-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
The locomotor deficits associated with Parkinson disease result from the death of a specific subset of dopamine neurons in the ventral part of the midbrain. The reason for the greater sensitivity to degeneration of those, relative to other, neurons is not clear. Prior work showed that the Pitx3 transcription factor is specifically expressed in these neurons where it has a survival role during development. The present work identified a cell signaling component, Rgs6, that is also restricted to the sensitive neurons in the midbrain and that exerts a protective function, particularly late in life. While the loss of Rgs6 function may predispose or contribute to Parkinson disease, its stimulation may provide a novel therapeutic avenue to treat Parkinson disease.
Published in the journal: . PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004863
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004863Summary
The locomotor deficits associated with Parkinson disease result from the death of a specific subset of dopamine neurons in the ventral part of the midbrain. The reason for the greater sensitivity to degeneration of those, relative to other, neurons is not clear. Prior work showed that the Pitx3 transcription factor is specifically expressed in these neurons where it has a survival role during development. The present work identified a cell signaling component, Rgs6, that is also restricted to the sensitive neurons in the midbrain and that exerts a protective function, particularly late in life. While the loss of Rgs6 function may predispose or contribute to Parkinson disease, its stimulation may provide a novel therapeutic avenue to treat Parkinson disease.
Introduction
Parkinson disease (PD) is characterised by the progressive loss of midbrain dopaminergic (mDA) neurons [1]. Although the clinical manifestations of PD can be variable, the appearance of motor deficits is the hallmark of this neurodegenerative disease. Similarly, the etiology of PD appears to be multifactorial but one consistent feature of this disease is the greater sensitivity of ventral substantia nigra compacta (vSNc) mDA neurons to degenerate [2] as opposed to mDA neurons of the dorsal SNc (dSNc) and ventral tegmental area (VTA). The molecular basis for this preferential sensitivity remains poorly understood although work in animal models has been useful [3].
Vertebrate animal models based on genetic causes of PD, which constitute 10–20% of PD cases, have not been overly successful in reproducing the selective neurodegeneration patterns of the mDA system [4]. For example, mice with transgenic expression of human autosomal dominant mutants of α-synuclein (SNCA) or leucine-rich repeat kinase 2 (LRRK2) rarely produce mDA neurodegeneration [5], [6]. Murine loss-of-function mutations in autosomal recessive gene products for PTEN induced putative kinase 1 (Pink) and Parkinson protein 2 (Park2), have not been enlightening either, with the recent exception of Parkinson protein 7 (Park7 or DJ-1). Indeed, DJ-1−/− mice show progressive adult degeneration of SNc mDA neurons upon backcrossing to an appropriate genetic background [7], indicating that many factors are necessary in order to model polygenic diseases such as PD. However, it is noteworthy that rat models of Pink1 and DJ-1 loss-of-function showed progressive loss of mDA neurons [8]. Both early-onset and late-onset forms of PD bear a major histopathological hallmark, the presence of Lewy bodies, which are α-synuclein-rich protein inclusions that are also found in non-dopaminergic brain regions depending on the stage of disease progression. Many familial PD genes have widespread brain expression, without any preferential expression in mDA subpopulations [5]. In general, they all participate in similar inter-related cellular processes such as in mitochondrial function (PINK1, DJ-1, SNCA), the secretory pathway (PARK2, LRRK2) and the ubiquitin-proteasome degradation pathway (PINK1, SNCA, PARK2).
Rodent animal models based on genes that participate in development and survival of mDA neurons (Pitx3, Nurr1, Girk2, En1/2, Otx2, etc) have proved extremely useful, especially in defining the candidate cellular pathways underlying the respective differential vulnerability of SNc versus VTA mDA neuron subpopulations to toxin-induced neurodegeneration and in human pathology [9]–[11]. Notably, mouse mutants for the homeobox transcription factor Pitx3 (Entrez gene ID: 5309) are unique in that they display a selective and stereotypic pattern of mDA cell loss that resembles typical PD [12]–[14]. In particular, Pitx3-deficient mice exhibit developmental loss of Pitx3-positive Calbindin (Calb)-negative mDA neurons of the vSNc (Pitx3-dependent) while Pitx3-negative Calb-positive mDA neurons of dSNc and VTA (Pitx3-independent) remain essentially unaffected by Pitx3 deficiency [15]. This cell loss is associated with a loss of spontaneous movement that can be partially rescued by L-dopa treatment [16], [17]. Human PITX3 polymorphisms are associated with sporadic PD [18]. Pitx3 mediates its effects by regulating the expression of many genes (Aldh1a1, DAT, Drd2, TH, Bdnf) in a subset specific fashion [19]. A well-studied Pitx3 target gene in mDA neurons of the SNc is Aldh1a1, which is important for retinoic acid production and subsequent neuronal maturation and protection through regulation of TH expression [19]–[21]. Other Pitx3 target genes, such as the classical dopaminergic markers DAT, Vmat2, Drd2 are important for neurotransmitter identity and are subject to the cooperative action of Pitx3 with Nurr1 [22]. On the other hand, Pitx3 expression has been reported to be itself regulated by GDNF, especially during development [23]. GDNF is the only neurotrophic factor for which conditional inactivation in the adult mouse has provided strong evidence of its absolute requirement for the cell-autonomous survival of brain cathecholaminergic neurons, including mDA neurons [24]. Although, many studies described the implication of Pitx3 in post-natal maturation and developmental survival of mDA neurons, Pitx3 has not been conclusively linked to mechanisms of survival and maintenance of mDA neurons in the adult, especially as it pertains to degenerative processes. We hypothesized that Pitx3-controlled genes and pathways, in addition to their known role in development, may also be implicated in the neuroprotective pathway required to maintain the integrity of specific subset of mDA neurons throughout adulthood.
In a screen of expression profiling data comparing Pitx3-dependent and -independent FACS-purified mDA neurons of SNc and VTA, the Regulator of G-protein Signaling 6 (Rgs6) (Entrez Gene ID: 9628) was identified as a putative survival factor that is preferentially expressed in vSNc mDA neurons and whose expression is positively regulated by Pitx3. Rgs6 belongs to the R7 subfamily of Rgs and functions as a GTPase activating protein to terminate signaling downstream of ligand-bound G-protein coupled receptors (GPCR). It does so by accelerating the conversion from the active Gα-GTP bound state (dissociated from Gβγ subunit) to the inactive Gα-GDP bound state (associated to Gβγ subunit). The R7 subfamily of Rgs regulators, including Rgs6, are known to have preference for catalysis of pertussis toxin-sensitive Gi/o heterotrimeric G-proteins through recognition of their Gαi by a C-terminal Rgs protein domain [25]. Activated Gαi subunits inhibit adenylate cyclase such that cAMP production from ATP is halted and PKA/cAMP-dependent protein kinase pathways are inhibited. Conversely, the activated Gβγ subunit opens Girk channels to allow efflux of potassium ions outside the cell resulting in hyperpolarization [26]. The neuronal GPCRs previously associated with Gi/o proteins include dopamine receptors (Drd2, Drd3), acetylcholine receptors (m2, m4), GABAB receptor, metabotropic glutamate receptors (mGluR2, 3, 4, 6, 7, 8). Thus in cerebellum and heart, phenotypes resulting from inactivation of Rgs6 are consistent with over-activation of signaling downstream of GABAB, serotonin 5-HT1A, M2 acetylcholine receptors, respectively. [26]–[29].
In the present work, we identified Rgs6 and investigated its role in the midbrain dopaminergic system. Rgs6 is shown to be preferentially expressed in vSNc mDA neurons and its knockout in mice results in progressive loss and alterations of Pitx3-positive mDA neurons specifically within the vSNc of aged animals. This late-onset degeneration is associated with markers of increased Drd2 signaling, down-regulation of Pitx3 expression and deregulated expression of its target genes, Aldh1a1, Bdnf, Vmat2, TH and Fgf10. Further, the pattern of mDA degeneration observed in Rgs6−/− mice is a close phenocopy of DJ-1−/− mice suggesting that these two genes may act through similar pathways.
Results
A regulator of G-protein signaling restricted to vSNc neurons
In order to identify genes responsible for the differential vulnerability of vSNc mDA neurons, we devised a strategy to isolate FACS-purified Pitx3-dependent and Pitx3-independent mDA neurons and compare their transcriptomes (Fig. 1). By birth, the SNc of Pitx3−/− pups is completely depleted of Pitx3-positive neurons but the dorsal Pitx3-negative neurons are spared [12]. After dissection of SN and VTA from midbrain slices of mice expressing TH-EGFP, FACS-sorting of TH-EGFP-positive neurons yielded a pure dSNc Pitx3-negative population from Pitx3−/− brains and mixed (∼80% Pitx3-positive and ∼20% Pitx3-negative) SN mDA populations from wild-type animals. The comparison of their transcriptomes defined vSNc - and dSNc-enriched genes (Fig. 2A). In VTA, Pitx3 deficiency leaves the 50% Pitx3-expressing mDA neurons intact at birth but they die within the next three months [12]. Comparison of VTA TH-EGFP cell expression profiles from Pitx3−/− and WT mice will thus identify genes which have Pitx3-dependent expression. RNA extracted from FACS-sorted cells (Fig. 1) was used to generate probes for hybridization in duplicates to Affymetrix Mouse Gene 1.0ST microarrays and determination of expression profiles. Unbiased clustering of the 1813 differentially expressed genes (fold changes >1.5, p≤0.05 and signal ≥60) into seven clusters defined genes that are expressed in specific subsets of mDA neurons and/or that are Pitx3-dependent in VTA (Fig. 2A). qRT-PCR analyses confirmed the expected enrichment for vSNc (Girk2, DAT), dSNc (Calb1) and VTA (Otx2, Calb1) markers (Fig. 2B). Further, many genes previously characterized for their subset-specific expression (marked by stars in Fig. 2A) validate the profiling data; these include Calb1/2 for dSNc, Otx2 for VTA, Kcnj6 (Girk2) for vSNc, Lpl for VTA, Aldh1a1 for vSNc, Slc6a3 (DAT) for vSNc, Lix1 for SNc [20], [30]–[32]. The complete list of genes in each cluster is provided in Table S1.
Fig. 1. Strategy for isolation of FACS-purified Pitx3-dependent (red) and Pitx3-independent (white) mDA neurons for expression profiling analysis. 
Dissected SN and VTA from newborn TH-EGFP transgenic Pitx3+/+ and Pitx3−/− mice were used for FACS purification of catecholaminergic neurons. The EGFP+ cells consists in various proportions (approximate % shown) of Pitx3+ (red), Pitx3− (white) and Pitx3del (yellow) mDA neurons, depending on the region dissected and mDA neuronal loss resulting from Pitx3 inactivation. RNA from four cell preparations (SNc WT, VTA WT, SNc KO, VTA KO) were analyzed by hybridization in duplicates to Affymetrix Mouse Gene 1.0ST microarrays. Fig. 2. Subset-specific expression signatures of mDA neurons. 
(A) Differentially expressed probesets were clustered in an unbiased manner into three-dimensional Cartesian-type coordinates defined according to the relative enrichment attributes for each probeset in the comparisons between SNc WT/VTA WT, SNc KO/SNc WT and VTA KO/VTA WT. Each cluster was named according to the observed preferential expression pattern (label and shown in red in diagrams). The heatmaps show relative enrichments for 20 genes that are highly enriched and/or that have documented functions (the complete gene lists for each subset is provided in Table S1). The total number of genes (not probesets) in each cluster is indicated together with the number of those that are either activated or repressed by Pitx3 based on the VTA KO/WT comparison. (B) qRT-PCR quantification of mRNAs with preferential expression in either SN or VTA assessed in FACS-sorted cells from WT or Pitx3−/− (KO) tissues. mRNA levels are normalized relative to Gapdh mRNA. Data are shown as means ± S.D. We chose to investigate genes enriched in vSNc and Pitx3-dependent. Rgs6 immediately appeared as an interesting candidate because it is known to negatively modulate signaling downstream of heterotrimeric Gi protein-coupled receptors through its instrinsic GTPase-stimulating protein activity [26], [28], [29]. The Rgs6 protein was detected by immunohistofluorescence in TH-positive SNc mDA neurons and not in VTA (Fig. 3A). Most dSNc mDA neurons were negative for Rgs6 (Fig. 3B upper left, arrowheads) as were VTA cells (Fig. 3B, bottom left). The SNc distribution of Rgs6 is very similar to that of Pitx3 but they differ in VTA (Fig. 3B). Triple immunohistofluorescence staining against TH, Calb1 and Pitx3 (Fig. 3C) showed that the majority of Pitx3-positive vSNc mDA neurons are negative for Calb1 whereas dSNc mDA neurons are calbindin-positive. The SNc thus has two major subsets of mDA neurons with differential vulnerability to Pitx3 knockout: TH+/Pitx3+/Rgs6+/Calb − neurons in vSNc (Pitx3-dependent, PD vulnerable) and TH+/Pitx3−/Rgs6−/Calb+ cells in dSNc (Pitx3-independent, PD resistant).
Fig. 3. Restricted expression of Rgs6 in Pitx3-positive (Pitx3+) dopaminergic neurons of ventral SNc. 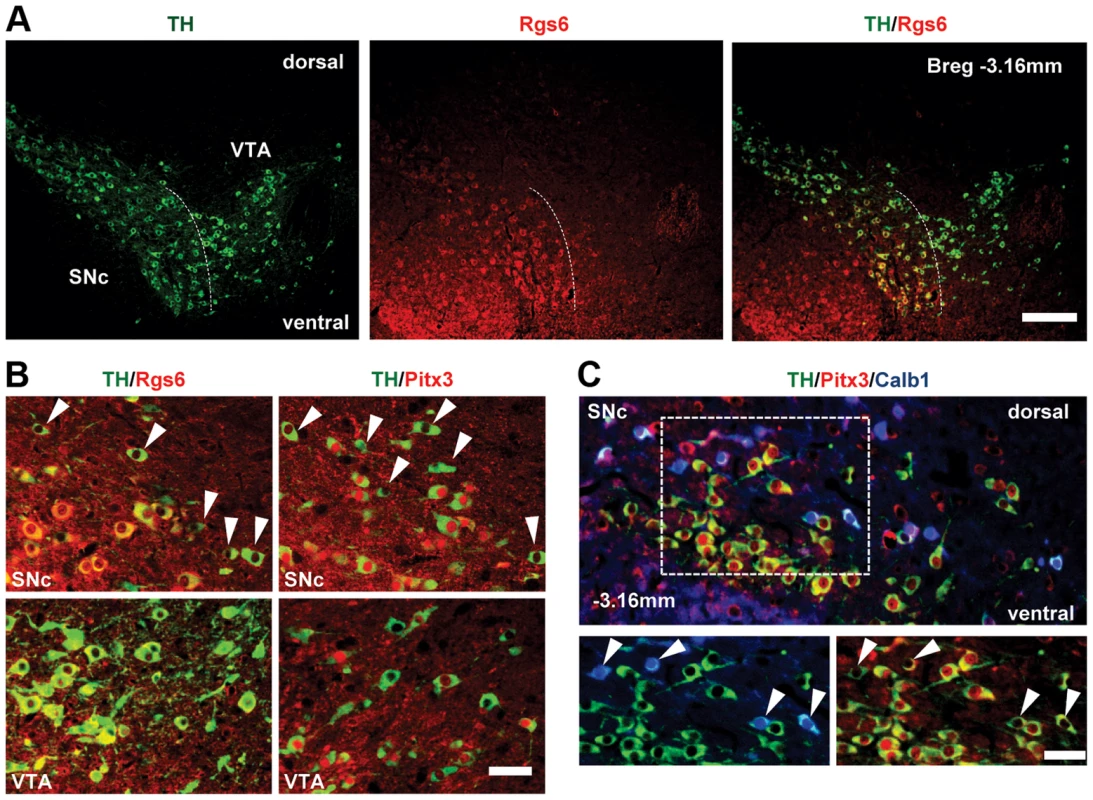
(A) Rgs6 expression revealed by immunohistofluorescence (red) together with TH (green) in mDA neurons of SNc but not VTA. Scale bar 410 µm. (B) Co-immunofluorescence analysis of TH (green) and Rgs6 or Pitx3 (red, as indicated) in SNc and VTA of adult mice. Arrowheads point to Pitx3-negative or Rgs6-negative mDA neurons in dorsal SNc. Scale bar 100 µm. (C) Triple immunofluorescence staining for TH (green), Pitx3 (red) and Calb1 (blue) on tissue sections of adult mice indicates that the majority of TH+Pitx3− cells of dSNc (arrowheads) are positive for Calb1, while TH+Pitx3+ cells of vSNc are negative for Calb1, consistent with depiction in A. Scale bar 100 µm. Neuronal degeneration in vSNc of Rgs6−/− mice
In order to define the in vivo role of Rgs6, we investigated the mDA system of Rgs6−/− mice by TH immunohistochemistry at 6, 180 and 356 days of age (Table S2). Only 1 y-old Rgs6−/− midbrains were markedly different from controls and we could identify two major phenotypes in different mice.
The first phenotype was a partial loss of SNc TH-positive cells on one side of the brain (random unilateral) (Fig. 4A and Figure S1). Quantification of TH-positive cells in SNc and VTA indicated a loss of about 35±6% (SD) in the SNc of the affected side (Fig. 4B). The loss of TH-positive cells was further supported by fewer Nissl-positive cells in SNc of Rgs6−/− mice (Fig. 4C). In addition, the Nissl stain revealed the presence of cells with abnormal elongated morphology that were found unilaterally within the SNc (Fig. 4C). The loss of TH-positive cells is correlated with a loss of Pitx3-positive cells (Fig. 4D).
Fig. 4. Unilateral loss of Pitx3-positive dopaminergic neurons in ventral SNc of Rgs6−/− mice. 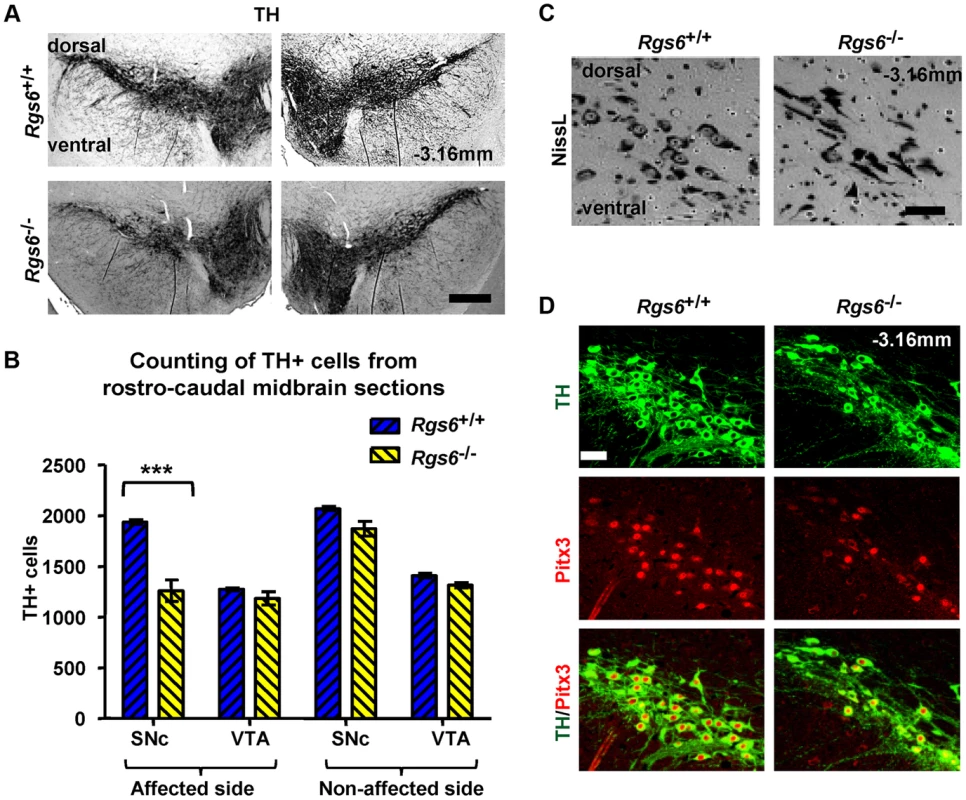
(A) Immunoperoxidase staining for TH on representative coronal midbrain sections showing less SNc TH+ neurons on one side of Rgs6−/− mice at 1 year of age compared to sib control. Sections are identified with Bregma position. Scale bar 400 µm. (B) Number of TH+ cells in SNc and VTA of TH-stained coronal sections across midbrain (every 30 µm). Cell counts are represented as means +/− S.D. (***p<0.005). (C) Nissl staining of vSNc sections contiguous to A shows fewer cell bodies and abnormal elongated neurons in Rgs6−/− mice compared to control. Scale bar 100 µm. (D) Double immunofluorescence staining for TH (green) and Pitx3 (red) on sections contiguous to A showing a marked loss of TH+Pitx3+ cells in Rgs6−/− mice. Scale bar 100 µm. A second group of 1 y-old Rgs6−/− mice exhibited dysmorphic mDA neurons that displayed low levels of TH immunoreactivity (THlow), aberrant morphology, pronounced cell shrinkage and disrupted TH-positive fiber network (Fig. 5A and Figure S2). These dysmorphic neurons were all localized in the vSNc, while mDA neurons in dSNc and VTA had normal appearance and unaffected Calb1 expression (Figure S3).
Fig. 5. Unilateral degeneration of vSNc neurons in a subset of Rgs6−/− mice. 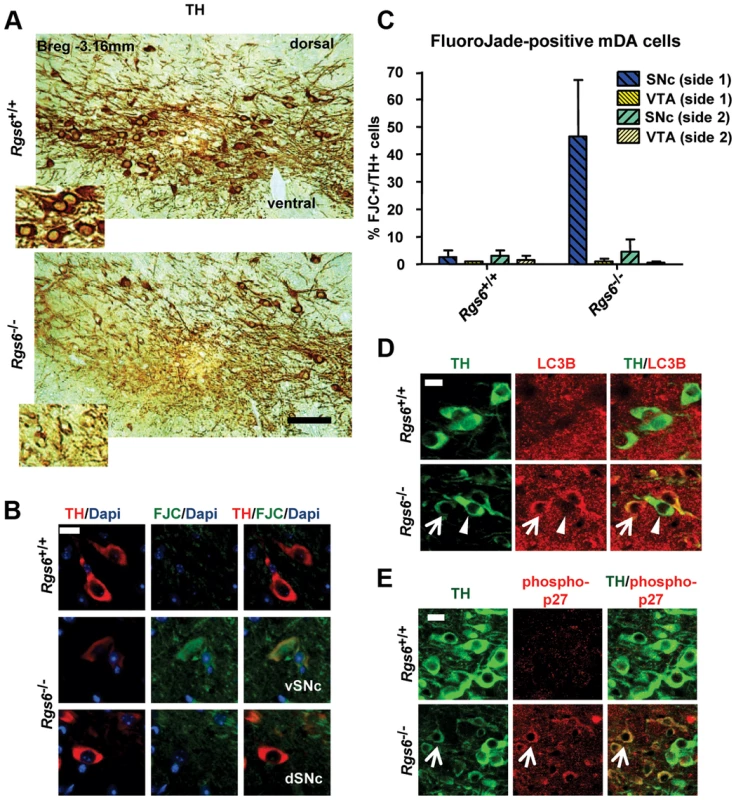
(A) Immunoperoxidase staining for TH on representative coronal midbrain sections showing dysmorphic TH+ neurons (low TH staining, THlow, inset) in ventral SNc of Rgs6−/− mice. The dSNc and VTA mDA neurons are unaffected (strong/normal TH staining, inset). Scale bar 200 µm. (B) Triple staining for TH (red), Dapi (blue) and Fluoro-Jade C (FJC, green) showing presence of degenerating THlow cells in vSNc of 1 y-old Rgs6−/− mice (middle panels) and not in control vSNc (upper panels) or in dorsal SNc (lower panels). Scale bar 20 µm. (C) Bilateral cell counts of FJC+ mDA neurons in SNc and VTA of coronal sections from control and 1 y-old Rgs6−/− mice. (D) Co-immunostaining for TH (green) and LC3B (red) in vSNc of WT and Rgs6−/− mice. Scale bar 20 µm. Arrowheads indicate unaffected neurons while arrows point to THlow cells. (E) Co-immunostaining for TH (green) and phosphorylated p27Kip1 (phospho-p27, red) in vSNc of WT and Rgs6−/− mice. Scale bar 20 µm. We then determined whether the Rgs6−/− dysmorphic mDA neurons are undergoing degeneration by staining with Fluoro-Jade C (FCJ), as previously shown in the MPTP or 6-OHDA-induced mouse PD models [33], [34] and zitter mutant rats [34]. A high proportion of the dysmorphic mDA neurons stained positive for FJC (Fig. 5B, C) and are THlow. Cell counts indicated that degeneration is mostly unilateral and limited to the vSNc (Fig. 5C). Some 180 days-old Rgs6−/− mice exhibited mild unilateral degeneration in the most lateral part of SNc, while newborn mice did not, supporting the progressive appearance of degeneration with age (Table S2).
Expression of pathological markers and familial PD gene products in Rgs6−/− vSNc neurons
We then characterized degenerating mDA neurons in Rgs6−/− mice for the presence of pathologocial markers observed in PD and in other neurodegenerative diseases. Notably since degenerating neurons in PD suffer from oxidative stress and increased autophagocytosis due to the presence of protein aggregates, we assessed and observed increased expression of LC3B, a marker of activated autophagosome in degenerating neurons (Fig. 5D). In order to further characterize the degenerating neurons, we verified expression of key genes implicated in development of familial forms of PD [5]. For example, DJ-1 and PINK1 are two genes whose mutated forms cause PD in an autosomal recessive manner and LRRK2 is the most frequently mutated gene causing PD and it acts in an autosomal-dominant fashion. Pink1 and Lrrk2 mouse null mutants do not however display degeneration of mDA neurons [5], [35] but a rat model of Pink1 knockout does [8]. Surprisingly, the THlow neurons of Rgs6−/− vSNc specifically exhibit decreased DJ-1 expression, while Pink1 and Lrrk2 expression is increased in those same neurons (Fig. 6); this contrasts with the fairly widespread expression of DJ-1 [36], Pink1 and Lrrk2 [5], [37] in midbrain neurons. Interestingly, the late-onset degeneration observed in Rgs6−/− midbrain appears to be a close phenocopy of the DJ-1−/− mice [7], especially in terms of the initial unilateral nature of defects in aging SNc mDA neurons and the kinetics of cell loss. The loss of DJ-1 may thus contribute to Rgs6-dependent degeneration.
Fig. 6. Expression of familial PD genes is altered in degenerating neurons of vSNc. 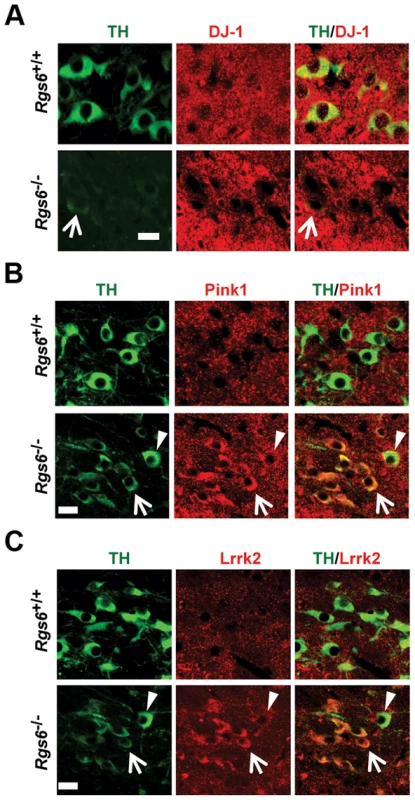
Double immunofluorescence staining against TH (green) and (A) DJ-1 (red) or (B) Pink1 (red) or (C) Lrrk2 (red) in SNc of control and 1 y-old Rgs6−/− mice that display dysmorphic THlow mDA neurons. Arrowheads indicate unaffected neurons while arrows point to THlow cells. Scale bar 20 µm. Degenerating neurons were shown to overexpress the phosphorylated cell cycle inhibitor p27Kip1 in Alzheimer's disease [38]. Interestingly, Rgs6 was implicated in control of cell cycle and apoptosis [39] and we observed cytoplasmic accumulation of phospho-p27Kip1 only in degenerating THlow mDA neurons of one year-old Rgs6−/− mice (Fig. 5E).
Reduced Pitx3-dependent gene expression in Rgs6−/− vSNc neurons
In order to define molecular correlates of Rgs6-dependent degeneration, we assessed Pitx3 expression that was previously shown to have a survival function in SNc [12]. Nuclear Pitx3 was greatly diminished in the dysmorphic mDA neurons while cytoplasmic Pitx3 staining increased, suggesting a shift in sub-cellular localization observed with two different polyclonal Pitx3 antibodies in different cells of the same sections (Fig. 7A). We assessed protein levels of some Pitx3 target genes by immunohistochemistry: these include Bdnf, Aldh1a1 (Adh2), TH, Drd2, DAT, Vmat2 and Fgf10 [19], [23], [40]. The expression of Vmat2 and Bdnf is decreased in THlow neurons (Fig. 7B, C). Expression of Aldh1a1 (Adh2) is also decreased in degenerating vSNc mDA neurons (Fig. 7D): this decrease may in part account for the low TH expression [19]. Our profiling data suggested that Fgf10 expression is repressed by Pitx3 in VTA and indeed, we found de-repression of Fgf10 expression only in THlow degenerating mDA neurons (Fig. 7D). At post-natal day 6 (not shown), we did not observe any change in expression of these genes consistent with a late onset phenotype.
Fig. 7. Reduced expression of Pitx3 and its target genes in degenerating neurons. 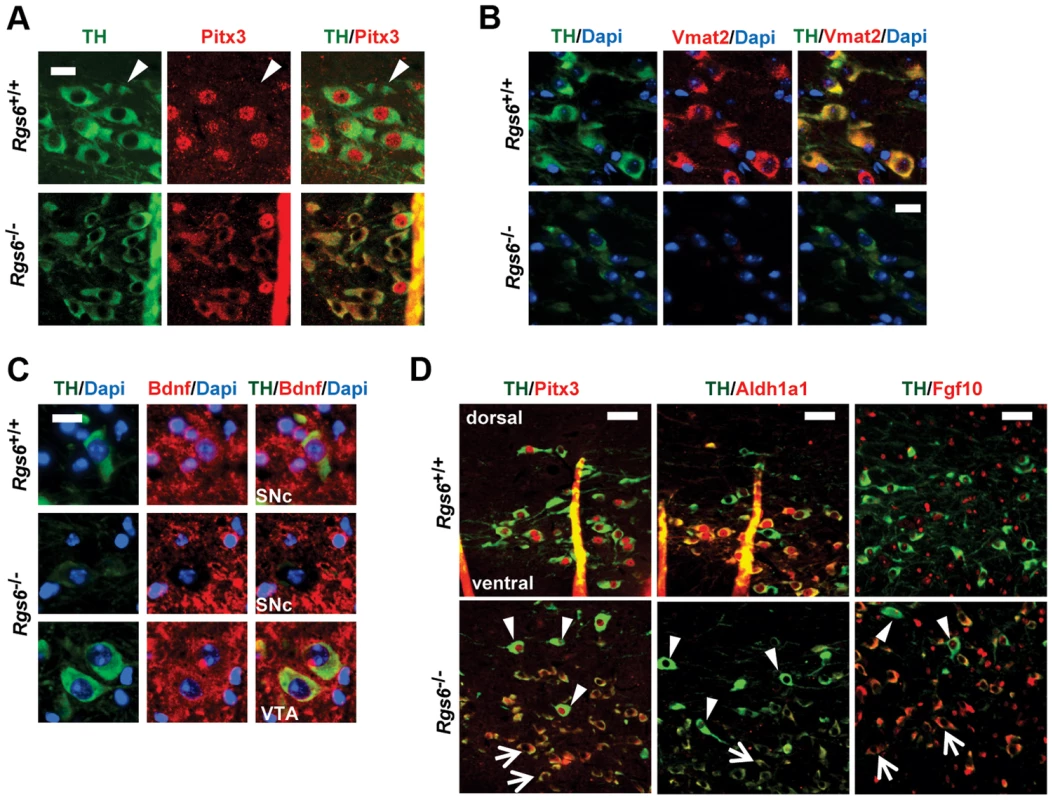
(A) Double immunofluorescence staining against TH (green) and Pitx3 (red) in SNc of control and 1 y-old Rgs6−/− mice that display dysmorphic mDA neurons. Arrowheads indicate Pitx3-negative neurons of dSNc. Scale bar 20 µm. (B) Co-immunofluorescence staining against TH (green), nuclear Dapi (blue) and Vmat2 (red) in SNc of WT and Rgs6−/− mice. Scale bar 20 µm. (C) Co-immunofluorescence staining against TH (green), nuclear Dapi (blue) and Bdnf (red) in SNc and VTA of WT and Rgs6−/− mice. Scale bar 20 µm. (D) Double immunofluorescence staining against TH (green) and Pitx3, Aldh1a1, Fgf10 (red) in control and 1 y-old Rgs6−/− mice that display dysmorphic mDA neurons. Arrowheads indicate unaffected dSNc neurons and arrows point to affected vSNc neurons. Scale bar 100 µm. Markers of dopaminergic signalling in Rgs6−/− midbrains
The regulatory action of Rgs6 was associated with various GPCRs, in particular the dopamine receptor D2 (Drd2) [25]. Ventral SNc mDA neurons are subject to regulatory negative feedback mediated by Drd2 autoreceptors. Expression of Drd2 itself is not affected in vSNc mDA neurons of Rgs6−/− mice (Fig. 8A) despite its dependence on Pitx3 [19]. Dopamine signalling in these neurons leads to activation of Erk1/2 [41] and accordingly, we observed significant phospho-Erk1/2 only in THlow neurons of Rgs6−/− vSNc (Fig. 8B). In addition, enhanced DA signalling downstream of Drd2 [42] would be expected to increase expression of the dopamine transporter DAT (SLC6A3) which is otherwise dependent on Pitx3. It was indeed observed that activated glycosyl-DAT is high in THlow neurons of Rgs6−/− vSNC (Fig. 8C). Thus, high DAT would presumably increase intracellular DA levels in these neurons by promoting DA uptake [43]. Cytoplasmic DA would be further enhanced by the decrease of Pitx3-dependent [43] Vmat2 (Fig. 7B) which is responsible for sequestration of DA into vesicles. Thus, the combined elevation of DAT with decreased Vmat2 is very likely to maintain high levels of free DA that may be toxic [44] and contribute to the degenerative process and cell death [45]. Increased DA signalling thus constitutes a putative mechanism to explain the late-onset neurodegeneration observed in Rgs6−/− vSNc mDA neurons (Fig. 9A).
Fig. 8. Drd2-related changes of gene expression in degenerating neurons of vSNc. 
(A) Immunohistofluorescence staining for TH and Drd2 in coronal sections of 1 y-old Rgs6−/− mice and WT controls. mDA neurons of vSNc express higher levels of glycosylated dopamine receptor D2 (Drd2) than those of dSNc (arrowheads). Scale bar 50 µm. (B) Phospho-Erk1/2 (red) staining is only present in degenerating vSNc THlow (green) neurons of Rgs6−/− mice and not in WT controls. Scale bar 20 µm. Arrowheads indicate unaffected neurons. (C) DAT (red) staining is stronger in degenerating vSNc THlow (green) neurons of Rgs6−/− mice than in WT controls. Scale bar 20 µm. Fig. 9. Rgs6−/− mice as a model for Parkinsonian neurodegeneration. 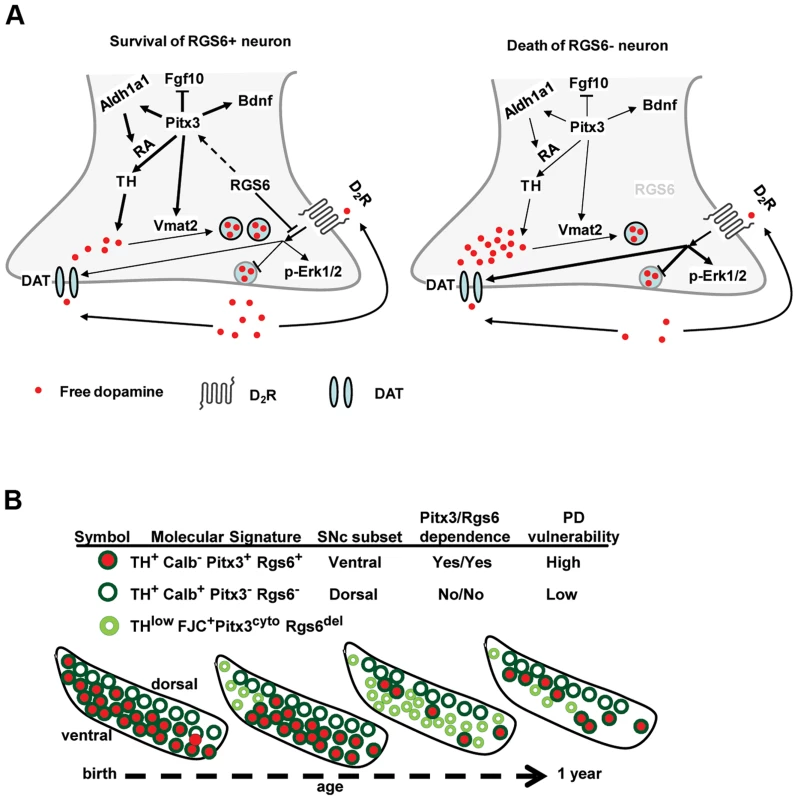
(A) Rgs6-dependent signaling pathways in vSNc mDA neurons control Pitx3 expression, which itself controls expression of Aldh1a1, TH, Fgf10, Vmat2 and Bdnf. Together, Rgs6 and Pitx3 define a survival pathway in these neurons. The dopamine receptor Drd2 may be a target of Rgs6 action and consistent with this hypothesis, expression of the dopamine transporter DAT is up-regulated in Rgs6−/− vSNc. (B) Schematic representation of major subsets of SNc mDA neurons present at different ages in midbrains of Rgs6−/− mice showing progressive degeneration (small pale green) followed by loss of vSNc mDA neurons. The two Rgs6−/− phenotypes appear to reveal incremental penetrance of similar defects: in this case, it would be expected that the contralateral midbrain of affected mice will be affected at some point. We thus further scrutinized the affected Rgs6−/− midbrains with the clear unilateral deficits described above and found two that exhibited on the contralateral side, small clusters of mDA neurons of relatively normal appearance but that are THlow, have cytoplasmic Pitx3, high glycosylated DAT and enhanced phospho-Erk1/2 expression (Figure S4). Such cells were not observed in control midbrains. These data suggest that elevation of DA signalling subsequent to the loss of Rgs6 is closely associated with translocation of Pitx3 to the cytoplasm.
In summary, the loss of Rgs6 is associated with three phenotypes each individually associated with mDA neuron degeneration, cell loss and Parkison's disease namely, 1) the loss of Pitx3 expression and of its target genes, 2) the loss of DJ-1 and finally 3) excessive dopaminergic tone.
Discussion
A few gene expression profiling studies have compared gene expression in SNc versus VTA [46]–[48]. These studies identified large numbers of SN or VTA restricted genes but did not include criteria to relate these specificities to function. Our reliance on Pitx3 gene dependence to identify genes with preferential expression in Pitx3-independent dSNC versus Pitx3-dependent vSNc neurons allowed the prioritization of candidate genes based on the pro-survival Pitx3 gene. This approach also allowed definition of the unique expression profiles for dorsal compared to ventral SNc mDA neurons. We focused on vSNc-enriched and Pitx3-dependent genes in order to identify candidates for role(s) in vSNc mDA neurons survival. The list of 10 Pitx3-activated and 6 Pitx3-repressed genes in this subset includes only one known gene encoding a regulator of a signaling pathway, Rgs6. Further, Rgs6 is the most dependent on Pitx3 for expression in VTA. The list also includes a transcriptional co-regulator Lmo3 that may contribute to Pitx3-dependent survival pathways but it's putative involvement in survival appeared less likely than Rgs6 to contribute to an age-dependent phenotype.
Thus, the present study focused on characterization of neuronal loss and on molecular features of degenerating vSNc neurons as a result of Rgs6 inactivation. We observed late-onset degeneration of vSNc mDA neurons in Rgs6−/− midbrains (Fig. 9B). This phenotype is detected at about 6 months of age and becomes more important in 1y-old mice. At that age, clear mDA cell loss is observed in a subset of mice (Table S2). It is likely that the degenerating THlow mDA phenotype (Fig. 5) and the mDA cell loss (Fig. 4) represent progressive steps of the same defects; accordingly, THlow neurons are also observed in the vSNc of midbrains with cell loss.
This late-onset degeneration is similar to another mouse model of monogenic PD, the DJ-1 (Parkin 7) mutant, that also initially exhibits unilateral defects [7]. Indeed, DJ-1−/− mice present unilateral loss of mDA cell bodies as early as 2 months after birth, with a transition to bilateral cell loss occurring at 1 year of age. Our study of Rgs6−/− mice showed unilateral cell loss, as evidence by decreased number of TH+ and NissL+ neurons (Fig. 4A–C, Table S2) at 12 months after birth, while evidence of THlow degenerating neurons is readily apparent earlier at 6 months of age (Fig. 5A, Table S2). The comparison of phenotypes for DJ-1 and Rgs6 knockout mice indicates that: 1) they both have selective degeneration of SNc, but not VTA, mDA neurons, 2) they both have progressive degeneration and loss of mDA neurons, 3) degeneration begins unilaterally, 4) degeneration eventually becomes bilateral with earlier transition in DJ-1−/− than Rgs6−/− mice. A distinguishing feature of the Rgs6−/− model is the bias towards degeneration of Calb-negative vSNc mDA neurons compared to Calb-positive dSNc mDA neurons that remain largely unaffected (Fig. 5A, Figure S4), as is usually observed in PD.
The slow degeneration of THlow vSNc neurons provided an opportunity to define the molecular features that accompany the dysmorphology. These neurons display increased expression of markers previously associated with pathological changes such as FluroJade C, LC3B and phospho-p27Kip1. Moreover, degenerating THlow vSNc neurons show decreased DJ-1 and elevated Pink1 and Lrrk2 protein expression, suggesting a relationship between Rgs6 signaling and pathways implicated in PD pathology (Fig. 6). Future studies should address the relationships between DJ-1, Pink1 and Lrrk2 in degeneration pathways of Rgs6−/− mice in terms of their known roles in mitochondria dynamics, calcium, balance, redox state and cell signaling.
Collectively, the data show that Rgs6 signaling is necessary for maintenance of vSNc mDA neurons in the aging animal and that its downstream action may be mediated, at least in part, by Pitx3-dependent mechanisms (Fig. 9A). Indeed, the THlow vSNc neurons exhibit low levels of Pitx3 and of its target gene products TH, Aldh1a1, Bdnf, Vmat2, together with enhanced expression of Pitx3-repressed Fgf10 (Fig. 7). The THlow neurons also exhibit cytoplasmic Pitx3 staining suggesting that there may be regulation of nuclear-cytoplasmic localization: this effect could be mediated through phosphorylation of Pitx3 as it was suggested that phosphorylated Pitx1 has greater affinity for nuclear DNA binding than its de-phosphorylated form [49]. One of the documented Pitx3-activated factors, Bdnf, is an important mediator of the neuroprotective action of Pitx3 during development and could contribute to trophic impairment in adult degenerating neurons. The degenerating neurons also exhibit a loss of determinants of postmitotic dopaminergic identity (TH, Aldh1a1, Vmat2) and this likely affects their neuronal activity. Aberrant neuronal activity is a hallmark [45] of the pre-symptomatic stage of PD and this likely transitions to major cellular disruptions (proteosome dysfunction, mitochondrial integrity, calcium permeability…) associated with degeneration and cell death.
What could be the target of Rgs6 action? One likely possibility is the dopamine receptor D2 (Drd2). Indeed, Rgs6 is a negative modulator of GPCR activity, including the dopamine Drd2 receptor [25]. Drd2 expression itself was not affected in degenerating Rgs6−/− mDA neurons (Fig. 8A). However, the vSNc THlow neurons showed evidence of increased DA signaling, namely accumulation of phospho-Erk1/2 (Fig. 8B) and enhanced glycosylated dopamine transporter (Slc6a3/DAT) expression in the mutant (Fig. 8C), consistent with a putative loss of negative Rgs6 input on dopamine signaling [50]. Since the Pitx3−/− midbrain exhibits decreased DAT and Drd2 [19], the observed increase in DAT together with phospho-Erk1/2 are consistent with a primary action of Rgs6 inactivation on DA signaling. Rgs6 may thus contribute to the auto-regulatory negative feedback of the dopaminergic system and its absence may lead to dopamine-dependent oxidative stress and neuronal loss [2], [50]. The enhanced phospho-p27Kip1 and FluoroJade staining support the interpretation that these cells are under stress.
Alternatively, Rgs6 may have GPCR-independent actions: those could involve the GDNF pathway that is essential for catecholaminergic neuron survival [24] or involve direct action on apoptotic pathways [39].
An important aspect of the expression changes discussed above is that they only occur in THlow dysmorphic neurons that normally express Rgs6 and not in other mDA neurons of VTA and dSNc that are negative for Rgs6 (Fig. 3). Therefore, the concordance between Rgs6 midbrain expression and observed cell degeneration patterns suggests that the changes are cell-autonomous and directly related to Rgs6-dependent signaling operating in Rgs6+Pitx3+ vSNc neurons. We cannot however rule out the contribution of other brain systems affected by Rgs6 deficiency.
Collectively, the data show that Rgs6 signaling is necessary for maintenance of vSNc mDA neurons in the aging animal and that its downstream action may be mediated, at least in part, by Pitx3-dependent mechanisms (Fig. 9). The present work identified a critical signalling pathway that controls survival of the mDA neuron subset that preferentially degenerates in PD. Further dissection of this pathway may lead to therapeutically useful insights on the unique properties of this group of mDA neurons.
Materials and Methods
Ethics statement
All experimental procedures with laboratory animals were approved by the IRCM Animal Protection Committee and followed guidelines and regulations of the Canadian Council of Animal Care.
Animal models
All mice were maintained as heterozygous carriers in the C57Bl/6J background and maintained on a 12 h light-dark cycle with food and water ad libitum. Rgs6-null [26] and TH-EGFP [51] mice were described previously. Pitx3-null mice were generated in this laboratory [52].
Dissections and flow cytometry
Ventral midbrain dissections were performed on WT and Pitx3−/− newborn mice (P1–P4) crossed onto TH-EGFP heterozygous background. Mouse brains were quickly washed in ice-cold PBS and then placed into cold Hibernate-A/1%B27 solution (Gibco) to dissect EGFP+ ventral midbrain (vMB) tissue under the fluorescence stereoscope (Leica DFC300 FX). Tissue blocks of vMB were then further microdissected so as to separate SNc (lateral) from VTA (medial). VTA and SNc tissue blocks were digested using the papain dissociation kit (Worthington). Dissociated cells were then resuspended in warm Hibernate-A/1%B27 solution containing propidium iodide (PI, 1 µg/ml), passed through 100 µm mesh and sorted by flow cytometry using the MOFLO™ instrument (Beckman Coulter). PI−/EGFP+ live sorted cells were deposited in 30 µl of RNAlater solution (Ambion) (max. of 3000 cells per 30 µl of RNAlater) to preserve RNA integrity.
RNA extraction, quantitative real-time PCR (qRT-PCR) and microarray analyses
Sorted EGFP+ cells in RNAlater were processed in batches of approximately 5000 cells for purification of total RNA using RNeasy Micro kit (Qiagen). Briefly, 350 µl of RLT lysis buffer was added per 30 µl RNAlater-suspended cells. After vortexing for 1 min, 1 volume of 70% ethanol was added and the content loaded into single pre-equilibrated RNeasy MinElute column. This was done in duplicate for each of the four different preparations of EGFP+ cells (Pitx3+/+ SNc, Pitx3+/+ VTA, Pitx3−/− SNc, Pitx3−/− VTA). Column-bound RNA was washed as recommended and eluted with 14 µl of RNAse-free water. Quality of total RNA was verified with the Agilent RNA 6000 Nano kit adapted for Agilent 2100 Bioanalyzer.
For RT-qPCR, first-strand cDNA was synthesized using Superscript III RT enzyme and accompanying kit (Invitrogen). Primers for PCR amplification are displayed in Table S3. qPCR was performed using Perfecta reagents (Quanta) on a MX-3005 device (Stratagene), and results were analyzed using the accompanying software. All quantifications were relative to Gapdh mRNA.
For microarray hybridization, total RNA with minimal degradation was used. Prior to hybridization onto Affymetrix Gene1.0ST expression arrays, which was done at the Genome Quebec/McGill Innovation Centre, total RNA was linearly amplified using WT-Ovation Pico RNA Amplification kit (NuGen). Gene expression summary values were computed by RMA Express (Bolstad et al, 2003) and raw data was normalized with the LPE algorithm embedded in the FlexArray suite of programs (Genome Quebec). Differentially expressed genes were chosen on the basis of their p-value≥0.05, fold-enrichment ≥1.5 and raw array signal ≥60. Hierarchical clustering and heat map display was done using Genesis (Institute for Genomics and Bioinformatics, Austria)
Immunohistochemistry
Mice were anesthetized and perfused intra-cardially with fresh 4% paraformaldehyde/PBS buffer. Brains were collected and post-fixed for 24 h at 4°C. After inclusion in paraffin, brains were cut in 5–6 µm coronal sections using microtome and mounted on Superfrost Plus (Fisher Scientific) slides. Immunonohistochemistry was performed after paraffin removal and hydration through xylene and graded alcohol series. Antigen retrieval was performed in 10 mM sodium citrate (pH 8.5) at 80°C water bath for 30 min. Sections in citrate solution were left to cool to room-temperature (RT) after which a step of endogenous biotin block was performed (Streptavidin/biotin kit, Vector Labs). Blocking with 5% normal serum for 1 h at RT preceded primary antibody incubation (overnight at 4°C). Primary antibodies used were against Pitx3 (rabbit home-made, 1∶400), Rgs6 (rabbit home-made 1∶25), Th (Millipore MAB318, 1∶1000), Th (Millipore AB152, 1∶500), Calb1 (R&D Systems AF3320, 1∶40), Fgf10 (Millipore ABN44, 1∶1000), Slc6a3 (Santa Cruz sc-32258, 1∶250), Drd2 (Santa Cruz sc5303, 1∶100), Aldh1a1 (Abcam ab24343, 1∶400), BDNF (Abcam ab108319, 1∶25), phospho-p27 (Abcam ab32096, 1∶50), phospho-Erk1/2 (Cell Signaling 4376, 1∶25), DJ-1 (Abcam ab18257, 1∶500) Lrrk2 (Epitomics 3514-1, 1∶50), Pink1 (Novus Biologicals BC100-494, 1∶50), LC3B (Cell Signaling 3868, 1∶50), and Vmat2 (Millipore AB1598P, 1∶200). Secondary antibodies were either biotinylated (Vector Labs, 1∶250) or directly coupled to fluorochromes such as AlexaFluor 488/546 (Invitrogen, 1∶250). For ABC method of amplification, AlexaFluor350/480/546 - or HRP-conjugated (PerkinElmer NEL750, 1∶1000) streptavidin compounds were used. For immunoperoxidase staining, 1% hydrogen peroxide treatment was done just after antigen retrieval step. Mounting of sections was in Mowiol (Sigma) (fluorescence) or in Permount (Fisher Scientific) (DAB chromogen reaction). Immunofluorescence sections were observed on Leica DM6000B light microscope and Carl Zeiss LSM700 confocal microscope.
Fluoro-Jade (FJC) and Nissl staining
FJC (Molecular Probes, 0.01% stock in water) staining was performed just after the immunohistochemical procedure [33], [34]. Briefly, sections were washed in water, dipped for 5 min in 0.06% of potassium permanganate, rinsed again in water and placed in 0.0001% FJC/0.1% acetic/0.0001% DAPI solution for 10 min. Finally, slides were rinsed in water and mounted in 0.1% acetic acid/80% glycerol (v/v). The Fluorescein/FITC filter system was used to visualize FJC. Nissl staining was performed on deparaffinized sections by immersion into warm 0.1% cresyl violet solution for 10 min, rinsing three times in distilled water and differentiating in 95% ethanol. Slides were then dehydrated in 100% alcohol, cleared in xylene and mounted with Permount.
Cell counts and statistics
Cell counts were performed using ImageJ (National Institutes of Health). For cell counts of degenerating neurons, TH-stained or TH/FJC/Dapi-stained coronal sections were loaded on ImageJ; the sections spanned regular intervals (30 or 100 µm) across rostro-caudal extent of midbrain of WT (n = 2) and 1-yo Rgs6−/− mice (n = 2). For each section, total numbers of TH+, TH+/FJC+ and TH−/FJC+ cells were separately counted for SNc and VTA in both hemispheres. The percentage of TH+ degenerating neurons for each anatomical region reflects the ratio between the total number of TH+/FJC+ events and the total number of TH+ events for all rostro-caudal series. Values are reported as means +/−S.D. Statistical significance was calculated using Student T-test.
Supporting Information
Zdroje
1. JellingerKA (2012) Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord 27 : 8–30.
2. Gonzalez-HernandezT, Cruz-MurosI, Afonso-OramasD, Salas-HernandezJ, Castro-HernandezJ (2010) Vulnerability of mesostriatal dopaminergic neurons in Parkinson's disease. Front Neuroanat 4 : 140.
3. ObesoJA, Rodriguez-OrozMC, GoetzCG, MarinC, KordowerJH, et al. (2010) Missing pieces in the Parkinson's disease puzzle. Nat Med 16 : 653–661.
4. DawsonTM, KoHS, DawsonVL (2010) Genetic animal models of Parkinson's disease. Neuron 66 : 646–661.
5. WesterlundM, HofferB, OlsonL (2010) Parkinson's disease: Exit toxins, enter genetics. Prog Neurobiol 90 : 146–156.
6. LiY, LiuW, OoTF, WangL, TangY, et al. (2009) Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci 12 : 826–828.
7. RousseauxMW, MarcogliesePC, QuD, HewittSJ, SeangS, et al. (2012) Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc Natl Acad Sci U S A 109 : 15918–15923.
8. DaveKD, De SilvaS, ShethNP, RambozS, BeckMJ, et al. (2014) Phenotypic characterization of recessive gene knockout rat models of Parkinson's disease. Neurobiol Dis 70 : 190–203..
9. LissB, NeuA, RoeperJ (1999) The weaver mouse gain-of-function phenotype of dopaminergic midbrain neurons is determined by coactivation of wvGirk2 and K-ATP channels. J Neurosci 19 : 8839–8848.
10. SmitsSM, BurbachJP, SmidtMP (2006) Developmental origin and fate of meso-diencephalic dopamine neurons. Prog Neurobiol 78 : 1–16.
11. SimeoneA, Di SalvioM, Di GiovannantonioLG, AcamporaD, OmodeiD, TomasettiC (2011) The role of otx2 in adult mesencephalic-diencephalic dopaminergic neurons. Mol Neurobiol 43 : 107–113.
12. van den MunckhofP, LukKC, Sainte-MarieL, MontgomeryJ, BlanchetPJ, et al. (2003) Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130 : 2535–2542.
13. HwangDY, ArdayfioP, KangUJ, SeminaEV, KimKS (2003) Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res 114 : 123–131.
14. NunesI, TovmasianLT, SilvaRM, BurkeRE, GoffSP (2003) Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA 100 : 4245–4250.
15. LukKC, RymarVV, van den MunckhofP, NicolauS, SteriadeC, et al. (2013) The transcription factor Pitx3 is expressed selectively in midbrain dopaminergic neurons susceptible to neurodegenerative stress. J Neurochem 125 : 932–943.
16. van den MunckhofP, GilbertF, ChamberlandM, LévesqueD, DrouinJ (2006) Striatal neuroadaptation and rescue of locomotor deficit by L-dopa in aphakia mice, a model of Parkinson's disease. J Neurochem 96 : 160–170.
17. HwangDY, FlemingSM, ArdayfioP, Moran-GatesT, KimH, et al. (2005) 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci 25 : 2132–2137.
18. FuchsJ, MuellerJC, LichtnerP, SchulteC, MunzM, et al. (2007) The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol Aging 30 : 731–8..
19. JacobsFM, VeenvlietJV, AlmirzaWH, HoekstraEJ, von OerthelL, et al. (2011) Retinoic acid-dependent and -independent gene-regulatory pathways of Pitx3 in meso-diencephalic dopaminergic neurons. Development 138 : 5213–5222.
20. JacobsFM, SmitsSM, NoorlanderCW, von OerthelL, van der LindenAJ, et al. (2007) Retinoic acid counteracts developmental defects in the substantia nigra caused by Pitx3 deficiency. Development 134 : 2673–2684.
21. LiuG, YuJ, DingJ, XieC, SunL, et al. (2014) Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J Clin Invest 124 : 3032–3046.
22. JacobsFM, van ErpS, van der LindenAJ, von OerthelL, BurbachJP, et al. (2009) Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development 136 : 531–540.
23. PengC, AronL, KleinR, LiM, WurstW, et al. (2011) Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. J Neurosci 31 : 12802–12815.
24. PascualA, Hidalgo-FigueroaM, PiruatJI, PintadoCO, Gomez-DiazR, et al. (2008) Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11 : 755–761.
25. AndersonGR, PosokhovaE, MartemyanovKA (2009) The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys 54 : 33–46.
26. YangJ, HuangJ, MaityB, GaoZ, LorcaRA, et al. (2010) RGS6, a modulator of parasympathetic activation in heart. Circ Res 107 : 1345–1349.
27. PosokhovaE, WydevenN, AllenKL, WickmanK, MartemyanovKA (2010) RGS6/Gbeta5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ Res 107 : 1350–1354.
28. MaityB, StewartA, YangJ, LooL, SheffD, et al. (2012) Regulator of G protein signaling 6 (RGS6) protein ensures coordination of motor movement by modulating GABAB receptor signaling. J Biol Chem 287 : 4972–4981.
29. StewartA, MaityB, WunschAM, MengF, WuQ, et al. (2014) Regulator of G-protein signaling 6 (RGS6) promotes anxiety and depression by attenuating serotonin-mediated activation of the 5-HT1A receptor-adenylyl cyclase axis. FASEB J 28 : 1735–1744.
30. FuY, YuanY, HallidayG, RusznakZ, WatsonC, et al. (2012) A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct Funct 217 : 591–612.
31. Di SalvioM, Di GiovannantonioLG, AcamporaD, ProsperiR, OmodeiD, et al. (2010) Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci 13 : 1481–1488.
32. MoellerC, YaylaogluMB, Alvarez-BoladoG, ThallerC, EicheleG (2002) Murine Lix1, a novel marker for substantia nigra, cortical layer 5, and hindbrain structures. Brain Res Gene Expr Patterns 1 : 199–203.
33. BianGL, WeiLC, ShiM, WangYQ, CaoR, et al. (2007) Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine-treated C57BL/6 mice. Brain Res 1150 : 55–61.
34. EharaA, UedaS (2009) Application of Fluoro-Jade C in acute and chronic neurodegeneration models: utilities and staining differences. Acta Histochem Cytochem 42 : 171–179.
35. Oliveras-SalvaM, Van RompuyAS, HeemanB, Van denHC, BaekelandtV (2011) Loss-of-function rodent models for parkin and PINK1. J Parkinsons Dis 1 : 229–251.
36. XieZ, ZhuangX, ChenL (2009) DJ-1 mRNA anatomical localization and cell type identification in the mouse brain. Neurosci Lett 465 : 214–219.
37. TaymansJM, Van denHC, BaekelandtV (2006) Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem 98 : 951–961.
38. OgawaO, LeeHG, ZhuX, RainaA, HarrisPL, et al. (2003) Increased p27, an essential component of cell cycle control, in Alzheimer's disease. Aging Cell 2 : 105–110.
39. MaityB, YangJ, HuangJ, AskelandRW, BeraS, et al. (2011) Regulator of G protein signaling 6 (RGS6) induces apoptosis via a mitochondrial-dependent pathway not involving its GTPase-activating protein activity. J Biol Chem 286 : 1409–1419.
40. LebelM, GauthierY, MoreauA, DrouinJ (2001) Pitx3 activates mouse tyrosine hydroxylase promoter via a high affinity binding site. J Neurochem 76 : 1–11.
41. BolanEA, KivellB, JaligamV, OzM, JayanthiLD, et al. (2007) D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol 71 : 1222–1232.
42. SondersMS, ZhuSJ, ZahniserNR, KavanaughMP, AmaraSG (1997) Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci 17 : 960–974.
43. VaughanRA, FosterJD (2013) Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci 34 : 489–496.
44. CaudleWM, RichardsonJR, WangMZ, TaylorTN, GuillotTS, et al. (2007) Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci 27 : 8138–8148.
45. LipskiJ, NisticoR, BerrettaN, GuatteoE, BernardiG, et al. (2011) L-DOPA: a scapegoat for accelerated neurodegeneration in Parkinson's disease? Prog Neurobiol 94 : 389–407.
46. GrimmJ, MuellerA, HeftiF, RosenthalA (2004) Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci U S A 101 : 13891–13896.
47. GreeneJG, DingledineR, GreenamyreJT (2005) Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis 18 : 19–31.
48. ChungCY, SeoH, SonntagKC, BrooksA, LinL, et al. (2005) Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet 14 : 1709–1725.
49. MelamedP, KohM, PreklathanP, BeiL, HewC (2002) Multiple mechanisms for Pitx-1 transactivation of a luteinizing hormone beta subunit gene. J Biol Chem 277 : 26200–26207.
50. Afonso-OramasD, Cruz-MurosI, AlvarezdlR, AbreuP, GiraldezT, et al. (2009) Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson's disease. Neurobiol Dis 36 : 494–508.
51. SawamotoK, NakaoN, KobayashiK, MatsushitaN, TakahashiH, et al. (2001) Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci USA 98 : 6423–6428.
52. L'HonoréA, CoulonV, MarcilA, LebelM, Lafrance-VanasseJ, et al. (2007) Sequential expression and redundancy of Pitx2 and Pitx3 genes during muscle development. Dev Biol 307 : 421–433.
Štítky
Genetika Reprodukční medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



