-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Inflammation: Gone with Translation
article has not abstract
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004442
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004442Summary
article has not abstract
Inflammation (derived from the Latin inflammo, which means “I set alight”) is a beneficial response of our immune system that protects us against infection and tissue injury. Like all immune responses, it needs to be tightly controlled: excessive inflammatory responses can lead to both acute diseases, such as septic shock, and chronic diseases, such as rheumatoid arthritis, atherosclerosis, and cancer. Until recently, it was thought that the negative regulatory loops that kick in early to counteract inflammation were mainly a result of changes in mRNA levels either through transcriptional activation of inhibitory proteins or posttranscriptional repression of proinflammatory molecules by RNA-binding proteins (RBP) or microRNAs. A paper by Georg Stoecklin and colleagues at the German Cancer Research Center now challenges this notion by demonstrating that, in the early stages of macrophage activation, translational derepression is a major mechanism that induces feedback inhibitors to dampen inflammation [1].
Although neutrophils are the first cells that localise to sites of tissue injury or infection, it is macrophages that orchestrate the multiple components of the inflammatory response through their ability to sense microbial products such as lipopolysaccharides (LPS), which bind toll-like receptor 4 (TLR4). TLR4 ligation initiates a signalling cascade in macrophages that leads to the production of proinflammatory molecules, of which tumor necrosis factor (TNF) is one of the most important [2]. TLR4 signalling and TNF signalling activate several transcription factors, most prominently NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells), that are important for the elimination of pathogens, irritants, or dead cells. Equally important for the healing of tissues later in the inflammatory response is the production of negative regulators of the NF-κB pathway and other inflammatory pathways, which limit the amount of inflammatory mediators and curtail the response in a timely manner [3].
Over the last few years, a number of studies have investigated global gene activation induced by pathogen-derived stimuli to gain insights into the modes of induction of the inflammatory mediators and regulators. Studies focusing on the early stages of inflammation only assessed changes in mRNA levels [4] and identified the importance of mRNA stability in the temporal control of inflammatory molecules. Parallel studies that specifically interrogated translational changes only looked at later stages of the response and were performed in cells other than macrophages [5], [6]. Of note, these latter studies reported translational inhibition of ribosomal proteins and RNAs encoding chemoattractants and their receptors. The present study by Schott et al. [1] is the first to investigate translational regulation in the early phase of macrophage activation and to identify translational derepression as an important mechanism underpinning negative feedback of the inflammatory response.
In assessing translation, sucrose density gradient centrifugation of cell extracts is often used to separate cellular mRNAs by their association with ribosomes. In combination with microarray analysis, this approach is known as translation state array analysis (TSAA). TSAA measures the proportion of mRNAs in dense polysomal complexes as an indicator of their translational activity [7]. The investigators treated cultures of the murine RAW264.7 macrophage cell line with LPS for 1 h and performed TSAA, in addition to measuring mRNA steady-state levels in parallel by RNA-seq. This combination allowed them to focus on mRNAs with an active change in ribosome load, identifying 59 cases of translational up-regulation and 55 cases of down-regulation, many of which could be validated by quantitative polymerase chain reaction (qPCR) not only in the original cell line but also in primary cultured macrophages. Four cytokine mRNAs scored as up-regulated (Tnf, Cxcl2, Il23a, and Tnfsf9) while, surprisingly, several mRNAs encoding proteins classed as feedback inhibitors of TLR4 signalling were also actively derepressed in their translation (Figure 1). Among the latter were those encoding likely inhibitors of NF-κB activation (IκBδ, IER3, and NR4A1), the p38 mitogen-activated protein kinase (MAPK) pathway inhibitor dual specificity phosphatase 1 (DUSP1), as well as the RBPs ZFP36 and ZC3H12A, known inhibitors of cytokine expression. Most cytokine mRNAs were scarce in resting macrophages and strongly induced. By contrast, feedback inhibitor mRNAs tended to already be abundant and showed relatively modest increases in steady-state level upon activation.
Fig. 1. Different modes of mRNA regulation in inflammation. 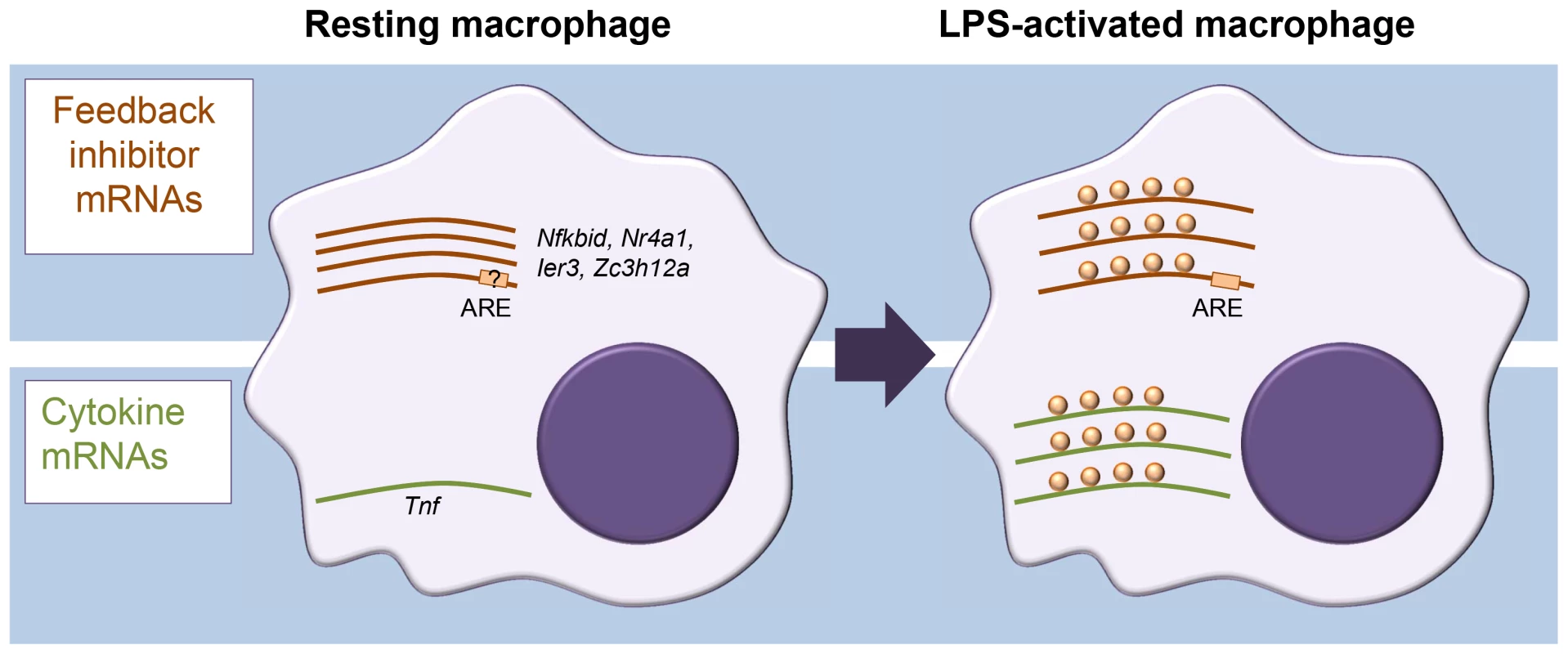
Diagram representing differential control of feedback inhibitor mRNAs and cytokine mRNAs in resting versus activated macrophages. In the resting state, feedback inhibitor mRNAs are being transcribed but translationally repressed; they become derepressed in the early phase of activation. By contrast, most cytokine mRNAs, which are generally unstable, are not expressed in resting macrophages, and their steady-state levels increase quickly upon activation. A motif search by Schott et al. [1] identified an overrepresentation of the adenylate-uridylate-rich element (AU-rich element; ARE) within the 3′ untranslated regions (UTRs) of translationally up-regulated mRNAs. AREs are present in the 3′ UTR of many cytokine mRNAs, including the Tnf mRNA, and recruit a range of RBPs to regulate mRNA stability and translation [8]. The authors further investigated the example of IER3 mRNA 3′ UTR to show that the ARE element was required for repression in resting cells as well as derepression after LPS induction. The present study makes no further inroads into identifying the molecular players and mechanism involved in this ARE-mediated repression/derepression. One intriguing possibility we see is that it could be driven by a switch to aerobic glycolysis commonly seen in activated immune cells [9] and involve ARE-binding by the glycolysis enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as reported for interferon γ (IFNγ) mRNA during T cell activation [10]. Binding of metabolic enzymes to RNA has been repeatedly reported and might underpin a broader crosstalk between metabolism and gene regulation and offer novel therapeutic possibilities [11].
The corollary from the studies by Schott et al. [1] is that, upon macrophage activation, expression of several anti-inflammatory inhibitors is driven by translational up-regulation of preexisting mRNAs. That cells choose the relatively resource-costly approach of stockpiling these mRNAs highlights their need to swiftly mount a feedback response to contain the inflammatory response. By contrast, induction of proinflammatory cytokines is predominately achieved by changes in mRNA abundance. This adds a new facet to the multilevel gene regulatory network controlling the inflammatory response. While we have known about the post-transcriptional regulation of cytokine mRNAs for some time, there is now a need to better understand how cells ensure the swift production of feedback inhibitors using translational control.
Zdroje
1. SchottJ, ReitterS, PhilippJ, HanekeK, SchäferH, et al. (2014) Translational regulation of specific mRNAs controls feedback inhibition and survival during macrophage activation. PLoS Genet 10: e1004368 doi: 10.1371/journal.pgen.1004368
2. BradleyJR (2008) TNF-mediated inflammatory disease. J Pathol 214 : 149–160.
3. RulandJ (2011) Return to homeostasis: downregulation of NF-kappaB responses. Nat Immunol 12 : 709–714.
4. HaoS, BaltimoreD (2009) The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol 10 : 281–288.
5. CeppiM, ClavarinoG, GattiE, SchmidtEK, de GassartA, et al. (2009) Ribosomal protein mRNAs are translationally-regulated during human dendritic cells activation by LPS. Immunome Res 5 : 5.
6. VyasK, ChaudhuriS, LeamanDW, KomarAA, MusiyenkoA, et al. (2009) Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol Cell Biol 29 : 458–470.
7. BeilharzTH, PreissT (2004) Translational profiling: the genome-wide measure of the nascent proteome. Brief Funct Genomic Proteomic 3 : 103–111.
8. SchottJ, StoecklinG (2010) Networks controlling mRNA decay in the immune system. Wiley Interdiscip Rev RNA 1 : 432–456.
9. Palsson-McDermottEM, O'NeillLA (2013) The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays 35 : 965–973.
10. ChangCH, CurtisJD, MaggiLBJr, FaubertB, VillarinoAV, et al. (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153 : 1239–1251.
11. HentzeMW, PreissT (2010) The REM phase of gene regulation. Trends Biochem Sci 35 : 423–426.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



