-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
In plants, sugars function as signaling molecules that control important processes such as photosynthesis, growth, carbon distribution over different organs and the production of storage compounds. Sugar signaling requires the phytohormone abscisic acid (ABA) and the ABA-induced regulatory transcription factor ABI4. In this study, a genetic analysis identified the transcription factor ANAC060 as an important component in establishing sugar sensitivity. It was found that, in natural Arabidopsis thaliana populations, the ANAC060 protein may occur as a long or a short version due to differential ANAC060 mRNA splicing caused by a single-nucleotide polymorphism (SNP). The long ANAC060 protein with an intact transmembrane domain (TMD) is excluded from the nucleus, whereas the short version lacking the TMD is always present in the nucleus, where it regulates gene expression. Functional analyses indicated that Col ANAC060 is involved in a novel negative feedback loop in the sugar-ABA signaling pathway. In this feedback loop model, ABI4 activates ANAC060 expression, but the nuclear presence of Col ANAC060 suppresses Glc-induced ABA accumulation and ABI4 expression, thereby reducing responsiveness to sugar signals.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004213
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004213Summary
In plants, sugars function as signaling molecules that control important processes such as photosynthesis, growth, carbon distribution over different organs and the production of storage compounds. Sugar signaling requires the phytohormone abscisic acid (ABA) and the ABA-induced regulatory transcription factor ABI4. In this study, a genetic analysis identified the transcription factor ANAC060 as an important component in establishing sugar sensitivity. It was found that, in natural Arabidopsis thaliana populations, the ANAC060 protein may occur as a long or a short version due to differential ANAC060 mRNA splicing caused by a single-nucleotide polymorphism (SNP). The long ANAC060 protein with an intact transmembrane domain (TMD) is excluded from the nucleus, whereas the short version lacking the TMD is always present in the nucleus, where it regulates gene expression. Functional analyses indicated that Col ANAC060 is involved in a novel negative feedback loop in the sugar-ABA signaling pathway. In this feedback loop model, ABI4 activates ANAC060 expression, but the nuclear presence of Col ANAC060 suppresses Glc-induced ABA accumulation and ABI4 expression, thereby reducing responsiveness to sugar signals.
Introduction
Plant growth and development depends on the energy and carbon building blocks provided by soluble sugars. For efficient carbon nutrient utilization, prokaryotic and eukaryotic organisms have evolved a range of sophisticated signal transduction pathways that link sugar status to growth and reproduction [1]–[3]. In plants, such sugar-sensing and -signaling systems regulate the expression of thousands of genes involved in the control of metabolic processes, growth, development and responses to the environment [4], [5]. Sugar signaling pathways closely interact with other signaling pathways, including those for light [6], phytohormones [7], stress [8], defense [9] and other nutrients, such as nitrogen and phosphate [10].
Seedling growth and greening is inhibited when high concentrations of sugars are added to the medium. In Arabidopsis thaliana, this phenomenon of early seedling development repression has been widely used as a convenient way of identifying mutants affected in sugar-sensing or signaling processes [11], [12]. Mutants that are insensitive or oversensitive to different sugars have been isolated in this way, and the genes involved have been identified. However, such genes function in a variety of biological processes [11]–[18], and it is thus challenging to understand how these genes participate specifically in sugar signaling pathways. Prominent among these genes are HEXOKINASE1 (HXK1), which functions as a glucose (Glc) sensor [7], and genes involved in ABA and ethylene synthesis and signaling [11], [12], [17], [19]. The sugar-ABA cascade regulates many genes involved in photosynthesis and metabolism and is antagonized by ethylene signaling via the EIN3 protein [20], [21]. A central regulator in sugar-responsive gene expression in plants is the ABI4 gene, which encodes an ERF/AP2 transcription factor [11], [13], [22]–[24]. ABI4 is a regulator of seed germination, plastid-to-nucleus signaling and photosynthesis, redox status, lipid biosynthesis and breakdown, lateral root development and cell wall modification (reviewed in [25], [26]). ABI4 is a versatile transcription factor, acting as both an activator and a repressor of gene expression. ABI4 binds directly to the identified ABI4 binding motif in the promoters of target genes [27]–[29].
Natural variation analysis provides an important tool for analyzing complex biological processes [30]. Previously, quantitative trait locus (QTL) mapping for seedling sugar sensitivity in Ler/Cvi recombinant inbred line (RIL) populations led to the identification of several loci and genes controlling sugar sensitivity. For example, Glc sensing QTL5 (GSQ5) [31] encodes the delay of germination 1(DOG1) gene, a major dormancy determinant. The sugar-ABA cascade induces GSQ5/DOG1 expression, and the Cvi GSQ5/DOG1 allele effectively enhances sugar-ABA signaling, thereby increasing sugar sensitivity [31]. Arabidopsis fructose (Frc) sensing QTL6 (FSQ6) is another interesting QTL that is Frc specific, and the study of this QTL revealed an HXK1-independent Frc signaling pathway [32]. FSQ6 encodes the ANAC089 transcription factor, and the Cvi ANAC089 allele specifies a dominant fructose insensitivity trait [32].
In this study, a novel sugar-sensing QTL (GSQ11) was identified in a Col X C24 F2 population using a selective genotyping approach. GSQ11 associates with the segregation distortion region in highly Glc-insensitive F2 individuals, and the QTL was confirmed in near-isogenic lines (NILs). Further studies showed that GSQ11 encodes the Arabidopsis NAC family transcription factor 060 (ANAC060). The Col ANAC060 allele gene harbors a single-nucleotide polymorphism (SNP) that affects intron splicing, leading to a truncated protein that lacks the C-terminal membrane anchor domain. This truncated ANAC060 protein is constitutively located in the nucleus. ANAC060 is likely a direct ABI4 target, but, interestingly, the Col ANAC060 allele attenuates sugar-induced ABA accumulation and renders seedlings insensitive to sugar.
Results
Selective genotyping identification of a Glc-sensing QTL in the Col X C24 F2 population
The sugar sensitivity of Col and C24 seedlings was investigated on half-strength MS medium supplemented with increasing Glc concentrations, and it was found that C24 is substantially more sensitive to Glc than Col. On media supplemented with 5.5% Glc, the Col seedlings turned green and developed normally (Figure 1A), whereas the C24 seedlings were developmentally arrested and remained pale (Figure 1B). The growth of both accessions on 5.5% sorbitol (Sor) medium was not inhibited, showing that the sugar effect was not due to an osmotic effect (Figure 1C, D).
Fig. 1. Identification of the Glc-sensing QTL GSQ11 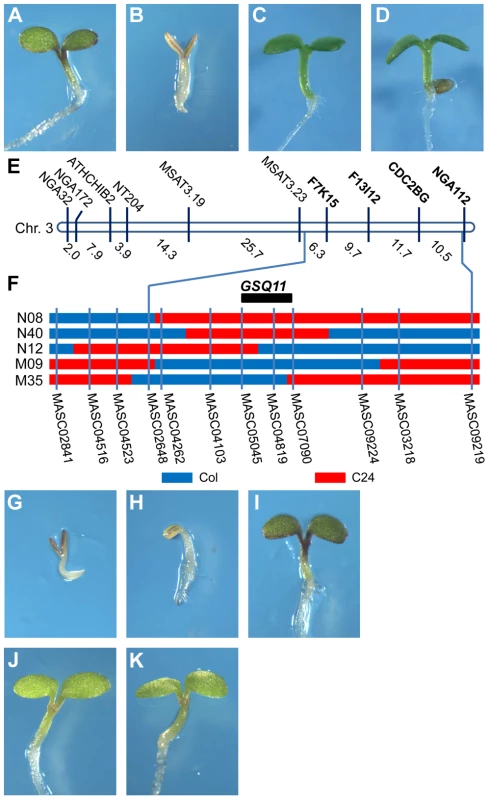
. Glc-sensitivity phenotypes of Col (A, C) and C24 (B, D) accessions. Seedlings were grown on agar-solidified 1/2 MS containing 5.5% Glc (A, B) or 5.5% Sor osmotic control (C, D) for 9 d at 22°C under continuous illumination. (E) Location of segregation distortion markers on chromosome 3. (F) Genotypes of the NILs and candidate region of GSQ11. Blue and red denote the Col and C24 accession genomic regions, respectively, (G–K) Glc sensitivities of the NILs (G) N08, (H) N40, (I) N12, (J) M09 and (K) M35 grown on agar-solidified 1/2 MS containing 5.5% glucose for 9 d at 22°C under continuous illumination. The selective genotyping strategy [33] was followed to identify QTLs for Glc sensitivity in the Col X C24 F2 population. Approximately 2000 F2 seeds were sown on 1/2 MS with 7% Glc, and 106 Glc insensitive seedlings were obtained. The genotypes of the insensitive F2 individuals were established using 81 polymorphic simple sequence length polymorphism (SSLP) markers (http://www.arabidopsis.org, http://www.inra.fr/vast/msat.php, http://amp.genomics.org.cn/). A segregation distortion region including SSLP markers F7K15, F13I12, CDC2BG and NGA112 was found on the long arm of chromosome 3 (Figure 1E, Table S1). The segregation ratios of these markers fit the 1∶2∶1 ratio in an unselected F2 population using 94 individuals (Table S1). Thus, it is likely that a Glc-sensing QTL (GSQ) is located in this region.
Confirmation and fine mapping of the Glc-sensing QTL
Near-isogenic lines (NILs) were used to further study the GSQ identified on chromosome 3. NILs N08, N12 and N40 have single genomic regions from C24 integrated into the Col background ([34], Figure 1F). Conversely, NILs M35 and M09 have single genomic regions from Col integrated into the C24 background ([34], Figure 1F). These NILs were tested for seedling Glc sensitivity. Of the NILs with a Col background, N08 and N40 showed Glc-sensitive phenotypes (Figure 1G, H), whereas NIL N12 showed Glc insensitivity comparable to Col (Figure 1I). Of the NILs with a C24 background, both M35 and M09 showed Glc insensitivity compared to C24 (Figure 1J, K). These results confirm the presence of a Glc-sensing QTL in this genomic region, with the Col sequence presenting Glc insensitivity. The QTL was named GSQ11, and its confidence interval was deduced from the NIL genotypes and Glc-sensing phenotypes. GSQ11 was localized to a 1.86-Mb region between markers MASC05045 (14.3 Mb) and MASC07090 (16.16 Mb) (Figure 1F).
GSQ11 encodes the transcription factor ANAC060
A total of 458 annotated genes are located in the GSQ11 candidate region. Conspicuously present among these genes is ANAC060 (At3g44290), which belongs to the OsNAC8 subgroup of group I NAC transcription factors that includes ANAC089 and ANAC040 [35]. ANAC089 was previously identified as determining seedling Frc sensitivity. The Cvi ANAC089 allele specifically suppresses Frc signaling, allowing seedling development to proceed on high-fructose-containing media [32]. Therefore, the effect of ANAC060 on Glc sensitivity and the possible association between ANAC060 and GSQ11 were investigated further.
A T-DNA insertion mutant of anac060 (Salk_012554C, Col background), in which a T-DNA insert is present in the second intron of the gene, was obtained (Figure S1A), and the insertion was confirmed by a PCR analysis (Figure S1B). No ANAC060 mRNA could be detected by RT-PCR in this mutant (Figure S1C). Interestingly, the growth of anac060 on 5.5% Glc resulted in seedling developmental arrest (Figure 2A), whereas Col is Glc insensitive. The Glc-sensitive phenotype of anac060 was found not to be the result of osmotic stress, as indicated by normal growth on 5.5% Sor (Figure 2B).
Fig. 2. GSQ11 encodes ANAC060 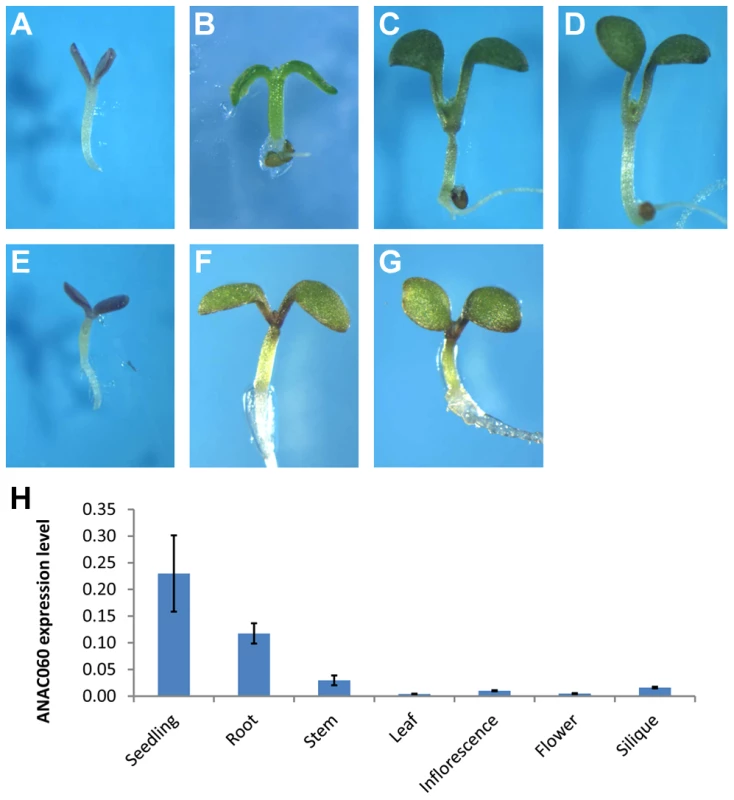
. (A) Glc sensitivity of anac060. Mutant seedlings were grown on agar-solidified 1/2 MS containing 5.5% Glc for 9 d at 22°C under continuous light. (B) anac060 growth on 5.5% Sor allows greening and development. Seedlings were grown on agar-solidified 1/2 MS containing 5.5% Sor for 9 d at 22°C under continuous light. (C–E) Allelism tests. Glc-sensitivity phenotypes of Col X anac060 F1 (C), Col X N40 F1 (D) and NIL N40 X anac060 F1 (E). Seedlings were grown on agar-solidified 1/2 MS containing 5.5% Glc for 9 d at 22°C under continuous light. (F–G) Transgenic complementation. (F) Glc sensitivity of the transgenic anac060 mutant transformed with the Col ANAC060 gene and (G) transgenic NIL N40 transformed with the Col ANAC060 gene. Seedlings were grown on agar-solidified 1/2 MS containing 5.5% Glc for 9 d at 22°C under continuous light. (H) ANAC060 expression levels in 7-day-old seedlings and in different organs of 30-day-old plants. Allelism tests for GSQ11 and ANAC060 were performed, and the F1 seedlings of Col X anac060 and Col X N40 were both found to be insensitive to 5.5% Glc, similar to Col. This finding further indicated that both the C24 GSQ11 allele and anac60 are recessive (Figure 2C, D). Importantly, the F1 seedlings of the anac060 X N40 cross were found to be Glc sensitive, as are the N40 and anac060 parents (Figure 2E), strongly suggesting that GSQ11 and ANAC060 are allelic.
GSQ11 and ANAC060 allelism was confirmed by transferring the Col ANAC060 gene into N40 and anac060 using the floral dip transformation method. Seedlings of the N40 and anac060 lines transformed with the Col ANAC060 gene were Glc insensitive, unlike the Glc-sensitive N40 and anac060 parents (Figure 2F, G). These results identify ANAC060 as GSQ11.
The Frc sensitivities of anac060 and of anac060 transformed with Col ANAC060 were then investigated. Seedling development of anac060 was arrested on media containing 6.5% Frc (Figure S2B), whereas the seedlings of anac60 transformed with Col ANAC060 and of wild-type Col were green and developed normally (Figure S2A, C). The Frc-sensitive phenotype of the anac060 seedlings also reverted to Frc insensitivity in anac060 harboring the Col ANAC060 transgene (Figure S2C). These results show that ANAC060 is involved in both Glc and Frc signaling.
ANAC060 expression levels were determined in different plant tissues by qPCR. ANAC060 was found to be highly expressed in both seedlings and roots and weakly expressed in the leaf, stem, flower and silique (Figure 2H).
Differential mRNA splicing renders Col GSQ11/ANAC060 insensitive to Glc
ANAC060 is annotated as a NAC transcription factor with a transmembrane domain (TMD) and was named NAC with transmembrane motif 1-like 5 (NTL5) [36]. The TAIR (www.arabidopsis.org)-annotated ANAC060 protein consists of 335 amino acids with a C-terminally located TMD. Remarkably, the Col ANAC060 full-length cDNA clone GSLTLS7ZC02 (www.ncbi.nlm.nih.gov/nucleotide/BX823820) encodes a protein of 228 amino acids that lacks the TMD. In yeast, the Col ANAC060 protein fused to the GAL4 DNA-binding protein strongly activated a GAL4 UAS promoter-containing reporter gene (Figure S3), suggesting that ANAC060 functions as a transcriptional activator. Serial deletions of the Col ANAC060 protein were tested for transcriptional activity in yeast, resulting in the identification of a region including V196 D197 F198 that is conserved in ANAC060 and ANAC089 as essential for transcriptional activity (Figure S3). An alignment analysis showed that, in the Col ANAC060 mRNA coding sequence, 20 base pairs (bp) are inserted that include an in-frame TAA stop codon, explaining the Col ANAC060 protein truncation. These 20 bp are absent in the C24 ANAC060 mRNA. It appears that the Col and C24 ANAC060 mRNAs are spliced differently (Figure 3A).
Fig. 3. A QTN alters the splicing pattern of Col and C24 ANAC060 mRNAs. 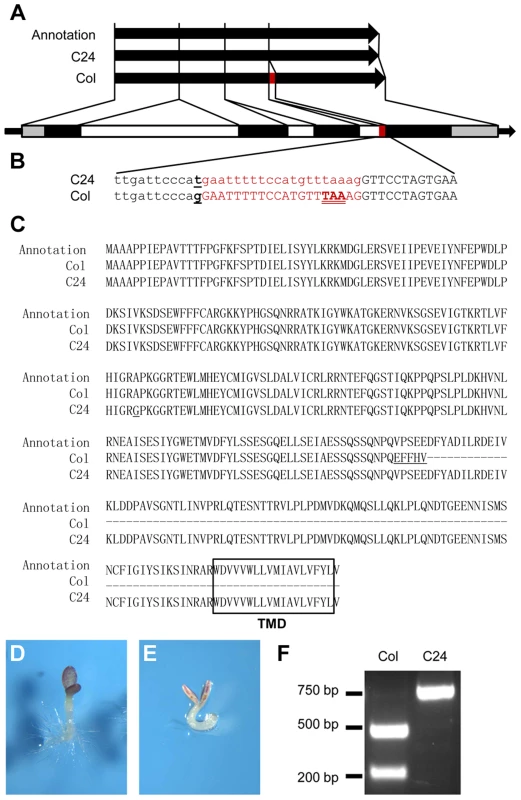
(A and B) Splicing patterns of Col and C24 ANAC060 mRNA compared to the TAIR annotation. The red bar indicates the extra 20 nucleotides in the Col accession. The black and open bars indicate the exons and introns, respectively. The grey bars represent the 5′ and 3′ UTRs. (B) Details of the SNP and splicing patterns. The underlined letter indicates the SNP. The red print indicates the sequence of the 20-nucleotide fragment that is absent (small caps) in the C24 mRNA and present (large caps) in the Col mRNA. The in-frame TAA stop codon in Col is double underlined. (C) Amino acid sequences of the TAIR annotation (top), Col (middle) and C24 (bottom) GSQ11/ANAC060 proteins. The transmembrane domain (TMD) is boxed. The underlined letters indicate the amino acid changes compared to the TAIR annotation. (D–E) Glc sensitivities of transgenic anac060 (D) and N40 (E) transformed with the mutated Col ANAC060G-T gene. Seedlings were grown on agar-solidified 1/2 MS containing 5.5% Glc for 9 days at 22°C under continuous illumination. (F) The CAPS marker for detecting the QTN between Col and C24. The primer sequences were CAPS-F 5′ GGAAAGCCACAGGAAAAGAGC 3′ and CAPS-R 5′ CACCAACACGGCTATCATAACGAG 3′. The Col-type alleles are digested by ScrFI, whereas the Cvi-type alleles are not. Comparing the genomic sequences of the Col and C24 ANAC060 genes revealed the probable cause for the differential splicing. An SNP is located precisely at the 3′ end of the 3rd intron splicing acceptor site of the Col ANAC060 gene. In Col, a G nucleotide is located at this position, whereas this is a T in C24 (Figure 3B). This G to T transversion affects the splicing pattern: a functional AG acceptor splice site is present in Col and a non-functional AT site is present in C24 (Figure 3B). However, the annotation in the TAIR database does not include this AG acceptor splice site, producing the 335-amino acid-long version of the ANAC60 protein that includes the TMD (Figure 3C).
The association between the ANAC060 splicing pattern and Glc sensitivity was illustrated by introducing a G to T point mutation in the Col ANAC060 gene and transforming this mutated Col gene into anac060 and NIL N40. cDNA derived from the mRNA of transgenic anac060 harboring the Col ANAC060G-T construct was sequenced. Importantly, the Col ANAC060G-T lines showed the same splicing pattern as C24 (Figure S4A). The splicing pattern in the transgenic anac060 lines transformed with the wild-type Col ANAC060 gene was identical to that of the Col accession (Figure S4B). As expected, the introduced mutated ANAC060G-T gene did not complement the Glc-sensitivity phenotypes of anac060 and NIL N40 (Figure 3D, E). These results confirm that the G-T SNP is responsible for the differential splicing in Col and C24 and establishes this SNP as a quantitative trait nucleotide (QTN) for sugar sensitivity.
The frequency of the natural G-T substitution causing the Glc-sensitive phenotype was investigated in Arabidopsis accessions. A cleaved amplified polymorphic sequence (CAPS) marker that specifically detects this SNP was developed (Figure 3F), and the haplotypes of 66 accessions were established. Six of the 66 accessions tested were of the Col type, and the remaining 60 accessions were of the C24 type (Table S2). The ANAC060 SNP was further analyzed using public data on 507 resequenced Arabidopsis accessions (http://signal.salk.edu/atg1001/3.0/gebrowser.php). The haplotypes of 436 accessions were T, including C24; those of 69 accessions were G, including Col (Table S3). Accessions Got-22 and Got-7 have a C nucleotide at the splice site position, differing from both Col and C24.
Differential localization of the Col and C24 ANAC060 proteins
The absence of a TMD in the truncated Col-type ANAC060 protein most likely results in differential protein localization compared to the C24-type ANAC060 protein, which includes the TMD. Differential localization of Col and C24 ANAC060 proteins was investigated by fusing these proteins N-terminally to GFP, followed by transient expression in Arabidopsis mesophyll protoplasts using the PA7 vector (created by Dr. Katrin Czempinski, Potsdam University, Germany). As expected, the PA7 vector expressing only the GFP protein was localized by confocal laser-scanning microscopy in the cytosol and nucleus (Figure 4A–D). Interestingly, the Col GFP::ANAC060 fusion protein was located exclusively in the nucleus (Figure 4E–H), whereas the C24 GFP::ANAC060 fusion protein was located in the cytosol (Figure 4I–L). Therefore, the presence of the TMD effectively retains the C24 ANAC060 protein in the cytosol, most likely in association with endomembranes [37].
Fig. 4. Localization of the Col and C24 ANAC060 proteins. 
(A–D) Localization of GFP expressed from the control vector PA7, (A) GFP fluorescence image, (B) chloroplast fluorescence, (C) bright-field image and (D) merged image of A–C. (E–H) Col GFP::ANAC060 fusion protein localization, (E) GFP fluorescence, (F) chloroplast fluorescence, (G) bright-field image and (H) merged image of E–G. (I–L) C24 GFP::ANAC060 fusion protein localization, (I) GFP fluorescence, (J) chloroplast fluorescence, (K) bright-field image and (L) merged image of I–K. Glc induction of ANAC060 requires ABA signaling
The Glc responsiveness of ANAC060 was investigated, and it was found that this gene is strongly induced by Glc (Figure 5A). Many Glc-responsive genes depend on ABA synthesis and signaling. Interestingly, ANAC060 does not respond to Glc in the aba2-1 ABA-deficient mutant and the abi4-1 ABA signaling mutant (Figure 5A). Thus, ANAC060 is responsive to the sugar-ABA signaling cascade. The genetic interaction between the ABA signaling pathway and GSQ11/ANAC060 in determining Glc sensitivity was investigated in anac060 aba2 and anac060 abi4 double mutants. Both the anac060 aba2 and anac060 abi4 double mutants showed sugar-insensitive phenotypes similar to the aba2 and abi4 single mutants (Figure 5B, C). These results show that ABA2 and ABI4 are epistatic to GSQ11/ANAC060.
Fig. 5. Glc induction of ANAC060 requires ABA signaling. 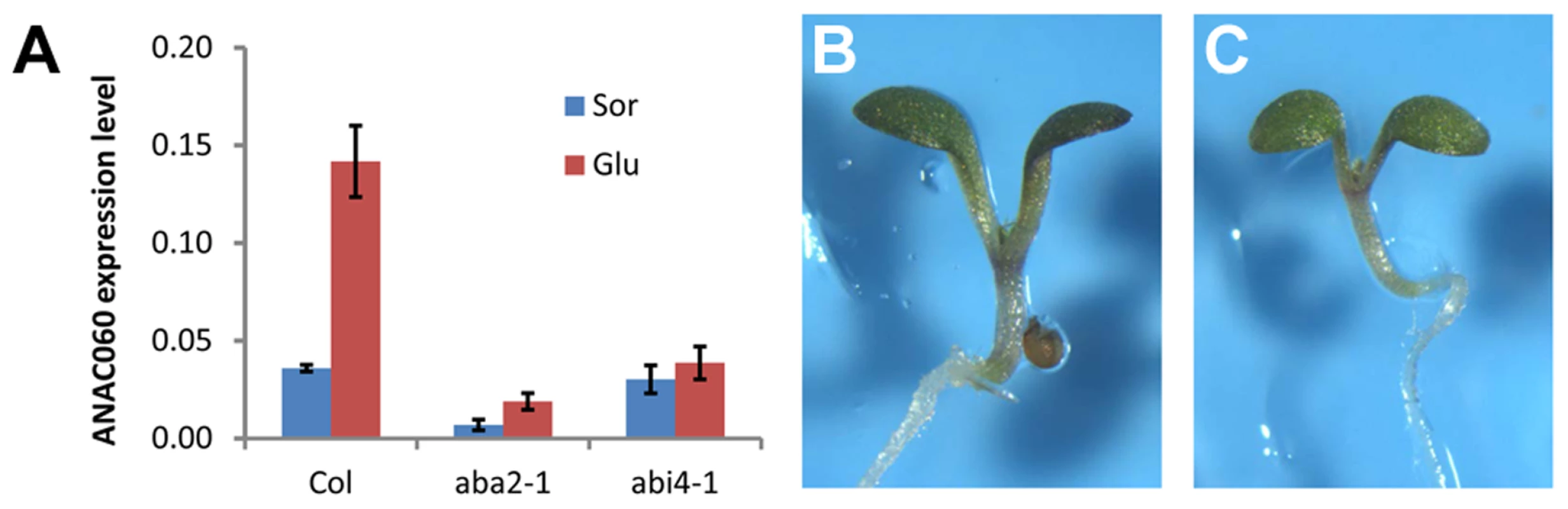
(A) ANAC060 mRNA levels in Col wild-type, aba2-1 and abi4-1 mutants grown at 22°C under continuous illumination for 7 days in 5.5% Glc or 5.5% Sor. The values represent the average of three technical repeats from a representative experiment. The bars indicate the standard errors. Similar results were obtained in three independent experiments. (B) Glc sensitivities of the anac060 aba2 double mutant and (C) anac060 abi4 double mutant. Seedlings were grown on agar-solidified 1/2 MS containing 5.5% Glc for 9 d at 22°C under continuous light. ABI4 binds to and activates the ANAC060 promoter
ABI4 regulates many sugar-responsive genes by direct promoter binding via the ABI4 binding motif [28]. In ANAC060, a CACCG ABI4 binding motif is located 1649 bp upstream of the ATG initiation codon (Figure 6A), suggesting that ABI4 induces ANAC060 by directly binding to the promoter. Accordingly, direct ABI4 binding to the ANAC060 promoter was investigated using ChIP (chromatin immunoprecipitation)-qPCR in a 35S-ABI4::GFP transgenic line (Col accession) [38]. This transgenic line showed a Glc-sensitive phenotype, indicating that the ABI4::GFP fusion protein retained its biological function (Figure S5A–B). The ANAC060 mRNA level in this ABI4-overexpressing line grown on glucose was approximately 5-fold higher than in wild-type (). Fragmented chromatin was immunoprecipitated using an anti-GFP antibody, and ANAC060 promoter fragments were quantitated using qPCR. In these experiments, a close to four-fold enrichment was observed for the CACCG-containing region (P1) in the ANAC060 promoter relative to the control; no such enrichment was found in a control region (P2) of the promoter (Figure 6B). Thus, it is likely that ABI4 binds directly to the CACCG region of the ANAC060 promoter in vivo.
Fig. 6. ABI4 directly activates ANAC060. 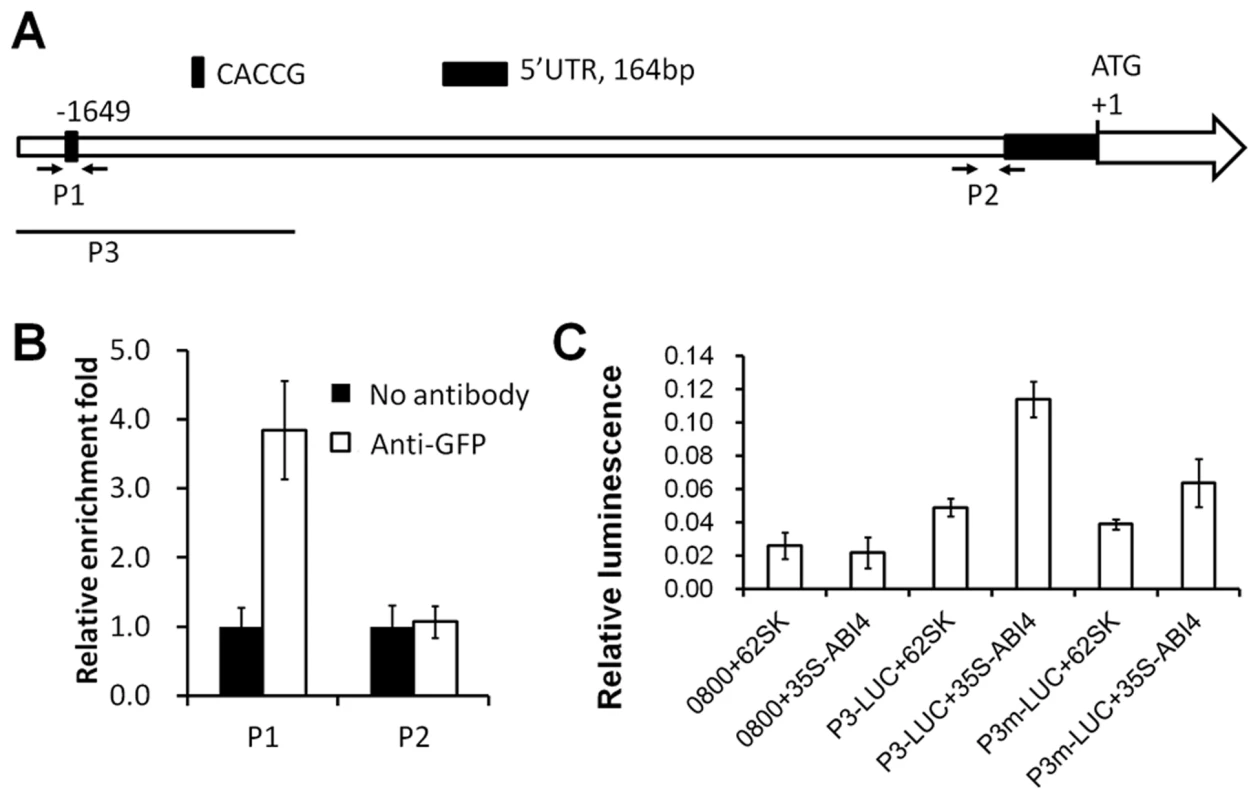
(A) The ANAC060 promoter region: P1 indicates the CACCG motif bound by ABI4, and P2 is proximal to the transcription initiation site. The arrows indicate the primers used for the P1 and P2 region ChIP-qPCR analyses. P3 indicates the fragment used in the transient expression assays. (B) P1 and P2 promoter fragment enrichment following ChIP-qPCR performed in the absence (No antibody) or presence (Anti-GFP) of anti-GFP antibodies. (C) Protoplast transient expression assay using the ANAC060 P3 promoter fragment (A). The luciferase luminescence intensity was quantitated following transfection with different vectors. 0800+62SK indicates protoplast transfection with the empty pGreenII 0800-LUC and pGreenII 62-SK vectors. 0800+35S-ABI4 indicates protoplast transfection with the empty pGreenII 0800-LUC vector and pGreenII 35S-ABI4. P3-LUC+62SK indicates protoplast transfection with pGreenII with the ANAC060 P3 promoter fragment fused to LUC and the empty pGreenII 62-SK vector. P3-LUC+35S-ABI4 indicates protoplast transfection with pGreenII with the ANAC060 P3 promoter fragment fused to LUC and pGreenII 35S-ABI4. P3m-LUC+62SK indicates protoplast transfection of pGreenII with the mutated (CACCG to TTTAA) ANAC060 P3 promoter fragment fused to LUC and the empty pGreenII 62-SK vector. P3m-LUC+35S-ABI4 indicates protoplast transfection with pGreenII with the mutated (CACCG to TTTAA) ANAC060 P3 promoter fragment fused to LUC and pGreenII 35S-ABI4. Three biological repeats were performed, and cotransfection with 35S-Renilla LUC was used for normalization. The regulation of ANAC060 expression by ABI4 was investigated using Arabidopsis mesophyll protoplasts and the dual luciferase transient expression system. A luciferase reporter plasmid was constructed containing the P3 part of the ANAC060 promoter fused to LUC (P3-LUC) in the pGreenII vector (Figure 6A). High relative luciferase activity was detected only when the reporter construct was co-transfected with the 35S-ABI4 construct in the pGreenII vector (35S-ABI4) (Figure 6C). The transfection of vectors lacking either 35S-ABI4 or the P3 target promoter element resulted in low relative luciferase activity comparable to single transfections of 35S-ABI4 or P3-LUC (Figure 6C). The role of the CACCG ABI4 binding motif in ANAC060 promoter activation was investigated by mutational analysis. Cotransfection of 35S-ABI4 and the P3 element harboring a mutated CACCG sequence (P3m-LUC) caused reduced luciferase activity compared to the wild-type P3-LUC sequence (Figure 6C). These findings, together with the ChIP-qPCR results, support the conclusion that ABI4 induces ANAC060 transcription in vivo.
ANAC060 attenuates Glc-induced ABA accumulation and ABA sensitivity
ABA levels were quantitated in 5-day-old Col and anac060 seedlings grown on 5.5% Glc or on a Sor osmotic control. Growth on Glc increased the ABA content both in Col and anac060 (Figure 7A) compared to the osmotic control. Interestingly, the ABA levels in the Glc-grown anac060 seedlings were ∼twofold higher than in Col (Figure 7A). As expected, Glc induced ABI4 expression in Col, but a nearly fourfold higher ABI4 mRNA level was detected in anac060 (Figure 7B). The increases in ABA and ABI4 expression in anac060 likely explain the anac060 Glc-sensitive phenotype.
Fig. 7. ANAC060 attenuates Glc-induced ABA accumulation and ABA sensitivity. 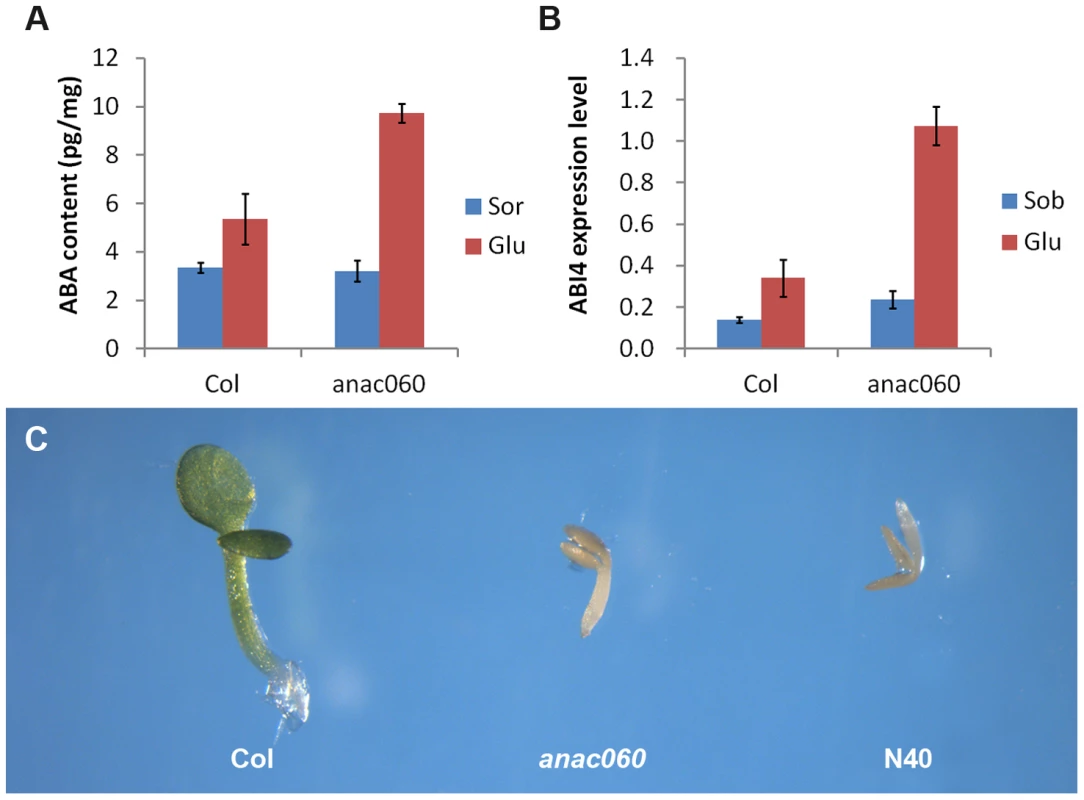
(A) ABA content of Col and anac060 seedlings grown at 22°C under continuous illumination for 5 days on 5.5% Glc or 5.5% Sor. (B) ABI4 expression level of Col and anac060 seedlings grown at 22°C under continuous illumination for 5 days on 5.5% Glc or 5.5% Sor. (C) Col, anac060 and NIL N40 seedlings grown on 1/2 MS with 0.5 µmol/L ABA for 5 days under continuous illumination. ABA sensitivity was investigated in Col, anac060 and NIL N40 in the absence of added sugars. Supplementing the 1/2 MS growth medium with 0.5 µmol/L ABA repressed seedling development in anac060 and NIL N40 compared to a control medium, whereas Col seedling development was unaffected (Figure 7C, Figure S6). These results indicate that the Col ANAC060 reduces sensitivity to ABA.
Discussion
The preparation of RIL populations for QTL mapping is laborious and time consuming. In contrast, selective genotyping represents a rapid and convenient way to locate QTLs [33], [39]. Selective genotyping has been used to map QTLs in maize [40], wheat [41], barley [42], tomato [43]–[45] and rat [46]. Glc sensitivities of C24 and Col accessions were compared, and it was found that C24 is substantially more sensitive to Glc than is Col. Glc insensitivity was investigated in Col X C24 F2 individuals, and a segregation distortion region was identified. The segregation distortion-associated QTL, GSQ11, was confirmed in NIL lines with Col and C24 backgrounds.
Previously, QTL analysis for sugar sensitivity was undertaken in the Ler/Cvi RIL population, and nine QTLs specifying Glc or Frc sensitivity were identified [31], [32]. The GSQ11 locus identified here represents a novel locus not previously observed in the Ler/Cvi RIL population. GSQ11 is identical to ANAC060, a novel gene that determines sugar sensitivity. In natural populations, ANAC060 is present in two variants: the Col variant, encoding a 228-amino acid-long protein that lacks the TMD, and a C24 variant encoding a 335-amino acid-long protein that includes the TMD. In the Col ANAC060 allele, an SNP creates a functional AG splice acceptor site, resulting in differential splicing that introduces a premature stop codon; conversely, an AT sequence is present at this location in C24, resulting in normal splicing and a full-length protein. As a consequence, the Col protein is constitutively localized to the nucleus, whereas the C24 protein is retained in the cytosol by its TMD.
Membrane-tethered transcription factors (MTTFs) are found in virtually all major transcription factor families in plants [47]. The TMDs of MTTFs are essential for membrane insertion, thereby excluding the proteins from the nucleus. However, developmental, metabolic and environmental stimuli can induce proteolytic cleavage of the TMD in MTTFs, resulting in their nuclear migration, where they can regulate target genes [48]. This mechanism allows for rapid responses to changing conditions. For example, membrane-attached ANAC089 TMD cleavage is controlled by the cellular redox status, and reducing conditions promote the nuclear migration of this protein [37]. At least 18 NAC transcription factors with TMDs are encoded by the Arabidopsis genome, including ANAC060 and ANAC089 [47]. Previously, a natural variation analysis identified the Cvi allele of the ANAC089/FSQ6 transcription factor as promoting seedling Frc insensitivity. This Cvi ANAC089 allele lacks a TMD, similar to Col ANAC060, and the truncated Cvi ANAC089 protein is also localized to the nucleus. However, truncated ANAC089 is specifically Frc insensitive, whereas truncated ANAC060 suppresses the response to both Glc and Frc. Further investigations are needed to understand the mechanism by which nuclear-localized ANAC060 and ANAC089 proteins establish different responses to sugars and the role of ABA signaling in this process. Such investigations include the identification of genes differentially regulated by Glc and Frc in variants of ANAC060 and ANAC089.
Glc induces GSQ11/ANAC060 expression, and this induction requires an intact ABA signaling pathway. The Glc sensitivity of the anac060 mutant depends on an intact ABA signaling pathway, and anac060 becomes Glc insensitive when combined with ABA biosynthesis (aba2) or signaling (abi4) mutants. The ABA-induced transcription factor ABI4 is a central regulator of sugar-responsive gene expression in plants. ABI4 induces ANAC060 expression by interacting with the ABI4 binding motif in the ANAC060 promoter. Two - to threefold higher Glc-induced ABA accumulation was reported in the Glc-treated gin6/abi4 mutant [13], suggesting that ABI4 induction inhibits ABA accumulation in the sugar-ABA signaling pathway. In the present study, enhanced sugar-induced ABA accumulation was also observed in the anac060 mutant. Therefore, ANAC060 localized to the nucleus also appears to be part of a negative feedback loop in the ABA-mediated sugar signaling pathway. The sugar-ABA signaling pathway induces the expression of ANAC060 through ABI4, whereas the nuclear presence of the ANAC060 protein represses sugar-ABA signaling, rendering seedlings insensitive to sugars.
Materials and Methods
Plant materials
The F2 population derived from a cross between Col and C24 was used for the QTL analysis. N08, N12 and N40 are NILs with single genomic regions of C24 integrated into the Col background; M35 and M09 are NILs with single genomic regions of Col integrated into the C24 background [34]. The anac060 T-DNA insertion mutant, Salk_012554C, was obtained from ABRC. 35S-ABI4::GFP transgenic line was provided by Dr. Xie [38].
Analysis of sugar sensitivity and ABA sensitivity
Sterilized seeds were placed on 0.1% agarose for 4 days at 4°C in the dark for stratification and then plated on solidified 1/2 MS medium, pH 5.8. Sugars, sorbitol and ABA were added at the concentrations indicated. The plates were incubated at 22°C under continuous fluorescent light for 9 days, and the sugar - and ABA-sensitivity phenotypes were scored.
QTL analysis by selective genotyping
A total of 106 Col X C24 F2 individuals insensitive to 7% Glc were selected and genotyped together with an unselected F2 population of 94 individuals. Eighty-one polymorphic SSLP markers between Col and C24 were used for genotyping (http://www.arabidopsis.org, http://www.inra.fr/vast/msat.php, http://amp.genomics.org.cn/). The statistical approach for QTL identification was as previously described [44].
Constructs
The promoter (1794 bp upstream of the start codon) and coding regions (2111 bp) of ANAC060 were amplified separately from Col genomic DNA using the primers presented in Table S4 and individually cloned into the pCAMBIA1301 to construct complementation vectors. The G-T point mutation was introduced into the complementation vector using the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene). The coding sequences of the ANAC060 gene from C24 (1028 bp) and Col (687 bp) were amplified from cDNA using the primers presented in Table S5 and subcloned into PA7 to construct vectors for the protein localization assay in protoplasts.
Transactivation activity assay in yeast
The transactivation activity assays in yeast were performed using the Matchmaker GAL4 Two-Hybrid System 3 (Clontech), with modifications as described [49]. The full-length ANAC060 cDNA (clone GSLTLS7ZC02) was used to create pANAC060ΔC1, pANAC060ΔC2 and pANAC060ΔC3 by PCR amplification using the primers presented in Table S6. The PCR products were digested with BamHI and PstI and inserted into PGBKT7, resulting in an in-frame fusion of ANAC060 sequences with the GAL4 DNA-binding domain. The plasmids were transformed into yeast strain PJ69-4A, and the yeast cultures were grown overnight in liquid medium and diluted to an OD600 of 0.5. Serially dilutions were dropped onto either -Trp SD media or -Trp/-His/-Ade SD media.
ChIP-qPCR
Seeds of Col and the 35S-ABI4::GFP transgenic line were grown on 1/2 MS medium for approximately 2 weeks. Seedlings (3 g) were harvested and crosslinked with 1% formaldehyde for 15 minutes under vacuum; the crosslinking was stopped by the addition of 0.125 M glycine. The seedlings were homogenized in liquid nitrogen, and nuclei were isolated. The isolated nuclei were treated with ultrasonic waves (Bioruptor UCD-200) to fragment the chromatin into 200–300-bp fragments. Immunoprecipitations were performed with an anti-GFP antibody (Abcam) and protein A beads (Millipore). Mock immunoprecipitations in the absence of anti-GFP served as controls. DNA was precipitated by isopropanol, washed with 70% ethanol and dissolved in 35 µl water containing 10 µg/µl RNase. The qPCR analysis was performed using primers (Table S7) corresponding to different promoter regions of ANAC060. The relative enrichment of each fragment was calculated by normalizing the values for transgenic plants against the values for wild-type, as described [50].
Dual luciferase essay
The Arabidopsis protoplast isolation and transfection followed the protocols of Dr. Sheen's laboratory [51]. The P3 region of the ANAC060 promoter was amplified (Table S8) and cloned into the pGreenII 0800-LUC (firefly luciferase) vector [52] to construct the pGreenII P3-LUC vector. The mutated P3 fragment (P3m) was artificially synthesized (Shanghai Generay Biotech Co., Ltd.) and cloned into the pGreenII 0800-LUC vector, similar to the P3 fragment. The ABI4 cDNA was fused to the pGreenII 62-SK vector [52] to construct the pGreenII 35S-ABI4 vector. In each co-transfection assay, 6 µg pGreenII P3-LUC plasmid and 12 µg 35S-ABI4 plasmid were used. Protoplasts that had been incubated for 16 h were subjected to luciferase assays using the Promega dual-luciferase reporter assay system and the GloMax 20/20 luminometer.
Real-time RT-PCR
Total RNA was isolated using the Plant RNA Kit from Yuanpinghao Bio (www.yph-bio.com). Aliquots (1 µg) were reverse transcribed using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech). Five µl of cDNA was used per real-time PCR reaction with 20 µl GoTaq q-PCR Master Mix from Promega (http://www.promega.com) and 0.8 µl of primers diluted to 10 pM (Table S9). PCR was performed using the Roche LightCycler 96 SW 1.0 Real-Time PCR system sequence detector. The expression levels of the ANAC060 and ABI4 genes were calculated relative to the PP2A [53] levels using the Q-gene method, which takes the relative efficiencies of the different primer pairs into account [54], [55]. The sequences of the primers used for the amplification of ANAC060 and ABI4 are presented in Table S9.
Quantification of endogenous ABA
Seedlings were ground in liquid nitrogen, and 150 mg of the powder was homogenized and extracted for 24 h in methanol containing D6-ABA (OIChemIm Co. Ltd.) as an internal standard. ABA was purified using an Oasis Max solid-phase extraction cartridge (150 mg/6 cc; Waters) and eluted with 5% formic acid in methanol. The eluate was dried, and ABA was quantitated as described [38] using a liquid chromatography–tandem mass spectrometry system with an Acquity ultra-performance liquid chromatograph (Acquity UPLC; Waters) and a triple quadruple tandem mass spectrometer (Quattro Premier XE; Waters). Three biological replications were performed for each treatment.
Supporting Information
Zdroje
1. GibsonS (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8 : 93–102.
2. RollandF, Baena-GonzalezE, SheenJ (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 : 675–709.
3. SmeekensS (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 : 49–81.
4. EvelandAL, JacksonDP (2012) Sugars, signalling, and plant development. J Exp Bot 63 : 3367–3377.
5. PriceJ, LaxmiA, St MartinSK, JangJC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16 : 2128–2150.
6. DijkwelPP, HuijserC, WeisbeekPJ, ChuaNH, SmeekensSC (1997) Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell 9 : 583–595.
7. MooreB, ZhouL, RollandF, HallQ, ChengWH, et al. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 : 332–336.
8. Baena-GonzalezE, RollandF, TheveleinJM, SheenJ (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448 : 938–942.
9. Bolouri MoghaddamMR, den EndeWV (2013) Sugars, the clock and transition to flowering. Front Plant Sci 4 : 22.
10. LeiM, LiuD (2011) Sucrose regulates plant responses to deficiencies in multiple nutrients. Plant Signal Behav 6 : 1247–1249.
11. LabyR, KincaidM, KimD, GibsonS (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23 : 587–596.
12. ZhouL, JangJ, JonesT, SheenJ (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95 : 10294–10299.
13. Arenas-HuerteroF, ArroyoA, ZhouL, SheenJ, LeonP (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14 : 2085–2096.
14. CarvalhoRF, CarvalhoSD, DuqueP (2010) The plant-specific SR45 protein negatively regulates glucose and ABA signaling during early seedling development in Arabidopsis. Plant Physiol 154 : 772–783.
15. ChenJG, JonesAM (2004) AtRGS1 function in Arabidopsis thaliana. Methods Enzymol 389 : 338–350.
16. CuiH, HaoY, KongD (2012) SCARECROW has a SHORT-ROOT-independent role in modulating the sugar response. Plant Physiol 158 : 1769–1778.
17. GibsonSI, LabyRJ, KimD (2001) The sugar-insensitive1 (sis1) Mutant of Arabidopsis Is Allelic to ctr1. Biochemical and Biophysical Research Communications 280 : 196–203.
18. HuangY, LiCY, PattisonDL, GrayWM, ParkS, et al. (2010) SUGAR-INSENSITIVE3, a RING E3 ligase, is a new player in plant sugar response. Plant Physiol 152 : 1889–1900.
19. HuangY, LiCY, BiddleK, GibsonS (2008) Identification, cloning and characterization of sis7 and sis10 sugar-insensitive mutants of Arabidopsis. BMC Plant Biology 8 : 104.
20. DekkersB, SchuurmansJ, SmeekensS (2008) Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Molecular Biology 67 : 151–167.
21. YanagisawaS, YooSD, SheenJ (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425 : 521–525.
22. FinkelsteinR, WangM, LynchT, RaoS, GoodmanH (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an Apetala2 domain protein. Plant Cell 10 : 1043–1054.
23. HuijserC, KortsteeA, PegoJ, WeisbeekP, WismanE, et al. (2000) The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J 23 : 577–586.
24. RookF, CorkeF, CardR, MunzG, SmithC, et al. (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26 : 421–433.
25. LeonP, GregorioJ, CordobaE (2012) ABI4 and its role in chloroplast retrograde communication. Front Plant Sci 3 : 304.
26. WindJJ, PevianiA, SnelB, HansonJ, SmeekensSC (2013) ABI4: versatile activator and repressor. Trends Plant Sci 18 : 125–132.
27. Acevedo-HernándezGJ, LeónP, Herrera-EstrellaLR (2005) Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. The Plant Journal 43 : 506–519.
28. BossiF, CordobaE, DupreP, MendozaMS, RomanCS, et al. (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59 : 359–374.
29. ReevesWM, LynchTJ, MobinR, FinkelsteinRR (2011) Direct targets of the transcription factors ABA-Insensitive(ABI)4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol 75 : 347–363.
30. Alonso-BlancoC, AartsMG, BentsinkL, KeurentjesJJ, ReymondM, et al. (2009) What has natural variation taught us about plant development, physiology, and adaptation? Plant Cell 21 : 1877–1896.
31. TengS, RognoniS, BentsinkL, SmeekensS (2008) The Arabidopsis GSQ5/DOG1 Cvi allele is induced by the ABA-mediated sugar signalling pathway, and enhances sugar sensitivity by stimulating ABI4 expression. Plant J 55 : 372–381.
32. LiP, WindJJ, ShiX, ZhangH, HansonJ, et al. (2011) Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc Natl Acad Sci U S A 108 : 3436–3441.
33. DarvasiA, SollerM (1992) Selective genotyping for determination of linkage between a marker locus and a quantitative trait locus. Theoretical and Applied Genetics 85 : 353–359.
34. TorjekO, MeyerRC, ZehnsdorfM, TeltowM, StrompenG, et al. (2008) Construction and analysis of 2 reciprocal Arabidopsis introgression line populations. J Hered 99 : 396–406.
35. OokaH, SatohK, DoiK, NagataT, OtomoY, et al. (2003) Comprehensive Analysis of NAC Family Genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10 : 239–247.
36. KimS-Y, KimS-G, KimY-S, SeoPJ, BaeM, et al. (2007) Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucl Acids Res 35 : 203–213.
37. KleinP, SeidelT, StockerB, DietzKJ (2012) The membrane-tethered transcription factor ANAC089 serves as redox-dependent suppressor of stromal ascorbate peroxidase gene expression. Front Plant Sci 3 : 247.
38. ShuK, ZhangH, WangS, ChenM, WuY, et al. (2013) ABI4 Regulates Primary Seed Dormancy by Regulating the Biogenesis of Abscisic Acid and Gibberellins in Arabidopsis. PLoS Genet 9: e1003577.
39. SunY, WangJ, CrouchJ, XuY (2010) Efficiency of selective genotyping for genetic analysis of complex traits and potential applications in crop improvement. Molecular Breeding 26 : 493–511.
40. FarkhariM, KrivanekA, XuY, RongT, NaghaviMR, et al. (2013) Root-lodging resistance in maize as an example for high-throughput genetic mapping via single nucleotide polymorphism-based selective genotyping. Plant Breeding 132 : 90–98.
41. PrasadM, VarshneyRK, KumarA, BalyanHS, SharmaPC, et al. (1999) A microsatellite marker associated with a QTL for grain protein content on chromosome arm 2DL of bread wheat. Theoretical and Applied Genetics 99 : 341–345.
42. AyoubM, MatherDE (2002) Effectiveness of selective genotyping for detection of quantitative trait loci: an analysis of grain and malt quality traits in three barley populations. Genome 45 : 1116–1124.
43. FooladMR, StoltzT, DervinisC, RodriguezRL, JonesRA (1997) Mapping QTLs conferring salt tolerance during germination in tomato by selective genotyping. Molecular Breeding 3 : 269–277.
44. FooladMR, ZhangLP, LinGY (2001) Identification and validation of QTLs for salt tolerance during vegetative growth in tomato by selective genotyping. Genome 44 : 444–454.
45. ZhangLP, LinGY, Niño-LiuD, FooladMR (2003) Mapping QTLs conferring early blight (Alternaria solani) resistance in a Lycopersicon esculentum×L. hirsutum cross by selective genotyping. Molecular Breeding 12 : 3–19.
46. CarrLG, ForoudT, BiceP, GobbettT, IvashinaJ, et al. (1998) A Quantitative Trait Locus for Alcohol Consumption in Selectively Bred Rat Lines. Alcoholism: Clinical and Experimental Research 22 : 884–887.
47. KimS-G, LeeS, SeoPJ, KimS-K, KimJ-K, et al. (2010) Genome-scale screening and molecular characterization of membrane-bound transcription factors in Arabidopsis and rice. Genomics 95 : 56–65.
48. ChenYN, SlabaughE, BrandizziF (2008) Membrane-tethered transcription factors in Arabidopsis thaliana: novel regulators in stress response and development. Curr Opin Plant Biol 11 : 695–701.
49. HuangX-Y, ChaoD-Y, GaoJ-P, ZhuM-Z, ShiM, et al. (2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes & Development 23 : 1805–1817.
50. ZhouCM, ZhangTQ, WangX, YuS, LianH, et al. (2013) Molecular basis of age-dependent vernalization in Cardamine flexuosa. Science 340 : 1097–1100.
51. YooSD, ChoYH, SheenJ (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2 : 1565–1572.
52. HellensRP, AllanAC, FrielEN, BolithoK, GraftonK, et al. (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1 : 13.
53. CzechowskiT, StittM, AltmannT, UdvardiMK, ScheibleW-R (2005) Genome-Wide Identification and Testing of Superior Reference Genes for Transcript Normalization in Arabidopsis. Plant Physiol 139 : 5–17.
54. MullerPY, JanovjakH, MiserezAR, DobbieZ (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32 : 1372–1374, 1376, 1378–1379.
55. SimonP (2003) Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19 : 1439–1440.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



