-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
Transposable elements (TEs) have the potential to act as controlling elements to influence the expression of genes and are often subject to heterochromatic silencing. The current paradigm suggests that heterochromatic silencing can spread beyond the borders of TEs and influence the chromatin state of neighboring low-copy sequences. This would allow TEs to condition obligatory or facilitated epialleles and act as controlling elements. The maize genome contains numerous families of class I TEs (retrotransposons) that are present in moderate to high copy numbers, and many are found in regions near genes, which provides an opportunity to test whether the spreading of heterochromatin from retrotransposons is prevalent. We have investigated the extent of heterochromatin spreading into DNA flanking each family of retrotransposons by profiling DNA methylation and di-methylation of lysine 9 of histone 3 (H3K9me2) in low-copy regions of the maize genome. The effects of different retrotransposon families on local chromatin are highly variable. Some retrotransposon families exhibit enrichment of heterochromatic marks within 800–1,200 base pairs of insertion sites, while other families exhibit very little evidence for the spreading of heterochromatic marks. The analysis of chromatin state in genotypes that lack specific insertions suggests that the heterochromatin in low-copy DNA flanking retrotransposons often results from the spreading of silencing marks rather than insertion-site preferences. Genes located near TEs that exhibit spreading of heterochromatin tend to be expressed at lower levels than other genes. Our findings suggest that a subset of retrotransposon families may act as controlling elements influencing neighboring sequences, while the majority of retrotransposons have little effect on flanking sequences.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003127
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003127Summary
Transposable elements (TEs) have the potential to act as controlling elements to influence the expression of genes and are often subject to heterochromatic silencing. The current paradigm suggests that heterochromatic silencing can spread beyond the borders of TEs and influence the chromatin state of neighboring low-copy sequences. This would allow TEs to condition obligatory or facilitated epialleles and act as controlling elements. The maize genome contains numerous families of class I TEs (retrotransposons) that are present in moderate to high copy numbers, and many are found in regions near genes, which provides an opportunity to test whether the spreading of heterochromatin from retrotransposons is prevalent. We have investigated the extent of heterochromatin spreading into DNA flanking each family of retrotransposons by profiling DNA methylation and di-methylation of lysine 9 of histone 3 (H3K9me2) in low-copy regions of the maize genome. The effects of different retrotransposon families on local chromatin are highly variable. Some retrotransposon families exhibit enrichment of heterochromatic marks within 800–1,200 base pairs of insertion sites, while other families exhibit very little evidence for the spreading of heterochromatic marks. The analysis of chromatin state in genotypes that lack specific insertions suggests that the heterochromatin in low-copy DNA flanking retrotransposons often results from the spreading of silencing marks rather than insertion-site preferences. Genes located near TEs that exhibit spreading of heterochromatin tend to be expressed at lower levels than other genes. Our findings suggest that a subset of retrotransposon families may act as controlling elements influencing neighboring sequences, while the majority of retrotransposons have little effect on flanking sequences.
Introduction
A substantial fraction of most eukaryotic genomes is composed of transposable elements (TEs) [1]–[4]. While these TEs are sometimes referred to as “junk” DNA, there is evidence for potential functional roles in some instances [5]. Indeed, Barbara McClintock used the term “controlling elements” to describe the potential for these sequences to affect the regulation of endogenous genes [6]–[7]. Mobile genetic elements include class I retrotransposons and class II DNA transposons [2]. The class I TEs transpose via an RNA intermediate while class II TEs utilize a DNA intermediate for transposition. There are a variety of sub-families of both types of TEs [2] that differ in structure, activity, and integration patterns.
TEs could influence neighboring genes by providing regulatory elements or promoters that would alter expression levels or patterns [8]–[9]. Alternatively, TEs may be targeted for silencing and this silencing could spread to affect neighboring sequences potentially including endogenous genes or regulatory elements [10]–[12]. There are several examples in which heterochromatic silencing of TEs can influence expression of nearby genes, including the agouti and Axin locus in mouse [13]–[15], FLC [16], FWA [17] and BNS [18] in Arabidopsis and sex-determination in melons [19]. While there are examples of heterochromatin spreading from retrotransposons to neighboring sequences, it is unclear how general this phenomenon is. Whole genome profiling of DNA methylation in Arabidopsis [20] found that the level of DNA methylation often had sharp boundaries at the edge of repeats although some inverted repeats did exhibit spreading. Another study [21] found limited (200–500 bp) spreading of DNA methylation surrounding some TEs in Arabidopsis. There is evidence that highly methylated TEs are under-represented near genes in Arabidopsis and it has been suggested that the silencing of TEs located near genes might have deleterious consequences [21]–[23]. There is evidence for variation in the spreading of heterochromatin for different families of TEs in mouse [24] and evidence that differences in TE insertions contribute to gene expression variation in other rodents [25].
The complex organization of the maize genome, with interspersed genes and TEs [26]–[28], provides an excellent system in which to study the effects of retrotransposons on neighboring DNA. Many model organisms have relatively small, compact genomes with relatively few retrotransposons. Since these genomes do not have a number of moderate-high copy retrotransposon families it can be difficult to assess the variation in spreading of heterochromatin to neighboring low-copy sequences. The maize genome is more representative of the organization of sequences observed within most flowering plants and is similar to the organization of many mammalian genomes as well. There are a large number of distinct families of retrotransposons within the maize genome and many of these families are moderate to high copy number [28]–[31]. In addition, haplotypes differ substantially with regard to the presence or absence of specific retrotransposon insertions [31]–[34]. The majority of repetitive sequences, including retrotransposons, in the maize genome are highly methylated [26], [35]–[38].
The existence of heavily silenced retrotransposons interspersed with genes throughout the maize genome provides ample opportunities for TEs to exert epigenetic regulation on surrounding sequences. We were interested in further documenting the extent of heterochromatin spreading from maize retrotransposons to neighboring sequencings. Genomic profiling of DNA methylation and H3K9me2 found that heterochromatic spreading is only observed for a small number of specific retrotransposon families. These families tend to be enriched in pericentromeric regions of chromosomes. The analysis of haplotypes lacking specific retrotransposon insertions provides evidence that the adjacent heterochromatin is the result of spreading rather than insertion site bias.
Results
Heterochromatin spreads from some retrotransposons within the maize genome
DNA methylation and chromatin modifications were profiled for low-copy sequences in the maize genome using methylated DNA immunoprecipitation (meDIP) and chromatin-immunoprecipitation (ChIP) with antibodies specific for H3K9me2 or H3K27me3, respectively. The fractions of the genome enriched for DNA or histone modifications were hybridized to a high-density microarray containing ∼2.1 million long oligonucleotide probes derived from the unmasked, non-repetitive fraction of the maize genome. The probes are spaced every 200 bp in the low-copy portions of the maize genome and can provide a profile for the chromatin state in these regions [39]. Our analyses focused on a subset of ∼1.4 million probes that are single-copy (no other sequences with at least 90% identity within maize genome sequence). While this approach does not provide information on the chromatin state within repetitive sequences it can assess how retrotransposons impact neighboring sequences [39]. An independent whole-genome bisulphite sequencing dataset (∼7× coverage) was used to further confirm the patterns that we observed in the meDIP-chip experiments. This independent approach was able to assess DNA methylation within retrotransposons as well as low-copy sequences. The enrichment for sequences associated with H3K9me2 was validated using a set of known sequences (Figure S1A) and several sequences identified by the profiling experiments (Figure S1B).
A large number of class I TEs (retrotransposons) have been identified within the maize genome [30]. These retrotransposons tend to be highly methylated in CG and CHG sequence contexts (Figure S2). We assessed whether heterochromatic chromatin modifications would be enriched in the single-copy regions that flank these retrotransposons. The chromatin state of sequences adjacent to any specific insertion of a retrotransposon is influenced by regulatory and insulator sequences as well as any potential effects of nearby retrotransposons. By assessing the average level of chromatin modifications near all of the retrotransposons of the same family it is possible to identify whether retrotransposon families vary in their influence on local chromatin state. Single-copy probes that are located within 4 kb of all retrotransposons were identified and used to assess the level of chromatin modifications in 200 bp bins of low-copy sequences adjacent to superfamilies, such as gypsy or copia (Figure S3) and individual families of retrotransposons (Figure 1). Many of the retrotransposon families exhibit elevated levels of DNA methylation and H3K9me2 in the 200 bp immediately adjacent to their insertion sites (Figure S3). Because the meDIP-chip profiling of DNA methylation has a resolution of 300–500 bp it is likely that some of the apparent increase in DNA methylation levels very close to retrotransposons represents DNA methylation within the repeats themselves.
Fig. 1. Heterochromatin spreading is restricted to some retrotransposon families. 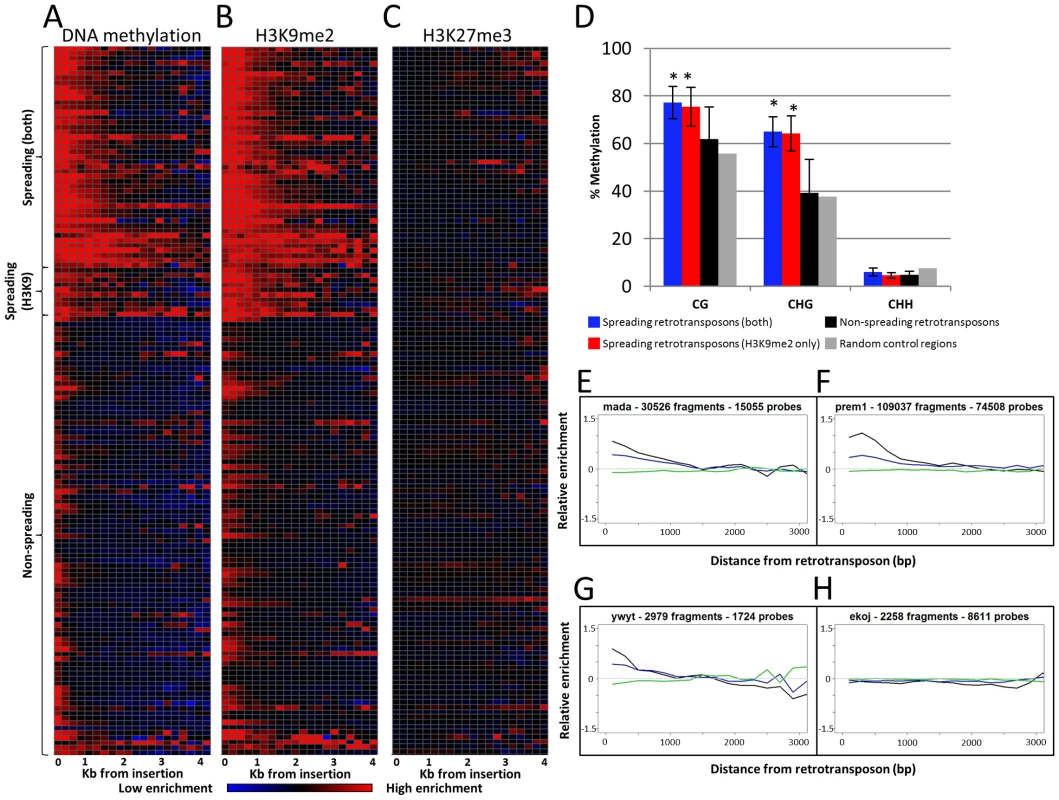
The 144 families of class I retrotransposons represented by at least 1000 probes within the adjacent 4 kb were identified and the average level of H3K9me2, 5-methylcytosine and K3K27me3 enrichment was determined for 200 bp bins. The average values are calculated from both sides of annotated elements collapsed. (A) The relative level of DNA methylation in each 200 bp bin is shown for each of the 144 families. The red color indicates enrichment for the modification while blue indicates depletion of the mark. Black indicates levels of the modification similar to genome-wide average values. The color intensity is based on the average log ratio of immunoprecipitated DNA relative to input DNA. The retrotransposons are grouped according to whether they show spreading for DNA methylation and H3K9me2, H3K9me2 only or neither of the marks. Similar plots are shown for H3K9me2 (B) and H3K27me3 (C). (D) Whole-genome bisulphite sequencing data was used to assess the methylation level in different cytosine contexts in the low-copy (based on the absence of repetitive sequences) 1 kilobase regions flanking spreading (both), spreading (H3K9), non-spreading retrotransposons and for 10,000 random genomic regions. The error bars indicate standard deviation among retrotransposon families and “*” indicate significant (p<0.001) differences for a group relative to the non-spreading families. The level of CG and CHG methylation is higher for spreading retrotransposons than for non-spreading retrotransposons. (E–H) show plots of representative family(s) for each class. The relative abundance of DNA methylation (black), H3K9me2 (blue) and H3K27me3 (green) is shown for the first 3 kb of low-copy DNA flanking the retrotransposon family on both sides. The y-axis indicates enrichment relative to genome-wide average (value of 0 is equal to average of permuted data). A subset of the retrotransposon families also exhibit elevated levels of DNA methylation and H3K9me2 in regions more than 200 bp away from their insertion sites. In general, levels of H3K9me2 and DNA methylation were well correlated, but there were some families with different enrichment for these two marks. As expected, there was no evidence for enrichment (or depletion) of the facultative heterochromatin mark, H3K27me3, in regions flanking the retrotransposons (Figure 1C). To identify retrotransposon families associated with significant levels of spreading of heterochromatic chromatin modifications in adjacent low-copy sequences we compared the distribution of methylation levels in each 200 bp bin with a set of randomly permuted data (10,000 randomly assigned “insertions”) and defined whether each 200 bp bin had significantly higher levels of a chromatin modification than random genomic sequences. Retrotransposon families that exhibit significant (p<0.001) enrichment for a chromatin modification for each bin up through at least 800 bp were classified as spreading families. There are 39 retrotransposon families that exhibit significant enrichments of DNA methylation and H3K9me2 within each of the first four 200 bp bins adjacent to their insertion sites. These families will hereafter classified as “spreading (both)” families (Figure 1A–1B, 1E–1F and Figure S4). Another 10 retrotransposon families had significant levels of H3K9me2 but did not have at least 800 bp of significant enrichment for DNA methylation. These families will hereafter be classified as “spreading (H3K9)” (Table S1; Figure 1A–1B, 1G and Figure S5). Many of these H3K9 only spreading families have elevated levels of DNA methylation in these same regions (Figure S4), but do not pass the significance threshold for all bins within the adjacent 800 base pairs. The remaining 95 retrotransposon families did not exhibit significant enrichment for either DNA methylation or H3K9me2 (example in Figure 1H). There was no evidence for significant enrichment of H3K27me3 in regions near any retrotransposon families (Figure 1C). The initial classification of retrotransposon families was based upon chromatin profiles from B73 seedling tissue. However, very similar patterns were observed for other genotypes and tissues. Specifically, the same families have significant enrichments of DNA methylation in Mo17 seedling, B73 endosperm and B73 embryo tissue (Figure S6). The H3K9me2 patterns are quite similar in both B73 and Mo17 seedlings (Figure S7A–S7B) and there was no evidence for enrichment for H3K27me3 in any of the tissues or genotypes assessed (Figure S7C–S7E).
The analysis of the whole-genome bisulphite sequencing data supports the classifications of different retrotransposon families (Figure 1D and Figure S2). Both CG and CHG DNA methylation levels are higher in low-copy regions flanking spreading (both) and spreading (H3K9) families (Figure 1D). The level of DNA methylation is higher in sequences flanking spreading (both) retrotransposon families than for sequences flanking spreading (H3K9) retrotransposons. The sequences flanking the non-spreading families have DNA methylation levels that are similar to randomly selected genomic regions (Figure 1D). The analysis of internal (within the repeat itself) DNA methylation levels (Figure S2) reveals that the levels of CG methylation within retrotransposons with, or without spreading are similar. However, the spreading (both) and spreading (H3K9) retrotransposon families have slightly elevated levels of CHG methylation at internal sequences. Interestingly, the non-spreading retrotransposon families tend to have higher levels of internal CHH methylation than do spreading families (Figure S2). The relative levels of H3K9me2 within retrotransposons was assessed by qPCR for 10 of the families, including six spreading (both) and four non-spreading families (Figure S8). There was no evidence for higher levels of H3K9me2 within the families that exhibit heterochromatic spreading than for those that do not (Figure S8). The elevated levels of DNA methylation and/or H3K9me2 in low copy sequences flanking the insertion sites observed for a subset of the retrotransposon families are largely confined to the region within 800–1,600 bp of the insertion site (Figure 1A–1B). A closer examination of the levels of DNA methylation and H3K9me2 near each spreading family indicates a fairly sharp drop to non-significant levels of the modifications within 2 kb of the insertion site (Figure 1E–1G; Figures S4, S5) for spreading families. The visualization of individual spreading families (Figures S4, S5) reveals that the distance of heterochromatin spreading varies for different retrotransposon families. This analysis provides clear evidence for diversity in the prevalence of heterochromatin found in low-copy regions flanking different families of retrotransposons in the maize genome.
Spreading of heterochromatin does not require CMT or Mop1
The mechanistic basis for the spreading of heterochromatin is not well defined. It is possible that the interplay between DNA methylation and histone modifications [40]–[41] would result in spreading of chromatin modifications beyond the specific target. To probe the mechanistic basis of spreading we profiled DNA methylation levels in several maize mutants that are known, or expected, to affect DNA methylation patterns. In plants, one pathway that impacts DNA methylation is RNA-directed, and requires the activity of multiple RNA polymerases (RNA PolIV and PolV), an RNA dependent RNA polymerase (RDR2), a dicer like protein, and multiple chromatin modifiers [42]. The mop1 mutant of the maize Rdr2 gene [43]–[45] exhibits variable expression of specific retrotransposon families in mutant relative to wild-type tissue [46]. However, we found no evidence for a consistent effect of the mop1 mutation on the expression levels of spreading or non-spreading retrotransposon families. Indeed, spreading retrotransposon families include examples of both up - and down-regulation in mop1 mutant individuals relative to wild-type (Table S1). In addition, there were examples of non-spreading retrotransposon families that do, and do not, exhibit altered expression in mop1 plants. The levels of DNA methylation in low-copy sequences neighboring retrotransposon families was analyzed in the mop1 mutant to assess whether the spreading of heterochromatin might be affected (Figure 2). There was no evidence for a reduction in the distance or magnitude of the spreading of DNA methylation in the mop1 mutants relative to wild-type plants. The small RNA profile of spreading and non-spreading retrotransposon families was assessed using a recently published small RNA profile based on B73 shoot tissue [47]. The average count of small RNAs per retrotransposon and coverage of retrotransposon did not vary between spreading (both), spreading (H3K9) or non-spreading retrotransposon families (Figure S9).
Fig. 2. DNA methylation enrichment near retrotransposons is not affected by mop1 or zmet2-m1 mutations. 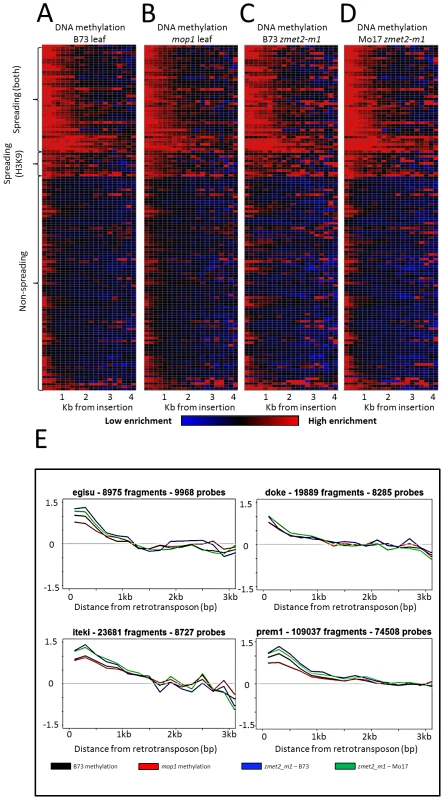
The relative level of DNA methylation in each 200 bp bin is shown for each of the 144 retrotransposon families. Modification levels calculated include probes flanking both ends of retrotransposns. The red color indicates enrichment for the modification while blue indicates depletion of the mark. Black indicates levels of the modification similar to genome-wide average values. The retrotransposons are grouped according to whether they show spreading for DNA methylation and H3K9me2, just H3K9me2 or neither of the marks. The profiles are shown for B73 leaf (A), homozygous mop1 mutant (B), homozygous mutant zmet2-m1 in B73 (C) and Mo17 (D) genetic backgrounds. Example plots of methylation levels in the same backgrounds (E) indicate minimal differences between mutant backgrounds on spreading. Spreading retrotransposons exhibit higher levels of CHG methylation within the retrotransposon themselves (Figure S2). Spreading levels were assessed in plants that were homozygous for mutations in the maize chromomethylase zmet2 (GRMZM2G025592) gene, which contributes substantially to CHG methylation [48]–[49]. While there were examples of locus-specific alterations in DNA methylation levels in this mutant, there was no evidence for a reduction in the spreading of DNA methylation in low copy sequences flanking spreading retrotransposon families (Figure 2).
Analysis of empty sites
The observation that certain families of retrotransposons have high levels of heterochromatic modifications in adjacent regions could reflect insertion site biases for these families or indicate that these families cause local spreading of heterochromatin. Examples of “empty” sites in the Mo17 haplotypes were identified and used to assess whether the high levels of DNA methylation would be observed in these regions when the retrotransposon was absent. Mo17 whole-genome shotgun WGS) sequences (generated by the DOE's Joint Genome Institute (JGI) and downloaded from ftp://ftp.jgipsf.org/pub/JGI_data/Zea_mays_Mo17/) were aligned to the B73 reference genome sequence. Empty sites were defined as being those as which at least three Mo17 sequence reads cover a low-copy sequence flanking an insertion but do not align to the retrotransposon itself and for which no Mo17 reads cover the junction between the low-copy sequence and the retrotransposon. In total, 668 empty sites were identified for the spreading (both) retrotransposon families and 29 empty sites for the spreading (H3K9) retrotransposon families for which we had DNA methylation data in the unique regions flanking the insertion. The lack of the specific insertion in Mo17 was confirmed at 13 of the 14 empty sites that were tested using site-specific PCR primers to confirm the presence/absence of specific insertions. This suggests that there is a low false-positive rate in the identification of empty sites in Mo17. However, given the low coverage of the WGS data and challenges associated with aligning polymorphic sequences it is likely that many of the true empty sites were not identified in this analysis.
The level of DNA methylation at the probe nearest to the empty site was used to assess relative DNA methylation levels with (B73) and without (Mo17) each insertion (Figure 3). The low-copy DNA flanking many of the empty sites showed differences in DNA methylation levels between B73 and Mo17 in 34.7% of the empty sites flanking spreading (both) retrotransposons and in 43.5% of the empty sites flanking spreading (H3K9) retrotransposons (Figure 3A). Over 95% of the empty sites with differential methylation had higher DNA methylation levels in B73 (the genotype with the insertion) than in Mo17. While 35–43% of the probes flanking the empty sites for spreading retrotransposons had variable DNA methylation in B73 and Mo17, only 3% of genome-wide probes assayed show significantly different levels of DNA methylation in B73 and Mo17 and these differences include equal frequencies of higher methylation levels in each genotype. This suggests that the insertion of the retrotransposon conditioned higher levels of DNA methylation and was responsible for the observed DNA methylation polymorphisms. In contrast, DNA methylation levels were similar (and frequently quite high) between B73 and Mo17 when the retrotransposon insertion was present in both genotypes (Figure 3A). Closer inspection of several of the empty sites provides evidence for enrichment of DNA methylation or H3K9me2 in regions flanking the sites in B73 but these modifications were not observed in the Mo17 haplotype that lacks the retrotransposon (Figure 3B). The presence of the insertion as well as the enrichment for DNA methylation was also assessed in five other inbred genotypes of maize (Figure 3B). The presence of insertions was strongly correlated with the presence of high levels of DNA methylation in these other genotypes as well. These results suggest that the high level of heterochromatin observed around these spreading retrotransposon families is an outcome of TE insertion rather than insertion site bias.
Fig. 3. Heterochromatic marks are associated with presence of retrotransposons. 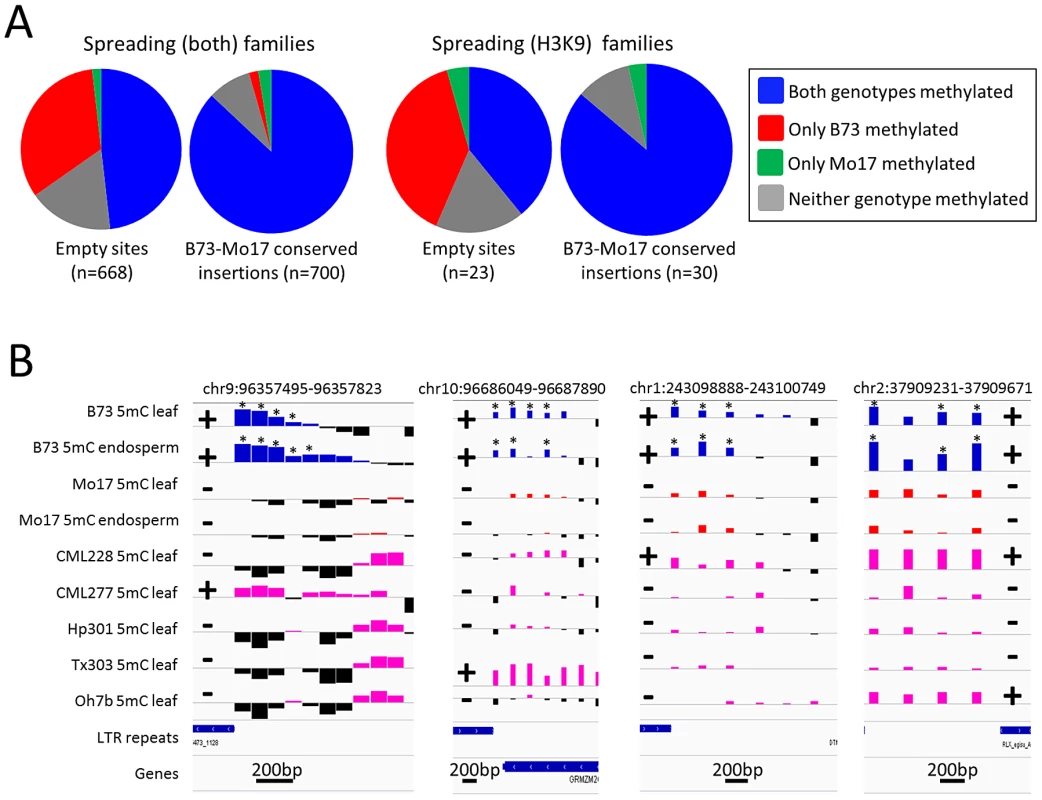
(A) Whole genome shotgun sequence data for Mo17 was used to identify retrotransposon insertions that are present within the B73 haplotype but missing in Mo17. The relative level of DNA methylation at the probe nearest the empty site was assessed in B73 and Mo17 for empty sites of spreading (both) and for spreading (H3K9) retrotransposon families. For each set of families we compared the distribution of methylation patterns to a similar number of insertion sites that are conserved in both B73 and Mo17. (B) The DNA methylation levels for four of these “empty” sites (the coordinates specify the retrotransposon present in B73) are shown for two tissues of B73 (blue bars) and Mo17 (red bars) as well as for a single tissue of five other maize genotypes; CML228, CML277, Hp301, Tx303 and Oh7b (pink bars). Black bars for all genotypes indicate depletion of methylation signal. The location of the retrotransposon and its presence or absence are indicated by + or − symbols, respectively. The genotypes containing the insertion of the retrotransposon all exhibit enrichment for DNA methylation while the genotypes without the insertion do not. The “*” indicates probes with significantly (P<0.01) higher DNA methylation levels in B73 relative to the same tissue in Mo17. The horizontal gray line indicates the genome-wide average for each of the modifications. Higher values indicate higher levels of DNA methylation and all plots are show on the same scale for the y-axis. The scale for black line near the bottom of each plot indicates the base pair scale for the x-axis. Characterization of retrotransposon families that induce local spreading of heterochromatin
The finding that only a subset of maize class I retrotransposon families are associated with local spreading of heterochromatin suggested that there might be intrinsic differences among different retrotransposon families that would explain this variation. We proceeded to characterize these families to ascertain whether there were specific common attributes of spreading families. None of the LINE families exhibit evidence for spreading of heterochromatic marks. RLG (gypsy) families are over-represented among spreading (both) retrotransposon families, while the spreading (H3K9) retrotransposons have more RLC (copia) families than expected (Figure 4A). Spreading (both or H3K9) retrotransposons exhibit significantly higher copy number and comprise a greater fraction of the genome (Table S1, attributes from [29]) than do non-spreading retrotransposon families (Figure S10A–S10B). While there are significant differences in copy number and total Mb within the genome there are examples of families with spreading that have lower copy numbers (Figure S10A). In addition, spreading (both) retrotransposon families have significantly higher average fragment lengths than do non-spreading families (Table S1). Spreading families do not have a significant difference in their mean insertion date relative to non-spreading families (Table S1). However, the analysis of average insertion date for each family (Figure S10C) shows that while non-spreading retrotransposon families include both old and young families the spreading (both) retrotransposon families only include younger families. The analysis of several characteristics of the retrotransposon families with and without spreading provides evidence for some significant differences but none of these factors are sufficient for predicting whether or not spreading occurs. Previous studies that had assessed expression of some retrotransposons in maize tissues [50]–[51] did not find unusually high or low abundance for transcripts of the families with heterochromatin spreading relative to other families.
Fig. 4. Characterization of retrotransposons that exhibit heterochromatin spreading. 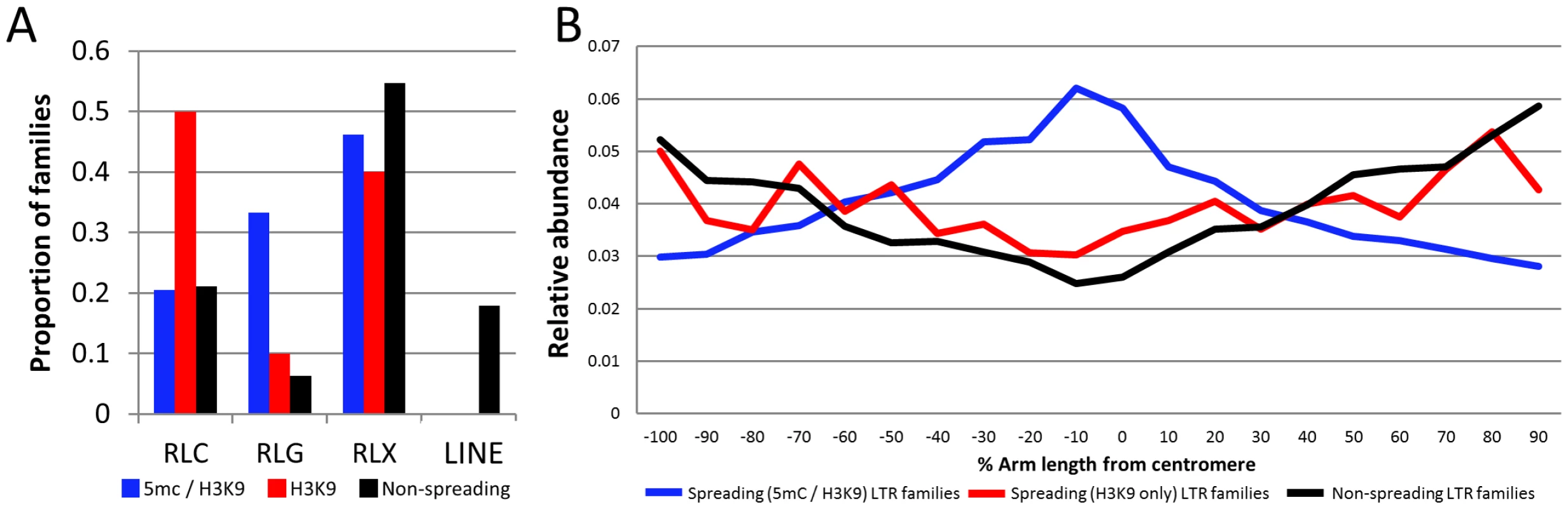
(A) The proportion of families within each of the superfamily designations [RLC – copia; RLG – gypsy; RLX – unknown retrotransposon; LINE – LINE elements] is shown for retrotransposons with spreading of both DNA methylation and H3K9me2 (blue), families with H3K9 spreading only (red) and families without spreading (black). (B) The relative abundance of the retrotransposons within each category was determined according to chromosomal position. The retrotransposons in families with spreading (both) are found throughout the maize chromosomes but are enriched in pericentromeric regions relative to the other families. The y-axis provides a normalized estimate of TE abundance along the chromosome (normalized relative to total copy number for each family). The relative abundance of spreading (both) retrotransposons is higher in the middle of the chromosome than the other families suggesting that these retrotransposons may be enriched in pericentromeric regions (Figure 4B). However, it should be noted that there are other retrotransposon families also preferentially located in pericentromeric regions [29] but that do not show spreading of heterochromatin to low-copy adjacent regions. Hence, the pericentromeric enrichment is insufficient for heterochromatin spreading. The observation that the spreading (both) retrotransposon families are enriched in pericentromeric regions suggested the possibility that the higher levels of DNA methylation in flanking sequences may be due to sampling bias. Because pericentromeric regions tend to have higher levels of DNA methylation [39] it is possible that higher sampling of these regions led to the observation of spreading. However, an analysis of the levels of DNA methylation in low-copy flanking regions relative to chromosome position provides evidence that low-copy sequences flanking spreading (both) retrotransposons is substantially higher than the corresponding regions flanking non-spreading families throughout the chromosome in both CG and CHG contexts (Figure S11). The levels of CG and CHG DNA methylation in spreading (H3K9) retrotransposon families are intermediate (Figure S11).
Genes located near retrotransposon with spreading of heterochromatic marks tend to have lower expression
The finding that some retrotransposon families exhibit spreading of heterochromatic marks to surrounding sequences while others do not led us to hypothesize that these families may influence expression of nearby genes. RNAseq was used to estimate transcript abundance in three tissues of B73 and Mo17 including the identical leaf tissue samples used for profiling DNA methylation levels. All maize genes were annotated to identify the first retrotransposon 5′ of the transcription start site and to determine the distance between the retrotransposon and the transcription start site. Genes that are located near retrotransposons that exhibit spreading (both or H3K9) have significantly (p<0.001) lower expression levels in all genotypes and tissue examined (Figure 5; Figure S12A). This reduction in expression is most severe when we examine genes with retrotransposons inserted within 500 bp of the transcription start site. As the distance between the insertion site and the transcription start site increases there is less evidence for an effect on expression levels, suggesting a limited range within which retrotransposons can influence gene expression. The genes located near spreading (both) and spreading (H3K9) retrotransposons frequently have no detectable expression (Figure S12B). However, even if we exclude genes with no expression, the mean expression of genes near spreading retrotransposons is lower (p<0.001) (Figure S12C).
Fig. 5. Genes near spreading retrotransposons show lower expression than genes near non-spreading retrotransposons. 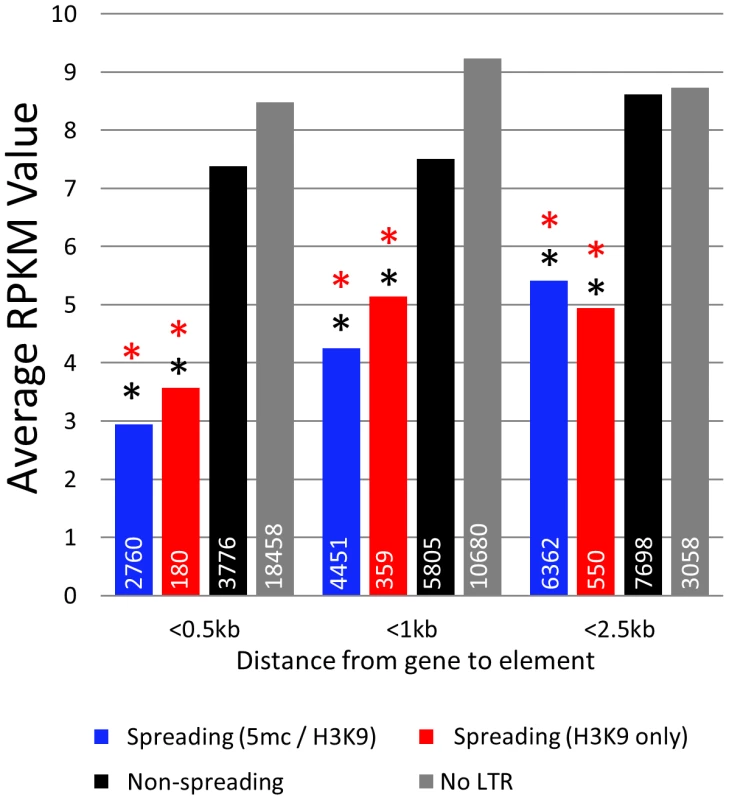
Average RPKM values (from B73 leaf) for all genes falling within 0.5 kb, 1 kb, and 2.5 kb from the respective class of retrotransposons were developed. White numbers within bars indicate the total number of genes within each category. Red asterisks indicate highly significant (p<0.001) difference from the non-spreading retrotransposons (black) within the distance classification. Black asterisks indicate significant (p<0.001) difference from genes not near any retrotransposons (grey). Discussion
Epigenetic variation in low-copy sequences can be the result of pure epigenetic changes (no correlation with DNA sequence polymorphisms) or occur in a facilitated or obligatory fashion such that DNA sequence differences contribute to the epigenetic changes [10]. A handful of examples in which epigenetic differences that impact phenotype has been shown to involve TEs inserted near genes [13]–[19], [52] and genomic profiling of DNA methylation in Arabidopsis has revealed some examples of heterochromatin spreading from TEs [20], [21]. However, it has not been clear whether all TEs have similar effects on neighboring chromatin or whether there are family-specific attributes that affect the spreading of heterochromatin. A recent study analyzed several families of retrotransposons in mouse and found that there is variation in the level of heterochromatin spreading [24] and there have been suggestions of variation in the effects of different repetitive elements on nearby gene expression in Arabidopsis [22], [23]. The complex organization of the maize genome with interspersed TEs and genes provides the opportunity to examine differences among class I retrotransposon families. The chromatin state of any low-copy region of a genome is likely influenced by nearby sequences including regulatory elements and insulator elements. In addition, it is quite likely that TEs will exert an influence on the chromatin state. By examining the average level of chromatin modifications in low-copy sequences neighboring families of retrotransposons we found evidence for heterochromatic spreading from a subset of the moderate to high-copy retrotransposon families in maize. Even in these families the heterochromatic marks spread only 600–1,000 base pairs from the retrotransposon. It is worth noting that there may be other mechanisms through which retrotransposons influence flanking regions. Our assessment is based upon only two chromatin marks, H3K9me2 and DNA methylation. These marks are frequently associated with heterochromatin, but there may be other specific types of chromatin marks that spread from these and transposon families.
There is also evidence that differences in interspecific variation in transposon insertions contributes to gene expression diversity between related species [22], [23]. Here we provide evidence that transposon insertions can also contribute to differences in DNA methylation patterns and gene expression levels within a species. Many TE insertions are exhibit presence/absence variation among maize haplotypes [31]–[34]. The retrotransposons that cause spreading of heterochromatin are expected to result in obligatory epigenetic variation in the low-copy sequences that flank insertions. Indeed, we found that the levels of DNA methylation and H3K9me2 were quite different in B73 and Mo17 at regions that exhibit presence/absence variation for an insertion of a retrotransposon from one of the spreading families. Specifically, these retrotransposons with spreading of heterochromatin may contribute to obligatory and facilitated epialleles, as defined by Richards [10], among different genotypes. Genomic resequencing is often used to identify SNPs as a means to explain phenotypic variation. However, it might be important to also use resequencing data to identify retrotransposon insertion polymorphisms, especially for the retrotransposon families that exhibit spreading of heterochromatic marks. The polymorphism for these insertions may lead to functional variation in the expression of nearby genes.
Barbara McClintock proposed the concept that transposons could serve as “controlling” elements that would influence nearby genes [6]–[7] and this could be extended to include the potential for retrotransposons to influence nearby genes as well. There are examples in which transposons contain regulatory elements or cryptic promoters that can influence the expression of nearby genes [9], [53]. There is also evidence that some transposons can act as controlling elements by “seeding” heterochromatin that spreads to adjacent low copy sequences [10]–[12]. Here we have shown that this activity is not a generic feature of all retrotransposons but is instead limited to a subset of retrotransposons. Hollister and Gaut [22] provide evidence that the presence of heavily silenced TEs near genes may lead to reduced expression and result in fitness consequences. This would suggest that many TEs would evolve to have minimal effects on neighboring genes to reduce their fitness costs. There is evidence that some Drosophila retrotransposons contain insulator elements that reduce the spreading of chromatin states [54]. Alternatively, studies at the bns locus in Arabidopsis have suggested the presence of an active mechanism to prevent the spreading of heterochromatin from retrotransposons [55]. It might be expected that different families of TEs would vary in their ability to limit potential spreading of heterochromatin through the presence of insulators or the recruitment of factors that limit spreading. Hollister and Gaut [22] noted heterogeneity among families of Arabidopsis class I retrotransposons for their distance to the nearest gene and suggested that this may reflect family specific differences in heterochromatin spreading. The analysis of the large families of retrotransposons in maize permitted us to identify several families of retrotransposons with high levels of spreading. These retrotransposon families may be considered as bad “neighbors” for genes. Indeed we find that many genes located near retrotransposons with spreading tend to be silenced or expressed at lower levels. We might predict that insertions of retrotransposons from these families will be more strongly selected against when inserted near genes, especially if they affect gene expression. Therefore, our observed expression differences will only report effects that have been tolerated during natural and artificial selection of maize lines. Consistent with this possibility, our observation that these retrotransposon families are enriched in relatively gene-poor pericentromeric regions may reflect selection against insertions of these retrotransposons when they are near genes. Further research efforts to understand the basis of this difference will be important in providing the ability to predict which retrotransposon families are likely to condition spreading of heterochromatin and understanding the consequences of the spreading of heterochromatin.
Materials and Methods
Epigenomic profiling
DNA methylation profiling on three replicates of 3rd leaf tissue of B73 and Mo17 was performed as described [39 – GSE29099]. Briefly, methylated DNA was immunoprecipitated with an anti-5-methylcytosine monoclonal antibody from 400 ng sonicated DNA using the Methylated DNA IP Kit (Zymo Research, Orange, CA; Cat # D5101). For each replication and genotype, whole genome amplification was conducted on 50–100 ng IP DNA and also 50–100 ng of sonicated DNA (input control) using the Whole Genome Amplification kit (Sigma Aldrich, St. Louis, MO, Cat # WGA2-50RXN). For each amplified IP input sample, 3 ug amplified DNA were labeled using the Dual-Color Labeling Kit (Roche NimbleGen, Cat # 05223547001) according to the array manufacturer's protocol (Roche NimbleGen Methylation UserGuide v7.0). Each IP sample was labeled with Cy5 and each input/control sonicated DNA was labeled with Cy3. H3K9me2 and H3K27me3 profiling were performed on three replicates of B73 and Mo17 seedlings using antibodies specific for H3K27me3 (#07-449) and H3K9me2 (#07-441) purchased from Millipore (Billerica, USA). For each replicate, 1 g of plant material was harvested on ice, rinsed with water, and crosslinked with 1% formaldehyde for 10 minutes under vacuum. Cross-linking was quenched by adding glycine solution to a final concentration of 0.125 M under vacuum infiltration for 5 minutes. Treated tissue was frozen in liquid nitrogen and stored at −800 C until chromatin extraction. Chromatin extractions were performed using EpiQuik Plant ChIP Kit (Epigentek, Brooklyn, USA) according to manufacturer's recommendations. Extracted chromatin was sheared in 600 µl of the EpiQuik buffer CP3F with 5 10-second pulses on a sonicator. To test and optimize sonication conditions, cross-linking was reversed in a sample of sheared chromatin and the resulting products were analyzed on agarose gel. Sonication conditions were optimized to yield predominantly 200–500 bp DNA samples. Chromatin immunoprecipitations, reverse cross-linking, and DNA cleanup was performed using EpiQuik Plant ChIP Kit (Epigentek) according to manufacturer's recommendations. For each genotype, antibody, and replicate, 50–100 ng of input and immunoprecipitated (IP) DNA was amplified with a whole genome amplification kit (WGA2, Sigma, St. Louis, USA). The amplification of no antibody control (negative control) was always 5–10 fold less efficient confirming specificity of immunoprecipitation. For each amplified IP and input sample, 3 ug of amplified DNA were labeled using the Dual-Color Labeling Kit (Roche NimbleGen, Cat # 05223547001) according to the array manufacturer's protocol (Roche NimbleGen Methylation User - Guide v7.0). Each IP sample was labeled with Cy5 and each input/control sonicated DNA was labeled with Cy3. Samples were hybridized to the custom 2.1 M probe array (GEO Platform GPL13499) for 16–20 hrs at 42 C. Slides were washed and scanned according to NimbleGen's protocol. Images were aligned and quantified using NimbleScan software (Roche NimbleGen) producing raw data reports for each probe on the array. The histone modification and methylation mutants array data can be obtained from GEO accession (GSE39460). The resulting microarray data were imported into the Bioconductor statistical environment (http://bioconductor.org/). Microarray data channels were assigned the following factors: B73 immunoprecipitation, Mo17 immunoprecipitation, B73 input, or Mo17 input depending on sample derivation. Non-maize probes and vendor-supplied process control probes were configured to have analytical weights of zero. Variance-stabilizing normalization was used to account for array-specific effects. Factor-specific hybridization coefficients were estimated by fitting fixed linear model accounting for dye and sample effects to the data using the limma package [56]. The probes were each annotated with respect to their location relative to repeats from the ZmB73_5a_MTEC_repeats file available from www.maizesequence.org. Each probe was only associated with the closest repeat and all probes located within 5 kb of a repeat were retained for further analyses. The probes were assigned based on distance to the retrotransposon and include both upstream (5′) and downstream (3′) sequences together. The distribution of retrotransposons along the length of the chromosome was performed as described in [57]. Data formatted for the Integrative Genomics Viewer (IGV) can be downloaded from http://genomics.tacc.utexas.edu/data/rte_methylation_spreading/.
Bisulphite sequencing
DNA was extracted from the outer tissues of B73 ears whose silks had emerged but had not been fertilized. Sodium bisulfite-treated Illumina sequencing libraries were prepared using a method similar to that of Lister et al [58]. Alignment to the genome (AGPv2) and identification of methylated cytosines was performed using BS Seeker [59]. A total of 198,333,982 single-end reads with unique alignments specifically on the ten chromosomes were obtained, with an average genome-matching read length of 72.8 bases (7.0× coverage, SRA accession SRA050144.1). The level of methylation in CG, CHG and CHH contexts and the total proportion of DNA methylation was calculated for non-repeat masked sequences (as annotated within ZmB73_5a_MTEC_repeats) located within 1 kb of each retrotransposon family. Percent methylation is defined as the number of methylated Cs per total number of Cs for a region. BEDTools [60] was used to identify low-copy sequences flanking retrotransposons.
Identification and analysis of empty sites
Approximately 63M Mo17 454 whole-genome shotgun sequencing reads generated by the DOE's Joint Genome Institute (JGI) were trimmed and aligned to Maize B73 reference genome (AGPv2) and reads aligned uniquely (single loci) were filtered for subsequent analysis. A retrotransposon insertion site was classified as empty if we identified at least 3 WGS reads supporting the site that aligned to the insertion site that included>50 bp of aligned sequence outside of the repeat region in B73 with similarity of ≥94%, relatively short unaligned tails (≤20 bp), and contained a long overhang of >20 bp that begins ±3 bp from the annotated retrotransposon insertion site. PCR primers were designed to amplify the sequence at the “empty” sites using the B73 sequence (which contained the insertion) and the Mo17 sequence (which lacks the insertion) (Table S2). These same primers were also used to assess the presence or absence of the insertion in several other maize genotypes including CML228, CML277, Hp301, Tx303 and Oh7b. Seeds for these genotypes were obtained from the USDA North Central Regional Plant Introduction Station. PCR and gel electrophoresis was conducted as described [61].
RNA–seq and expression analysis
RNA–seq was performed on three biological replicates of four tissues (3rd leaf, embryo, endosperm, and immature ear) for both B73 and Mo17. Samples were prepared at the University of Minnesota BioMedical Genomics Center in accordance with the TruSeq library creation protocol (Illumina). Samples were sequenced on the HiSeq 2000 developing 6–17 million reads per replicate. Raw reads were filtered to eliminate poor quality reads using CASAVA (Illumina). Transcript abundance was calculated by mapping reads to the maize reference genome (AGPv2) using TopHat under standard parameters [62]. Counts of mapped reads across the exon space of the maize genome reference working gene set (ZmB73_5a) were developed using ‘BAM to Counts’ within the iPlant Discovery Environment (www.iplantcollaborative.org). RPKM values were calculated per gene. All genes within 500, 1000, 2500, and 5000 bases of the closest upstream annotated transposable element (ZmB73_5a) using BEDtools [60] were grouped by the spreading class of the nearest TE: spreading (5mc/H3K9), spreading (H3K9 only), non-spreading, and no TE within distance. Genes were also classified as expressed for any RPKM value >0. The proportions of genes showing expression for each distance and spreading class combination were calculated. Average RPKM values for each distance and spreading class combination were also calculated. Significance testing was performed non-parametrically through Wilcox rank-sum tests. Sequencing data is available from the NCBI short read archive under studies SRP013432 and SRP009313.
Supporting Information
Zdroje
1. BiemontC, VieiraC (2006) Genetics: Junk DNA as an evolutionary force. Nature 443(7111): 521–524 10.1038/443521a.
2. WickerT, SabotF, Hua-VanA, BennetzenJL, CapyP, et al. (2007) A unified classification system for eukaryotic transposable elements. Nat Rev Genet 8 : 973–982.
3. LevinHL, MoranJV (2011) Dynamic interactions between transposable elements and their hosts. Nat Rev Genet 12 : 615–627 10.1038/nrg3030; 10.1038/nrg3030.
4. LischD, BennetzenJL (2011) Transposable element origins of epigenetic gene regulation. Curr Opin Plant Biol 14 : 156–161 10.1016/j.pbi.2011.01.003.
5. BiemontC (2010) A brief history of the status of transposable elements: From junk DNA to major players in evolution. Genetics 186 : 1085–1093 10.1534/genetics.110.124180.
6. McClintockB (1984) The significance of responses of the genome to challenge. Science 226 : 792–801.
7. ComfortNC (2001) From controlling elements to transposons: Barbara McClintock and the nobel prize. Trends Genet 17 : 475–478.
8. GirardL, FreelingM (1999) Regulatory changes as a consequence of transposon insertion. Dev Genet 25 : 291–296. 2–5.
9. FeschotteC (2008) Transposable elements and the evolution of regulatory networks. Nat Rev Genet 9 : 397–405 10.1038/nrg2337.
10. RichardsEJ (2006) Inherited epigenetic variation–revisiting soft inheritance. Nat Rev Genet 7 : 395–401.
11. WeilC, MartienssenR (2008) Epigenetic interactions between transposons and genes: Lessons from plants. Curr Opin Genet Dev 18 : 188–192.
12. LischD (2009) Epigenetic regulation of transposable elements in plants. Annu Rev Plant Biol 60 : 43–66 10.1146/annurev.arplant.59.032607.092744.
13. MichaudEJ, van VugtMJ, BultmanSJ, SweetHO, DavissonMT, et al. (1994) Differential expression of a new dominant agouti allele (aiapy) is correlated with methylation state and is influenced by parental lineage. Genes Dev 8 : 1463–1472.
14. MorganHD, SutherlandHG, MartinDI, WhitelawE (1999) Epigenetic inheritance at the agouti locus in the mouse. Nat Genet 23 : 314–318.
15. RakyanV, WhitelawE (2003) Transgenerational epigenetic inheritance. Curr Biol 13: R6.
16. LiuJ, HeY, AmasinoR, ChenX (2004) siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in arabidopsis. Genes Dev 18 : 2873–2878 10.1101/gad.1217304.
17. SoppeWJ, JacobsenSE, Alonso-BlancoC, JacksonJP, KakutaniT, et al. (2000) The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol Cell 6 : 791–802.
18. SazeH, KakutaniT (2007) Heritable epigenetic mutation of a transposon-flanked arabidopsis gene due to lack of the chromatin-remodeling factor DDM1. EMBO J 26 : 3641–3652 10.1038/sj.emboj.7601788.
19. MartinA, TroadecC, BoualemA, RajabM, FernandezR, et al. (2009) A transposon-induced epigenetic change leads to sex determination in melon. Nature 461 : 1135–1138 10.1038/nature08498.
20. CokusSJ, FengS, ZhangX, ChenZ, MerrimanB, et al. (2008) Shotgun bisulphite sequencing of the arabidopsis genome reveals DNA methylation patterning. Nature 452 : 215–219.
21. AhmedI, SarazinA, BowlerC, ColotV, QuesnevilleH (2011) Genome-wide evidence for local DNA methylation spreading from small RNA-targeted sequences in arabidopsis. Nucleic Acids Res 39 : 6919–6931 10.1093/nar/gkr324.
22. HollisterJD, GautBS (2009) Epigenetic silencing of transposable elements: A trade-off between reduced transposition and deleterious effects on neighboring gene expression. Genome Res 19 : 1419–1428 10.1101/gr.091678.109.
23. HollisterJD, SmithLM, GuoYL, OttF, WeigelD, et al. (2011) Transposable elements and small RNAs contribute to gene expression divergence between arabidopsis thaliana and arabidopsis lyrata. Proc Natl Acad Sci U S A 108 : 2322–2327 10.1073/pnas.1018222108.
24. RebolloR, KarimiMM, BilenkyM, GagnierL, Miceli-RoyerK, et al. (2011) Retrotransposon-induced heterochromatin spreading in the mouse revealed by insertional polymorphisms. PLoS Genet 7: e1002301 doi:10.1371/journal.pgen.1002301.
25. PereiraV, EnardD, Eyre-WalkerA (2009) The effect of transposable element insertions on gene expression evolution in rodents. PLoS ONE 4: e4321 doi:10.1371/journal.pone.0004321.
26. BennetzenJL, SchrickK, SpringerPS, BrownWE, SanMiguelP (1994) Active maize genes are unmodified and flanked by diverse classes of modified, highly repetitive DNA. Genome 37 : 565–576.
27. SchnablePS, WareD, FultonRS, SteinJC, WeiF, et al. (2009) The B73 maize genome: Complexity, diversity, and dynamics. Science 326 : 1112–1115.
28. SanMiguel P, Vitte C. (2009) The LTR-retrotransposons of maize.
29. MeyersB, TingeyS, MorganteM (2001) Abundance, distribution, and transcriptional activity of repetitive elements in the maize genome. Genome Res 11 : 1660–1676.
30. BaucomRS, EstillJC, ChaparroC, UpshawN, JogiA, et al. (2009) Exceptional diversity, non-random distribution, and rapid evolution of retroelements in the B73 maize genome. PLoS Genet 5: e1000732 doi:10.1371/journal.pgen.1000732.
31. DuC, FefelovaN, CaronnaJ, HeL, DoonerHK (2009) The polychromatic helitron landscape of the maize genome. Proc Natl Acad Sci U S A 106 : 19916–19921 10.1073/pnas.0904742106.
32. FuH, DoonerHK (2002) Intraspecific violation of genetic colinearity and its implications in maize. Proc Natl Acad Sci U S A 99 : 9573–9578.
33. BrunnerS, FenglerK, MorganteM, TingeyS, RafalskiA (2005) Evolution of DNA sequence nonhomologies among maize inbreds. Plant Cell 17 : 343–360.
34. WangQ, DoonerHK (2006) Remarkable variation in maize genome structure inferred from haplotype diversity at the bz locus. Proc Natl Acad Sci U S A 103 : 17644–17649.
35. RabinowiczPD, SchutzK, DedhiaN, YordanC, ParnellLD, et al. (1999) Differential methylation of genes and retrotransposons facilitates shotgun sequencing of the maize genome. Nat Genet 23 : 305–308.
36. PalmerLE, RabinowiczPD, O'ShaughnessyAL, BalijaVS, NascimentoLU, et al. (2003) Maize genome sequencing by methylation filtration. Science 302 : 2115–2117.
37. WhitelawCA, BarbazukWB, PerteaG, ChanAP, CheungF, et al. (2003) Enrichment of gene-coding sequences in maize by genome filtration. Science 302 : 2118–2120 10.1126/science.1090047.
38. EmbertonJ, MaJ, YuanY, SanMiguelP, BennetzenJL (2005) Gene enrichment in maize with hypomethylated partial restriction (HMPR) libraries. Genome Res 15 : 1441–1446 10.1101/gr.3362105.
39. EichtenSR, Swanson-WagnerRA, SchnableJC, WatersAJ, HermansonPJ, et al. (2011) Heritable epigenetic variation among maize inbreds. PLoS Genet 7: e1002372 doi:10.1371/journal.pgen.1002372.
40. LippmanZ, GendrelAV, BlackM, VaughnMW, DedhiaN, et al. (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430 : 471–476.
41. BernatavichuteYV, ZhangX, CokusS, PellegriniM, JacobsenSE (2008) Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in arabidopsis thaliana. PLoS ONE 3: e3156 doi:10.1371/journal.pone.0003156.
42. HaagJR, PikaardCS (2011) Multisubunit RNA polymerases IV and V: Purveyors of non-coding RNA for plant gene silencing. Nat Rev Mol Cell Biol 12 : 483–492 10.1038/nrm3152; 10.1038/nrm3152.
43. DorweilerJE, CareyCC, KuboKM, HollickJB, KermicleJL, et al. (2000) Mediator of Paramutation1 is required for establishment and maintenance of paramutation at multiple maize loci. Plant Cell 12 : 2101–2118.
44. LischD, CareyCC, DorweilerJE, ChandlerVL (2002) A mutation that prevents paramutation in maize also reverses mutator transposon methylation and silencing. Proc Natl Acad Sci U S A 99 : 6130–6135.
45. AllemanM, SidorenkoL, McGinnisK, SeshadriV, DorweilerJE, et al. (2006) An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442 : 295–298.
46. JiaY, LischDR, OhtsuK, ScanlonMJ, NettletonD, et al. (2009) Loss of RNA-dependent RNA polymerase 2 (RDR2) function causes widespread and unexpected changes in the expression of transposons, genes, and 24-nt small RNAs. PLoS Genet 5: e1000737 doi:10.1371/journal.pgen.1000737.
47. BarberWT, ZhangW, WinH, VaralaKK, DorweilerJE, et al. (2012) Repeat associated small RNAs vary among parents and following hybridization in maize. Proc Natl Acad Sci U S A 109 : 10444–10449 10.1073/pnas.1202073109.
48. PapaCM, SpringerNM, MuszynskiMG, MeeleyR, KaepplerSM (2001) Maize chromomethylase zea methyltransferase2 is required for CpNpG methylation. Plant Cell 13 : 1919–1928.
49. MakarevitchI, StuparRM, IniguezAL, HaunWJ, BarbazukWB, et al. (2007) Natural variation for alleles under epigenetic control by the maize chromomethylase Zmet2. Genetics 177 : 749–760.
50. OhtsuK, SmithMB, EmrichSJ, BorsukLA, ZhouR, et al. (2007) Global gene expression analysis of the shoot apical meristem of maize (zea mays L.). Plant J 52 : 391–404 10.1111/j.1365-313X.2007.03244.x.
51. VicientCM (2010) Transcriptional activity of transposable elements in maize. BMC Genomics 11 : 601 10.1186/1471-2164-11-601.
52. MartienssenR, BarkanA, TaylorWC, FreelingM (1990) Somatically heritable switches in the DNA modification of mu transposable elements monitored with a suppressible mutant in maize. Genes Dev 4 : 331–343.
53. KashkushK, FeldmanM, LevyAA (2003) Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat Genet 33 : 102–106 10.1038/ng1063.
54. GdulaDA, GerasimovaTI, CorcesVG (1996) Genetic and molecular analysis of the gypsy chromatin insulator of drosophila. Proc Natl Acad Sci U S A 93 : 9378–9383.
55. SazeH, ShiraishiA, MiuraA, KakutaniT (2008) Control of genic DNA methylation by a jmjC domain-containing protein in arabidopsis thaliana. Science 319 : 462–465 10.1126/science.1150987.
56. Smyth GK. (2005) Limma: Linear models for microarray data. In: Anonymous Bioinformatics and Computational Biology Solutions using R and Bioconductor New York: Springer. pp. 397–420.
57. GentJI, DongY, JiangJ, DaweRK (2012) Strong epigenetic similarity between maize centromeric and pericentromeric regions at the level of small RNAs, DNA methylation and H3 chromatin modifications. Nucleic Acids Res 40 : 1550–1560 10.1093/nar/gkr862.
58. ListerR, PelizzolaM, DowenRH, HawkinsRD, HonG, et al. (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462 : 315–322 10.1038/nature08514.
59. ChenPY, CokusSJ, PellegriniM (2010) BS seeker: Precise mapping for bisulfite sequencing. BMC Bioinformatics 11 : 203 10.1186/1471-2105-11-203.
60. QuinlanAR, HallIM (2010) BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26 : 841–842 10.1093/bioinformatics/btq033.
61. Swanson-WagnerRA, EichtenSR, KumariS, TiffinP, SteinJC, et al. (2010) Pervasive gene content variation and copy number variation in maize and its undomesticated progenitor. Genome Res 20 : 1689–1699 10.1101/gr.109165.110.
62. TrapnellC, WilliamsBA, PerteaG, MortazaviA, KwanG, et al. (2010) Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28 : 511–515 10.1038/nbt.1621.
63. KentWJ (2002) BLAT–the BLAST-like alignment tool. Genome Res 12 : 656–664 10.1101/gr.229202. Article published online before March 2002.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



