-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
Human induced pluripotent stem (hiPS) cells are a potentially powerful model system for studying human disease and development, and a resource for personalized medicine. However, it has been reported that hiPS cells exhibit substantial heterogeneity which could limit their use as model systems. Clearly, knowledge of the source of heterogeneity is key for deeper understanding of the use of human iPS cells for basic and therapeutic applications. One source of this heterogeneity has been presumed to be “memory” of the adult somatic cell from which the hIPS cells were derived, but the evidence to support this view is scant. We have generated a set of human iPS cells from a set of somatic cell types from different donors. Our study shows that cell lines from different somatic sources but from the same donor (i.e. with the same genome) are more similar than cell lines isolated from the same tissue type but from different donors. Once genetic changes are accounted for, all aspects of gene expression, including mRNA levels, splicing and imprinting are highly similar between iPS cells derived from different human tissues. Thus, most of the previously described transcriptional variation between cell lines is likely to be genetic in origin.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004432
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004432Summary
Human induced pluripotent stem (hiPS) cells are a potentially powerful model system for studying human disease and development, and a resource for personalized medicine. However, it has been reported that hiPS cells exhibit substantial heterogeneity which could limit their use as model systems. Clearly, knowledge of the source of heterogeneity is key for deeper understanding of the use of human iPS cells for basic and therapeutic applications. One source of this heterogeneity has been presumed to be “memory” of the adult somatic cell from which the hIPS cells were derived, but the evidence to support this view is scant. We have generated a set of human iPS cells from a set of somatic cell types from different donors. Our study shows that cell lines from different somatic sources but from the same donor (i.e. with the same genome) are more similar than cell lines isolated from the same tissue type but from different donors. Once genetic changes are accounted for, all aspects of gene expression, including mRNA levels, splicing and imprinting are highly similar between iPS cells derived from different human tissues. Thus, most of the previously described transcriptional variation between cell lines is likely to be genetic in origin.
Introduction
Induced pluripotent stem cells (iPSCs) are the subject of tremendous interest as model systems for studying human disease and development [1], [2]. However, cellular reprogramming to iPSCs is an inefficient process in which stochastic events during clonal selection may fix a variety of alternative epigenetic and transcriptional states[3]. Some reports have described significant variation between iPS cells and ES cells, while others have suggested that iPS cells retain a memory of the somatic tissue from which they were derived that may negatively affect their differentiation efficiency into certain cell lineages [4]–[10]. However, comparisons between human iPS cells and ES cells are confounded with differences in genetic background because the lines are derived from different donors. Likewise, because collection of multiple primary tissues from the same individual is frequently impractical, studies of cellular memory in hiPS cells have often confounded iPS source tissue type and donor genetic background. This is important because many cellular phenotypes, including transcription and methylation, are substantially impacted by genetic differences between individuals [11]–[13].
In this study we set out to understand the basis of this variation by establishing a set of iPS cells from a panel of tissues isolated in parallel from several different donors. RNA-seq data sets from these lines, the corresponding adult somatic cells and human ES cells have been systematically compared. This has enabled us to investigate patterns of expression, splicing and imprinting between these iPS cells, their adult cell progenitors and compare these with hES cells. Using a statistical model we estimated the relative contributions of genetic background and tissue of origin to transcriptional variability between human iPS cell lines.
Results
We established primary fibroblast, keratinocyte and endothelial progenitor cell (EPC) somatic cell lines from three healthy male organ donors, labeled S2, S5 and S7, and one healthy female donor (S4). From each primary adult tissue cell line, we derived at least three independent iPS cell lines for each donor. For the adult cell cultures we extracted RNA following each of three passages to give a total of 18 RNA samples from adult donor cells (6 fibroblast, 3 keratinocyte and 9 EPCs). We also extracted RNA from the iPS cell lines derived from each of these tissues to give a total of 9 RNA samples from fibroblast-derived iPSCs (F-iPSCs), 6 from keratinocyte-derived iPSCs (K-iPSCs) and 10 EPC-derived iPSCs (E-iPSCs). Finally, we also extracted 4 RNA samples from two ES cell lines, H9 and Val9. Throughout our study highly standardized conditions were used. This included the isolation, derivation and culture of primary cell cells as well as use of the same batches of reprogramming viruses, serum and media. Each of the primary cell lines were reprogrammed using the four Yamanaka factors to generate a series of 25 iPS cell lines derived from each of the three adult tissues (hereafter F-, K - and E-iPS cells). The differentiation properties of our iPS cell lines were checked by differentiating a subset to endoderm, mesoderm and neuroectoderm derived cell types (Figure S1). Polyadenylated mRNA was prepared from each iPS cell line after 10 passages, the somatic cell lines at passage 3 and from two human ES cell lines which was subjected to high-depth RNA-seq (Fig. 1a). We checked a subset of lines for Sendai virus persistence using RT-PCR with virus-specific primers, but found no evidence for viral presence following passage (Figure S2). For each sample, we performed 75 bp paired end sequencing on the Illumina HiSeq2000 platform. In total we generated 7.3 billion reads, with between 85.3 and 229.8 million reads sequenced in each sample (Figure S3). We mapped reads to assembly h37 of the human genome using Bowtie2 [14] and constructed spliced alignments using Tophat2 [15]. Following read alignment and QC filtering, between 49% and 89% of reads mapped uniquely to the human genome (Figure S4).
Fig. 1. Experimental design and variance components analysis of transcription in iPSCs, somatic progenitors, H9 and Val9 embryonic stem cells. 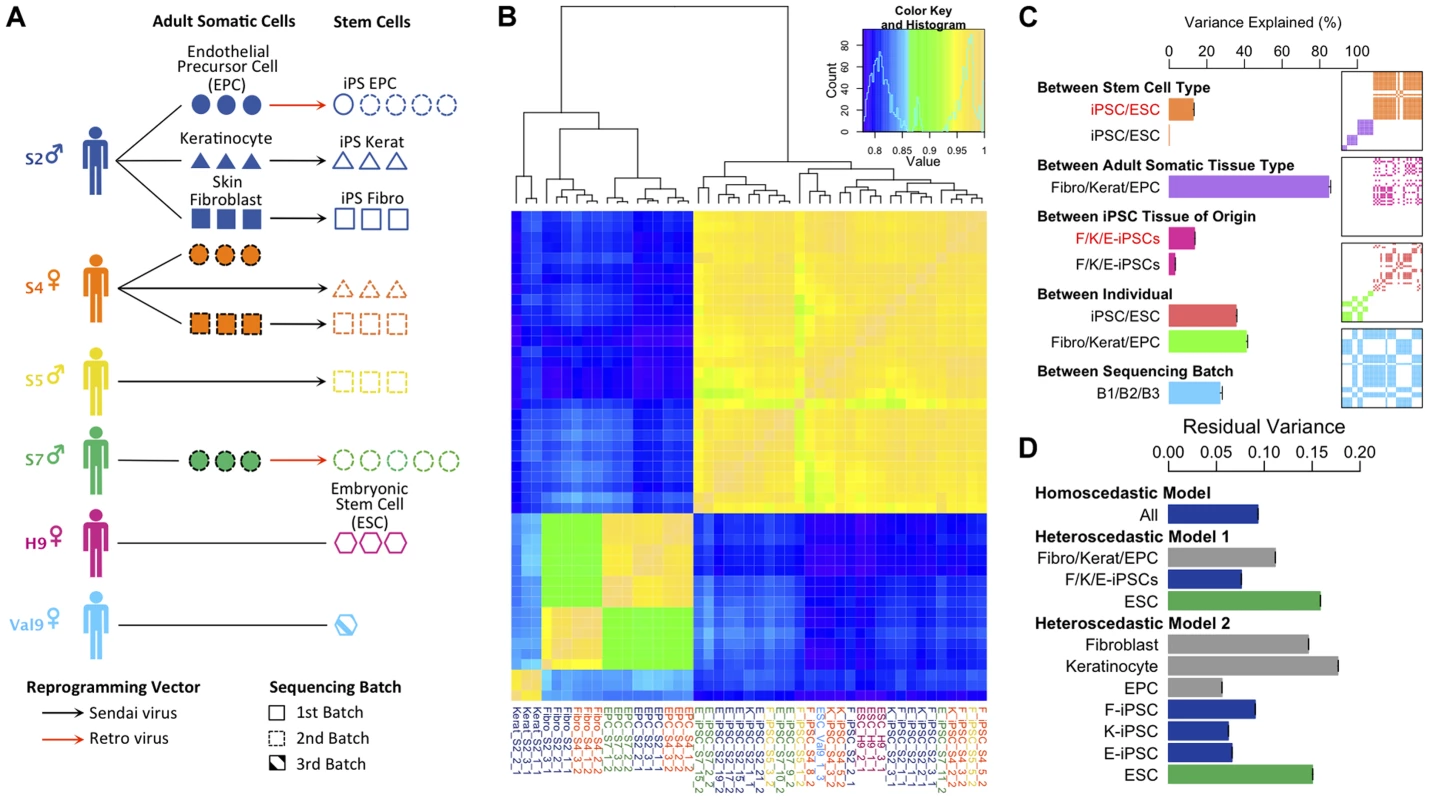
(a) Schematic showing experimental design. (b) Heatmap of Pearson correlation coefficients of log2 FPKMs (fragments per kilobase of exon per million fragments mapped) across all genes in all samples (N = 47). Complete-linkage clustering on the correlation coefficients was performed to order samples by similarity. (c) Decomposition of transcriptional variation using a linear mixed model. Each bar shows the percentage of transcriptional variance explained (%VE) by each of the components, holding all other factors constant (see Text S1 section 1.3 for details). Bars show the percentage variance explained between iPS and ES cells δ222/(δ222+σ2) (orange); between adult tissues δ221/(δ221+σ2) (purple); between iPSC somatic tissue of origin δ232/(δ232+σ2) (pink); between individual (iPSCs) δ241/(δ242+σ2) (red); between individual (adult tissues) δ241/(δ241+σ2) (green) and between sequencing batches δ25/(δ25+σ2)(blue). δ2 and σ2 parameters are explained in section 1.3 of Text S1. Red text denotes variance explained by a component in a model that excluded an individual component, black text denotes variance explained in a model including an individual component. Panels show layered covariance matrices Zj Dj Zj for the variance components j (j = 2…5) of which the correlation heatmap in Figure 1b consists. The color corresponds to each of variance components in the barchart of Figure 1c and white color corresponds to covariance of 0. The sample order of the layered matrices is compatible with Figure 1b. (d) Bars show the estimate of residual variance parameters estimated from a homoscedastic and two heteroscedastic models. “Homoscedastic model” represents a model with a single error term shared over all samples in the data set, and the estimate of this error term is labeled “All”. The heteroscedastic models 1 and 2 are described in the main text and in the Supplement (Section 1.3.3. “Heteroscedastic Model”. Each bar shows the estimate of a residual error parameter as outlined the Supplement section 1.3.3. Initially we examined the global pattern of transcription in the different cell lines. Previous work has suggested that embryonic stem cells are more transcriptionally active than differentiated cells [16]. Within protein coding regions there was a clear bimodal distribution of gene expression levels in all samples reflecting abundantly expressed and transcriptionally repressed genes (Figure S5). Adult cell lines exhibited more completely repressed and very highly expressed genes compared with hiPS cells and hES. Relative to differentiated lineages approximately twice as many genes could be classified as coming from the repressed mode of the distribution in the adult cell lines compared with hiPS cells and ES cells (Figure S 5, inset). We also found that more transcription appeared to be originate from repetitive elements in pluripotent stem cells (Figure S6), although it is unclear whether this arises from a slightly more relaxed chromatin structure in stem cells, or is an artifact resulting from the slight excess of very highly expressed genes in somatic cells relative to pluripotent stem cells.
Variance component analysis of transcription levels
Next, we sought to quantify the contribution of multiple biological and experimental factors to transcriptional variation in hiPSCs. Hierarchical clustering clearly placed adult somatic cells and pluripotent cells in two distinct clades (Fig. 1b). However other sources of variation, such as tissue of origin, were more difficult to discern. To quantify the relative importance of different sources of global transcriptional variation more precisely we employed a variance component analysis. Here, transcriptional variation was decomposed into five separate components. These components comprised: (1) a random intercept term (2) a component to capture variation in transcription between the three adult somatic tissues, hESCs and hiPSCs (3) a component modeling differences between F-, K - and E-iPSCs (4) a component capturing transcriptional variation between different donors or genetic backgrounds and (5) a component captures differences between the two sequencing batches in our data set (see Text S1). We quantified the contribution of each of the five components using intraclass correlation, which measures the proportion of total transcriptional variance explained (VE) by different experimental groups holding other model factors constant. As such, the estimated VEs for each component are not constrained to sum to 100%. Throughout, we modeled the effect of sequencing batch to disentangle its potential influence from the other variance components in the model.
We found that inter-individual transcriptional variation in hiPS cells (VE ∼38%) is considerably larger than that between somatic tissue of origin (VE∼4%) with an even smaller fraction of transcriptional variation (<1%) explained by differences between iPSCs and ESCs (Fig. 1c). Strikingly, when we didn't correct for variation between individuals, transcriptional variation between iPSCs and ESCs and between different iPSC tissues of origin appeared to be much larger (<1% vs 12.7%, iPSCs vs ESCs, 4% vs 13.5%, iPSC tissue of origin, with versus without individual included in the model, respectively) (Fig1c). This suggests that some previous observations of cellular memory and transcriptional differences between iPSCs and ESCS may in fact arise from changing genetic backgrounds rather than experimental effects. Confounding with donor genetic background seems particularly plausible for comparisons between iPSCs and ESCs where controlling for genetic differences is often impossible [4], [17], or in cases where iPSC tissue of origin is confounded with donor genetic background [9], [18]. We found that fibroblast - and keratinocyte-derived iPS cells (F - and K-iPSCs) were highly similar at the transcriptional level with tissue of origin explaining <1% of the transcriptional variation in the between them. In fact, the majority of the tissue of origin effect we observe arises from differences between F/K-iPS cells and EPC-derived iPS cells (E-iPS cells). Some of this signal could reflect transcriptional memory. However it is possible that the reprogramming method could account for this difference because the EPCs were resistant to Sendai virus infection and were therefore derived using the retroviral method. Individual transcriptional variation was slightly greater in adult somatic cells (VE∼42%) than in stem cells. We speculate that this could potentially be explained by non-genetic differences between individuals, such as varying methylation status, which are present in somatic cells but erased during cellular reprogramming.
We also examined the amount of residual transcriptional variation that remained unexplained by any of the known factors using a heteroscedastic model (Text S1). This extension of the model allowed us to capture differences in residual variance between different subsets of our data set. In our experiment, the residual variance captures transcriptional variation between different cell lines from the same donor, either as growths of different IPS cell lines derived from the same donor, from different growths of either the ESCs or as different passages of adult cells from the same donor. We compared two heteroscedastic models with a homoscedastic model, which contained a single error term for the entire data set (Figure 1d, “All”). Heteroscedastic model 1 (“model 1”) contained three terms representing variation between biological replicates of adult cells, IPS cells and ESCs. The results of this analysis illustrate that the residual variation between replicates of IPS cells is lower than that in adult cells and ESCs (Figure 1d: Heteroscedastic Model 1 “F/K/E-iPSCs” versus “ESC”). Heteroscedastic model 2 further divided error into components for each of the three different adult cell types, for each of the three IPS cell types and for ESCs. This analysis demonstrated that variation between biological replicates of IPS lines derived from different tissues was relatively consistent (Figure 1d: Heteroscedastic Model 2). Overall, our data suggest that transcriptional variation between biological replicates of iPS cells was not substantially, and may be somewhat lower, different from that between passages of an established hES cell line or of adult primary cells.
Given that our variance component analysis was based on FPKM values, we also reanalyzed data excluding highly expressed genes, as this may impact our results. We found that our component estimates and correlation heatmap were qualitatively very similar when the top 1 and 5% of genes were removed from the data set (Figure S7, S8). We also attempted a similar analysis of mitochondrial gene expression. We found similar proportions of reads coming from mitochondrial genes in adult, IPS and ES cells (Figure S9). However, the very low number of expressed genes (13) resulted in extremely noisy estimates of the correlation between sample gene expression profiles (Figure S10) and prohibited variance components analysis, due to failure of the model to converge. Our differential expression analysis classified all mitochondrial genes as “invariant expression” (data not shown). Our experiment did not include multiple replicates from the same cell line, and so we were unable to formally address the issue of variation between lines from the same donor. Visual inspection of our read coverage plots did suggest that some cell lines might be more variable than others. Heatmaps of the differentially expressed genes illustrate that while most cell lines were quite consistent, one cell line derived from keratinocytes formed an outgroup (K-iPSC-S2-1) with other keratinocyte cell lines (Figure S11).
Cellular memory and aberrant reprogramming are rare in hIPS cells
Although our variance components analysis suggested relatively small global effects of tissue of origin, this could mask effects at individual genes. We next sought to identify those genes whose expression in hiPS cells more closely resembled their somatic progenitors or were improperly silenced or activated, relative to ES cells (Fig. 2a). For each of the three somatic tissues in turn, we performed a three-way comparison of expression levels in the somatic tissue, in the hiPS cells derived from that tissue, and in the hES cells. At each gene we tested for departures from a null hypothesis of equal expression levels in the somatic cell, the iPS cells and the ES cells using a negative binomial generalized linear model similar to that outlined in [19] (Text S1). When the null hypothesis (“invariant expression”) was rejected we further classified genes into one of four possible categories, “correctly reprogrammed”, “transcriptional memory”, “aberrantly reprogrammed” and “complex” by selecting the alternative with the highest log likelihood ratio.
Fig. 2. Effects of cell type and tissues of origin on expression levels in iPSCs. 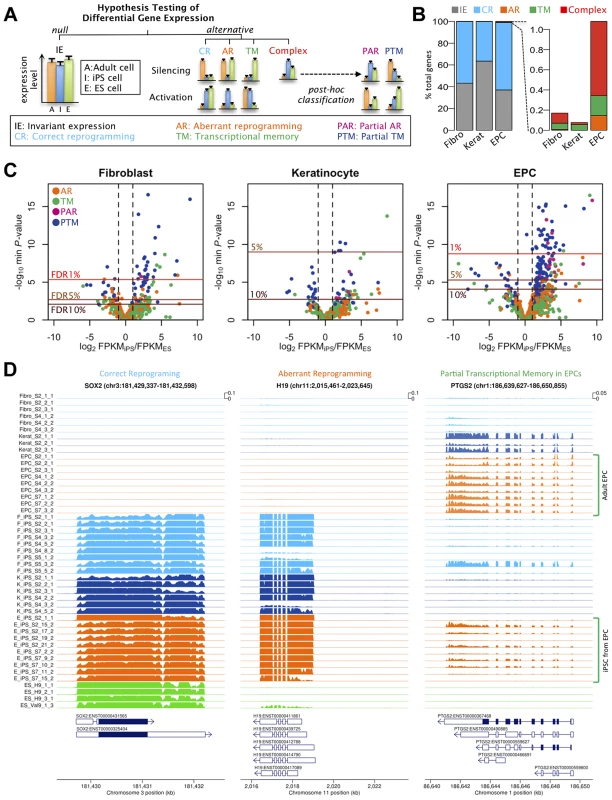
(a) Schematic of hypothesis testing approach to identify genes with invariant expression (IE) from genes that we defined as correctly reprogrammed (CR), aberrantly reprogrammed (AR), with transcriptional memory (TM) and showing complex expression. Genes showing complex expression are further classified into partial AR (PAR) and partial TM (PTM) according to the expression patterns. (b) Percentages of genes assigned to alternative differential expression groups based on a hierarchical model (see Text S1 1.4.3). (c) Volcano plots of differentially expressed genes. Plots show log10 of minimum P-values among the four alternative hypotheses against log2 fold change of average expression levels between iPSCs and ESCs. The colours of points indicate the differential expression categories into which a gene was classified. Dashed lines show twofold enrichment of mean expression levels between iPSCs and ESCs. FDR thresholds were calculated by permutation. (d) Read coverage depth in three examples of differentially expressed genes; SOX2, was categorised as correctly reprogrammed in all three tissues (F-iPSCs: p<1×10−24; K-iPSCs: p<1.2×10−16;E-iPSCs: p<4.6×10−25), H19 was categorised as aberrantly reprogrammed in all three tissues (F-iPSCs: p<1.2×10−6; K-iPSCs: p<2.7×10−4;E-iPSCs: p<1.5×10−16) and TYW3 gene was categorised as partial transcriptional memory in E-iPSCs (p<7.1×10−8), invariant expression in F-iPSCs (p>0.1) and correctly reprogrammed in K-iPSCs (p<1.4×10−3). Coverage depth was truncated at 1000 reads per bp. We used a hierarchical model (Text S1), similar to that in [20], to estimate the true proportion of transcribed genes in each category without setting a threshold on statistical significance or effect size. Our results suggested that transcriptional memory is very uncommon, occurring at 0.06, 0.06 and 0.20% of all expressed genes in F-, K - and E-iPS cells (Fig 2b). Aberrant or complex expression patterns were also infrequent, although aberrant expression appeared slightly more often in E-iPSCs (0.15% of genes) than in the other two tissues. The fractions falling into the complex category were similarly low (0.10, 0.02 and 0.74% of genes, respectively). The remaining genes either showed invariant expression or were classified as correctly reprogrammed (99.83, 99.92 and 98.92%). Core pluripotency markers, including Sox2, Nanog and Oct4, were all classified as correctly reprogrammed (Figure S12). Our analysis suggested that the fraction of correctly reprogrammed genes is approximately 30-60% of expressed genes. Although comparison between different studies and methodologies difficult, this is not dissimilar to fraction of genes that are differentially expressed in reprogramming found by other studies [21]).
Next we identified individual loci that were differentially expressed at a genome-wide false discovery rate (FDR) of 5% (estimated from permuted data), and that exhibited a 1.5-fold or greater change in expression level between hiPS cells and hES cells. Using these criteria we identified a total of 61, 5 and 103 transcribed regions that showed some form of differential expression in F-, K-, or E-iPS cells respectively (Table 1), the majority of which exhibited either complete or partial transcriptional memory (“TM” or “PTM”). Most genes we identified were very weakly expressed in IPS cells, with mean FPKMs 74–79% lower than the average (Figure S13). The most enriched gene ontology (GO) terms in the TM and PTM gene sets were for mesodermal migration in F-iPS cells (enrichment q-value <0.002;), for hemidesmosome development in K-iPS cells (q<0.03) and for inflammatory and defense response in E-iPS cells (q<3×10−5) (Figure S14). Aberrantly expressed genes were less frequent than genes exhibiting transcriptional memory. One interesting exception to this was the long noncoding RNA, H19, which was highly expressed in the majority of hiPS cells in our data set relative to the hES cells (Fig. 2d). Our differential expression analysis also suggested that aberrant activation occurs more often than aberrant silencing (21 versus 6 genes at FDR 5%), and that transcriptional memory more frequently involves memory of an active rather than a silenced gene (101 versus 19 genes at FDR 5%) (Table 1). However, this may simply reflect better power to detect differential expression when a gene is highly expressed rather than silenced. Removal of highly expressed genes had almost no impact on our differential expression analysis (Figures S6, S7). Finally, differential expression analysis of mitochondrial genes classified all 13 genes we found be expressed in any of our samples as “invariant expression”. Overall, our results suggest that, although transcriptional memory and aberrant reprogramming do occur occasionally, relatively few genes are involved, those that are affected are weakly expressed.
Tab. 1. Numbers of significantly (FDR%) differentially expressed genes in each differential expression category. 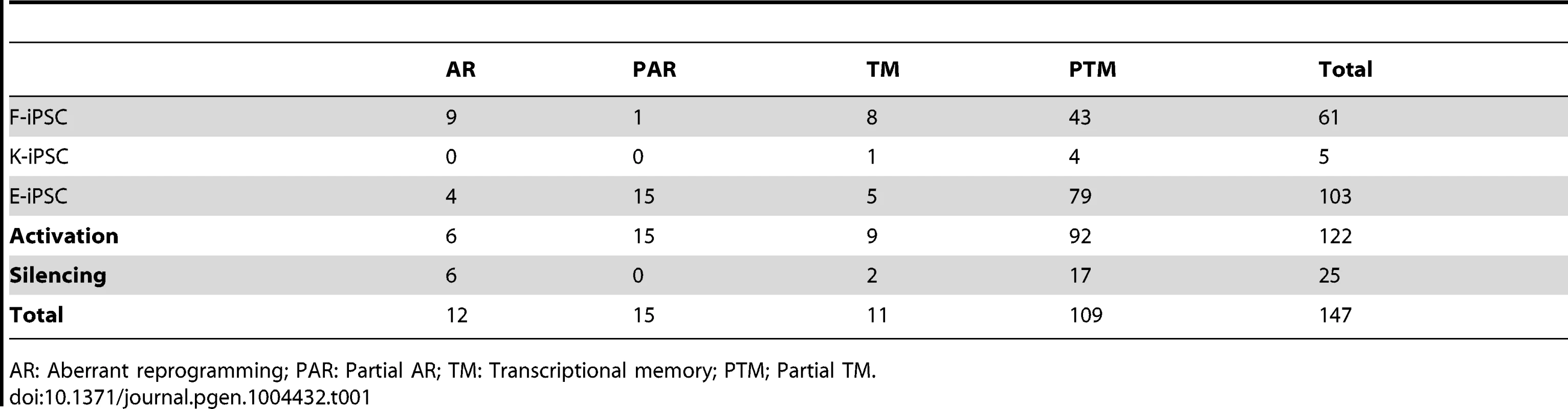
AR: Aberrant reprogramming; PAR: Partial AR; TM: Transcriptional memory; PTM; Partial TM. RNA splicing patterns in hIPS cells resemble ES cells
Although whole gene expression levels appeared to be relatively stable across different tissues of origin, cellular memory could also manifest at the level of RNA splicing. Using the statistical framework we developed for gene expression levels, we next tested whether isoform abundance ratios showed evidence of memory of their cell type of origin (Text S1). In this analysis, we tested the null hypothesis that the ratio of the top two most abundant isoforms was equal in adult tissues, iPS cells and ES cells. We computed abundances using two popular approaches, Cufflinks2 and MISO [22], [23], and analysed the subset of genes where the ranking of isoform abundances agreed between the two methods (Figure S15). We found no significant memory of adult cell splice patterns or aberrant alternative splicing in IPS at an FDR of 5% estimated from permuted data (Figure S16). This suggests that transcriptional memory of cellular splice patterns or aberrant splicing induced during reprogramming is a relatively weak effect, smaller than that observed at the level of whole gene expression, and potentially masked by larger technical and genetic effects.
Imprinting is conserved between iPS cells and their somatic cell of origin
Previous studies have suggested that imprinted gene expression patterns may be unstable in hES cells and hIPS cells [24]. We genotyped the four individuals from whom our hIPS cell lines were derived using an Illumina Omni2.5 genotyping chip. Genotypes were phased, and SNP genotypes were imputed using Beagle [25]. Using the phased haplotypes, we computed estimates of allele-specific expression for the paternal and maternal chromosomes of each individual. We began by investigating whether patterns of imprinting observed in the adult somatic tissue remained conserved in the iPS cells that were derived from them in a set of 210 putatively imprinted genes obtained from the http://www.geneimprint.com/database. We found that many genes were expressed at a low level or lacked sufficient coverage of heterozygous SNPs, which were subsequently excluded. The remaining genes (26, 23 and 23 in donor S2, S4 and S7, respectively) exhibited allelic expression patterns in adult cells that were conserved in their derived iPS cell lines (Pearson r2 = 0.46; p<6.3×10−10), Fig 3. Many genes did not demonstrate the characteristic mono-allelic expression of imprinted genes in either adult or pluripotent cells (Fig 3). In a small number of cases, such as the paternally expressed zinc finger gene, ZDBF2, we observed a loss of imprinting and reversion to bi-allelic expression in many IPS lines. We note that, in the cases where we observe loss or alteration of imprinting, the genes involved are relatively weakly expressed in either the adult or the IPS cell, making ascertainment of imprinting status more difficult.
Fig. 3. Imprinting effects on transcription in adult and iPS cells. 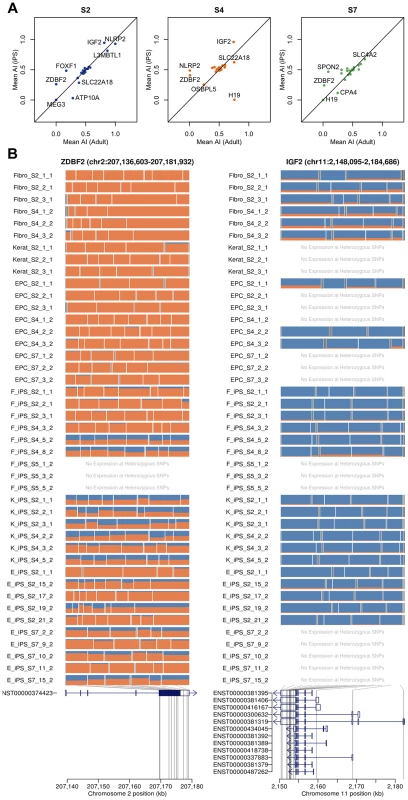
(a) Allelic imbalance between adult cells and iPS cells for each donor (S2, S4 and S7). (b) The mosaic plots for each cell line show the allelic imbalance at multiple heterozygous SNP loci throughout the transcribed region. Each row corresponds to a sample, and each box corresponds to a single heterozygous SNP. Box width is proportional to the total nucleotide count at the heterozygous SNP, with the box bisected in proportion to the paternal and maternal nucleotide counts (blue and orange) based on the phasing haplotype information. Examples show consistent loss of imprinting in iPSCs (ZDBF2) and in adult cells (IFG2). Detecting known genetic effects on gene expression in hIPSCs
Finally we returned to the effects of the genetic background in our hiPS cells. Extensive maps of genetic variants whose genotype correlates with gene expression (expression quantitative trait loci, eQTLs) have been generated in adult human tissues and cell lines [26]. We investigated whether similar effects could be observed in our iPS cells. Since the number of individuals in our data set was small, a standard eQTL mapping experiment was not possible. Instead, we tested whether genetic associations ascertained in lymphoblastoid cell lines (LCLs), a model system for eQTL detection in humans, were also detectable in iPS cells.
We reanalyzed an existing LCL RNA-seq dataset derived from the same source population (162 GBR+CEU individuals) as our hiPS cells (Figure S17) and identified 4,350 eQTLs at an FDR of 5% [27]. Variance component analysis revealed a greater amount of variation between individuals in genes that were ascertained to have an eQTL in LCLs, than in genes where the null of no eQTL could not be rejected (Fig 4a). A substantial fraction (17%) of this variation could be explained by the lead eQTL SNPs (eSNPs). We tested whether the direction of the genetic effect at the LCL eQTL genes replicated in iPS cells by grouping the four individuals in our data set according to their eSNP genotype. We found that the expression level of genes with an eQTL ascertained in LCLs follows the expected direction in hiPS cells (Fig. 4b). Likewise, for individuals in our dataset that are heterozygous at the eSNP we see a corresponding, highly significant allelic imbalance also in the expected direction (Fig. 4c). The correlation between genotype and expression level at ascertained eQTLs in hiPS cells was highly significant (Pearson r = 0.44, p<9.8×10−68), as was the allelic imbalance at heterozygous ascertained eSNPs (Student t, p<1.3×10−8). Although our data set is small, we do find convincing examples where a correlation between genotype and gene expression replicated a known eQTL identified in LCLs such as the exonic eSNP, rs1059307, located within the noncoding RNA gene SNHG5. At this gene, we also observe clear allelic imbalance in iPS cells derived from S5 and S7 individuals, who are heterozygous for this eSNP (Fig. 4e), which is in the same direction as the eQTL effect in LCLs (Fig. 4f). These genetic effects on gene expression in iPS cells were detectable across multiple independent iPS cells from the same genetic background, despite the variety of different tissues sources and reprogramming methods.
Fig. 4. Genetic effects on transcription in iPSCs. 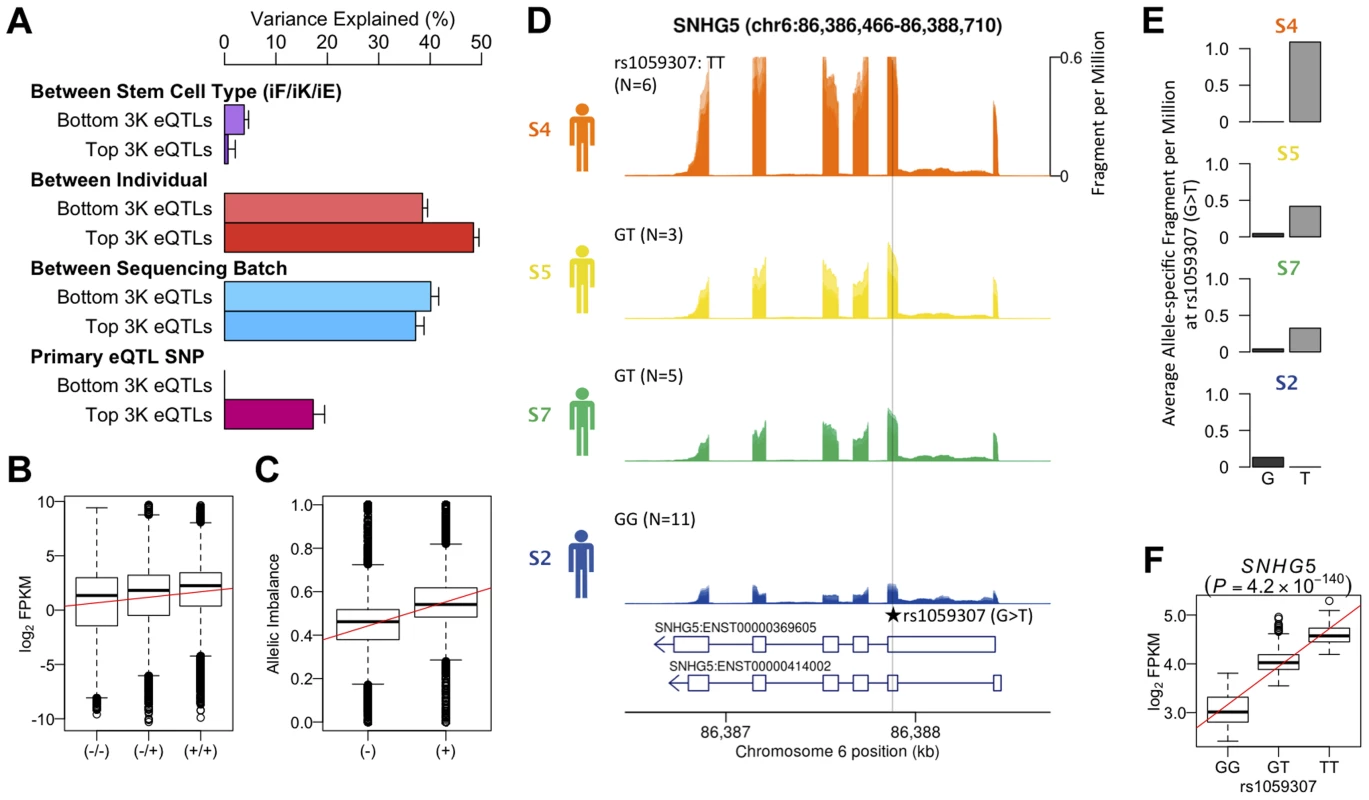
(a) Variance component of known eQTL genes showing primary eQTL SNPs being able to explain 17% of FPKM variation on average. Top and bottom 3,000 eQTL genes and SNPs were determined by means of P-values using gEUVADIS RNA-seq data [27]. (b) Box- plot of log2 FPKM aggregated across 462 eQTL genes (FDR 5%) stratified into high (+/+), medium (+/−) and low (−/−) genotypes at the minimum p-value eQTL SNPs ascertained from [2] (FDR 5%). Pearson correlation r = 0.44, p<9.8×10−68. (c) Boxplot of allelic imbalance at high-expression (+) and low-expression (−) haplotypes across all eQTL genes ascertained from [2]. The red line is at 0.5 indicating the expected fraction under the null. Student t p<1.3×10−8. (d) Mean RNA-Seq coverage depth in iPSCs, averaged across all iPSC lines from each individual, at a putative example eQTL for the noncoding RNA SNHG5 gene. Individuals are grouped by their genotype at the putatively causal variant rs1059307. Individual genotypes are shown with the number of RNA-seq samples in parentheses. (e) Average coverage depth of fragments coming from the high-expression haplotype (allele T) and low- expression haplotype (allele G) at rs1059307. (f) eQTL association of SNHG5 gene at rs1059307 in lymphoblastoid cell lines from [2] showing distributions of log2 FPKM against SNP genotypes at rs1059307. The red line shows the best-fit linear regression line. Discussion
We have shown that epigenetic memory of the adult progenitor cell is a rare phenomenon in hIPS cells, and that cellular heterogeneity between different hIPS lines is more likely to be driven by changing genetic background. Our study has important implications for future attempts to use iPSCs as cellular model systems for drug discovery and other applications. Encouragingly, our results suggest that genetic effects are readily detected in hIPSCs and that cell phenotypes are highly reproducible within individuals. Equally important, however, is the fact that the noise introduced by genetic background could potentially obscure small genetic signals of interest in small samples. A clear implication of this result is that, in iPSC-based studies of genetic disease, most effort should be expended on collection of samples from different donors rather than generation of large numbers of lines from the same individual. Collection of multiple individuals, perhaps with a shared genotype at a single locus of interest, will allow the effects of genetic background to be averaged over and separated from that of the putatively causal locus.
Our study also highlights how the effects of genetic background cannot be ignored when considering cellular variability between pluripotent stem cell lines. Previous studies have attributed cellular variability in IPS to a range of sources, including epigenetic memory [5]–[10], [17], inherent differences between IPSCs and ESCs [4], [28], artifacts of reprogramming [7] or lab environment [29]. Perhaps surprisingly, the effects of genetic background have been less well appreciated, although more recent work has highlighted its potential importance in differentiation [30]. It seems likely that at least some of variability previously reported to exist in IPS cells could in fact have arisen from genetic differences. This is particularly true of comparisons of IPS with ES that are typically derived from different individuals. It is also notable that studies including larger numbers of donors tend to find fewer transcriptional differences between IPS and ES [29], [31]–[33]. Studies that have not controlled for genetic background when investigating epigenetic memory, such as by confounding tissue of origin and donor, may also have mistakenly attributed genetically driven differences in transcription to epigenetic memory. Our study explicitly incorporates multiple tissues from the same donors, allowing us to correct for the effects of changing genetic background. This is likely to explain why we do not find extensive apparent epigenetic memory. We note, however, that other studies that have also explicitly controlled for genetic background still report some variation in transcription, methylation and differentiation efficiencies that appear to arise from cell type of origin effects [5], [8], [10]. A possible explanation for the discrepancy between the results of these studies and our own is that we have also taken our samples from cells at between 10 and 13 passages and epigenetic memory effects may be transient and disappear following multiple passages [8].
An important caveat for our study is that influential cellular differences may simply not manifest as transcriptional variation but reside at, for example, the epigenetic level as changes in methylation status or histone tail modifications. Such differences may harbor a “hidden” functional role that only becomes apparent upon differentiation into a specific cell lineage. Our study suggests that epigenetic differences are likely to be more plausible candidates as drivers of variation in IPS cell differentiation ability. However, our results also illustrate that current iPS cell technology is robust enough to enable detection of genetic effects on important cellular phenotypes such as mRNA levels. Although further technological hurdles remain, an exciting area for future work will be detection of regulatory variation that influences transcription during cell lineage specification and differentiation, employing iPS cells as a model system.
Materials and Methods
Samples
All primary tissue samples and blood for this project were obtained from adult cadaveric organ transplant donors referred to the Eastern Organ Donation Services Team (part of NHS Blood and Transplant). Ethics approval was obtained from the local Research Ethics Committee (REC No. 09/H306/73).
Derivation of fibroblasts and keratinocytes from skin samples
For each subject included in this study, a sample of skin was excised from the midline surgical incision. The skin was transported to the lab and washed in iodine and ethanol and was cut into approximately 1 mm3 pieces. These were dispersed evenly on a 90 mm plate and incubated with fibroblast media (Knockout DMEM and 10% FBS). Outgrowths of fibroblasts and keratinocytes from the skin explants were usually apparent at around 14 days. The cells were separately harvested using 5 min treatment with Versene (15040-066, Invitrogen), which detached the fibroblasts leaving the keratinocytes on the plate. The fibroblasts were cultured on non-coated plates using fibroblast media and keratinocytes were cultured on plates coated with matrix (R011K, Invitrogen) and using EpiLife media plus Defined Growth Supplement (M-EPI-500-CA and S-012-5, Invitrogen).
Derivation of Endothelial Progenitor Cells (EPCs)
Endothelial Progenitor Cells were derived from 100 mL of peripheral blood as previously described [11]. Briefly, the mononuclear cells of the blood sample were separated using Ficoll. The cells were cultured on collagen-coated plates using EPC media (EGM-2MV supplemented with growth factors plus 20% Hyclone serum; CC-3202, Lonza and HYC-001-331G; Thermo Scientific Hyclone respectively). Colonies of EPCs appeared at around 10 days.
Generation of EPC-hiPSCs using retroviruses
Four pseudotyped Moloney murine leukemia retroviruses containing the coding sequences each of human OCT-4, SOX-2, KLF-4 and C-MYC were obtained from Vectalys (Toulouse, France). A multiplicity of infection of 10 was used in all retroviral reprogramming experiments. For each hiPS cell derivation, 1×105 EPCs were transfected with the 4 viruses in the presence of 10 ug/mL of polybrene (TR-1003-G, Millipore). After 24 hrs the viruses were washed off with PBS and the cells were re-fed with EPC media that remained for the next 4 days. On day 5 after transduction, the cells were re-plated using trypsin onto a 10 cm dish of fresh MEF feeders. After 2 days the media was changed from primary cell-specific to hiPSC media (KSR + FGF-2). The media was changed every 2 days until colonies emerged after which the media was changed daily. Colonies were picked once they had reached sufficient size, typically from day 25 following transduction. The colony was split into quarters and the segments gently lifted off the plate and transferred to one well of a 12 well plate of fresh MEF feeders containing hiPS cell media (KSR + FGF2) supplemented with ROCK inhibitor (Y-27632, Sigma).
Generation of F-hiPS cells and K-hiPS cells using Sendai virus
Four Sendai viruses containing the coding sequences of each of human OCT-4, SOX-2, KLF-4 and C-MYC were obtained from DNAVec (Ibaraki, Japan). The protocol for reprogramming was identical to that of retroviruses with the exceptions that 5×105 primary cells (fibroblasts or keratinocytes) were used at MOI 3 and polybrene was not used.
hiPS cell culture
hiPS cells were grown on irradiated MEF feeders, using human embryonic stem cell media (termed KSR + FGF-2): Advanced DMEM (12634-010, Invitrogen) was supplemented a follows: 10% Knockout Serum Replacement (10828028, Invitrogen), 2 mM L-glutamine (25030024, Invitrogen, 0.1 mM β-mercaptoethanol (M6250, Sigma-Aldrich) and 4 ng/µL of recombinant human basic Fibroblast Growth Factor-2 (233-FB-025, R&D systems, Minneapolis, MO, USA). Media was changed daily and the cells were passaged every 5–10 days depending on the confluence of the plates. To passage hiPS cells, the plates were washed in PBS and colonies detached using collagenase and dispase (Collagenase IV 1 mg/mL, Invitrogen 17104-019; Dispase 1 mg/mL, Invitrogen 17105-041). The colonies were washed in media and mechanically broken up before being re-plated onto fresh MEF feeders.
RNA extraction
Total RNA was extracted using the RNeasy Mini Kit protocol (Qiagen, Hilden, Germany). RNA-seq libraries were constructed according the manufacturers guidelines, with minor modifications, using the Illumina mRNA-seq and TruSeq mRNA sample preparation kits (Illumina, Inc., San Diego, CA). Briefly, mRNA was enriched from total RNA using oligo dT beads before fragmentation via zinc and heat hydrolysis. mRNA was subject to first and second strand cDNA synthesis before end repairing and A-tailing. Double-stranded cDNA was then adapter-ligated before size-selecting fragments with inserts ranging from 200–300 bp using a LabChipR XT (Perkin Elmer, Waltham, MA). Size-selected material was then PCR-amplified using KAPA HiFi polymerase (Kapa Biosystems, Boston, MA) before sequencing on an Illumina HiSeq2000 (Illumina, Inc., San Diego, CA).
Computational and statistical analysis
We mapped reads to assembly h37 of the human genome using Bowtie2 [14] and constructed spliced alignments using Tophat2 [34] with default settings. We also used known gene annotation information given by Ensembl release 69 as a guide for the alignment. Following read mapping, we selected fragments (read-pairs) where at least one of mate-pairs had a quality score of >10, aligned with no gaps, with three base mismatches or less. Any read pairs with an insert size less than 150 bp or greater than 1 Mb, or on different chromosomes, were excluded from subsequent analyses. Computational analysis was carried out using a combination of existing packages, such as DESeq [19] and our own analysis tools. For the variance components analysis, transcription level at each gene j was modeled as a linear combination of five normally distributed random effects (b1–5) and a single error term:
where is a vector of log normalized fragments per kilobase per million reads sequenced (FPKMs) for gene j in each of the 46 samples in our data set, b1 is an intercept term, b2 models variation in transcription between the three adult somatic tissues, hESCs and hiPSCs, b3 models differences between F-, K - and E-iPSCs, b4 captures transcriptional variation between different donors, b5 captures differences between the two sequencing batches in our data set, ε is the error term and Z1-Z5 are design matrices. For full details of computational and statistical analyses, see Text S1.Data
Our raw sequence data are available from the European Genotype Archive under study ID EGAS00001000367. A variety of processed data, including raw read counts, log2 FPKMS and the results of our differential expression analysis are available from our lab website (http://www.sanger.ac.uk/research/projects/genomicsofgeneregulation/) under the “Data” tab.
Supporting Information
Zdroje
1. TakahashiK, TanabeK, OhnukiM, NaritaM, IchisakaT, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131 : 861–872.
2. WuS, HochedlingerK (2011) Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature Cell Biology 13 : 497–505.
3. LundR, NarvaE, LahesmaaR (2012) Genetic and epigenetic stability of human pluripotent stem cells. Nature Reviews Genetics 13 : 732–744.
4. ChinM, MasonM, XieW, VoliniaS, SingerM, et al. (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5 : 111–123.
5. KimK, DoiA, WenB, NgK, ZhaoR, et al. (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467 : 285–290.
6. KimK, ZhaoR, DoiA, NgK, UnternaehrerJ, et al. (2011) Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature Biotechnology 29 : 1117–1119.
7. ListerR, PelizzolaM, KidaY, HawkinsR, NeryJ, et al. (2011) Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471 : 68–73.
8. PoloJ, LiuS, FigueroaM, KulalertW, EminliS, et al. (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature Biotechnology 28 : 848–855.
9. OhiY, QinH, HongC, BlouinL, PoloJ, et al. (2011) Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature Cell Biology 13 : 541–549.
10. Bar-NurO, RussH, EfratS, BenvenistyN (2011) Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9 : 17–23.
11. SkellyD, RonaldJ, AkeyJ (2009) Inherited variation in gene expression. Annual Review of Genomics and Human Genetics 10 : 313–332.
12. BellJ, PaA, PickrellJ, GaffneyD, Pique-RegR, et al. (2011) DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biology 12: R10.
13. GibbsJ, van der BrugM, HernandezD, TraynorB, NallsM, et al. (2010) Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genetics 6: e1000952.
14. LangmeadB, SalzbergS (2012) Fast gapped-read alignment with Bowtie 2. Nature Methods 9 : 357–359.
15. TrapnellC, PachterL, SalzbergS (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111.
16. EfroniS, DuttaguptaR, ChengJ, DehghaniH, HoeppnerD, et al. (2008) Global transcription in pluripotent embryonic stem cells. Cell Stem Cell 2 : 437–447.
17. MarchettoM, YeoG, KainohanaO, MarsalaM, GageF, et al. (2009) Transcriptional signature and memory retention of human-induced pluripotent stem cells. PloS One 4: e7076.
18. GhoshZ, WilsonK, WuY, HuS, QuertermousT, et al. (2010) Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PloS one 5: e8975.
19. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biology 11: R106.
20. VeyrierasJ-B, KudaravalliS, KimS, DermitzakisE, GiladY, et al. (2008) High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genetics 4: e1000214.
21. PoloJ, AnderssenE, WalshR, SchwarzB, NefzgerC, et al. (2012) A Molecular Roadmap of Reprogramming Somatic Cells into iPS Cells. Cell 151 : 1617–1632.
22. TrapnellC, WilliamsB, PerteaG, MortazaviA, KwanG, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology 28 : 511–515.
23. KatzY, WangE, AiroldiE, BurgeC (2010) Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nature Methods 7 : 1009–1015.
24. PickM, StelzerY, Bar-NurO, MaysharY, EdenA, et al. (2009) Clone - and gene-specific aberrations of parental imprinting in human induced pluripotent stem cells. Stem Cells 27 : 2686–2690.
25. BrowningB, BrowningS (2009) A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. American Journal of Human Genetics 84 : 210–223.
26. GaffneyD (2013) Global properties and functional complexity of human gene regulatory variation. PLoS Genetics 9: e1003501.
27. LappalainenT, SammethM, FriedlanderM, t HoenP, MonlongJ, et al. (2013) Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501 : 506–511.
28. WangA, HuangK, ShenY, XueZ, CaiC, et al. (2011) Functional modules distinguish human induced pluripotent stem cells from embryonic stem cells. Stem Cells and Development 20 : 1937–1950.
29. NewmanA, CooperJ (2010) Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell 7 : 258–262.
30. KajiwaraM, AoiT, OkitaK, TakahashiR, InoueH, et al. (2012) Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America 109 : 12538–12543.
31. GuentherM, FramptonG, SoldnerF, HockemeyerD, MitalipovaM, et al. (2010) Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell 7 : 249–257.
32. BockC, KiskinisE, VerstappenG, GuH, BoultingG, et al. (2011) Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144 : 439–452.
33. YamanakaS (2012) Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10 : 678–684.
34. KimD, PerteaG, TrapnellC, PimentelH, KelleyR, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology 14: R36.
35. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics 38 : 904–909.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




