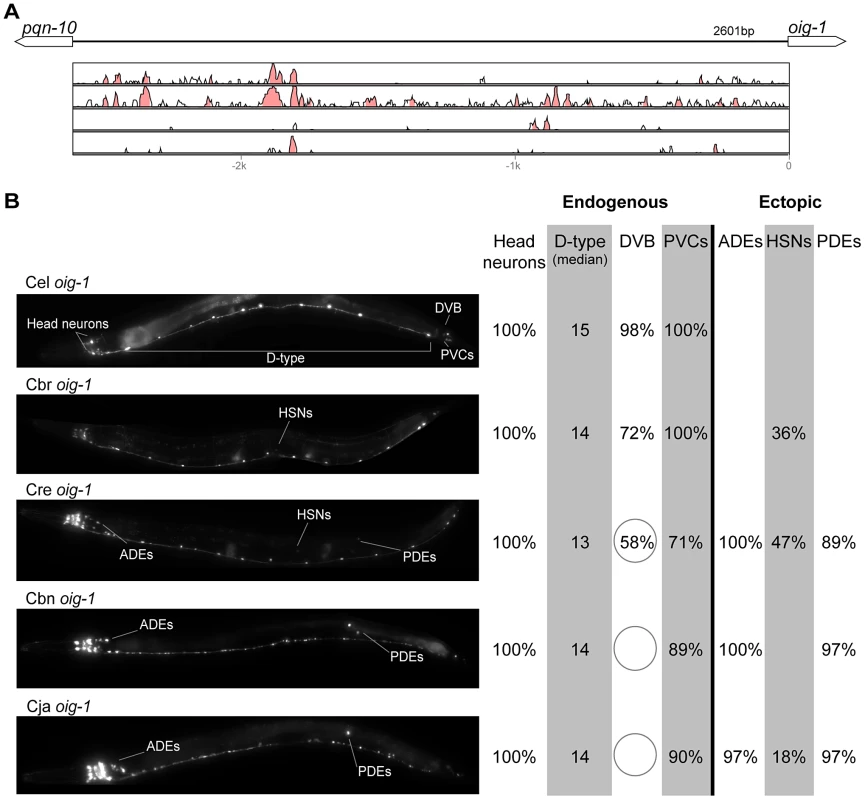
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of oig-1, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), C. brenneri (Cbn), and C. japonica (Cja) CREs of oig-1. For all cells, frequency of expression is indicated, except for D-type neurons for which the median number of expressing cells in shown. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: Head neurons (large cluster of head neurons, including ALAL/R, SMDVL/R, RMDVL/R, RIAL/R, AVAL/R, RIML/R, RMDDL/R and IL1s), PVCs (PVCL/R), ADEs (ADEL/R), HSNs (HSNL/R), PDEs (PDEL/R). Reductions and losses of expression compared to the endogenous pattern are circled. Detailed data are shown in Table S9. Fig. 9. Functional conservation and divergence of mod-5 regulation. 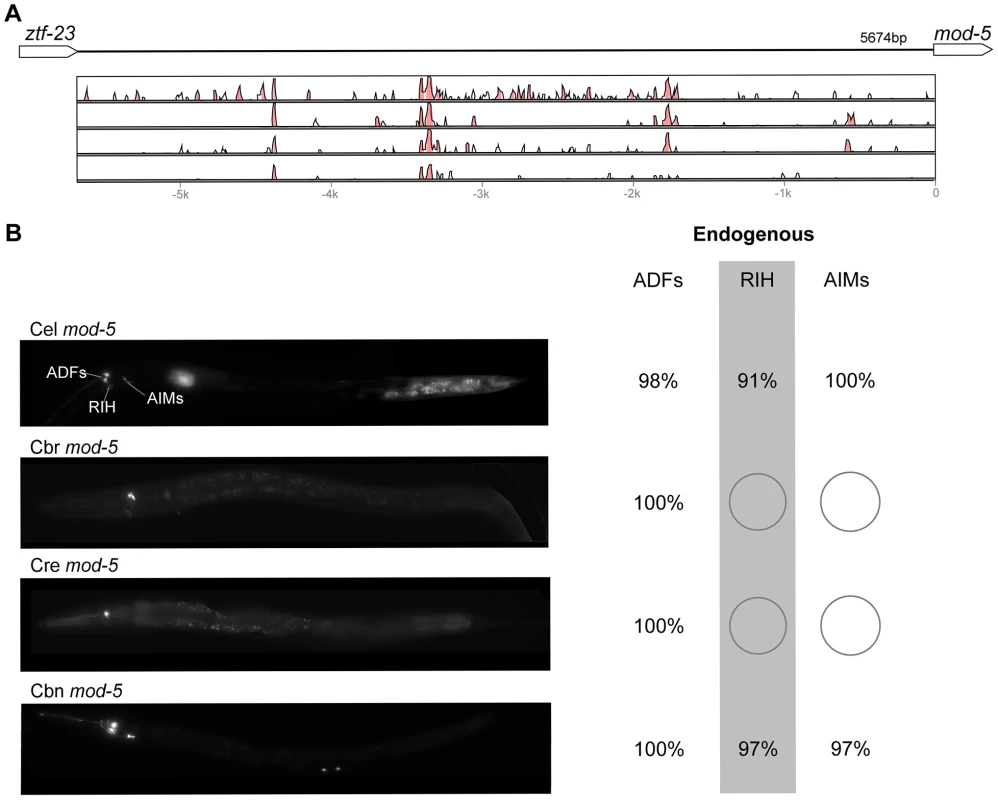
(A) Vista plots represent primary sequence conservation in the intergenic region upstream of mod-5, relative to C. elegans. Window size = 20 bp, threshold: 70%. From top to bottom: C. briggsae, C. remanei, C. brenneri, C. japonica. (B) Expression patterns driven by the C. elegans (Cel), C. briggsae (Cbr), C. remanei (Cre), and C. brenneri (Cbn) CREs of mod-5. For all cells, frequency of expression is indicated. For groups of multiple cells, percentages represent frequency of expression in at least one of these cells: ADFs (ADFL/R), AIMs (AIML/R). Losses of expression compared to the endogenous pattern are circled. Detailed data are shown in Table S10. Pervasive functional divergence in cis elements
Expression patterns directed in C. elegans by the orthologous cis elements of the eight studied genes were largely similar (Figures 2–9; detailed descriptions of the observed patterns are presented in Text S1). However, patterns driven by heterologous CREs were indistinguishable from those directed by their C. elegans orthologs in only three instances: C. brenneri unc-25 (Figure 6B), C. remanei gpa-5 (Figure 7B), and C. brenneri mod-5 (Figure 9B). In the rest of the cases, the expression patterns of heterologous CREs differed from their C. elegans counterparts. Some failed to direct expression in some of the cells in which C. elegans cis elements were active, others drove expression in additional cells. For reasons of brevity, in the following we will refer to the former as “losses” and to the latter as “gains” or ectopic expression, without the implication that these reflect differences in endogenous expression patterns. They do, however, reveal instances of divergence of the regulatory mechanisms controlling expression of orthologous genes in the examined species.
“Losses” of expression in the endogenous pattern typically affected single cell types. In two cases (unc-46 and unc-25; Figures 2B and 6B), the expression patterns driven by the C. elegans CREs were completely recapitulated by all heterologous CREs. In three instances (unc-47, gpa-5, and oig-1; Figures 4B, 7B, and 8B), while the patterns were qualitatively conserved, portions directed by one or more heterologous CREs were markedly decreased, in frequency or intensity. For example, the C. remanei cis element of unc-47 drives weak and inconsistent expression in the neuron RIS (Figure 4B), the C. briggsae and C. brenneri CREs of gpa-5 direct weak and inconsistent expression in AWAL/R (Figure 7B), and the C. remanei, C. brenneri and C. japonica CREs of oig-1 are expressed inconsistently in DVB (Figure 8B). The C. japonica CRE of acr-14 fails to direct expression in several cell types in the ventral nerve cord, only maintaining expression in D-type neurons, while expression in AVAL/R is much weaker than with other species' CREs (Figure 3B). The C. remanei and C. brenneri CREs of kat-1 fail to drive expression in the gonadal sheath (Figure 5B), the somatic tissue enveloping the proximal gonad. In the most severe case, mod-5, the CREs from C. briggsae and C. remanei only support expression in ADFL/R (Figure 9B).
In addition to “losses” of expression in subsets of endogenous patterns, most heterologous cis elements also drove ectopic expression. Indeed, only six tested CREs did not show any evidence of “gain” of expression: C. remanei unc-47 (Figure 4B), C. brenneri unc-25 (Figure 6B), C. remanei gpa-5 (Figure 7B), and all three heterologous cis elements of mod-5 (Figure 9B). Ectopic expression was seen in as few as one and as many as five different cell types, depending on the gene. In some cases, this expression was driven in the same cells or tissues by all heterologous CREs of a given gene: unidentified lateral ganglion neurons in the head (unc-46, Figure 2B), AVnL/R neurons in the lateral ganglion (acr-14, Figure 3B), and head muscles (kat-1, Figure 5B). In other instances, only some of the orthologous elements directed co-occurring expression: HSNL/R for unc-46 (Figure 2B), hypodermis for kat-1 (Figure 5B), DVB for gpa-5 (Figure 7B), and ADEL/R, PDEL/R, HSNL/R with oig-1 (Figure 8B).
The results described above reveal pervasive divergence in cis-regulatory function. However, divergence can also stem from changes in trans regulators [2], [3]. To test whether the trans environments were functionally equivalent between species, we compared spatial expression patterns driven by four C. briggsae CREs in C. elegans and C. briggsae. Although expression patterns generated by these sequences were qualitatively similar between the two species, in every instance there were reproducible differences as well (Figure S2). These results further reinforce the notion that divergence has taken place in both cis - and trans-regulatory mechanisms.
Discussion
We carried out functional comparisons of orthologous regulatory elements of eight genes from Caenorhabditis nematodes. Our experimental paradigm, placing orthologous cis elements into the common trans-regulatory environment of C. elegans, allows inferences to be made about the extent of functional divergence between C. elegans CREs and their orthologs from other species. Because we selected genes expressed in relatively simple patterns, we were able to detect even subtle differences. Our results support four notable conclusions.
Divergence is pervasive
Most of the orthologous cis elements we analyzed directed patterns of expression in C. elegans that either substantially or completely matched the expression patterns of the orthologous C. elegans CREs (Figures 2–8; with the possible exception of mod-5, Figure 9). This result, supported by 30 transgenes, suggests that the mechanisms controlling orthologous gene expression are largely conserved among the studied species. Yet, in the vast majority of these cases (27/30), orthologous CREs directed expression patterns that differed from their C. elegans counterparts. These differences were fairly subtle, typically affecting only a few cells, as previously reported in other species [65]–[67] highlighting the value of detailed, focused, multi-gene analyses to reveal trends. Differences in the lengths of tested cis elements did not appear to correlate with the observed differences in expression patterns (Figure S3).
We observed “losses”, as well as “gains” of expression, as compared to the patterns generated by the C. elegans CREs. Even cis elements from two closely related species, C. briggsae and C. remanei, often differed in the expression patterns they directed, indicating that divergence could accumulate relatively quickly. Because in most instances it is difficult to establish the precise endogenous expression patterns of the genes, the observed differences either reflect lineage-specific changes in gene expression or divergence in the mechanisms that regulate conserved expression. In several cases, however, compelling indirect evidence points to the latter scenario.
Three of the eight genes in this study, unc-25, unc-46, and unc-47, are terminal effectors of the GABAergic neuronal fate. Immunostaining for GABA in C. elegans [68], Ascaris suum [69], and C. briggsae and C. remanei (AB & IR, unpublished data) revealed very similar patterns. Furthermore, the expression driven by the C. briggsae unc-47 CRE in its endogenous trans-regulatory environment is identical to that driven by the C. elegans unc-47 CRE in C. elegans [22]. Similarly, patterns of immunostaining for serotonin in C. elegans, C. briggsae, and C. remanei were identical [61], [70]. These results suggest that the number and relative position of GABAergic and serotonergic neurons, and thus the expression patterns of key genes defining these neuronal fates (the three GABA genes above and mod-5), are conserved among these Caenorhabditis nematodes. Thus, differences in cis regulatory elements of these four genes (Figures 2B, 4B, 6B, 9B) likely reveal changes in the specific ways in which these conserved expression patterns are encoded. This interpretation stresses noticeable divergence in gene regulation even between closely related lineages, consistent with what has been seen in others species [71], [72]. This view suggests that changes in trans-regulatory mechanisms and cis-regulatory elements accumulate in a somewhat compensatory fashion to ensure that the overall expression patterns of genes remain conserved [22], [35], [42], [73]. The different expression patterns of four C. briggsae CREs in C. elegans and C. briggsae (Figure S2) support the idea that trans-regulatory divergence is prevalent.
Sequence conservation and functional divergence
Consistent with previous reports [73]–[75], we saw no obvious correspondence between the extent of large-scale sequence conservation and functional conservation. For example, while the CREs of unc-25 and oig-1 show relatively scant primary sequence conservation, their functions appear to be conserved no less well (Figures 6, 8) than those of genes with apparently greater sequence conservation (e.g. unc-46, Figure 2). Sequence comparisons in noncoding regions, particularly when these are of different length, are notoriously challenging. Other metrics of sequence similarity, like the portion of the CRE that is conserved, also failed to reveal a discernible relationship to functional conservation (Figure S3, Table S1). We also tested shorter cis elements of mod-5 and unc-25 that excluded the majority of conserved sequence blocks; their expression patterns were qualitatively similar to those of their longer counterparts (data not shown). These findings are consistent with previous reports that conserved expression patterns can be driven by highly divergent regulatory elements [76]–[83]. Previous research suggested that at least in some instances, long tracts of conserved sequences in cis elements may reflect particular features of regulatory organization, rather than unusually stringent selection for the maintenance of expression patterns [84].
Collectively, these results suggest that we may need to reevaluate a common reliance on large-scale sequence conservation when using comparative sequence data to identify cis-regulatory elements. Presence or absence of transcription factors binding sites, their arrangement and spacing may be more informative, although harder to detect [73], [85]–[89].
We did not detect greater functional divergence of CREs from the more distant C. japonica compared to C. briggsae, C. remanei, and C. brenneri. Among the six genes that have been tested from all four of these species, C. japonica cis elements show approximately the same number of “gains” and “losses” as their orthologs from other species (Table S2). It is possible that the ∼2-fold difference in the phylogenetic distance [40] separating, on the one hand, C. elegans and C. japonica and, on the other hand, C. elegans and C. briggsae/C. remanei/C. brenneri, does not offer enough power to test this hypothesis. Examining more distantly related pairs of species may be required. Finally, the complexity of the expression pattern of a gene does not seem to be correlated with the amount of functional divergence in its cis element (Figure S3).
“Gains” are more common than “losses”
One striking pattern evident in our results is that a substantial majority of functional differences between orthologous cis elements is due to “gain”, rather than “loss” or reduction, of expression relative to the pattern directed by the C. elegans CREs. Put another way, when tested in C. elegans, heterologous regulatory elements more commonly directed expression in more rather than fewer cells, compared to the C. elegans-driven patterns. When all experiments reported here are considered together, the total number of “gains” was nearly three-fold higher than the number of “losses” (51 vs. 18). Even when minor differences in patterns are counted as “losses”, their number (23) is still less than half than that of “gains” (51). This phenomenon does not appear to be due to greater power to detect “gains” compared to “losses” (Figure S4). Restricting comparisons only to those genes for which all four non-C. elegans species were tested, does not substantially alter this conclusion (12 vs. 44 or 16 vs. 44, if “losses” are counted more liberally). Therefore, our results suggest that the two regulatory modalities, namely one directing expression in certain cells and another repressing inappropriate expression, evolve at different rates. The molecular mechanisms and evolutionary forces that could account for this observation remain to be investigated. It is possible, however, that the positive and negative regulatory aspects of gene regulation evolve under different regimes, because of the difference in the ways in which they are encoded within cis elements.
Recurrent divergence patterns suggest developmental bias in evolutionary trajectories
The relatively large number of cases in which heterologous cis elements directed ectopic expression when in C. elegans, allowed us to investigate whether these “gains” followed a pattern. Notably, for the neuronal genes unc-46, acr-14, unc-47, unc-25, oig-1, and gpa-5, nearly all “gains” occurred in neurons (Figures 2–4, 6–8). This tropism suggests that the regulatory architecture of neuronal CREs – some transcriptional inputs are pan-neuronal in nature [90], [91] – may restrict ectopic expression to neurons. We further noted that in several instances, CREs of different genes or from different species directed ectopic expression in the same cells (Figure 10). The cells “gaining” expression do not appear to be transcriptionally promiscuous, because ectopic expression is seen in several different cells not previously noted for indiscriminate expression (Text S1). Furthermore, the “gain” of expression is not likely to be due to effects of vector sequences. We used a standard vector utilized by us and others thousands of times. Previous studies using this vector documented ectopic expression in the intestine and pharynx [49], [92], not specific subsets of neurons, as we reported here. Instead, we favor a hypothesis that the cis elements themselves could be sharing certain characteristics that make them more likely to direct expression in particular cells. The recurrent “gains” of expression were seen for unc-46, acr-14, unc-47, and oig-1, which are coexpressed in a subset of GABAergic neurons and are know to be coregulated by at least one transcription factor, UNC-30 [58]. It is therefore plausible that these cis elements share some features, for example transcription factor binding sites or general organization, and that this similarity may bias the trajectories that evolution could follow [15]. This may in part account for the commonly observed instances of parallel evolution [33], [93]–[95].
Fig. 10. Recurrent “gains” of expression by different CREs. 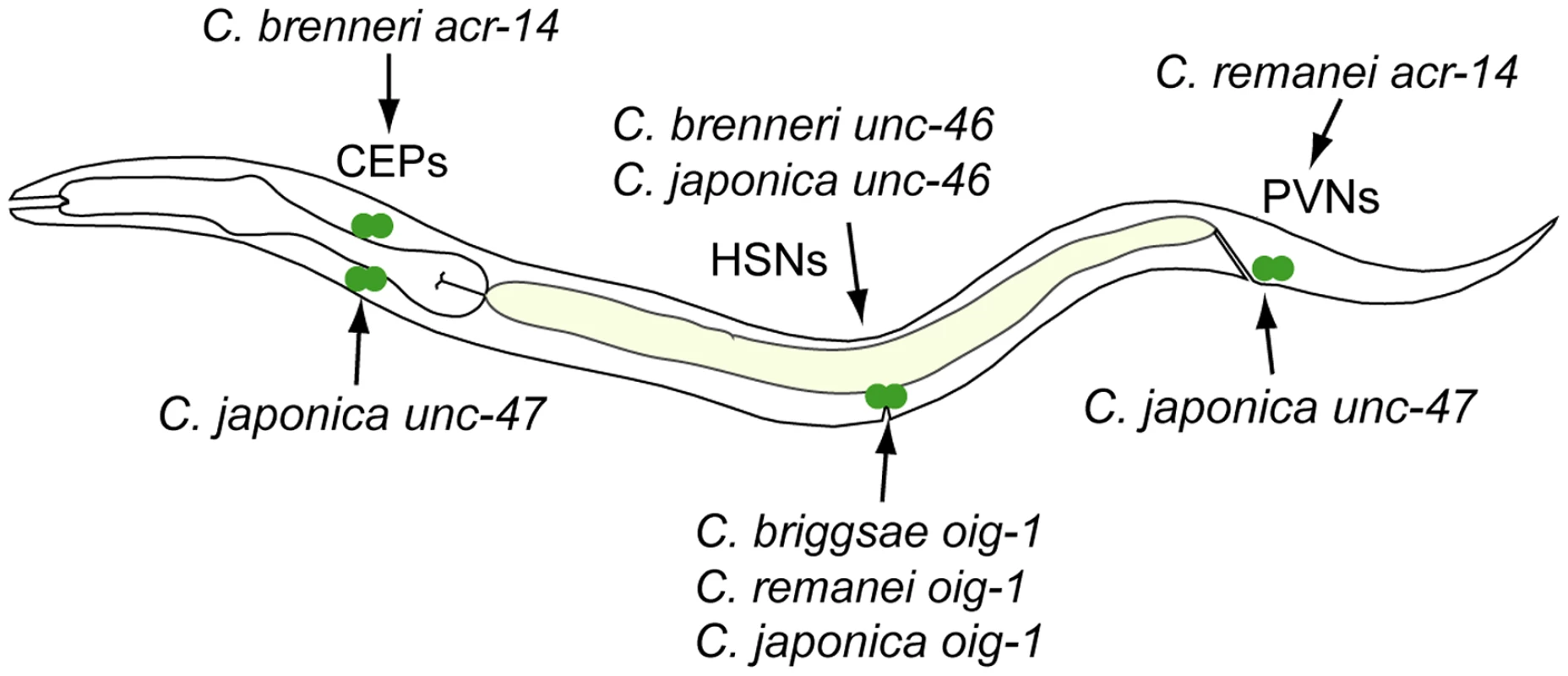
CREs of acr-14 from C. brenneri (Figure 3B) and unc-47 from C. japonica (Figure 4B) drive expression in CEP neurons in the head. CREs of unc-46 from C. brenneri and C. japonica (Figure 2B) and oig-1 from C. briggsae, C. remanei and C. brenneri (Figure 8B) drive expression in HSN neurons in the mid-body. CREs of acr-14 from C. remanei (Figure 3B) and unc-47 from C. japonica (Figure 4B) drive expression in PVN neurons in the tail. With this survey, we established several trends of functional conservation and divergence of cis-regulatory elements. We found pervasive functional divergence in transcriptional regulatory mechanisms, both in cis and in trans. More strikingly, we identified inherent biases in the nature and functional consequences of this divergence, hinting at possible mechanisms underlying repeated evolution.
Materials and Methods
Cloning of cis-regulatory elements
Putative cis-regulatory elements (extending from the first exon to the nearest upstream gene) were PCR amplified from genomic DNA using Phusion polymerase and cloned upstream of GFP into the pPD95.75 plasmid, routinely used for analysis of gene expression in C. elegans [96]. Cloned fragments were sequenced to ensure accuracy. C. elegans CREs of unc-46, acr-14, kat-1, unc-25, and oig-1, were also cloned into the plasmid HYM153 (kind gift of H.-Y. Mak) upstream of the mCherry reporter gene as controls.
Strains
C. elegans transgenic lines were established by injecting into pha-1(e2123) worms cocktails consisting of 5 ng/µL CRE::GFP reporter constructs with 5 ng/µL rescue plasmid [97] and 100 ng/µL salmon sperm DNA; this is thought to facilitate the formation of complex transgenic constructs as extrachromosomal arrays [98]. For five genes (unc-46, acr-14, kat-1, unc-25, and oig-1), plasmids carrying C. elegans CREs fused to mCherry were coinjected with the plasmids carrying orthologous CREs from each of the five species fused to GFP. C. briggsae transgenic lines were established by injecting cocktails consisting of 5 ng/µL CRE::GFP reporter constructs with 5 ng/µL rescue plasmid and 100 ng/µL salmon sperm DNA into Cbr-unc-119 (nm67) worms [99].
Microscopy
Mixed-stage populations of transgenic worms were grown with abundant food and L4-stage larvae or young adults were selected. These were immobilized on agar slides with 10 mM sodium azide in M9 buffer. The slides were examined on a Leica DM5000B compound microscope under 400-fold magnification. Worms without any visible GFP expression were assumed to have lost the transgene. Each photograph showing worms in figures is composed of several images of the same individual capturing anterior, middle, and posterior sections.
Analysis
At least fifty individuals from no fewer than two independent strains were analyzed for each transgene. The plasmid pPD95.75 has been used extensively by the C. elegans community over the last two decades. It has been reported to direct low-level background expression in the pharynx and anterior and posterior intestine [49], [92], [96]. We have previously reported that extrachromosomal arrays direct expression patterns that are concordant with those of integrated and single-copy transgenes [22], [100]. Still, to obtain conservative estimates of expression differences between CREs from C. elegans and other species, we only counted discrepancies (missing or extra expression) observed in two or more strains. Data on consistency of expression patterns between strains and individuals are presented in Tables S1, S2, S3, S4, S5, S6, S7, S8 and S10.
Supporting Information
Zdroje
1. WrayGA, HahnMW, AbouheifE, BalhoffJP, PizerM, et al. (2003) The evolution of transcriptional regulation in eukaryotes. Mol Biol Evol 20 : 1377–1419 doi:10.1093/molbev/msg140
2. LynchVJ, WagnerGP (2008) Resurrecting the role of transcription factor change in developmental evolution. Evolution 62 : 2131–2154 doi:10.1111/j.1558-5646.2008.00440.x
3. HsiaCC, McGinnisW (2003) Evolution of transcription factor function. Curr Opin Genet Dev 13 : 199–206 doi:10.1016/S0959-437X(03)00017-0
4. TuchBB, GalgoczyDJ, HerndayAD, LiH, JohnsonAD (2008) The evolution of combinatorial gene regulation in fungi. PLoS Biol 6: e38 doi:10.1371/journal.pbio.0060038
5. WrayGA (2007) The evolutionary significance of cis-regulatory mutations. Nat Rev Genet 8 : 206–216 doi:10.1038/nrg2063
6. CarrollSB (2000) Endless forms: the evolution of gene regulation and morphological diversity. Cell 101 : 577–580.
7. BremRB, YvertG, ClintonR, KruglyakL (2002) Genetic dissection of transcriptional regulation in budding yeast. Science 296 : 752–755 doi:10.1126/science.1069516
8. Stern DL (2010) Evolution, development, and the predictable genome. 1st ed. Roberts & Company Publishers.
9. WittkoppPJ (2005) Genomic sources of regulatory variation in cis and in trans. Cell Mol Life Sci 62 : 1779–1783 doi:10.1007/s00018-005-5064-9
10. GruberJD, VogelK, KalayG, WittkoppPJ (2012) Contrasting properties of gene-specific regulatory, coding, and copy number mutations in Saccharomyces cerevisiae: frequency, effects, and dominance. PLoS Genet 8: e1002497 doi:10.1371/journal.pgen.1002497
11. LandryCR, LemosB, RifkinSA, DickinsonWJ, HartlDL (2007) Genetic properties influencing the evolvability of gene expression. Science 317 : 118–121 doi:10.1126/science.1140247
12. Davidson EH (2001) Genomic Regulatory Systems: Development and Evolution. Academic Press.
13. PollardDA, MosesAM, IyerVN, EisenMB (2006) Detecting the limits of regulatory element conservation and divergence estimation using pairwise and multiple alignments. BMC Bioinformatics 7 : 376 doi:10.1186/1471-2105-7-376
14. HoMCW, JohnsenH, GoetzSE, SchillerBJ, BaeE, et al. (2009) Functional evolution of cis-regulatory modules at a homeotic gene in Drosophila. PLoS Genet 5: e1000709 doi:10.1371/journal.pgen.1000709
15. BrownCD, JohnsonDS, SidowA (2007) Functional Architecture and Evolution of Transcriptional Elements That Drive Gene Coexpression. Science 317 : 1557–1560 doi:10.1126/science.1145893
16. CameronRA, DavidsonEH (2009) Flexibility of transcription factor target site position in conserved cis-regulatory modules. Dev Biol 336 : 122–135 doi:10.1016/j.ydbio.2009.09.018
17. GordonKL, RuvinskyI (2012) Tempo and mode in evolution of transcriptional regulation. PLoS Genet 8: e1002432 doi:10.1371/journal.pgen.1002432
18. WangX, ChamberlinHM (2002) Multiple regulatory changes contribute to the evolution of the Caenorhabditis lin-48 ovo gene. Genes Dev 16 : 2345–2349 doi:10.1101/gad.996302
19. WangX, GreenbergJF, ChamberlinHM (2004) Evolution of regulatory elements producing a conserved gene expression pattern in Caenorhabditis. Evol Dev 6 : 237–245 doi:10.1111/j.1525-142X.2004.04029.x
20. MarcelliniS, SimpsonP (2006) Two or four bristles: functional evolution of an enhancer of scute in Drosophilidae. PLoS Biol 4: e386 doi:10.1371/journal.pbio.0040386
21. SungH-M, WangT-Y, WangD, HuangY-S, WuJ-P, et al. (2009) Roles of trans and cis variation in yeast intraspecies evolution of gene expression. Mol Biol Evol 26 : 2533–2538 doi:10.1093/molbev/msp171
22. BarrièreA, GordonKL, RuvinskyI (2012) Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration. PLoS Genet 8: e1002961 doi:10.1371/journal.pgen.1002961
23. ChiangDY, MosesAM, KellisM, LanderES, EisenMB (2003) Phylogenetically and spatially conserved word pairs associated with gene-expression changes in yeasts. Genome Biol 4: R43 doi:10.1186/gb-2003-4-7-r43
24. GradYH, RothFP, HalfonMS, ChurchGM (2004) Prediction of similarly acting cis-regulatory modules by subsequence profiling and comparative genomics in Drosophila melanogaster and D.pseudoobscura. Bioinformatics 20 : 2738–2750 doi:10.1093/bioinformatics/bth320
25. RebeizM, CastroB, LiuF, YueF, PosakonyJW (2012) Ancestral and conserved cis-regulatory architectures in developmental control genes. Dev Biol 362 : 282–294 doi:10.1016/j.ydbio.2011.12.011
26. HareEE, PetersonBK, IyerVN, MeierR, EisenMB (2008) Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genet 4: e1000106 doi:10.1371/journal.pgen.1000106
27. CandeJ, GoltsevY, LevineMS (2009) Conservation of enhancer location in divergent insects. Proc Natl Acad Sci 106 : 14414–14419 doi:10.1073/pnas.0905754106
28. PanD, ValentineSA, CoureyAJ (1994) The bipartite D. melanogaster twist promoter is reorganized in D. virilis. Mech Dev 46 : 41–53.
29. HauenschildA, RingroseL, AltmutterC, ParoR, RehmsmeierM (2008) Evolutionary plasticity of polycomb/trithorax response elements in Drosophila species. PLoS Biol 6: e261 doi:10.1371/journal.pbio.0060261
30. KalayG, WittkoppPJ (2010) Nomadic enhancers: tissue-specific cis-regulatory elements of yellow have divergent genomic positions among Drosophila species. PLoS Genet 6: e1001222 doi:10.1371/journal.pgen.1001222
31. FrankelN, ErezyilmazDF, McGregorAP, WangS, PayreF, et al. (2011) Morphological evolution caused by many subtle-effect substitutions in regulatory DNA. Nature 474 : 598–603 doi:10.1038/nature10200
32. LudwigMZ, BergmanC, PatelNH, KreitmanM (2000) Evidence for stabilizing selection in eukaryotic enhancer element. Nature 403 : 564–567.
33. GompelN, Prud'hommeB, WittkoppPJ, KassnerVA, CarrollSB (2005) Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433 : 481–487 doi:10.1038/nature03235
34. Prud'hommeB, GompelN, RokasA, KassnerVA, WilliamsTM, et al. (2006) Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440 : 1050–1053 doi:10.1038/nature04597
35. TrueJR, HaagES (2001) Developmental system drift and flexibility in evolutionary trajectories. Evol Dev 3 : 109–119.
36. SulstonJE, HorvitzHR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56 : 110–156.
37. SulstonJE, SchierenbergE, WhiteJG, ThomsonJN (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100 : 64–119.
38. ZhaoZ, BoyleTJ, BaoZ, MurrayJI, MericleB, et al. (2008) Comparative analysis of embryonic cell lineage between Caenorhabditis briggsae and Caenorhabditis elegans. Dev Biol 314 : 93–99 doi:10.1016/j.ydbio.2007.11.015
39. ElementoO, TavazoieS (2005) Fast and systematic genome-wide discovery of conserved regulatory elements using a non-alignment based approach. Genome Biol 6: R18 doi:10.1186/gb-2005-6-2-r18
40. Kiontke K, Fitch DHA (2005) The phylogenetic relationships of Caenorhabditis and other rhabditids. WormBook : the online review of C. elegans biology. pp. 1–11. doi:10.1895/wormbook.1.11.1.
41. TiroshI, BarkaiN (2011) Inferring regulatory mechanisms from patterns of evolutionary divergence. Mol Syst Biol 7 : 530 doi:10.1038/msb.2011.60
42. LandryCR, WittkoppPJ, TaubesCH, RanzJM, ClarkAG, et al. (2005) Compensatory cis-trans evolution and the dysregulation of gene expression in interspecific hybrids of Drosophila. Genetics 171 : 1813–1822 doi:10.1534/genetics.105.047449
43. WittkoppPJ, HaerumBK, ClarkAG (2008) Regulatory changes underlying expression differences within and between Drosophila species. Nat Genet 40 : 346–350 doi:10.1038/ng.77
44. TiroshI, ReikhavS, LevyAA, BarkaiN (2009) A yeast hybrid provides insight into the evolution of gene expression regulation. Science 324 : 659–662 doi:10.1126/science.1169766
45. HillierLW, MillerRD, BairdSE, ChinwallaA, FultonLA, et al. (2007) Comparison of C. elegans and C. briggsae genome sequences reveals extensive conservation of chromosome organization and synteny. PLoS Biol 5: e167 doi:10.1371/journal.pbio.0050167
46. BarrièreA, YangS-P, PekarekE, ThomasCG, HaagES, et al. (2009) Detecting heterozygosity in shotgun genome assemblies: Lessons from obligately outcrossing nematodes. Genome Res 19 : 470–480 doi:10.1101/gr.081851.108.470
47. KennedyBP, AamodtEJ, AllenFL, ChungMA, HeschlMFP, et al. (1993) The Gut Esterase Gene (ges-1) From the Nematodes Caenorhabditis elegans and Caenorhabditis briggsae. J Mol Biol 229 : 890–908.
48. MaduroMF, PilgrimD (1996) Conservation of function and expression of unc-119 from two Caenorhabditis species despite divergence of non-coding DNA. Gene 183 : 77–85.
49. RuvinskyI, RuvkunG (2003) Functional tests of enhancer conservation between distantly related species. Development 130 : 5133–5142 doi:10.1242/dev.00711
50. PetalcorinMIR, JoshuaGW, AgapowP-M, DolphinCT (2005) The fmo genes of Caenorhabditis elegans and C. briggsae: characterisation, gene expression and comparative genomic analysis. Gene 346 : 83–96 doi:10.1016/j.gene.2004.09.021
51. MarriS, GuptaBP (2009) Dissection of lin-11 enhancer regions in Caenorhabditis elegans and other nematodes. Dev Biol 325 : 402–411 doi:10.1016/j.ydbio.2008.09.026
52. KiontkeKC, FélixM-A, AilionM, Rockman MV, BraendleC, et al. (2011) A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol Biol 11 : 339 doi:10.1186/1471-2148-11-339
53. KiontkeK, GavinNP, RaynesY, RoehrigC, PianoF, et al. (2004) Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci 101 : 9003–9008 doi:10.1073/pnas.0403094101
54. Poinar Jr. GO (2011) The evolutionary history of nematodes. Leiden, the Netherlands: Brill.
55. JinY, JorgensenEM, HartwiegE, HorvitzHR (1999) The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci 19 : 539–548.
56. SchuskeK, PalfreymanMT, WatanabeS, JorgensenEM (2007) UNC-46 is required for trafficking of the vesicular GABA transporter. Nat Neurosci 10 : 846–853 doi:10.1038/nn1920
57. McIntireSL, ReimerRJ, SchuskeK, EdwardsRH, JorgensenEM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389 : 870–876 doi:10.1038/39908
58. CinarH, KelesS, JinY (2005) Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Curr Biol 15 : 340–346 doi:10.1016/j.cub.2005.02.025
59. JansenG, ThijssenK, WernerP (1999) The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet 21 : 414–419.
60. RanganathanR, SawinER, TrentC, HorvitzHR (2001) Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci 21 : 5871–5884.
61. LoerCM, RivardL (2007) Evolution of Neuronal Patterning in Free-Living Rhabditid Nematodes I : Sex - Specific Serotonin-Containing Neurons. J Comp Neurol 502 : 736–767 doi:10.1002/cne
62. BerdichevskyA, NedelcuS, BouliasK, BishopNA, GuarenteL, et al. (2010) 3-Ketoacyl thiolase delays aging of Caenorhabditis elegans and is required for lifespan extension mediated by sir-2.1. Proc Natl Acad Sci 107 : 18927–18932 doi:10.1073/pnas.1013854107
63. MakHY, NelsonLS, BassonM, JohnsonCD, RuvkunG (2006) Polygenic control of Caenorhabditis elegans fat storage. Nat Genet 38 : 363–368 doi:10.1038/ng1739
64. FrazerKA, PachterL, PoliakovA, RubinEM, DubchakI (2004) VISTA: computational tools for comparative genomics. Nucleic Acids Res 32: W273–9 doi:10.1093/nar/gkh458
65. LudwigMZ, PalssonA, AlekseevaE, BergmanCM, NathanJ, et al. (2005) Functional evolution of a cis-regulatory module. PLoS Biol 3: e93 doi:10.1371/journal.pbio.0030093
66. CrockerJ, TamoriY, ErivesA (2008) Evolution acts on enhancer organization to fine-tune gradient threshold readouts. PLoS Biol 6: e263 doi:10.1371/journal.pbio.0060263
67. WunderlichZ, BragdonMD, EckenrodeKB, Lydiard-MartinT, Pearl-WasermanS, et al. (2012) Dissecting sources of quantitative gene expression pattern divergence between Drosophila species. Mol Syst Biol 8 : 604 doi:10.1038/msb.2012.35
68. McIntireSL, JorgensenEM, KaplanJ, HorvitzHR (1993) The GABAergic nervous system of Caenorhabditis elegans. Nature 364 : 337–341.
69. GuastellaJ, JohnsonCD, StrettonAO (1991) GABA-immunoreactive neurons in the nematode Ascaris. J Comp Neurol 307 : 584–597 doi:10.1002/cne.903070406
70. RivardL, SrinivasanJ, StoneA, OchoaS, SternbergPW, et al. (2010) A comparison of experience-dependent locomotory behaviors and biogenic amine neurons in nematode relatives of Caenorhabditis elegans. BMC Neurosci 11 : 22 doi:10.1186/1471-2202-11-22
71. McManusCJ, CoolonJD, O'DuffM, Eipper-MainsJ, GraveleyBR, et al. (2010) Regulatory divergence in Drosophila revealed by mRNA-seq. Genome Res 20 : 816–825 doi:10.1101/gr.102491.109
72. FowlkesCC, EckenrodeKB, BragdonMD, MeyerM, WunderlichZ, et al. (2011) A conserved developmental patterning network produces quantitatively different output in multiple species of Drosophila. PLoS Genet 7: e1002346 doi:10.1371/journal.pgen.1002346
73. TiroshI, WeinbergerA, BezalelD, KaganovichM, BarkaiN (2008) On the relation between promoter divergence and gene expression evolution. Mol Syst Biol 4 : 159 doi:10.1038/msb4100198
74. McGaugheyDM, VintonR, HuynhJ (2008) Metrics of sequence constraint overlook regulatory sequences in an exhaustive analysis at phox2b. Genome Res 18 : 252–260 doi:10.1101/gr.6929408.1
75. RitterDI, LiQ, KostkaD, PollardKS, GuoS, et al. (2010) The importance of being cis: evolution of orthologous fish and mammalian enhancer activity. Mol Biol Evol 27 : 2322–2332 doi:10.1093/molbev/msq128
76. TakahashiH, MitaniY, SatohG, SatohN (1999) Evolutionary alterations of the minimal promoter for notochord-specific Brachyury expression in ascidian embryos. Development 126 : 3725–3734.
77. Oda-IshiiI, BertrandV, MatsuoI, LemaireP, SaigaH (2005) Making very similar embryos with divergent genomes: conservation of regulatory mechanisms of Otx between the ascidians Halocynthia roretzi and Ciona intestinalis. Development 132 : 1663–1674 doi:10.1242/dev.01707
78. DutilhBE, HuynenMA, SnelB (2006) A global definition of expression context is conserved between orthologs, but does not correlate with sequence conservation. BMC Genomics 7 : 10 doi:10.1186/1471-2164-7-10
79. FisherS, GriceEA, VintonRM, BesslingSL, McCallionAS (2006) Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science 312 : 276–279 doi:10.1126/science.1124070
80. CooperGM, BrownCD (2008) Qualifying the relationship between sequence conservation and molecular function. Genome Res 18 : 201–205 doi:10.1101/gr.7205808.4
81. TaherL, McGaugheyDM, MaraghS, AneasI, BesslingSL, et al. (2011) Genome-wide identification of conserved regulatory function in diverged sequences. Genome Res 21 : 1139–1149 doi:10.1101/gr.119016.110
82. SwansonCI, SchwimmerDB, BaroloS (2011) Rapid Evolutionary Rewiring of a Structurally Constrained Eye Enhancer. Curr Biol 21 : 1–11 doi:10.1016/j.cub.2011.05.056
83. NelsonAC, WardleFC (2013) Conserved non-coding elements and cis regulation: actions speak louder than words. Development 140 : 1385–1395 doi:10.1242/dev.084459
84. BullaugheyK (2011) Changes in selective effects over time facilitate turnover of enhancer sequences. Genetics 187 : 567–582 doi:10.1534/genetics.110.121590
85. ErivesA, LevineM (2004) Coordinate enhancers share common organizational features in the Drosophila genome. Proc Natl Acad Sci 101 : 3851–3856 doi:10.1073/pnas.0400611101
86. CrockerJ, ErivesA (2008) A closer look at the eve stripe 2 enhancers of Drosophila and Themira. PLoS Genet 4: e1000276 doi:10.1371/journal.pgen.1000276
87. KimJ, CunninghamR, JamesB, WyderS, GibsonJD, et al. (2010) Functional characterization of transcription factor motifs using cross-species comparison across large evolutionary distances. PLoS Comput Biol 6: e1000652 doi:10.1371/journal.pcbi.1000652
88. SwansonCI, EvansNC, BaroloS (2010) Structural rules and complex regulatory circuitry constrain expression of a Notch - and EGFR-regulated eye enhancer. Dev Cell 18 : 359–370 doi:10.1016/j.devcel.2009.12.026
89. LibermanLM, StathopoulosA (2009) Design flexibility in cis-regulatory control of gene expression: synthetic and comparative evidence. Dev Biol 327 : 578–589 doi:10.1016/j.ydbio.2008.12.020
90. RuvinskyI, OhlerU, BurgeCB, RuvkunG (2007) Detection of broadly expressed neuronal genes in C. elegans. Dev Biol 302 : 617–626 doi:10.1016/j.ydbio.2006.09.014
91. HobertO (2011) Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol 27 : 681–696 doi:10.1146/annurev-cellbio-092910-154226
92. Boulin T, Etchberger JF, Hobert O (2006) Reporter gene fusions. WormBook : the online review of C. elegans biology. pp. 1–23. doi:10.1895/wormbook.1.106.1.
93. SternDL, OrgogozoV (2009) Is genetic evolution predictable? Science 323 : 746–751.
94. RajakumarR, San MauroD, DijkstraMB, HuangMH, WheelerDE, et al. (2012) Ancestral developmental potential facilitates parallel evolution in ants. Science 335 : 79–82 doi:10.1126/science.1211451
95. RogersWA, SalomoneJR, TacyDJ, CaminoEM, DavisKA, et al. (2013) Recurrent modification of a conserved cis-regulatory element underlies fruit fly pigmentation diversity. PLoS Genet 9: e1003740 doi:10.1371/journal.pgen.1003740
96. Xu S, Fire A, Seydoux G, Okkema P (1995) Fire Lab Vector Kit - June 1995.
97. GranatoM, SchnabelH, SchnabelR (1994) pha-1, a selectable marker for gene transfer in C. elegans. Nucleic Acids Res 22 : 1762–1763.
98. KellyW, XuS, MontgomeryM, FireA (1997) Distinct requirements for somatic and germline expression of a generally expressed Caenorhabditis elegans gene. Genetics 146 : 227–238.
99. WoodruffGC, EkeO, BairdSE, FélixM-A, HaagES (2010) Insights into species divergence and the evolution of hermaphroditism from fertile interspecies hybrids of Caenorhabditis nematodes. Genetics 186 : 997–1012 doi:10.1534/genetics.110.120550
100. BarrièreA, GordonKL, RuvinskyI (2011) Distinct Functional Constraints Partition Sequence Conservation in a cis-Regulatory Element. PLoS Genet 7: e1002095 doi:10.1371/journal.pgen.1002095
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



