-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
Drosophila mating behaviors serve as an attractive model to understand how external sensory cues are detected and used to generate appropriate behavioral responses. Pheromones present on the cuticle of Drosophila have important roles in stimulating male courtship toward females and inhibiting male courtship directed at other males. Recently, stimulatory pheromones emitted by females and inhibitory pheromones emitted by males have been shown to stimulate distinct subsets of gustatory neurons on the legs. We have previously shown that a DEG/ENaC ion channel subunit, ppk25, is involved in male courtship toward females but not in inhibition of male-male courtship. Here we show that ppk25 is specifically expressed and functions in a subset of gustatory neurons that mediate physiological and behavioral responses to female-specific stimulatory pheromones. Furthermore, ppk25 is also required for the function of those neurons to activate male courtship in response to other pheromones that are not female-specific. In addition to their roles in males, we find that ppk25, and the related DEG/ENaC subunits ppk23 and ppk29, also stimulate female mating behavior. In conclusion, these results show that, in both sexes, ppk25 functions in a group of neurons with a specialized role in stimulating mating behaviors.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004238
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004238Summary
Drosophila mating behaviors serve as an attractive model to understand how external sensory cues are detected and used to generate appropriate behavioral responses. Pheromones present on the cuticle of Drosophila have important roles in stimulating male courtship toward females and inhibiting male courtship directed at other males. Recently, stimulatory pheromones emitted by females and inhibitory pheromones emitted by males have been shown to stimulate distinct subsets of gustatory neurons on the legs. We have previously shown that a DEG/ENaC ion channel subunit, ppk25, is involved in male courtship toward females but not in inhibition of male-male courtship. Here we show that ppk25 is specifically expressed and functions in a subset of gustatory neurons that mediate physiological and behavioral responses to female-specific stimulatory pheromones. Furthermore, ppk25 is also required for the function of those neurons to activate male courtship in response to other pheromones that are not female-specific. In addition to their roles in males, we find that ppk25, and the related DEG/ENaC subunits ppk23 and ppk29, also stimulate female mating behavior. In conclusion, these results show that, in both sexes, ppk25 functions in a group of neurons with a specialized role in stimulating mating behaviors.
Introduction
Ever since the identification of the first pheromone, Bombykol, as the sexual attractant of the silkmoth more than fifty years ago [1], the mechanisms underlying the detection of pheromones and their regulation of animal behavior have been an important area of inquiry. The expanding understanding of the molecular basis of pheromone detection has been aided by studies in Drosophila melanogaster, a species in which males perform a series of highly stereotyped behaviors toward females eventually culminating in mating [2], [3]. A number of pheromones that modulate male courtship have been identified [4], [5]. 7,11-heptacosadiene (7,11 - HD) and 7,11-nonacosadiene (7,11-ND) are the major excitatory compounds selectively produced by mature females, while 7-Tricosene (7-T) and the volatile cis-Vaccenyl acetate (cVA) are produced by mature males and inhibit male-male courtship. While olfaction is involved in the inhibition of courtship by cVA (reviewed in [6]) and stimulation of courtship by unknown fly odors [7], as well as by food odors [8], most known Drosophila pheromones are low volatility cuticular hydrocarbons believed to be detected by direct contact with gustatory organs [9].
Recently, several laboratories have independently reported that three members of the DEG/ENaC family of ion channel subunits, ppk25, ppk23 and ppk29, are required for the gustatory detection of pheromones that modulate male courtship behavior [10]–[14]. ppk23 expression marks a subset of gustatory neurons, two per chemosensory hair, both of which also express fruitless (fru), a key transcription factor that regulates sexually dimorphic development of neurons involved in sex-specific behaviors (reviewed in [15]). Importantly, ppk23-expressing cells respond to pheromones [13], [14], and the two cells present in a single chemosensory hair detect distinct compounds [13]. F cells (female-sensing) respond to female stimulatory pheromones, while M cells (male-sensing) respond to inhibitory male pheromones. ppk25 is also required for courtship behavior, but unlike ppk23, ppk25 is only expressed in one fru-positive gustatory neuron per chemosensory bristle. Furthermore, while loss of ppk23 function decreases courtship of females and also increases courtship directed at other males [13], [14], mutations in ppk25 decrease male courtship of females, but do not increase courtship of other males [10], [16]. Together, these results raised the possibility that ppk25 neurons represent a functionally specialized subset of pheromone-sensing neurons [17].
Here, we show that ppk25 specifically marks the F cell subset of ppk23-expressing cells and is required for their response to stimulatory female-specific pheromones. In contrast, ppk23-expressing M cells do not express ppk25 or require ppk25 function to detect inhibitory male pheromones. Furthermore, we show that the function of ppk25 is not restricted to gustatory detection of female-specific pheromones. ppk25 and ppk25-expressing neurons are also required for stimulation of courtship by pheromones present on immature males [18], and by pheromones present on males of the Tai2 strain [19]. Finally, we show that in addition to regulating male courtship behavior, ppk25 and ppk25-expressing gustatory neurons also regulate female mating behavior, suggesting their involvement in detection of male pheromones that stimulate female receptivity.
Results
Sensory neurons expressing ppk25 detect female pheromones that stimulate male courtship
To evaluate the ligand specificity of ppk25 cells, expression of the genetically-encoded calcium indicator, G-CaMP3 [20], was targeted using the ppk25-Gal4 driver [10]. Single bristles on the front legs of both males and females were stimulated with two female pheromones that had been previously shown to stimulate the F (female-sensing) subset of ppk23-expressing cells (7,11-HD and 7,11-ND), and three compounds produced by males that stimulate M (male-sensing) cells (7-tricosene (7T), 7-Pentacosene (7P) and cVA) [13]. As shown previously for F cells [13], ppk25-expressing cells in both males and females showed robust calcium responses to the female pheromones, 7,11-HD and 7,11-ND, but not to the male compounds, 7T, 7P or cVA (Fig. 1A). Importantly, this response requires ppk25, as ppk25 null mutants no longer responded to the female cues and targeted expression of ppk25 in mutants rescued this defect (Fig. 1A). To confirm that ppk25 is required in cells that detect female pheromones but not in those that detect male pheromones, ppk23-Gal4 was used to drive expression of G-CaMP3 in all pheromone-sensing cells in a ppk25 null mutant background. As described previously for flies with normal ppk25 [13], ppk23-Gal4 labeled two cells under each bristle in ppk25 mutants. However, while one of these cells responded specifically to male compounds as previously described for M cells [13], the second cell, did not respond to female compounds as expected of F cells (Fig. 1B). Thus, ppk25 is essential for the recognition of courtship-stimulating pheromones produced by females but not of courtship-inhibiting pheromones produced by males.
Fig. 1. Calcium imaging reveals that ppk25 cells respond specifically to female pheromones. 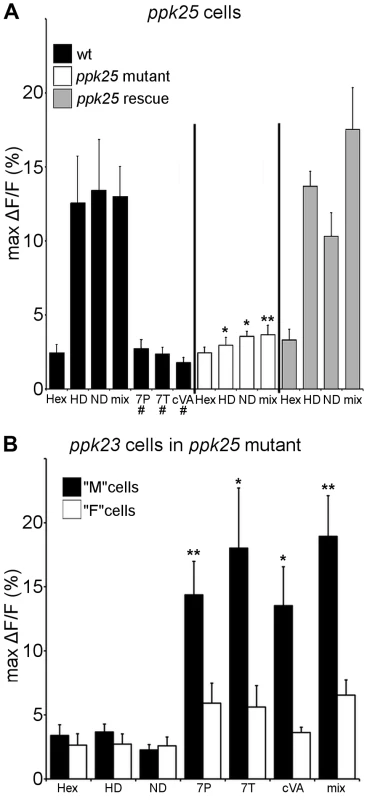
A. Solutions (100 ng/µl in 10%hexane, 90% water) of 7,11-HD (HD), 7,11-ND (ND), 7T, cVA, a mixture of all pheromones (mix) or 10% hexane, 90% water solution alone (hex) were applied to single leg bristles of ppk25-Gal4, 20×UAS-GCaMP3 flies. “wt” flies contained one copy of the normal ppk25 gene. ppk25 null mutants were heterozygous for two different deletions of the ppk25 locus [16], and “ppk25 rescue” flies are ppk25 mutants carrying UAS-ppk25 and ppk25-Gal4 transgenes to target ppk25 expression to ppk25 cells [10]. “#” denotes pheromones that did not elicit responses significantly higher than hexane alone in “wt” flies and were not tested further. n = 7–10; Mean ± SEM; ttest to wt, *p<0.05, **p<0.01. B. The same pheromone solutions as in A were applied to single leg bristles of ppk25 mutants carrying the ppk23-Gal4 and 20×UAS-GCaMP3 transgenes. As previously observed in flies with normal ppk25 genes [13], one population of ppk23 cells, the M cells, respond specifically to male pheromones. In contrast to wt males however, in ppk25 null mutants the second population of ppk23 cells, corresponding to F cells does not respond to any pheromone. n = 8; Mean ± SEM; ttest to wt,*p<0.05,**p<0.01. To test whether ppk25 is essential for behavioral responses to individual pheromones, control and ppk25 mutant males were paired with oenocyte-lacking (oe-) flies painted with single cuticular hydrocarbons in courtship assays. In oe - flies, the pheromone-producing cells called oenocytes have been genetically ablated, such that only residual hydrocarbons are present on their cuticle. oe - individuals therefore serve as pheromone-blank flies to which a single synthetic pheromone can be added to test its effect on male courtship [21]. For better comparison with previous studies on the effects of ppk23 mutations [13], the number of wing extensions in a 20-minute observation period was used as a measure of male courtship. As observed previously [13], painting oe - females with 7,11 HD, the excitatory female-specific pheromone, increased the levels of courtship displayed by control males. In contrast, ppk25 mutant males were not affected by the presence of 7,11 HD, and this stimulatory effect was restored by targeted expression of ppk25 (Fig. 2A). In contrast to the inability of ppk25 mutants to detect stimulatory female pheromones, painting oe - males with 7-T inhibited wing extensions of ppk25 mutant and normal males to similar extents (Fig. 2B). Thus, ppk25 mutant males respond to male cuticular hydrocarbons but behave as though they are selectively blind to female cuticular hydrocarbons. These data demonstrate that ppk25 is specifically required for the stimulatory effect of 7,11 HD, the major female taste pheromone, but is not required for detection of the major male inhibitory taste pheromone, 7T.
Fig. 2. ppk25 is required for courtship stimulation by 7,11HD but not for inhibition of courtship by 7-T. 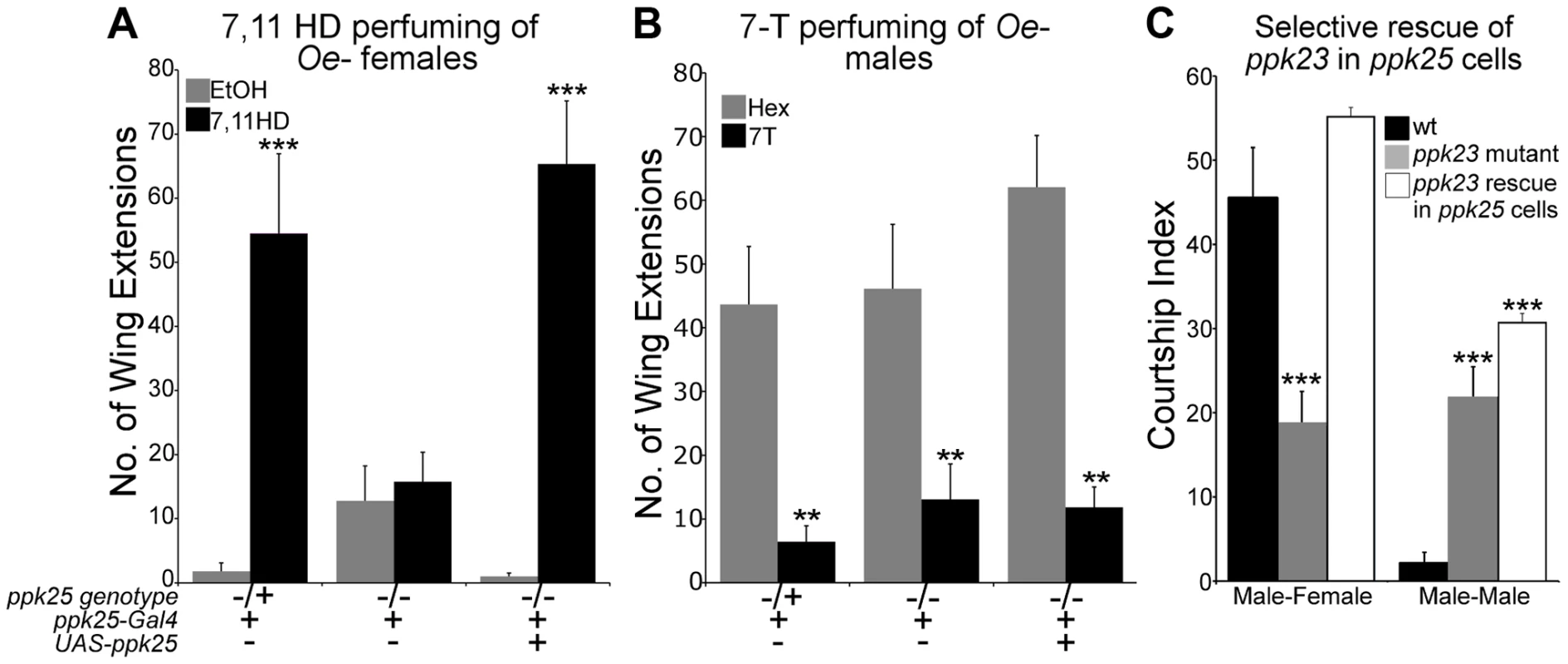
A. The number of wing extensions performed by males with normal or mutant copies of ppk25, or by ppk25 mutant males in which ppk25 function is restored specifically in ppk25 cells, was measured in the presence of oe- females painted either with solvent alone or with a single female pheromone, 7,11HD. oe- females were pierced through the head with forceps, contributing to the low courtship background. These and all following assays involving perfumed oe- targets were conducted under normal laboratory lights [13]. n = 19–24; Mean ± SEM; ***p<0.001 (perfumed relative to solvent control). Error bars indicate the SEM for number of wing extensions and statistical significance was determined using the Kruskal-Wallis test followed by Dunn's post hoc test. B. The number of wing extensions displayed by males with normal or mutant ppk25 was measured in the presence of oe- males painted either with solvent alone or with a single male pheromone, 7T. In this experiment, males were raised in groups as this resulted in higher baseline courtship toward oe- targets, thereby allowing inhibition to be measured. n = 24–28; Mean ± SEM; **p<0.01; (Kruskal-Wallis test followed by Dunn's post hoc test to solvent control). C. The Courtship Index (CI, percentage of a ten minute observation time during which the male is courting [16]) was measured for control w1118 males, males mutant for ppk23, and ppk23 mutant males in which expression of ppk23 is targeted to F cells using the ppk25-Gal4 driver. Male-female courtship was measured in the presence of decapitated w1118 females to reduce behavioral feedback. Male-male courtship was performed with intact male targets in the light since ppk23 mutants display robust male-male courtship under these conditions [13], [14]. n = 30–40; Mean ±SEM; ***p<0.001 to wt. Error bars indicate the SEM for CI and statistical significance was determined using the Kruskal-Wallis test followed by Dunn's post hoc test. In addition to decreasing male courtship toward females, loss of ppk23 also increases male courtship toward other males and both phenotypes are efficiently rescued by expressing ppk23 in ppk23-Gal4 cells [13], [14]. To test whether the ppk25-positive cells are involved in both courtship defects of ppk23 mutants, we rescued ppk23 function exclusively in ppk25-Gal4 cells in a ppk23 mutant background. Male flies expressing functional ppk23 only in ppk25 cells showed normal courtship toward females but, like ppk23 mutant males, displayed abnormally high levels of male-male courtship as measured using the courtship index (CI, the percentage of a ten minute observation time during which the male is courting [16]) (Fig. 2C). These experiments show that ppk25 cells are required for normal recognition of female-specific pheromones and for their stimulatory effects on male courtship whereas the ppk23-positive, ppk25-negative cells are required for recognition of inhibitory male pheromones.
ppk25 is required for detection of courtship-stimulating pheromones present in young males and Tai2 males
Given the requirement of ppk25 for detection of female stimulatory pheromones but not male inhibitory pheromones, we investigated whether ppk25 might also be required for detection of stimulatory pheromones that are not specific to the female. Immature Drosophila of either sex, while lacking adult female-specific pheromones, efficiently elicit courtship from adult males, most likely as a result of the unusual long-chain hydrocarbons present on their cuticle [18]. We therefore tested whether ppk25 is needed for male courtship of immature males (Fig. 3). As expected, males with one functional copy of ppk25 showed strong courtship toward young males. In contrast, ppk25 homozygous mutant males performed very little courtship as measured by either CI or fraction of males initiating courtship, but courtship was restored in ppk25 mutants when ppk25 expression was driven with the ppk25-Gal4 driver (Fig. 3A). As shown previously [10], mutations in ppk25 do not cause a generalized defect in behavior since the Total Behavior Index (TBI - the fraction of the observation time the male spends performing any observable behavior: courtship, walking or preening) was not affected in ppk25 mutants (Fig. 3A).
Fig. 3. Male courtship of immature males requires ppk25 function. 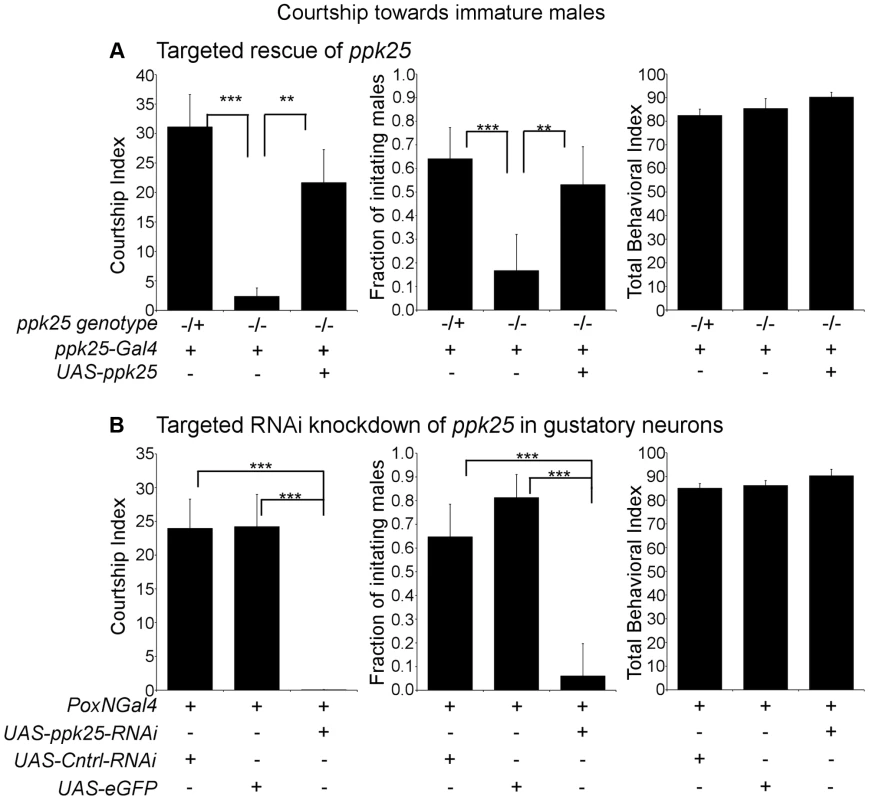
A. ppk25 expression in gustatory neurons defined by ppk25-Gal4 is required for male courtship of immature males. CI, fraction of males initiating courtship toward decapitated immature males and Total Behavioral Index (TBI) were calculated for ppk25 mutant males, control males with one wt copy of ppk25 and for ppk25 null mutant males where ppk25 expression has been restored in cells expressing ppk25-Gal4. N = 32–39; Mean ± SEM; ***p<0.001; **p<0.01 (Error bars indicate the SEM for the fraction of males initiating courtship and statistical significance was determined by Fisher's exact test. Error bars and statistical significance for TBI was determined as described previously for CI). Newly eclosed Canton-S males with light body pigmentation and unfurled wings were used as immature male targets. B. Targeted RNAi knockdown of ppk25 in gustatory neurons with Poxn-Gal4 reduces courtship toward Tai2 males. CI, fraction of males initiating courtship toward decapitated Tai2 males and TBI were calculated for males expressing ppk25 RNAi in gustatory neurons. Control males expressed eGFP or a control RNAi targeting CG13895, a gene with no known involvement in mating behavior [58] in all gustatory neuron. N = 32–34; Mean ± SEM; ***p<0.001. To further test the role of ppk25 in courtship of immature males and to identify the type of cell involved, we knocked-down ppk25 mRNA using targeted expression of a UAS-ppk25-RNAi transgene [10] with Poxn-Gal4, a driver expressed specifically and at high levels in all gustatory neurons [22]. In control males, Poxn-Gal4 drove expression of either GFP, or of an RNAi that targets an unrelated gene (CG13895). Compared to control males, males with gustatory neuron-specific knockdown of ppk25 courted young males at significantly reduced levels, as reflected by a reduction in both CI and fraction of initiating males, while the TBI remained unchanged (Fig. 3B). Together, these results indicate that ppk25 function in gustatory neurons marked by expression of ppk25-Gal4, is required for activation of courtship not only by female-specific pheromones [10], but also by pheromones present on immature males.
In addition to females and immature males, males of the naturally occurring Tai2 strain of Drosophila melanogaster also stimulate male courtship, likely as a result of their unusual pheromone profile [19], [23]. As expected, control males displayed high levels of male-male courtship when paired with Tai2 males (Fig. 4A). In contrast, courtship of Tai2 males was largely eliminated in ppk25 mutant males and rescued by targeted expression of ppk25 using ppk25-Gal4 (Fig. 4A). Finally, knockdown of ppk25 in all gustatory neurons using Poxn-Gal4-driven RNAi results in a similarly severe decrease in male courtship of Tai2 males (Fig. 4B). Together, these results show that courtship stimulation by Tai2 males requires ppk25 in the subset of gustatory neurons defined by expression of ppk25-Gal4.
Fig. 4. ppk25 function in gustatory neurons is required for courtship stimulation by Tai2 males or 7-pentacosene. 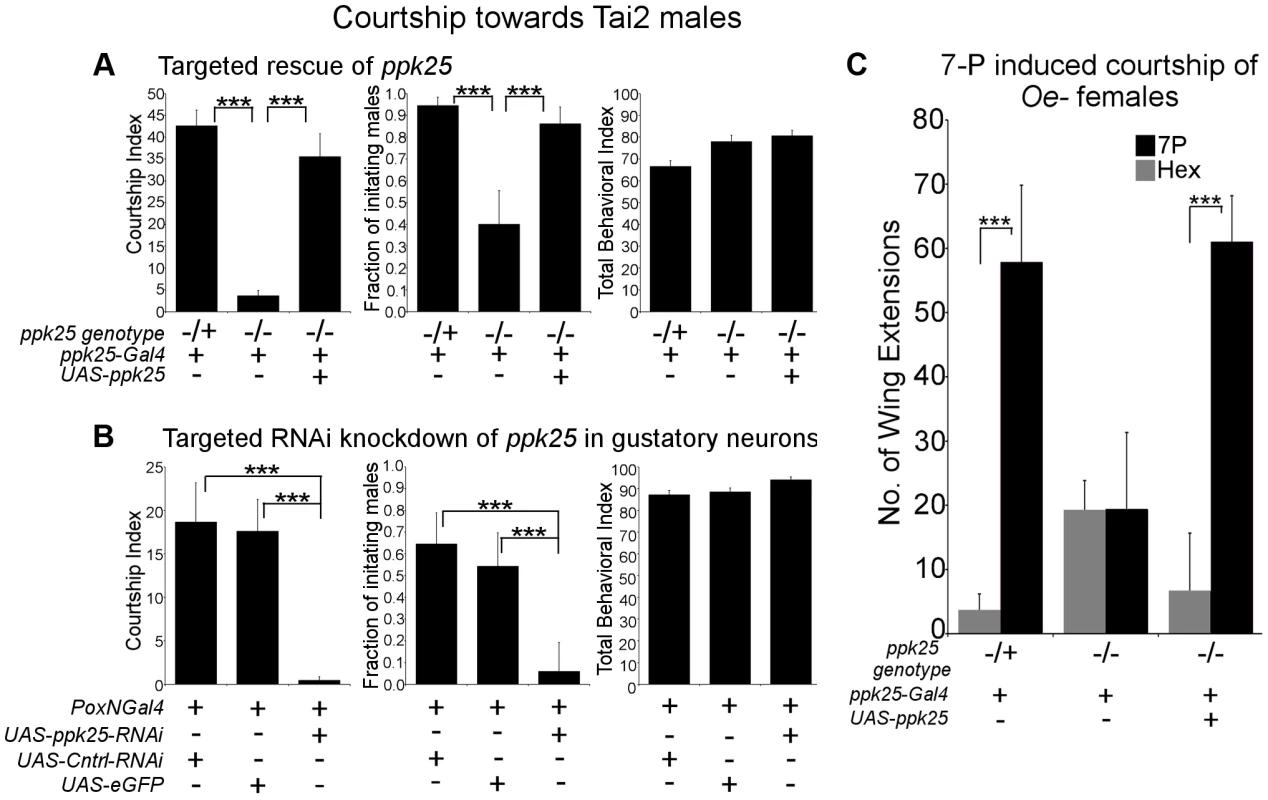
A. ppk25 function in cells defined by ppk25-Gal4 expression is required for male courtship directed at Tai2 males. CI, fraction of males initiating courtship toward decapitated Tai2 males and TBI were calculated for ppk25 null mutant males, control males with one wild-type copy of ppk25, and ppk25 mutant males where ppk25 expression had been restored in ppk25 cells. N = 36–40; Mean ± SEM; ***p<0.001. B. Targeted RNAi knockdown of ppk25 in all gustatory neurons reduces courtship toward Tai2 males. CI, fraction of males initiating courtship toward decapitated Tai2 males and TBI were calculated for males expressing ppk25 RNAi in gustatory neurons using Poxn-Gal4. Control males expressed eGFP or a control RNAi targeting CG13895 under control of Poxn-Gal4. Error bars are SEM; N = 31–34; ***p<0.001. C. The number of wing extensions performed by males with normal or mutant copies of ppk25, or by ppk25 mutant males in which ppk25 function is restored in ppk25 cells, was measured in the presence of oe- females painted either with solvent alone or with 7-Pentacosene. oe- females were pierced through the head with forceps, contributing to the low courtship background and assays were conducted in the light. n = 20–24; Mean ± SEM; ***p<0.001. One of the significant differences in the pheromone profile of Tai2 males is the elevated level of 7-Pentacosene (7P) [19], [23], which can stimulate male courtship behavior [24] and may therefore underlie courtship stimulation by Tai2 males. We therefore tested whether ppk25 is required for the stimulation of courtship by 7-P. 7-P perfuming of oe - females resulted in a significant increase in the wing extensions for control males, but not for males lacking functional ppk25, and the stimulatory effect of 7-P was restored in ppk25 mutants by targeted expression of ppk25 with ppk25-Gal4 (Fig. 4C). These results show that, as is the case for 7,11-HD (Fig. 2), the stimulatory effect of 7-P on male courtship requires ppk25 function in ppk25-Gal4 cells.
Thus, in addition to functioning in the detection of female-specific pheromones that stimulate male courtship, ppk25 and ppk25-expressing neurons are important for detecting at least two other types of excitatory pheromonal cues that promote courtship: pheromones emitted by immature Drosophila, and 7-P, likely accounting for its requirement in the courtship of Tai2 males. In contrast, and unlike ppk23, ppk25 is not required for detection of the major male inhibitory pheromone, 7T, indicating that ppk25-expressing neurons represent a subset of pheromone-sensing neurons specialized in detecting pheromones that stimulate male courtship behavior.
ppk25 is involved in female receptivity
Since ppk25 is required for the detection of a variety of stimulatory pheromones in males, we considered the possibility that it may also be involved in regulating female behavior. Indeed, expression of reporters under the control of ppk25-Gal4 [10], as well as ppk23-Gal4 and ppk29-Gal4 [11]–[14] is seen in chemosensory neurons of females. Furthermore, chemical senses, in addition to vision and hearing, likely control female receptivity to mating [25]–[27]. To test the role of DEG/ENaC channels in regulating female receptivity, we paired wild-type (Canton-S) males with control females or females that were homozygous mutant for ppk23, ppk25 or ppk29, and used the fraction of females that mated within thirty minutes as a measure of female receptivity [28]. Females with homozygous mutations in ppk25, ppk23 or ppk29 displayed showed similar levels of receptivity as control females (Fig. 5A). However, we considered the possibility that the apparent lack of requirement for ppk25, ppk23 and ppk29 in female receptivity observed under our experimental conditions could result from the redundant action of multiple sensory cues. Indeed, olfactory and/or acoustic signals detected by the antennae play a crucial role in controlling female receptivity [25], [26], [29], [30]. We therefore tested the effect of ppk mutations on the receptivity of females whose antennae had been inactivated by surgical removal of the third antennal segment. Even after antennal inactivation, more than 60% of control females mated within 30 minutes. In sharp contrast, females that were homozygous mutant for ppk25, ppk29 or ppk23 displayed little, if any, receptivity (Fig. 5B). In order to further explore the redundant antennal cues driving receptivity, we looked at the effect of ppk25 and ppk23 mutations on the receptivity of females that were only lacking the aristae rather than the whole antennae, thereby impairing auditory function but leaving olfactory function intact [31]. Here again, we find that while the receptivity of control females remains high after removal of the arista, mutations in ppk23 and ppk25 significantly decrease receptivity in aristaless females (Fig. 5C). These data suggest that acoustic stimuli are sufficient to promote high levels of female receptivity in the absence of functional ppk25 or ppk23. The effect of ppk25 inactivation on mating does not result from a reduced sexual attractiveness of the mutant females, as levels of male courtship during these female receptivity assays are similar in the presence of control and ppk25 mutant females (Fig. 5D, left panel). To further rule out any possible effect of the female genotype on male behavior, we conducted male courtship assays using decapitated ppk25 mutant females and controls, thereby reducing behavioral feedback from females. In these assays, males courted both sets of females with equal intensity (Fig. 5D, right panel).
Fig. 5. Antenna-less females mutant for ppk25, ppk23 or ppk29 are unreceptive to males. 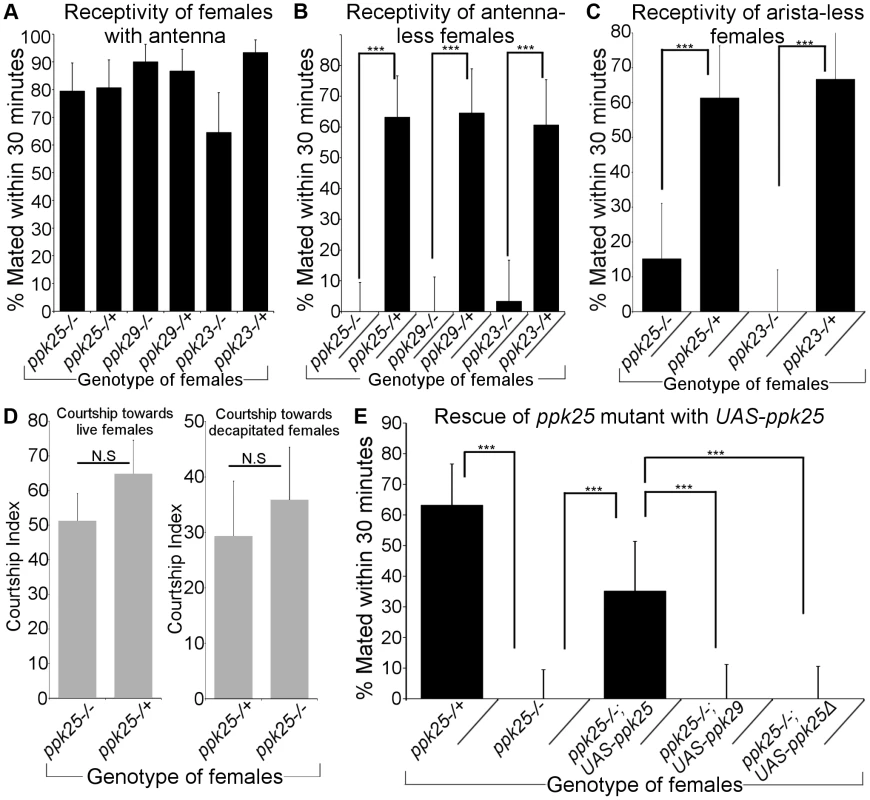
A. The percentage of females that mated within 30(“Receptivity”) was calculated for females with homozygous null mutations in ppk25, ppk23 or ppk29 [11], [13], [16], and for control females with one normal copy of ppk25, ppk23 and ppk29. N = 30–34. For receptivity in Fig. 5 and 6, error bars indicate the SEM. B. Antenna-less females mutant for ppk25, ppk23 or ppk29 show reduced receptivity to males. Receptivity was measured for mutants and control females as in A but after removal of antennae. N = 30–38; ***p<0.001. (Fisher's exact test). C. Arista-less females mutant for ppk23 or ppk25 show reduced receptivity to males. Receptivity was measured for mutants and control females as in A but after removal of arista. N = 21–32; ***p<0.001. (Fisher's exact test). D. wt males find ppk25 mutant and control females equally attractive. CI measured for Canton-S males toward females that were either alive (left) or decapitated (right). CI of males toward live females was quantified by measuring male courtship behavior during the first 10 minutes of the receptivity assay shown in Fig. 5B. Females were either homozygous null mutants for ppk25 or contained one normal copy of ppk25. N = 20. E. Introduction of a ppk25 transgene increases receptivity of ppk25 mutants but not of ppk29 mutants. Receptivity was measured for antenna-less females carrying an UAS-ppk25 transgene in either a ppk25 mutant or ppk29 mutant background and compared to ppk25 mutant and controls shown earlier (panel A). N = 30–38; ***p<0.001 (Fisher's exact test). Surprisingly, given that the UAS-ppk25 transgene by itself does not rescue the courtship defect of ppk25 mutant males [10], we find that the presence of UAS-ppk25 significantly increases the receptivity of ppk25 mutant females (Fig. 5E). This observation suggests that leaky expression from UAS-ppk25, as seen with other UAS transgenes [11], [32], [33], is sufficient to partially rescue ppk25 function in females, further supporting the role of ppk25 in female receptivity. Indeed, a similar transgene with a truncated version of ppk25, UAS-ppk25Δ [10], or a UAS-ppk29 rescue transgene [11] do not increase the receptivity of ppk25 mutant females (Fig. 5E), and the UAS-ppk25 transgene does not increase the receptivity of ppk29 mutant females (data not shown). Taken together, these results suggest that all three DEG/ENaC genes play critical roles in female receptivity that can be obscured by the presence of redundant sensory inputs from the antennae.
Since in males, ppk25 and ppk23 function within defined subsets of gustatory neurons [10]–[14], we sought to clarify the relationships between the expression of these ppks in female gustatory neurons, and to test their function in regulating female receptivity. Expression of ppk25 and ppk23 in females was visualized by driving expression of GFP using ppk25-Gal4 and ppk23-Gal4. Consistent with the sexual dimorphism in the total number of taste hairs on the front legs [34] as well as in the number of ppk23-expressing taste hairs [12], [13], there are fewer ppk25-expressing hairs on the front legs of females compared to males (data not shown). As seen in males [12]–[14], ppk23-positive hairs have two fru-positive chemosensory neurons labeled by fru-LexA-driven expression of Red Fluorescent Protein (RFP), both of which also express GFP under the control of ppk23-Gal4 (Fig. 6A). In contrast, ppk25-Gal4-driven expression of GFP co-localizes with only one of the two fru-positive cells within any particular taste hair (Fig. 6B). Therefore, as in males, ppk23-Gal4 is expressed in each of the two fru-positive taste neurons present in a subset of chemosensory bristles, one of which also expresses ppk25-Gal4.
Fig. 6. Female ppk25-Gal4 neurons represent a subset of ppk23-Gal4 neurons and are involved in female receptivity. 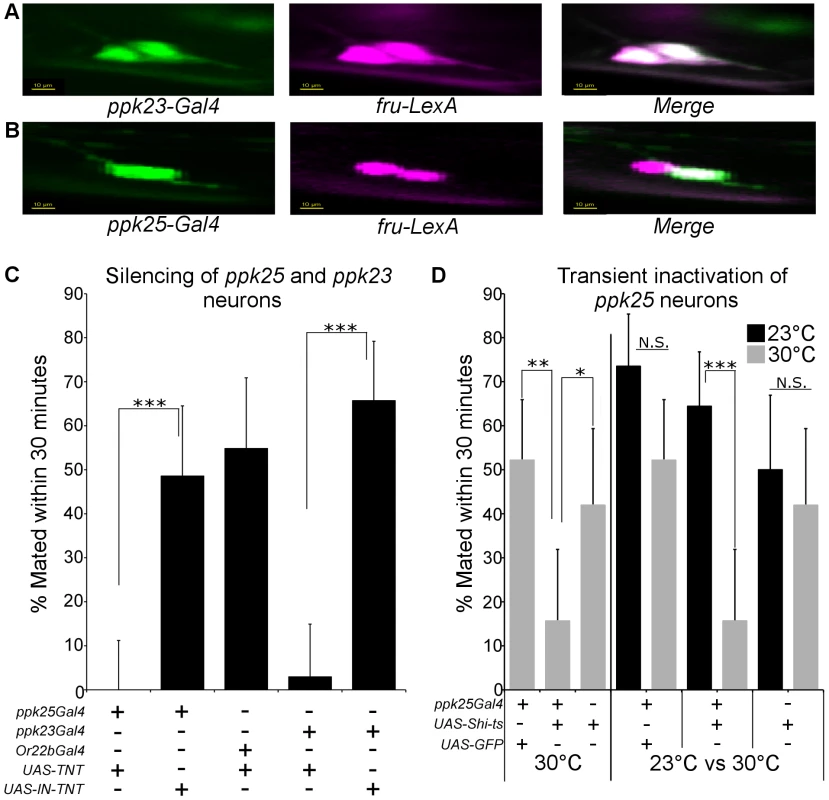
A. Segment of female front legs showing expression of ppk23-Gal4 (green) in each of the two fru-positive cells (fru-LexA, magenta) under each chemosensory bristle. Scale 10 µm. B. Segment of female front legs showing expression of ppk25-Gal4 (green) in only one of the two fru-positive cells (fru-LexA, magenta) present under each chemosensory bristle. Scale 10 µm. C. Silencing of ppk25-Gal4 or ppk23-Gal4 neurons reduces female receptivity. Receptivity was calculated for females expressing active (UAS-TNT) or inactive (UAS-IN-TNT) forms of TNT under control of either ppk23-Gal4 or ppk25-Gal4. Additional control females contained UAS-TNT driven by Or22b-Gal4, which is expressed in a neuronal subset not associated with mating behaviors [36]. N = 30–35; ***p<0.001 (Fisher's exact test). D. Transient inactivation of ppk25-Gal4 neurons inhibits female receptivity. Females expressing the temperature-sensitive dominant Dynamin allele shits [37] under control of ppk25-Gal4 were incubated at either permissive (23°C) or non-permissive (30°C) temperature for 20 minutes prior to, and during the test. Receptivity for females expressing GFP in ppk25-Gal4 neurons or females with UAS-Shi(ts) alone was measured under identical conditions. N = 30–45; ***p<0.001;**p<0.01;*p<0.05 (Fisher's exact test). To test the function of neurons expressing ppk25 or ppk23, we used the same drivers to target expression of tetanus toxin (TNT), a synaptic transmission blocker [35]. For both ppk25-Gal4 and ppk23-Gal4, targeted expression of TNT, but not of an inactive variant (IN-TNT), resulted in a severe loss of receptivity. (Fig. 6C). In contrast, expression of TNT within Or22b-expressing olfactory neurons that have no known role in taste or pheromone reception [36], had no effect. To discriminate between developmental and acute requirements of neuronal function, we expressed the temperature-sensitive version of Drosophila dynamin, shibire (Shits) [37] in ppk25-Gal4 neurons and shifted females to the restrictive temperature of 30°C a few minutes before and during the receptivity assay. When assayed at 30°C, females expressing Shits in ppk25-Gal4 neurons were significantly less receptive than females expressing GFP in the same neurons or females carrying UAS-Shits but lacking the Gal4 driver (Fig. 6D). Furthermore, ppk25-Gal4>UAS-Shits females placed at the permissive temperature of 23°C had significantly higher receptivity compared to genetically identical females maintained at the non-permissive temperature (Fig. 6D). Together, these data suggest that the cells identified by ppk25 - and ppk23-Gal4 expression are directly involved in female receptivity to mating, most likely in response to male pheromones.
Discussion
Expression and function of ppk25 define a subset of gustatory neurons that stimulate male courtship in response to a variety of pheromones
GCaMP-based calcium imaging of ppk23-expressing, pheromone-sensing gustatory neurons identified two types of neurons with different response specificities: F cells respond to female-specific pheromones that stimulate male courtship, while M cells respond to pheromones enriched in males that inhibit male courtship [13]. Here, we present several lines of evidence indicating that ppk25 expression specifically labels F cells. First, GCaMP imaging shows that ppk25-expressing cells respond to female-specific stimulatory pheromones but not to male inhibitory pheromones. Second, in ppk25 mutants, F cells lose their response to pheromones while the response of M cells is unchanged. Finally, behavioral responses to 7,11HD, a female-specific pheromone detected by F cells, requires ppk25 function in ppk25-Gal4 neurons, while behavioral response to 7T, a male inhibitory pheromone detected by M cells, does not. These findings are also consistent with previous results showing that ppk25 is required for stimulation of male courtship by females but not for inhibition of male-male courtship [10]. Together, these observations identify a functionally specialized group of gustatory neurons defined by ppk25 function and expression that detects female-specific pheromones and mediates their stimulatory effect on male courtship. Furthermore, mutations in ppk23 or silencing of ppk23 neurons result in loss of both the physiological and behavioral effects of male inhibitory pheromones [13], [14], while mutations in ppk25 or silencing of ppk25 neurons do not ([10] and this report). Consistent with those observations, we find that ppk23 mutant males in which ppk23 function is rescued specifically in ppk25 neurons have normal responses to females but still display increased courtship of other males. Together, these data suggest that pheromone-sensing neurons fall into two functionally complementary types. F cells are defined by expression of ppk25, respond to female-specific pheromones, and mediate the stimulatory effects of those pheromones on courtship. In contrast, M cells express ppk23 but not ppk25, respond to male pheromones, and mediate the inhibitory effects of those pheromones on courtship.
In addition to female-specific pheromones, pheromones found on immature Drosophila [18] as well as 7-Pentacosene [24] can activate male courtship. Both targeted rescue and knockdown experiments demonstrate that the activation of courtship by these pheromones or pheromone blends requires functional ppk25 within the subset of gustatory neurons that also mediate responses to adult female pheromones ([10] and this report). The requirement of ppk25 cells for courtship activation by 7P was surprising given that, in both males or females, while 7P elicits a response from cells that respond to male inhibitory pheromones ([13] and this report), ppk25 cells display no detectable response to this hydrocarbon (Fig. 1). Compared to their responses to female-specific pheromones, ppk25 cells may respond to 7P with either lower intensity or slower kinetics, preventing detection in our GCaMP assay. Indeed, the physiological responses of olfactory neurons to their cognate olfactory neurons vary in both magnitude and kinetics, neither of which correlates with the strength of the behavioral response [38]. Furthermore, the volatile pheromone cVA was originally shown to strongly activate Or67d neurons [39] but an improved odor testing paradigm revealed that cVA also activates Or65a neurons weakly [40] and subsequent work showed that Or65a neurons are critical for cVA's chronic effects on Drosophila aggression [41]. Finally, while we have tested responses from ppk25-expressing cells at different positions on the front legs, we cannot rule out the possibility that a subset of ppk25 cells, perhaps in a less accessible part of the front leg, or on the second or third pairs of legs, display a detectable GCaMP response to 7P. Alternatively, the lack of GCaMP signal may accurately reflect the fact that ppk25 neurons do not directly detect 7P. By analogy with the integration of olfactory information resulting from non-synaptic interactions between neighboring olfactory neurons [42], 7-P activation of male courtship may require non-synaptic interactions between ppk25 cells and the neighboring M cells which detect 7P [13].
Whether it is through the direct detection of pheromones, or in a more indirect regulatory role, ppk25 neurons are required for the stimulation of courtship by several different pheromones. In contrast, ppk25 neurons are not required for the responses to at least two different courtship-inhibiting pheromones, suggesting that they are functionally specialized in courtship activation. This specialized function is analogous but opposed to that of the previously described but non-overlapping subsets of neurons on the front legs of males that express Gr32a [7], [43], [44] or Gr66a [45], [46] and detect pheromones that inhibit male courtship.
ppk25 gustatory neurons contribute to redundant sensory inputs that control female receptivity
In addition to their roles in pheromonal control of male courtship, we show here that ppk25, ppk23 and ppk29 also play critical roles in regulating female mating behavior. During the Drosophila courtship ritual, females actively assess the courting male by detecting a variety of cues, including male pheromones and appropriate sensory stimulation of the female is required for mating to occur [47]. Here, we show that ppk25, ppk23 and ppk29 are all required for antenna-less Drosophila females to become receptive to mating. Furthermore, as in males, ppk23-Gal4 and ppk25-Gal4 are strongly expressed in gustatory neurons of female legs ([10], [13] and present work). While in addition to its expression in gustatory neurons, ppk25 is also expressed at lower levels in the olfactory system [10], ppk23 and ppk29 are only detectably expressed in gustatory neurons [11]–[14]. Therefore, the dramatic loss of receptivity observed for antenna-less females carrying mutations in any of the three ppks, or whose ppk23- or ppk25-expressing neurons have been silenced suggests that all three ppks function in a common subset of pheromone-sensing gustatory neurons that regulate female receptivity to mating. Finally, while the identity of the pheromone(s) involved remains to be determined, the role of ppk25 in promoting female receptivity is consistent with the involvement of this DEG/ENaC subunit in the responses to multiple pheromones.
These data provide, to the best of our knowledge, the first evidence that female receptivity to mating in Drosophila is regulated by gustatory detection of pheromones. Furthermore, gustatory pheromone detection is at least partially redundant with other sensory stimuli, in particular auditory stimuli, as mutations in ppk genes only affect the receptivity of females whose aristae have been removed. Similar redundancy exists in sensory stimulation of male courtship behavior in Drosophila [9], [16], [44], [48] and, more generally in sensory detection of signals that drive sexual behavior in many other species, with potential evolutionary and ecological advantages [49]. While male pheromones, in particular cVA and 7T, have been shown to regulate female mating receptivity, detection of these two pheromones has been reported to involve olfactory rather than gustatory organs; for cVA through the antennal Or67d olfactory receptor [26] and for 7T through unknown receptors on the antennae [25]. The male pheromone that stimulates female receptivity and is detected by ppk25 gustatory neurons therefore remains to be identified.
In conclusion, we show that a subset of pheromone-sensing neurons, identified by the expression and function of ppk25, have a specialized role in stimulating male courtship and female receptivity in Drosophila. The identification and manipulation of these neurons may lead to a better understanding of how gustatory neural circuits drive not only courtship and mating, but also other Drosophila behaviors that depend on the detection of conspecific pheromones [50].
Finally, these results are relevant to the consideration of two mutually exclusive models for the molecular role of DEG/ENaC channels in pheromone detection [17]. Channel gating may result from direct interaction with pheromones or pheromones-protein complexes. Alternatively, these channels may have an indirect role, modulating the excitability of specific subsets of pheromone-sensing neurons. The existence of multiple pheromones requiring ppk25 function is surprising, given the high specificity of known pheromone receptors [51], [52]. However, the three identified ppk25-dependent pheromones are unsaturated linear hydrocarbons with chain-lengths varying from C25 to C29 with a double-bond at position C7, while ppk25 function is not required for 7-T, another hydrocarbon with a double-bond at C7, but with a shorter chain of 23 carbons. The presence of ppk25 in a heterotrimeric DEG/ENaC could therefore modify channel specificity by requiring a ligand with a longer hydrocarbon chain. However, our results are also compatible with the possibility that ppk25, ppk23 and ppk29 function less directly by modulating the excitability of pheromone-sensing neurons whose ligand specificity is dictated by as yet unidentified pheromone receptors. The specific function of ppk25 in pheromone detection should prove invaluable for dissecting the molecular mechanisms underlying the function of ppk25, ppk23 and ppk29 in pheromone response, with implications for the roles of DEG/ENaCs in a number of other sensory processes [53], [54].
Materials and Methods
Drosophila stocks
Mutations in ppk25, ppk23 and ppk29 were described previously [11], [13], [16]. ppk25 null mutant flies were heterozygous for two different deletions of the ppk25 gene; an imprecise excision of a P-element that removes the regulatory regions and the first half of the ppk25 gene (Δ5–22) and a deletion spanning 20 genes in the ppk25 regions(Δ42E) [16]. ppk29 mutants were homozygous for a transposable element insertion in exon 5 of the ppk29 gene [11]. ppk23 mutants were generated by deletion of an 8.3 kb ppk23-containing region through FLP-FRT-mediated recombination of two piggybac transposons [13]. Targeted expression of ppk23 or ppk25 was achieved using the ppk25-Gal4, UAS-ppk23 and UAS-ppk25 transgenes [10], [13]. The UAS-ppk25 RNAi line was obtained from the Transgenic RNAi Project at Harvard Medical School (stock number JF02434). Poxn-Gal4, fru-LexA, and lexAop-FRT-tdTomato::nls; UAS-stinger lines were gifts from David Mellert and UAS-Shi(ts) was a gift from Kathy Siwicki. All other lines were obtained from the Bloomington Stock Center at Indiana University.
G-CaMP imaging
G-CaMP imaging was performed on single leg chemosensory bristles as described previously [13], except for the use of the 20×UAS-GCAMP3 transgene [55]. Briefly, single bristles were stimulated by bringing them in contact with a custom-built glass capillary filled with ∼5 µL of a solution of synthetic pheromones (Cayman Chemical, Ann Harbor, MI). Pheromones were diluted to 100 ng/µl in 10% hexane∶90% water solution. Calcium-induced fluorescent increases of one or two cells under a single bristle were monitored by spinning disk confocal microscopy. Errors bars represent the SEM and t-tests were used for statistical significance.
Behavioral assays
Flies were raised at 25°C, 50% relative humidity in a 12 h∶12 h light∶dark cycle. For testing male courtship, males were raised individually for 4–8 days post-eclosion unless noted otherwise, while females and males used as courtship targets were raised in groups. In perfuming experiments, synthetic pheromones (Cayman Chemical, Ann Harbor, MI) were applied to oe - males or females lacking cuticular hydrocarbons [21] as described [13]. All assays were conducted under infrared lights and monitored using infrared-sensitive cameras, except perfuming assays with oe - targets and male-male courtship assays, which were performed as previously described [13], [14]. Courtship directed at decapitated females or males with normal cuticular hydrocarbons was measured for 10 minutes and quantitated using the Courtship Index (CI, the fraction of time the male spends performing any courtship behavior ×100 [56]). As a measure of total behavioral activity, we used the Total Behavioral Index (TBI, the fraction of time during which the male courts, walks, or preens ×100 [10]). For better comparison with previous work [13], courtship behavior toward perfumed oe - females and males was measured as the number of times the male extends its wings in a 20-minute observation period. For measures of the number of wing extensions, CI and TBI, error bars indicate the SEM, and the Kruskal-Wallis test followed by Dunn's post hoc test was used to determine statistical significance. For the fraction of males initiating courtship, error bars indicate the SEM, and statistical significance was calculated using Fisher's exact test.
For female receptivity assays, females were aged for 4–8 days before removal of the third antennal segment with fine forceps at least two days before testing. Canton-S males aged 3–6 days were used for all female receptivity assays. Males and females were aspirated into plexiglass chambers, their behavior recorded for 30 minutes in the light and the number of females copulating within 30 minutes was determined. Neuronal inactivation with Shibirets was achieved by placing females in a 30° Celsius room for 20 minutes prior to, and during the 30-minute receptivity assay. Error bars for female receptivity indicate the SEM, and statistical significance was calculated using Fisher's exact test. All behaviors were scored blind and analyzed either manually or using the LIFESONG X software (version 0.8) [57].
Zdroje
1. ButenandtA, BeckmannR, StammD, HeckerE (1959) Concerning the sexual attractant of the silkmoth Bombyx mori. Purification and composition Z Naturforschg 14b: 283–284.
2. DicksonBJ (2008) Wired for sex: the neurobiology of Drosophila mating decisions. Science 322 : 904–909.
3. VillellaA, HallJC (2008) Neurogenetics of courtship and mating in Drosophila. Adv Genet 62 : 67–184.
4. Wicker-ThomasC (2007) Pheromonal communication involved in courtship behavior in Diptera. J Insect Physiol 53 : 1089–1100.
5. YewJ, CodyR, KravitzE (2008) Cuticular hydrocarbon analysis of an awake behaving fly using direct analysis in real-time time-of-flight mass spectrometry. Proc Natl Acad Sci USA 105 (20) 7135–40.
6. VosshallL (2008) Scent of a Fly. Neuron 59 : 685–689.
7. WangL, HanX, MehrenJ, HiroiM, BilleterJ-C, et al. (2011) Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci 14 (6) 757–62.
8. GrosjeanY, RytzR, FarineJ-P, AbuinL, CortotJ, et al. (2011) An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478 : 236–240.
9. KrsticD, BollW, NollM (2009) Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE 4: e4457.
10. StarostinaE, LiuT, VijayanV, ZhengZ, SiwickiKK, et al. (2012) A Drosophila DEG/ENaC Subunit Functions Specifically in Gustatory Neurons Required for Male Courtship Behavior. J Neurosci 32 : 4665–4674.
11. LiuT, StarostinaE, VijayanV, PikielnyCW (2012) Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J Neurosci 32 : 11879–11889.
12. LuB, LamoraA, SunY, WelshMJ, Ben-ShaharY (2012) ppk23-Dependent Chemosensory Functions Contribute to Courtship Behavior in Drosophila melanogaster. PLoS Genet 8: e1002587.
13. ThistleR, CameronP, GhorayshiA, DennisonL, ScottK (2012) Contact Chemoreceptors Mediate Male-Male Repulsion and Male-Female Attraction during Drosophila Courtship. Cell 149 : 1140–1151.
14. TodaH, ZhaoX, DicksonBJ (2012) The Drosophila Female Aphrodisiac Pheromone Activates ppk23+ Sensory Neurons to Elicit Male Courtship Behavior. Cell Reports 1–9.
15. ManoliDS, FanP, FraserEJ, ShahNM (2013) Neural control of sexually dimorphic behaviors. Current Opinion in Neurobiology 23 : 330–338.
16. LinH, MannKJ, StarostinaE, KinserRD, PikielnyCW (2005) A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci USA 102 : 12831–12836.
17. PikielnyCW (2012) Sexy DEG/ENaC channels involved in gustatory detection of fruit fly pheromones. Sci Signal 5: pe48.
18. TompkinsL, HallJC, HallLM (1980) Courtship-stimulating volatile compounds from normal and mutant Drosophila. J Insect Physiol 26 : 689–697.
19. SureauG, FerveurJF (1999) Co-adaptation of pheromone production and behavioural responses in Drosophila melanogaster males. Genet Res 74 : 129–137.
20. TianL, HiresS, MaoT, HuberD, ChiappeM, et al. (2009) Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Meth 6 (12) 875–81.
21. BilleterJ-C, AtallahJ, KruppJJ, MillarJG, LevineJD (2009) Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461 : 987–991.
22. BollW, NollM (2002) The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129 : 5667–5681.
23. SiwickiKK, RiccioP, LadewskiL, MarcillacF, DartevelleL, CrossSA, FerveurJF (2005) The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn Mem 12 : 636–645.
24. AntonyC, DavisT, CarlsonD, PechineJ, JallonJ (1985) Compared behavioral responses of maleDrosophila melanogaster (Canton S) to natural and synthetic aphrodisiacs. Journal of chemical ecology 11 : 1617–1629.
25. GrilletM, DartevelleL, FerveurJ-F (2006) A Drosophila male pheromone affects female sexual receptivity. Proc Biol Sci 273 : 315–323.
26. KurtovicA, WidmerA, DicksonBJ (2007) A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446 : 542–546.
27. RonderosDS, SmithDP (2010) Activation of the T1 neuronal circuit is necessary and sufficient to induce sexually dimorphic mating behavior in Drosophila melanogaster. J Neurosci 30 : 2595–2599.
28. GaileyDA, LacailladeRC, HallJC (1986) Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav Genet 16 : 375–405.
29. SchilcherF, ManningA (1975) Courtship song and mating speed in hybrids betweenDrosophila melanogaster and Drosophila simulans. Behav Genet 5 (4) 395–404.
30. TalynBC, DowseHB (2004) The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Animal Behaviour 68 : 1165–1180.
31. GöpfertMC, RobertD (2001) Biomechanics. Turning the key on Drosophila audition. Nature 411 : 908.
32. WaddellS, ArmstrongJD, KitamotoT, KaiserK, QuinnWG (2000) The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 103 : 805–813.
33. FergestadT, SaleH, BostwickB, SchafferA, HoL, et al. (2010) A Drosophila behavioral mutant, down and out (dao), is defective in an essential regulator of Erg potassium channels. Proc Natl Acad Sci USA 107 : 5617–5621.
34. PossidenteDR, MurpheyRK (1989) Genetic control of sexually dimorphic axon morphology in Drosophila sensory neurons. Developmental Biology 132 : 448–457.
35. SweeneyST, BroadieK, KeaneJ, NiemannH, O'KaneCJ (1995) Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14 : 341–351.
36. HallemEA, HoMG, CarlsonJR (2004) The molecular basis of odor coding in the Drosophila antenna. Cell 117 : 965–979.
37. KitamotoT (2001) Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol 47 : 81–92.
38. MathewD, MartelliC, Kelley-SwiftE, BrusalisC, GershowM, et al. (2013) Functional diversity among sensory receptors in a Drosophila olfactory circuit. Proc Natl Acad Sci USA 110: E2134–E2143.
39. HaTS, SmithDP (2006) A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci 26 : 8727–8733.
40. van der Goes van NatersW, CarlsonJR (2007) Receptors and neurons for fly odors in Drosophila. Curr Biol 17 : 606–612.
41. LiuW, LiangX, GongJ, YangZ, ZhangY-H, et al. (2011) Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci 14 : 896–902.
42. SuC-Y, MenuzK, ReisertJ, CarlsonJR (2012) Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492 : 66–71.
43. MiyamotoT, AmreinH (2008) Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci 11 : 874–876.
44. FanP, ManoliDS, AhmedOM, ChenY, AgarwalN, et al. (2013) Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell 154 : 89–102.
45. LacailleF, HiroiM, TweleR, InoshitaT, UmemotoD, et al. (2007) An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2: e661.
46. LacailleF, EveraertsC, FerveurJ-F (2009) Feminization and alteration of Drosophila taste neurons induce reciprocal effects on male avoidance behavior. Behav Genet 39 : 554–563.
47. FerveurJ-F (2010) Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Current Opinion in Neurobiology 20 : 764–769.
48. RobertsonH (1983) Chemical stimuli eliciting courtship by males inDrosophila melanogaster. Cellular and Molecular Life Sciences 39 : 333–335.
49. Bro-JørgensenJ (2010) Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol Evol (Amst) 25 : 292–300.
50. DahanukarA, RayA (2011) Courtship, aggression and avoidance: pheromones, receptors and neurons for social behaviors in Drosophila. Fly 5 : 58–63.
51. HagaS, HattoriT, SatoT, SatoK, MatsudaS, et al. (2010) The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466 : 118–122.
52. SakuraiT, MitsunoH, HauptSS, UchinoK, YokohariF, et al. (2011) A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth Bombyx mori. PLoS Genet 7: e1002115.
53. Ben-Shahar Y (2011) Sensory Functions for Degenerin/Epithelial Sodium Channels (DEG/ENaC). Advances in Genetics. Advances in Genetics. Elsevier, Vol. 76. pp. 1–26.
54. EastwoodAL, GoodmanMB (2012) Insight into DEG/ENaC channel gating from genetics and structure. Physiology (Bethesda, Md) 27 : 282–290.
55. PfeifferBD, NgoT-TB, HibbardKL, MurphyC, JenettA, et al. (2010) Refinement of tools for targeted gene expression in Drosophila. Genetics 186 : 735–755.
56. HallJC (1978) Courtship among males due to a male-sterile mutation in Drosophila melanogaster. Behav Genet 8 : 125–141.
57. VillellaA, FerriSL, KrystalJD, HallJC (2005) Functional analysis of fruitless gene expression by transgenic manipulations of Drosophila courtship. Proc Natl Acad Sci USA 102 : 16550–16557.
58. BenchabaneH, XinN, TianA, HaflerBP, NguyenK, et al. (2011) Jerky/Earthbound facilitates cell-specific Wnt/Wingless signalling by modulating β-catenin-TCF activity. EMBO J 30 : 1444–1458.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



