-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
Inferring the function of proteins and the role they play in cellular processes is essential for our understanding of cell biology, genetics and biology in general. Standard genetic approaches use large perturbations to cells such as gene knockout, knockdown or over expression of genes. Such methods are powerful, but have the drawback of taking the cell far from its normal working point. Here, we provide a new and much milder approach, which uses the natural cell-cell variation in protein level and expression pattern as a source of mild perturbation. We monitor individual live cancer cells under the microscope and correlate their protein levels and localization with phenotype in the same cells. We use the motility of human cancer cells as a model system that is highly important for understanding metastasis in cancer. We find that our approach uncovers most of the known motility proteins, as well as new ones which we validate using knockdown experiments. Our novel approach is widely applicable to any phenotype that can be visualized in individual cells, and for any organism for which one can measure proteins in individual cells.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004176
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004176Summary
Inferring the function of proteins and the role they play in cellular processes is essential for our understanding of cell biology, genetics and biology in general. Standard genetic approaches use large perturbations to cells such as gene knockout, knockdown or over expression of genes. Such methods are powerful, but have the drawback of taking the cell far from its normal working point. Here, we provide a new and much milder approach, which uses the natural cell-cell variation in protein level and expression pattern as a source of mild perturbation. We monitor individual live cancer cells under the microscope and correlate their protein levels and localization with phenotype in the same cells. We use the motility of human cancer cells as a model system that is highly important for understanding metastasis in cancer. We find that our approach uncovers most of the known motility proteins, as well as new ones which we validate using knockdown experiments. Our novel approach is widely applicable to any phenotype that can be visualized in individual cells, and for any organism for which one can measure proteins in individual cells.
Introduction
To understand which proteins contribute to a biological phenomenon, current approaches use perturbations such as gene knockdown, over-expression or knockout. These approaches have provided the basis for much of what we know about cell biology. However, such perturbations also have drawbacks. Perturbations currently used are usually large - a protein expression is either markedly reduced or increased, and the measurement is therefore far from the cells normal working condition. This can lead to artificial off-target effects or to masking of the perturbation by changes in the cell that compensate for the loss of a protein. It is thus possible that some of the information about protein function has remained hidden due to these features of current methods.
To offer a complementary way to understand protein function, we present an approach called noise genetics. Noise genetics uses the natural cell-cell variation in protein levels and localization [1]–[8] as a source of mild perturbations to reveal protein function. Since natural fluctuations are mild, the risk of compensation is reduced. The idea is to correlate the protein levels and localization in individual cells to the phenotype in the same cells. Notably, cell-cell variation in protein level changes slowly over time: cells keep their individual levels for about a cell generation [7]. Thus, the noise we use is a type of cell individuality (Figure S1). Cells have individual character in many of their phenotypes as well, that also last for about a cell generation [9], [10].
Previous studies used noise for understanding regulatory interactions between a few proteins in bacteria [1], [11], whereas here we screen hundreds of proteins. As a model system, we use the motility phenotype of human cancer cells. Motility of cancer cells is of general interest both as a well-studied biological phenotype [12], and as a feature of normal physiology and cancer metastasis [13]. Wide scale genetic screens, including siRNA knockdowns, have revealed numerous genes involved in motility [14]–[16]. Moreover, natural phenotypic variability and fluorescent microscopy were used to study the shape of motile cells [10] and the cytoskeleton dynamics [17].We use a library of human cancer cell clones each with a different protein fluorescently tagged at its endogenous chromosomal locus [18], [19] to follow the natural variability of proteins, and the natural variability of motility in the same cell. Proteins whose level or localization correlate with motility are identified as candidate motility proteins.
We find that about 15% of the 566 highly expressed proteins that we tested exhibit a significantly high correlation between their protein features and motility in individual cells. This correlation can suggest that the protein has a role in cell motility. About half of these candidate proteins were previously known to be involved in cell motility. We validated a sample of these candidates using mild siRNA knockdown.
Results
Level and localization of 566 unique proteins were analyzed in time-lapse movies
To study natural variability between individual cells, we used the LARC library of human clones with tagged proteins [18]–[20]. The library is made of clones of a parental human lung cancer cell line, H1299. In each clone, a full-length protein is fluorescently tagged with YFP as an internal exon (Figure 1A). The protein is tagged at its endogenous chromosomal locus, preserving the natural promoter and regulatory sequences (Figure 1B). Previous studies suggest that most (70–80%) of the tagged proteins preserve their wild-type dynamics and localization [18], [20].
Fig. 1. Overview of the noise genetics approach. 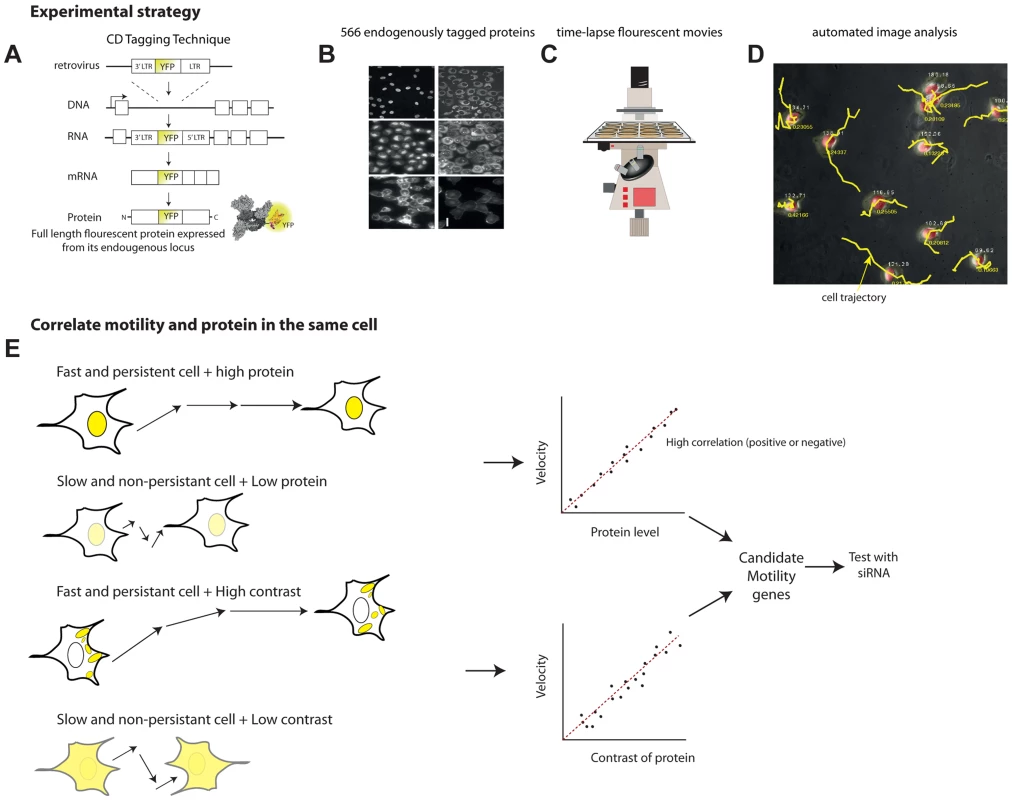
(a) We used a library of endogenously tagged proteins in human H1299 cells. Briefly, a retrovirus introduced YFP as an artificial exon into the introns of genes. Fluorescent clones were selected and sequenced. The clones express full length protein under endogenous control, with an internal YFP tag. (b) We selected 566 unique proteins with high quality movies and correct localization, and performed, or used existing, 24 h time-lapse movies(c) under controlled conditions. (d) Automated image analysis enabled by a mCherry tag in the parental clone enabled automatic tracking of protein level and localization as well as motility of each individual cell over time. (e) To find candidate motility genes, we sought proteins with high absolute correlation between protein level or localization (contrast, texture) and motility parameters (velocity, persistence). We tested a sample of the candidate motility genes by siRNA knockdown. The parental clone also expresses proteins tagged with red florescence using mCherry. This red fluorescence is used for image analysis of time lapse movies (Figure 1C), allowing automated segmentation and tracking of the nucleus and cytoplasm in all clones (Figure 1D). The tagged proteins are XRCC5 and DAP1, both not known to be involved in motility.
A previous study employed this library to follow 1260 clones with different tagged proteins as they responded to an anti-cancer drug using time-lapse movies [18]. Here, we re-analyzed these movies, that also included the 24 h period before drug addition, together with movies from a recent study on protein half-lives using the same library and microscopy system (Eden et al, 2011) and chose 704 unique proteins with high quality movies for further analysis (movies chosen had 4 fields of view totaling at least 20 cells at each time-point). Of these, we chose only known proteins (as opposed to ESTs) with subcellular localization matching the literature. This results in a final set of 566 different proteins. These proteins have diverse cellular localizations and functions (Supplementary File S1).
Correlation between protein and motility features was tested for each cell
We tracked the protein level and localization in each cell, and also the motility of the same cells. Protein level is given by the summed YFP fluorescence of all pixels in the cell. Most proteins in our dataset did not show large translocation events between cell compartments such as nucleus and cytoplasm. To parameterize protein localization, we therefore characterized the spatial distribution inside the cell, using two well-known measures from image analysis: contrast (the existence of sharp changes in intensity) and texture (also called texture correlation, the linear dependency of grey levels on those of neighboring pixels) [21]–[23].
Cell motility was quantified in terms of speed and angle change of the motion (Figure 2A). Cell velocity was measured as the change in cell center of mass between frames (20 min). The angle change of cell movement in frame i was calculated based on the deviation of the cell in frame i+1 from its movement between frame i-1 and frame i. Persistent motion results in low values of angle change (Figure 2B). We found that angle change was negatively correlated with cell velocity (Figure S2).
Fig. 2. Candidate motility genes were found with high correlation between protein and motility features in individual cells. 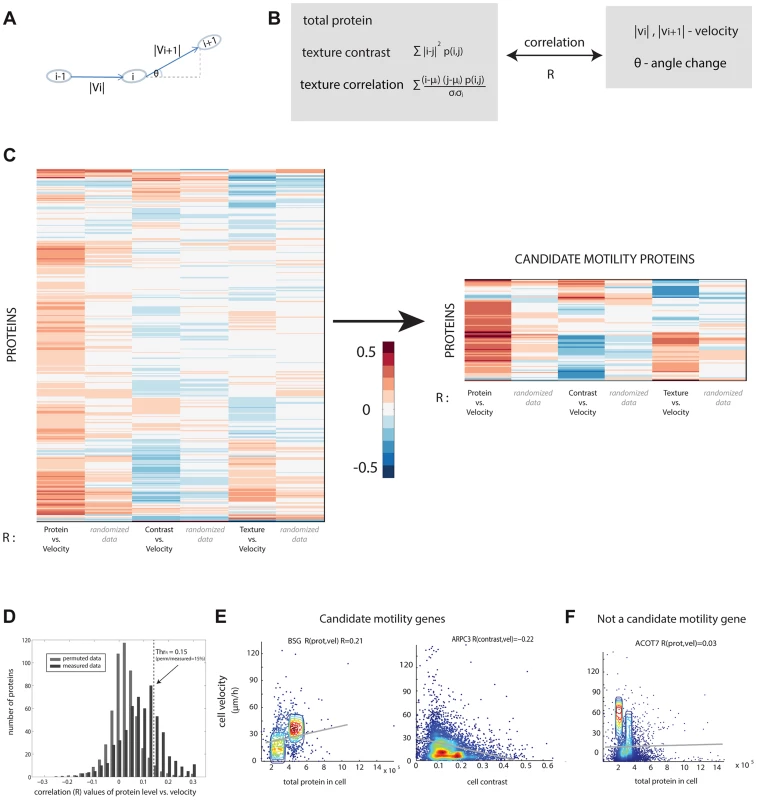
(a) Cell velocity and angle change (persistence) for a cell in three consecutive frames i−1,i and i+1. (b) Three protein features were compared to two motility features. Contrast and texture sum over pixels in a cell and employs the occurrence matrix p (i, j) equal to the probability that pixels of intensity i and j are adjacent. Texture uses the mean and standard deviation (std) of pixel intensity levels mu and sigma. (c) Pearson correlation coefficients R between protein and motility features show a group of proteins with high absolute correlation. Blue/red denotes low/high R values. (d) Randomized data (gray) showing lower correlations than real data (black) is used to establish a threshold correlation (dashed line) for detecting motility candidate genes with low false discovery rates. (e) Candidate motility genes show positive or negative correlation to protein level or localization measures. Data is shown in density plot along with the regression line of the linear fit. (f) An example for a non-motility candidate that show low absolute correlation. If a protein is involved in motility, we expect a significant positive or negative correlation between at least one of the protein parameters and one of the motility parameters. For example, if cells that express protein X at high levels move faster, whereas cells that express it at lower levels move slower, we predict that protein X is a candidate motility gene (Figure 1E). A similar conclusion is reached if contrast or texture correlate with motility. For example, if cells that show a homogenous spatial distribution of protein Y move slower than cells that express protein Y in more punctuate manner (high contrast of fluorescence across the cell), one may predict that Y is a candidate motility gene. The contrast and texture differences we observe between cells are subtle, not gross changes such as transitions between organelles. Images of cells are provided in S3–S5.
We compared the three protein properties (protein level, contrast and texture) to the two motility parameters (speed, angle change) in each cell and each time-point using Spearman and Pearson correlations (Figure 2C, Figure S6). The observed correlation values are centered at around zero and range from −0.4 to 0.4.
In order to test the significance of the calculated correlation values, we compared them to correlations in randomized data. To make a stringent comparison, we note that data from different time-points of the same cell are not independent. Furthermore, cells from the same field of view are potentially more dependent than cells in other fields of view, due to possible systematic effects in the experiment. We thus constructed the randomized dataset by associating the motility parameters for cell i at all time points with the protein properties from a different cell j at the same time points in the same field of view, with i and j randomly chosen. This provides a randomized control of the same size as the original data.
The permuted datasets showed correlations between protein and motility parameters mainly (90%) in the range −0.1 to 0.1 (gray bars in Figure 2D). Comparing the randomized distribution to the measured data (black bars in Figure 2D) shows proteins with correlations higher or lower than expected by chance. In this study, we define a candidate motility gene if its absolute correlation coefficient |R| exceeds 0.15 in both Pearson and Spearman correlations. Based on the comparison to randomized data, the rate of false positives is expected to be 15% for comparisons of protein level to cell velocity (Figure S7), 23% for contrast versus velocity, 30% for texture versus velocity. To test this, we conducted a new set of time-lapse microscopy experiments on a random sample of 19 candidate motility proteins, and found that 16/19 showed the same above-threshold correlations as in the original movie dataset, consistent with a false-detection rate of about 15% (see Supplementary File S3).
Similar false discovery rates were obtained for the comparison between angle change and the protein properties (Figure S7). Several examples of proteins with positive and negative high correlations are shown (Figure 2E) along with examples of proteins that did not show a significant correlation between the protein level and the motility in individual cells (Figure 2F).
Many of the motility candidate genes are known to play a role in motility
We found 74 candidate motility genes (Supplementary File S1, S2). Of these, 31 (41%) correlate by protein level. The rest of the proteins correlate with motility by contrast (25%) or texture (23%), and 15% of the candidate proteins correlate by more than one measure.
The candidate genes are highly enriched in genes previously known to play a role in motility: 41% (30/74) were previously characterized as motility genes (hypergeometric p = 0.0009) according to the Genecards database (see Methods). Among the candidate genes are actin regulators in the ARP complex (ARPC3 [24]), two actin related proteins (ACTR2 and ACTR1A), RAC1 [25] that is essential for cell migration and WASF2 [26] that is part of the WAVE complex that regulates lamellipodia formation [12]. (Other examples are described in Figure 3A). Some of the candidate genes have no known role in motility (Figure 3B).
Fig. 3. Candidate motility genes recover many of the known motility proteins in the screen. 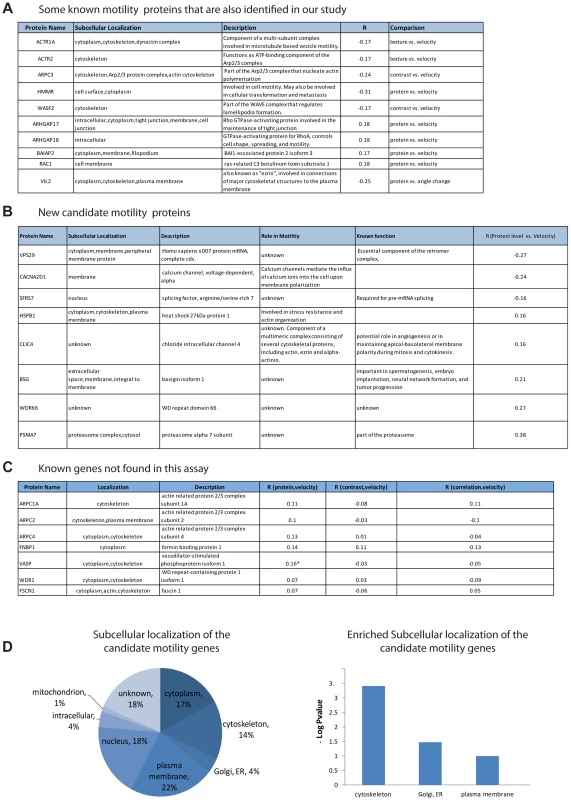
(a) Examples of known motility genes found in the present assay, with their correlation coefficient. (b) New candidate motility genes found in the assay. (c) Known motility genes included in the screen but not found in the assay. Note that three of the genes have marginal R values. (d) Sub-cellular localization of candidate motility genes is enriched for cytoskeleton, Golgi/ER and plasma membrane, similar to all known motility genes (gene-cards). The subcellular localization of the candidate genes is enriched in the cytoskeleton (hypergeometric p-value = 0.0008), the plasma membrane (p = 0.02) as well as the ER/golgi (p = 0.06) (Figure 3D).
In an attempt to estimate the false-negative rate of this assay, we considered the 13 genes out of the 566 in this study that were listed as motility genes in a recent review of motility (Ridley, 2011). Among these 13 relatively well-characterized genes, 7 genes were not identified as candidate motility genes in this study (Figure 3C). This suggests a false-negative rate on the order of 50%. However, some of these false negative genes showed a relatively high correlation in one of the examined comparisons and would be scored positive in a less stringent threshold choice. A more extensive false-negative test compared the present assay to all 110 genes in our set of 566 that are listed as motility related in the Genecards database. Of these, only 29 are found in our assay. Our analysis is not expected to find all motility genes due to several limitations that will be addressed below.
Mild knockdown experiments were used to validate some of the candidate genes
In order to test the involvement of the candidate genes in the motility process, we used siRNA directed against the YFP tag to lower the expression level of the tagged proteins. Since we introduced YFP as an exon to all clones, the anti-YFP siRNA can be used to knockdown expression in any clone from the library. The knockdown is mild (at most half-knockdown) because only one allele is tagged with YFP. Thus, a 50% reduction in YFP corresponds to a 25% reduction in total protein due to the expression from the untagged allele. We chose 11 candidate genes that had positive correlation between protein level and motility or a significant correlation between a protein feature and motility. Of these candidate genes, 4 are previously known motility genes and 7 are novel, randomly chosen from the candidate list. We also tested 4 control genes that did not correlate with motility. We took time-lapse movies for 48 hours starting 24 hours after the siRNA infection (Figure 4A).
Fig. 4. Novel motility genes are validated by mild knockdown experiments. 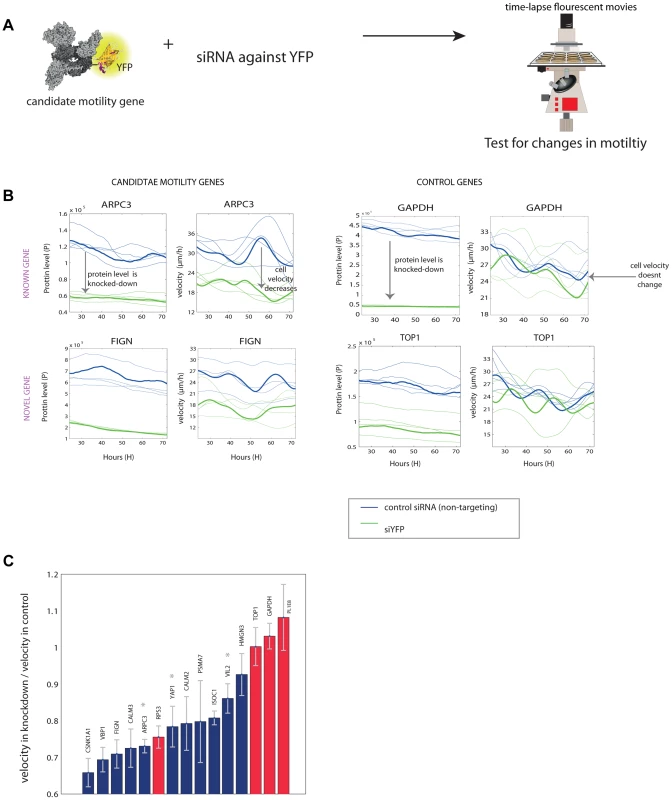
(a) We used anti-YFP siRNA to produce knockdown of the tagged gene in each clone, and tested protein level and motility in 48 time-lapse movies. (b) Examples of known and novel candidate motility genes after knockdown, as well as control genes, that are not known to be involved in the motility process. On the left, the effect of the knockdown on the expression of the protein is shown. In all knockdown experiments, protein fluorescence level was reduced at least by half. On the right, the effect of the knockdown of the gene on cell speed is shown. In the examples shown, candidate motility genes showed a reduction in velocity after knockdown compared to mock treatment, whereas control genes showed no significant reduction in motility. (c) Velocity reduction in knockdown experiments compared to mock treatment shows that 10/11 candidate genes showed a motility defect upon knockdown (blue bars), in contrast to control genes not known to be involved in motility, for which 3 out of 4 showed no significant defect (red bars). Stars denote known motility genes also found in our assay. Error bars stands for standard deviations (SD). We used the YFP fluorescence to observe the extent of knockdown (Figure 4B). The mCherry labeling of the cells was not influenced by the siRNA (Figure S8). 10 out of the 11 candidate motility gene clones showed significantly lowered velocity (reduction of 15–35%), whereas 3 of the control genes (GAPDH and TOP1 and one gene of unknown function) showed no measurable reduction in velocity (Figure 4C).
One control gene – the ribosomal gene RPS3, not known to affect motility, showed a motility defect upon mild knockdown. It was not picked up as a candidate motility gene in the noise genetics assay based on cell-cell variations. This may point to a difference between knockdown that affects only a single gene in a module such a ribosome, and noise genetics, where fluctuations in all genes in a module (e.g. all ribosomal genes) are expected to be correlated [7], [8].
In total, candidate motility genes, both previously known and novel were validated by mild knockdown at a level of about 90%. We further tested proteins with a negative correlation between their protein level and motility and the results are summarized in the supplementary information (see Supplementary File S4).
Discussion
In this study, we presented ‘noise genetics’ - an approach to assign function to proteins that uses the natural noise in protein level and localization and correlates it to the variation in phenotype of the same individual cells. We demonstrated this using the motility phenotype of cancer cells. Noise genetics recovers 30 of the known motility genes in our clone library and also 43 novel motility candidates, of which 10 were validated by siRNA knockdown (Figure 4).
Noise genetics can complement standard genetic perturbations. Among its advantages are the non-invasive and mild nature of the natural variations used, which keep the cell near its normal working point. Proteins whose knockdown is lethal are hard to evaluate using standard genetics, but can potentially be picked up by the assay. Similarly, proteins whose knockdown effects are masked by compensation from other proteins in the same module may be picked up by noise genetics, because one expects the entire module to show correlated noise [7], [8]. Noise genetics compares individual cells from the same field of view, and therefore contains a type of internal control for systematic errors. In standard perturbation assays, one needs to compare perturbed cell populations to a separate experiment with unperturbed cell populations in order to control for experimental systematic errors.
Among the limitations of noise genetics as implemented here are the need for a fluorescent cell library or other means of observing cell-cell variation in both protein and phenotype. Such libraries exist, for example, in S. cerevisae [27], E. coli [28], [29], C. elegans [30] and Zebrafish [31]. If the phenotype of interest can be observed in fixed cells, individual cell imaging of proteins [32] or mRNA [33]–[35] might be used for noise genetics. Such an approach has been used with pre-selected genes, for example, to explore the effect of protein variability on stem-cell differentiation [36], sporulation timing in Bacillus subtilis [37] and meiosis timing in Saccharomyces cerevisiae [38]. We currently tested only linear correlation; more elaborate time-series analysis methods or non-linear correlation analysis may be able to improve the resolution of this approach [39].
The cells in this study are diploid (or multiploid), but only one copy of each gene was labeled with YFP. Therefore, the fluorescence measurement does not necessarily reflect the total protein level or distribution. Previous work with the present cell system showed that there is high correlation in the expression of two alleles of the same ribosomal gene [7]. Future work is needed to test the present approach with all alleles tagged.
Noise genetics can miss proteins whose effect on phenotype is small in the working point of the cell. Such proteins can be picked up by standard genetics which makes large perturbations. Similarly, if natural variations in proteins or phenotypes are very small, noise genetics may not be applicable. Importantly, noise genetics on its own can only detect correlations, and additional experiments such as the mild knockdown performed here, are needed to gain evidence for causality.
Noise genetics uses natural cell-cell variation in proteins to discover links with phenotypes. Additional phenotypes that can be readily studied include cell size and shape, and any other phenotype measurable by time-lapse microscopy.
Methods
Time-lapse microscopy movies
Movies from previous studies [18], [20] were used for this analysis, as well as new movies on 19 clones. In the previous studies, 4 movies (fields of view) were taken for each of the 1,000 clones totaling about 4,000 movies. In each movie, 10–20 cells were tracked over 24 hours at least, every 20 minutes. Some of these clones were filmed more than once and some clones represent the same protein. For the present analysis, we combined all data for the same protein from all relevant movies. Each time point included transmitted light image (phase contrast) and two fluorescent channels (red and yellow). Of the original movie sets, we chose 566 unique protein clones as described in the text. The 566 known proteins that were used in our analysis tend to have high expression levels (so that they are picked up in the LARC library construction which used FACS to select for fluorescent clones).
Image analysis of time-lapse movies
We used the image analysis software described in [18] with minor modifications. The main steps in this software include background correction (flat field and background subtraction), segmentation, cell tracking, and automated identification of cell phenotypes (mitosis and cell death). Cell and nuclei segmentation was based on the red fluorescent images of the two red tagged proteins found in all clones, localized to the cytoplasm (DAP1) and nucleus (XRCC5), with intensity which is very uniform across cells and clones. Segmentation used global image threshold and seeded watershed segmentation. The cell-tracking procedure maps each cell to the appropriate cells in the preceding and following frames as described [40]. Texture parameters (contrast and correlation) of the proteins were measured for each cell in each time point based on the YFP image of the tagged protein as described below.
In our previous studies with the same movies, we also analyzed the protein concentration by taking the average or median fluorescent intensity inside the cell, as opposed to the total intensity. We find this measure to be more sensitive to image outlier pixels - even when using the median pixel intensity [23]; we therefore use total fluorescent intensity in the present study.
Texture and contrast calculation
To calculate texture and contrast, we first evaluated a gray-level (fluorescence intensity) co-occurrence matrix (GLCM) from each fluorescent image of the cells [41]. Each element (i, j) in GCLM specifies the number of times that the pixel with gray-level i occurred horizontally adjacent to a pixel with gray-level j.
From the matrix one can compute the various texture features. For example, contrast is giving a value of 0 for a constant intensity image and high values when adjacent pixels have different intensity. Our second measure, ‘texture’ or ‘correlation texture’ is . The correlation texture measures the linear dependency of grey levels on those of neighboring pixels. The two measures are weakly anti-correlated (Fig. 2C). We verified that the texture and contrast values that were calculated after removing the background and after rescaling to 64 gray levels have a low sensitivity to rotations (Figure S5).
Correlation analysis
For each protein, we collected all cells in all fields of view at all time frames. For each cell, 3 protein parameters (total protein, contrast and texture) and 2 motion parameters (velocity and angle change) (See Figure 2A) were calculated. Then, 6 correlation values (between all pairs) were calculated using Pearson and Spearman correlation. No binning was used to compute these correlations. The correlation values are summarized in Supplementary File S1. We tested other texture features as well (homogeneity and energy), but these did do not add known genes to the list of candidates (data not shown). Next, we collected only cells that were tracked from the beginning of the movie until its end and repeated the same calculation.
In order to establish a threshold that minimizes the false positive rate, we generated permuted datasets. In each permuted dataset, for each of the protein parameter and for each of the motion parameter, the protein values from one cell time trace were correlated to the motion values in another cell time trace in the same field of view. We repeated this permutation 10 times for each of the 6 comparisons (and both for Pearson and Spearman calculations) and generated 6 correlation distributions, for all proteins. These correlation values are summarized in the Supplementary File S3. We chose to use both Spearman and Pearson correlations since using only Spearman correlation results in marginally higher false positives (Data not shown). A correlation value of R = 0.15 (or similar) was chosen in order to minimized false positives, while maximizing potential true candidates (See Figure S7). We further tested for all the proteins with significant correlation values in which the correlation still holds even when calculating it only in one field of view (FOV) for most of its FOVs. The final candidate list includes only proteins that passed this test.
The shape of the cell is known to affect its motility [10]; in order to estimate this effect in our dataset, we calculated the correlations between the aspect ratio (major axis/minor axis) of each cell and the cell velocity and found no significant correlation in the examined clones (Figure S9).
Validation analysis
We conducted new experiments by performing time-lapse movies for 19 of the candidate clones. Of the 19, 13 proteins were chosen randomly out of the list of novel candidate proteins, and the other were candidates that were also known motility genes. 16 out of the 19 showed a correlation that is similar to the previous calculated correlation. (Results are shown in Supplementary File S3).
Function analysis
Several databases were used to annotate whether genes are known to be involved in motility (Supplementary File S2). We used GeneCards (http://www.genecards.org/) to download all genes with the keywords “motility” or “migration”. We further considered genes that were identified as part of the “adhesome” (http://www.adhesome.org/). Finally, well-studied characterized genes listed in the Review by [12] were also considered as motility genes.
Subcellular localization assignment and enrichment test
We used subcellular localization according to GeneCards (http://www.genecards.org/) and other databases as provided in the LARC database (http://www.weizmann.ac.il/mcb/UriAlon/DynamProt/). All clones used here have localization in the experiment that agrees with the previously known localization. When more than one localization was assigned to a protein, the first localization in the list was used for the category assignment. The subcellular information and categories are list in the “Candidate_genes.xls” file. Hypergeometric p-value [42] was used to calculate the enrichment of specific subcellular localization categories in the candidate genes group over all the 566 genes used in this analysis.
Mild knockdown experiments
To knockdown the expression of the tagged protein in clones from our library, we used siRNA against GFP (QIAGEN, 1022064) transfection using lipofectamin (Invitrogen) as described in their protocol. As a control siRNA, we used the non-targeting siRNA (Dharmacon, D-001810-10-05). No significant difference between siRNA used from QIAGEN or Dharmacon was detected in our system (Figure S10).
Briefly, 2×104 cells were grown on 12-well glass bottom MatTek plates. The next day, siRNA transfection was performed. We used 24 pmol of si-RNA for each well and 0.8 ul lipofectamin and incubated it for 6 hours, then we replaced with fresh media and let cells grow overnight. The next day, we took a time-lapse movie of the plate for 48 hours. We took fields of view from each well. We used the same exposure time for the well with the non-targeting siRNA (where no decrease in expression is expected) and the well with the si-GFP (which showed a decrease in the YFP fluorescent and not in the mCherry fluorescent).
Supporting Information
Zdroje
1. EldarA, ElowitzMB (2010) Functional roles for noise in genetic circuits. Nature 467 : 167–173.
2. LestasI, VinnicombeG, PaulssonJ (2010) Fundamental limits on the suppression of molecular fluctuations. Nature 467 : 174–178.
3. RaserJM, O'SheaEK (2005) Noise in Gene Expression: Origins, Consequences, and Control. Science 309 : 2010–2013.
4. ZengL, SkinnerSO, ZongC, SippyJ, FeissM, et al. (2010) Decision making at a subcellular level determines the outcome of bacteriophage infection. Cell 141 : 682–691.
5. RajA, RifkinSA, AndersenE, Oudenaarden Avan (2010) Variability in gene expression underlies incomplete penetrance. Nature 463 : 913–918.
6. BlakeWJ, KAErnM, CantorCR, CollinsJJ (2003) Noise in eukaryotic gene expression. Nature 422 : 633–637.
7. SigalA, MiloR, CohenA, Geva-ZatorskyN, KleinY, et al. (2006) Variability and memory of protein levels in human cells. Nature 444 : 643–646.
8. Stewart-OrnsteinJ, WeissmanJS, El-SamadH (2012) Cellular noise regulons underlie fluctuations in Saccharomyces cerevisiae. Mol Cell 45 : 483–493.
9. SpudichJL, KoshlandDEJr (1976) Non-genetic individuality: chance in the single cell. Nature 262 : 467–471.
10. KerenK, PincusZ, AllenGM, BarnhartEL, MarriottG, et al. (2008) Mechanism of shape determination in motile cells. Nature 453 : 475–480.
11. DunlopMJ, CoxRS3rd, LevineJH, MurrayRM, ElowitzMB (2008) Regulatory activity revealed by dynamic correlations in gene expression noise. Nat Genet 40 : 1493–1498.
12. RidleyAJ (2011) Life at the leading edge. Cell 145 : 1012–1022.
13. Weinberg RA (2007) The biology of cancer. New York: Garland Science.
14. Winograd-KatzSE, ItzkovitzS, KamZ, GeigerB (2009) Multiparametric analysis of focal adhesion formation by RNAi-mediated gene knockdown. J Cell Biol 186 : 423–436.
15. SimpsonKJ, SelforsLM, BuiJ, ReynoldsA, LeakeD, et al. (2008) Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol 10 : 1027–1038.
16. NeumannB, WalterT, HérichéJ-K, BulkescherJ, ErfleH, et al. (2010) Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464 : 721–727.
17. DanuserG, Waterman-StorerCM (2006) Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu Rev Biophys Biomol Struct 35 : 361–387.
18. CohenAA, Geva-ZatorskyN, EdenE, Frenkel-MorgensternM, IssaevaI, et al. (2008) Dynamic proteomics of individual cancer cells in response to a drug. Science 322 : 1511–1516.
19. SigalA, DanonT, CohenA, MiloR, Geva-ZatorskyN, et al. (2007) Generation of a fluorescently labeled endogenous protein library in living human cells. Nat Protoc 2 : 1515–1527.
20. EdenE, Geva-ZatorskyN, IssaevaI, CohenA, DekelE, et al. (2011) Proteome half-life dynamics in living human cells. Science 331 : 764–768.
21. ChebiraA, BarbotinY, JacksonC, MerrymanT, SrinivasaG, et al. (2007) A multiresolution approach to automated classification of protein subcellular location images. BMC Bioinformatics 8 : 210.
22. BolandMV, MurphyRF (2001) A neural network classifier capable of recognizing the patterns of all major subcellular structures in fluorescence microscope images of HeLa cells. Bioinformatics 17 : 1213–1223.
23. Farkash-AmarS, EdenE, CohenA, Geva-ZatorskyN, CohenL, et al. (2012) Dynamic Proteomics of Human Protein Level and Localization across the Cell Cycle. PLoS ONE 7: e48722.
24. WelchMD, DePaceAH, VermaS, IwamatsuA, MitchisonTJ (1997) The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol 138 : 375–384.
25. HanG, FanB, ZhangY, ZhouX, WangY, et al. (2008) Positive regulation of migration and invasion by vasodilator-stimulated phosphoprotein via Rac1 pathway in human breast cancer cells. Oncol Rep 20 : 929–939.
26. SuetsuguS, MikiH, TakenawaT (1999) Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem Biophys Res Commun 260 : 296–302.
27. NewmanJRS, GhaemmaghamiS, IhmelsJ, BreslowDK, NobleM, et al. (2006) Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 441 : 840–846.
28. TaniguchiY, ChoiPJ, LiG-W, ChenH, BabuM, et al. (2010) Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329 : 533–538.
29. ZaslaverA, BrenA, RonenM, ItzkovitzS, KikoinI, et al. (2006) A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat Methods 3 : 623–628.
30. SarovM, MurrayJI, SchanzeK, PozniakovskiA, NiuW, et al. (2012) A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell 150 : 855–866.
31. TrinhLA, HochgrebT, GrahamM, WuD, Ruf-ZamojskiF, et al. (2011) A versatile gene trap to visualize and interrogate the function of the vertebrate proteome. Genes Dev 25 : 2306–2320.
32. ChanEM, RatanasirintrawootS, ParkI-H, ManosPD, LohY-H, et al. (2009) Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nature Biotechnology 27 : 1033–1037.
33. TrcekT, ChaoJA, LarsonDR, ParkHY, ZenklusenD, et al. (2012) Single-mRNA counting using fluorescent in situ hybridization in budding yeast. Nat Protoc 7 : 408–419.
34. Shav-TalY, DarzacqX, ShenoySM, FuscoD, JanickiSM, et al. (2004) Dynamics of single mRNPs in nuclei of living cells. Science 304 : 1797–1800.
35. ItzkovitzS, LyubimovaA, BlatIC, MaynardM, van EsJ, et al. (2012) Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol 14 : 106–114.
36. ChangHH, HembergM, BarahonaM, IngberDE, HuangS (2008) Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453 : 544–547.
37. EldarA, CharyVK, XenopoulosP, FontesME, LosónOC, et al. (2009) Partial penetrance facilitates developmental evolution in bacteria. Nature 460 : 510–514.
38. NachmanI, RegevA, RamanathanS (2007) Dissecting timing variability in yeast meiosis. Cell 131 : 544–556.
39. MontanaG, BerkM, EbbelsT (2011) Modelling short time series in metabolomics: a functional data analysis approach. Adv Exp Med Biol 696 : 307–315 doi:_10.1007/978-1419-7046-6_31
40. Geva-ZatorskyN, IssaevaI, MayoA, CohenA, DekelE, et al. (2012) Using bleach-chase to measure protein half-lives in living cells. Nat Protoc 7 : 801–811.
41. HaralickRM (1979) Statistical and structural approaches to texture. Proceedings of the IEEE 67 : 786–804.
42. RivalsI, PersonnazL, TaingL, PotierM-C (2007) Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics 23 : 401–407.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



