-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
Hormones play major roles in initiating major developmental transitions, such as puberty and metamorphosis. However, how organisms coordinate changes across multiple hormones remains unclear. In this study, we show that silencing the POU domain transcription factor Ventral veins lacking (Vvl)/Drifter in the red flour beetle Tribolium castaneum leads to precocious metamorphosis and an inability to molt. We show that Vvl regulates the biosynthesis and signaling of two key insect developmental hormones, juvenile hormone (JH) and ecdysteroids. Vvl therefore appears to act as a potential central regulator of developmental timing by influencing two major hormones. Because POU factors are known as a major regulator of the onset of puberty, POU factors play a major role during sexual maturation in both vertebrates and insects.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004425
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004425Summary
Hormones play major roles in initiating major developmental transitions, such as puberty and metamorphosis. However, how organisms coordinate changes across multiple hormones remains unclear. In this study, we show that silencing the POU domain transcription factor Ventral veins lacking (Vvl)/Drifter in the red flour beetle Tribolium castaneum leads to precocious metamorphosis and an inability to molt. We show that Vvl regulates the biosynthesis and signaling of two key insect developmental hormones, juvenile hormone (JH) and ecdysteroids. Vvl therefore appears to act as a potential central regulator of developmental timing by influencing two major hormones. Because POU factors are known as a major regulator of the onset of puberty, POU factors play a major role during sexual maturation in both vertebrates and insects.
Introduction
Many organisms, including amphibians, echinoderms, marine invertebrates, vertebrates, and insects, undergo dramatic morphological and behavioral changes when they enter metamorphosis or puberty. In holometabolous insects, or insects that undergo complete metamorphosis, the larva molts several times before transforming into a pupa and ultimately into an adult. In mammals, puberty is also associated with morphological changes and reproductive maturation. These dramatic transformations are orchestrated by neuroendocrine changes that occur during postembryonic development of an organism. While the central nervous system (CNS) is known to regulate these endocrine changes [1]–[6], the link between the CNS and the endocrine centers remains poorly understood.
Members of the POU family have been shown to influence the neuroendocrine system during puberty and early development of vertebrates [7]–[13]. POU proteins have a highly conserved POU homeodomain and regulate gene expression by binding to high-affinity octamer sites [8], [14]. Many known POU factors are expressed in cell - or region-specific patterns within the developing CNS, suggesting a role in neural development. In addition, POU factors regulate the onset of puberty in mammals [13], [15].
Because POU domain transcription factors have been found in both vertebrates and invertebrates, their neuroendocrine functions may be conserved across species. Here, we investigated a homolog of the POU domain transcription factor, Ventral veins lacking (Vvl)/Drifter, in the holometabolous insect, Tribolium castaneum. Vvl has been shown to regulate the development of the tritocerebrum, CNS, peripheral nervous system and trachea in Drosophila embryos [16]–[22]. In addition, Vvl has been shown to regulate the expression of Diapause hormone-pheromone biosynthesis-activating neuropeptide (DH-PBAN) coding gene in the silkworm, Bombyx mori, and in the cotton bollworm, Helicoverpa armigera [23], [24]. More recently, it has been shown that in Drosophila, the endocrine glands, which synthesize metamorphic hormones, and trachea, share the same developmental origin, and that the progenitor cells that give rise to these structures express Vvl [25]. These studies suggest that Vvl, like vertebrate POU factors, may act within the neuroendocrine organs to regulate the biosynthesis of metamorphic hormones.
Many insects undergo three distinct phases of development: the larval, pupal and adult stages. The larval stage is primarily a feeding stage marked by several larval-larval molts (shedding of the cuticle) that enables the larva to grow to a sufficient size. Ecdysteroids and JH are two major developmental hormones involved in the transition from a larva to a pupa [3]. Ecdysteroidogenesis occurs in the prothoracic gland and involves the conversion of cholesterol into precursors of 20-hydroxyecdysone (20E), the primary ecdysteroid involved in molting. These precursors are ecdysone (E) in Drosophila and beetles, or 3-dehydroecdysone in Manduca sexta and a number of other lepidopteran species [26]–[29]. The secretion/synthesis of ecdysone is in turn triggered by prothoracicotropic hormone (PTTH), a neuropeptide released from the corpora cardiaca [3], [30], and by insulin signaling [31], [32]. Inside the prothoracic gland, these signaling pathways regulate the expression of several Halloween family genes, such as spook (spo), phantom (phm), disembodied (dib) and shadow (sad), which code for cytochrome P450 enzymes necessary for catalyzing a series of reactions that ultimately convert cholesterol into E [26], [33]–[35]. Mutations in these genes lead to lowered ecdysteroid titers in Drosophila [33]. The expressions of these genes are regulated dynamically by both PTTH and insulin signaling [34] and correlate with the ecdysteroid titer. Once released into the hemolymph, E is converted to 20E in the peripheral tissues by shade (shd) [36].
At the target tissues, ecdysteroids act by binding to the nuclear hormone receptor Ecdysone receptor (EcR), a heterodimer with the RXR homolog Ultraspiracle (Usp). Knockdown of EcR or Usp expression leads to disrupted molting and metamorphosis [37]–[40]. Once 20E binds to EcR, a series of ecdysone response genes are activated. Among the first genes to be activated are so-called primary response genes, which include E74 and E75 [41]–[44]. Subsequently, these early response genes activate the expression of delayed early genes, such as HR3 [45], [46]. Silencing the expression of these ecdysone response genes leads to disrupted molting and if silenced during the final instar, the larvae typically arrest their development during the prepupal period, indicating that metamorphosis is incomplete [47]–[49]. These studies show that ecdysteroid signaling is essential for molting and the completion of metamorphosis.
The nature of this ecdysteroid-induced molting depends on JH, a sesquiterpenoid hormone that is secreted from the corpora allata [50]. JH is known as a “status quo” hormone because it prevents progression to the next life stage after a molt [51]. In holometabolous insects, JH is present at high levels during the larval stages (instars) and prevents progression to the pupal stage during a molt. JH titers are known to be regulated by a complex interplay between JH synthesis, degradation and sequestration by JH binding proteins [52]. Only when JH levels drop in the last instar can ecdysone induce metamorphosis. That JH decline is essential for the initiation of metamorphosis has been illustrated through several distinct approaches. First, in many insects, application of JH during the penultimate instar induces a supernumerary molt [53]. [54]–[56]. Second, previous studies have shown that the removal of the JH-producing corpora allata can induce precocious metamorphosis with larvae exhibiting pupal characteristics before entering the final larval instar [57]. In addition, recent studies have demonstrated that the basic helix-loop-helix (bHLH) - Per-Arnt-Sim (PAS) domain protein encoded by Methoprene-tolerant (Met) plays a key role in mediating sensitivity to JH and likely acts as a JH receptor in Tribolium [58]–[60]. When Met is knocked down in Tribolium, larvae undergo precocious metamorphosis, a few larval molts earlier than normal [59], [61], [62], and develop into miniature adults. Finally, silencing of the JH biosynthesis gene, JH acid methyltransferase 3 (jhamt3), in Tribolium leads to precocious metamorphosis, again after fewer larval molts than normal, resulting in the formation of miniature adults [63]. The expression of this gene tracks the JH titer closely [63], and JHAMT is thought to act on the rate-limiting step for the series of biochemical reactions that ultimately results in the formation of active JH [64]. The regulation of JH biosynthesis is clearly an integral part of the regulation of timing of metamorphosis. However, how the expression of the key JH biosynthetic enzymes is regulated remains poorly understood. To summarize, the timing of metamorphosis is regulated by the dynamic titers of ecdysteroids and JH. Ecdysteroids are required for molting and their removal leads to disrupted molting and failure to initiate or complete metamorphosis. In contrast, JH is required to maintain the larval stage and its removal leads to precocious metamorphosis because in its absence, ecdysone induces metamorphosis even if the larva has undergone fewer larval-larval molts than in the wildtype.
In this study, we investigated the role of the POU domain transcription factor Vvl in coordinating the metamorphic hormones in Tribolium. Tribolium is well suited for the study of metamorphic regulation because of its sequenced genome and amenability to RNA interference (RNAi). Moreover, JH in Tribolium plays a prominent role in determining the number of molts prior to metamorphosis, facilitating the study of the role of developmental hormones in the regulation of metamorphic timing [59], [61], [63]. In contrast, Drosophila has a fixed number of instars, and topical application of JH does not lead to additional larval molts [65].
To determine the function of Vvl in Tribolium, we knocked down the expression of vvl and found that this resulted in precocious metamorphosis. These animals also had lowered expression of the JH response gene, krüppel-homolog 1 (kr-h1), which could be restored with topical application of JH. Knockdown of vvl also resulted in the reduced expression of jhamt3, a key regulator of JH biosynthesis. In addition, vvl knockdown led to an inability to molt and a corresponding reduction in ecdysone levels, and the expressions of ecdysone biosynthesis genes and the ecdysone-response gene, HR3. The expression of HR3 but not the molting defects could be rescued by injection of 20E. Our results suggest that Vvl may act as a nexus between JH and ecdysone biosynthesis.
Results
Vvl ortholog in Tribolium
We have identified one single ortholog of Vvl in the Tribolium Genome Base (http://www.beetlebase.org). Phylogenetic analysis of Tribolium POU factors confirms that the Tribolium Vvl clusters with both Drosophila and Bombyx Vvl homologs (Figure S1). To compare the Vvl ortholog from Tribolium to those of other invertebrates and vertebrates, the Tribolium Vvl protein sequence was identified in the Tribolium Genome Base and blasted in Geneious (http://www.geneious.com/). The POU region of the Tribolium Vvl consists of a POU domain and a homeodomain. The amino acid sequence of this region is highly conserved with those found in other species, such as Vvl in Drosophila melanogaster (98% sequence identity), POU-M2 in Bombyx mori (98% sequence identity), POU3F4 in Mus musculus (93% sequence identity), Xenopus laevis (93% sequence identity) and Homo sapiens (93% sequence identity) (Figure 1; Figure S2).
Fig. 1. Sequence alignment of the conserved regions of Tribolium Vvl and the vertebrate POU3F4 proteins. 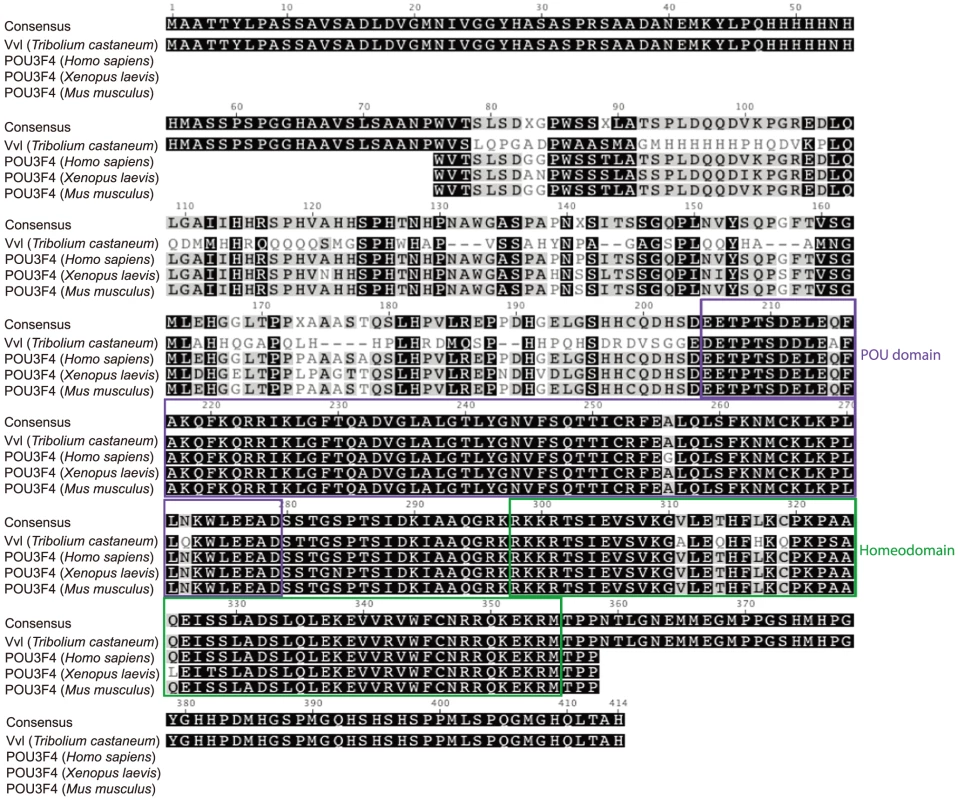
Sequences were downloaded from NCBI and aligned using the Geneious 6 software. The POU domains and homeodomains are highlighted. Expression of vvl in wildtype Tribolium
To determine the expression profile of vvl in Tribolium, qPCR was used to amplify vvl in cDNA obtained from the whole body mRNA extracts of the sixth and final instar larvae, and prepupae (Figure 2A). The expression of vvl was the highest on day 0 of the sixth instar and then dropped to a low level by day 3. The expression then increased to a high level at the time of the molt to the final instar. During the final instar, the vvl expression decreased gradually until the larva entered the prepupal stage when vvl expression increased again to a high level. The fluctuations observed are similar to the fluctuations of ecdysteroids and JH observed during the penultimate and final instars in other insects [51]. Thus, vvl expression might be correlated with endocrine signaling.
Fig. 2. Expression profile of vvl and knockdown verification for RNA interference. 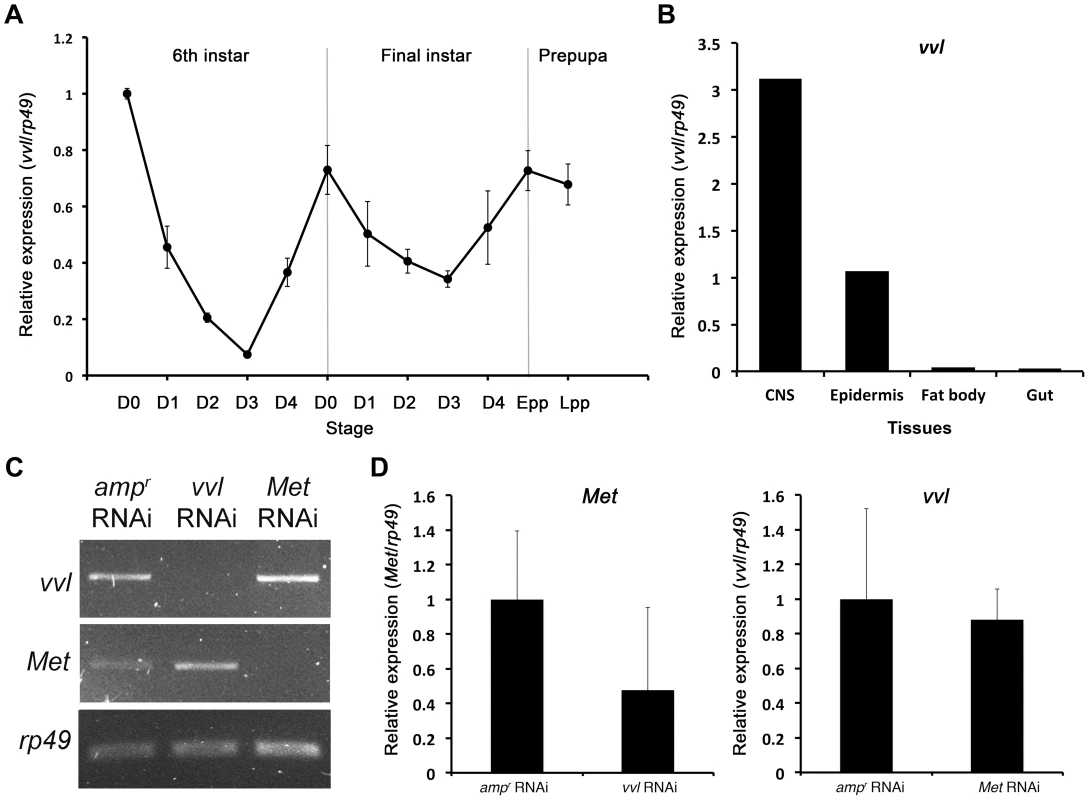
(A) Expression profile of vvl during the late larval and prepupal stages of Tribolium. Expression profile was determined by qPCR of vvl from whole body sixth and seventh instars and prepupae. Ribosomal protein 49 (rp49) was used as the internal control. For all of the treatments, each sample consisted of RNA pooled from five sixth instars, three seventh instars and three prepupae. Three biological replicates were used per treatment, and each sample was run in triplicates. (B) Expression of vvl in the CNS, epidermis, fat body and gut of day 0 seventh instar larvae. mRNA was isolated from tissues pooled from 20 individuals. (C) Knockdown verification of vvl and Met in early prepupae. Expression profiles for vvl, Met, and rp49 (control) in ampr, vvl, and Met dsRNA-injected animals. Cycle numbers for vvl, Met and rp49 were 34, 35, and 28, respectively. (D) Quantitative real-time PCR data showing the expression of Met (left) and vvl (right) in vvl and Met knockdown prepupae, respectively. To further investigate tissue specific expression of vvl, vvl expression was analyzed in the CNS/corpora allata complex, epidermis, fat body and gut of day 0 seventh instar larvae using qPCR. The expression of vvl was the highest in the CNS/corpora allata complex (Figure 2B). Vvl was also expressed in the epidermis. Very low amounts of vvl mRNA were detected in the fat body and the gut. If vvl influences endocrine signaling, then one might expect that its removal would disrupt molting and/or the metamorphic transition.
vvl knockdown causes precocious metamorphosis
To investigate the functions of Vvl during Tribolium development, vvl double-stranded RNA (dsRNA) was injected into day 0 fifth instar Tribolium larvae. All animals injected with vvl dsRNA initiated precocious metamorphosis and entered the prepupal stage without molting (n = 15; Figures 3 and 4A; Table 1). In contrast, control larvae injected with ampicillin-resistance (ampr) dsRNA at the beginning of the fifth instar molted at least two more times before initiating metamorphosis, typically at the end of the seventh instar stage (n = 16). After larvae were injected with ampr dsRNA, the larvae took approximately 12 days to enter the quiescent stage; in contrast, when animals were injected with 0.5 µg of vvl dsRNA, the timing of metamorphosis was shifted about four days earlier (Figure 4A). Because the animals injected with vvl did not molt to subsequent larval instars but rather initiated metamorphosis without molting (Table 1), the vvl dsRNA-injected prepupae were much smaller than the ampr dsRNA-injected prepupae (Figure 3A). The larvae injected with vvl dsRNA arrested at the prepupal stage, but the pupal characters, such as compound eyes and gin traps, eventually developed under the larval cuticle (Figures 3B–I). When these animals were sectioned, the old larval cuticle and the newly synthesized pupal cuticle appeared to be attached to each other, indicating that these animals fail to complete apolysis (Figure 3M). The vvl dsRNA-injected animals never developed into adults. To determine whether this effect was indeed due to the specific effect of vvl knockdown, dsRNA targeted to another region of the vvl gene was injected into fifth instar larvae. Similar precocious metamorphosis was observed (Figure S3), indicating that the precocious metamorphosis observed in this study is due to knockdown of vvl and not another gene.
Fig. 3. Phenotypic effect of dsRNA injections in fifth instar larvae. 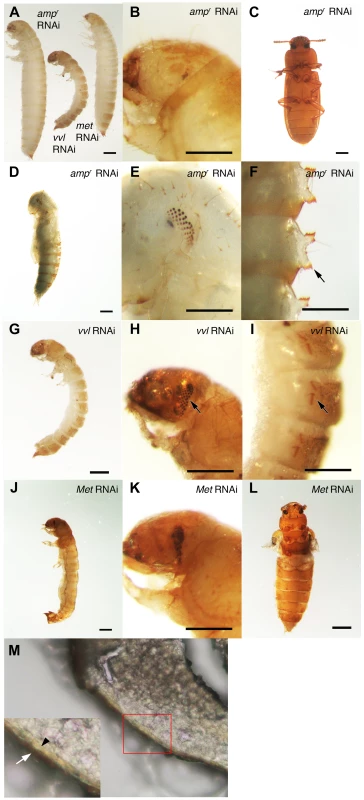
(A) Size comparison of ampr, vvl and Met dsRNA-injected prepupae. (B) ampr dsRNA-injected prepupal eyes. (C) ampr dsRNA injected normal adult. (D) ampr dsRNA-injected pupa. (E) ampr dsRNA-injected pupal eyes. (F) ampr dsRNA-injected pupal gin traps (arrow). (G) vvl dsRNA-injected prepupa. (H) vvl dsRNA-injected prepupa with compound eyes (arrow) developing underneath the cuticle. (I) vvl dsRNA-injected prepupa with pupal gin traps (arrow) developing underneath the cuticle. (J) Met dsRNA-injected prepupa. (K) Met dsRNA-injected prepupal eyes. (L) Met dsRNA-injected eclosed adult. (M) Cross section of a vvl dsRNA-injected prepupa that failed to pupate. (Inset) The old darker larval cuticle (white arrow) is attached to the newly synthesized pupal cuticle (black arrowhead). Scale bars indicate 0.5 mm. Fig. 4. The timing of metamorphosis initiation in larvae treated with vvl dsRNA and 5 ng methoprene. 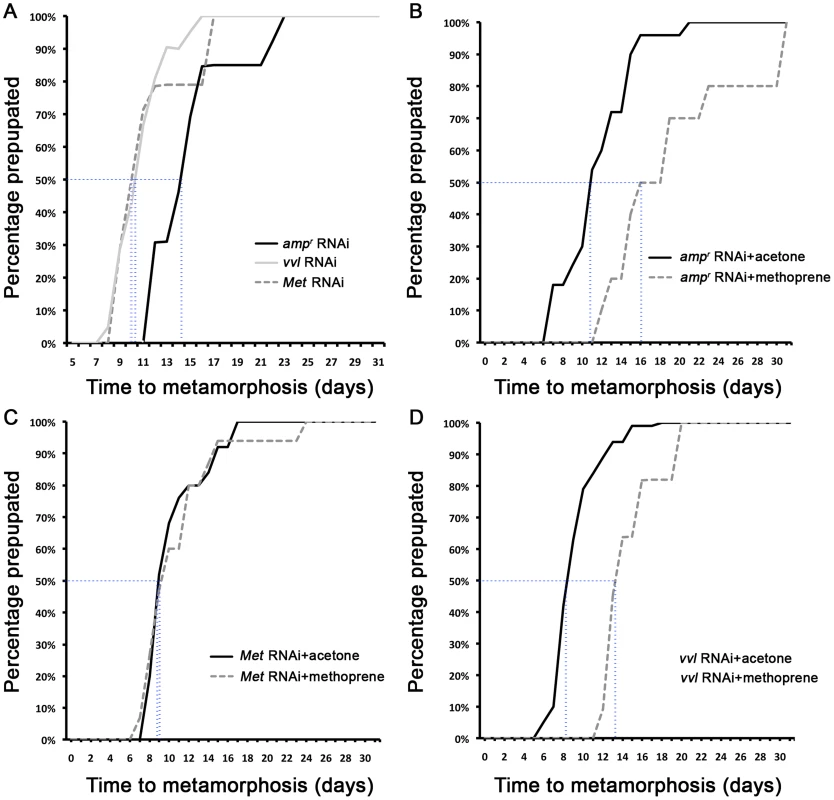
(A) Timing of prepupa formation in dsRNA injected fifth instars. (B–D) Timing of prepupa formation in dsRNA-injected fifth instars treated with acetone and methoprene. ampr RNAi (B), Met RNAi (C) and vvl RNAi (D) indicate animals injected with ampr dsRNA, Met dsRNA and vvl dsRNA, respectively. The time to the onset of prepupal period was recorded. All animals were maintained at 29°C and 50% humidity. Tab. 1. Phenotypic effects of dsRNA injection into fifth instar larvae. 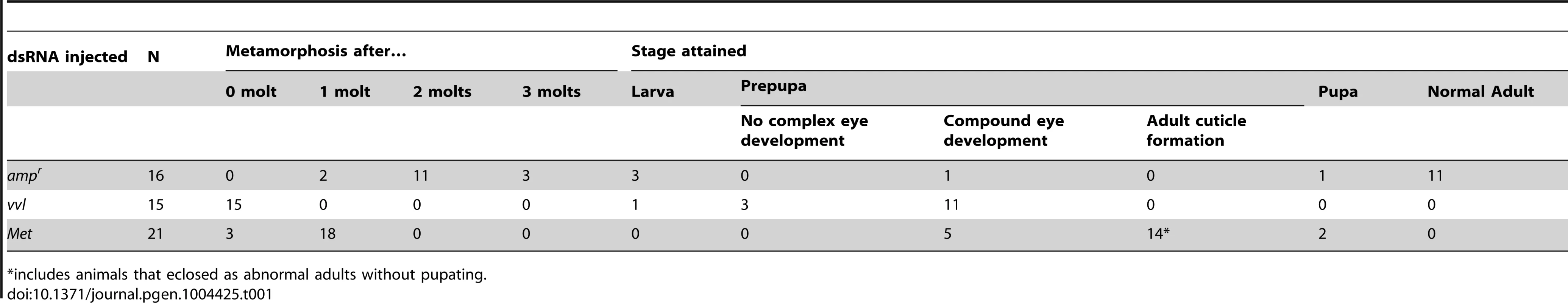
*includes animals that eclosed as abnormal adults without pupating. The precocious metamorphosis seen in vvl dsRNA-injected larvae suggested the possibility that JH signaling might be affected. To determine how vvl dsRNA-injected larvae compare with larvae that have reduced expression of the JH receptor Met, Met dsRNA was injected into day 0 fifth instar larvae. Met dsRNA-injected larvae molted precociously, and a comparison of the mean time to metamorphosis showed that the mean time to metamorphosis in vvl and Met dsRNA-injected larvae was not significantly different from each other whereas they metamorphosed significantly earlier compared to ampr dsRNA-injected animals (p<0.005, ANOVA with Tukey HSD). However, in contrast to the vvl dsRNA injected animals, most larvae injected with Met dsRNA successfully completed a single molt before precociously entering metamorphosis (Table 1). Furthermore, the Met dsRNA-injected animals did not arrest their development at the prepupal stage (Figures 3J–L). Nearly 67% of Met dsRNA-injected animals (n = 21) began to develop adult tissues in the head and thoracic regions under the old larval cuticle and eventually eclosed as an abnormal adult, similar to the phenotypes reported by Parthasarathy et al (2008) ([61]; Table 1; Figures 3J–L). These observations suggest that vvl not only influences JH signaling but might also regulate the molting process.
Semi-quantitative RT-PCR was used to verify knockdown of vvl expression in early prepupae after last instar larvae were injected with 0.5 µg of dsRNA. In addition, semi-quantitative RT-PCR and qPCR were used to determine whether knockdown of either vvl or Met leads to changes in Met or expression, respectively. vvl mRNA expression level was lower in animals injected with vvl dsRNA than in the control larvae injected with ampr dsRNA and larvae injected with Met dsRNA (Figures 2C and 2D). In addition, Met expression level was lower in animals injected with Met dsRNA than in those injected with ampr dsRNA or vvl dsRNA (Figure 2C and 2D). These results demonstrate that the vvl and Met expression was effectively reduced and that Met and Vvl do not regulate the mRNA expression of each other.
vvl knockdown leads to a reduction of jhamt3 expression
The precocious metamorphosis suggested that JH biosynthesis might be impaired in vvl knockdown animals. A key JH biosynthesis enzyme is encoded by JHAMT, an enzyme that converts JH acid into JH. This step has been proposed to act as the rate-limiting step of JH biosynthesis [64], and its knockdown in Tribolium results in precocious metamorphosis [63]. The vvl expression profile described above follows closely the published expression profile of jhamt3 [63]. Thus, we examined whether the removal of Vvl affects the expression of jhamt3.
To determine whether Vvl plays a role in JH biosynthesis, qPCR was performed on day 4 fifth instar Tribolium larvae that were injected with either vvl or ampr dsRNA on day 0. The expression of jhamt3 was significantly decreased when the larvae were injected with vvl dsRNA (Figure 5A). In contrast, the expression of Met did not differ between vvl and ampr dsRNA-injected larvae (Figure 5B). Thus, vvl appears to play a crucial role in JH biosynthesis by influencing the expression of jhamt3.
Fig. 5. Effect of vvl knockdown on genes involved in JH and ecdysone signaling. 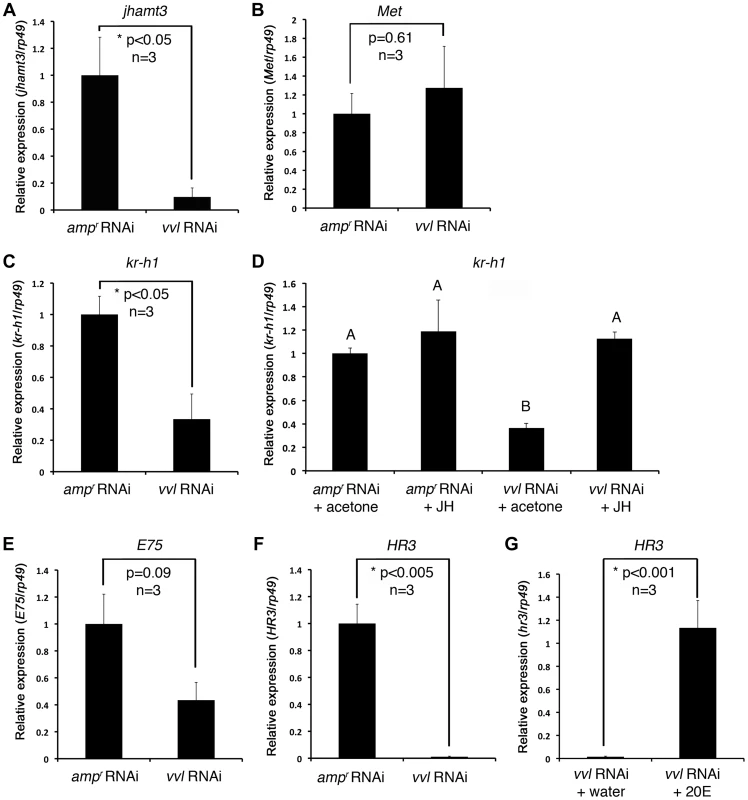
(A–C) Effect of knockdown of vvl on the expression of jhamt3 (A), Met (B) and the JH response gene, kr-h1 (C), in fifth instar larvae. Three biological replicates were used per treatment, and each sample was run in triplicates. (D) Effect of methoprene application on the expression of kr-h1 in vvl knockdown larvae. vvl dsRNA-injected larvae were treated with either acetone (control) or methoprene (15 µg) on day 0 of the fifth instar stage. ampr dsRNA-injected animals treated similarly were used as a comparison. Four biological replicates were used per treatment, and each sample was run in triplicates. Means not sharing the same letter are significantly different (p<0.05, ANOVA with Tukey HSD test). (E, F) Effect of knockdown of vvl on the expression of E75 (E) and HR3 (F). (G) Effect of 20E injection on the expression of HR3 in vvl knockdown larvae. Three biological replicates were used per treatment, and each sample was run in triplicates. For (A)–(F), each sample consisted of RNA pooled from five larvae. For (G), each biological replicate consisted of RNA pooled from three larvae. Data are represented as mean +/− SEM. Precocious metamorphosis of vvl dsRNA-injected larvae is rescued by methoprene
If JH biosynthesis, and not JH sensitivity, is affected by vvl knockdown, topical application of methoprene should restore the normal timing of metamorphosis. We therefore ectopically applied the JH analog, methoprene, to day 0 fifth instar larvae injected with vvl dsRNA. Acetone treatments were used as controls, and ampr and Met dsRNA-injected larvae were also treated similarly for comparison.
All controls injected with ampr dsRNA and treated with acetone molted into pupae and formed normal adults (Table 2; Figures 6A and 6C). Application of 5 ng methoprene to day 0 fifth instar larvae injected with ampr dsRNA caused supernumerary molts (extra larval molts after the eighth instar) in seven out of 14 larvae (Table 2). Five of the methoprene-treated ampr dsRNA-injected animals failed to progress beyond the larval stage, but nine were able to undergo metamorphosis (Figure 6E); however, most larvae that underwent metamorphosis arrested their development and died as either prepupae or pupae (Table 2; Figure 5G). The time to metamorphosis was significantly delayed relative to those treated with acetone (Figure 4B; p<0.005, Student's t-test). When 15 µg of methoprene was applied to day 0 fifth instar larvae, the majority of the larvae stayed in the larval stage and did not pupate (n = 13/14).
Fig. 6. Phenotypic effect of dsRNA injection with methoprene and acetone treatments. 
(A–D) Acetone-treated animals. (A) Size comparison of ampr, vvl and Met dsRNA injected prepupae treated with acetone. (B, left) vvl dsRNA-injected prepupa. (B, middle) vvl dsRNA-injected prepupa with gin traps (arrow) developing underneath the cuticle. (B, right) vvl dsRNA-injected prepupa with complex eyes (arrowhead) developing underneath the cuticle. (C) ampr dsRNA-injected adult. (D) Met dsRNA-injected eclosed adult. (E–H) Methoprene-treated prepupae. (E) Size comparison of ampr, vvl and Met dsRNA-injected animals treated with methoprene. (F, left) vvl dsRNA-injected prepupa. (F, middle) vvl dsRNA-injected prepupa with gin traps (arrow) developing underneath the cuticle. (F, right) vvl dsRNA-injected prepupa with complex eyes (arrowhead) developing underneath the cuticle. (G) ampr dsRNA-injected arrested pupa. (H) Met dsRNA-injected eclosed adult. Scale bars represent 0.5 mm. Tab. 2. Effects of ectopic application of methoprene on developmental timing in larvae injected with dsRNA as fifth instars. 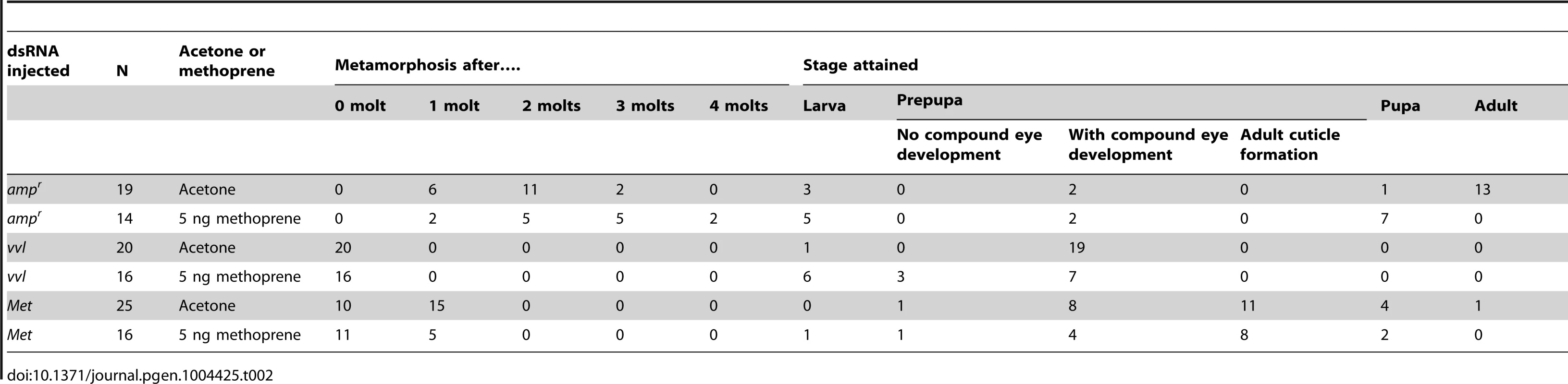
In contrast, day 0 fifth instar larvae injected with vvl dsRNA and treated with 5 ng methoprene were still unable to molt (n = 16), and most died as prepupae (n = 10; Table 2). These prepupae ultimately developed pupal-like characteristics underneath the larval cuticle (Figures 6B and 6F). Ectopic application of methoprene delayed the timing of metamorphosis relative to those treated with acetone (Figure 4D; p<0.0001, Student's t-test). When the concentration of methoprene was increased to 15 µg, none of the larvae initiated metamorphosis and eventually died without ever molting (n = 9). The larvae survived on average for 23.9 days without molting.
As a comparison, day 0 fifth instar larvae were also injected with Met dsRNA and treated with acetone or 5 ng methoprene. For both treatments, the majority of Met dsRNA-injected animals underwent metamorphosis and developed into prepupae or eclosed as adults (Table 2; Figures 6A, 6D, 6E and 6H). In agreement with previous studies suggesting that Met is a receptor for JH, there was no significant difference between the timing of metamorphosis in larvae treated with acetone or 5 ng methoprene (Figure 4C; p = 0.61, Student's t-test), indicating that Met knockdown animals are insensitive to JH. Similarly, all Met knockdown animals treated with 15 µg of methoprene metamorphosed precociously, unlike those injected ampr or vvl dsRNA and treated with 15 µg of methoprene (n = 13/14). Taken together, the results support the notion that Vvl acts on JH biosynthesis but not JH reception to regulate the timing of metamorphosis.
Expression of kr-h1 is reduced in vvl dsRNA-injected larvae
To determine whether Vvl influences the expression of a downstream target gene of the JH pathway, qPCR analysis was performed on day 4 fifth instar Tribolium larvae that were previously injected with vvl dsRNA and treated with either acetone (control) or 15 µg methoprene on day 0 of the fifth instar. ampr dsRNA-injected animals treated similarly were used as a comparison. kr-h1 expression was reduced in the vvl dsRNA-injected larvae in comparison to control ampr dsRNA-injected larvae (Figure 5C). In larvae that were ectopically treated with 15 µg methoprene following injection of vvl dsRNA on day 0 of the fifth instar, the expression level of kr-h1 was restored to a level similar to that of the ampr dsRNA-injected animals treated with methoprene (Figure 5D). Thus, knockdown of vvl results in the downregulation of kr-h1 expression which can be rescued with the ectopic application of methoprene. These results further support the notion that JH biosynthesis rather than reception is influenced by Vvl.
vvl knockdown larvae have reduced expression of ecdysone response genes
The inability of vvl dsRNA-injected larvae to molt indicated a potential disruption of ecdysteroid signaling pathway, which is required for molting. To determine whether Vvl is required for the activation of ecdysone response genes that are associated with molting, we examined the expression of two such genes, E75 and HR3 [48], in vvl dsRNA - and ampr dsRNA-injected larvae four days after injection, just prior to their molt to the sixth instar in control larvae. The expression level of the ecdysone inducible early gene, E75, was not significantly reduced in vvl dsRNA-injected larvae (Figure 5E). However, the ecdysone-inducible gene, HR3, was dramatically reduced in vvl dsRNA-injected larvae (Figure 5F). We do not know why E75 did not decrease significantly in vvl knockdown animals. One reason might be that HR3, further downstream in the transcriptional cascade, is more sensitive than E75 to sustained Vvl-mediated changes in ecdysteroid secretion or action. Nevertheless, our results taken together suggest that ecdysteroid signaling is disrupted in vvl knockdown larvae. Thus, Vvl may have a dual role as both an activator of JH signaling and as a regulator of molting through its influence on ecdysteroid signaling.
Vvl knockdown larvae have lowered ecdysteroid titer
To determine whether ecdysteroid titer was affected in response to vvl knockdown, ecdysteroid titer was observed on day 4 of the fifth instar after larvae were injected with either vvl dsRNA or ampr dsRNA on day 0 of the fifth instar. We observed that larvae injected with vvl dsRNA have a lower ecdysteroid titer relative to ampr dsRNA-injected larvae (Figure 7; p<0.01, Student's t-test). Together with the lack of molting phenotype and the lowered expression of ecdysone response genes, the lowered ecdysone titer indicates that Vvl influences ecdysteroid biosynthesis.
Fig. 7. Vvl removal leads to decreased ecdysteroid titer. 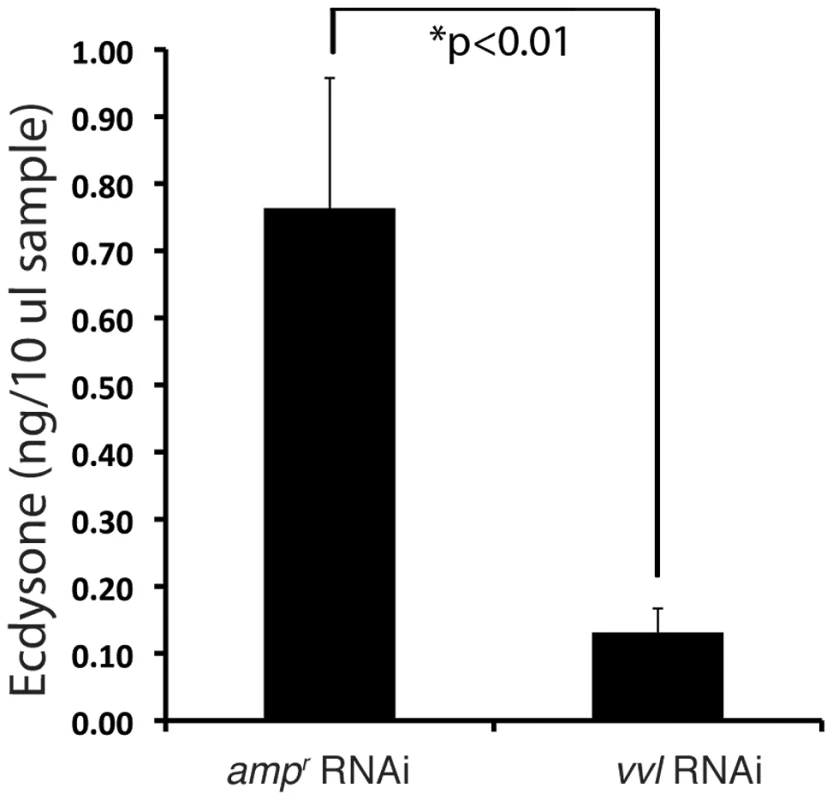
Ecdysone titers in ampr and vvl dsRNA-injected fifth instar larvae on day 4. A 10 µl sample represents two larval equivalents of extracted ecdysteroids. A recent study by Burns et al (2012) demonstrated that a GFP enhancer trap line that expresses GFP under the control of the vvl enhancer (strain KT817) expresses GFP in the oenocytes during the embryonic and early larval stages [66]. We examined the late sixth instar larvae and found GFP expression in the abdominal structures that most likely correspond to oenocytes (Figures 8A–8A″). Since oenocytes have been shown to be capable of synthesizing ecdysone from cholesterol in another tenebrionid beetle, Tenebrio molitor [67], [68], we wondered if the reduction of ecdysone titer might result from the reduced expression of vvl in the oenocytes.
Fig. 8. vvl expression in embryos and sixth instar larvae. 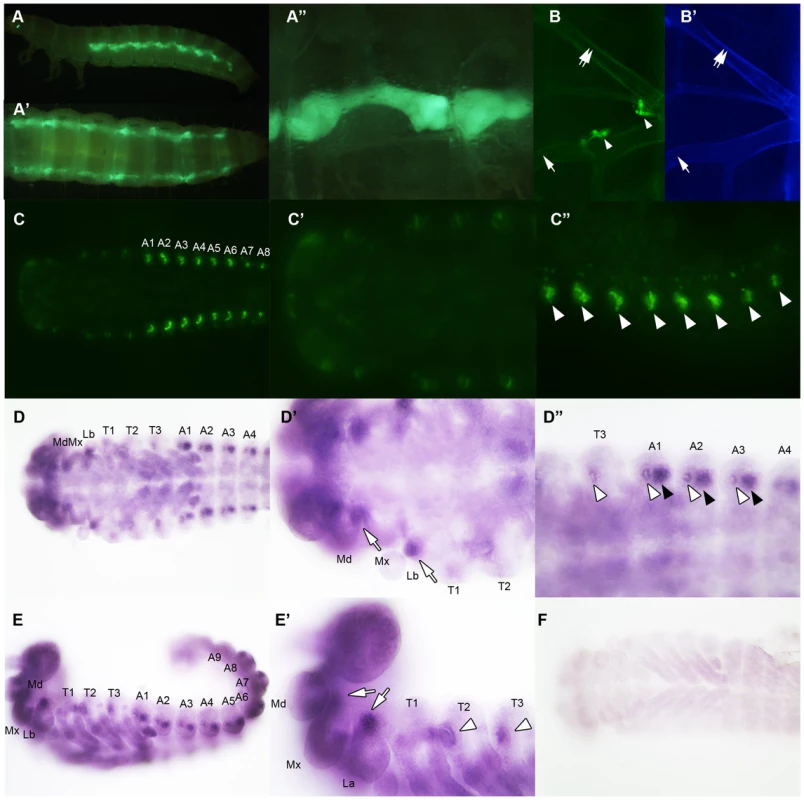
(A–A″) Vvl-GFP expression in the oenocytes of the sixth instar KT817 enhancer trap line. GFP expression in the oenocytes of this strain is driven by a vvl enhancer [66]. Lateral (A), dorsal (A′) and close-up (A″) views are shown. (B) Vvl-GFP expression in the dorsal and ventral tracheal trunks in the prothorax of a sixth instar KT817 larva. Double arrow indicates the dorsal tracheal trunk, and single arrow points to the ventral tracheal trunk. Arrowheads point to GFP-positive cells on the trachea. (B′) Autofluorescence of the same structures shown in (B). (C–C″) GFP expression in the embryo of a KT817 enhancer trap line. Ventral view (C), close-up of the anterior end (C′) and close-up of the posterior end (C″) are shown. Arrowheads indicate the oenocytes. (D–D″, E, E′) Ventral (D–D″) and lateral views (E, E′) of vvl mRNA expression in the day 1 embryo as detected by in situ hybridization. (D′, D″, E′) Close-up of the embryos shown in D and E. White arrows indicate vvl expression detected in the maxillary and labial segments. White arrowheads indicate tracheal pits. Black arrowheads indicate the oenocytes. (F) Embryos probed with a control vvl sense probe. To determine whether ecdysone biosynthesis was altered in the prothoracic gland or the oenocytes, larvae injected with vvl or ampr dsRNA were bisected and assayed for ecdysone biosynthesis gene expression. We found that the expression of spo was significantly reduced in the posterior half of the vvl knockdown larvae (Figure 9A). The expression of spo in the anterior portion of vvl knockdown larvae was not significantly different from that of ampr knockdown larvae (Figure 9A). To see if expression of spo was lowered in the oenoctyes of vvl knockdown larvae, day 0 fifth instar KT817 larvae were injected with vvl or ampr dsRNA and oenocytes along with the associated tissues were collected on day 4. We found that the expression of spo was lowered in the oenocytes of vvl knockdown larvae relative to the ampr knockdown larvae (Figure 9F). In contrast, we found that phm expression was reduced in the anterior portion of vvl dsRNA-injected animals relative to those injected with ampr dsRNA (Figure 9B). The posterior expression of phm remained unchanged. The expression of sad, shd and dib did not differ between the vvl and ampr dsRNA-injected animals in both the anterior and posterior portions of the body (Figures 9C–9E). These results suggest that Vvl regulates ecdysone biosynthesis in different glands by influencing the expression of different ecdysone biosynthesis genes.
Fig. 9. vvl knockdown leads to decreased expression of ecdysone biosynthesis genes. 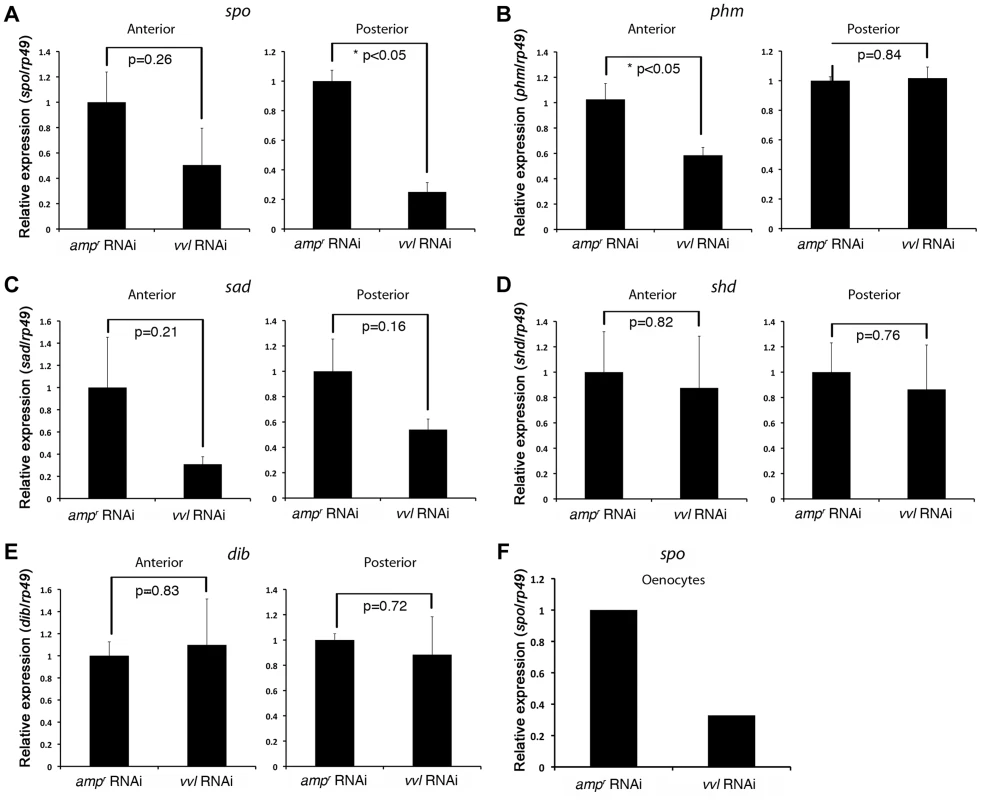
(A–E) Ecdysone response gene expression in ampr and vvl dsRNA-injected fifth instar larvae on day 4. Anterior represents expression in the head and thorax. Posterior represents expression in the abdomen. Three biological replicates were used per treatment, and each sample was run in triplicates. Each biological sample consisted of pooled RNA from five larvae. Error bards represent standard error. (F) Relative expression of spo expression in the oenocytes of day 4 ampr and vvl dsRNA-injected fifth instar KT817 larvae. Oenocytes from 15 larvae were pooled per treatment. We also looked for expression of GFP in the anterior portion of the larvae. In Coleoptera, prothoracic glands have been found to be associated with the dorsal tracheal trunk and the ventral tracheal trunk [69]. No obvious expression of GFP was observed in the cells lining the dorsal and ventral tracheal trunks except a few cells, which may correspond to Inka cells (Figures 8B and 8B′). The absence of GFP expression in the PG cells may be due to the KT817 enhancer trap not reflecting the entire repertoire of tissues where the vvl gene is active. To determine whether GFP is expressed in all the cells that express vvl, in situ hybridization was performed on day 1 embryos. In the anterior region, vvl mRNA was detected at the base of the maxillary and labial segments as well as the tracheal pits in the T2 and T3 segments (Figures 8D, 8D′, 8E and 8E′). These locations fail to express GFP in the KT817 strain (Figures 8C and 8C′). Similarly, in the abdomen, vvl mRNA was detected in the tracheal pits as well as the oenocytes even though no GFP expression was detected in the tracheal pits of the KT817 strain (Figures 8C, 8C″, 8D and 8D″). No staining was observed when the control sense probe was used (Figure 8F). These results suggest that there are additional enhancers that drive expression of vvl besides the one that drives GFP expression in the KT817 strain. The expression of vvl at the base of the labial and maxillary segments is noteworthy since in the Drosophila, vvl expressing cells in these segments give rise to the future endocrine glands [25]. While we attempted to stain for vvl in the prothoracic glands during the larval stages, we were unable to obtain convincing staining to due to excess background staining inherent to this stage.
20E and ecdysteroid agonist RH-2845 are unable to induce a molt in vvl knockdown larvae
To determine whether exogenous ecdysteroid agonist can rescue molting defects in vvl knockdown larvae, vvl dsRNA-injected larvae were treated with either 20E or RH-2845 two days after injection (Table 3). Injection of 1.5 µg or 0.15 µg of 20E led to death in vvl dsRNA-injected larvae (Table 3). When the concentration was reduced to 0.015 µg, a few larvae (n = 2/5) were able to survive but did not molt and developed into prepupae. ampr dsRNA-injected larvae also had high mortality although even at the highest concentration of 20E (1.5 µg), a few larvae survived to undergo a larval-larval molt. Thus, 20E does not appear to rescue the normal molting phenotype in vvl knockdown larvae. However, the high number of dead larvae relative to water injected controls (Table 3) suggests that vvl dsRNA injected larvae are able to sense 20E even though they cannot molt.
Tab. 3. Effects of 20E or RH-2485 treatments on larvae injected with dsRNA as fifth instars. 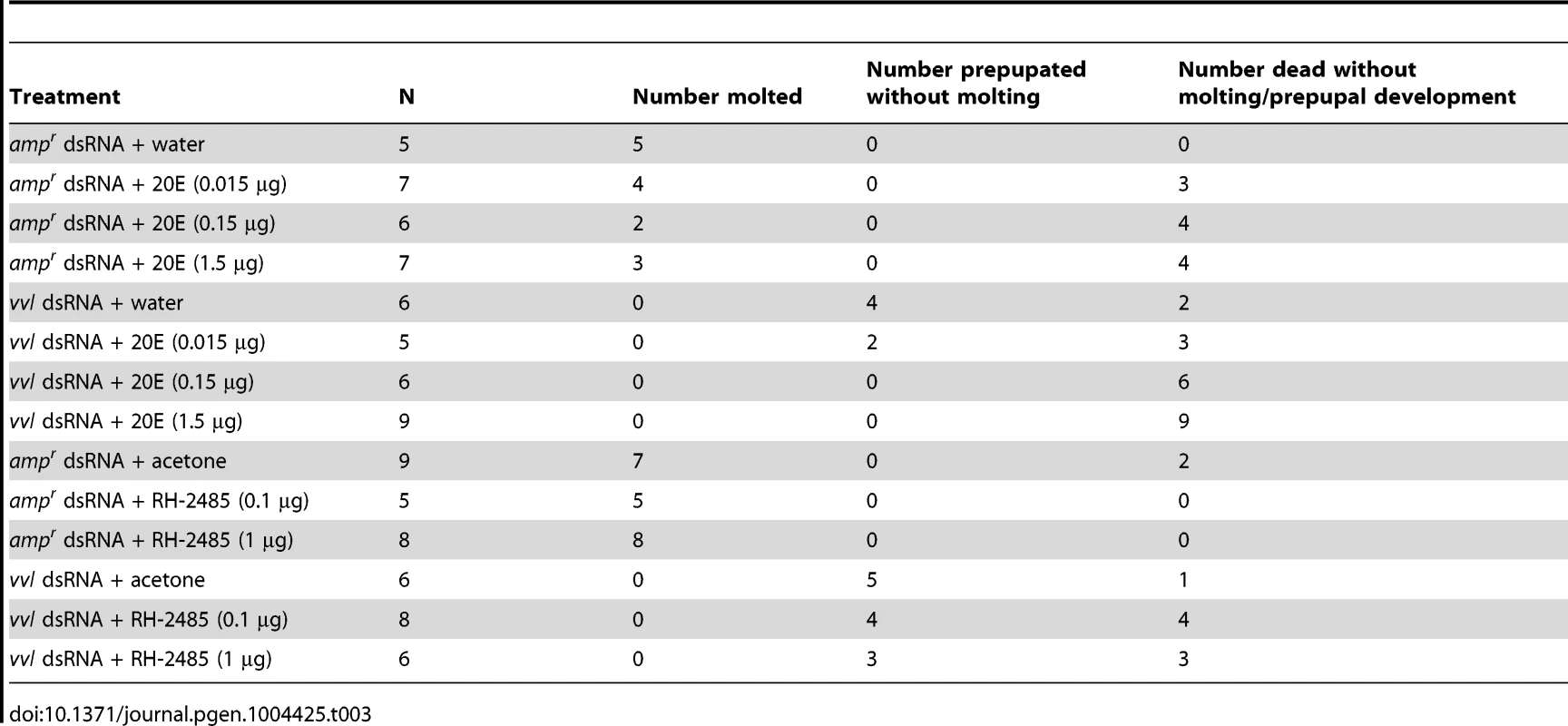
To determine whether 20E treatment can rescue HR3 expression, day 0 fifth instar larvae were injected with vvl dsRNA. Two days later, these larvae were injected with either 0.15 µg 20E or water. We found that larvae injected with 20E had significantly higher levels of HR3 expression (Figure 5G). Thus, exogenous 20E can rescue HR3 expression in vvl knockdown larvae. Our findings show that vvl knockdown leads to lowered ecdysteroid titer, which in turn leads to lowered HR3 expression. However, the failure of 20E to rescue the molting phenotype indicates that vvl knockdown impacts the molting process downstream of EcR/Usp.
Since injection of 20E just two days after dsRNA injection is potentially traumatic for the larvae, we decided to also investigate the effect of topical application of an ecdysteroid mimic. In Tribolium adults, 1 µg of RH-2845 upregulates the expression of ecdysone response genes, EcR and E75, indicating that this compound activates of ecdysteroid signaling [70]. ampr dsRNA-injected larvae molted after 1.6±0.4 days or 3.5±0.6 days when treated with either 0.1 µg or 1 µg of RH-2845, respectively (Table 3). In contrast, larvae injected with vvl dsRNA could not molt even when they were treated with either 0.1 µg or 1 µg of RH-2845 (Table 3). A high rate of mortality was also observed when these larvae were treated with RH-2845 (n = 4/8 and 3/6 for 0.1 µg and 1 µg of RH-2845, respectively). The remaining larvae all became prepupae without molting (n = 4/8 and 3/6 for 0.1 µg and 1 µg of RH-2845, respectively). While the mechanism by which RH-2485 acts as an ecdysteroid agonist remains unknown, the fact that RH-2485 is unable to induce a molt in the absence of Vvl supports the notion that Vvl is in part required for mediating the organism's molting behavior, in addition to its role in regulating ecdysone biosynthesis.
Discussion
In this study, we determined the effects of knocking down vvl in Tribolium and elucidated a possible mechanism by which the gene interacts with the physiological regulation of development. Use of dsRNA-mediated expression knockdown revealed that suppression of vvl results in precocious metamorphosis. We also propose that Vvl acts upstream of JH signaling and that its expression is required for jhamt3 expression. In addition, we found that Vvl influences ecdysteroid biosynthesis and signaling to regulate molting.
Vvl regulates the onset of metamorphosis by regulating JH biosynthesis
In the present study, we found that fifth instar animals injected with vvl dsRNA underwent premature metamorphosis. The precocious metamorphosis observed in vvl dsRNA-injected larvae indicates that these larvae have disrupted JH signaling which results in precocious pupal commitment (Figure 10). Consistent with this interpretation, we found that knockdown of vvl expression in the fifth instars causes a down-regulation of the expression of the JH-response gene kr-h1 [71]. In addition, we found that vvl knockdown animals were not resistant to methoprene as seen in Met knockdown animals. The delay in metamorphosis observed in these methoprene-treated vvl knockdown larvae suggests that vvl does not affect the sensitivity to JH. Similarly, ectopic application of methoprene was able to upregulate kr-h1 expression in vvl knockdown animals. Because kr-h1 is a direct downstream target of Met [58], [71], the down-regulation of kr-h1 observed in vvl knockdown animals and the rescue of its expression with ectopically applied methoprene indicate that vvl knockdown causes a decrease in JH biosynthesis by acting upstream of kr-h1. Because the expression of jhamt3, a JH biosynthesis enzyme, is decreased in vvl RNAi animals, Vvl likely influences the rate of JH biosynthesis by influencing the transcription of jhamt3 directly or by regulating an upstream regulator of jhamt3 expression. In agreement with this view, vvl and jhamt3 whole body expression profiles are similar (this study; [63]). Our study suggests that this transcription factor plays a key role in regulating the timing of JH biosynthesis. In support of this, we have detected high levels of vvl mRNA in day 0 seventh instar CNS/corpora allata complex. While we were unable to distinguish between the expression of vvl in the CNS and that in the corpora allata, our finding suggests an intriguing possibility that Vvl might function as a mediator between the nervous system and JH production, potentially linking the two systems to coordinate the onset of metamorphosis.
Fig. 10. Proposed mechanism of Vvl action. 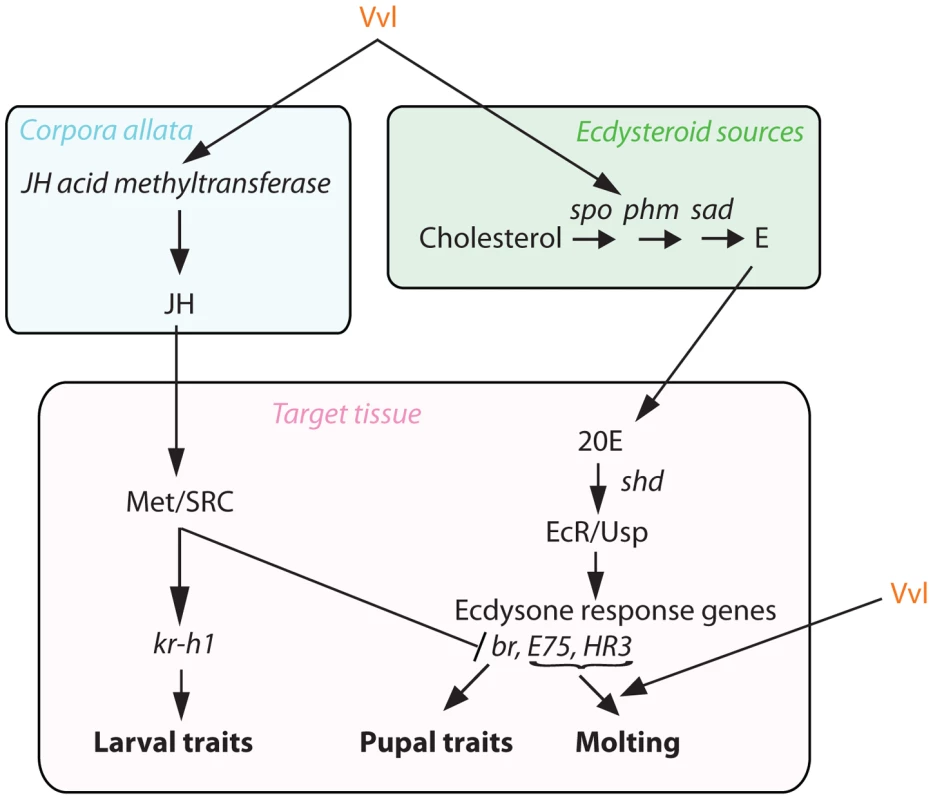
Vvl influences JH production via jhamt expression and hence the timing of metamorphosis. Vvl also regulates the biosynthesis of ecdysteroids and the molting pathway. Vvl influences ecdysone biosynthesis and its signaling
All larvae injected with vvl dsRNA underwent precocious metamorphosis without molting, while Met dsRNA-injected animals almost always molted before metamorphosis (Table 1). In addition, although jhamt3 dsRNA-injected larvae undergo precocious metamorphosis as seen in vvl knockdown larvae, jhamt3 knockdown animals do not show any molting defects and eclose into miniature but externally complete adults [63]. Thus, Vvl suppression might have an additional inhibitory effect on the ecdysteroid signaling pathway, a key regulator of molting. Consistent with this notion, we found that ecdysone titer and the expression of the ecdysone response gene HR3 were reduced in vvl knockdown larvae. This lowered level of HR3 could be rescued by injection of 20E, further supporting the notion that the vvl is required for ecdysteroid biosynthesis. In Bombyx, a homolog of Vvl, BmPOUM2, has been shown to be required for metamorphosis. However, when the expression of this gene was knocked down during the wandering stage of Bombyx, the expression of ecdysone response genes was not altered [72]. This difference may be due to a difference in the species used or because Vvl removal during wandering is too late to detect an effect on ecdysone biosynthesis or response genes.
We also observed that the expression of the ecdysone biosynthesis genes phm and spo were lowered in the anterior and posterior halves of the vvl knockdown larvae, respectively (Figure 9). The latter observation is consistent with the expression of GFP in the oenocytes of the KT817 GFP enhancer trap line, which expresses GFP under the control of a vvl enhancer [66]. In several insect species, including another tenebrionid beetle, Tenebrio molitor, oenocytes are capable of producing ecdysone from cholesterol [67]. We have found that when vvl is knocked down, spo expression decreases. Thus, our observations support the notion that Vvl in the oenocytes regulates ecdysteroid production. However, it is possible that Vvl might target additional ecdysteroidogenic tissues besides oenocytes [68], [73].
We were unable to detect GFP expression in the cells lining the ventral and dorsal tracheal trunk where prothoracic gland cells are typically found [69]. The comparison between embryonic vvl expression and embryonic GFP expression in the KT817 line suggests that vvl expression in the prothoracic glands and oenocytes are regulated by distinct enhancers. The expression of vvl in the labial and maxillary segment of the embryo is similar to what has been observed in Drosophila. In Drosophila, these vvl-expressing cells give rise to the prothoracic glands and the corpora allata. Thus, we suggest that the vvl expressing cells in the labial and maxillary segments likely also contribute to the endocrine organs in Tribolium.
While we cannot be certain about the mechanism by which ecdysone biosynthesis gene expression is modulated in the different glands, our findings suggest that ecdysone biosynthesis genes are regulated differently in the various endocrine structures. This is not surprising considering that in adults, ecdysone biosynthesis genes are activated differently in the accessory glands and the ovaries [35]. The modular nature of gland specific regulation of hormonal production is probably very common. Future studies should investigate the relative contributions of ecdysteroids from the prothoracic gland and other endocrine sources during larval development.
In addition, the inability of exogenous 20E and RH-2485 to rescue the molting defects despite being able to rescue HR3 expression suggests that Vvl might affect a component of the ecdysis pathway, such as ecdysis triggering hormone or eclosion hormone [74]–[77]. Our observation of vvl-GFP expression in the Inka cell-like cells in the KT817 line is also consistent with this notion since Inka cells have been shown to produce ecdysis triggering hormone in a several insects [77], [78]. The expression of ecdysis triggering hormone is influenced by circulating levels of ecdysteroids and EcR [76], [79], [80]. Preliminary findings show that Vvl binds to EcR/Usp through a GST-pulldown assay (Figure S4). This suggests a possibility that Usp and EcR heterodimerize with Vvl to regulate the expression of ecdysis triggering hormone (Figure S4). However, additional studies, such as ChIP assays, are necessary to definitively determine how Vvl modulates target genes.
POU factors may regulate metamorphosis in a similar manner as reproductive maturation in vertebrates
Our findings show that Vvl regulates both ecdysone and JH signaling, the two hormonal signaling pathways that regulate metamorphosis (Figure 10). Thus, Vvl may couple the two hormonal pathways to coordinate the timing of metamorphosis in insects. Specifically, we propose that a switch in the expression of Vvl signals the initiation of metamorphosis by regulating the dynamic fluctuations of both of these developmental hormones. Whether Vvl regulates the two hormones separately or relays a change from one to another remains unclear at present.
It has been suggested that puberty in humans and other mammals is another form of metamorphosis. Although seemingly different, both processes are marked by dramatic changes in physiology. Puberty in humans is initiated by endocrine changes in response to increased pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus. In vertebrates, POU factors have been shown to regulate neuropeptide expression. In particular, GnRH expression is regulated by POU factors, which bind to the promoter and enhancer sequences of the GnRH gene. For example, the POU domain transcription factor Oct-6 (POU3F1) represses GnRH activity, delaying the onset of puberty [10]. In the absence of Oct-6, GnRH is expressed, triggering pubertal development.
Our study shows an intriguing similarity between the transcriptional regulation of the key neuroendocrine regulators of insect metamorphosis and mammalian puberty. Most certainly, these two processes have evolved independently and are not homologous. However, given the substantial sequence conservation of POU domain proteins across various metazoan taxa, future studies should investigate whether POU factors play a role in reproductive maturation in other metazoan taxa. Since the corpora allata and the prothoracic gland along with the trachea all express vvl during Drosophila embryogenesis, it has been suggested that these Vvl-specified structures have a common origin in a proto-arthropod [25], [81]. In light of the endocrine functions we identified in our study, we suggest that vvl had an ancient endocrine function, and its expression in these endocrine structures was subsequently retained as corpora allata and prothoracic glands evolved.
Materials and Methods
Animal husbandry
Wildtype Tribolium strain GA-1 was obtained from Dr. Richard Beeman (USDA ARS Biological Research Unit, Grain Marketing & Production Research Center, Manhattan, Kansas). The KT817 GFP enhancer trap line was obtained from the Kansas State University Tribolium Stock Center [82]. All beetles were raised on organic whole-wheat flour fortified with 5% nutritional yeast at 29°C and 50% humidity.
RNA isolation and cDNA synthesis
Tribolium RNA from various larval instars was isolated by homogenizing the tissues in TRIzol (Life Technologies, Carlsbad, CA). The RNA sample was treated with DNAse and converted to complementary DNA (cDNA) using reverse transcriptase (Thermo Scientific, Waltham, MA). The vvl gene sequence was obtained from NCBI (NM_001145913). Primers were designed to amplify particular regions of interest in the synthesized cDNA using polymerase chain reaction (PCR) (Table 4).
Tab. 4. Forward (FW) and reverse (RV) primers used for dsRNA synthesis, expression profiling and knockdown verification. 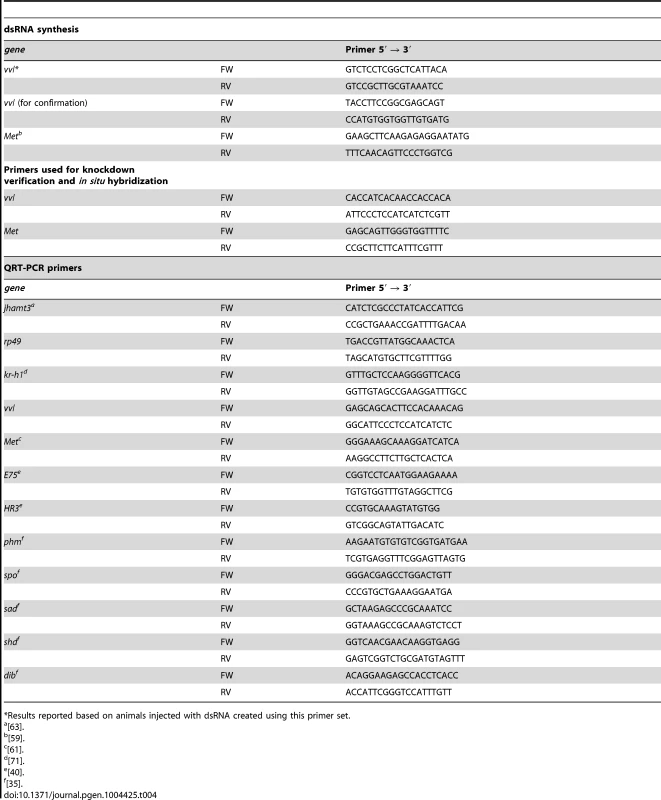
*Results reported based on animals injected with dsRNA created using this primer set. Cloning and dsRNA synthesis
The PCR product was subsequently cloned into the TOPO-TA cloning vector (Life Technologies, Carlsbad, CA), and cells were transformed with this plasmid. The purified plasmid DNA was sequenced to confirm the identity of the cloned gene and the accuracy of the insertion into the TOPO vector. The plasmid DNA was linearized using Spe1 and Not1 restriction enzymes (New England Biolabs, Ipswich, MA) and subsequently used for single-stranded RNA (ssRNA) synthesis. ssRNA was synthesized using MEGAscript T3 and T7 kits (Life Technologies, Carlsbad, CA), according to the manufacturer's instructions. The complementary ssRNA were then combined and annealed to form a 2 µg/µl dsRNA solution using a standard annealing protocol [83]. The final annealed product was analyzed via gel electrophoresis to ensure proper annealing.
dsRNA injection and treatment
To characterize the role of vvl, day 0 fifth instar Tribolium larvae were injected with dsRNA. Using a pulled 10 µl glass capillary needle connected to a syringe, dsRNA was manually injected into each animal. dsRNA was injected until the abdomen began to stretch (approximately 0.25 µl). Controls were injected with the similar volume of bacterial ampr dsRNA. Animals were also injected with Met dsRNA as a comparison to the vvl knockdown animals.
To determine the effect of JH on vvl knockdown animals, a subset of the injected larvae was also topically treated with 0.5 µl JH analog methoprene (5 ng or 15 µg) (Sigma-Aldrich, St. Louis, MO) dissolved in acetone. The same amount of acetone was applied to control larvae. All solutions were applied to the dorsal side of the animals immediately following injection with dsRNA (day 0 fifth instar) to mimic a constant level of JH. All experimentally treated animals were maintained at 29°C and 50% humidity. The animals were examined every other day and characterized in comparison to ampr dsRNA-injected animals treated with acetone. Initiation of metamorphosis was identified by observation of the characteristic J-shape (“J-hangers”) of the larva, immobile legs and the position of the larval eyes, which begin to migrate towards the posterior of the head segment.
20E and RH-2845 treatments
To determine the effect of vvl knockdown on ecdysone sensitivity, larvae injected with either vvl or ampr dsRNA as day 0 fifth instar larvae were treated with either 20E or an ecdysteroid mimic, RH-2485 methoxyfenozide (Sigma-Aldrich, St. Louis, MO) on day 2 of the fifth instar. For 20E, larvae were injected with 0.3 µl of 5 µg/µl, 0.5 µg/µl or 0.05 µg/µl 20E dissolved in water. Water injection was used as a control. For RH-2485, 0.5 µl of 0.2 or 2 µg/µl RH-2485 in acetone was topically applied to the larva. The latter amount of RH-2485 has previously been shown to activate ecdysone response genes in adult Tribolium [70]. Acetone was used as a control. Treated larvae were checked daily for signs of molting, prepupal development or death.
Ecdysone quantification assay
To determine the effect of vvl knockdown on ecdysone titer, day 0 fifth instar larvae were injected with ampr or vvl dsRNA. On day 4, six larvae were collected and frozen at −80°C. Pooled larvae were homogenized in 100% methanol, centrifuged, and the supernatant was dried, then resuspended in 35 µl Grace's media. Triplicate 10 µl samples were assayed for ecdysteroids, and averaged. The radioimmunoassay was conducted as previously described using an antibody generously provided by Dr. Lawrence Gilbert that cross-reacted equivalently with ecdysone and 20-hydroxyecdysone [84]. Ecdysone was used as a standard.
Quantitative RT-PCR
To determine the developmental expression profile of vvl, RNA was isolated from the whole body of sixth and seventh instar GA-1 strain larvae, and prepupae. Total RNA was isolated from the whole body of day 4 fifth instar larvae using Trizol extraction and treated with DNAse to remove genomic DNA. cDNA was synthesized from 1 µg of RNA as described above. Each biological sample consisted of pooled RNA from five fifth instar larvae or three seventh instar larvae or prepupae. Three biological replicates of each treatment were prepared, and SsoAdvanced SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) was used for qPCR analyses.
To assess the effect of vvl on the expression of E75, HR3, kr-h1, Met and jhamt3, day 0 fifth instar GA-1 strain larvae were injected with vvl dsRNA or ampr dsRNA, and their RNA was isolated. A subset of larvae was also treated with 0.5 µl of methoprene (30 µg/µl) or acetone. The effects of vvl or Met knockdown on Met and vvl expression, respectively, were also assayed during the prepupal stage. For this, two animals injected with either vvl, Met or amprdsRNA were pooled per biological sample, and three biological replicates were created.
The effect of vvl on the expression of ecdysone biosynthesis genes spo, phm, shd, dib and sad, was assayed by injecting day 0 fifth instar larvae with vvl dsRNA or ampr dsRNA, and splitting the larvae into the anterior (containing the thorax) and posterior (containing the abdomen) halves. RNA was pooled from five animals per biological sample, and three biological samples were prepared as described above. In addition, to determine whether vvl knockdown influences spo expression in the oenocytes, day 0 fifth instar KT817 strain larvae were injected with either vvl or ampr dsRNA. Oenocytes were visualized by GFP and isolated from day 4 fifth instar larvae. RNA was pooled from 15 larvae per treatment.
To determine the tissue specific expression of vvl, CNS with the associated structures including the CA, the fat body, the gut and the epidermis were isolated from 20 day 0 seventh instar GA-1 strain larvae. The tissues were pooled and RNA was extracted as described above. cDNA was created from 1 µg RNA.
To determine the effect of 20E on HR3 expression in vvl dsRNA-injected animals, day 0 fifth instar GA-1 strain larvae were injected with vvl dsRNA. Two days later, larvae were injected with either 0.3 µl of water as a control or 0.3 µl of 0.5 µg/µl 20E. After 6 hrs, larvae were harvested for RNA isolation and subsequent cDNA synthesis as described above. Three larvae were pooled per biological replicate and three biological replicates were created. Each sample was run in triplicate with no-template controls. rp49 was used as an internal control for all qPCRs.
Semi-quantitative RT-PCR
In order to perform semi-quantitative RT-PCR, early prepupae were collected. Last instars injected with ampr, vvl, or Met dsRNA were collected as they prepupated. Three animals for each treatment were pooled in Trizol and prepared for total RNA isolation, and cDNA was synthesized from 1 µg of RNA as described above. PCR products were run on a gel and visualized using a UV-illuminator.
In situ hybridization and visualization of GFP in KT817 strain
To create the probes, vvl gene was cloned into a TOPO-TA cloning vector and restriction digested as described above. The purified linearized plasmid DNA was used to create probes as described previously [85]. To examine the expression of vvl in GA-1 strain embryos, day 1 eggs were collected and dechorionated in 25% bleach. After heptane/formaldehyde fixation, eggs were cracked in heptane/methanol. Embryos were individually dissected out of the egg shell. Standard in situ hybridization protocol was followed. The sense probe was used as a control. To visualize GFP expression in KT817 embryos, eggs were dechorionated and fixed as above. Embryos were manually dissected out and mounted in 70% glycerol in PBS.
Supporting Information
Zdroje
1. TrumanJW, RiddifordLM (1974) Physiology of insect rhythms. 3. The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol 60 : 371–382.
2. TrumanJW (1972) Physiology of insect rhythms. 1. Circadian organization of endocrine events underlying molting cycle of larval tobacco hornworms. J Exp Biol 57 : 805–820.
3. Nijhout HF (1998) Insect Hormones. Princeton, NJ: Princeton University Press.
4. RoaJ, Garcia-GalianoD, CastellanoJM, GaytanF, PinillaL, et al. (2010) Metabolic control of puberty onset: new players, new mechanisms. Mol Cell Endocrinol 324 : 87–94.
5. ClarksonJ, HanSK, LiuX, LeeK, HerbisonAE (2010) Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol 324 : 45–50.
6. McBrayerZ, OnoH, ShimellM, ParvyJP, BecksteadRB, et al. (2007) Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell 13 : 857–871.
7. AndersenB, RosenfeldMG (2001) POU domain factors in the neuroendocrine system: Lessons from developmental biology provide insights into human disease. Endocr Rev 22 : 2–35.
8. RosenfeldMG (1991) Pou-domain transcription factors - pou-er-ful developmental regulators. Genes Dev 5 : 897–907.
9. RamkumarT, AdlerS (1999) A requirement for the POU transcription factor, Brn-2, in corticotropin-releasing hormone expression in a neuronal cell line. Mol Endocrinol 13 : 1237–1248.
10. WiermanME, XiongXY, KepaJK, SpauldingAJ, JacobsenBM, et al. (1997) Repression of gonadotropin-releasing hormone promoter activity by the POU homeodomain transcription factor SCIP/Oct-6/Tst-1: A regulatory mechanism of phenotype expression? Mol Cell Biol 17 : 1652–1665.
11. EralySA, NelsonSB, HuangKM, MellonPL (1998) Oct-1 binds promoter elements required for transcription of the GnRH gene. Mol Endocrinol 12 : 469–481.
12. ClarkME, MellonPL (1995) The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol Cell Biol 15 : 6169–6177.
13. OjedaSR, HillJ, HillDF, CostaME, TapiaV, et al. (1999) The Oct-2 POU domain gene in the neuroendocrine brain: A transcriptional regulator of mammalian puberty. Endocrinology 140 : 3774–3789.
14. HerrW, ClearyMA (1995) The POU domain: versatility in transcriptional regulation by a flexible two-in-one DNA-binding domain. Genes Dev 9 : 1679–1693.
15. OjedaSR, DubayC, LomnicziA, KaidarG, MatagneV, et al. (2010) Gene networks and the neuroendocrine regulation of puberty. Mol Cell Endocrinol 324 : 3–11.
16. AndersonMG, PerkinsGL, ChittickP, ShrigleyRJ, JohnsonWA (1995) Drifter, a Drosophila Pou-domain transcription factor, is required for correct differentiation and migration of tracheal cells and midline glia. Genes Dev 9 : 123–137.
17. MaY, CertelK, GaoYP, NiemitzE, MosherJ, et al. (2000) Functional interactions between Drosophila bHLH/PAS, Sox, and POU transcription factors regulate CNS midline expression of the slit gene. J Neurosci 20 : 4596–4605.
18. CertelK, AndersonMG, ShrigleyRJ, WayneAJ (1996) Distinct variant DNA-Binding sites determine cell-specific autoregulated expression of the Drosophila POU domain transcription factor drifter in midline glia or trachea. Mol Cell Biol 16 : 1813–1823.
19. InbalA, LevanonD, SalzbergA (2003) Multiple roles for u-turn/ventral veinless in the development of Drosophila PNS. Development 130 : 2467–2478.
20. MeierS, SprecherSG, ReichertH, HirthF (2006) ventral veins lacking is required for specification of the tritocerebrum in embryonic brain development of Drosophila. Mech Dev 123 : 76–83.
21. CertelSJ, ThorS (2004) Specification of Drosophila motoneuron identity by the combinatorial action of POU and LIM-HD factors. Development 131 : 5429–5439.
22. AndersonMG, CertelSJ, CertelK, LeeT, MontellDJ, et al. (1996) Function of the Drosophila POU domain transcription factor Drifter as an upstream regulator of Breathless receptor tyrosine kinase expression in developing trachea. Development 122 : 4169–4178.
23. ZhangTY, KangL, ZhangZF, XuWH (2004) Identification of a POU factor involved in regulating the neuron-specific expression of the gene encoding diapause hormone and pheromone biosynthesis-activating neuropeptide in Bombyx mori. Biochem J 380 : 255–263.
24. ZhangTY, XuWH (2009) Identification and characterization of a POU transcription factor in the cotton bollworm, Helicoverpa armigera. BMC Mol Biol 10 : 25 doi: 10.1186/1471-2199-10-25
25. Sanchez-HiguerasC, SotillosS, Castelli-Gair HombriaJ (2014) Common origin of insect trachea and endocrine organs from a segmentally repeated precursor. Curr Biol 24 : 76–81.
26. GilbertLI, RybczynskiR, WarrenJT (2002) Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol 47 : 883–916.
27. WarrenJT, PetrykA, MarquesG, JarchoM, ParvyJP, et al. (2002) Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A 99 : 11043–11048.
28. WarrenJT, SakuraiS, RountreeDB, GilbertLI, LeeSS, et al. (1988) Regulation of the ecdysteroid titer of Manduca-sexta - reappraisal of the role of the prothoracic glands. Proc Natl Acad Sci U S A 85 : 958–962.
29. AribiN, PitoizetN, QuennedeyA, DelbecqueJP (1997) 2-deoxyecdysone is a circulating ecdysteroid in the beetle Zophobas atratus. Biochem Biophys Acta 1335 : 246–252.
30. NijhoutHF, WilliamsCM (1974) Control of molting and metamorphosis in tobacco hornworm, Manduca sexta (L): Growth of last-instar larva and decision to pupate. J Exp Biol 61 : 481–491.
31. MirthC, TrumanJW, RiddifordLM (2005) The role of the prothoracic gland in determining critical weight to metamorphosis in Drosophila melanogaster. Curr Biol 15 : 1796–1807.
32. Smith WA, Rybczynski R (2011) Prothoracicotropic hormone. In: Gilbert LI, editor. Insect Endocrinology. New York: Academic Press. pp. 1–62.
33. RewitzKF, O'ConnorMB, GilbertLI (2007) Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem Mol Biol 37 : 741–753.
34. WalkiewiczMA, SternM (2009) Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS ONE 4(4): e5072 doi:10.1371/journal.pone.0005072
35. HentzeJL, MoellerME, JorgensenAF, BengtssonMS, BordoyAM, et al. (2013) Accessory gland as a site for prothoracicotropic hormone controlled ecdysone synthesis in adult male insects. PLoS ONE 8(2): e55131 doi:10.1371/journal.pone.0055131
36. PetrykA, WarrenJT, MarquesG, JarchoMP, GilbertLI, et al. (2003) Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A 100 : 13773–13778.
37. HallBL, ThummelCS (1998) The RXR homolog Ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development 125 : 4709–4717.
38. LiTR, BenderM (2000) A conditional rescue system reveals essential functions for the ecdysone receptor (EcR) gene during molting and metamorphosis in Drosophila. Development 127 : 2897–2905.
39. MartinD, MaestroO, CruzJ, Mane-PadrosD, BellesX (2006) RNAi studies reveal a conserved role for RXR in molting in the cockroach Blattella germanica. J Insect Physiol 52 : 410–416.
40. TanA, PalliSR (2008) Edysone receptor isoforms play distinct roles in controlling molting and metamorphosis in the red flour beetle, Tribolium castaneum. Mol Cell Endocrinol 291 : 42–49.
41. SegravesWA, HognessDS (1990) The E75 ecdysone-inducible gene responsible for the 75B early puff in Drosophila encodes two new members of the steroid receptor superfamily. Genes Dev 4 : 204–219.
42. BurtisKC, ThummelCS, JonesCW, KarimFD, HognessDS (1990) The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell 61 : 85–99.
43. King-JonesK, ThummelCS (2005) Nuclear receptors - a perspective from Drosophila. Nat Rev Genet 6 : 311–323.
44. ThummelCS (1995) From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell 83 : 871–877.
45. LamGT, JiangC, ThummelCS (1997) Coordination of larval and prepupal gene expression by the DHR3 orphan receptor during Drosophila metamorphosis. Development 124 : 1757–1769.
46. HuetF, RuizC, RichardsG (1995) Sequential gene activation by ecdysone in Drosophila melanogaster: the hierarchical equivalence of early and early late genes. Development 121 : 1195–1204.
47. LamG, HallBL, BenderM, ThummelCS (1999) DHR3 is required for the prepupal-pupal transition and differentiation of adult structures during Drosophila metamorphosis. Dev Biol 212 : 204–216.
48. TanA, PalliSR (2008) Identification and characterization of nuclear receptors from the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 38 : 430–439.
49. FletcherJC, BurtisKC, HognessDS, ThummelCS (1995) The Drosophila E74 gene is required for metamorphosis and plays a role in the polytene chromosome puffing response to ecdysone. Development 121 : 1455–1465.
50. NijhoutHF, WilliamsCM (1974) Control of molting and metamorphosis in tobacco hornworm, Manduca sexta (L): Cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol 61 : 493–501.
51. RiddifordLM (1996) Juvenile hormone: The status of its “status quo” action. Arch Insect Biochem Physiol 32 : 271–286.
52. Goodman WG, Granger NA, Editors-in-Chief: Lawrence IG, Kostas I, Sarjeet SG(2005) The Juvenile Hormones. Comprehensive Molecular Insect Science. Amsterdam: Elsevier. pp. 319–408.
53. FutahashiR, FujiwaraH (2008) Juvenile hormone regulates butterfly larval pattern switches. Science 319 : 1061–1061.
54. SuzukiY, TrumanJW, RiddifordLM (2008) The role of broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development 135 : 569–577.
55. WilliamsCM (1961) The juvenile hormone. II. Its role in the endocrine control of molting, pupation, and adult development in the Cecropia silkworm. Biol Bull 121 : 572–585.
56. KamimuraM, KiuchiM (2002) Applying fenoxycarb at the penultimate instar triggers an additional ecdysteroid surge and induces perfect extra larval molting in the silkworm. Gen Comp Endocrinol 128 : 231–237.
57. FukudaS (1944) The hormonal mechanism of larval molting and metamorphosis in the silkworm. J Fac Sci Tokyo Imp Univ 4 : 477–532.
58. KayukawaT, MinakuchiC, NamikiT, TogawaT, YoshiyamaM, et al. (2012) Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci U S A 109 : 11729–11734.
59. KonopovaB, JindraM (2007) Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci U S A 104 : 10488–10493.
60. CharlesJP, IwemaT, EpaVC, TakakiK, RynesJ, et al. (2011) Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci U S A 108 : 21128–21133.
61. ParthasarathyR, TanAJ, PalliSR (2008) bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech Dev 125 : 601–616.
62. KonopovaB, JindraM (2008) Broad-complex acts downstream of Met in juvenile hormone signaling to coordinate primitive Holometabolan metamorphosis. Development 135 : 559–568.
63. MinakuchiC, NamikiT, YoshiyamaM, ShinodaT (2008) RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J 275 : 2919–2931.
64. KinjohT, KanekoY, ItoyamaK, MitaK, HirumaK, et al. (2007) Control of juvenile hormone biosynthesis in Bombyx mori: Cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem Mol Biol 37 : 808–818.
65. RiddifordLM, AshburnerM (1991) Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol 82 : 172–183.
66. BurnsKA, GutzwillerLM, TomoyasuY, GebeleinB (2012) Oenocyte development in the red flour beetle Tribolium castaneum. Dev Genes Evol 222 : 77–88.
67. RomerF, EmmerichH, NowockJ (1974) Biosynthesis of ecdysones in isolated prothoracic glands and oenocytes of Tenebrio molitor in vitro. J Insect Physiol 20 : 1975–1987.
68. DelbecqueJP, WeidnerK, HoffmannKH (1990) Alternative sites for ecdysteroid production in insects. Inv Rep Dev 18 : 29–42.
69. SrivastavaUS (1958) Prothoracic glands in Tenebrio molitor L. (Coleoptera: Tenebrionidae). Nature 181 : 1668–1668.
70. ParthasarathyR, TanA, SunZ, ChenZ, RankinM, et al. (2009) Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech Dev 126 : 563–579.
71. MinakuchiC, NamikiT, ShinodaT (2009) Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol 325 : 341–350.
72. DengHM, ZhangJL, LiY, ZhengSC, LiuL, et al. (2012) Homeodomain POU and Abd-A proteins regulate the transcription of pupal genes during metamorphosis of the silkworm, Bombyx mori. Proc Natl Acad Sci U S A 109 : 12598–12603.
73. DelbecqueJP, MeisterMF, QuennedeyA (1986) Conversion of radiolabelled 2,22,25-tri-deoxyecdysone in Tenebrio pupae. Insect Biochem 16 : 57–63.
74. EwerJ, GammieSC, TrumanJW (1997) Control of insect ecdysis by a positive-feedback endocrine system: Roles of eclosion hormone and ecdysis triggering hormone. J Exp Biol 200 : 869–881.
75. ParkY, FilippovV, GillSS, AdamsME (2002) Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development 129 : 493–503.
76. ParkY, ZitnanD, GillSJ, AdamsME (1999) Molecular cloning and biological activity of ecdysis-triggering hormones in Drosophila melanogaster. FEBS Lett 463 : 133–138.
77. ZitnanD, ZitnanovaI, SpalovskaI, TakacP, ParkY, et al. (2003) Conservation of ecdysis-triggering hormone signalling in insects. J Exp Biol 206 : 1275–1289.
78. ZitnanD, KinganTG, HermesmanJL, AdamsME (1996) Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science 271 : 88–91.
79. GauthierSA, HewesRS (2006) Transcriptional regulation of neuropeptide and peptide hormone expression by the Drosophila dimmed and cryptocephal genes. J Exp Biol 209 : 1803–1815.
80. GauthierSA, VanHaaftenE, CherbasL, CherbasP, HewesRS (2012) Cryptocephal, the Drosophila melanogaster ATF4, is a specific coactivator for ecdysone receptor isoform B2. PLoS Genetics 8(8): e1002883 doi:10.1371/journal.pgen.1002883
81. GrilloM, CasanovaJ, AverofM (2014) Development: a deep breath for endocrine organ evolution. Curr Biol 24: R38–40.
82. TraunerJ, SchinkoJ, LorenzenMD, ShippyTD, WimmerEA, et al. (2009) Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol 7 : 73.
83. HughesCL, KaufmanTC (2000) RNAi analysis of Deformed, proboscipedia and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the Hemipteran head. Development 127 : 3683–3694.
84. WarrenJT, SmithW, GilbertLI (1984) Simplification of the ecdysteroid radioimmunoassay by the use of protein A from Staphylococcus aureus. Experientia 40 : 393–394.
85. SuzukiY, SquiresDC, RiddifordLM (2009) Larval leg integrity is maintained by Distal-less and is required for proper timing of metamorphosis in the flour beetle, Tribolium castaneum. Dev Biol 326 : 60–67.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



