-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
Genome-wide association studies have identified hundreds of genetic variants associated to autoimmune diseases. To understand the mechanisms and pathways affected by these variants, follow-up studies of molecular phenotypes and functions are required. Given the diversity of cell types and specialization of functions within the immune system, it is crucial that such studies focus on specific and relevant cell types. Here, we studied genetic and cellular traits of CD4-positive effector memory T (CD4+ TEM) cells, which are particularly important in the onset of rheumatoid arthritis, celiac disease, and type 1 diabetes. In a cohort of healthy individuals, we purified CD4+ TEM cells, assayed genome-wide single nucleotide polymorphisms (SNPs), abundance of CD4+ TEM cells in blood, proliferation upon T cell receptor stimulation, and 215 gene transcripts in resting and stimulated states. We found that expression levels of 46 genes were regulated by nearby SNPs, including disease-associated SNPs. Many of these expression quantitative trait loci were not previously seen in studies of more heterogeneous peripheral blood cells. We demonstrated that relative abundance and proliferative response of CD4+ TEM cells varied in the population, however disease alleles are unlikely to confer risk by modulating these traits in this cell type.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004404
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004404Summary
Genome-wide association studies have identified hundreds of genetic variants associated to autoimmune diseases. To understand the mechanisms and pathways affected by these variants, follow-up studies of molecular phenotypes and functions are required. Given the diversity of cell types and specialization of functions within the immune system, it is crucial that such studies focus on specific and relevant cell types. Here, we studied genetic and cellular traits of CD4-positive effector memory T (CD4+ TEM) cells, which are particularly important in the onset of rheumatoid arthritis, celiac disease, and type 1 diabetes. In a cohort of healthy individuals, we purified CD4+ TEM cells, assayed genome-wide single nucleotide polymorphisms (SNPs), abundance of CD4+ TEM cells in blood, proliferation upon T cell receptor stimulation, and 215 gene transcripts in resting and stimulated states. We found that expression levels of 46 genes were regulated by nearby SNPs, including disease-associated SNPs. Many of these expression quantitative trait loci were not previously seen in studies of more heterogeneous peripheral blood cells. We demonstrated that relative abundance and proliferative response of CD4+ TEM cells varied in the population, however disease alleles are unlikely to confer risk by modulating these traits in this cell type.
Introduction
Memory T cells are an important component of the adaptive immune system. They circulate between lymphoid organs, blood, and peripheral tissues, and facilitate faster and more aggressive immune response to antigens after re-exposure. CD4-positive effector memory T (CD4+ TEM) cells are known to migrate to peripheral sites of inflammation upon activation, and rapidly produce both Th1 and Th2 cytokines [1]. Investigators have long suggested their involvement in autoimmune diseases including rheumatoid arthritis (RA), type I diabetes (T1D), and celiac disease (CeD) [2]–[5]. However, whether changes in cell population subsets and functions are causal or reactive to disease is uncertain. One strategy to answer this question is to examine potential intermediate molecular phenotypes, and identify those modulated by genetic variants. In order to understand the pathogenic roles of CD4+ TEM cells in autoimmunity, we aimed to characterize the variation in their phenotypic and functional markers in a healthy population, and to identify whether these markers intersect with the genetic basis for autoimmunity.
The majority of autoimmune disease risk variants are located in non-coding regions of the genome. It is reasonable to hypothesize that a subset of them causes disease by altering gene regulatory mechanisms as expression quantitative trait loci (eQTL) [6]–[9]. So far, studies of gene regulation have largely been carried out in cell lines and primary resting blood cells including undifferentiated CD4+ T cells, B cells, monocytes, and dendritic cells [8], [10]–[12]. However, to understand the pathogenic mechanisms of risk variants, especially when studying the immune system where cells are highly diverse and functionally specialized, it is crucial to focus on relevant cell types and stimulated cellular states.
We have previously shown that genes within RA risk loci were most specifically expressed in CD4+ TEM cells, compared to more than 200 other immune cell types of various lineages and developmental stages (p = 1.00×10−8; Figure S1) [13]. Celiac disease and T1D loci were also enriched for genes specifically expressed in CD4+ TEM cells (p = 1.43×10−5 and 1.29×10−4, respectively; Figure S1) [13]. Non-coding single nucleotide polymorphisms (SNPs) associated with RA significantly overlap chromatin marks of trimethylation of histone H3 at lysine 4 (H3K4me3) specifically in CD4+ regulatory and memory T cells (p = 1.3×10−4 and 7.0×10−4, respectively) [14].
We hypothesized that the risk alleles of these conditions might influence CD4+ TEM quantitative molecular phenotypes: 1) the expression of immune-related genes; 2) the relative abundance of CD4+ TEM cells in peripheral blood; and 3) proliferative response to T cell receptor (TCR) stimulation. To this end, we undertook a large immunoprofiling study in a healthy population of 174 European-descent individuals, by cross-analyzing genotype, transcription, abundance, and proliferative response in primary CD4+ TEM cells. Because the post-stimulation activation of CD4+ TEM cells is presumably crucial for their autoimmune response, we assayed cells not only at rest, but also after T cell receptor (TCR) stimulation with anti-CD3/CD28 beads. As such, this study is the first to our knowledge to map expression quantitative trait loci and examine immunological cellular traits in primary CD4+ TEM cells under multiple states.
Using the ImmunoChip platform, investigators recently densely genotyped 186 loci disease that originally arose through genome-wide association studies (GWAS) in case-control samples for RA, CeD, and inflammatory bowel disease [15]–[17], as well as T1D (unpublished data). Dense genotyping allowed localization of association signals within these disease loci to a set of alleles that are very likely to be causal. Within these loci, we have a greater ability to identify co-localization between alleles driving variation in molecular phenotypes (such as eQTLs) and the disease risk alleles. However, in instances where multiple variants are in perfect linkage, we cannot pinpoint the exact causal variant without functional evaluation.
Results
The experimental protocol (Figure 1) is described in detail in Methods and Text S1. Briefly, we obtained peripheral blood mononuclear cells (PBMCs) from the whole blood of healthy individuals via Ficoll-Paque centrifugation, and then used magnetic - and fluorescence-activated cell sorting to isolate CD4+ TEM cells at a high degree of purity (>90%; see Figure S2A). We acquired genome-wide genotype data of about 640,000 SNPs on Illumina Infinium Human OmniExpress Exome BeadChips [18]. For each individual we then measured three quantitative phenotypes: 1) the expression of 215 genes (see Table S1) before and after T cell receptor (TCR) stimulation by anti-CD3/CD28 antibody beads; 2) the relative abundance of CD4+ TEM cells (CD45RA−/CD45RO+/CD62L−/low) as a proportion of total CD4+ T cells; and 3) proliferation upon stimulation. Since we had low numbers of primary cells for expression profiling, we used the highly sensitive NanoString nCounter assay to avoid biases potentially induced by cDNA preparation. Out of the 215 genes assayed, 115 were within densely genotyped disease risk loci (see Tables S2 and S3). We quantified CD4+ TEM cell abundance with X-Cyt, an automated statistical method that accurately identifies cell populations in cytometry data [19].
Fig. 1. Schematic of the experimental workflow. 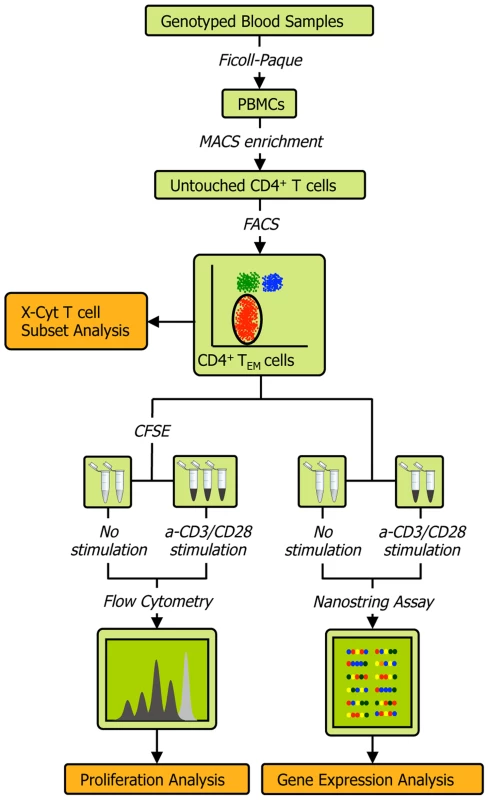
We collected four types of data from each individual: 1) quality-controlled genome-wide SNP data containing 638,347 markers collected on Illumina Infinium Human OmniExpress Exome BeadChips, 2) abundance of CD4 TEM cells as a percentage of all CD4 T cells obtained by FACS and quantified by X-Cyt, 3) average cell division upon T cell receptor stimulation by anti-CD3/CD28 commercial beads, measured using a CFSE (carboxyfluorescein succinimidyl ester) dye dilution assay, and 4) expression of 215 genes measured by NanoString nCounter. We repeated each proliferation assay in two-three technical replicates. Cell sorting purity and replication correlations for CD4 TEM abundance, division index, and proliferation index are shown in Figure S2. Mapping cis-eQTLs that regulate genes in risk loci
We first aimed to identify SNP variants that regulated expression of genes in cis. To best localize eQTL signals, we imputed 1000 Genomes variants within 250 kb from the transcription start site (TSS) of each gene (excluding five HLA genes and five long non-coding RNAs). We tested SNPs in gene-coding and non-coding regions in both resting and stimulated CD4+ TEM cells. We included gender and the top five principal components of the genotype data (calculated by EIGENSTRAT) as covariates in regression. To adjust for multiple hypothesis testing, we conducted 10,000 permutations within each gene region to calculate empirical p-values, and then reported associations at a false discovery rate of 5%.
In total, we observed 46 genes (22.4%) with cis-eQTL signals, including 17 in resting cells and 43 in stimulated cells (Tables 1 and 2, Figure 2A). For 14 of the 46 genes (30.4%), we detected eQTL signals in both resting (14/17, 82.4%) and stimulated (14/43, 32.6%) states. In four of these 14 genes (FHL3, GRB10, IL18R1, and PIGC), the lead eQTL SNPs across resting and stimulated states were identical. In another five genes (C1QTNF6, PRDM1, SKAP2, DDX6, and LYRM7), the lead SNPs are in tight LD (r2 = 0.80∼1; based on 1000 Genomes Release 2, European samples). For the remaining five genes (BLK, TMPRSS3, CD101, ORMDL3, and GSDMB), the lead SNPs from the two states were in partial LD (0.42<r2<0.56). In these five cases, we could not be confident that the eQTL SNPs across stimulation states were tagging the same variant.
Fig. 2. State-specific effects of eQTL SNPs. 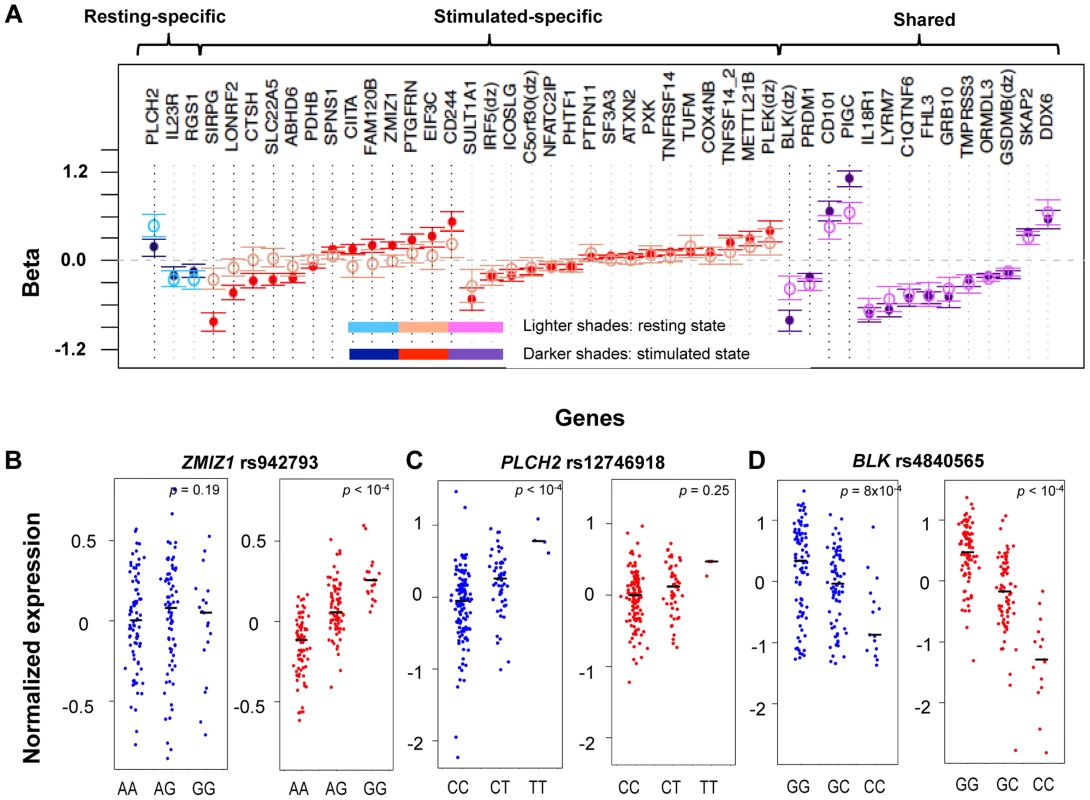
A) For a subset of genes, the correlation effects (β) of the top associated SNP across resting and stimulated cells differed. The genes shown with a black-dotted vertical lines had significantly different effect sizes across states. Black horizontal segments in B)–D) denote median values. Blue panels show resting-state (normalized) expression values; red panels show stimulated expression values. B) rs942793G significantly increased the expression of ZMIZ1 only in stimulated cells. C) rs12746918T was correlated with increased expression of PLCH2 in resting cells only. D) rs4840565C decreased BLK expression in stimulated cells nearly twice as much as in resting cells [βrest(SE) = −0.366(0.085), βstim = −0.805(0.071)]. Tab. 1. Cell-state specific eQTLs. 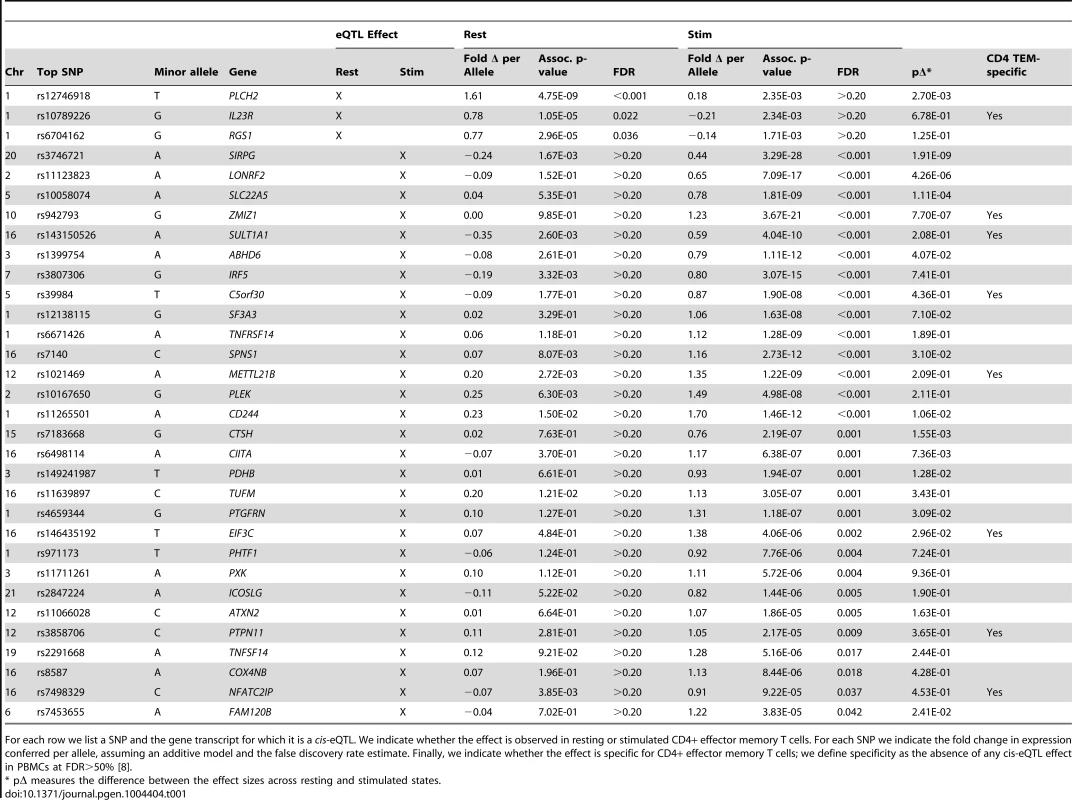
For each row we list a SNP and the gene transcript for which it is a cis-eQTL. We indicate whether the effect is observed in resting or stimulated CD4+ effector memory T cells. For each SNP we indicate the fold change in expression conferred per allele, assuming an additive model and the false discovery rate estimate. Finally, we indicate whether the effect is specific for CD4+ effector memory T cells; we define specificity as the absence of any cis-eQTL effect in PBMCs at FDR>50% [8]. Tab. 2. Genes with resting and stimulated eQTLs. 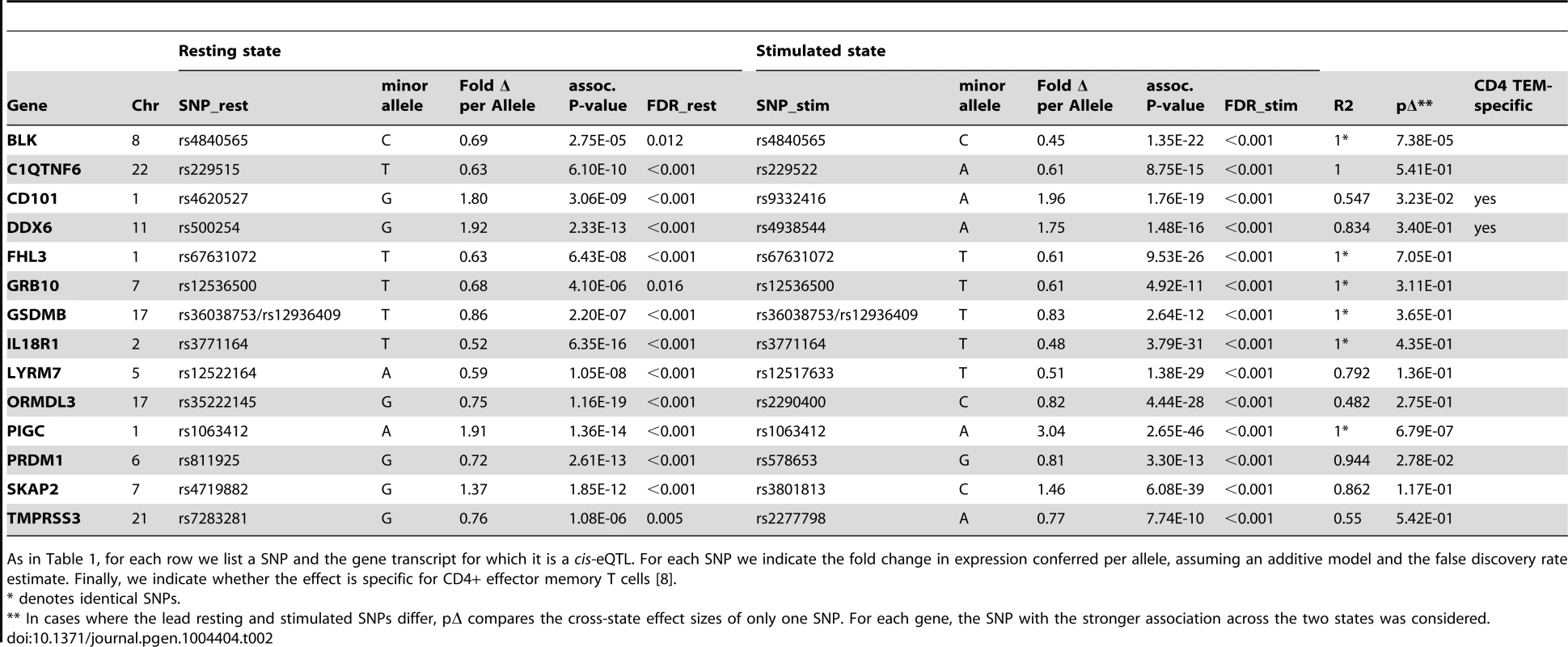
As in Table 1, for each row we list a SNP and the gene transcript for which it is a cis-eQTL. For each SNP we indicate the fold change in expression conferred per allele, assuming an additive model and the false discovery rate estimate. Finally, we indicate whether the effect is specific for CD4+ effector memory T cells [8]. Three genes (IL23R, PLCH2, and RGS1) had statistically significant eQTLs exclusively in the resting state, while 29 genes had statistically significant eQTLs exclusively in stimulated cells, such as rs942793 associated with ZMIZ1 expression (Figure 2B). One possibility is that some SNPs failed to reach significance threshold due to the small sample size or low expression levels in resting cells. However, we observed many genes with truly state-specific eQTLs, where the estimated effect sizes (β) of the eQTL SNP differed significantly across resting and stimulated states. To systematically compare the βrest and βstim for each gene, we used a z-statistic to quantify the probability that they differ. We then reported the p-value (two-tailed) assuming that z is distributed as standard normal, considering p<0.05 to be significantly different (“state-specific”; see Tables 1 and 2). For example, rs12746918T increased the expression of PLCH2 significantly only in resting cells; and βrest was approximately twice as large as βstim (Figure 2C). We note that 1 of the 3 eQTLs in resting cells was state-specific (p<0.05), and 13 out the 29 eQTLs seen in stimulated cells were state-specific (p<0.05). Of the 14 eQTLs that were shared between resting and stimulated cells, only 4 of them, BLK (Figure 2D), CD101, PIGC, and PRDM1, had different β's across states. The abundance of eQTLs detected exclusively in stimulated cells underscores the importance of studying cells in different cellular states.
We wanted to assess whether the eQTLs might act by altering gene regulatory elements in CD4+ TEM cells. To this end we asked whether the eQTL SNPs co-localized with marks of active promoters or enhancers. We utilized H3K4me3 marks from the NIH Roadmap Epigenomics Mapping Consortium [20] measured by ChIP-seq in primary CD4+ memory T cells. For the SNP with the strongest association to each gene, we queried the distance of the nearest H3K4me3 mark to this SNP or its LD partners (r2>0.8). We compared this distance measure between two sets of SNPs: the 46 SNPs with significant eQTL associations (FDR<5%, resting or stimulated), and the SNPs most strongly correlated with the other 159 genes but did not reach significance threshold. Indeed, the 46 significant eQTL SNPs were located at smaller distances to H3K4me3 marks (p = 1.10×10−7, one-sided Mann-Whitney test, Figure S3A). In addition, we queried the height of each H3K4me3 mark's peak, which reflected the number of reads at a given position compared to genomic controls as defined by the MACS software package. A tall peak gives us confidence that the mark is present in a large proportion of cells. Comparing the marks nearest to the two sets of SNPs, we saw that the 46 eQTL SNPs were also located near taller peaks (p = 9.56×10−8, Figure S3B).
Many eQTLs are CD4+ TEM cell-specific
We compared the cis-eQTLs we discovered to those found in heterogeneous peripheral blood mononuclear cells (PBMC) in a large genome-wide eQTL meta-study (n = 5,331) conducted by Westra et al. [8]. At 5% FDR, eleven of the 46 eQTL genes we identified showed no detectable signal in PBMCs at 50% FDR. We saw significant associations in 131 genes at 50% FDR, 53 of which had no signal in PBMCs at 50% FDR (Tables 1 and 2). We hypothesized that these genes tended to be more specifically expressed in CD4+ TEM cells, thus making eQTLs readily detectable in the purified cell population. To assess this, we examined cell-specific expression of the genes the ImmGen dataset, which assayed the genome-wide expression in 247 murine mouse immunological cell types [13], [21]. We found that the genes with CD4+ TEM cell-specific eQTLs (at 50% FDR) were more specifically expressed in CD4+ TEM cells than genes with eQTLs detected in both datasets (p = 0.044, one-sided Mann-Whitney test).
Autoimmune disease alleles affect the transcription of genes in cis
We then focused on 115 genes near 96 risk alleles of RA, T1D, and/or CeD in densely genotyped loci (182 gene-SNP pairs, including two risk alleles shared by at least two diseases, see Tables S2 and S3). We discovered that eleven (11.4%) disease-associated SNPs (6 of 24 RA SNPs, 5 of 37 T1D SNPs, and 3 of 37 CeD SNPs) correlated significantly with the expression of ten genes in either resting or stimulated state (Table S3). In addition, there was substantial enrichment of nominally significant associations (p<0.05) among disease SNPs. By random chance, we expected about nine SNP-gene pairs to reach nominal association in each stimulation state. However, we observed 26 pairs (14.2%) with nominal association in resting cells (p = 4.67×10−7, one-tailed binomial test). Even more strikingly, we observed 45 pairs (24.7%) with nominal association in stimulated cells (p<10−15, one-tailed binomial test).
To identify those instances where the disease-associated SNP could explain the entire eQTL signal in the gene region, we applied conditional analysis to identify any residual signals after controlling for the disease SNP. In five of the ten genes (BLK, C5orf30, GSDMB, IRF5, PLEK), conditioning on the disease SNP obviated any remaining eQTL signal in the region (no SNP with permutation p-value <0.05; Figure 3), suggesting that there was a single variant (the disease-associated SNP or one in very high LD to it) that drove variation in expression. Interestingly, as previously noted, the lead SNPs in resting and stimulated states for BLK and GSDMB were in partial linkage to each other. The absence of residual eQTL signal upon conditioning on the same risk allele might suggest that the lead SNPs were indeed tagging the same causal SNP in each of these genes. In each of the other five genes (ORMDL3, SKAP2, TMPRSS3, TNFRSF14, and ZMIZ1), evidence of independent eQTL effect remained after conditional analysis. In these instances the disease-associated SNP and remaining lead signal are in partial linkage disequilibrium (r2 = 0.36–0.73). In these cases, we could not conclude whether the disease SNPs drove the alteration in expression, or whether the true causal SNPs were in partial linkage and caused spurious associations. It is probable that disease risk alleles were indeed causal, yet we could not confidently fine-map the effect due to experimental noise in expression assays or inadequate sampling.
Fig. 3. Five disease risk alleles explained the eQTL associations with five genes. 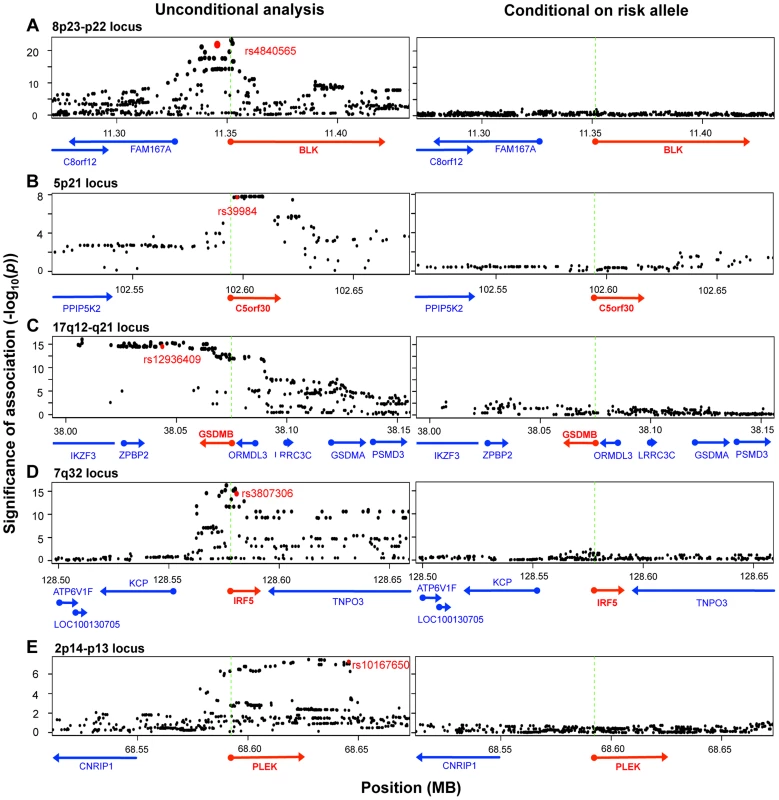
The left-sided panels show unconditional SNP-expression association results. Green dashed lines mark the TSS of the eQTL gene. The red dots indicate the risk alleles associated with the expression of respective genes shown as red arrows. The right-sided panels show adjusted association results after conditioning for the respective risk alleles. In each of the five loci, conditioning on the disease SNP obviated signals in the entire region, such that no association more significant than p = 0.05 remains. We note that another 26 genes within disease loci associated contained cis-eQTL signals, but that these cis-eQTL signals did not co-localize with RA, T1D, or CeD alleles. As these loci had been fine-mapped using Immunochip, the lack of overlap strongly suggested that these cis-eQTLs and disease-causing variants were distinct. For example, rs798000 is an RA risk allele located in a non-coding region upstream of CD2, CD58, and PTGFRN. However, it was not associated with the expression of any of these genes (p>0.5). Another example was rs6911690, an RA allele located about 60 kb 5′ of PRDM1, that was not associated with the expression of the gene at rest or after stimulation (p>0.5). The lead eQTL SNP associated to PRDM1 was rs578653 (FDR<10−3), which was not in LD with the disease allele (r2<0.05).
The genetic basis of CD4+ TEM cell proliferation
The relative peripheral abundance of CD4+ TEM cells varied between individuals (mean = 9.57%; SD = 4.85%), and was reproducible 35 individuals with two separate blood draws more than one month apart (Pearson's r = 0.87, p = 1.77×10−11, see also Figure S2B). Consistent with other studies, we observed that the relative proportion of CD4+ TEM cells increased with age by 0.11% per year (page = 1.92×10−3) [22]. We also observed that on average men had 2.22% more CD4+ TEM cells than women (pgender = 3.80×10−2; see Figure S4). Upon anti-CD3/CD28 stimulation, there was a substantial inter-individual variation in proliferation measured by both division index (DI, average number of divisions undergone by all cells; mean = 1.46, SD = 0.35), and proliferation index (PI, average number of divisions undergone only by dividing cells; mean = 2.16, SD = 0.21). Proliferation metrics were also reproducible in the 35 individuals (Pearson's rDI = 0.57; Pearson's rPI = 0.62, Figures S2C and S2D). Interestingly, proliferation was negatively correlated to the proportion of CD4+ TEM cells (pDI = 1.28×10−3, pPI = 1.93×10−3), but was not associated to age or gender (p>0.3). This negative correlation needs to be replicated in an independent dataset. Effector functions of TEM cells with higher proliferative capacities need to be examined to understand whether they represent a hyperactive subset whose abundance is controlled to maintain immune homeostasis. Possibly individuals with a lower proportion of TEM cells are relatively enriched for these subsets.
We tested genome-wide SNPs for association to relative abundance, division index, and proliferation index, considering p<5×10−8 as the threshold for significance. For abundance, we included gender, age, and the top five principal components of genotypes as covariates. Given the correlation with proliferation, we also included the measured CD4+ TEM relative abundance as an additional covariate. We observed associations to division index in several loci, including 13q34 led by rs389862 (p = 4.75×10−8; Figure 4A). This SNP is a non-coding variant located 30 kb upstream of RASA3, and 70 kb upstream from CDC16. Both genes have known roles in regulating cell proliferation or differentiation [23], [24]. This SNP was also strongly associated with proliferation index (p = 2.75×10−7). Additionally, there was a strongly suggestive association to rs3775500 on chromosome 4, located in the intron of DAPP1, which encodes the Bam32 protein (p = 5.40×10−7; Figure 4B), which is an adaptor protein expressed solely in antigen presenting B cells. Interestingly, mutations in this gene have been shown by several groups to affect T cell activation [25], [26], suggesting the possibility that B cells may indirectly regulate T cell function in autoimmunity. We did not observe any significant association with the relative abundance of CD4+ TEM cells.
Fig. 4. Genome-wide association to division index (the average number of division undergone by all cells). 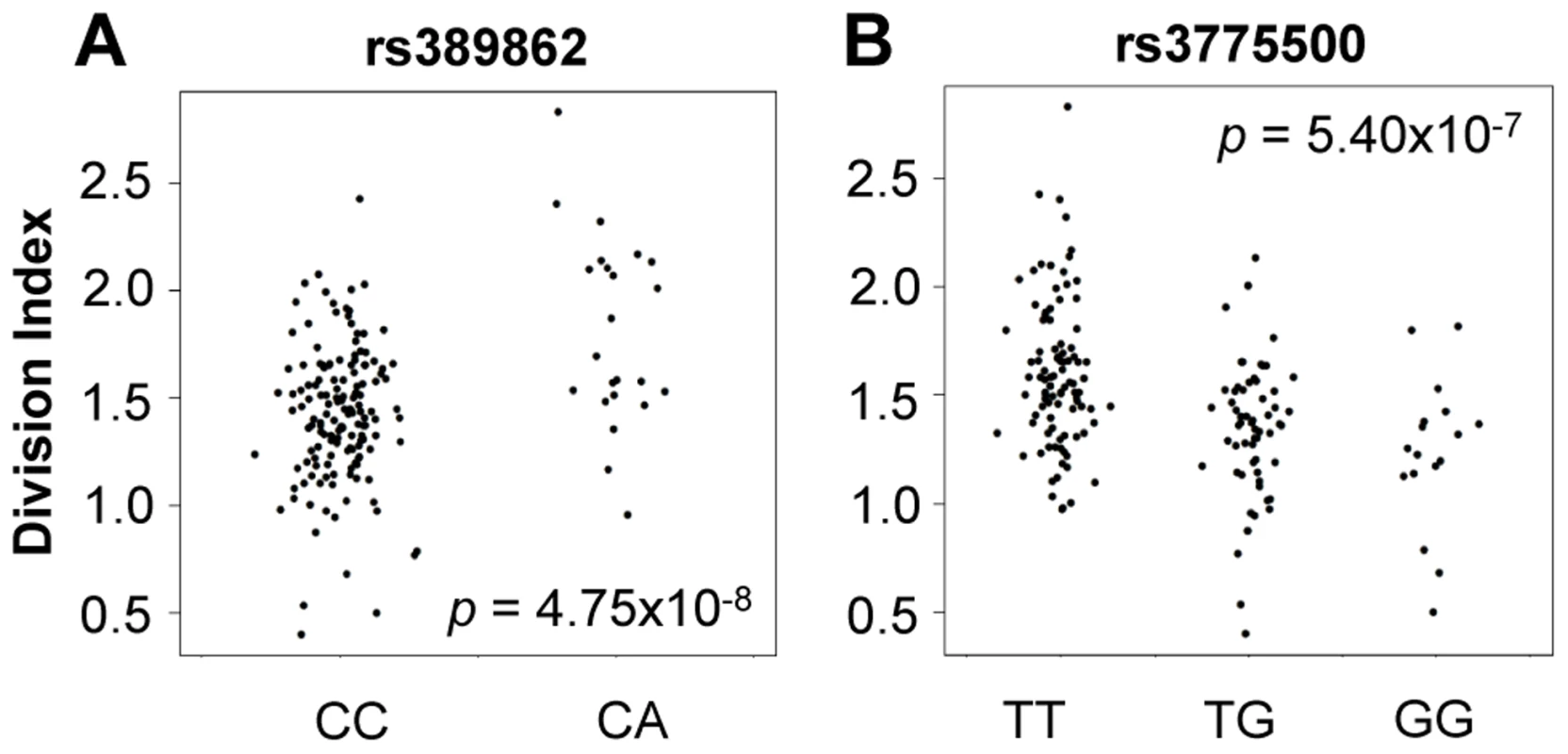
A) rs389862A on chromosome 13 was significantly associated to increased division index at p = 4.75×10−8, and is located in a non-coding region 30 kb upstream of RASA3, and 70 kb upstream from CDC16. B) rs3775500G on chromosome 4 shows a strongly suggestive association at p = 5.40×10−7, and is located within the DAPP1 (Bam32) gene. When we extracted the association statistics of 118 densely genotyped risk alleles of CeD, RA, and/or T1D, they showed no inflation in association p-values for relative abundance of CD4+ TEM cells (Figure 5A, Table S2). This suggested that risk variants did not modify risk via modulation of CD4+ TEM peripheral abundance. We recognized that the power to detect significant associations might have been limited in our study by the sample size. However, this negative finding was corroborated by results from a recently published study with data from ∼2800 individuals, in which the same set of risk alleles also showed no significant association to CD4+ TEM (see Figure S5) [27]. Similarly, the same set of risk alleles did not show significant association to proliferative response (Figure 5B, Table S2). Based on these data, it was unlikely that SNP variants associated to RA, T1D, or CeD conferred risk through modulation of CD4+ TEM cell abundance or proliferation.
Fig. 5. Risk alleles of CeD, RA, and T1D, showed no significant association to CD4 TEM cell abundance or proliferation. 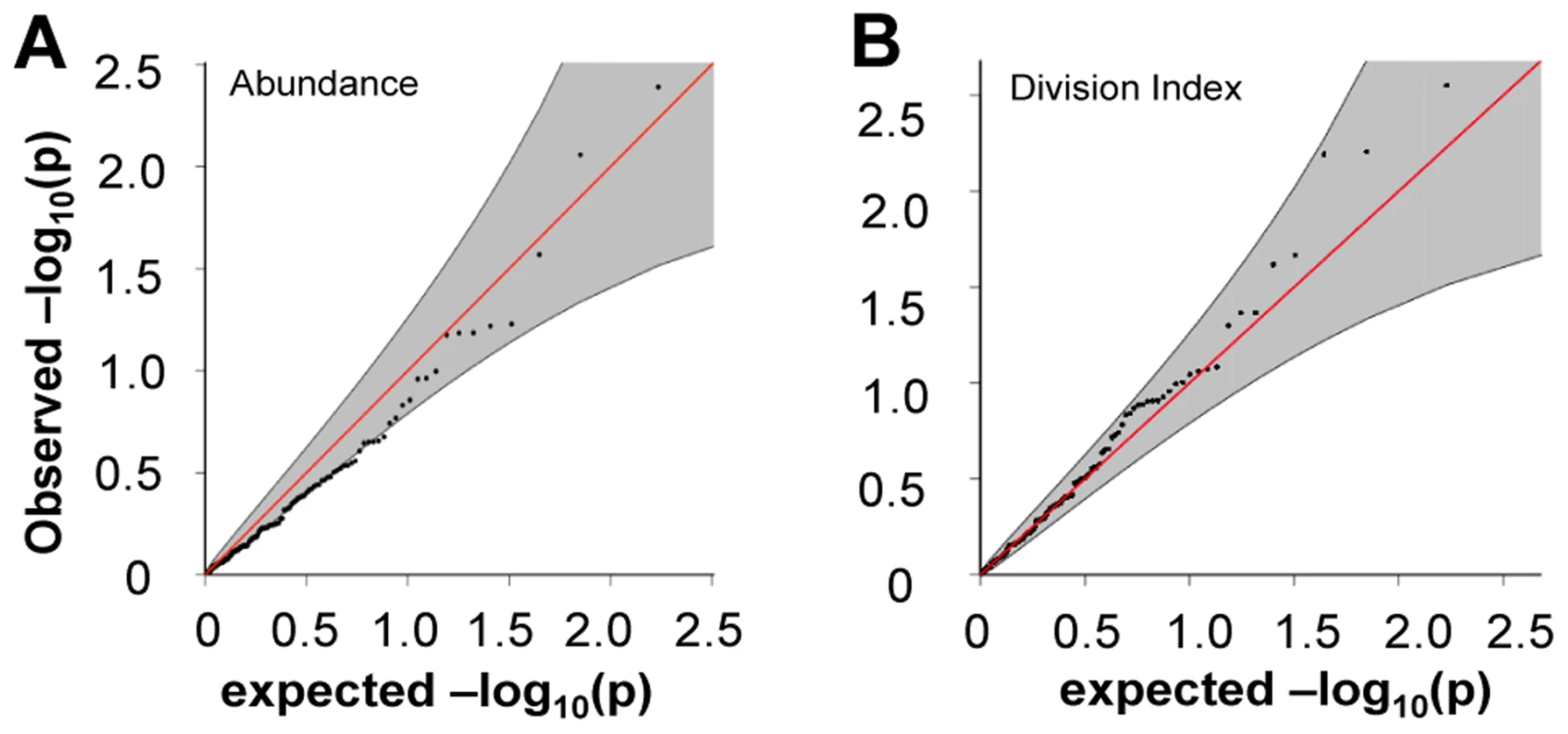
A) The 118 SNPs with association to diseases in densely genotyped regions on Immunochip platform were not significantly associated to CD4 TEM cell abundance. The shaded region shows 95% confidence interval. See also Figure S5. B) The same set of 118 risk alleles also showed no inflation in association with proliferative response measured as division index. Gene expression in resting cells predicted post-TCR stimulation proliferation
After stimulation we observed that 122 genes showed significant changes in expression in response to stimulation, including 78 whose expression at least doubled or decreased by 50% (Table S1). The gene with the greatest post-stimulation induction was GZMB (average fold change = 93.48), which encodes granzyme B, a protein involved in the apoptosis of target cells during cell-mediated immune response in cytotoxic and memory lymphocytes. The most significantly down-regulated gene was GRB10 (average fold change = 0.18), which is near rs6944602 associated with T1D and encodes growth factor receptor-bound protein 10, whose function in the immune system is unclear.
We observed that relative gene expression at rest predicted proliferative response. In 182 individuals with both proliferation and gene expression data, 17 of the 215 genes were associated with proliferation index (p<0.01, two-tailed test by permuting proliferation data, Figure 6, Table S1). Increased expression of 15 of the 17 genes including CCR5, IL2RB, PRR5L, and TBX21, were correlated with reduced proliferative response, while CCR9 and the lncRNA XLOC_003479 showed significant correlation with increased proliferation. This number of correlated genes was far in excess of random chance based on a null distribution consisting of 1000 permutations (p<10−3, median 2, maximum 15). The weighted sum of the 17 genes served as a “proliferation potential signature”, where we weighted the positively - and negatively-correlated genes as +1 and −1, respectively. This signature strongly predicted proliferation index (r = 0.55). We show the correlation between each of the 17 genes as well as the aggregate signature to proliferation as a heatmap (Figure 6A). To assess if we were overfitting the data, we applied a two way cross-validation, where we defined the proliferation signature based on genes from half of the individuals and tested correlation to proliferation in the remaining half of the individuals. In both instances we again observed significant prediction of proliferation (r = 0.41,one tailed p<10−3 by permutation; r = 0.39, p<10−3).
Fig. 6. Relationship between baseline expression and post-stimulatory response. 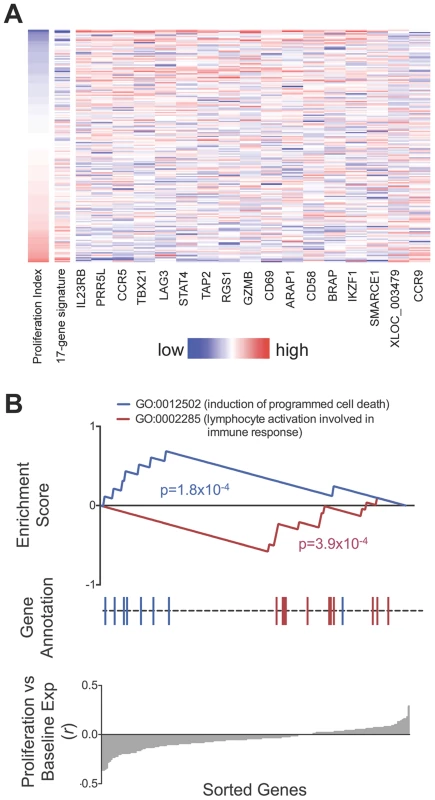
A) Baseline expression of 17 genes correlated with post-stimulation proliferation. Rows in the heatmap are ordered from top to bottom by ascending proliferation index. Genes/columns are ordered from the most negatively correlated (IL23RB) to the most positively correlated (CCR9). The 17-gene signature was calculated as the weighted sum of the 17 genes, where the negatively-correlated genes were given a weight of −1, and the positively-correlated genes were given a weight of +1. Table S1 lists the correlation coefficient and p-value for each gene. B) Genes correlated with proliferative response were enriched for apoptosis and lymphocyte activation pathways. Genes correlated to lower proliferative response (proliferative index) were enriched for Gene Ontology code GO:0012502 (induction of programmed cell death, p = 1.8×10−4). Conversely, genes correlated to higher proliferative response were enriched for GO:0002285 (lymphocyte activation, p = 3.9×10−4). To search for biological pathways underlying genes correlated to proliferation, we applied gene set enrichment analysis (GSEA) to test for enrichment for 1,008 functional gene sets based on Gene Ontology codes [28] (Figure 6B). Genes correlated to reduced proliferation were most significantly enriched for GO:0012502 (induction of programmed cell death; one tailed p = 1.8×10−4); those correlated with increased proliferation were most significantly enriched for GO:0002285 (lymphocyte activation involved in immune response, one tailed p = 3.9×10−4).
Using data from 29 individuals each with two samples collected at least one month apart, we replicated the observed correlation. In these samples we performed a cross-visit analysis, and observed that the same 17-gene signature from the first visit significantly predicted proliferation indices on the second visit (r = 0.65, p = 0.0006, 1-tailed permutation), and vice versa (r = 0.55, p = 0.0019).
Discussion
To fine-map and link risk loci to their pathogenic mechanisms, we investigated molecular and immune phenotypes potentially leading to disease end-points. The immune system is particularly complex, and different cells under various activation states have specialized functions that may not be adequately captured by examining PBMCs. Therefore, we focused on one purified cell population that had been shown to be important for the pathogenesis of several autoimmune diseases. We quantified population variation in several traits, including peripheral abundance, proliferative response to TCR stimulation, and expression of genes within autoimmune disease loci at rest and after stimulation. In Tables 1, 2, and S2, we provide significant cis-eQTLs and genome-wide association results.
To our knowledge this study was a first cross-examination of genetic-, transcriptional-, and cellular-level quantitative traits in CD4+ TEM cells. It demonstrated the importance of focusing functional studies in a purified cell population under relevant developmental and stimulation states. By examining the proliferative response upon TCR stimulation, we identified a subset of genes whose baseline expression predicted proliferative potential. Intriguingly, these genes were involved in programmed cell death and lymphocyte activation. Whether variation in proliferative abilities correlated with cytokine production and other signaling functions, thus affecting susceptibility to autoimmunity, remains a question to be addressed by future studies.
Of the 205 genes in disease loci that we examined, 46 had cis-eQTLs. Notably, eleven of these were specific to stimulated CD4+ TEM cells, and not previously found in PBMCs. We noted that approximately 10% of genes within risk loci of diseases had cis-eQTLs. However in many instances the lead eQTL SNPs were unrelated to the disease-associated SNPs. One example of a disease allele that functioned as cis-eQTL was rs39984, which was associated to lower risk of RA, and regulated the expression of C5orf30 encoding an UNC119-binding protein. This SNP variant is located in the first intron of C5orf30, and indeed explained the entire cis-eQTL signal in this gene (see Figure 2B). This eQTL effect was previously undetected in PBMCs, and the protein's functional role in the immune system is largely unknown. However, a recent study showed that rs26232 (the lead GWAS SNP prior to fine-mapping, r2 = 0.988 to rs39984) was correlated with lower severity of radiologic damage in RA, independent of previously established biomarkers [29]. Another gene in the locus, GIN1, is located 140 kb from rs39984; however its expression showed no correlation with the SNP (p>0.5).
Another CD4+ TEM cell-specific eQTL gene was DDX6, which encodes DEAD-box RNA helicase 6. However, in this case, the lead eQTL SNP (rs4938544) associated to increased expression of DDX6 in stimulated cells was not in LD with the known CeD risk allele (rs10892258, r2<0.1) or the RA risk allele (rs4938573, r2<0.1). Neither risk allele showed significant association to DDX6 expression (p = 0.19 and 0.26, respectively). Both risk alleles are also located near CXCR5, BCL9L, and TREH; none of these genes had reported cis-eQTLs in PBMCs [8]. However, we did not assay these three genes in this study, therefore could not confirm the role of disease alleles in regulating their expression in CD4+ TEM cells.
Although we did not assay all genes or test for trans-acting eQTLs, based on the level of co-localization between eQTL SNPs and risk alleles observed in the study, we found it unlikely that all non-coding risk variants caused disease by altering gene expression within resting or stimulated CD4+ TEM cells. In addition, while changes in proportions of lymphocyte subsets had been observed in patients of autoimmune disorders [30]–[35], we did not find evidence to support disease alleles' roles in directly modulating CD4+ TEM cell abundance or proliferative response. Ultimately, other cell states and cell types will need to be investigated.
We recognize several limitations to the current study. In order to conduct a focused study on a small amount of purified primary cells we used the NanoString nCounter assay system. This avoided potential biases and artifacts arising from cDNA synthesis required for microarray or RNA-seq studies, but restricted our analysis to a subset of candidate genes within risk loci of CeD, RA, and T1D, rather than a genome-wide expression analysis. Consequently we could not identify trans-eQTLs, splice variants, or epistatic effects on expression regulation. Additionally, anti-CD3/CD28 stimulation for memory T cells is not antigenic, especially while in isolation from a “natural” multi-cellular environment, thus it was only partially physiological.
This and other cell-specific studies on population variation in molecular phenotypes are only a beginning of examining potential intermediate phenotypes. Post-activation cytokine production by CD4+ TEM cells are likely crucial in driving autoimmunity. Therefore, it is critical that future studies of molecular phenotypes include proteomic assays to quantify functional markers of immune response. Finally, functional experiments will need to be conducted in the future to determine whether these molecular phenotypes are indeed intermediary to disease.
Materials and Methods
Ethics statement
All research was approved by our Institutional Review Board, and informed consent was obtained from each volunteer.
Study sample
We enrolled 225 healthy volunteers (134 females, 91 males) of non-Hispanic Caucasian descent that proved informed consent through the Phenogenetics Project at Brigham and Women's Hospital. Subjects' ages ranged from 19 to 57 years with average female and male ages of 28.8 years and 34.9 years, respectively. Thirty-five subjects (18 females, 17 males) returned for a second study visit one to nine months after their initial visits.
Genotyping
We genotyped each subject using the Illumina Infinium Human OmniExpress Exome BeadChip. In total, we genotyped 951,117 SNPs, of which 704,808 SNPs are common variants (minor allele frequency [MAF]>0.01) and 246,229 are part of the exome. After quality control, 638,347 common SNPs remained. Of all subjects, 174 subjects had abundance, proliferation, gene expression, and quality controlled genotype data. Detailed quality control criteria are described in Text S1.
SNP imputation
For each gene, we selected a 500 kb region (250 kb each in the 3′ and 5′ directions) around the transcription start site and imputed 1000 Genomes SNPs into the genome-wide SNP data using BEAGLE Version 3.3.2. We used the European samples from 1,000 Genomes as the reference panel. We excluded markers that had MAF<0.05 in the reference panel as well as all insertion/deletions. After imputation, we excluded markers with a BEAGLE R2<0.4 or MAF<0.01 in the imputed samples.
CD4+ TEM cell isolation and stimulation
We isolated peripheral blood mononuclear cells (PBMC) from whole blood using a Ficoll density gradient (GE Healthcare). We then isolated CD4+ effector memory T cells from PBMCs first by magnetic-activated cell sorting to enrich for CD4+ T cells, followed by fluorescent-activated cell sorting using labeled antibodies against CD45RA, CD45RO, and CD62L.
We stimulated CD4+ TEM cells by incubation with commercial anti-CD3/CD28 beads for 72 hours. For proliferation studies, we labeled cells with carboxyfluorescein diacetate succinimidyl ester (CFSE; eBioscience), and measured proliferation by dye dilution. Detailed isolation and purification methods are described in Text S1 (also see Figure S2A).
Gene expression
We designed the NanoString codeset based on GWAS SNPs associated with CeD, RA, and T1D as of April 2012. This list of SNPs can be found in Supplementary Table S4. As the numbers of associated loci with autoimmune diseases continuously expand, we refer the reader to ImmunoBase (https://www.immunobase.org) for up-to-date disease regions. For each locus, we defined a region of interest implicated by the GWAS lead SNP [36]. We identified the furthest SNPs in LD in the 3′ and 5′ directions (r2>0.5). We then extended outward in each direction to the nearest recombination hotspot. If no genes were found in this region, we extended an additional 250 kb in each direction. All genes overlapping this region were considered implicated by the locus. The final NanoString codeset (prior to expression data quality control) included 312 genes, including 270 genes near SNPs associated with 157 RA, CeD, and T1D through GWAS, 26 genes of immunological interest, and 15 reference genes with minimal change in expression after TCR stimulation (see Supplementary Table S1).
After quality control, 215 genes remained. Of all 225 subjects in the study, 187 subjects passed gene expression quality control for both resting and stimulated cells. Specific normalization and quality control procedures are described in Text S1.
Genotype principal component analysis
To control for any potential population stratification, we adjusted all association tests using the top five principal components of our genome-wide SNP data. Principal components were generated via EIGENSTRAT using unsupervised analysis (no reference populations were used). The top five PCs explained 6.88% (2.08%, 1.27%, 1.20%, 1.17%, and 1.16%, respectively) of the total variance. After controlling for these five PCs, the lambda GC for CD4 TEM proportion association was 1.008; that of division index was 1.001.
Cis-eQTL analysis
For each gene-SNP pair, we applied linear regression using the first five principal components of the genotype data and gender as covariates. As such, normalized expression = β0+β1*allelic dosage+β2*PC1+β3*PC2+β4*PC3+β5*PC4+β6*PC5+β7*(factor)gender. To adjust for multiple hypothesis testing while taking into consideration the correlation among SNPs within each locus, we calculated a permutation-based p-value for each SNP. We performed 10,000 permutations of the residual expression values. We reported each SNP's p-value the proportion of permutation P value smaller than the analytical p-value. For conditional analysis, the vector of allelic dosages of the disease-associated SNP was included as an additional covariate.
Quantification of CD4+ TEM cells and proliferative response
We defined CD4+ TEM cells as CD45RA−, CD45RO+, and CD62Llow/−. In all samples CD4+ TEM cells were quantified using X-Cyt, a mixture-modeling based clustering program for automated cell population identification (see Figure S6) [19]. We fit proliferation division peaks with one-dimensional Gaussian mixture models (see Figure S7). Detailed protocol and algorithms are described in Text S1.
Statistical analysis
All linkage disequilibrium calculations (r2) were based on 1000 Genomes Release 3 European samples. All association tests were performed using Plink v1.07. We considered p<5×10−8 to be genome-wide significant; p<5×10−5 was considered as suggestive. CD4+ TEM abundance and proliferation correlations with age and gender were calculated by multivariate linear model implemented in R-3.0. We calculated two-sample comparisons (CD4+ TEM cell-specific expression between genes, and H3K4me3 h/d scores between SNPs) with the Mann-Whitney test. Details of statistical analyses are described in Text S1.
Data access
We make all phenotypic data (expression, peripheral abundance, and proliferation) along with eQTL results publicly available online (http://immunogenomics.hms.harvard.edu/CD4eqtl.html). Genome-wide genotype data will become available through dbGAP and through the ImmVar project. These data are potentially useful to investigators wishing to assess the potential of genetic variants in altering these molecular phenotypes.
Supporting Information
Zdroje
1. MasopustD, VezysV, MarzoAL, LefrancoisL (2001) Preferential localization of effector memory cells in nonlymphoid tissue. Science 291 : 2413–2417.
2. FritschRD, ShenX, IlleiGG, YarboroCH, PrussinC, et al. (2006) Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum 54 : 2184–2197.
3. ZhouX, Bailey-BucktroutSL, JekerLT, PenarandaC, Martinez-LlordellaM, et al. (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10 : 1000–1007.
4. OlingV, ReijonenH, SimellO, KnipM, IlonenJ (2012) Autoantigen-specific memory CD4+ T cells are prevalent early in progression to Type 1 diabetes. Cell Immunol 273 : 133–139.
5. SattlerA, WagnerU, RossolM, SieperJ, WuP, et al. (2009) Cytokine-induced human IFN-gamma-secreting effector-memory Th cells in chronic autoimmune inflammation. Blood 113 : 1948–1956.
6. NicaAC, MontgomerySB, DimasAS, StrangerBE, BeazleyC, et al. (2010) Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS Genet 6: e1000895.
7. NicolaeDL, GamazonE, ZhangW, DuanS, DolanME, et al. (2010) Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet 6: e1000888.
8. WestraHJ, PetersMJ, EskoT, YaghootkarH, SchurmannC, et al. (2013) Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet 45 : 1238–1243.
9. TrynkaG, RaychaudhuriS (2013) Using chromatin marks to interpret and localize genetic associations to complex human traits and diseases. Curr Opin Genet Dev 23 : 635–641.
10. FairfaxBP, MakinoS, RadhakrishnanJ, PlantK, LeslieS, et al. (2012) Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet 44 : 502–510.
11. LeeMN, YeC, VillaniA-C, RajT, LiW, et al. (2014) Common Genetic Variants Modulate Pathogen-Sensing Responses in Human Dendritic Cells. Science 343 DOI:10.1126/science.1246980
12. StrangerBE, MontgomerySB, DimasAS, PartsL, StegleO, et al. (2012) Patterns of cis regulatory variation in diverse human populations. PLoS Genet 8: e1002639.
13. HuX, KimH, StahlE, PlengeR, DalyM, et al. (2011) Integrating autoimmune risk loci with gene-expression data identifies specific pathogenic immune cell subsets. Am J Hum Genet 89 : 496–506.
14. TrynkaG, SandorC, HanB, XuH, StrangerBE, et al. (2013) Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat Genet 45 : 124–130.
15. TrynkaG, HuntKA, BockettNA, RomanosJ, MistryV, et al. (2011) Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet 43 : 1193–1201.
16. EyreS, BowesJ, DiogoD, LeeA, BartonA, et al. (2012) High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 44 : 1336–1340.
17. JostinsL, RipkeS, WeersmaRK, DuerrRH, McGovernDP, et al. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491 : 119–124.
18. RajT, RothamelK, MostafaviS, YeC, LeeMN, et al. (2014) Polarization of the Effects of Autoimmune and Neurodegenerative Risk Alleles in Leukocytes. Science 344 : 519–523.
19. HuX, KimH, BrennanPJ, HanB, Baecher-AllanCM, et al. (2013) Application of user-guided automated cytometric data analysis to large-scale immunoprofiling of invariant natural killer T cells. Proc Natl Acad Sci U S A 110 : 19030–19035.
20. BernsteinBE, StamatoyannopoulosJA, CostelloJF, RenB, MilosavljevicA, et al. (2010) The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol 28 : 1045–1048.
21. HyattG, MelamedR, ParkR, SeguritanR, LaplaceC, et al. (2006) Gene expression microarrays: glimpses of the immunological genome. Nat Immunol 7 : 686–691.
22. SauleP, TrauetJ, DutriezV, LekeuxV, DessaintJP, et al. (2006) Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev 127 : 274–281.
23. NafisiH, BanihashemiB, DaigleM, AlbertPR (2008) GAP1(IP4BP)/RASA3 mediates Galphai-induced inhibition of mitogen-activated protein kinase. J Biol Chem 283 : 35908–35917.
24. TugendreichS, TomkielJ, EarnshawW, HieterP (1995) CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 81 : 261–268.
25. SommersCL, GursonJM, SuranaR, Barda-SaadM, LeeJ, et al. (2008) Bam32: a novel mediator of Erk activation in T cells. Int Immunol 20 : 811–818.
26. Al-AlwanM, HouS, ZhangTT, MakondoK, MarshallAJ (2010) Bam32/DAPP1 promotes B cell adhesion and formation of polarized conjugates with T cells. J Immunol 184 : 6961–6969.
27. OrruV, SteriM, SoleG, SidoreC, VirdisF, et al. (2013) Genetic variants regulating immune cell levels in health and disease. Cell 155 : 242–256.
28. SubramanianA, TamayoP, MoothaVK, MukherjeeS, EbertBL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102 : 15545–15550.
29. TeareMD, KnevelR, MorganMD, KleszczA, EmeryP, et al. (2013) Allele-dose association of the C5orf30 rs26232 variant with joint damage in rheumatoid arthritis. Arthritis Rheum 65 : 2555–2561.
30. FaureG, KahnMF, BachMA, BachJF (1982) T cell subsets in the blood of rheumatoid arthritis patients in clinical remission. Arthritis Rheum 25 : 1507–1509.
31. HendersonLA, KingSL, AmeriS, MartinSD, SimmonsBP, et al. (2014) A161: Novel 3-Dimensional Explant Method Facilitates the Study of Lymphocyte Populations in the Synovium and Reveals a Large Population of Resident Memory T cells in Rheumatoid Arthritis. Arthritis Rheumatol 66 Suppl 11: S209.
32. HusseinMR, FathiNA, El-DinAM, HassanHI, AbdullahF, et al. (2008) Alterations of the CD4(+), CD8 (+) T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: preliminary observations. Pathol Oncol Res 14 : 321–328.
33. KotzinBL, KansasGS, EnglemanEG, HoppeRT, KaplanHS, et al. (1983) Changes in T-cell subsets in patients with rheumatoid arthritis treated with total lymphoid irradiation. Clin Immunol Immunopathol 27 : 250–260.
34. MatsukiF, SaegusaJ, MiyamotoY, MisakiK, KumagaiS, et al. (2013) CD45RA-Foxp3(high) activated/effector regulatory T cells in the CCR7+CD45RA-CD27+CD28+central memory subset are decreased in peripheral blood from patients with rheumatoid arthritis. Biochem Biophys Res Commun 438 : 778–783.
35. SyrjanenSM, SyrjanenKJ (1984) Enumeration of T cell subsets with monoclonal antibodies in minor salivary glands of patients with rheumatoid arthritis. Scand J Dent Res 92 : 275–281.
36. RaychaudhuriS, PlengeRM, RossinEJ, NgAC, PurcellSM, et al. (2009) Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet 5: e1000534.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



