-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Recurrent Loss of Specific Introns during Angiosperm Evolution
The spliceosomal introns are nucleotide sequences that interrupt coding regions of eukaryotic genes and are removed by RNA splicing after transcription. Recent studies have reported several examples of possible recurrent intron loss or gain, i.e., introns that are independently removed from or inserted into the identical sites more than once in an investigated phylogeny. However, the frequency, evolutionary patterns or other characteristics of recurrent intron turnover remain unknown. We provide results for the first comprehensive analysis of recurrent intron turnover within a plant family and show that recurrent intron loss represents a considerable portion of all intron losses identified and intron loss events far outnumber intron gain events. We also demonstrate that recurrent intron loss is non-random, affecting only a small number of introns that are repeatedly lost, and that different lineages show significantly different rates of intron loss. Our results suggest a possible role of DNA methylation in the process of intron loss. Moreover, this study provides strong support for the model of intron loss by reverse transcriptase mediated conversion of genes by their processed mRNA transcripts.
Published in the journal: . PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004843
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004843Summary
The spliceosomal introns are nucleotide sequences that interrupt coding regions of eukaryotic genes and are removed by RNA splicing after transcription. Recent studies have reported several examples of possible recurrent intron loss or gain, i.e., introns that are independently removed from or inserted into the identical sites more than once in an investigated phylogeny. However, the frequency, evolutionary patterns or other characteristics of recurrent intron turnover remain unknown. We provide results for the first comprehensive analysis of recurrent intron turnover within a plant family and show that recurrent intron loss represents a considerable portion of all intron losses identified and intron loss events far outnumber intron gain events. We also demonstrate that recurrent intron loss is non-random, affecting only a small number of introns that are repeatedly lost, and that different lineages show significantly different rates of intron loss. Our results suggest a possible role of DNA methylation in the process of intron loss. Moreover, this study provides strong support for the model of intron loss by reverse transcriptase mediated conversion of genes by their processed mRNA transcripts.
Introduction
Spliceosomal introns (called introns hereafter) are noncoding DNA segments within eukaryotic genes that are removed after transcription. Although the presence of introns is one of the universal features of eukaryotes, and a large number of intron positions are highly conserved in orthologous genes across species, family and even kingdom boundaries [1], [2], intron functions and evolutionary origins continue to be a subject of much debate (see reviews in [3], [4]). The number of introns varies dramatically among organisms (see reviews in [5], [6]). Accumulating evidence suggests that the common ancestors of at least several eukaryotic supergroups were intron rich [1], [2], [7], [8] and the great interspecies difference in intron density was caused by considerably different rates of lineage-specific intron loss and/or gain [3], [5].
Patterns of intron loss and gain have been investigated extensively in numerous subclades of the eukaryotic tree of life with different levels of taxon sampling (see review in [3]). To date, vast numbers of single loss and gain events (events inferred as occurring only once in the phylogeny investigated (Fig. 1, top) have been well-documented. Some studies also document cases of recurrent loss [9]–[11] and/or recurrent gain (otherwise called parallel gain) [12]–[14], terms describing introns that are independently removed from or inserted into the identical sites more than once in an investigated phylogeny (Fig. 1, middle).
Fig. 1. Patterns of intron loss and gain. 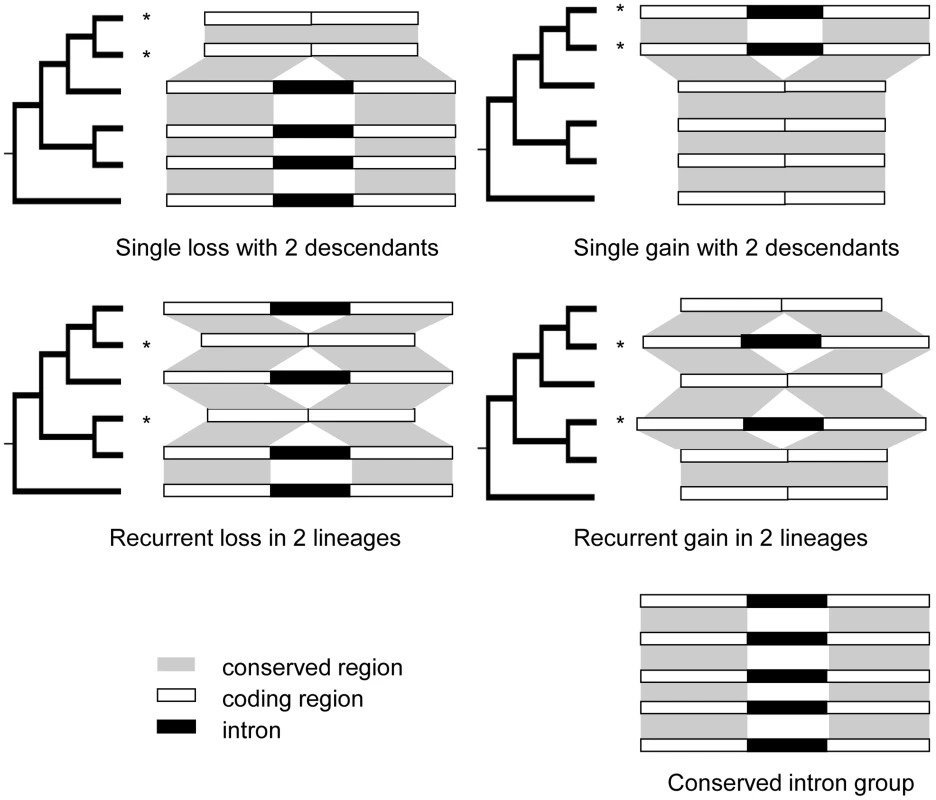
The history of intron loss and/or gain is inferred by comparing the observed pattern of intron presence-absence with the phylogeny of the conserved genes exhibiting intron presence/absence variation using the parsimony principle. “*” denotes reconstructed loss or gain event. Bottom right example: intron exists in all genes studied, and no loss or gain has occurred. Middle left: the presence of an intron in the outgroup and two apparent losses in two lineages. Any other reconstruction requires at least 3 events. Applying the same logic, the other three patterns located at top left, top right, and middle right can be reconstructed. Early examples of potential recurrent intron gain came from small scale studies of single genes, including the Chironomus globin gene [14] and the fruit fly and plant xanthine dehydrogenase (xdh) genes [13]. One of the reasons that recurrent intron gain has attracted particular attention is that it has been proposed as a possible explanation for the presence of introns at the same sites in orthologous genes from distant lineages under the proto-splice site model [15], [16].
Recent studies employing genome-scale data have tried to evaluate the importance of recurrent gain through broad taxon sampling [17], [18]. These studies have argued that recurrent gain is rare and that shared introns are primarily due to evolutionary conservation. In 2009, [12] reported 4 cases of recurrent intron gain in a Daphnia pulex population based on the most parsimonious reconstruction of intron history and supporting structural evidence, suggesting that intron gain occurs with high specificity and at a high rate in this species.
Recurrent loss of introns has been reported in the mammalian glyceraldehyde-3-phosphate dehydrogenase gene [10], dipteran white gene [9], and Drosophila and mosquito multidrug resistance protein MRP1 genes [11]. Although the possibility of extensive recurrent loss in animal evolution has been proposed [4], little is known about the frequency, patterns or other characteristics of recurrent intron loss from orthologous genes since no comprehensive investigation of this phenomenon in any lineage of organisms has been reported yet.
Here we report the results of genome-wide computational identification and analysis of potential recurrent intron loss and/or gain events in five sequenced grass genomes by performing parsimonious reconstruction (Fig. 1) on trees of conserved genes and using Arabidopsis as the initial outgroup species (with additional outgroups used to confirm detected cases of intron presence/absence variation). The data show that recurrent intron loss accounts for at least 10% of all detected intron presence/absence variation sites. In contrast, we did not detect any clear case of recurrent gain. We further studied rate differentiation of recurrent loss in lineages, frequency of adjacent loss, position of lost introns in affected genes, expression patterns and functional enrichment of affected genes, intron size at turnover sites and their local DNA composition. The results of this comprehensive analysis yielded several observations that had previously been made in two-species comparisons, analyses of single gene families or broad multi-kingdom investigations, for instance that smaller introns were preferentially lost [10], [19], [20] and that rates of intron loss and/or gain varied between lineages [8], [21]. In addition, the observations that no recurrent intron gain events were detected, that genes with intron loss exhibited preferential expression in embryonic and/or gametophytic tissues, that lost introns had a high TG/CG ratio indicative of extensive CG methylation and that intron loss rates were similar across all chromosome regions have not been reported in any previous study of intron dynamics.
Results
Number and classification of presence/absence (PA) intron groups
Our intron loss and gain detection method identified 990 intron sites at which an intron was polymorphic for presence/absence in at least one of the five grass genomes (called PA intron groups hereafter; Table 1) and 24,567 introns that were present at the same sites in homologous genes in all six genomes (conserved intron groups). The PA intron groups belong to 762 OrthoMCL clusters (that is, predicted orthologous gene families) of which the number of member genes in the six genomes ranges from 3 to 141 genes. Among them, 195 clusters (containing 230 PA intron groups) had 6 member genes and 179 (212 groups) out of the 195 clusters had single copy genes in each of the six species, which gave the peak value in the cluster size histogram (Figure S1). Information on intron turnover in the 179 single-copy OrthoMCL clusters is shown in Table S1.
Tab. 1. Summary of detected intron losses and gains. 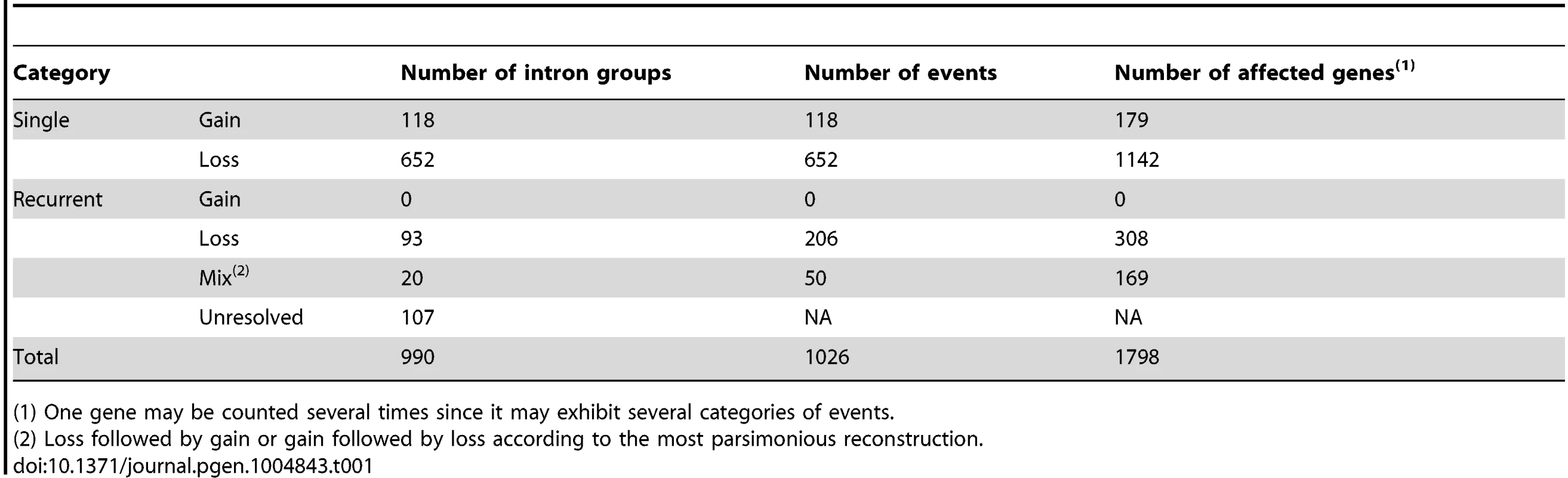
(1) One gene may be counted several times since it may exhibit several categories of events. By mapping the PA intron groups onto the phylogeny of the corresponding genes and performing parsimonious reconstructions, we tried to resolve the intron loss and gain history in PA intron groups (Fig. 1 and see Methods). An intron group was called well-resolved if the minimum number of intron loss or gain events gave a unique history. To study the effects of taxon sampling on the inference of recurrent intron turnover, we added orthologous genes from banana, spikemoss, and moss, and then re-analyzed the intron loss and/or gain history in groups that appeared to have undergone recurrent intron turnover based on the analysis of the six species. We found that (1) adding these outgroups helped reduce the number of unresolved histories by providing information on the ancient states of introns in some genes (examples are shown in Figure S2). (2) Moreover, adding the banana outgroup supported the conclusion for all but 4 recurrent loss groups that the intron was present in the common ancestor of the grasses. In these 4 cases, loss of introns occurred in Arabidopsis as well as one or more of the grasses. For these 4 cases, adding the outgroup did not change but further supported the intron turnover history that was modeled from analysis without the outgroup. (3) In a few cases, using additional outgroups suggested a different gain/loss history than that obtained using only Arabidopsis as outgroup. For instance, we found 2 cases (one example is shown in Figure S3) of an apparent recurrent intron gain that was the most parsimonious reconstruction in the gene clusters composed of the five grass and Arabidopsis genomes alone, but adding outgroups indicated that several recurrent losses were the correct interpretation of these data.
More precision can be gained if additional outgroups are used, but this essentially infinite task would still leave some ambiguities unresolved because of haplotype extinctions. As one further test to evaluate the accuracy of our reconstructions, we downloaded grape (Vitis vinifera) gene annotations [22] for 50 randomly selected PA intron groups and manually checked for any change in the reconstruction of intron turnover history. In all but 1 of these cases, the intron status of grape genes fully supported our reconstructions based on the 5 grasses and Arabidopsis alone. In the exceptional case, the most parsimonious reconstruction as a single gain event in rice was replaced by a more likely two losses, one in Arabidopsis and one in the common ancestor of the grasses. This difference and re-interpretation had already been detected by the comparison to M. acuminata, which also contains an intron in this location.
A summary of the properties of identified PA intron groups is shown in Table 1. We found 770 groups that experienced only one loss or one gain (652 losses and 118 gains; called single event groups) and 220 groups, accounting for 22% of all PA intron groups, that required at least two events to have independently occurred. Out of these 220, 113 groups were well-resolved, including 93 groups experiencing recurrent loss (recurrent loss groups), and 20 mixed events groups (i.e., loss followed by gain (3 groups) or gain followed by loss (17 groups)).
Intron loss is predominant
In the single event groups, the number of losses was found to be 5.5 times higher than that of intron gain (652∶118) and, in recurrent event groups, we did not detect any confirmed recurrent intron gains. When using the gene set only from the six genomes (five grasses plus Arabidopsis), 5 intron groups appeared to show recurrent gain. However, adding more distant outgroups indicated that 2 of them were more likely exhibiting recurrent loss and the other 3 were unresolved in the larger gene sets. In contrast, adding outgroups supported recurrent loss in most (93 out of 97) cases. In four cases, adding banana genes altered the gene tree topology and they became unresolved. Hence, recurrent gain, if it occurs at all, is much less frequent than single gain (0 vs 118 in our analysis).
Number and orthology of recurrent loss events in groups
Because the scale of our phylogenetically analysis limited the number of recurrent events that could be detected, it was not surprising to observe that most (82 out of 93; 88%) of the recurrent loss groups experienced only 2 independent losses of any specific intron (Table S2). In fact, the maximum number of recurrent events that can be detected in the five grass genomes if an orthoMCL cluster consists entirely of five orthologous genes is 3. According to the parsimony principle, events in paralogous genes that resulted from a species-specific duplication event were counted only once. An example is shown in Figure S4, where an intron was absent in both members of this rice tandem gene pair, but only 1 loss was counted.
We found that independent intron losses occurred between orthologs in 46 recurrent loss groups and between paralogs in 41 groups, respectively. Because this analysis only captured recent paralogs due to the homology criteria involved in creating OrthoMCL clusters, these relative numbers do not provide a comparable analysis of overall intron stability in orthologs versus paralogs. In the other 6 groups, loss or gain occurred between both orthologs and paralogs. Examples of these three patterns are shown in Figure S5.
Recurrent intron loss is not random
Overall, we detected 858 intron loss events (652 single +206 recurrent loss events, Table 1) out of a total of 176,078 intron locations analyzed which were members of 25,557 intron groups (990 PA + 4,567 conserved groups). The 858 loss events resulted in the absence of 1635 introns in affected genes. 8782 (212 PA (Table S1) +8570 conserved) out of the 25,557 intron groups had gene tree topologies identical to the topology of the species tree. In this intron group subset, the total number of intron locations was 43,910. There were 182 intron loss events (including 37 recurrent losses, Table S1) and these resulted in 257 intron losses. To determine whether some introns are preferentially lost, we randomly assigned 257 intron absences to the 43,910 intron locations and counted the number of recurrent intron loss events. This random assignment process was repeated 10,000 times and the average and maximum number of recurrent intron losses were found to be 3.5 and 19, respectively. Because the actual number of recurrent losses in this data set was 37, this indicates that the observed recurrent loss is significantly higher (p-value <0.0001) than expected based on the hypothesis that intron loss was fully random in this intron group subset.
Lineage differentiation of events
We identified 1026 intron loss and/or gain events in the 883 resolved PA intron groups, including 879 loss and 147 gain events. The number of events in the 93 recurrent loss groups was 206, or ∼20% of all events. We investigated lineage differentiation of the average frequency of intron loss (measured by the number of events/branch length) in the grass family and our results (Fig. 2 and Figure S6) revealed that (1) single loss occurred at a higher frequency than recurrent loss in all branches (counts/time are tested; log-linear model with time as offset, p-value <0.0001); (2) sorghum and maize had significantly (counts/time are tested; log-linear model with time as offset, p-value <0.0001) higher frequency of intron loss than foxtail millet, rice or Brachypodium in both single loss and recurrent loss groups; and (3) in the BEP clade, Brachypodium had a higher (not significant, p-value = 0.22) frequency than rice.
Fig. 2. Intron turnover frequencies and rates of (a) recurrent loss, (b) all loss and (c) single gain in five grass genomes. 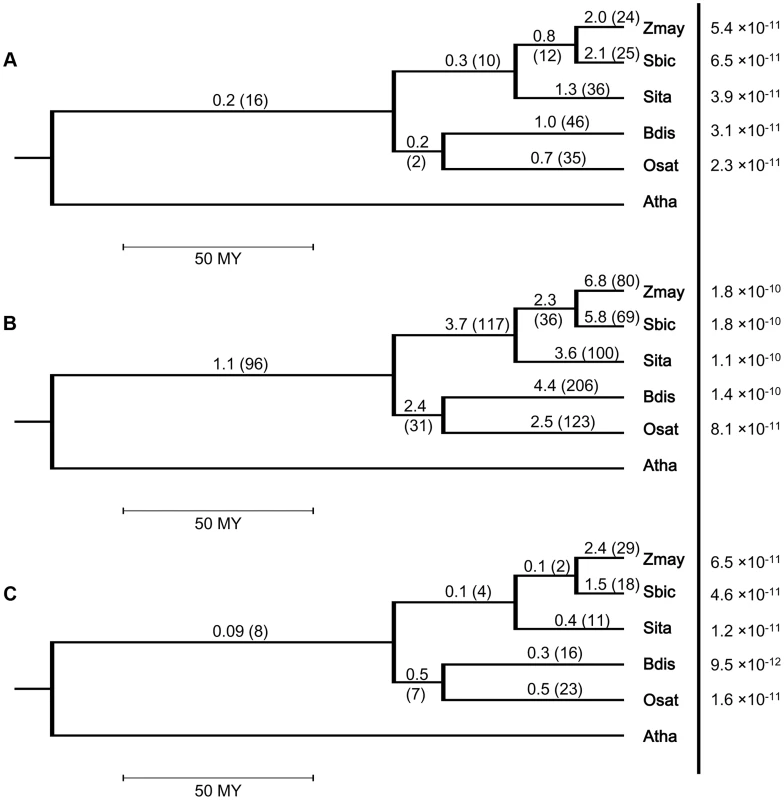
Branches of species tree represent evolutionary time. Frequency is defined as the number of events divided by the branch length and the unit branch length is million years (MY). Rate in a terminal node is measured by the number of events per intron per year. Rates are shown to the right of the vertical line and in the same line with the corresponding species codes. The 4-letter genome codes used are Zmay: Zea mays; Sbic: Sorghum bicolor; Sita: Setaria italica; Bdis: Brachypodium distachyon; Osat: Oryza sativa; Atha: Arabidopsis thaliana. Another interesting trend was that the frequency of detected recurrent loss tended to be lower in more ancient branches. In the 5 terminal branches, the frequency was higher than or close to 1/MY, while in the three older branches, the Panicoid, BEP and grass values were 0.3, 0.2 and 0.2, respectively. This might be because the smaller number of ancient branches and useful outgroups are expected to yield a higher frequency of intron changes that are not resolvable at the older nodes. The lower intron loss frequency was less obvious in the single loss groups (Figure S6a), where only the branch representing the common ancestor of all grasses had a statistically significant lower (counts/time are tested; log-linear model with time as offset, p-value <0.0001) frequency than the other branches. The predicted lower intron loss frequency in ancient lineages could also be seen when calculating the frequencies for all single gain (Fig. 2c) or for all turnover events (Figure S6b).
Information on the presence or absence of a target intron in an ancestral organism can only be obtained from analyzing sister or ancestral taxa. Hence, the closer to the root of the phylogeny an event occurred, the fewer such taxa are available, thus making it more difficult to resolve more ancient events. This is expected to contribute to the lower observed frequency of intron loss observed, when compared to recent branches. Further study with increased taxon sampling will be needed to draw solid conclusions about intron loss and gain frequencies in the most ancestral stages of these lineages.
Because we know the total number of current introns that we were able to properly align, it is possible to estimate the rate of intron loss or gain events measured per intron per year in the terminal nodes. The overall number of analyzed introns in Brachypodium, rice, sorghum, foxtail millet and maize genomes were 31685, 30685, 32386, 33230 and 37119, respectively, and these numbers were used to derive the rates indicated in the terminal nodes for Fig. 2 and Figure S6.
Intronless copies caused by retroposition or full length cDNA conversion events
Besides recurrent events, we also detected 10 OrthoMCL clusters (Table S3) that might have experienced retroposition or conversion between the intronless cDNA and the original gene copy. In their ancestral forms, the genes in these ten clusters contained at least 8 introns each. However, all introns were absent in all members of specific clades in the gene trees (Table S3). Both reverse-transcriptase-mediated (RT-mediated) intron loss (basically, cDNA-based gene conversion) [5], [23] and retroposition can lead to simultaneous loss of multiple introns, with all introns routinely being removed by the latter process. Retroposition [24], [25] usually generates an intronless copy that is located at a different locus from the original copy through the activity of retroelements like LINEs. RT-mediated intron loss, however, removes introns in the original gene but does not change its physical position in the genome. This observation provides the opportunity to discriminate between the two types of events. We found that intronless members of 2 gene clusters were located in syntenic blocks [26], [27] and thus were probably modified by cDNA conversion. Intronless members of the other 8 clusters were in new (non-syntenic) locations. In all of these cases. all introns were removed, suggesting retroposition. However, given the frequent movements of single genes to new locations that have been documented in the grasses [28]–[30], it is also possible that some of these are cases of cDNA conversion with prior or subsequent gene movement. As a control, we determined that only 33% of intron-retaining grass members of the 8 clusters were in non-sytenic blocks. Therefore, the 8/10 fully intronless genes in new locations were at a frequency suggesting that at least some lost their introns during retroposition.
Frequencies of adjacent intron loss
In a gene, several introns may experience loss and/or gain events, and this corresponds to multiple PA intron groups in one OrthoMCL cluster. We found that 628 clusters contained 1 PA intron group and 134 contained 2 to 7 lost or gained introns. The PA intron groups at different locations exhibited identical presence/absence polymorphic patterns for introns in non-sister lineages in only 4 of these 134 clusters, suggesting recurrent loss of the same set of introns independently. In the other 130 multiple-intron-loss clusters, PA intron groups at different locations had at least two different presence/absence patterns. Furthermore, 87% of the 130 clusters contained 2 or 3 PA intron groups (Table S4) and the total number of PA intron groups in the 130 clusters was 364.
We further investigated the frequency of adjacent intron loss. We found 84 neighboring PA intron groups in the 25,557 intron groups (PA + conserved) analyzed. Among them, 57 were confirmed adjacent intron losses from the same lineage, suggesting origin in a single event. These 57 adjacent pairs belonged to 40 OrthoMCL gene clusters (Table S5). We found 11 cases of> = 3 adjacent intron losses. If adjacent triple PA intron groups (those with losses of three introns in a row in the same gene) were counted as 2 pairs, and if adjacent intron turnover events were independent, the probability of turnover of a neighbor intron could be described by a binomial distribution. A statistical test of the observed number of adjacent intron loss groups rejected the independent turnover hypothesis at the 0.05 level.
In cases where adjacent introns were lost from the same lineage, a size-limited model of intron loss (for instance, by nuclear cDNA conversion or aberrant double-strand break repair) would suggest that adjacent introns with a smaller intervening exon would be more likely to be lost in a single event. Surprisingly, we found that the sizes of intervening exons was not significantly different between adjacent introns lost in a single lineage (presumed single events) when compared to the intervening exon sizes of adjacent introns lost in two independent events (Figure S7).
Intron turnover location in a gene
When intron turnover occurred in a terminal node of the gene tree, the gene that underwent intron loss and/or gain was the affected gene of that event. When intron turnover occurred in an internal node, all descendants of that node were taken as affected genes. Therefore, one event might have more than one affected gene. The total number of genes that underwent intron turnover was 1798, including 308 involved in the category of recurrent loss, and 1142, 179, and 169 in single loss, single gain and gain/loss mixtures, respectively (Table 1). Some genes were counted more than once because intron turnovers occurred at multiple locations in some gene clusters. For example, if two introns in a gene family experienced recurrent loss and single loss, respectively, this gene was counted twice. If all genes were counted only once, the total number of affected genes of intron turnover was 1720.
We normalized for gene size and investigated the distribution of intron loss or gain along each gene (Fig. 3 and Figure S8). The locations of all introns in all gene models of the six genomes showed a relatively even distribution, with the two termini, i.e. (0, 0.1) and (0.9, 1.0) of the total length of a gene when calculating from the 5′ end, exhibiting lower values (Fig. 3a). Under-representation of intron loss and/or gain at the gene termini was also observed in recurrent (Fig. 3b) and PA intron groups (Fig, 3c): the percentage of recurrently lost and PA introns located at (0, 0.1) and PA introns at (0.9, 1.0) was lower than expected based on the mean and sd from 1000 resamplings from all introns. In the all loss (recurrent + single) groups, we observed (0.2, 0.3) had a percentage higher than expected based on the resampling results (Figure S8).
Fig. 3. Intron locations in genes from five grass genomes: (a) all introns, (b) recurrent loss introns and (c) PA introns. 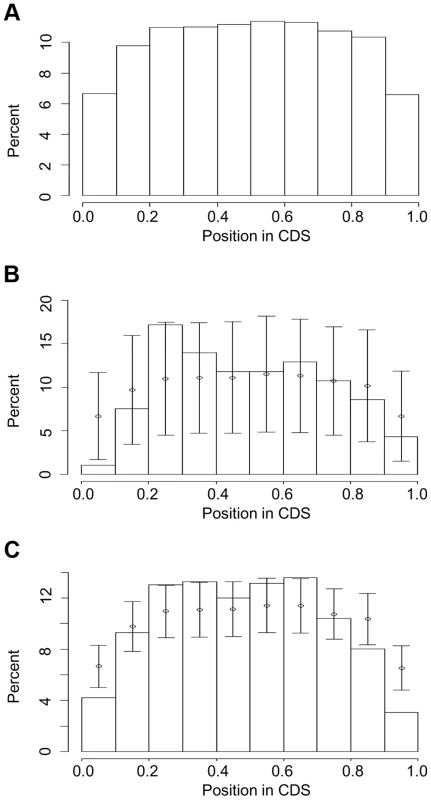
The CDS lengths in genes are normalized to 1 and the positions of introns are calculated as (length from translation start)/(total size of CDS). The normalized gene is partitioned into 10 intervals (X-axis) and Y-axis values are the percentage of introns in these intervals. Error bars in (b) and (c) represent one sd from interval mean values (circle), where mean and sd are calculated by resampling with replacement (1000 times) from the entire intron set. Chromosomal distribution of genes exhibiting intron turnover
We investigated the distribution of intron turnover across chromosomes (Fig. 4 and Figure S9) by normalization of chromosome size. The centromere was located at 0; and then the short arms and long arms of chromosomes were normalized separately, with the short and long arm termini located at -1 and 1, respectively. Locations of genes belonging to the same intron group were counted independently. The distribution of the whole gene set of the five genomes (Fig. 4a) showed a smooth “V” shape with the lowest gene density located at the centromeric/pericentromeric region. The distributions of genes with detected recurrent loss (Fig. 4b) and total intron turnover (Fig. 4c) exhibited a similar overall trend. However, it should be noted that plant genes are highly mobile over evolutionary time, and even centromeres can be found in different positions in closely related lineages [31], so the current genomic location is not a perfect predictor for any single gene of its location when an intron was gained or lost.
Fig. 4. Chromosomal locations of (a) all genes in five grass genomes and those that have undergone (b) recurrent intron loss or (c) any PA intron variation (gain or loss). 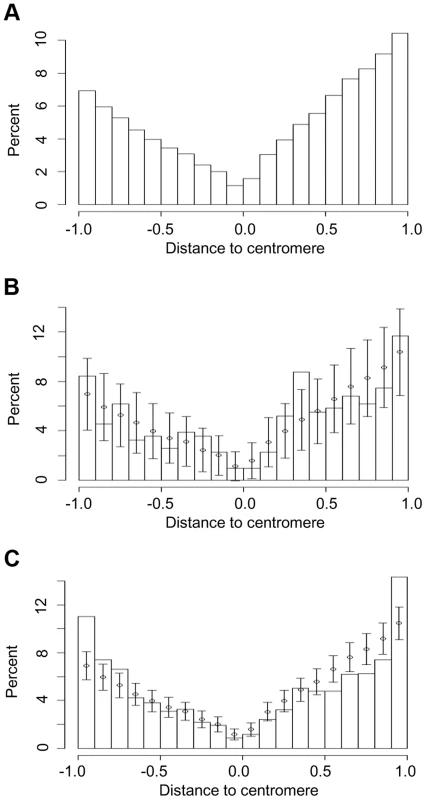
Short arms and long arms of chromosomes are normalized separately. The centromere is located at 0 and the short and long arm termini at -1 and 1, respectively. The locations of genes are calculated as distance from centromere divided by total length of chromosome arm, where distance is a negative number for the short arm. The normalized chromosome is partitioned into 20 intervals (X-axis) and Y-axis values are the percentage of genes in these intervals. Error bars in (b) and (c) represent one sd from interval mean values (circle), where mean and sd are calculated by resampling with replacement (1000 times) from the entire intron set. Resampling showed that the percentage of genes that underwent recurrent intron loss located in (0.3, 0.4), the region close to the middle of a normalized long arm, was higher than expected from the resampling mean and sd (Fig. 4b). The density ratio calculated as the density (number of genes per normalized unit length of a chromosome) of genes with recurrent intron loss divided by the density of the entire gene set exhibited a peak at this region (Figure S10), suggesting hotspots for intron recurrent loss events at these regions. When considering all PA introns, a higher than expected percentage of affected genes was located at the chromosome arm ends (−1, −0.9) and (0.9, 1) (Fig. 4c).
Frequency of intron turnover in genes with single and multiple introns
We found that 698 (91.6%) of the 762 OrthoMCL clusters with intron turnover events were derived from genes with multiple introns and the other 64 (8.4%) were from ancestral genes with a single intron. The 93 resolved recurrent loss intron groups belonged to 88 gene clusters, 4 (4.5%) of which were from single-intron ancestral genes. The frequency of all loss and recurrent loss occurring in single intron genes is 8.4% and 4.5%, respectively. A Pearson's Chi-square test (p-value = 0.2918) indicated that, compared to the PA intron groups, the number of single-intron genes was neither over-represented nor under-represented in the recurrent loss groups.
A total of 1026 confirmed intron turnover events (Table 1) were found among all introns analyzed in the grasses (588,669) and 65 of these events were in one of the 18,226 single-intron genes. A Pearson's Chi-square test (p-value = 1.75e-8) indicated that the rate of intron turnover in single intron genes (65/18,226 or 0.36%) is significantly higher than the rate of intron turnover overall (1026/588,669 or 0.17%).
Phase distribution of intron turnover
We investigated the codon phase distribution of intron turnover events and found that 58% (575/990 PA intron groups), 20% (195) and 22% (220) of turnover events involved introns in phase 0 (intron-exon boundaries located between two codons), phase 1 (between the first and second base of a codon), and phase 2 (between the second and third base of a codon), respectively. The excess of phase 0 introns has been well-documented (see review in [3]) and intron phase distribution in rice has been estimated at 57∶22∶21 for phase0: phase1: phase2 [32], very close to our estimation for intron turnover in the five grass genomes (56∶21∶23). Statistical analysis (Pearson's Chi-square) indicated no significant difference between the overall intron phase and PA intron phase data.
Expression patterns of genes that underwent intron loss and/or gain
We investigated the expression patterns of the 289 rice genes that exhibited intron turnover because publicly available expression data are relatively abundant for rice. A total of 283 out of the 289 genes matched probes in the rice 57K Affymetrix GeneChip (http://www.affymetrix.com/). 283 probes were selected through PLExdb's “Gene List Suite” tool using these genes as queries. Each probe corresponded to a single gene. When a gene matched multiple GeneChip probes, the probe with highest BLAST bit score was used to represent the gene. We extracted gene expression information for eleven stages in early embryogenesis and six for pollen development from multiple experiments deposited in PLEXdb (Table S6; see Methods). Since expression values are not comparable between different experiments, we only focused on whether genes that underwent intron turnover were detectably expressed or not in a given tissue. As shown in Table 2, compared to total nuclear genes, significantly higher (Pearson's Chi-square test, see Table 2) percentages of genes with intron loss events were expressed in both early embryogenesis and pollen development both when considering the average percentage of genes expressed in all developmental stages and the percentage of genes expressed in at least one of the developmental stages. Genes in the PA category, which includes all genes that underwent intron loss and/or gain, also were expressed significantly more frequently in these two expression categories than total nuclear genes. The fact that genes that have undergone intron loss exhibit a higher frequency of germ line or early embryogenesis expression than observed for the complete transcriptome suggests that expression in heritable tissues is associated with transmission of the intron removal outcome.
Tab. 2. Expression pattern of host genes of intron groups. 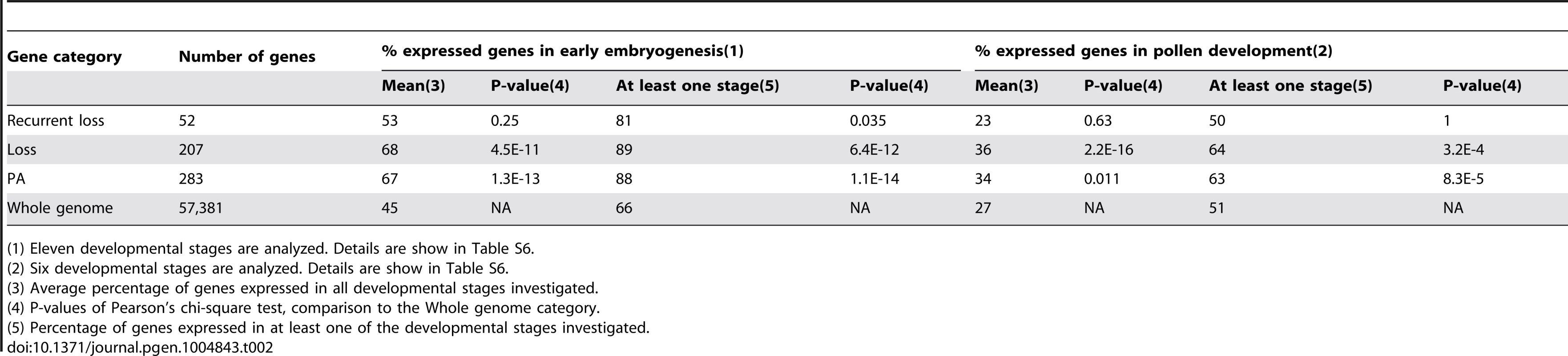
(1) Eleven developmental stages are analyzed. Details are show in Table S6. GO analysis of genes in all intron gain and/or loss categories indicated numerous terms that were over-represented at a level considered statistically significant by the GO analysis software (see Methods). These over-represented categories are shown in Figure S11. Among them, the top five most significant GO terms were from the following functional categories: ‘catalytic activity’, ‘oxidoreductase activity’, ‘metabolic process’, ‘omega-3 fatty acid desaturase activity’ and ‘positive regulation of protein modification process’.
Intron size
We compared the size and composition of PA intron groups to that of all intron groups. The average sizes of introns in recurrent loss intron groups, all PA groups and conserved groups were 212 bp, 266 bp and 360 bp, respectively. The average size of PA introns (total intron count = 7950) and recurrent loss introns (1097) were both significantly shorter than that of conserved introns (201,660) (Mann-Whitney test, p-value <2.2e-16). The preference for loss of short introns has been reported previously [10], [19], [20]. It is not clear why smaller introns might be more easily lost, although two distinct possibilities come to mind. Smaller introns may be less likely to contain important regulatory modules, so that their loss would be less likely to detrimentally affect gene function. A second possible explanation is that intron removal (perhaps by a cDNA conversion process or aberrant NHEJ repair) is tolerated only if complete because partial removal would leave an unspliceable intron fragment, and thus smaller introns would be more likely to be fully removed in any time-constrained process. A third possibility is selection for smaller transcript size or fewer introns so that genes might be more rapidly transcribed and/or matured to increase gene expression [33]. However, we observed that small genes were just as likely to lose and/or recurrently lose introns as large genes (Figure S12), indicating that transcription rate is not a clear factor in any possible selection for intron loss. We also did not observe any correlation between organismal genome size and overall intron number or intron loss rate (Figs. 2, Figure S6), nor exceptionally frequent loss of introns from genes with large intron numbers (Figure S13), so a model suggesting selection against introns per se is not supported by these observations. The observation in Figure S13 that introns are most frequently lost from genes with few introns does not provide any obvious mechanistic model for this preferential loss, but does suggest that many of these genes with very few introns are the ongoing products of especially frequent intron loss. Consistent to our observation of non-random loss of introns, Figure S13 suggests that not all introns in a gene have an equal likelihood of being removed.
Although recurrently lost introns, like single-loss introns, are smaller in size than the average intron, this fact does not by itself explain recurrent loss. That is, if one compares the size of recurrently lost introns, there is no significant different in their size compared to single loss introns (Figure S14).
DNA composition
We investigated the nucleotide compositions of different categories of intron groups (recurrent loss, PA, and conserved intron groups) as well as their flanking exonic sequences in the five grass genomes. As depicted in Table S7, the mean G+C content of recurrent loss introns, PA introns and conserved introns identified in OrthoMCL groups were very similar (39%) and lower than the genome-wide G+C content (44%) or the average G+C content of exons (55%). The lower G+C content of introns compared to exons in plants is a well-known phenomenon (e.g. [34]–[36]) and is believed to be related to the high G+C content of plant triplet codons [37], [38]. The mean G+C contents of the last 20 bp of the upstream flanking exon and the first 20 bp of the downstream flanking exon were similar in recurrent loss and PA intron categories (55%). This value is identical to the genome-wide G+C average for exons. Interestingly, the mean G+C content of the 20 bp of exonic sequences flanking conserved introns (47%) was about 8% less than that of the 20 bp of exonic sequences flanking PA introns. Pearson's Chi-square test indicates that this difference is highly significant (p-value <2.2e-16).
We investigated dinucleotide frequencies for all PA and conserved introns. The three intron categories (recurrent loss, PA and conserved) exhibited similar frequencies of different dinucleotides (Table S8). In all three categories, TT, AT, AA, TG and TA were the most abundant dinucleotides, while GC, CC, GG and CG were the least abundant dinucleotides. A similar tendency was found in the genome-wide dinucleotide frequency, where CG had the lowest frequency and AA, TT and AT had the highest frequencies. While CG was also the least frequent dinucleotide in exons flanking conserved introns, it was TA that had the lowest frequency in exons flanking lost introns, in agreement with the higher AT-richness of exons flanking conserved introns mentioned above. Within introns, TG was the fifth (recurrent loss), fourth (PA) and third (conserved) most abundant dinucleotide across the three different categories. Interestingly, the TG/CG ratio was 2.4, 2.9 and 4.2 in recurrent loss, PA and conserved categories (Tables S9 and S10). The differences in the TG/CG ratios among the three intron categories were highly significant in terms of Pearson's Chi-square test with Bonferroni correction (Table S10). A low “CG” and high “TG” frequency suggests the process of “C” to “T” transition that is enhanced by 5-methylation at cytosine bases [39], [40]. Introns in general are observed to be relatively less methylated than exons in plants, especially at cytosine bases [41]. This result suggests that introns with a history of less CG methylation are more likely to be removed.
Flanking exons of conserved introns also had a relatively higher TG/CG ratio than flanking exons of PA and recurrent loss introns (TG/CG = 2.0 vs. 0.9 and 0.77; Table S9). Hence, these results suggest that conserved introns and their flanking exons were relatively highly methylated while intron turnover tended to occur where the degree of cytosine-5 methylation in both the flanking exons and introns was relatively low.
Intron-exon junction sequences associated with intron PA variation
Although the GT….AG terminal intron dinucleotides are most abundant in all studied eukaryotes, including plants, rarer junction sequences are also found [42]. Table S11 shows the terminal intron dinucleotides associated with conserved introns and PA introns. No significant differences were observed between conserved or PA introns, regardless of whether the intron location had the added precision of confirmation by transcript analysis.
Discussion
Frequency of intron loss is far higher than gain
Many previous parsimonious reconstructions of intron loss and/or gain [2], [43] were based on Dollo parsimony, which assumes that every intron arose only once along the tree and thus explicitly excludes parallel gains. Recent studies, however, suggest that this assumption is not valid [8]. Prohibiting recurrent intron gain is also a characteristic of some probabilistic methods [44]–[46]. However, one of our aims was to provide an unbiased assessment of the frequency of intron gain. Hence, a cladistic parsimonious counting strategy (See Fig. 1 and Methods) was employed that initially modeled the fewest intron turnover events in the tree but did not restrict the occurrence of recurrent gain or loss. The parsimony method was valid as a first step in the analysis as we had no prior knowledge on the frequency of gain or loss. Our results indicate that intron loss is much more frequent than intron gain (858 losses (206 recurrent events +652 single events): 118 gains = 7.2 : 1) (Table 1), consistent with results from several previous investigations in plants [32], [47], [48].
Use of a simple cladistic approach to predict the origin of a rearrangement is not valid when the rates of the two mechanisms of rearrangement are quite different. In our case, for example, where intron gain occurs at a 7-fold lower rate than intron loss, parsimony tends to overestimate gain and underestimate loss since it cannot be guaranteed that any of the events judged as intron gains are not actually cases of multiple independent losses that could not be resolved due to a lack of phylogenetic power. In fact, in all unresolved and mixed groups, the gain events predicted by the parsimony principle could also be explained by two or more recurrent intron losses. In 18% (21 out of 118) of single gain groups, the gain can be substituted by 2 losses. If every gain is allowed to be substituted by at most 3 losses, the percentage reaches 31% (37 out of 118). Given the at least 7.2 fold higher frequency of losses than gains that we observed under the parsimony model, even models with 3, 4, 5 or 6 recurrent losses are more likely than single gain events, thus allowing all of the gains to be potentially explained by recurrent loss.
Proposed mechanisms suggest a low rate of intron gain
The origin of new introns requires the insertion of precisely positioned functional splicing signals inside (and, presumably by chance, flanking) the new intron. Unlikely as this seems, many models (see [49] for a short summary) of frequent intron gain have been proposed, including (1) modification of self-splicing introns [50]; (2) intronization of coding sequences [51], [52]; (3) transposon insertions that become precise introns [53]–[55]; (4) tandem duplication of coding sequence with an AGGT motif to create an intron terminal signal [50]; (5) nonhomologous end-joining (NHEJ) of DNA segments [12], [56]; and (6) intron transposition [57]–[59]. In plants, only one of these models, a transposon insertion turned intron, has been supported by in vivo evidence [53]–[55]. One case has been observed in which a new intron did not dramatically debilitate gene function because it was precisely processed [55]. However, none of the proposed intron gains that have been fixed in a species have been associated with a transposable element insertion/structure, so rare intraspecies variation for this trait may be more an indication of mutational neutrality than a major mechanism of evolutionary change by intron gain.
Does recurrent gain ever occur?
The rarity of intron gain implies that the chance of recurrent gain should be even lower. Previous reports of recurrent intron gains [12]–[14] were based exclusively on a cladistic approach with few investigated taxa. The main reason that these investigators preferred recurrent gains is that the intron distribution in their phylogeny could be explained more parsimoniously by invoking less independent intron gains than losses: 2 gains v.s. 8 losses in the globin gene analyzed by [14] and 2 gains v.s. 14 losses in the xdh genes analyzed by [13]. As mentioned above, when the frequency of loss is much higher than gain — and this has been supported by the current study as well as by previous analyses [10], [32], [47], [48] — the cladistic approach tends to mistake recurrent losses as individual or recurrent gains. Furthermore, as is shown in our study, using a single species as outgroup may lead to incorrect assumptions on the ancestral state of an intron. In short, more convincing evidence (such as that provided in [12]) of recurrent intron gain is needed to determine whether this proposed phenomenon has a solid foundation in plants. However, phylogenetically deep and/or molecularly detailed studies of intron gain have recently been published for a number of organisms [12], [56], [60]–[64], so it will be interesting to see if any future plant studies uncover similar phenomena.
Recurrent loss may be going on at a higher frequency than reported
Including outgroups, the phylogenetic scope of the three previously reported cases of recurrent intron loss were in single genes in vertebrates [10], Bilateria [9] or Pancrustacea [11]. In our research, the scope is extended to the last common ancestor of monocots and dicots. During the ∼150 MY evolution of the grass family since their divergence from a common ancestor with Arabidopsis, we have identified 93 (initial parsimony result) to 200 (all possible cases, including those currently unresolved) polymorphic intron sites that are likely to have experienced recurrent losses. The rate of recurrent intron loss thus is 2.4−5.2×10−5/MY/intron site (total analyzed intron sites = 25,535). The counting of recurrent loss is affected by the depth of phylogeny and taxon sampling. For example, recurrent losses in rice and maize will appear as a single loss in each species if BEP clade and PACCAD clade species were investigated separately. Here BEP is an abbreviation representing the clade of grass subfamilies Bambusoideae, Ehrhartoideae, and Pooideae; and PACCAD an abbreviation representing the clade of grass subfamilies Panicoideae, Arundinoideae, Chloridoideae, Centothecoideae, Aristidoideae and Danthonioideae. As a further investigaton of this point, we extended the intron loss analysis for six sets of orthologous genes that contained a total of 9 recurrent loss groups and 18 intron loss events by manually aligning the orthologs from the five grass species. These were analyzed with the orthologs from one additional species (2 genes) or two additional species (4 genes) for which sequence information was available, and this identified 3 additional recurrent loss events, all in the same gene (unpub. obs). Thus, adding more taxa will lead to the detection of more recurrent losses, indicating that the current estimates are very conservative minima.
Can selection explain recurrent loss?
Our results show that recurrent loss accounts for a considerable proportion of all detected intron losses. Moreover, loss is not random, but involves a small subset of introns that are lost over and over again in multiple lineages. Our analyses indicate that some of these introns may have been lost repeatedly because of their (1) small size, (2) expression in tissues that contribute to the germ line or (3) history of less 5-methylation in regional CG dinucleotides. Fawcett and colleagues [48] suggested that the high rate of intron loss in a small genome might be caused by strong selection for genome reduction in their study of two Arabidopsis species. However, consistent with previous studies [10], [19], [20], our results shows that short introns are preferentially lost, a result not consistent with selection for intron loss as a mode of genome size reduction. Moreover, we observed a higher frequency of intron loss in larger compared to smaller grass genomes. Both observations suggest that selection is not on the basis of an effect on genome size. Given that the loss of a single intron in plants will only decrease genome size by a few hundred bp in most cases, it is difficult to see how this would be detected and/or significant within a several gigabase genome. Another theory is that loss of an intron could lead to more efficient transcription [33], but this model is also inconsistent with a preference for short intron removal.
Based on our observation that lowly methylated introns are more likely to be removed than highly methylated introns, it is possible that there might be selection related to the epigenetic status of genes. Methylation of DNA is associated with specific chromatin compositions/conformations and an epigenetic status that tends to have negative effects on transcription [65], [66]. Hence, loss of an intron with a particular level of DNA methylation might be expected to have a selected epigenetic outcome, perhaps leading to an altered level or timing of gene expression.
Mechanisms that drive intron loss
Although natural selection might explain some or all recurrent losses, it is also possible that coincidental features of intron structure, gene location and/or gene expression might explain a high rate of removal for specific introns. Two general mechanisms for intron loss have been proposed, but neither has gained comprehensive support yet [49]. One proposed model is intron loss by genomic deletion [4], [5] via NHEJ [48] or other molecular mechanisms, leading to exact [48], [67] or inexact [20], [68] intron removal. The other proposed mechanism is an RT-mediated intron loss model. The standard version of this mechanism predicts that introns located at 3′ ends of genes are more likely to be lost because reverse transcriptase reads/polymerizes from 3′ to 5′ along the RNA template and often produces incomplete transcripts [5], [69], [70]. However, a lack of 3′ bias of intron loss has been reported in several species, including plants [32], [48], animals [43], [71] and fungi [46], [60]. So, modified RT-mediated intron loss models involving self-primed reverse transcription have been proposed [72]–[74], but there is not yet any compelling evidence to support these models [43], [60], [75].
Our data indicated that large grass genomes (sorghum and maize) have a significantly higher intron removal rate and that short introns are more commonly removed. These observations are compatible with the hypothesis that RT-mediated intron loss plays a role in driving intron loss because large genomes contain more class I TEs and thus may have higher reverse transcriptase activity. Furthermore, smaller introns are more likely to be lost by RT if the enzyme is prone to incomplete template coverage. In addition, several other results in this study provide indirect support for an RT-mediated intron loss mechanism, including (1) adjacent intron loss occurring at higher frequency than expected by chance, (2) genes exhibiting intron loss are enriched in germline and early embryogenesis transcriptomes; and (3) deletions of introns are exact. The lack of a 3′ bias for intron loss detected in our study, however, conflicts with the simplest models of cDNA conversion for intron loss, as does the observation that the concurrent loss of adjacent introns does not seem to be highly affected by intervening exon size. Another model, involving ncRNAs that direct DNA rearrangement (including deletion) [76], does not necessarily require RT activity or 3′-end priming at an mRNA polyA, but still would predict a more frequent loss of adjacent introns if they were separated by smaller exons. To increase our understanding of these issues, studies are needed to investigate de novo intron loss, and these studies would be best performed with introns in genes that exhibit a history of recurrent loss in other lineages and in genetic backgrounds that are enriched for RT and/or for NHEJ activities.
Materials and Methods
Genomic sequences and centromere positions
The genomic sequences and annotation data for maize (Zea mays, version 5b.50) and rice (Oryza sativa, IRGSP/RAP Build5) were downloaded from MaizeSequence (http://www.maizesequence.org/index.html), IRGSP (http://rgp.dna.affrc.go.jp/E/IRGSP/) and RAP-DB (http://rapdb.dna.affrc.go.jp/) databases. Data for the sorghum (Sorghum bicolor, version 1.4), foxtail millet (Setaria italica, JGI release 164) and Brachypodium (Brachypodium distachyon, JGI release 114) genomes were obtained from the Phytozome website at DOE-JGI (http://www.phytozome.net/). Data for Arabidopsis thaliana (version TAIR10) were retrieved from TAIR (http://www.arabidopsis.org/). Raw reads for the five grass genomes were downloaded from the NCBI Trace Archive Database (ftp://ftp.ncbi.nih.gov/pub/TraceDB/) and the reads for Arabidopsis thaliana were from the 1001 Genomes project website (http://1001genomes.org/). Besides these six species, sequences and annotation of banana (Musa acuminata, version 1), was downloaded from The Banana Genome Hub (http://banana-genome.cirad.fr/); data of spike moss (Selaginella moellendorffii, JGI release 91) and moss (Physcomitrella patens, JGI release 152) were also downloaded from Phytozome v8.0.
The estimated positions of maize, sorghum, foxtail millet, Brachypodium and Arabidopsis centromeres were directly extracted from several earlier publications [77]–[81]. The positions of rice centromeres were collected from the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/annotation_pseudo_centromeres.shtml), and sequences around these regions were compared to IRGSP/RAP Build5 to find the corresponding locations.
Detection of intron loss and gain events
Intron group identification: The workflow of intron group identification is shown in Figure S15. Firstly, the representative gene repertoires of the five grasses and Arabidopsis were extracted and orthologous clusters (called OrthoMCL clusters) were built using OrthoMCL [82] with default parameter settings. Here, we used the longest transcript as a representative sequence for each gene. Secondly, protein alignment-guided multiple sequence alignments (MSA) of coding DNA sequences (CDS) of each OrthoMCL cluster were constructed using TranslatorX [83] with default parameter settings. Next, OrthoMCL clusters too large or small (>200 or <3 member genes) or clusters of which>30% of the members matched known transposon proteins were excluded. This last step was designed to remove from consideration those gene models where transposable elements (TEs) were found inside coding sequence, because we have noted that most of these are mis-annotations (especially pseudogenes mis-annotated as genes) ([84], H. Wang and J. Bennetzen, unpub. res.). The analyzed genes that remained included many cases of introns (both conserved and PA variants) that contained TEs.
Subsequently, we extracted intron positions from gene models annotated in the various genomes and mapped these positions to the above protein-guided CDS alignment. An intron in the genomic sequence thus was mapped to a position between two consecutive bases corresponding to the last base of exon k (base i) and the first base of exon k+1 (base i+1) in a CDS (Figure S15, top right). In the CDS alignment, whenever a position between two consecutive bases had break points, we called this position an intron group. If all member genes in the intron groups had break points, this indicated that each member gene had an intron at the same position and this group was judged a conserved intron group. If only some member genes had break points, the group was called a PA intron group (Figure S15, middle left). We identified all intron group candidates for all OrthoMCL clusters with this method. We further required that the up - and downstream exons of detected intron groups were well-aligned (flanking exons in each member gene exhibited ≥60% identity to consensus sequences of the flanking exon alignments) (Figure S15, middle right). This homology restriction led to the selection of conserved intron sites and excluded artificial intron groups caused by MSA algorithms. One possible issue was “intron sliding” [3], [85], where an intron was not actually lost or gained, but moved one to several nucleotides away due to a shift in the intron/exon boundaries. With an inappropriate detection method, “intron sliding” might be perceived as an intron gain, intron loss or (most likely) reciprocal intron gain and loss in two different lineages. Hence, we required that the alignment be perfect at the exact ends of the intron/exon junction, and that no two intron PAs were allowed to be within <20 bp of each other. We manually checked all 990 of our PA intron groups to see if any were due to intron sliding, and none were. Next, we excluded intron groups containing very short introns (≤10 bp) to avoid artifacts generated by incorrect intron annotation (Figure S15, bottom). Finally, selected intron groups were compared with raw reads to confirm that the presence or absence of introns in the groups was not caused by assembly errors (Figure S15, bottom and Figure S16).
Another possible issue with these analyses involves the quality of the gene annotations that we accepted from the published genomes investigated. In particular, it was not clear whether we would see any differences in intron presence/absence variation properties in cases where introns were confirmed by transcript analysis. Transcript data (e.g. ESTs) covered the breakpoint plus at least 20 bp upstream and downstream of the intron boundary for 946 of the 990 PA intron groups in at least one of the species investigated. Although the number of PA introns without transcript support was too small to allow statistically significant values to be obtained when comparing PA intron properties to those PA introns with transcript support, in all cases the trends were in the same direction.
Gain or loss event resolutions: For every PA intron group, we mapped the intron presence/absence pattern on the corresponding gene tree. The history of intron turnover of the group was reconstructed according to the parsimony principle which assumes that the history with the lowest number of intron turnover events has the highest likelihood of representing the true chain of events (Fig. 1). If the parsimonious reconstruction corresponded to more than one possible intron loss and/or gain history, the intron group was called an unresolved group. For every group for which recurrent events were inferred from the six genome analysis, we added in orthologous genes from banana, spike moss and moss and redid the intron loss and/or gain history inference. This analysis allowed us to confirm reconstructions based on fewer species and demonstrate that none of the initial recurrent gains calls were supported by the broader cladistics analysis. Detailed information for all intron loss events identified in this study is provided in Table S12.
Gene tree construction
TreeBeST (http://treesoft.sourceforge.net/treebest.shtml) was used to build the gene trees for the OrthoMCL clusters. The program constructed trees with the Maximum Likelihood method under guidance of the species tree. The species tree topology used in this study was (((((((Zmay, Sbic)Andropogoneae, Sita)Panicoid,(Bdis, Osat)BEP)Grass, Muca)Monocot, Atha)Angiosperm, Smoe), Ppat). The 4-letter genome codes used were Zmay: Zea mays; Sbic: Sorghum bicolor; Sita: Setaria italica; Bdis: Brachypodium distachyon; Osat: Oryza sativa; Muca: Musa acuminata; Atha: Arabidopsis thaliana; Smoe: Selaginella moellendorffii; Ppat: Physcomitrella patens.
Rates of occurrence of intron loss or gain
Using previous estimations of the divergence time of the five grass species, i.e. 150 million years (MY) between Arabidopsis and grasses [86], [87]; 60 MY between BEP and Panicoids [87], [88], 47 MY between rice and Brachypodium [77], 26 MY between foxtail millet and Andropogoneae [78], and 12 MY between sorghum and maize [89], branch lengths of the grass species tree could be scaled as evolutionary time. The mean rates of intron loss or gain in branches were calculated as the number of events divided by the branch length.
Gene ontology analysis
GO annotation and enrichment analysis of genes exhibiting intron loss or gain were performed in AgriGO (http://bioinfo.cau.edu.cn/agriGO/) [90] using “suggested backgrounds” as references. These backgrounds were the GO annotation of whole gene sets of organisms. For Brachypodium, sorghum and foxtail millet, only one background was available. For rice and maize, we chose the annotation labeled as “MSU 7.0 nonTE” and “Zea mays ssp.”, respectively. In all analyses, statistical tests were performed using the Fisher exact test and the multi-test adjustment method according to Yekutieli [91]; the significance level was set to 0.05; and complete GO was chosen as gene ontology type.
Expression pattern analysis
Rice expression data were downloaded from PLEXdb (http://www.plexdb.org/). Normalized expression data from various experiments (Table S6) were extracted for early embryogenesis and germ line cells. Details of these experiments can be found at the PLEXdb website under the “Expression Atlases” link. We identified probes corresponding to the rice genes exhibiting intron turnover with the “Gene List Suite” tool (http://www.plexdb.org/modules/glSuite/gl_main.php).
Supporting Information
Zdroje
1. FedorovA, MericanAF, GilbertW (2002) Large-scale comparison of intron positions among animal, plant, and fungal genes. Proc Natl Acad Sci U S A 99 : 16128–16133.
2. RogozinIB, WolfYI, SorokinAV, MirkinBG, KooninEV (2003) Remarkable interkingdom conservation of intron positions and massive, lineage-specific intron loss and gain in eukaryotic evolution. Curr Biol 13 : 1512–1517.
3. RogozinIB, CarmelL, CsurosM, KooninEV (2012) Origin and evolution of spliceosomal introns. Biol Direct 7 : 11.
4. Rodriguez-TrellesF, TarrioR, AyalaFJ (2006) Origins and evolution of spliceosomal introns. Annu Rev Genet 40 : 47–76.
5. RoySW, GilbertW (2006) The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet 7 : 211–221.
6. JeffaresDC, MourierT, PennyD (2006) The biology of intron gain and loss. Trends Genet 22 : 16–22.
7. CsurosM, RogozinIB, KooninEV (2008) Extremely intron-rich genes in the alveolate ancestors inferred with a flexible maximum-likelihood approach. Mol Biol Evol 25 : 903–911.
8. CsurosM, RogozinIB, KooninEV (2011) A detailed history of intron-rich eukaryotic ancestors inferred from a global survey of 100 complete genomes. PLoS Comput Biol 7: e1002150.
9. KrzywinskiJ, BesanskyNJ (2002) Frequent intron loss in the white gene: a cautionary tale for phylogeneticists. Mol Biol Evol 19 : 362–366.
10. Coulombe-HuntingtonJ, MajewskiJ (2007) Characterization of intron loss events in mammals. Genome Res 17 : 23–32.
11. ZhanLL, DingZ, QianYH, ZengQT (2012) Convergent intron loss of MRP1 in Drosophila and mosquito species. J Hered 103 : 147–151.
12. LiW, TuckerAE, SungW, ThomasWK, LynchM (2009) Extensive, recent intron gains in Daphnia populations. Science 326 : 1260–1262.
13. TarrioR, Rodriguez-TrellesF, AyalaFJ (2003) A new Drosophila spliceosomal intron position is common in plants. Proc Natl Acad Sci U S A 100 : 6580–6583.
14. HankelnT, FriedlH, EbersbergerI, MartinJ, SchmidtER (1997) A variable intron distribution in globin genes of Chironomus: evidence for recent intron gain. Gene 205 : 151–160.
15. DibbNJ, NewmanAJ (1989) Evidence that introns arose at proto-splice sites. EMBO J 8 : 2015–2021.
16. QiuWG, SchislerN, StoltzfusA (2004) The evolutionary gain of spliceosomal introns: sequence and phase preferences. Mol Biol Evol 21 : 1252–1263.
17. SverdlovAV, RogozinIB, BabenkoVN, KooninEV (2005) Conservation versus parallel gains in intron evolution. Nucleic Acids Res 33 : 1741–1748.
18. CarmelL, RogozinIB, WolfYI, KooninEV (2007) Patterns of intron gain and conservation in eukaryotic genes. BMC Evol Biol 7 : 192.
19. RoySW, FedorovA, GilbertW (2003) Large-scale comparison of intron positions in mammalian genes shows intron loss but no gain. Proc Natl Acad Sci U S A 100 : 7158–7162.
20. ChoS, JinSW, CohenA, EllisRE (2004) A phylogeny of caenorhabditis reveals frequent loss of introns during nematode evolution. Genome Res 14 : 1207–1220.
21. StajichJE, DietrichFS, RoySW (2007) Comparative genomic analysis of fungal genomes reveals intron-rich ancestors. Genome Biol 8: R223.
22. JaillonO, AuryJM, NoelB, PolicritiA, ClepetC, et al. (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 : 463–467.
23. DerrLK (1998) The involvement of cellular recombination and repair genes in RNA-mediated recombination in Saccharomyces cerevisiae. Genetics 148 : 937–945.
24. BrosiusJ (1991) Retroposons—seeds of evolution. Science 251 : 753.
25. McCarreyJR, ThomasK (1987) Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326 : 501–505.
26. BennetzenJL, FreelingM (1997) The unified grass genome: synergy in synteny. Genome Res 7 : 301–306.
27. GaleMD, DevosKM (1998) Comparative genetics in the grasses. Proc Natl Acad Sci U S A 95 : 1971–1974.
28. IlicK, SanMiguelPJ, BennetzenJL (2003) A complex history of rearrangement in an orthologous region of the maize, sorghum, and rice genomes. Proc Natl Acad Sci U S A 100 : 12265–12270.
29. LaiJ, MaJ, SwigonovaZ, RamakrishnaW, LintonE, et al. (2004) Gene loss and movement in the maize genome. Genome Res 14 : 1924–1931.
30. WickerT, BuchmannJP, KellerB (2010) Patching gaps in plant genomes results in gene movement and erosion of colinearity. Genome Res 20 : 1229–1237.
31. WangH, BennetzenJL (2012) Centromere retention and loss during the descent of maize from a tetraploid ancestor. Proc Natl Acad Sci U S A 109 : 21004–21009.
32. LinH, ZhuW, SilvaJC, GuX, BuellCR (2006) Intron gain and loss in segmentally duplicated genes in rice. Genome Biol 7: R41.
33. Castillo-DavisCI, MekhedovSL, HartlDL, KooninEV, KondrashovFA (2002) Selection for short introns in highly expressed genes. Nat Genet 31 : 415–418.
34. YuJ, HuS, WangJ, WongGK, LiS, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296 : 79–92.
35. ZhuL, ZhangY, ZhangW, YangS, ChenJQ, et al. (2009) Patterns of exon-intron architecture variation of genes in eukaryotic genomes. BMC Genomics 10 : 47.
36. CarelsN, HateyP, JabbariK, BernardiG (1998) Compositional properties of homologous coding sequences from plants. J Mol Evol 46 : 45–53.
37. CampbellWH, GowriG (1990) Codon usage in higher plants, green algae, and cyanobacteria. Plant Physiol 92 : 1–11.
38. KawabeA, MiyashitaNT (2003) Patterns of codon usage bias in three dicot and four monocot plant species. Genes Genet Syst 78 : 343–352.
39. GruenbaumY, SzyfM, CedarH, RazinA (1983) Methylation of replicating and post-replicated mouse L-cell DNA. Proc Natl Acad Sci U S A 80 : 4919–4921.
40. SanMiguelP, GautBS, TikhonovA, NakajimaY, BennetzenJL (1998) The paleontology of intergene retrotransposons of maize. Nat Genet 20 : 43–45.
41. FengS, CokusSJ, ZhangX, ChenPY, BostickM, et al. (2010) Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A 107 : 8689–8694.
42. SharpPA, BurgeCB (1997) Classification of introns: U2-type or U12-type. Cell 91 : 875–879.
43. YenerallP, KrupaB, ZhouL (2011) Mechanisms of intron gain and loss in Drosophila. BMC Evol Biol 11 : 364.
44. RoySW, GilbertW (2005) Complex early genes. Proc Natl Acad Sci U S A 102 : 1986–1991.
45. RoySW, GilbertW (2005) Rates of intron loss and gain: implications for early eukaryotic evolution. Proc Natl Acad Sci U S A 102 : 5773–5778.
46. NielsenCB, FriedmanB, BirrenB, BurgeCB, GalaganJE (2004) Patterns of intron gain and loss in fungi. PLoS Biol 2: e422.
47. RoySW, PennyD (2007) Patterns of intron loss and gain in plants: intron loss-dominated evolution and genome-wide comparison of O. sativa and A. thaliana. Mol Biol Evol 24 : 171–181.
48. FawcettJA, RouzeP, Van de PeerY (2011) Higher intron loss rate in Arabidopsis thaliana than A. lyrata is consistent with stronger selection for a smaller genome. Mol Biol Evol 29 : 849–859.
49. CohenNE, ShenR, CarmelL (2011) The role of reverse transcriptase in intron gain and loss mechanisms. Mol Biol Evol 29 : 179–186.
50. RogersJH (1989) How were introns inserted into nuclear genes? Trends Genet 5 : 213–216.
51. IrimiaM, RukovJL, PennyD, VintherJ, Garcia-FernandezJ, et al. (2008) Origin of introns by 'intronization' of exonic sequences. Trends Genet 24 : 378–381.
52. CataniaF, LynchM (2008) Where do introns come from? PLoS Biol 6: e283.
53. WesslerSR, BaranG, VaragonaM (1987) The maize transposable element Ds is spliced from RNA. Science 237 : 916–918.
54. FridellRA, PretAM, SearlesLL (1990) A retrotransposon 412 insertion within an exon of the Drosophila melanogaster vermilion gene is spliced from the precursor RNA. Genes Dev 4 : 559–566.
55. GirouxMJ, ClancyM, BaierJ, InghamL, McCartyD, et al. (1994) De novo synthesis of an intron by the maize transposable element Dissociation. Proc Natl Acad Sci U S A 91 : 12150–12154.
56. FarlowA, MeduriE, DolezalM, HuaL, SchlottererC (2010) Nonsense-mediated decay enables intron gain in Drosophila. PLoS Genet 6: e1000819.
57. Cavalier-SmithT (1985) Selfish DNA and the origin of introns. Nature 315 : 283–284.
58. SharpPA (1985) On the origin of RNA splicing and introns. Cell 42 : 397–400.
59. RoySW, IrimiaM (2009) Mystery of intron gain: new data and new models. Trends Genet 25 : 67–73.
60. ZhangLY, YangYF, NiuDK (2010) Evaluation of models of the mechanisms underlying intron loss and gain in Aspergillus fungi. J Mol Evol 71 : 364–373.
61. WordenAZ, LeeJH, MockT, RouzeP, SimmonsMP, et al. (2009) Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science 324 : 268–272.
62. TorrianiSF, StukenbrockEH, BrunnerPC, McDonaldBA, CrollD (2011) Evidence for extensive recent intron transposition in closely related fungi. Curr Biol 21 : 2017–2022.
63. van der BurgtA, SeveringE, de WitPJ, CollemareJ (2012) Birth of new spliceosomal introns in fungi by multiplication of introner-like elements. Curr Biol 22 : 1260–1265.
64. VerhelstB, Van de PeerY, RouzeP (2013) The complex intron landscape and massive intron invasion in a picoeukaryote provides insights into intron evolution. Genome Biol Evol 5 : 2393–2401.
65. KassSU, PrussD, WolffeAP (1997) How does DNA methylation repress transcription? Trends Genet 13 : 444–449.
66. JonesPA, TaylorSM (1980) Cellular differentiation, cytidine analogs and DNA methylation. Cell 20 : 85–93.
67. RobertsonHM (1998) Two large families of chemoreceptor genes in the nematodes Caenorhabditis elegans and Caenorhabditis briggsae reveal extensive gene duplication, diversification, movement, and intron loss. Genome Res 8 : 449–463.
68. LlopartA, ComeronJM, BrunetFG, LachaiseD, LongM (2002) Intron presence-absence polymorphism in Drosophila driven by positive Darwinian selection. Proc Natl Acad Sci U S A 99 : 8121–8126.
69. DerrLK, StrathernJN (1993) A role for reverse transcripts in gene conversion. Nature 361 : 170–173.
70. FinkGR (1987) Pseudogenes in yeast? Cell 49 : 5–6.
71. DenoeudF, HenrietS, MungpakdeeS, AuryJM, Da SilvaC, et al. (2010) Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science 330 : 1381–1385.
72. FeiberAL, RangarajanJ, VaughnJC (2002) The evolution of single-copy Drosophila nuclear 4f-rnp genes: spliceosomal intron losses create polymorphic alleles. J Mol Evol 55 : 401–413.
73. NiuDK, HouWR, LiSW (2005) mRNA-mediated intron losses: evidence from extraordinarily large exons. Mol Biol Evol 22 : 1475–1481.
74. SharptonTJ, NeafseyDE, GalaganJE, TaylorJW (2008) Mechanisms of intron gain and loss in Cryptococcus. Genome Biol 9: R24.
75. ZhuT, NiuDK (2013) Frequency of intron loss correlates with processed pseudogene abundance: a novel strategy to test the reverse transcriptase model of intron loss. BMC Biol 11 : 23.
76. FangW, LandweberLF (2013) RNA-mediated genome rearrangement: hypotheses and evidence. Bioessays 35 : 84–87.
77. VogelJP, GarvinDF, MocklerTC, SchmutzJ, RokhsarD, et al. (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463 : 763–768.
78. BennetzenJL, SchmutzJ, WangH, PercifieldR, HawkinsJ, et al. (2012) Reference genome sequence of the model plant Setaria. Nat Biotechnol 30 : 555–561.
79. PatersonAH, BowersJE, BruggmannR, DubchakI, GrimwoodJ, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457 : 551–556.
80. SchnablePS, WareD, FultonRS, SteinJC, WeiF, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326 : 1112–1115.
81. WolfgruberTK, SharmaA, SchneiderKL, AlbertPS, KooDH, et al. (2009) Maize centromere structure and evolution: sequence analysis of centromeres 2 and 5 reveals dynamic Loci shaped primarily by retrotransposons. PLoS Genet 5: e1000743.
82. LiL, StoeckertCJJr, RoosDS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13 : 2178–2189.
83. AbascalF, ZardoyaR, TelfordMJ (2010) TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res 38: W7–13.
84. BennetzenJL, ColemanC, LiuR, MaJ, RamakrishnaW (2004) Consistent over-estimation of gene number in complex plant genomes. Curr Opin Plant Biol 7 : 732–736.
85. StoltzfusA, LogsdonJMJr, PalmerJD, DoolittleWF (1997) Intron "sliding" and the diversity of intron positions. Proc Natl Acad Sci U S A 94 : 10739–10744.
86. ChawSM, ChangCC, ChenHL, LiWH (2004) Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. J Mol Evol 58 : 424–441.
87. WolfeKH, GouyM, YangYW, SharpPM, LiWH (1989) Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc Natl Acad Sci U S A 86 : 6201–6205.
88. ChalupskaD, LeeHY, FarisJD, EvrardA, ChalhoubB, et al. (2008) Acc homoeoloci and the evolution of wheat genomes. Proc Natl Acad Sci U S A 105 : 9691–9696.
89. SwigonovaZ, LaiJ, MaJ, RamakrishnaW, LlacaV, et al. (2004) Close split of sorghum and maize genome progenitors. Genome Res 14 : 1916–1923.
90. DuZ, ZhouX, LingY, ZhangZ, SuZ (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–70.
91. BenjaminiY, YekutieliD (2001) The control of the false discovery rate in multiple testing under dependency. Annals of Statistics 29 : 1165–1188.
Štítky
Genetika Reprodukční medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 12- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



