-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
Protein folding is fundamentally important for development and responses to environmental stresses in eukaryotes. When excess misfolded proteins are accumulated in the endoplasmic reticulum (ER), the unfolded protein response (UPR) is triggered to promote cell survival through optimizing protein folding, and also promote programmed cell death (PCD) when the stress is severe. However, the link from ER-stress-sensing to PCD is largely unknown. Here, we report the identification of one membrane-associated transcription factor NAC089 as an important regulator of ER stress-induced PCD in plants. We have established a previously unrecognized molecular connection between ER stress sensors and PCD regulators. We have shown that organelle-to-organelle translocation of a transcription factor is important for its function in transcriptional regulation. Our results have provided novel insights into the molecular mechanisms of PCD in plants, especially under ER stress conditions.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004243
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004243Summary
Protein folding is fundamentally important for development and responses to environmental stresses in eukaryotes. When excess misfolded proteins are accumulated in the endoplasmic reticulum (ER), the unfolded protein response (UPR) is triggered to promote cell survival through optimizing protein folding, and also promote programmed cell death (PCD) when the stress is severe. However, the link from ER-stress-sensing to PCD is largely unknown. Here, we report the identification of one membrane-associated transcription factor NAC089 as an important regulator of ER stress-induced PCD in plants. We have established a previously unrecognized molecular connection between ER stress sensors and PCD regulators. We have shown that organelle-to-organelle translocation of a transcription factor is important for its function in transcriptional regulation. Our results have provided novel insights into the molecular mechanisms of PCD in plants, especially under ER stress conditions.
Introduction
In eukaryotic cells, ER is a major site for the production of secreted, plasma membrane and organelle proteins. Cells have evolved a sophisticated quality control system to ensure the accuracy of protein folding through optimizing the protein-folding machinery and ER-associated degradation (ERAD) [1], [2], [3]. To coordinate protein-folding capacity with protein-folding demand, a collection of phylogenetically conserved signaling pathways, termed the UPR, senses the accumulation of misfolded proteins in the ER and sustains homeostatic balance according to the protein folding needs which change constantly depending on different developmental programs and/or environmental conditions [1], [4], [5].
Three arms of UPR signaling pathways, namely inositol requiring enzyme 1 (IRE1), double-stranded RNA-activated protein kinase (PKR) like ER kinase (PERK), and activating transcription factor 6 (ATF6), were identified in mammalian cells that have the ability to promote cell survival by reducing misfolded protein accumulation in the ER. IRE1 is a key component in the most conserved branch, which acts by splicing messenger RNA encoding transcription factor Hac1p in yeast or XBP1 in mammalian cell, respectively [6], [7], [8]. Recently, the equivalent pathways were discovered in plants (e.g. the IRE1-bZIP60 pathway in Arabidopsis), which also play important roles in heat stress response, as well as in plant immune response [9], [10], [11], [12], [13], [14]. PERK is an ER-localized kinase and its activation upon ER stress leads to the attenuation of bulk protein translation in metazoan cells [15]. ATF6 is an ER membrane-associated bZIP transcription factor; its activation requires ER-to-Golgi translocation and regulated intramembrane proteolysis (RIP) [16]. Although the plant PERK ortholog has not yet been reported, the ER membrane-associated Arabidopsis bZIP28 was found to be the functional homolog of mammalian ATF6, which is activated in a manner similar to ATF6 [17], [18], [19], [20], [21].
Severe or chronic ER stress can also lead to PCD, a process that kills unwanted cells under ER stress conditions to protect other cells [22]. In contrast to what is known about how UPR protects cells, less is known about the mechanisms that link UPR to PCD, especially in plants [23]. In mammalian cells, IRE1 can trigger PCD by activating the Jun amino-terminal kinase (JNK) pathway [24]. Phosphorylation of JNK leads to the activation of pro-death protein BIM and inhibition of anti-death protein BCL-2 [25]. Mammalian IRE1 also binds to BAX and BAK, two cell-death-inducing proteins involved in the mitochondrial cell death pathway [26]. The activation of mammalian IRE1 is able to cause rapid decay of selected microRNAs (miRs -17, -34a, -96, and -125b) that normally repress translation of caspase-2 mRNA, and thus sharply elevates protein levels of this initiator protease in the mitochondrial cell death pathway [27]. The ER stress-induced mammalian bZIP transcription factor CHOP is one of the major players that induces PCD, most probably through suppression of the pro-survival protein BCL-2 and up-regulation of ERO1α to further perturb the cellular redox state [28]. CHOP is a downstream target of all three aforementioned UPR signaling pathways in mammals [4]. Recently, transcriptional induction through ATF4 and CHOP was shown to increase protein synthesis leading to oxidative stress and PCD [29]. Orthologs of mammalian JNK, BAX, BAK, CHOP and ATF4 are not found in the Arabidopsis genome [30]. However, ER stress-induced PCD is reported in plants with the hallmark of DNA segmentation, and the conserved BAX inhibitor-1 (BI-1) plays important roles in suppression of ER stress-induced PCD in Arabidopsis plant [31], [32], [33], [34], [35], [36], [37], [38]. When animal cells are subjected to severe ER stress, IRE1 loses its specificity and begins to degrade mRNAs in a process called regulated IRE1-dependent decay (RIDD) [39]. IRE1 in Arabidopsis also has similar function in the RIDD process in the UPR for degradation of mRNA encoding proteins in the secretory pathway to decrease the amount of proteins entering the ER [40]. Different from the animal system, knock-outs of both IRE1s in Arabidopsis impairs UPR and enhances PCD upon ER stress, indicating that RIDD may play a negative role in PCD in plants [40]. Despite the emerging evidence on ER stress-mediated PCD in plants, the underlying molecular mechanisms of PCD in plant UPR is still largely unknown. In soybean plants, prolonged ER stress and osmotic stress synergistically activate N-rich proteins (NRPs) to induce the expression of NAC6/NAC30 to regulate PCD together with NAC081 [33], [41], [42], however, the link from ER stress-sensing machinery to these NRPs is still missing.
Here we show that NAC089 plays important roles in regulating ER-stress-induced PCD in the model plant Arabidopsis. NAC089 relocates from the ER membrane to the nucleus during ER stress response. Inducible expression of a truncated form of NAC089 promotes PCD with increased caspase 3/7-like activity and DNA fragmentation. Down-regulation of NAC089 confers ER stress tolerance and impairs ER-stress-induced caspase 3/7-like activity. Several UPR downstream genes including the PCD regulators MC5, BAG6 and NAC094 are shown to be regulated by NAC089 under ER stress condition. NAC089 itself is also up-regulated by ER stress, which is directly controlled by bZIP28 and bZIP60. Therefore, NAC089 is an important PCD regulator in plant UPR, linking ER stress-sensing to downstream PCD regulators during ER stress response in plants.
Results
Up-regulation of NAC089 by ER stress is controlled by both bZIP28 and bZIP60
An ER-stress-related NAC (for NAM, ATAF, and CUC) transcription factor NAC089 (also known as ANAC089) was identified from our previous microarray analysis [43]. Its expression was up-regulated rapidly by ER stress inducers tunicamycin (TM) and dithiothreitol (DTT) (Figure 1A and Figure S1). Knock-out of either bZIP28 or bZIP60 partially suppressed, while knock-outs of both bZIP28 and bZIP60 in zip28zip60 double mutant completely abolished the up-regulation of NAC089 under ER stress condition (Figure 1B). Previously two ER stress responsive cis-elements UPRE and ERSE-I were identified as the binding sites of bZIP28 and bZIP60 [21], [43], [44]. We searched the NAC089 promoter region and found that one copy of UPRE and one copy of ERSE-I like (one mismatch) cis-elements are present over the segment [−95, −49] relative to the TSS site of NAC089. To assess the activation of NAC089 promoter by bZIP28 and bZIP60, an effector-reporter dual-luciferase transient assay was set up. The NAC089 promoter fragment containing the aforementioned cis-elements was fused to the firefly luciferase reporter and tested in Arabidopsis leaf protoplasts. As expected, the reporter was activated by either TM or DTT treatment (Figure 1C). Using this assay system, co-expression of either bZIP28D or bZIP60S dramatically enhanced the firefly luciferase reporter activity (Figure 1D). To demonstrate the direct binding of bZIP28 or bZIP60 to the NAC089 promoter, electrophoretic mobility shift assays (EMSAs) were performed with the biotin-labeled NAC089 promoter DNA. When the truncated form of either bZIP28 or bZIP60 was incubated with the biotin-labeled DNA probe, a band shift was observed reflecting the formation of the respective complex. To show the binding specificity, excess un-labeled probe was added and shown to be an effective competitor for the formation of each complex. On the contrary, the un-labeled mutated UPRE probe could not compete with the binding (Figure 1E–F). Through further mutation analysis, it was found that neither bZIP28 nor bZIP60 binds to the ERSE-I like cis-element presented in the probe (Figure S2). Thus, the expression of NAC089 is up-regulated by ER stress, which is directly controlled by both bZIP28 and bZIP60 through the UPRE cis-element.
Fig. 1. Up-regulation of NAC089 by ER stress is directly controlled by bZIP28 and bZIP60. 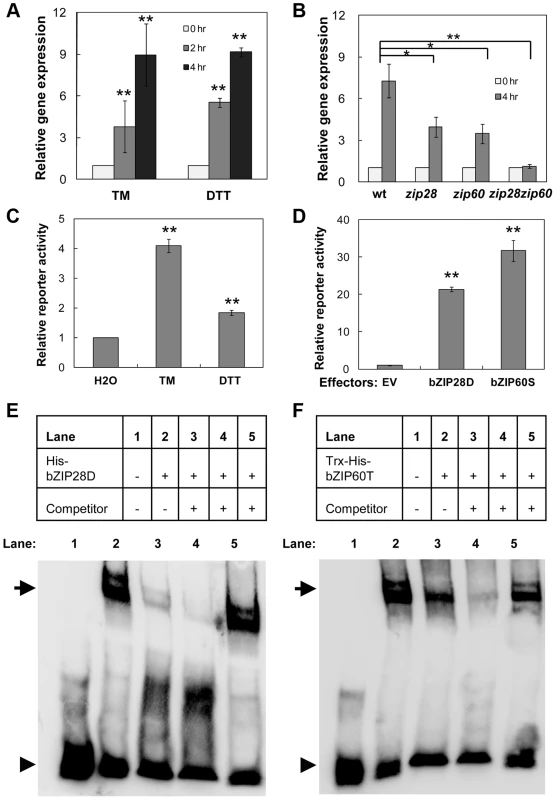
(A–B) Up-regulation of NAC089 in the wild-type (wt) plants by ER-stress inducers tunicamycin (TM, 5 µg/ml) and dithiothreitol (DTT, 2 mM) (A) and in the wt, bZIP28 single mutant (zip28), bZIP60 single mutant (zip60) or double mutant (zip28zip60) by TM (5 µg/ml) treatment (B). The expression of NAC089 is normalized to the expression of the internal control actin. (C–D) Transactivation of NAC089 promoter in the dual-luciferase leaf protoplast assays. Activations of the NAC089 promoter by ER stress treatments (C) and by co-expression of either activated bZIP28 (bZIP28D) or activated bZIP60 (bZIP60S) (D). Relative reporter activity is the firefly luciferase activity normalized to the renilla luciferase activity. Bars depict SE (n = 3) in A–D. The empty vector (EV) was used as a negative control. ** P<0.01, * P<0.05. (E–F) EMSA experiments to detect the protein-DNA binding. Either the purified His-bZIP28D (E) or Trx-His-bZIP60T (F) was incubated with the biotin-labeled pNAC089 DNA. Lane 3, 50× un-labeled pNAC089; lane 4, 200× un-labeled pNAC089; lane 5, 200× un-labeled mutated form pNAC089M1. Arrows and arrow heads point to the positions of shifted bands and free probes, respectively. NAC089 relocates from the ER membrane to the nucleus under ER stress condition
NAC089 is predicted to be a membrane-associated transcription factor [45] with the N-terminal DNA-binding domain facing the cytoplasm (Figure 2A). It has transcriptional activation activity and forms homodimers [46] (Figure S3). To confirm the membrane association of NAC089 and also to investigate the possible membrane-to-nucleus translocation of NAC089 in response to ER stress, 4X MYC tag was fused to NAC089 at the N-terminus and the fusion protein was expressed in Arabidopsis plants. Total proteins were extracted from transgenic seedlings and MYC-NAC089 was detected with the western blotting analysis. Without TM or DTT treatment, one prominent band reacted with the anti-MYC antibody. After TM or DTT treatment for 6 hr, one excess band with smaller molecular weight was induced, with a similar migration rate to the truncated form NAC089D, in which the C-terminal 24 amino acids of NAC089 were replaced with the 4X MYC tag (Figure 2B). To track the movement of NAC089 in response to ER stress, mGFP-NAC089 was expressed in Arabidopsis and observed under confocal laser scanning microscopy. In the mock (H2O) treatment, most of the mGFP-NAC089 signals were observed in the ER (Figure 2C–D); after either TM or DTT treatment for 6 hr, the fluorescence signals were largely found in the nuclei of the Arabidopsis leaf protoplasts and root cells (Figure 2C–D). The nuclear relocation of mGFP-NAC089 was not observed in the root cells when the transgenic plants were treated with TM (5 µg/ml) for short period of time (e.g. 2 hr). The ER-to-nucleus movement was also confirmed in the protein fractionation studies (Figure S4). Taken together, NAC089 is an ER membrane-associated protein and it relocates from the ER membrane to the nucleus in response to ER stress.
Fig. 2. NAC089 relocates from the ER membrane to the nucleus during ER stress responses. 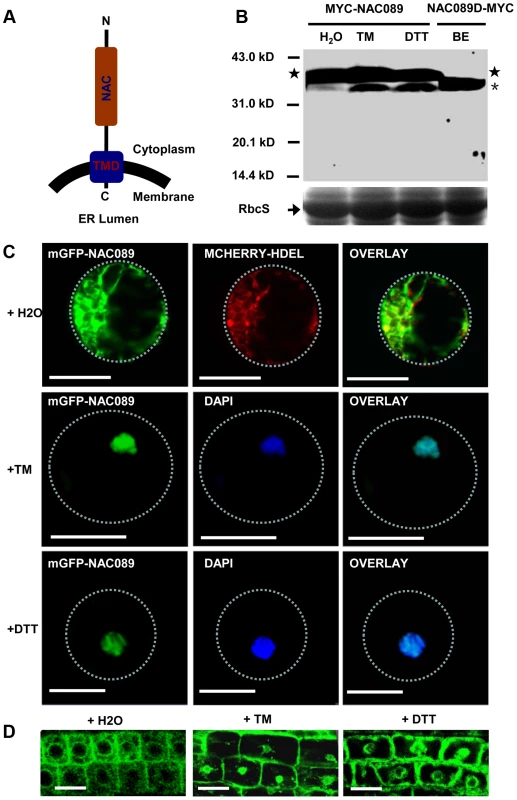
(A) Topology of NAC089 protein. (B) Activation of MYC-NAC089 in response to ER stress. Plant seedlings were treated with H2O (control), tunicaymin (TM, 5 µg/ml) or DTT (2 mM) for 6 hr. Star and asterisk represent the precursor and the processed form, respectively. The beta-estradiol (BE) induced truncated form NAC089D-MYC was used as the migration marker. Coomassie blue staining of RbcS serves as the loading control. (C–D) Nuclear relocation of mGFP-NAC089 in response to ER stresses in Arabidopsis leaf protoplast (C) and root cells (D). TM = 5 µg/ml in C and TM = 10 µg/ml in D. Dashed lines highlight the cell boundary. Bar = 50 µm. Down-regulation of NAC089 confers ER stress tolerance in plants
To investigate the biological function of NAC089 in the ER stress response, we created partial loss-of-function mutants by RNA interference (RNAi) and chimeric repressor silencing technology (CRES-T). NAC089 knock-down plants (RNAi089) grew as well as the wild-type (wt) control under normal growth condition, but they were more tolerant to ER stress than the wt (Figure 3A–B, Figure S5). More greenish big (G-B) plants and less yellowish small (Y-S) plants were observed in the RNAi089 plants than that in the wt under ER stress conditions (Figure 3B). In CRES-T system, fusion of an EAR-motif repression domain to a transcription factor converts an activator into a repressor, which results in partial loss function of the transcription factor [47]. We replaced the C-terminal hydrophobic tail of NAC089 with the EAR-motif, and expressed the chimerical fusion protein NAC089D-EAR in Arabidopsis with NAC089's native promoter. NAC089D-EAR expression did not affect seedling development under normal growth condition, but also conferred ER stress tolerance in plants (Figure S6A–D). ER stress should be built-up in plants which had been grown on solid growth medium with low concentrations of tunicamycin for a long period of time, as ascertained by the up-regulation of UPR marker genes in the wild-type plants (Figure S6E). All together, we concluded that partial loss-of-function of NAC089 in Arabidopsis increases chronic ER stress tolerance.
Fig. 3. Down-regulation of NAC089 confers ER stress tolerance. 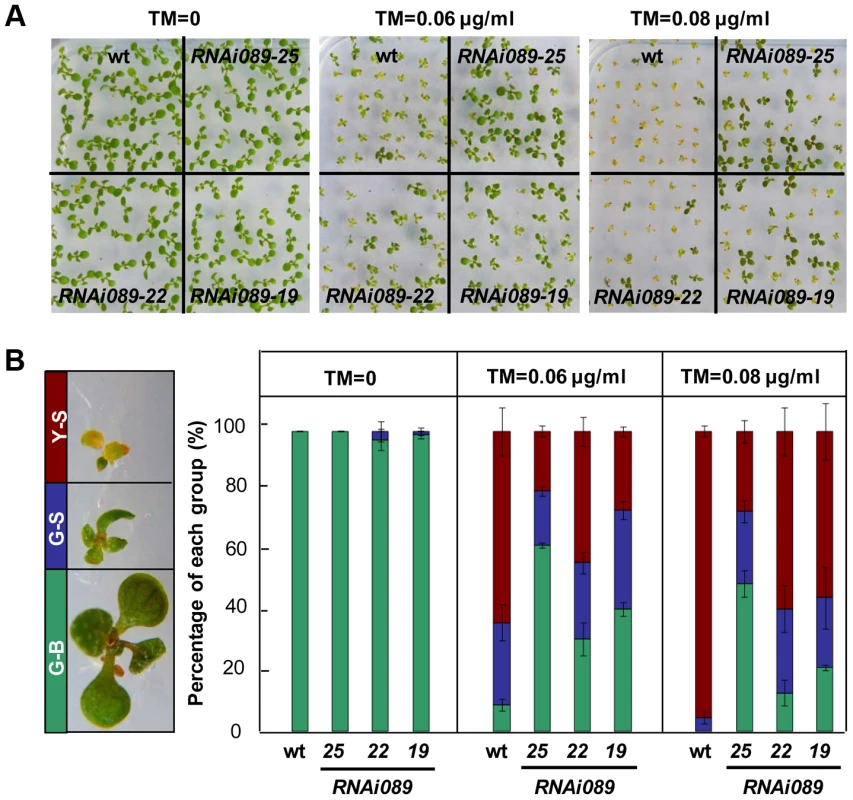
(A–B) Sensitivity of NAC089 RNAi plants to ER stress. Photos were taken with 10-day-old Arabidopsis seedlings grown on 1/2 MS medium supplied without or with different concentrations of tunicamycin (TM) (A) and percentage of green-big (G-B), green-small (G-S) and yellow-small (Y-S) plants was calculated (B). Bars depict SE (n = 3). The difference between the wild-type (wt) and each NAC089 RNAi line is significant (P<0.05). NAC089 promotes programmed cell death in plants
To gain insight into mechanisms by which NAC089 operates, we conditionally expressed a MYC-tagged truncated form of NAC089 (NAC089D-MYC) with the beta-estradiol (BE) inducible system [48] (Figure S7). This truncated form co-migrates with the ER stress-induced nuclear form of MYC-NAC089, has the transcriptional activation activity and localizes in the nucleus as mentioned above. The wt control and NAC089D-MYC expressing plants (XVE089D) were transferred to growth medium supplemented with or without BE. There was no obvious difference between the wt and XVE089D plants on the growth medium without BE (Figure 4A). However, when BE was included in the growth medium, root growth of the XVE089D plants was inhibited, and chlorotic leaves were observed, representing a typical PCD phenotype (Figure 4B–C). Cysteine-dependent aspartate-directed proteases (caspases) are the key regulators of PCD in animals, of which caspase-3 is the crucial executioner of PCD and recognize tetra-peptide motif DEVD [49]. Although the ortholog of animal caspase-3 is absent in plants, caspase 3/7-like activity has been reported in many examples involved in plant development and adaptation to environmental stresses [50]. To investigate whether the NAC089D-MYC-induced PCD was associated with the caspase-like activity, we performed caspase-3/7 activity assays using the same tetra-peptide substrate as previously reported [32], [33], [41]. It was found that the expression of NAC089D-MYC considerably induced caspase 3/7-like activity in the XVE089D plants (Figure 4D). The caspase 3/7 activity was also checked in the wt control and NAC089 RNAi plants (line RNAi089-25). ER stress gradually induced caspase 3/7-like activity in both the wt and RNAi089-25 plants in response to chronic ER stress. However, the caspase 3/7-like activity in the RNAi089-25 plants was about half of that in the wt plants after 3 days of TM treatment (Figure 4E), suggesting that NAC089-regulated caspase 3/7-like activity is stress severity-dependent. It is possible that other pathways also regulate such caspase 3/7-like activity. Loss of cell viability, accumulation of H2O2 and rupture of plasma membrane are often associated with PCD [30]. To assess cell viability, roots of XVE089D plants were stained with fluorescein diacetate (FDA), which is a substrate for many endogenous esterases. NAC089D-MYC expression dramatically reduced the endogenous esterase activities (Figure 4F–G). Further 3, 3′-diaminobenzidine (DAB) staining demonstrated that H2O2 was accumulated in the roots when NAC089D-MYC was induced (Figure 4H–I). Propidium iodide (PI) binds to DNA, but it is often used to stain plant cell wall or plasma membrane (Figure 4J) because it is membrane impermeant. When NAC089D-MYC was induced, PI signals were observed in both the cytoplasm and nucleus (Figure 4K), indicating that expression of NAC089D-MYC reduced the rigidity of cell membrane. Another characteristic of PCD is the morphological changes in the nucleus which could be revealed by 4, 6′-diamidino-2-phenylindole (DAPI) staining. Intact and round nuclei were found in most of the XVE089D root cells without BE treatment (Figure 4L). In contrast, nuclei with stretches and speckles were observed in the XVE089D root cells when the plants were treated with BE (Figure 4M–N). Cleavage of genomic DNA at internucleosomal sites by endogenous nucleases is always associated with PCD and terminal deoxynucleotidyl transferase-mediated dUTP nick and labeling (TUNEL) assay is frequently used to label the fragmentation of nuclear DNA in situ [31]. Compared to the low level of background green fluorescence in normal-grown roots (Figure 4O), strong TUNEL-positive signals were observed in the XVE089D root cells when the plants were treated with BE (Figure 4P–Q). BE treatment had no obvious effect on the aforementioned histochemical staining in the wt control plants (Figure S8). These results suggest that NAC089 has the ability to promote PCD in plants.
Fig. 4. NAC089 promotes programmed cell death in plants. 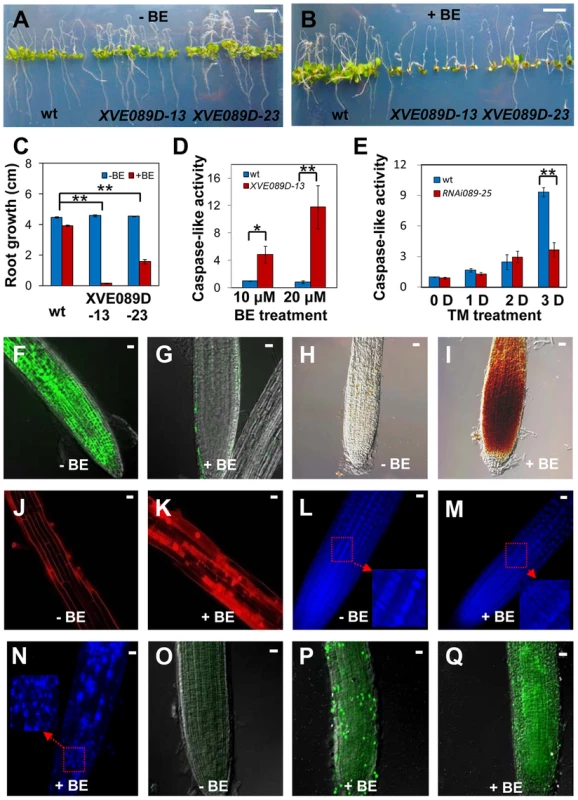
(A–C) Photos of Arabidopsis NAC089D-MYC transgenic lines (XVE089D) and wild-type (wt) control plants transferred to 1/2 MS medium supplied without (A) or with (B) beta-estradiol (BE) for additional 5 days with the quantitative data in (C). (D–E) Caspase 3/7-like activity in protein extracts from NAC089D-MYC overexpression plants treated with BE (D) or in the wt and NAC089 RNAi plants treated with TM (0.5 µg/ml) (E). The caspase-like activity was normalized to the activity of the wt control under normal condition. Bars depict SE (n = 3) in C–E. ** P<0.01, * P<0.05. (F–I) Esterase activities (F–G) and H2O2 accumulation (H–I) in the XVE089D-13 roots as revealed by FDA and DAB staining without (F, H) or with (G, I) BE treatment for 3 days. (J–N) Membrane rigidity and nucleus morphology in the XVE089D-13 roots as reflected by PI (J–K) and DAPI (L–N) staining without (J, L) or with (K, M–N) BE treatment for 5 days. (O–Q) DNA breakage in the XVE089D-13 roots as detected by TUNEL assay without (O) or with (P–Q) BE treatment for 5 days. Bar = 10 mm in A–B and bar = 10 µm in F–Q. NAC089 regulates many downstream genes including PCD regulators under ER stress condition
PCD is a genetically controlled process that plays important roles in plant development and responses to abiotic stress or pathogens [51], [52]. Many PCD regulators that have been well characterized in humans, worms and flies are absent from the Arabidopsis genome, indicating that plants may use different regulators to execute PCD [52], [53]. To understand how NAC089 regulates plant PCD, we performed microarray (Agilent 4X44K) experiments with the BE inducible gene expression system [48]. BE treatment did not affect much of the gene expression in the wt plants as reported by other colleagues [54] (Figure S9), but up-regulated 1363 probes (fold change >2, P<0.01) in the NAC089D-MYC expressing plants (XVE089D-13) (Dataset S1). Gene ontology (GO) analysis revealed that the most significant GO term among the NAC089D-MYC-regulated genes is the transcription factor activity (Figure S10), indicating that NAC089 is an important transcriptional regulator. To validate the microarray expression data, 23 genes were selected from the microarray data and their expression was examined by qRT-PCR in two NAC089D-MYC expressing lines. It was found that the expression of these genes was highly induced in both transgenic lines, especially in line XVE089D-13 (Figure 5). Among the 23 selected genes, 13 of them were up-regulated more than two fold by the prolonged ER stress, especially after 12 hr TM treatment (Figure S11). The up-regulation of these 13 genes by ER stress was also checked in the wt and NAC089 knock-down plants (line RNAi089-25). Previously, the BAX inhibitor 1 (BI-1) was reported to be an important modulator of ER stress-mediated PCD in Arabidopsis [31]. The transcription factor WRKY33, which is required for resistance to necrotrophic pathogens, plays critical roles in autophagy [55]. These two PCD markers along with other cell survival UPR markers were also included in the expression study. It was found that the up-regulation of 6 NAC089D-MYC-regulated genes (i.e. AT1G69325, encoding remorin-like protein; AT1G79330, encoding metacaspase MC5; AT2G46240, encoding BCL-2-associated athanogene BAG6; AT3G52350, encoding unknown protein; AT4G30880, encoding lipid transfer protein; and AT5G39820, encoding transcription factor NAC094) and autophagy-related gene WRKY33 was impaired in the RNAi089-25 plants under ER stress condition comparing to the wt control (Figure 6A–B). These results indicate that NAC089 plays critical roles in regulating these ER-stress-induced genes including several PCD-related genes. ER stress also up-regulates several cell survival UPR marker genes and BI-1 in both the wt and RNAi089-25 plants (Figure 6B), suggesting that NAC089 plays minor role in regulating the expression of these genes. In order to know whether NAC089 directly regulates these downstream genes, chromatin immunoprecipitation (ChIP) experiments were carried out with NAC089D-MYC plants (line XVE089D-13) using anti-MYC antibody. It was found that NAC089D-MYC was enriched significantly with fold change greater than 2 at the promoter regions of 7 genes (i.e. AT1G65240, AT1G71390, AT1G79330, AT2G46240, AT4G30880, AT5G39820 and AT5G40010) (Figure 7), indicating that these genes might be the direct targets of NAC089. Among the 7 NAC089 possible targets, the up-regulation of 3 genes (i.e. AT1G65240, encoding aspartyl protease; AT1G71390, encoding receptor-like protein RLP11 and AT5G40010, encoding AAA ATPase 1) by ER stress was not suppressed in the NAC089 knock-down mutants (Figure 6A), suggesting that other factors may also up-regulate these genes under ER stress condition. We concluded that NAC089 has the ability to regulate some of the UPR downstream genes, including the PCD regulatory genes MC5, BAG6 and NAC094, and the autophagy regulatory gene WRKY33. The function of other NAC089 downstream genes in ER stress-induced PCD needs to be investigated in the future.
Fig. 5. NAC089 regulates many downstream genes. 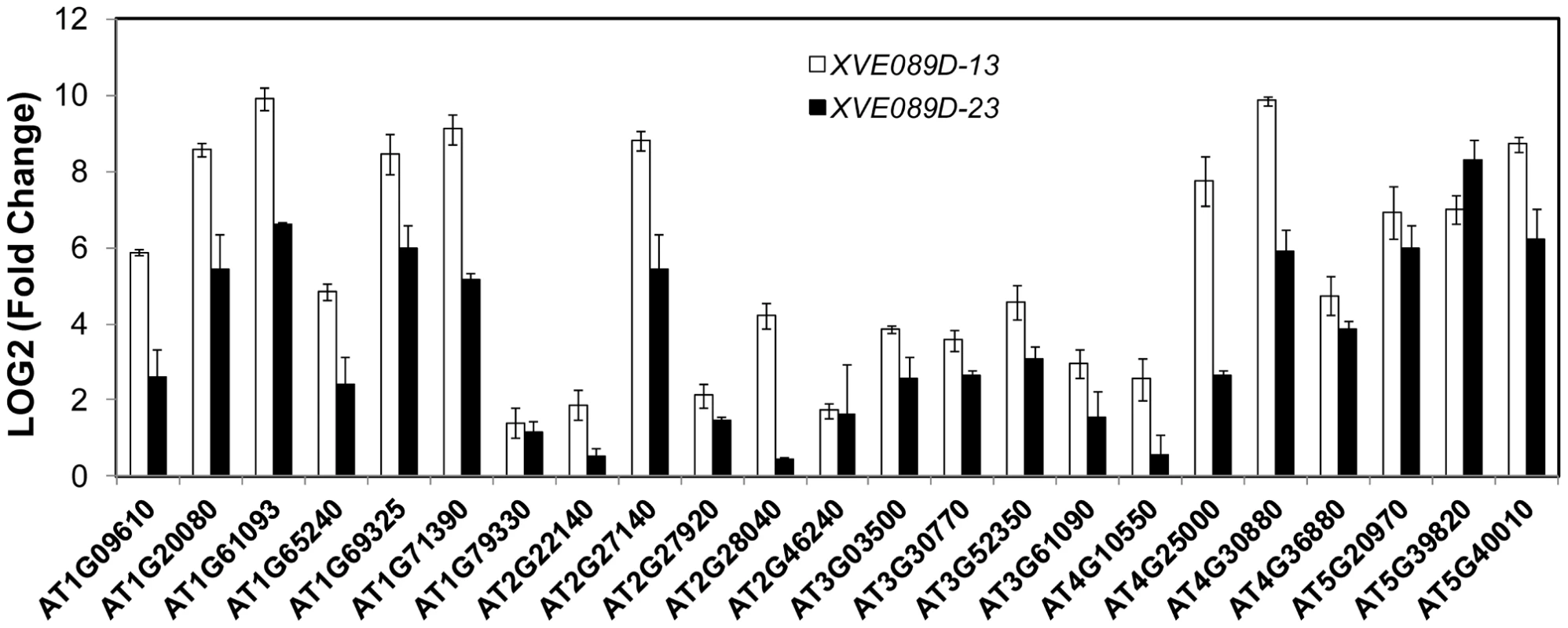
Expression of down-stream genes identified through microarray analysis was examined with qRT-PCR in two lines of NAC089D-MYC plants. Fold change is the gene expression level of plants treated with beta-estradiol (BE) for 16 hr divided by that of plants treated with DMSO (solvent control), both of which were normalized to the expression of actin. Bars depict SE (n = 3). The fold change for each gene is significant (P<0.01) in line XVE089D-13. Fig. 6. Up-regulation of NAC089D-MYC-regulated genes by ER stress is impaired in the NAC089 RNAi plants. 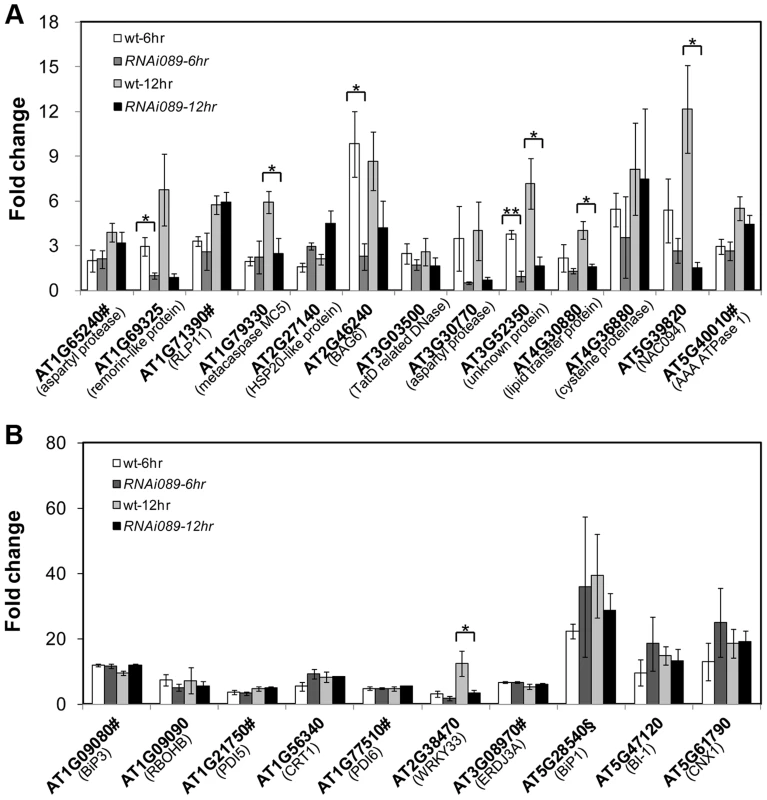
The expressions of NAC089D-MYC downstream genes (A) and other UPR markers (B) were quantified with qRT-PCR in the wild-type control (wt) and NAC089 knock-down plants (line RNAi089-25). Fold change is the gene expression value in TM (5 µg/ml) -treated plants divided by the value in non-treated plants, both of which were normalized to the expression of actin. Bars depict SE (n = 3). Values for the gene locus with # are log2 values. Fold change of BiP1 (AT5g28540, highlighted with §) also includes that of BiP2 (AT5G42020). ** P<0.01, * P<0.05. Fig. 7. NAC089 binds to the promoter regions of target genes. 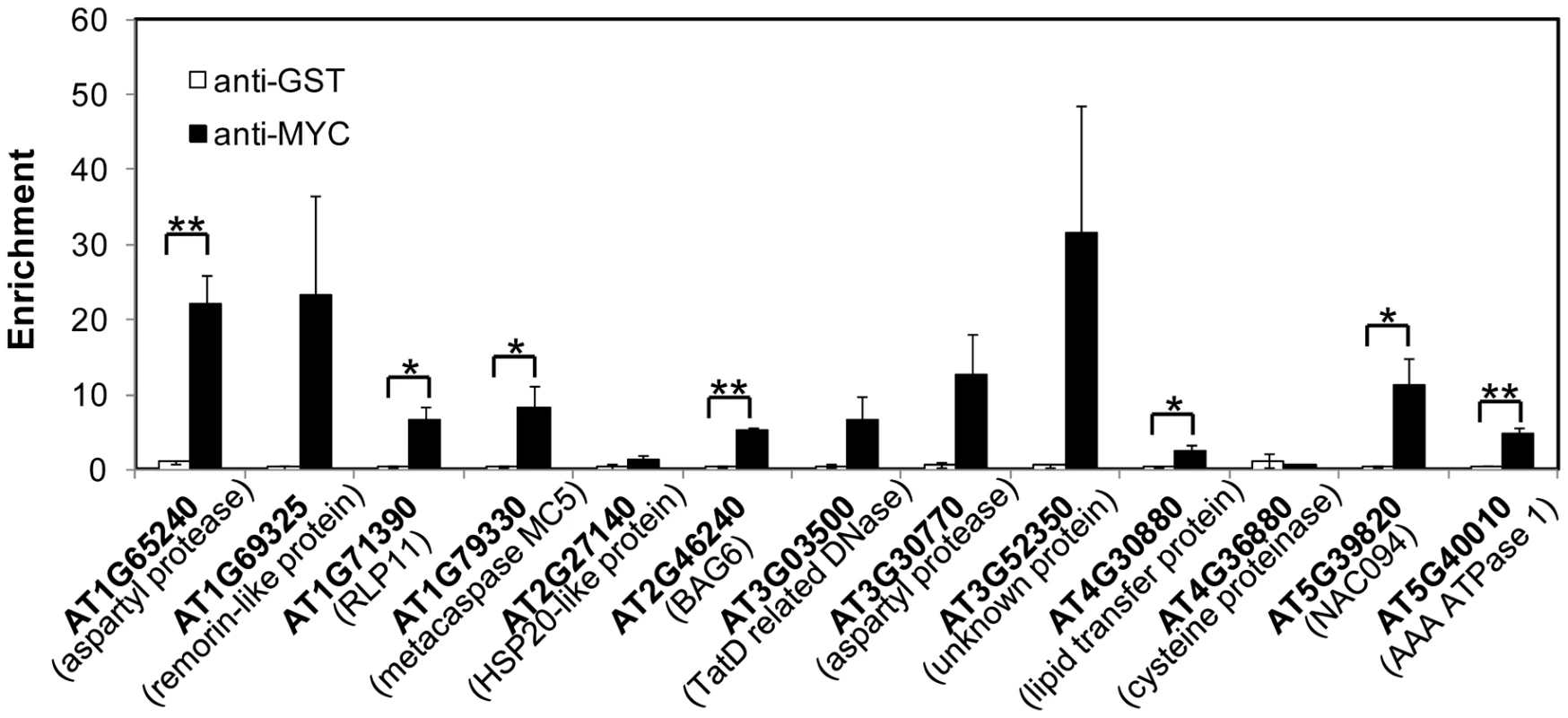
Enrichment is the DNA level of each fragment in beta-estradiol (BE)-treated sample divided by that in DMSO-treated sample, both of which were normalized to the level of TA3. Anti-MYC antibody was used to precipitate NAC089D-MYC and anti-GST was used as a negative antibody control. Bars depict SE (n = 3). ** P<0.01, * P<0.05 except for AT1G79330 (p = 0.057). Discussion
The unmitigated ER stress is believed to induce PCD in animals [3], as well as in plants [31], [56]. Given that PCD components are not highly conserved between animals and plants [30], [57], our knowledge on ER stress-induced PCD in plants is very limited [58]. Previously, BI-1 and IRE1 were reported to be the negative regulators of PCD in plants [31], [40]. In the current study, a membrane-associated transcription factor NAC089 was identified as an important transcriptional regulator of plant PCD under ER stress condition based on the following evidences: 1) NAC089 is up-regulated by UPR regulators bZIP28 and bZIP60 under ER stress condition; 2) NAC089 relocates from the ER membrane to the nucleus in response to ER stress; 3) Inducible expression of the truncated form of NAC089 induces PCD; 4) Partial loss-of-function of NAC089 confers resistance to chronic ER stress with reduced caspase 3/7-like activity; 5) NAC089 has transcriptional activity and binds to the promoter of many downstream targets; 6) Knock-down NAC089 suppresses the ER-stress-induced expression of several PCD regulators.
As in animals, plant development and adaptations to environmental stresses are intimately connected to PCD [59], [60]. In mammals, PCD is controlled predominately through functionally conserved proteins such as CED9/BCL-2 and BAX, but such genes have not been identified in plants [52]. Interestingly, the heterotrimeric G protein signaling was reported to be involved in ER stress-associated PCD. Null mutants of G beta subunit (AGB1) were more resistant to ER stress than either the wt plants or null mutants of G alpha subunit, but the underlying molecular mechanism was not known yet [61]. On the contrary, Chen and Brandizzi recently reported that the null AGB1 mutants were more sensitive to ER stress [62]. The function of AGB1 in ER stresses response needs to be further clarified. Caspases are cysteine-aspartic proteases that play essential roles in PCD in animals [4]. Plant caspase homologs are not found so far, and the metacaspases were demonstrated to have similar function in plant PCD [53], [63], [64]. Caspase-like activity has been detected in plant PCD associated with xylem formation and adaptations to heavy metal stress, pathogen infection, as well as exposure to ultraviolent-C [50], [65], [66], [67], [68]. In the current study, chronic ER stress induced caspase 3/7-like activity, and such induction was impaired in the NAC089 knock-down plants. Several NAC089 downstream targets including some known PCD regulators were also indentified in the current study. Among them, one metacaspase (MC5) and several other proteases were induced by ER stress, which was suppressed in the NAC089 knock-down plants. BAG (BCL2-associated athanogene) family proteins were originally identified as the anti-cell-death protein in mammals [69]. Among the seven animal BAG homologs found in Arabidopsis [70], overexpression of BAG6 induced PCD in yeast and plants [71], indicating that BAG6 is a pro-death protein in plants. In the current study, BAG6 was induced by ER stress in the Arabidopsis wt plants, which was impaired in the NAC089 knock-down plants. Previously, the soybean NAC transcription factor NAC6/NAC30 was shown to induce caspase 3-like activity and promote extensive DNA fragmentation when it was overexpressed in soybean protoplasts [33]. Here in the current study, we found that one of the direct targets of NAC089, NAC094, is the close-related homolog of soybean NAC6/NAC30 in Arabidopsis. ER stress-induced expression of NAC094 was greatly suppressed in the NAC089 knock-down plants. These results support that NAC089 controls the expression of several PCD-related downstream genes in Arabidopsis under ER stress condition. Interestingly, the autophagy-related gene WRKY33 [55]was also up-regulated by ER stress, which was dependent on NAC089. Other NAC089 downstream genes such as genes encoding protease and nuclease were also identified in the current study. The identification of NAC089 as a PCD regulator provides more opportunities for further understanding new molecular components involved in plant PCD, especially under ER stress condition.
NAC089 is regulated by ER stress at both transcriptional and post-translational levels. At the transcriptional level, NAC089 is up-regulated by ER stress, which is directly controlled by bZIP28 and bZIP60, two important regulators in plant UPR [9], [18], [44], [72]. At the protein level, NAC089 is an ER membrane-associated transcription factor (MTF) and it relocates from the ER to the nucleus under ER stress condition. Interestingly, bZIP28 and bZIP60 are also ER MTFs. bZIP28 is activated through regulated proteolysis. In response to ER stress, bZIP28 relocates from the ER to the Golgi where it is cleaved by two Golgi-resident proteases S1P and S2P, and the C-terminal lumen-facing domain is thought to be responsible for the sensing of ER stress [17], [18], [19], [72], [73]. The activation mechanism for bZIP60 is unconventional, and the activation of bZIP60 is dependent on the ER membrane-localized IRE1 proteins. Under ER stress conditions, bZIP60 mRNA is spliced by IRE1, which results in an open reading frame (ORF) shift and elimination of the transmembrane domain [9], [11], [12]. The N-terminal part of yeast IRE1 is inserted into the ER lumen and plays important role in direct sensing the unfolded proteins in the ER in yeast [74]. Recently, at least 13 MTFs in NAC family are found in Arabidopsis, of which some are activated during development and adaptations to environmental stresses [45], [75], [76], [77], [78]. However, the activation mechanisms of these NAC MTFs are still largely unknown. The NAC089 mRNA does not have the predicted double stem-loop structure that has been shown to be very important for IRE1 splicing [9]. Furthermore, there is no alternative spliced transcript of NAC089 observed in the ER stressed wt seedlings (Figure S12), suggesting that NAC089 might be activated in a manner different from bZIP60. The C-terminal ER lumen facing tail of NAC089 is very short and does not have the canonical S1P cutting site, which implicates that NAC089 might not be proteolytically processed in the same way as bZIP28. We did not include protease inhibitors in the NAC089 activation experiments because most of the protease inhibitors are not permeable to live plant cells. Further investigation of the activation mechanism of NAC089 will improve our understanding of MTFs in plants. Surprisingly, one rare nucleotide polymorphism caused by natural variation in the Arabidopsis Cvi ecotype results in premature stop and constitutive nuclear localization of NAC089, in which the C-terminus (114 AA) including the hydrophobic tail is not translated. Although Cvi ecotype is much more sensitive to fructose than Ler ecotype, expressing the Cvi NAC089 suppresses fructose sensitivity in Ler seedlings [79]. The truncated form (114 AA deletions) of NAC089 was found in the nucleus, however, the activation or nuclear relocation of NAC089 in response to fructose treatment is not reported, and how the truncated form of NAC089 represses fructose signaling is not clear. Since the deletion occurred in Cvi was found neither in over 100 Arabidopsis accessions nor in the Arabidopsis Genome 1001 sequence collections, the biological function of NAC089 in fructose signaling other than in the Cvi ecotype is elusive [79]. Recently, it was reported that fructose feeding induced ER stress in mice [80]. It would be interesting to determine whether the high concentration of fructose could also induce ER stress in Arabidopsis plant. Recently, NAC089 was reported to be involved in redox regulation [81]. Besides its effect on redox status, DTT also inhibits disulphide bond formation and therefore promotes protein misfolding. However, TM is a more specific ER stress inducer because of its specific effect on blocking protein N-glycosylation in the ER. In the current study, both TM and DTT treatments were employed to demonstrate the specific role of NAC089 in ER stress response. Our current study has also advanced the understanding of the function of NAC089 in ER-stress-induced plant PCD and the underlying molecular mechanisms.
How cells make the cell fate selection between life and death remains enigmatic. In human cells, ER stress activates all the three arms of UPR pathways; each branch has different effect on cell survival or cell death, but attenuation of each branch is different. Switch between cell survival and cell death outputs lies in part in the duration of individual branch activity, which guides the cell toward survival or demise [82]. In plants, except the PERK pathway, the bZIP28 and bZIP60 branches of signaling pathway have been previously discovered to regulate downstream genes involved in promoting cell survival [83]. Knock-outs of both branches in the zip28zip60 double mutant causes high sensitivity to ER stresses and accelerated PCD under prolonged ER stress condition [72]. IRE1A and IRE1B redundantly control the activation of bZIP60 and RIDD; knock-outs of both IRE1s in Arabidopsis also promotes PCD while knock-out of single bZIP60 gene has no PCD phenotype [40]. The ER stress-induced up-regulation of NAC089 is dependent on both bZIP28 and bZIP60. Different from the zip28zip60 mutant, knock-down NAC089 confers ER stress tolerance and over-expression of the truncated form of NAC089 promotes PCD. These results may not necessarily be controversial. Firstly, bZIP28 and bZIP60 regulate many survival genes whose expressions are almost completely abolished in the zip28zip60 mutant [72]. Lacking the expression of survival genes in the zip28zip60 mutant may lead to the accelerated PCD. Secondly, NAC089 has substantial constitutive expression and the NAC089 pathway may still operate for PCD in the zip28zip60 mutant even without further NAC089 up-regulation. Thirdly, it is possible to have other PCD pathways turned on to execute PCD in the zip28zip60 mutant. Recently, heterotrimeric G protein signaling [61], vacuolar processing enzyme (VPE)-triggered cell death [42], [84] and IRE1-mediated autophagy [85] pathways were reported to be involved in the ER stress-induced PCD in plants.
A hypothetical working model has been emerged from the current study (Figure 8). When Arabidopsis cells are confronted with ER stress, both bZIP28 and bZIP60 pathways are activated to mitigate the stress by up-regulation of genes involved in protein folding or ERAD to improve survival [1], [83]. The activated bZIP60 also induces its own transcription [44] and another transcription factor NAC103 [86] to amplify the cell survival signal. The ER-localized IRE1 protects cell through a process called RIDD to reduce the protein folding demand [40]. In the meantime, besides the constitutive high expression of NAC089 under normal condition, both bZIP28 and bZIP60 up-regulate the expression of NAC089 under ER stress condition. Up-regulation of NAC089 mRNA may increase the protein level of the membrane-associated NAC089 precursor. However, nuclear relocation of NAC089 is tightly controlled, in which bZIP28 and bZIP60 may play negative roles. When the ER stress is severe, NAC089 is activated and relocates from the ER to the nucleus, inducing the expression of PCD regulators to promote cell death. It is possible that the stress intensity and/or duration of ER stress might determine the signaling output and the final cell fate. Further understanding on how cells balance the cell survival and cell death effects in UPR is of great fascination.
Fig. 8. Hypothetical working model of cell survival and cell death pathways in plant UPR. 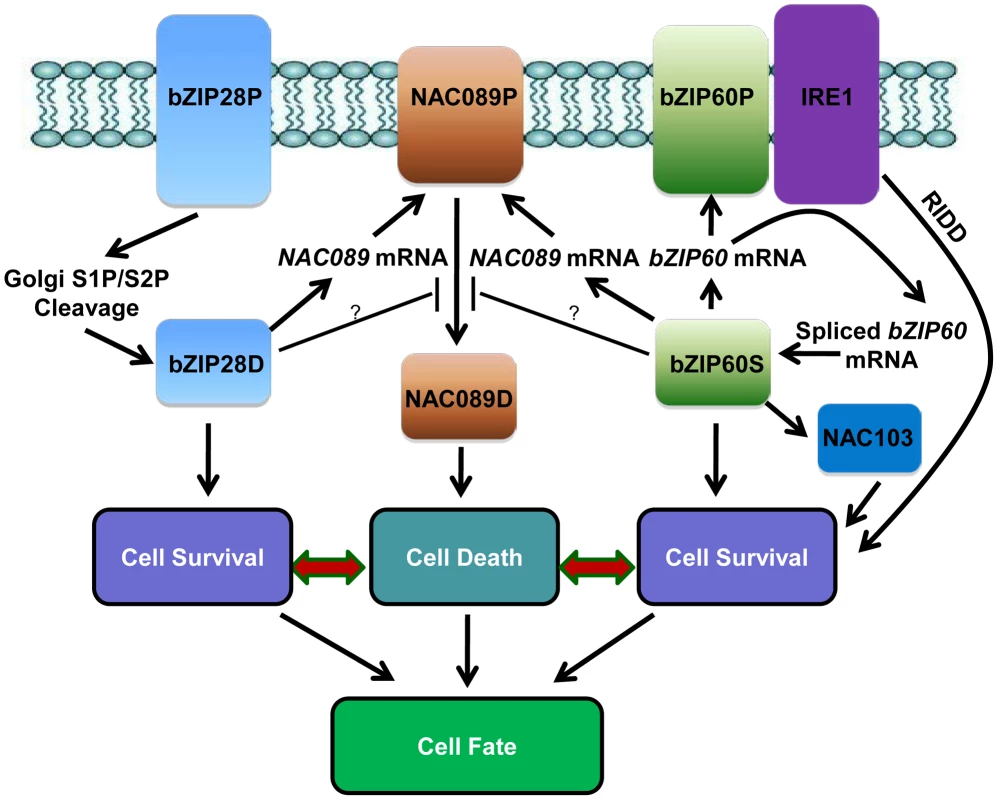
In response to ER stress, the ER-localized bZIP28P is activated through S1P/S2P sequential cleavage. The precursor bZIP60P is also ER membrane-associated under normal growth condition; ER stress activates the ER-localized IRE1, which splices the bZIP60 mRNA to produce a shorter bZIP60 mRNA encoding the activated form bZIP60S. Both the activated bZIP28D and bZIP60S enter the nucleus and up-regulate downstream genes such as molecular chaperones and ERAD components to ensure cell survival. The activated bZIP60S also induces its own transcription and another transcription factor NAC103 to amplify the cell survival signal. IRE1 could also protect cells through RIDD to reduce the entry of secretory proteins into the ER lumen. On the other hand, the ER membrane-localized NAC089P is activated to produce the nuclear form NAC089D when the ER stress is severe. NAC089D activates several downstream PCD-related genes to promote cell death. Both bZIP28D and bZIP60S control the up-regulation of NAC089 under ER stress condition and may also control the expression of negative regulators for NAC089 activation. Thus, both pro-survival and pro-death signals are controlled by bZIP28 and bZIP60, and the balance between their outputs probably decides the life-or-death cell fate in Arabidopsis plants under ER stress conditions. Materials and Methods
Plant materials, growth conditions and phenotypic analysis
All Arabidopsis (Arabidopsis thaliana) wild-type, T-DNA mutants and transgenic plants in the current study were in the Columbia (Col-0) ecotype background. The double mutant zip28zip60 was made as previously reported [72]. Methods for plant growth were described previously [43]. For short time treatment, different concentrations of TM (5 µg/ml), DTT (2 mM) or BE (10 µM) were added in the half-strength MS medium unless mentioned in the text. For long time treatment, 10 µM BE or various low concentrations of TM were supplied in the solid growth medium. Root length was measured and emergence rate of true leaves was calculated. Total chlorophylls were extracted from seedlings with 80% (v/v) acetone at 4°C overnight and measured from A663 and A646 readings for each sample [87]. All the data in the paper were subjected to Student's t-test or two-way ANOVA (analysis of variance) analysis.
Plasmid construction and generation of transgenic plants
The coding sequence of NAC089 was amplified with PCR and inserted into pSKM36 after digestion with AscI and SpeI restriction enzymes to produce the vector Pro35S:NAC089. Modified green fluorescence protein (mGFP) tag and 4X MYC tag were amplified and inserted into Pro35S:NAC089 at AscI site to generate the Pro35S:mGFP-NAC089 and Pro35S:MYC-NAC089 constructs, respectively. For RNAi construct preparation, part of the NAC089 gene sequence covering cDNA 651–1150 was inserted into pHANNIBAL in both sense and antisense orientations separated by an intron sequence. The entire RNAi cassette was cut with NotI and inserted into pART27 to make the RNAi expression vector. To express the dominant negative fusion protein ProNAC089:NAC089D-EAR, the sequence encoding EAR motif (QDLDLELRLGFA) was synthesized and firstly inserted into pCAMBIA1300; about 1 kb upstream sequence of NAC089 and sequence encoding the truncated form of NAC089 (NAC089D, aa 1–316) were amplified and subsequently inserted. To generate the conditionally overexpression construct [48], nucleotides encoding NAC089D was amplified and inserted into pER10M. For dual luciferase activity assay, fragment of the NAC089 promoter (−98 bp to −46 bp relative to the TSS site) was synthesized and inserted into pGreen0800-II after the 35S minimal promoter was introduced. Constructs expressing bZIP28D and bZIP60S were made as described [9], [18]. For protein expression in E. coli, the respective sequence of bZIP18D (aa 1–321) or bZIP60T (aa 87–217) was amplified and inserted into pET28 and pET32, respectively. All the primers were listed in Table S1 and error-free clones were introduced into plants by either transient expression or stable transformation.
Protein extraction and immunoblotting analysis
Total protein was extracted from plants with extraction buffer described previously [17]. Membrane fraction and nuclear fraction were prepared with sucrose gradient centrifuge according to the standard protocol [88]. Proteins were resolved on 8–10% SDS-PAGE gels and visualized by western blotting using antibodies against c-MYC (Santa Cruz Biotechnology), nuclear protein marker histone H3 (Abcam) and ER protein marker BiP (Santa Cruz Biotechnology).
Caspase-like activity and luciferase reporter assays
Caspase-like activity was measured with luminescent assays based on DEVD short peptides with Caspase-Glo 3/7 Assay Kit (Promega). Both caspase 3 and caspase 7 recognize the same DEVD substrate. Briefly, seedlings were harvested after various treatments and total proteins were extracted with liquid nitrogen in a buffer containing 100 mM sodium acetate, pH 5.5, 100 mM NaCl, 1 mM EDTA, and 5 mM DTT. To measure caspase-3/7 activity, 30 µl caspase-3/7 luminogenic substrate (Z-DEVD-aminoluciferin) was added to 50 µg protein extracts and incubated at 22°C for 1 hr protected from light. The luminescence of each sample was measured with the Synergy 2 Multi-Mode Microplate Reader (BioTek). For dual-luciferase activity assays [89], Arabidopsis leaf protoplasts were isolated from 4-week-old soil-grown seedlings and transfected according to a standard protocol [43] with various reporter constructs or cotranstransfected with different effectors. Firefly and renilla luciferase were quantified with Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer's instructions in the Synergy 2 Multi-Mode Microplate Reader (BioTek).
Histochemistry and microscopy
For FDA and DAB staining, seedlings were stained with 2.5 µg/ml FDA (Sigma-Aldrich) in phosphate-buffered saline for 10 min or 1 mg/ml DAB (pH 5.5, Sigma-Aldrich) for 2 hr at room temperature, immersed into boiled ethanol for 10 min according to the standard protocol [31]. For PI and DAPI staining, samples were stained using DAPI (Sigma-Aldrich) at 1 µg/ml in 0.1% (v/v) Triton X-100 for 10 min or PI (Sigma-Aldrich) at 10 µg/ml for 1 min, and washed twice with water. For in situ TUNEL staining, roots were stained in microcentrifuge tubes (1.5 ml) using the in situ cell death detection kit (Takara) according to the manufacturer' instructions. Except DAB staining, which was observed under differential interference contrast (DIC) microscopy, other staining, BiFC and subcellular localization of mGFP-NAC089 were visualize with laser confocal fluorescence microscopy (Zeiss LSM A710).
Chromatin immunoprecipitation (ChIP) and gene expression analysis
ChIP was performed according to the standard protocols. Briefly about 3 g of 2-week-old XVE089D transgenic seedlings were treated with either 10 µM BE or DMSO (solvent control) for 16 hr and fixed with 1.0% formaldehyde for 10 min subsequently. Antibodies against c-MYC (Santa Cruz Biotechnology) and GST (IgG control, Abmart) were used for immunoprecipitation. Protein-A-agarose beads were blocked with salmon sperm DNA and used to pull down the protein-DNA complex. Equal amounts of starting plant material and the ChIP products were used for quantitative PCR. Primers were selected in the promoter regions of each selected gene. DNA levels were calculated relative to TA3 (AT1G37110) using a comparative threshold cycle method. The ChIP experiments were performed 3 times with biological replications and similar results were obtained. For microarray analysis or qRT-PCR, the wt control, XVE089D and NAC089 RNAi plants were grown vertically on agar plates for one week and then transferred to 1/2 MS liquid supplied with 10 µM BE or DMSO or TM for a period of time as noted. Total RNA was extracted and purified according to the manufacturer's instructions [43]. Agilent Arabidopsis gene chips (4X44K) were used to compare the gene expression profiles with three independent replications. P-values were calculated and used to select the genes that are up-regulated by NAC089D-MYC (cut-off: P<0.01, fold change >2). Microarray data from this article can be found in ArrayExpress under the accession number E-MTAB-1377. Quantitative PCR and RT-PCR were routinely conducted [43] and all the primers are listed in Table S1. GO analysis was performed with AgriGO (http://bioinfo.cau.edu.cn/agriGO/analysis.php).
Electrophoretic mobility shift assay (EMSA)
EMSA was performed using a LightShift Chemiluminescent EMSA Kit (Pierce), according to the manufacturer's protocols [43]. Briefly, each 20 µL binding reaction contained 2 µl binding buffer, 0.3 µl Poly (dI-dC), 4 µg purified protein, 0.83 µmol biotin-labeled probe or certain amount of unlabeled probe as the competitor. The pNAC089 wt or pNAC089M1-M3 probes were created by annealing together complementing oligonucleotides and biotinylated with a labeling kit (Pierce). His-tagged bZIP28D or Trx-His-tagged bZIP60T proteins were expressed in E. coli strain BL21 and purified with Ni-NTA agarose beads (Qiagen). The binding reactions were allowed to incubate on ice for 1 hr and run on a 5% polyacrylamide mini-gel (37.5∶1 acrylamide-bisacrylamide in 0.5× Tris-Borate-EDTA (TBE) containing 3% glycerol). The complex was transferred to a membrane and developed according to a standard protocol.
Supporting Information
Zdroje
1. LiuJX, HowellSH (2010) Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22 : 2930–2942.
2. SmithMH, PloeghHL, WeissmanJS (2011) Road to ruin: Targeting proteins for degradation in the endoplasmic reticulum. Science 334 : 1086–1090.
3. WalterP, RonD (2011) The unfolded protein response: From stress pathway to homeostatic regulation. Science 334 : 1081–1086.
4. RonD, WalterP (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8 : 519–529.
5. VitaleA, BostonRS (2008) Endoplasmic reticulum quality control and the unfolded protein response: Insights from plants. Traffic 9 : 1581–1588.
6. CoxJS, ShamuCE, WalterP (1993) Transcriptional induction of genes encoding endoplasmic-reticulum resident proteins requires a transmembrane protein-kinase. Cell 73 : 1197–1206.
7. ShenXH, EllisRE, LeeK, LiuCY, YangK, et al. (2001) Complementary signaling pathways regulate the unfolded protein response and are required for C-elegans development. Cell 107 : 893–903.
8. CalfonM, ZengHQ, UranoF, TillJH, HubbardSR, et al. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415 : 92–96.
9. DengY, HumbertS, LiuJX, SrivastavaR, RothsteinSJ, et al. (2011) Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc Natl Acad Sci USA 108 : 7247–7252.
10. LuSJ, YangZT, SunL, SunL, SongZT, et al. (2012) Conservation of IRE1-regulated bZIP74 mRNA unconventional splicing in rice (Oryza sativa L.) involved in ER stress responses. Mol Plant 5 : 504–514.
11. NagashimaY, MishibaKI, SuzukiE, ShimadaY, IwataY, et al. (2011) Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci Rep 1 : 29.
12. MorenoAA, MukhtarMS, BlancoF, BoatwrightJL, MorenoI, et al. (2012) IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PloS One 7: e31944.
13. HayashiS, WakasaY, TakahashiH, KawakatsuT, TakaiwaF (2012) Signal transduction by IRE1-mediated splicing of bZIP50 and other stress sensors in the endoplasmic reticulum stress response of rice. Plant J 69 : 946–956.
14. DengY, SrivastavaR, HowellSH (2013) Protein kinase and ribonuclease domains of IRE1 confer stress tolerance, vegetative growth, and reproductive development in Arabidopsis. Proc Natl Acad Sci USA 110 : 19633–19638.
15. HardingHP, ZhangYH, RonD (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397 : 271–274.
16. HazeK, YoshidaH, YanagiH, YuraT, MoriK (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10 : 3787–3799.
17. LiuJX, SrivastavaR, CheP, HowellSH (2007) Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J 51 : 897–909.
18. LiuJX, SrivastavaR, CheP, HowellSH (2007) An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 19 : 4111–4119.
19. CheP, BussellJD, ZhouWX, EstavilloGM, PogsonBJ, et al. (2010) Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in Arabidopsis. Sci Signal 3 (141) ra69.
20. GaoH, BrandizziF, BenningC, LarkinRM (2008) A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc Natl Acad Sci USA 105 : 16398–16403.
21. TajimaH, IwataY, IwanoM, TakayamaS, KoizumiN (2008) Identification of an Arabidopsis transmembrane bZIP transcription factor involved in the endoplasmic reticulum stress response. Biochem Biophy Res Commun 374 : 242–247.
22. SitiaR, BraakmanI (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426 : 891–894.
23. TabasI, RonD (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13 : 184–190.
24. UranoF, WangXZ, BertolottiA, ZhangYH, ChungP, et al. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287 : 664–666.
25. ShoreGC, PapaFR, OakesSA (2011) Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 23 : 143–149.
26. HetzC, BernasconiP, FisherJ, LeeAH, BassikMC, et al. (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1 alpha. Science 312 : 572–576.
27. UptonJP, WangL, HanD, WangES, HuskeyNE, et al. (2012) IRE1 alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science 338 : 818–822.
28. McCulloughKD, MartindaleJL, KlotzLO, AwTY, HolbrookNJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bc12 and perturbing the cellular redox state. Mol Cell Biol 21 : 1249–1259.
29. HanJ, BackaSH, HurJ, LinY-H, GildersleeveR, et al. (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15 : 481.
30. van DoornWG, BeersEP, DanglJL, Franklin-TongVE, GalloisP, et al. (2011) Morphological classification of plant cell deaths. Cell Death Differ 18 : 1241–1246.
31. WatanabeN, LamE (2008) BAX inhibitor-1 modulates endoplasmic reticulum stress-mediated programmed cell death in Arabidopsis. J Biol Chem 283 : 3200–3210.
32. ZuppiniA, NavazioL, MarianiP (2004) Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J Cell Sci 117 : 2591–2598.
33. FariaJAQA, ReisPAB, ReisMTB, RosadoGL, PinheiroGL, et al. (2011) The NAC domain-containing protein, GmNAC6, is a downstream component of the ER stress - and osmotic stress-induced NRP-mediated cell-death signaling pathway. BMC Plant Biology 11 : 129.
34. AlvesMS, ReisPAB, DadaltoSP, FariaJAQA, FontesEPB, et al. (2011) A novel transcription factor, ERD15 (Early Responsive to Dehydration 15), connects endoplasmic reticulum stress with an osmotic stress-induced cell death signal. J Biol Chem 286 : 20020–20030.
35. IshikawaT, WatanabeN, NaganoM, Kawai-YamadaM, LamE (2011) Bax inhibitor-1: a highly conserved endoplasmic reticulum-resident cell death suppressor. Cell Death Differ 18 : 1271–1278.
36. Kawai-YamadaM, JinLH, YoshinagaK, HirataA, UchimiyaH (2001) Mammalian Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1 (AtBl-1). Proc Natl Acad Sci USA 98 : 12295–12300.
37. Kawai-YamadaM, OhoriY, UchimiyaH (2004) Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 16 : 21–32.
38. SanchezP, ZabalaMD, GrantM (2000) AtBI-1, a plant homologue of Bax Inhibitor-1, suppresses Bax-induced cell death in yeast and is rapidly upregulated during wounding and pathogen challenge. Plant J 21 : 393–399.
39. HollienJ, LinJH, LiH, StevensN, WalterP, et al. (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186 : 323–331.
40. MishibaKI, NagashimaY, SuzukiE, HayashiN, OgataY, et al. (2013) Defects in IRE1 enhance cell death and fail to degrade mRNAs encoding secretory pathway proteins in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA 110 : 5713–5718.
41. CostaMDL, ReisPAB, ValenteMAS, IrsiglerAST, CarvalhoCM, et al. (2008) A new branch of endoplasmic reticulum stress signaling and the osmotic signal converge on plant-specific asparagine-rich proteins to promote cell death. J Biol Chem 283 : 20209–20219.
42. MendesGC, ReisPAB, CalilIP, CarvalhoHH, AragaoFJL, et al. (2013) GmNAC30 and GmNAC81 integrate the endoplasmic reticulum stress - and osmotic stress-induced cell death responses through a vacuolar processing enzyme. Proc Natl Acad Sci USA 110 : 19627–19632.
43. LiuJX, HowellSH (2010) bZIP28 and NF-Y transcription factors are activated by ER Stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22 : 782–796.
44. IwataY, KoizumiN (2005) An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc Natl Acad Sci USA 102 : 5280–5285.
45. KimSY, KimSG, KimYS, SeoPJ, BaeM, et al. (2007) Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucl Acid Res 35 : 203–213.
46. LiJ, ZhangJ, WangX, ChenJ (2010) A membrane-tethered transcription factor ANAC089 negatively regulates floral initiation in Arabidopsis thaliana. Sci China-Life Sci 53 : 1299–1306.
47. HiratsuK, MatsuiK, KoyamaT, Ohme-TakagiM (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34 : 733–739.
48. ZuoJR, NiuQW, ChuaNH (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24 : 265–273.
49. PorterAG, JanickeRU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6 : 99–104.
50. HanJJ, LinW, OdaY, CuiKM, FukudaH, et al. (2012) The proteasome is responsible for caspase-3-like activity during xylem development. Plant J 72 : 129–141.
51. KuriyamaH, FukudaH (2002) Developmental programmed cell death in plants. Curr Opin Plant Biol 5 : 568–573.
52. LamE, KatoN, LawtonM (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411 : 848–853.
53. CollNS, VercammenD, SmidlerA, CloverC, Van BreusegemF, et al. (2010) Arabidopsis type I metacaspases control cell death. Science 330 : 1393–1397.
54. Ohashi-ItoK, OdaY, FukudaH (2010) Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell 22 : 3461–3473.
55. LaiZ, WangF, ZhengZ, FanB, ChenZ (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J 66 : 953–968.
56. CrostiP, MalerbaM, BianchettiR (2001) Tunicamycin and Brefeldin A induce in plant cells a programmed cell death showing apoptotic features. Protoplasma 216 : 31–38.
57. HoeberichtsFA, WolteringEJ (2003) Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. Bioessays 25 : 47–57.
58. EichmannR, SchaferP (2012) The endoplasmic reticulum in plant immunity and cell death. Front Plant Sci 3 : 200–200.
59. CacasJL (2010) Devil inside: does plant programmed cell death involve the endomembrane system? Plant Cell Environ 33 : 1453–1473.
60. LoveAJ, MilnerJJ, SadanandomA (2008) Timing is everything: regulatory overlap in plant cell death. Trend Plant Sci 13 : 589–595.
61. WangS, NarendraS, FedoroffN (2007) Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA 104 : 3817–3822.
62. ChenY, BrandizziF (2012) AtIRE1A/AtIRE1B and AGB1 independently control two essential unfolded protein response pathways in Arabidopsis. Plant J 69 : 266–277.
63. TsiatsianiL, Van BreusegemF, GalloisP, ZavialovA, LamE, et al. (2011) Metacaspases. Cell Death Differ 18 : 1279–1288.
64. LamE, ZhangY (2012) Regulating the reapers: activating metacaspases for programmed cell death. Trend Plant Sci 17 : 487–494.
65. YeY, LiZ, XingD (2013) Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ 36 : 1–15.
66. ZhangL, XuQ, XingD, GaoC, XiongH (2009) Real-time detection of caspase-3-like protease activation in vivo using fluorescence resonance energy transfer during plant programmed cell death Induced by ultraviolet C overexposure. Plant Physiol 150 : 1773–1783.
67. DanonA, RotariVI, GordonA, MailhacN, GalloisP (2004) Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against Apoptotic Death. J Biol Chem 279 : 779–787.
68. HatsugaiN, IwasakiS, TamuraK, KondoM, FujiK, et al. (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens. Gene Dev 23 : 2496–2506.
69. TakayamaS, SatoT, KrajewskiS, KochelK, IrieS, et al. (1995) Cloning and functional-anlaysis of BAG-1 - A novel BCL-2-binding protein with anti-cell death activity. Cell 80 : 279–284.
70. DoukhaninaEV, ChenS, van der ZalmE, GodzikA, ReedJ, et al. (2006) Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem 281 : 18793–18801.
71. KangCH, JungWY, KangYH, KimJY, KimDG, et al. (2006) AtBAG6, a novel calmodulin-binding protein, induces programmed cell death in yeast and plants. Cell Death Differ 13 : 84–95.
72. SunL, LuSJ, ZhangSS, ZhouSF, SunL, et al. (2013) The lumen-facing domain is important for the biological function and organelle-to-organelle movement of bZIP28 during ER Stress in Arabidopsis. Mol Plant 6 : 1605–1615.
73. SrivastavaR, DengY, ShahS, RaoAG, HowellSH (2013) BINDING PROTEIN is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell 25 : 1416–1429.
74. GardnerBM, WalterP (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333 : 1891–1894.
75. KimYS, KimSG, ParkJE, ParkHY, LimMH, et al. (2006) A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell 18 : 3132–3144.
76. KimSG, LeeAK, YoonHK, ParkCM (2008) A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. Plant J 55 : 77–88.
77. NgS, IvanovaA, DuncanO, LawSR, Van AkenO, et al. (2013) A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25 : 3450–3471.
78. De ClercqI, VermeirssenV, Van AkenO, VandepoeleK, MurchaMW, et al. (2013) The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 25 : 3472–3490.
79. LiP, WindJJ, ShiX, ZhangH, HansonJ, et al. (2011) Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc Natl Acad Sci USA 108 : 3436–3441.
80. ZhangC, ChenX, ZhuRM, ZhangY, YuT, et al. (2012) Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxi Lett 212 : 229–240.
81. KleinP, SeidelT, StockerB, DietzKJ (2012) The membrane-tethered transcription factor ANAC089 serves as redox-dependent suppressor of stromal ascorbate peroxidase gene expression. Front Plant Sci 3 : 247–247.
82. LinJH, LiH, YasumuraD, CohenHR, ZhangC, et al. (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318 : 944–949.
83. HowellSH (2013) Endoplasmic reticulum stress responses in plants. Annual Rev Plant Biol 64 : 477–499.
84. QiangX, ZechmannB, ReitzMU, KogelK-H, SchaeferP (2012) The mutualistic fungus piriformospora indica colonizes Arabidopsis roots by inducing an endoplasmic reticulum stress-triggered caspase-dependent cell death. Plant Cell 24 : 794–809.
85. LiuY, BurgosJS, DengY, SrivastavaR, HowellSH, et al. (2012) Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 24 : 4635–4651.
86. SunL, YangZT, SongZT, WangMJ, LuSJ, et al. (2013) The plant-specific transcription factor NAC103 is induced by bZIP60 through a new cis-regulatory element to modulate the unfolded protein response in Arabidopsis. Plant J 76 : 274–286.
87. LiuJX, SrivastavaR, HowellSH (2008) Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Plant Cell Environ 31 : 1735–1743.
88. IwataY, FedoroffNV, KoizumiN (2008) Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20 : 3107–3121.
89. HellensRP, AllanAC, FrielEN, BolithoK, GraftonK, et al. (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1 : 13.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



