-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
Sequencing technologies allow identification of genetic variants down to single base resolution for a whole human genome. The vast majority of these variants (over 90%) are rare, with population frequencies less than 1%. Furthermore, in a specific study, many of the variants identified are not associated with the disease of interest, and identification of the small proportion of truly causal variants is a difficult task. Clearly, for causal variants that are rare enough to only appear a few times in a study, observed frequencies in cases and controls are not enough to distinguish them from the vast majority of random variation, and rich functional annotations can help identify the causal variants. Here we propose to develop a set of statistical methods that leverage diverse functional genomics annotations with sequencing data to identify a small set of potentially causal variants and estimate their effects. Pinpointing a subset of potentially causal variants is crucial for understanding precise biological mechanisms, and for further experimental functional studies.
Published in the journal: . PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004729
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004729Summary
Sequencing technologies allow identification of genetic variants down to single base resolution for a whole human genome. The vast majority of these variants (over 90%) are rare, with population frequencies less than 1%. Furthermore, in a specific study, many of the variants identified are not associated with the disease of interest, and identification of the small proportion of truly causal variants is a difficult task. Clearly, for causal variants that are rare enough to only appear a few times in a study, observed frequencies in cases and controls are not enough to distinguish them from the vast majority of random variation, and rich functional annotations can help identify the causal variants. Here we propose to develop a set of statistical methods that leverage diverse functional genomics annotations with sequencing data to identify a small set of potentially causal variants and estimate their effects. Pinpointing a subset of potentially causal variants is crucial for understanding precise biological mechanisms, and for further experimental functional studies.
Introduction
The tremendous progress in massively parallel sequencing (aka ‘next generation’ sequencing) technologies enables investigators to obtain genetic information down to single base resolution on a genome-wide scale in a rapid and cost efficient manner [1], [2], [3]. The resulting datasets are high dimensional and very sparse, with millions of genetic variants, the vast majority of which are rare in the population. For example, in any genetic region, it is expected that over 90% of genetic variants have a frequency in the population of less than 1% [4]. Therefore in any given study, most variants are only observed a small number of times (e.g. many of them are singletons or doubletons). This sparse nature of the data poses nontrivial statistical difficulties, and traditional statistical methods employed for association testing with common variants are not powerful in this context [5].
Both empirical and theoretical studies suggest that rare genetic variants are an important contributor to disease risk [6], [7], [8], [9]. Over the past few years several statistical tests have been proposed to test for association with rare variants in a small genetic region, such as a gene [10], [11], [12], [13], [14], [15], [16], [17]. The proposed association tests are based on the idea of grouping together variants in the gene, and testing for association at the gene rather than variant level. While these methods attempt to increase power by cumulating the signal across a larger region, they compromise precision and, in particular, it is not possible to pinpoint individual causal variants and estimate their effects on disease. Prioritizing a small number of plausible causal variant candidates is very important for further follow-up functional studies, since experimental analyses are difficult to implement and expensive for large number of variants [18], [19]. Furthermore, identification of causal variants is essential for understanding the precise molecular mechanisms of disease. Despite its importance, this problem is only now possible to address due to the increasing availability of large-scale sequencing data and the advances in computational methods for predicting the functional effects of genetic variation [19].
The fundamental challenge in pinpointing rare causal variants is that these variants are observed very infrequently in any given dataset and these sparse frequencies on their own are insufficient to provide meaningful risk predictions. In particular, for singletons or doubletons it will be necessary to incorporate prior functional and evolutionary conservation information in order to prioritize them as likely causal. Hierarchical modeling offers a natural strategy to leverage collective evidence from rare variants with sparse data. This can be accomplished in the presence of hierarchical covariates that are associated with disease risk and which can be used for implicitly aggregating the rare variants to permit stronger inferences about individual variants. These hierarchical covariates are characteristics of the variants themselves, such as the degree of conservation across species, the position in the gene, and other features that can be represented using bioinformatic measures. Indeed, many annotation tools (such as ANNOVAR [20], PolyPhen-2 [21], SIFT [22], GERP++ [23]) exist to predict the possible impact of a variant on the function of a human protein, or the level of evolutionary constraint for a variant. Even though such bioinformatic predictions of deleteriousness are not extremely accurate and are continuously being improved, they can provide useful information on the prior likelihood that a variant is causal, especially when multiple such predictors are used, as we show in this work. In earlier work we have developed a hierarchical modeling approach that is capable of estimating odds ratios for variants that occur infrequently in the dataset [24], [25]. Hierarchical regression techniques have also been adopted in a Bayesian framework with the goal of detecting rare causal variants [26], [27], however they can be computationally intensive and can be dependent on the choice of the prior weights [27]. More recently, Pickrell [28] has used hierarchical models to combine rich functional genomics annotations (as generated by the ENCODE project [29]) and summary statistics from GWAS to identify types of genomic elements enriched among disease susceptibility loci.
Here we propose and investigate the performance of two complementary statistical methods that are able to incorporate prior information on the putative function of individual variants in a gene in order to (1) identify a list of likely causal variants, and (2) estimate the effects of these variants on disease. The first approach is a backward elimination procedure based on groupwise association tests that leads to the identification of a small set of “interesting” variants in the gene, which are enriched in causal variants. The second approach complements the first by employing hierarchical models [24],[25] that can incorporate diverse functional and evolutionary conservation annotations, and in turn provides effect size estimates and confidence intervals for individual variants.
Methods
First, we review the basics of groupwise association tests, and then we describe in detail the two complementary methods we propose for prioritizing variants for follow-up functional studies.
Groupwise association tests for sequencing data
We assume that n subjects have been sequenced in a region of interest (e.g., a gene), that contains m variants. Let be the genotype matrix. We consider the regression model
where is a link function, and can be set to be the identity function when traits are continuous, or the logistic function when traits are dichotomous; are regression coefficients for the covariates that we want to adjust for. is the vector of genotypes for the ith individual, and is its trait value. are regression coefficients for the m genetic variants.We are interested in testing the null hypothesis of no genetic effects: Testing each individual or using multiple df tests can lack power because of the sparsity of the data and the many variants in a gene. Therefore, we need to impose certain assumptions on 's to make the test more powerful. For example, one of the most widely used tests, the Burden test, assumes that all β's have essentially the same value, say , and the regression model in (1) amounts to . More generally, Lee et al. [15] assume that is a random variable with , and for different j and k. To test the null hypothesis of no genetic effects the variance-component score statistic has been proposed [15]:
where and specifies an exchangeable correlation matrix, and is a diagonal weight matrix, where each weight can be related, for example, to the predicted functional effect of a variant (e.g. PolyPhen-2 or SIFT score); for a dichotomous trait, is a vector of estimated probabilities of under the null model. Although this class of tests is more general, the two commonly used tests are the Burden test () and the SKAT test (). These score statistics are easy to compute and can be written simply asThe null distribution of is approximated by a mixture of distributions. Davies' method [30] or moment matching can be employed to calculate the p value. The relative performance of the two tests will depend on the true underlying disease model. The Burden test tends to be more powerful when disease associated variants are all of the same type (risk or protective) and with effects of similar magnitude. The SKAT test tends to be more powerful when there is a mixture of risk and protective variants, and also when only a small percentage of variants in a region are causal. A parallel framework for family-based designs has also been proposed [16].
Backward elimination procedure
The groupwise association tests described above test for association at a gene level, but are not able to pinpoint individual causal variants in the gene. However, once a gene has been shown to contain variants associated with disease (e.g. using the Burden or SKAT tests), identifying the individual causal variants among the many variants in a gene is of considerable interest as it can lead to a better understanding of the molecular mechanisms underlying a complex trait, and is essential for further experimental validation work.
Starting with a groupwise association test, one natural way to identify causal variants that are individually of weak effect is to evaluate their contribution to a given set of variants by removing the variant from the set, and assessing the resulting effect, e.g. the p value for the reduced set. The following iterative algorithm (essentially a backward elimination procedure) is designed for this purpose.
Backward Elimination Algorithm: Step 1. Start with a set of variants . The current set is . Compute the score statistic in eq. (2) (either or ) for this current set , and compute the p value: .
Step 2. Remove each of the variants one at a time from , i.e. consider the sets with , and then compute the corresponding score statistic and p value for each of these reduced sets: .
Step 3. If then remove the variant that leads to the smallest p value:
The current set becomes and repeat steps 2 & 3. If the current p value cannot be improved, then go to step 4.
Step 4. Return the current set of variants.
The results on a typical simulated example are shown in Figure S1. We show there the effect of removing a causal variant on the p value of the reduced set (i.e. in Step 2 above), compared to removing a non-causal variant. As shown, the removal of causal variants will tend to result in an increase in the p value for the reduced set, as desired. There is a highly significant difference in the p values for the reduced sets when removing causal vs. non-causal variants (bootstrap Kolmogorov-Smirnov test p value ).
This algorithm is applicable when the number of variants we start with in Step 1 is not too large (otherwise, the contribution of a weak variant to a large set is difficult to evaluate). However, sequencing a gene in thousands of individuals can lead to the detection of potentially hundreds of variants, or more. Therefore, we use a resampling procedure, whereby each time a small number of variants is chosen (say ) from the large number of variants identified in a gene, and then the above algorithm is applied to such small sets a large number of times (in our examples we use 2000 such re-samplings, although this number can be increased in the case of a large number of variants in the gene). At the end, for each variant in the gene we calculate the number of times it was returned in Step 4; we call this number the return count for a variant. A similar resampling procedure has been applied before in the context of gene-by-gene interaction [31]. Our goal is to use the sample of return counts to partition variants into two groups: “interesting” (higher return counts) and “non-interesting” (lower return counts), with the “interesting” category expected to be enriched in disease causing variants. We use nonparametric EM-like methods [32] to identify the two subgroups (see Text S2 for more details).
Integrating functional annotation into the above algorithm
It is well recognized that certain functional categories are more likely to be enriched among causal variants than others [28], [33]. An obvious example is rare non-synonymous variants, which are known to be enriched among disease causing variants. Similarly, loss-of-function (LoF) variants, including nonsense, splice-site and frameshift mutations, are heavily enriched among causal variants [18]. Therefore stratifying variants by different functional categories can improve false discovery rates. We can incorporate information on functional annotation in the backward elimination algorithm above. This can be done by simply applying the algorithm within different classes, say non-synonymous and synonymous. Furthermore, other functional or conservation scores (such as PolyPhen-2, SIFT and GERP++ scores) can be explicitly incorporated in the Burden and SKAT score statistics themselves (as weights associated with individual variants in eq. (2)), although only one such score can be incorporated at a time.
Hierarchical model to estimate odds ratios of individual rare variants
A complementary approach to the backward elimination procedure described above is a hierarchical model. Hierarchical modeling has several important advantages in the analysis of rare variant data, because it can naturally integrate various functional prediction scores for individual variants. Such prior knowledge will be essential in pinpointing the likely causal variants in a gene, especially for causal variants that are rare enough to only appear a few times in a study (e.g. singletons and doubletons). For such variants, observed frequencies in cases and controls are clearly not enough to distinguish them from the vast majority of random variation (in the Nelson et al. study [4], more than 74% of variants were singletons or doubletons). Information on the putative functional effect of a variant on the protein or the degree of evolutionary conservation can be an important indicator on the likelihood of a variant being causal.
Such functional information can be incorporated through a hierarchical model [24], [25]. In the first stage, the trait value is related to the genotypes and possible confounders via the following model:
with notations similar to those in model (1) above.A second stage model relates the individual variant risks to prior (e.g. functional annotation) information known about the variants:
where Z is an matrix for the k variant covariates (e.g. functional information); is a vector of regression parameters for the second stage covariates, and is a vector of normally distributed residual effects, assumed (for convenience) to be statistically independent. A principal advantage of the hierarchical modeling framework is that it can easily incorporate multiple functional annotations.Combining the two models, one obtains the following generalized linear mixed effects model:
The parameters of this model can be estimated using a hybrid Bayesian pseudo-likelihood approach which performs Bayesian estimation of the variance component of the model and then conducts pseudo-likelihood estimation of the fixed and random effects using this estimated random effects variance [24], [25]. We can use the resulting estimates for the odds ratios and their standard errors to rank variants in a gene. Naturally the most difficult to identify are causal variants that occur only a few times. The odds ratio estimates for such variants will heavily depend on the higher level covariates, such as information on the predicted functional effect for a variant. For example, for a variant that occurs infrequently in a dataset (e.g. 2 times in cases and 0 times in controls), knowing that it is a LoF variant increases its likelihood to be a causal variant compared with a synonymous variant with the same frequency.
Backward elimination procedure and hierarchical model
We combine the two complementary methods, the backward elimination procedure and the hierarchical modeling framework, as follows. We identify the list of “interesting” variants from the backward elimination procedure, and for each of these variants we report the effect size estimate, and the associated standard error obtained from the hierarchical model (when applied to all variants, not just to the “interesting” list). The variants in the “interesting” list can be naturally ranked according to these effect estimates. We show below that restricting attention to only the list of “interesting” variants can improve the ranking of causal variants, and that this combined approach performs well in the scenarios we investigated.
Both methods are computationally efficient and have been implemented in software available on the authors' website (http://www.columbia.edu/~ii2135/).
Results
We evaluate the performance of the proposed methods using simulated data and then apply them to two sequencing studies, the Dallas Heart Study and a study on Autism Spectrum Disorders.
Simulated data
We simulated sequence data on 10,000 haplotypes in one genomic region of length 1 Mb under a coalescent model using the software package COSI [34]. The model used in the simulations was the calibrated model for the European population. For our purposes, we randomly sampled small subregions of size 10 kb, and simulated datasets with individuals (equal number of cases and controls). The number of variants and the minor allele frequency (MAF) distribution varies depending on the subregion sampled.
We considered two disease models (Table 1). In these two models, the odds ratio (OR) is a decreasing function of the MAF. For both models, we assume that of the variants with MAF ≤0.05 in the 10 kb region under investigation are causal variants.
Tab. 1. Two disease models M1 and M2. 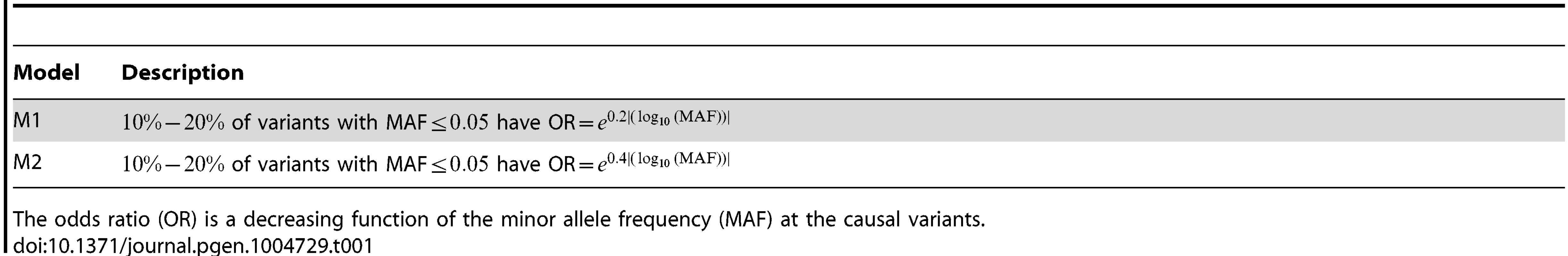
The odds ratio (OR) is a decreasing function of the minor allele frequency (MAF) at the causal variants. For a dichotomous trait, we assumed the logistic model:
with chosen such that the disease prevalence was 0.05.We have also simulated bioinformatic covariates for variants to be used in the backward elimination algorithm, as well as to be incorporated in the hierarchical model. A first bioinformatic covariate we simulate is a binary variable, such as whether a variant is non-synonymous or not. Based on empirical studies [4], we consider the non-synonymous to synonymous ratio (NS:S) for the rare variants in the region to be between (depending on the strength of the purifying selection in the region). We assume that 80% of causal variants are non-synonymous, and then using Bayes' rule we calculate the proportion of non-causal variants that are non-synonymous (see Table 2). Given these settings, the proportions of causal variants among non-synonymous and synonymous variants can be easily derived and are reported for completeness in Table 2.
Tab. 2. Simulation scenarios. 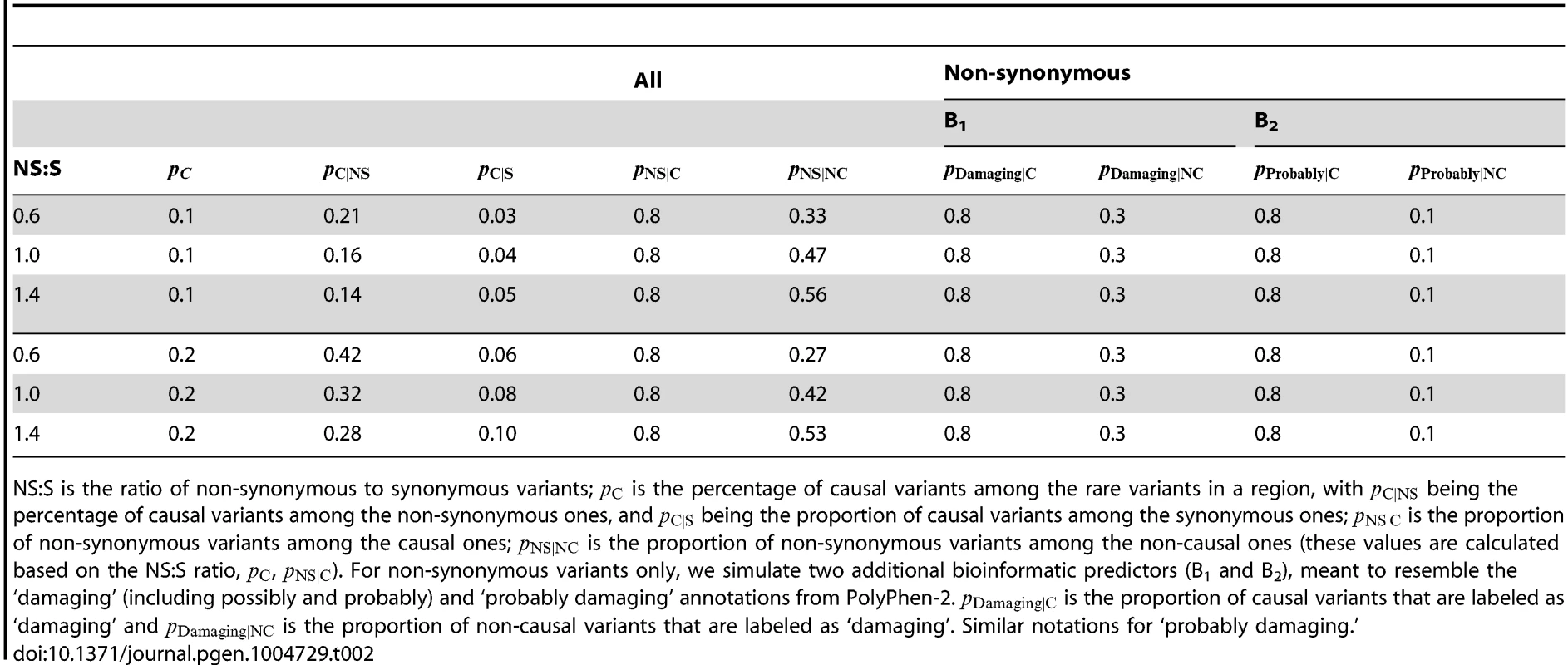
NS:S is the ratio of non-synonymous to synonymous variants; is the percentage of causal variants among the rare variants in a region, with being the percentage of causal variants among the non-synonymous ones, and being the proportion of causal variants among the synonymous ones; is the proportion of non-synonymous variants among the causal ones; is the proportion of non-synonymous variants among the non-causal ones (these values are calculated based on the NS:S ratio, , ). For non-synonymous variants only, we simulate two additional bioinformatic predictors (B1 and B2), meant to resemble the ‘damaging’ (including possibly and probably) and ‘probably damaging’ annotations from PolyPhen-2. is the proportion of causal variants that are labeled as ‘damaging’ and is the proportion of non-causal variants that are labeled as ‘damaging’. Similar notations for ‘probably damaging.’ Furthermore, additional variant annotation tools for non-synonymous variants exist, and are able, for example, to predict the damaging effect of an amino acid substitution (PolyPhen-2 and SIFT), and to assess the extent of evolutionary conservation at a position (GERP++). Therefore, for non-synonymous variants we simulate two additional predictors, as follows. The first bioinformatic predictor (B1) for non-synonymous variants is defined as a binary indicator whether a variant is predicted to be damaging or not. Following the empirical results in Cooper et al. [18] we assume that 30% of non-synonymous, non-causal variants are damaging (possibly or probably), and that 80% of non-synonymous, causal variants are damaging (Table 2). A second bioinformatic predictor (B2) is also defined as a binary indicator whether a variant is predicted to be probably damaging or not. Again, as in Cooper et al. [18] we assume that 10% of non-synonymous, non-causal variants are probably damaging, and that 80% of non-synonymous, causal variants are probably damaging (Table 2). To assess the effect of using a non-informative predictor, we also simulate a binary predictor with 50% non-synonymous causal and 50% non-synonymous non-causal variants having a value of 1 for this non-informative predictor.
The main goal of the proposed methods is to combine sequencing data with functional predictions about the deleteriousness of variants to identify a set of promising variants, enriched in causal variants. Furthermore, the selected variants can be ranked according to their return counts from the backward elimination procedure, or the estimated effects from the hierarchical model (ranking based on Z scores gave similar results). We use several measures to assess the performance of the methods. The main measures are: (1) the overall ranking of the true causal variants among the variants in the gene, and (2) the bias and coverage accuracy in the estimation of effect sizes for the variants from the hierarchical model.
Ranking of causal variants: Simulation results
Nonparametric mixture modeling of return counts
In what follows, we apply the backward elimination procedure described in Methodsseparately to non-synonymous and synonymous variants. Since the non-synonymous variants tend to be enriched in causal variants, the sample distribution of return counts obtained for the non-synonymous variants tends to exhibit two separate subgroups (a “non-interesting” group, enriched in null variants, and an “interesting” group, enriched in causal variants). We identify these two groups using an expectation-maximization (EM) algorithm for nonparametric estimation of mixture models and declare as “interesting” those variants belonging to the right tail component (which corresponds to the larger return counts). This is illustrated in Figure S2 which depicts for a simulated example the histogram of return counts for non-synonymous variants with the fitted two-component mixture overlaid.
For synonymous variants, since only a small proportion of causal variants are expected to be synonymous, the sample distribution of return counts often fails to exhibit separate groups in our simulations; therefore we select the variants with the top 20% return counts as “interesting” to be further investigated; although we expect less than 20% of synonymous variants to be causal (indeed in our simulations only 3%–10% of the synonymous variants were causal), we conservatively choose the threshold of 20% to increase the probability of selecting causal variants, especially since for synonymous variants, unlike the non-synonymous ones, we do not make use of additional information in prioritizing variants.
Ranking of causal variants Non-synonymous vs. synonymous
We employ the backward elimination procedure as well as fit a hierarchical model including the full set of variants and assuming a single functional predictor in the second stage, namely whether a variant is non-synonymous or synonymous. Due to the expected difference in enrichment of causal variants among non-synonymous versus synonymous variants we evaluate the overall ranking of the causal variants separately among non-synonymous and synonymous variants. More explicitly, among the non-synonymous variants selected as “interesting” by the backward elimination procedure we rank the causal variants based on their return counts (this approach is denoted as BE in the figures below). Furthermore, we also use the estimates obtained from the hierarchical model to rank the causal variants among all non-synonymous variants (HM) as well as among the non-synonymous variants selected as “interesting” by the backward elimination procedure (HMS). For each simulation we take the median of the ranks of the causal variants involved and then compute the median of these estimates across simulations. The ranking for the synonymous variants is done similarly.
Figures 1(a) and S3(a) present the median ranks of the causal non-synonymous variants based on the different ranking procedures (HM, BE, and HMS). The hierarchical model (titled “HM” in the figure) results in higher median rank (worse performance) than that of the backward elimination procedure (titled “BE” in the figure). This is of course expected due to the smaller number of variants that the backward elimination procedure returns as “interesting” (Figures 1(a) and S3(a)). However, despite excluding a substantial proportion of variants in the backward elimination process (the “non-interesting” category), we show that the top ranked causal variants in the hierarchical model are kept in the selected list (Figures 1(b) and S3(b)). For the scenarios investigated, the number of causal, non-synonymous variants in the top 10 ranked variants varies between 5 and 8 for the case when the percentage of causal variants in a region is 20% (Figure 1(b)), and 3–6 for the case with only 10% causal variants (Figure S3(b)). When looking only among the “interesting” variants from the backward elimination procedure, the overall ranking of causal variants based on the hierarchical model estimates (titled “HMS”) is similar to the one based on return counts in the backward elimination procedure (Figures 1 and S3). Therefore the backward elimination method can be used as an effective tool to select and rank a set of promising variants, and reduce the overall list of variants to a smaller, more manageable list, followed by further characterization of these variants’ effects within the framework of the hierarchical model. For all methods, and regardless of disease model, the performance tends to decrease as the non-synonymous to synonymous ratio increases from 0.6 to 1.4 (as the effect of purifying selection becomes weaker).
Fig. 1. (a) Median rank of causal variants among the non-synonymous variants for two disease models (M1 and M2) and three values for the NS:S ratio (R = {0.6, 1.0, 1.4}). 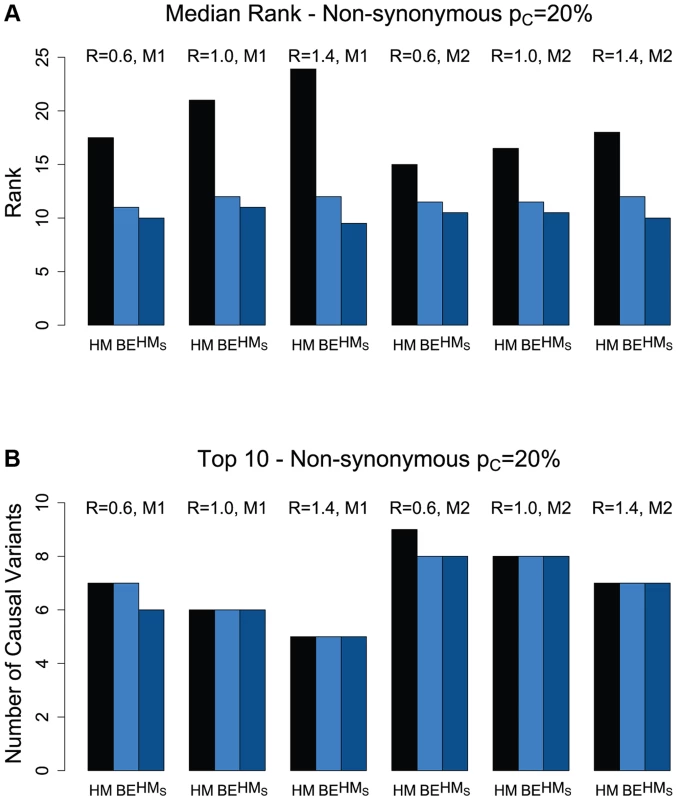
The proportion of causal variants in the region is 20%. HM refers to the original hierarchical model with ranking of the causal variants among the non-synonymous variants, based on their estimated effects; BE refers to the backward elimination procedure for non-synonymous variants; and HMS refers to the ranking of causal variants only among those non-synonymous variants selected by the backward elimination procedure, with ranks based on the estimated effects from the hierarchical model. (b) The number of causal variants in Top 10 for non-synonymous variants. When looking at the synonymous variants separately, the results are qualitatively similar to the non-synonymous case. However, because only 20% of the causal variants are assumed synonymous, the overall ranking of the few causal variants among the synonymous variants is noticeably worse compared with non-synonymous variants, as expected (Figures S4 and S5). For example, in a region with 20% causal variants, overall we detect between 2 and 3 causal synonymous variants among the top 10 ranked variants, and only 1–2 in a region with 10% causal variants. Due to these high false-discovery rates for synonymous variants, it may be more effective to focus initial efforts for causal variant identification among the functional (non-synonymous and LoF) variants. As genomic annotations become richer for synonymous variants, we expect the discovery of causal variants among synonymous variants to become more accurate.
Multiple bioinformatic predictors for non-synonymous variants
We evaluate the effect on ranking the causal variants among non-synonymous variants when additional bioinformatic predictors are added to the hierarchical model (in addition to the indicator whether the variant is non-synonymous vs. synonymous). Note that synonymous variants were assigned a bioinformatic predictor of 0.
We restrict attention to ranking only among the “interesting” variants, as selected by the backward elimination procedure. As shown in Figure 2, when we add one bioinformatic predictor (B1 or B2; see Table 2), the ranking of causal variants improves significantly compared to the original hierarchical model that only uses a binary predictor (whether a variant is non-synonymous or not). The improvement is more pronounced with predictor B2, due to the higher specificity of this predictor. For example, for model M1, a non-synonymous to synonymous ratio of 1.4 and 20% causal variants in a region, the median number of causal variants among the top 10 ranked non-synonymous variants increases from 5 (in the original hierarchical model) to 8 when using bioinformatic predictor B2. Since we do not always know which of several available bioinformatic predictors may have higher accuracy, the hierarchical model allows us to combine multiple bioinformatic predictors. When combining three bioinformatic predictors (two predictors with the same sensitivity and specificity as B1 and one predictor B2, all independent), we find that the ranking of causal variants is now similar or superior to the ranking obtained when using only the better of the two bioinformatic predictors (i.e. B2). Similarly, when using a combination of four bioinformatic predictors (four predictors with the same sensitivity and specificity as B1), the ranking of causal variants is better than using just a single predictor B1, and similar to using the more accurate predictor B2. These results suggest that using multiple bioinformatic predictors with different accuracies (even multiple weak predictors) can help detection of the causal variants. Similar results are obtained when the proportion of causal variants in a region is 10% (Figure S6).
Fig. 2. The effect of multiple bioinformatic predictors for non-synonymous variants. 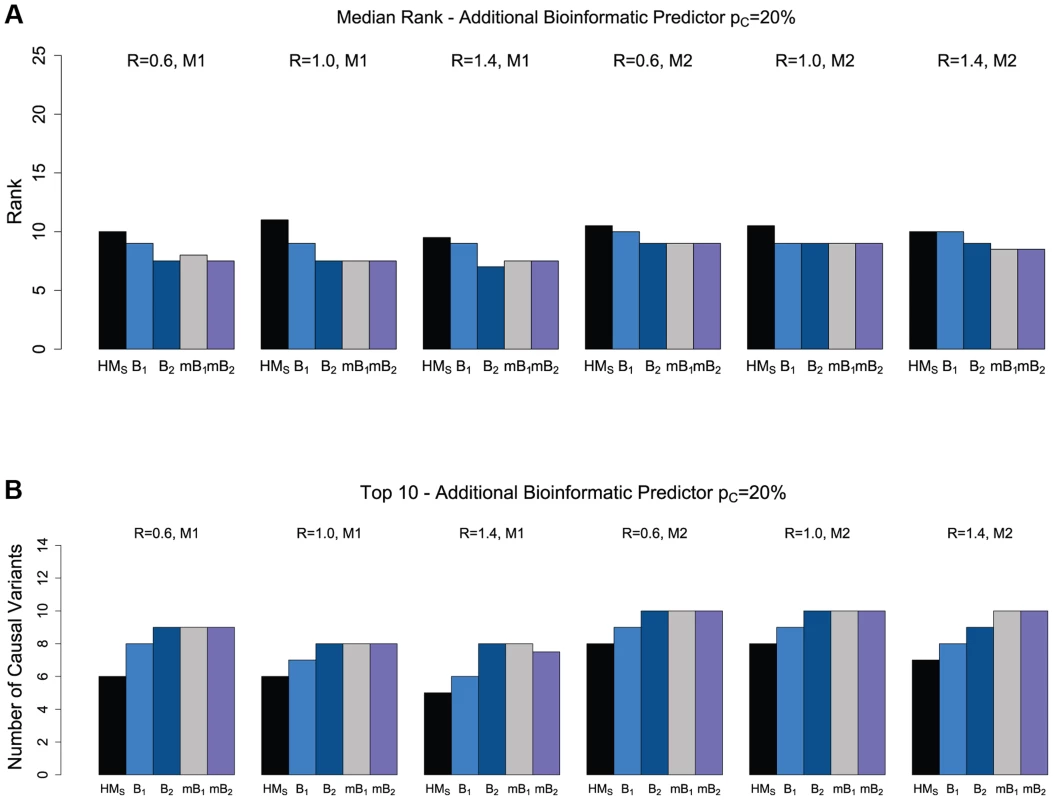
Ranking is done only within the set of variants selected by the backward elimination procedure. (a) Median rank of causal variants for two disease models (M1 and M2) and three values for the NS:S ratio (R = {0.6, 1.0, 1.4}). The proportion of causal variants in the region is 20%. HMS refers to the hierarchical model with ranking of the causal variants among the selected non-synonymous variants, based on their estimated effects, B1 refers to the hierarchical model with one bioinformatic predictor (B1, Table 2), B2 refers to the hierarchical model with one bioinformatic predictor (B2), mB1 refers to the hierarchical model with three bioinformatic predictors (B1, B1, and B2), and mB2 refers to the hierarchical model with four bioinformatic predictors (four B1s). (b) The number of causal variants in Top 10 for non-synonymous variants. We have also evaluated the effect of including a non-informative predictor in the analysis, although in practice we expect that functional annotations are correlated with the causal status of a variant. The results are reported in Figures S11 and S12. As shown, including a random (non-informative) bioinformatic predictor does lead to worse performance compared to when such a predictor is not included, although combining an informative predictor (B1) together with a non-informative one does help improve the performance. Again, the ability of the hierarchical model to incorporate multiple functional predictors of varying accuracy is an important feature when the best predictors are not known a priori.
It is possible to incorporate one bioinformatic predictor, such as B1 or B2, in the backward elimination procedure directly (as a weight in the Burden test statistic). We found that for the case of only one bioinformatic predictor, the backward elimination procedure performed similarly with (or slightly worse than) the hierarchical model (Figures S7). However, in general, it is not clear how to choose one single functional annotation from several annotations available. Therefore, the hierarchical model has the important advantage that multiple bioinformatic predictors can be included, and, as shown above, the ranking of the causal variants improves with the addition of several predictors of varying accuracy.
Effect size estimation for variants in the hierarchical model
As already mentioned, the hierarchical model has distinct advantages when multiple functional predictions are available for variants. In particular, it is possible to provide effect size estimates and standard errors for individual variants, that take into account such diverse functional predictions. As seen in Table S1, for disease model M1 (Table 1), absolute biases of the log odds ratio estimates from the hierarchical modeling approach are similar among the different scenarios while coverages are close to the nominal level of 95%. In comparison, bias is further increased and coverages are under the nominal level of 95% for disease model M2 (which assumes higher odds ratios than model M1, Table 1), though there is a trend towards reduced bias and improved coverage with the addition of stronger bioinformatic predictor(s).
The biases observed here are due to several causes. One main source of bias is the shrinkage phenomenon that occurs with hierarchical models: in this setting of sparse data the model relies heavily on the higher level covariates and as a result the estimated risks of the non-causal variants with high bioinformatic predictor scores will be biased upwards, while the risks of the causal variants with low bioinformatic predictor values will be shrunk down, resulting in increased bias and loss of power, respectively. As the frequency of carriers increases, the model overrides the misclassifications of the higher level covariate, yielding less biased estimates (data not shown). This shrinkage is even more pronounced for model M2, which assumes higher odds ratios for the causal variants (compared to M1), resulting in the poorer performance noted with model M2. An additional source of bias comes from our analyses being conditional on the groupwise (gene-based) test being significant.
Application to the Dallas Heart Study and ANGPTL4
We first show an application of the proposed methods to a well studied re-sequencing dataset for ANGPTL4 for 3,551 individuals of varied ethnicity from the Dallas Heart Study. Rare and low-frequency variants in this gene have been previously associated with low serum triglyceride levels [35]. We consider log-transformed triglyceride level as our phenotype, and adjust for gender and ethnicity. As in the original study [35], we dichotomize the phenotype by considering the individuals in the lowest quartile as cases, and the individuals in the highest quartile as controls, for a total of 898 individuals with variation in this gene. We identify 20 functional variants (missense, nonsense, and frameshift).
In Table 3 we report the functional variants, ranked by the estimated effects from the hierarchical model taking into account their PolyPhen-2 and GERP_RS scores. Also reported is the return count from the backward elimination procedure (due to the small number of variants in this gene we do not fit the nonparametric mixture model in this example; instead we simply rank all variants). All the top ranked variants that appear only in cases (i.e. the lowest quartile) have been shown by Romeo et al. [36] to severely compromise the function of the protein. In particular, the top ranked variant, p.Lys217Ter, is a nonsense variant that appears only once in an affected individual, and is assumed to interfere with protein synthesis. The second, fourth, seventh and eighth variants have been shown using functional studies to lead to impaired protein secretion. The fifth variant showed reduced ability to inhibit LPL (lipoprotein lipase) activity in vitro, while the sixth variant introduced a premature termination codon [36]. The third variant in the list, p.Glu40Lys, is a missense variant (classified as probably damaging by PolyPhen-2 and as evolutionarily conserved site by GERP_RS), with a frequency of 1.3% in this dataset, and has been shown to be significantly associated with plasma triglyceride levels [35]. However, due to its presence even among controls (i.e. the highest quartile), this variant was not investigated in the functional studies in Romeo et al. [36].
Tab. 3. The 20 functional (non-synonymous, nonsense and frameshift) variants in ANGPTL4, with PolyPhen-2 and GERP_RS scores included in the hierarchical model. 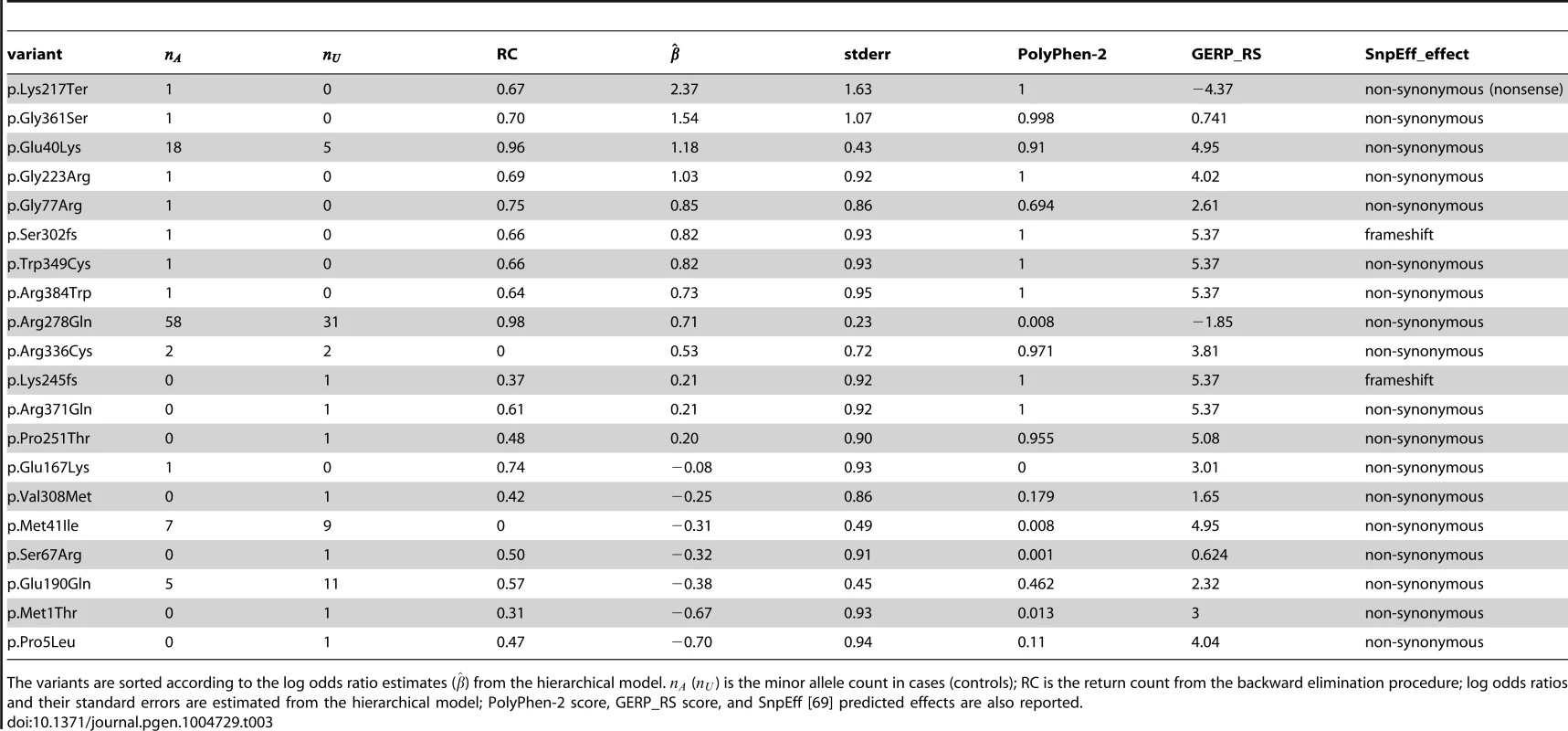
The variants are sorted according to the log odds ratio estimates () from the hierarchical model. () is the minor allele count in cases (controls); RC is the return count from the backward elimination procedure; log odds ratios and their standard errors are estimated from the hierarchical model; PolyPhen-2 score, GERP_RS score, and SnpEff [69] predicted effects are also reported. We next show an application to a gene with a larger number of functional variants, and for which not much is known on the likely causal variants. Hence the next application is a more difficult example for the proposed methods.
Application to the Cohen syndrome and VPS13B
The Vacuolar Protein Sorting 13 homolog B (VPS13B, also known as COH1, MIM #607817) is a gene associated with Cohen syndrome (CS, OMIM #216550), a rare autosomal recessive neurodevelopmental disorder overrepresented in Finland and common in Amish, Irish travelers and Greek/Mediterranean founder populations [37], [38]. At least 200 affected individuals of diverse ethnic background have been reported so far with diverse VPS13B mutations, including nonsense, missense, splicing, indels, microdeletions and microduplications [38]. Despite clinical heterogeneity in part related to ethnic background, the disorder has core features, including non-progressive intellectual disability, motor clumsiness, postnatal microcephaly, a typical facial gestalt, hypotonia, intermittent neutropenia, and chorioretinal dystrophy [39]. Behavioral disturbances are common among CS individuals, and autistic traits have been reported in cases of greek/mediterranean descent [40]. Furthermore, VPS13B mutations have been found in individuals with autism [41] and non-syndromic intellectual disability [42]. It is worth noting that mutations in another member of the VPS13 gene family (VPS13A or CHAC, MIM #605978, encoding for a protein known as Chorein), cause chorea-acanthocytosis [43] (MIM #200150), a recessive disorder of acanthocytosis and adult-onset choreic involuntary movements with significant co-morbidity with psychiatric illness [44].
VPS13B is also an intolerant gene with a Residual Variation Intolerance Score [45] of −2.44 (top 0.55% most intolerant genes) in Europeans and a similar score for African Americans. We applied the proposed methods to the 166 VPS13B variants identified in a whole-exome sequencing autism spectrum disorders (ASD) case/control dataset (; more details on this dataset can be found in Text S1). We tested for association with functional (non-synonymous, nonsense and splice-sites) rare variants in this gene and the Burden test p value was 0.01. We then used the backward elimination algorithm to identify a set of “interesting” (i.e. potentially causal) variants, and for each of these variants we report effect size estimates and standard errors from the hierarchical model. Note that the ratio of non-synonymous to synonymous variants in this gene is 0.84, hence towards the lower end of values in our simulated scenarios.
Of the 166 variants in this gene, we focus on 74 that are non-synonymous, nonsense or splice-sites (two variants affecting the invariant splice acceptor site of the intron between exons 51 and 52 have been excluded from further analyses because they did not validate by Sanger sequencing). Of these, the backward elimination procedure selects 42 : 2 of them are LoF (one nonsense and one variant affecting an essential splice site), and of the missense PolyPhen-2 predicts that 14 are probably damaging, 1 possibly damaging, and 25 benign (Figure 3). In Figure 4(a) we show the drop in p value each time a variant is being removed in step 3 of the backward elimination procedure; the process stops when the p value starts to increase as one tries to remove any of the remaining variants. Also shown in Figure 4(b) is the distribution of return counts (from the re-sampling procedure), and overlaid is the fitted mixture with two distinct components. The 42 selected variants belong to the second component of the fitted mixture (these are the “interesting” variants). As a comparison, applying the backward elimination algorithm to the remaining 90 synonymous variants results in no distinguishable “interesting” component (and markedly smaller average return counts compared to the non-synonymous case; Figure S8).
Fig. 3. Predicted deleteriousness scores are shown for 71 rare functional variants (non-synonymous, nonsense and splice-sites). 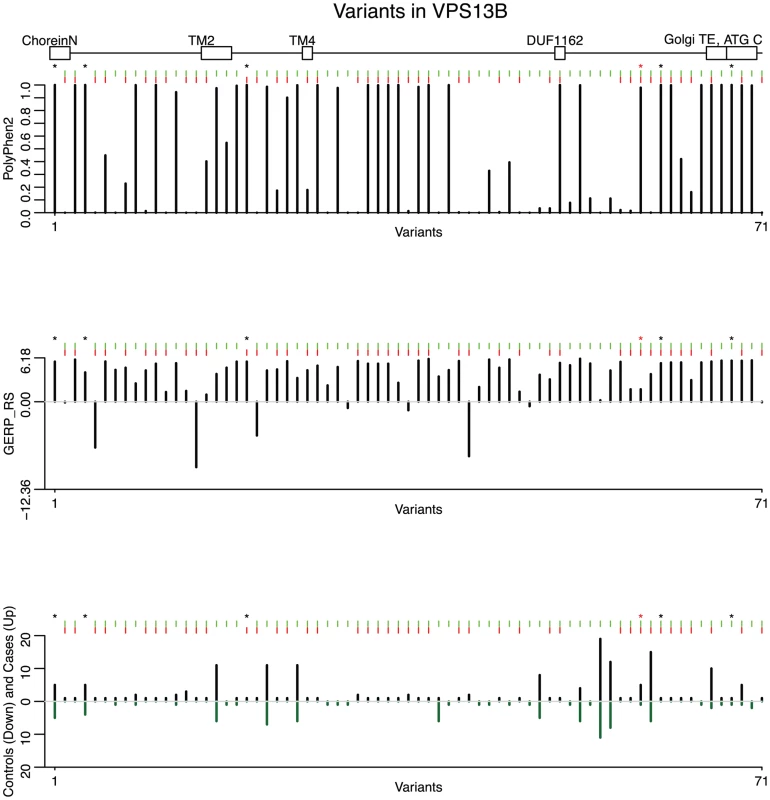
From the top, the first plot depicts the PolyPhen-2 score for each variant, the second depicts the GERP_RS score, and the third depicts variant counts for cases (up) and controls (down). Green tick marks indicate a variant contained in an exon, and red ticks indicate that a variant is selected by the backward elimination procedure. LoF variants are marked by a black asterisk; the homozygous probably damaging variant is marked by a red asterisk. The location of five protein domains (ChoreinN, TM2, TM4, DUF1162, Golgi targeting element, and ATG C) are depicted by boxes at the top of the plot (see Figure S10 for a complete view of VPS13B protein domains). Variants are plotted equidistantly on the x-axis. Fig. 4. Results from the backward elimination procedure for non-synonymous and splice site variants in VPS13B. 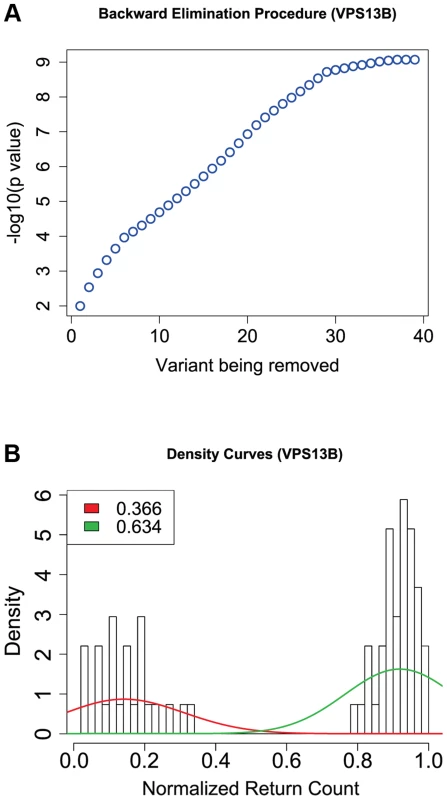
(a) The change in p value is shown as variants are being removed one by one (when the backward elimination procedure is run once on all non-synonymous variants). (b) Distribution of return counts for non-synonymous and splice site variants in VPS13B; overlaid is a fitted mixture with two components. In Table 4 we report the top 20 variants among the selected functional variants (ranked by the estimated effects from the hierarchical model), along with their PolyPhen-2 and GERP_RS scores. Noticeably, among the top ranked variants there is a probably damaging variant (, p.Arg3198Trp, annotated on NM_152564.4 and Q7Z768-2, respectively) with 5 variant copies in cases and 1 in controls, with one case being homozygous at the position. Furthermore, the top two variants in the list have both very high C-scores [46] (36 and 35, top 0.1%), based on the recently introduced measure of deleteriousness Combined Annotation-Dependent Depletion (CADD) that integrates diverse genome annotations. The two LoF variants (one nonsense (, p.Ser3383Ter) and one splice site ()) have been seen only once in cases (i.e. singletons). Notably, the splice variant affects the splice donor site of the intron between exons 18 and 19, and a homozygous mutation in the splice acceptor site of the same intron has been identified in an individual suffering from CS [47]. As a first step toward the characterization of the variants, we used Sanger sequencing to validate two cases with the variant and the cases with and and study their inheritance pattern. This analysis is of particular relevance for singletons, considering that the false discovery rate among those can be high. All variants were validated and found to be inherited (Figure S9). In one family with the , both affected children are homozygous and inherit the variant from their parents (father homozygous and mother heterozygous - Figure S9A). In a second family with , the variant is transmitted from heterozygous parents to one affected child, and untransmitted to the unaffected child (Figure S9B). The variant is inherited from the mother (Figure S9C), and the variant is paternally transmitted to both affected children (Figure S9D).
Tab. 4. Top 20 functional (non-synonymous, nonsense and splice site) variants in VPS13B (among those selected by the backward elimination procedure), with PolyPhen-2 and GERP_RS scores included in the hierarchical model. 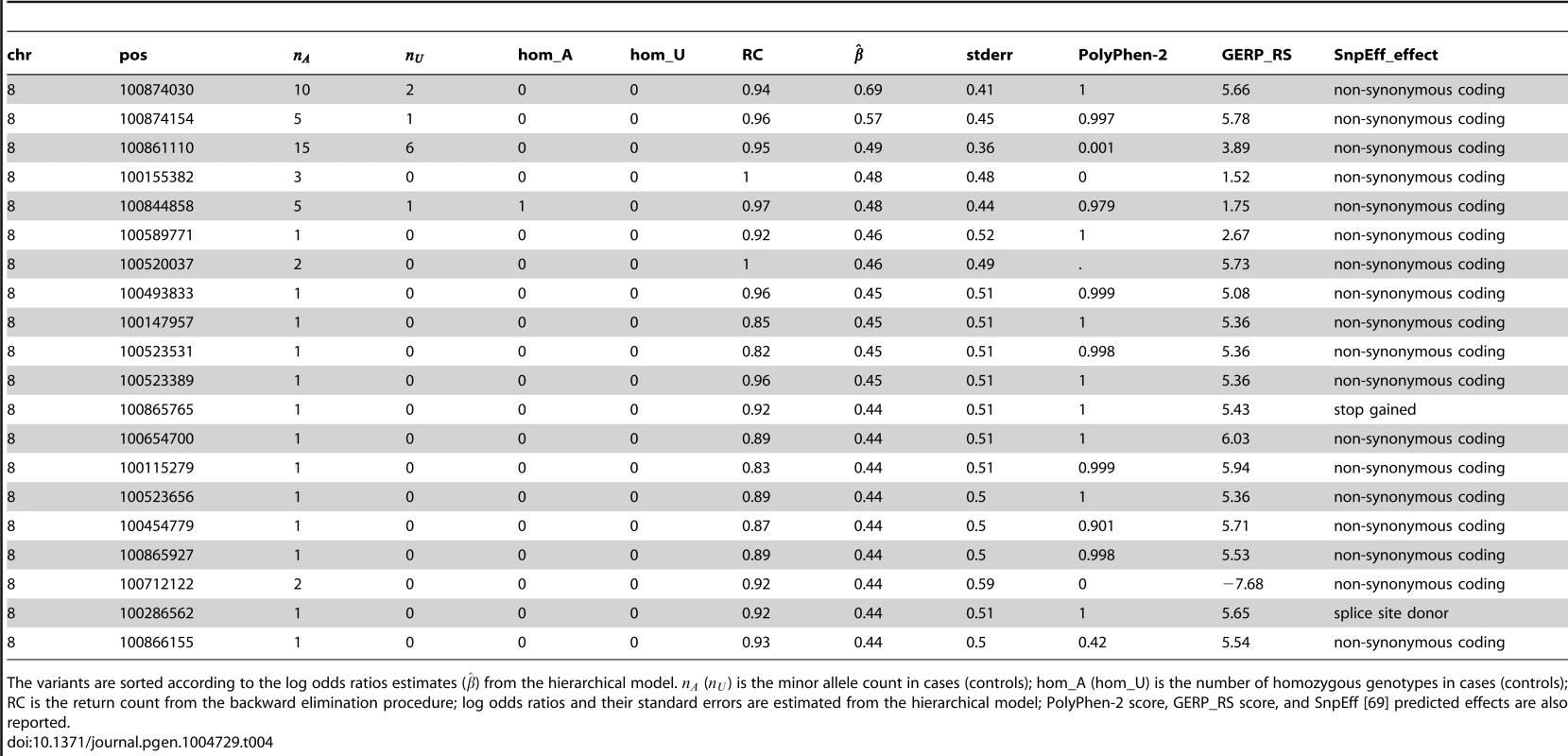
The variants are sorted according to the log odds ratios estimates () from the hierarchical model. () is the minor allele count in cases (controls); hom_A (hom_U) is the number of homozygous genotypes in cases (controls); RC is the return count from the backward elimination procedure; log odds ratios and their standard errors are estimated from the hierarchical model; PolyPhen-2 score, GERP_RS score, and SnpEff [69] predicted effects are also reported. To understand the impact of the variants on the molecular functions of VPS13B, all 42 variants deemed “interesting” by the backward elimination procedure were projected on the protein topology, reconstructed with the Pfam domains (N-terminal region of Chorein, DUF1162, ATG C-terminal domain), the experimentally ascertained Golgi targeting domain [47], and 11 transmembrane domains predicted with TMPred [48] (Figure S10). Benign variants appear scattered along the protein topology, while some of the predicted damaging variants map to known domains, including a missense in the DUF1162 domain and two missense in the Golgi targeting domain. Prediction of the structural changes that can result from the variants using MutPred [49] further revealed two top deleterious missense variants (p.Tyr1428His, predicted to cause gain of disorder (p = 0.006), loss of beta-sheet (p = 0.008) and gain of alpha-helix (p = 0.049); and p.Asp1475Gly, predicted to cause gain of alpha-helix (p = 0.049)). The LoF variants are upstream the Golgi domain, thus they are likely to cause premature insertion of a stop codon, activating nonsense-mediated mRNA decay or producing protein isoforms lacking the Golgi targeting domain. Although the pathological mechanisms caused by VPS13B insufficiency or mutations are still unknown, fibroblasts isolated from individuals with CS show severe fragmentation of the Golgi apparatus into ministacks [47], a defect observed in neurodegenerative disorders [50] and hypothesized to precede neuronal cell death [51]. Therefore, the LoF variants might prevent proper localization of VPS13B and disruption of its molecular functions on Golgi assembly or maintenance, triggering the pathological cascades underlying Cohen syndrome and/or autism.
Most of the variants selected are singletons (34 out of 42). As previously mentioned, for singletons accurate bioinformatic predictors about their likely functional effects are essential in order to identify such variants as promising, and hierarchical modeling is a natural framework to incorporate such information. Naturally the false discovery rate among these singletons can be high, and dependent on the sensitivity and specificity of the bioinformatic predictors used to characterize the variants in the hierarchical model. For comparison, in Table S2 we show the top 20 variants among the functional variants selected by the backward elimination procedure with ranking based on return count. No functional prediction score was used in this analysis. Although the more common variants still occur among the top variants in this analysis, for singletons, the hierarchical model gives higher priority to variants with high scores for both PolyPhen-2 and GERP_RS (Table 4). This ability of the hierarchical model to prioritize low frequency variants by taking into account multiple functional predictions is a distinct advantage over ranking based on return count alone (with no consideration of the PolyPhen-2 and GERP_RS scores for the variants).
Discussion
Pinpointing the rare causal variants among a large number of variants that occur in a genetic region is a difficult challenge, but crucial for follow-up functional studies, and for a better understanding of the molecular mechanisms that lead to disease. For many causal variants that occur only a few times in a dataset, incorporation of external information characterizing the variants (such as bioinformatic predictions on the deleteriousness of a variant) is essential to help prioritize these rare variants. We have described here two complementary statistical methods, that are able to integrate diverse functional annotations on individual variants in a region, and produce a selected list of candidates for causal variants, ranked according to their estimated effect sizes. The backward elimination procedure offers a natural way to select a set of promising variants, while using multiple functional predictors in the hierarchical modeling approach provides more in depth characterization of variants’ effects on disease, and can help boost the power to identify causal variants. We have focused attention here on some of the commonly used annotations for coding regions; however we acknowledge that there are other possible functional genomics annotations available both for the coding and non-coding regions [28] and with continued efforts to improve these functional predictions this list will further expand.
We illustrate the proposed methods through an application to a gene implicated in Cohen syndrome and autism, VPS13B. For this gene, we show that among the top selected variants are two LoF variants, and one rare, probably damaging variant that is homozygous in one affected individual. Autosomal recessive mutations associated with autism have been recognized for decades [52]. Recently, whole-exome sequencing has provided strong evidence that rare, recessive LoF variation is a major contributor to risk [53]. It is likely that many recessive missense variants contribute to ASD as well, although there has been insufficient power in whole-exome studies carried out to date to fully explore such variation. VPS13B is indispensable for the Golgi apparatus, and genes important for Golgi morphology and function have been linked to autism disorders, including RAB39B, mutated in a X-linked intellectual disability associated with autism, epilepsy and macrocephaly [54], and UBE3A, responsible of Angelman syndrome [55]. In addition, disturbances in pathways linked to Golgi, e.g. autophagy [56] and protein glycosylation [57], have been associated with autism etiology. Our findings extend the mutational landscape of VPS13B in Cohen syndrome and autism and further strengthen the connection between Golgi homeostasis and autism.
A rather large number of selected variants in the backward elimination procedure are singletons. Causal variants that appear as singletons in a dataset are difficult to distinguish from random genetic variation, and accurate functional predictions on such variants are crucial and will help in identifying those singletons more likely to be causal. Currently, it is not uncommon for predictions on the deleteriousness of a variant to be discordant (e.g. predictions from PolyPhen-2 and SIFT), and combining such multiple predictors can be difficult, although aggregate deleteriousness scores, such as Condel [58] and C-score [46], are available. Since the hierarchical model can easily incorporate multiple functional predictions, it has a distinct advantage over methods that cannot consider multiple predictions at once. Indeed, most of the existing groupwise association tests (including the Burden and SKAT tests discussed here) can only use one functional score at a time, and therefore it is not clear how multiple scores (that are sometimes discordant) can be taken into account. It is also worth noting that in the case of existing Burden (or SKAT) tests, a variant with a low functional score (e.g. PolyPhen-2 score close to 0) will be excluded from analysis regardless of the evidence of association that the data suggests (frequency in cases vs. controls). In contrast, in the presence of sufficient case-control frequencies, the hierarchical model places more weight on the larger case-control frequencies overriding the information from the bioinformatic predictors when that information does not support the likelihood of an increased risk. Therefore, the hierarchical model has an advantage also over simple methods to prioritize based on one functional score (e.g. PolyPhen-2) alone.
The use of next-generation sequencing technologies may lead to higher error rates compared to a traditional Sanger sequencing platform. Sequencing errors may be disproportionately present among singletons or very rare variants, especially for larger sample sizes, although for a single gene the number of errors is expected to be relatively small. Therefore, as a first step toward the characterization of the top ranked variants, Sanger sequencing can be used to validate the variants.
Classical variable selection methods (such as ridge regression [59] and LASSO [60]) are natural tools to employ in this setting in which the causal variants are expected to be just a small subset of all sequenced variants. Such methods have recently been applied to sequencing data [61], [62]. However, because these methods have not been developed to handle such sparse data, they have difficulty in selecting very rare variants (such as singletons). Furthermore, it is not clear how one can take into account multiple functional predictions for variants. Further work in this area needs to be done to assess the ability of these classical variable selection approaches to identify rare causal variants. Other existing methods one could use for causal variant prioritization fall into two extremes. Some methods use only data on observed case-control frequencies. For example, in KBAC [11], variants are weighted using data-adaptive weights, reflecting the estimated effect of a variant on the phenotype, and these weights can be potentially used to rank variants. However, as explained above, for rare and low frequency variants it is essential to make use of rich functional genomics annotations. At the other extreme, one can rank variants based on a functional score alone. This latter class of methods has the important drawback, especially in the case of complex traits, that it ignores case-control frequencies, and relies heavily on the accuracy of the bioinformatics predictor. Based on simulation studies, we have shown that hierarchical modeling that takes into account both association evidence coming from the sequencing data, and available functional genomics predictions, have better performance compared with ranking based on a single bioinformatic predictor alone (Text S3 and Figure S13).
We have focused here on the selection of variants that increase risk to disease. However, one can in principle use the proposed methods to identify protective variants. Instead of a Burden type statistic, one can use a SKAT statistic in the backward elimination procedure (also implemented in our software). Similarly, for the hierarchical model, variants with low functional score and higher frequency in controls compared with cases will be candidates for protective variants.
Further improvements to the backward elimination procedure are possible. For example, instead of partitioning variants into two groups based on a binary bioinformatic predictor, such as non-synonymous and synonymous, an alternative would be to calculate stratified false discovery rates [63] or possibly covariate-modulated local false discovery rates [64]. The advantage of such an approach would be that more than one covariate can be added to the backward-elimination procedure, although this point requires further work. Furthermore, information on the location of a variant within a gene or region of interest, e.g. what functional domain it affects, can be important, especially for missense variants. We have previously described scan statistic approaches to identify clusters of rare disease associated variants, and have shown applications to both autism and schizophrenia studies [65], [66], suggesting that incorporating such location information into the backward elimination procedure could improve the identification of causal variants.
The estimates of a rare variant's effect on disease from the hierarchical model can have substantial bias. This happens because in any particular gene or region only a small proportion of variants are expected to be disease causing, and most of the variants represent random genetic variation. Therefore, when estimating odds ratios of causal variants, there is a strong shrinkage effect toward the overall estimate. Incorporation of accurate functional predictors in the hierarchical model is one possible way to help attenuate this bias; further work is needed on finding better ways to reduce the bias.
In addition to their ability to pinpoint likely causal variants, the proposed methods can be used to prioritize variants for genotyping in independent datasets for the purpose of replication or validation. This is relevant when re-sequencing the gene or region in additional datasets is too expensive, and one chooses instead to genotype variants discovered in the original study [67]. Moreover, the proposed approaches can be used at a genome-wide scale, by first selecting the promising genes based on the overall gene-based test or other criteria (e.g. good biological candidate) followed by the backward elimination and hierarchical model approach to prioritize the variants within the genes identified as promising. Such a genome-wide analysis can, for example, identify classes of functional elements or domains enriched among the top variants in the selected genes.
The proposed methods are applicable to case-control or population-based designs. However, family-based designs represent a natural way to identify causal variants. For example, in multiplex families, significant sharing of a non-synonymous mutation among multiple affected relatives can be an important indication of causality. Bayesian approaches in this context have been developed before [68], and further work in this area is worth pursuing.
In summary, we have proposed and investigated two complementary statistical methods to identify causal variants among the naturally occurring genetic variation at a locus. They are able to incorporate sequencing data with various functional predictors on variants, and select a small number of variants that are enriched in causal variants. In the current study, we applied the proposed methods to a gene known to contain risk variants for ASD as proof-of-principle, and identify several interesting variants, including two LoF variants and a homozygous probably damaging variant likely important to autism risk.
Supporting Information
Zdroje
1. MardisER (2008) The impact of next-generation sequencing technology on genetics. Trends Genet 24 : 133–141.
2. MetzkerML (2010) Sequencing technologies - the next generation. Nat Rev Genet 11 : 31–46.
3. ZhangJ, ChiodiniR, BadrA, ZhangG (2011) The impact of next-generation sequencing on genomics. J Genet Genomics 38 : 95–109.
4. NelsonMR, WegmannD, EhmMG, KessnerD, St JeanP, et al. (2012) An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science 337 : 100–104.
5. Ionita-LazaI, ChoMH, LairdNM (2013) Statistical Challenges in Sequence-Based Association Studies with Population-and Family-Based Designs. Statistics in Biosciences 5 : 54–70.
6. PritchardJK (2001) Are rare variants responsible for susceptibility to common diseases? Am J Hum Genet 69 : 124–137.
7. PritchardJK, CoxNJ (2002) The allelic architecture of human disease genes: common disease-common variant… or not? Hum Mol Genet 11 : 2417–2423.
8. KryukovGV, ShpuntA, StamatoyannopoulosJA, SunyaevSR (2009) Power of deep, all-exon resequencing for discovery of human trait genes. Proc Natl Acad Sci USA 106 : 3871–3876.
9. BonnefondA, ClementN, FawcettK, YengoL, VaillantE, et al. (2012) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 44 : 297–301.
10. LiB, LealSM (2008) Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet 83 : 311–321.
11. LiuDJ, LealSM (2010) A novel adaptive method for the analysis of next-generation sequencing data to detect complex trait associations with rare variants due to gene main effects and interactions. PLoS Genet 6: e1001156.
12. Ionita-LazaI, BuxbaumJ, LairdNM, LangeC (2011) New testing strategy to identify rare variants with risk or protective effect on disease. PLoS Genet 7: e1001289.
13. WuMC, LeeS, CaiT, LiY, BoehnkeM, et al. (2011) Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 89 : 82–93.
14. Ionita-LazaI, MakarovV, YoonS, RabyB, BuxbaumJ, et al. (2011) Finding disease variants in Mendelian disorders by using sequence data: methods and applications. Am J Hum Genet 89 : 701–712.
15. LeeS, WuMC, LinX (2012) Optimal tests for rare variant effects in sequencing association studies. Biostatistics 13 : 762–775.
16. Ionita-LazaI, LeeS, MakarovV, BuxbaumJD, LinX (2013) Family-based association tests for sequence data, and comparisons with population-based association tests. Eur J Hum Genet doi: 10.1038/ejhg.2012.308
17. Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X (2013) Sequence Kernel Association Tests for the Combined Effect of Rare and Common Variants. Am J Hum Genet doi: pii: S0002-9297(13)00176-6.
18. CooperGM, ShendureJ (2011) Needles in stacks of needles: finding disease-causal variants in a wealth of genomic data. Nat Rev Genet 12 : 628–640.
19. SunyaevSR (2012) Inferring causality and functional significance of human coding DNA variants. Hum Mol Genet 21(R1): R10–7.
20. WangK, LiM, HakonarsonH (2010) ANNOVAR: Functional annotation of genetic variants from next-generation sequencing data. Nucleic Acids Research 38: e164.
21. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–249.
22. KumarP, HenikoffS, NgPC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4(7): 1073–81.
23. Davydov EV GoodeDL, SirotaM, CooperGM, SidowA, et al. (2010) Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput. Biol 6: e1001025.
24. CapanuM, OrlowI, BerwickM, HummerAJ, ThomasDC (2008) The use of hierarchical models for estimating relative risks of individual genetic variants: an application to a study of melanoma. Stat Med 27 : 1973–1992.
25. CapanuM, BeggCB (2011) Hierarchical modeling for estimating relative risks of rare genetic variants: properties of the pseudo-likelihood method. Biometrics 67 : 371–380.
26. QuintanaMA, ContiDV (2013) Integrative variable selection via Bayesian model uncertainty. Stat Med doi: 10.1002/sim.5888
27. LongN, DicksonSP, MaiaJM, KimHS, ZhuQ (2013) Leveraging prior information to detect causal variants via multi-variant regression. PLoS Comput Biol 9: e1003093.
28. PickrellJK (2014) Joint Analysis of Functional Genomic Data and Genome-wide Association Studies of 18 Human Traits. Am J Hum Genet 94 : 559–573.
29. ENCODE Project Consortium, BernsteinBE, BirneyE, DunhamI, GreenED (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74.
30. DaviesRB (1977) Hypothesis testing when a nuisance parameter is present only under the alternative. Biometrika 64 : 247–254.
31. LoSH, ZhengT (2004) A demonstration and findings of a statistical approach through reanalysis of inflammatory bowel disease data. Proc Natl Acad Sci USA 101 : 10386–10391.
32. BenagliaT, ChauveauD, HunterDR (2009) An EM-like algorithm for semi - and non-parametric estimation in multivariate mixtures. Journal of Computational and Graphical Statistics 18 : 505–526.
33. SchorkAJ, ThompsonWK, PhamP, TorkamaniA, RoddeyJC (2013) All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet 9: e1003449.
34. Schaffner SF FooC, GabrielS, ReichD, DalyMJ, et al. (2005) Calibrating a coalescent simulation of human genome sequence variation. Genome Res 15 : 1576–1583.
35. RomeoS, PennacchioLA, FuY, BoerwinkleE, Tybjaerg-HansenA, et al. (2007) Population-based re-sequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 39 : 513–516.
36. Romeo YinW, KozlitinaJ, PennacchioLA, BoerwinkleE, et al. (2009) Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest 119 : 70–79.
37. KolehmainenJ, BlackGC, SaarinenA, ChandlerK, Clayton-SmithJ, et al. (2003) Cohen syndrome is caused by mutations in a novel gene, COH1, encoding a transmembrane protein with a presumed role in vesicle-mediated sorting and intracellular protein transport. Am J Hum Genet 72 : 1359–1369.
38. DouzgouS, PetersenMB (2011) Clinical variability of genetic isolates of Cohen syndrome. Clinical genetics 79 : 501–506.
39. Kivitie-KallioS, NorioR (2001) Cohen syndrome: essential features, natural history, and heterogeneity. American journal of medical genetics 102 : 125–135.
40. HowlinP, KarpfJ, TurkJ (2005) Behavioural characteristics and autistic features in individuals with Cohen Syndrome. European child & adolescent psychiatry 14 : 57–64.
41. YuTW, ChahrourMH, CoulterME, JiralerspongS, Okamura-IkedaK, et al. (2013) Using whole-exome sequencing to identify inherited causes of autism. Neuron 77 : 259–273.
42. de LigtJ, WillemsenMH, van BonBW, KleefstraT, YntemaHG, et al. (2012) Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 367 : 1921–1929.
43. UenoS, MarukiY, NakamuraM, TomemoriY, KamaeK, et al. (2001) The gene encoding a newly discovered protein, chorein, is mutated in chorea-acanthocytosis. Nature genetics 28 : 121–122.
44. WalterfangM, EvansA, LooiJC, JungHH, et al. (2011) The neuropsychiatry of neuroacanthocytosis syndromes. Neuroscience and biobehavioral reviews 35 : 1275–1283.
45. PetrovskiS, WangQ, HeinzenEL, AllenAS, GoldsteinDB (2013) Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes. PLoS Genetics doi: 10.1371/journal.pgen.1003709
46. Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, et al. (2014) A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014 Feb 2. doi: 10.1038/ng.2892.
47. SeifertW, KuhnischJ, MaritzenT, HornD, HauckeV, et al. (2011) Cohen syndrome-associated protein, COH1, is a novel, giant Golgi matrix protein required for Golgi integrity. J Biol Chem 286 : 37665–37675.
48. HofmannK, StoffelW, eds. ( TMbase - A database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler 374 : 166 (1993).
49. LiB, KrishnanVG, MortME, XinF, KamatiKK, et al. (2009) Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics 25 : 2744–2750.
50. GonatasNK, StieberA, GonatasJO (2006) Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. Journal of the neurological sciences 246 : 21–30.
51. NakagomiS, BarsoumMJ, Bossy-WetzelE, SutterlinC, MalhotraV, et al. (2008) A Golgi fragmentation pathway in neurodegeneration. Neurobiology of disease 29 : 221–231.
52. BetancurC (2011) Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res 1380 : 42–77.
53. LimET, RaychaudhuriS, SandersSJ, StevensC, SaboA, et al. (2013) Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron 77 : 235–242.
54. GiannandreaM, BianchiV, MignognaML, SirriA, CarrabinoS, et al. (2010) Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. American journal of human genetics 86 : 185–195.
55. CondonKH, HoJ, RobinsonCG, HanusC, EhlersMD (2013) The Angelman syndrome protein Ube3a/E6AP is required for Golgi acidification and surface protein sialylation. J Neurosci 33 : 3799–3814.
56. PoultneyCS, GoldbergAP, DrapeauE, KouY, Harony-NicolasH, et al. (2013) Identification of Small Exonic CNV from Whole-Exome Sequence Data and Application to Autism Spectrum Disorder. American journal of human genetics 93 : 607–619.
57. van der ZwaagB, FrankeL, PootM, HochstenbachR, SpierenburgHA, et al. (2009) Gene-network analysis identifies susceptibility genes related to glycobiology in autism. PloS one 4: e5324.
58. Gonzlez-PrezA, Lpez-BigasN (2011) Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am J Hum Genet 88 : 440–449.
59. HoerlAE, KennardR (1970) Ridge regression: biased estimation for nonorthogonal problems. Technometrics 12 : 55–67.
60. TibshiraniR (1996) Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society, Series B 58 : 267–288.
61. XuC, LadouceurM, DastaniZ, RichardsJB, CiampiA, et al. (2012) Multiple regression methods show great potential for rare variant association tests. PLoS One 7: e41694.
62. ZhouH, SehlME, SinsheimerJS, LangeK (2010) Association screening of common and rare genetic variants by penalized regression. Bioinformatics 26 : 2375–2382.
63. SunL, CraiuRV, PatersonAD, BullSB (2006) Stratified false discovery control for large-scale hypothesis testing with application to genome-wide association studies. Genet Epidemiol 30 : 519–530.
64. FerkingstadE, FrigessiA, RueH, ThorleifssonG, KongA (2008) Unsupervised empirical Bayesian multiple testing with external covariates. Ann. Appl. Stat 2 : 714–735.
65. Ionita-LazaI, MakarovV, ARRA Autism Sequencing Consortium (2012) BuxbaumJD (2012) Scan-statistic approach identifies clusters of rare disease variants in LRP2, a gene linked and associated with autism spectrum disorders, in three datasets. Am J Hum Genet 90 : 1002–1013.
66. Ionita-Laza I, Xu B, Makarov V, Buxbaum J, Louw Roos J, et al. (2013) A Scan-Statistic Based Analysis of Exome Sequencing Data Identifies FAN1 at 15q13.3 as a Susceptibility Gene for Schizophrenia and Autism Proceedings of the National Academy of Sciences USA, in press.
67. LiuDJ, LealSM (2010) Replication strategies for rare variant complex trait association studies via next-generation sequencing. Am J Hum Genet 87 : 790–801.
68. PetersenGM, ParmigianiG, ThomasD (1998) Missense mutations in disease genes: a Bayesian approach to evaluate causality. Am J Hum Genet 62 : 1516–1524.
69. CingolaniP, PlattsA, Wang leL, CoonM, NguyenT, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3 Fly (Austin). 6 : 80–92.
Štítky
Genetika Reprodukční medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 12- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání












