-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
Mutations in the BREVIPEDICELLUS (BP) gene of Arabidopsis thaliana condition a pleiotropic phenotype featuring defects in internode elongation, the homeotic conversion of internode to node tissue, and downward pointing flowers and pedicels. We have characterized five mutant alleles of BP, generated by EMS, fast neutrons, x-rays, and aberrant T–DNA insertion events. Curiously, all of these mutagens resulted in large deletions that range from 140 kbp to over 900 kbp just south of the centromere of chromosome 4. The breakpoints of these mutants were identified by employing inverse PCR and DNA sequencing. The south breakpoints of all alleles cluster in BAC T12G13, while the north breakpoint locations are scattered. With the exception of a microhomology at the bp-5 breakpoint, there is no homology in the junction regions, suggesting that double-stranded breaks are repaired via non-homologous end joining. Southwestern blotting demonstrated the presence of nuclear matrix binding sites in the south breakpoint cluster (SBC), which is A/T rich and possesses a variety of repeat sequences. In situ hybridization on pachytene chromosome spreads complemented the molecular analyses and revealed heretofore unrecognized structural variation between the Columbia and Landsberg erecta genomes. Data mining was employed to localize other large deletions around the HY4 locus to the SBC region and to show that chromatin modifications in the region shift from a heterochromatic to euchromatic profile. Comparisons between the BP/HY4 regions of A. lyrata and A. thaliana revealed that several chromosome rearrangement events have occurred during the evolution of these two genomes. Collectively, the features of the region are strikingly similar to the features of characterized metazoan chromosome fragile sites, some of which are associated with karyotype evolution.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003136
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003136Summary
Mutations in the BREVIPEDICELLUS (BP) gene of Arabidopsis thaliana condition a pleiotropic phenotype featuring defects in internode elongation, the homeotic conversion of internode to node tissue, and downward pointing flowers and pedicels. We have characterized five mutant alleles of BP, generated by EMS, fast neutrons, x-rays, and aberrant T–DNA insertion events. Curiously, all of these mutagens resulted in large deletions that range from 140 kbp to over 900 kbp just south of the centromere of chromosome 4. The breakpoints of these mutants were identified by employing inverse PCR and DNA sequencing. The south breakpoints of all alleles cluster in BAC T12G13, while the north breakpoint locations are scattered. With the exception of a microhomology at the bp-5 breakpoint, there is no homology in the junction regions, suggesting that double-stranded breaks are repaired via non-homologous end joining. Southwestern blotting demonstrated the presence of nuclear matrix binding sites in the south breakpoint cluster (SBC), which is A/T rich and possesses a variety of repeat sequences. In situ hybridization on pachytene chromosome spreads complemented the molecular analyses and revealed heretofore unrecognized structural variation between the Columbia and Landsberg erecta genomes. Data mining was employed to localize other large deletions around the HY4 locus to the SBC region and to show that chromatin modifications in the region shift from a heterochromatic to euchromatic profile. Comparisons between the BP/HY4 regions of A. lyrata and A. thaliana revealed that several chromosome rearrangement events have occurred during the evolution of these two genomes. Collectively, the features of the region are strikingly similar to the features of characterized metazoan chromosome fragile sites, some of which are associated with karyotype evolution.
Introduction
Genome integrity depends upon the coordination of replicon and centriole duplication, chromatin condensation, and the assembly and action of the spindle apparatus. Several checkpoints regulate the progression of the chromosomal and cytoskeletal events [1], and repair systems are recruited as needed to correct replication errors and lesions caused by intrinsic and extrinsic mutagens. Intrinsic mutations may result from the interaction of DNA with reactive metabolites (e.g. hydroxyl radicals) and through the activation of mobile genetic elements. Forward genetics proceeds by employing mutagens, which can range from simple chemical mutagens such as ethyl methanesulfonate (EMS), that typically induce base substitutions, to insertional mutagens such as viral and T-DNA integration, to ionizing radiation that is often associated with rearrangements and/or deletions. Characterization of naturally occurring and induced mutants has dramatically accelerated our understanding of all cellular processes and has led to the discovery of small regulatory RNA molecules and epigenetic modifications of chromatin, two areas of intense investigation in contemporary biology.
The eukaryotic nucleus is organized hierarchically. The basic level of chromatin organization centers on the nucleosome and through various levels of organization and compaction, nucleosome strings are organized into large loop domains that are anchored to the nuclear matrix [2]. Superimposed on this, specific chromosome boundaries or territories exist within the nucleus, further defining the association of specific inter - and intrachromosomal domains [3]. Double strand breaks (DSB), created in the context of recombination activities, or due to mutagen exposure, must be repaired to ensure chromosome integrity. The juxtapositioning of specific chromosomes likely underpins the recurrent nature of specific rearrangements, for example the reciprocal translocation between human chromosomes 9 and 22 associated with chronic myelogenous leukemia.
The evolution of chromosomes has been intensively studied in many systems and is being revolutionized by high throughput sequencing technologies. In plants, genome duplication followed by translocations, inversions, centromere shifts, and the activity of endogenous mobile elements are deemed to be responsible for dispersed blocks of synteny that are observed between distantly related species [4], [5]. The breakpoints for some of these rearrangements have now been mapped onto reference genomes, and this has facilitated a high-resolution comparison between Arabidopsis thaliana and A. lyrata [6], which are believed to have evolved from a common ancestor. A series of chromosome fusion and other rearrangement events, coupled with DNA loss, has reduced the chromosome number from eight to five and the amount of DNA by approximately 40%. Within the Brassicaceae, it is clear that 24 conserved chromosomal blocks have been rearranged during evolution to constitute the genomes of mustard family members [7]. At present, it is unknown whether structures at the boundaries of the blocks promote recombination/rearrangement events or if repressive structures exist elsewhere to maintain the syntenic blocks.
We previously reported that the brevipedicellus phenotype of Arabidopsis is due to loss-of function of the KNAT1 homeodomain protein-encoding gene and that unusually large deletions occur with a high frequency [8], [9]. Here we report the characterization of the breakpoint junctions of five bp deletion alleles and the conservation of their features with metazoan chromosome fragile sites, some of which are associated with chromosome breakage events that occurred during the evolution of eukaryotic karyotypes.
Results
Delineation of Deletion Breakpoints and Analyses of Sequence Junctions
Our previous work documented that five different brevipedicellus alleles were not simple alterations in the gene sequence, but rather were due to large deletions of at least 150 kbp [8]. Information on all reported bp alleles can be found in Table S1. The original bp-1 mutant, isolated by Koornneef and coworkers [10], was generated by EMS mutagenesis, an alkylating agent that typically induces G to A transition mutations. We identified the bp-2 and bp-3 mutants from a fast neutron mutagenized population; bp-5 is the result of an aberrant T-DNA insertion, and bp-11 is an x-ray induced mutant. All five of these alleles exhibit large deletions. The other characterized bp mutants appear to be simple base changes induced by EMS (bp-4, 6–8, and 10), or are insertional mutants (bp-9). Lastly, Venglat et al. [11] reported the isolation of a bp-2 mutant from a promoter tagged population; this mutant was later characterized as a point mutation.
Delineation of the boundaries of these deletion alleles would permit an analysis of breakpoint regions and perhaps provide clues as to why the region appears to be prone to such extreme segmental deletion events. We therefore employed a six-phase strategy to determine the breakpoints of the five deletions, followed by in-silico analyses to search for motifs that might be important determinants of either generating the deletion or limiting the extent of the lesion. First, for each allele, at least one breakpoint was generally localized by employing PCR, using sets of primers that span a region of approximately 1.2 Mbp. The absence of a PCR amplification product was interpreted to mean that the region was part of the deletion (Figure 1). Next, DNA gel blotting was employed to find a restriction fragment length polymorphism (RFLP) between the mutant and parental (either Columbia or Ler) DNA. In phase 3, mutant DNA was cleaved with the enzyme identified by RFLP analysis and the DNA was ligated under conditions designed to produce intramolecular events, generating circular products. This DNA was used as a template for inverse PCR (iPCR) to amplify sequences adjacent to known DNA, including the repaired breakpoints. DNA sequencing was then employed to determine the breakpoint junction sequences. Based on this information, new primers were designed to amplify mutant DNA across the breakpoints to give rise to products of predicted size and sequence and thus validate the deletion (phase 5 analysis). Lastly, computer algorithms were employed to analyze the breakpoint junctions, to search for common features/motifs.
Fig. 1. Localization of bp allele breakpoints by PCR. 
DNA from Columbia (C), Landsberg erecta (L), and bp alleles, bp-1, 2, 3, 5 and 11 (designated 0) were amplified with primer sets spanning approximately 1.2 Mbp of chromosome 4. Note that Ler DNA is polymorphic for some loci, leading to either no amplification or to products of a different size than found for Columbia (AFLPs). For the bp alleles the absence of a PCR product is interpreted as representing a deletion of that region of the genome. Primer information and data for additional primer sets that punctuate those shown in the figure can be consulted in Table S2. Table 1 summarizes the five bp deletion alleles. Sequencing of iPCR products revealed that both bp-1 and bp-2 north junction sequences are composed of highly repeated sequences that are found predominantly in centromeric regions on all five chromosomes. For bp-1, the breakpoint occurs within a tandem (AG)n motif that is broadly distributed and may be a member of the LIMPET transposon family [12]. The north junction sequence could not be unequivocally localized for the following reasons. First, we found that a substantial number of primer sets, spanning BACs F5K24, F10A2, T3E15, and F28D6, did not give rise to PCR products with Landsberg erecta DNA templates (and therefore were PCR negative for all bp alleles in an Ler background; see Tables S2, S3, S4 for primer sequences, locations and amplicon status). Other primer sets generated AFLPs wherein the PCR product size differed between Ler and Col, but which can be useful tools for map-based cloning (e.g. primer sets 76/77 and T3E15-2 in Figure 1). Second, sequencing of iPCR products revealed that the junction sequence adjacent to the south bp-1 breakpoint is most similar to sequences found on other chromosomes, particularly chromosomes 1 and 3. Intriguingly, the percent match for all of these sequences is only 80–91%. The best matches on chromosome 4 include a sequence near 1 Mb, which could be interpreted as an inversion involving the centromere. Additionally there is homology to a region centered at about 4.3 Mb that has a polarity opposite that expected from sequencing the iPCR product. Subsequent pachytene chromosome in situ hybridization discounted the first possibility as cytological landmarks and the distances between fluorescent probe signals are normally distributed in bp-1 (see below). Although a complex rearrangement may be involved, it is more likely that sequence divergence between the Columbia and Landsberg erecta genomes is responsible for this disparity. Based on the locations of the primer sets, bp-1 has suffered a deletion of at least 400.6 kbp (south breakpoint to primer set F1K3) and possibly as much as 900 kbp (south breakpoint to homologous region at 4.3 Mb).
Tab. 1. Summary of bp deletion alleles. 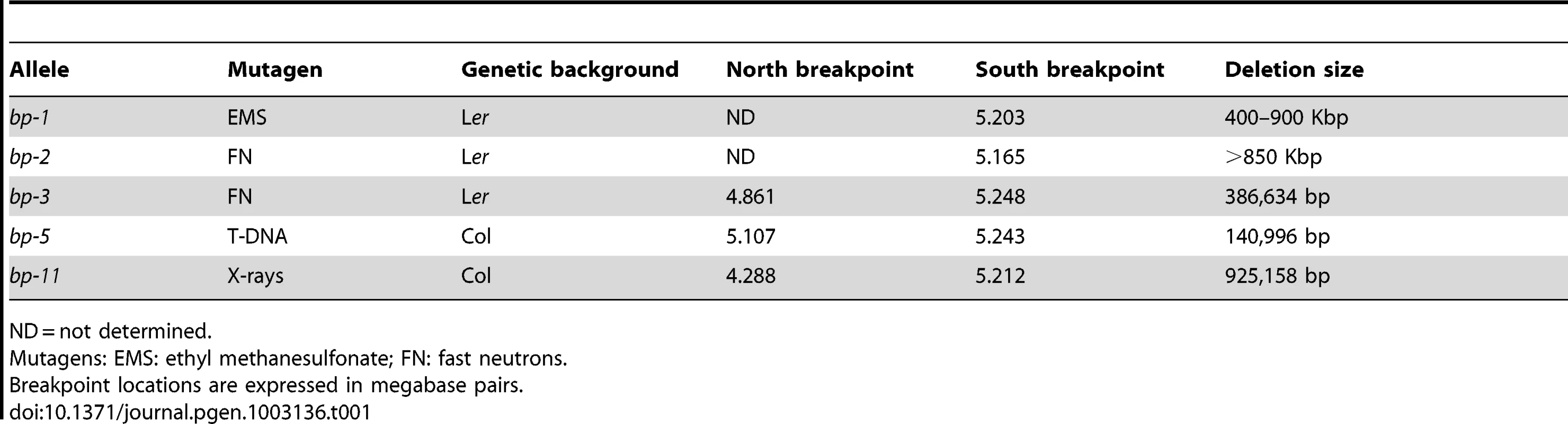
ND = not determined. The bp-2 south breakpoint is located 13.7 kbp south of the BP gene, within the third intron of At4g01870, encoding a putative IP3 kinase. The sequence north of this breakpoint exhibits significant homology to a centromeric satellite sequence that is highly repeated on all chromosomes. The best match on chromosome 4 is to BAC F13J5 (94%), followed by the adjacent BAC, F15N16 (93%). It is noteworthy that the bp-2 allele, also in the Ler background, is PCR positive for three primer sets within BACs T3E15, F28D6 and F14G16, all of which are south of F15N16 according to the AGI reference sequence. The simplest explanation of these data is that either Columbia or Ler has suffered an inversion that positions the F13J5 and F15N16 sequences closer to the BP locus. Additionally or alternatively, sequence divergence between the centromeric satellites in Col vs. Ler might account for the less than perfect sequence homology we found. As is the case for bp-1, the repetitive nature of the bp-2 flanking sequence prohibits a phase 5 PCR analysis to amplify across the breakpoints and thus the extent of the bp-2 deletion cannot be unequivocally known. However, with the Columbia BAC tiling path as a reference, the deletion in bp-2 is at least 851 kbp based on the localized south breakpoint at AGI coordinate 5164664, and the first PCR positive fragment on the north end (in BAC T3E15 centering at 4.3 Mbp).
The locations of both junctions for bp-3, bp-5 and bp-11 were unequivocally determined. bp-3, another fast neutron generated allele, suffered a precise deletion of 386,634 bp, with a single guanosine residue inserted between the two breakpoints. bp-5, which presumably arose due to an aberrant T-DNA integration event, suffered a 140,996 bp deletion, but possesses a 19 bp ‘filler’ sequence (5′ TCCATGTAGTAAGGTAATT3′) at the junction that is 90% identical to a sequence on chromosome 3. The phase 5 PCR product sequence validates the deletion boundaries and the foreign insertion sequence, but its origin is unknown. Lastly, bp-11 is an x-ray induced allele in which the precise excision of 925,158 bp occurred.
FISH Analyses Confirm Cytological Landmarks and Reveal Large-Scale Ecotype-Specific Chromosome Polymorphisms
To provide complementary data on the breakpoint locations determined by our molecular analyses, and to investigate the extent of the deletions in bp-1 and bp-2, we coupled cytological analyses with pachytene chromosome fluorescence in situ hybridization (FISH), using five probesets that span chromosome 4 from 0.7 Mbp to 6.4 Mbp (Figure 2). In Columbia, an inversion event involving pericentric heterochromatin has resulted in a heterochromatic knob in this ecotype, which has no counterpart in Ler [13], [14]. Cytological examination of DAPI stained chromosomes enabled us to measure distances between the NOR4 ribosomal gene cluster at the north end and other cytological landmarks: the heterochromatic knob (hk4S, in Columbia backgrounds), and CEN4 (Table 2). In addition, the size of hk4S and CEN4 could be evaluated. These analyses revealed that the distance from NOR4 to CEN4 and the size of CEN4 are approximately equal in both parental lines and in all bp alleles. In the Columbia based alleles, the size of hk4S is also similar to the wildtype parent line, as was expected.
Fig. 2. Chromosomal landscapes for Columbia, Landsberg erecta, and the bp alleles reveal ecotype specific and allele specific differences. 
A. Cytological map of the north end of chromosome 4 showing the nucleolar organizer (NOR4), the heterochromatic knob that exists in Columbia (hk4S), and the centromere region (CEN4). The locations of the BP gene and the red and green in situ hybridization probes and their AGI coordinates are shown. The figure is drawn to scale, with the exceptions that NOR4 and CEN4 are only allotted 1000 bp in the AGI numbering scheme. Cytologically, these are large regions, occupying 2–4 Mbp and have been represented as such to enable comparisons of DAPI stained landmarks vs. FISH signals. B. Representative pachytene in situ chromosome hybridization patterns for the samples listed. Scale bars represent 10 microns. Tab. 2. Cytological and FISH analysis of Columbia, Landsberg erecta, and the bp alleles. 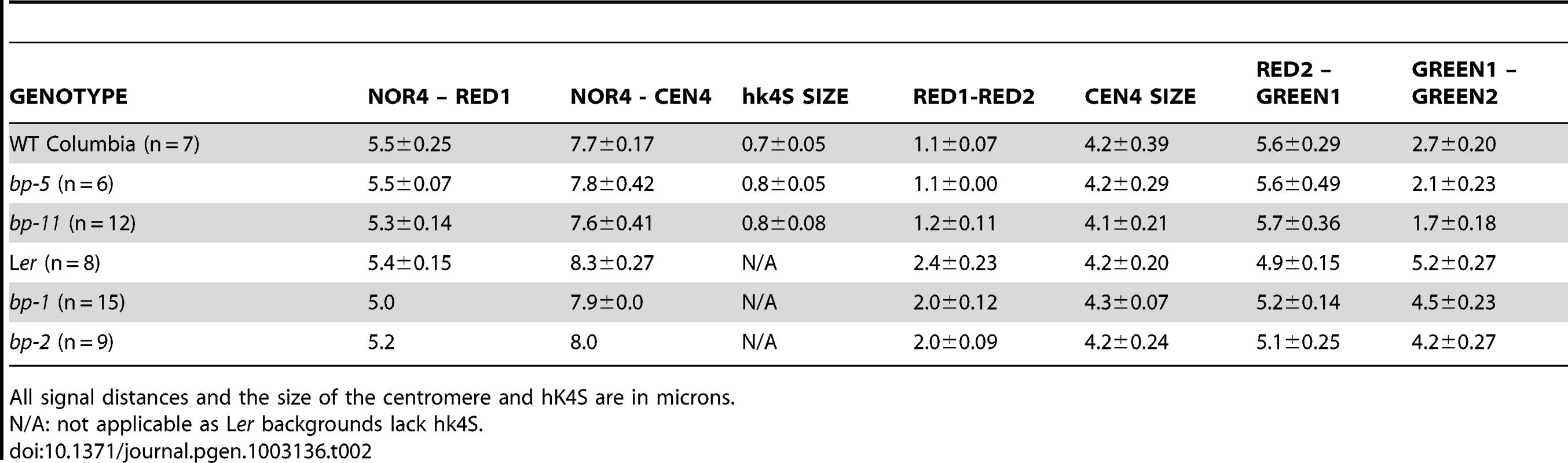
All signal distances and the size of the centromere and hK4S are in microns. The probesets for FISH were chosen to evaluate distances between distinct cytological landmarks and the probe target sequences. The two rhodamine-associated probesets RED1 and RED2 bracket hk4S in Columbia (Figure 2a) and measurements of NOR4 to RED1 revealed no significant differences in either of the two parental ecotypes, or in the bp alleles. As expected, due to the pericentric inversion in Columbia that generated hk4S, the RED1 to RED2 interval is smaller in Columbia than in Ler, and the distances for the bp alleles are similar to their parent ecotypes. We conclude that the bp-associated deletions do not involve chromatin north of the centromere. In this regard, the possibility that the bp-1 north junction sequence, which exhibits homology to the 1 Mbp region suggestive of an inversion involving the centromere, can be ruled out in favor of an event occurring south of the centromere in a region that has diverged between Columbia and Ler.
To correlate the size of the deletions with cytological measurements, three additional probesets were employed. GREEN 1 sequences are located very near CEN4 at 4.3 Mbp, while two additional GREEN probes bind in the 6–6.4 Mbp region, south of the BP locus at 5.15 Mbp (Figure 2). We expected that the extent of the deletions, as gauged by molecular analyses and sequence comparisons, could be roughly correlated with changes in the distance between GREEN1 and GREEN2 (G1/G2). Indeed, this is the general trend we observed as bp-5 (141 Kbp) and bp-11 (925 Kbp) deletions gave intersignal distances of 2.1 µm and 1.7 µm, respectively, compared to the wildtype Columbia distance of 2.7 µm. For the Ler-based alleles bp-1 and bp-2, for which the north breakpoints could not be established, we observed similar reductions in signal distances compared to the Ler parental background with bp-2 exhibiting a shorter G1/G2 length, and thus a larger deletion, than bp-1.
To our surprise, we found that the G1/G2 distance in Columbia is markedly different from Ler, being on average about 2.7 µm in Columbia, but over 5 µm for Ler. These measurements were reproducible and statistically significant, implying that a major polymorphism exists between these two ecotypes. Because the RED2 to GREEN1 distances do not vary significantly between Col and Ler and because GREEN1 localizes very close to the DAPI stained centromere in both ecotypes, it seems likely that the G1/G2 polymorphism is due to an indel occurring between them, or possibly an inversion that moved the G2/G3 probeset sequences in a manner similar to the event that created hk4S. The latter possibility might be resolved by using different colored G2/G3 probes in future experiments.
Bioinformatic Analyses of Breakpoint Regions
Simple BLASTn searches coupled with gene annotations available through the TAIR database permit the identification of genetic elements at breakpoint regions. As the bp deletions are found in the pericentromeric region, there are many occurrences of transposons, pseudogenes, and repetitive sequences. Some of these may be expressed, based on annotated cDNAs and/or expressed sequence tags that map to these sequences, but due to their repetitive nature, it is not clear if they are the actively expressed copies. Discounting transposon/repeat-associated sequences, in the region of 4.2 Mbp to 5.25 Mbp, there are 31 annotation units for which cDNA/EST annotations exist, and another 16 predicted genes for which there are no cDNA/EST sequences. The largest deletion, bp-11, which spans over 925 kbp, has lost 27 genes, while bp-5, the shortest deletion allele spanning 141 kbp, is missing 10 genes (see Table S5). Within the set of 31 genes, there are two pseudogenes, nine that encode unknown proteins, and three that encode proteins with conserved domains of unknown function. Genes with more complete annotations are mostly members of gene families, though a few are single copy genes. Under normal growth conditions, the Ler based alleles are indistinguishable from one another, and the Columbia based alleles are also similar to one another. It must be appreciated that the bp phenotype is enhanced in the Ler background due to the absence of the ERECTA protein kinase [9]. Our initial work with bp-2 demonstrated that the bp mutant phenotype can be rescued by transformation with a wildtype gene [8]; thus under normal growth conditions, the deleted genes seem to be dispensable.
A suite of bioinformatics algorithms (BLAST, MEME, RepeatMasker) was used to interrogate the breakpoint sequences to discover commonalities that might inform our understanding of how the lesions were generated/repaired. Our strategy was to analyze north and south donor sequences. A north donor consists of 1 kbp of DNA north of the north breakpoint linked to 1 kbp of adjacent DNA that was deleted. Similarly, a south donor consists of 1 kbp of DNA that was deleted adjacent to 1 kbp of DNA south of the south breakpoints. As the breakpoint regions lie in the pericentromeric chromatin, most of the breakpoint donor sequences were found to possess one or more known repeats and/or transposon remnants (Figure 3). There is no common sequence motif shared by all alleles, but several short repeats of 20–50 nucleotides (motifs 1–5, see also Figure S1 for alignments and their p-values) are conserved in three to four alleles. The north flanking sequences are predominated by satellite (bp-2), Athila/gypsy transposons (bp-3, bp-5, bp-11) or LIMPET elements (bp-1), most of which are abundant and dispersed throughout the pericentromeric region. The south flanking regions, which cluster within 80 kbp of each other in BAC T12G13, tend to be sparse in repetitive sequences, but are A/T rich and 80% possess motif 5, a T-rich element that is also found in two of the north flanking regions. Statistically, the p-values for motifs 1–5 range from 2.8×10−22 to 5×10−6, lending credibility to their association with lesion formation and/or repair.
Fig. 3. Bioinformatic analysis of bp alleles. 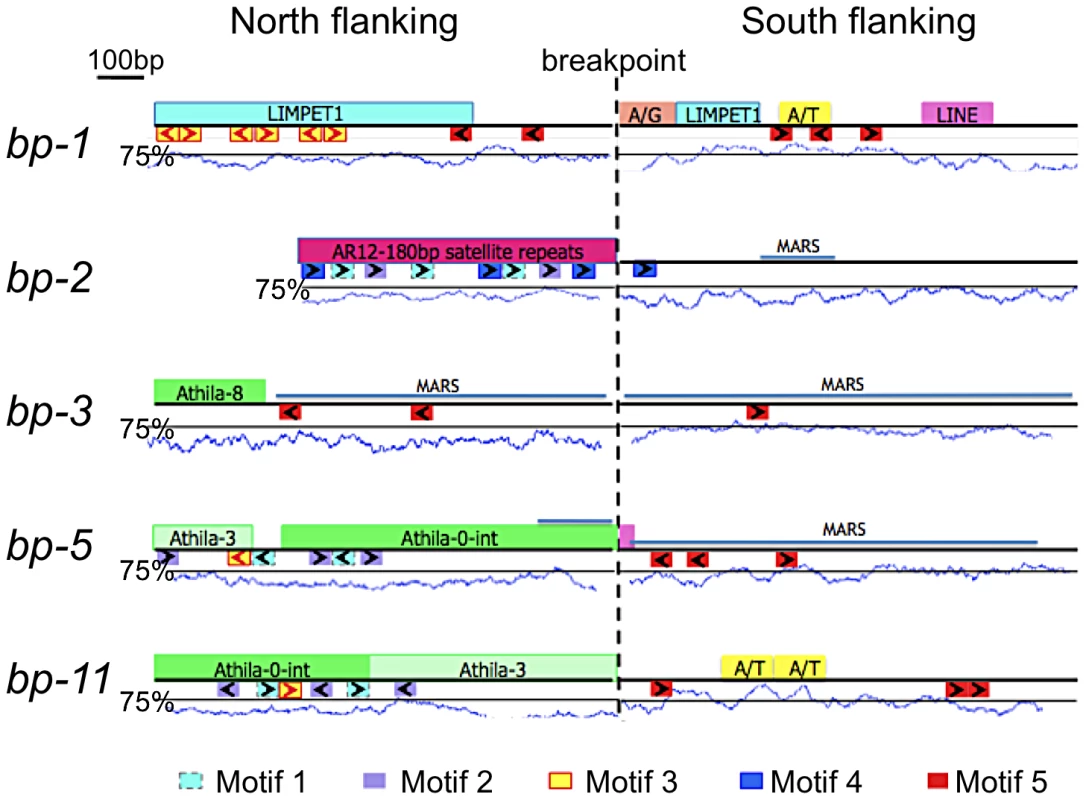
One kilobase of flanking sequence north and south of the breakpoint is represented. Above each line is a summary of Repeatmasker analysis that identified common repetitive elements and low complexity regions. A/T represents an adenine/thymine rich region, while A/G represents an adenine/guanine repeat. The A/T content is graphed below the boxes with the solid line positioned at the 75% threshold for a window size of 50. Below each line is a summary of MEME analysis, which identified common sequences represented as colored boxes (motifs 1–5). The orientation of these repeats is given by the arrows within the boxes. Figure S1 shows the MEME sequence alignments and their p-values. The locations of predicted MARS binding sites are shown as labeled blue lines. Sequences at the breakpoints offer little evidence of homologous recombination. Although both the north and south flanking regions of bp-1 contain sequences with homology to LIMPET elements, the junctions are not a continuous sequence in the repaired product, which argues against homologous recombination. A simplistic explanation for this lesion is that it results from illegitimate recombination between (AG)n rich repeat sequences located at approximately 4.2 and 5.2 Mbp, but the significance of the flanking LIMPET sequences cannot be evaluated. Additionally, the close proximity of a (CT)n rich sequence juxtaposes two complementary sequences that could generate a long hairpin structure with the breakpoint occurring in a loop of four nucleotides at its apex. Complex secondary structures are predicted at or near all of the donor sequences, with the exception of bp-1 (see Figure S2).
The apparent lack of homology between the paired north and south flanking sequences indicates that the DNA damage is likely repaired by non-homologous end joining (NHEJ). In only one instance (bp-5) is there microhomology between the 5′ and 3′ donor sequence junctions, which might portend the involvement of microhomology-mediated recombination.
Breakpoint Junctions Associate with a Nuclear Matrix Protein In Vitro
Matrix attachment region (MAR) prediction algorithms suggested the possibility that some of the sequences near the breakpoint junctions contain binding sites for the nuclear matrix. There are several known components of the nuclear matrix, including AHL1, an AT hook domain containing protein that has been shown by biochemical and cytological assays to be associated with the nuclear matrix [15]. We cloned an AHL1 cDNA behind an inducible promoter and expressed it as a His-tagged protein in E. coli. Southwestern blot analysis was then undertaken with end-labeled probes, including a positive control (a plastocyanin gene, PC, [16]), a negative control (a histone H1 gene fragment, At1g06760) and several fragments near the breakpoint junctions (2S, 3N, 3S, 5S, 5N, and 11S). Figure 4 shows that the histone H1 probe does not bind to the AHL1 protein. The positive control PC1 probe as well as the 2S, 3N and 5N probes bound weakly, but above background levels, while the 3S, 5S and 11S probes exhibited strong binding to AHL1. We conclude that these probes, representing regions predicted to contain MAR binding sites, do indeed bind to a known matrix associated protein. Intriguingly, the 3S, 5S and 11S probes are located within 26 kbp of each other at the south end of BAC T12G13, which harbors all of the south end deletion breakpoints. It is conceivable that BAC T12G13 contains sequences that organize chromatin loops and that in the bp deletion mutants, resection of the initial lesions is limited by either a complex chromatin structure (e.g. boundary element possessing extensive secondary structure) and/or an attachment point on the nuclear matrix.
Fig. 4. Breakpoint junction regions bind the nuclear matrix protein AHL1. 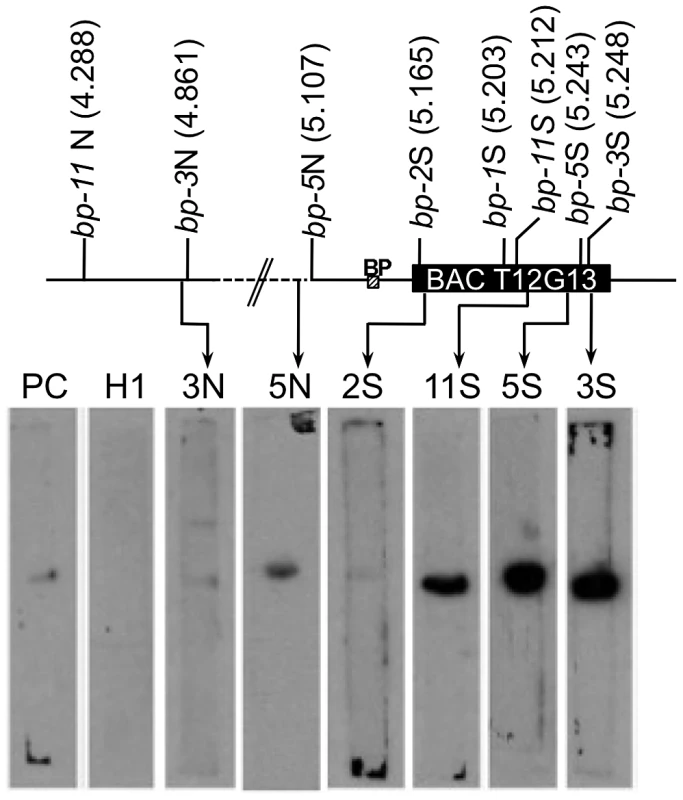
Aliquots of proteins from induced E. coli harboring HIS-tagged AHL1 were subjected to SDS-PAGE and blotting, and membrane strips were probed with end labeled DNA fragments. 2S, 3N, 3S, 5N, 11S represent probes near the north or south (N or S) borders of the breakpoints of each bp allele as shown on the accompanying map with AGI coordinates in Mbp. PC represents a positive control from the plastocyanin gene [16] while H1 represents the histone H1 gene (At1g06760) probe, used as a negative control. The locations of the BP gene and BAC T12G13 are shown. Crossover Frequency and Genome Polymorphisms Are Indexed by the Bp Breakpoints
The lack of an ordered array of large clones of Ler DNA precludes both direct Col/Ler synteny comparisons as well as the construction of genome array chips for chromatin immunoprecipitation analysis. Nevertheless, as two of our bp alleles are derived from Columbia, we suspected that data mining might prove fruitful for correlating the breakpoint regions with established chromatin features and genetic data. We reasoned that segments of the genome that are active in recombination might have features that could predispose them for rearrangement events. High-resolution recombination mapping along chromosome 4 revealed very little recombination in the hk4S and CEN4 regions, as expected, but also identified regions which exhibit high levels of recombination (hotspots, [17]). Figure 5 shows that the 5–6 Mbp region is very active in recombination, with one of the hotspots in the same region where the south breakpoints of all bp alleles cluster. Interestingly, this region also includes the HY4 locus, where multiple large deletion alleles have been reported [18].
Fig. 5. Colocalization of breakpoints with recombination hotspots and chromosome rearrangement events. 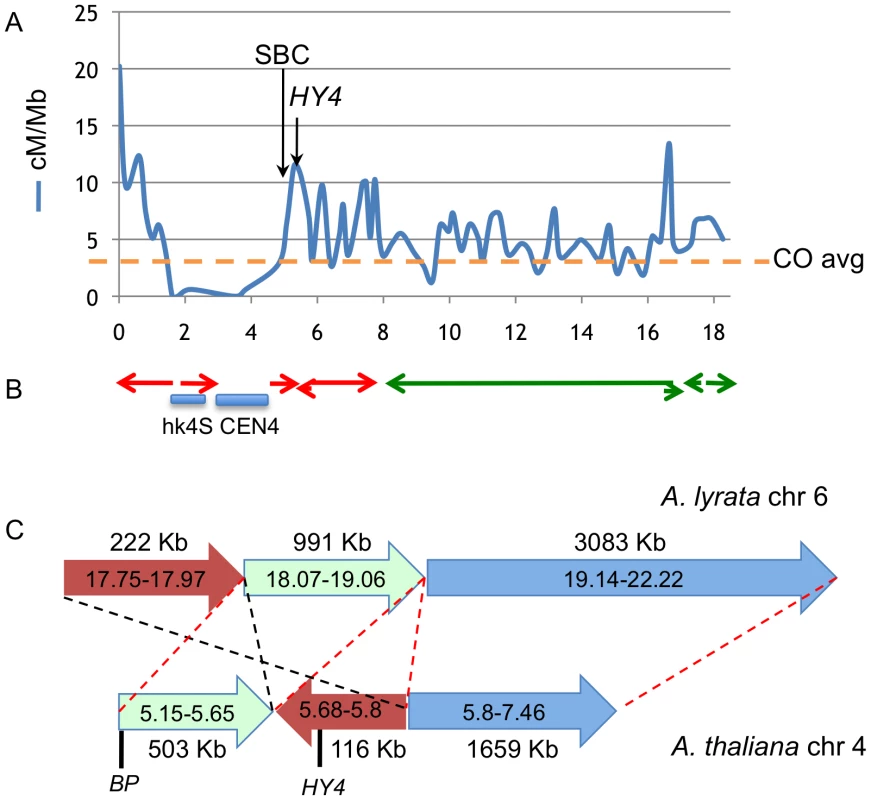
A. Recombination frequency (in cM/Mbp) along chromosome 4, recharted from data of Drouaud et al [17]. The horizontal axis values are megabase pairs, with the north end of the chromosome on the left. The average recombination frequency (CO) is shown by the horizontal hatched line. B. Locations of gross chromosomal fusion and rearrangement events in Arabidopsis progenitors that gave rise to A. lyrata and A. thaliana. The locations of A. lyrata chromosomes 6 (red) and 7 (green) segments are depicted as the colored arrows [6]. The direction of the arrows indicates the polarity of rearranged segments. Not all fusions/inversions are shown. Note the general correlation between CO frequency, the cluster of bp south breakpoint deletions (SBC), and the location of chromosome rearrangements. The HY4 arrow points to a region around 5.7 Mbp where numerous HY4 deletion mutants exist (see the Discussion and [18]). C. High resolution sequence comparison of A. lyrata chromosome 6 (top) and A. thaliana chromosome 4 (bottom) in the pericentric region where BP and HY4 are located. Three A. lyrata genome segments are shown along with their lengths and are color coded. The corresponding A. thaliana regions are shorter and ancestral rearrangement events have repositioned the BP/HY4 loci relative to the progenitor genome. The red hatched lines and segment polarity arrows indicate colinearity/synteny between the two genome segments, while the black hatched lines highlight a putative inversion/transposition event(s) involving the brown colored segments. In addition to the rearrangement events, note that significantly more DNA exists in this region in A. lyrata relative to A. thaliana, implying that additional remodeling events have also occurred. Sequences to the left of each chromosome represent pericentromeric DNA that cannot be accurately aligned due to the repetitive nature of the region. A. thaliana and A. lyrata likely evolved from a common ancestor and several chromosomal fusion and rearrangement events have occurred to reduce both the size of the genome and the number of linkage groups in A. thaliana [6], [7]. Comparisons of the genomic sequences of the two species reveal that several regions of the ancestral chromosomes six and seven were fused to generate A. thaliana chromosome 4 [6], [7 and references therein]. Importantly, some of the major rearrangement events also map to the region where the south breakpoints are clustered and in other areas, for example in the 16–17 Mbp region where other rearrangement events have occurred, recombination hotspots also exist (Figure 5B). Comparative sequence analysis of the BP/HY4 region of the A. thaliana and A. lyrata genomes indicate that one or more segments of these genomes underwent transposition/inversion events, providing additional evidence that the region is recombinogenic and prone to chromosome rearrangement events that are possibly associated with speciation (Figure 5C).
Epigenomic data mining revealed that the defined north end breakpoints (bp-3, bp-5 and bp-11) possess common histone modifications, specifically H3K27me1, H3K9me2 and H4K20me1, and in addition exhibit 5–15% 5-methylcytosine (Figure 6, see also Figure S3). This combination of chromatin modifications is associated with transposable element rich regions found in pericentric heterochromatin [19]. The south end breakpoints, with the exception of bp-1, differ markedly from the north ends, sharing no chromatin marks and exhibiting very low levels of 5-methylcytosine. The bp-2 south breakpoint, located within an expressed gene, contains ubiquitinated H2B, H3K27me3 and H3K4me2, all typically associated with euchromatin [19]. The other clustered south breakpoints contain either of the two latter modifications (bp-5, bp-11), but are generally devoid of chromatin marks. However, the local regions possess a variety of modifications and the four basic chromatin states [19] are interspersed (see Figure S3). It is conceivable that a particular combination of chromatin modifications may promote genome instability, or, as we observed for the clustered south breakpoints, a dearth of chromatin modifications and lack of 5-methylcytosine may be indicative of a chromatin state that underpins genomic instability/recombinogenic potential. In any event, features inherent to BAC T12G13 represent a boundary element or transition zone in which the chromatin state switches from one bearing heterochromatic marks to one indicative of a more euchromatic state (Figure 6B).
Fig. 6. Chromatin modifications at the breakpoint junctions. 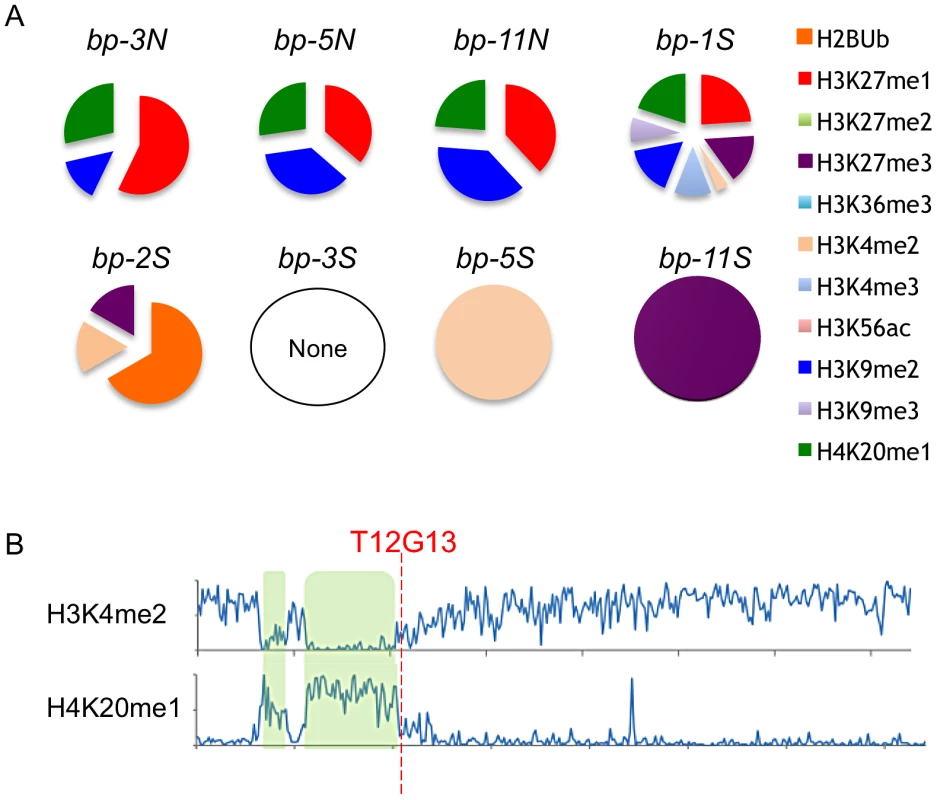
A. North breakpoints occur in pericentric heterochromatin and bear H3K9me2, H4K20me1 and H3K27me1 signatures. South breakpoint modifications exhibit no epigenetic marks or euchromatic marks. B. Global view of chromosome 4 euchromatic (H3K4me2) and heterochromatic (H4K20me1) modification patterns. hk4S and CEN4 positions are highlighted in green; the position of BAC T12G13 at the transition zone is indicated (data from [19]). The BP/HY4 Region Is Unstable Relative to Many Other Loci
The large deletions that we document for the BP locus, as well as the close proximity of the HY4 locus where numerous large deletions have also been reported [18], encourages speculation that the region is generally unstable and prone to deletions and other gross chromosomal rearrangement events. We therefore examined literature reports and employed mutant germplasm search engines to catalog the locations of deletions greater than 25 bp to ascertain if the BP/HY4 region is overrepresented in this data set. Figure S4 shows that the 5 bp alleles and the 15 hy4 alleles reported by Bruggemann and coworkers [18], constitute 20 of the 120 deletion mutations associated with structural genes. We conclude that the BP/HY4 region is on average more prone to deletion/rearrangement events than most other regions of the genome.
Discussion
Genome evolution is driven by a variety of factors, not the least of which are gross chromosome rearrangements that are sponsored by the activity of mobile genetic elements, first reported by Barbara McClintock [20]. More recently, mechanistic analyses of structural variation in a variety of organisms have focused on errors in replication and recombination as causative [21]–[23]. Supporting evidence is provided by the existence of sequences with homology to transposons and/or repeat motifs at breakpoint junctions, evoking homologous recombination or microhomology mediated non-homologous end joining (NHEJ) mechanisms for lesion repair. In the absence of evidence for homologous recombination and outside of the context of meiosis, NHEJ is a pathway to repair [24], and ligation of free ends is a stochastic process, often resulting in translocations. The pericentric chromatin in which BP resides would seem to be inherently unstable due to the presence of autonomous and non-autonomous transposons of various types, numerous repetitive sequences, and sequences of unusual base composition that may impose constraints on replication fidelity due to the formation of secondary structures.
In instances where mutagenesis is employed and which result in gross chromosomal rearrangements, it is not clear if the initial event is associated with DSB formation followed by resection of the free ends and final repair, or alternatively, if there are two DSBs that upon repair result in loss of the intervening sequence. With respect to the bp deletions, it is intriguing that several different mutagenic agents (EMS, fast neutrons, T-DNA insertion and x-rays) produce the same effect: large deletions. Four of the five bp alleles possess Athila LTR retrotransposon elements at or near the north breakpoint junction and the fifth contains satellite repeats at the junction. As there is no corresponding transposon/satellite DNA south of the breakpoint, there is little evidence to support transposon movement, homology-based recombination or replication slippage. An exception is bp-5, where the microhomology found at the junction could suggest one of the latter two processes may have been involved. In the absence of evidence for these types of events in the other alleles, we speculate that the initial break likely occurred during DNA replication, and that resection, likely mediated by the MRN complex [25], extended the region. The extent of DNA loss could be limited by either a change in the structure of DNA (e.g. alternating dinucleotides or elaborate secondary structures, as is observed for several bp alleles), a change in the chromatin microenvironment (e.g. accessibility changes due to differences in histone modification patterns and binding by their associated proteins), and/or a repressive influence of protein factors (e.g. encountering a nuclear matrix protein complex as we report for the south breakpoint cluster).
Breakpoint Junction Sequences Exhibit Features of Metazoan Fragile Sites
Chromosome fragile sites have long been recognized in metazoans as regions which are prone to breakage when replication stress occurs, and activation (breakage) of some are implicated in various disease states and cancer [26], [27]. Some of the common fragile sites that have been characterized at the molecular level are associated with breakpoints that delineate homologous syntenic blocks that have persisted during the evolution of eukaryote karyotypes [28]. Although a wealth of data correlates recurrent deletions and rearrangements with these fragile sites, very little is known about the initial breakage events, but rather only the sequences of junction regions provide clues as to the repair pathway. Some fragile site breakpoints possess microhomologies indicative of replication slippage or recombination based repair, whereas others possess no homology at the junctions. In the latter case, NHEJ must occur and likely involves structural features (e.g. attachment points on the nuclear matrix) that juxtapose free ends. Common fragile sites are A/T rich, possess various repeats and classes of repetitive DNAs (particularly LINE/LTR type elements), are gene-poor regions, and occupy large tracts of eukaryotic genomes, sometimes extending over hundreds of thousands of nucleotides [29], [30]. Our analyses of the SBC revealed that four of the five alleles harbor Athila/LTR retrotransposon elements at or near the breakpoints, which are A/T rich. The A/T rich sequences may contribute to secondary structure formation (see below) and could serve to anchor the chromatin fiber to MAR regions, which are known to be enriched for enzymes of DNA metabolism [31]. Indeed, the inhibition of topoisomerase I by camptothecin almost completely eliminates CFS breakage in cultured mammalian cells [32].
Fragile sites are found at the interface of chromosome R and G bands, classically defined as being early and late replicating, respectively [33]. Recent studies suggest that CFS activation (breakage) may be due to differential utilization of replication origins such that the CFS zone may experience replication stalling or fork collapse [34], [35], and secondary structures likely contribute to this process [36]. The paucity of replicon initiation in these regions necessitates the use of more distant origins and delays replicon completion, in part explaining their location at R/G boundaries. In Arabidopsis, high resolution profiling of replication in suspension cultured cells has revealed the locations of origins and their replication timing [37], [38]. The south breakpoint cluster in T12G13 is situated between two distantly separated origins that span 271 Kbp; for comparison, the interorigin median and mean distances along chromosome 4 are 51.1 and 77.2 kbp, respectively. We propose that the south breakpoint cluster possesses the hallmark features of metazoan chromosome fragile sites, harboring sequences that promote replication fork collapse, and that loss of DNA may be limited by association of local sequences with the nuclear matrix, where topoisomerase/ligase activities can coordinate repair by a NHEJ (or other) mechanism. In addition to the deletions associated with BP/HY4, this stochastic process may occasionally generate rearrangements and chromosomal fusion events that underpin chromosome evolution.
Genome Variation among Accessions and Chromosome Evolution
Arabidopsis has been extensively used for comparative evolutionary genomics studies in plants. The 1001 Genomes Project has generated a wealth of sequence information, and numerous SNPs and indels are known to exist in many ecotypes [39]–[41]. The vast majority of this work has employed high throughput sequencing technologies, generating short sequence reads that are mapped onto scaffolds of reference genomes. While this strategy is useful and saturation can be achieved, it has two major disadvantages. First, some syntenic relationships may be masked, as many inversions and translocations cannot be accurately mapped. This is also the case for sequences associated with repetitive DNAs. Second, some copy number variants and their locations may go undetected and unrecognized, depending on the extent of divergence amongst them and their flanking sequences. While paired end mapping can be used to discover and verify structural variation [42], only through the sequencing of contiguous long clones (e.g. BACs) can such sequence anomalies be accurately located and quantified. In Arabidopsis, several such studies have revealed striking departures from the Columbia reference genome. For example, comparative sequence analysis of a region around 100 map units on chromosome 1 in Columbia vs. Ler revealed that the region in Columbia is approximately 135 kbp, while the comparable region in Ler is only 71 kbp [43]. This appears to be due to several gross rearrangement events including two different ecotype specific duplications, a deletion and several ecotype specific transposition events that ultimately led to the shorter Ler region having fewer R genes than its Columbia counterpart [43]. In a similar vein, Lai et al. [44] resequenced a 371 kbp region of chromosome 3 from Columbia and Ler, mapping these reads onto both the Columbia reference genome as well as the more recent Wellcome Trust generated Ler draft genome, and discovered 61 misassemblies and large structural variants not represented by the draft genome. Lastly, the quartet mutation has afforded the possibility of tetrad analysis in Arabidopsis, and Lu and coworkers [45] sequenced the F1 genomes of a Col/Ler cross, reporting numerous known and heretofore unknown SNPs and indels. Analysis of the indels revealed that a deletion of 19.2 kbp encompasses the region of the bp-11 north breakpoint. These high resolution sequencing projects reveal that the genomes of Arabidopsis accessions are highly polymorphic and subject to rapid change as a result of normal meiotic recombination events and other rearrangements that may be due to replication errors and mobile element activity.
While the power and economics of high throughput sequencing technologies cannot be disputed, discovering and mapping large structural variation in comparative genomics studies ultimately will depend upon more painstaking mapping and the generation and analysis of long clones. Fluorescent in situ hybridization or chromosome painting offers a middle ground for analyzing gross chromosomal architecture. Our experiments reveal that the two most commonly used Arabidopsis ecotypes, Columbia and Landsberg erecta, possess at least two major structural differences on chromosome 4. The heterochromatic knob, which can be readily detected by DAPI staining, arose in Columbia via a pericentric inversion, moving a heterochromatic region towards the north end of the chromosome [13], [14]. South of the centromere, there appears to be an indel that generates a significant length difference between the two GREEN probeset signals located at 4.3 Mbp and 6 Mbp of the Columbia reference genome. The precise nature of this polymorphism is unknown, but a comparison of homologous regions in A. lyrata and A. thaliana (Columbia) indicates that on two contigs that span the region, A. lyrata possesses over 600 kbp more DNA than Columbia. It is possible that this region has persisted in the Ler genome, accounting for the longer GREEN1/GREEN2 intervals that we observed. Given that similar polymorphisms are likely to exist on the other chromosomes, which could involve the mobilization of large blocks of sequence to perhaps distant sites (e.g. an inversion involving over 1 Mbp), map-based cloning of some genes could be complicated and require protracted efforts.
Does the Chromatin State Determine the Integrity of the Genome?
Advances in chromatin immunoprecipitation have permitted high throughput approaches for elucidation of the histone codes associated with genome elements. Roudier et al., [19] conducted integrative epigenomic mapping in Arabidopsis, tracking 12 chromatin modifications to define four primary chromatin states associated with different coding and noncoding sequences. As in other organisms, the Arabidopsis pericentromeric DNA is enriched for several histone modifications, in particular methylation at H3K27, H3K9, and at H4K20 residues. In the context of maintaining genome integrity, H4K20 modifications are intriguing. In mammals, the H4K20 methylation state plays important roles in DNA damage repair as well as in class switch recombination (CSR) during the maturation of antibody producing cells [46]. CSR employs a NHEJ mechanism to exchange constant regions of immunoglobulin genes during B cell differentiation [47]. In mouse cells in which H4K20 methyl transferases are silenced, H4K20me1 accumulates, and this is associated with translocations and deletions of the IgH locus [46]. To our knowledge, there are no reports in Arabidopsis on the distribution and role of either di - or trimethylated H4K20, but these modifications might prove to associate with the instability that we observe.
Methods
Biological Materials
The alleles bp-1, bp-2, bp-3, and bp-5 were described by Douglas et al. [8]. bp-11 was obtained through ABRC (CS3161). Table S1 contains information on all characterized bp mutants. Plants were grown in environmental growth chambers with a 16 hr day/8 hr night cycle at 22°C under fluorescent lighting of approximately 100 µE/m2. Bacterial artificial chromosome clones were obtained from ABRC and DNA was prepared by employing Qiagen midi-prep columns.
Molecular Techniques
General molecular techniques were carried out as described by Sambrook et al. [48]. Genomic DNA for PCR was prepared using Sigma GeneElute columns. A list of primers used for determining the presence or absence of a locus, as well as for use in inverse PCR and phase 5 PCR is given in Tables S2, S3, S4. Breakpoint junctions were cloned by employing inverse PCR. Genomic DNA was digested with a restriction enzyme that was shown by DNA gel blotting to give rise to an RFLP. The digested DNA was purified, diluted to approximately 1 µg/ml and subjected to overnight ligation to promote recircularization. Inverse PCR primers were then employed in PCR reactions to generate products containing the breakpoint junctions. These molecules were cloned into pJet1.2 (Fermentas) and sequenced (The Centre for Applied Genomics, Toronto).
For Southwestern analysis, a cDNA encoding the MAR binding protein AHL1 [15] was cloned by conducting RT-PCR on silique RNA, using oligo dT to prime first strand synthesis, and two primers: MAR Forward Nhe: 5′ AGGCTAGCGTCTTAAATATGGAGTCTACC 3′ and MAR Backward Bgl II: 5′ AAAGATCTGATTTCAAGTTACATTGACATTAATATCGG 3′. The underlined sequences represent engineered Nhe I and Bgl II sites that were used to clone the cDNA into the expression vector pRSET B (Invitrogen). Authenticated clones were mobilized into BL21 cells and expression of AHL1 induced by the addition of IPTG to a final concentration of 1 mM. After several hours of growth, induced and uninduced cultures were harvested by centrifugation and resuspended in Laemmli buffer (100 µl per 1 ml of culture), boiled, and stored at −20°C until needed. For Southwestern blotting, aliquots of protein extracts from induced and uninduced cultures were subjected to SDS-PAGE and transferred to nitrocellulose. The membrane was placed in 3% gelatin/TBS and kept at 4°C overnight. Protein refolding and blocking was carried out for 2 hours in a solution of 5% non-fat dry milk (Carnation), 20 mM Tris, pH7.6, 150 mM NaCl, 10 mM MgCl2, 0.25 mM DTT, 0.05% Tween-20, and 10 µg/ml salmon sperm DNA. DNA fragments to be used for probes were generated by PCR (see Table S6 for primer set information) and cloned into pJet1.2 (Fermentas). Probe DNA was prepared by fill-in reactions using alpha 32P-dCTP and the Klenow fragment of DNA polymerase I (Invitogen) on restriction fragments. Binding was performed in the refolding buffer except that the non-fat milk concentration was reduced to 0.5%. The membranes were gently agitated at room temperature for 2 hours in approximately 3 ml of liquid containing the radiolabeled probes, then washed four times in 20 mMTris pH7.5, 200 mM NaCl, 10 mM MgCl2, 0.05% Tween-20, 0.1% Triton ×100, 0.25 mM DTT and 10 µg/ml salmon sperm DNA. Autoradiography was then employed to detect binding. In no case was binding detected using uninduced extracts. Probe labeling was assessed by gel electrophoresis and autoradiography and all probes were deemed to be of comparable specific activity.
Bioinformatics and Data Mining
The BLASTn search tool, the MEME algorithm (National Biomedical Computation Resource website; version 4.9.0; http://meme.sdsc.edu/meme/cgi-bin/meme.cgi; Accessed 2012 Nov 7 [49]) and the RepeatMasker algorithm (Institute for Systems Biology website, open-3.3.0. Available: http://www.repeatmasker.org/cgi-bin/WEBRepeatMasker. Accessed 2012 Nov 7. [50]) were employed to identify common motifs and repetitive elements. Potential secondary structures were determined by employing the Mold algorithm [51]. The EPIGARA database (Arabidopsis epigenetics and epigenomics group website; version 1.69; http://epigara.biologie.ens.fr/index.html. Accessed 2012 Nov 7.) of chromatin modifications was interrogated for locations of modifications and their proximity to the bp breakpoint junctions. Data was extracted from ChIP/chip studies conducted by Roudier et al. [19] and array based profiling of methylated DNA [52]. The pie charts in Figure 6 were generated by taking the IP/input log ratio for each region and dividing this by the sum of all IP/inputs for the region.
Fluorescence In Situ Hybridization (FISH)
Staged floral buds of wildtype Columbia and the Columbia based bp-5 and bp-11 alleles, along with Landsberg erecta (Ler) and the Ler derived bp-1 and bp-2 alleles were used as the starting materials. Pachytene chromosome spreads were prepared and identified according to the method of Stronghill and Hasenkampf [53]. Spreads were then subjected to fluorescence in situ hybridization as described by Lysak et al. [54]. Five chromosome 4 probesets, comprised of bacterial artificial chromosomes (BACs), were used to determine the contour distances between these five sequences, which span chromosome 4 from 0.8 Mbp to 6.4 Mbp (AGI coordinates). Probes were generated by employing a Nick Translation Kit (Roche). Two of the five probesets (north of the centromere) were biotin-labeled BAC clones bracketing the heterochromatic knob region in Columbia: Red 1: BACs T7B11, T2H3 and Red 2: BACs T4B21, T1J1, T32N4. Detection of these signals was facilitated by goat anti-biotin antibodies (Vector Laboratories) and a secondary donkey anti-goat Cy3 conjugated antibody (Jackson Immunoresearch). Three additional probesets were DIG-labeled BAC clones (south of centromere), bracketing the region of the BP locus: Green 1: BACs F28D6, T3E15; Green 2: BACs T15G18, T25P22 and Green 3: T9A4, F24G24. These hybridization signals were detected by employing a mouse anti-DIG primary antibody and a donkey anti-mouse FITC conjugated secondary antibody (Jackson Immunoresearch). Spreads were examined using a Zeiss axiophot epifluorescent microscope and a Plan-Neofluar 100×/1.3NA oil immersion objective lens. Northern Eclipse 5.0 software was used to capture images and measure the distance between FISH signals. Merged images were created using Photoshop CS5 software.
Supporting Information
Zdroje
1. BranzeiD, FoianiM (2010) Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol 11 : 208–219.
2. KantidzeOL, RazinSV (2009) Chromatin loops, illegitimate recombination, and genome evolution. Bioessays 31 : 278–286.
3. CremerT, CremerM (2010) Chromosome Territories. Cold Spring Harb Per 2: a003889.
4. SchubertI, LysakMA (2011) Interpretation of karyotype evolution should consider chromosome structural constraints. Trends Genet 27 : 207–216.
5. SalseJ (2012) In silico archeogenomics unveils modern plant genome organisation, regulation and evolution. Curr Opin Plant Biol 15 : 122–130.
6. HuTT, PattynP, BakkerEG, CaoJ, ChengJ-F, et al. (2011) The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat Genet 43 : 476–481.
7. SchranzME, LysakMA, Mitchell-OldsT (2006) The ABC's of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci 11 : 535–542.
8. DouglasSJ, ChuckG, DenglerRE, PelecandaL, RiggsCD (2002) KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell 14 : 547–558.
9. DouglasSJ, RiggsCD (2005) Pedicel development in Arabidopsis thaliana: contribution of vascular positioning and the role of the BREVIPEDICELLUS and ERECTA genes. Dev Biol 284 : 451–463.
10. KoornneefM, VanedenJ, HanhartCJ, StamP, BraaksmaFJ, et al. (1983) Linkage map of Arabidopsis thaliana. J Hered 74 : 265–272.
11. VenglatSP, DumonceauxT, RozwadowskiK, ParnellL, BabicV, et al. (2002) The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci U S A 99 : 4730–4735.
12. KlimyukVI, JonesJD (1997) AtDMC1, the Arabidopsis homologue of the yeast DMC1 gene: characterization, transposon-induced allelic variation and meiosis-associated expression. Plant J 11 : 1–14.
13. FranszPF, ArmstrongS, de JongJH, ParnellLD, van DrunenC, et al. (2000) Integrated cytogenetic map of chromosome arm 4S of A. thaliana: Structural organization of heterochromatic knob and centromere region. Cell 100 : 367–376.
14. McCombieWR, de la BastideM, HabermannK, ParnellL, DedhiaN, et al. (2000) The complete sequence of a heterochromatic island from a higher eukaryote. Cell 100 : 377–386.
15. FujimotoS, MatsunagaS, YonemuraM, UchiyamaS, AzumaT, et al. (2004) Identification of a novel plant MAR DNA binding protein localized on chromosomal surfaces. Plant Mol Biol 56 : 225–239.
16. vanDrunenCM, OosterlingRW, KeultjesGM, WeisbeekPJ, vanDrielR, et al. (1997) Analysis of the chromatin domain organisation around the plastocyanin gene reveals an MAR-specific sequence element in Arabidopsis thaliana. Nucleic Acids Res 25 : 3904–3911.
17. DrouaudJ, CamilleriC, BourguignonPY, CanaguierA, BerardA, et al. (2006) Variation in crossing-over rates across chromosome 4 of Arabidopsis thaliana reveals the presence of meiotic recombination “hot spots”. Genome Res 16 : 106–114.
18. BruggemannE, HandwergerK, EssexC, StorzG (1996) Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J 10 : 755–760.
19. RoudierF, AhmedI, BerardC, SarazinA, Mary-HuardT, et al. (2011) Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO Journal 30 : 1928–1938.
20. McClintockB (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26 : 234–282.
21. HuangJT, DoonerHK (2008) Macrotransposition and other complex chromosomal restructuring in maize by closely linked transposons in direct orientation. Plant Cell 20 : 2019–2032.
22. SasakiM, LangeJ, KeeneyS (2010) Genome destabilization by homologous recombination in the germ line. Nat Rev Mol Cell Biol 11 : 182–195.
23. ChenJM, CooperDN, FerecC, Kehrer-SawatzkiH, PatrinosGP (2010) Genomic rearrangements in inherited disease and cancer. Semin Cancer Biol 20 : 222–233.
24. HuefnerND, MizunoY, WeilCF, KorfI, BrittAB (2011) Breadth by depth: expanding our understanding of the repair of transposon-induced DNA double strand breaks via deep-sequencing. DNA Repair (Amst) 10 : 1023–1033.
25. AmiardS, CharbonnelC, AllainE, DepeigesA, WhiteCI, et al. (2010) Distinct Roles of the ATR Kinase and the Mre11-Rad50-Nbs1 Complex in the Maintenance of Chromosomal Stability in Arabidopsis. Plant Cell 22 : 3020–3033.
26. DurkinSG, GloverTW (2007) Chromosome fragile sites. Annu Rev Genet 41 : 169–192.
27. BignellGR, GreenmanCD, DaviesH, ButlerAP, EdkinsA, et al. (2010) Signatures of mutation and selection in the cancer genome. Nature 463 : 893–898.
28. Ruiz-HerreraA, CastresanaJ, RobinsonTJ (2006) Is mammalian chromosomal evolution driven by regions of genome fragility? Genome Biol 7: R115.
29. BruecknerLM, SagulenkoE, HessEM, ZhegloD, BlumrichA, et al. (2012) Genomic rearrangements at the FRA2H common fragile site frequently involve non-homologous recombination events across LTR and L1 (LINE) repeats. Hum Genet 131 : 1345–1359.
30. MitsuiJ, TakahashiY, GotoJ, TomiyamaH, IshikawaS, et al. (2010) Mechanisms of genomic instabilities underlying two common fragile site associated loci, PARK2 and DMD, in germ cell and cancer cell lines. Am J Hum Genet 87 : 75–89.
31. JacksonJA, TrevinoAV, HerzigMC, HermanTS, WoynarowskiJM (2003) Matrix attachment region (MAR) properties and abnormal expansion of AT island minisatellites in FRA16B fragile sites in leukemic CEM cells. Nuc Acids Res 31 : 6354–6364.
32. ArltMF, GloverTW (2010) Inhibition of topoisomerase prevents chromosome breakage at common fragile sites. DNA Repair 9 : 678–689.
33. El AchkarE, Gerbault-SeureauM, MulerisM, DutrillauxB, DebatisseM (2005) Premature condensation induces breaks at the interface of early and late replicating chromosome bands bearing common fragile sites. Proc Natl Acad Sci U S A 102 : 18069–18074.
34. Le TallecB, DutrillauxB, LachagesAM, MillotGA, BrisonO, et al. (2011) Molecular profiling of common fragile sites in human fibroblasts. Nat Struct Mol Biol 18 : 1421–1423.
35. LetessierA, MillotGA, KoundrioukoffS, LachagesAM, VogtN, et al. (2011) Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature 470 : 120–124.
36. BurrowAA, MarulloA, HolderLR, WangY-H (2010) Secondary structure formation and DNA instability at fragile site FRA16B. Nuc Acids Res 38 : 2865–2877.
37. LeeT-J, PascuzziPE, SettlageSB, ShultzRW, TanurdzicM, et al. (2010) Arabidopsis thaliana Chromosome 4 Replicates in Two Phases That Correlate with Chromatin State. PLoS Genet 6: e1000982 doi:10.1371/journal.pgen.1000982.
38. CostasC, SanchezMD, StroudH, YuY, OliverosJC, et al. (2011) Genome-wide mapping of Arabidopsis thaliana origins of DNA replication and their associated epigenetic marks. Nat Struct Mol Biol 18 : 395–400.
39. CaoJ, SchneebergerK, OssowskiS, GuntherT, BenderS, et al. (2011) Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43 : 956–U960.
40. GanXC, StegleO, BehrJ, SteffenJG, DreweP, et al. (2011) Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477 : 419–423.
41. WeigelD (2012) Natural Variation in Arabidopsis: From Molecular Genetics to Ecological Genomics. Plant Physiol 158 : 2–22.
42. KorbelJO, UrbanAE, AffourtitJP, GodwinB, GrubertF, et al. (2007) Paired-End Mapping Reveals Extensive Structural Variation in the Human Genome. Science 318 : 420–426.
43. KatoA, KatoH, ShidaT, SaitoT, KomedaY (2009) Evolutionary Process of the Genomic Sequence Around the 100 Map Unit of Chromosome 1 in Arabidopsis thaliana. J Plant Biol 52 : 616–624.
44. LaiAG, Denton-GilesM, Mueller-RoeberB, SchippersJHM, DijkwelPP (2011) Positional Information Resolves Structural Variations and Uncovers an Evolutionarily Divergent Genetic Locus in Accessions of Arabidopsis thaliana. Genome Biol Evol 3 : 627–640.
45. LuPL, HanXW, QiJ, YangJG, WijeratneAJ, et al. (2012) Analysis of Arabidopsis genome-wide variations before and after meiosis and meiotic recombination by resequencing Landsberg erecta and all four products of a single meiosis. Genome Res 22 : 508–518.
46. SchottaG, SenguptaR, KubicekS, MalinS, KauerM, et al. (2008) A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Gene Dev 22 : 2048–2061.
47. KotnisA, DuL, LiuC, PopovSW, Pan-HammarstromQ (2009) Non-homologous end joining in class switch recombination: the beginning of the end. Philos Trans R Soc Lond B Biol Sci 364 : 653–665.
48. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, NY.
49. BaileyTL, BodenM, BuskeFA, FrithM, GrantCE, et al. (2009) MEME Suite: tools for motif discovery and searching. Nucleic Acids Res 37: W202–W208.
50. Smit AFA, Hubley R, Green P (1996–2010) RepeatMasker Open-3.0. http://repeatmaskerorg.
51. ZukerM (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31 : 3406–3415.
52. VaughnMW, TanurdzicM, LippmanZ, JiangH, CarrasquilloR, et al. (2007) Epigenetic natural variation in Arabidopsis thaliana. PLoS Biol 5: e174 doi:10.1371/journal.pbio.0050174.
53. StronghillPE, HasenkampfCA (2007) Analysis of substage associations in prophase I of meiosis in floral buds of wild-type Arabidopsis thaliana (Brassicaceae). Am J Bot 94 : 2063–2067.
54. LysakM, FranszP, SchubertI (2006) Cytogenetic analyses of Arabidopsis. Methods Mol Biol 323 : 173–186.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



