-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
, a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
The anterior chamber is the space within the eye which is bound by the cornea, and the anterior surfaces of the iris and lens. Anterior chamber depth (ACD) is the distance measured along the eye's optical axis, from the cornea to the lens surface. ACD is an important risk factor for primary angle closure glaucoma (PACG), a major cause of irreversible blindness worldwide, and in particular, individuals of Asian ethnicity. In order to identify the genes that underlie PACG susceptibility, we conducted a two-staged study. We first conducted a large scale genetic study on a total of 5,308 population-based individuals of Asian descent to identify the genetic variants that influence ACD. This was followed by testing for associations between the identified genetic variant and PACG in another independent collection of 4,276 PACG cases and 18,801 controls. We found that a genetic variant within ABCC5 was associated with an increased risk of having PACG. Our findings suggest that the increase in PACG risk could in part be mediated by genetic sequence variants that influence the anterior chamber dimensions of the eye.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004089
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004089Summary
The anterior chamber is the space within the eye which is bound by the cornea, and the anterior surfaces of the iris and lens. Anterior chamber depth (ACD) is the distance measured along the eye's optical axis, from the cornea to the lens surface. ACD is an important risk factor for primary angle closure glaucoma (PACG), a major cause of irreversible blindness worldwide, and in particular, individuals of Asian ethnicity. In order to identify the genes that underlie PACG susceptibility, we conducted a two-staged study. We first conducted a large scale genetic study on a total of 5,308 population-based individuals of Asian descent to identify the genetic variants that influence ACD. This was followed by testing for associations between the identified genetic variant and PACG in another independent collection of 4,276 PACG cases and 18,801 controls. We found that a genetic variant within ABCC5 was associated with an increased risk of having PACG. Our findings suggest that the increase in PACG risk could in part be mediated by genetic sequence variants that influence the anterior chamber dimensions of the eye.
Introduction
Primary angle closure glaucoma (PACG) remains a major cause of irreversible blindness, particularly in Asian countries such as China [1], Mongolia [2], Singapore [3], and India [4] with up to 80% of the estimated 15 million people afflicted with PACG resident in Asia [5]. We recently conducted a genome-wide association study (GWAS) on PACG with 3,771 PACG cases and 18,551 controls, and identified 3 strongly associated genetic variants: rs11024102 in PLEKHA7, rs3753841 in COL11A1 and rs1015213 located between PCMTD1 and ST18 on Chromosome 8q [6]. As these 3 sequence variants only explained <2 percent of PACG risk, we looked into other methodologies besides the GWAS based approaches to identify more genes that underlie PACG susceptibility. The clinical heterogeneity of PACG suggests that disease-related endophenotypes/quantitative traits may help elucidate true disease genes. Quantitative phenotypes allow individuals to be viewed along the continuum of risk, and may provide additional information which could complement dichotomous measures of affection status [7], [8]. Such an approach has been used in the study of genetic variants controlling lipid traits and susceptibility to coronary artery disease [7], [8].
Smaller anterior segment dimensions are a hallmark of PACG, with shallower anterior chamber depth (ACD), the cardinal feature associated with increased susceptibility to PACG [9], [10]. Eyes with an ACD of less than 2.80 mm were more inclined to have angle closure when compared to eyes with an ACD of at least 3 mm (odds ratio (OR), 42.5; 95% confidence interval (CI), 27.4–66.2) [11]. There is also a greater likelihood of developing glaucomatous optic neuropathy in persons with the shallowest anterior chambers [12].
ACD, an easily and precisely quantified measure by ocular imaging techniques, is a normally distributed quantitative trait within the general population. It displays high heritability with a coefficient as high as 0.90 [13], [14], and can be considered an endophenotype for PACG.
To identify genetic variants that significantly influence ACD, and to determine if such genes (if any) affects PACG risk, we conducted a two-staged study, first a GWAS on a total of 4,484 population-based individuals of Indian and Malay ethnicity from Singapore, and Chinese from Beijing, China. Secondly, the identified QTLs for ACD were assessed for association in PACG case cohorts.
Results
Identification of a QTL for ACD
After sample and genotyping QC, a total of 1752 (Singapore Malay Eye Study, SiMES), 1860 (Singapore Indian Eye Study, SINDI), and 872 (Beijing Eye Study, BES) individuals with complete data for ACD measurements, age and gender were available for GWA analysis. We measured the association between ACD and individual SNP genotypes using linear regression, modeling for a trend-per-copy effect on the minor allele. Additional adjustments were made for age, gender, and the significant axes of genetic stratification. We noted a significant excess of small P-values at the extreme tail of the quantile-quantile distribution (Figure S1) accompanied by a background of minimal genomic inflation, thus indicating that there could be genuine associations between SNP genotypes and ACD. Highly suggestive evidence of association (P = 1.92×10−7) was observed at a sequence variant within ABCC5 (rs1401999) on Chromosome 3 (Figure S2). We were able to replicate of this observation in a further 824 population-based samples of Chinese descent from Beijing, China (P = 0.011) using Sanger sequencing, leading to genome-wide significant association with ACD upon meta-analysis of all 5,308 population-based samples (β = −0.045 mm ACD per-copy of the minor allele (C allele), P = 8.17×10−9; Table 1). Furthermore, attesting to the robustness of our findings, we observed a similar magnitude of association when using left eye ACD measurements of SiMES and SINDI cohorts (β = −0.051, P = 8.15×10−4 and β = −0.045, P = 8.11×10−5 respectively), where left eye ACD data were also available.
Tab. 1. Quantitative trait analysis between ABCC5 rs1401999 and anterior chamber depth in SIMES, SINDI, and BES. 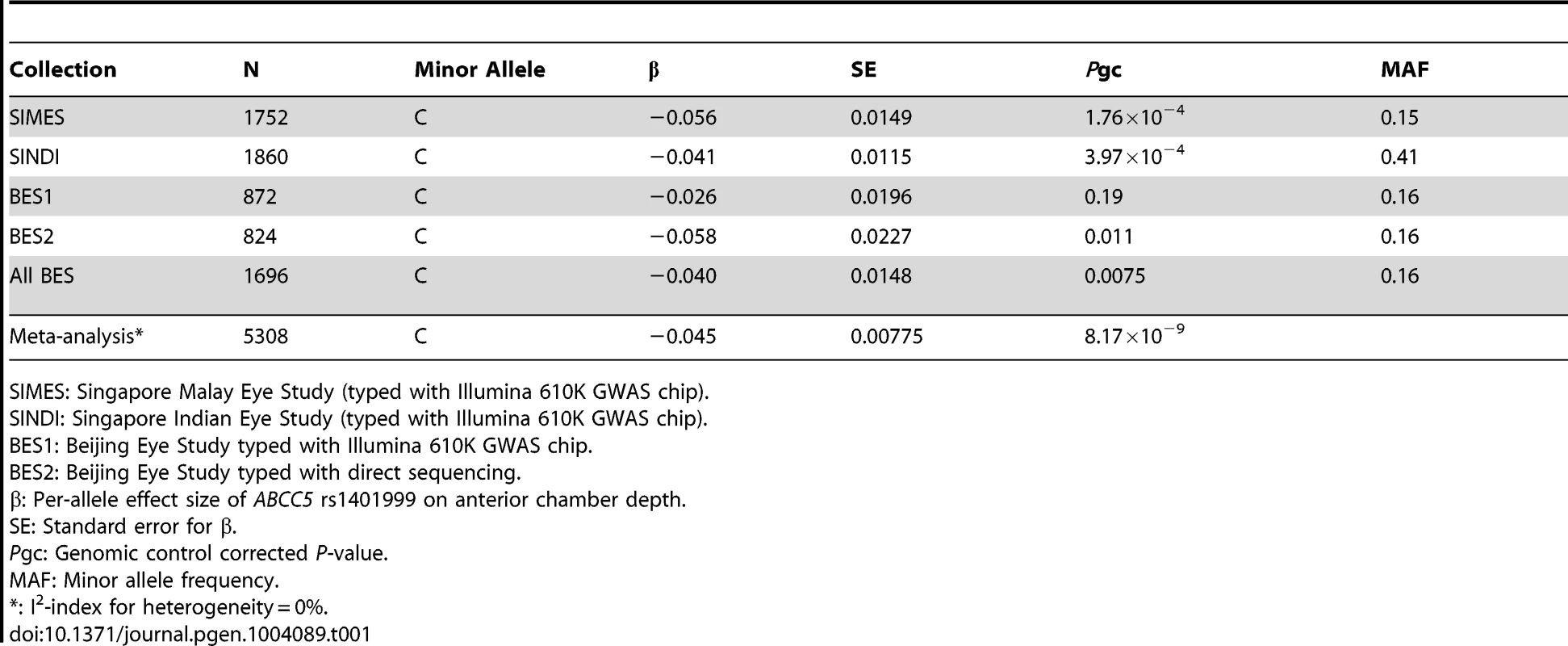
SIMES: Singapore Malay Eye Study (typed with Illumina 610K GWAS chip). Additionally, as both ACD and axial length are distance measurements on the axial direction of the eye globe, previously described to share genetic factors to a certain degree [15], we also assessed the effect of ABCC5 rs1401999 on axial length. We found the association between rs1401999 and axial length to be much weaker compared to that observed with ACD (P-meta = 0.000615, Table S1).
Association between ABCC5 rs1401999 and PACG
For the second analysis, we examined if this variant was associated with PACG, and proceeded to conduct analysis of 1,854 PACG cases and 9,608 controls from 5 cohorts (Table S2), all genotyped with Illumina SNP-arrays, and a further 2,422 cases and up to 9,193 controls from 7 independent collections genotyped using the Sequenom MassArray or Taqman platforms. As there was significant heterogeneity for the effect of ABCC5 rs1401999 and PACG risk between the 12 sample collections (Pheterogeneity = 0.0047, I2-index = 60.6%; Figure 1 and Table 2), we looked for sources of possible heterogeneity within the sample collections [16], the most obvious of which are the use of clean, open angle controls in some collections (see ‘Selection of controls without angle closure’ in methods), and un-ascertained population-based controls in others. This could be important in the context of this study as the prevalence of at-risk population with inherent angle closure is as high as 10% in Asian populations [3], [17]. Overall, we noted modest evidence of association (per-allele odds ratio = 1.13, 95% confidence interval = 1.06–1.22; P = 0.00046) between the minor allele (C allele) of rs1401999 and PACG when all 12 case-control collections were considered, and this association was augmented when we only included pre-selected sample collections where the controls had definite open angles (N = 3,458 PACG cases and N = 3,831 controls, per-allele OR = 1.30, P = 7.45×10−9; Figure 1 and Table 2).
Fig. 1. Association analysis between ABCC5 rs1401999 and susceptibility to primary angle closure glaucoma (PACG). 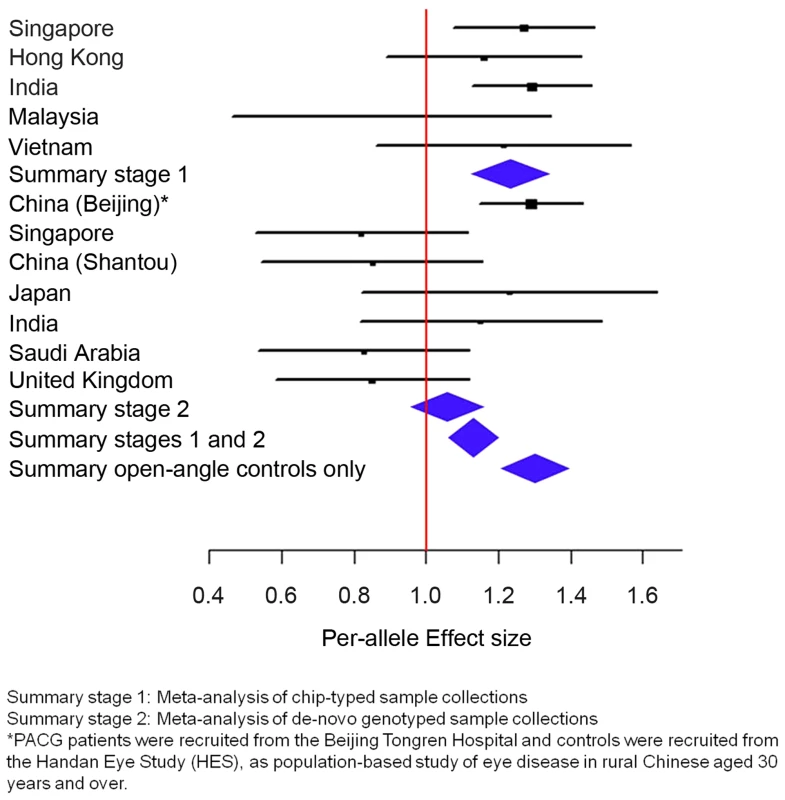
The PACG sample collections have been described elsewhere [6]. The vertical line represents a per-allele odds ratio of 1.00. The oblongs represent point estimates (referring to the per-allele odds ratio), with the height of the oblongs inversely proportional to the standard error of the point estimates. Horizontal lines indicate the 95% confidence interval for each point estimate. Meta-analyses of samples are reflected by blue diamonds. The width of the diamonds indicates their 95% confidence intervals. All point estimates in Stage 1 have been adjusted for the top axes of genetic stratification using logistic regression. Tab. 2. Association analysis between ABCC5 rs1401999 and primary angle closure glaucoma in all chip-typed sample collections (top panel), de-novo genotyped sample collections (middle panel), and PACG cases and clinically certified controls with open angles (bottom panel). 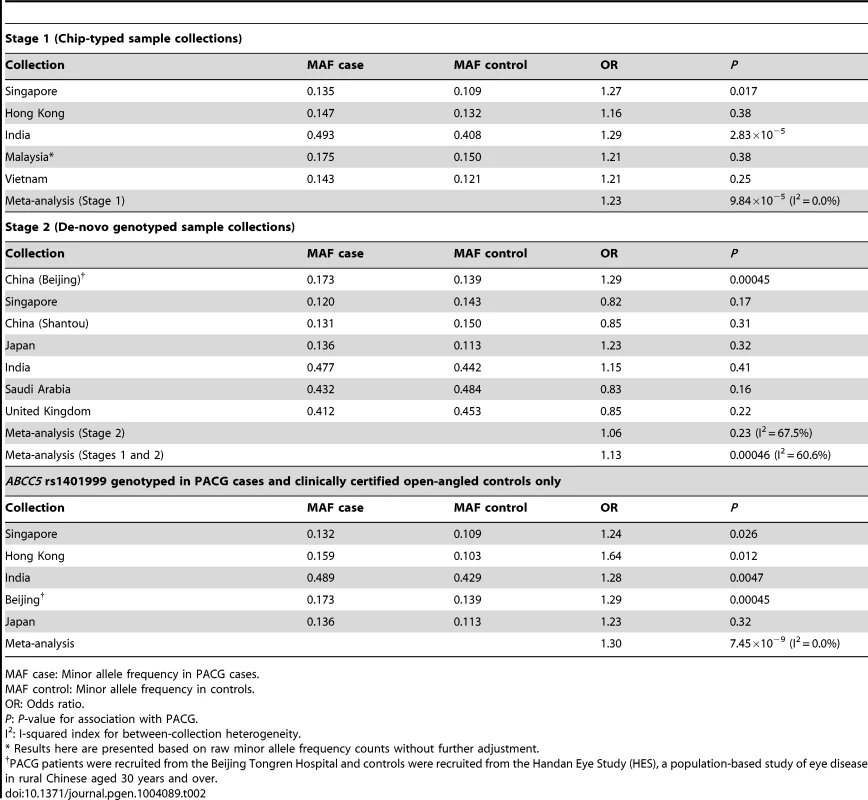
MAF case: Minor allele frequency in PACG cases. We also performed conditional analyses of the ABCC5 variant with the previously implicated PACG loci [6] and observed no change in either the odds ratio or p-values of the association. (Table S3).
Expression of ABCC5 in eye tissues
RT-PCR analysis on human ocular tissues demonstrated that ABCC5 is expressed in anterior segment structures relevant to PACG such as the iris, ciliary body, and lens (Figure S3). Additionally, Abcc5 message and protein were confirmed in mouse ocular tissues using in situ hybridization and immunohistochemistry (Figure S4, S5).
Discussion
In this study, a GWAS of ACD in Singaporean Indians and Malays and Chinese from Beijing China found rs1401999 within ABCC5 to contribute to the normal variation of ACD, a quantitative trait relevant to PACG. Interestingly, none of the previously identified PACG-associated genetic variants [6] were significantly associated with ACD [18]. We also demonstrated the association of rs1401999 with PACG using case-control cohorts from multiple populations across Asia. Importantly this association surpassed GWAS significance when the analysis was confined to control pre-selected to have open angles.
ABCC5, also known as multidrug resistance protein 5 (MRP5), has been shown to participate in tissue defense and cellular signal transduction through efflux of anticancer drugs, toxicants and a second messenger cGMP [19]. [20], [21]. It is expressed in most human tissues, including the cornea [22], retinal pigment epithelium and retina of the eye [23]. We also noted ABCC5 expression in ocular structures relevant to PACG such as the iris, ciliary body, and lens. However, its exact role in the context of PACG is not yet known. The significant association between ABCC5 rs1401999 with a shallower ACD argues favorably for a role in eye growth, particularly that of the anterior segment. Intriguingly, a study in zebrafish suggested that Abcc5 may play an active role in eye development through the regulation of intracellular cGMP levels. Zebrafish Abcc5, which shares 73% amino acid sequence identity with human ABCC5, is highly expressed in the lens of the developing eye [24]. Notably, the blockage of endogenous ABCC5 activity by its dominant-negative was shown to retard development, producing smaller eyes as well as overall reduction of body length and pigmentation of embryos [24]. Abcc5 knockout mice have been generated but an evaluation of their eyes was not reported [25]. A developmental role for ABCC5 in mammalian eyes therefore remains to be defined and will require further detailed studies in model organisms.
In addition, the linkage disequilibrium (LD) block that includes rs1401999 and ABCC5, also includes the presenilin-associated rhomboid-like (PARL) gene, 5-hydroxytryptamine receptor 3D (HTR3D) gene and the 5-hydroxytryptamine receptor 3C (HTR3C) gene (Figure S6). It is thus possible that rs1401999 might simply be in LD with an as yet unidentified causal variant, and it remains unclear whether the causal alleles or group of alleles influence ABCC5 or any of the neighboring genes to influence ACD. However, the clear expression within ocular tissues and a possible role in eye development make ABCC5 a rather attractive candidate gene for ACD. Indeed, re-sequencing of the region will be necessary to identify novel potentially functional polymorphisms related to PACG pathogenesis.
The prevalence of the pre-cursor stage of PACG, namely narrow angles, is about 10% in many Asian populations [3], [17]. Given the association between angle closure and shallow ACD [11] it is therefore appropriate to remove the ‘at-risk individuals’ from the control population in order to assess the true relationship between ACD QTLs and PACG. Unsurprisingly, the evidence of association between rs1401999 and PACG was augmented when we only included sample collections where the controls had definite open angles. Similar observations have also been seen with very recent studies on Alzheimer's disease, where the inclusion of general population-based controls resulted in significant underestimation of the odds ratio conferred by the disease-associated SNP compared to when properly matched, risk-free controls were applied, [26]. [27], [28]. We caution that the modest statistical evidence reported here for rs1401999, when examined in a total of 4,276 PACG cases and 18,801 controls, is at least 5 orders of magnitude below that of the three PACG-associated variants from our recent GWAS study [6]. Clinical studies have shown that ACD is only a modest determinant of angle width [29]. Therefore, this may explain why an ACD controlling gene such as ABCC5 would only confer a relatively small effect on PACG disease itself. Incidentally, the proportion of PACG risk explained by ABCC5 rs1401999 is 0.35% (95% confidence interval = 0.01 to 1.2%). Our study highlights the fact that even larger sample sizes may be necessary to dissect and conclusively identify the possible modifiers of genetic risk conferred by variants of modest effects, particularly when they exert their action on disease pathogenesis via endophenotypes. The following observations can also be drawn from this study. It is important that all sample collections are included and assessed transparently especially when drawn from diverse populations. Secondly, despite the broad-based success in the use of large numbers of unselected, population-based controls in genetic studies [30], [31], [32], [33], [34], [35] the deployment of controls with proper clinical phenotyping and documentation will often assist in more definitive identification of susceptibility genes. A comprehensive examination of all variation around ABCC5 using targeted deep re-sequencing is now necessary to parse the true association signal in an effort to more completely understand the role of this gene in ACD and PACG.
In summary our study identified a common genetic variant within ABCC5 as being significantly associated with ACD, which was also associated with a modest risk of PACG. Our findings are largely in keeping with the anatomical risk factors of individual susceptibility to PACG in the eye, whereby shallower ACD is a cardinal clinical and pathogenic feature, predisposing the eye to more ‘crowded’ anterior segment and thus increasing the risk of PACG. Our study provides further clues to genetic mechanisms underlying this major global cause of blindness.
Materials and Methods
Sample collections analyzed for Anterior Chamber Depth
Anterior chamber depth (ACD) measurements were derived from three population based samples: the Singapore Malay Eye Study (SiMES), the Singapore Indian Eye Study (SINDI) and the Beijing Eye Study (BES).
SiMES
The Singapore Malay Eye Study (SiMES) was a population-based, cross-sectional study of 3280 Malay adults aged 40 to 79 years. Details of the SiMES design, sampling plan, and methods have been reported elsewhere [36]. In brief, an age-stratified random sampling of all Malay adults, aged 40 to 80 years, residing in 15 residential districts in the south - western part of Singapore was drawn from the computer-generated random list of 16,069 Malay names provided by the Ministry of Home Affairs. A total of 1400 names from each decade of age (40–49, 50–59, 60–69, and 70–79 years), or 5600 names, were selected. Of these, 4168 individuals (74.4%) were determined to be eligible to participate. A person was considered ineligible if he or she had moved from the residential address, had not lived there in the past 6 months, was deceased, or was terminally ill. Of the 4168 eligible individuals, 3280 participants (78.7%) took part in the study. The study was conducted from August, 2004 to June, 2006.
SINDI
As with SiMES, the Singapore Indian Eye Study (SINDI) was a population-based, cross-sectional epidemiological study, but of ethnic Indian adults aged between 40 and 80+ years residing in Singapore. The Ministry of Home Affairs provided an initial computer-generated list of Indian names derived from a simple random sampling of all ethnic Indian adults aged 40–80+ years of age residing in 15 residential districts in south-western Singapore. From this list, a final sampling frame of 6,350 ethnic Indian residents was derived using an age-stratified random sampling strategy similar to SiMES. SINDI was conducted from March, 2007 to December, 2009 and recruited 3,400 (75% response rate) participants [37].
BES
The Beijing Eye Study was a population-based, cross-sectional study of Chinese adults aged 40+ years and residing in 4 communities in the urban district of Haidian in the North of Central Beijing and in 3 communities in the village area of Yufa of the Daxing District south of Beijing [38]. At the time of the first survey in the year 2001, the 7 communities had a total population of 5324 individuals aged 40 years or older and eligible to take part in the study. In total, 4439 individuals participated in the eye examination (83.4% response rate). In the year 2006, when blood samples were taken, the study was repeated by re-inviting all participants from the survey from 2001 to be re-examined with 3251 subjects participating (73.3% response rate).
Measurement and analysis of Anterior Chamber Depth (ACD)
ACD was measured using the IOLMaster (Carl Zeiss Meditec, Dublin, CA). Five readings were obtained and the average computed. The signal-to-noise ratio for all readings were >2.0, which indicate that a clear signal was obtained when performing the measurement. All the readings were within 0.05 mm of the one with the highest signal-to-noise ratio.
The ACD measurements used in the GWAS excluded any measurements from any eye which were pseudophakic or aphakic. For collections with data from two phakic eyes (SiMES, SINDI), individuals were excluded whose ACD measurements between the two eyes differed more than 0.2 mm (which represented the top ∼20th percentile of symmetrical data) which gave a good correlation between the left and the right eye (r2>0.95 in both SiMES and SINDI). However, given that BES only had measurements for the right eye; final meta-analysis used ACD measurements taken from the right eye in all three cohorts.
PACG case-control cohorts
The subjects for the PACG case-control study were compiled from 11 independent sample collections enrolled from 8 different countries; and have been described previously [6]. Furthermore, we have included an additional PACG case-control collection from Japan (136 cases and 419 controls) as well as an additional 436 PACG cases from the Beijing site. The PACG cases and controls were defined using the same criteria as described previously [6].
Selection of controls without angle closure
These controls were selected from within the population-based samples based on robust clinical criteria. A control was defined as having an intraocular pressure (IOP)<21 mmHg with open angles (on gonioscopy) in all quadrants, healthy optic nerves and normal visual fields, and no previous intraocular surgery.
Ethics
All involved studies were conducted in accordance with the principles of the Declaration of Helsinki. Study procedures and protocols were approved by the Institutional Review Board of each local institution involved in the study, and all study participants provided written informed consent at the recruitment into the studies.
Genotyping
ACD GWAS
Genotyping in the following sample collections (SIMES, N = 1752; SINDI, N = 1860; BES1, N = 872) was performed using the Illumina 610K Quad BeadChips following manufacturer instructions after genomic DNA were extracted from participants using standard laboratory techniques. Genotyping of SNP ABCC5 rs1401999 in an additional 824 participants from the Beijing Eye Study (termed BES2) was performed using direct capillary sequencing.
PACG sample collections
Genome-wide genotyping was performed for a total of 1,854 PACG cases and 9,608 controls using Illumina SNP-arrays. A further 1,917 PACG cases and up to 8,943 controls were genotyped using the Sequenom MassArray and Taqman real-time PCR method (Table S2).
Statistical analysis
Genome-wide per-cohort and meta-analysis of ACD for all three sample collections was performed using standard procedures as previously described [39], [40], [41]. A selection of stringent QC filters were applied to remove poorly performing SNPs and samples using tools implemented in PLINK version 1.7 [42]. The QC criteria were as follows: SNPs that had >5% of missing genotypes, gross departure from Hardy-Weinberg equilibrium (test for HWE showing P<10−6) or were of minor allele frequency below 1% were excluded from downstream analysis. For sample QC, samples with an overall genotyping call rate of <95% were excluded from analysis. Principal component (PC) analysis was undertaken to account for spurious associations resulting from ancestral differences of individual SNPs. PC plots were performed using the R statistical program package (www.r-project.org/).
For the GWAS on ACD, linear regression was performed to test for association between SNP genotypes and ACD as implemented by PLINK (version 1.06). Individual SNP genotypes were coded according to the number of copies of the minor allele present: 0 for the wild-type genotype, 1 for heterozygotes, and 2 for homozygote variants. A trend test using linear regression was used for primary association testing between genotypes and ACD as a quantitative trait, adjusting for age, gender, and the significant axes of genetic stratification. Meta-analysis across SiMES, SINDI and BES was performed using the inverse-variance, fixed effects model in order to obtain a combined point estimate of the overall effect size (β) coefficients and its corresponding standard error (SE). Inter-cohort heterogeneity was assessed with the Cochran's Q statistic and its accompanying I2 index. Quantile-quantile (QQ) and Manhattan plots were created using the software R (www.r-project.org). After sample and genotyping QC, a total of 1752, 1860, 872 individuals with complete data for ACD measurements, age and gender were available for SiMES, SINDI and BES individual GWAS. The overall genomic inflation factor for the meta-analysis of the three sample collections was minimal (λgc = 1.036; see Figure S1). We considered P<5×10−8 as genome-wide significant, and the previously used threshold for genome-wide significance (P<5×10−7) as ‘highly suggestive evidence of association [43].
Descriptions of the GWAS datasets used in the current study, principal component analysis, and adjustment for population stratification have been described elsewhere (Table S4) [39], [40], [41].
For the PACG sample collections, the analysis was performed as previously described, with associations between ABCC5 rs1401999 and PACG modeled using logistic regression.
Power calculations
A power calculation was conducted for bringing forward genome-wide significant SNPs from the ACD quantitative trait analysis to the PACG case control analysis (for 4,276 cases and 18,801 controls) (Supplementary Table S5). The power calculation is consistent with the findings we report in the current manuscript.
Expression analysis
RT-PCR in human ocular tissues
Expression of ABCC5 was assessed by semi quantitative reverse transcription PCR (RT-PCR) using ABCC5 specific primers (forward 5′ - ATTGGCATTGTGGGGCGGAC -3′ and reverse 5′ - CCTCTCCAGGGCATCCCAAATC -3′) on total RNA extracted from a variety of ocular tissues (anterior sclera, cornea, iris, ciliary body, trabecular meshwork, lens, lens capsule, retina and retinal pigment epithelium, optic nerve head and optic nerve) with TRIzol Reagent (Invitrogen, Carlsbad, California) in accordance with the manufacturer's protocol. First-strand cDNA synthesis was performed with SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, California). Semi quantitative RT-PCR was performed according to manufacturer's protocol, with the SYBR Green Master Mix (Invitrogen, Carlsbad, California) using the above ABCC5 primers. The resulting PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining. The ubiquitously expressed beta-actin (ACTB) gene was amplified using specific primers (forward 5′ - CCAACCGCGAGAAGATGA -3′ and reverse 5′ - CCAGAGGCGTACAGGGATAG-3′) and used as amplification and normalizing control. The ABCC5 primer sequences were derived from NCBI Reference Sequence: NM_005688.2.
In situ hybridization
In situ hybridization was performed on 12-µm-thick 4% paraformaldehyde (PFA) fixed ocular sections using Dig-labeled riboprobe. Digoxigenin-labeled (DIG-labeled) riboprobes for mouse Abcc5 were transcribed from cDNA clones (Open Biosystems clone ID: 6839816). For anti-sense probe generation, the plasmid was digested with EcoRI and transcribed with T3 polymerase. While for the sense probe Not1 and T7 were used. For this procedure, C57BL/6J or A/J mice were perfused transcardially with 4% PFA in Phosphate buffered saline (PBS). Eyes were postfixed in 4% PFA overnight, cryoprotected in 30% sucrose, and embedded in Optimal Cutting Temperature embedding medium (Tissue-Tek O.C.T. Compound, Sakura Finetek U.S.A., Inc., Torrance, CA). Frozen sections were air dried (10 min), postfixed (4% PFA; 10 min) and acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (TEA). Intercalated washes were done with PBS and after the last wash the sections were incubated overnight at 65°C with hybridization solution [50% formamide, 1× Hybe solution (Sigma-Aldrich, St. Louis, MO), 1 mg/ml yeast RNA] containing 1 µg/ml Dig-labeled riboprobes. After hybridization, slides were washed with 0.2× SSC at 72°C for 1 h, and endogenous peroxidases were quenched with a solution of 0.1% sodium azide and for 10 min. Bound probes were detected with an POD-conjugated anti-Dig antibody. The detection of hybridized mRNA in sections was performed using the Cy-3 Tyramide Signal Amplification System (PerkinElmer, Waltham, MA).
Immunohistochemistry
Enucleated eyes from A/J mice were embedded in Optimal Cutting Temperature embedding medium (Tissue-Tek O.C.T. Compound, Sakura Finetek U.S.A., Inc., Torrance, CA). The eyes were cryo-sectioned (14 µm) and transferred to glass slides. Cryosections were air dried for 10 min at room temperature, fixed for 10 min in 4% paraformaldehyde, followed by two washes (5 min each) in phosphate buffered saline (PBS). Sections were blocked 30 min at room temperature with 10% normal donkey serum. Primary antibodies were applied for 1 hr at room temperature using polyclonal goat anti-ABCC5 antibody (diluted 1∶300; Catalog No. sc-5781, Santacruz Biotechnology Inc., Santacruz, CA). Primary antibody was removed by three washes (5 min each) in PBS and the sections were treated for 1 hr at room temperature with AlexaFluor conjugated secondary antibodies (1∶200 dilution, Jackson-ImmunoResearch, West Grove, PA) diluted in 1% normal donkey serum and 10 mg/mL BSA in PBS. After three washes in PBS, the sections were mounted (Fluormount, Sigma-Aldrich, St. Louis, MO) and viewed by fluorescence microscopy. All photomicrographs were taken with identical camera settings. For peptide blocking experiments, the antibody was preincubated with 10× concentration of the blocking peptide (sc-5781P, Santacruz Biotechnology Inc., Santacruz, CA), incubated for 2 h at room temperature prior to treating it with the sections.
Supporting Information
Zdroje
1. FosterPJ, JohnsonGJ (2001) Glaucoma in China: how big is the problem? Br J Ophthalmol 85 : 1277–1282.
2. FosterPJ, BaasanhuJ, AlsbirkPH, MunkhbayarD, UranchimegD, et al. (1996) Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol 114 : 1235–1241.
3. FosterPJ, OenFT, MachinD, NgTP, DevereuxJG, et al. (2000) The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 118 : 1105–1111.
4. DandonaL, DandonaR, MandalP, SrinivasM, JohnRK, et al. (2000) Angle-closure glaucoma in an urban population in southern India. The Andhra Pradesh eye disease study. Ophthalmology 107 : 1710–1716.
5. QuigleyHA, BromanAT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90 : 262–267.
6. VithanaEN, KhorCC, QiaoC, NongpiurME, GeorgeR, et al. (2012) Genome-wide association analyses identify three new susceptibility loci for primary angle closure glaucoma. Nat Genet 44 : 1142–1146.
7. CohenJC, BoerwinkleE, MosleyTHJr, HobbsHH (2006) Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354 : 1264–1272.
8. KathiresanS (2008) Myocardial Infarction Genetics Consortium (2008) A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med 358 : 2299–2300.
9. FosterPJ, DevereuxJG, AlsbirkPH, LeePS, UranchimegD, et al. (2000) Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol 84 : 186–192.
10. DevereuxJG, FosterPJ, BaasanhuJ, UranchimegD, LeePS, et al. (2000) Anterior chamber depth measurement as a screening tool for primary angle-closure glaucoma in an East Asian population. Arch Ophthalmol 118 : 257–263.
11. LavanyaR, WongTY, FriedmanDS, AungHT, AlfredT, et al. (2008) Determinants of angle closure in older Singaporeans. Arch Ophthalmol 126 : 686–691.
12. AungT, NolanWP, MachinD, SeahSK, BaasanhuJ, et al. (2005) Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol 123 : 527–532.
13. HeM, WangD, ZhengY, ZhangJ, YinQ, et al. (2008) Heritability of anterior chamber depth as an intermediate phenotype of angle-closure in Chinese: the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci 49 : 81–86.
14. LyhneN, SjølieAK, KyvikKO, GreenA (2001) The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol 85 : 1470–6.
15. HeM, HurYM, ZhangJ, DingX, HuangW, et al. (2008) Shared genetic determinant of axial length, anterior chamber depth, and angle opening distance: the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci 49 : 4790–4794.
16. McAteerJB, PrudenteS, BacciS, LyonHN, HirschhornJN, et al. (2008) ENPP1 Consortium (2008) The ENPP1 K121Q polymorphism is associated with type 2 diabetes in European populations: evidence from an updated meta-analysis in 42,042 subjects. Diabetes 57 : 1125–1130.
17. HeM, FosterPJ, GeJ, HuangW, ZhengY, et al. (2006) Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Invest Ophthalmol Vis Sci 47 : 2782–2788.
18. NongpiurME, WeiX, XuL, PereraSA, WuRY, et al. (2013) Lack of association between primary angle-closure glaucoma susceptibility Loci and the ocular biometric parameters anterior chamber depth and axial length. Invest Ophthalmol Vis Sci 54 : 5824–5828.
19. JedlitschkyG, BurchellB, KepplerD (2000) The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275 : 30069–30074.
20. WijnholdsJ, MolCA, van DeemterL, de HaasM, SchefferGL, et al. (2000) Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A 97 : 7476–7481.
21. PrattS, ShepardRL, KandasamyRA, JohnstonPA, PerryW, et al. (2005) The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther 4 : 855–863.
22. KarlaPK, QuinnTL, HerndonBL, ThomasP, PalD, et al. (2009) Expression of multidrug resistance associated protein 5 (MRP5) on cornea and its role in drug efflux. J Ocul Pharmacol Ther 25 : 121–132.
23. StojicJ, StohrH, WeberBH (2007) Three novel ABCC5 splice variants in human retina and their role as regulators of ABCC5 gene expression. BMC Mol Biol 8 : 42.
24. LongY, LiQ, LiJ, CuiZ (2011) Molecular analysis, developmental function and heavy metal-induced expression of ABCC5 in zebrafish. Comp Biochem Physiol B Biochem Mol Biol 158 : 46–55.
25. de WolfCJ, YamaguchiH, van der HeijdenI, WielingaPR, HundscheidSL, et al. (2007) cGMP transport by vesicles from human and mouse erythrocytes. FEBS J 274 : 439–450.
26. JonssonT, AtwalJK, SteinbergS, SnaedalJ, JonssonPV, et al. (2012) A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature 488 : 96–99.
27. JonssonT, StefanssonH, SteinbergS, JonsdottirI, JonssonPV, et al. (2013) Variant of TREM2 Associated with the Risk of Alzheimer's Disease. N Engl J Med 368 : 107–116.
28. GuerreiroR, WojtasA, BrasJ, CarrasquilloM, RogaevaE, et al. (2013) Alzheimer Genetic Analysis Group (2013) TREM2 variants in Alzheimer's disease. N Engl J Med 368 : 117–127.
29. FooLL, NongpiurME, AllenJC, PereraSA, FriedmanDS, et al. (2012) Determinants of angle width in Chinese Singaporeans. Ophthalmology 119 : 278–282.
30. Wellcome Trust Case Control Consortium (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 : 661–78.
31. MorrisAP, VoightBF, TeslovichTM, FerreiraT, SegrèAV, et al. (2012) Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 44 : 981–990.
32. KhorCC, ChauTN, PangJ, DavilaS, LongHT, et al. (2011) Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet 43 : 1139–1141.
33. ZhangF, LiuH, ChenS, LowH, SunL, et al. (2011) Identification of two new loci at IL23R and RAB32 that influence susceptibility to leprosy. Nat Genet 43 : 1247–1251.
34. UK IBD Genetics Consortium (2009) BarrettJC, LeeCJ, LeesCW, PrescottNJ, AndersonCA, et al. (2009) Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 41 : 1330–1334.
35. KhorCC, DavilaS, BreunisWB, LeeYC, ShimizuC, et al. (2011) Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet 43 : 1241–1246.
36. FoongAW, SawSM, LooJL, ShenS, LoonSC, et al. (2007) Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic Epidemiol 14 : 25–35.
37. LavanyaR, JeganathanVS, ZhengY, RajuP, CheungN, et al. (2009) Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol 16 : 325–336.
38. ZhangH, XuL, ChenC, JonasJB (2008) Central corneal thickness in adult Chinese. Association with ocular and general parameters. The Beijing Eye Study. Graefes Arch Clin Exp Ophthalmol 246 : 587–592.
39. VithanaEN, AungT, KhorCC, CornesBK, TayWT, et al. (2011) Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet 20 : 649–658.
40. KhorCC, RamdasWD, VithanaEN, CornesBK, SimX, et al. (2011) Genome-wide association studies in Asians confirm the involvement of ATOH7 and TGFBR3, and further identify CARD10 as a novel locus influencing optic disc area. Hum Mol Genet 20 : 1864–1872.
41. CornesBK, KhorCC, NongpiurME, XuL, TayWT, et al. (2012) Identification of four novel variants that influence central corneal thickness in multi-ethnic Asian populations. Hum Mol Genet 21 : 437–445.
42. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
43. van HeelDA, FrankeL, HuntKA, GwilliamR, ZhernakovaA, et al. (2007) A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet 39 : 827–829.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



